The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
-
Huaizhu Liu
Abstract
This study presents a novel approach for the reuse of uncontaminated fracturing flowback fluids to improve the inhibitory and lubricating properties of water-based drilling fluids (WBFs), curb environmental pollution arising from flowback fluids, and substantially mitigate the expenses associated with WBFs. The experimental design was optimized using orthogonal experiments and range analyses, whereby the modified rubber powder was set at 2.0%, xanthan gum at 0.15%, and a plant phenol to modified complexing agent ratio of 1:0.01. The assessment of the performance evaluation tests indicated that the use of uncontaminated fracturing flowback fluids as the base water can remarkably enhance the inhibitory and lubricating properties of WBFs. Precisely, this approach reduces the linear expansion rate from 62.31% to 21.25%, the reduction rate of extreme pressure lubrication coefficient by 87.98%, and the reduction rate of mud cake sticking factor by 59.86%. This investigation has established the potential environmental and economic benefits of reusing clean fracturing flowback fluids in WBFs.
Nomenclature
- AV
-
apparent viscosity
- FLAPI
-
API fluid loss
- PV
-
plastic viscosity
- DSC
-
differential scanning calorimeter
- KPAM
-
potassium polyacrylamide
- TGA
-
thermal gravimetric analysis
- t g
-
lubrication coefficient
- XC
-
xanthate gum
- YP
-
dynamic shear force
- YP/PV
-
dynamic shear force ratio
- WBFs
-
water-based drilling fluids
1 Introduction
Hydraulic fracturing stands as a critically vital technique for augmenting the productivity of oil and gas fields [1,2,3]. Notably, the gradual growth of hydraulic fracturing in oil and gas fields has led to a marked escalation in the volumes of fracturing flowback fluids. In contrast to other wastewaters emanating from oil and gas fields, the intricate makeup of fracturing flowback fluids renders them highly complex [4,5,6,7,8]. The flowback fluid is comprised of a multitude of chemical additives, sediments, bacteria, heavy metals, and other substances that are present in the formation during the flowback process. As such, fracturing fluid is characterized by a complex composition, high viscosity, strong stability, elevated salinity, and a high content of suspended solids. These factors contribute to the challenges associated with the disposal of fracturing flowback fluids [9,10]. Discharging untreated fracturing fluid directly would result in significant pollution of the soil and surrounding water. Harmless treatment and recycling of fracturing flowback fluids are crucial for solving the pollution caused by fracturing flowback fluids.
The flowback fluids that result from hydraulic fracturing are notably complex in nature, consisting of a multitude of different chemical compounds. In particular, the fundamental constituents of these fluids are heavily concentrated melon gum, various polymers, and a range of other chemical substances [11,12,13,14]. Additionally, the flowback fluid contains elements such as S and Fe, as well as some bacteria. Several treatment methods have been developed for the primary components of fracturing flowback fluids. Standard treatment technologies for fracturing flowback fluids comprise physical, chemical, and biological methods [15,16,17,18,19,20]: The physical method targets suspended contaminants in the fracturing flowback fluids and uses gravity separation, filtration, centrifugal separation, and other physical methods for treatment. Physical treatment offers the advantages of a simple process, low investment, and rapid effect. Lu et al. were the first to use ultrasound to treat fracturing flowback fluids and found that ultrasound treatment was even more effective than chemical treatment in reducing fluids’ viscosity. However, the physical method can only treat a small number of contaminants and has limited use for some soluble materials. In the chemical treatment, chemicals are added to the fracturing flowback fluids to react chemically with organic contaminants and cause decomposition or precipitation. The homogeneous Sono-Fenton process decomposes hydroxypropyl guar rubber in fracturing flowback fluids, achieving a COD removal rate of 81.15% [21,22,23]. However, the treatment effect of this method is easily influenced by various factors, such as field operation and process. The biological method is a process that uses the metabolism of microorganisms to oxidize and decompose organic matter and then performs the conversion of organic matter to stabilize inorganic matter [24,25,26]. Although good results can be achieved with the biological treatment of fracturing flowback fluids, it is difficult to find suitable microorganisms for fracturing flowback fluids.
The proper disposition of fracturing flowback fluids has gained in importance due to the heightened environmental regulations imposed by governments. There has been a surge in the number of firms and scholars that are delving into the realm of recycling fracturing flowback fluids [1,2,3,17,18,19,20]. Currently, clean fracturing fluids are used in large oil fields because of their environmental friendliness, low viscosity, lower residues, and less damage to the reservoir. However, clean fracturing fluids may contain a variety of surfactants and other additives during the flowback process and cannot be discharged directly. Additionally, large amounts of water are consumed during fracturing, and direct discharge of water resources results in significant waste generation. Therefore, recycling the clean fracturing fluid must be considered. The reflux of the clean fracturing fluid usually contains a large amount of quaternary ammonium salt, inhibiting clay hydration expansion. At the same time, the surfactant components contained in the clean fracturing fluid may have good lubricating properties.
In this study, an assessment was conducted on the feasibility of producing WBFs through the utilization of unadulterated fracturing flowback fluids. Additionally, the optimization of WBFs was achieved through the employment of orthogonal experimental techniques, followed by a comprehensive evaluation of the drilling fluid performance.
2 Materials and methods
2.1 Materials
Sodium-bentonite was purchased from Xi’an Fengyun Chemical Co. Ltd. Polyaluminum chloride was purchased from Yangzhou Runda Oilfield Chemical Co., Ltd. Modified plant phenol was purchased from Lingshi County Hengxing Co., Ltd., Shanxi Province. Xanthan gum and modified gum were from Changqing oilfield site. The clean fracturing flowback fluids were provided by Tangshan Jiyou Ruifeng, Chemical Colimmted Company, Jidong Oilfield, Petro China.
2.2 Clean fracturing flowback fluids preparation of water-based drilling fluid (WBF) method
Complexing agents and modified plant phenol were added to the clean fracturing flowback fluids to make the pH alkaline. Mix water with treated clean fracturing rejection fluid at 1:1, 1:2, 1:3, 1:4, and 1:5. After mixing well, add 4% sodium bentonite to the mixture to prepare the base slurry and age it at room temperature for 12 h before use.
2.3 Determination of the surfactant content
The standard curve was fitted according to the relationship between Abs and the mass concentration of the clean fracturing fluid [27]. The absorbance value (Abs) of standard clean fracturing fluid with different concentrations was determined by UV2802 UV spectrophotometer (Shanghai Dapping Instrument Co., Ltd).
2.4 Component analysis of the clean fracturing flowback fluids
According to the “Oilfield Water Analysis Method” (SY/T 5523-2006) and other industry standards and commonly used experimental test methods to perform the component analysis of clean fracturing flowback water samples.
2.5 Drilling fluid performance test
The drilling fluid was prepared by mixing the clean fracturing flowback fluids with the base mud of different concentrations in a particular ratio with xanthan gum as a sealant [28]. According to the test standard GB/T 16783.1-2014, the apparent viscosity (AV), plastic viscosity (PV), dynamic shear force (YP), dynamic shear force ratio (YP/PV), density (ρ), API fluid loss (FLAPI), lubrication coefficient (t g), and other performance parameters of the drilling fluid were determined.
2.6 Evaluation of drilling fluid inhibition and lubrication
According to the inhibition evaluation standard SY/T6335-1997, the inhibition effect of drilling fluid on clay swelling was evaluated by measuring the change in bentonite swelling data within 2 h. The mass ratio of bentonite to water was 2:1 to produce mud balls of approximately 10 g mass and the mud balls were immersed in the same volume of different treatment agent solutions, and the appearance and morphological changes of the mud balls were recorded by photography after a particular time [29]. The prepared drilling fluid was centrifugally dried, and 5–10 g samples were added to the thermogravimetric analyzer (TGA/DSC1, METTLER TOLEDO, Germany). The N2 flow rate was set to 10 mL·min−1, the temperature rise rate was set to 10°C·min−1, and the mass changes of the measured samples were recorded. Referring to the enterprise standard Q/SHCG 4-2011 of China Petroleum and Chemical Corporation, the extreme pressure lubrication coefficient of drilling fluid was measured by using an extreme pressure lubrication meter (112-00-01, Osfit Test Equipment Corporation, America).
3 Results
3.1 Detection of surfactant content in clean fracturing flowback fluids
The basis of the clean fracturing fluid is mainly a viscoelastic surfactant system, the main components of which are various surfactants [30]. When using drilling fluids with clean fracturing flowback fluids, the surfactant content in clean fracturing flowback fluids should be measured. The relationship between absorption and surfactant content of clean fracturing fluid was determined by measuring the absorption of standard fluid with different surfactant contents. The standard curve of the clean fracturing fluid is shown in Figure 1.
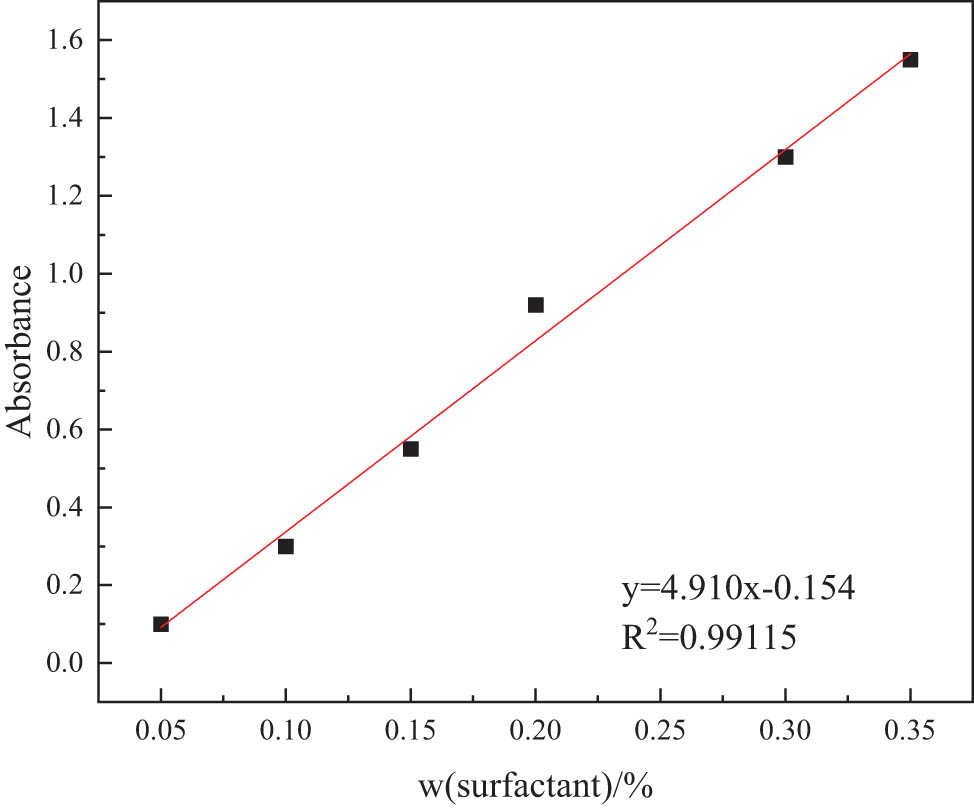
Standard curve of clean fracturing fluid.
The fitting equation of the standard curve for clean fracturing fluid is y = 4.910x − 0.154, and the linear correlation coefficient R 2 = 0.99115. The curve exhibits a good linear relationship and conforms to Beer’s law. If the mass fraction of surfactant in the flowback solution is within the range shown in Figure 1, it can be calculated directly using the fitting equation. If the mass fraction is above this range, it can be measured by dilution conversion.
The clean fracturing flowback fluids are first pretreated at room temperature by natural sedimentation, high-speed centrifugation, and membrane filtration. The effective surfactant content in the pretreated clean fracturing flowback fluids was determined. The experimental results are shown in Table 1. The mean effective mass fraction of surfactant in the clean fracturing flowback fluids was 0.343% after repeated tests on the same batch of samples.
Determination of surfactant content in clean fracturing flowback fluids
| Sample | Absorbance | w (surfactant) (%) |
|---|---|---|
| 1 | 1.663 | 0.37 |
| 2 | 1.550 | 0.35 |
| 3 | 1.368 | 0.31 |
3.2 Components of fracturing flowback fluids
Clean fracturing flowback fluids contain a variety of additives and impurities derived from the formation [31]. Therefore, a water quality analysis should be performed during reuse. The results of the water quality analysis of clean fracturing flowback fluids are shown in Table 2.
Analysis of fracture flowback fluids components
| Type | Result |
|---|---|
| pH | 6.0 |
| CI− (mg·L−1) | 8,421 |
|
|
214 |
| Ca2+ (mg·L−1) | 952 |
| Mg2+ (mg·L−1) | 19 |
| Na+ (mg·L−1) | 3,246 |
| K+ (mg·L−1) | 126 |
| Fe2+ and Fe3+ (mg·L−1) | 30 |
| Ba2+ (mg·L−1) | 25 |
| Total mineralization | 13,464 |
| Oil (mg·L−1) | 300 |
| Suspended matter content (SS, mg·L−1) | 597 |
| Chemical oxygen demand (COD, mg·L−1) | 5,000 |
| Total dissolved solid (TDS, mg·L−1) | 60,000 |
| Turbidity (NTU, mg·L−1) | 367 |
| Viscosity (MPa·s) | 5.0 |
The composition and content of fracturing flowback fluids produced in different zones vary widely. The content of sodium ions in the above fracturing flowback fluids is high. This is because sodium chloride is commonly used as a clay stabilizer in fracturing fluids. Sulfate ions in the fracturing flowback fluids come from the formation water and the original fracturing fluid. Iron ions in the fracturing flowback fluids may be due to corrosion in the pipeline. Due to the large number of organics and crude oil in the clean fracturing flowback fluids and the complex formation environment, the level of chemical oxygen demand and suspended solids in the clean fracturing flowback fluids is high. The salt content and total dissolved solids in the fracturing flowback fluids are high, so it is necessary to treat various harmful salt ions in the flowback fluids.
3.3 Screening of mixing ratio between clean fracturing flowback fluids and base mud
According to the method in Section 2.2, fracturing flowback fluids was mixed with base mud at different concentrations and proportions to produce a drilling fluid. The properties of the drilling fluid were investigated, and the experimental results are shown in Table 3.
Performance evaluation of drilling fluids formulated with a mixture of water and different ratios of clean fracturing rejection fluid
| Ratio | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV | pH | ρ (g·cm−3) | t g | FLAPI (mL) |
|---|---|---|---|---|---|---|---|---|
| 1:1 | 18.5 | 7.6 | 1.9 | 0.25 | 9.35 | 1.027 | 0.2024 | 22.6 |
| 1:2 | 17.8 | 7.2 | 2.2 | 0.31 | 9.26 | 1.036 | 0.2358 | 21.8 |
| 1:3 | 16.7 | 5.8 | 2.0 | 0.36 | 9.14 | 1.039 | 0.1975 | 17.2 |
| 1:4 | 14.9 | 3.6 | 1.0 | 0.29 | 9.25 | 1.042 | 0.2257 | 19.9 |
| 1:5 | 15.3 | 4.5 | 1.2 | 0.27 | 9.38 | 1.047 | 0.2261 | 23.4 |
According to Table 3, the filtration loss of the drilling fluid prepared by mixing water with clean fracture rejection fluid at 1:3 was 17.2 mL. The dynamic–plastic ratio of the drilling fluid prepared at this mixing ratio is 0.36, indicating that the drilling fluid has suitable shear dilution. The friction of the cake is reduced to 0.1975, indicating that the lubrication performance is relatively good. To ensure that the drilling fluid configured with fracturing flowback fluids has good inhibition performance and lubrication, the following experiments are conducted by this ratio.
3.4 Drilling fluid formulation optimization
The main component of the clean fracturing fluid is quaternary ammonium salt, which strongly inhibits the hydration expansion of clay and cannot be directly used to prepare drilling fluids [32]. Therefore, when cleaning fracturing flowback fluids to prepare drilling fluid, it is necessary to add a colloid protection reagent to maintain the stability of the drilling fluid. XC (xanthan gum) and modified gum powder were used as protective agents to adjust the mixing ratio of plant phenol and complexing agent. An orthogonal test was conducted to optimize the drilling fluid formula. The table of the orthogonal experimental design is shown in Table 4. The test results of the drilling fluid are shown in Table 5.
L9 (33) orthogonal experimental design
| Number | Modified rubber powder | XC (%) | Plant phenol and complexing agent |
|---|---|---|---|
| 1 | 0.5 | 0.05 | 1:0.01 |
| 2 | 0.5 | 0.10 | 1:0.02 |
| 3 | 0.5 | 0.15 | 1:0.03 |
| 4 | 1.0 | 0.05 | 1:0.02 |
| 5 | 1.0 | 0.10 | 1:0.03 |
| 6 | 1.0 | 0.15 | 1:0.01 |
| 7 | 1.5 | 0.05 | 1:0.03 |
| 8 | 1.5 | 0.10 | 1:0.01 |
| 9 | 1.5 | 0.15 | 1:0.02 |
The performance of drilling fluid
| Number | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV | pH | ρ (g·cm−3) | t g | FLAPI (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 21.4 | 19.4 | 6.0 | 0.31 | 9.14 | 1.031 | 0.1125 | 13.6 |
| 2 | 18.8 | 17.6 | 5.1 | 0.29 | 9.26 | 1.044 | 0.1263 | 14.9 |
| 3 | 16.7 | 14.3 | 5.4 | 0.38 | 9.23 | 1.035 | 0.0957 | 18.6 |
| 4 | 19.4 | 14.9 | 4.7 | 0.32 | 9.02 | 1.042 | 0.1123 | 15.4 |
| 5 | 23.6 | 16.3 | 6.0 | 0.37 | 8.86 | 1.041 | 0.1354 | 14.6 |
| 6 | 26.8 | 17.6 | 5.1 | 0.29 | 8.79 | 1.038 | 0.1268 | 13.2 |
| 7 | 25.3 | 19.4 | 6.0 | 0.31 | 8.64 | 1.046 | 0.1397 | 12.9 |
| 8 | 32.5 | 16.9 | 3.4 | 0.20 | 8.82 | 1.038 | 0.1428 | 14.5 |
| 9 | 33.4 | 16.5 | 3.9 | 0.24 | 8.74 | 1.044 | 0.1385 | 15.8 |
According to the data in Table 5, the range method was used to analyze the experimental results and optimize the reaction conditions. The corresponding mean values of AV and PV in the orthogonal test are shown in Tables 6 and 7.
Mean corresponding table of AV
| Level | Modified rubber powder | XC | Plant phenoll and complexing agent |
|---|---|---|---|
| 1 | 18.967 | 22.033 | 21.967 |
| 2 | 20.600 | 20.633 | 21.900 |
| 3 | 23.733 | 20.633 | 19.533 |
| Range | 4.766 | 1.400 | 1.367 |
| SupRank | 1 | 2 | 3 |
Mean corresponding table of PV
| Level | Modified rubber powder | XC | Plant phenol and complexing agent |
|---|---|---|---|
| 1 | 17.400 | 17.900 | 17.967 |
| 2 | 15.367 | 16.933 | 16.933 |
| 3 | 18.200 | 16.133 | 16.667 |
| Range | 2.833 | 1.767 | 1.634 |
| SupRank | 1 | 2 | 3 |
From the orthogonal test orthogonal tests and range analysis, modified gum powder is the main influencing factor for AV and PV on drilling fluid properties, as shown in Tables 6 and 7. The ratio of xanthan gum and plant phenol complexing agent had little effect. Therefore, in the following experiment, the concentration of xanthan gum was set at 0.05%, and the mixing ratio of plant phenol to complexing agent was set at 1:0.01 to investigate the effects of the concentration of the modified gum powder on the properties of the drilling fluid. The experimental results are shown in Table 8.
Effect of modified rubber powder concentration on drilling fluid properties
| Concentration (%) | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV | pH | ρ (g·cm−3) | t g | FLAPI (mL) |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 23.2 | 11.4 | 1.4 | 0.12 | 8.56 | 1.022 | 0.1254 | 12.6 |
| 1.0 | 25.6 | 13.3 | 2.1 | 0.16 | 8.48 | 1.031 | 0.1348 | 13.5 |
| 1.5 | 27.6 | 15.7 | 4.1 | 0.26 | 8.96 | 1.053 | 0.1025 | 11.8 |
| 2.0 | 33.5 | 16.8 | 5.2 | 0.31 | 9.10 | 1.041 | 0.1261 | 10.3 |
| 2.5 | 38.2 | 26.3 | 7.6 | 0.29 | 8.23 | 1.039 | 0.1358 | 11.4 |
As shown in Table 8, AV and PV are significantly affected by the concentration of the modified rubber powder. When the concentration of modified rubber powder was increased to 2.0%, the PV and AV of the drilling fluid were more suitable and exhibited good shear thinning. At this concentration, the fluid loss was reduced to 10.3 mL. Therefore, the optimum dosage of modified rubber powder is 2.0%.
Orthogonal tests and range analyses were conducted to investigate the effects of each material on t g and FLAPI in the drilling fluid formulation. The corresponding mean values of AV and PV in the orthogonal test are shown in Tables 9 and 10.
Mean corresponding table of t g
| Level | Modified rubber powder | XC | Plant phenol and complexing agent |
|---|---|---|---|
| 1 | 0.112 | 0.121 | 0.127 |
| 2 | 0.125 | 0.135 | 0.126 |
| 3 | 0.140 | 0.120 | 0.124 |
| Range | 0.028 | 0.015 | 0.014 |
| SupRank | 1 | 2 | 3 |
Mean corresponding table of FLAPI
| Level | Modified rubber powder | XC | Plant phenol and complexing agent |
|---|---|---|---|
| 1 | 9.700 | 7.967 | 7.767 |
| 2 | 8.400 | 8.667 | 9.367 |
| 3 | 8.400 | 9.867 | 9.367 |
| Range | 1.300 | 1.900 | 1.600 |
| SupRank | 3 | 1 | 2 |
Table 9 shows that modified rubber powder in the drilling fluid formulation is the main factor affecting t g. According to Table 8, the optimum formula of drilling fluid with modified gum powder 2.0%, xanthan gum 0.1%, and plant phenol:complexing agent = 1:0.01 was determined. Table 10 shows that xanthine has the most apparent effect on FLAPI in the drilling fluid formulation. Therefore, the modified gum powder content of 2.0% and plant phenol: complexing agent = 1:0.01 were used in the solid drilling fluid formulation to investigate the effect of the content of XC on fluid loss performance. The experimental results are shown in Table 11.
Effect of XC concentration on drilling fluid properties
| Concentration (%) | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV | pH | ρ (g·cm−3) | t g | FLAPI (mL) |
|---|---|---|---|---|---|---|---|---|
| 0.05 | 20.2 | 9.0 | 1.4 | 0.22 | 8.67 | 1.027 | 0.1053 | 8.5 |
| 0.10 | 23.5 | 11.1 | 2.1 | 0.30 | 8.51 | 1.045 | 0.1526 | 8.2 |
| 0.15 | 28.2 | 15.3 | 4.1 | 0.34 | 8.85 | 1.033 | 0.0842 | 7.0 |
| 0.20 | 33.6 | 18.6 | 5.2 | 0.26 | 8.12 | 1.052 | 0.1268 | 7.3 |
| 0.25 | 32.1 | 25.3 | 7.6 | 0.32 | 9.35 | 1.046 | 0.1324 | 9.8 |
As shown in Table 11, it can be seen that AV and PY showed an increasing trend with the increase of xanthan content. When the xanthan content increased to 0.15%, the fluid loss decreased to 7.0 mL. Under these conditions, the rheological properties of the drilling fluid can be well maintained. The t g value (filter cake friction) was reduced to 0.0842, indicating good lubrication performance. Therefore, the formula for the drilling fluid was set as follows: modified gum powder 2.0%, xanthan gum 0.15%, plant phenol: modified complexing agent = 1:0.01. This formula was used for the subsequent tests.
3.5 Effect of temperature on properties of drilling fluids treated with fracturing flowback fluids
The effect of temperature on the performance of the drilling fluid is shown in Table 12. As temperature increases, the AV of the drilling fluid first increases and then decreases while the PV decreases. When the temperature increased to 150℃, the drilling fluid with xanthan gum as the colloid protection reagent still performed well. When the temperature rose to 180°C, the filtration loss of the drilling fluid reached 22.6 mL, which could no longer meet the standard drilling requirements.
Effect of temperature on drilling fluid properties
| Temperature (°C) | AV (mPa·s) | PV (mPa·s) | YP (Pa) | YP/PV | pH | ρ (g·cm−3) | t g | FLAPI (mL) |
|---|---|---|---|---|---|---|---|---|
| 30 | 28.2 | 15.3 | 4.1 | 0.34 | 8.85 | 1.033 | 0.0842 | 7.0 |
| 90 | 19.5 | 10.6 | 2.6 | 0.25 | 8.12 | 1.024 | 0.1365 | 9.8 |
| 120 | 17.2 | 9.2 | 2.2 | 0.24 | 7.46 | 1.018 | 0.1563 | 10.3 |
| 150 | 13.3 | 8.3 | 1.7 | 0.21 | 7.24 | 1.023 | 0.1829 | 12.0 |
| 180 | 7.6 | 6.5 | 1.0 | 0.15 | 8.35 | 1.031 | 0.1721 | 22.6 |
3.6 Evaluation of inhibition of drilling fluids produced with clean fracturing flowback fluids
3.6.1 Linear expansion ratio
The linear expansion ratio of the drilling fluid and the supernatant prepared with the best formula was measured to evaluate the inhibition of clay. The experimental results are shown in Figure 2.
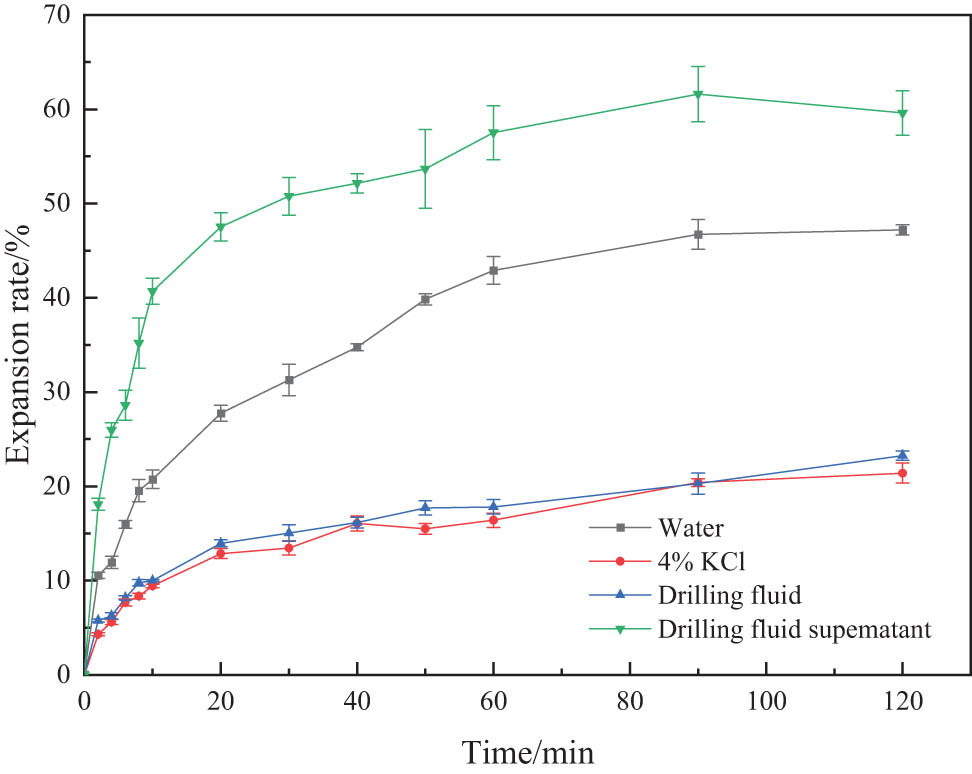
Effect of drilling fluid on the linear expansion rate of clay.
As shown in Figure 2, the drilling fluid and supernatant produced by clean fracturing flowback fluids have an excellent inhibition effect on the hydration expansion of clay. After 120 min, the expansion rate of the drilling fluid-treated clay is only 21.25%, which is better than that of the 4% KCl solution. When linear swelling experiments were performed using drilling fluid supernatant, the swelling of the clay was reduced from 62.31% to 23.64%. The reason is that the polar groups of quaternary ammonium salt contained in the clean fracturing flowback fluids can efficiently adsorb the clay, forming an adsorption layer. This creates an adsorption layer on the clay surface that further slows the flow of water molecules into the shale. Modified rubber powder prevents the penetration of free water into the clay by hydrogen bonds formed by a large number of hydroxyl groups in the molecular chain.
3.6.2 Mud ball tests
The inhibitory effect of the drilling fluid was tested by a mud ball test. The experimental results are shown in Figure 3. As shown in Figure 3a, the mud ball collapsed after it was soaked in tap water. The mud ball’s surface, soaked with the drilling fluid’s supernatant, remains intact and smooth. This indicates that the fluid prepared with fracturing flowback fluids can significantly inhibit the hydration expansion of the clay. The drilling fluid under this formulation can effectively inhibit water molecules from entering inside the mud ball, and its inhibition effect is better than 3% KCl and 1% KPAM.
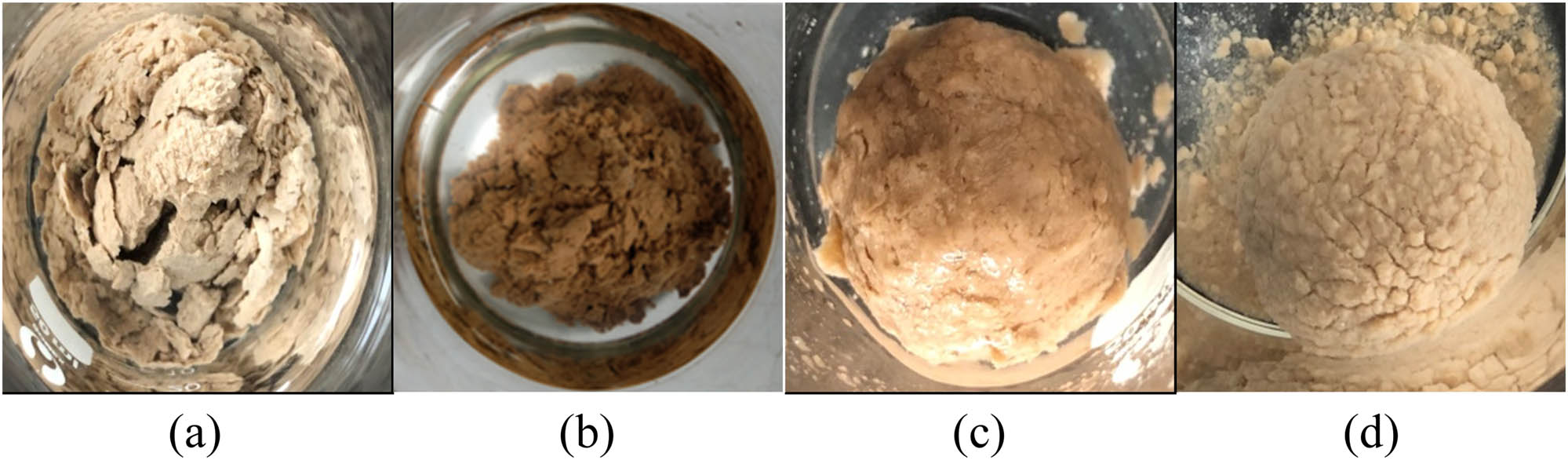
The mud balls are soaked in different treating agents for 24 h: (a) water, (b) 3% KCl, (c) 1% KPAM, and (d) the drilling fluid.
3.6.3 Thermogravimetric analysis
The bentonite in the drilling fluid was centrifuged and dried for thermogravimetric analysis. The experimental results are shown in Figure 4. As the temperature increases, the clay particles become weightless to a certain extent. When the temperature increased to 100℃, the mass loss rate of the bentonite hydrated with tap water reached 3.73%. At the same temperature, the mass loss rate of the fracturing flowback fluids was only 0.99%. The main reason for this is that the quaternary ammonium salts contained in the clean fracturing flowback fluids effectively slow down the penetration of water molecules into the clay layer. When the temperature rises to 300℃, the mass fraction of clay decreases significantly. This may be due to the thermal decomposition of surfactant components, xanthan gum, and modified guar gum in the drilling fluid.
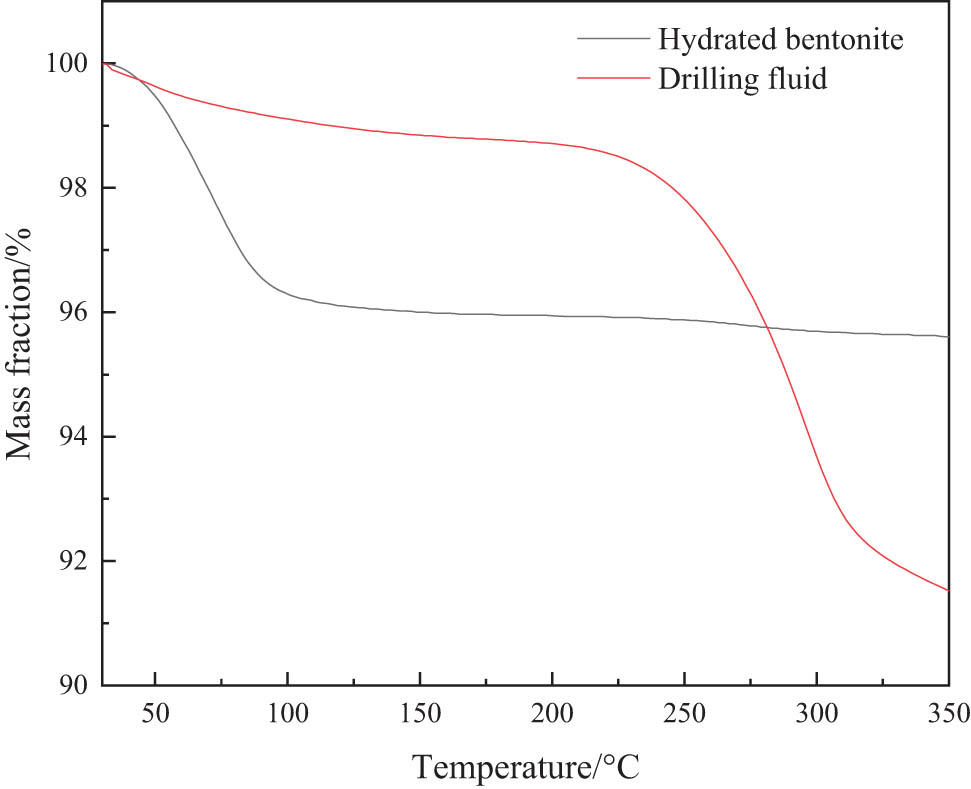
Thermogravimetric curve of clay in drilling fluid prepared with fracturing flowback fluids.
3.7 Evaluation of lubrication of drilling fluids produced with clean fracturing flowback fluids
The surfactants in clean fracturing flowback fluids ensure that the drilling fluid is prepared with lubrication. Therefore, the extreme pressure lubrication coefficient of the drilling fluid prepared with fracturing flowback fluids was measured to evaluate the lubrication performance. The experimental results are shown in Figure 5.
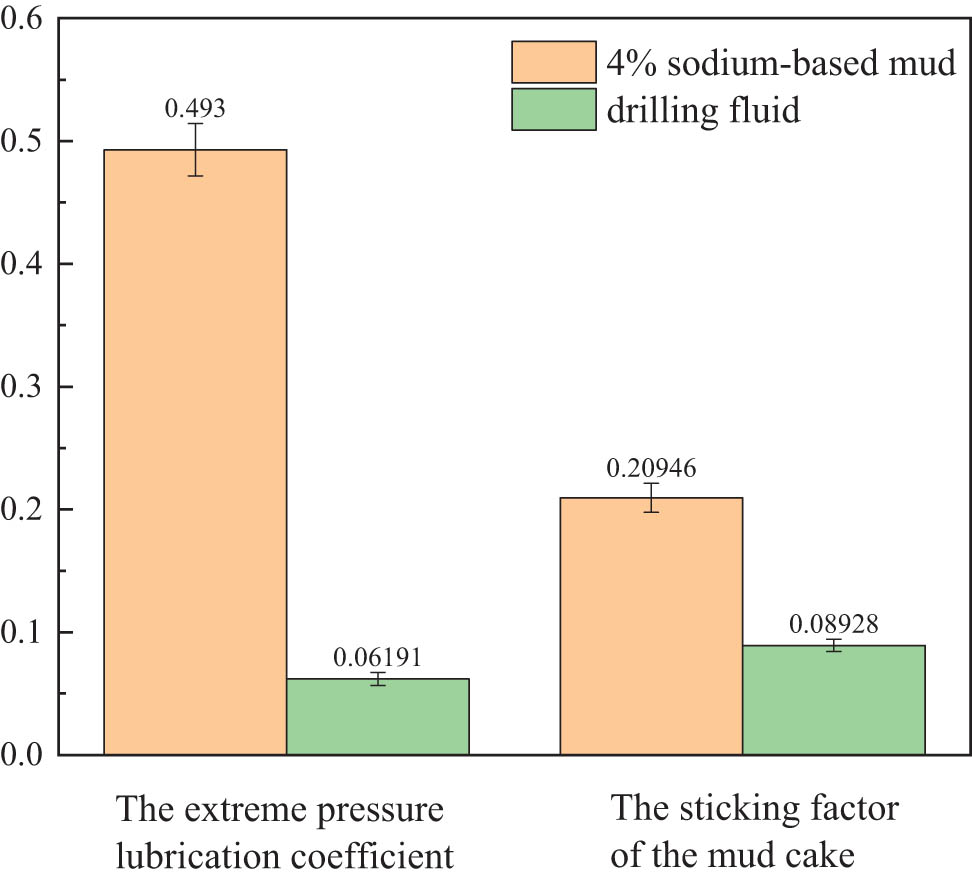
Lubrication performance evaluation of drilling fluid prepared with fracturing flowback fluids.
At room temperature, the extreme pressure lubrication coefficient of 4% sodium-based mud is 0.5100. The extreme pressure lubrication coefficient of drilling fluid prepared with clean fracturing flowback fluids was 0.0613, and the extreme pressure lubrication coefficient reduction rate was 87.98%. The sticking factor of the mud cake decreased from 0.2152 to 0.0864, and the sticking factor of the mud cake decreased by 59.86%. The extreme pressure lubrication coefficient of the drilling fluid decreases significantly and has good lubrication performance.
4 Conclusions
This study delved into the possible impeding and smoothing outcomes of purified fracturing flowback fluids in WBFs. The formula was optimized through a series of tests including orthogonal, range, and single-factor testing. The resulting optimized formula consists of 2.0% modified gum powder, 0.15% xanthan gum, and a plant phenol to modified complexing agent ratio of 1:0.01. The experimental findings indicate that utilizing purified fracturing flowback fluids as the fundamental water source can enhance the impeding and smoothing properties of the WBFs. Specifically, it leads to a reduction in linear expansion rate from 62.31% to 21.25%, an 87.98% decrease in the reduction rate of extreme pressure lubrication coefficient, and a 59.86% decrease in the reduction rate of mud cake sticking factor. The organic salts and surfactants found in clean fracturing flowback fluids possess active components that are capable of enhancing the inhibition and lubrication characteristics of WBFs. Furthermore, there exists the possibility of potential environmental and economic benefits when clean fracturing flowback fluids are reused.
-
Funding information: This dissertation is based on the project provided by the National Natural Science Foundation of China (52204011); Shaanxi Natural Science Basic Research Program Youth Program (2022JQ-493); and Technical service for Investigation of inhibition and lubrication mechanism of fracturing flowback fluid, Ruifeng Chemical Company.
-
Author contributions: Huaizhu Liu: data curation, methodology and formal analysis, writing; Dong Chen and Kangning Zhao: investigation, validation. supervision, writing – review and editing; Binbin Hu: data collection and processing; Jianjia Zhang: experimental design, investigation; Yang Ning: data statistics, chart production; Tong Shan: literature collation and analysis; Jie Zhang: literature review, experimental program design; Wangyuan Zhang: formal analysis, data curation; Fan Zhang: writing – review and editing, resources, funding acquisition.
-
Conflicts of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
[1] Osiptsov AA. Fluid mechanics of hydraulic fracturing: a review. J Petrol Sci Eng. 2017;156:513–35. 10.1016/j.petrol.2017.05.019.Suche in Google Scholar
[2] Zhong C, Zolfaghari A, Hou D, Goss GG, Lanoil BD, Gehman J, et al. Comparison of the hydraulic fracturing water cycle in China and North America: a critical review. Environ Sci Technol. 2021;55(11):7167–85. 10.1021/acs.est.0c06119.Suche in Google Scholar PubMed
[3] Yazdan MMS, Ahad MT, Jahan I, Mazumder M. Review on the evaluation of the impacts of wastewater disposal in hydraulic fracturing industry in the United States. Technologies. 2020;8(4):67. 10.3390/technologies8040067.Suche in Google Scholar
[4] Zeng F, Zhou H, Lin X, Li YH, Liang YP, Xie QL, et al. Enhanced remediation of fracturing flowback fluids by the combined application of a bioflocculant/biosurfactant-producing Bacillus sp. SS15 and its metabolites. Chemosphere. 2022;302:134870. 10.1016/j.chemosphere.2022.134870.Suche in Google Scholar PubMed
[5] Su HF, Lin JF, Chen Q, Wu CQ, Xu FL. A novel approach to reducing the biological toxicity of shale gas fracturing flowback fluid through recycling of high-value nano-BaSO4. J Clean Prod. 2023;414:137443. 10.1016/j.jclepro.2023.137443.Suche in Google Scholar
[6] Heath G, Hadley D, Okesanya T. Improving Sustainability Through the Utilization of Recycled Produced Water for Drilling Operations and the Associated Benefits//SPE Canadian Energy Technology Conference and Exhibition: Day 1 Wed, March 15, 2023. D021S003R002. 10.2118/212814-ms.Suche in Google Scholar
[7] Syam Babu D, Anantha Singh TS, Nidheesh PV, Suresh Kumar M. Industrial wastewater treatment by electrocoagulation process. Separation Sci Technol. 2020;55(17):3195–227. 10.1080/01496395.2019.1671866.Suche in Google Scholar
[8] Yang XJ, Mao JC, Zhang H, Zhang ZY, Zhao JZ. Reutilization of thickener from fracturing flowback fluid based on Gemini cationic surfactant. Fuel. 2019;235:670–6. 10.1016/j.fuel.2018.08.059.Suche in Google Scholar
[9] Nur W, Sheng JJ. Surfactant selection criteria with flowback efficiency and oil recovery considered. J Petrol Sci Eng. 2020;192:107305. 10.1016/j.petrol.2020.107305.Suche in Google Scholar
[10] Meng MM, Ge HK, Shen YH, Hu QH, Li LL, Gao ZY, et al. The effect of clay-swelling induced cracks on imbibition behavior of marine shale reservoirs. J Nat Gas Sci Eng. 2020;83:103525. 10.1016/j.jngse.2020.103525.Suche in Google Scholar
[11] Guan B, Liang L, Cheng F, Liu J, Xue X, Xu Y, et al. Application of recycling technology for fracturing flowback fluid. Acta Petrolei Sin. 2017;38(1):99–104. 10.1080/09593330.2021.1876171.Suche in Google Scholar PubMed
[12] Yan ZH, Dai CL, Zhao MW, Sun YP, Zhao G. Development, formation mechanism and performance evaluation of a reusable viscoelastic surfactant fracturing fluid. J Ind Eng Chem. 2016;37:115–22. 10.1016/j.jiec.2016.03.012.Suche in Google Scholar
[13] Alba C, Viviani CO, Raquel S, Rubén R, Eric SF, José AC, et al. Optimal pretreatment system of flowback water from shale gas production. Ind Eng Chem Res. 2017;56(15):4386–98. 10.1021/acs.iecr.6b04016.Suche in Google Scholar
[14] Jenna LL, Michael G. Organic compounds in hydraulic fracturing fluids and wastewaters: A review. Water research. 2017;123:536–48. 10.1016/j.watres.2017.07.012.Suche in Google Scholar PubMed
[15] Zhang N, You LJ, Kang YL, Xu JM, Li KM, Cheng QY, et al. The investigation into oxidative method to realize zero flowback rate of hydraulic fracturing fluid in shale gas reservoir. J Petrol Sci and Eng. 2022;209:109918. 10.1016/j.petrol.2021.109918.Suche in Google Scholar
[16] Boya X, Selina R, Bethany P, Zachary DM, Benjamin F, Travis LT, et al. Polyacrylamide in hydraulic fracturing fluid causes severe membrane fouling during flowback water treatment. J Membr Sci. 2018;560:125–31. 10.1016/j.memsci.2018.04.055.Suche in Google Scholar
[17] Li R, Yang J, Pan J, Zhang L, Qin WL. Effect of immobilization on growth and organics removal of chlorella in fracturing flowback fluids treatment. J Environ Manage. 2018;226:163–8. 10.1016/j.jenvman.2018.08.046.Suche in Google Scholar PubMed
[18] Huang QM, Liu SM, Wang G, Wu B, Zhang YZ. Coalbed methane reservoir stimulation using guar-based fracturing fluid: A review. J Nat Gas Sci Eng. 2019;66:107–25. 10.1016/j.jngse.2019.03.027.Suche in Google Scholar
[19] Oetjen K, Chan KE, Gulmark K, Christensen JH, Blotevogel B, Borch T, et al. Temporal characterization and statistical analysis of flowback and produced waters and their potential for reuse. Sci Total Environ. 2018;619:654–64. 10.1016/j.scitotenv.2017.11.078.Suche in Google Scholar PubMed
[20] Yu T, He RR, Sun X, Miao F, Kou B, Zhang XF, et al. Removal of scaling ions from catalytic oxidation and flocculation-treated fracking flowback fluids. J Inst Eng (India): Ser E. 2021;102(2):311–7. 10.1007/S40034-021-00222-4.Suche in Google Scholar
[21] Xiong BY, Miller Z, Selina R, Tasker T, Farina BJ, Piechowicz B, et al. Chemical degradation of polyacrylamide during hydraulic fracturing. Environ Sci Technol. 2018;52(1):327–36. 10.1021/acs.est.7b00792.Suche in Google Scholar PubMed
[22] Geng Y, Sun JS, Wang JH, Wang R, Yang J, Wang QB, et al. Modified nanopolystyrene as a plugging agent for oil-based drilling fluids applied in shale formation. Energy Fuels. 2021;35(20):16543–52. 10.1021/acs.energyfuels.Suche in Google Scholar
[23] Liao B, Wang JT, Han XP, Wang R, Lv KH, Bai YJ. Microscopic molecular insights into clathrate methane hydrates dissociation in a flowing system. Chem Eng J. 2021;430:133098. 10.1016/J.CEJ.2021.133098.Suche in Google Scholar
[24] Ewa K, Katarzyna C, Łukasz L, Sławomir W. Reuse of flowback water from hydraulic fracturing for drilling mud preparation and secondary hydrocarbon recovery. Energies. 2021;14:5921. 10.3390/EN14185921.Suche in Google Scholar
[25] Boschee P. Produced and flowback water recycling and reuse: economics, limitations, and technology. Oil Gas Facil. 2014;3(1):16–21. 10.2118/0214-0016-OGF.Suche in Google Scholar
[26] Saleh TA. Experimental and analytical methods for testing inhibitors and fluids in water-based drilling environments. TrAC, Trends Anal Chem. 2022;149:116543. 10.1016/J.TRAC.2022.116543.Suche in Google Scholar
[27] Zhang F, Sun JS, Li Q, Lv KH, Wang JT, Wang ZY. Mechanism of organosilicate polymer as high-temperature resistant inhibitor in water-based drilling fluids. Colloids Surf A. 2022;641:128489. 10.1016/j.colsurfa.2022.128489.Suche in Google Scholar
[28] Zhang F, Sun JS, Chang XF, Xu Z, Zhang XF, Huang XB, et al. A novel environment-friendly natural extract for inhibiting shale hydration. Energy Fuels. 2019;33:7118–26. 10.1021/acs.energyfuels.9b01166.Suche in Google Scholar
[29] Zhao G, Yan ZH, Qian F, Sun HN, Lu X, Fan HM. Molecular simulation study on the rheological properties of a pH-responsive clean fracturing fluid system. Fuel. 2019;53:677–84. 10.1016/j.fuel.2019.05.027.Suche in Google Scholar
[30] Tang Y, Ren HM, Yang PW, Li H, Zhang J, Qu CT, et al. Treatment of fracturing fluid waste by Fenton reaction using transition metal complexes catalyzes oxidation of hydroxypropyl guar gum at high pH. Environ Chem Lett. 2019;17(1):559–64. 10.1007/s10311-018-0805-9.Suche in Google Scholar
[31] Chieng ZH, Mohyaldinn ME, Hassan AM, Bruining H. Experimental investigation and performance evaluation of modified viscoelastic surfactant (VES) as a new thickening fracturing fluid. Polymers. 2020;12(7):1470. 10.3390/polym12071470.Suche in Google Scholar PubMed PubMed Central
[32] Zhang WL, Mao JC, Yang XJ, Zhang H, Zhang ZY, Yang B, et al. Study of a novel gemini viscoelastic surfactant with high performance in clean fracturing fluid application. Polymers. 2018;10(11):1215. 10.3390/polym10111215.Suche in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

