The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
-
Jingjing Sun
, Xuefeng Mao
Abstract
The heterogeneous supported Lewis acid catalyst prepared by immobilization technology has high reaction activity. It is an environment-friendly catalyst. Using Lewis acid immobilized as the catalyst, 2-methyl-6-propionyl naphthalene is synthesized by Friedel–Crafts reaction with 2-methylnaphthalene and propionic anhydride, which has a good development prospect. A variety of AlCl3 catalysts supported by H-zeolite molecular sieves are prepared using the solvent reflux method in the paper. It is found that AlCl3/Hβ has better catalytic performance. The results showed that AlCl3/Hβ catalyst is mainly composed of L acid. The acid content of B acid and the specific surface area increase, and the pore volume and pore size decreases. With the increase in AlCl3 concentration, the acid content of strong acid, medium strong acid, and weak acid increases, but the solubility of AlCl3 in CHCl3 is limited. When the concentration of AlCl3 is too high, too much AlCl3 is deposited on the surface of the molecular sieve, which is useless to its binding with Si–OH. AlCl3/Hβ’s activity is higher when the concentration of AlCl3 is 3 g·L−1, and the solvent is refluxed for 8 h and calcined at 550°C for 3 h. Under these conditions, the conversion of 2-methylnaphthalene is 85.86%, and the yield of β,β-methyl propyl naphthalene is increased to 81.19%.
1 Introduction
β,β-Methyl propyl naphthalene is an important organic chemical raw material. The 2-methyl-6-propionyl naphthalene (2,6-MPN) is oxidized with ethylene glycol to obtain polyethylene 2,6-naphthalene dicarboxylate (PEN). It is a high-end polyester with excellent physical and chemical properties [1,2,3,4,5,6]. The synthesis method of 2,6-MPN mainly uses Lewis acid anhydrous AlCl3 as the catalyst to catalyze the Friedel–Crafts acylation reaction between 2-methylnaphthalene (2-MN) and propyl chloride [7,8,9]. Li et al. [10] used an anhydrous AlCl3 catalyst to carry out the 2-MN acylation reaction in a stainless-steel microchannel reactor, which had a high heat transfer rate. The yield of 2,6-MPN is 85.8%, and the selectivity is 87.5%. Although anhydrous AlCl3 has good catalytic activity, it also has many shortcomings, such as large consumption, severe pollution, equipment corrosion, non-regeneration, and poor safety [11,12,13]. Therefore, it is urgent to develop a new acylation catalyst and green synthesis process of 2,6-MPN. The immobilized AlCl3 catalyst can maintain the high catalytic activity of AlCl3 and overcome the shortcomings of AlCl3 decomposition in water, which is an ideal heterogeneous catalyst [14,15,16,17,18,19,20,21,22]. There are two methods to prepare an immobilized AlCl3 catalyst: the physical adsorption method and the chemical bonding method [23,24,25,26]. Chemical bonding refers to the bonding between AlCl3 and the hydroxyl group on the surface and the removal of the HCl molecule to obtain a heterogeneous catalyst containing AlCl3.
There are two main methods to immobilize AlCl3 by chemical bond: the gas phase method [27] and the liquid phase method [22,28,29,30]. AlCl3 is easy to sublimate, and N2 is used to react with zeolite molecular sieve and other carriers to form the immobilized AlCl3. The experimental operation is simple, but the equipment is too high to realize. The liquid phase method dissolves AlCl3 in halogenated alkane solvent and reacts with the carrier for a period to generate the embedded AlCl3. The solvent used in the liquid phase reaction is toxic, but the operation is simple. Both methods of immobilized AlCl3 have been presented, and the prepared immobilized AlCl3 catalyst has good catalytic activity in many organic reactions, especially Friedel–Crafts reactions. In this article, a series of catalysts were prepared using H-zeolite molecular sieves as carriers and using the solvent reflux method to immobilize AlCl3 under different conditions. The preparation conditions and the catalytic performance of the 2-MN acylation reaction were studied. This is a new green catalytic process, which can accelerate the research and production process of PEN and improve people’s quality of life.
2 Experimental
2.1 Preparation of AlCl3 supported catalyst
The zeolite molecular sieve was pretreated in a muffle furnace at 600°C for 3 h. Anhydrous AlCl3 was weighed and placed in a 250 mL three-way flask. Then, 100 mL CHCl3 solution was added. After nitrogen was pumped in and the air was emptied, the metal bath controller was turned on to stir. After AlCl3 was fully dissolved, 10 g of pretreated zeolite molecular sieve (sieve agent ratio 1:10) was added and stirred at a high temperature to reflux the solvent. After a while, the heating was stopped. The three-end flask was cooled to room temperature, filtered, and washed with CHCl3. The solid was placed in a vacuum oven at 50°C overnight. After grinding, it was roasted at 550°C for 3 h in a muffle furnace and cooled to room temperature. Finally, the immobilized AlCl3 catalyst was obtained.
2.2 Characterization of the supported catalyst
Acidity was determined using an automatic temperature-programmed chemisorption (NH3-TPD) unit developed by Micromeritics (AutoChem II 2920) with a thermal conductivity detector (TCD). The 0.2 g catalyst sample was first flushed with helium (30 mL·min−1) at 700°C for 3 h, cooled to 150°C, saturated with NH3 until equilibrated, and then flushed again with helium until the baseline of the integrator was stable. The NH3-TPD treatment quickly starts from 150°C to 700°C, and the heating rate was 15°C·min−1. Surface morphology was observed using the TESCAN MIRA4 ultra-high resolution field emission scanning electron microscope (SEM) manufactured in the Czech Republic. The electron gun was a Schottky field emission gun, the magnification was 2–50 k, and the test voltage was 30 kV.
Specific surface area and pore volume aperture (BET) were measured using Micromeritics ASAP 2460 3.01. About 0.12 g of the sample was weighed into a sample tube, vacuum-dried, and then de-vacuumed (300°C degassing for 3 h), and then N2 was adsorbed in liquid nitrogen −196°C. After the test was completed, the specific surface area of the catalyst was calculated by the BET method. The BJH model calculated the pore size distribution.
A PANalytical Axios X-ray fluorescence spectrometer (XRF) produced by Philips was used to determine the contents of each component in the catalyst. The instrument type was multi-channel, and the power of the X-ray was 4 kW. Pyridine absorption infrared spectroscopy (Py-IR) used a Thermo Fisher Nicolet iS50 infrared spectrometer to measure the concentration of Brönsted and Lewis acids in the samples. The transmission method was used for testing, the number of infrared scanning was 32 times, the scanning wavelength was 400–4,000 cm−1, and the resolution was 4 cm−1.
2.3 Catalyst evaluation
A certain amount of 2-MN and propionic anhydride (PA) were added to a three-necked round-bottomed flask equipped with a condenser tube, a thermometer, a drying tube, and a metal bath. Then, an appropriate amount of solvent was added. After stirring evenly, the catalyst was added, and the temperature was raised and stirred continuously for a while. Then, the catalyst was separated by filtration, and the reaction solution was analyzed using a gas chromatograph.
GC-2014C (SHIMADZU, Japan) was equipped with an FID detector. The temperature program mode (215°C for 30 min) was used to analyze the extracted reaction solution. The chromatographic column was HP-5 50 m × 0.20 mm. N2 was used as carrier gas at the nitrogen pressure of 0.5 MPa. The detector temperature was 300°C, and the injection port temperature was 300°C. The injection quantity was 0.2 μL.
3 Results and discussion
3.1 Effect of different molecular sieve carriers on 2-MN acylation catalyzed by AlCl3
Five H-zeolite molecular sieves USY (SiO2/Al2O3 = 6), HY (SiO2/Al2O3 = 6), meridians (MOR: SiO2/Al2O3 = 10), HZSM-5 (SiO2/Al2O3 = 25), and Hβ (SiO2/Al2O3 = 25) were used as carriers to prepare the immobilized AlCl3 catalyst under the same conditions, which was used to catalyze the acylation reaction of 2-MN with PA. Tetramethylene sulfone (TS) was used as the solvent. The results are shown in Table 1.
Effect of different zeolite carriers on 2-MN propionyl catalyzed by AlCl3
| Catalysts | Conversion (%) | Selective (%) | ||
|---|---|---|---|---|
| 2,6-MPN | 2,7-MPN | Others | ||
| AlCl3/USY | 49.6 | 41.8 | 25.7 | 32.5 |
| AlCl3/HY | 55.0 | 34.3 | 12.5 | 53.2 |
| AlCl3/MOR | 48.1 | 21.7 | 9.8 | 68.5 |
| AlCl3/HZSM-5 | 49.7 | 23.4 | 23.5 | 53.1 |
| AlCl3/Hβ | 76.2 | 48.0 | 46.4 | 5.6 |
Reaction conditions: 2-MN 0.0125 mol, PA 0.025 mol, TS 10 g, catalyst (calcination at 600°C for 3 h) 3 g, 190°C, 10 h.
Hβ zeolite molecular sieve as a carrier of AlCl3 had significantly more vigorous catalytic activity than other zeolite molecular sieves, with a higher conversion of 2-MN and a selectivity of β,β-MPN up to 94.4%. There were two possible reasons: on the one hand, Hβ had open three-dimensional channels, the pore size was much larger than HZSM-5, PA could freely access Hβ channels, AlCl3/Hβ surface-active center was completely contacted, and chemical adsorption could form a large number of acyl carbocation, conducive to the reaction; on the other hand, Hβ itself had a large number of medium and strong acids that promoted Friedel–Crafts acylation reaction between 2-MN and PA, and its acidity was much more than HY, USY, MOR, and HZSM-5, which was the main reason why AlCl3/Hβ had more vital catalytic activity than other zeolite molecular sieves immobilized AlCl3. However, other zeolite molecular sieves had less strong acid and limited AlCl3 load, so the active intermediates generated on the active center were inadequate to attack the inert aromatic ring, resulting in low catalytic activity. Therefore, the Hβ zeolite molecular sieve (Si/Al ratio of 25) was used as the carrier to prepare AlCl3/Hβ catalyst.
3.2 Characterization analysis of AlCl3/Hβ catalyst
3.2.1 XRD characterization of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
The AlCl3/Hβ catalysts prepared by the solvent reflux method with different concentrations of AlCl3 were characterized by XRD. According to the results in Figure 1, the XRD diffraction peaks of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3 showed the standard diffraction peaks of Hβ (PDF # 48-0074). Moreover, the peak pattern was sharp, indicating that the crystal phase of Hβ was undamaged in the modification process, and the crystal phase was intact. There was no diffraction peak of AlCl3 in the figure, and the skeleton structure of the catalyst was still Hβ, indicating that AlCl3 existed in an amorphous form in the AlCl3/Hβ catalyst and AlCl3 was well dispersed in Hβ. Compared with Hβ, the diffraction peak intensity of the catalyst with AlCl3 immobilized was more vigorous overall. The diffraction peak intensity of the catalyst immobilized with 1 g·L−1 AlCl3 had a non-significant difference from the unmodified Hβ. The diffraction peak of AlCl3/Hβ catalyst with an AlCl3 concentration of 1 g·L−1 was inconspicuous from that of unmodified Hβ.
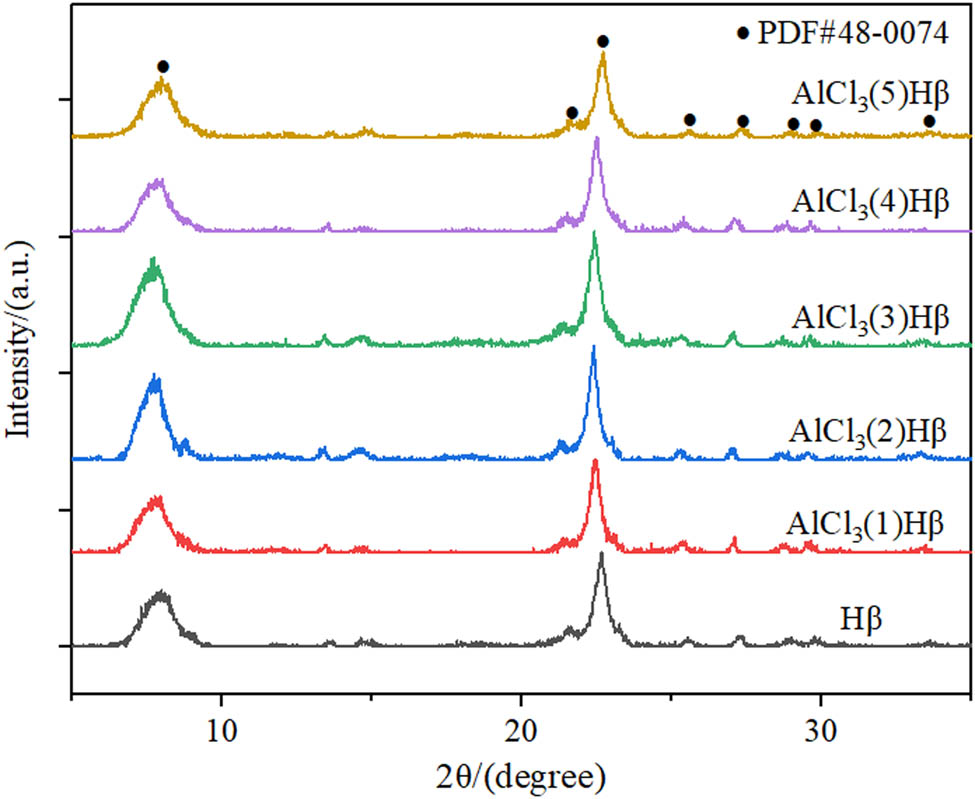
XRD patterns of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3.
3.2.2 SEM characterization of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
SEM characterized the AlCl3/Hβ catalysts obtained with different concentrations of AlCl3, and the results are shown in Figure 2.
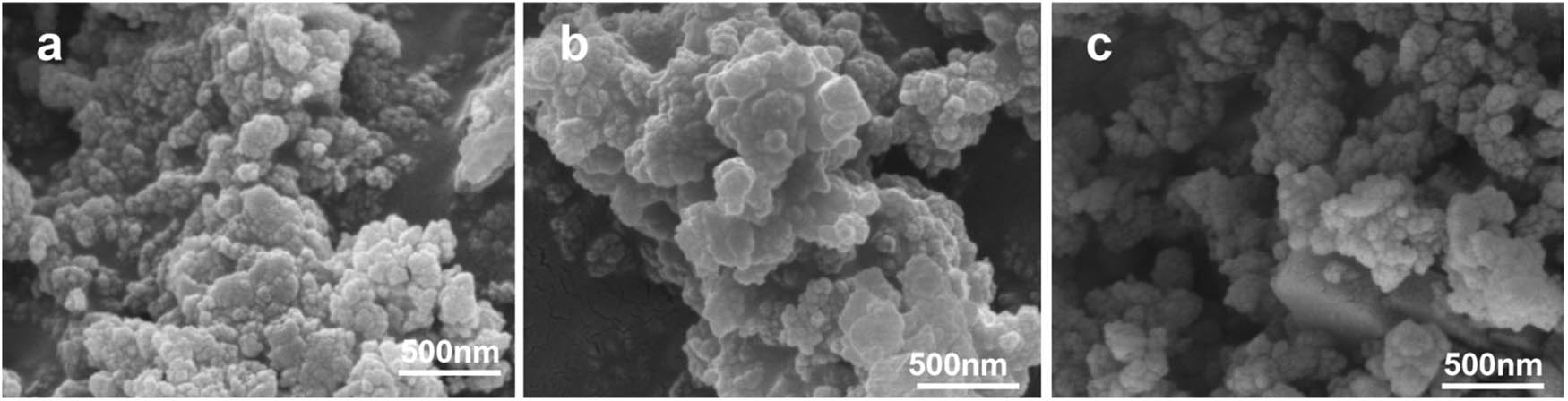
SEM of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3: (a) AlCl3 (1 g·L−1)/Hβ, (b) AlCl3 (3 g·L−1)/Hβ, and (c) AlCl3 (5 g·L−1)/Hβ.
The AlCl3/Hβ catalyst particles were small cubes, but the size distribution of surface particles was uneven, and there was an agglomeration phenomenon. With the increase in AlCl3 concentration, the tiny particles immobilized on the surface of the 3 g·L−1 AlCl3-modified Hβ became more extensive. The distribution was relatively uniform compared with that of 1 g·L−1 void became smaller. As the concentration of AlCl3 increased, the surface particles increased, the voids continued to decrease, and some pores were blocked, which was adverse to catalyzing the 2-MN acylation reaction.
3.2.3 BET characterization of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
The effect of different concentrations of AlCl3 loading on the specific surface area, pore volume, and pore size distribution of Hβ was studied, and the BET characterization was carried out. The results are shown in Table 2.
BET characterization results of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
| Catalysts | Surface area (m2·g−1) | Micropore area (m2·g−1) | External surface area (m2·g−1) | Pore volume (cm3·g−1) | Micropore volume (cm3·g−1) | Mesoporous volume (cm3·g−1) | Pore size (nm) |
|---|---|---|---|---|---|---|---|
| Hβ | 530 | 358 | 172 | 0.45 | 0.17 | 0.29 | 4.63 |
| AlCl3(1)/Hβ | 534 | 362 | 172 | 0.44 | 0.17 | 0.27 | 4.63 |
| AlCl3(3)/Hβ | 559 | 376 | 183 | 0.45 | 0.16 | 0.25 | 4.50 |
| AlCl3(5)/Hβ | 543 | 371 | 172 | 0.33 | 0.15 | 0.18 | 4.26 |
Compared with the unmodified Hβ, the Hβ-specific surface area of AlCl3 supported by solvent reflux increased significantly with AlCl3 concentration. The reason can be explained that the load increases with the concentration increasing of AlCl3. Combined with SEM results, it was shown that the number of small particles on the surface increased, resulting in an increase in specific surface area and a decrease in pore volume and pore size. It may be due to the reaction between AlCl3 and Si–OH on the surface of zeolite, resulting in the reduction of pore size. When the concentration of AlCl3 continued to increase, the particles on the surface gradually increased. The bonding between the bonds leads to a decrease in the specific surface area, pore volume, and aperture. In addition, due to the high concentration of AlCl3 load, AlCl3/Hβ catalyst appeared to sinter after calcination, which was also one reason for reducing the specific surface area. At the same time, the results characterized by BET also showed that the specific surface area was the largest, and the void space of the catalyst was rich and uniform when the AlCl3 concentration was 3 g·L−1.
3.2.4 NH3-TPD characterization of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
NH3-TPD spectra of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3 are shown in Figure 3.
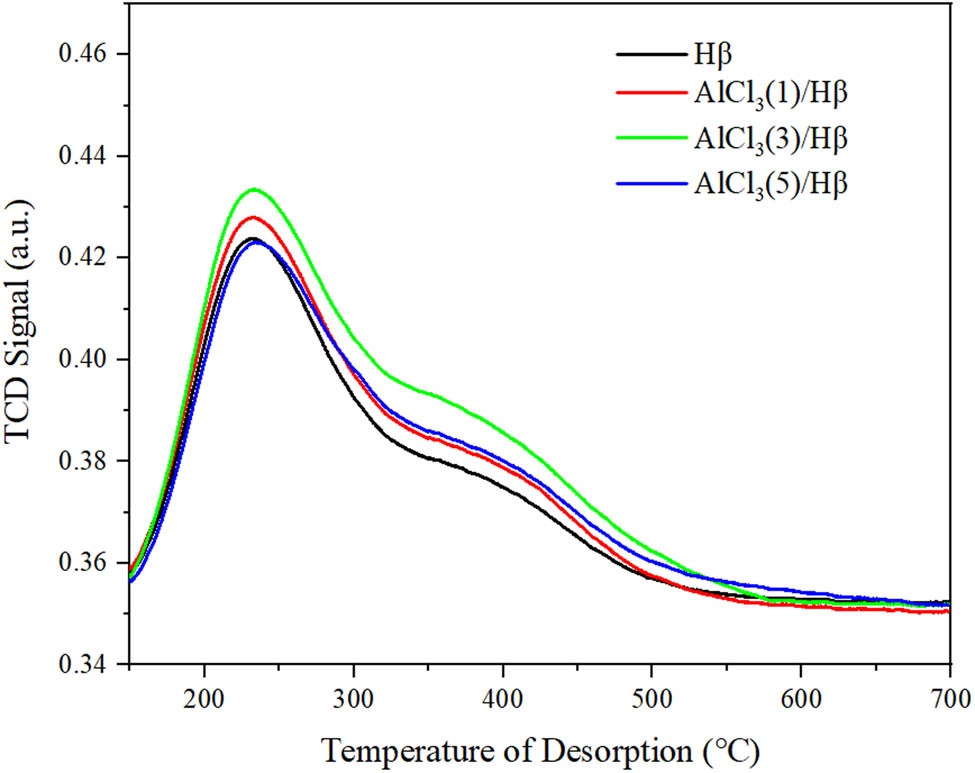
NH3-TPD spectra of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3.
Compared with the unmodified Hβ zeolite molecular sieve, with the increase in AlCl3 concentration, the strong acid strength and acid content of AlCl3/Hβ only changed slightly. In general, there was still an increase in acid content. It may be due to the increase in the number of L-acid sites, which happened to be caused by strong acid sites, thus increasing acid strength and acid content. However, as the concentration of AlCl3 continued to increase, the strength of strong acid was unchanged, but the acid content increased slightly. The strength of medium–strong acid increased compared with Hβ, but it was still slightly decreased compared with the Hβ molecular sieve loaded with 3 g·L−1 AlCl3. The reason may be that the concentration of AlCl3 was too high. The undissolved AlCl3 was deposited on the surface of Hβ in a free state. Part of the AlCl3 was sintered during calcining, destroying the part of the acid center.
3.2.5 Py-IR characterization of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
Pyridine adsorption infrared data of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3 at different temperatures are shown in Figure 4.
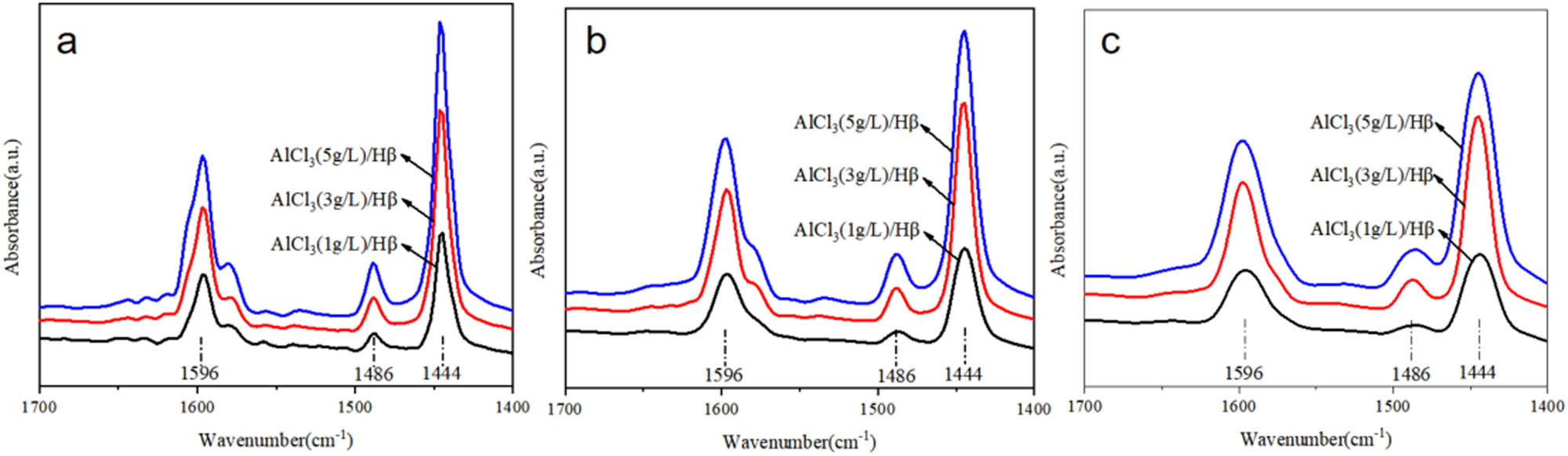
Py-IR spectra of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3: (a) 150°C, (b) 300°C, and (c) 450°C.
To more intuitively compare the acidity of Hβ molecular sieves loaded with AlCl3, the data of the pyridine infrared spectrum were summarized, as shown in Table 3.
Py-IR data of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
| Catalysts | Strong acid (μmol·g−1) | Mediate strong acid (μmol·g−1) | Weak acid (μmol·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bronsted acid | Lewis acid | Total acid | Bronsted acid | Lewis acid | Total acid | Bronsted acid | Lewis acid | Total acid | |
| AlCl3(1)/Hβ | 1.74 | 14.20 | 15.93 | 2.68 | 71.03 | 73.71 | 3.48 | 262.03 | 265.31 |
| AlCl3(3)/Hβ | 2.06 | 65.69 | 67.75 | 6.45 | 176.15 | 182.60 | 9.49 | 401.30 | 410.79 |
| AlCl3(5)/Hβ | 2.76 | 147.30 | 150.06 | 6.48 | 174.21 | 180.69 | 18.00 | 413.42 | 431.42 |
Figure 4a–c corresponds to the changes in acid content and acid strength of AlCl3/Hβ catalysts prepared at different concentrations of AlCl3 at 150°C, 300°C, and 450°C, respectively. With the increase in AlCl3 concentration, compared with strong acid and medium–strong acid, the increasing trend of weak acid was the largest, and the increasing trend of strong acid was also more extensive. The increasing trend of Lewis acid was more prominent than that of Bronsted acid. The main reason was that strong acid sites increased Lewis acid quantity, and AlCl3 itself was a Lewis acid. With the further increase of AlCl3 concentration, the medium and strong acid contents decreased, while the change in the Bronsted acid content was minor. As the concentration of AlCl3 continued to increase, part of AlCl3 attached to the surface of Hβ did not form chemical bonds with the surface elements. It is also reflected in the preparation process of increasing bonds mainly for Hβ molecular sieve increased the Bronsted acid center. The ratio of Bronsted acid and Lewis acid determines the increase of medium strong acid.
3.2.6 XRF characterization of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3
For the supported zeolite catalyst, the Si/Al ratio and other components of the supported zeolite have a significant influence on the activity of the catalyst. Table 4 compares the percentage content and ratio of each component in AlCl3/Hβ catalysts supported by different concentrations of AlCl3.
Comparison of percentage content and ratio of AlCl3/Hβ catalysts supported by different concentrations of AlCl3
| Catalysts | Al2O3 (wt%) | SiO2 (wt%) | Cl (wt%) | SiO2/Al2O3 | Si (wt%) | Al (wt%) | Si/Al |
|---|---|---|---|---|---|---|---|
| Hβ | 6.665 | 92.802 | 0 | 23.628 | 43.379 | 3.528 | 11.814 |
| AlCl3(1)/Hβ | 9.709 | 89.562 | 0.094 | 15.654 | 88.600 | 10.219 | 8.328 |
| AlCl3(3)/Hβ | 10.652 | 88.129 | 0.113 | 14.040 | 86.898 | 11.108 | 7.514 |
| AlCl3(5)/Hβ | 11.716 | 87.592 | 0.111 | 12.687 | 86.702 | 12.178 | 6.838 |
The Si/Al ratio of Hβ immobilized with AlCl3 decreased, the SiO2/Al2O3 ratio of the modified zeolite decreased from 23.628 to 12.687, and the content of Cl remained unchanged. The percentage content of Cl increased gradually with the increase in AlCl3 concentration. It indicated that the immobilized AlCl3 was combined with the Si–OH on the surface of the Hβ molecular sieve. Qi et al. [26] used the 27Al MAS NMR method to study the possible binding forms of AlCl3 and Si–OH after immobilization in the molecular sieve, as shown in Figure 5.
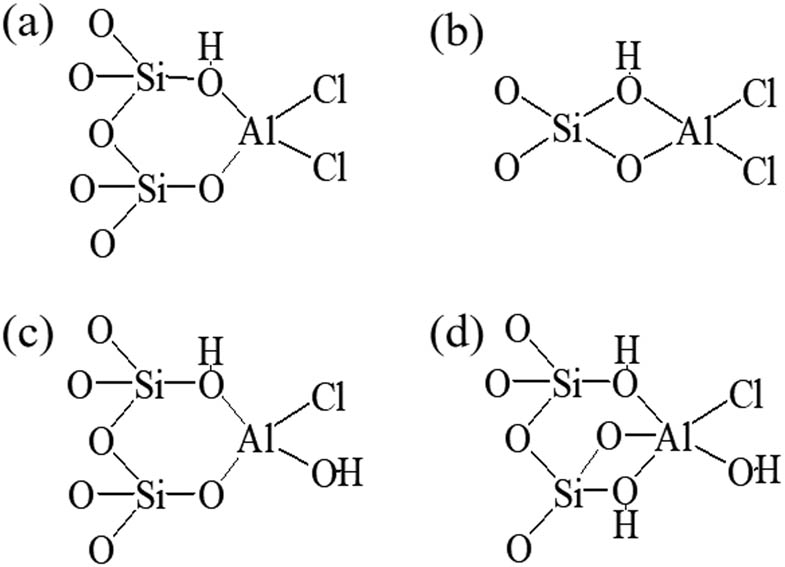
Possible binding form of AlCl3 and Si–OH after loading on molecular sieve.
Different concentrations of AlCl3 may predominate AlCl3/Hβ in one or more forms shown in Figure 5a–d. However, either form increased Cl in terms of acidity and acid strength, thus modulating the acidity of the catalyst, catalytic activity, and selectivity to the target product β,β-MPN.
3.3 Effect of preparation conditions on propionyl of 2-MN catalyzed by AlCl3/Hβ catalyst
3.3.1 Effect of AlCl3 concentration on the catalytic performance of AlCl3/Hβ
The effect of AlCl3/Hβ catalysts prepared with different concentrations of AlCl3 on the acylation reaction of 2-MN and PA is shown in Figure 6.
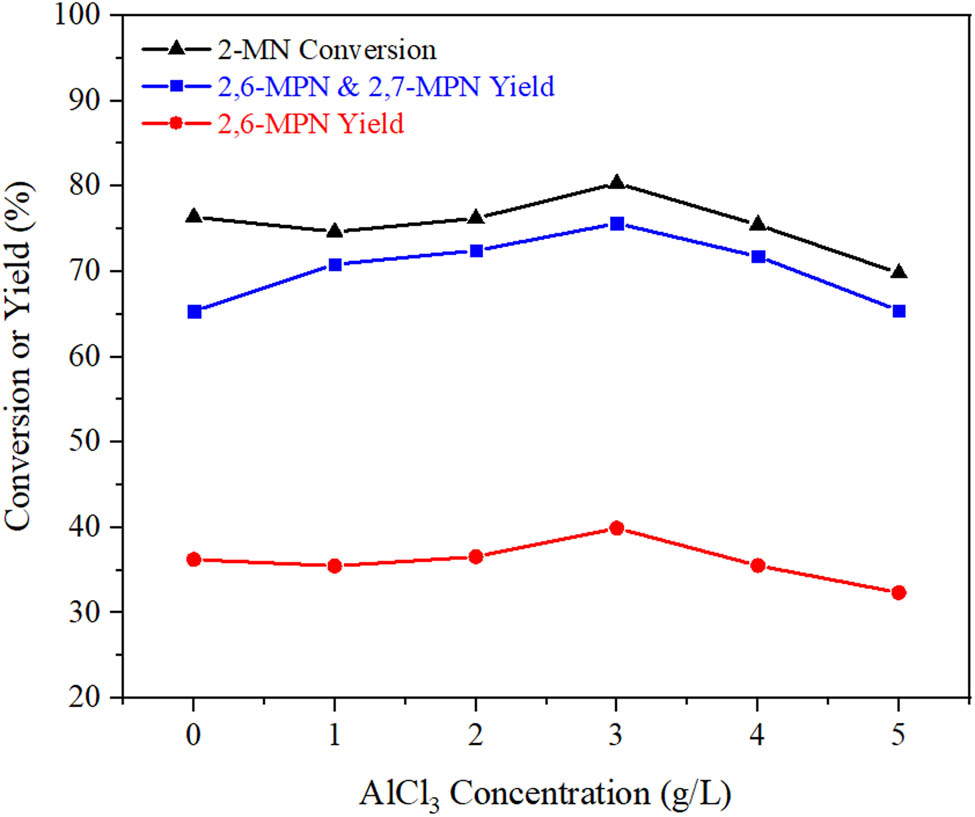
Effect of AlCl3 concentration on the catalytic performance of AlCl3/Hβ. Reaction conditions: n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ (reflux for 8 h and calcination at 550°C for 3 h) 3 g, 190°C, 10 h.
As shown in Figure 6, with the increase of AlCl3 concentration, the conversion of 2-MN gradually increased and reached the highest point at 3 g·L−1, which may be the maximum AlCl3 loading capacity of the catalyst prepared at this concentration. However, when AlCl3 was immobilized with Hβ, two B-acid sites were converted into one L-acid site. The total acid content showed an increasing trend, so the catalytic activity increased. In addition, the reaction between AlCl3 and part of Si–OH in Hβ channels increased the pore size and the shape selectivity of AlCl3/Hβ, which was conducive to the formation of 2,6-MPN. To further increase the concentration of AlCl3, due to the high concentration part of AlCl3 exhalation, deposition on the surface of Hβ, after calcining sintered parts, affects the catalytic activity of catalysts. It can be seen from the SEM in the figure that a high concentration of AlCl3 Hβ surface structure of the load was affected, selectivity of the catalyst declined. Therefore, the appropriate concentration of AlCl3 was 3 g·L−1.
3.3.2 Effect of reflux time on the catalytic performance of AlCl3/Hβ
The effect of AlCl3/Hβ catalysts prepared at different load times on the 2-MN acylation reaction is shown in Figure 7. When Hβ zeolites were reflux reacted in CHCl3 solution of AlCl3 for 9 h, the conversion was the highest, 82.2%, but the selectivity of the target product 2,6-MPN was slightly reduced. When the load time was 8 h, the selectivity for 2,6-MPN was up to 49.7%, although the catalytic activity decreased. It may be because when the load time was 9 h, AlCl3 had completely reacted with Si–OH on the pore surface, and part of AlCl3 was attached to the surface. Although a large amount of AlCl3 can catalyze the acylation of 2-MN, the decrease of pore size resulted in some β,β-MPN with larger molecular diameters trapped in the pore, and an increase of other by-products. Therefore, the appropriate load time was 9 h.
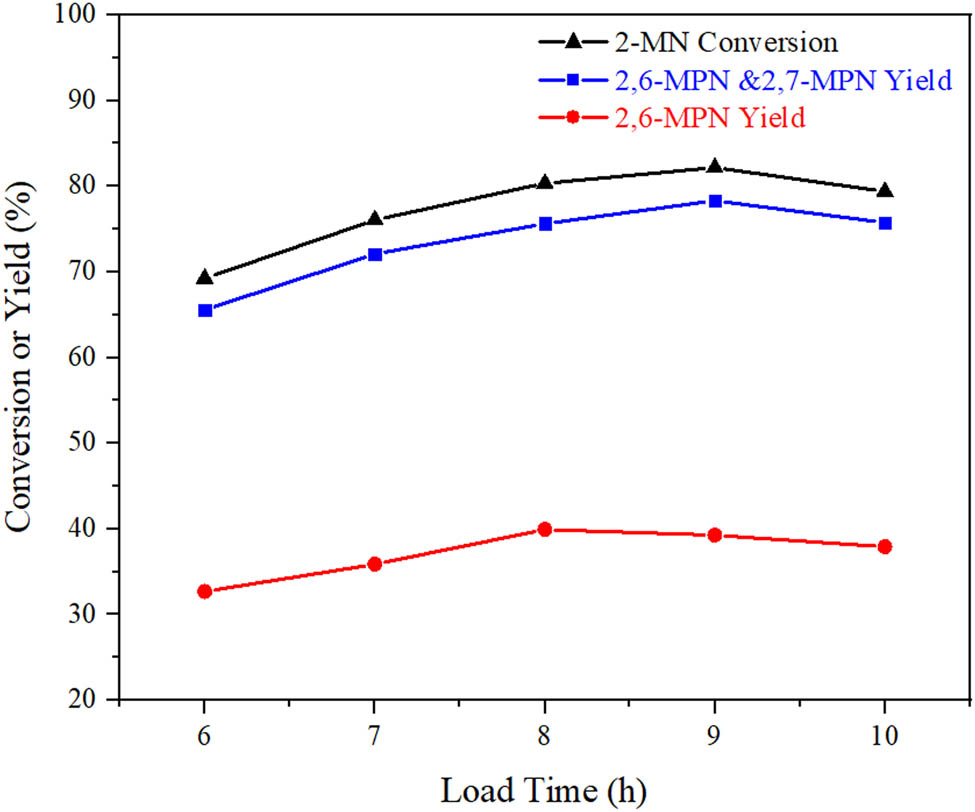
Influence of reflux time on catalytic performance of AlCl3/Hβ. Reaction conditions: n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ (AlCl3 3 g·L−1 and calcination at 550°C for 3 h) 3 g, 190°C, 10 h.
3.3.3 Effect of calcination temperature on the catalytic performance of AlCl3/Hβ
The precursor of AlCl3/Hβ catalyst prepared with AlCl3 concentration of 3 g·L−1 at 60°C for 8 h was calcined in a muffle furnace. The effect of calcination temperature was investigated, and the results are shown in Figure 8. The conversion of 2-MN was almost unchanged with calcination temperature increasing, but the yield of 2,6-MPN increased gradually. When the calcination temperature was over 550°C, the yield of 2,6-MPN decreased, while the yield of β,β-MPN was almost unchanged. The reason may be that the calcination temperature was too high, and AlCl3/Hβ could be partially sintered, destroying the surface acid center and affecting the catalytic activity. Therefore, the appropriate roasting temperature was 550°C.
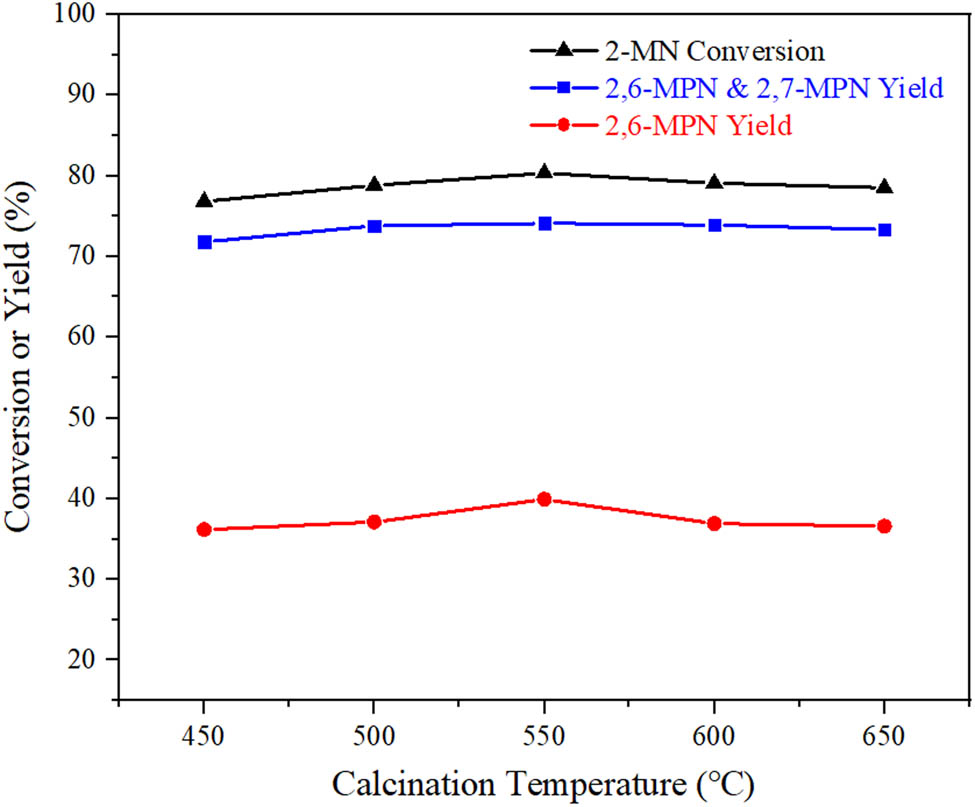
Influence of calcination temperature on the performance of 2-MN propionyl catalyzed by AlCl3/Hβ. Reaction conditions: n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ (AlCl3 g·L−1, reflux for 9 h and calcination for 3 h) 3 g, 190°C, 10 h.
3.3.4 Effect of calcination time on the catalytic performance of AlCl3/Hβ
The results of calcination time are shown in Figure 9. It can be seen that calcination time had little effect on AlCl3/Hβ. The conversion of 2-MN and the yield of β,β-MPN were the highest at 3 h. It showed that the structure of the carrier Hβ had been very stable. Therefore, the calcination time of 3 h was suitable.
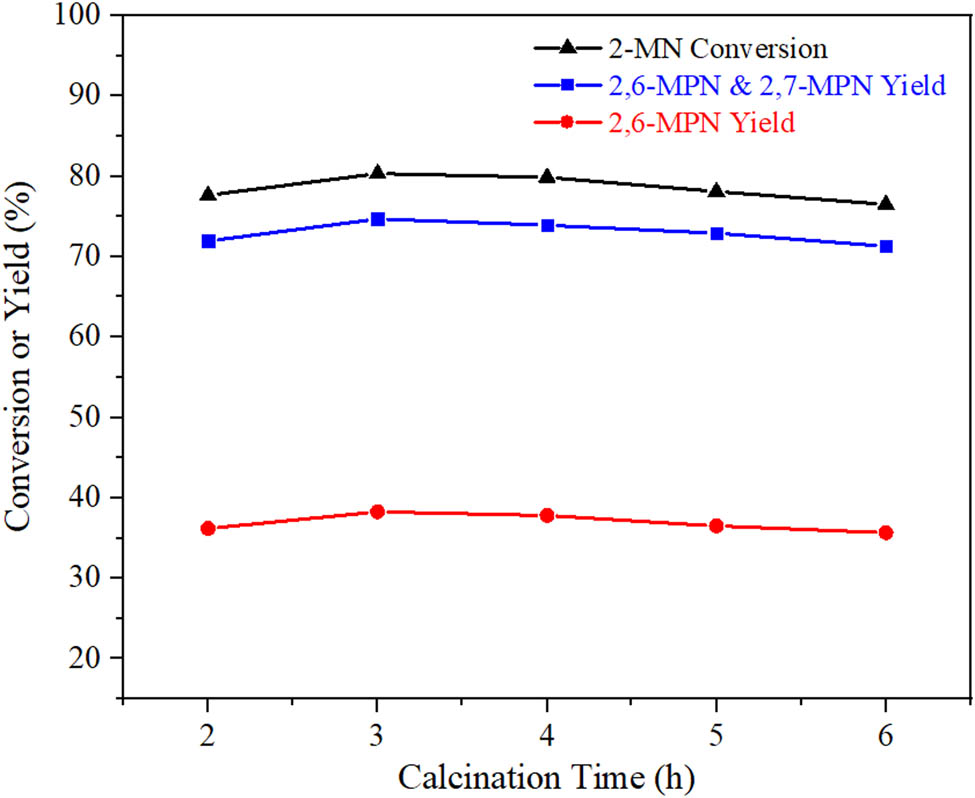
Effect of calcination time on the performance of 2-MN propionyl catalyzed by AlCl3/Hβ. Reaction conditions: n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ (AlCl3 3 g·L−1, reflux for 9 h and calcination at 550°C) 3 g, 190°C, 10 h.
3.4 Optimization of propionyl reaction conditions of 2-MN catalyzed by AlCl3/Hβ catalyst
AlCl3/Hβ catalyst was prepared with the AlCl3 concentration of 3 g·L−1 at 60°C for 8 h and calcination at 550°C for 3 h to optimize the reaction conditions of 2-MN propyl acylation.
3.4.1 Effect of catalyst dosage on 2-MN propionyl reaction
The effect of catalyst dosage on 2-MN propionyl reaction was investigated, and the results are shown in Figure 10. When the dosage of AlCl3/Hβ was 2 g, the conversion of 2-MN was only 53.2%, and the yield of β,β-MPN was also very low. With the increase of the dosage of AlCl3/Hβ, the conversion of 2-MN showed a trend of continuous increase because the increase of AlCl3/Hβ accelerated the acylation reaction of 2-MN with PA. The yield of 2-MPN was the highest when the dosage was 3 g. The 2-MN conversion increased, but the by-products increased obviously. Furthermore, too much catalyst would accelerate the occurrence of side reactions and significantly increase the cost of the reaction. Overall consideration, when the dosage of AlCl3/Hβ was 3 g, it was suitable.
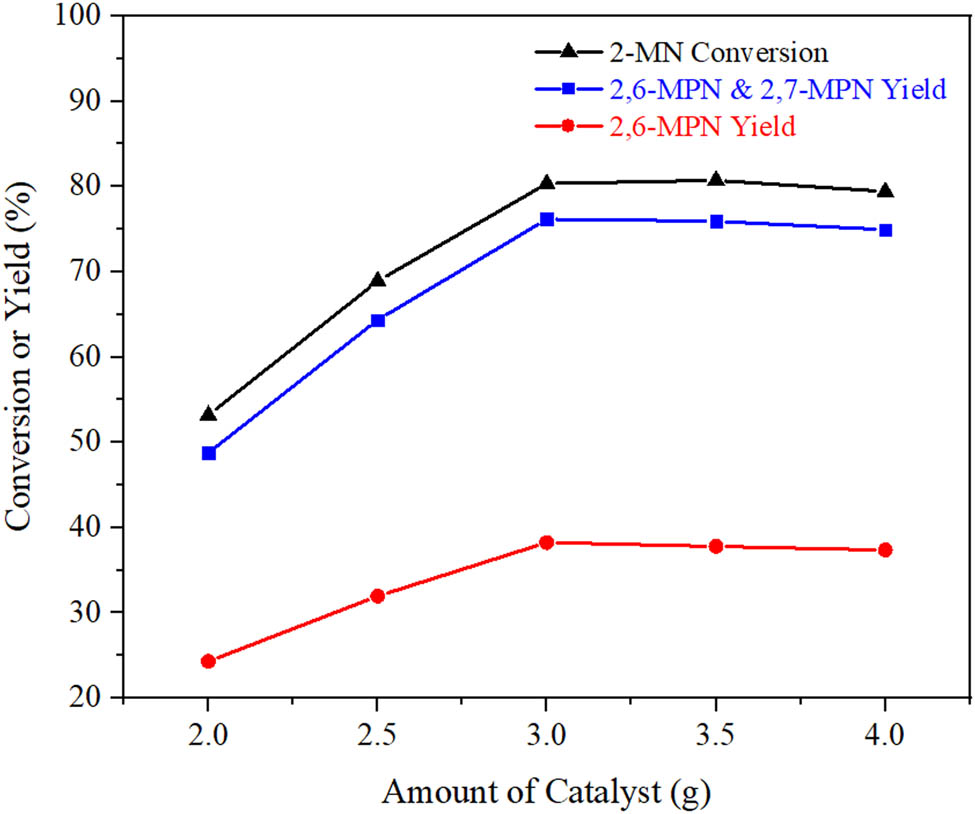
Influence of catalyst dosage on 2-MN propionylation reaction. Reaction conditions: n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ (AlCl3 3 g·L−1, reflux for 9 h and calcination at 550°C for 3 h), 190°C, 10 h.
3.4.2 Effect of reaction temperature on propionyl of 2-MN
The acylation of 2-MN is an exothermic reaction. The temperature has a certain influence on the acylation reaction. Under the conditions of n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ 3 g, and reaction time 9 h, the effects of reaction temperature on the conversion of 2-MN and the selectivity of β,β-MPN were investigated. The experimental results are shown in Figure 11.
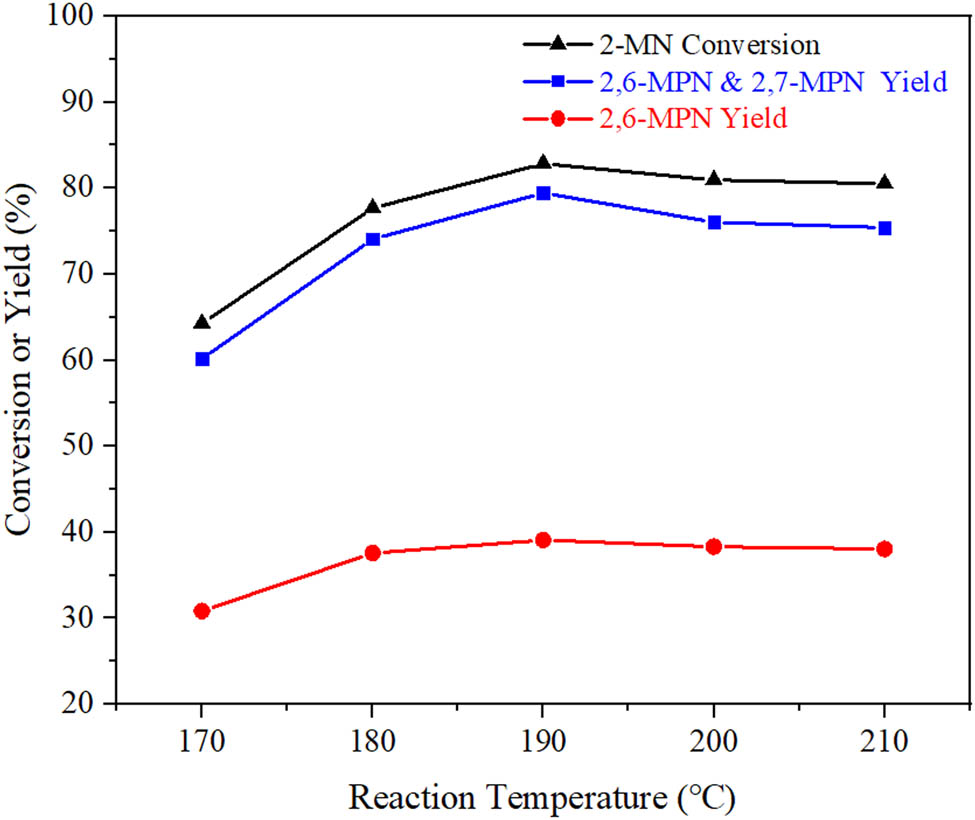
Effect of reaction temperature on propionylation of 2-MN. Reaction conditions: n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ (AlCl3 3 g·L−1, reflux for 9 h and calcination at 550°C for 3 h) 3 g, 10 h.
With the increase in reaction temperature, the transformation of 2-MN first increased and then decreased; the highest conversion was 82.9% at 190°C. It was because when the reaction temperature was increased in the range of 170–190°C, the movement rate of molecules in the reaction was accelerated, which made the reactants 2-MN, PA, and products 2,6-MPN, 2,7-MPN molecules more easily desorbed from AlCl3/Hβ and reduced the accumulation of macromolecules. It could improve the catalytic performance of the AlCl3/Hβ catalyst. Therefore, the reaction temperature of 190°C was appropriate.
3.4.3 Effect of reaction time on propionyl of 2-MN
Under the conditions of n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ 3 g, reaction temperature 190°C, the samples of different times were taken for GC detection to investigate the effect of reaction time on the conversion of 2-MN and the selectivity of β,β-MPN. The result is shown in Figure 12.
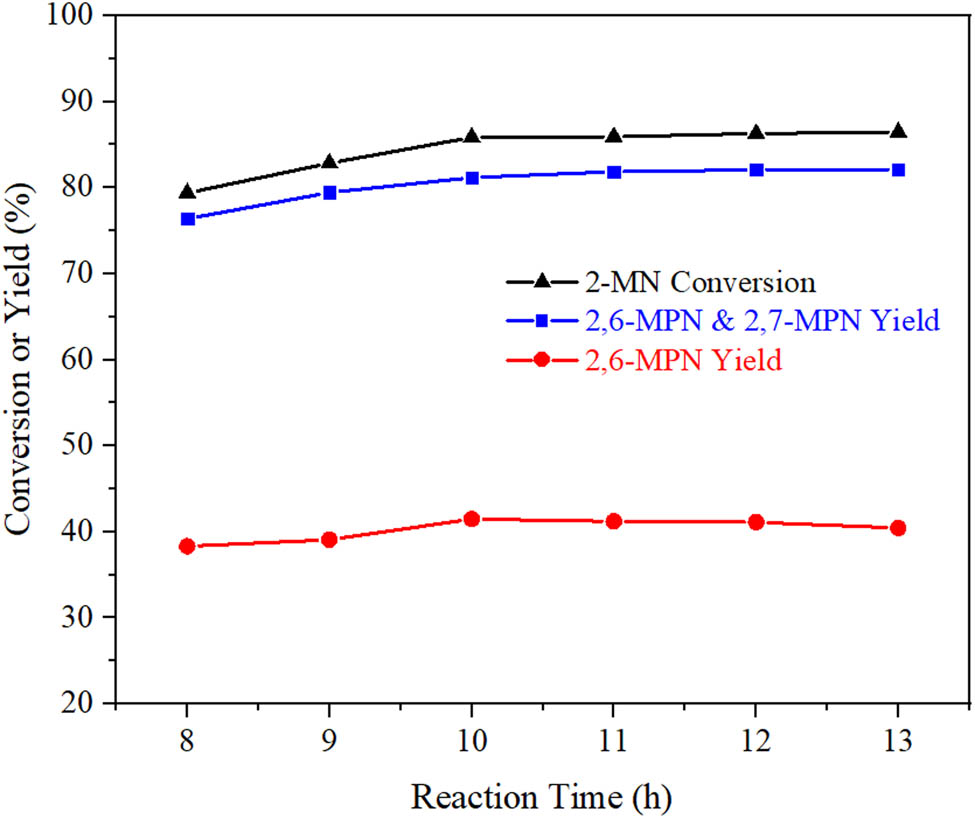
Effect of reaction time on propionylation of 2-MN. Reaction conditions: n (2-MN):n (PA) = 1:1.8, TS 10 g, AlCl3/Hβ (AlCl3 3 g·L−1, reflux for 9 h and calcination at 550°C for 3 h) 3 g, 190°C, 10 h.
With the prolongation of reaction time, the conversion of 2-MN and the yield of β,β-MPN increased gradually. When the reaction time was 10 h, the maximum yield of 2,6-MPN was 41.5%. After that, when the reaction time was 11 h, the conversion of 2-MN and the yield of other β,β-MPN continued to increase, while the yield of 2,6-MPN decreased by 0.3%. As the reaction time continued to increase, the results were predictable: The conversion of 2-MN and other β,β-MPN yields continued to increase, while the yields of 2,6-MPN gradually decreased. It may be because the propionyl reaction of 2-MN in the reaction was intermittent, and 2,6-MPN, 2,7-MPN, 2,3-MPN, and other products coexisted in the product. As the reaction time increased, the generated products would be converted into other by-products, reducing 2,6-MPN [9]. Therefore, after careful consideration, the appropriate reaction time was 10 h.
3.5 Catalytic reaction feasible mechanism
The reaction mechanism of Friedel–Crafts acylation of 2-MN catalyzed by zeolite molecular sieve is still ambiguous. Yuan et al. [9] proposed that in the catalytic process of zeolite molecular sieve catalyst, medium strong acid played a significant role. The ratio of Bronsted acid to Lewis acid could effectively catalyze the acylation reaction of the aromatic ring within an appropriate range. And the acylation reaction process of 2-MN and anhydride catalyzed by zeolite molecular sieve was proposed, as shown in Figure 13.
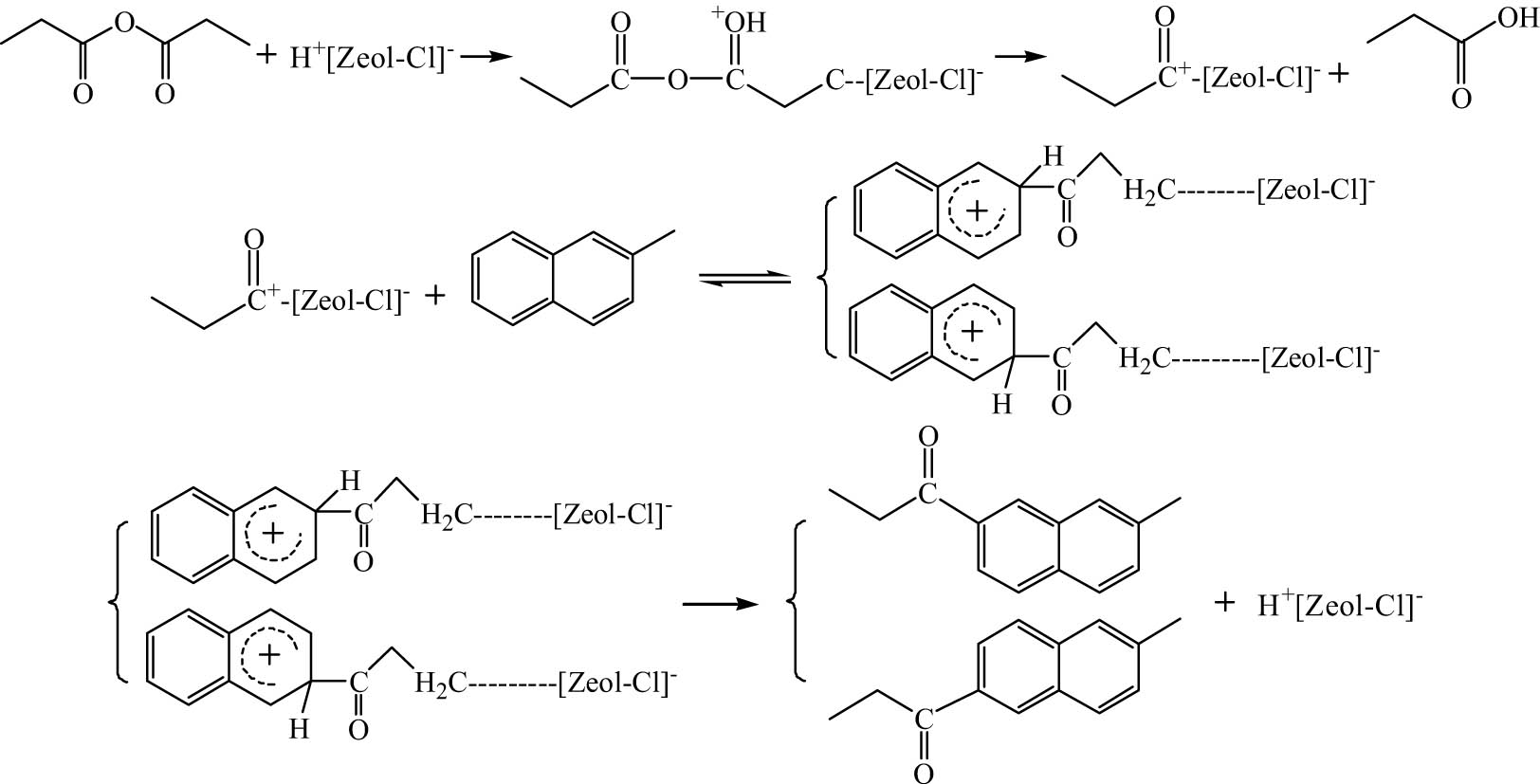
Mechanism of 2-MN propionyl catalyzed by zeolite molecular sieve.
The reaction mechanism of Hβ zeolite immobilized by AlCl3 can be divided into two types according to the type of acid active center. One is a new acid active center formed by AlCl3 on the surface of zeolite; The other is the existing acid active center on the surface of the zeolite molecular sieve. Although the reaction mechanism of this reaction is complicated with the previous zeolite molecular sieve catalysis, there are still many similarities in the overall reaction process. As shown in Figure 13, the anhydride first chemically adsorbed on the acid active center of the molecular sieve to form acyl carbocation, then attacked 2-MN to form active complex intermediates, and finally desorbed from the surface of the molecular sieve to form products. In addition, it can be seen from the characterization results that the acid content of the AlCl3/Hβ catalyst increases with the increase of Cl content, indicating that the effective acid amount of the acid center formed by the bond between the molecular sieve surface and Cl is more, among which the medium and strong acids are very beneficial to the reaction.
4 Conclusion
In this article, the AlCl3/Hβ catalysts are prepared by solvent reflux method using Hβ zeolite (Si/Al ratio of 25) as support. XRD results show that the prepared AlCl3/Hβ catalyst has typical characteristic peaks and complete structure. SEM characterization shows that the molecular sieve loaded with AlCl3 presents a small cube structure. With the increase in AlCl3 concentration, more small particles are attached to the surface. BET results showed that the specific surface area of Hβ zeolite is increased, and pore volume and pore size are decreased by AlCl3 fixation. XRF results show that immobilized AlCl3 increases Al2O3 and Cl. NH3-TPD and Py-IR showed that the AlCl3/Hβ catalyst is mainly L-acid, and the acid content of B-acid also increases. With the increase in AlCl3 concentration, the acid content of strong acid, medium strong acid, and weak acid all increase.
In the acylation of 2-MN with PA over AlCl3/Hβ, the reaction activity and selectivity decrease with pore size and specific surface area and increase with Cl content and acid content. The results showed that when the concentration of AlCl3 was 3 g·L−1, the concentration of reflux solvent was 8 h, and calcination at 550°C for 3 h, the catalytic activity of AlCl3/Hβ was better. Under atmospheric pressure, AlCl3/Hβ catalyst has higher catalytic activity in propionyl of 2-MN with TS as the solvent and 190°C for 10 h. Under this condition, the reaction has higher activity and selectivity. The conversion of 2-MN is 85.86%, and the yield of β,β-MPN is 81.19%.
In general, AlCl3/Hβ catalyst can catalyze the acylation of 2-methylnaphthalene effectively. Compared with the traditional AlCl3 catalyst, the amount of AlCl3 used is greatly reduced, and the production cost is reduced. Moreover, the immobilization catalyst can be directly filtered and separated from the solution, simplifying the post-treatment steps, and reducing the generation of a large amount of waste acid and waste gas. It is a relatively environmentally friendly green catalyst.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (91634101) and The Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20180508).
-
Author contributions: Jingjing Sun: writing – original draft, writing – review and editing, methodology, formal analysis; Haibo Jin: writing – review and editing, resources, funding acquisition, conceptualization; Xuefeng Mao: formal analysis; Guangxiang He: writing – review and editing, methodology; Junfang Li: resources, validation; Zihao Yan: validation; Fating Hu: investigation; Lei Ma: formal analysis, investigation; Xiaoyan Guo: supervision; Suohe Yang: software.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Zhu H, Meyer MP. Cationic intermediates in Friedel-Crafts acylation: Structural information from theory and experiment. Chem Commun. 2010;47:409–1111. 10.1039/c0cc02286a.Suche in Google Scholar PubMed
[2] Kreitman KM, Brewer SE, Rodden JB, Blackbourn RL, Brownscombe TF, Buechele JL et al. An integrated process for the production of 2,6-naphthalene dicarboxylic acid. US: 6448436; 2002.Suche in Google Scholar
[3] Xu HS, Song CY, Zhao JH, Wang LC, Gan W. Study on methylation of 2-methylnaphthalene with methanol over MFI type zeolite catalyst. Acta Petrolei Sin. 1999;15(1):52–6.Suche in Google Scholar
[4] Zhang CR, Zhou B. Progress in synthesis of 2,6-naphthalene dicarboxylic acid. Chem Adhes. 2012;34(06):54–517.Suche in Google Scholar
[5] Li JF, Mao XF, Hu FT, Li WB. Synthesis and characterization of 2-methyl-6-propoylnaphthalene. J China Coal Soc. 2020;45(12):4184–90.Suche in Google Scholar
[6] Ren XH, Yang HH, Zhe DM, Gao YJ. Application status and the prospect of polyethylene naphthalenediate. Petrkchem Techno. 2021;50(06):616–21.Suche in Google Scholar
[7] Pivsa-Art S, Okuro K, Miura M, Murata S, Nomura M. Acylation of 2-methoxynaphthalene with acyl chlorides in the presence of a catalytic amount of lewis acids. J Chem Soc Perkin Trans 1. 1994;13:1703–7. 10.1039/P19940001703.Suche in Google Scholar
[8] Wang P, Jian XG. Synthesis of 2-methyl-6-acylnaphthalene. J Dalian Univ Technol. 2008;1:99–102. 10.16411/j.cnki.issn1006-7736.2008.01.007.Suche in Google Scholar
[9] Yuan B, Li ZS, Liu YJ, Zhang SS. Liquid phase acylation of 2-methylnaphthalene catalyzed by H-beta zeolite. J Mol Catal A-Chem. 2007;280(1):210–8.10.1016/j.molcata.2007.11.004Suche in Google Scholar
[10] Li WP, Yang SH, Guo XY, He GX, Jin HB. The effect of operating conditions on acylation of 2-methylnaphthalene in a microchannel reactor. Chin J Chem Eng. 2018;26(6):1307–11.10.1016/j.cjche.2018.01.004Suche in Google Scholar
[11] Mu MM, Chen LG. Research progress in Friedel-Crafts acylation of aromatic hydrocarbons catalyzed by solid acids. Fine Chem. 2017;34(4):361–7 + 406. 10.13550/j.jxhg.2017.04.001.Suche in Google Scholar
[12] Wu YH, Tian FQ, He M, He M, Cai TX. Isomerization of α-pinene over immobilized AlCl3 catalysts. Chin J Catal. 2011;32(6–8):1138–42.10.1016/S1872-2067(10)60244-6Suche in Google Scholar
[13] Petre AL, Hoelderich WF, Gorbaty ML. Dodecylbenzene transformations: Dealkylation and disproportionation over immobilized ionic liquid catalysts. Appl Catal A Gen. 2009;363(1):100–8.10.1016/j.apcata.2009.04.047Suche in Google Scholar
[14] Zhang FF, Wu GW, Li H, Zhou YB. Research progress of Friedel-Crafts acylation catalysts. Appl Chem Ind. 2020;49(7):1823–8 + 1834.Suche in Google Scholar
[15] Qiao XL, Hu XY, Fang Y. Research progress of Friedel-Crafts acylation catalysts. Chem Ind Eng Prog. 2012;31(12):2702–7.Suche in Google Scholar
[16] Bykov VI, Belyaev BA. A new preparation method for the alkylation catalysts of aromatic compounds based on immobilized AlCl3. Kinet Catal. 2021;62(2):328–30.10.1134/S0023158421020026Suche in Google Scholar
[17] Cai TC, He M, Shi XZ, Wang XP, Han DX, Lv LH. A study on structural suitability of immobilized aluminum chloride catalyst for isobutene polymerization. Catal Today. 2001;69:291–6.10.1016/S0920-5861(01)00381-9Suche in Google Scholar
[18] Cai TC, He M. New approaches to immobilization of aluminum chloride on γ-Alumina and its regeneration after deactivation. Catal Lett. 2003;86:17–23.10.1023/A:1022609304596Suche in Google Scholar
[19] Hu XC, Foo ML, Chuah GK, Jaenicke S. Pore size engineering on MCM-41: Selectivity tuning of heterogenized AlCl3: For the synthesis of linear alkyl benzenes. J Catal. 2000;195(2):412–5.10.1006/jcat.2000.2965Suche in Google Scholar
[20] Tang H, Liu ZJ, Ji M, Jia CY, He M, Cai TC. Alkylation of naphthalene with propylene over a fixed AlCl3 catalyst. Ind Catal. 2010;18(11):62–5.Suche in Google Scholar
[21] Lu PF, Jiang L, Mi PK, Mi PK, Huang FL, Chen Q, et al. Study on the structure of AlCl3 catalyst supported by Al2O3. Petrkchem Techno. 2010;39(6):616–9.Suche in Google Scholar
[22] Wang NN, Jiang S, Ji M, He M, Lin J, Cai TC. Preparation of AlCl3 supported catalyst and its catalytic performance for 1-decene polymerization. Petrkchem Techno. 2006;5:479–82.Suche in Google Scholar
[23] Dube D, Royer S, On DT, Beland F, Kaliaguine S. Aluminum chloride grafted mesoporous molecular sieves as alkylation catalysts. Micropor Mesopor Mate. 2005;79:137–44.10.1016/j.micromeso.2004.11.002Suche in Google Scholar
[24] Han X, Guo YJ, Wu W. Progress in preparing and applying of supported Lewis acid catalysts. Petrkchem Techno. 2006;35(1):88–93.Suche in Google Scholar
[25] Wu YH, Tian FQ, He M, Cai TX. Isomerization of a-pinene over immobilized AlCl3 catalysts. Chin J Catal. 2011;32(7):1138–42.10.1016/S1872-2067(10)60244-6Suche in Google Scholar
[26] Qi LL, Ji M, Wang XK, Min H, Cai T. AlCl3/MCM-41 as a catalyst for isomerization of Endo-tricyclodecane. Chin J Catal. 2010;31(4):383–5.10.1016/S1872-2067(09)60057-7Suche in Google Scholar
[27] Wen J, You K, Zhao F, Jian J, Liu P, Ai Q, et al. AlCl3 immobilized on silicic acid as efficient Lewis acid catalyst for highly selective preparation of dicyclohexylamine from the vapor phase hydroamination of cyclohexene with cyclohexylamine. Catal Commun. 2020;145:106–12.10.1016/j.catcom.2020.106112Suche in Google Scholar
[28] Jadhav AH, Chinnappan A, Hiremath V, Seo JG. Synthesis and characterization of AlCl3 impregnated molybdenum oxide as heterogeneous nano-catalyst for the Friedel-Crafts acylation reaction in ambient condition. JNN. 2015;15(10):8243–50.10.1166/jnn.2015.11253Suche in Google Scholar PubMed
[29] Jiang T, Cheng J, Liu W. Sulfuric acid functional zirconium(or aluminum) incorporated mesoporous MCM-48 solid acid catalysts for alkylation of phenol with tertbutylalcohol. J Solid State Chem. 2014;218(2):71–80.10.1016/j.jssc.2014.06.021Suche in Google Scholar
[30] Bai G, Han J, Zhang H, Liu C, Lan X, Tian F, et al. Friedel-Crafts acylation of anisole with octanoic acid over acid-modified zeolites. RSC Adv. 2014;4(52):27116–21.10.1039/C4RA02278ESuche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

