Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
-
Tiruhi Gomktsyan
and Vergush Pivazyan
Abstract
Eco-friendly ultrasound-assisted synthesis of a series of 3-N-substituted 6-((4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)pyridazin-3(2H)-one derivatives and in silico study to predict their biological activities were carried out. Physicochemical and pharmacokinetic properties were obtained. Absorption, distribution, metabolism, excretion and toxicity properties and bioavailability index were calculated. A comparative analysis of structural similarity based on the Tanimoto coefficient was carried out.
Graphical abstract
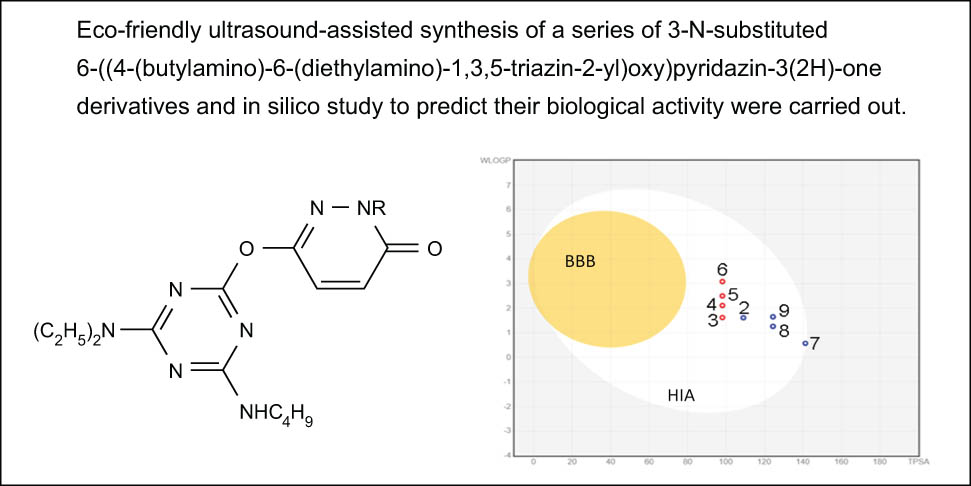
1 Introduction
Heterocyclic compounds containing a symmetrical 1,3,5-triazine moiety are an interesting class of compounds with a wide spectrum of biological activity. In medical practice, several drugs are used: the respiratory stimulant almitrin (duxil), the antitumor drugs altretamine (hexalen), dioxadet and tretamine, the myorelaxant isocyuronium bromide, the trypanocidal drugs melarsen oxide and melarsomine, the antimalarial drug cycloguanil, and the antiulcer drug irsogladine.
Given the breadth of the spectrum of physiological action, targeted syntheses and studies of the biological activity of new derivatives of 1,3,5-triazine continue. This series includes compounds with antimicrobial [1,2,3,4], anti-tuberculosis [5,6], anti-inflammatory [7,8], antiviral [9], antifungal [10], anticancer [11,12,13,14], anti-HIV [15,16], antitrypanosomal [17,18], and antimalarial [19,20,21] activities. Some derivatives of 1,3,5-triazine inhibit monoamine oxidase [22] and are blockers of neuronal sodium channels [23].
Much attention is also paid to the search for new biologically active derivatives of pyridazine. In the last two decades, among them, the compounds with analgesic and anti-inflammatory [24,25], antimicrobial [26,27,28], antifungal [29], antitumor [30,31], and antinociceptive [32] activities have been found. Some substances have shown inhibitory effects on α1-α2-adrenoceptors [33], c-Met kinase [34], and plant growth [35].
Derivatives of 1,3,5-triazine and pyridazine are also widely used in agriculture. Among the 1,3,5-triazine derivatives, fungicides (anilazine), and herbicides (dipropetryn, trihydroxy triazine, a large number of substituted chlorotriazines, fluoroalkyltriazines, methoxytriazines, methylthiotriazines, triazinones, and also a wide number of triazinylsulfonylurea derivatives) are known [36].
The arsenal of pesticides based on pyridazine includes mainly herbicides (credazune, pyridafol, pyridate, brompyrazon, chloridazon, dimidazon, flufenpyr, metflurazon, norflurazon, oxapyrazon, and pudanon) [36]. Our early studies also identified compounds that have a stimulating effect on plant growth [37,38,39,40].
However, the acquisition by harmful organisms of resistance to the substances used necessitates a systematic replenishment of their assortment with new drugs with different mechanisms of action.
In this regard, the purpose of this study was the targeted synthesis of new compounds with a combination of 1,3,5-triazine and pyridazine cycles in the molecule, which can lead to new biologically active derivatives, to which the indicated resistance has not yet been formed.
2 Materials and methods
2.1 General
An ultrasonic generator I10-840 with an operating frequency of 22 kHz ± 10% and a maximum pulse power of 1,000 W was used to carry out the sonochemical syntheses. In all experiments, the following conditions were applied: irradiation power – 30% (300 W), exposure time – 30 min. The vessel with the reagents subjected to irradiation was placed in a water bath, which was maintained at room temperature (25°C).
The structure and purity of the compounds synthesized were confirmed by 1H and 13C NMR spectra, obtained at 30°C on a Varian Mercury-300 NMR spectrometer (300 and 75 MHz, respectively) in a mixture of CCl4/DMSO-d6 solvents (3:1), using a standard pulse sequence; TMS was used as an internal standard. The progress of the reactions and the purity of the obtained compounds were checked by TLC on Silufol UV-254 plates; an acetone/hexane mixture (2:1 or 1:1) was used as the eluent. Elemental analysis was performed on a Eurovector EA3000 CHN analyzer. Melting points were determined on a Stuart SMP10 apparatus and were uncorrected.
2.2 US-assisted syntheses of compounds 2–9
2.2.1 6-((4-(Butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)pyridazin-3(2H)-one (2)
The mixture of compound 1 (0.02 mol), and potassium salt of 6-hydroxypyridazin-3(2H)-one (0.02 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield 83%, m.p., 160–161°C. IR, ν, cm−1: 1,676 (C═O). 1H NMR, δ, ppm (J, Hz): 0.95 (3H, t, J = 7.1, CH 3-Bu); 1.10–1.20 (6H, t, J = 7.1, (CH3)2-Et); 1.33 and 1.50 (4H, m, CH2CH2); 3.20 and 3.24 (2H, m, CH 2 NH); 3.40–3.58 (4H, m, (NCH2)2-Et); 6.82 and 7.20 (2H, d, J = 9.0, CH═CH); 6.80 and 7.50 (1H, brs, NH-Bu); 12.45 (1H, brs, NH-pyrid.). 13C NMR, δ, ppm: 12.6, 12.9, 13.0, 13.4, 13.5, 19.38, 19.45, 19.5, 30.8, 31.5, 40.1, 40.3, 40.8, 40.9, 130.5,130.8, 148.2, 159.8, 164.8, 166.0, 169.0. Anal. calculated for C15H23N7O2: C, 54.04; H, 6.95; N, 29.41. Found: C, 54.14; H, 6.90; N, 29.55.
2.2.2 6-((4-(Butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)-2-methylpyridazin-3(2H)-one (3)
The mixture of compound 1 (0.02 mol) and potassium salt of 6-hydroxy-2-methylpyridazin-3(2H)-one (0.02 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield, 75%; m.p., 133–135°C. IR, ν, cm−1: 1,667 (C═O). 1H NMR, δ, ppm (J, Hz): 0.95 (3H, t, J = 7.1, CH 3-Bu); 1.12–1.20 (6H, t, J = 7.1, (CH3)2-Et); 1.32 and 1.50 (4H, m, CH2CH2); 3.19 and 3.24 (2H, m, CH 2NH); 3.43 and 3.55 (4H, m, (NCH2)2-Et); 3.60 (3H, s, NCH3); 6.25 and 7.18 (1H, t, J = 4.8, NH); 6.90 and 7.23 (2H, d, J = 9.0, CH═CH). 13C NMR, δ, ppm: 12.76, 12.83, 13.4, 19.4, 30.8, 38.7, 39.7, 40.8, 40.9, 130.0, 146.9, 147.40, 158.5, 164.8, 166.0, 169.0. Anal. calculated for C16H25N7O2: C, 55.31; H, 7.25; N, 28.22. Found: C, 55.25; H, 7.20; N, 28.11.
2.2.3 6-((4-(Butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)-2-phenylpyridazin-3(2H)-one (4)
The mixture of compound 1 (0.02 mol) and potassium salt of 6-hydroxy-2-phenylpyridazin-3(2H)-one (0.02 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield, 65%; m.p., 162–164°C. IR, ν, cm−1: 1,679 (C═O). 1H NMR, δ, ppm (J, Hz): 0.95 (3H, t, J = 7.1, CH 3-Bu); 1.12–1.20 (6H, t, J = 7.1, (CH3)2-Et); 1.32 and 1.50 (4H, m, CH 2CH2); 3.20 and 3.25 (2H, m, CH2NH); 3.44 and 3.57 (4H, m, (NCH2)2-Et); 6.91 and 7.23 (1H, t, J = 4.8, NH); 7.00–7.71 (7H, m, C6H5 and CH═CH). 13C NMR, δ, ppm: 12.6, 12.9, 13.4, 19.4, 30.8, 39.7, 40.8, 41.0, 124.4, 126.9, 127.8, 130.1, 131.7, 140.7, 148.2, 157.9, 164.8, 166.1, 169.0. Anal. calculated for C21H27N7O2: C, 61.60; H, 6.65; N, 23.94. Found: C, 61.8; H, 6.69; N, 24.02.
2.2.4 6-((4-(Butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)-2-propylpyridazin-3(2H)-one (5)
The mixture of compound 2 (0.02 mol), KOH (0.02 mol), and propylbromide (0.022 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield, 71%; m.p., 95–97°C. IR, ν, cm−1: 1,672 (C═O). 1H NMR, δ, ppm (J, Hz): 0.96–1.23 (12H, m, (CH3)4); 1.27–1.57 (4H, m, CH2CH2); 1.76 (2H, m, CCH2C-Pr); 3.18 and 3.25 (2H, m, CH 2 NH); 3.43 and 3.55 (4H, m, (NCH2)2-Et); 3.96 (2H, t, J = 7.2, NCH2-Pr); 6.80 and 7.18 (1H, t, J = 4.8, NH); 6.84 and 7.20 (2H, d, J = 9.0, CH═CH). 13C NMR, δ, ppm: 10.5, 12.6, 12.8, 13.4, 19.36, 19.44, 20.9, 29.0, 30.8, 31.1, 36.7, 40.5, 40.8, 41.0, 51.7, 129.6, 129.9, 130.2, 130.5, 130.7, 147.4, 148.2, 158.3, 159.7, 164.7, 166.1, 169.0. Anal. calculated for C18H29N7O2: C, 57.58; H, 7.79; N, 26.11. Found: C, 57.66; H, 7.84; N, 26.27.
2.2.5 6-((4-(Butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)-2-ethylpyridazin-3(2H)-one (6)
The mixture of compound 2 (0.02 mol), KOH (0.02 mol), and ethyliodide (0.022 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield, 72%; m.p., 120–122°C. IR, ν, cm−1: 1,666 (C═O). 1H NMR, δ, ppm (J, Hz): 0.96 (3H, t, J = 7.1, CH 3-Bu); 1.10–1.20 (6H, t, J = 7.1, (CH3)2-Et); 1.30 (3H, t, J = 7.0, NCH2CH 3-Et); 1.27–1.57 (4H, m, CH2CH2); 3.20 and 3.27 (2H, m, CH 2NH); 3.43 and 3.55 (4H, m, (NCH2)2-Et); 4.03 (2H, q, J = 7.0, NCH2-Et); 6.92 and 7.20 (1H, t, J = 4.8, NH); 6.94 and 7.22 (2H, d, J = 9.0, CH═CH). 13C NMR, δ, ppm: 12.6, 12.8, 12.9, 13.3, 19.4, 30.8, 40.8, 41.0, 45.4, 129.7, 130.2, 147.6, 158.0, 164.7, 166.1, 169.0. Anal. calculated for C17H27N7O2: C, 56.49; H, 7.53; N, 27.13. Found: C, 56.40; H, 7.47; N, 27.01.
2.2.6 2-(3-((4-(Butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)-6-oxopyridazin-1(6H)-yl)acetamide (7)
The mixture of compound 2 (0.02 mol), KOH (0.02 mol), and 2-chloroacetamide (0.022 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield, 87%; m.p., 188–190°C. IR, ν, cm−1: 1,676 (C═O), 1,680 (C═O). 1H NMR, δ, ppm (J, Hz): 0.96 (3H, t, J = 7.2, CH 3-Bu); 1.11 and 1.17 (6H, t, J = 7.1, (CH 3)2-Et); 1.35 and 1.50 (4H, m, CH2CH2); 3.20 and 3.25 (2H, m, CH2NH); 3.43 and 3.55 (4H, m, (NCH2)2-Et); 4.53 (2H, s, NCH2CO); 7.32 (1H, t, J = 6.0, NH); 6.91 and 7.26 (2H, d, J = 9.8, CH═CH); 6.93 and 7.36 (2H, brs, NH2). 13C NMR, δ, ppm: 12.7, 12.9, 13.4, 19.5, 30.8, 40.76, 40.78, 40.94, 130.2, 130.3, 147.5, 158.5, 164.8, 166.0, 167.4, 168.8. Anal. calculated for C17H26N8O3: C, 52.30; H, 6.71; N, 28.70. Found: C, 52.22; H, 6.76; N, 28.81.
2.2.7 Methyl 2-(3-((4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)-6-oxopyridazin-1(6H)-yl)acetate (8)
The mixture of compound 2 (0.02 mol), KOH (0.02 mol), and methyl 2-chloroacetate (0.022 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield, 70%; m.p., 93–95°C. IR, ν, cm−1: 1,683 (C═O), 1,751 (C═O). 1H NMR, δ, ppm (J, Hz): 0.93 (3H, t, J = 7.1, CH 3-Bu); 1.12–1.20 (6H, t, J = 7.1, (CH3)2-Et); 1.34 and 1.50 (4H, m, CH2CH2); 3.20 and 3.25 (2H, m, CH2NH); 3.42 and 3.53 (4H, m, (NCH2)2-Et); 3.77 (3H, s, OCH3); 4.72 (2H, s, NCH2CO); 6.85 and 7.25 (1H, t, J = 5.0, NH); 6.93 and 7.33 (2H, d, J = 9.0, CH═CH). 13C NMR, δ, ppm: 12.3, 12.6, 13.1, 19.1, 30.5, 39.8, 40.5, 40.6, 40.7, 51.3, 51.7, 129.9, 130.7, 147.6, 158.0, 164.4, 165.8, 166.5, 168.5. Anal. calculated for C18H27N7O4: C, 53.32; H, 6.71; N, 24.18. Found: C, 53.38; H, 6.77; N, 24.03.
2.2.8 Ethyl 2-(3-((4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)-6-oxopyridazin-1(6H)-yl)acetate (9)
The mixture of compound 2 (0.02 mol), KOH (0.02 mol), and ethyl 2-chloroacetate (0.022 mol) in 20 mL of DMF was subjected to ultrasound irradiation. Yield, 68%; m.p., 68–70°C. IR, ν, cm−1: 1,676 (C═O), 1,761 (C═O). 1H NMR, δ, ppm (J, Hz): 0.95 (3H, t, J = 7.1, CH 3-Bu); 1.13–1.20 (6H, t, J = 7.1, (CH3)2-Et); 1.30 (3H, t, J = 7.1, OCH2CH 3); 1.34 and 1.50 (4H, m, CH2CH2); 3.21 and 3.24 (2H, m, CH 2 NH); 3.42 and 3.54 (4H, m, (NCH2)2-Et); 4.20 (2H, q, J = 7.1, OCH2 CH 3); 4.68 (2H, s, NCH2CO); 6.85 and 7.23 (1H, t, J = 5.0, NH); 6.93 and 7.33 (2H, d, J = 9.0, CH═CH). 13C NMR, δ, ppm: 12.6, 12.8, 13.3, 13.7, 19.4, 30.8, 39.7, 40.8, 40.9, 52.1, 60.5, 130.1, 130.9, 147.8, 158.3, 164.7, 166.0, 166.2, 167.8. Anal. calculated for C19H29N7O4: C, 54.40; H, 6.97; N, 23.37. Found: C, 54.39; H, 6.82; N, 23.17.
3 Results and discussion
3.1 Chemistry
The initial goal of this work was the target synthesis of triazinyloxypyridazines, which should be obtained by substituting the chlorine atom in the second position of 2-chloro-4-butylamino-6-diethylamino-1,3,5-triazine with an oxypyridazine fragment. However, taking into account that such a reaction proceeds under rather severe conditions, at first the chlorosubstituted derivative was transformed into 4-(butylamino)-6-(diethylamino)-N,N,N-trimethyl-1,3,5-triazine-2-aminium chloride (1) and then the subsequent reactions of latter were carried out with potassium salts of the previously obtained 6-hydroxypyridazin-3(2H)-one and its 1-methyl- and 1-phenyl substituted derivatives. As a result of these reactions, the corresponding triazinyloxypyridazines (2–4) were synthesized (Scheme 1).
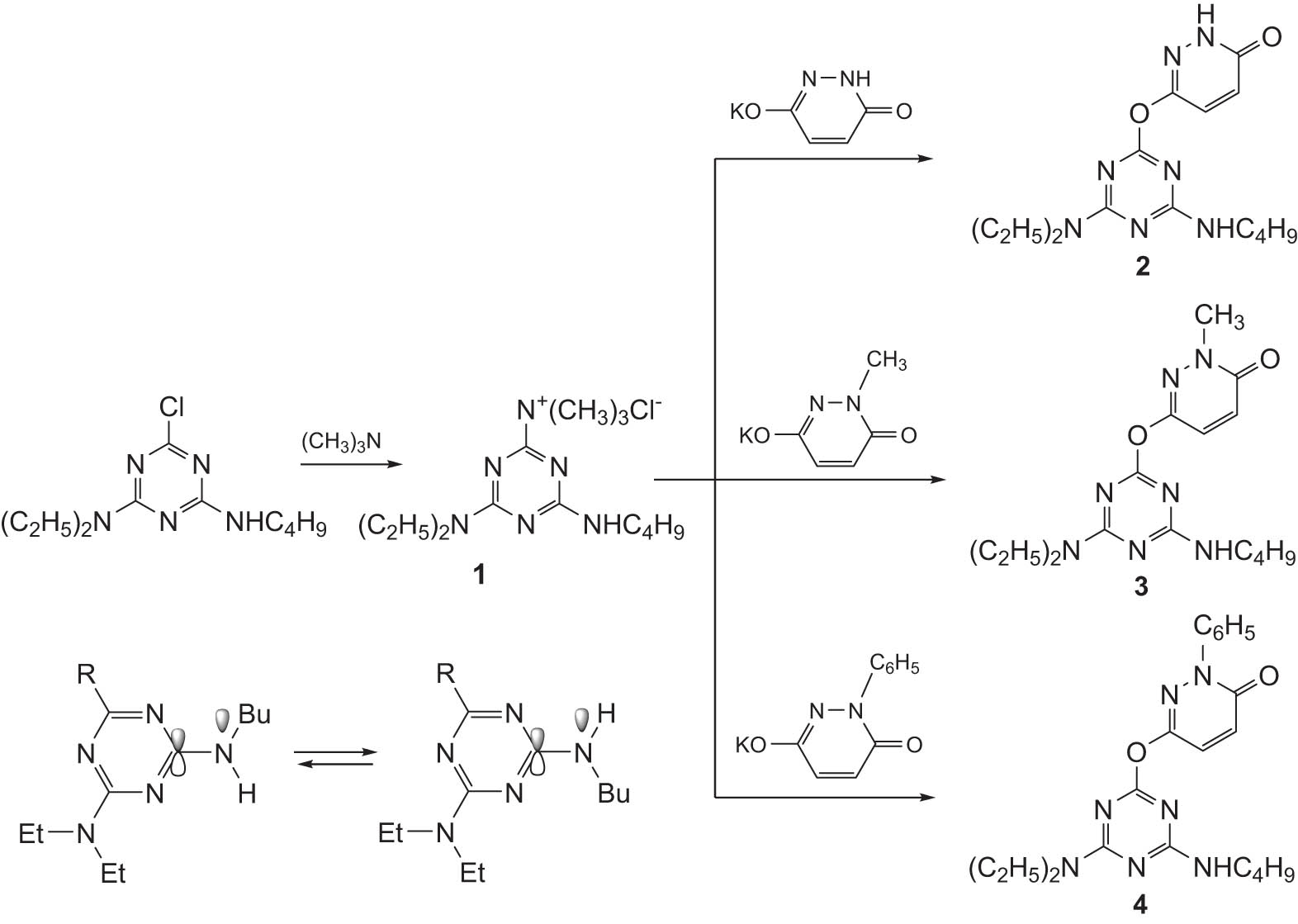
Synthesis of triazinyloxypyridazine derivatives.
Compounds 3 and 4 can also be obtained from 6-((4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)pyridazin-3(2H)-one (2). At the same time, the latter can exist in two different tautomeric forms, and, as a result, the substitution reactions can proceed both at the cyclic nitrogen atom (2A) or the oxygen atom of the hydroxyl group (2B). Compound 2 was alkylated with various alkyl halides (Scheme 2).
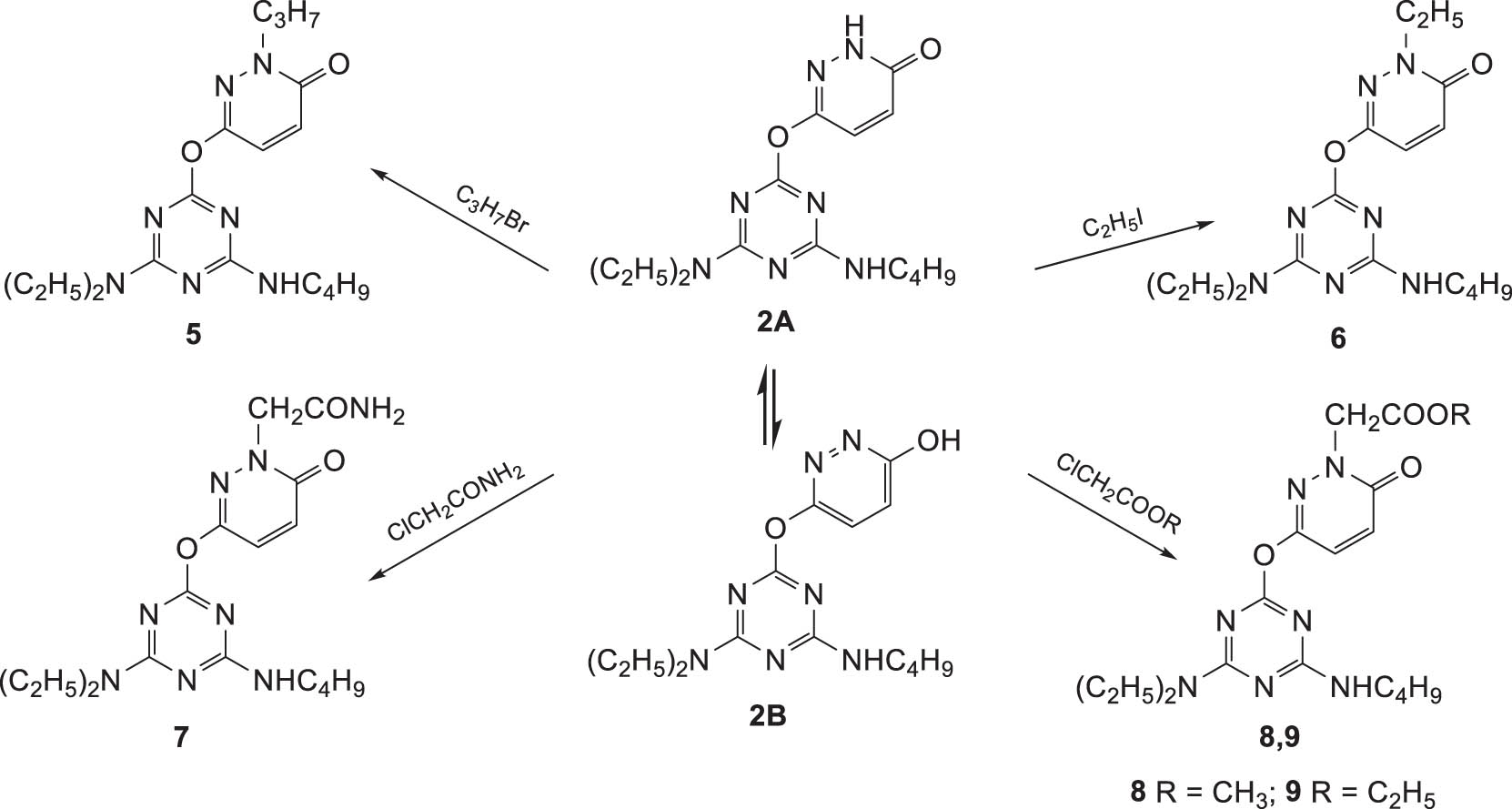
Synthesis of N-substituted derivatives of 6-((4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)pyridazin-3(2H)-one (2).
In the IR spectra the absorptions at 1,672 cm−1 (compound 5) and 1,666 cm−1 (compound 6), corresponding to the C═O carbonyl groups were obtained, which is consistent with the substitution at the nitrogen atom of the pyridazine ring (A). In the IR spectra of compounds 7–9, for each compound, two absorptions related to the C═O groups are observed, which also indicates N-alkylation (A). The structure of compounds 5–9 is also confirmed by the 1H NMR and 13C NMR spectra, in which the chemical shifts of the signals of the alkyl substituents methylene groups are consistent with N-substitution.
It should be noted that the 1H NMR spectra of all synthesized compounds 2–9 contain two sets of signals corresponding to N-alkyl groups (4-butylamino and 6-diethylamino), which is explained by the hindered internal rotation around the N-heterocycle bond. The increase in the order of this bond is associated with the interaction of the p-electron orbital of the carbon atom of the triazine ring with the n-orbital of the exocyclic nitrogen atom. This effect was described in detail in our earlier article [41]. For this reason, the number of signals in the 13C NMR spectra of these compounds is greater than the number of carbon atoms in the molecule. The yield and melting points of synthesized compounds are listed in Table 1.
Characteristics of synthesized compounds 2–9
| No. | Yield (%) | m.p. (°C) | No. | Yield (%) | m.p. (°C) |
|---|---|---|---|---|---|
| 2 | 83 | 160–161 | 6 | 72 | 120–122 |
| 3 | 75 | 133–135 | 7 | 87 | 188–190 |
| 4 | 65 | 162–164 | 8 | 70 | 93–95 |
| 5 | 71 | 95–97 | 9 | 68 | 68–70 |
3.2 In silico study
Absorption, distribution, metabolism, excretion and toxicity (ADMET) parameter values were calculated using SwissADME [42] and ADMETlab v.2 [43] platforms. The prediction of possible toxicity was made using the AdmetSAR [44] and PeoTox [45] platforms. Possible biological activities were obtained based on PASS online [46]. Structural similarity parameters based on the Tanimoto coefficient and data clustering were performed using the online platform http://chemmine.ucr.edu/ (Figure 1).
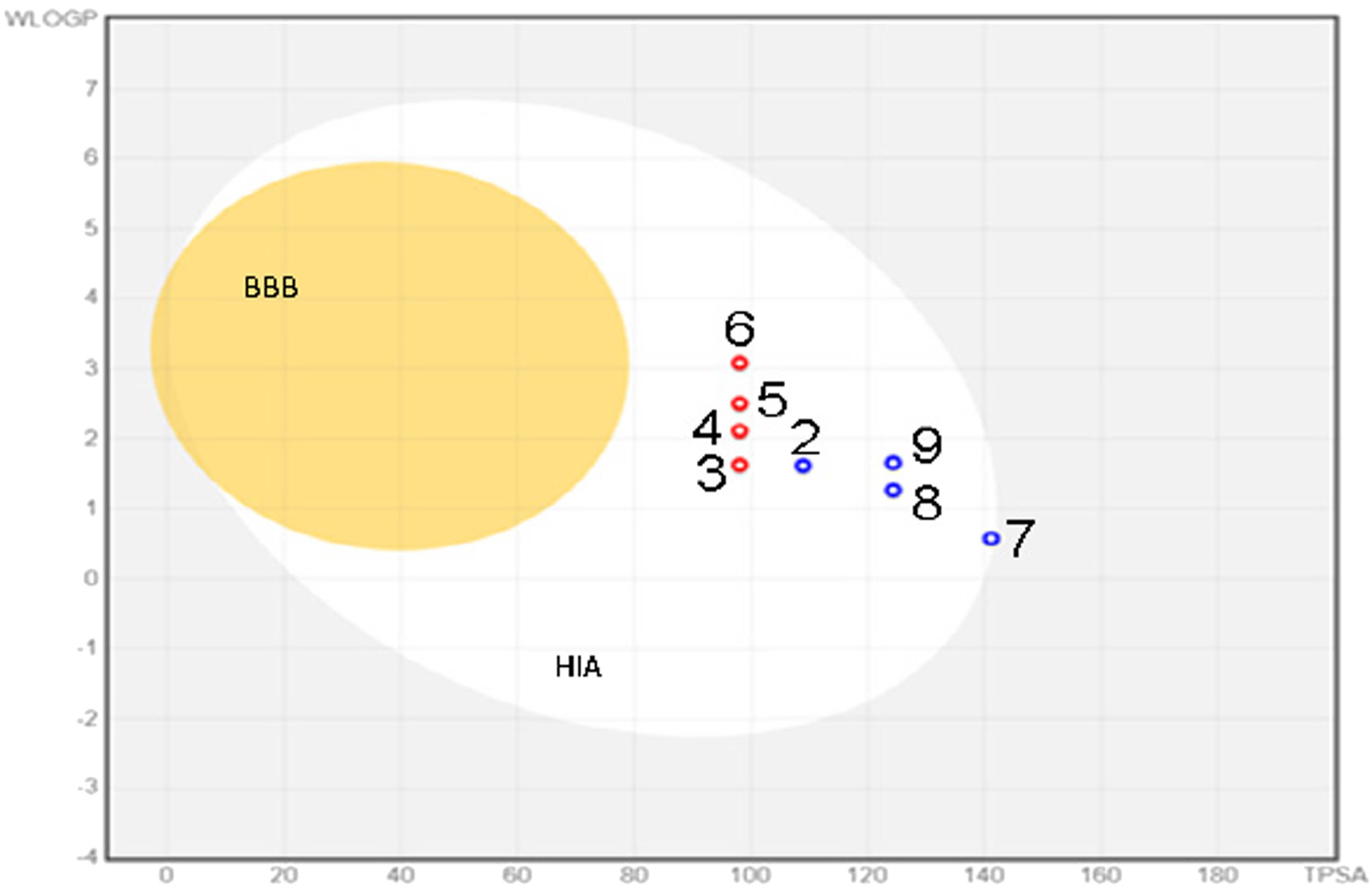
Map of the absorption of compounds through the GIT and BBB. BBB – passage through the BBB, HIA – absorption through the GIT, red dots – active transport, blue dots – passive transport.
All studied compounds meet the criteria of Lipinski’s “Rule of Five” [47]. The calculated values of absorption through the gastrointestinal tract (GIT) and the blood–brain barrier (BBB) indicate that all compounds are well absorbed through the GIT while showing negative values of passage through the BBB. Permeability through the skin is also low at −6.21 cm·s−1.
It is known that the methodology that reveals the correlation between the structural features of compounds and their activity based on the similarity coefficient is one of the tools of modern drug design. By using QSAR and QSPR methods based on molecular fingerprints, it is possible to compare and group similar compounds in an “all-versus-all” comparison. Data clustering and its visualization simplify data analysis and increase the statistical validity of the experiment [48]. We carried out a comparative analysis and visualization of the structural similarity between the studied compounds. Our result shows that compounds 6 and 9 exhibit the maximum values of the Tanimoto coefficient of 60%.
Clustering based on structural similarity revealed three clusters: compounds 2–5 form cluster 1, 7–9 form cluster 2, and compound 6 forms cluster 3 (Figure 2).
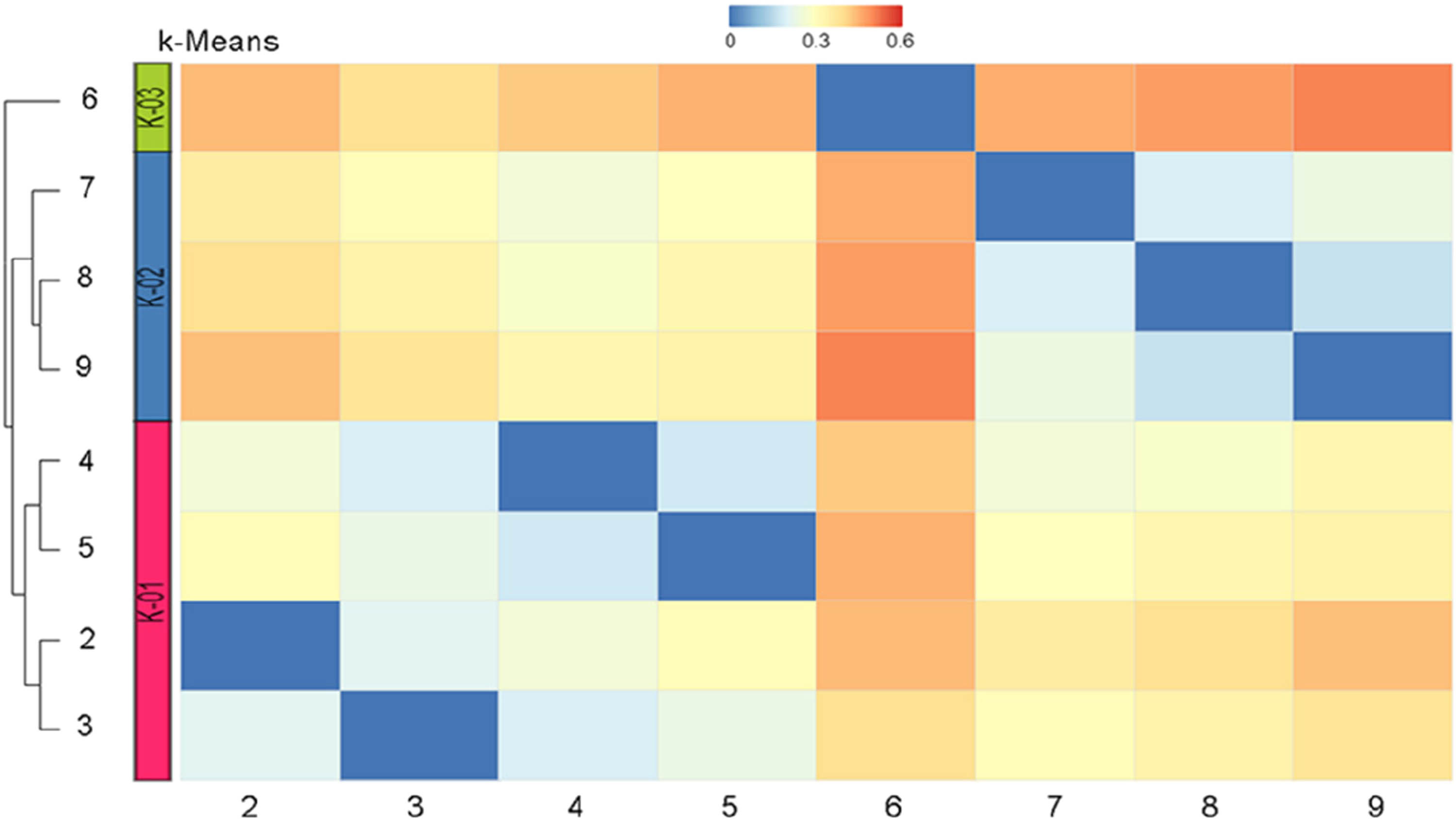
The resulting heat map of the similarity of molecular models based on the Tanimoto coefficient.
From the point of view of possible biological activity, the compounds included in cluster 2 exhibit an antianginal type of possible activity. Cluster 1 has a similar picture to cluster 2, except for compound 4, which may exhibit an inhibitory effect on the proteasome ATPase. In the studied series, only compound 6 appears to be a possible enhancer of HMGCS2 expression. It is known that such compounds are therapeutic agents for the treatment of malignant neoplasms of the GIT [49].
Prediction of toxicities based on mutagenicity, carcinogenicity, hepatotoxicity, and ecotoxicity revealed that all the studied compounds can exhibit mutagenicity and high hepatotoxicity. Ecotoxicity values obtained are above average for both pesticides and insecticides. The maximum value of 4.169 is observed for compound 2 with an acceptable standard of ≥11 μg [50]. For other compounds, the value varies from 5.151 to 5.681.
4 Conclusions
In summary, a sonochemical method for the synthesis of a series of novel 3-N-substituted 6-((4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxy)pyridazin-3(2H)-one derivatives was carried out, which corresponds to the principles of “green chemistry.” Comparison of this technique with the traditional methods for the synthesis of the researched, as well as previously obtained compounds with a similar structure [51,52,53,54], shows that the reactions with ultrasonic activation of molecules proceed quickly and in high yields. Since industrial ultrasonic reactors exist, the synthesis of these potentially bioactive compounds can be carried out on a large scale, which will have a significant economic effect.
Based on the data of in silico analysis, the predicted diversity of the biological activity of the synthesized compounds was established. Our results show that the studied compounds show similar activity within the group. Compound 6 is different and appears to be a possible enhancer of HMGCS2 expression. From the point of view of possible toxicity, the studied compounds may have pesticidal and insecticidal properties.
-
Funding information: This work was supported by the SCS of the Republic of Armenia within the framework of scientific project No. 21T-1D165.
-
Author contributions: Tiruhi Gomktsyan, Vergush Pivazyan: conceptualization, data curation, validation, investigation, visualization, methodology; Angelina Khachatryan, Diana Avakyan: resources, software, formal analysis, visualization, methodology; Lernik Hunanyan: conceptualization, supervision, investigation, methodology, resources, software, formal analysis, visualization, writing – original draft; Roza Shainova, Armen Karapetyan, Emma Ghazaryan, Asya Vorskanyan: resources, data curation, formal analysis, validation, investigation, visualization, methodology; Siranush Harutyunyan, Yana Gharibyan: formal analysis, validation, investigation, visualization; Aleksandr Yengoyan: conceptualization, supervision, validation, investigation, methodology, writing – original draft, project administration, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Patel D, Patel R, Kumari P, Patel N. Microwave assisted synthesis and in vitro antimicrobial assessment of quinolone based s-triazines. Heterocycl Commun. 2011;17(1–2):33–41. 10.1515/HC.2011.006.Search in Google Scholar
[2] Cholera AY, Ladva KD. A convenient synthesis of trisubstituted 1,3,5-triazine derivatives and their antimicrobial screening. Der Pharm Chem. 2018;10(4):57–61. www.derpharmachemica.com.Search in Google Scholar
[3] Al-Rasheed HH, Al Alshaikh M, Khaled JM, Alharbi NS, El-Faham A. Ultrasonic irradiation: synthesis, characterization, and preliminary antimicrobial activity of novel series of 4,6-disubstituted-1,3,5-triazine containing hydrazine derivatives. J Chem. 2016;9:Article ID 3464758. 10.1155/2016/3464758.Search in Google Scholar
[4] Vembu S, Pazhamalai S, Gopalakrishnan M. Potential antibacterial activity of triazinedendrimer: Synthesis and controllable drug release properties. Biorg Med Chem. 2015;23(15):4561–6. 10.1016/j.bmc.2015.06.009.Search in Google Scholar PubMed
[5] Sunduru N, Gupta L, Chaturvedi V, Dwivedi R, Sinha S, Chauhan PMS. Discovery of new 1,3,5-triazine scaffolds with potent activity against mycobacterium tuberculosis H37Rv. Eur J Med Chem. 2010;45:3335–45. 10.1016/j.ejmech.2010.04.017.Search in Google Scholar PubMed
[6] Avupati VR, Yejella RP, Parala VR, Killari KN, Papasani VMR, Cheepurupalli P, et al. Synthesis, characterization and in vitro biological evaluation of some novel 1, 3, 5-triazine-Schiff base conjugates as potential antimycobacterial agents. Biorg Med Chem Lett. 2013;23(21):5968–70. 10.1016/j.bmcl.2013.08.063.Search in Google Scholar PubMed
[7] Barakat A, El-Senduny FF, Almarhoon Z, Al-Rasheed HH, Badria FA, Al-Majid AM, et al. Synthesis, X-ray crystal structures, and preliminary antiproliferative activities of new s-triazine-hydroxybenzylidenehydrazone derivatives. J Chem. 2019;10:Article ID 9403908. 10.1155/2019/9403908.Search in Google Scholar
[8] Sun D, Melman G, Letourneau N, Hays AM, Melman A. Synthesis and antiproliferating activity of iron chelators of hydroxyamino-1,3,5-triazine family. Bioorg Med Chem Lett. 2010;20(2):458–60. 10.1016/j.bmcl.2009.11.130.Search in Google Scholar PubMed
[9] Krecmerová M, Holý A, Pískala A, Masojídková M, Andrei G, Naesens L, et al. Antiviral activity of triazine analogues of 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine (cidofovir) and related compounds. J Med Chem. 2007;50(5):1069–77. 10.1021/jm061281.Search in Google Scholar
[10] Ghaib A, Menager S, Verite P, Lafont O. Synthesis of variously 9,9-dialkylated octahydropyrimido [3,4-a]-s-triazines with potential antifungal activity. Il Farmaco. 2002;57(2):109–16. 10.1016/s0014-827x(01)01181-8.Search in Google Scholar PubMed
[11] Kumar R, Gupta L, Pal P, Khan Sh, Singh N, Katiyar SB, et al. Synthesis and cytotoxicity evaluation of (tetrahydro-β-carboline)-1,3,5-triazine hybrids as anticancer agents. Eur J Med Chem. 2010;45(6):2265–76. 10.1016/j.ejmech.2010.02.001.Search in Google Scholar PubMed
[12] Makowska A, Saczewski F, Bednarski PJ, Saczewski J, Balewski L. Hybrid molecules composed of 2,4-diamino-1,3,5-triazines and 2-imino-coumarinsand coumarins. Synthesis and cytotoxic properties. Molecules. 2018;23(7):1616–32. 10.3390/molecules23071616.Search in Google Scholar PubMed PubMed Central
[13] Patel AB, Chikhalia KH, Kumari P. An efficient synthesis of new thiazolidin-4-one fused s-triazines as potential antimicrobial and anticancer agents. J Saudi Chem Soc. 2014;18(5):646–56. 10.1016/j.jscs.2014.02.002.Search in Google Scholar
[14] Liu B, Sun T, Zhou Z, Du L. A systematic review on antitumor agents with 1,3,5-triazines. Med Chem. 2015;5:131–48. 10.4172/2161-0444.1000255.Search in Google Scholar
[15] Sakakibara N, Balboni G, Congiu C, Onnis V, Demizu Y, Misawa T, et al. Design, synthesis, and anti-HIV-1 activity of 1-substituted 3-(3,5-dimethylbenzyl)triazine derivatives. Antivir Chem Chemother. 2015;24(2):62–71. 10.1177/2040206615612208.Search in Google Scholar PubMed PubMed Central
[16] Xiong Y-Z, Chen F-E, Balzarini J, De Clercq E, Pannecouque Ch. Structural modulation of diaryltriazine with potent anti-HIV activity. Eur J Med Chem. 2008;43:1230–6. 10.1016/j.ejmech.2007.08.001.Search in Google Scholar PubMed
[17] Klenke B, Stewart M, Barrett MP, Brun R, Gilbert IH, Gorka JB. Synthesis and biological evaluation of s-triazine substituted polyamines as potential new anti-trypanosomal drugs. J Med Chem. 2001;44:3440–52. 10.1021/jm010854+.Search in Google Scholar PubMed
[18] Baliani A, Bueno GJ, Stewart ML, Yardley V, Brun R. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J Med Chem. 2005;48(17):5570–9. 10.1021/jm050177+.Search in Google Scholar PubMed
[19] Kumar A, Srivastava K, Kumar SR, Puri SK, Chauhan PMS. Synthesis and bioevaluation of hybrid 4-aminoquinoline triazines as a new class of antimalarial agents. Bioorg Med Chem. 2008;18:6530–3. 10.1016/j.bmcl.2008.10.049.Search in Google Scholar PubMed
[20] Sunduru N, Sharma M, Srivastava K, Rajakumar S, Puri S, Saxena JK, et al. Discovery of new 1,3,5-triazine scaffolds with potent activity against mycobacterium tuberculosis H37Rv. Biorg Med Chem. 2009;17(17):6451–62. 10.1016/j.bmc.2009.05.075.Search in Google Scholar PubMed
[21] Agarwal A, Srivastava K, Puri S, Chauhan PM. Syntheses of 2,4,6-trisubstituted triazines as antimalarial agents. Biorg Med Chem Lett. 2005;15(3):531–3. 10.1016/j.bmcl.2004.11.052.Search in Google Scholar PubMed
[22] Khattab SN, Khalil HH, Bekhit AA, Abd El-Rahman MM, El-Faham A, Albericio F. Synthesis and preliminary biological evaluation of 1,3,5-triazine amino acid derivatives to study their MAO inhibitors. Molecules. 2015;20(9):15976–88. 10.3390/molecules200915976.Search in Google Scholar PubMed PubMed Central
[23] Ma X, Poon T-Y, Wong PTH, Chui W-K. Synthesis and in vitro evaluation of 2,4-diamino-1,3,5-triazine derivatives as neuronal voltage-gated sodium channel blockers. Bioorg Med Chem Lett. 2009;19(19):5644–7. 10.1016/j.bmcl.2009.08.052.Search in Google Scholar PubMed
[24] Sahin MF, Badiçoglu B, Gökçe M, Küpeli E, Yeşilada E. Synthesis and analgesic and antiinflammatory activity of methyl 6-substituted-3(2H)-pyridazinone-2-ylacetate derivatives. Arch Pharm. 2004;337(8):445–52. 10.1002/ardp.200400896.Search in Google Scholar PubMed
[25] Lakshmayya AS, Asif M. Analgesic and anti-inflammatory activities of several 4-substituted-6-(3’-nitrophenyl)pyridazin-(2H)-3-one derivatives. Braz J Pharm Sci. 2013;49(4):903–9. 10.1590/S1984-82502013000400030.Search in Google Scholar
[26] Kandile NG, Mohamed MI, Zaky H, Mohamed H. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur J Med Chem. 2009;44:1989–96. 10.1016/j.ejmech.2008.09.047.Search in Google Scholar PubMed
[27] Abd El-Salam NM, Mostafa MS, Ahmed GA, Othman YA. Synthesis and antimicrobial activities of some new heterocyclic compounds based on 6-chloropyridazine-3(2H)-thione. J Chem. 2013;2013:Article ID 890617. 10.1155/2013/890617.Search in Google Scholar
[28] Behalo MS, Gad El-karim IA, Issac YA, Farag MAJ. Synthesis of novel pyridazine derivatives as potential antimicrobial agents. Sulf Chem. 2014;35:661–73. 10.1080/17415993.2014.950661.Search in Google Scholar
[29] Wu J, Song B, Chen H, Bhadury P, Hu D. Synthesis and antifungal activity of 5-chloro-6-phenylpyridazin-3(2H)-one derivatives. Molecules. 2009;14(9):3676–87. 10.3390/molecules14093676.Search in Google Scholar PubMed PubMed Central
[30] Kim C, Kim S-B, Park M-S. Synthesis of novel 3-allylseleno-6-alkylthiopyridazines: their anticancer activity against MCF-7 cells. Arch Pharm Res. 2014;37:452–8. 10.1007/s12272-013-0244-x.Search in Google Scholar PubMed
[31] Kim C, Kim S-B, Jung J, Park M-S. Design, synthesis, and biological evaluation of selenium-incorporated aminopyridazines as anticancer agents. Bull Korean Chem Soc. 2015;36(6):1669–75. 10.1002/bkcs.10319.Search in Google Scholar
[32] Dogruer DS, Sahin MF, Unlü S, Ito S. Studies on some 3(2H)-pyridazinone derivatives with antinociceptive activity. Arch Pharm (Weinh). 2000;333(4):79–86. 10.1002/(sici)1521-4184(20004)333:4<79:aid-ardp79>3.0.co;2-s.Search in Google Scholar
[33] Corsano S, Strapoghetti G, Barbaro R. Synthesis of new pyridazinone derivatives and their affinity towards α1–α2-adrenoceptors. Bioorg Med Chem. 1999;7(5):933–41. 10.1016/s0968-0896(99)00046-2.Search in Google Scholar
[34] Kim E-Y, Kang S-T, Jung H, Park CH, Yun C-S, Hwang JY, et al. Discovery of substituted pyrazol-4-yl pyridazinone derivatives as novel c-Met kinase inhibitors. Arch Pharm Res. 2016;39(4):453–64. 10.1007/s12272-015-0703-7.Search in Google Scholar
[35] Zhang M, Hu F-Z, Zhao T, Yang L-Q, Yang H-Z. Synthesis and herbicidal evaluation of 3-N-substituted amino-6-benzyloxypyridazine derivatives. J Heterocycl Chem. 2014;51(5):1404–9. 10.1002/jhet.1831.Search in Google Scholar
[36] http://www.alanwood.net/pesticides/class_pesticides.html.Search in Google Scholar
[37] Yengoyan AP, Shainova RS, Gomktsyan TA, Karapetyan AV. Synthesis of novel 6-(3,5-dimethyl-1H-pyrazol-1-yl)pyridazin-3(2H)-one derivatives and their preliminary biological evaluation. J Chem Res. 2018;42(10):535–9. 10.3184/174751918X15389922302823.Search in Google Scholar
[38] Shainova RS, Gomktsyan TA, Karapetyan AV, Yengoyan AP. Synthesis and biological evaluation of 3-O-substituted 1-benzyl-6-oxo-1,6- dihydropyridazine derivatives. J Chem Res. 2019;43(9–10):352–8. 10.1177/1747519819866402.Search in Google Scholar
[39] Shainova RS, Gomktsyan TA, Karapetyan AV, Yengoyan AP. Synthesis and biological properties of novel 3-(pyrazol-1-yl)-6-oxopyridazine derivatives. J Chem Res. 2020;26(5–6):271–6. 10.1177/1747519819897523.Search in Google Scholar
[40] Gomktsyan TA, Shainova RS, Karapetyan AV, Yengoyan AP. Synthesis and biological activity of new 3-pyrazolyl-6-hydrazinylpyridazine derivatives. Rus J Gen Chem. 2021;91(10):2019–24. 10.1134/S1070363221100145.Search in Google Scholar
[41] Yengoyan AP, Mamyan SS, Gomktsyan TA, Hambardzumyan EN, Vorskanyan AS, Eliazyan KA, et al. Hindered Internal Rotation about a C-N Bond in Some Trisubstituted 1,3,5-Triazines. Chem Heterocycl Comp. 2005;41(8):1059–61.10.1007/s10593-005-0279-0Search in Google Scholar
[42] Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7 Article number42717. 10.1038/srep42717.Search in Google Scholar
[43] Xiong G, Wu Z, Yi J, Fu L, Yang Z, Hsieh C, et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021;49(W1):W5–W14. 10.1093/nar/gkab255.Search in Google Scholar PubMed PubMed Central
[44] Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, et al. admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35(6):1067–9. 10.1093/bioinformatics/bty707.Search in Google Scholar PubMed
[45] Kamaryan VS, Hunanyan LS, Popugaeva EA. XXVII Symposium on Bioinformatics and Computer-Aided Drug Discovery. 2021. p. 58. (in Russia). http://way2drug.com/dr/download/BCADD/KamaryanVS_BCADD_2021-04-06.pdf.Search in Google Scholar
[46] Filimonov DA, Lagunin AA, Gloriozova TA, Rudik AV, Druzhilovskii DS, Pogodin PV, et al. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem Heterocycl Comp. 2014;50(3):444–57. 10.1007/s10593-014-1496-1.Search in Google Scholar
[47] Lipinski ChA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today Technol. 2004;1(4):337–41. 10.1016/j.ddtec.2004.11.007.Search in Google Scholar PubMed
[48] Vogt M, Bajorath J. ccbmlib – a Python package for modeling Tanimoto similarity value distributions. [version 2; peer review: 2 approved]. F1000 Res. 2020;10:9:Chem Inf Sci-100:1–14. 10.12688/f1000research.22292.2.Search in Google Scholar PubMed PubMed Central
[49] Chen SW, Chou CT, Chang CC, Li Y-J, Chen S-T, Lin I-Ch, et al. HMGCS2 enhances invasion and metastasis via direct interaction with PPARα to activate Src signaling in colorectal cancer and oral cancer. Oncotarget. 2017;8(14):22460–76. 10.18632/oncotarget.13006.Search in Google Scholar PubMed PubMed Central
[50] Xu X, Zhao P, Wang Z, Zhang X, Wu Z, Li W, et al. In silico prediction of chemical acute contact toxicity on honey bees via machine learning methods. Toxicol Vitro. 2021;72:105089. 10.1016/j.tiv.2021.105089.Search in Google Scholar PubMed
[51] Dovlatyan VV, Gomktsyan TA, Oganisyan MG, Khachatryan LA, Yengoyan AP. Synthesis of azinyloxypyridazinyl derivatives of oxyacids and aryloxyethanols. Hayastani Kimiakan H (Chem J Armen). 2003;56(4):75–9. (Language: Russian).Search in Google Scholar
[52] Dovlatyan VV, Gomktsyan TA, Oganisyan MG. (Azinyloxy)pyridazines. Hayastani Kimiakan H (Chem J Armen). 2003;56(4):68–74. (Language: Russian).Search in Google Scholar
[53] Dovlatyan VV, Gomktsyan TA, Oganisyan MG, Yengoyan AP, Khachatryan LA. O-,N-Derivatives of azinyloxypyridazines. Hayastani Kimiakan H (Chem J Armen). 2003;56(1–2):96–101. (Language: Russian).Search in Google Scholar
[54] Dovlatyan VV, Gomktsyan TA, Karapetyan AV, Yengoyan AP. Synthesis and some reactions of hydroxypyridazinone derivatives. Hayastani Kimiakan H (Chem J Armen). 2006;59(3):95–104. (Language: Russian).Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

