Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
Abstract
The synthesis of rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide, cleverly adorned on a poly(1-H pyrrole) (P1HP) matrix (MoS3–MoO3/P1HP), is achieved through a one-pot preparation method. This process occurs under the pyrrole oxidation employing the oxidizing agent Na2MoO4. Notably, this oxidation process facilitates the direct incorporation of the inorganic constituents into the polymer matrix. Of particular significance is the material’s bandgap, which is optimally situated at 1.4 eV, rendering it highly suitable for its intended applications. The material assumes a rod-like structure, characterized by an average length of 400 nm and width of 30 nm, further enhancing its desirability. In practice, this thin film serves as an exceptionally promising photoelectrode. It finds its forte in the generation of hydrogen from sewage water, achieving an impressive efficiency rate of 12.66%, specifically at 340 nm. In addition to that, it boasts a remarkable hydrogen generation rate of 1.2 moles·h−1·cm−2. Moreover, the material exhibits remarkable versatility in its response to light. Its sensitivity to monochromatic light across a broad optical spectrum (UV till IR), underscores its potential for hydrogen generation applications for industrial applications.
1 Introduction
Conductive polymers represent a captivating and innovative category of electronic materials [1,2]. The allure of conductive polymers lies in their unique amalgamation of metallic and polymeric properties, rendering them exceptionally versatile and adaptable for a myriad of applications. Among the plethora of conductive polymers, poly(1-H pyrrole) (P1HP) stands out as an exceptionally compelling choice. Its allure is underscored by a multitude of research publications delving into its electrical properties and the broad spectrum of potential applications it offers. With a conductivity range of about 1.14 S·cm−1, P1HP has earned its place as a frontrunner in the realm of conductive polymers [3]. However, its appeal extends far beyond its electrical prowess; this remarkable material boasts a repertoire of exceptional physicochemical properties that render it a promising candidate for intelligent and adaptive materials.
Capitalizing on its exceptional electrical properties, P1HP extends its influence into the vast domain of the ultraviolet (UV) spectrum. This extension is closely linked to the intriguing phenomenon of electron transitions from the highest unoccupied molecular orbital (HOMO) to lowest unoccupied molecular orbital (LUMO) states, giving rise to optical behaviors that span the UV region [4]. Researchers have diligently pursued avenues to augment the optical characteristics of P1HP, channeling their efforts in two primary directions: Morphological Mastery: The first avenue to enhance the optical performance of P1HP revolves around the meticulous control of its morphological features. Researchers employ strategies to craft this polymer into structures that exhibit exceptional optical properties within the UV range [4]. These endeavors encompass the fabrication of P1HP with distinct morphologies that can efficiently capture and interact with UV light. In addition to the synergetic Composites: The second, equally compelling route involves the integration of P1HP with highly optically active inorganic materials. By forming composite materials that combine the unique attributes of P1HP with the optical prowess of inorganic components like NiO, CuO, MnO, MnS, or PbS [5,6,7,8], researchers create materials that open up a vast panorama of opportunities in the realms of optoelectronics and photocatalysis. These composite materials emerge as stellar candidates for a diverse array of optoelectronic and photocatalytic applications, representing a fusion of organic and inorganic worlds to deliver enhanced optical performance and functionality. This synergy not only augments the UV optical behavior but also extends the application spectrum of these materials into hitherto uncharted territories [9,10,11,12,13]. The catalytic behavior of the nanocomposite facilitates electron transitions between the composite materials, following a distinctive Z- or S-shaped trajectory as the electron undergoes its transition pathway. Subsequently, the electrons or holes act as effective carriers, instigating supplementary reactions, which may encompass the splitting of water molecules [14,15].
In the realm of renewable energy, with a specific focus on hydrogen generation, P1HP material has found its niche as a photocatalytic substance. However, an inherent limitation of this material lies in the relatively modest quantities of hydrogen gas it can generate, as indicated by the corresponding current density. While P1HP exhibits promising photocatalytic properties for the conversion of water to H2, the volume of hydrogen gas produced is constrained by the current density it can generate. This limitation is a critical consideration in the practical application of P1HP-based photocatalysis for hydrogen production. Efforts are underway to optimize and enhance the performance of P1HP photocatalysts, with a particular focus on increasing the current density to augment hydrogen gas yields. Researchers are exploring various strategies, such as modifying the material’s composition, improving its morphological characteristics, or integrating it with complementary materials to boost its photocatalytic efficiency [16,17]. Overcoming this limitation is pivotal for realizing the full potential of P1HP as a photocatalytic material for green H2. As advancements continue in the field of renewable energy, addressing this constraint will be a key step in harnessing P1HP’s capabilities to contribute significantly to the sustainable production of hydrogen, a vital component in the transition to a cleaner and greener energy landscape [18,19].
In this study, a groundbreaking composite material known as MoS3–MoO3/P1HP rods takes the spotlight as an exceptionally promising nanocomposite photoelectrode. This innovative material exhibits remarkable potential for the efficient generation of green hydrogen, and it is put to the test in the context of sewage water treatment. The investigation leaves no stone unturned as it delves into a comprehensive array of analyses. These encompass optical assessments, morphological examinations, elemental characterizations, and crystalline structure evaluations.
The study scrutinizes the photoelectrode’s performance under varying conditions, including complete darkness, exposure to white light, and the utilization of monochromatic wavelengths for the hydrogen generation reaction. Furthermore, the investigation extends its scope to calculate two crucial parameters. The incident photon to current efficiency (IPCE) that is evaluated to gauge the photoelectrode’s ability to convert incident photons into electrical current. Additionally, the quantity of hydrogen gas produced is meticulously quantified in terms of moles. This comprehensive exploration of the MoS3–MoO3/P1HP nanocomposite photoelectrode opens up exciting prospects for sustainable hydrogen production from sewage water. By shedding light on the material’s behavior in different light conditions and quantifying its performance through IPCE and hydrogen production measurements, the study paves the way for innovative solutions in wastewater treatment and renewable energy generation.
2 Experimental part
2.1 Materials
Pyrrole and hydrochloric acid (HCl) were acquired from the reputable supplier Merck Co. located in Germany. Meanwhile, dimethyl formamide, an essential component in the process, was provided by Sigma Aldrich Co., a trusted source based in the United States. The procurement extended globally, as sodium molybdate (Na2MoO4) was imported from the United Kingdom through Winlab Co. Lastly, K2S2O8, a critical reagent for the experiment, was sourced from Pio-Chem Co., an established supplier situated in Egypt.
2.2 MoS3–MoO3/P1HP thin film nanocomposite synthesis
The synthesis of the MoS3–MoO3/P1HP thin film nanocomposite involves a polymerization process initiated by the action of Na2MoO4 and K2S2O8 on a 0.06 M solution of 1-H pyrrole monomer. Both these oxidants collectively have a concentration of 0.15 M. To facilitate this polymerization, the monomer is thoroughly dissolved in a 0.5 M solution of HCl. During the polymerization process, the oxidants are rapidly introduced to initiate oxidation. It is at this stage that the MoS3–MoO3 inorganic filler is incorporated into the growing polymer network. This results in the formation of the MoS3–MoO3/P1HP thin film.
Conversely, in the absence of the Na2MoO4 oxidant, a separate process occurs for P1HP thin film formation. In this scenario, the polymerization process proceeds, but the MoS3–MoO3 inorganic filler is not introduced into the polymer network.
2.3 Photoelectrochemical reaction of MoS3–MoO3/P1HP thin film photocathode for hydrogen generation
The photoelectrochemical reaction of the MoS3–MoO3/P1HP thin film photocathode, aimed at hydrogen generation, is conducted by employing this photocathode as the main electrode. In this configuration, another electrode is introduced, specifically a graphite electrode, serving as the auxiliary electrode. Additionally, a calomel electrode is utilized as the reference electrode. This electrochemical cell is connected to the CHI608E workstation for the precise measurement and monitoring of the electrochemical reactions.
The electrolyte used in this setup consists of sewage water, which has undergone a thorough treatment process within the Beni-Suef city company responsible for drinking and salination. This treated water exhibits a pH level of 7.2. It is important to note that this water has undergone rigorous purification and treatment procedures.
The experiments are conducted under a metal halide lamp. To precisely control the wavelengths of light, optical filters are employed. This enables the systematic study of the photocathode’s performance under different lighting conditions. The configuration of the cell used in these experiments is visually represented in Figure 1.
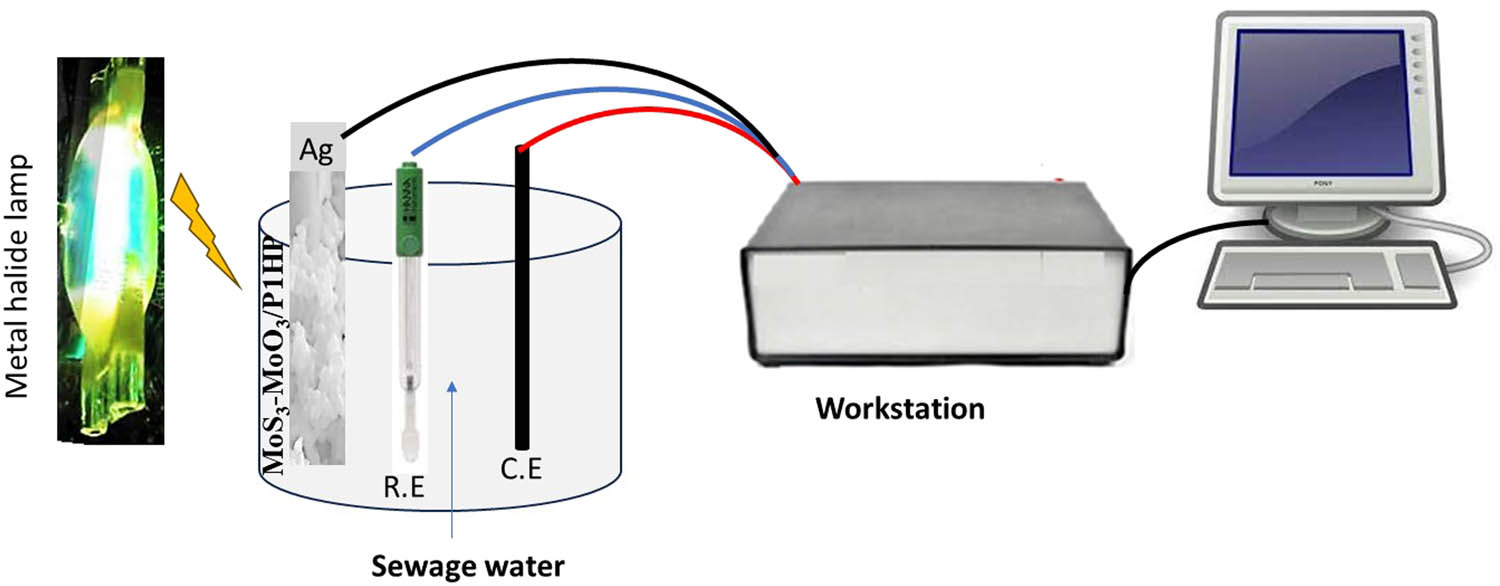
Three-electrode cell diagram used for the green hydrogen generation using the MoS3–MoO3/P1HP thin film photocathode for green H2 production.
3 Results and discussion
3.1 Analysis processes
Figure 2(a) provides the FTIR transmittance spectra for both P1HP and MoS3–MoO3/P1HP, offering insights into their molecular compositions and characteristic peaks. Let us examine the primary peaks and their attributions in these spectra:
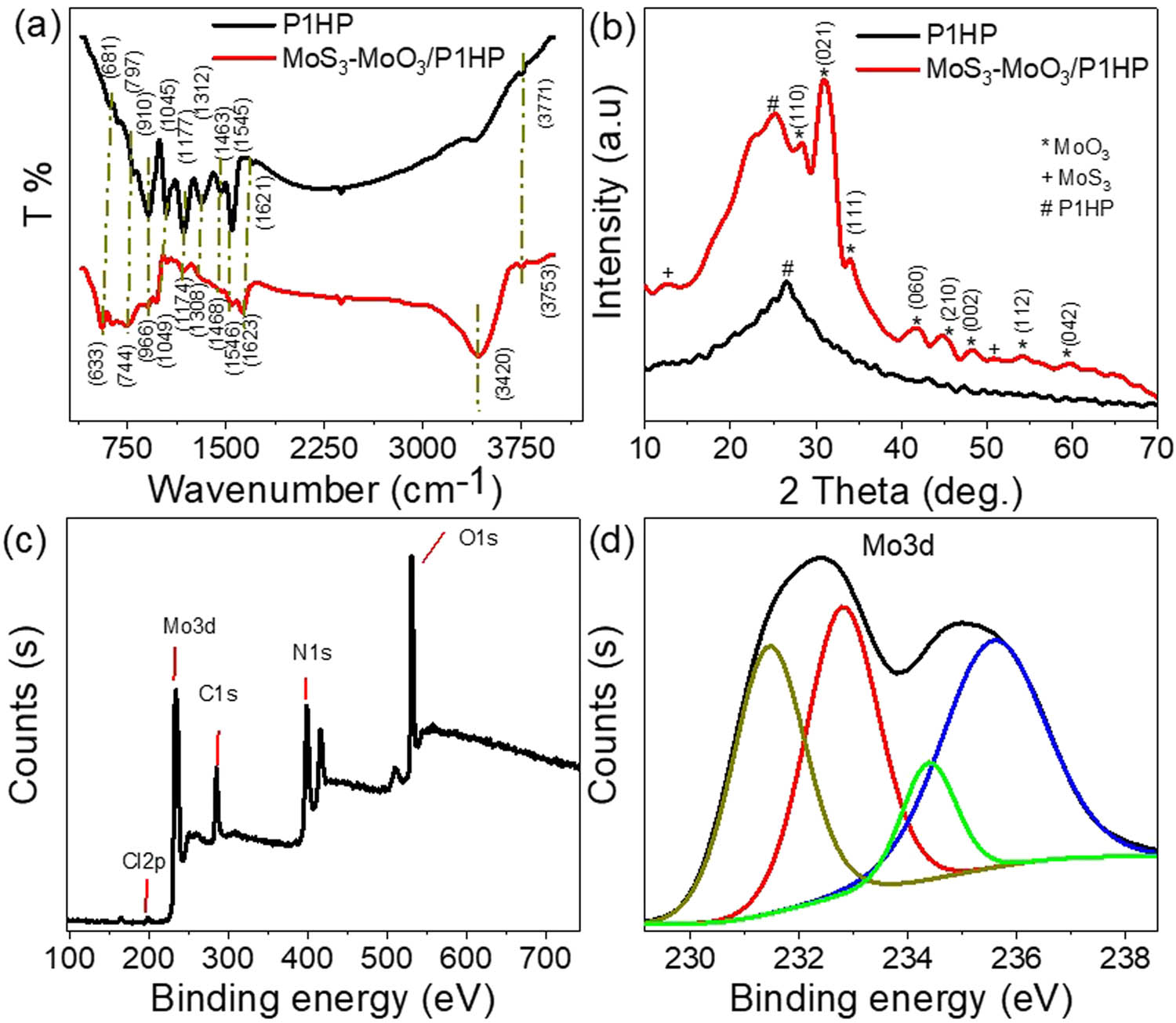
Chemical structure through vibration bonds, crystalline behavior, and elemental structure of the fabricated MoS3–MoO3/P1HP nanocomposite: (a) FTIR, (b) XRD, and (c and d) XPS analyses.
In the spectrum of pyrrole (P1HP), absorptions are observed at 681 and 797 cm−1 related to the pare substituted P1HP ring. Prominent absorption peaks centered at 1,045 and 1,177 cm−1 are for the C–H bonds [20]. The dominant absorption peak is located around 3,420 cm−1 for the N–H bonds present in the pyrrole amines [21]. Peaks centered at 1,621 cm−1 are for C═C bonds within the molecular structure. A peak at 1,545 cm−1 can be assigned to the C═C stretching vibrations originating from the pyrrole rings. The peak appearing at 1,463 cm−1 is linked to the conjugated pyrrole C–N. An intermediate-intensity band at 1,312 cm−1 is for the valence vibrations of the pyrrole C–N bond [22]. Overall, the observed FTIR spectrum is in excellent agreement with the proposed molecular structure of pyrrole. These characteristic peaks and their assignments confirm the presence of key functional groups and molecular bonds within the P1HP molecule. Furthermore, the FTIR spectrum of MoS3–MoO3/P1HP emerges as a valuable instrument for evaluating the structural modifications and interactions occurring in the course of nanocomposite formation with additional shifts compared to the pristine P1HP polymer related to the interaction of the inorganic components with the pristine polymer during the polymer formation [23]. This analytical tool aids in clarifying the impact of introducing MoS3–MoO3 on the vibrational properties of the polymer, as evidenced by enhanced peaks at 681 and 797 cm−1 in the composite. These peaks signify the vibrational characteristics associated with Mo–O and Mo–S, shedding light on the alterations induced by the inclusion of MoS3–MoO3 in the composite material.
The crystalline characteristics of the MoS3–MoO3/P1HP nanocomposite are demonstrated through the XRD pattern presented in Figure 2(b). This figure provides great evidence of the nanocomposite’s crystalline nature, as evidenced by the discernible peaks associated with MoO3. These peaks at the specific angles: 28.4°, 31.0°, 34.0°, 41.9°, 44.8°, 48.3°, 54.3°, and 59.7°. These angles correspond to the Miller indices of (110), (012), (111), (060), (210), (002), and (042), respectively, as per JCPDS-05-0508 [24]. These peaks are indicative of the crystalline structure of MoO3 within the composite.
However, it is noteworthy that both P1HP and MoS3 exhibit an amorphous nature in this composite. This amorphous behavior is consistent with the known properties of these materials, which have been documented in previous literature. For instance, the broad peaks observed at 12.66° and 51.0° for MoS3 substantiate its amorphous character with its sulfur constituents [25,26]. Similarly, the XRD curve for P1HP exhibits broad bands, reinforcing the amorphous nature associated with this polymer material with limited shifts in these broad peaks after the composite formation, related to the behavior of the inorganic materials on the chain network of the pristine P1HP [27]. To determine the crystalline size (D), the Scherrer equation (Eq. 1) is employed [28], utilizing the full-width at half maximum (W) of the high-intensity peak observed at 2ϴ 31.0°, concerning the wavelength of the incidence XRD (λ). The calculation yields a crystalline size value of 65 nm.
For a comprehensive understanding of the fabricated MoS3–MoO3/P1HP nanocomposite’s chemical composition, elemental constituents, and oxidation states, XPS analyses were conducted, and the results are shown in Figure 2(c) and (d). Figure 2(c) provides valuable insights into the chemical structure of P1HP. This is evident from the evaluation of the binding energies associated with C and N elements. The binding energies for C and N are identified at 285.4 and 400.3 eV, respectively. Furthermore, the presence of chloride ions (Cl) is detected at 200.4 eV. This presence can be related to the use of hydrochloric acid as both the acid medium and solvent for the monomer during the synthesis process. The presence of Cl− ions is particularly noteworthy for boosting the conductivity of the entire composite due to their favorable interaction with the polymer’s nitrogen atoms through Van der Waals forces. Figure 2(d) delves into the characterization of MoS3 and MoO3 within the nanocomposite. The binding energies associated with the Mo3d orbital are used to confirm the presence of these compounds. Specifically, Mo3d5/2 and Mo3d3/2 binding energies are at 231.4 and 234.3 eV, respectively, which aligns with the characteristics of MoS3 [29]. Meanwhile, for MoO3, the Mo3d5/2 and Mo3d3/2 binding energies are at 232.8 and 235.6 eV, respectively [30].
The surface topography and morphological characteristics of both the synthesized P1HP and the MoS3–MoO3/P1HP nanocomposite are vividly portrayed through SEM images, as depicted in Figure 3(a) and (b), respectively. Let us delve into the observations and insights provided by these images.
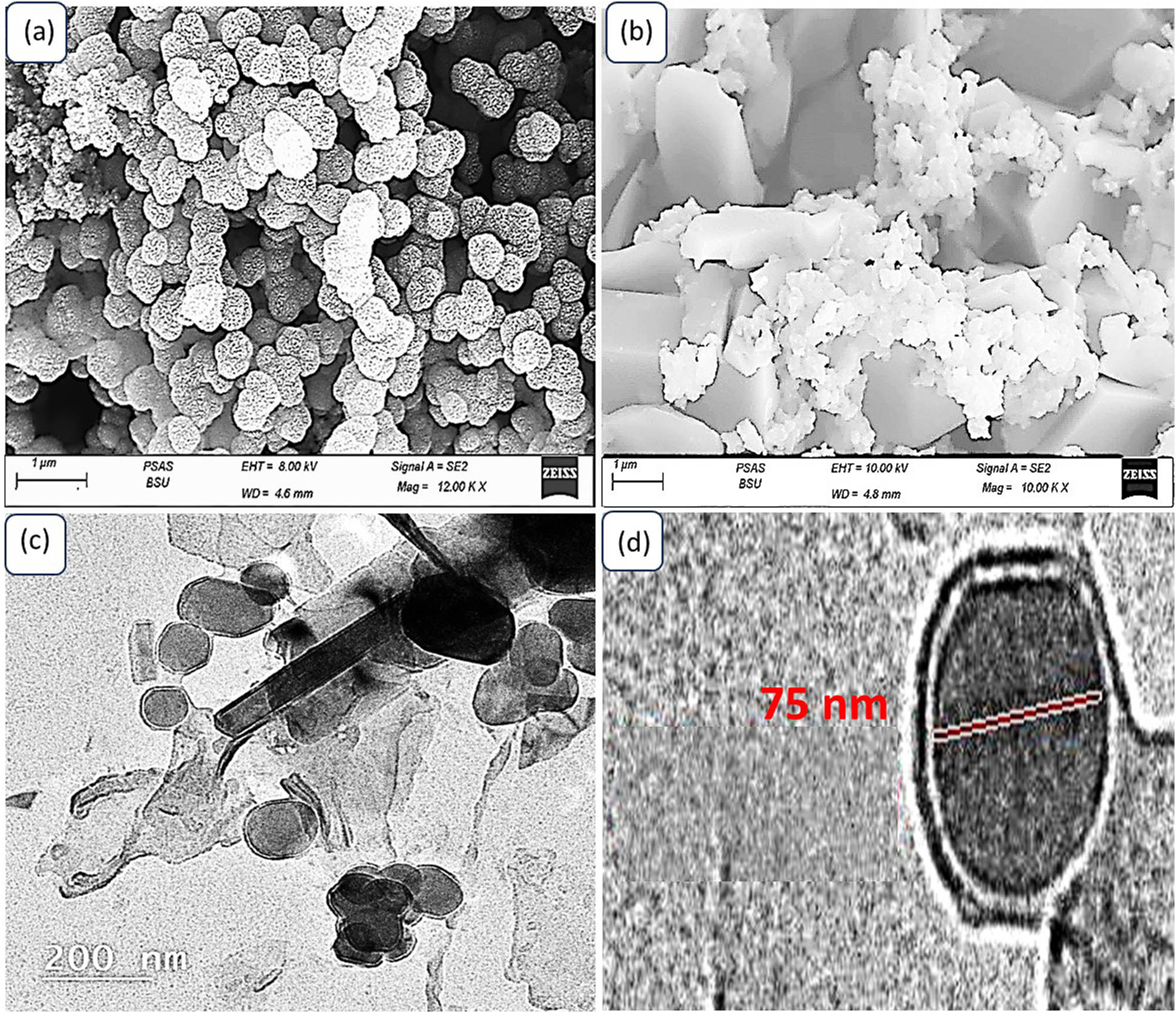
Morphology and topography of the synthesized nanomaterials: (a) SEM of P1HP, (b) SEM of MoS3–MoO3/P1HP, and (c and d) TEM of this nanocomposite.
In Figure 3(a), the SEM image of P1HP reveals the presence of uniform semi-spherical particles with approximately 250 nm. These particles exhibit a porous nature, and there is a notable degree of porosity between them. This porous structure is particularly advantageous for facilitating the formation of the nanocomposite [31,32].
Upon the formation of the MoS3–MoO3/P1HP nanocomposite, as illustrated in Figure 3(b), a substantial alteration in morphology becomes evident. The composite material displays a distinctive transformation, with the emergence of rod-like particles. This transformation in morphology can be attributed to the interaction between the inorganic components, MoS3–MoO3, and the organic component, P1HP, during the polymerization process. This interaction leads to the creation of a novel nanocomposite structure characterized by a distinct diameter [33].
Figure 3(c) offers a closer look at the nanocomposite’s morphology through a transmission electron microscopy (TEM) image. In this image, the nanocomposite is composed of rod-shaped particles with an average length of approximately 400 nm and a width of about 30 nm. These nanorods exhibit an intriguing arrangement adorned with additional semi-spherical particles – these semi-spherical particles are of 75 nm. To gain a more detailed view of these semi-spherical particles, Figure 3(d) provides an enlarged perspective. This image clearly reveals that the semi-spherical particles are not isolated; rather, they are enveloped by an additional thin film. This structural complexity further underscores the intricate and finely tuned composition of the MoS3–MoO3/P1HP nanocomposite.
So, the SEM and TEM images presented in Figure 3(a)–(d) offer valuable insights into the topography and morphological evolution of both P1HP and the MoS3–MoO3/P1HP nanocomposite.
In Figure 4(a), the absorbance of pristine P1HP and the MoS3–MoO3/P1HP nanocomposite are presented, offering critical insights into the materials’ optical properties. The P1HP displays two distinct peaks at approximately 295 and 480 nm. These peaks correspond to ᴨ–ᴨ* transitions, aligning with previous findings [34,35,36]. The absorbance spectrum of the MoS3–MoO3/P1HP nanocomposite, on the contrary, exhibits significant changes. There is a pronounced increase in absorbance intensity, with shifts in the second peak position from 480 to 740 nm, suggesting the successful integration of MoS3–MoO3 nanorods into the P1HP matrix.
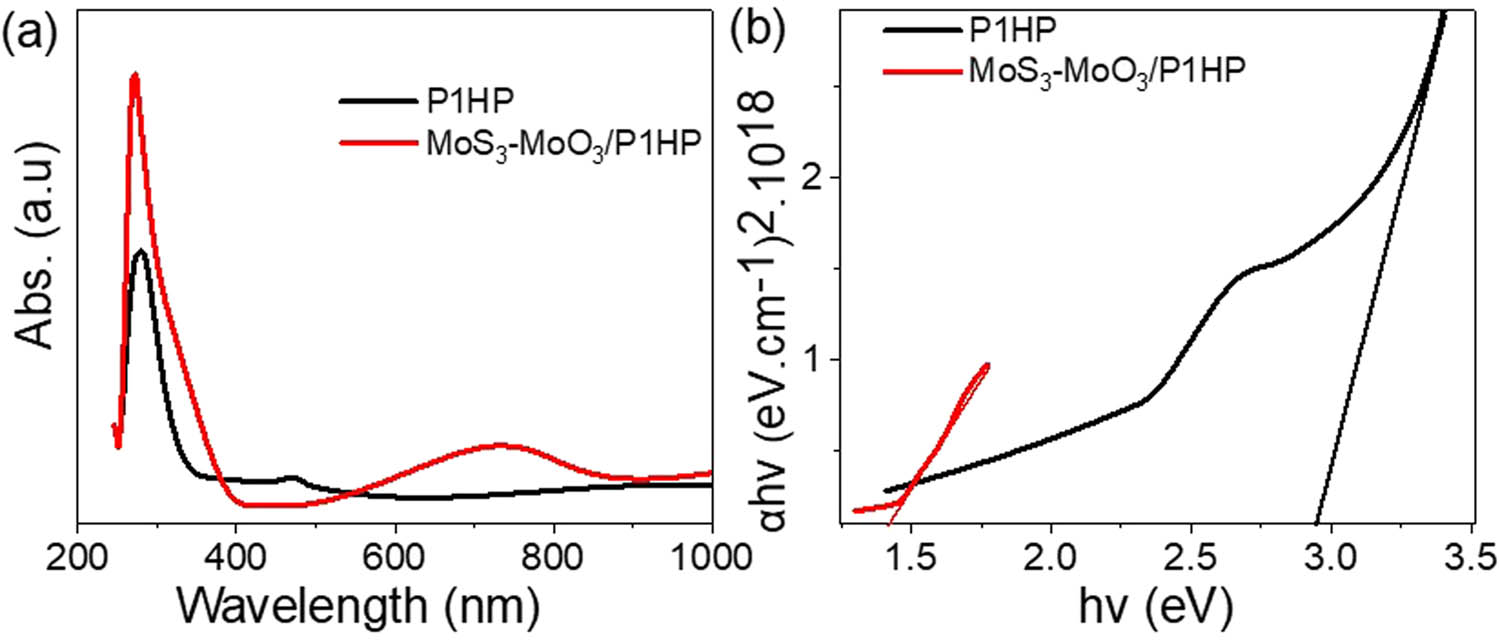
Optical analyses of the P1HP and MoS3–MoO3/P1HP nanocomposite: (a) absorbance and (b) bandgap.
In Figure 4(b), the bandgap of the synthesized materials is addressed. The bandgap calculation, performed using Tauc’s equation [37], yields an E g value of 2.9 eV for P1HP. However, upon the incorporation of MoS3–MoO3 rods, a significant decrease in bandgap to 1.4 eV is observed; this behavior is due to nanorod incorporation [34,38].
3.2 Photoelectrochemical testing of the MoS3–MoO3/P1HP nanocomposite photocathode for green hydrogen generation
The photoelectrochemical assessment of the MoS3–MoO3/P1HP nanocomposite photocathode for the production of green hydrogen is performed using sewage water with a chemical composition outlined in Table 1. This specific type of sewage water serves as an electrolyte, eliminating the need for any additional synthetic electrolytes. In this unique process, the study capitalizes on the contaminants present in the sewage water as a source of renewable energy. This innovative approach represents an environmentally friendly and sustainable means of harnessing energy from a previously underutilized resource.
Compositions of the used wastewater for green hydrogen production
| Material/element | Conc. (mg·L−1) |
|---|---|
| Fe3+ | 1.5 |
| Pb2+ | 0.5 |
| Phenols | 0.015 |
| Ba3+ | 2.0 |
| F− | 1.0 |
| Cr3+ | 1.0 |
| NH3 | 5.0 |
| Hg2+ | 0.005 |
| Cu2+ | 1.5 |
| Cd3+ | 0.05 |
| As3+ | 0.05 |
| Al3+ | 3.0 |
| CN−1 | 0.1 |
| Ni3+ | 0.1 |
| Zn2+ | 5.0 |
| Mn2+ | 1.0 |
| Co2+ | 2.0 |
| Ag+ | 0.1 |
| Pesticides | 0.2 |
| Industrial washing | 0.5 |
| Other cations | 0.1 |
| Coli groups | 4,000/100 cm3 |
Furthermore, the utilization of sewage water in this context presents a highly valuable and abundant resource. Sewage water is both readily available and abundant in virtually every corner of the world. Its ubiquity makes it an incredibly valuable asset for this energy conversion process. By tapping into this resource, the study not only addresses the challenge of wastewater management but also transforms a traditionally discarded and problematic substance into a valuable energy source.
In essence, the photoelectrochemical testing of the MoS3–MoO3/P1HP nanocomposite photocathode in sewage water, as described, not only demonstrates the potential for green hydrogen generation but also showcases a sustainable approach to addressing wastewater challenges. It exemplifies an ingenious way to repurpose and extract value from a resource that is both readily available and abundant, offering significant promise for a more sustainable and energy-efficient future.
Figure 5(a) depicts the current–voltage relationship observed for the MoS3–MoO3/P1HP nanocomposite photocathode under two distinct conditions: darkness and white light illumination. The graph clearly illustrates discernible current values for the photocathode, denoted as the current density during light exposure (J ph) and in the absence of light (J o), highlighting the differences between these contrasting conditions. Specifically, the photocathode yields J ph and J o values of 4.6 and 1.4 mA·cm−2, respectively, when exposed to white light, whereas these values decrease significantly in the absence of light at −0.85 V.
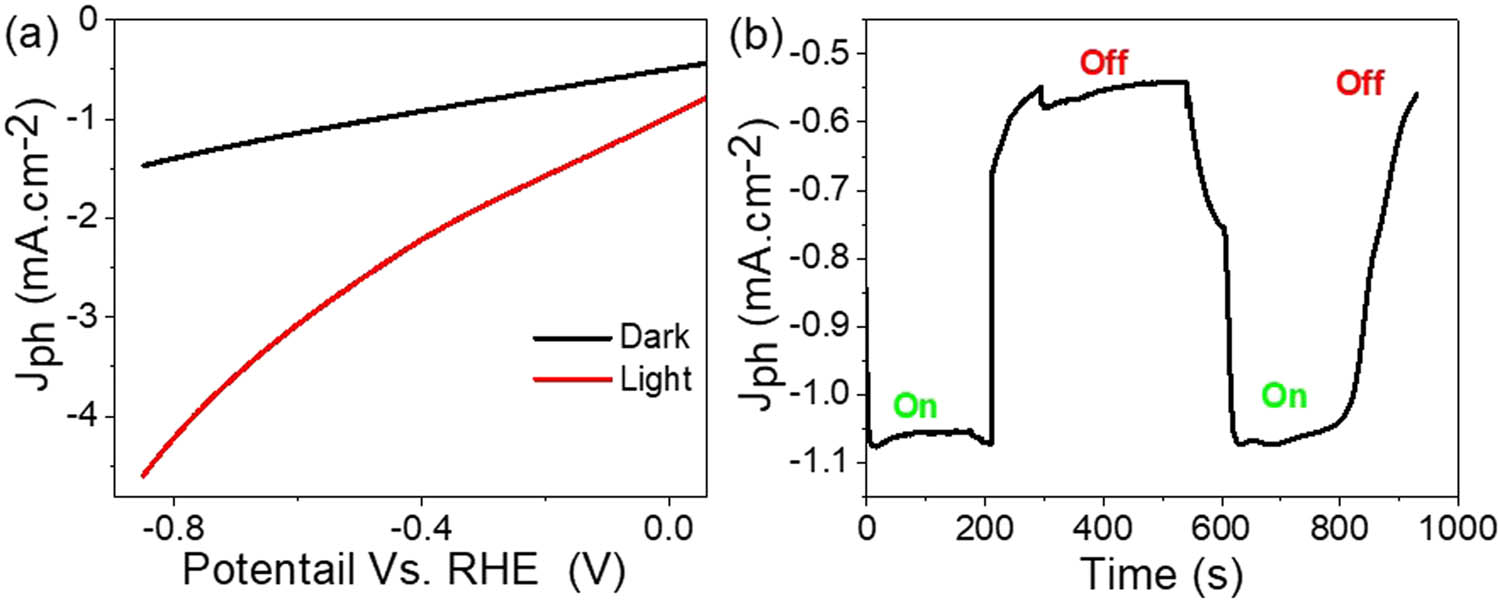
Photoelectrochemical response of the MoS3–MoO3/P1HP nanocomposite photocathode (a) under darkness and light and (b) chopped on/off the light.
Figure 5(b) effectively illustrates this behavior by showcasing the impact of alternating light conditions, denoted as chopping on/off light. During this process, the produced current demonstrates a direct correlation with the presence or absence of incident light. This correlation is exemplified by the J ph values, which exhibit substantial changes as the light source is toggled on and off. Notably, the J ph values experience a notable sequential decrease, shifting from −1.07 mA·cm−2 when the light is on to −0.57 mA·cm−2 when the light is off. This pronounced alteration highlights two critical aspects of the synthesized MoS3–MoO3/P1HP nanocomposite photocathode.
First, it underscores the exceptional stability exhibited by the photocathode, as reflected in the consistent current levels observed in both light and dark conditions. This stability speaks to the robust performance and reliability of the photocathode under varying environmental conditions. Second, it emphasizes the photocathode’s remarkable sensitivity to incident photons and its capacity to respond dynamically to changes in light exposure. The increase in J ph under light clearly illustrates this heightened sensitivity. This responsiveness is attributed to the unique light-absorbing properties of the constituent materials, namely MoS3, MoO3, and P1HP. These materials exhibit diverse absorbance behaviors across different optical regions, effectively capturing photons with varying energies.
The sensitivity of the synthesized MoS3–MoO3/P1HP nanocomposite photocathode to incident photons is effectively assessed by examining the J ph values [13,39] when subjected to monochromatic light, as depicted in Figure 6(a). Additionally, Figure 6(b) displays the resulting J ph values obtained under these monochromatic light conditions. A notable observation is that the produced J ph values exhibit a strong dependency on the frequency or wavelength of the incident light. The optimal J ph value is achieved at a wavelength of 340 nm, where it reaches −4.2 mA·cm−2. Subsequently, it is evident that 730 nm light has a more significant effect on the photocathode for hydrogen generation compared to 540 nm light, with J ph values of −3.5 and −2.2 mA·cm−2, respectively. This behavior confirms the optical absorbance curve (Figure 4(a)), through which the wavelength at 730 nm has a much higher absorbance than 540 nm. These correlations are depicted in Figure 5(b) through the wavelength-produced current relationship at −0.85 V.
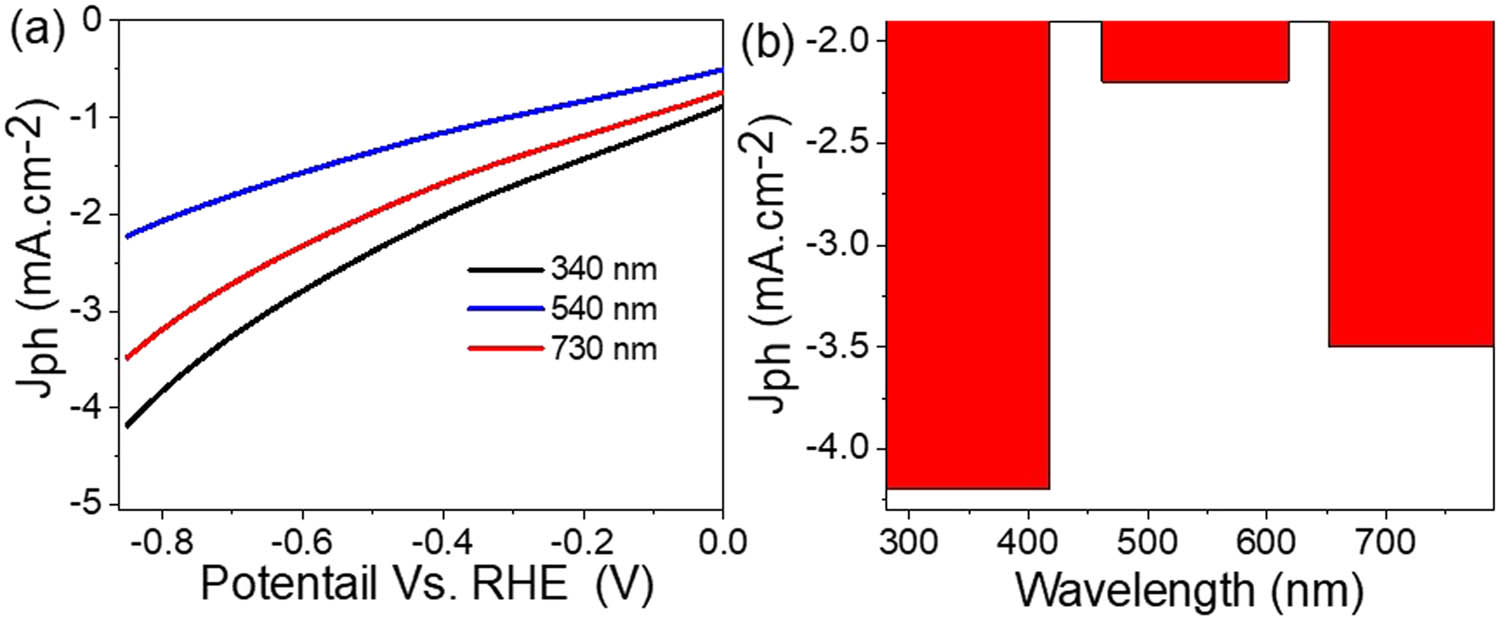
(a) Responsivity of the MoS3–MoO3/P1HP photocathode to different monochromatic light and (b) the estimated J ph value at −0.85 V.
These data clearly illustrate that the prepared photocathode possesses a remarkable efficiency for generating hydrogen from sewage water across a wide range of optical regions, extending from the UV to the infrared (IR) regions. This exceptional performance can be attributed to the diverse compositions of the synthesized photocathode, encompassing MoS3, MoO3, and P1HP. These materials have been skillfully combined within the nanocomposite to facilitate light absorption and uniformly respond to photons spanning various optical regions.
By equation, E = hv, it is crucial to note that the energy of incident light at 340, 540, and 730 nm amounts to 3.6, 2.3, and 1.6 eV, respectively. Importantly, all of these energy levels surpass the nanocomposite’s bandgap value of 1.4 eV. Moreover, all incident photons possess adequate energy to stimulate hot electrons and propel them into the conducting band. These electrons finally stay in the aqueous wastewater, where they facilitate the hydrogen generation reaction, ultimately contributing to the production of J ph.
The IPCE generated by the synthesized MoS3–MoO3/P1HP nanocomposite photocathode is meticulously calculated across a range of wavelengths, as illustrated in Figure 7(a) using Eq. 2 [40]. This analysis reveals that the MoS3–MoO3/P1HP nanocomposite photocathode attains its peak IPCE at 340 nm, achieving an impressive efficiency of 12.66%. This efficiency is noteworthy for two key reasons: First, the hydrogen production process is conducted using sewage water as the source material. This approach effectively converts contaminants present in sewage water into a valuable source of renewable energy. The ability to harness energy from such a waste product underscores the environmental and sustainability benefits of this technology.
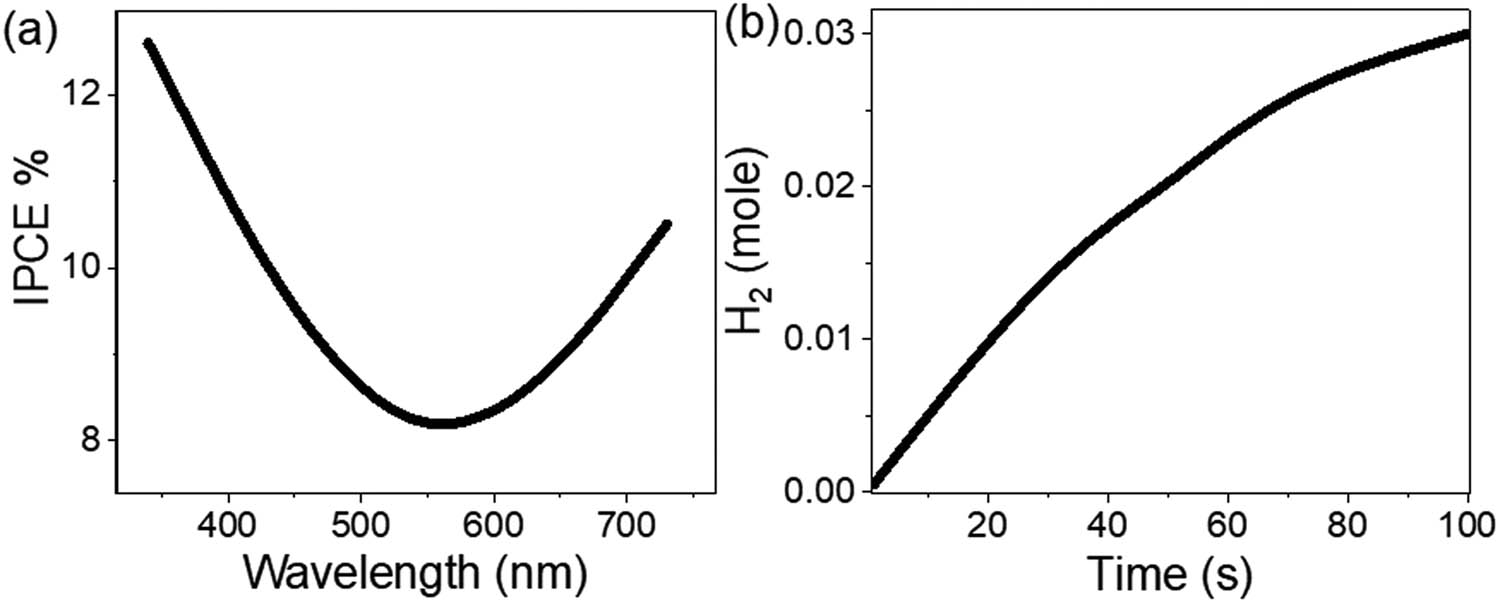
(a) IPCE and (b) the hydrogen mole produced for the MoS3–MoO3/P1HP nanocomposite photocathode inside the three electrodes for green hydrogen generation.
Second, the photocathode itself is constructed from a cost-effective MoS3–MoO3/P1HP nanocomposite material supported on a glass slide. This cost-effective nature of the photocathode highlights the potential for large-scale and economically viable applications. Thus, this study demonstrates the production of highly efficient products through cost-effective techniques, further enhancing its practicality and relevance.
Figure 7(b) provides additional insight into this phenomenon by depicting the quantity of hydrogen molecules produced, which amounts to 1.2 moles·h−1·cm−2. These substantial hydrogen production figures serve as further evidence of the efficiency of the MoS3–MoO3/P1HP nanocomposite photocathode. The calculation of these quantities is accomplished through the application of the Nernstian equation, Eq. 3 [41].
So, the MoS3–MoO3/P1HP nanocomposite photocathode achieves an outstanding level of efficiency, as demonstrated by its peak IPCE of 12.66% at 340 nm. This efficiency is particularly remarkable due to the utilization of sewage water as the source of hydrogen production and the cost-effective composition of the photocathode. Furthermore, the significant quantities of hydrogen molecules produced underscore the effectiveness and practicality of this innovative technology
The synthesized MoS3–MoO3/P1HP nanocomposite, shown in Figure 8, serves as a highly promising photocathode for H2 generation using sewage water. This promin composite is related to the interaction of the MoS3–MoO3 and P1HP during the polymerization reaction under the formation of coordination bonds.
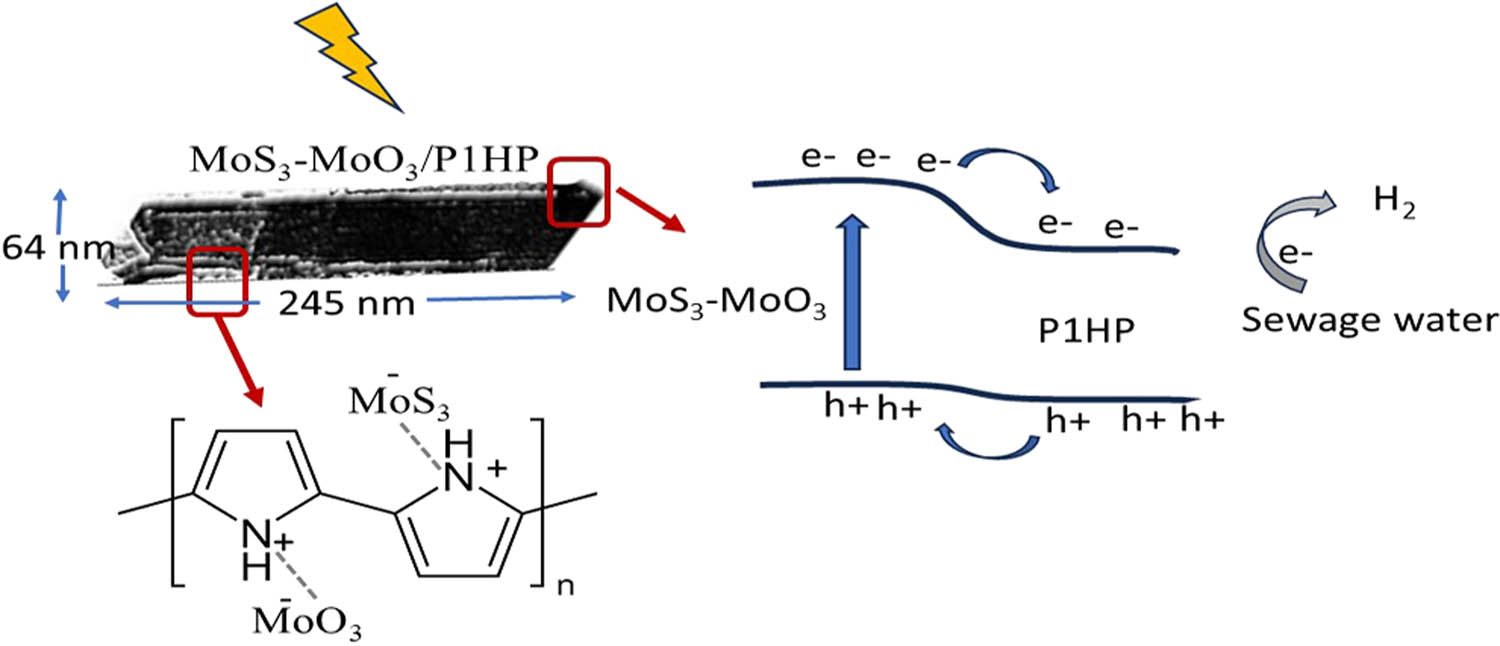
Schematic mechanism for the synthesized MoS3–MoO3/P1HP photocathode for the hydrogen generation from wastewater (through the rod-shaped particle) and mention the chemical formula of this nanocomposite.
The schematic mechanism provides a comprehensive overview of the nanocomposite’s theoretical modeling and its photoactive nature. In the illustration, the nanocomposite exhibits rod-like structures with dimensions specified at 245 nm in length and 64 nm in width. Notably, the rod structure reveals a porous nature, indicative of the incorporation of inorganic materials MoS3–MoO3 within the polymer network. This integration gives rise to distinct dark and faint regions within the nanocomposite.
Under light illumination, the photocatalytic properties of the material come into play. External level splitting occurs, initiating the generation of photogenerated carriers, specifically electron–hole pairs. The electrons undergo accumulation on the surface of the P1HP, facilitated by their migration from the MoS3–MoO3 materials, while the holes travel in the opposite direction. As the process unfolds, the hot electrons become attached to the wastewater, marking significant steps in the photocatalytic cycle [42,43]. Ultimately, a series of reactions culminated in the production of H2 gas. This multifaceted mechanism demonstrates the nanocomposite’s efficiency in harnessing light energy to drive the photocatalytic generation of hydrogen from sewage water, offering a promising avenue for sustainable and environmentally friendly H2 production.
4 Conclusions
The synthesis of rod-shaped MoS3–MoO3/P1HP involves a one-pot preparation method. In this process, the pyrrole monomer is oxidized using Na2MoO4 as the oxidizing agent. This oxidation process is crucial for the integration of the inorganic components into the polymer matrix. The resulting material undergoes a comprehensive range of analyses, collectively providing a profound understanding of its properties. These investigations encompass an exploration of its chemical structure, crystalline characteristics, elemental composition, oxidation states, as well as its optical and electrical behavior.
One of the standout features of this material is its bandgap, which is optimally situated at 1.4 eV. This places it in a highly favorable position for its intended applications. Furthermore, its rod-like structure, with an average length of 400 nm and a width of 30 nm, enhances its appeal. In practical applications, the MoS3–MoO3/P1HP thin film serves as an exceptionally promising photoelectrode. Its primary strength lies in its ability to efficiently generate hydrogen from sewage water. It achieves an impressive efficiency rate of 12.66%, specifically at 340 nm. Additionally, it boasts an extraordinary hydrogen generation rate of 1.2 moles·h−1·cm−2.
The exceptional performance and unique properties of this composite material have prompted experts to recommend its adoption for hydrogen generation in industrial settings. Its efficiency and capability to produce hydrogen from wastewater make it a highly promising candidate for sustainable and environmentally friendly industrial applications. This breakthrough could pave the way for advancements in the field of hydrogen production, offering a cleaner and more efficient alternative to traditional methods.
Acknowledgments
Researchers Supporting Program Number (RSPD2024R845), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: This research was funded by Researchers Supporting Program Number (RSPD2024R845), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Mohamed Rabia: experimental, analyses, and writing; Eman Aldosari, Ahmed Adel A. Abdelazeez: Supervision, revision, and ordering the work.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Sevastyanova LG, Klyamkin SN, Stupnikov VA, Ilyukhina AV, Bulychev BM. On mechanism of hydrogen generation at oxidation of activated aluminum in aqueous solutions. Int J Hydrogen Energy. 2023;54:428–36.10.1016/j.ijhydene.2023.07.224Suche in Google Scholar
[2] Gonuguntla S, Kamesh R, Pal U, Chatterjee D. Dye sensitization of TiO2 relevant to photocatalytic hydrogen generation: Current research trends and prospects. J Photochem Photobiol C: Photochem Rev. 2023;57:100621. 10.1016/J.JPHOTOCHEMREV.2023.100621.Suche in Google Scholar
[3] Fraser SA, van Zyl WE. In situ polymerization and electrical conductivity of polypyrrole/cellulose nanocomposites using Schweizer’s reagent. RSC Adv. 2022;12:22031–43. 10.1039/d2ra04320c.Suche in Google Scholar PubMed PubMed Central
[4] Sacchidan S, Kher J, Kulkarni M. Influence of dodecylbenzene sulfonic acid doping on structural, morphological, electrical and optical properties on polypyrrole/3C-SiC nanocomposites. J Nanomed Nanotechnol. 2015;6:1000313–7. 10.4172/2157-7439.1000313.Suche in Google Scholar
[5] Xue J, Xie Z, Wang B, Zhu Y, Wu Z, Nie Y, et al. Enhanced visible-light photocatalytic performance by PPy/CN composites for reduction of UO22+. J Solid State Chem. 2022;315:123440. 10.1016/J.JSSC.2022.123440.Suche in Google Scholar
[6] Xue J, Zhang H, Shen Q, Zhang W, Gao J, Li Q, et al. Enhanced photoelectrocatalytic hydrogen production performance of porous MoS2/PPy/ZnO film under visible light irradiation. Int J Hydrogen Energy. 2021;46:35219–29. 10.1016/J.IJHYDENE.2021.08.083.Suche in Google Scholar
[7] Chen Y, Zhang M, Chen T, Zhang G, Xu H, Sun H, et al. Facile fabrication of rGO/PPy/nZVI catalytic microreactor for ultrafast removal of p-nitrophenol from water. Appl Catal B: Environ. 2023;324:122270. 10.1016/J.APCATB.2022.122270.Suche in Google Scholar
[8] Cheng X, Liao J, Xue Y, Lin Q, Yang Z, Yan G, et al. Ultrahigh-flux and self-cleaning composite membrane based on BiOCl-PPy modified MXene nanosheets for contaminants removal from wastewater. J Membr Sci. 2022;644:120188. 10.1016/J.MEMSCI.2021.120188.Suche in Google Scholar
[9] Siva V, Murugan A, Shameem A, Thangarasu S, Kannan S, Raja A. Gel combustion synthesized NiMoO4 anchored polymer nanocomposites as a flexible electrode material for solid state asymmetric supercapacitors. Int J Hydrogen Energy. 2023;48:18856–70. 10.1016/J.IJHYDENE.2023.01.295.Suche in Google Scholar
[10] Telfah A, Al-Akhras MA, Al-Izzy KA, Ahmad AA, Ababneh R, Ahmad MJ, et al. Dielectric relaxation, XPS and structural studies of polyethylene oxide/iodine complex composite films. Polym Bull. 2022;79:3759–78. 10.1007/S00289-021-03593-1/METRICS.Suche in Google Scholar
[11] Wang LS, Xu S, Gopal S, Kim E, Kim D, Brier M, et al. Facile fabrication of antibacterial and antiviral perhydrolase-polydopamine composite coatings. Sci Rep. 2021;11:1–11. 10.1038/s41598-021-91925-6.Suche in Google Scholar PubMed PubMed Central
[12] Zhou J, Qiao Q, Tan Y, Wu C, Hu J, Qiu X, et al. The improvement of polymer photodetector based on 1D-ZnO nanorod arrays/0D-ZnO quantum dots composite film. Opt Mater. 2023;142:114086. 10.1016/J.OPTMAT.2023.114086.Suche in Google Scholar
[13] Ecer Ü, Zengin A, Şahan T. Hydrogen generation from NaBH4 hydrolysis catalyzed by cobalt (0)-Deposited cross-linked polymer brushes: Optimization with an experimental design approach. Int J Hydrogen Energy. 2023;48:12814–25. 10.1016/J.IJHYDENE.2022.12.224.Suche in Google Scholar
[14] Burlakovs J, Kaczala F, Orupõld K, Bhatnagar A, Vincevica-Gaile Z, Rudovica V, et al. Field-portable X-ray fluorescence spectrometry as rapid measurement tool for landfill mining operations: comparison of field data vs. laboratory analysis. Int J Environ Anal Chem. 2015;95:609–17. 10.1080/03067319.2015.1036865.Suche in Google Scholar
[15] Tkalec M, Stefanić PP, Cvjetko P, Sikić S, Pavlica M, Balen B. The effects of cadmium-zinc interactions on biochemical responses in tobacco seedlings and adult plants. PLoS ONE. 2014;9:87582. 10.1371/JOURNAL.PONE.0087582.Suche in Google Scholar PubMed PubMed Central
[16] Rabia M, Trabelsi ABG, Elsayed AM, Alkallas FH. Porous-spherical Cr2O3-Cr(OH)3-polypyrrole/polypyrrole nanocomposite thin-film photodetector and solar cell applications. Coatings. 2023;13:1240. 10.3390/COATINGS13071240.Suche in Google Scholar
[17] Alkallas FH, Elsayed AM, Trabelsi ABG, Rabia M. Quantum dot supernova-like-shaped Arsenic (III) sulfide-oxide/polypyrrole thin film for optoelectronic applications in a wide optical range from ultraviolet to infrared. Catalysts. 2023;13:1274. 10.3390/CATAL13091274.Suche in Google Scholar
[18] Yamani K, Berenguer R, Benyoucef A, Morallón E. Preparation of polypyrrole (PPy)-derived polymer/ZrO 2 nanocomposites: Effects of nanoparticles interface and polymer structure. J Therm Anal Calorim. 2019;135:2089–100. 10.1007/S10973-018-7347-Z/FIGURES/8.Suche in Google Scholar
[19] Kulandaivalu S, Suhaimi N, Sulaiman Y. Unveiling high specific energy supercapacitor from layer-by-layer assembled polypyrrole/graphene oxide|polypyrrole/manganese oxide electrode material. Sci Rep. 2019;9:1–10. 10.1038/s41598-019-41203-3.Suche in Google Scholar PubMed PubMed Central
[20] Hameed SA, Ewais HA, Rabia M. Dumbbell-like shape Fe2O3/poly-2-aminothiophenol nanocomposite for two-symmetric electrode supercapacitor application. J Mater Sci: Mater Electron. 2023;34:1–8. 10.1007/S10854-023-10586-5/METRICS.Suche in Google Scholar
[21] Rabia M, Elsayed AM, Salem AM, Alnuwaiser MA. Highly uniform multi-layers reduced graphene oxide/poly-2-aminobenzene-1-thiol nanocomposite as a promising two electrode symmetric supercapacitor under the effect of absence and presence of porous-sphere polypyrrole nanomaterial. Micromachines. 2023;14:1424. 10.3390/MI14071424.Suche in Google Scholar PubMed PubMed Central
[22] Azzam EMS, Abd El-Salam HM, Aboad RS. Kinetic preparation and antibacterial activity of nanocrystalline poly(2-aminothiophenol). Polym Bull. 2019;76:1929–47. 10.1007/S00289-018-2405-Z/FIGURES/14.Suche in Google Scholar
[23] Rabia M, Elsayed AM, Abdallah Alnuwaiser M, Abdelazeez AAA. Ag2S-Ag2O-Ag/poly-2-aminobenzene-1-thiol nanocomposite as a promising two-electrode symmetric supercapacitor: Tested in acidic and basic mediums. Micromachines. 2023;14:1423. 10.3390/MI14071423.Suche in Google Scholar
[24] Sen SK, Dutta S, Khan MR, Manir MS, Dutta S, Al Mortuza A, et al. Characterization and antibacterial activity study of hydrothermally synthesized h-MoO3 nanorods and α-MoO3 nanoplates. BioNanoScience. 2019;9:873–82. 10.1007/S12668-019-00671-7/TABLES/3.Suche in Google Scholar
[25] Shirota G, Nasu A, Deguchi M, Sakuda A, Tatsumisago M, Hayashi A. Electrode performance of amorphous MoS3 in all-solid-state sodium secondary batteries. J Power Sources Adv. 2021;10:100061. 10.1016/J.POWERA.2021.100061.Suche in Google Scholar
[26] Ye H, Ma L, Zhou Y, Lu J. Amorphous MoS3 as the sulfur-equivalent cathode material for room-temperature Li–S and Na–S batteries. Proc Natl Acad Sci U S A. 2017;114:13091–6. 10.1073/PNAS.1711917114/SUPPL_FILE/PNAS.201711917SI.PDF.Suche in Google Scholar
[27] Atta A, Negm H, Abdeltwab E, Rabia M, Abdelhamied MM. Facile fabrication of polypyrrole/NiOx core-shell nanocomposites for hydrogen production from wastewater. Polym Adv Technol. 2023;34:1633–41. 10.1002/PAT.5997.Suche in Google Scholar
[28] Burton AW, Ong K, Rea T, Chan IY. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009;117:75–90. 10.1016/J.MICROMESO.2008.06.010.Suche in Google Scholar
[29] Cheng CK, Lin JY, Huang KC, Yeh TK, Hsieh CK. Enhanced efficiency of dye-sensitized solar counter electrodes consisting of two-dimensional nanostructural molybdenum disulfide nanosheets supported Pt nanoparticles. Coatings. 2017;7:167. 10.3390/COATINGS7100167.Suche in Google Scholar
[30] Elsayed AM, Alkallas FH, Trabelsi ABG, Rabia M. Highly uniform spherical MoO2-MoO3/polypyrrole core-shell nanocomposite as an optoelectronic photodetector in UV, Vis, and IR domains. Micromachines. 2023;14:1694. 10.3390/MI14091694.Suche in Google Scholar
[31] Wu S, Xu J, Zou L, Luo S, Yao R, Zheng B, et al. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat Commun. 2021;12:1–14. 10.1038/s41467-021-23069-0.Suche in Google Scholar PubMed PubMed Central
[32] Mohan A, Al-Sayah MH, Ahmed A, El-Kadri OM. Triazine-based porous organic polymers for reversible capture of iodine and utilization in antibacterial application. Sci Rep. 2022;12:1–10. 10.1038/s41598-022-06671-0.Suche in Google Scholar PubMed PubMed Central
[33] Rabia M, Elsayed AM, Alnuwaiser MA. Preparation and characterization of polyhedron Mn(III) oxide/-β-Mn(IV) oxide/poly-o-chloroaniline porous nanocomposite for electroanalytical photon detection. Processes. 2023;11:2375. 10.3390/PR11082375.Suche in Google Scholar
[34] Kumar U, Yang YH, Deng ZY, Lee MW, Huang WM, Wu CH. In situ growth of ternary metal sulfide based quantum dots to detect dual gas at extremely low levels with theoretical investigations. Sens Actuators B: Chem. 2022;353:131192. 10.1016/J.SNB.2021.131192.Suche in Google Scholar
[35] Nayak J, Mahadeva SK, Kim J. Characteristics of flexible electrode made on cellulose by soluble polypyrrole coating. Proc Inst Mech Eng Part C: J Mech Eng Sci. 2012;226:2605–9. 10.1177/0954406212439965.Suche in Google Scholar
[36] Maruthapandi M, Nagvenkar AP, Perelshtein I, Gedanken A. Carbon-dot initiated synthesis of polypyrrole and polypyrrole@CuO micro/nanoparticles with enhanced antibacterial activity. ACS Appl Polym Mater. 2019;1:1181–6. 10.1021/ACSAPM.9B00194.Suche in Google Scholar
[37] Sendi RK, Al-Harbi N, Atta A, Rabia M, Abdelhamied MM. Copper oxide and copper nanoparticles insertion within a PPy matrix for photodetector applications. Opt Quantum Electron. 2023;55:1–15. 10.1007/S11082-023-05226-5/METRICS.Suche in Google Scholar
[38] Reda SM, Al-Ghannam SM, Reda SM, Al-Ghannam SM. Synthesis and electrical properties of polyaniline composite with silver nanoparticles. Adv Mater Phys Chem. 2012;2:75–81. 10.4236/AMPC.2012.22013.Suche in Google Scholar
[39] Dou Y, Yang X, Wang Q, Yang Z, Wang A, Zhao L, et al. Efficient hydrogen generation of a cobalt porphyrin-bridged covalent triazine polymer. J Colloid Interface Sci. 2023;644:256–63. 10.1016/J.JCIS.2023.04.082.Suche in Google Scholar
[40] Rabia M, Mohamed HSH, Shaban M, Taha S. Preparation of polyaniline/PbS core-shell nano/microcomposite and its application for photocatalytic H2 electrogeneration from H2O. Sci Rep. 2018;8:1107. 10.1038/s41598-018-19326-w.Suche in Google Scholar PubMed PubMed Central
[41] Babic U, Suermann M, Büchi FN, Gubler L, Schmidt TJ. Critical review–identifying critical gaps for polymer electrolyte water electrolysis development. J Electrochem Soc. 2017;164:387.10.1149/2.1441704jesSuche in Google Scholar
[42] Mallikarjuna K, Rafiqul Bari GAKM, Vattikuti SVP, Kim H. Synthesis of carbon-doped SnO2 nanostructures for visible-light-driven photocatalytic hydrogen production from water splitting. Int J Hydrogen Energy. 2020;45:32789–96. 10.1016/J.IJHYDENE.2020.02.176.Suche in Google Scholar
[43] Zhang Y, Guo S, Xin X, Song Y, Yang L, Wang B, et al. Plasmonic MoO2 as co-catalyst of MoS2 for enhanced photocatalytic hydrogen evolution. Appl Surf Sci. 2020;504:144291. 10.1016/J.APSUSC.2019.144291.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

