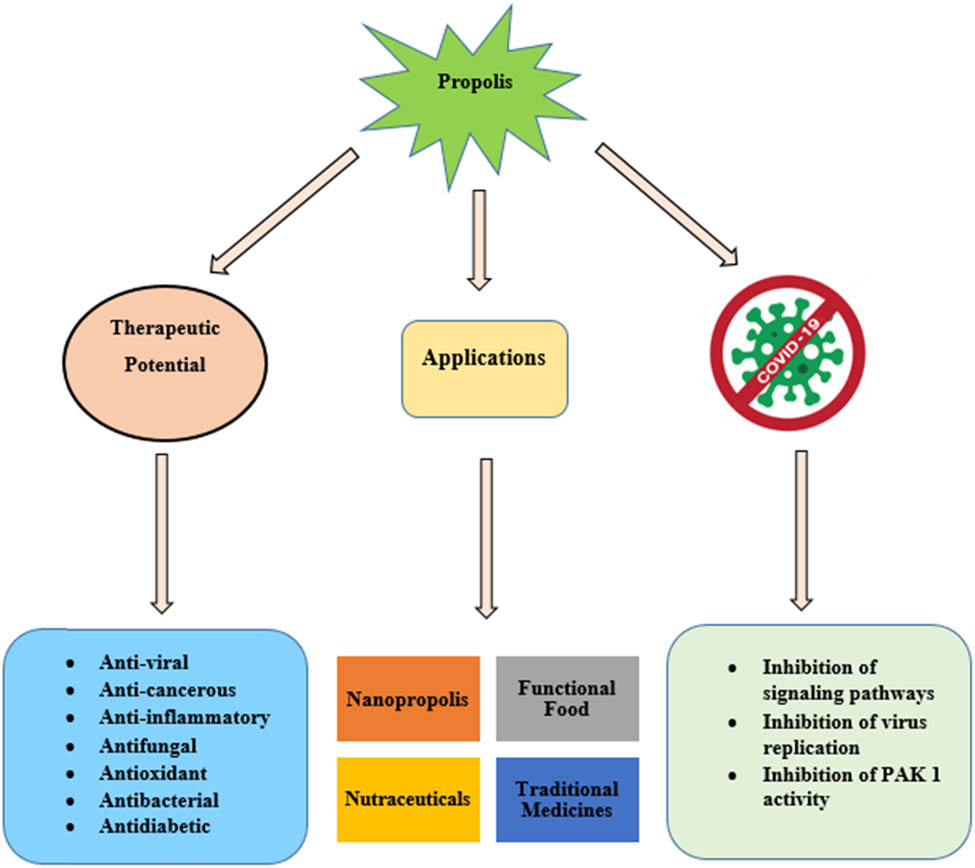
Abstract
The most fascinating product of honeybee is propolis. It has an immense role in dentistry, dermatology, and otorhinolaryngology. The increased popularity of propolis as an important remedy is due to its constituents, which have anti-inflammatory, immunomodulatory, antihepatotoxic, anti-cancerous, antifungal, antioxidant, antidiabetic, and antiviral activities. The diverse biological and pharmacological activities of propolis have piqued the interest of many scientists. Many techniques like gas chromatography-mass spectrometry, chromatography, and spectroscopy are being used to identify different propolis constituents. Flavonoids, phenolic acids, and their esters are the most pharmacologically active molecules of propolis and are known to disrupt the replication machinery of the virus corroborating the anti-coronavirus activity of propolis. The main aim of this article is to provide an insight of the increasing theragnostic uses of propolis and its nanoparticles, including their chemical analysis, diverse biological activities, and the necessity for chemical standardization. In this review, we have focused at the promising effects of propolis, its optimization, and its liposomal formulation as a therapeutic intervention for COVID-19 and its accompanying comorbidities.
Graphical abstract
1 Introduction
Bees evolved from Apoidea approximately 140 million years ago. In earlier times, the role of bee products was considered essential only for the survival and development of the bees. With time, the role of bee products in medicines, various technologies, and in cosmetics got popularized. The major bee products are honey, bee wax, royal jelly, and propolis. Term apitherapy is very popular nowadays and is used to treat illness, pain from acute and chronic injuries by using honey bee products [1–3]. Illness like multiple sclerosis and rheumatoid arthritis can be treated with apitherapy [4]. One of the most innovative and rapidly evolving field is nanotechnology, which has the potential to increase the efficacy of the bee products. Synthesis of nanoparticles involves two main approaches, i.e. top-down approach (chemical method and physical method) and bottom-up approach (biological method) as given in Figure 1. Plants, bacteria, algae, and fungi are the main agents which are employed in the biological method for nanoparticles synthesis. Biogenetic synthesis of the nanoparticles is now gaining popularity leading toward the green chemistry approach [5]. In this article, the role of propolis and its nanoparticles, composition, analytical methods, possible research areas, and its chemical standardization is discussed.
Methods for the synthesis of nanoparticles.
1.1 What is propolis?
The Greek word propolis means pro, for or defense, and polis, the city, i.e., “defense of the hive” [3,6–9]. The word propolis was coined by Aristotle [10]. It is also referred as “Bee Glue” [7]. Propolis is a resinous mixture and it is produced from honeybees by mixing saliva and beeswax with exudate collected from the tree buds [11]. Propolis is lipophilic in nature [12]. Propolis is collected by the worker bees and then carried back to their colony packed on their hind leg [13]. The United States Department of Agriculture’s United States Standards for Grades of Extracted Honey, effective May 23, 1985 describes propolis as “Propolis means a gum that is gathered by bees from various plants. It may vary in color from light yellow to dark brown. It may cause staining of the comb or frame and may be found in extracted honey” [7]. Variation of colour is due to different plant sources [9]. The melting point of propolis is 25–45°C. Propolis is a soft, pliable, and sticky substance at room temperature. Above 45°C, it becomes sticky and gummy, and from 60°C to 70°C, it becomes liquid. In frozen conditions, it becomes hard and brittle [12,13]. The viscosity of propolis decreases upon heating to about 40°C, and hardening is observed below 10°C [6,8]. Propolis is used by the bees to seal the holes in their honeycombs, and also to cover carcasses of intruders who die inside the hive so that the decomposition of dead organisms does not harm or lead to any bacterial or other infections in their hives [6,11,14]. Against pathogenic microorganisms propolis also acts as a chemical weapon of bees [10]. Antiseptic and antimicrobial properties of propolis help bees to protect the colony from diseases [2,3,6,7,15]. Bees also use propolis to reduce air flow into the hive to retain heat [16]. Apis dorsata (giant honey bee) uses propolis to reinforce the hive’s adhesion, although Apis cerana does not use it at all. Maximum use of propolis is mostly made by Apis mellifera [17]. Activity of propolis gets affected by the methods of extraction. Solid–liquid extraction is the most common method in which different concentrations of ethanol, methanol, or water are used [18]. It is necessary to purify propolis by using extraction with solvents as it is not possible to commercialize them as raw materials [19]. The most common solvent is absolute ethanol, which is used to prepare propolis extracts; also extraction with aqueous ethanol (70–95%) can result in wax-free tinctures containing higher amounts of phenolic substances [20,21]. Some other solvents which are used for extraction are water, methanol, chloroform, dichloromethane, acetone, and ether [12,18]. About 70% of ethanol extract of propolis is also called propolis balsam, which includes phenolic acids (caffeic acid and ferulic acid), flavonols (galangin, kaempferol, and quercetin), flavones (chrysin, apigenin, luteolin, and tectochrysin), and flavanones (pinocembrin) [22].
According to Przybyłek and Karpiński [14], propolis is composed of flavonoid group (chrysin, pinocembrin, apigenin, galangin, kaempferol, quercetin, tectochrysin, and pinostrobin), aromatic acids (ferulic acid, cinnamic acid, caffeic acid, benzoic acid, salicylic acid, and ρ-cumaric acids), terpenes (terpineol, camphor, geraniol, nerol, and farnesol), and some micro- and macroelements (Mn, Fe, Si, Mg, Zn, Se, Ca, K, Na,) along with vitamins (B1, B2, B6, C, E). Propolis, which is mostly made up of plant secretions, is a good source of cinnamic acid and esters [14]. Raw propolis is typically composed of 50% plant resins, 30% waxes, 10% essential and aromatic oils, 5% pollens, and 5% other organic substances [10,23,24]. In six Indian propolis samples observed by authors of ref. [25], 93 compounds were studied out of which 14 were new for propolis. Indian propolis samples were characterized by the presence of significant amount of carboxylic acid (20.4%), terpenoids (15.0%), steroids (11.5%), hydrocarbons (9.6%), sugars (6.4%), alkaloids (6.4%), flavonoids (4.3%), phenols (3.2%), ketones (2.1%), amino acid (2.1%), vitamins (2.1%), and other compounds (15.0%) [25] (Figure 2). Variations in chemical compositions of propolis are observed due to different geographical regions. Chemical composition of propolis for Africa and Europe was given by Bankova et al. [26] as shown in Figure 2.
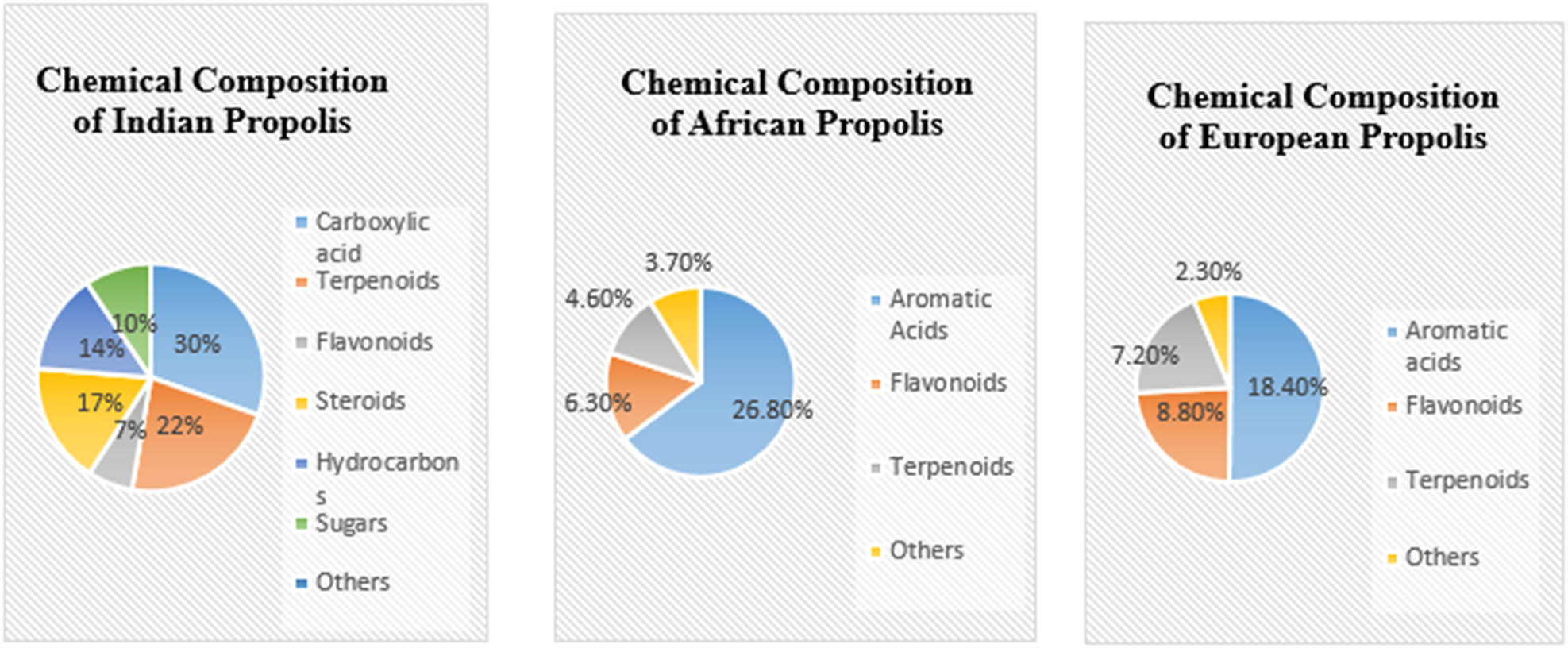
Chemical composition of propolis from different geographical regions.
1.2 Botanical sources
Quality and composition of propolis depend on the source plants or the flora at the site of collection [1,8,27]. Bees use variety of plants as a source of propolis, which includes poplars, resins of conifers, birch, alder, willow, palm, Dalbergia ecastaphyllum, etc. [27].
1.3 Types of propolis
Catchpole et al. [16] stated that there are around seven types of propolis. Mountford-McAuley et al. [15] and Catchpole et al. [16] categorized propolis on the basis of their geographical distribution and on the basis of plant from which resin is collected (Table 1).
Different types of propolis
| S. no. | Type of propolis | Botanical source | Main bioactive compound | Geographical origin | References |
|---|---|---|---|---|---|
| 1 | Poplar | Populus spp. (poplar) | Polyphenols | Europe, China, America, New Zealand, Albania, and non-tropic regions of Asia | [1,10,12,30,31] |
| Populus nigra | |||||
| Populus italica | |||||
| Populus tremula | |||||
| Populus alba | |||||
| 2 | Red propolis | D. ecastaphyllum (coin vine) | Isoflavonoids and pterocarpans | Cuba, Mexico, and Northern Brazil | [30] |
| 3 | Brazilian green | Baccharis dracunculifolia (alecrim), Araucaria angustifolia, Araucaria heterophylla, Clusia minor | Prenylated p-coumaric acids, diterpenic acids, and flavanoids | Brazil | [1,12,14,15,32–35] |
| Eucalyptus citriodora | |||||
| D. ecastophyllum | |||||
| Hyptis divaricata | |||||
| 4 | Birch | Betula spp. (birch) | Polyphenols | Russia | [12] |
| 5 | Mediterranean | Conifers (e.g., pine) | Flavonoids, terpenes | Greece, Sicily, Crete, and Malta | [12] |
| 6 | Clusia/Cuban | Clusia spp. | Polyprenylated benzophenones | Cuba and Venezuela | [1,7–9,12,15] |
| Clusia rosea | |||||
| Clusia minor | |||||
| 7 | Pacific | c-Prenylflavanones furofuran lignans | Okinawa, Taiwan, Indonesia, and parts of Japan | [12] | |
| 8 | Taiwanese propolis | Prenylflavones | Taiwan | [1,36] | |
| 9 | Netherland propolis | — | CAPE | Netherland | [1] |
1.4 Factors affecting the propolis production
There are different factors which affect the propolis production, viz. botanical sources, genetics of honey bee, structure of hive, seasons, food availability, environmental factors, diseases, and colony strength. Both the quantity as well as the quality of propolis get affected by these factors [11,15,28]. Variation in composition of European propolis was reported by Bankova et al. [26] as shown in Figure 3. It can be inferred from Figure 3 that in spring season maximum amount of aromatic acids was present in European propolis and terpenoids were completely absent in the propolis samples.
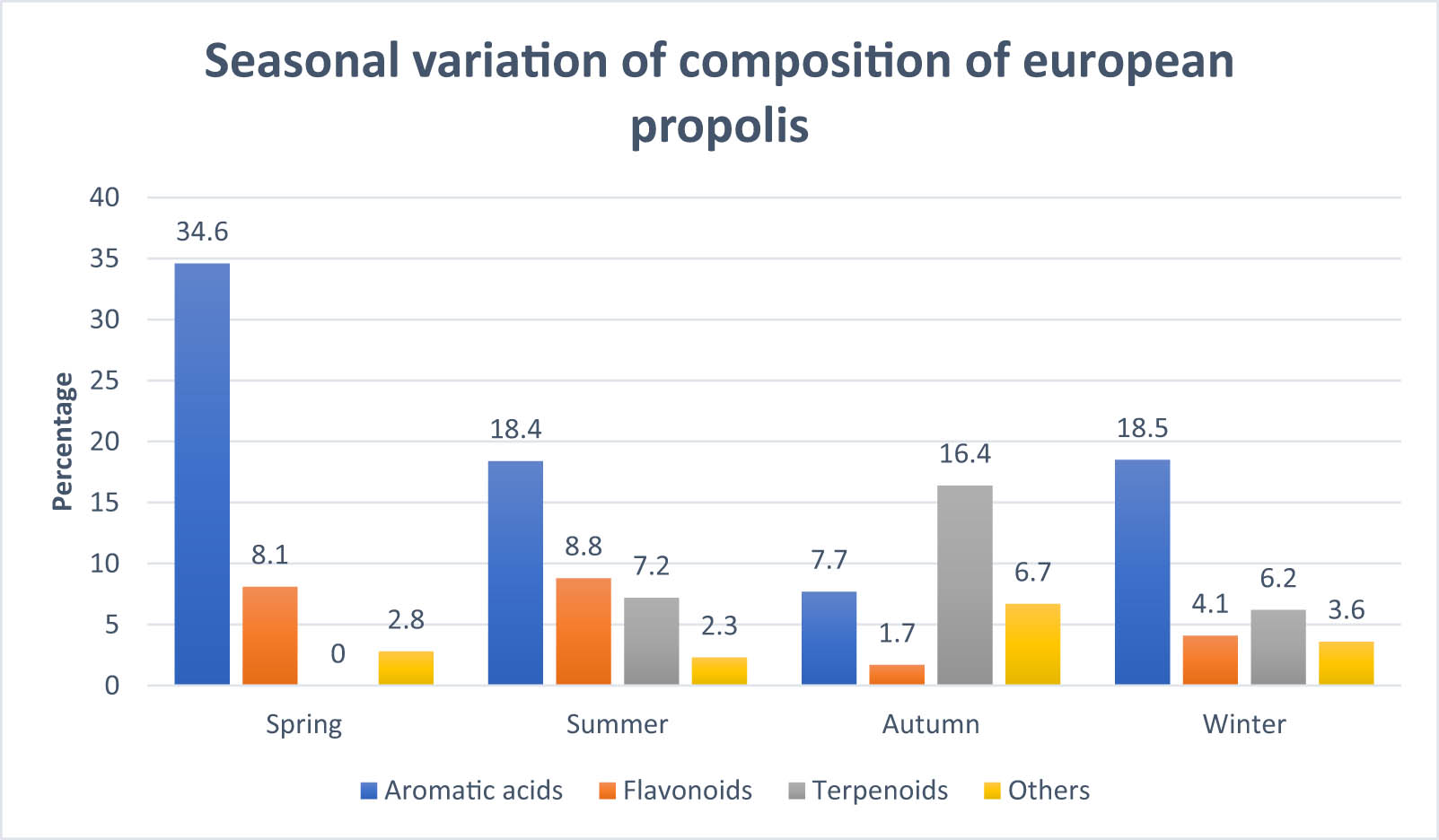
Seasonal variation of composition of European propolis.
1.5 Harvesting of propolis
Collection of propolis samples can be done by direct scraping of the substance from the wooden hive parts [13]. At the end of every season beekeeper cleans up the boxes properly so that they can be used in next season also. Therefore, at the end of the season scraping propolis from the frame rest, edges, inside the boxes produces very good quantity of propolis and it can be collected in multiple seasons [11]. It has been concluded from different papers that nowadays propolis traps (thick plastic sheets having 1.6 mm grooved slits over the entire surface) or mats with grid slots are placed into the hives by beekeepers for collecting propolis to be used in commercial scale [16]. The extraction process has a direct impact on the yield and selectivity of particular compounds. It has been reported that the properties of same propolis from different extracts can vary [29]. The different methods used for the extraction of propolis were cold extraction method, supercritical fluid extraction method, Soxhlet extraction, and sonication extraction. Cold extraction method was most commonly used for the preparation of propolis extract to be used in the chemical analysis of propolis. Collected propolis samples were placed in a freezer at low temperature (from −20°C to 4°C). Using grinding device, frozen propolis was powdered to get a size of 10–80 μm, and further using different solvents (chloroform, methanol, ethanol, water, olive oil, and propylene glycol) successive solvent extracts were prepared. For preparing ethanol extract, 70% ethanol (1:30 w:v) was added and placed for 5–7 days in dark and shaken only once in a day. Further drying was done after filtering it using Whatman’s filter paper. The obtained extract was ready to use in further experiments. The same method was followed for various solvents. Weigh the dried extract to determine the extract yield [18]. For the preparation of propolis extract the most commonly used solvent is ethanol but nowadays many cases have been reported of allergy to ethanol, which limits its use in extract preparation [24]. European (poplar type) propolis has an allergenic property due to the presence of 3,3-dimethylallyl caffeate [1,6]. Application of silicone barrier lotions before exposure to propolis is advised in cases of propolis allergies [6]. Propolis may inflict some immunological responses in few people. So further investigation is required before implementing their use in medical sciences.
2 Biological aspects of propolis
Propolis can be used extensively in pharmaceutical sciences due to its immense potential nutraceutical value and biological properties. There is a need for chemical standardization of propolis before considering its use in medical sciences as its constituents vary according to the geographical region and availability of botanical sources. Important constituents of propolis have increased the chances of its use against COVID-19, in nanomedicines, and in apitherapy. More clinical trials are needed to commercialize the use of propolis in its different forms.
In ancient times, propolis was famous for its general healing qualities, for the cure of some lesions of the skin, to heal sores and ulcers, and recently it is also used in tissue regeneration [6]. Due to the diversity in the chemical composition of propolis, it has been reported to possess various biological activities, namely anti-cancerous, antioxidant, anti-inflammatory, antibiotic, antifungal, antihepatotoxic, and antidiabetic [14,15,37,38].
2.1 Nutraceutical value of propolis
Propolis is supposed to have nutraceutical benefits due to its high amount of different amino acids, vitamins, and minerals [39]. There has been very less attempt to examine the nutraceutical potential of Indian propolis as of now [39]. Although propolis has less nutritional value, they have higher antioxidant effects than other bee products such as royal jelly and honey [40,41]. It contains about 1% amino acids, with arginine and proline accounting for nearly half of this quantity [40]. Literature surveyed for the nutritional analysis of propolis revealed that methanolic extract of propolis (MEEP) had 283.33 ± 51.31 g·kg−1 lipids, 30.07 ± 7.30 g·kg−1 fibres, 102.56 ± 2.84 g·kg−1 proteins, and 389.36 ± 57.50 g·kg−1 carbohydrates with a calorie content of 38,409.33 ± 6,169.80 kJ·kg−1. MEEP had a greater carbohydrate content with 493.6 ± 54.69 g·kg−1. It was likewise high in energy (40,406.14 ± 4,801.12 kJ·kg−1) [39]. It was observed that propolis sample was rich in calcium (19.2 ppm), zinc (4.72 ppm), iron (1.3 ppm), manganese (0.09 ppm), boron (0.19 ppm), rubidium (0.08 ppm), strontium (0.01 ppm), molybdenum (0.02 ppm), barium (2.84 ppm), aluminium (3.2 ppm), and lithium (0.03 ppm) [39]. An experiment was carried out to explore the impact of alcoholic extract of propolis on the performance of Ross (308) broiler chicks. A statistical comparison was done for weight gain, feed consumption, feed conversion ratio, and mortality rate. The results showed that average weight increase, feed consumption, and feed efficiency were significantly greater for propolis fed birds in all periods, and the presence of propolis also reduced the mortality rate in comparison to the control diet. Authors discovered that administering propolis extract on a daily basis altered chickens’ blood concentrations of cholesterol, total proteins, and amino acids. It also activated the immune system, which resulted in lower mortality when compared to the control [42]. It was reported from different studies that effectiveness of bee propolis on physical fitness and other parameters was assessed in rats after adding propolis to their diet as a supplement. In rats, fed with propolis, the authors noted an increase in haemoglobin regeneration efficiency, calcium and phosphorus absorption, iron utilization, and weight gain [43].
3 Chemical composition
Composition of propolis depends upon the geographical region as well as on the methods of extraction [12]. More than 300 complexes have already been identified [44]. To know about the composition of propolis, propolis extracts or samples were analysed by gas-chromatography, gas chromatography-mass spectrometry (GC-MS), and thin layer chromatography (TLC). Total content of flavonoids in propolis was determined by complementary colorimetric aluminium chloride method [18,37,45,46]. Folin–Ciocalteu method and phenolic sulphuric acid method were used for the determination of total polyphenol content and total polysaccharide content, respectively [30,37,39,47,48,49].
It was reported from the literature surveyed that phenolic compounds were the main components of propolis, which included flavonoids, aromatic acids, and benzopyranes [2]. Propolis extract was composed of cinnimic acids, caffeic acid, terpenes, phenolic acids, amino acids, flavonoids, and phenolic acid esters. Authors in refs [15,23,26,50] stated that the species from which resin was collected can be identified by the chemical composition of propolis. Pinocembrin was present in highest amount among flavonoid group, i.e., 4.7% [14,45]. It was reported that ethanolic extracts of propolis had a greater content of artepillin C (3,5-diprenyl-p-coumaric acid, a phenolic compound) [14]. The most effective flavonoid agents noted were galangin, pinocembrin, and pinostrobin (Figure 4) [8].
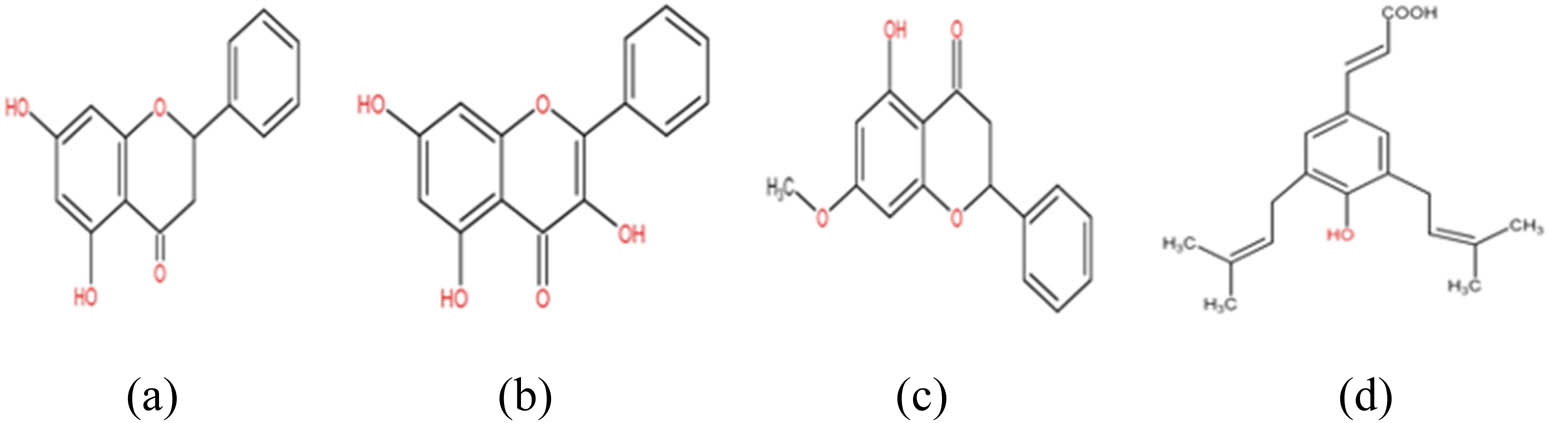
Chemical structures of important phenolic and flavonoid agents: (a) pinocembrin, (b) galangin, (c) pinostrobin, and (d) artepillin C.
It was also observed that content of diterpenes was maximum in propolis extract from both Kazan and Marmaris regions of Turkey. Steroid compounds were absent in propolis extract of Marmaris, whereas it was 14.99% in propolis extract from Kazan. In the propolis extract from Marmaris, 20.44% of caffeic acid isomers was also observed [44]. In Indian propolis, maximum concentration of carboxylic acids was observed, i.e. 20.4% [25]. In Bulgarian propolis maximum amount of pinocombrin (23%) was observed among flavonoids, while traces of acetophenone derivatives were also found. In Mongolian propolis maximum amount of 3-methyl-2 butenyl caffeate (∼24%) was observed [51]. When chemical analysis of red type Mexican propolis was carried out by Lotti et al. [52], they found three new compounds:
1-(3′,4′-dihydroxy-2′methoxyphenyl)-3-(phenyl)propane,
(Z)-1-(2′-methoxy-4′,5′-dihydroxyphenyl)-2-(3-phenyl)propene, and
3-hydroxy-5,6-dimethoxyflavan.
In Egyptian propolis, a total of 57 compounds were discovered [53]. In European propolis, among aromatic acids 3,5-diprenyl-p-coumaric acid was present in maximum concentration, i.e., 5.8%, and kaempferid was 2.8% among the flavonoids [26]. Chemical constituent of propolis varies according to the different regions (as shown in Table 2) and their varying components are responsible for different biological activities (Table 3).
Propolis from different nations and their chemical constituents
| Propolis | Chemical constituents | References |
|---|---|---|
| Indian propolis | Carboxylic acids (20.4%) | [25] |
| Terpenoids (15%) | ||
| Steroids (11.5%) | ||
| Hydrocarbon (9.6%) | ||
| Sugars (6.4%) | ||
| Alkaloids (6.4%) | ||
| Flavonoids (4.3%) | ||
| Phenols (3.2%) | ||
| Ketones (2.1%) | ||
| Amino acids (2.1%) | ||
| Vitamins (2.1%) | ||
| Other compounds (15%) | ||
| African propolis | Aromatic acids (26.8%) | [26] |
| Flavonoids (6.3%) | ||
| Di and tri-terpenes (4.6%) | ||
| Others (3.7%) | ||
| *Turkey (Kazan) | Aliphatic acids (5.79) | [44] |
| Aromatic acids (0.83) | ||
| Sesquiterpenes (1.72) | ||
| Diterpenes (34.89) | ||
| Sugars (3.24) | ||
| Steroid compounds (14.99) | ||
| Other compounds (13.64) | ||
| *Turkey (Marmaris) | Aliphatic acids (5.78%) | [44] |
| Aromatic acids (21.03%) | ||
| Monoterpenes (0.23%) | ||
| Sesquiterpenes (0.52%) | ||
| Diterpenes (33.17%) | ||
| Sugars (1.14%) | ||
| Other compounds (0.78%) | ||
| *Bulgarian propolis | Flavonoids (∼42%) | [51] |
| Derivatives of cinnamic acid (∼31%) | ||
| Acetophenones derivatives (traces) | ||
| Unknown compounds (∼15%) | ||
| *Mongolian propolis | Flavonoids (∼25%) | [51] |
| Derivatives of cinnamic acid (∼34%) | ||
| Unknown compounds (∼4%) | ||
| Mexican propolis (red type) | 1-(3′,4′-Dihydroxy-2′methoxyphenyl)-3-(phenyl)propane | [30,52] |
| (Z)-1-(2′-methoxy-4′,5′-dihydroxyphenyl)-2-(3-phenyl)propene | ||
| 3-Hydroxy-5,6-dimethoxyflavan | ||
| Flavanones, isoflavans, and pterocarpans | ||
| Polyprenylated benzophenones | ||
| Egyptian propolis | Pterin-6-carboxylic acid | [53] |
| Cinnamic acid | ||
| Dodecanoic acid | ||
| Ascorbyl palmitate | ||
| Flavanones | ||
| Cinnamic acid derivatives | ||
| Haramaya propolis (Ethiopia) | Benzenamine | [10] |
| N,N-dibutyl (21.94%) | ||
| Paromomycin (9.74%) | ||
| 4-Aminobutyramide, N-methyl-N-[4-(1-pyrrolidinyl)-2-butynyl] (9.26%) | ||
| dl-tryptophan, 5-methoxy (7.43%) | ||
| Imidazole, 2-fluoro-5-(2-carboxyvinyl) (4.66%) | ||
| 2,7-Dioxatricyclo (4.4.0.0(3, 8) decan-4-amino (6.25%) | ||
| European propolis | Aromatic acids (18.4%) | [26,30,34] |
| Flavonoids (8.8%) | ||
| Di- and tri-terpenes (7.2%) | ||
| Phenolic acid esters (CAPE) | ||
| Others (2.3%) | ||
| Brazilian propolis (green propolis) | Prenylated phenylpropanoids (e.g., artepillin C) | [30] |
| Chlorogenic acid | ||
| Benzoic acids | ||
| Triterpenoids | ||
| Brazilian propolis (red propolis) | Pterocarpans | [30] |
| Isoflavonoids | ||
| Chalcones | ||
| Prenylated benzophenones | ||
| Phenyl propanoids | ||
| Anatolian propolis | Aromatic alcohols (5.5%) | [54] |
| Aromatic acids (3.65%) | ||
| Flavanones (36.08%) | ||
| Fatty acids (3.26%) | ||
| Linear hydrocarbons and their acids (1.34%) | ||
| Cinnamic acid and its esters (2.7%) | ||
| Others (24%) | ||
| Nigerian propolis | Phlobatannins | [43] |
| Glycosides | ||
| Tannins | ||
| Anthraquinones | ||
| Flavonoids | ||
| Alkaloids | ||
| Saponins |
- *
Percentage of total ion current, GC-MS.
Different compounds of propolis and their biological activities
| Compounds | Biological activity | References |
|---|---|---|
| Ferulic acid | Antibacterial | [1,6,8,30] |
| Hepatoprotective | ||
| Antiproliferative | ||
| Pinocembrin | Antimicrobial | [6,8] |
| Antibacterial | ||
| Stilbenes | Fungicidal and fungistatic toward wood rotting fungi | [6] |
| Pterostilbene | Antifungal | [6] |
| Gelangin | Antibacterial | [6,8,38] |
| Antiproliferative | ||
| Caffeic acid | Antibacterial | [1,6–8,55] |
| Anti-tumor | ||
| Hepatoprotective | ||
| Antioxidant | ||
| Flavonoids | Antibiotic | [1,6,8,56] |
| Anti-inflammatory | ||
| Antiviral | ||
| Hepatoprotective | ||
| Antioxidant | ||
| Flavonones | Antibacterial | [1,34] |
| Anti-inflammatory | ||
| Flavones | Antibacterial | [1] |
| Anti-inflammatory | ||
| Phenolic acids and their esters | Antibacterial | [1] |
| Anti-inflammatory | ||
| Antioxidant | ||
| Prenylated p-coumaric acid | Antibacterial | [1,34] |
| Anti-tumor | ||
| Hepatoprotective | ||
| Antioxidant | ||
| Labdane diterpenes | Antibacterial | [1,34] |
| Clerodane diterpene | Anti-tumor | [1] |
| Prenylated benzophenones | Antibacterial | [1] |
| Anti-tumor | ||
| Antioxidant | ||
| CAPE | Anti-tumor | [1,6–8,55] |
| Antioxidant | ||
| Hepatoprotective | ||
| Anti-inflammatory | ||
| Antibacterial | ||
| Antiviral | ||
| Benzofuranes | Anti-tumor | [1] |
| Prenylated flavanones | Anti-tumor | [1] |
| Antioxidant | ||
| Lignans | Hepatoprotective | [1] |
| Caffeoylquinic acids | Hepatoprotective | [1] |
| Cinnamic acid | Antiproliferative | [38] |
4 Chemical standardization of propolis and the problem of standardization
As the composition of propolis varies according to the source plant and local flora, it can create difficulty in its chemical standardization and medical use [8,57]. Chemical standardization of propolis is very necessary in order to get accepted officially in the main streams of healthcare system. The most important active principle component in propolis is caffeic acid phenethyl ester (CAPE), which can be used for the standardization of propolis. But in tropical samples of propolis CAPE is not present. So, we need a universal analytical method for standardization of propolis. We can do this by categorizing the propolis into different types according to their plant source and their corresponding chemical profile [1,38].
4.1 Standardization of poplar-type propolis based on the biologically active substances
Identification of poplar-type propolis is necessary. According to the information given for the chemical composition of propolis by Bankova et al. [27], seven phenolic compounds were selected as markers and proceeded with TLC procedure. Three parameters have been used for the characterization of chemical profile of poplar propolis: total flavone, flavonol content, total flavanone and dihydroflavanol content, and total phenolic content. For the quantification of these three main groups spectrophotometric assay was done. For quantification of total flavones/flavonols ammonium chloride complex-based spectrophotometric assay is used. After that colorimetric method with 2,4-dinitrophenylhydrazine was applied for the quantification of flavanones and dihydroflavonols as their amount was high in poplar propolis. Folin–Ciocalteu procedure was used for the final measurement of phenolic content. Final results were verified by HPLC procedure [1].
5 Therapeutic applications of propolis
5.1 Traditional uses of propolis
Propolis has been employed as traditional medicine for thousands of years. Egyptians use propolis in the practice of mummifying corpses [58]. Propolis is being used from the ancient times in the treatment of gastroduodenal ulcers (internal use) and as an antiseptic (external use) [3]. Propolis was extensively used in several Soviet clinics during the Second World War for its antibacterial and anti-inflammatory properties [12]. Polyanthus, a perfume for Greeks featured propolis as a key component [59]. Wound healing and antibacterial properties were discussed in earlier times by Aristotle, Galen, and Pliny. Since the eighteenth century propolis has been categorized as an official medication by London pharmacopoeia.
Nowadays, propolis can be used for the treatment of many bacterial diseases like tuberculosis [60]. It is also known to possess antiviral, analgesic, and wound healing properties [8], and is used to treat various types of dermatitis, which are caused by bacteria and fungi. It is also available in the form of capsules (either in pure form or combined with aloe gel and Rosa canina or pollen), as an extract, as a mouthwash, and in powdered form [3]. Propolis is also known to lower blood pressure and cholesterol levels, and for the treatment of atherosclerosis [23]. Data given by Pillai et al. [61] also indicate that Indian propolis has ulcer-preventive as well as ulcer-curative characteristics. It is also known to show regenerative effects on biological tissues and exhibit anti-neoplastic activity against cancer cells [3]. Propolis helps to reduce the lung problems and in appetite recovery. Extracts or ointments of propolis are beneficial in the treatment of genital HSV infection [3]. One of the important components of propolis, CAPE, is known to provide protection against ischemic spinal cord injury after infrarenal aortic occlusion in rabbit. It has been reported that the use of an alcoholic solution of propolis and sulphopen (potassium penicillin G, benzene sulphonamide hydrochloride, and sulphonilamide) and Framykoin (neomycin with zinc bacitracin) can stimulate tissue regeneration [6]. Efficacy of nanopropolis is even more than propolis due to its smaller size.
5.2 Role of propolis as a functional food ingredient
Hundreds of nutritious products have entered the market, but contribution of propolis as a functional food ingredient is still largely insignificant. In many of the most recent studies, spray drying is recommended as an effective method for transforming the propolis ethanolic extract into a powder that can be used in food systems without changing its characteristics [62]. Propolis should be used as a nutritional supplement or functional food in the fight against the COVID-19 pandemic due to its diversified properties and inhibitory potential [63].
5.3 Role of propolis against COVID-19
Presence of the CAPE and other phenolic acids confirms the anti-inflammatory and antiviral properties of propolis. Chemical constituents of propolis especially flavonoids such as chrysin, kaempferol, quercetin, and its derivatives are known to inhibit different signaling pathways and in vitro virus replication. Investigational studies confirmed the anti-corona effects of propolis highlighting the need for clinical trials, further standardization, and investigation covering its prophylactic effects in high-risk groups. Potentially active components of propolis help to alleviate symptoms of COVID-19 [64]. The potential of propolis liposomes was revealed as the most promising approach for the treatment against COVID-19 [65]. As per the standards adopted by the World Health Organization, propolis is non-toxic and does not interact with the primary liver enzymes or with other important enzymes; as a result, propolis can be used alongside the main medications without running the risk of potentiation or inactivation [66,67]. Due to the biological properties of propolis, with some antibiotics it can exert a synergistic action in the prophylactic effect of COVID-19. Bioinformatics approaches can also provide fresh perspectives [67]. Trace elements such as zinc ions, selenium, vitamin A, vitamin B12, and retinoic acid present in propolis have an immense role in maintaining adaptive immune system. Zinc ions block viral enzymes necessary for the virus to replicate in the host cells. Selenium inhibits proinflammatory cytokines [69]. It was revealed in many studies that propolis inhibits PAK-1 and interacts with ACE2 and TMPRSS2, which may aid in reducing or preventing SARS-CoV-2 host cell invasion as given in Table 4 [74]. It was revealed in a study that the main constituents of propolis such as caffeic acid, uercetin, kaempferol, and myricetin had a close interaction with the ACE2 protein and thus block the binding of COVID-19 virus with ACE2 protein as given in Figure 5 [63].
Role of propolis constituents in inhibiting COVID-19
| S. no. | Propolis constituent | Role | References |
|---|---|---|---|
| 1. | CAPE | Inhibits PAK1 activity | [68–70] |
| Artepillin C | |||
| 2. | Glyasperin A | Inhibits the binding of ACE-2 and SARS-CoV-2 | [71] |
| Broussoflavonol F | |||
| Sulabiroins A | |||
| Isorhamnetin | |||
| 3. | Phenolic compounds | Reduce the adsorption and entrance of the virus into the host cells | [72] |
| 4. | Flavonoids | Inhibits the activity of ACE | [69] |
| 5. | Genistin | Inhibits main protease and spike protein | [73] |
| Methylophiopogonanone A |
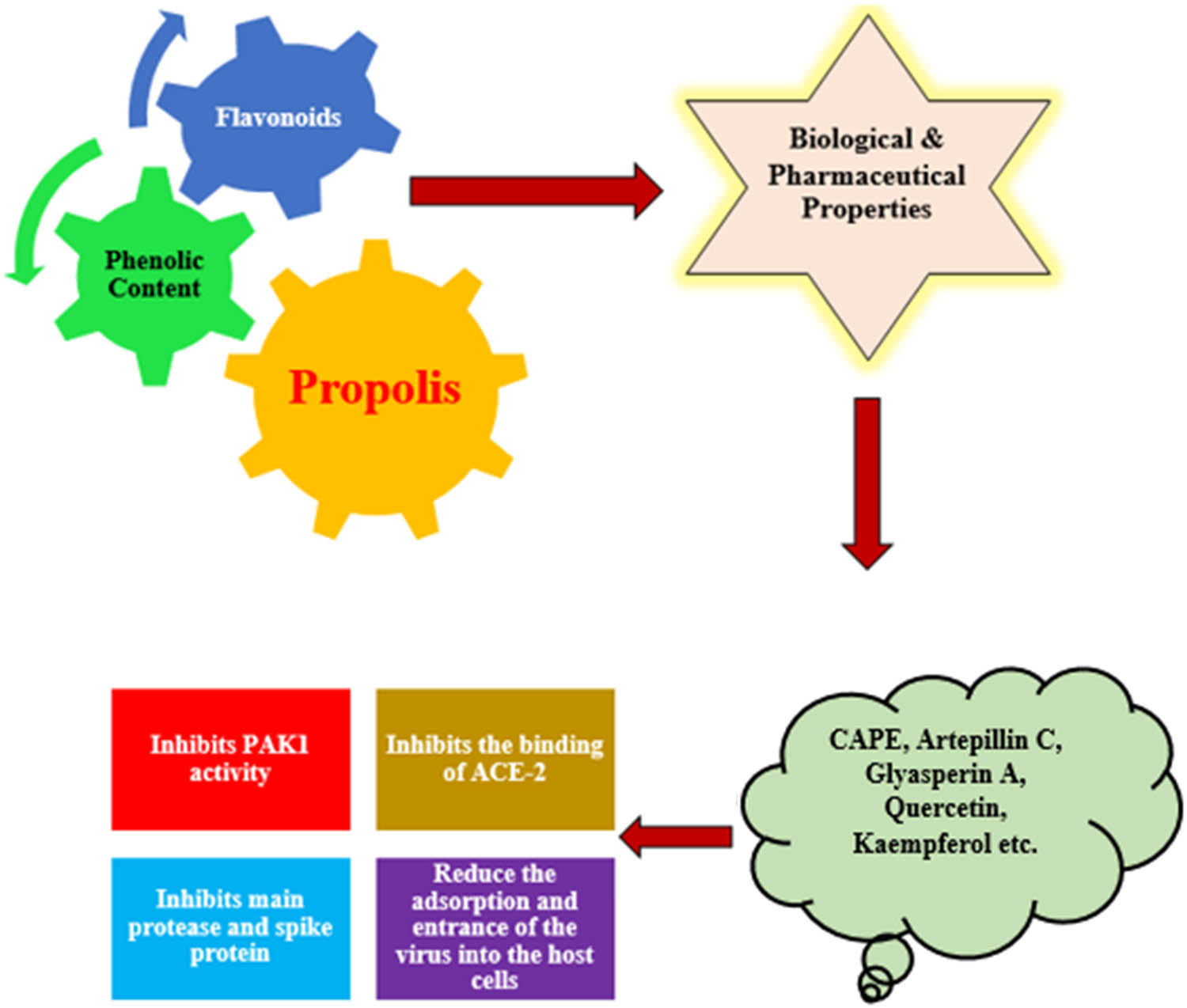
Role of propolis constituents in mitigating COVID-19.
5.4 Role of nanotechnology and green chemistry in apitherapy
Nanotechnology has substantial impact in apitherapy. Utilization of free form of propolis is restricted due to its low absorption and low solubility. Nowadays, the production of nanopropolis by various nano-encapsulation technologies has become popular to increase the efficacy of propolis. Size of nanopropolis is 1–100 nm in diameter and due to this smaller size absorption of nanopropolis is easier by the body [75]. Nanopropolis exhibits more effective pharmaceutical properties than propolis. To treat the cancer, nanopropolis can be used as nanofood. More research is required on propolis prepared as nanoparticles to demonstrate their effectiveness in the treatment of diseases [76]. Considering the benefits of green chemistry propolis nanoparticles are being prepared. Propolis nanoparticles biosynthesized by green chemistry have promising effect in the treatment of human ailments. Green chemistry has several advantages, including cost effectiveness, requiring less energy, being non-hazardous, non-toxic, eco-friendly, and facilitating ease of procedures and adaptability [77]. Propolis nanoparticles exhibit remarkable antibacterial [75,78], antifungal [78], and antidiabetic [79] properties. Due to these properties they have broad range of applications such as in nanomedicine, pharmaceuticals, drug delivery, veterinary medicines, and cosmetics [75].
6 Conclusion
Propolis and its nanoparticles (nanopropolis) have been considered to be a possible source of natural antioxidants that can combat the effects of oxidative stress, which is implicated in the aetiology of many diseases. These have enormous nutritional and medicinal potential but still more research and standardization are required for its clinical and nutraceutical use. The complex, varied composition of propolis according to geographical region, as well as the variety of analytical methods employed to examine their antioxidant properties, are responsible for the broad range of results reported by various researchers. This emphasizes the need for chemical standardization of propolis. This review indicates a potential impact of propolis as an adjuvant therapy on COVID-19. For prevention and treatment of COVID-19 further investigations and clinical trials are required to emphasize the understanding of potential therapeutic benefits from propolis. Usage of propolis products as a health-promoting supplement in human medicine is restricted in many nations because they are not standardized and vary in their components and biological activity. Propolis has enticing therapeutic potential and can be employed to treat COVID-19. As the majority of the findings were non-clinical studies, or reviews, there is a necessity of more clinical trials. Nanoparticles generated with the green approach are eco-friendly, non-toxic, and have successfully been used in various applications.
7 Research possibilities
The use of propolis and its nanoparticles in alimentary supplements and bio-cosmetics is increasing day by day. This has driven the interest of researchers to work on the quality as well as quantity of propolis, and also on the chemical standardization of propolis [15]. More research is needed to better define the parameters for using propolis in food formulations or in the pharmaceutical industry [29]. The efficiency of mouth rinses containing propolis samples on oral bacteria was not shown to be as effective as chlorhexidine (CHX). Also, propolis samples were shown to be less cytotoxic on human gingival fibroblasts than CHX. At sufficient doses, standardized propolis formulations can be used as a mouth rinse. Advanced research is required to acquire a standardized chemical composition [80].
Use of propolis in medicines is a very popular topic of research worldwide. However, research into Indian propolis and its nanoparticles is still in its infancy. In India studies on propolis have not been reported extensively except in few states like Maharashtra, Madhya Pradesh, West Bengal, Tamil Naidu, and Gujarat, so its popularity among researchers also opens the door for research in other states also [12]. Role of CAPE in cancer treatment also requires more experimental studies [3].
Propolis helps to alleviate the pathophysiological effects of COVID-19 infection due to its antioxidant, antimicrobial, antiviral, and immunity enhancing properties. Additional research pertaining to its standardization and dosage, as well as an increase in its production, should be done [81]. Medical trials are required to use propolis and its nanopaticles in the medical sciences. The advancement of science and technology has resulted in a plethora of improvements to all facets of human life [82], which further can be enhanced by incooperating bee products with modern technologies like nanomedicine [83], biomarkers [84], and biosensors [85].
Acknowledgements
Throughout the writing of this review article, we have received a great deal of support and assistance. We would like to thank the supervisor Dr Priyanka Kumari whose expertise was invaluable in formulating this review article.
-
Funding information: Authors state no funding involved.
-
Author contributions: Bindiya Barsola and Shivani Saklani have made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data from research papers. Priyanka Kumari has given final approval of the version to be published. Priyanka Kumari, Avtar K. Sidhu, and Anjoo Dhar have been involved in drafting the manuscript or revising it critically for important intellectual content.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Availability of data and material is not applicable to this article as no datasets were generated or analysed during the current study.
References
[1] Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2018;100(1–2):114–7. 10.1016/j.jep.2005.05.004.Suche in Google Scholar PubMed
[2] Sforcin JM. Propolis and the immune system: a review. J Ethnopharmacol. 2007;113(1):1–14. 10.1016/j.jep.2007.05.012.Suche in Google Scholar PubMed
[3] Castaldo S, Capasso F. Propolis an old remedy used in modern medicine. Fitoterapia. 2002;73(1):S1–6. 10.1016/S0367-326X(02)00185-5.Suche in Google Scholar PubMed
[4] Hellner M, Winter D, von Georgi R, Münstedt K. Apitherapy: usage and experience in German beekeepers. Evid Based Complement Altern Med. 2008;5(4):475–9. 10.1093/ecam/nem052.Suche in Google Scholar PubMed PubMed Central
[5] Goutam SP, Saxena G, Roy D, Yadav AK, Bharagava RN. Green synthesis of nanoparticles and their applications in water and wastewater treatment. Singapore: Springer Nature; 2019.10.1007/978-981-13-1891-7_16Suche in Google Scholar
[6] Ghisalberti EL. Propolis: a review. Bee World. 1979;60(2):59–84. 10.1080/0005772X.1979.11097738.Suche in Google Scholar
[7] Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol. 1998;36(4):347–63. 10.1016/S0278-6915(97)00145-2.Suche in Google Scholar PubMed
[8] Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26(2):83–99. 10.1051/apido:19950202.Suche in Google Scholar
[9] Ristivojević P, Trifković J, Andrić F, Milojković-Opsenica D. Poplar-type propolis: chemical composition, botanical origin and biological activity. Nat Prod Commun. 2015;10(11):1869–76. 10.1177/1934578X1501001117.Suche in Google Scholar
[10] Haile K, Dekebo A. Chemical composition and antimicrobial activity of Haramaya propolis (bee glue). Ethiopia Int J Pharm Sci Res. 2013;4(2):734–40. 10.13040/IJPSR.0975-8232.4(2).734-40.Suche in Google Scholar
[11] Bankova V, Bertelli D, Borba R, Conti BJ, da Silva Cunha IB, Danert C, et al. Standard methods for Apis mellifera propolis research. J Apic Res. 2019;58(2):1–49. 10.1080/00218839.2016.1222661.Suche in Google Scholar
[12] Wagh VD. Propolis: a wonder bees product and its pharmacological potentials. Adv Pharmacol Sci. 2013;2013:1–11. 10.1155/2013/308249.Suche in Google Scholar PubMed PubMed Central
[13] Jain S, Rai R, Sharma V, Batra M. Propolis in oral health: a natural remedy. World J Pharm Sci. 2014;2014(2):90–4. http://www.wjpsonline.com/.Suche in Google Scholar
[14] Przybyłek I, Karpiński TM. Antibacterial properties of propolis. Molecules. 2019;24(11):2047. 10.3390/molecules24112047.Suche in Google Scholar PubMed PubMed Central
[15] Mountford-McAuley R, Prior J, Clavijo McCormick A. Factors affecting propolis production. J Apic Res. 2021;2021:1–9. 10.1080/00218839.2021.1938456.Suche in Google Scholar
[16] Catchpole O, Mitchell K, Bloor S, Davis P, Suddes A. Antiproliferative activity of New Zealand propolis and phenolic compounds vs human colorectal adenocarcinoma cells. Fitoterapia. 2014;106(2014):167–74. 10.1016/j.fitote.2015.09.004.Suche in Google Scholar PubMed
[17] Kumar V. Propolis in dentistry and oral cancer management. N Am J Med Sci. 2014;6(6):250–9. 10.4103/1947-2714.134369.Suche in Google Scholar PubMed PubMed Central
[18] Pujirahayu N, Ritonga H, Uslinawaty Z. Properties and flavonoids content in propolis of some extraction method of raw propolis. Int J Pharm Sci. 2014;6(6):338–40. https://www.researchgate.net/publication/267031425.Suche in Google Scholar
[19] Alvarez-Suarez JM, editor. Bee products—chemical and biological properties. Cham: Springer; 2017. https://link.springer.com/book/10.1007/978-3-319-59689-1.10.1007/978-3-319-59689-1Suche in Google Scholar
[20] Park YK, Ikegaki M. Preparation of water and ethanolic extracts of propolis and evaluation of the preparations. Biosci Biotechnol Biochem. 1998;62(11):2230–2. 10.1271/bbb.62.2230.Suche in Google Scholar PubMed
[21] Cunha I, Sawaya AC, Caetano FM, Shimizu MT, Marcucci MC, Drezza FT, et al. Factors that influence the yield and composition of Brazilian propolis extracts. J Braz Chem Soc. 2004;15(6):964–70. 10.1590/S0103-50532004000600026.Suche in Google Scholar
[22] Cheng PC, Wong G. Honey bee propolis: prospects in medicine. Bee World. 1996;77(1):8–15. 10.1080/0005772X.1996.11099278.Suche in Google Scholar
[23] Kumar N, KK MA, Dang R, Husain A. Antioxidant and antimicrobial activity of propolis from Tamil Nadu zone. J Med Plants Res. 2008;2(12):361–4. 10.5897/JMPR.9000208.Suche in Google Scholar
[24] Biscaia D, Ferreira SR. Propolis extracts obtained by low pressure methods and supercritical fluid extraction. J Supercrit Fluids. 2009;51(1):17–23. 10.1016/j.supflu.2009.07.011.Suche in Google Scholar
[25] Ramnath S, Venkataramegowda S, Singh C. Chemical composition of bee propolis collected from different regions in India by GCMS analysis. Int J Pharmacogn Phytochem. 2015;30(1):1319–28. https://www.researchgate.net/publication/278414517.Suche in Google Scholar
[26] Bankova V, Boudourova-Krasteva G, Popov S, Sforcin JM, Funari SRC. Seasonal variations of the chemical composition of Brazilian propolis. Apidologie. 1998;29(4):361–7. 10.1051/apido:19980406.Suche in Google Scholar
[27] Bankova VS, de Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31(1):3–15. 10.1051/apido:2000102.Suche in Google Scholar
[28] El Adaouia Taleb R, Djebli N, Chenini H, Sahin H, Kolayli S. In vivo and in vitro anti‐diabetic activity of ethanolic propolis extract. J Food Biochem. 2020;44(7):e13267. 10.1111/jfbc.13267.Suche in Google Scholar PubMed
[29] Dantas Silva RP, Machado BAS, Barreto GdA, Costa SS, Andrade LN, Amaral RG, et al. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE. 2017;12(3):e0172585. 10.1371/journal.pone.0172585.Suche in Google Scholar PubMed PubMed Central
[30] Righi AA, Alves TR, Negri G, Marques LM, Breyer H, Salatino A. Brazilian red propolis: unreported substances, antioxidant and antimicrobial activities. J Sci Food Agric. 2011;91(13):2363–70. 10.1002/jsfa.4468.Suche in Google Scholar PubMed
[31] Daugsch A, Moraes CS, Fort P, Park YK. Brazilian red propolis—chemical composition and botanical origin. Evid Based Complement Altern Med. 2008;5(4):435–41. 10.1093/ecam/nem057.Suche in Google Scholar PubMed PubMed Central
[32] Ferreira JM, Fernandes – Silva CC, Salatino A, Negri G, Message D. New propolis type from north-east Brazil: chemical composition, antioxidant activity and botanical origin. J Sci Food Agric. 2017;97(11):3552–8. 10.1002/jsfa.8210.Suche in Google Scholar PubMed
[33] Midorikawa K, Banskota AH, Tezuka Y, Nagaoka T, Matsushige K, Message D, et al. Liquid chromatography–mass spectrometry analysis of propolis. Phytochem Anal Int J Plant Chem Biochem Tech. 2001;12(6):366–73. 10.1002/pca.605.Suche in Google Scholar PubMed
[34] De Marco S, Piccioni M, Pagiotti R, Pietrella D. Antibiofilm and antioxidant activity of propolis and bud poplar resins versus Pseudomonas aeruginosa. Evid Based Complement Altern Med. 2017;2017(2017):11. 10.1155/2017/5163575.Suche in Google Scholar PubMed PubMed Central
[35] Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50(9):2502–6. 10.1021/jf011432b.Suche in Google Scholar PubMed
[36] Chen CN, Wu CL, Shy HS, Lin JK. Cytotoxic prenylflavanones from Taiwanese propolis. J Nat Prod. 2003;66(4):503–6. 10.1021/np0203180.Suche in Google Scholar PubMed
[37] Laskar RA, Sk I, Roy N, Begum NA. Antioxidant activity of Indian propolis and its chemical constituents. Food Chem. 2010;122(1):233–7. 10.1016/j.foodchem.2010.02.068.Suche in Google Scholar
[38] Banskota AH, Nagaoka T, Sumioka LY, Tezuka Y, Awale S, Midorikawa K, et al. Antiproliferative activity of the Netherlands propolis and its active principles in cancer cell lines. J Ethnopharmacol. 2002;80(1):67–73. 10.1016/S0378-8741(02)00022-3.Suche in Google Scholar PubMed
[39] Sadhana N, Lohidasan S, Mahadik KR. Marker-based standardization and investigation of nutraceutical potential of Indian propolis. J Integr Med. 2017;15(6):483–94. 10.1016/S2095-4964(17)60360-1.Suche in Google Scholar PubMed
[40] Fontana JD, Adelmann J, Passos M, Maraschin M, de Lacerda CA, Lanças FM. Chemical micro-heterogeneity and bioactivity. In: Walker M, series editor. NJ: Humana Totowa; 2004. p. 203.Suche in Google Scholar
[41] Nakajima Y, Tsuruma K, Shimazawa M, Mishima S, Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med. 2009;9(4):1–9. 10.1186/1472-6882-9-4.Suche in Google Scholar PubMed PubMed Central
[42] Shalmany SK, Shivazad M. The effect of diet propolis supplementation on Ross broiler chick’s performance. Int J Poult Sci. 2006;5(1):84–8. 10.3923/ijps.2006.84.88.Suche in Google Scholar
[43] Yuksel S, Akyol S. The consumption of propolis and royal jelly in preventing upper respiratory tract infections and as dietary supplementation in children. J Intercult Ethnopharmacol. 2016;5(3):308–11. 10.5455/jice.20160331064836.Suche in Google Scholar PubMed PubMed Central
[44] Kartal M, Kaya S, Kurucu S. GC-MS analysis of propolis samples from two different regions of Turkey. Z Naturforsch C. 2002;57(9–10):905–9. 10.1515/znc-2002-9-1025.Suche in Google Scholar PubMed
[45] Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–82. 10.38212/2224-6614.2748.Suche in Google Scholar
[46] Campana R, Patrone V, Franzini ITM, Diamantini G, Vittoria E, Baffone W. Antimicrobial activity of two propolis samples against human Campylobacter jejuni. J Med Food. 2009;12(5):1050–6. 10.1089/jmf.2008.0173.Suche in Google Scholar PubMed
[47] Kasote DM, Pawar MV, Bhatia RS, Nandre VS, Gundu SS, Jagtap SD, et al. HPLC, NMR based chemical profiling and biological characterisation of Indian propolis. Fitoterapia. 2017;122(2017):52–60. 10.1016/j.fitote.2017.08.011.Suche in Google Scholar PubMed
[48] Naik DG, Vaidya HS, Behera BC. Antioxidant properties of Indian propolis. J ApiProd ApiMed Sci. 2009;1(2009):110–20. 10.3896/IBRA.4.01.4.03.Suche in Google Scholar
[49] Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J. 2007;1(1):1–4. 10.1186/1752-153X-1-13.Suche in Google Scholar PubMed PubMed Central
[50] Greenaway W, Scaysbrook T, Whatley FR. The composition and plant origins of propolis: a report of work at Oxford. Bee World. 1990;71(3):107–18. 10.1080/0005772X.1990.11099047.Suche in Google Scholar
[51] Bankova V, Dyulgerov A, Popov S, Evstatieva L, Kuleva L, Pureb O, et al. Propolis produced in Bulgaria and Mongolia: phenolic compounds and plant origin. Apidologie. 1992;23(1):79–85. 10.1051/apido:19920109.Suche in Google Scholar
[52] Lotti C, Campo Fernandez M, Piccinelli AL, Cuesta-Rubio O, Marquez Hernandez I, Rastrelli L. Chemical constituents of red Mexican propolis. J Agric Food Chem. 2010;58(4):2209–13. 10.1021/jf100070w.Suche in Google Scholar PubMed
[53] Abozid MM, Ahmed AA. Chemical composition of Egyptian and commercial propolis and its effects on liver function and lipid profiles in albino rats. J Biol Chem Environ Res. 2013;8(2013):323–40.Suche in Google Scholar
[54] Uzel A, Önçağ Ö, Çoğulu D, Gençay Ö. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol Res. 2005;160(2):189–95. 10.1016/j.micres.2005.01.002.Suche in Google Scholar PubMed
[55] McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73(1):73–84. 10.1016/S0308-8146(00)00288-0.Suche in Google Scholar
[56] Martinello M, Mutinelli F. Antioxidant activity in bee products: a review. Antioxidants. 2021;10(2021):71. 10.3390/antiox10010071.Suche in Google Scholar PubMed PubMed Central
[57] Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84(3):329–39. 10.1016/S0308-8146(03)00216-4.Suche in Google Scholar
[58] Wali AF, Mushtaq A, Rehman MU, Akbar S, Masoodi MH. Bee propolis (Bee’s Glue): a phytochemistry review. J Crit Rev. 2017;4(2017):9–13. 10.22159/jcr.2017v4i4.20135.Suche in Google Scholar
[59] Kuropatnicki AK, Szliszka E, Krol W. Historical aspects of propolis research in modern times. eCAM. 2013;2013:964149. 10.1155/2013/964149.Suche in Google Scholar PubMed PubMed Central
[60] Grange JM, Davey RW. Antibacterial properties of propolis (bee glue). J R Soc Med. 1990;83(3):159–60. 10.1177/014107689008300310.Suche in Google Scholar PubMed PubMed Central
[61] Pillai SI, Kandaswamy M, Subramanian S. Antiulcerogenic and ulcer healing effects of Indian propolis in experimental rat ulcer models. JAAS. 2010;2(1):21–8. 10.3896/IBRA.4.02.1.02.Suche in Google Scholar
[62] Irigoiti Y, Navarro A, Yamul D, Libonatti C, Tabera A. The use of propolis as a functional food ingredient: a review. Trends Food Sci Technol. 2021;115(2021):297–306. 10.1016/j.tifs.2021.06.041.Suche in Google Scholar
[63] Kosedag M, Gulaboglu M. Effectiveness of propolis in the treatment of Covid-19. Int J PharmATA. 2022;2(3):11–5.Suche in Google Scholar
[64] Naggar YA, Giesy JP, Abdel-Daim MM, Ansari MJ, Al-Kahtani SN, Yahya G. Fighting against the second wave of COVID-19: can honeybee products help protect against the pandemic? Saudi J Biol Sci. 2020;28(3):1519–27. 10.1016/j.sjbs.2020.12.031.Suche in Google Scholar PubMed PubMed Central
[65] Refaat H, Mady FM, Sarhan HA, Rateb HS, Alaaeldin E. Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19. Int J Pharm. 2021;592:120028. 10.1016/j.ijpharm.2020.120028.Suche in Google Scholar PubMed PubMed Central
[66] Berretta AA, Silveira MA, Capcha JM, Jong DD. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed Pharmacother. 2020;131:110622. 10.1016/j.biopha.2020.110622.Suche in Google Scholar PubMed PubMed Central
[67] Ripari N, Sartori AA, Honorio Md, Conte FL, Tasca KI, Santiago KB, et al. Propolis antiviral and immunomodulatory activity: a review and perspectives for COVID-19 treatment. J Pharm Pharmacol. 2021;73(3):281–99. 10.1093/jpp/rgaa067.Suche in Google Scholar PubMed PubMed Central
[68] Maruta H, He H. PAK1-blockers: potential therapeutics against COVID-19. Med Drug Discov. 2020;100039. 10.1016/j.medidd.2020.100039.Suche in Google Scholar PubMed PubMed Central
[69] Meltem U. The importance of propolis in combating COVID-19. J Apith Nat. 2021;4(1):22–40. 10.35206/jan.932050.Suche in Google Scholar
[70] Górecka AK, Dragon KW, Felitti R, Buchta AN, Baron S, Olczyk P. The influence of propolis on dental plaque reduction and the correlation between dental plaque and severity of COVID-19 complications—a literature review. Molecules. 2021;26(18):5516. 10.3390/molecules26185516.Suche in Google Scholar PubMed PubMed Central
[71] Khayrani AC, Irdiani R, Aditama R, Pratami DK, Lischer K, Ansari MJ, et al. Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study. J King Saud Univ Sci. 2021;33(2):101297. 10.1016/j.jksus.2020.101297.Suche in Google Scholar PubMed PubMed Central
[72] Lima WG, Brito JC, Nizer WS. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2). Phytother Res. 2020;35(2):743–50. org/10.1002/ptr.6872.Suche in Google Scholar
[73] Harisna AH, Nurdiansyah R, Syaifie PH, Nugroho DW, Saputro KE, Prakoso CD, et al. In silico investigation of potential inhibitors to main protease and spike protein of SARS-CoV-2 in propolis. Biochem Biophys Rep. 2021;26(1):100969. 10.1016/j.bbrep.2021.100969.Suche in Google Scholar PubMed PubMed Central
[74] Kalia A, Morya S, Neumann A. Health from the hive: therapeutic potential of propolis—a review. J Food Bioact. 2022;18(18):77–84. 10.31665/JFB.2022.18310. Suche in Google Scholar
[75] Seven PT, Seven I, Baykalir BG, Mutlu SI, Salem AZ. Nanotechnology and nano-propolis in animal production and health: an overview. Ital J Anim Sci. 2018;17(4):921–30. 10.1080/1828051X.2018.1448726.Suche in Google Scholar
[76] Abou-Shaara HF, Staron M, Staroňová D. Potential applications of nanotechnology in apiculture. Entomol Appl Sci Lett. 2020;7(4):1–8.Suche in Google Scholar
[77] Barsola B, Kumari P. Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: a review. Green Process Synth. 2022;11(1):659–73. 10.1515/gps-2022-0059.Suche in Google Scholar
[78] Afrouzan H, Amirinia C, Mirhadi SA, Ebadollahi A, Vasej N, Tahmasb G. Evaluation of antimicrobial activity of propolis and nanopropolis against Staphylococcus aureus and Candida albicans. Afr J Microbiol Res. 2012;6(2):421–5. 10.5897/AJMR11.1183.Suche in Google Scholar
[79] Chung N-K, Cho Y-C, Ha C-S, Kim H-S. Hypoglycemic effects of nano powder propolis on streptozotocin-induced diabetic rats. Korean J Vet Serv. 2010;33(2):199–206.Suche in Google Scholar
[80] Ozan F, Sümer Z, Polat ZA, Er K, Ozan U, Deger O. Effect of mouthrinse containing propolis on oral microorganisms and human gingival fibroblasts. Eur J Dent. 2007;1(4):195–201. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2609911/.10.1055/s-0039-1698339Suche in Google Scholar
[81] Carmona GS, Sousa IL, Laurentino RV, Junior DL, Pantoja AV, Mendonça RZ, et al. Propolis and its components can help reduce the physiopathological consequences of Covid-19 infection. Int J Dev Res. 2020;10(10):41067–9. 10.37118/ijdr.20132.10.2020.Suche in Google Scholar
[82] Saklani S, Barsola B, Kumari P, Pathania D. Organic nitrogen application on algal growth for biodiesel applications. Mater Today Proceed. 2022. 10.1016/j.matpr.2022.10.024.Suche in Google Scholar
[83] Pathania D, Sharma M, Thakur P, Chaudhary V, Kaushik A, Furukawa H, et al. Exploring phytochemical composition, photocatalytic, antibacterial, and antifungal efficacies of Au NPs supported by Cymbopogon flexuosus essential oil. Sci Rep. 2022;12(1):1–15. 10.1038/s41598-022-15899-9.Suche in Google Scholar PubMed PubMed Central
[84] Barsola B, Kumari P, Saklani S, Pathania D. Circulatory RNA based non-invasive detection and diagnosis of breast cancer. Mater Today Proceed. 2022. 10.1016/j.matpr.2022.10.206.Suche in Google Scholar
[85] Sonu, Chaudhary V. A paradigm of internet-of-nano-things inspired intelligent plant pathogen-diagnostic biosensors. ECS Sens Plus. 2022;1(3):031401. 10.1149/2754-2726/ac92ed.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Artikel in diesem Heft
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

