Abstract
Objective
This study aimed to clarify the roles and underlying mechanisms of luteolin in the progression of cerebral ischemia/reperfusion injury (CIRI).
Methods
A mouse model of CIRI was established using the middle cerebral artery occlusion (MCAO) method, after which luteolin was administered. Subsequently, neuronal apoptosis and pyroptosis were measured and the brain tissues of each group were subjected to RNA sequencing.
Results
Luteolin alleviated MCAO-induced brain infarction, apoptosis, and pyroptosis. RNA sequencing identified 3,379, 2,777, and 3,933 differentially expressed genes (DEGs) in the MCAO vs sham, MCAO vs MCAO + luteolin, and MCAO + luteolin vs sham groups, respectively. The identified DEGs showed enrichment in multiple processes, including pattern specification, forebrain development, anion transport, leukocyte migration, regulation of cell–cell adhesion, and positive regulation of the response to external stimuli, as well as the calcium, PI3K-AKT, JAK-STAT, NF-kappa B, IL-17, cAMP, cGMP-PKG, and Wnt signaling pathways. In addition, Ccl2 and Angpt2 interacted more with the other top 30 DEGs with high interaction weights. Finally, RT-qPCR results showed that MCAO induction significantly up-regulated the expression of Stoml3, Eomes, and Ms4a15 and down-regulated Nms, Ttr, and Avpr1a; however, luteolin could partially reverse the expression caused by MCAO.
Conclusion
Luteolin can alleviate brain infarction, apoptosis, and pyroptosis in CIRI, and may improve MCAO-induced CIRI by targeting the identified DEGs and their enriched pathways.
1 Introduction
Stroke is the second leading cause of death and third leading cause of disability worldwide [1]. It is estimated that over 80% of stroke cases are ischemic stroke (IS), with approximately 14 million cases occurring annually [2,3]. IS is predominantly caused by cerebral artery occlusion, resulting in glucose and oxygen deficiencies in brain cells, triggering oxidative stress, inflammation, apoptosis, and cell death [4,5]. Restoration of the cerebral blood flow perfusion to the ischemic area is the primary treatment strategy for IS [4,6]. However, rapid reperfusion commonly leads to secondary brain tissue damage, known as cerebral ischemia–reperfusion injury (CIRI) [4,6]. Several physiological mechanisms, including atherosclerosis and acute myocardial infarction, promote ischemia and lead to hypoxia and hypoperfusion [7,8]. The obstruction of arterial blood flow leads to hypoxia and dysfunction of the mitochondrial electron transport chain [9]. Reduced ATP production [10] in the mitochondria can induce anaerobic metabolism, sodium potassium pump dysfunction, and ribosome detachment. During reperfusion, the blood flow to the ischemic tissue is restored by providing oxygen through red blood cells; however, due to the low concentration of antioxidants in ischemic cells, the production of reactive oxygen species increases, causing oxidative stress, and promoting endothelial dysfunction, DNA damage, and local inflammatory responses [8]. Currently, intravenous thrombolytic drugs, such as recombinant tissue plasminogen activator, are the only drug treatments approved by the US Food and Drug Administration/European Medicines Agency for patients with acute IS; however, their efficacy is limited by therapeutic time windows, bleeding complications, and single-target approaches [11,12]. As such, it is crucial to develop novel therapeutic strategies for CIRI.
Luteolin (3,4,5,7-tetrahydroxy-flavone) is a flavonoid compound widely found in fruits, herbs, and green vegetables [13,14]. In traditional Chinese medicine, plants rich in luteolin have been extensively used to treat high blood pressure, inflammatory diseases, and cancer, among others [15]. Increasing evidence has shown that luteolin exerts a variety of pharmacological effects, including antioxidant, anti-inflammatory, anticancer, and apoptotic regulatory effects [16,17]. Further, Xu et al. [18] reported that pretreatment with luteolin both in vitro and in vivo inhibits cell apoptosis via the JPX/miR-146b axis, eventually improving myocardial ischemia–reperfusion injury. A previous study by Jiang et al. [19] also demonstrated that luteolin could suppress inflammation, autophagy, and cell apoptosis via modulation of the ERK/PPARα pathway, thereby alleviating the hepatic cell injury caused by ischemia reperfusion. Another study showed that luteolin protects against renal ischemia–reperfusion injury by regulating pro-inflammatory cytokines, oxidative stress, and cell apoptosis, thereby benefiting kidney transplantation [20]. These reports suggest that luteolin exerts protective effects against ischemia/reperfusion injury. In addition, recent studies have shown that luteolin can provide neuroprotective roles in CIRI [21,22]. For example, Fan et al. [23] illustrated that luteolin-7-O-β-d-glucuronide could mitigate CIRI by improving the permeability of the blood–brain barrier. Another investigation showed that luteolin could exhibit neuroprotective effects on CIRI-induced hippocampal inflammation and autophagy by activating PPARγ in rats [24]. However, the molecular mechanisms underlying the protective effects of luteolin against CIRI remain unclear.
Pyroptosis is a form of programmed cell death that plays a key role in host defense against pathogen infections under normal physiological conditions [25]. During this process, the activated NLRP3 inflammasome and procaspase 1 bind to the inflammatory complex via apoptosis-associated spot-like proteins and promote the activation of caspase 1 [25,26]. Activated caspase 1 drives the cleavage of Gasdermin D (GSDMD) to GSDMD-N, thereby processing IL-1β and IL-18, and inducing pyroptosis and corresponding inflammation [25,26]. Xiao et al. [27] further demonstrated that Astragaloside IV alleviates CIRI by activating Nrf2 to inhibit NLRP3 inflammasome-mediated pyroptosis. Another study showed that down-regulation of XBP-1 could protect neurons by inhibiting pyroptosis via action on the classical NLRP3/Caspase-1/GSDMD pathway, thereby mitigating CIRI. Together, these studies revealed that cell pyroptosis plays an essential role in CIRI progression, while modulation of cell pyroptosis could be considered an effective therapeutic strategy for CIRI [28,29]. Furthermore, luteolin has been shown to induce pyroptosis in HT-29 cells by activating the Caspase-1/GSDMD pathway, thereby inhibiting the proliferation of colon cancer cells [30]. However, whether luteolin regulates pyroptosis in CIRI remains unclear.
Given this context, in the present study, we constructed a mouse CIRI model using middle cerebral artery occlusion (MCAO) and used luteolin to treat MCAO-induced mice to explore the role of cell pyroptosis in luteolin-mediated alleviation of CIRI. The brain tissues of mice in different groups were subjected to high-throughput sequencing to uncover other specific molecular mechanisms of luteolin-relieving CIRI. This study provides novel insights into the treatment of CIRI with luteolin, and lays the theoretical foundation for the development of novel therapeutic targets for CIRI.
2 Materials and methods
2.1 Animals and grouping
A total of 18 male C57BL/6 mice weighing 20 ± 2 g (6–8 weeks) were purchased from the SLAC Laboratory Animal Center of Shanghai (Shanghai, China). All mice were housed under controlled temperature (24 ± 2°C) and humidity (50 ± 5%), with a 12 h light/dark cycle, with access to food and water provided ad libitum. After acclimatization for 7 days, 18 mice were randomly and equally divided into three groups (n = 6 per group): sham, MCAO, and MCAO + luteolin.
Mice in the MCAO and MCAO + luteolin groups underwent induction of CIRI in vivo using MCAO. In brief, mice were anesthetized with 3% pentobarbital sodium, and an incision was made in the midline of the neck to expose the left common carotid artery, external carotid artery, and internal carotid artery. Subsequently, a 3.0 nylon suture was advanced from the external carotid artery to the internal carotid artery until the middle cerebral artery was blocked. After 1 h, the suture was retracted to initiate reperfusion, and the wound was closed. The mice in the sham group underwent sham operation without ligation. Mice in the MCAO + luteolin group were intraperitoneally injected with 5 mg/kg luteolin (Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China) following suture removal. After 24 h, all mice were euthanized by CO2 inhalation, and their brain tissue was collected.
-
Ethical approval: The research related to animal use has been complied with the National Medical Advisory Committee guidelines using procedures approved by the Institutional Animal Care and Use Committee at East Hospital, Tongji University School of Medicine.
2.2 2,3,5-Triphenyltetrazolium chloride (TTC) staining
Brain infarction in different groups was assessed using TTC staining. In brief, after the mice were euthanized, the cerebrum was extracted and maintained at −20°C. Subsequently, 2 mm coronal sections were obtained and immersed in 0.5% TTC reagent (Shanghai Yuanye Biotechnology Co. Ltd, Shanghai, China) for 30 min before fixation in 4% paraformaldehyde. The brain infarct volumes of the mice were measured using ImageJ software.
2.3 TUNEL staining
Neuronal apoptosis in different groups was detected using TUNEL staining, as previously described [31]. In brief, the collected brain tissues were fixed, embedded, and prepared into 4 µm slices. The slices were then deparaffinized and rehydrated, and endogenous peroxidase was blocked. Subsequently, the slices were used to evaluate neuronal apoptosis using the DAB (SA-HRP) TUNEL Cell Apoptosis Detection Kit (Servicebio, Wuhan, China), following the manufacturer’s protocols. Apoptosis in different groups was measured under a microscope.
2.4 Real-time quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from the brain tissues of different groups using RNAiso Plus (Takara, Tokyo, Japan), and reverse transcribed into cDNA using PrimeScript RT Master Mix (Takara). The RT-qPCR assay was conducted using 2× Universal SYBR Green Fast qPCR Mix (ABclonal, Wuhan, China). The sequences of all primers are shown in Table 1. GAPDH was used as an internal reference. The relative mRNA expressions of related genes were calculated using the 2−ΔΔCt method.
Sequences of all primers
| Primer | Sequence (5′–3′) |
|---|---|
| TNF-α-F | CTGAACTTCGGGGTGATCGG |
| TNF-α-R | GGCTTGTCACTCGAATTTTGAGA |
| IL-1β-F | TGCCACCTTTTGACAGTGATG |
| IL-1β-R | TGATGTGCTGCTGCGAGATT |
| NLRP3-F | ATTACCCGCCCGAGAAAGG |
| NLRP3-R | TCGCAGCAAAGATCCACACAG |
| Caspase1-F | ACAAGGCACGGGACCTATG |
| Caspase1-R | TCCCAGTCAGTCCTGGAAATG |
| GSDMD-F | CCATCGGCCTTTGAGAAAGTG |
| GSDMD-R | ACACATGAATAACGGGGTTTCC |
| GAPDH-F | GGTGAAGGTCGGTGTGAACG |
| GAPDH-R | CTCGCTCCTGGAAGATGGTG |
| Stoml3-F | GATTCACCGGAGAAACTGGAG |
| Stoml3-R | TCCATACTGAGATTGGGAAGGT |
| Eomes-F | GCGCATGTTTCCTTTCTTGAG |
| Eomes-R | GGTCGGCCAGAACCACTTC |
| Ms4a15-F | GCGCCCAGAGCTACTCAAC |
| Ms4a15-R | GTGGATGAGACCGATCAGGAT |
| Scgn-F | ATGGACAACGCACGCAGAAA |
| Scgn-R | TCCTCAGTGCCAGATTTTGCC |
| Nms-F | CCAATCCTGTTCATCTACTGCTT |
| Nms-R | CGGGAGAATCAGCTAAAGGTG |
| Ttr-F | CTGCTGTAGACGTGGCTGTAA |
| Ttr-R | CTTCCAGTACGATTTGGTGTCC |
| Avpr1a-F | GCTGGCGGTGATTTTCGTG |
| Avpr1a-R | GCAAACACCTGCAAGTGCT |
| Nkx2-1-F | ATGAAGCGCCAGGCTAAGG |
| Nkx2-1-R | GGTTTGCCGTCTTTGACTAGG |
2.5 Western blot
Total protein was isolated from the brain tissues of different groups using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) and quantified using a BCA protein assay kit (Beyotime Biotechnology). Subsequently, protein samples (20 μg) were separated by 10% SDS-PAGE and transferred to PVDF membranes. After being blocked with 5% skim milk at 37°C for 2 h, membranes were incubated with the following primary antibodies: anti-MMP9 (1:1,000, 10375-2-AP; Proteintech), anti-NLRP3 (1:2,000, 68102-1-Ig; Proteintech), anti-IL-6 (1:1,000, 26404-1-AP; Proteintech), anti-IL-1β (1:800, A16288; Abclonal), and anti-GAPDH (1:5,000, 60004-1-Ig; Proteintech) at 4°C overnight, before incubation with the relevant secondary antibody (1:10,000; Proteintech) at 37°C for 1 h. After washing, the blots were visualized using an ECL chemiluminescence kit (Beyotime), and quantified using ImageJ software.
2.6 Library construction and sequencing
RNA from the sham, MCAO, and MCAO + luteolin groups was sent to Yanzai Biotechnology (Shanghai) Co. Ltd (Shanghai, China) for RNA sequencing. In brief, RNA samples were checked by RNase-free agarose gel electrophoresis and quantified using a NanoDrop 2000. Subsequently, sequencing transcriptome libraries were prepared using the TruSeq™ RNA Sample Prep Kit (Illumina, San Diego, CA, USA), and RNA sequencing was carried out using the NovaSeq 6000 S4 Reagent Kit v1.5 (300 cycles) (Illumina, San Diego, CA, USA) and the Illumina NovaSeq2500 (Illumina, San Diego, CA, USA) system, following the manufacturer’s instructions.
2.7 Data analysis and bioinformatic analysis
Raw sequencing reads were subjected to optimization processes, such as de-sequencing splices, quality splicing, short sequence filtering, and removal of ribosomal contamination. The processed reads were compared to the mouse reference genome (GRCm39.106). Subsequently, differentially expressed genes (DEGs) among the different groups were identified based on the following thresholds: adjusted P value <0.05 and |log2 fold change (FC)| ≥ 1. The identified DEGs were then subjected to gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses, and protein–protein interaction (PPI) networks were constructed using the STRING tool.
2.8 Statistical analysis
Data are shown as mean ± standard deviation, and analyzed by GraphPad Prism 8.4.2. Differences between the groups were evaluated using one-way analysis of variance followed by Tukey’s post hoc test. Statistical significance was set at P < 0.05.
3 Results
3.1 Luteolin alleviates MCAO-induced brain infarction, cell apoptosis, and pyroptosis
To clarify the role of luteolin in CIRI progression, a mouse model of CIRI was established using MCAO, after which luteolin was applied, as shown in Figure 1a. The brain infarct volumes in the different groups were then assessed. Compared to the sham group, MCAO induction significantly increased the brain infarct volume (P < 0.01), whereas luteolin administration reduced the brain infarct volume induced by MCAO (P < 0.01, Figure 1b and c), indicating that luteolin could alleviate brain infarction induced by MCAO, thereby exerting a protective role against CIRI.

Luteolin alleviates MCAO-induced brain infarction. (a) A surgical diagram outlining MCAO model establishment. (b) Cerebral infarction in the MCAO model after luteolin treatment evaluated by TTC staining. **P < 0.01 vs sham group; ## P < 0.01 vs MCAO group.
Herein, we examined the effects of luteolin on apoptosis and pyroptosis. Apoptosis was significantly higher in the MCAO group than in the sham group (P < 0.05), whereas luteolin treatment inhibited MCAO-induced apoptosis (P < 0.05, Figure 2a). Subsequently, the expression levels of inflammatory cytokines (TNF-α, IL-1β, and IL-6) and pyroptosis-related factors (NLRP3, Caspase-1, GSDMD, and MMP9) were analyzed by RT-qPCR and western blot. RT-qPCR showed significant up-regulation of the mRNA expressions of TNF-α, IL-1β, and NLRP3 in MCAO mice compared with sham mice (P < 0.05); while luteolin administration reversed this expression induced by MCAO (P < 0.05, Figure 2b). However, no significant differences were found in the mRNA expression levels of Caspase-1 and GSDMD among the sham, MCAO, and MCAO + luteolin groups (P > 0.05, Figure 2b). Furthermore, western blot analysis showed significant increases in the protein levels of MMP9, NLRP3, IL-1β, and IL-6 following MCAO induction relative to the sham mice (P < 0.05), which was partially abrogated by luteolin (P < 0.05, Figure 2c). These results indicate that luteolin can mitigate MCAO-induced apoptosis and partially restore the expression of pro-inflammatory cytokines and pyroptosis-related factors induced by MCAO.

Luteolin alleviates MCAO-stimulated cell apoptosis and pyroptosis. (a) Cell apoptosis in the brain tissues of MCAO mice after luteolin treatment assessed using TUNEL staining. (b) The mRNA expression levels of TNF-α, IL-1β, NLRP3, Caspase-1, and GSDMD in brain tissues of MCAO mice after luteolin treatment measured by RT-qPCR. (c) The protein expression of MMP9, NLRP3, IL-1β, and IL-6 in brain tissues of MCAO mice after luteolin treatment detected using western blot. *P < 0.05, **P < 0.01 vs sham group; # P < 0.05, ## P < 0.01 vs MCAO group.
3.2 Identification of DEGs among different groups
To elucidate the potential molecular mechanisms through which luteolin protects against CIRI, the total RNA of brain tissues in the sham, MCAO, and MCAO + luteolin groups was subjected to RNA sequencing. Based on the established criteria (adjusted P value < 0.05 and |log2 FC| ≥ 1), 3,379 (2,137 up-regulated and 1,242 down-regulated in MCAO), 2,777 (1,109 up-regulated and 1,668 down-regulated in MCAO), and 3,933 (2,595 up-regulated and 1,338 down-regulated in MCAO + luteolin) DEGs were identified in the comparison of MCAO vs sham, MCAO vs MCAO + luteolin, and MCAO + luteolin vs sham, respectively (Figure 3a). In the comparison between MCAO and sham, the top five up-regulated DEGs were Slfn4, Mmp3, Chil3, Gm20431, and Cxcr2, while the top five down-regulated DEGs were Shox2, Commd1b, Oxt, Irx2, and Pmch (Table 2). In the comparison between the MCAO and MCAO + luteolin, the top five up-regulated DEGs were Stoml3, Foxd3, Gdpd4, Gm49388, and Tyrp1, and the top five down-regulated DEGs were Commd1b, Gm28539, Xlr4a, Fam71a, and Gm614 (Table 1). In addition, Mmp3, Mmp12, Chil3, Cxcl2, and Cxcr2 were the top five up-regulated DEGs, whereas Fezf1, Lhx5, Shox2, Sim1, and Mab21l2 were the top five down-regulated DEGs in the MCAO + luteolin group compared to the sham group (Table 1). In addition, the bidirectional hierarchical clustering heatmaps of the identified DEGs in the different comparison groups are displayed in Figure 3b, which show that the identified DEGs in the different comparison groups could significantly distinguish the two different groups.

Identification of DEGs among different groups. (a) Volcano plots of DEGs in the comparison groups of MCAO vs sham, MCAO vs MCAO + luteolin, and MCAO + luteolin vs sham. (b) Bidirectional hierarchical clustering heatmaps of the identified DEGs in the different comparison groups of MCAO vs sham, MCAO vs MCAO + luteolin, and MCAO + luteolin vs sham.
Top five DEGs with up-regulation and down-regulation in the comparison groups of MCAO vs sham, MCAO + luteolin vs MCAO, and MCAO + luteolin vs sham
| DEGs | Comparison | |||||
|---|---|---|---|---|---|---|
| MCAO vs sham | MCAO + luteolin vs MCAO | MCAO + luteolin vs sham | ||||
| Up-regulation (log2 FC) | Down-regulation (log2 FC) | Up-regulation (log2 FC) | Down-regulation (log2 FC) | Up-regulation (log2 FC) | Down-regulation (log2 FC) | |
| 1 | Slfn4 (11.9) | Shox2 (−14.6) | Stoml3 (11.9) | Commd1b (−9.6) | Mmp3 (14.9) | Fezf1 (−11.2) |
| 2 | Mmp3 (11.8) | Commd1b (−11.7) | Foxd3 (11.5) | Gm28539 (−8.4) | Mmp12 (13.8) | Lhx5 (−10.7) |
| 3 | Chil3 (11.4) | Oxt (−10.9) | Gdpd4 (11.3) | Xlr4a (−8.2) | Chil3 (13.5) | Shox2 (−10.0) |
| 4 | Gm20431 (11.2) | Irx2 (−10.4) | Gm49388 (10.8) | Fam71a (−7.5) | Cxcl2 (13.5) | Sim1 (−9.9) |
| 5 | Cxcr2 (11.1) | Pmch (−10.4) | Tyrp1 (10.5) | Gm614 (−7.4) | Cxcr2 (13.1) | Mab21l2 (−9.3) |
3.3 GO term analysis of the identified DEGs in the different comparison groups
DEGs identified in the different comparison groups were subjected to GO term analysis, including biological processes (BP), cellular components (CC), and molecular functions (MF). As shown in Tables S1 and S2, the up-regulated DEGs in the comparison of MCAO vs sham were enriched in “positive regulation of response to external stimulus,” “regulation of immune effector process,” “cytokine-mediated signaling pathway,” “negative regulation of immune system process,” and “leukocyte migration”; while the down-regulated DEGs were enriched in “adenylate cyclase-modulating G protein-coupled receptor signaling pathway,” “regulation of membrane potential,” “hormone transport,” “cilium movement,” and “locomotory behavior” in BP. In CC, the up-regulated DEGs between the sham and MCAO groups largely participated in “collagen-containing extracellular matrix,” “apical part of cell,” “receptor complex,” “membrane microdomain,” and “membrane raft”; while the down-regulated DEGs were associated with “synaptic membrane,” “motile cilium,” “transmembrane transporter complex,” “transporter complex,” and “ion channel complex” (Tables S1 and S2). At the MF level, the up-regulated DEGs between the sham and MCAO groups were closely related to “endopeptidase activity,” “cytokine activity,” “cytokine receptor binding,” “cell adhesion molecule binding,” and “sulfur compound binding”; while the decreased DEGs were correlated with “channel activity,” “passive transmembrane transporter activity,” “ion channel activity,” “gated channel activity,” and “metal ion transmembrane transporter activity” (Tables S1 and S2).
In the comparison between the MCAO and MCAO + luteolin groups, the up-regulated DEGs were significantly enriched in the pattern specification process “pattern specification process,” “forebrain development,” “anion transport,” “visual system development,” and “sensory system development” in BP; “apical part of cell,” “synaptic membrane,” “transporter complex,” “transmembrane transporter complex,” and “collagen-containing extracellular matrix” in CC; and “channel activity,” “passive transmembrane transporter activity,” “ion channel activity,” “metal ion transmembrane transporter activity,” and “anion transmembrane transporter activity” in MF (Table S3). Further, the down-regulated DEGs were correlated with “leukocyte migration,” “regulation of cell–cell adhesion,” “positive regulation of response to external stimulus,” and “leukocyte cell–cell adhesion in BP; “collagen-containing extracellular matrix,” “receptor complex,” “secretory granule,” “apical part of cell,” and “membrane raft” in CC; and “cytokine receptor binding,” “G protein-coupled receptor binding,” “channel activity,” “cytokine activity,” and “enzyme inhibitor activity” in MF (Table S4).
In the comparison of MCAO + luteolin vs sham, up-regulated DEGs were significantly enriched in “positive regulation of response to external stimulus,” “leukocyte migration,” “positive regulation of defense response,” “cytokine-mediated signaling pathway,” and “regulation of immune effector process” in BP; “collagen-containing extracellular matrix,” “membrane microdomain,” “membrane raft,” and “receptor complex” in CC; and “cytokine activity,” “immune receptor activity,” and “cytokine binding” in MF (Table S5). Furthermore, the identified down-regulated DEGs were closely related to “neuropeptide signaling pathway,” “catecholamine transport,” “forebrain development,” and “positive regulation of cytosolic calcium ion concentration” in BP, “ion channel complex,” “transporter complex,” and “transmembrane transporter complex” in CC, and “gated channel activity,” “ion channel activity,” and “G protein-coupled peptide receptor activity” in MF (Figure S6).
3.4 KEGG pathway analysis of the identified DEGs in the different comparison groups
KEGG analysis was further conducted to study the enrichment pathways of the identified DEGs. This analysis revealed that the up-regulated DEGs in the MCAO group compared to the sham group were primarily involved in “chemokine signaling pathway,” “TNF signaling pathway,” “JAK-STAT signaling pathway,” “NF-kappa B signaling pathway,” “IL-17 signaling pathway,” and “Toll-like receptor signaling pathway” (Figure 4a); while the down-regulated DEGs in the MCAO group compared to the sham group were related to “cAMP signaling pathway,” “calcium signaling pathway,” “cGMP-PKG signaling pathway,” and “neuroactive ligand–receptor interaction” (Figure 4b). The identified DEGs between the MCAO and MCAO + luteolin groups were significantly enriched in “neuroactive ligand–receptor interaction,” “cAMP signaling pathway,” “calcium signaling pathway,” “PI3K-Akt signaling pathway,” “JAK-STAT signaling pathway,” “TNF signaling pathway,” “IL-17 signaling pathway,” “NF-kappa B signaling pathway,” and “Th17 cell differentiation” (Figure 4c and d). Additionally, for the identified DEGs between the MCAO + luteolin and sham groups, the pathways enriched in up-regulated DEGs included the “chemokine signaling pathway,” “Toll-like receptor signaling pathway,” “TNF signaling pathway,” “IL-17 signaling pathway,” and “NF-kappa B signaling pathway” (Figure 4e); while the pathways enriched in the down-regulated DEGs included “neuroactive ligand–receptor interaction,” “cAMP signaling pathway,” “calcium signaling pathway,” and “Wnt signaling pathway” (Figure 4f).

KEGG pathways of the identified DEGs in the different comparison groups. (a) KEGG analysis of the up-regulated DEGs between the sham and MCAO groups. (b) KEGG analysis of the down-regulated DEGs between the sham and MCAO groups. (c) KEGG analysis of the up-regulated DEGs between the MCAO and MCAO + luteolin groups. (d) KEGG analysis of the down-regulated DEGs between the MCAO and MCAO + luteolin groups. (e) KEGG analysis of the up-regulated DEGs between the sham and MCAO + luteolin groups. (f) KEGG analysis of the down-regulated DEGs between the sham and MCAO + luteolin groups.
3.5 Analysis of the constructed PPI networks
DEGs identified in the different comparison groups were used to construct PPI networks using the STRING database. The top 30 DEGs were sorted based on their interaction weights from the highest to lowest among the three comparison groups (MCAO vs sham, MCAO vs MCAO + luteolin, and MCAO + luteolin vs sham). The top 30 DEGs with higher interaction weights between the sham and MCAO groups are presented in Figure 5a; in this comparison the top three up-regulated DEGs were IL6, Tfap2c, and Ccl2, while the top three down-regulated DEGs were Sim1, Avp, and Tyr. In the PPI network of the identified DEGs in the comparison of MCAO vs MCAO + letuolin, the top three up-regulated DEGs were Nme3, Kcnh6, and Egf, while the top three down-regulated DEGs were Zp1, Figia, and Cd14; among which Ccl2 and Angpt2 presented more interactions with other DEGs (Figure 5b). Additionally, in the PPI network of the identified DEGs in the comparison of MCAO + letuolin vs sham, the top three up-regulated DEGs were Il6, Tnfsf14, and Ccl2, while the top three down-regulated DEGs were Sim1, Trh, and Trpc3, among which Ccl2 interacted more other DEGs (Figure 5c).

Analysis of the constructed PPI networks. (a) The PPI network of the top 30 DEGs between the sham and MCAO groups. (b). The PPI network of the top 30 DEGs between the MCAO and MCAO + luteolin groups. (c) The PPI network of the top 30 DEGs between the sham and MCAO + luteolin groups.
3.6 Validation of the sequencing results by RT-qPCR
Finally, DEGs with significant fold changes and different expression trends in different groups were selected for RT-qPCR validation. In the current study, Stoml3, Eomes, Ms4a15, and Scgn (up-regulated by MCAO but down-regulated after luteolin treatment), as well as Nms, Ttr, Avpr1a, and Nkx2-1 (down-regulated in MCAO but up-regulated after luteolin treatment) were chosen for validation by RT-qPCR. Compared to the sham group, the mRNA expressions of Stoml3, Eomes, and Ms4a15 were significantly up-regulated in MCAO-induced mice (P < 0.05), and down-regulated after luteolin treatment (P < 0.05, Figure 6). However, Scgn mRNA expression showed no significant differences among the sham, MCAO, and MCAO + luteolin groups (P > 0.05; Figure 6). Analysis of Nms, Ttr, and Avpr1a revealed marked decreases in expression after MCAO induction compared to the sham group (P < 0.05), which was enhanced by luteolin administration (P < 0.05, Figure 6). However, Nkx2-1 expression was significantly up-regulated by MCAO (P < 0.05), but was down-regulated by luteolin (P < 0.05, Figure 6). After comparison with the sequencing data, we found that the expression tendencies of Stoml3, Eomes, Ms4a15, Nms, Ttr, and Avpr1a determined by RT-qPCR were consistent with those detected by RNA sequencing, yielding a consistency rate of 70% between the RT-qPCR and RNA sequencing results, indicating a relatively high reliability of RNA sequencing.
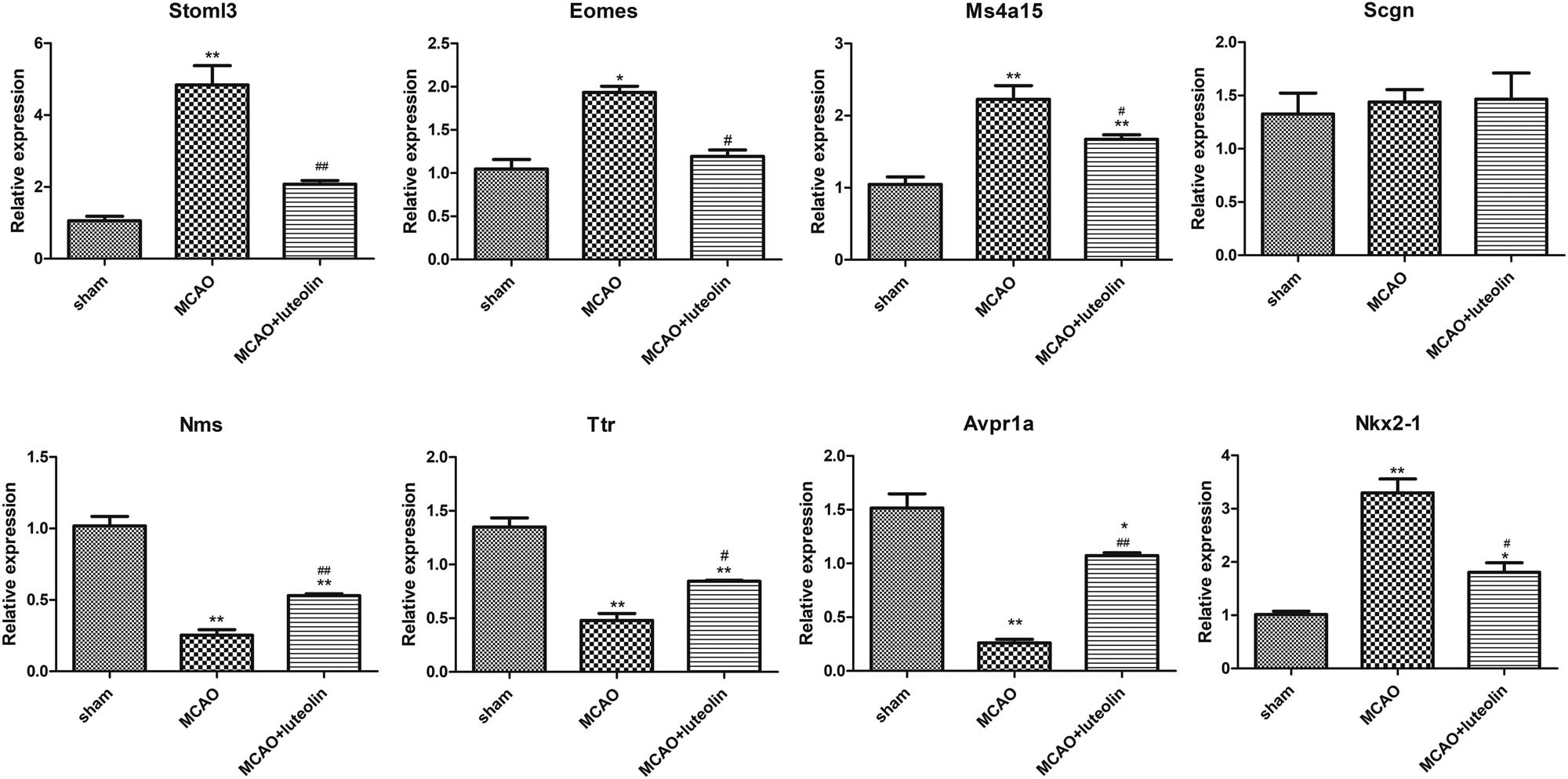
Validation of the RT-qPCR sequencing data. The mRNA expression levels of Stoml3, Eomes, Ms4a15, Scgn, Nms, Ttr, Avpr1a, and Nkx2-1 in the different groups. *P < 0.05, **P < 0.01 vs sham group; # P < 0.05, ## P < 0.01 vs MCAO group.
4 Discussion
CIRI is a complicated disease associated with high disability and mortality rates that can seriously impact the health and life of patients [32]. Luteolin, a flavonoid compound, has been shown to freely penetrate the blood–brain barrier, providing neuroprotection against brain injury [33]. However, the role of luteolin in CIRI has not been thoroughly investigated. Therefore, the present study comprehensively clarified the potential mechanisms underlying the role of luteolin in CIRI progression. To investigate the mechanisms of action of luteolin in CIRI, a mouse model of CIRI was established by MCAO and then treated with luteolin. The MCAO model is one of the models that most closely simulate human IS, and is widely used in IS research [34]. Our study showed that MCAO induction caused brain infarction in mice and that luteolin significantly alleviated this phenomenon. A previous study by Tan et al. reported that luteolin decreased the brain infarct area and neuronal injury in an MCAO rat model [21]. Liu et al. further revealed that luteolin could decrease the cerebral edema and infarction volumes, thereby alleviating neurological injury [22]. These results indicate that luteolin could reduce the brain infarct area in MCAO-induced mice, thereby alleviating neuronal injury.
Apoptosis and pyroptosis are the two most common forms of programmed cell death. Cell apoptosis is critical for disease development and tissue homeostasis, and has an important impact on CIRI progression [35,36]. A previous study by Xie et al. [37] showed that cellular apoptosis was exacerbated in rat brain tissues following MCAO, whereas dihydromyricetin alleviated the apoptosis induced by MCAO. Our study further showed that MCAO significantly enhanced apoptosis, which was alleviated by luteolin treatment. Our results are consistent with those of a previous study [22]. Pyroptosis is caspase-1-dependent and inflammation-related programmed cell death pathway that plays a role in CIRI pathogenesis [25]. Our study found that MCAO up-regulated the expression of TNF-α, IL-1β, IL-6, MMP9, and NLRP3; whereas luteolin could reverse these increases. TNF-α, IL-1β, and IL-6 are pro-inflammatory cytokines, closely associated with pyroptosis. Li et al. [38] previously demonstrated that isoliquiritin could improve neuronal survival and morphology, and reduce pyroptosis-related neuronal cell death by decreasing the levels of TNF-α, IL-1β, IL-6, cleaved Caspase-1, NLRP3, and GSDMD-N, thereby ameliorating depression. MMP9 is a component of the extracellular matrix that increases after stroke. This disturbance is associated with disruption of the blood–brain barrier, an increased risk of bleeding complications, and worsening outcomes [39]. A previous study showed that MMP9 is highly expressed in human lung transplantation and rat lung ischemia–reperfusion tissues, and could contribute to lung ischemia–reperfusion injury by activating cell pyroptosis [40]. The NLRP3 inflammasome participates in the inflammatory response, and its assembly and activation lead to the Caspase-1-dependent release of pro-inflammatory cytokines (IL-1β and IL-18) [41]. It has further been reported that the role of the NLRP3 inflammasome in CIRI primarily involves the release and pyroptosis of NLRP3 inflammasome-dependent cytokines, indicating that NLRP3 may be a therapeutic target in CIRI [42]. Based on these results, we speculate that luteolin may suppress cell apoptosis and pyroptosis by down-regulating the expression of TNF-α, IL-1β, IL-6, MMP9, and NLRP3, thereby improving CIRI.
To further understand the molecular mechanisms by which luteolin improves CIRI, brain tissue samples were subjected to high-throughput sequencing. This analysis identified 3,379, 2,777, and 3,933 DEGs in the MCAO vs sham, MCAO vs MCAO + luteolin, and MCAO + luteolin vs sham comparison groups, respectively. The increased DEGs in the MCAO model after luteolin treatment was predominantly enriched in BP of “pattern specification process,” “forebrain development,” and “anion transport”; while the down-regulated DEGs in the MCAO model after luteolin treatment were primarily enriched in BP of “leukocyte migration,” “regulation of cell–cell adhesion,” and “positive regulation of response to external stimulus.” This pattern specification process can modulate cell differentiation during development and early patterning of the mesoderm [43]. Li et al. also found that the pattern specification process was primarily enriched in DEGs in hepatic I/R injury [44]. Anion transport critically affects CIRI development [45,46]. Dai et al. showed that chloride (Cl–) efflux-regulated cerebral circulation after neonatal hypoxia–ischemia can contribute to brain injury [45]. Kohut et al. further showed that anion transport inhibitors inhibit ischemic brain injury [46]. Leukocytes contribute to tissue damage following cerebral ischemia and can adhere to the wall of pial postcapillary venules at the start of reperfusion and then migrate to the brain tissue [47]. Cell adhesion plays a critical role in the assembly of individual cells into tissues [48]. Previous studies have shown that cell adhesion is involved in CIRI [49,50]. For example, leukocyte–endothelial cell adhesion occurs following CIRI [50]. In addition, we showed that the DEGs in the MCAO model after luteolin treatment were related to positive regulation of the response to external stimuli. Similarly, Liu et al. found that the DEGs in celastrol-treated MCAO mice were enriched in the positive regulation of responses to external stimuli [51]. These reports, together with the results of our study, suggest that the processes of pattern specification, forebrain development, anion transport, leukocyte migration, regulation of cell–cell adhesion, and positive regulation of responses to external stimuli might exert critical functions in the recovery of CIRI.
In addition, KEGG analysis showed a close association of the identified DEGs with the calcium, PI3K-AKT, JAK-STAT, NF-kappa B, IL-17, cAMP, cGMP-PKG, and Wnt signaling pathways. Calcium homeostasis plays a vital role in maintaining excitation–contraction coupling, and drugs associated with the calcium-signaling pathway have been reported to alleviate cerebrovascular diseases, including CIRI [52]. PI3K-AKT signaling pathway is also known as a critical pathway that facilitates the survival of ischemic nerve cells in CIRI [53]. As such, this pathway is considered an effective target for CIRI therapy [54]. The JAK-STAT pathway mediates neuroinflammation, and is involved in the regulation of physiological activities such as cell proliferation, differentiation, and apoptosis, which play important roles in CIRCI [55]. Li et al. previously reported that quercetin could facilitate the M2 polarization of microglia/macrophages by regulating the PI3K/Akt/NF-κB signaling pathway, thereby ameliorating CIRI [56]. A previous study also showed that papaverine exhibited anti-inflammatory immunomodulatory effects on CIRI via the IL-17 pathway, with the activation of IL-23, NF-κB, RANKL, and p38MAPK [57]. Wnt signaling pathway includes canonical Wnt/β-catenin, non-canonical Wnt/PCP, and Wnt/Ca2+ pathways, which are pivotal for CIRI [58]. Chen et al. [59] further demonstrated that the suppression of LMP2 could improve the blood–brain barrier dysfunction induced by IR through activation of the Wnt signaling pathway. Neuronal apoptosis regulated by the cAMP/PKA signaling pathway is an important neuronal transduction pathway that plays an important role in the cognitive, learning, and memory functions of organisms [60]. Lidocaine has also been shown to improve CIRI in rats through the cAMP/PKA signaling pathway [60]. The cGMP-PKG pathway plays a key role in regulating cell growth, metabolism, and many other intracellular processes. The cGMP/PKG pathway in cardiomyocytes is inhibited by IR and preserved by ischemic post-regulation, which largely contributes to post-regulatory protection [61]. Combined with our findings, we conclude that luteolin may improve CIRI by regulating the calcium, PI3K-AKT, JAK-STAT, NF-kappa B, IL-17, cAMP, cGMP-PKG, and Wnt signaling pathways.
Finally, our RT-qPCR results showed that MCAO induction significantly up-regulated expression of the genes Stoml3, Eomes, and Ms4a15, while down-regulating Nms, Ttr, and Avpr1a; however, luteolin could partially reverse these changes in expression. In addition, the constructed PPI networks showed that Ccl2 and Angpt2 interacted with other DEGs. Stoml3 is necessary for normal mechanoreceptor function; as such targeting Stoml3 may be an effective way to reduce pain caused by harmful stress on bones and/or painful inflammatory pathology [62]. Eomes has been shown to positively correlate with the cytotoxic functions of cytotoxic CD4 T cells (CD4 CTLs). A previous study showed that Eomes-expressing CD4+ CTLs can act as biomarkers for the diagnosis and prognosis of secondary progressive multiple sclerosis with over 80% accuracy [63]. Another study suggested that Eomes is a potential biomarker for distinguishing radiation-induced brain injury-associated CD4 CTLs [64]. Ms4a15 is an uncharacterized four-pass membrane protein, and its overexpression can significantly alter Ca2+ homeostasis and suppress IP3R1 expression, leading to extensive lipid remodeling [65]. Previous research has shown that Nms is down-regulated in experimental traumatic brain tissues, whereas progesterone can alleviate brain edema and induce an increase in Nms and its receptors [66]. Ttr is a tetramer transporter protein that plays a role in neuroprotection and promotes neurite growth in response to injury [67]. Gao et al. [68] previously demonstrated that Avpr1a was up-regulated following MCAO, and that modified constraint-induced movement therapy could down-regulate Avpr1a expression induced by MCAO. In addition, Ccl2, a member of the cytokine–cytokine receptor interaction family, plays a vital role in stroke pathophysiology [69]. Wu et al. [70] previously reported that Ccl2 exerted a critical effect on the blood–brain barrier integrity during CIRI. Angpt2, an angiogenic factor, is considered a promising biomarker for early screening of IS [71]. A previous study found that Angpt2 could facilitate brain repair and attenuate cerebral ischemic injury via angiogenesis [72]. Based on these studies and our own results, we speculate that Stoml3, Eomes, Ms4a15, Nms, Ttr, Avpr1a, Ccl2, and Angpt2 may mediate the beneficial effects of luteolin on CIRI; however, this remains to be verified in the future.
This study has some limitations which should be mentioned. First, the use of a single animal model and the potential variability in luteolin effects across different species or conditions should be acknowledged. Second, the specific mechanisms of pyroptosis in luteolin-induced CIRI should be investigated in detail. Additionally, the roles and exhaustive mechanisms of the identified DEGs (Stoml3, Eomes, Ms4a15, Nms, Ttr, Avpr1a, Ccl2, and Angpt2) as well as the enrichment of the calcium, PI3K-AKT, JAK-STAT, NF-kappa B, IL-17, cAMP, cGMP-PKG, and Wnt signaling pathways in CIRI progression need to be further verified through both in vitro and in vivo experiments.
5 Conclusion
Overall, the present study showed that luteolin alleviates brain infarction, apoptosis, and pyroptosis in MCAO-induced CIRI. Additionally, our analysis is the first to clarify the gene expression profiles of CIRI following luteolin treatment, from which it can be inferred that luteolin may improve MCAO-induced CIRI by targeting the identified DEGs (Stoml3, Eomes, Ms4a15, Nms, Ttr, Avpr1a, Ccl2, and Angpt2) and regulating the calcium, PI3K-AKT, JAK-STAT, NF-kappa B, IL-17, cAMP, cGMP-PKG, and Wnt signaling pathways. These findings provide a basis for the future comprehensive elucidation of the mechanisms underlying the role of luteolin in CIRI, and lay a theoretical foundation for the development of novel therapeutic targets and pathways for CIRI.
-
Funding information: This study was supported by Pudong New Area Science and Technology Development Fund Public Institutions Livelihood Research Special Medical and Health Project (No. PKJ2022-Y55).
-
Author contributions: Conception and design of the research: Fei Yu, Guangxue Wang, Hui Hu, and Liang Wei; acquisition of data: Fei Yu, Guangxue Wang, Xingyi Chen, and Yanfei Zhang; analysis and interpretation of data: Fei Yu, Guangxue Wang, and Cheng Yang; statistical analysis: Xingyi Chen, Yanfei Zhang, and Cheng Yang; obtaining funding: Hui Hu and Liang Wei; drafting the manuscript: Fei Yu and Guangxue Wang; revision of manuscript for important intellectual content: Hui Hu and Liang Wei. All authors read and approved the final manuscript.
-
Conflict of interest: The authors declare that they have no conflict of interest.
-
Data availability statement: The dataset used and/or analyzed during the current study are available from the corresponding author on a reasonable request.
References
[1] Shekhar S, Liu Y, Wang S, Zhang H, Fang X, Zhang J, et al. Novel mechanistic insights and potential therapeutic impact of TRPC6 in neurovascular coupling and ischemic stroke. Int J Mol Sci. 2021;22:2074.10.3390/ijms22042074Search in Google Scholar PubMed PubMed Central
[2] She R, Liu D, Liao J, Wang G, Ge J, Mei Z. Mitochondrial dysfunctions induce PANoptosis and ferroptosis in cerebral ischemia/reperfusion injury: from pathology to therapeutic potential. Front Cell Neurosci. 2023;17:1191629.10.3389/fncel.2023.1191629Search in Google Scholar PubMed PubMed Central
[3] Skagen K, Skjelland M, Zamani M, Russell D. Unstable carotid artery plaque: new insights and controversies in diagnostics and treatment. Croat Med J. 2016;57:311–20.10.3325/cmj.2016.57.311Search in Google Scholar PubMed PubMed Central
[4] Huang J, Chen L, Yao ZM, Sun XR, Tong XH, Dong SY. The role of mitochondrial dynamics in cerebral ischemia–reperfusion injury. Biomed Pharmacother. 2023;162:114671.10.1016/j.biopha.2023.114671Search in Google Scholar PubMed
[5] Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci: Off J Italian Neurol Soc Italian Soc Clin Neurophysiol. 2017;38:1167–86.10.1007/s10072-017-2938-1Search in Google Scholar PubMed
[6] Xu S, Huang P, Yang J, Du H, Wan H, He Y. Calycosin alleviates cerebral ischemia/reperfusion injury by repressing autophagy via STAT3/FOXO3a signaling pathway. Phytomed: Int J Phytother Phytopharmacol. 2023;115:154845.10.1016/j.phymed.2023.154845Search in Google Scholar PubMed
[7] Fang X, Zhang J, Roman RJ, Fan F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? GeroScience. 2022;44:1879–83.10.1007/s11357-022-00591-7Search in Google Scholar PubMed PubMed Central
[8] Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46:1650–67.10.1159/000489241Search in Google Scholar PubMed
[9] Dai J, Gao J, Dong H. Prognostic relevance and validation of ARPC1A in the progression of low-grade glioma. Aging (Albany NY). 2024;16:11162–84.10.18632/aging.205952Search in Google Scholar PubMed PubMed Central
[10] Straub JS, Nowotarski MS, Lu J, Sheth T, Jiao S, Fisher MPA, et al. Phosphates form spectroscopically dark state assemblies in common aqueous solutions. Proc Natl Acad Sci USA. 2023;120:e2206765120.10.1073/pnas.2206765120Search in Google Scholar PubMed PubMed Central
[11] Al-Mufti F, Amuluru K, Roth W, Nuoman R, El-Ghanem M, Meyers PM. Cerebral ischemic reperfusion injury following recanalization of large vessel occlusions. Neurosurgery. 2018;82:781–9.10.1093/neuros/nyx341Search in Google Scholar PubMed
[12] Zhao J, Ma M, Li L, Fang G. Oxysophoridine protects against cerebral ischemia/reperfusion injury via inhibition of TLR4/p38MAPK‑mediated ferroptosis. Mol Med Rep. 2023;27:44.10.3892/mmr.2023.12931Search in Google Scholar PubMed PubMed Central
[13] Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586–92.10.1016/j.biopha.2018.09.161Search in Google Scholar PubMed
[14] Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–58.10.1016/j.jep.2018.05.019Search in Google Scholar PubMed
[15] Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612.10.1016/j.biopha.2019.108612Search in Google Scholar PubMed
[16] López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009;9:31–59.10.2174/138955709787001712Search in Google Scholar PubMed
[17] Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008;74:1667–77.10.1055/s-0028-1088314Search in Google Scholar PubMed
[18] Xu T, Zhang Y, Liao G, Xuan H, Yin J, Bao J, et al. Luteolin pretreatment ameliorates myocardial ischemia/reperfusion injury by lncRNA-JPX/miR-146b axis. Anal Cell Pathol (Amst). 2023;2023:4500810.10.1155/2023/4500810Search in Google Scholar PubMed PubMed Central
[19] Jiang Y, Yang W, Ding J, Ji J, Wu L, Zheng Y, et al. Luteolin pretreatment attenuates hepatic ischemia–reperfusion injury in mice by inhibiting inflammation, autophagy, and apoptosis via the ERK/PPARα pathway. PPAR Res. 2022;2022:8161946.10.1155/2022/8161946Search in Google Scholar PubMed PubMed Central
[20] Liu Y, Shi B, Li Y, Zhang H. Protective effect of luteolin against renal ischemia/reperfusion injury via modulation of pro-inflammatory cytokines, oxidative stress and apoptosis for possible benefit in kidney transplant. Med Sci Monit. 2017;23:5720–7.10.12659/MSM.903253Search in Google Scholar PubMed PubMed Central
[21] Tan L, Liang C, Wang Y, Jiang Y, Zeng S, Tan R. Pharmacodynamic effect of luteolin micelles on alleviating cerebral ischemia reperfusion injury. Pharmaceutics. 2018;10:248.10.3390/pharmaceutics10040248Search in Google Scholar PubMed PubMed Central
[22] Liu S, Su Y, Sun B, Hao R, Pan S, Gao X, et al. Luteolin protects against CIRI, potentially via regulation of the SIRT3/AMPK/mTOR signaling pathway. Neurochem Res. 2020;45:2499–515.10.1007/s11064-020-03108-wSearch in Google Scholar PubMed
[23] Fan X, Song J, Zhang S, Lu L, Lin F, Chen Y, et al. Luteolin-7-O-β-d-glucuronide attenuated cerebral ischemia/reperfusion injury: involvement of the blood–brain barrier. Biomedicines. 2024;12:1366.10.3390/biomedicines12061366Search in Google Scholar PubMed PubMed Central
[24] Li L, Pan G, Fan R, Li D, Guo L, Ma L, et al. Luteolin alleviates inflammation and autophagy of hippocampus induced by cerebral ischemia/reperfusion by activating PPAR gamma in rats. BMC Complement Med Ther. 2022;22:176.10.1186/s12906-022-03652-8Search in Google Scholar PubMed PubMed Central
[25] Wu Y, Zhang J, Yu S, Li Y, Zhu J, Zhang K, et al. Cell pyroptosis in health and inflammatory diseases. Cell Death Discov. 2022;8:191.10.1038/s41420-022-00998-3Search in Google Scholar PubMed PubMed Central
[26] Yang F, Wang X, Qi J, Zhang K. Glucagon-like peptide 1 receptor activation inhibits microglial pyroptosis via promoting mitophagy to alleviate depression-like behaviors in diabetic mice. Nutrients. 2022;15:38.10.3390/nu15010038Search in Google Scholar PubMed PubMed Central
[27] Xiao L, Dai Z, Tang W, Liu C, Tang B. Astragaloside IV alleviates cerebral ischemia–reperfusion injury through NLRP3 inflammasome-mediated pyroptosis inhibition via activating Nrf2. Oxid Med Cell Longev. 2021;2021:9925561.10.1155/2021/9925561Search in Google Scholar PubMed PubMed Central
[28] Shi M, Chen J, Liu T. Protective effects of remimazolam on cerebral ischemia/reperfusion injury in rats by inhibiting of NLRP3 inflammasome-dependent pyroptosis. Drug Des Devel Ther. 2022;16:413–23.10.2147/DDDT.S344240Search in Google Scholar PubMed PubMed Central
[29] Bai W, Huo S, Zhou G, Li J, Yang Y, Shao J. Biliverdin modulates the Nrf2/A20/eEF1A2 axis to alleviate cerebral ischemia–reperfusion injury by inhibiting pyroptosis. Biomed Pharmacother. 2023;165:115057.10.1016/j.biopha.2023.115057Search in Google Scholar PubMed
[30] Chen Y, Ma S, Pi D, Wu Y, Zuo Q, Li C, et al. Luteolin induces pyroptosis in HT-29 cells by activating the caspase1/gasdermin D signalling pathway. Front Pharmacol. 2022;13:952587.10.3389/fphar.2022.952587Search in Google Scholar PubMed PubMed Central
[31] Zhang B, Zhang HX, Shi ST, Bai YL, Zhe X, Zhang SJ, et al. Interleukin-11 treatment protected against cerebral ischemia/reperfusion injury. Biomed Pharmacother. 2019;115:108816.10.1016/j.biopha.2019.108816Search in Google Scholar PubMed
[32] Yuan Q, Yuan Y, Zheng Y, Sheng R, Liu L, Xie F, et al. Anti-cerebral ischemia reperfusion injury of polysaccharides: a review of the mechanisms. Biomed Pharmacother. 2021;137:111303.10.1016/j.biopha.2021.111303Search in Google Scholar PubMed
[33] Dong R, Huang R, Shi X, Xu Z, Mang J. Exploration of the mechanism of luteolin against ischemic stroke based on network pharmacology, molecular docking and experimental verification. Bioengineered. 2021;12:12274–93.10.1080/21655979.2021.2006966Search in Google Scholar PubMed PubMed Central
[34] Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Dev Ther. 2015;9:3445–54.10.2147/DDDT.S56071Search in Google Scholar PubMed PubMed Central
[35] Zhang Q, Jia M, Wang Y, Wang Q, Wu J. Cell death mechanisms in cerebral ischemia–reperfusion injury. Neurochem Res. 2022;47:3525–42.10.1007/s11064-022-03697-8Search in Google Scholar PubMed
[36] Zhao N, Gao Y, Jia H, Jiang X. Anti-apoptosis effect of traditional Chinese medicine in the treatment of cerebral ischemia–reperfusion injury. Apoptosis: Int J Program Cell Death. 2023;28:702–29.10.1007/s10495-023-01824-6Search in Google Scholar PubMed
[37] Xie J, Zhang T, Li P, Wang D, Liu T, Xu S. Dihydromyricetin attenuates cerebral ischemia reperfusion injury by inhibiting SPHK1/mTOR signaling and targeting ferroptosis. Drug Des Dev Ther. 2022;16:3071–85.10.2147/DDDT.S378786Search in Google Scholar PubMed PubMed Central
[38] Li Y, Song W, Tong Y, Zhang X, Zhao J, Gao X, et al. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J Neuroinflammation. 2021;18:1.10.1186/s12974-020-02040-8Search in Google Scholar PubMed PubMed Central
[39] Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. 2016;10:56.10.3389/fncel.2016.00056Search in Google Scholar PubMed PubMed Central
[40] Zhou P, Song NC, Zheng ZK, Li YQ, Li JS. MMP2 and MMP9 contribute to lung ischemia–reperfusion injury via promoting pyroptosis in mice. BMC Pulm Med. 2022;22:230.10.1186/s12890-022-02018-7Search in Google Scholar PubMed PubMed Central
[41] Franke M, Bieber M, Kraft P, Weber ANR, Stoll G, Schuhmann MK. The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav Immun. 2021;92:223–33.10.1016/j.bbi.2020.12.009Search in Google Scholar PubMed
[42] Wang L, Ren W, Wu Q, Liu T, Wei Y, Ding J, et al. NLRP3 inflammasome activation: a therapeutic target for cerebral ischemia–reperfusion injury. Front Mol Neurosci. 2022;15:847440.10.3389/fnmol.2022.847440Search in Google Scholar PubMed PubMed Central
[43] Wyles SP, Li X, Hrstka SC, Reyes S, Oommen S, Beraldi R, et al. Modeling structural and functional deficiencies of RBM20 familial dilated cardiomyopathy using human induced pluripotent stem cells. Hum Mol Genet. 2016;25:254–65.10.1093/hmg/ddv468Search in Google Scholar PubMed PubMed Central
[44] Li X, Su Q, Li W, Zhang X, Ran J. Analysis and identification of potential key genes in hepatic ischemia–reperfusion injury. Ann Transl Med. 2022;10:1375.10.21037/atm-22-6171Search in Google Scholar PubMed PubMed Central
[45] Dai Y, Tang J, Zhang JH. Role of Cl- in cerebral vascular tone and expression of Na+–K+–2Cl− co-transporter after neonatal hypoxia–ischemia. Can J Physiol Pharmacol. 2005;83:767–73.10.1139/y05-076Search in Google Scholar PubMed
[46] Kohut JJ, Bednar MM, Kimelberg HK, McAuliffe TL, Gross CE. Reduction in ischemic brain injury in rabbits by the anion transport inhibitor L-644,711. Stroke. 1992;23:93–7.10.1161/01.STR.23.1.93Search in Google Scholar PubMed
[47] Sienel RI, Kataoka H, Kim SW, Seker FB, Plesnila N. Adhesion of leukocytes to cerebral venules precedes neuronal cell death and is sufficient to trigger tissue damage after cerebral ischemia. Front Neurol. 2021;12:807658.10.3389/fneur.2021.807658Search in Google Scholar PubMed PubMed Central
[48] Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57.10.1016/S0092-8674(00)81279-9Search in Google Scholar
[49] Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia: experimental bases and therapeutic perspectives. Arterioscler Thromb Vasc Biol. 1998;18:503–13.10.1161/01.ATV.18.4.503Search in Google Scholar
[50] Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromol Med. 2010;12:193–204.10.1007/s12017-009-8074-1Search in Google Scholar PubMed PubMed Central
[51] Liu J, Guo X, Yang L, Tao T, Cao J, Hong Z, et al. Effect of celastrol on LncRNAs and mRNAs profiles of cerebral ischemia–reperfusion injury in transient middle cerebral artery occlusion mice model. Front Neurosci. 2022;16:889292.10.3389/fnins.2022.889292Search in Google Scholar PubMed PubMed Central
[52] Wang R, Wang M, He S, Sun G, Sun X. Targeting calcium homeostasis in myocardial ischemia/reperfusion injury: an overview of regulatory mechanisms and therapeutic reagents. Front Pharmacol. 2020;11:872.10.3389/fphar.2020.00872Search in Google Scholar PubMed PubMed Central
[53] Sun J, Wang J, Hu L, Yan J. K-3-Rh protects against cerebral ischemia/reperfusion injury by anti-apoptotic effect through PI3K-Akt signaling pathway in rat. Neuropsychiatr Dis Treat. 2020;16:1217–27.10.2147/NDT.S233622Search in Google Scholar PubMed PubMed Central
[54] Li Z, Cao X, Xiao L, Zhou R. Aloperine protects against cerebral ischemia/reperfusion injury via activating the PI3K/AKT signaling pathway in rats. Exp Ther Med. 2021;22:1045.10.3892/etm.2021.10478Search in Google Scholar PubMed PubMed Central
[55] Song T, Zhang Y, Zhu L, Zhang Y, Song J. The role of JAK/STAT signaling pathway in cerebral ischemia–reperfusion injury and the therapeutic effect of traditional Chinese medicine: a narrative review. Medicine (Baltimore). 2023;102:e35890.10.1097/MD.0000000000035890Search in Google Scholar PubMed PubMed Central
[56] Li L, Jiang W, Yu B, Liang H, Mao S, Hu X, et al. Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-κB signaling pathway. Biomed Pharmacother. 2023;168:115653.10.1016/j.biopha.2023.115653Search in Google Scholar PubMed
[57] Guan S, Liu Q, Gu H, Zhang YY, Wei PL, Qi YF, et al. Pluripotent anti-inflammatory immunomodulatory effects of papaverine against cerebral ischemic-reperfusion injury. J Pharmacol Sci. 2020;144:69–75.10.1016/j.jphs.2020.07.008Search in Google Scholar PubMed
[58] Zhang M, Liu Q, Meng H, Duan H, Liu X, Wu J, et al. Ischemia–reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9:12.10.1038/s41392-023-01688-xSearch in Google Scholar PubMed PubMed Central
[59] Chen XY, Wan SF, Yao NN, Lin ZJ, Mao YG, Yu XH, et al. Inhibition of the immunoproteasome LMP2 ameliorates ischemia/hypoxia-induced blood–brain barrier injury through the Wnt/β-catenin signalling pathway. Military Med Res. 2021;8:62.10.1186/s40779-021-00356-xSearch in Google Scholar PubMed PubMed Central
[60] Liu Y, Zhang J, Zan J, Zhang F, Liu G, Wu A. Lidocaine improves cerebral ischemia–reperfusion injury in rats through cAMP/PKA signaling pathway. Exp Ther Med. 2020;20:495–9.10.3892/etm.2020.8688Search in Google Scholar PubMed PubMed Central
[61] Inserte J, Garcia-Dorado D. The cGMP/PKG pathway as a common mediator of cardioprotection: translatability and mechanism. Br J Pharmacol. 2015;172:1996–2009.10.1111/bph.12959Search in Google Scholar PubMed PubMed Central
[62] Morgan M, Thai J, Nencini S, Xu J, Ivanusic JJ. Stomatin-like protein 3 modulates the responses of Aδ, but not C fiber bone afferent neurons to noxious mechanical stimulation in an animal model of acute experimental bone pain. Mol Pain. 2023;19:17448069231222407.10.1177/17448069231222407Search in Google Scholar PubMed PubMed Central
[63] Raveney BJE, Sato W, Takewaki D, Zhang C, Kanazawa T, Lin Y, et al. Involvement of cytotoxic eomes-expressing CD4( +) T cells in secondary progressive multiple sclerosis. Proc Natl Acad Sci U S A. 2021;118:e2021818118.10.1073/pnas.2021818118Search in Google Scholar PubMed PubMed Central
[64] Ma X, Zuo Y, Hu X, Chen S, Zhong K, Xue R, et al. Terminally differentiated cytotoxic CD4(+) T cells were clonally expanded in the brain lesion of radiation-induced brain injury. CNS Neurosci Ther. 2024;30:e14682.10.1111/cns.14682Search in Google Scholar PubMed PubMed Central
[65] Xin S, Mueller C, Pfeiffer S, Kraft VAN, Merl-Pham J, Bao X, et al. MS4A15 drives ferroptosis resistance through calcium-restricted lipid remodeling. Cell Death Differ. 2022;29:670–86.10.1038/s41418-021-00883-zSearch in Google Scholar PubMed PubMed Central
[66] Khaksari M, Maghool F, Asadikaram G, Hajializadeh Z. Effects of sex steroid hormones on neuromedin S and neuromedin U2 receptor expression following experimental traumatic brain injury. Iran J Basic Med Sci. 2016;19:1080–9.Search in Google Scholar
[67] Liz MA, Coelho T, Bellotti V, Fernandez-Arias MI, Mallaina P, Obici L. A narrative review of the role of transthyretin in health and disease. Neurol Ther. 2020;9:395–402.10.1007/s40120-020-00217-0Search in Google Scholar PubMed PubMed Central
[68] Gao BY, Xu DS, Liu PL, Li C, Du L, Hua Y, et al. Modified constraint-induced movement therapy alters synaptic plasticity of rat contralateral hippocampus following middle cerebral artery occlusion. Neural Regen Res. 2020;15:1045–57.10.4103/1673-5374.270312Search in Google Scholar PubMed PubMed Central
[69] Shi J, Li W. CCL2 (C–C motif chemokine ligand 2) biomarker responses in central versus peripheral compartments after focal cerebral ischemia. Stroke. 2021;52:3670–9.10.1161/STROKEAHA.120.032782Search in Google Scholar PubMed PubMed Central
[70] Wu W, Wu G, Cao D. Acteoside presents protective effects on cerebral ischemia/reperfusion injury through targeting CCL2, CXCL10, and ICAM1. Cell Biochem Biophys. 2021;79:301–10.10.1007/s12013-020-00965-8Search in Google Scholar PubMed
[71] Luo J, Chen D, Mei Y, Li H, Qin B, Lin X, et al. Comparative transcriptome findings reveal the neuroinflammatory network and potential biomarkers to early detection of ischemic stroke. J Biol Eng. 2023;17:50.10.1186/s13036-023-00362-8Search in Google Scholar PubMed PubMed Central
[72] Lv LL, Du YT, Chen X, Lei Y, Sun FY. Neuroprotective effect of angiopoietin2 is associated with angiogenesis in mouse brain following ischemic stroke. Brain Sci. 2022;12:1428.10.3390/brainsci12111428Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- EDNRB inhibits the growth and migration of prostate cancer cells by activating the cGMP-PKG pathway
- STK11 (LKB1) mutation suppresses ferroptosis in lung adenocarcinoma by facilitating monounsaturated fatty acid synthesis
- Association of SOX6 gene polymorphisms with Kashin-Beck disease risk in the Chinese Han population
- The pyroptosis-related signature predicts prognosis and influences the tumor immune microenvironment in dedifferentiated liposarcoma
- METTL3 attenuates ferroptosis sensitivity in lung cancer via modulating TFRC
- Identification and validation of molecular subtypes and prognostic signature for stage I and stage II gastric cancer based on neutrophil extracellular traps
- Novel lumbar plexus block versus femoral nerve block for analgesia and motor recovery after total knee arthroplasty
- Correlation between ABCB1 and OLIG2 polymorphisms and the severity and prognosis of patients with cerebral infarction
- Study on the radiotherapy effect and serum neutral granulocyte lymphocyte ratio and inflammatory factor expression of nasopharyngeal carcinoma
- Transcriptome analysis of effects of Tecrl deficiency on cardiometabolic and calcium regulation in cardiac tissue
- Aflatoxin B1 induces infertility, fetal deformities, and potential therapies
- Serum levels of HMW adiponectin and its receptors are associated with cytokine levels and clinical characteristics in chronic obstructive pulmonary disease
- METTL3-mediated methylation of CYP2C19 mRNA may aggravate clopidogrel resistance in ischemic stroke patients
- Understand how machine learning impact lung cancer research from 2010 to 2021: A bibliometric analysis
- Pressure ulcers in German hospitals: Analysis of reimbursement and length of stay
- Metformin plus L-carnitine enhances brown/beige adipose tissue activity via Nrf2/HO-1 signaling to reduce lipid accumulation and inflammation in murine obesity
- Downregulation of carbonic anhydrase IX expression in mouse xenograft nasopharyngeal carcinoma model via doxorubicin nanobubble combined with ultrasound
- Feasibility of 3-dimensional printed models in simulated training and teaching of transcatheter aortic valve replacement
- miR-335-3p improves type II diabetes mellitus by IGF-1 regulating macrophage polarization
- The analyses of human MCPH1 DNA repair machinery and genetic variations
- Activation of Piezo1 increases the sensitivity of breast cancer to hyperthermia therapy
- Comprehensive analysis based on the disulfidptosis-related genes identifies hub genes and immune infiltration for pancreatic adenocarcinoma
- Changes of serum CA125 and PGE2 before and after high-intensity focused ultrasound combined with GnRH-a in treatment of patients with adenomyosis
- The clinical value of the hepatic venous pressure gradient in patients undergoing hepatic resection for hepatocellular carcinoma with or without liver cirrhosis
- Development and validation of a novel model to predict pulmonary embolism in cardiology suspected patients: A 10-year retrospective analysis
- Downregulation of lncRNA XLOC_032768 in diabetic patients predicts the occurrence of diabetic nephropathy
- Circ_0051428 targeting miR-885-3p/MMP2 axis enhances the malignancy of cervical cancer
- Effectiveness of ginkgo diterpene lactone meglumine on cognitive function in patients with acute ischemic stroke
- The construction of a novel prognostic prediction model for glioma based on GWAS-identified prognostic-related risk loci
- Evaluating the impact of childhood BMI on the risk of coronavirus disease 2019: A Mendelian randomization study
- Lactate dehydrogenase to albumin ratio is associated with in-hospital mortality in patients with acute heart failure: Data from the MIMIC-III database
- CD36-mediated podocyte lipotoxicity promotes foot process effacement
- Efficacy of etonogestrel subcutaneous implants versus the levonorgestrel-releasing intrauterine system in the conservative treatment of adenomyosis
- FLRT2 mediates chondrogenesis of nasal septal cartilage and mandibular condyle cartilage
- Challenges in treating primary immune thrombocytopenia patients undergoing COVID-19 vaccination: A retrospective study
- Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway
- Phospholipid transfer protein ameliorates sepsis-induced cardiac dysfunction through NLRP3 inflammasome inhibition
- Postoperative cognitive dysfunction in elderly patients with colorectal cancer: A randomized controlled study comparing goal-directed and conventional fluid therapy
- Long-pulsed ultrasound-mediated microbubble thrombolysis in a rat model of microvascular obstruction
- High SEC61A1 expression predicts poor outcome of acute myeloid leukemia
- Comparison of polymerase chain reaction and next-generation sequencing with conventional urine culture for the diagnosis of urinary tract infections: A meta-analysis
- Secreted frizzled-related protein 5 protects against renal fibrosis by inhibiting Wnt/β-catenin pathway
- Pan-cancer and single-cell analysis of actin cytoskeleton genes related to disulfidptosis
- Overexpression of miR-532-5p restrains oxidative stress response of chondrocytes in nontraumatic osteonecrosis of the femoral head by inhibiting ABL1
- Autologous liver transplantation for unresectable hepatobiliary malignancies in enhanced recovery after surgery model
- Clinical analysis of incomplete rupture of the uterus secondary to previous cesarean section
- Abnormal sleep duration is associated with sarcopenia in older Chinese people: A large retrospective cross-sectional study
- No genetic causality between obesity and benign paroxysmal vertigo: A two-sample Mendelian randomization study
- Identification and validation of autophagy-related genes in SSc
- Long non-coding RNA SRA1 suppresses radiotherapy resistance in esophageal squamous cell carcinoma by modulating glycolytic reprogramming
- Evaluation of quality of life in patients with schizophrenia: An inpatient social welfare institution-based cross-sectional study
- The possible role of oxidative stress marker glutathione in the assessment of cognitive impairment in multiple sclerosis
- Compilation of a self-management assessment scale for postoperative patients with aortic dissection
- Left atrial appendage closure in conjunction with radiofrequency ablation: Effects on left atrial functioning in patients with paroxysmal atrial fibrillation
- Effect of anterior femoral cortical notch grade on postoperative function and complications during TKA surgery: A multicenter, retrospective study
- Clinical characteristics and assessment of risk factors in patients with influenza A-induced severe pneumonia after the prevalence of SARS-CoV-2
- Analgesia nociception index is an indicator of laparoscopic trocar insertion-induced transient nociceptive stimuli
- High STAT4 expression correlates with poor prognosis in acute myeloid leukemia and facilitates disease progression by upregulating VEGFA expression
- Factors influencing cardiovascular system-related post-COVID-19 sequelae: A single-center cohort study
- HOXD10 regulates intestinal permeability and inhibits inflammation of dextran sulfate sodium-induced ulcerative colitis through the inactivation of the Rho/ROCK/MMPs axis
- Mesenchymal stem cell-derived exosomal miR-26a induces ferroptosis, suppresses hepatic stellate cell activation, and ameliorates liver fibrosis by modulating SLC7A11
- Endovascular thrombectomy versus intravenous thrombolysis for primary distal, medium vessel occlusion in acute ischemic stroke
- ANO6 (TMEM16F) inhibits gastrointestinal stromal tumor growth and induces ferroptosis
- Prognostic value of EIF5A2 in solid tumors: A meta-analysis and bioinformatics analysis
- The role of enhanced expression of Cx43 in patients with ulcerative colitis
- Choosing a COVID-19 vaccination site might be driven by anxiety and body vigilance
- Role of ICAM-1 in triple-negative breast cancer
- Cost-effectiveness of ambroxol in the treatment of Gaucher disease type 2
- HLA-DRB5 promotes immune thrombocytopenia via activating CD8+ T cells
- Efficacy and factors of myofascial release therapy combined with electrical and magnetic stimulation in the treatment of chronic pelvic pain syndrome
- Efficacy of tacrolimus monotherapy in primary membranous nephropathy
- Mechanisms of Tripterygium wilfordii Hook F on treating rheumatoid arthritis explored by network pharmacology analysis and molecular docking
- FBXO45 levels regulated ferroptosis renal tubular epithelial cells in a model of diabetic nephropathy by PLK1
- Optimizing anesthesia strategies to NSCLC patients in VATS procedures: Insights from drug requirements and patient recovery patterns
- Alpha-lipoic acid upregulates the PPARγ/NRF2/GPX4 signal pathway to inhibit ferroptosis in the pathogenesis of unexplained recurrent pregnancy loss
- Correlation between fat-soluble vitamin levels and inflammatory factors in paediatric community-acquired pneumonia: A prospective study
- CD1d affects the proliferation, migration, and apoptosis of human papillary thyroid carcinoma TPC-1 cells via regulating MAPK/NF-κB signaling pathway
- miR-let-7a inhibits sympathetic nerve remodeling after myocardial infarction by downregulating the expression of nerve growth factor
- Immune response analysis of solid organ transplantation recipients inoculated with inactivated COVID-19 vaccine: A retrospective analysis
- The H2Valdien derivatives regulate the epithelial–mesenchymal transition of hepatoma carcinoma cells through the Hedgehog signaling pathway
- Clinical efficacy of dexamethasone combined with isoniazid in the treatment of tuberculous meningitis and its effect on peripheral blood T cell subsets
- Comparison of short-segment and long-segment fixation in treatment of degenerative scoliosis and analysis of factors associated with adjacent spondylolisthesis
- Lycopene inhibits pyroptosis of endothelial progenitor cells induced by ox-LDL through the AMPK/mTOR/NLRP3 pathway
- Methylation regulation for FUNDC1 stability in childhood leukemia was up-regulated and facilitates metastasis and reduces ferroptosis of leukemia through mitochondrial damage by FBXL2
- Correlation of single-fiber electromyography studies and functional status in patients with amyotrophic lateral sclerosis
- Risk factors of postoperative airway obstruction complications in children with oral floor mass
- Expression levels and clinical significance of serum miR-19a/CCL20 in patients with acute cerebral infarction
- Physical activity and mental health trends in Korean adolescents: Analyzing the impact of the COVID-19 pandemic from 2018 to 2022
- Evaluating anemia in HIV-infected patients using chest CT
- Ponticulus posticus and skeletal malocclusion: A pilot study in a Southern Italian pre-orthodontic court
- Causal association of circulating immune cells and lymphoma: A Mendelian randomization study
- Assessment of the renal function and fibrosis indexes of conventional western medicine with Chinese medicine for dredging collaterals on treating renal fibrosis: A systematic review and meta-analysis
- Comprehensive landscape of integrator complex subunits and their association with prognosis and tumor microenvironment in gastric cancer
- New target-HMGCR inhibitors for the treatment of primary sclerosing cholangitis: A drug Mendelian randomization study
- Population pharmacokinetics of meropenem in critically ill patients
- Comparison of the ability of newly inflammatory markers to predict complicated appendicitis
- Comparative morphology of the cruciate ligaments: A radiological study
- Immune landscape of hepatocellular carcinoma: The central role of TP53-inducible glycolysis and apoptosis regulator
- Serum SIRT3 levels in epilepsy patients and its association with clinical outcomes and severity: A prospective observational study
- SHP-1 mediates cigarette smoke extract-induced epithelial–mesenchymal transformation and inflammation in 16HBE cells
- Acute hyper-hypoxia accelerates the development of depression in mice via the IL-6/PGC1α/MFN2 signaling pathway
- The GJB3 correlates with the prognosis, immune cell infiltration, and therapeutic responses in lung adenocarcinoma
- Physical fitness and blood parameters outcomes of breast cancer survivor in a low-intensity circuit resistance exercise program
- Exploring anesthetic-induced gene expression changes and immune cell dynamics in atrial tissue post-coronary artery bypass graft surgery
- Empagliflozin improves aortic injury in obese mice by regulating fatty acid metabolism
- Analysis of the risk factors of the radiation-induced encephalopathy in nasopharyngeal carcinoma: A retrospective cohort study
- Reproductive outcomes in women with BRCA 1/2 germline mutations: A retrospective observational study and literature review
- Evaluation of upper airway ultrasonographic measurements in predicting difficult intubation: A cross-section of the Turkish population
- Prognostic and diagnostic value of circulating IGFBP2 in pancreatic cancer
- Postural stability after operative reconstruction of the AFTL in chronic ankle instability comparing three different surgical techniques
- Research trends related to emergence agitation in the post-anaesthesia care unit from 2001 to 2023: A bibliometric analysis
- Frequency and clinicopathological correlation of gastrointestinal polyps: A six-year single center experience
- ACSL4 mediates inflammatory bowel disease and contributes to LPS-induced intestinal epithelial cell dysfunction by activating ferroptosis and inflammation
- Affibody-based molecular probe 99mTc-(HE)3ZHER2:V2 for non-invasive HER2 detection in ovarian and breast cancer xenografts
- Effectiveness of nutritional support for clinical outcomes in gastric cancer patients: A meta-analysis of randomized controlled trials
- The relationship between IFN-γ, IL-10, IL-6 cytokines, and severity of the condition with serum zinc and Fe in children infected with Mycoplasma pneumoniae
- Paraquat disrupts the blood–brain barrier by increasing IL-6 expression and oxidative stress through the activation of PI3K/AKT signaling pathway
- Sleep quality associate with the increased prevalence of cognitive impairment in coronary artery disease patients: A retrospective case–control study
- Dioscin protects against chronic prostatitis through the TLR4/NF-κB pathway
- Association of polymorphisms in FBN1, MYH11, and TGF-β signaling-related genes with susceptibility of sporadic thoracic aortic aneurysm and dissection in the Zhejiang Han population
- Application value of multi-parameter magnetic resonance image-transrectal ultrasound cognitive fusion in prostate biopsy
- Laboratory variables‐based artificial neural network models for predicting fatty liver disease: A retrospective study
- Decreased BIRC5-206 promotes epithelial–mesenchymal transition in nasopharyngeal carcinoma through sponging miR-145-5p
- Sepsis induces the cardiomyocyte apoptosis and cardiac dysfunction through activation of YAP1/Serpine1/caspase-3 pathway
- Assessment of iron metabolism and iron deficiency in incident patients on incident continuous ambulatory peritoneal dialysis
- Tibial periosteum flap combined with autologous bone grafting in the treatment of Gustilo-IIIB/IIIC open tibial fractures
- The application of intravenous general anesthesia under nasopharyngeal airway assisted ventilation undergoing ureteroscopic holmium laser lithotripsy: A prospective, single-center, controlled trial
- Long intergenic noncoding RNA for IGF2BP2 stability suppresses gastric cancer cell apoptosis by inhibiting the maturation of microRNA-34a
- Role of FOXM1 and AURKB in regulating keratinocyte function in psoriasis
- Parental control attitudes over their pre-school children’s diet
- The role of auto-HSCT in extranodal natural killer/T cell lymphoma
- Significance of negative cervical cytology and positive HPV in the diagnosis of cervical lesions by colposcopy
- Echinacoside inhibits PASMCs calcium overload to prevent hypoxic pulmonary artery remodeling by regulating TRPC1/4/6 and calmodulin
- ADAR1 plays a protective role in proximal tubular cells under high glucose conditions by attenuating the PI3K/AKT/mTOR signaling pathway
- The risk of cancer among insulin glargine users in Lithuania: A retrospective population-based study
- The unusual location of primary hydatid cyst: A case series study
- Intraoperative changes in electrophysiological monitoring can be used to predict clinical outcomes in patients with spinal cavernous malformation
- Obesity and risk of placenta accreta spectrum: A meta-analysis
- Shikonin alleviates asthma phenotypes in mice via an airway epithelial STAT3-dependent mechanism
- NSUN6 and HTR7 disturbed the stability of carotid atherosclerotic plaques by regulating the immune responses of macrophages
- The effect of COVID-19 lockdown on admission rates in Maternity Hospital
- Temporal muscle thickness is not a prognostic predictor in patients with high-grade glioma, an experience at two centers in China
- Luteolin alleviates cerebral ischemia/reperfusion injury by regulating cell pyroptosis
- Therapeutic role of respiratory exercise in patients with tuberculous pleurisy
- Effects of CFTR-ENaC on spinal cord edema after spinal cord injury
- Irisin-regulated lncRNAs and their potential regulatory functions in chondrogenic differentiation of human mesenchymal stem cells
- DMD mutations in pediatric patients with phenotypes of Duchenne/Becker muscular dystrophy
- Combination of C-reactive protein and fibrinogen-to-albumin ratio as a novel predictor of all-cause mortality in heart failure patients
- Significant role and the underly mechanism of cullin-1 in chronic obstructive pulmonary disease
- Ferroptosis-related prognostic model of mantle cell lymphoma
- Observation of choking reaction and other related indexes in elderly painless fiberoptic bronchoscopy with transnasal high-flow humidification oxygen therapy
- A bibliometric analysis of Prader-Willi syndrome from 2002 to 2022
- The causal effects of childhood sunburn occasions on melanoma: A univariable and multivariable Mendelian randomization study
- Oxidative stress regulates glycogen synthase kinase-3 in lymphocytes of diabetes mellitus patients complicated with cerebral infarction
- Role of COX6C and NDUFB3 in septic shock and stroke
- Trends in disease burden of type 2 diabetes, stroke, and hypertensive heart disease attributable to high BMI in China: 1990–2019
- Purinergic P2X7 receptor mediates hyperoxia-induced injury in pulmonary microvascular endothelial cells via NLRP3-mediated pyroptotic pathway
- Investigating the role of oviductal mucosa–endometrial co-culture in modulating factors relevant to embryo implantation
- Analgesic effect of external oblique intercostal block in laparoscopic cholecystectomy: A retrospective study
- Elevated serum miR-142-5p correlates with ischemic lesions and both NSE and S100β in ischemic stroke patients
- Correlation between the mechanism of arteriopathy in IgA nephropathy and blood stasis syndrome: A cohort study
- Risk factors for progressive kyphosis after percutaneous kyphoplasty in osteoporotic vertebral compression fracture
- Predictive role of neuron-specific enolase and S100-β in early neurological deterioration and unfavorable prognosis in patients with ischemic stroke
- The potential risk factors of postoperative cognitive dysfunction for endovascular therapy in acute ischemic stroke with general anesthesia
- Fluoxetine inhibited RANKL-induced osteoclastic differentiation in vitro
- Detection of serum FOXM1 and IGF2 in patients with ARDS and their correlation with disease and prognosis
- Rhein promotes skin wound healing by activating the PI3K/AKT signaling pathway
- Differences in mortality risk by levels of physical activity among persons with disabilities in South Korea
- Review Articles
- Cutaneous signs of selected cardiovascular disorders: A narrative review
- XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis
- A narrative review on adverse drug reactions of COVID-19 treatments on the kidney
- Emerging role and function of SPDL1 in human health and diseases
- Adverse reactions of piperacillin: A literature review of case reports
- Molecular mechanism and intervention measures of microvascular complications in diabetes
- Regulation of mesenchymal stem cell differentiation by autophagy
- Molecular landscape of borderline ovarian tumours: A systematic review
- Advances in synthetic lethality modalities for glioblastoma multiforme
- Investigating hormesis, aging, and neurodegeneration: From bench to clinics
- Frankincense: A neuronutrient to approach Parkinson’s disease treatment
- Sox9: A potential regulator of cancer stem cells in osteosarcoma
- Early detection of cardiovascular risk markers through non-invasive ultrasound methodologies in periodontitis patients
- Advanced neuroimaging and criminal interrogation in lie detection
- Maternal factors for neural tube defects in offspring: An umbrella review
- The chemoprotective hormetic effects of rosmarinic acid
- CBD’s potential impact on Parkinson’s disease: An updated overview
- Progress in cytokine research for ARDS: A comprehensive review
- Utilizing reactive oxygen species-scavenging nanoparticles for targeting oxidative stress in the treatment of ischemic stroke: A review
- NRXN1-related disorders, attempt to better define clinical assessment
- Lidocaine infusion for the treatment of complex regional pain syndrome: Case series and literature review
- Trends and future directions of autophagy in osteosarcoma: A bibliometric analysis
- Iron in ventricular remodeling and aneurysms post-myocardial infarction
- Case Reports
- Sirolimus potentiated angioedema: A case report and review of the literature
- Identification of mixed anaerobic infections after inguinal hernia repair based on metagenomic next-generation sequencing: A case report
- Successful treatment with bortezomib in combination with dexamethasone in a middle-aged male with idiopathic multicentric Castleman’s disease: A case report
- Complete heart block associated with hepatitis A infection in a female child with fatal outcome
- Elevation of D-dimer in eosinophilic gastrointestinal diseases in the absence of venous thrombosis: A case series and literature review
- Four years of natural progressive course: A rare case report of juvenile Xp11.2 translocations renal cell carcinoma with TFE3 gene fusion
- Advancing prenatal diagnosis: Echocardiographic detection of Scimitar syndrome in China – A case series
- Outcomes and complications of hemodialysis in patients with renal cancer following bilateral nephrectomy
- Anti-HMGCR myopathy mimicking facioscapulohumeral muscular dystrophy
- Recurrent opportunistic infections in a HIV-negative patient with combined C6 and NFKB1 mutations: A case report, pedigree analysis, and literature review
- Letter to the Editor
- Letter to the Editor: Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Erratum
- Erratum to “Bladder-embedded ectopic intrauterine device with calculus”
- Retraction
- Retraction of “XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis”
- Corrigendum
- Corrigendum to “Investigating hormesis, aging, and neurodegeneration: From bench to clinics”
- Corrigendum to “Frankincense: A neuronutrient to approach Parkinson’s disease treatment”
- Special Issue The evolving saga of RNAs from bench to bedside - Part II
- Machine-learning-based prediction of a diagnostic model using autophagy-related genes based on RNA sequencing for patients with papillary thyroid carcinoma
- Unlocking the future of hepatocellular carcinoma treatment: A comprehensive analysis of disulfidptosis-related lncRNAs for prognosis and drug screening
- Elevated mRNA level indicates FSIP1 promotes EMT and gastric cancer progression by regulating fibroblasts in tumor microenvironment
- Special Issue Advancements in oncology: bridging clinical and experimental research - Part I
- Ultrasound-guided transperineal vs transrectal prostate biopsy: A meta-analysis of diagnostic accuracy and complication rates
- Assessment of diagnostic value of unilateral systematic biopsy combined with targeted biopsy in detecting clinically significant prostate cancer
- SENP7 inhibits glioblastoma metastasis and invasion by dissociating SUMO2/3 binding to specific target proteins
- MARK1 suppress malignant progression of hepatocellular carcinoma and improves sorafenib resistance through negatively regulating POTEE
- Analysis of postoperative complications in bladder cancer patients
- Carboplatin combined with arsenic trioxide versus carboplatin combined with docetaxel treatment for LACC: A randomized, open-label, phase II clinical study
- Special Issue Exploring the biological mechanism of human diseases based on MultiOmics Technology - Part I
- Comprehensive pan-cancer investigation of carnosine dipeptidase 1 and its prospective prognostic significance in hepatocellular carcinoma
- Identification of signatures associated with microsatellite instability and immune characteristics to predict the prognostic risk of colon cancer
- Single-cell analysis identified key macrophage subpopulations associated with atherosclerosis
Articles in the same Issue
- Research Articles
- EDNRB inhibits the growth and migration of prostate cancer cells by activating the cGMP-PKG pathway
- STK11 (LKB1) mutation suppresses ferroptosis in lung adenocarcinoma by facilitating monounsaturated fatty acid synthesis
- Association of SOX6 gene polymorphisms with Kashin-Beck disease risk in the Chinese Han population
- The pyroptosis-related signature predicts prognosis and influences the tumor immune microenvironment in dedifferentiated liposarcoma
- METTL3 attenuates ferroptosis sensitivity in lung cancer via modulating TFRC
- Identification and validation of molecular subtypes and prognostic signature for stage I and stage II gastric cancer based on neutrophil extracellular traps
- Novel lumbar plexus block versus femoral nerve block for analgesia and motor recovery after total knee arthroplasty
- Correlation between ABCB1 and OLIG2 polymorphisms and the severity and prognosis of patients with cerebral infarction
- Study on the radiotherapy effect and serum neutral granulocyte lymphocyte ratio and inflammatory factor expression of nasopharyngeal carcinoma
- Transcriptome analysis of effects of Tecrl deficiency on cardiometabolic and calcium regulation in cardiac tissue
- Aflatoxin B1 induces infertility, fetal deformities, and potential therapies
- Serum levels of HMW adiponectin and its receptors are associated with cytokine levels and clinical characteristics in chronic obstructive pulmonary disease
- METTL3-mediated methylation of CYP2C19 mRNA may aggravate clopidogrel resistance in ischemic stroke patients
- Understand how machine learning impact lung cancer research from 2010 to 2021: A bibliometric analysis
- Pressure ulcers in German hospitals: Analysis of reimbursement and length of stay
- Metformin plus L-carnitine enhances brown/beige adipose tissue activity via Nrf2/HO-1 signaling to reduce lipid accumulation and inflammation in murine obesity
- Downregulation of carbonic anhydrase IX expression in mouse xenograft nasopharyngeal carcinoma model via doxorubicin nanobubble combined with ultrasound
- Feasibility of 3-dimensional printed models in simulated training and teaching of transcatheter aortic valve replacement
- miR-335-3p improves type II diabetes mellitus by IGF-1 regulating macrophage polarization
- The analyses of human MCPH1 DNA repair machinery and genetic variations
- Activation of Piezo1 increases the sensitivity of breast cancer to hyperthermia therapy
- Comprehensive analysis based on the disulfidptosis-related genes identifies hub genes and immune infiltration for pancreatic adenocarcinoma
- Changes of serum CA125 and PGE2 before and after high-intensity focused ultrasound combined with GnRH-a in treatment of patients with adenomyosis
- The clinical value of the hepatic venous pressure gradient in patients undergoing hepatic resection for hepatocellular carcinoma with or without liver cirrhosis
- Development and validation of a novel model to predict pulmonary embolism in cardiology suspected patients: A 10-year retrospective analysis
- Downregulation of lncRNA XLOC_032768 in diabetic patients predicts the occurrence of diabetic nephropathy
- Circ_0051428 targeting miR-885-3p/MMP2 axis enhances the malignancy of cervical cancer
- Effectiveness of ginkgo diterpene lactone meglumine on cognitive function in patients with acute ischemic stroke
- The construction of a novel prognostic prediction model for glioma based on GWAS-identified prognostic-related risk loci
- Evaluating the impact of childhood BMI on the risk of coronavirus disease 2019: A Mendelian randomization study
- Lactate dehydrogenase to albumin ratio is associated with in-hospital mortality in patients with acute heart failure: Data from the MIMIC-III database
- CD36-mediated podocyte lipotoxicity promotes foot process effacement
- Efficacy of etonogestrel subcutaneous implants versus the levonorgestrel-releasing intrauterine system in the conservative treatment of adenomyosis
- FLRT2 mediates chondrogenesis of nasal septal cartilage and mandibular condyle cartilage
- Challenges in treating primary immune thrombocytopenia patients undergoing COVID-19 vaccination: A retrospective study
- Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway
- Phospholipid transfer protein ameliorates sepsis-induced cardiac dysfunction through NLRP3 inflammasome inhibition
- Postoperative cognitive dysfunction in elderly patients with colorectal cancer: A randomized controlled study comparing goal-directed and conventional fluid therapy
- Long-pulsed ultrasound-mediated microbubble thrombolysis in a rat model of microvascular obstruction
- High SEC61A1 expression predicts poor outcome of acute myeloid leukemia
- Comparison of polymerase chain reaction and next-generation sequencing with conventional urine culture for the diagnosis of urinary tract infections: A meta-analysis
- Secreted frizzled-related protein 5 protects against renal fibrosis by inhibiting Wnt/β-catenin pathway
- Pan-cancer and single-cell analysis of actin cytoskeleton genes related to disulfidptosis
- Overexpression of miR-532-5p restrains oxidative stress response of chondrocytes in nontraumatic osteonecrosis of the femoral head by inhibiting ABL1
- Autologous liver transplantation for unresectable hepatobiliary malignancies in enhanced recovery after surgery model
- Clinical analysis of incomplete rupture of the uterus secondary to previous cesarean section
- Abnormal sleep duration is associated with sarcopenia in older Chinese people: A large retrospective cross-sectional study
- No genetic causality between obesity and benign paroxysmal vertigo: A two-sample Mendelian randomization study
- Identification and validation of autophagy-related genes in SSc
- Long non-coding RNA SRA1 suppresses radiotherapy resistance in esophageal squamous cell carcinoma by modulating glycolytic reprogramming
- Evaluation of quality of life in patients with schizophrenia: An inpatient social welfare institution-based cross-sectional study
- The possible role of oxidative stress marker glutathione in the assessment of cognitive impairment in multiple sclerosis
- Compilation of a self-management assessment scale for postoperative patients with aortic dissection
- Left atrial appendage closure in conjunction with radiofrequency ablation: Effects on left atrial functioning in patients with paroxysmal atrial fibrillation
- Effect of anterior femoral cortical notch grade on postoperative function and complications during TKA surgery: A multicenter, retrospective study
- Clinical characteristics and assessment of risk factors in patients with influenza A-induced severe pneumonia after the prevalence of SARS-CoV-2
- Analgesia nociception index is an indicator of laparoscopic trocar insertion-induced transient nociceptive stimuli
- High STAT4 expression correlates with poor prognosis in acute myeloid leukemia and facilitates disease progression by upregulating VEGFA expression
- Factors influencing cardiovascular system-related post-COVID-19 sequelae: A single-center cohort study
- HOXD10 regulates intestinal permeability and inhibits inflammation of dextran sulfate sodium-induced ulcerative colitis through the inactivation of the Rho/ROCK/MMPs axis
- Mesenchymal stem cell-derived exosomal miR-26a induces ferroptosis, suppresses hepatic stellate cell activation, and ameliorates liver fibrosis by modulating SLC7A11
- Endovascular thrombectomy versus intravenous thrombolysis for primary distal, medium vessel occlusion in acute ischemic stroke
- ANO6 (TMEM16F) inhibits gastrointestinal stromal tumor growth and induces ferroptosis
- Prognostic value of EIF5A2 in solid tumors: A meta-analysis and bioinformatics analysis
- The role of enhanced expression of Cx43 in patients with ulcerative colitis
- Choosing a COVID-19 vaccination site might be driven by anxiety and body vigilance
- Role of ICAM-1 in triple-negative breast cancer
- Cost-effectiveness of ambroxol in the treatment of Gaucher disease type 2
- HLA-DRB5 promotes immune thrombocytopenia via activating CD8+ T cells
- Efficacy and factors of myofascial release therapy combined with electrical and magnetic stimulation in the treatment of chronic pelvic pain syndrome
- Efficacy of tacrolimus monotherapy in primary membranous nephropathy
- Mechanisms of Tripterygium wilfordii Hook F on treating rheumatoid arthritis explored by network pharmacology analysis and molecular docking
- FBXO45 levels regulated ferroptosis renal tubular epithelial cells in a model of diabetic nephropathy by PLK1
- Optimizing anesthesia strategies to NSCLC patients in VATS procedures: Insights from drug requirements and patient recovery patterns
- Alpha-lipoic acid upregulates the PPARγ/NRF2/GPX4 signal pathway to inhibit ferroptosis in the pathogenesis of unexplained recurrent pregnancy loss
- Correlation between fat-soluble vitamin levels and inflammatory factors in paediatric community-acquired pneumonia: A prospective study
- CD1d affects the proliferation, migration, and apoptosis of human papillary thyroid carcinoma TPC-1 cells via regulating MAPK/NF-κB signaling pathway
- miR-let-7a inhibits sympathetic nerve remodeling after myocardial infarction by downregulating the expression of nerve growth factor
- Immune response analysis of solid organ transplantation recipients inoculated with inactivated COVID-19 vaccine: A retrospective analysis
- The H2Valdien derivatives regulate the epithelial–mesenchymal transition of hepatoma carcinoma cells through the Hedgehog signaling pathway
- Clinical efficacy of dexamethasone combined with isoniazid in the treatment of tuberculous meningitis and its effect on peripheral blood T cell subsets
- Comparison of short-segment and long-segment fixation in treatment of degenerative scoliosis and analysis of factors associated with adjacent spondylolisthesis
- Lycopene inhibits pyroptosis of endothelial progenitor cells induced by ox-LDL through the AMPK/mTOR/NLRP3 pathway
- Methylation regulation for FUNDC1 stability in childhood leukemia was up-regulated and facilitates metastasis and reduces ferroptosis of leukemia through mitochondrial damage by FBXL2
- Correlation of single-fiber electromyography studies and functional status in patients with amyotrophic lateral sclerosis
- Risk factors of postoperative airway obstruction complications in children with oral floor mass
- Expression levels and clinical significance of serum miR-19a/CCL20 in patients with acute cerebral infarction
- Physical activity and mental health trends in Korean adolescents: Analyzing the impact of the COVID-19 pandemic from 2018 to 2022
- Evaluating anemia in HIV-infected patients using chest CT
- Ponticulus posticus and skeletal malocclusion: A pilot study in a Southern Italian pre-orthodontic court
- Causal association of circulating immune cells and lymphoma: A Mendelian randomization study
- Assessment of the renal function and fibrosis indexes of conventional western medicine with Chinese medicine for dredging collaterals on treating renal fibrosis: A systematic review and meta-analysis
- Comprehensive landscape of integrator complex subunits and their association with prognosis and tumor microenvironment in gastric cancer
- New target-HMGCR inhibitors for the treatment of primary sclerosing cholangitis: A drug Mendelian randomization study
- Population pharmacokinetics of meropenem in critically ill patients
- Comparison of the ability of newly inflammatory markers to predict complicated appendicitis
- Comparative morphology of the cruciate ligaments: A radiological study
- Immune landscape of hepatocellular carcinoma: The central role of TP53-inducible glycolysis and apoptosis regulator
- Serum SIRT3 levels in epilepsy patients and its association with clinical outcomes and severity: A prospective observational study
- SHP-1 mediates cigarette smoke extract-induced epithelial–mesenchymal transformation and inflammation in 16HBE cells
- Acute hyper-hypoxia accelerates the development of depression in mice via the IL-6/PGC1α/MFN2 signaling pathway
- The GJB3 correlates with the prognosis, immune cell infiltration, and therapeutic responses in lung adenocarcinoma
- Physical fitness and blood parameters outcomes of breast cancer survivor in a low-intensity circuit resistance exercise program
- Exploring anesthetic-induced gene expression changes and immune cell dynamics in atrial tissue post-coronary artery bypass graft surgery
- Empagliflozin improves aortic injury in obese mice by regulating fatty acid metabolism
- Analysis of the risk factors of the radiation-induced encephalopathy in nasopharyngeal carcinoma: A retrospective cohort study
- Reproductive outcomes in women with BRCA 1/2 germline mutations: A retrospective observational study and literature review
- Evaluation of upper airway ultrasonographic measurements in predicting difficult intubation: A cross-section of the Turkish population
- Prognostic and diagnostic value of circulating IGFBP2 in pancreatic cancer
- Postural stability after operative reconstruction of the AFTL in chronic ankle instability comparing three different surgical techniques
- Research trends related to emergence agitation in the post-anaesthesia care unit from 2001 to 2023: A bibliometric analysis
- Frequency and clinicopathological correlation of gastrointestinal polyps: A six-year single center experience
- ACSL4 mediates inflammatory bowel disease and contributes to LPS-induced intestinal epithelial cell dysfunction by activating ferroptosis and inflammation
- Affibody-based molecular probe 99mTc-(HE)3ZHER2:V2 for non-invasive HER2 detection in ovarian and breast cancer xenografts
- Effectiveness of nutritional support for clinical outcomes in gastric cancer patients: A meta-analysis of randomized controlled trials
- The relationship between IFN-γ, IL-10, IL-6 cytokines, and severity of the condition with serum zinc and Fe in children infected with Mycoplasma pneumoniae
- Paraquat disrupts the blood–brain barrier by increasing IL-6 expression and oxidative stress through the activation of PI3K/AKT signaling pathway
- Sleep quality associate with the increased prevalence of cognitive impairment in coronary artery disease patients: A retrospective case–control study
- Dioscin protects against chronic prostatitis through the TLR4/NF-κB pathway
- Association of polymorphisms in FBN1, MYH11, and TGF-β signaling-related genes with susceptibility of sporadic thoracic aortic aneurysm and dissection in the Zhejiang Han population
- Application value of multi-parameter magnetic resonance image-transrectal ultrasound cognitive fusion in prostate biopsy
- Laboratory variables‐based artificial neural network models for predicting fatty liver disease: A retrospective study
- Decreased BIRC5-206 promotes epithelial–mesenchymal transition in nasopharyngeal carcinoma through sponging miR-145-5p
- Sepsis induces the cardiomyocyte apoptosis and cardiac dysfunction through activation of YAP1/Serpine1/caspase-3 pathway
- Assessment of iron metabolism and iron deficiency in incident patients on incident continuous ambulatory peritoneal dialysis
- Tibial periosteum flap combined with autologous bone grafting in the treatment of Gustilo-IIIB/IIIC open tibial fractures
- The application of intravenous general anesthesia under nasopharyngeal airway assisted ventilation undergoing ureteroscopic holmium laser lithotripsy: A prospective, single-center, controlled trial
- Long intergenic noncoding RNA for IGF2BP2 stability suppresses gastric cancer cell apoptosis by inhibiting the maturation of microRNA-34a
- Role of FOXM1 and AURKB in regulating keratinocyte function in psoriasis
- Parental control attitudes over their pre-school children’s diet
- The role of auto-HSCT in extranodal natural killer/T cell lymphoma
- Significance of negative cervical cytology and positive HPV in the diagnosis of cervical lesions by colposcopy
- Echinacoside inhibits PASMCs calcium overload to prevent hypoxic pulmonary artery remodeling by regulating TRPC1/4/6 and calmodulin
- ADAR1 plays a protective role in proximal tubular cells under high glucose conditions by attenuating the PI3K/AKT/mTOR signaling pathway
- The risk of cancer among insulin glargine users in Lithuania: A retrospective population-based study
- The unusual location of primary hydatid cyst: A case series study
- Intraoperative changes in electrophysiological monitoring can be used to predict clinical outcomes in patients with spinal cavernous malformation
- Obesity and risk of placenta accreta spectrum: A meta-analysis
- Shikonin alleviates asthma phenotypes in mice via an airway epithelial STAT3-dependent mechanism
- NSUN6 and HTR7 disturbed the stability of carotid atherosclerotic plaques by regulating the immune responses of macrophages
- The effect of COVID-19 lockdown on admission rates in Maternity Hospital
- Temporal muscle thickness is not a prognostic predictor in patients with high-grade glioma, an experience at two centers in China
- Luteolin alleviates cerebral ischemia/reperfusion injury by regulating cell pyroptosis
- Therapeutic role of respiratory exercise in patients with tuberculous pleurisy
- Effects of CFTR-ENaC on spinal cord edema after spinal cord injury
- Irisin-regulated lncRNAs and their potential regulatory functions in chondrogenic differentiation of human mesenchymal stem cells
- DMD mutations in pediatric patients with phenotypes of Duchenne/Becker muscular dystrophy
- Combination of C-reactive protein and fibrinogen-to-albumin ratio as a novel predictor of all-cause mortality in heart failure patients
- Significant role and the underly mechanism of cullin-1 in chronic obstructive pulmonary disease
- Ferroptosis-related prognostic model of mantle cell lymphoma
- Observation of choking reaction and other related indexes in elderly painless fiberoptic bronchoscopy with transnasal high-flow humidification oxygen therapy
- A bibliometric analysis of Prader-Willi syndrome from 2002 to 2022
- The causal effects of childhood sunburn occasions on melanoma: A univariable and multivariable Mendelian randomization study
- Oxidative stress regulates glycogen synthase kinase-3 in lymphocytes of diabetes mellitus patients complicated with cerebral infarction
- Role of COX6C and NDUFB3 in septic shock and stroke
- Trends in disease burden of type 2 diabetes, stroke, and hypertensive heart disease attributable to high BMI in China: 1990–2019
- Purinergic P2X7 receptor mediates hyperoxia-induced injury in pulmonary microvascular endothelial cells via NLRP3-mediated pyroptotic pathway
- Investigating the role of oviductal mucosa–endometrial co-culture in modulating factors relevant to embryo implantation
- Analgesic effect of external oblique intercostal block in laparoscopic cholecystectomy: A retrospective study
- Elevated serum miR-142-5p correlates with ischemic lesions and both NSE and S100β in ischemic stroke patients
- Correlation between the mechanism of arteriopathy in IgA nephropathy and blood stasis syndrome: A cohort study
- Risk factors for progressive kyphosis after percutaneous kyphoplasty in osteoporotic vertebral compression fracture
- Predictive role of neuron-specific enolase and S100-β in early neurological deterioration and unfavorable prognosis in patients with ischemic stroke
- The potential risk factors of postoperative cognitive dysfunction for endovascular therapy in acute ischemic stroke with general anesthesia
- Fluoxetine inhibited RANKL-induced osteoclastic differentiation in vitro
- Detection of serum FOXM1 and IGF2 in patients with ARDS and their correlation with disease and prognosis
- Rhein promotes skin wound healing by activating the PI3K/AKT signaling pathway
- Differences in mortality risk by levels of physical activity among persons with disabilities in South Korea
- Review Articles
- Cutaneous signs of selected cardiovascular disorders: A narrative review
- XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis
- A narrative review on adverse drug reactions of COVID-19 treatments on the kidney
- Emerging role and function of SPDL1 in human health and diseases
- Adverse reactions of piperacillin: A literature review of case reports
- Molecular mechanism and intervention measures of microvascular complications in diabetes
- Regulation of mesenchymal stem cell differentiation by autophagy
- Molecular landscape of borderline ovarian tumours: A systematic review
- Advances in synthetic lethality modalities for glioblastoma multiforme
- Investigating hormesis, aging, and neurodegeneration: From bench to clinics
- Frankincense: A neuronutrient to approach Parkinson’s disease treatment
- Sox9: A potential regulator of cancer stem cells in osteosarcoma
- Early detection of cardiovascular risk markers through non-invasive ultrasound methodologies in periodontitis patients
- Advanced neuroimaging and criminal interrogation in lie detection
- Maternal factors for neural tube defects in offspring: An umbrella review
- The chemoprotective hormetic effects of rosmarinic acid
- CBD’s potential impact on Parkinson’s disease: An updated overview
- Progress in cytokine research for ARDS: A comprehensive review
- Utilizing reactive oxygen species-scavenging nanoparticles for targeting oxidative stress in the treatment of ischemic stroke: A review
- NRXN1-related disorders, attempt to better define clinical assessment
- Lidocaine infusion for the treatment of complex regional pain syndrome: Case series and literature review
- Trends and future directions of autophagy in osteosarcoma: A bibliometric analysis
- Iron in ventricular remodeling and aneurysms post-myocardial infarction
- Case Reports
- Sirolimus potentiated angioedema: A case report and review of the literature
- Identification of mixed anaerobic infections after inguinal hernia repair based on metagenomic next-generation sequencing: A case report
- Successful treatment with bortezomib in combination with dexamethasone in a middle-aged male with idiopathic multicentric Castleman’s disease: A case report
- Complete heart block associated with hepatitis A infection in a female child with fatal outcome
- Elevation of D-dimer in eosinophilic gastrointestinal diseases in the absence of venous thrombosis: A case series and literature review
- Four years of natural progressive course: A rare case report of juvenile Xp11.2 translocations renal cell carcinoma with TFE3 gene fusion
- Advancing prenatal diagnosis: Echocardiographic detection of Scimitar syndrome in China – A case series
- Outcomes and complications of hemodialysis in patients with renal cancer following bilateral nephrectomy
- Anti-HMGCR myopathy mimicking facioscapulohumeral muscular dystrophy
- Recurrent opportunistic infections in a HIV-negative patient with combined C6 and NFKB1 mutations: A case report, pedigree analysis, and literature review
- Letter to the Editor
- Letter to the Editor: Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Erratum
- Erratum to “Bladder-embedded ectopic intrauterine device with calculus”
- Retraction
- Retraction of “XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis”
- Corrigendum
- Corrigendum to “Investigating hormesis, aging, and neurodegeneration: From bench to clinics”
- Corrigendum to “Frankincense: A neuronutrient to approach Parkinson’s disease treatment”
- Special Issue The evolving saga of RNAs from bench to bedside - Part II
- Machine-learning-based prediction of a diagnostic model using autophagy-related genes based on RNA sequencing for patients with papillary thyroid carcinoma
- Unlocking the future of hepatocellular carcinoma treatment: A comprehensive analysis of disulfidptosis-related lncRNAs for prognosis and drug screening
- Elevated mRNA level indicates FSIP1 promotes EMT and gastric cancer progression by regulating fibroblasts in tumor microenvironment
- Special Issue Advancements in oncology: bridging clinical and experimental research - Part I
- Ultrasound-guided transperineal vs transrectal prostate biopsy: A meta-analysis of diagnostic accuracy and complication rates
- Assessment of diagnostic value of unilateral systematic biopsy combined with targeted biopsy in detecting clinically significant prostate cancer
- SENP7 inhibits glioblastoma metastasis and invasion by dissociating SUMO2/3 binding to specific target proteins
- MARK1 suppress malignant progression of hepatocellular carcinoma and improves sorafenib resistance through negatively regulating POTEE
- Analysis of postoperative complications in bladder cancer patients
- Carboplatin combined with arsenic trioxide versus carboplatin combined with docetaxel treatment for LACC: A randomized, open-label, phase II clinical study
- Special Issue Exploring the biological mechanism of human diseases based on MultiOmics Technology - Part I
- Comprehensive pan-cancer investigation of carnosine dipeptidase 1 and its prospective prognostic significance in hepatocellular carcinoma
- Identification of signatures associated with microsatellite instability and immune characteristics to predict the prognostic risk of colon cancer
- Single-cell analysis identified key macrophage subpopulations associated with atherosclerosis

