Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
-
Tahira Sultana
, Zia-ur-Rehman Mashwani
und Abdulwahed Fahad Alrefaei
Abstract
Green nano-chemistry is an advanced research route covering eco-friendly fabrication approaches for synthesizing bimetallic nanocomposites (NCs) to enhance their therapeutic properties. The current study aims to phytofabrication, characterization, and bio-potential evaluation of novel selenium–iron (Se–Fe) NCs by utilizing garlic extract. The morphological and physicochemical features of Se–Fe NCs were evaluated by UV–visible spectroscopy, scanning electron microscopy, energy-dispersive X-ray, Fourier transform infrared, X-ray diffraction, and Zeta potential analysis. The findings showed that garlic cloves extract was a promising capping and reducing agent for the formulation of the NC. To explore the antioxidant potential of a bioinspired Se–Fe NC, 2,2-diphenyl-1-picrylhydrazyl and reducing power assays were performed. Furthermore, antioxidant efficacy was confirmed through antimicrobial activities against clinical pathogens. Phytosynthesized Se–Fe NCs (25, 50, 75, and 100 ppm) showed a dose-dependent response. Higher concentrations of Se–Fe NCs impose a more potent antioxidant and antimicrobial potential. The astonishing findings suggest that phytochemicals in Allium sativum extract are useful reducing agents in the formulation of well-defined Se–Fe NCs, and such NCs could act as competitive inhibitors against pathogens. To the extent of our understanding, Se–Fe NC is the first time synthesized and demonstrates the distinctiveness of green chemistry and will give multifunctional applications in nano-biotechnology.
Graphical abstract
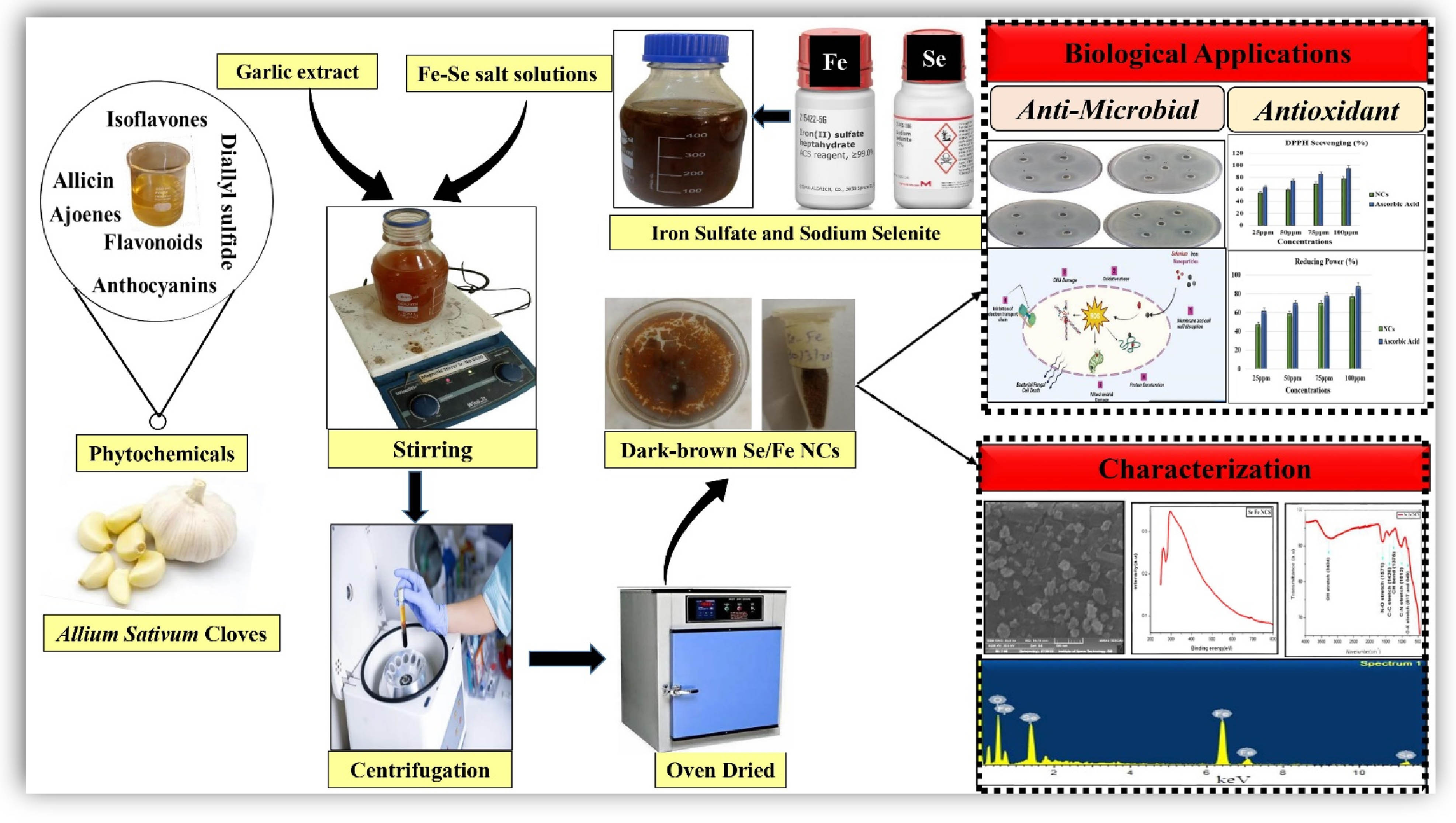
1 Introduction
Nanoscale materials are entities with a size of 1–100 nm, according to the definition given in the context of chemistry. In recent decades, these nanomaterials have contributed significantly to the exponential growth of nanoscience, green chemistry, and nano-biotechnology [1]. However, nanomaterials frequently deviate considerably from their macroscale counterparts regarding their biological, physical, and chemical characteristics despite possessing similar chemical compositions [2]. A new addition to the line of nanotechnology is the formulation of nanocomposites (NCs). An attempt to investigate the uniqueness of a single nanocomponent can be overloaded by adding multiple other nanocomponents that deliver a lot of practical synergistic potential in various biomedical fields [3]. Monometallic nanomaterial properties can be enhanced by combining the other material, resulting in the formation of hybrid NCs [4]. According to some reports, these are the materials of the twenty-first century because they possess special architectural features and character combinations that are not present in traditional composites. Therefore, bimetallic NCs are enormously essential and have gained special attention among scientists in the medicinal chemistry and biomedical area to treat many diseases [5] effectively. There is still much to learn about these characteristics in general [6], despite the fact that their first indication was recorded in 1992 [7]. Generally, different approaches have been recognized for the fabrication of metal nanoparticles (NPs), such as chemical reduction [8], electrochemical [9], irradiation [10], thermal evaporation, as well as mechanical approaches and the green phytochemistry synthetic route [11]. Recently, numerous chemical and classical physical methods have been applied in NP synthesis but have gained a detrimental impact on biological and environmental concerns due to the high energy utilization and use of excessive toxic chemicals [12,13]. In order to solve these demonstrated environmental issues, exceptional attention has been focused on developing environmentally benevolent green nanomaterials that are free from toxic chemicals, more stable, and cost-effective in their fabrication [14,15,16]. Consequently, there is growing enthusiasm and demand for efficient green nanochemistry [17,18] because of the enormous strides it has made to represent one of the top explored and prospering fields because of its applicability in diverse human well-being fields [19]. When nanomaterials are adequately prepared by green synthetic procedures, they show remarkable unique emerging properties and astonishing applications compared to their bulk materials [20,21,22]. The most extensively used approach for synthesizing biocompatible NPs is the fabrication of NPs through plant extracts. This widely explored method has a significant benefit in that plants are abundant, simple to get, considerably safer to handle, and act as a reservoir of several phytomolecules or secondary metabolites that act as potent biocatalysts [23,24]. Garlic is a well-known herb, known from ancient times as Allium sativum, which is the second-largest growing crop worldwide. The major account of natural bioactive constituents in garlic extract is organosulfur-based array of phytochemical compounds with a wide range of biological and modulated therapeutic potential and are targeted for the biogenic formulation and synthesize stable nanomaterials [25]. The extract of these cloves contains an array of phytochemical compounds that can act as reducing agents. Garlic-based NPs were mainly exploited in the therapeutics fields and, to a lesser extent, in agriculture, food, and related areas [26].
The literature review revealed that various monometallic and their oxide NPs are formulated by biological means effectively. Still, bioinspired hybrid, bi, or tri-metallic NCs are reported significantly less. Selenium and iron monometallic NPs fabricated by phytochemical-based approaches have gained much interest with increasing green chemistry enthusiasm because of their fascinating application in biochemistry, environmental remediation, biomedical fields such as drug delivery systems, pharmaceutical, food industries, antimicrobial, anticancer, antioxidants, neurodegenerative disease and also in the agricultural domain owing to non-toxicity as both are essential nutrients [27,28,29]. Iron NPs have immense application in the biomedical field due to their super-magnetic potential and excellent antimicrobial and antioxidant properties [30]. In one of the documented studies, Allium saralicum extract-assisted iron NPs showed significant antifungal, antibacterial, antioxidant, and wound healing potential. Remarkable results reported that Fe NPs inhibited the growth of all fungi and bacteria dose dependently and significantly showed antioxidant activity [31]. In another study, it is reported that Eucalyptus robusta extract-mediated iron NPs showed a remarkable antioxidant effect, this attribute making FeNPs potent candidate for many pharmacological applications where free radicals play a decisive role [32]. Similarly, selenium NPs have been introduced as a trace mineral in biomedical that is considered an alternative therapy for cellular damage and possesses antimicrobial and anticancer potential [33,34]. Many reported studies revealed that the green formulation of selenium NPs exhibited promising antimicrobial functionality against the Gram-positive and Gram-negative bacteria [35,36]. Previous in vivo and in vitro studies have shown that green-fabricated selenium NPs are potent antimicrobial agents at the nanoscale that suppress the growth of multicellular and unicellular fungi up to 70% [6,37]. Selenium is the primary constituent of antioxidant selenoproteins. Moreover, several in vitro antioxidant surveys highlight that plant-mediated Se NPs have remarkable antioxidant activity that confers the resistance against oxidative stress by scavenging of free radicals [38,39]. The mechanism behind antimicrobial behavior is that they react with thiol protein, which disrupts the membrane permeability and stability, and leads to the leakage of membranes. After internalization, these NPs damage the cellular organelles, and macromolecules ultimately cause cell death [40,41]. Because of the broad range of applications of selenium and iron NPs, the present study was devoted to fabricating NCs using phytoconstituents as reducing agents for getting synergistic modulated potential. Due to their high bioavailability, it is the first and foremost attempt for the synthesis of selenium–iron (Se–Fe) composites by bioreduction of sodium selenite and iron sulfate salts using A. sativum bud extract with synergistic characters which have never been achieved by other conventional methods. The garlic extract-mediated NCs were well characterized through various analytical techniques such as UV–visible spectrum was used to ensure the composite formation, morphology, and surface topography by scanning electron microscope (SEM), and stretching vibrations or functional groups were detected via Fourier transform infrared (FTIR) techniques. Energy-dispersive X-ray (EDX) spectroscopy was done to know the elemental composition present in prepared Se–Fe NCs. The novelty of our research study is based on the fact that there has yet to be any work reported on phytofabrication and application of Se–Fe NC. Moreover, the newly synthesized material was assessed for biopotential applications such as antioxidant and antimicrobial activities.
2 Materials and methods
The phytofabrication of Se–Fe NCs was carried out in the Nano-biotechnology Laboratory at the Department of Botany, PMAS Arid Agriculture University Rawalpindi. Furthermore, bioinspired Se–Fe NCs were evaluated for their bio-potential applications.
2.1 Materials
All reagents that were used in the current study are of analytical grade for accurate results. Hydrated salts used for NCs syntheses like sodium selenite and iron sulfate and chemicals for antimicrobial study such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, Mueller-Hinton nutrient agar media, potato dextrose agar (PDA), and methanol were purchased from Sigma-Aldrich. The deionized water was used throughout the whole study. All glasswares were properly washed and autoclaved. Garlic clove extract, selenium, and iron salt solutions were prepared in distilled water. The biological reduction of selenium and iron salt solutions for synthesizing its NCs was made by garlic extract. Agar well diffusion assay was used for the antimicrobial experiment.
2.2 Preparation of A. sativum plant extract
The preparation of garlic extract was initiated after the collection of garlic buds (shown in Figure 1) and peeled off the buds, then washed thoroughly with deionized water to remove all the dust and impurities. After drying the plant material, 20 g of fresh buds were crushed into a thick paste. The obtained content is boiled in 400 mL distilled water on a hot plate for 15 min and then cooled down at room temperature. The resulting extract was ultimately filtered through Whatman filter paper for the pure aqueous plant extract. The final extract was used to reduce the selenium and iron salts into NCs [42].
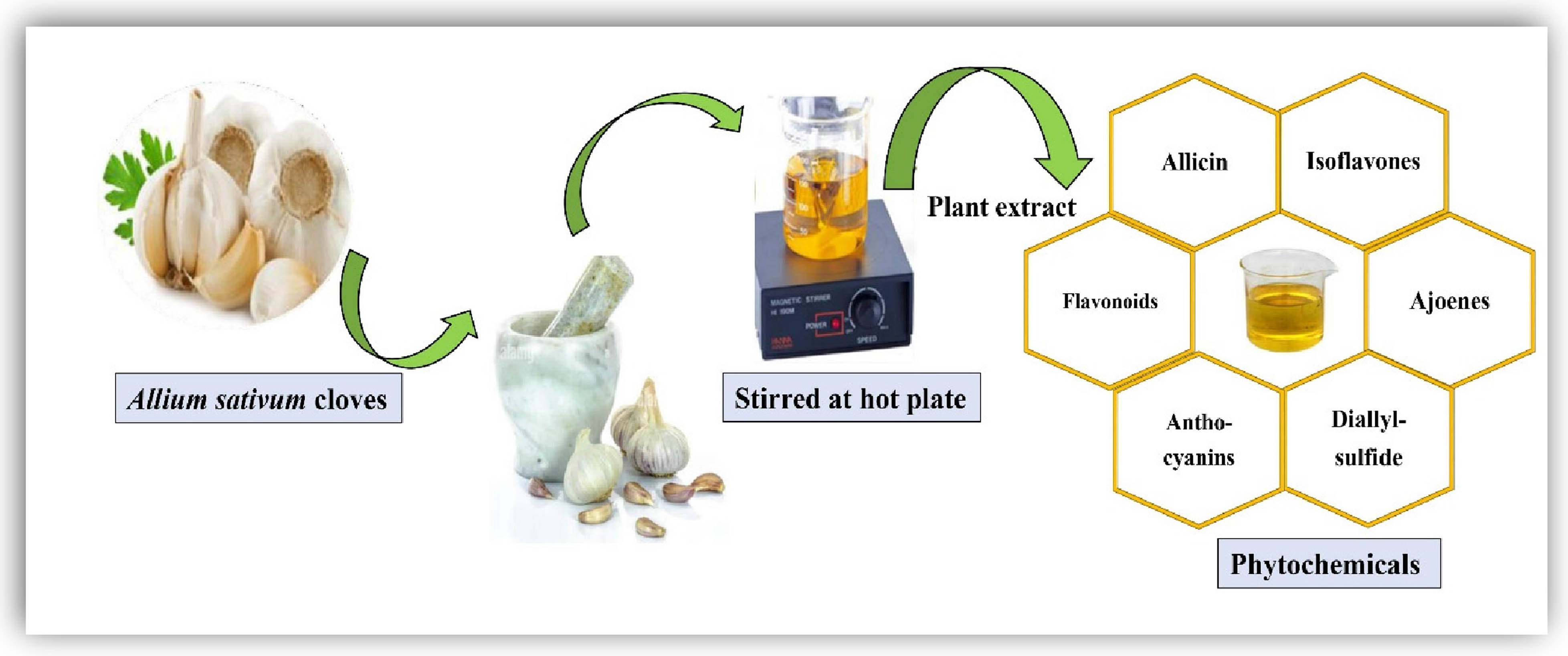
Schematic presentation of A. sativum plant extract.
2.3 Phytosynthesis of Se–Fe NC
To synthesize Se–Fe NCs, 5 mM aqueous salt solutions of sodium selenite and iron sulfate are freshly prepared by dissolving salts in distilled water. In total, 5 mM selenium and 5 mM iron salt solutions are mixed equally in the flask and kept on a magnetic stirrer to form a homogenous Se–Fe salt solution. About 100 mL of the prepared garlic bud extract was added continuously in aqueous salt solutions dropwise. This reaction mixture was continuously heated at 70°C and stirred until the color of the solution changed from clear salt solutions to light brown to dark brown, which represents the first indicative characteristic of Se–Fe NCs (as shown in Figure 2). Se–Fe NCs were visually confirmed by the sequential color change of the reaction mixture from transparent to light brown to brackish to dark brown, which indicates the reduction reaction. Selenium and iron from their respective salt solutions transformed into Se–Fe NCs at the required concentration of garlic extract. The minimum and optimal concentrations of garlic extract have an immense role in the reaction process. The obtained NC solution was centrifuged at 10,000 rpm for about 10 min to separate the NCs from impurities. The obtained NCs were rinsed in distilled water, resuspended in methanol, and centrifuged thrice. After complete purification, the pellet was collected and dried for 12 h in a drying oven at 400°C to obtain Se–Fe NC in powder form for further characterization, including, optical, morphological, and chemical characterization of the NC. The freshly phytosynthesized Se–Fe NCs were studied through various characterization techniques.
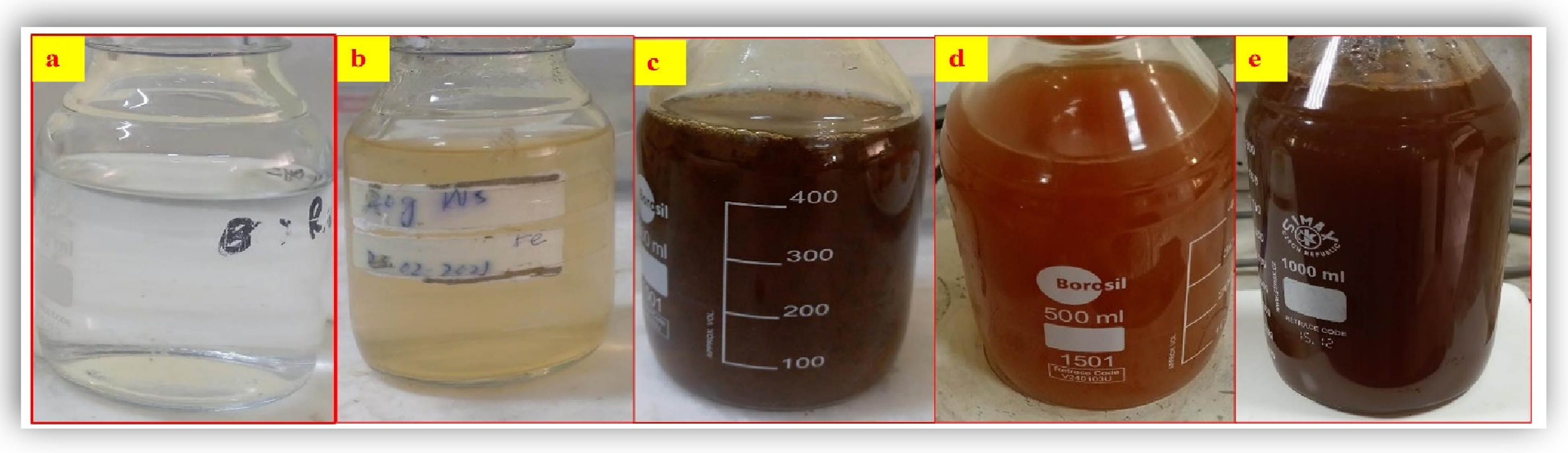
Visual observation of phytosynthesized Se–Fe NC solution: (a) selenium salt solution, (b) iron salt solution, (c) Se–iron salt solutions, (d) and (e); Se–Fe NCs solutions at different stirring times and increasing concentration of reducing agent (garlic extract).
2.4 Structural characterizations of bio-inspired Se–Fe NCs
2.4.1 UV–visible spectroscopy
The original formulation and optical characteristics of Se–Fe NCs were assessed using UV–visible spectrophotometry (Labomed UVD 3500, Los Angeles, CA, USA), which recorded absorbance from 200 to 900 nm.
2.4.2 FTIR spectroscopy
All transformations in the synthesis process were specifically analyzed through an FTIR spectrometer (Perkin-Elmer FTIR-Spectrum, Akron, Ohio, USA) to ensure the biomolecules naturally occurring in garlic extract, which plays a role in metal reduction, stability, and creation of Se–Fe NCs. The dried NC was homogenized with potassium bromide, and FTIR analysis from 400 to 4,000 cm−1 spectral range [43] was carried out.
2.4.3 SEM analysis
The topography or surface morphology of green synthesized Se–Fe NCs was determined through SEM (SIGMA) analysis. The SEM worked at 5 kV and at ×10k magnification. For the sample preparation, a drop coating method was adopted in which the sample was put on a copper grid that was coated by carbon. For drying, the resulting prepared sample was placed under the mercury lamp for 5 min. The extra solution was wiped off by blotting paper. The surface micrographs of the Se–Fe NC were observed at different magnifications [44].
2.4.4 EDX spectroscopy
The elemental analysis of bioinspired Se–Fe NCs was carried out by the EDX instrument (SIGMA). Thin carbon films were used for the sample preparation.
2.4.5 X-ray diffraction (XRD) characterization
Amorphous or crystalline state of the Se–Fe NCs was analyzed via XRD spectroscopy. The dried sample of Se–Fe NCs was loaded on XRD spectroscopy (Shimadzu XRD-6000) in 20–80° range at a 2θ angle.
2.4.6 Zeta potential analysis
Zeta potential analysis was performed to determine the charge distribution on the Se–Fe NC surface using the Zetasizer Nano Series (Malvern). For sample preparation, Se–Fe NCs were added in distilled water, and then the sample sonicated for 8 min. After sonication, about 0.5 mL of NC suspension was placed in a cuvette for determination of the electric charge distribution of the particles.
2.5 Antioxidant activity of bioinspired Se–Fe NCs
Free radical scavenging activity of novel green synthesized Se–Fe NCs was determined through the DPPH method. The DPPH stock solution of 1 mM concentration was prepared in methanol. Then, newly synthesized Se–Fe NC solutions were composed of various concentrations (25, 50, 75, and 100 ppm) and added to the DPPH solution separately. The resulting prepared solutions were finally incubated in the dark for about 30 min, and then the absorbance of blank (methanol), ascorbic acid (standard), and a sample was calculated through a UV–visible spectrometer (BioAquarius CE 7250, Cambridge, UK) at the 517 nm wavelength. The percentage of DPPH inhibition or free radical scavenging activity of Se–Fe NCs was calculated using the following formula [45]:
2.5.1 Reducing power assay
The reducing power of garlic extract-mediated Se–Fe NCs was analyzed by following the mentioned protocol [46]. In brief, various concentrations (25, 50, 75, and 100 ppm) of Se–Fe NCs were prepared and homogenized with 2.5 mL of phosphate buffer and 2.5 mL of 1% potassium ferricyanide. The resulting mixture was incubated for about 20 min at 500 °C and then cooled instantly. Subsequently, about 2.5 mL of 10% trichloroacetic acid was added to the above-mentioned resulting solution, which was then centrifuged at 3,000 rpm for 8 min. After that, equal amount (2.5 mL) of the supernatant layer was taken and mixed with distilled water. In the last step, 1 mL of 0.1% ferric chloride was added to the supernatant solution, and absorbance at 700 nm was measured using a spectrophotometer wavelength. In the experiment, ascorbic acid was taken as the standard. Reducing power percentage was noted by the following formula:
2.6 Antimicrobial applications of phytogenic Se–Fe NCs
2.6.1 Micro-organism strains
In the current study, for antimicrobial activity, several bacterial and fungal strains were cultured on media plates containing nutrient agar and PDA, respectively. Both media were autoclaved (HVE-50 HIRAYAMA) at 121 for 15 min. The antimicrobial activity of the Se–Fe NC was evaluated against tested bacteria Escherichia coli (Gram-negative), Staphylococcus aureus (Gram-positive), and fungal strains (Aspergillus flavus, F. oxysporum) using antimicrobial assays, which are defined by their ability to portray the antimicrobial activity. The selected microorganism strains were obtained from the Department of Botany at PMAS Arid Agriculture University Rawalpindi.
2.7 Antibacterial and antifungal activity
Phytosynthesized Se–Fe NCs were tested for antimicrobial activity against clinical pathogen strains using the agar well diffusion method [47]. The selected pathogen strains were introduced on the Mueller-Hinton agar media and then spread on plates uniformly. A sterile metal cork borer was used to drill about 6 mm diameter wells in the agar plates. Different concentrations of Se–Fe NCs (25, 50, 75, and 100 ppm) were prepared, and 60 µL NC solutions were poured into specified wells. The antimicrobial efficacy of NCs was determined by measuring the inhibition zone diameter in millimeters around the designed wells with a measuring scale. In the case of antifungal activity, freshly cultured fungal spores were collected and placed on PDA. The spore count was adjusted to 2 × 106 CFU·mL−1 with the help of a hemocytometer. Antibiotics (Streptomycin, Terbinafine) were used as control. Petri dishes with bacterial culture were incubated in a dry oven at 37°C for 24 h and fungal plates at about 25°C for 72 h. The entire experiment was conducted in a highly sterilized environment.
2.8 Statistical analysis
All the experiments were conducted in a completely randomized design. Each treatment had three replicates that were repeated three times, and the data were analyzed statistically by using SPSS ver. 16.0 software (Chicago, IL, USA). The mean values and significant differences in the data were calculated using DMRT at p < 0.05.
3 Results and discussion
3.1 Visual observation of Se–Fe NCs
The present study revealed that the color of the reaction mixture for Se–Fe NCs was changed. When a reducing agent (garlic extract) was added, it transformed from transparent salt solutions to an ultimately dark brown composite product that was continuously stirred (Figure 2). This is the first indicator for the formation of NC solution. The respective metal ions were eventually reduced when they were exposed to garlic bud extract within 24 h of the incubation period. The previous studies also revealed that garlic extract was determined to be a promising reducing agent [48]. This color change due to the complete reduction of metal ions can be ascribed to the excitation of surface plasmon vibrations in the NP [49].
3.2 UV–visible spectra
The initial synthesis and optical properties of plant-mediated newly synthesized Se–Fe NCs were further confirmed by the UV–Vis spectrum. UV–visible analysis is considered to be an important method to ascertain the stability and formulation of metal NPs present in the aqueous solution. Figure 3 shows the existence of selenium nanostructures because of the excitation of plasmon longitudinal vibration at a broad plasmon surface resonance band 262 nm. Aside from that, the figure also illustrates the second absorbance peak at 296–316 nm, which implies the presence of iron NPs. These absorption peaks were confirmed by previous studies for the green synthesis of monometallic selenium and iron nanostructures. According to the literature, a spectrum of nanostructures at 262 nm [50], at 265 nm [51], and broad spectra recorded within a wavelength of 200–500 nm display the presence of selenium NPs [52,53]. Jagathesan and Rajiv [54] stated that using UV–visible analysis revealed that Eichhornia-mediated FeNPs had a broad absorption spectrum at 379 nm. Throughout the whole spectral range, a wide absorbance band was found, particularly around 350 and 500 nm [55,56]. Respective wavelengths of selenium and iron are discrete and separated. The Se and Fe nanomaterials in a solution generally form aggregates and are discovered to be stable in suspension. SPR bands are often affected by the synthesized NPs’ shape, size, surface topography, elemental composition, and electrostatic environment [57].
UV–Visible spectrum of Se–Fe NCs.
3.3 FTIR spectroscopy
An evaluation of the FTIR spectrum of garlic clove extract with the Se–Fe NC FTIR spectrum shows many sharp peaks, indicating that the phenolic compounds of garlic extract interact with selenium and iron metal ions. These phytochemicals of extract counter for reducing, stabilizing, and capping agents that are responsible for the formulation of stabilized Se–Fe NCs. The FTIR spectra, represented in Figure 4, exploit the maximum information that confirms the bonding nature of garlic extract-mediated Se–Fe NC on the basis of identifying a number of functional groups residing on the surface of nanostructures. In order to confirm the NC’s structure, procedures from the literature were applied to identify the conspicuous peaks. The spectrum displays a number of prominent peaks at certain regions that are clearly distinguished from weak bands. From the spectrum, the most substantial peak was observed at 3,434 cm−1, which corresponds to (OH) the functionality of the hydroxyl group excessively found in phenolic compounds, which plays a crucial role in the stable formulation of NCs [58]. The range within 600 to 1,500 cm−1 was regarded as a fingerprint zone and is indicative of the compound. The sharp peaks in this trademark region for Se–Fe NCs were 1,012 cm−1 for C–N group stretching and also due to ether linkage. Moreover, the C–H distortion peak appeared at 1,375 cm−1, respectively. The absorption peak at 1,426 cm−1 in Se–Fe NC FTIR spectra represents the C═C stretch. The two absorption peaks observed at around 1,283 to 1,083 cm−1 range were due to the overlapping of stretch bands of the alcoholic O–H as well as vibrations of C–O–C ether linkage [1]. In addition, as depicted in Figure 3, most of the significant and conspicuous peak noticed at 1,571 cm−1 was ascribed to the bending frequencies of the amide group (N–O) NH. In addition, two weak peaks appear in spectra at 819 and 649 cm−1 due to out-of-plane C–H bending and C–X stretching found in alkyl halides, respectively. Ultimately, the FTIR spectra showed all the potential peaks that demonstrate the NC formation. It also illuminated the significance of phytochemicals present in plant extract as capping or reducing agents in stabilizing the overall NC structure. The absorption or stretching peaks due to various functional groups residing on nanomaterials are confirmed through all the reported literature; bands at 3,471 and 1,682 cm−1 elaborate stretching and bending vibrations of –OH. Furthermore, C–H stretching is linked to the sharp peaks that appeared at 2,359 and 1,483 cm−1. Consequently, two vast peaks observed at 2,999 and 2,359 cm−1 might be associated with O–H bending vibration. It was considered that water molecules adhered to the surface. The small peak at 1,132 cm−1 is attributable to the stretching vibration of the C–OH bond [59]. One of the studies documented that O–H, N–H, C═O, and C–H functional groups interact with selenium atoms to form NPs [60]. Another scientific study also conducted FTIR spectroscopy and verified that the presence of phenols and other alkenes in plant extract due to O–H, C–H and C–C stretching are involved in the formulation of iron NPs [61].
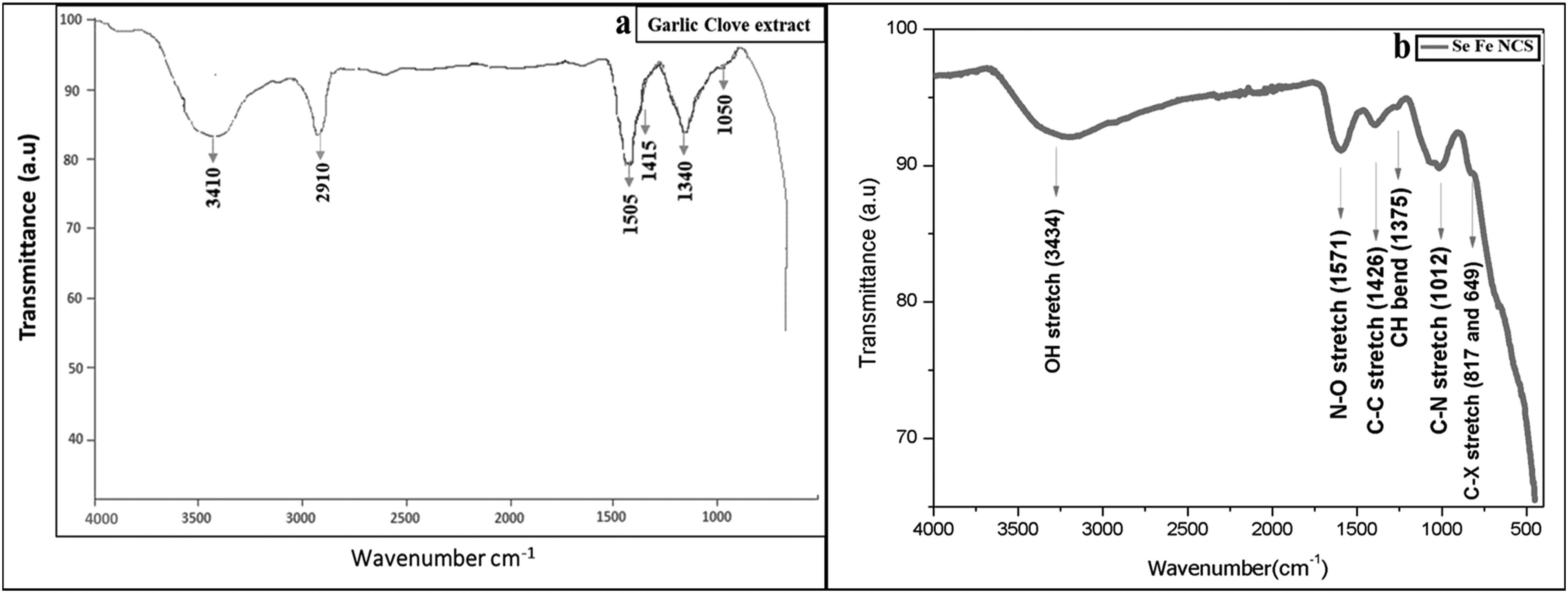
FTIR investigation of (a) garlic clove extract and (b) Se–Fe NCs.
3.4 SEM analysis
The surface topography of newly phytosynthesized Se–Fe NCs was investigated through SEM analysis. It is regarded as one of the most renowned analytical approaches for demonstrating the size and topography of NCs [5]. Figure 5 demonstrates typical SEM photographs of garlic extract-based Se–Fe NCs, representing the uniform surface topography and spherical shape with a fine crystallite nature having a particle size in the 50–80 nm range. Previous literature confirmed SEM results of biosynthesized iron oxide monometallic nanomaterials in the 70 nm range [62] and selenium in the 45–90 nm range [63]. These images depict that plant-mediated nanomaterial was highly uniform distributed and in a stable conformation. Furthermore, these micrographs revealed no indication of conglomeration with an unequivocal distribution of the particles, confirming the stable nature of synthesized NPs.
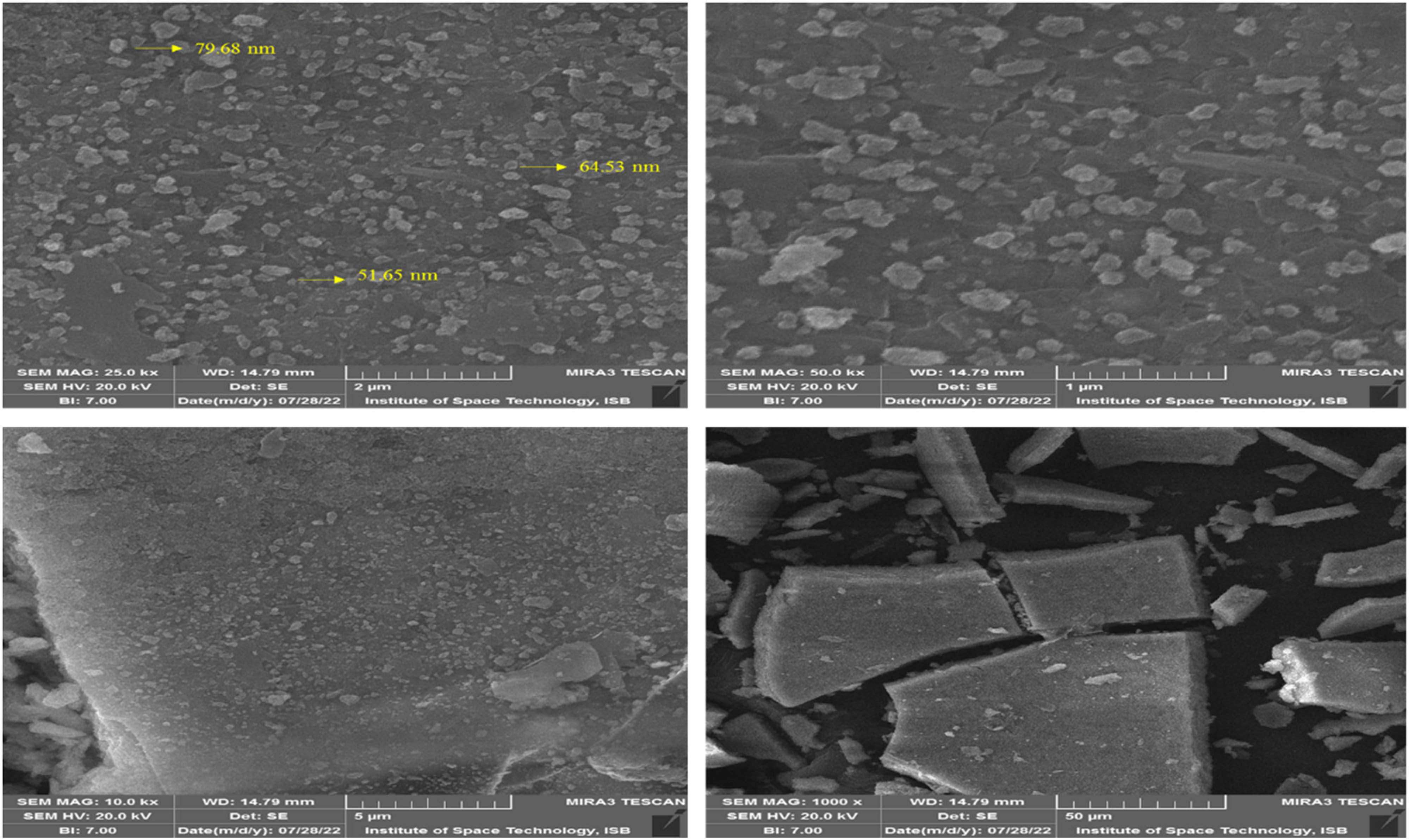
SEM images of Se–Fe NC at various magnifications.
3.5 EDS analysis
To determine the selenium and iron presence in the phytogenic synthesized Se–Fe nanomaterials, EDX spectroscopy technique was used. Figure 6 elucidates peaks comparable to oxygen, selenium, and iron elements. The resulting NC (Se–Fe) is pure because no residual impurities were noticed. The presence of Se, Fe, and O confirms the formation of Se–Fe NCs. According to recently reported literature, the NC formulation is readily explained by the indication for the oxygen atom that was also identified in Se–Fe NCs [5]. The proportion of all the existing elements in the Se–Fe nano-based composite is Fe (31.52%), O (30.08%), Se (15.11%), and C (23.28%) based on atomic% analysis.
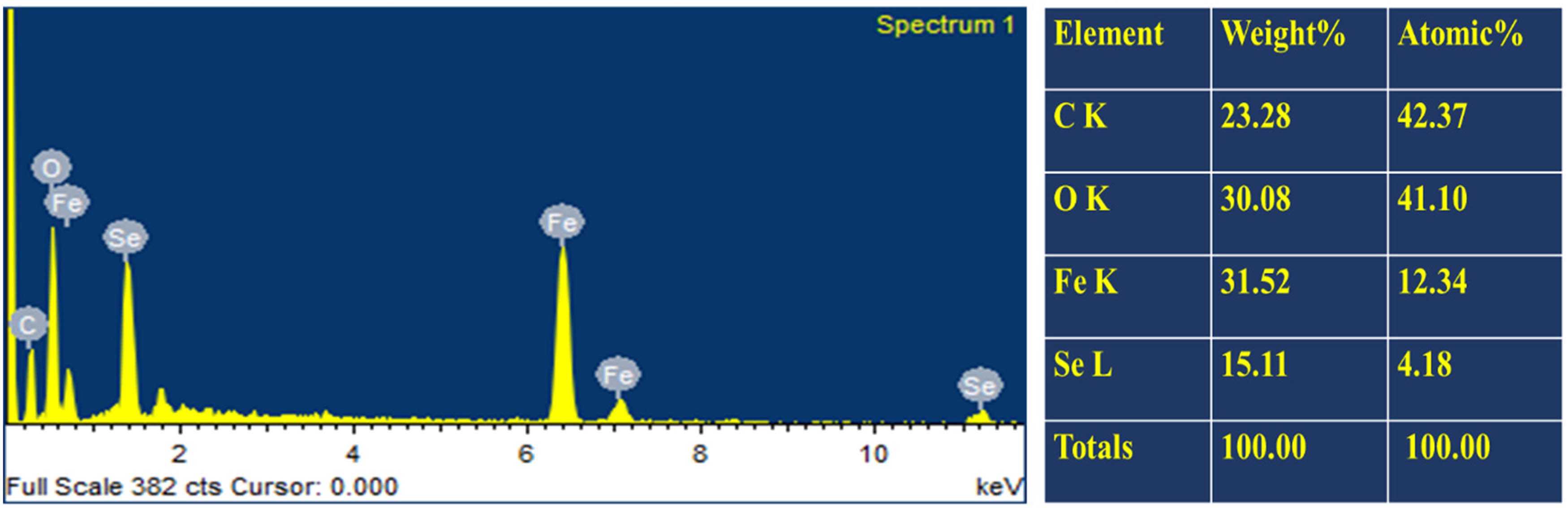
EDX analysis of Se–Fe NC.
3.6 XRD analysis
Figure 7 depicts the XRD pattern for the green synthesized Se–Fe NCs. The pattern represents the crystalline nature of synthesized NCs because of the presence of Bragg’s reflections. Peaks were identified using selenium diffraction signals (220) and (311) and iron signals (422), (511), (533), (620), and (622). The pattern shows d-spacing values for both selenium (JCPDS card No. 06-362) and iron (JCPDS card no. 39-1346). The EDX analysis explained that the presence of both selenium and iron peaks in a single pattern shows the formulation of Se–Fe NCs. Furthermore, the average crystallite size of Se–Fe NCs was estimated by Scherer’s equation: D = kλ/βcos θ, where k is constant = 0.94, λ is the X-ray wavelength (0.15406 nm), β is the peak half width, and θ is the half of the Bragg’s angle. By following the equation, the single crystal size of resulting NCs was predicted in the range of 14–20 nm. Moreover, narrow and sharp peaks in the spectrum illustrated that Se–Fe NCs have strong crystallinity.
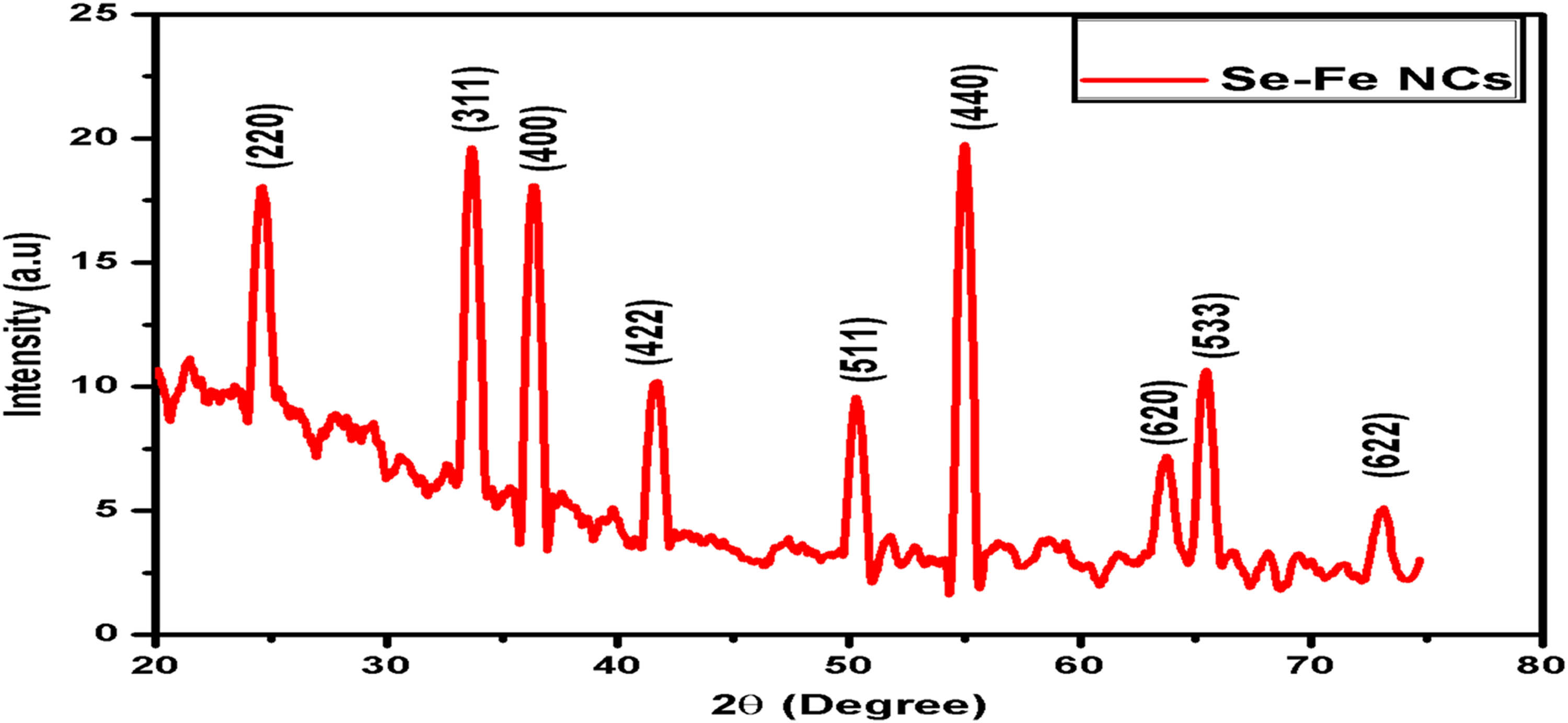
XRD analysis of Se–Fe NC.
3.7 Zeta potential spectroscopy
The zeta analysis is a useful characterization technique for knowing more about the stability of NPs. Zeta potential distribution amplitude represents the nanomaterial’s possible stabilization [64]. The zeta potential findings of the present study depicted the negative charge (−25.2 mV) on the Se–Fe NCs (Figure 8). In the suspension, if all the NPs have a positive or negative surface charge, then those particles will strongly repel to one another. There will be a minimum inclination for the NPs to join together, which represents their high stability [36]. The negative charge potential on formulated NCs probably leads to the strong stabilization of the Se–Fe NCs without aggregation. According to previous reports, the negative potential was found on green synthesized selenium (−24.4 mV) [34] and iron NPs (−16 mV) [62], respectively, and these stabilized nanomaterials do not turn to dark amorphous when they stored for a long time. The NPs with a higher zeta potential magnitude showed high stability because of stronger electrostatic repulsion among the NPs. Our results are aligned with the previous reported literature.
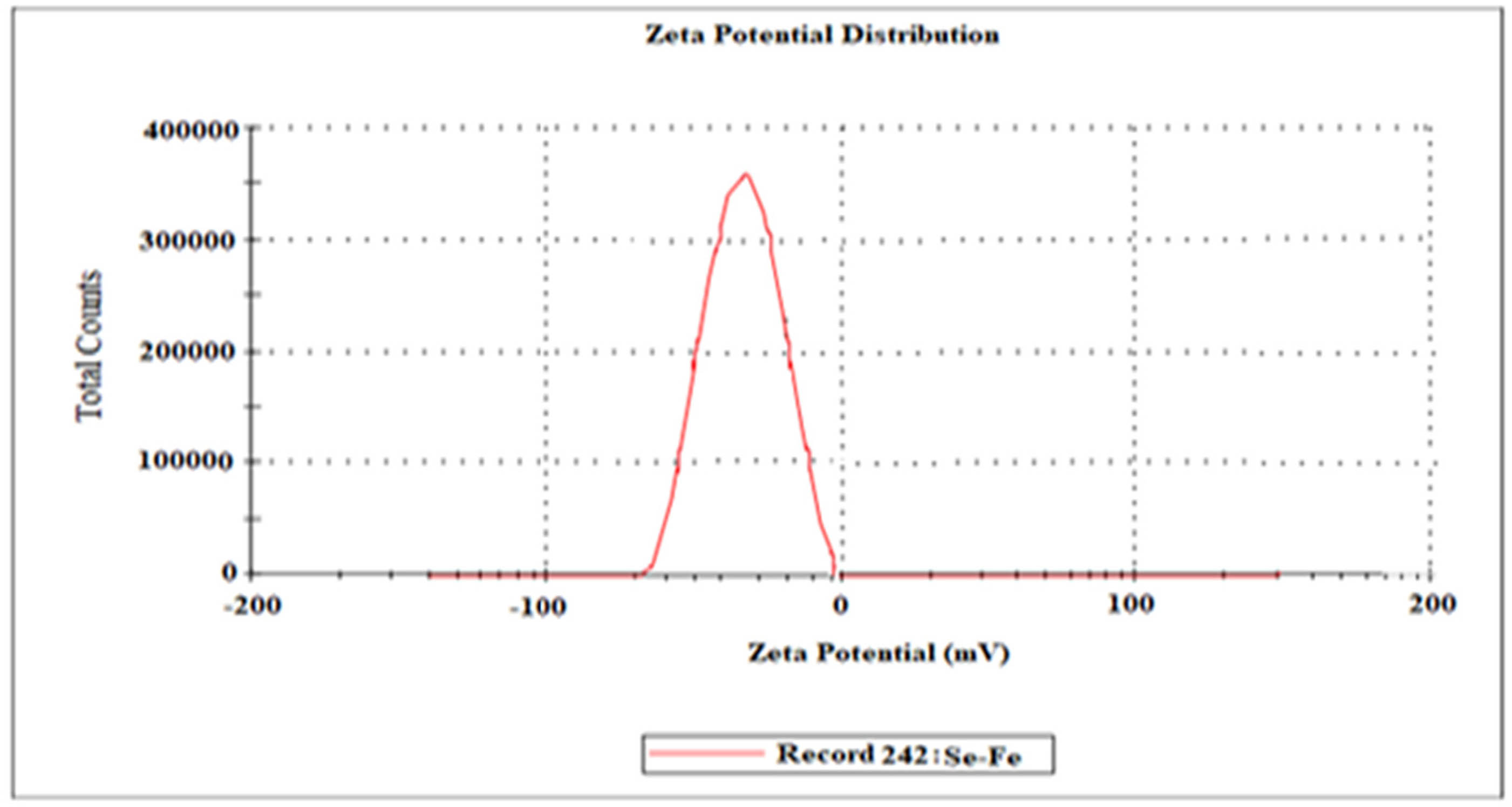
Zeta potential analysis of Se–Fe NC.
3.8 Bio-potential applications of biocompatible Se–Fe NCs
3.8.1 Antioxidant activity (DPPH)
One of the foremost promising and reliable methods for determining antioxidant effectiveness is the DPPH assay, which was designed using recognized and renowned practices. Owing to the accumulation of reactive oxygen species (ROS) in terms of free radicals, many types of persistent diseases, including oxidative damage, cancer, protein degradation, diabetes mellitus, and liver and brain dysfunctions, have emerged. In addition to this, the presence of ROS in the body is linked to a number of additional disorders, including aging, cataracts, and cardiovascular issues [65]. The DPPH efficacy of Se–Fe NCs was evaluated by observing a change in color. From Figure 9, it was observed that the DPPH scavenging method showed effective inhibition potential of Se–Fe NCs by increasing the concentration from 25 to 100 ppm. The maximum scavenging efficiency of biogenic Se–Fe NCs and standard (ascorbic acid) was 77.30% and 94.16%, respectively, at 100 ppm concentration. The potential of antioxidant activity of green synthesized nanomaterials was enhanced due to the influential association with various phytochemicals present in the bud extract of A. sativum, which are responsible for the synthesis of stable NCs. However, the protocol followed for the synthesis of Se–Fe nanomaterials tends to enhance the antioxidant potential and aid in the reduction of DPPH radicals. The plant extract phytochemicals adhered on nanomaterials are found to be electron-enriched species and neutralize the free radicals by donating electrons [66]. Current study outcomes were in line with the results of phytosynthesized iron and selenium NPs individually. Our results are strongly coherent with previous studies in which the average percentage DPPH antioxidant efficacy of green synthesized selenium NPs was 75% inhibition at 600 µg·mL−1 concentration [67]. Recently Kokila et al. [68] mentioned that plant-mediated 16 nm sized selenium NPs inhibit 50% scavenging potential at 22.5 µg·mL−1. Another study of biosynthesized iron NPs documented the DPPH scavenging activity 17.25% at 50 µg·mL−1. Iron NPs have a strong ability to donate an electron and serve as ROS scavengers or inhibitors; however, they act as primary antioxidants [69]. The DPPH scavenging activity assay outcomes in this current study show that plant-based NC was potently active.
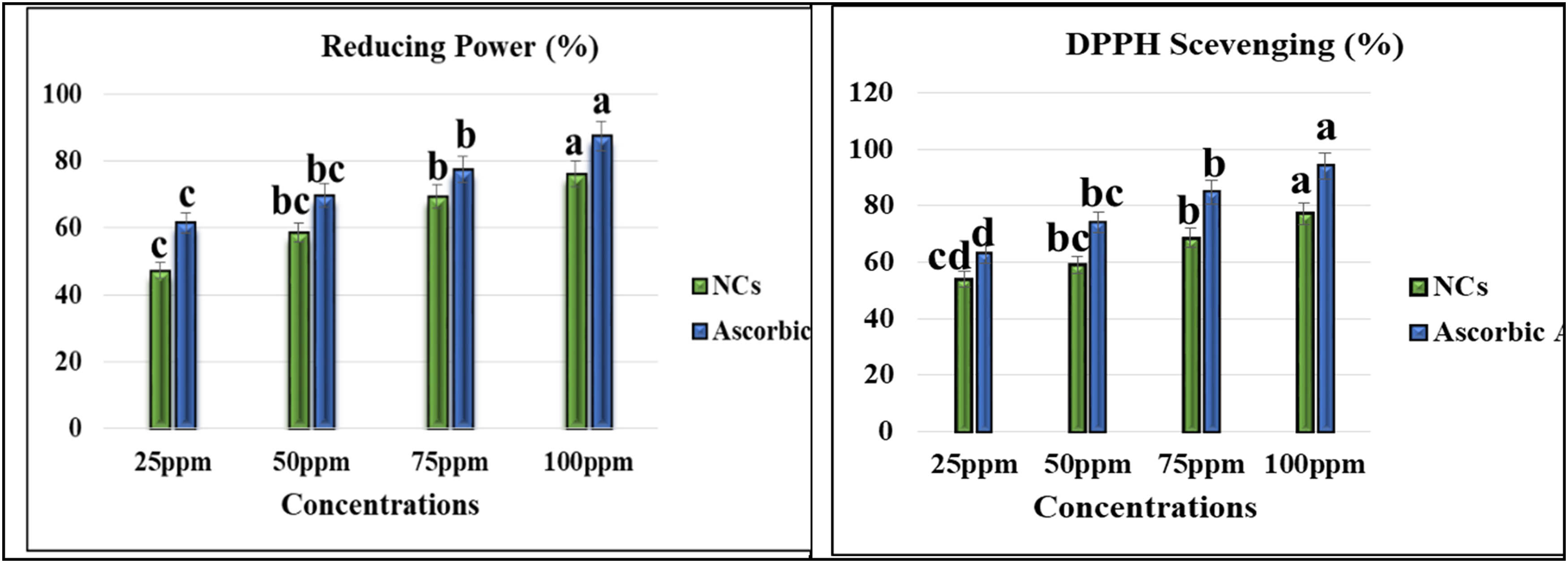
Antioxidant activities of Se–Fe NCs. Data represent the mean values of triplicates with ± standard error for each treatment in three repeated experiments. Annotation of columns data with different alphabet (s) represents the significance at (p = 0.05).
3.8.2 Reducing power assay
Figure 9 depicts the reducing power of the garlic extract-mediated Se–Fe dose-dependent NC response. By increasing the concentration of NCs and ascorbic acid (standard), reducing potential was increased consistently. The outcomes of the DPPH experiment revealed that Se–Fe NCs and ascorbic acid showed 76.17% and 87.52% scavenging activity through reducing assay, respectively. Surprisingly, Se–Fe NCs exhibited better results due to the excessive presence of an array of phytochemicals in the garlic extract. However, these secondary metabolites such as phenols, saponins, polyphenols, and sulfur-containing substances have antioxidant activity because these are electron donors [70]. Our study results are coherent with previous reports and observed that fenugreek seed-mediated iron NPs showed 60% reducing power at 110 μg·mL−1 [71]. Due to their strong antioxidant potential, these iron NPs are potent aspirants for supplementary pharmacological studies where ROS production plays a critical role [32]. A recent study documented that garlic extract-based selenium NPs showed a maximum reducing power of 80.37% at 400 ppm concentration, which is a very high dose of NPs [72].
3.8.3 Antimicrobial activity of Se–Fe NCs
In the present work, the antimicrobial potential of phytosynthesized Se–Fe NC was checked and measured through the agar well diffusion method against selected clinical microbial strains, which were E. coli, S. aureus, A. flavus, and Fusarium oxysporum. Different concentrations of NCs ranging from 25, 50, 75, and 100 ppm were used and evaluated zone of inhibition against selected pathogens as presented in Table 1. The results represent that at 100 ppm concentration of NC zone of inhibition against S. aureus is 29.04 mm, 26.89 for E. coli, 22.66 for A. flavus, and 24.32 for F. oxysporum. These outcomes documented better antimicrobial potential of garlic-mediated Se–Fe NCs than the garlic extract used as standard. At the same concentration (100 ppm) of A. sativum bud extract, several other inhibition zones, 26.38, 22.87, 18.11, and 19.93 mm, were observed, respectively. Outcomes of the study are confirmed with previous results in which iron NPs demonstrated that the maximum inhibition zone for Bacillus subtilis and Aspergillus niger was 19 mm [73], and Aloe vera-mediated selenium NPs showed inhibition zones 12 and 10 mm against S. aureus and E. coli, respectively [74]. In the current study, it was also observed that Gram-positive bacteria were shown to be more susceptible to Se–Fe NCs than Gram-negative. It could elucidate the sensitivity of bacteria to iron and selenium NPs because the thick peptidoglycan coating present on Gram-positive bacteria presumably allows for an adequate level of contact between pathogens and NPs. Comparatively, Gram-negative bacteria have a thin peptidoglycan coating in between their cytoplasmic and cell membranes, which serve as their boundaries. In this situation, getting Fe NPs into the thin layer is very challenging [75]. In reference to selenium NPs, they exhibit significant antagonistic effects on the membranes of Gram-negative bacteria and their polysaccharides. Due to this, bacterial fatality may require a more significant selenium nanomaterial accumulation on the surface of Gram-positive bacterial membranes. As a result, Gram-negative bacteria show strong resistance power against SeNPs [76]. From the results mentioned above, it was considered that Se–Fe NCs are potent and promising antimicrobial nano-products in the treatment of pathogenic infections (Figure 10).
Zone of inhibition of biogenic Se–Fe NCs for selected clinical pathogens
| Microbial strains | NC concentrations (ppm) | |||
|---|---|---|---|---|
| 25 | 50 | 75 | 100 | |
| Zone of inhibition (mm) | ||||
| E. coli | 13.88 | 18.1 | 23.09 | 26.89 |
| S. aureus | 15.06 | 19.89 | 24.96 | 29.04 |
| A. flavus | 10.11 | 13.26 | 17.96 | 22.66 |
| F. oxysporum | 13.11 | 16.74 | 21.1 | 24.32 |
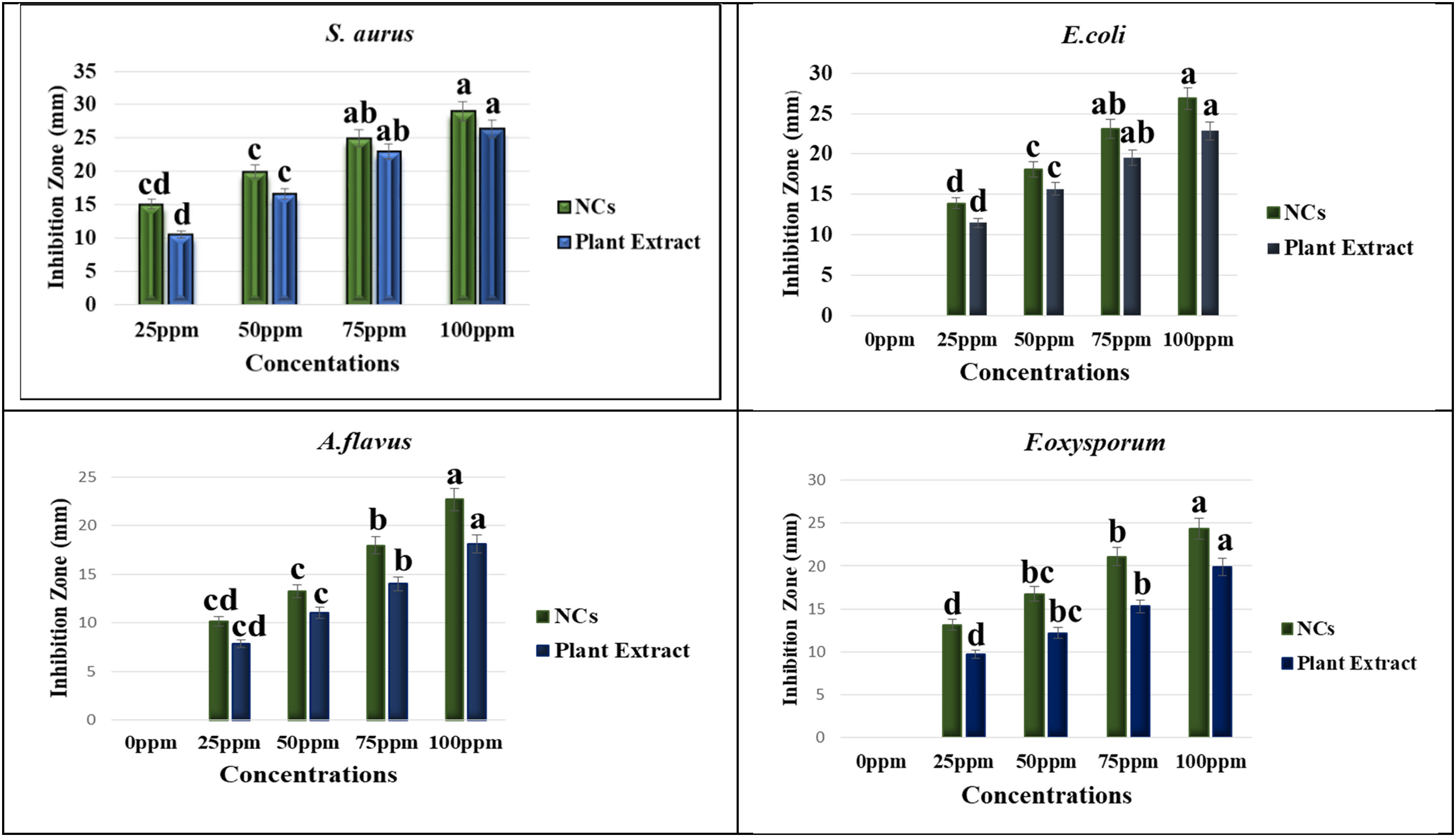
Antimicrobial activities of Se–Fe NCs. Data represent the mean values of triplicates with ± standard error for each treatment in three repeated experiments. Annotation of columns data with different alphabet (s) represents the significance at p = 0.05.
3.8.4 Antimicrobial mechanism of Se–Fe-based nanomaterials
In recent studies, different antimicrobial mechanisms of nanomaterials have been proposed. The antimicrobial action of NPs is generally defined as adhering to various models, such as NPs being causative agents for oxidative damage [77]. These release metal ions affect the antimicrobial potential of respective metal-based nanomaterials [78] and other non-oxidative approaches [79]. Eventually, these different mechanistic ways can happen concurrently. NPs inhibit the synthesis of the cell wall and cell’s membranous system, disturb energy transduction pathways, free radicals such as ROS are produced, photocatalysis, interference with enzyme activity, and degrade DNA, amino acids, and proteins [80]. The results of the present work elucidate the mechanism of Se–Fe NCs, which disinfect the pathogens in several ways. Monometallic selenium NPs have garnered significant interest in treating clinically significant pathogens such as bacteria, fungi, viruses, and other parasites, due to their excellent therapeutic proficiency and nearly completely devoid of detrimental effects [81]. In another study, a leakage test was performed and demonstrated that many proteins and some polysaccharides were released out of the cells after reacting with green synthesized SeNPs. It was discovered that the rupture of cell walls and alterations in membrane permeability were responsible for the leakages of proteins and polysaccharides. Additionally, the change in free radical concentration reveals that oxidative destruction may be a major factor in antimicrobial activities [82]. In contrast to the antimicrobial activity of iron monometallic NPs, the proposed mechanism is that particles accumulate in the cytoplasm and penetrate the cell wall of a pathogen in order to trigger the membrane to burst, resulting in the release of cellular substance and ultimately the death of the microorganisms [83]. The above-mentioned outcomes from the current study clearly depict the synergistic potential of Se–Fe NCs in an antimicrobial mechanism that disrupts the cell membranes and causes oxidative stress (Figure 11).
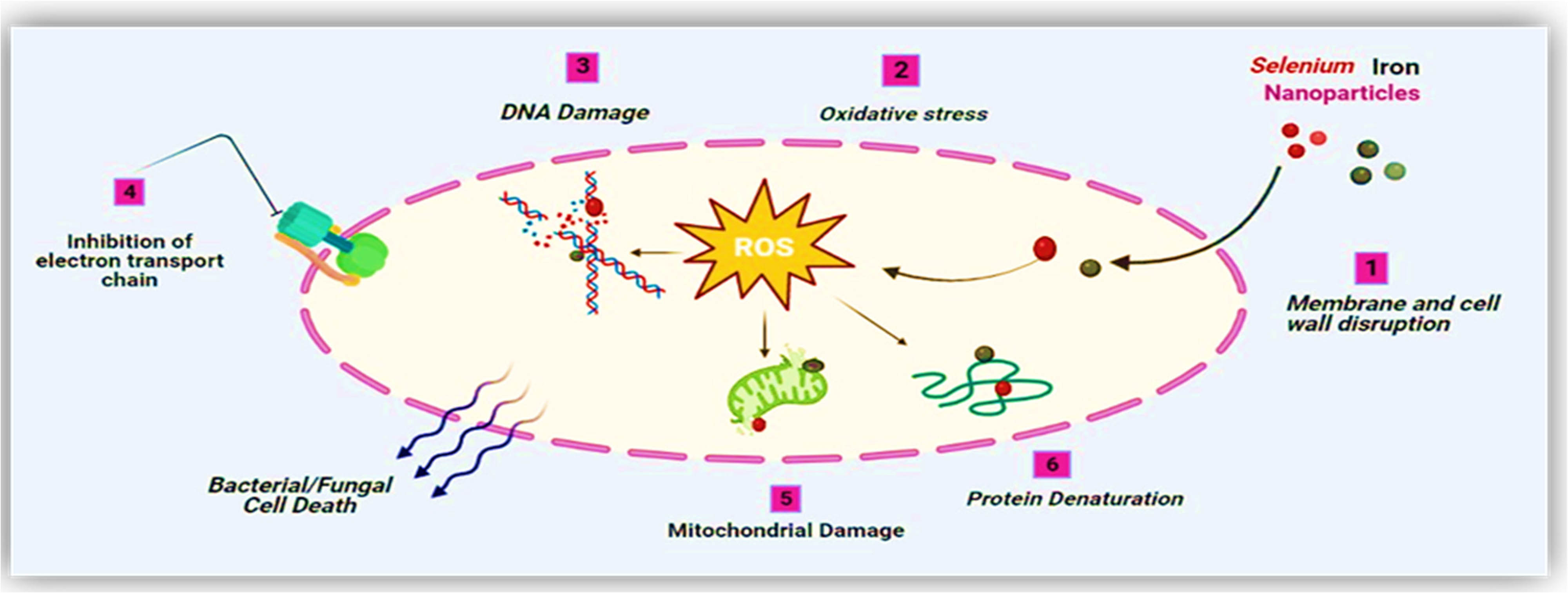
Antimicrobial mechanism of NPs.
4 Conclusion
This study successfully introduced green-synthesized Se–Fe NCs and their significant bio-potential applications for the first time, demonstrating the efficacy of green nano-chemistry in therapeutic applications. It is obvious from the results of the current investigation that A. sativum bud extract was proven to be an effective reducing agent, and it is an economically viable, efficient, and commercially feasible green synthesis method for the NC. The formation of Se–Fe NCs was confirmed through UV–Vis absorption spectra, which exhibit strong peaks in the range of 262–316 nm; surface morphology and particle size were identified from SEM, which showed that NCs were near to spherical shape and further crystallite size confirmed from XRD that was in 14–20 nm range. FTIR characterization confirmed the Se–O–Se and Fe–O–Fe types of bonding formation. Further elemental composition was investigated through the EDX spectrum, which confirmed the presence of Se, O, and Fe in the sample. The stability of particles was assessed by zeta potential analysis, which showed that NCs are negatively charged. Synthesized NCs showed strong stability and antioxidant and antimicrobial efficacy due to the synergistic effect of selenium, iron NC, and phytochemicals adhered to the nanomaterials. Finally, the biogenic approaches enrooted the green revolution and an economic threat-less way for designing antimicrobial nano-products with required biocompatibilities. Future directions for our research will focus on the resulting NC specific characteristics and detailed applications.
Acknowledgements
We appreciate the Researchers Supporting Project (no. RSP2023R218), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: We appreciate the Researchers Supporting Project (no. RSP2023R218), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Khafsa Malik and Tahira Sultana: devised the study; Tahira Sultana: performed the experiments; Tahira Sultana: wrote the first draft; Khafsa Malik, Naveed Iqbal Raja: supervised the study; Zia-ur-Rehman Mashwani, Amir Ali, Asma Hameed, Sohail, Muhammad Yousuf Jat Baloch and Abdulwahed Fahad Alrefaei: edited, reviewed, and revised the manuscript. All authors reviewed and endorsed the final version of the manuscript for submission.
-
Conflict of interest: Authors state no conflict of interest.
-
Institutional review board statement: Not applicable.
-
Informed consent statement: Not applicable.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Ahmad MB, Tay MY, Shameli K, Hussein MZ, Lim JJ. Green synthesis and characterization of silver/chitosan/polyethylene glycol nanocomposites without any reducing agent. Int J Mol Sci. 2011;12(8):4872–84.10.3390/ijms12084872Suche in Google Scholar PubMed PubMed Central
[2] Heidarpour F, Ghani WAWAK, Ahmadun FR, Sobri S, Zargar M, Mozafari MR. Nano silver-coated polypropylene water filter: I. Manufacture by electron beam gun using a modified balzers 760 machine. Dig J Nanomater Biostruct. 2010;5:787–96.Suche in Google Scholar
[3] Panáček A, Kolář M, Večeřová R, Prucek R, Soukupová J, Kryštof V, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30(31):6333–40.10.1016/j.biomaterials.2009.07.065Suche in Google Scholar PubMed
[4] Sharma G, Kumar D, Kumar A, Ala’a H, Pathania D, Naushad M, et al. Revolution from monometallic to trimetallic nanoparticle composites, various synthesis methods and their applications: A review. Mater Sci Eng C. 2017;71:1216–30.10.1016/j.msec.2016.11.002Suche in Google Scholar PubMed
[5] Khan AU, Khan AU, Li B, Mahnashi MH, Alyami BA, Alqahtani YS, et al. Biosynthesis of silver capped magnesium oxide nanocomposite using Olea cuspidata leaf extract and their photocatalytic, antioxidant and antibacterial activity. Photodiagnosis Photodynamic Ther. 2021;33:102153.10.1016/j.pdpdt.2020.102153Suche in Google Scholar PubMed
[6] Schmidt D, Shah D, Giannelis EP. New advances in polymer/layered silicate nanocomposites. Curr OpSolid State Mater Sci. 2002;6(3):205–12.10.1016/S1359-0286(02)00049-9Suche in Google Scholar
[7] Gleiter H. Materials with ultrafine microstructures: retrospectives and perspectives. Nanostruct Mater. 1992;1(1):1–19.10.1016/0965-9773(92)90045-YSuche in Google Scholar
[8] Nikam AV, Prasad BLV, Kulkarni AA. Wet chemical synthesis of metal oxide nanoparticles: a review. CrystEngComm. 2018;20(35):5091–107.10.1039/C8CE00487KSuche in Google Scholar
[9] Starowicz M, Stypuła B, Banaś J. Electrochemical synthesis of silver nanoparticles. Electrochem Commun. 2006;8(2):227–30.10.1016/j.elecom.2005.11.018Suche in Google Scholar
[10] Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J Drug Delivery Sci Technol. 2019;53:101174.10.1016/j.jddst.2019.101174Suche in Google Scholar
[11] Garibo D, Borbón-Nuñez HA, de León JND, García Mendoza E, Estrada I, Toledano-Magaña Y, et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci Rep. 2020;10:12805.10.1038/s41598-020-69606-7Suche in Google Scholar PubMed PubMed Central
[12] Ahmad A, Wei Y, Syed F, Imran M, Khan ZUH, Tahir K, et al. Size dependent catalytic activities of green synthesized gold nanoparticles and electro-catalytic oxidation of catechol on gold nanoparticles modified electrode. RSC Adv. 2015;5(120):99364–77.10.1039/C5RA20096BSuche in Google Scholar
[13] Ai J, Biazar E, Jafarpour M, Montazeri M, Majdi A, Aminifard S, et al. Nanotoxicology and nanoparticle safety in biomedical designs. Int J Nanomed. 2011;1117–27.10.2147/IJN.S16603Suche in Google Scholar PubMed PubMed Central
[14] Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8(11):7278–308.10.3390/ma8115377Suche in Google Scholar PubMed PubMed Central
[15] Lagashetty A. Green synthesis and characterization of silver nanoparticles using piper betel leaf extract. Bull Adv Sci Res. 2015;1(5):136–8.Suche in Google Scholar
[16] Amarendra DD. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extracts. Colloids Surf A. 2010;369(3):27–33.10.1016/j.colsurfa.2010.07.020Suche in Google Scholar
[17] Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–50.10.1039/c1gc15386bSuche in Google Scholar
[18] Vaidyanathan R, Gopalram S, Kalishwaralal K, Deepak V, Pandian SR, Gurunathan S. Enhanced silver nanoparticle synthesis by optimization of nitrate reductase activity. Colloids Surf B Biointerfaces. 2010;75(1):335–41.10.1016/j.colsurfb.2009.09.006Suche in Google Scholar PubMed
[19] Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, et al. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts. 2021;11(8):902.10.3390/catal11080902Suche in Google Scholar
[20] Hemanth NKS, Karthik KG, Bhaskara RKV. Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch Appl Sci Res. 2010;2(6):161–7.Suche in Google Scholar
[21] Basavaraja S, Balaji SD, Lagashetty A, Rajasab S, Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectumm. Mater Res Bull. 2008;43(5):1164–70.10.1016/j.materresbull.2007.06.020Suche in Google Scholar
[22] Ankamwar B, Ahmad DC, Sastry M. Biosynthesis of gold and silver nanoparticles using Emblica Officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol. 2005;5(10):1665–71.10.1166/jnn.2005.184Suche in Google Scholar PubMed
[23] Parashar UK, Saxena PS, Srivastava A. Bioinspired synthesis of silver nanoparticles. Dig J Nanomater Biostruct. 2009;4:159–66.Suche in Google Scholar
[24] Elumalai EK, Prasad TNVKV, Hemachandran J, Therasa SV, Thirumalai T, David E. Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci Res. 2010;2(9):549–54.Suche in Google Scholar
[25] Subbanna S, Gopenath TS, Basalingappa KM. Biogenic nanoparticles from Allium sativum and its bioactives applications. Eur J Mol Clin Med. 2020;7(08):212–32.Suche in Google Scholar
[26] Krishna SBN. Emergent Roles of Garlic-Based Nanoparticles for Bio-medical Applications-A Review. Curr Trends Biotechnol Pharm. 2021;15(3):349–60.Suche in Google Scholar
[27] Johnson J, Shanmugam R, Lakshmi T. A review on plant-mediated selenium nanoparticles and its applications. J Popul Therapeutics Clin Pharmacol = J de la Therapeutique des Popul et de la Pharmacologie Clin. 2022;28(2):29–40.10.47750/jptcp.2022.870Suche in Google Scholar PubMed
[28] Mondal P, Anweshan A, Purkait MK. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: a review. Chemosphere. 2020;259:127509.10.1016/j.chemosphere.2020.127509Suche in Google Scholar PubMed
[29] Chockalingam S, Preetha S, Jeevitha M, Pratap L. Antibacterial Effects of Capparis decidua Fruit Mediated Selenium Nanoparticles. J Evol Med Dent Sci. 2020;9:2947–51.10.14260/jemds/2020/646Suche in Google Scholar
[30] Zakariya NA, Majeed S, Jusof WHW. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sens Int. 2022;3:100164.10.1016/j.sintl.2022.100164Suche in Google Scholar
[31] Zangeneh A, Zangeneh MM, Moradi R. Ethnomedicinal plant‐extract‐assisted green synthesis of iron nanoparticles using Allium saralicum extract, and their antioxidant, cytotoxicity, antibacterial, antifungal and cutaneous wound‐healing activities. Appl Organomet Chem. 2020;34(1):e5247.10.1002/aoc.5247Suche in Google Scholar
[32] Vitta Y, Figueroa M, Calderon M, Ciangherotti C. Synthesis of iron nanoparticles from aqueous extract of Eucalyptus robusta Sm and evaluation of antioxidant and antimicrobial activity. Mater Sci Energy Technol. 2020;3:97–103.10.1016/j.mset.2019.10.014Suche in Google Scholar
[33] Shnoudeh AJ, Hamad I, Abdo RW, Qadumii L, Jaber AY, Surchi HS, et al. Synthesis, characterization, and applications of metal nanoparticles. In: Biomaterials and Bionanotechnology. MDPI: Academic Press; 2019. p. 527–612.10.1016/B978-0-12-814427-5.00015-9Suche in Google Scholar
[34] Gunti L, Dass RS, Kalagatur NK. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: antioxidant, antimicrobial, and biocompatibility. Front Microbiology. 2019;10:931.10.3389/fmicb.2019.00931Suche in Google Scholar PubMed PubMed Central
[35] Adibian F, Ghaderi RS, Sabouri Z, Davoodi J, Kazemi M, Ghazvini K, et al. Green synthesis of selenium nanoparticles using Rosmarinus officinalis and investigated their antimicrobial activity. BioMetals. 2022;35:147–58.10.1007/s10534-021-00356-3Suche in Google Scholar PubMed
[36] Sarkar J, Dey P, Saha S, Acharya K. Mycosynthesis of selenium nanoparticles. Micro Nano Lett. 2011;6(8):599–602.10.1049/mnl.2011.0227Suche in Google Scholar
[37] Safaei M, Mozaffari HR, Moradpoor H, Imani MM, Sharifi R, Golshah A. Optimization of green synthesis of selenium nanoparticles and evaluation of their antifungal activity against oral Candida albicans infection. Adv Mater Sci Eng. 2022;2022:1–8.10.1155/2022/1376998Suche in Google Scholar
[38] Al-Qaraleh SY, Al-Zereini WA, Oran SA, Al-Sarayreh AZ, Sa'ed M. Evaluation of the antioxidant activities of green synthesized selenium nanoparticles and their conjugated polyethylene glycol (PEG) form in vivo. Open Nano. 2022;8:100109.10.1016/j.onano.2022.100109Suche in Google Scholar
[39] Liu Y, Li F, Zhang L, Wu J, Wang Y, Yu H. Taurine alleviates lipopolysaccharide-induced liver injury by anti-inflammation and antioxidants in rats. Mol Med Rep. 2017;16(5):6512–7.10.3892/mmr.2017.7414Suche in Google Scholar PubMed PubMed Central
[40] Filipović N, Ušjak D, Milenković MT, Zheng K, Liverani L, Boccaccini AR, et al. Comparative study of the antimicrobial activity of selenium nanoparticles with different surface chemistry and structure. Front Bioeng Biotechnol. 2021;8:624621.10.3389/fbioe.2020.624621Suche in Google Scholar PubMed PubMed Central
[41] Truong LB, Medina-Cruz D, Mostafavi E, Rabiee N. Selenium nanomaterials to combat antimicrobial resistance. Molecules. 2021;26(12):3611.10.3390/molecules26123611Suche in Google Scholar PubMed PubMed Central
[42] Velsankar K, RM AK, Preethi R, Muthulakshmi V, Sudhahar S. Green synthesis of CuO nanoparticles via Allium sativum extract and its characterizations on antimicrobial, antioxidant, antilarvicidal activities. J Environ Chem Eng. 2020;8(5):104123.10.1016/j.jece.2020.104123Suche in Google Scholar
[43] Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A. Biomimetic synthesis of silver nanoparticles by Citrus limon (Lemon) aqueous extract and theoretical prediction of particle size. Colloid Surf B: Bioint. 2011 Jan;82:152–9.10.1016/j.colsurfb.2010.08.036Suche in Google Scholar PubMed
[44] javed B, Nadhman A, Mashwani Z. Optimization, characterization and antimicrobial activity of silvernanoparticles against plant bacterial pathogens phyto-synthesized by Mentha longifolia. Mater ResExpress. 2020;7:085406.10.1088/2053-1591/abaf19Suche in Google Scholar
[45] Wang W, Tang Q, Yu T, Li X, Gao Y, Li J, et al. Surfactant-free preparation of Au@ resveratrol hollow nanoparticles with photothermal performance and antioxidant activity. ACS Appl Mater Interfaces. 2017;9:3376–87.10.1021/acsami.6b13911Suche in Google Scholar PubMed
[46] Bhakya S, Muthukrishnan S, Sukumaran M, Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl Nanosci. 2016;6:755–66.10.1007/s13204-015-0473-zSuche in Google Scholar
[47] Senthilkumar SR, Sivakumar T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int J Pharm Pharm Sci. 2014;6(6):461–5.Suche in Google Scholar
[48] Baliah NT, Muthulakshmi P, Sheeba PC, Priyatharsini SL. Green synthesis and characterization of nanocomposites. Int Res J Eng Technol. 2018;5(12):179–86.Suche in Google Scholar
[49] Prasad KS, Patel H, Patel T, Patel K, Selvaraj K. Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf B: Biointerfaces. 2013;103:261–6.10.1016/j.colsurfb.2012.10.029Suche in Google Scholar PubMed
[50] Gangadoo S, Stanley D, Hughes RJ, Moore RJ, Chapman J. The synthesis and characterisation of highly stable and reproducible selenium nanoparticles. Inorg Nano-Metal Chem. 2017;47(11):1568–76.10.1080/24701556.2017.1357611Suche in Google Scholar
[51] Vahdati M, Tohidi Moghadam T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci Rep. 2020;10(1):1–10.10.1038/s41598-019-57333-7Suche in Google Scholar PubMed PubMed Central
[52] Fesharaki PJ, Nazari P, Shakibaie M, Rezaie S, Banoee M, Abdollahi M, et al. Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Braz J Microbiol. 2010;41:461–6.10.1590/S1517-83822010000200028Suche in Google Scholar
[53] Ramamurthy CH, Sampath KS, Arunkumar P, Kumar MS, Sujatha V, Premkumar K, et al. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst Eng. 2013;36(8):1131–9.10.1007/s00449-012-0867-1Suche in Google Scholar PubMed
[54] Jagathesan G, Rajiv P. Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatalysis Agric Biotechnol. 2018;13:90–4.10.1016/j.bcab.2017.11.014Suche in Google Scholar
[55] Tartaj P, del Puerto Morales M, Veintemillas-Verdaguer S, González-Carreño T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D: Appl Phys. 2003;36(13):R182.10.1088/0022-3727/36/13/202Suche in Google Scholar
[56] Abdullah JAA, Eddine LS, Abderrhmane B, Alonso-González M, Guerrero A, Romero A. Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain Chem Pharm. 2020;17:100280.10.1016/j.scp.2020.100280Suche in Google Scholar
[57] Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal anoparticles: The influence of size, shape and dielectric environment. J Phys Chem. 2003;107:668–77.10.1021/jp026731ySuche in Google Scholar
[58] Anu K, Singaravelu G, Murugan K, Benelli G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): biophysical characterization and cytotoxicity on vero cells. J Clust Sci. 2017;28:551–63.10.1007/s10876-016-1123-7Suche in Google Scholar
[59] Sree BS, Aparna Y, Babu TA, Krishna NV. Indium tin oxide nanocomposites-green synthesis, characterization, morphology and optical properties using Almond Gum. Lett Appl NanoBioScience. 2022;11(1):3134–41.10.33263/LIANBS111.31343141Suche in Google Scholar
[60] Prasad KS, Selvaraj K. Biogenic synthesis of selenium nanoparticles and their effect on As (III)-induced toxicity on human lymphocytes. Biol Trace Elem Res. 2014;157:275–83.10.1007/s12011-014-9891-0Suche in Google Scholar PubMed
[61] Batool F, Iqbal MS, Khan SUD, Khan J, Ahmed B, Qadir MI. Biologically synthesized iron nanoparticles (FeNPs) from Phoenix dactylifera have anti-bacterial activities. Sci Rep. 2021;11(1):22132.10.1038/s41598-021-01374-4Suche in Google Scholar PubMed PubMed Central
[62] Lakshminarayanan S, Shereen MF, Niraimathi KL, Brindha P, Arumugam A. One-pot green synthesis of iron oxide nanoparticles from Bauhinia tomentosa: Characterization and application towards synthesis of 1, 3 diolein. Sci Rep. 2021;11(1):1–13.10.1038/s41598-021-87960-ySuche in Google Scholar PubMed PubMed Central
[63] Alagesan V, Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9(1):105–16.10.1007/s12668-018-0566-8Suche in Google Scholar
[64] Nahari MH, Al Ali A, Asiri A, Mahnashi MH, Shaikh IA, Shettar AK, et al. Green synthesis and characterization of iron nanoparticles synthesized from aqueous leaf extract of vitex leucoxylon and its biomedical applications. Nanomaterials. 2022;12(14):2404.10.3390/nano12142404Suche in Google Scholar PubMed PubMed Central
[65] Zimmerman MT, Bayse CA, Ramoutar RR, Brumaghim JL. Sulfur and selenium antioxidants: challenging radical scavenging mechanisms and developing structure–activity relationships based on metal binding. J Inorg Biochem. 2015;145:30–40.10.1016/j.jinorgbio.2014.12.020Suche in Google Scholar PubMed
[66] Singh J, Kumar S, Dhaliwal A. Controlled release of amoxicillin and antioxidant potential of gold nanoparticles-xanthan gum/poly (Acrylic acid) biodegradable nanocomposite. J Drug Deliv Sci Technol. 2019;55:101384.10.1016/j.jddst.2019.101384Suche in Google Scholar
[67] Vyas J, Rana S. Antioxidant activity and green synthesis of selenium nanoparticles using allium sativum extract. Int J Phytomedicine. 2017;9(4):634–41.10.5138/09750185.2185Suche in Google Scholar
[68] Kokila K, Elavarasan N, Sujatha V. Disopyros montana leaf extract- mediated synthesis of selenium nanoparticles and their biological applications. N J Chem. 2017;41:7481–90. 10.1039/c7nj01124e.Suche in Google Scholar
[69] Chavan RR, Bhinge SD, Bhutkar MA, Randive DS, Wadkar GH, Todkar SS, et al. Characterization, antioxidant, antimicrobial and cytotoxic activities of green synthesized silver and iron nanoparticles using alcoholic Blumea eriantha DC plant extract. Mater Today Com. 2020;24:101320.10.1016/j.mtcomm.2020.101320Suche in Google Scholar
[70] Lin YL, Juan IM, Chen YL, Liang YC, Lin JK. Composition of polyphenols in fresh tea leaves and associations of their oxygen-radical-absorbing capacity with antiproliferative actions in fibroblast cells. J Agric Food Chem. 1996;44:1387–94.10.1021/jf950652kSuche in Google Scholar
[71] Deshmukh AR, Gupta A, Kim BS. Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activities. BioMed Res Int. 2019;2019:51–94.10.1155/2019/1714358Suche in Google Scholar PubMed PubMed Central
[72] Hassan HU, Raja NI, Abasi F, Mehmood A, Qureshi R, Manzoor Z, et al. Comparative Study of Antimicrobial and Antioxidant Potential of Olea ferruginea Fruit Extract and Its Mediated Selenium Nanoparticles. Molecules. 2022;27(16):5194.10.3390/molecules27165194Suche in Google Scholar PubMed PubMed Central
[73] Chau TP, Brindhadevi K, Krishnan R, Alyousef MA, Almoallim HS, Whangchai N, et al. A novel synthesis, analysis and evaluation of Musa coccinea based zero valent iron nanoparticles for antimicrobial and antioxidant. Environ Res. 2022;209:112770.10.1016/j.envres.2022.112770Suche in Google Scholar PubMed
[74] Fardsadegh B, Jafarizadeh-Malmiri H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process Synth. 2019;8(1):399–407.10.1515/gps-2019-0007Suche in Google Scholar
[75] Nwamezie OUIF. Green synthesis of iron nanoparticles using flower extract of Piliostigma thonningii and their antibacterial activity evaluation. Chem Int. 2018;4(1):60.Suche in Google Scholar
[76] Tran PA, O’Brien-Simpson N, Reynolds EC, Pantarat N, Biswas DP, O’Connor AJ. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology. 2015;27(4):045101.10.1088/0957-4484/27/4/045101Suche in Google Scholar PubMed
[77] Gurunathan S, Han JW, Dayem AA, Eppakayala V, Kim JH. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomed. 2012;7:5901.10.2147/IJN.S37397Suche in Google Scholar PubMed PubMed Central
[78] Nagy A, Harrison A, Sabbani S, Munson Jr RS, Dutta PK, Waldman WJ. Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. Int J Nanomed. 2011;6:1833.10.2147/IJN.S24019Suche in Google Scholar PubMed PubMed Central
[79] Leung YH, Ng AM, Xu X, Shen Z, Gethings LA, Wong MT, et al. Mechanisms of antibacterial activity of MgO: non‐ROS mediated toxicity of MgO nanoparticles towards Escherichia coli. Small. 2014;10(6):1171–83.10.1002/smll.201302434Suche in Google Scholar PubMed
[80] Weir E, Lawlor A, Whelan A, Regan F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst. 2008;133(7):835–45.10.1039/b715532hSuche in Google Scholar PubMed
[81] Martínez-Esquivias F, Guzmán-Flores JM, Pérez-Larios A, González Silva N, Becerra-Ruiz JS. A review of the antimicrobial activity of selenium nanoparticles. J Nanosci Nanotechnol. 2021;21(11):5383–98.10.1166/jnn.2021.19471Suche in Google Scholar PubMed
[82] Zhang H, Li Z, Dai C, Wang P, Fan S, Yu B, et al. Antibacterial properties and mechanism of selenium nanoparticles synthesized by Providencia sp. DCX. Environ Res. 2021;194:110630.10.1016/j.envres.2020.110630Suche in Google Scholar PubMed
[83] Devatha CP, Jagadeesh K, Patil M. Effect of Green synthesized iron nanoparticles by Azardirachta Indica in different proportions on antibacterial activity. Environ Nanotechnol Monit Manag. 2018;9:85–94.10.1016/j.enmm.2017.11.007Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

