Abstract
Aflatoxin B1 (AFB1) is a subsidiary poisonous metabolite, archetypally spawned by Aspergillus flavus and A. parasiticus, which are often isolated in warm or tropical countries across the world. AFB1 is capable of disrupting the functioning of several reproductive endocrine glands by interrupting the enzymes and their substrates that are liable for the synthesis of various hormones in both males and females. In men, AFB1 is capable of hindering testicular development, testicular degeneration, and reduces reproductive capabilities. In women, a direct antagonistic interaction of AFB1 with steroid hormone receptors influencing gonadal hormone production of estrogen and progesterone was responsible for AFB1-associated infertility. AFB1 is potentially teratogenic and is responsible for the development of malformation in humans and animals. Soft-tissue anomalies such as internal hydrocephalus, microphthalmia, cardiac defects, augmented liver lobes, reproductive changes, immune modifications, behavioral changes and predisposition of animals and humans to neoplasm development are AFB1-associated anomalies. Substances such as esculin, selenium, gynandra extract, vitamins C and E, oltipraz, and CDDO-Im are potential therapies for AFB1. Thus, this review elucidates the pivotal pathogenic roles of AFB1 in infertility, fetal deformities, and potential therapies because AFB1 toxicity is a key problem globally.
1 Introduction
Aflatoxin B1 (AFB1) is a subsidiary poisonous metabolite, archetypally spawned by Aspergillus flavus as well as A. parasiticus [1,2,3,4]. These species of molds are routinely isolated in warm or tropical countries across the world [1,5]. This type of fungi is extremely pathogenic to animals and humans [6]. The name B, “blue,” was adopted based on the blue light of ultraviolet (UV) radiation [6]. AFB1 has been isolated in various types of foods, such as cereals (corn, wheat, millet, and rice), oleaginous plants (soya, sunflower, and cotton), spices (chilly, black pepper and coriander), nut fruits (pistachio, coconut, almond, etc.), dried fruits and vegetables, milk, and other meat products [7,6,8,9,10,11,12].
It is worth noting that approximately 4.5 billion of the world’s population is exposed to aflatoxins [9,10]. Underdeveloped and poor countries in Africa and Asia have registered acute toxicity of AFB1 in humans, and chronic toxicity has been observed in people who consume food with minute concentrations of AFB1 contained in organisms [5,8–10]. Thus, the American Food and Drug Administration and the European Union have legally regulated normal or abnormal concentrations of aflatoxins in animal as well as human foods [13,14]. Notably, AFB1-contaminated food is often ingested via mouth into the gastrointestinal system through which the toxins gain access to targeted organs via the blood stream.
AFB1 has been implicated as a pathogenic factor in child underweight, neurologic injuries, hypoimmunity, cancer such as hepatocellular as well as high mortality [15,16]. Globally, human fertility is diminishing, a state that cannot be ascribed exclusively to an upsurge in contraception [9,17]. It was observed that in about 30% of infertility, pathology originated from men alone, and in another 20%, the pathology often originated from both men and women [18]. Thus, the male factor is accountable for infertility in about 50% of patients who present at the clinic [18]. However, there is currently no data suggesting that AFB1 affects males than females or vice versa in the clinic.
Also, regression in semen quality has a substantial negative influence on male fertility and is thus a public health concern. Also, follicle growth and atresia in the ovaries and fallopian tubes, resulting in severe infertility, are also major public health concerns. Environmental factors have been implicated as a major cause of regression in semen quality, follicle growth, and atresia of the ovaries and fallopian tubes, although the precise triggers are still a matter of debate [9,19]. Thus, this review elucidates the pivotal pathogenic roles of AFB1 in infertility, fetal deformities, and potential therapies.
The “Boolean logic” was used to search for articles on the subject matter in PubMed and PubMed central as well as Google scholar with search terms like AFB1 and associated diseases in humans and animals, AFB1 and infertility in humans and animals, AFB1 and reproductive hormones, AFB1 and deformities, and potential therapies to AFB1. Studies involving both humans and animals were included. Also, findings from both clinical research and basic research were critically reviewed.
2 AFB1 induces diseases in humans and animals
Interestingly, AFB1 has been linked to hepatotoxic, nephrotoxic, genotoxic, mutagenic, and immunotoxic [20,21]. Also, AFB1 triggered an immunosuppressive effect, resulting in a decrease in the natural and acquired resistance to diseases when ingested at very low levels [22,23]. Notably, AFB1 has been detected in the blood during the acute phase of illness after exposure, as well as in the liver of affected children [24–27]. Nevertheless, use of aspirin or phenothiazines was also assumed to be associated with the etiology [28]. Furthermore, AFB1 has been isolated in the blood of pregnant women, in neonatal umbilical cord blood, and in breast milk in African countries, with cyclical disparities [25,29]. Also, the highest concentrations of AFB1 ever observed in human tissue and fluids were detected in the umbilical cord blood at birth [24], which signify that AFB1 crosses the placenta and could trigger fetal deformities.
Intriguingly, AFB1 has been implicated as the etiological factor in encephalopathy and fatty degeneration of viscera, analogous to Reye syndrome in countries with a hot and humid climate [24,30]. Also, patients presented with pale, fatty liver, enlarged kidneys, and severe cerebral edema [24]. Notably, aflatoxicol was detected in the serum, liver, urine, and stools of children with kwashiorkor and marasmic kwashiorkor compared to marasmus and control children, where this metabolite was not detectable [24]. Moreover, as high as 80% of AFB1 was detected in the serum and 46% in the urine of infants with kwashiorkor and marasmus [31].
Remarkably, children with kwashiorkor and marasmic kwashiorkor who were fed an AFB1-free diet, had a very slow elimination of AFB1 during clinical investigations [32]. Also, AFB1 was isolated in the brain and lungs of children who had died from kwashiorkor, as well as children who had died from diverse diseases [24,27,33]. Furthermore, AFB1 was detected in the lungs of all children who suffered from pneumonia, regardless of the presence of kwashiorkor [24]. This may be due to a decrease in the eliminatory ability of the lungs in pulmonary diseases and/or due to contact via the respiratory route [24].
Interestingly, a correlation study on the existence of AFB1 in the serum and urine of children and the prognosis of acute lower respiratory infection did not yield any association [34]. Also, AFB1 was detected in the lungs of some textile workers and farmers who died from pulmonary interstitial fibrosis [24,35]. Furthermore, acute human aflatoxicosis was associated with liver failure and gastrointestinal bleeding in Southeast Asia and Africa [24]. Notably, AFB1 was connected to a specific AGG to AGT amino acid transversion mutation at codon 249 of the p53 gene in human hepatocellular carcinoma, specifying mechanistic evidence to a causal link between exposure and disease [36].
Interestingly, AFB1 was capable of triggering acute hepatic injury, resulting in the elevation of serum enzymes, such as lactate dehydrogenase, aspartate aminotransferase, glutamate dehydrogenase, gamma-glutamyltransferase, and alkaline phosphatase [37]. Furthermore, bilirubin, which indicates liver damage, and other biochemical factors like proteinuria, ketonuria, glycosuria, and hematuria were elevated in AFB1-triggered acute live injury [37]. Moreover, about 72% of kids had perceptible concentrations of AFB1-lys in their plasma at 24 months of age. However, no relation was established between the low AFB1 ingestions and growth impairment [38].
Moreover, the focal severe distraction of the renal cortex, which was not only limited to the renal tubules but also stretched into the renal corpuscles, resulting in a wide gap in the urinary spaces, has been associated with AFB1 [24]. It is worth noting that augmented collagen deposition and focal mononuclear cell infiltration were detected in renal system after AFB1 administration [39]. Furthermore, it was observed that AFB1 triggered focal necrosis and degeneration, predominantly at the renal tubules [40]. Also, AFB1 was capable of triggering lymphocytic infiltration, necrosis, and steatosis in the liver of ducklings [41].
Intriguingly, AFB1 was capable of triggering aberrations in the mitochondrial DNA of brain cells, resulting in malfunctioning oxidative phosphorylation [42,43]. The defective oxidative injury resulted in disruptions in key cellular macromolecules, such as DNA, lipids, and proteins [42,43]. Also, cellular fatty acids are freely oxidized by reactive oxygen species (ROS) induced by AFB1 to generate lipid peroxyl radicals, which in turn proliferate into malondialdehyde (MDA), and the resultant MDA interrelates with cellular DNA to form DNA–MDA, which influences the generation of energy in the brain [42,43]. Notably, AFB1 has been implicated to influence ovarian secretory cells via the hypothalamus–hypophysis–ovary axis [44].
Studies on the effect of AFB1 and key reproductive hormones mediated by brain regions are insufficient. Also, it is worth noting that via the above organs and associated diseases, AFB1 may migrate to productive organs to induce infertility. Thus, correlation studies between the above diseases and infertility are warranted.
3 Isolation of AFB1 in reproductive organs
Notably, mice fed with AFB1 diet showed histological alterations like germ cell loss in their testis, while aflatoxicosis was associated with reduction in sperm production and augmented sperm abnormalities in male mice [45–47]. Also, AFB1 exposure in the testis of male mouse triggered a reduction in sperm concentration as well as motility and an upsurge in aberrations leading to reduced fertility in the mice [46]. Notably, AFB1 associated spermatogenesis, with almost total absence of spermatids engaging in spermiogenesis, accompanied by the loss of immature germ cells leading to decreased sperm count, was detected histologically [46]. Interestingly, these pathological changes triggered by AFB1 in the testis were observed in the Leydig cells mice. Also, major histopathological changes in the epididymis were associated with AFB1 in the testis of mice [48]. Thus, a direct toxicity of AFB1 to the spermatogenic compartment is the key mechanism of action of AFB1 in the stimulation of abnormal sperms. Remarkably, AFB1 was associated with substantial upsurge in oxidative stress markers and reduction in anti-oxidant enzymes in the testicles of rats [49].
Notably, isolated AFB1 in the ovaries triggered damage in the ovary and increased the risk of ovarian disease; moreover, zearalenone has a dual effect on ovarian toxicity induced by AFB1 [50]. In the rat ovaries, the detected AFB1 inhibited follicle growth and atresia in the ovaries resulting in severe infertility [51]. Interestingly, the detected AFB1 triggered multiple signaling pathways in the ovary and induced oxidative stress, affecting genes associated with sterol, amino acid, and lipid synthesis during transcriptomic analysis [50]. Interestingly, the detected AFB1 in the ovaries inhibited the growth of oocytes, reduced the ovary size and weight, reduced oestradiol-17β concentration, and increased progesterone concentration in blood after AFB1 administration in female rats [44,52]. It is well established that mycotoxins are capable of crossing the placental barrier and have already been isolated in human umbilical cord samples [53,54]. Interestingly, injection of a single dose of AFB1 in the pre-implantation period affected uterine growth and triggered failure of fetal development [55].
4 Biotransformation of AFB1 in the body
Biotransformation or metabolism is the means by which a chemical substance is altered or transformed from one chemical state to another via successions of enzymatic or chemical response(s) inside the body and subsequently excretion of the byproducts or metabolites mostly via renal excretion [56–58]. In toxicology, biotransformation is very crucial in the defense mechanism via the excretion of toxic xenobiotics and body wastes in which they are transformed into less detrimental and polar substances that are easily excreted [56–58].
The process of elimination, which often encompasses metabolism and excretion of chemical substances from the body, comprises two principal phases [56,59]. Phase I involves the metabolism of chemical substances via the addition of small polar groups comprising of both positive and negative charges to xenobiotics of aflatoxins via the process of acetylation, oxidation, reduction, and hydrolysis, which render it harmless [56,59]. Phase I is mainly intermediated via the cytochrome P450 (CYP450) enzyme systems [56,59].
In contrast, phase II metabolism often encompasses glucuronide, glutathione, sulfate, and amino acid conjugation reactions [57,60]. The whole metabolic process permits Phase I products to “fit” into Phase II enzyme cascades where they are capable of combining with another substance to yield a polar or water-soluble substance that can certainly be excreted via the kidneys [57,59]. Nevertheless, in some instances, some of the chemical substances may be transformed into reactive or harmful products [56]. Microsomal enzymes metabolize AFB1 to distinctive metabolites via hydroxylation, hydration, demethylation, and epoxidation in the liver [61] and may migrate to the reproductive organs to induce infertility.
Interestingly, cytochrome P450-mediated metabolism is the primary focus of the biotransformation of AFB1 [56]. Nevertheless, AFB1 bioactivation via prostaglandin H synthase and lipoxygenase may be more essential than P450-catalyzed bioactivation in certain experimental systems [62,63]. Notably, AFB1 is bioactivated via epoxidation of the terminal furan ring double bond, producing an electrophilic intermediate, AFB1-8,9-epoxide, a stereoisomer that consists of both the exo and the endo configurations [64–66].
Interestingly, AFB1-exo-epoxide was proficient in alkylating nucleic acids and proteins, while the AFB1-endo-epoxide was a very weak mutagenic [64]. Furthermore, AFB1-exo-epoxide was easily crystallized in high quantities and steady in aprotic non-nucleophilic solvents [62,67]. Moreover, AFB1-exo-epoxide was capable of reacting with an extreme concentration of DNA, resulting in the formation of 98% of AFB1-DNA adducts although it had a half-life of approximately 1 s in an aqueous buffer [62,67].
AFB1 was also capable of stimulating 8-hydroxy-20-deoxyguanosine (8-OHdG) configuration in livers of rats and ducks during in vivo treatment [68–70]. Additionally, AFB1 triggered the elevation of 8-OHdG levels following the treatment of cultured woodchuck hepatocytes [62,69]. It was established that the most commonly detected mutation stimulated by AFB1 was DNA alkylation via AFB1-exo-epoxide followed by the AFB1-N7-Gua formation leading to G-T transversions [62,69].
Interestingly, AFB1-triggered ROS formation involves metabolism via cytochrome P450 to form AFB1-exo-epoxide and/or the hydroxylated metabolite AFM1 and requires both the participation of iron-catalyzed reactions and Kupffer cells in the rat livers in vivo and in rat hepatocytes [62,71,72]. Also, oxidative damage was capable of triggering AFB1 toxicity, resulting in a reduction in the anti-oxidant-capable parameters related to apoptosis [73,74]. Remarkably, AFB1 augmented apoptotic cells in spleen, broilers jejunum, and bursa fabricius [75–77]. Also, AFB1 augmented the secretion of fundamental liver apoptotic markers like Bcl-2-associated X protein (Bax), caspase-3, and p53 as well as reduced the secretion of key anti-apoptotic markers like B-cell lymphoma 2 (Bcl-2) [73]. Moreover, MDA was markedly elevated while superoxide dismutase (SOD) and the total anti-oxidant capacity (T-AOC) were much lower in AFB1-treated mice [16].
Notably, the MDA tissue content often reflects the degree of oxidative damage because it is a peroxide generated via free radicals [78]. Markedly, AFB1 stimulated oxidative stress, which was observed via the peroxidation of lipids and MDA in the serum [16]. Furthermore, AFB1 triggered the expression of free radicals, particularly superoxide anions, in kidney tissue where numerous T-AOC factors, such as SOD in the serum, were conscripted into the tissue, leading to downregulation of T-AOC and SOD in serum [16]. Thus, the effects of AFB1 on these factors are coherent with their stimulation of oxidative reactions in the mice [16].
It is worth noting that SOD is a typical anti-oxidant enzyme in diverse organisms, which translates superoxide anion radicals to hydrogen peroxide and safeguards organisms from oxidative injury. However, T-AOC is marker of total antioxidative activity and it reflects the activity of all the anti-oxidants in an organism [79]. Interestingly, proline dehydrogenase (ProDH) mRNA and protein were expressively elevated in AFB1-treated mice, and cell apoptosis in kidney tissues was similarly expressively stimulated and was associated with varying secretions of Bcl-2, Bax, and caspase-3 [16].
Moreover, proline levels were low in kidney tissue obtained from AFB1-treated mice, which was possibly due to ProDH upregulation [16]. Furthermore, ProDH siRNA was utilized to determine whether ProDH was a direct target of AFB1 [16]. In this experiment, it was established that the secretion of pyrroline-5-carboxylate synthase (P5CS), pyrroline-5-carboxylate reductase (P5CR), and proapoptotic factors were not distinctive in AFB1-treated and small interfering RNA (siRNA)-treated cells, and in cells that were treated with ProDH siRNA alone [16]. Thus, downstream apoptotic factors, such as Bcl-2, Bax, and caspase-3, were influenced by ProDH siRNA treatment [16].
It was established that AFB1 was capable of changing the metabolism of tryptophan in the brain, which decreases the levels of serotonin [80,81]. It was also observed that recurrent exposure to AFB1 resulted in the reduction of striatal dopamine and serotonin levels by 37% and 29%, respectively, signifying that AFB1 influenced dopaminergic and serotoninergic pathways, probably via selective triggering of the translation of tyrosine to biogenic catecholamine neurotransmitters [80,82]. Furthermore, acute AFB1 exposure reduced brain acetylcholinesterase, while the chronic exposure augmented adenohypophyseal acetylcholinesterase [80,83]. Thus, this indicates that neurotransmitters are influenced by AFB1, which leads to hormonal imbalance and maybe infertility. Further studies are needed in this direction.
5 AFB1 and reproductive hormones
AFB1 was capable of disrupting the functioning of several endocrine glands by interrupting the enzymes and their substrates that are liable for the synthesis of diverse hormones. Notably, AFB1 and generated ROS were capable of causing cancers in endocrine glands, such as pituitary gland, granulosa cell tumors of the ovary as well as adenomas and adenocarcinomas of the adrenal gland, ovaries, testes, kidneys, thyroid gland, parathyroid glands, and pancreas [84,85]. Interestingly, AFB1 is capable of inducing cancer as result of its capacity to generate several altered forms of DNA adducts. Thus, AFB1 was capable of adversely influencing the reproductive capacity of male and female animals [84,85].
Notably, AFB1 was capable of inducing a reduction in the sizes of ovaries and uterus as well as augmentation in the rates of fetal resorption, implantation loss, and intra-uterine death in AFB1-exposed female rats (Figure 1) [86]. Furthermore, AFB1 was capable of influencing sexual maturation, follicular growth and maturation, hormonal levels, pregnancy, and growth of fetus (Figure 1) [86]. Interestingly, in male mammals, 17β-estradiol is produced by Leydig cells as a result of a feedback reaction to luteinizing hormone (LH) and by Sertoli cells as a result of a feedback reaction to follicle-stimulating hormone (FSH) (Table 1) [87].
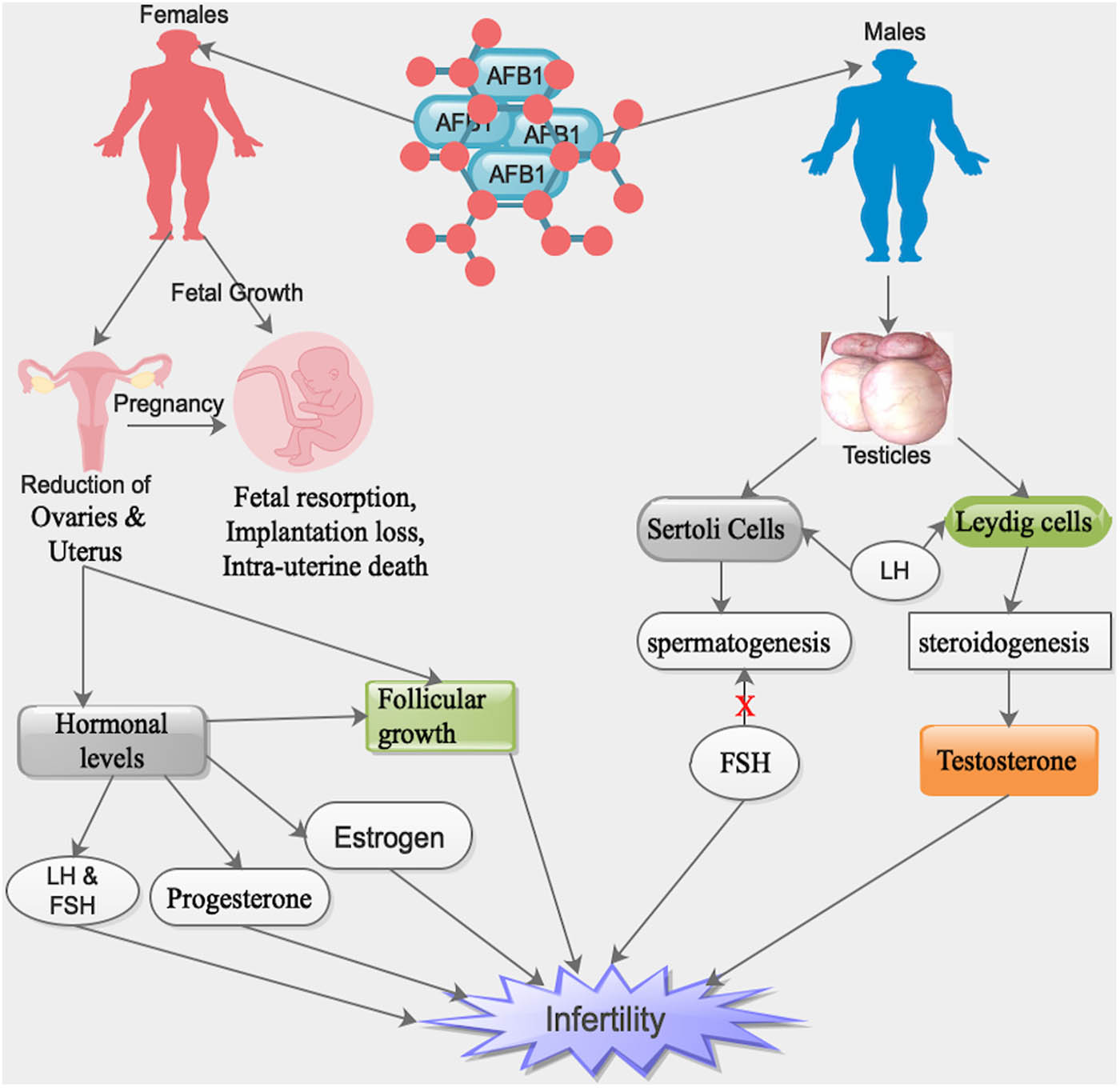
Influence of the ingested AFB1 by various ways in both male and female reproductive organs, resulting in infertility and/or fetal instability during pregnancy. X = inhibitory. Note: human symbols denote both humans/animals.
Various types of hormones influenced by AFB1 and their mechanisms
| Hormone | Effect of AFB1 on the hormone | Mechanism of action | References |
|---|---|---|---|
| Estrogen | Downregulation | Direct antagonistic interaction of AFB1 with steroid hormone receptors influencing the gonadal hormone production of estrogen, as a result of structural similarity of AFB1 and steroid hormones | [50,86,104] |
| Luteinizing hormone (LH) | Downregulation | Diminished serum testosterone concentration triggered a decreased sensitivity of Leydig cells to LH and/or direct blockade of testosterone production in rats exposed to AFB1 | [87,91,92,94] |
| Follicle-stimulating hormone (FSH) | Upregulation | Decreased levels of circulatory testosterone with higher concentrations of FSH during AFB1 exposure | [86,87,91,92,95] |
| Testosterone | Downregulation | Upsurge in cholesterol levels in the testis of AFB1-treated mice could be a result of inadequate utilization of cholesterol or impaired steroidogenesis | [87,90–92,108] |
| Progesterone | Upregulation | AFB1 in the placenta was capable of triggering an upsurge in progesterone synthesis | [50,97,104] |
| Direct antagonistic interaction of AFB1 with steroid hormone receptors influencing gonadal hormone production of progesterone, as a result of structural similarity of AFB1 and steroid hormones | |||
| Hepatic alphafetoprotein (AFP) | Upregulation | AFB1 adversely influences AFP production, which in turn inhibited the gonadal function resulting in a reduction in the concentrations of hormonal promoters | [98] |
Also, in male mammals, testosterone biosynthesis transpires in the Leydig cells of the testis as a result of a feedback reaction to LH produced by the pituitary gland (Table 1) [88]. Cholesterol is a substrate for steroidogenesis and it is transported into mitochondria via steroidogenic acute regulatory (StAR) proteins [89]. Cholesterol is converted into pregnenolone by cytochrome P450, a side-chain-cleaving enzyme in the mitochondria [89]. Subsequently, pregnenolone is transported into the smooth endoplasmic reticulum where it is converted into testosterone [88].
Remarkably, diminished steroidogenesis is a key phenomenon in AFB1-mediated reproductive toxicity [90,91]. Further studies on the exact mechanism via which AFB1-mediated reproductive toxicity occurs during steroidogenesis are warranted. Interestingly, an upsurge in cholesterol levels in the testis of AFB1-treated mice was observed [91]. Remarkably, the upsurge in cholesterol levels in the testis of AFB1-treated mice could be as result of inadequate utilization of cholesterol or impaired steroidogenesis (Table 1) [91].
Moreover, a reduction in serum testosterone concentrations in rats following exposure to AFB1 was observed (Table 1) [92,93]. The diminished serum testosterone concentration was probably due to a decreased sensitivity of Leydig cells to LH and/or direct blockade of testosterone production in rats exposed to AFB1 (Figure 1) [92,93]. Markedly, a substantial upsurge in serum concentrations of FSH with reduced testosterone levels in AFB1-treated rats was detected compared to controls (Table 1) [87]. It is worth noting that a decreased concentration of circulatory testosterone with higher concentrations of FSH and LH signifies an intact pituitary–testicular axis in AFB1-treated rats (Table 1) [88,92,93].
Intriguingly, FSH was capable of stimulating Sertoli and Leydig cells, resulting in the regulation of spermatogenesis and steroidogenesis, respectively (Figure 1) [94,95]. It is worth noting that an upsurge in the serum FSH concentration signifies an inhibition of spermatogenesis in AFB1-treated rats and suggests germ cell loss or impairment of Sertoli cells, resulting in impaired feedback regulation of FSH secretion (Figure 1 and Table 1) [88,96]. Furthermore, LH was also capable of stimulating Sertoli and Leydig cells, resulting in the regulation of spermatogenesis and steroidogenesis, respectively (Figure 1 and Table 1) [88,95].
Also, it was observed that chronic exposure to AFB1 was capable of triggering endocrine disruption in the human foetoplacental component because it was capable of influencing the secretion of aromatase enzymes, such as P450 or CYP enzymes (Figure 1) [97]. Thus, AFB1 was a hypothetical endocrine disruptor, which was capable of influencing steroid ovarian hormones levels, either directly or indirectly [97]. Furthermore, AFB1 was capable of augmenting the secretion of CYP19A1 in human placenta cells [97,98].
Furthermore, AFB1 was capable of influencing key genes in endocrine regulation in placental cells after being metabolized into aflatoxicol [97]. Also, AFB1 influenced placental steroid hormone production, metabolism, and conjugating enzymes, which triggered abnormalities in the foetoplacental hormonal homeostasis [98]. Moreover, CYPs have been implicated in steroid hormones production and the upsurge of the secretions of these enzymes by AFB1 in the placenta was capable of triggering an upsurge in progesterone synthesis (Figure 1) [97].
It was established that modifications in oestradiol-17β and/or progesterone levels during the luteal phase and/or the orchestrated oestrus had unfavorable influences like shortened cycles, lower fertility, negative influence on follicle maturation, ovulation, or the existence and/or the signs of the oestrus cycle on succeeding reproductive lifecycle of the animals (Figure 1) [44]. Moreover, blood oestradiol-17β and progesterone were expressively lower and higher, respectively, in rats exposed to AFB1 (Figure 1 and Table 1) [44]. Thus, AFB1 had a direct influence on ovarian secretory cells or on the hypothalamus–hypophysis–ovary axis [44].
Interestingly, after exposing AFB1 to male rats for 48 days, it was observed that the levels of blood serum LH, testosterone, and oestradiol-17β were expressively lower in the group of rats exposed to the highest dose of AFB1 (Figure 1 and Table 1) [87]. Thus, AFB1 had direct influence on testes secretory cells or on the hypothalamus–hypophysis–testis axis [87]. Notably, AFB1 adversely influenced hepatic alphafetoprotein (AFP) production, which is identified to inhibit gonadal function, resulting in the reduction in the concentrations of hormonal promoters above (Table 1) [99].
6 AFB1-associated infertility in humans
The disruptive abilities of AFB1 have been observed in the reproductive system in both male and female human beings after consumption of AFB1-contaminated foods [37]. Testicular damage has been reported in infertile men as a result of early accumulation of AFB1 in human systems [9]. Patients who are exposed to chronic AFB1 develop a lower percentage of sperm morphology even with the very low cut-off value for sperm morphology, as recommended in the new edition of the World Health Organization (WHO) semen analysis [9].
AFB1 was capable of hindering testicular development, testicular degeneration, reduced reproductive capabilities, morphological regressive modifications in the testis, and blockage of Leydig cell function in men (Figure 1) [9,100]. AFB1 was isolated in the blood and semen of infertile men [47]. Interestingly, isolated AFB1 was 25% in the semen of infertile patients as compared to 2.1% in controls [9]. Furthermore, detection of abnormal semen parameters, such as severe decrease in the sperm count, decreased motility, high percentage of abnormal morphology, and high viscosity in the semen of the infertile group compared to that in the fertile group as well as the WHO reference values for normal semen parameters (Figure 1) [9].
Notably, a similar study observed a prevalence rate of AFB1 in 40% of infertile men compared to 8% in fertile men [101]. Remarkably, 50% of the infertile men with high AFB1 semen levels also exhibited abnormalities in semen parameters. Also, AFB1 was capable of damaging chromosomes, genes, and forming aflatoxin–DNA complex, which triggered key anomalies in human sperms [102,103]. Furthermore, AFB1 was capable of generating ROS in the form of free radicals, which triggered the anomalies in human sperms [9].
ROS were able to influence macromolecules like protein, DNA, lipid of the sperm and testicular tissues, resulting in cellular/tissue damage after exhaustion of natural anti-oxidants [9]. However, selenium and/or vitamin E supplements in cases of idiopathic male infertility were capable of augmenting the quality of semen and boosting the production and protective effects on sperm motility [104]. Also, circulating ROS generated by aflatoxins in cases of idiopathic male infertility were neutralized by these above potent anti-oxidants [9].
Interestingly, a substantial increase in the mean ovarian volume in infertile females and a substantial reduction in the mean follicular size was observed (Figure 1) [105]. It is worth noting that two distinct adverse activities have been implicated as causes of AFB1-related female fertility [105]. These two actions are an indirect influence facilitated by AFB1-stimulated hypovitaminosis A and a direct antagonistic interaction of AFB1 with steroid hormone receptors influencing gonadal hormone production of estrogen and progesterone as a result of structural similarity of AFB1 and steroid hormones (Figure 1 and Table 1) [105].
7 AFB1-associated infertility in animals
AFB1 has been implicated in the disruption of reproductive systems in both male and female animals after ingestion of AFB1-contaminated foods [37]. Experimental studies in animals revealed that certain AFB1 was capable of influencing the reproductive abilities of both sexes resulting in anomalous sperm, low sperm count, sterility, and/or affect hormone activity leading to infertility [9,106]. Furthermore, AFB1 was capable of reducing motility of sperms obtained from ejaculation or epididymis (Figure 1) [9,106]. Thus, AFB1 is capable of decreasing the number of primary spermatocytes, spermatids, and the morphology of sperm cells produced (Figure 1) [107,108].
Notably, the concentration of plasma testosterone and 5α-dihydrotestosterone (5α-DHT) as well as absolute and relative testicular weights of experimental male animals exposed to AFB1 remained low in all age groups, and a tardiness in the onset of sexual maturation during aflatoxicosis (Table 1) [109]. Furthermore, AFB1 was capable of triggering pathological modifications, such as degeneration and necrosis of epithelial cells of sperm tubules and decrease in the number of sperms (Figure 1) [110]. Also, AFB1 was capable of decreasing the semen volume, testicular weight, spermatocrit, plasma testosterone, and a decrease in the egg output in poultry (Figure 1) [37,111].
Intriguingly, continuous feeding of male goats with diets containing AFB1 triggered testicular degeneration (Figure 1) [112]. Also, AFB1 was capable of delaying the physiological, behavioral sexual maturation, and testicular development in Japanese quail (Figure 1) [113]. Furthermore, AFB1 was capable of reducing the semen volume and testis weight, which resulted in the interference of the germinal epithelium in mature male white Leghorn chicks [114].
Moreover, degenerating alterations of diverse intensity in the germinal epithelium of the seminiferous tubules led to devastating dystrophic changes in the spermatogenic epithelium alongside edematous alterations in the interstitial tissue in adult male rats exposed to AFB1 diet for prolonged periods were observed (Figure 1) [90,115]. Similarly, degeneration in the epithelium lining of seminiferous tubules and congestion of testicular blood vessels with intertubular edema in the rats exposed to AFB1 was observed (Figure 1) [116].
Furthermore, coagulative necrosis of the whole epithelium lining of several seminiferous tubules, which were transformed into homogenous eosinophilic debris in their lumina, was also observed [116]. Also, AFB1 was capable of decreasing the number of Leydig cells, the height of seminiferous tubules, the number and the index of sertoli cells, the diameter of caput epididymis, and lumen caput epididymis (Figure 1) [110]. Moreover, the number of spermatogenesis, spermatocytes, and spermatids was also decreased [110].
In female experimental animals, AFB1 was capable of triggering pathological modifications in the form of coagulative necrosis, particularly in the growing and mature follicles, resulting in a reduction in the number and size of graffian and growing follicles with augmented number of atretic follicles and a slight portion of degenerative alterations (Figure 1) [37,105]. Also, in laboratory and domestic female animals, AFB1 was capable of triggering a decrease in ovarian and uterine sizes, augmented fetal resorption, implantation loss, and intra-uterine death in female rats exposed to AFB1 (Figure 1) [37,105].
Interestingly, AFB1 wields all types of teratogenic effects on growing and non-growing follicles, which result in the reduction of ovulatory follicles in rats (Figure 1) [9,51]. Furthermore, infertility parameters like disturbances of estrus cyclicity, blockade of lordosis, and decrease in conception rates and litter sizes were detected in rats exposed to AFB1 [117]. Also, mature domestic fowls exposed to AFB1 exhibited follicular atresia during histopathological examinations of their ovaries, which resulted in the cessation of egg production during the whole feeding period [111].
8 AFB1 and fetal deformities
AFB1 presents a potential hazard to animal and human health in view of their teratogenicity [118,119]. Notably, AFB1 could be accountable for the development of malformation in humans [120–122]. Several detrimental consequences like low birth weight, small litters, fetal death and resorption, bone and visceral deformities, reproductive changes, impact on immune capacity, and behavioral changes, and predisposition to neoplasm development are related to exposure to AFB1 during the prenatal period [5]. Specifically, fetal anomalies are observed when the pregnant animals are exposed to AFB1 via gavage or intramuscularly [123].
Notably, reduction in weight and absolute size of the viscera, decreased size of the heart sinusoid capillaries, as well as ventricular lumen, liver, and kidneys containing vacuoles as well as congestion, atrophy, glomerular degeneration, and disorganization of hepatocyte were detected in fetus exposed to AFB1 during the prenatal period [119,124]. Also, anomalies in organs like thymus presenting lymphoid depletion and decrease in epithelial differentiation, moderate degeneration of the testicles with atrophy, and the decrease of germ cells of seminiferous tubules were detected in fetus exposed to AFB1 during the prenatal period [125].
Interestingly, at the cellular level, AFB1 was capable of augmenting the numbers of both apoptotic cells and mitoses in the periportal regions; the nuclei of some cells were distended; hyperchromatic, and pleomorphic with a coarse chromatin pattern with cytoplasm’s that were markedly melanocytosic [119]. Notably, AFB1 was capable of triggering chromosome aberrations in bone marrow of rats [126–128]. Remarkably, mutagenicity of AFB1 resulted in the formation of covalent N7 guanine adducts, which interrupted DNA replication, leading to anomalies in the chromosomes [129,130]. Also, AFB1 was capable of stimulating chromosomal aberrations, such as centromeric attenuations, chromatid breaks, chromatid gaps, end-to-end associations, chromosomal fusions, ring chromosomes, dicentric chromosomes, fragments, deletions, centric fusions, stickiness, and hypoployploidy in the bone marrow cells of rats [119,131,132].
Markedly, AFB1 was capable of causing skeletal anomalies with incomplete ossification of skull bones and failure of ossification of small bones (Figure 2) [133,134]. These skeletal defects were seen mostly in the ribs and soft-tissue anomalies [133,134]. Furthermore, AFB1-associated deformities are a result of the formation of phenotypic abnormalities due to delayed metamorphosis [119]. Also, AFB1 triggered some errors during transcription of developmental genes and defects in homoeotic genes, which influenced the final ailment of imaginal discs [135].
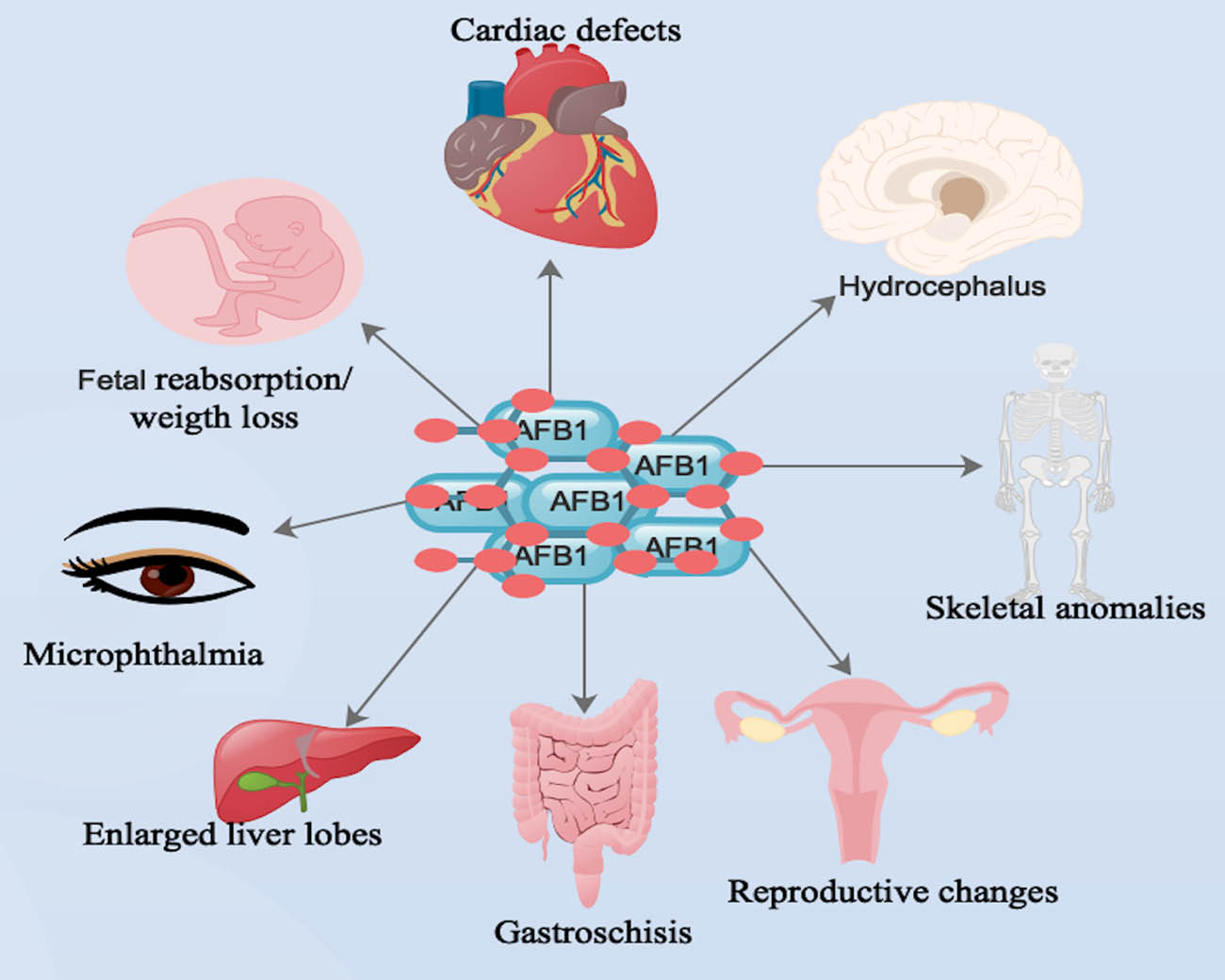
Main body organs that AFB1 was capable of triggering deformities during prenatal exposes. Note: human symbols denote both humans/animals.
Interestingly, daily consumption of a mixture of AFB1 and AFG1 by pregnant white rats from the 8th to the 12th day of gestation triggered a decrease in the number of implantation sites and fetal weight and augmented the reabsorption of fetuses (Figure 2) [136,137] as well as the absence of one or more coccygeal vertebrae and, in some cases, compaction of the vertebral column [119]. Furthermore, AFB1-associated bone defects were usually linked to ossification failures, alterations in bone size, and shape, and the absence or modification of some bone issues (Figure 2) [123].
Intriguingly, AFB1 was capable of influencing the transcription of genes linked to bone development, affecting activities like intramembrane mineralization and endochondral ossification [55,123,134,138]. Also, animals exposed to AFB1 during embryonic development were more prone to minor malformations, like those affecting bone fates was detected (Figure 2) [119]. These minor malformations were mostly limb defects, such as the absence of some metacarpal and metatarsal bones and some phalanges [119]. Furthermore, AFB1-triggered mineralization-associated defects were linked to the direct effect of AFB1 on the osteoblasts, osteoclasts, and periosteal cells [119].
Notably, impairment in the mineralization of osteoid tissues, such as bone matrix, affected bone maturation and prevention of periosteal new bone formation parameters like endochondral and intramembranous ossification [119,134]. Also, exencephaly and gastroschisis were observed in mice fetuses exposed to AFB1 [139]. Furthermore, soft-tissue anomalies, such as internal hydrocephalus, microphthalmia, cardiac defects, and augmented liver lobes were observed (Figure 2) [140]. Also, multilobulation of the liver in one fetus exposed to a higher dose of AFB1 was detected [140].
Notably, localization of 14C-labeled AFB1 by the pigment layer of fetal eye, liver, and heart, and continuous exposure of AFB1 during the organogenesis period was responsible for the manifestation of distinctive abnormalities of these organs (Figure 2) [141]. Also, AFB1 was associated with reproductive changes, immune modifications, behavioral changes, and predisposition of animals and humans to neoplasm development (Figure 2) [5,123].
9 Potential therapies for AFB1
Detoxification techniques generally used to destroy AFB1 are physical and chemical methods on contaminated foods [142]. Physical approaches, such as heat and gamma rays are the most typical techniques for neutralizing AFB1, while chemicals such as acids, bases, oxidizing agents, and reducing agents are the most typical techniques used to destroy or extinguish AFB1 on contaminated foods (Table 2) [142]. The use of plant extracts to degrade AFB1 and the inoculation of bacterial strains in food substrates are two main biotechnological techniques used to reduce the AFB1 levels in contaminated foods (Table 2) [142].
Agent/chemical/drug and the modes of actions used in the treatment of contaminated food, exposed to animals and/or human beings
| Agent/chemical/crug | Treatment (mode) food/animal/human | Mode of action of agent/chemical/drug | Reference |
|---|---|---|---|
| Physical (heat and gamma rays) | Contaminated food | Neutralization of AFB1 | [141] |
| Chemical (acids, bases, oxidizing agents, and reducing agents) | Contaminated food | Destruction or extinguish AFB1 | [141] |
| Biotechnological (plant extracts and bacterial strains) | Contaminated food | Degradation of AFB1 | [141] |
| Novasil clay minerals | Animal and humans (oral) | Absorption of AFB1 in vitro | [141] |
| Phyllanthus amarus | Humans (oral) | Augmentation lipid peroxidation, leading to downregulation of AFB1 in the liver | [143,144] |
| Black tea | Humans (oral) | Augmentation lipid peroxidation, leading to downregulation of AFB1 in the liver | [143,144] |
| Gynandra extract | Animals and humans (oral) | Anti-oxidant | [39,40,145] |
| Esculin | Animals and humans (oral) | Anti-oxidant | [39,40,145] |
| Selenium | Animals and humans (oral) | Anti-oxidant | [39,40,72,76,145] |
| Ascorbic acid (vitamin C) | Animals and humans (oral) | Anti-oxidant | [146] |
| Vitamin E | Animals and humans (oral) | Anti-oxidant | [38,90,148] |
| Oltipraz | Animals (oral) | Reduction of hepatic AFB1-derived DNA adducts | [150–152] |
| CDDO-Im | Animals and humans (oral) | Multifunctional agent with anti-inflammatory, antiproliferative, apoptotic, and cytoprotective activities | [156–159] |
However, novasil clay minerals have been proven to possess high affinity and combine well with AFB1 in the gastrointestinal tract [142]. Novasil clay minerals were capable of absorbing AFB1 in vitro in both animal models and human studies (Table 2) [142]. They were able to decrease the bioavailability of blood toxins, and their usage in humans did not influence the utilization of vitamins and trace elements in the body during clinical trials [142]. Natural plant products are synthetic antibacterial agents with biodegradability, biosafety, effectiveness, and regenerability capabilities [143].
Interestingly, the anti-oxidant influence of Phyllanthus amarus herbal extracts and black tea were capable of augmenting lipid peroxidation, which is often downregulated by AFB1 in the liver (Table 2) [144,145]. Also, studies have shown that substances, such as esculin, selenium, and gynandra extract, which have anti-oxidant functions, are capable of modifying AFB1-stimulated oxidative stress, resulting in the relieving of the resultant histological anomalies (Table 2) [40,41,146].
Intriguingly, standard anti-oxidants like ascorbic acid (vitamin C) were capable of neutralizing the harmful effects of AFB1 on most hematological, biochemical, and enzymatic parameters (Table 2) [147]. Additionally, selenium had protective effect in the spleen and liver against AFB1-stimulated toxicity by blocking oxidative stress and associated extreme apoptosis (Table 2) [73,77].
Moreover, selenium was capable of decreasing mitochondrial swelling and mitochondrial DNA mutations in ducklings that were exposed to AFB1 due to its anti-oxidant capabilities [41]. Also, AFB1 was capable of augmenting oxidative stress marker, MDA, in the kidneys and blood. In contrast, they also observed that AFB1 was capable of reducing the anti-oxidant capacities of markers, such as nonenzymatic (glutathione) and enzymatic (glutathione peroxidase, glutathione reductase, and glutathione-S-transferase) [39].
Interestingly, vitamin E was capable of neutralizing AFB1 levels and restoring the parameter values above nearly the control level (Table 2) [39]. It was established that vitamin E was capable of sustaining integrity of long-chain polyunsaturated fatty acids in the membranes of cells, resulting in the preservation of their signaling molecules that could be modified by oxidative stress [148]. Moreover, vitamin E was capable of ameliorating AFB1-stimulated lipid peroxidation in the testis of mice as a result of its higher enzymatic and nonenzymatic anti-oxidant capabilities (Table 2) [91].
Furthermore, vitamin E was capable of influencing signaling function in vascular smooth muscle cells, resulting in its role beyond the antioxidative function (Table 2) [149]. Additionally, vitamin E had precise blockade effect on protein kinase C and a gene like collagenase [150]. Similarly, vitamin E had stimulatory effects on one protein, phosphatase, and on other genes such as alpha-tropomyosin and connective tissue growth factor [150].
Notably, antischistosomal drug oltipraz was capable of decreasing the disease burden associated with AFB1 during preliminary cancer prevention bioassays in AFB1-exposed rats [151–153]. It was capable of substantial but inadequate reductions in quantities of hepatic AFB1-derived DNA adducts in these animals (Table 2). Also, the pharmacodynamic action of the medicine was suggestive of augmented detoxication of AFB1 (Table 2) [154,155]. Also, synthetic oleanane triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oylimidazole (CDDO-Im) was capable of blocking AFB1-stimulated tumorigenesis in the rat (Table 2) [151].
The actions of CDDO-Im were obvious in the reduction of the hepatic focal burden of the glutathione S-transferase placental form (GST-P positive foci) of preneoplastic lesions [156]. Interestingly, CDDO-Im was a potent stimulator of Keap1-NF-E2-related factor 2 (Nrf2) signaling, which was capable of triggering augmented conjugation of the 8,9-epoxide of AFB1 with glutathione via the action of glutathione S-transferases (GSTs), resulting in the reduction of DNA adducts formed from this ultimate carcinogenic electrophile [157,158].
Furthermore, studies established that the protection offered by CDDO-Im in this model was attained mainly via the interaction with signaling pathways facilitated by the transcription factor Nrf2 [156,158]. Also, hepatic secretion of Nrf2 target genes, such as aldo-keto reductase 7A1 and GSTs, which were implicated in AFB1 detoxication, was elevated after CDDO-Im administration [151]. Thus, CDDO-Im functions as a multifunctional agent with anti-inflammatory, antiproliferative, apoptotic, and cytoprotective activities, influenced multiple targets and pathways (Table 2) [159,160].
In our perspective, medications, such as esculin, selenium, gynandra extract, vitamins C and E, oltipraz, and CDDO-Im, which have anti-oxidant functions, are capable of modifying AFB1-stimulated oxidative stress, resulting in the relieving of resultant anomalies. Thus, these drugs have to be re-purposed for the treatment of AFB1-associated infertility. We also advocate clinical trials on these medications for treatment of AFB1-associated infertility. Thus far, future research on the treatment of AFB1-associated infertility in both males and females should focus on these medications, which are already in use and readily available.
10 Conclusion
AFB1 is able to distract the reproductive systems in both male and female animals. Also, AFB1 is capable of interfering with the functions of several endocrine glands via the disruption enzymes and their substrates that are liable for the synthesis of hormones. Additionally, AFB1 is capable of influencing the key genes in endocrine regulation in placental cells after being metabolized into aflatoxicol, resulting in fetal anomalies. Moreover, AFB1 is potentially teratogenic and it is responsible for the development of malformation in humans and animals. Thus, AFB1 is one of the crucial markers to investigate in couples with infertility.
Abbreviations
- AFB1
-
aflatoxin B1
- Bax
-
Bcl-2-associated X protein
- Bcl2
-
B-cell lymphoma 2
- CDDO-Im
-
1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oylimidazole
- CYP450
-
cytochrome P450
- DHT
-
5α-dihydrotestosterone
- FSH
-
Follicle-stimulating hormone
- GST
-
glutathione S-transferase
- LH
-
Luteinizing hormone
- MDA
-
malondialdehyde
- Nrf2
-
NF-E2-related factor 2
- 8-OHdG
-
8-hydroxy-20- deoxyguanosine
- P5CR
-
pyrroline-5-carboxylate reductase
- P5CS
-
pyrroline-5-carboxylate synthase
- ProDH
-
proline dehydrogenase
- ROS
-
reactive oxygen species
- siRNA
-
small interfering RNA
- SOD
-
superoxide dismutase
- StAR
-
steroidogenic acute regulatory
- T-AOC
-
total antioxidant capacity
- UV
-
ultraviolet
- Vitamin C
-
ascorbic acid
- WHO
-
World Health Organization
Acknowledgements
Not applicable.
-
Funding information: This work has no funding or financial source.
-
Author contributions: All authors contributed toward literature search, drafting, and critically revising the article and agree to be accountable for all aspects of the work.
-
Conflict of interest: None declared.
-
Data availability statement: No data were used in this article.
References
[1] Souza CF, Baldissera MD, Zeppenfeld CC, Descovi S, Stefani LM, Baldisserotto B, et al. Oxidative stress mediated the inhibition of cerebral creatine kinase activity in silver catfish fed with aflatoxin B1-contaminated diet. Fish Physiol Biochem. 2019;45(1):63–70.10.1007/s10695-018-0534-9Search in Google Scholar PubMed
[2] Hussain D, Mateen A, Gatlin DM III. Alleviation of aflatoxin B1 (AFB1) toxicity by calcium bentonite clay: Effects on growth performance, condition indices and bioaccumulation of AFB1 residues in Nile tilapia (Oreochromis niloticus). Aquaculture. 2017;475:8–15.10.1016/j.aquaculture.2017.04.003Search in Google Scholar
[3] Souto NS, Claudia Monteiro Braga A, Lutchemeyer de Freitas M, Rechia Fighera M, Royes LFF, Schneider Oliveira M, et al. Aflatoxin B1 reduces nonenzymatic anti-oxidant defenses and increases protein kinase C activation in the cerebral cortex of young rats. Nutritional Neurosci. 2018;21(4):268–75.Search in Google Scholar
[4] Richard SA, Manaphraim NY, Kortei NK. The novel neurotoxic and neuroimmunotoxic capabilities of aflatoxin B1 on the nervous system: a review. Adv Biosci Cl Med. 2020;8(3):1–8.10.7575/aiac.abcmed.v.8n.3p.1Search in Google Scholar
[5] Benkerroum N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int J Environ Res Public Health. 2020;17(2):423. 10.3390/ijerph17020423.Search in Google Scholar PubMed PubMed Central
[6] Lalah JO, Omwoma S, Orony D. Aflatoxin B1: Chemistry, environmental and diet sources and potential exposure in human in Kenya. Aflatoxin B1 occurrence, detection and toxicological effects. IntechOpen; 2020. 10.5772/intechopen.88773.Search in Google Scholar
[7] Kortei NK, Annan T, Richard SA, Boakye AA, Tettey CO, Essuman EK, et al. Dietary exposure to aflatoxins in some randomly selected foods and cancer risk estimations of cereals consumed on a Ghanaian market. J Food Qual. 2022;2022:5770836.10.1155/2022/5770836Search in Google Scholar
[8] Chandra P. Aflatoxins: Food safety, human health hazards and their prevention. Aflatoxins – Occurrence, Detoxification, Determination and Health Risks. IntechOpen; 2022. 10.5772/intechopen.96647.Search in Google Scholar
[9] Mohammed MN, Ameen MM, Mohammed OA, Al-Maghraby OM, Aziz OA, Ahmed SR, et al. The effect of aflatoxins on male reproduction. Med Arch. 2014;68(4):272.10.5455/medarh.2014.68.272-275Search in Google Scholar PubMed PubMed Central
[10] Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80(5):1106–22. 10.1093/ajcn/80.5.1106.Search in Google Scholar PubMed
[11] Kortei NK, Annan T, Akonor PT, Richard SA, Annan HA, Kyei-Baffour V, et al. The occurrence of aflatoxins and human health risk estimations in randomly obtained maize from some markets in Ghana. Sci Rep. 2021;11(1):1–13.10.1038/s41598-021-83751-7Search in Google Scholar PubMed PubMed Central
[12] Kortei NK, Annan T, Akonor PT, Richard SA, Annan HA, Wiafe-Kwagyan M, et al. Aflatoxins in randomly selected groundnuts (Arachis hypogaea) and its products from some local markets across Ghana: Human risk assessment and monitoring. Toxicol Rep. 2021;8:186–95.10.1016/j.toxrep.2021.01.002Search in Google Scholar PubMed PubMed Central
[13] Mahato DK, Lee KE, Kamle M, Devi S, Dewangan KN, Kumar P, et al. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front Microbiol. 2019;10:2266. 10.3389/fmicb.2019.02266.Search in Google Scholar PubMed PubMed Central
[14] Alvarado AM, Zamora-Sanabria R, Granados-Chinchilla F. A focus on aflatoxins in feedstuffs: levels of contamination, prevalence, control strategies, and impacts on animal health. Aflatoxin-Control, Analysis, Detection Health Risks. 2017;116–52.10.5772/intechopen.69468Search in Google Scholar
[15] Sabino M, Purchio A, Milanez TV. Aflatoxins B1, M1 and aflatoxicol in tissues and urine of calves receiving aflatoxin. Food Addit Contam. 1995;12(3):467–72. 10.1080/02652039509374331.Search in Google Scholar PubMed
[16] Li H, Xing L, Zhang M, Wang J, Zheng N. The toxic effects of aflatoxin B1 and aflatoxin M1 on kidney through regulating L-proline and downstream apoptosis. Biomed Res Int. 2018;2018:9074861. 10.1155/2018/9074861.Search in Google Scholar PubMed PubMed Central
[17] Akim M, Kembo J. Falling fertility and increase in use of contraception in Zimbabwe. Afr J Reprod Health. 2011;15(2):31–44.Search in Google Scholar
[18] Ozoemena O, Ezugworie J, Mbah A, Esom E, Ayogu B, Ejezie F. Abnormality of pituitary gonadal axis among Nigerian males with infertility: study of patterns and possible etiologic interrelationships. Open Access J Urol. 2011;3:133–7. 10.2147/oaju.S22916.Search in Google Scholar
[19] Girela JL, Gil D, Johnsson M, Gomez-Torres MJ, De Juan J. Semen parameters can be predicted from environmental factors and lifestyle using artificial intelligence methods. Biol Reprod. 2013;88(4):99. 10.1095/biolreprod.112.104653.Search in Google Scholar PubMed
[20] Abdel-Wahhab MA, Aly SE. Anti-oxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005;25(3):218–23. 10.1002/jat.1057.Search in Google Scholar PubMed
[21] Stoev SD. Foodborne mycotoxicoses, risk assessment and underestimated hazard of masked mycotoxins and joint mycotoxin effects or interaction. Env Toxicol Pharmacol. 2015;39(2):794–809. 10.1016/j.etap.2015.01.022.Search in Google Scholar PubMed
[22] Herzog-Soares JD, Freire RB. Effect of citrinin and in association with aflatoxin B(1) on the infectivity and proliferation of Toxoplasma gondii in vitro. Braz J Infect Dis. 2004;8(1):101–8. 10.1590/s1413-86702004000100007.Search in Google Scholar PubMed
[23] Raisuddin S, Singh KP, Zaidi SI, Paul BN, Ray PK. Immunosuppressive effects of aflatoxin in growing rats. Mycopathologia. 1993;124(3):189–94. 10.1007/bf01103737.Search in Google Scholar
[24] Peraica M, Radić B, Lucić A, Pavlović M. Toxic effects of mycotoxins in humans. Bull World Health Organ. 1999;77(9):754–66.Search in Google Scholar
[25] Coulter JB, Lamplugh SM, Suliman GI, Omer MI, Hendrickse RG. Aflatoxins in human breast milk. Ann Trop Paediatr. 1984;4(2):61–6. 10.1080/02724936.1984.11748311.Search in Google Scholar PubMed
[26] Lamplugh SM, Hendrickse RG, Apeagyei F, Mwanmut DD. Aflatoxins in breast milk, neonatal cord blood, and serum of pregnant women. Br Med J (Clin Res Ed). 1988;296(6627):968. 10.1136/bmj.296.6627.968.Search in Google Scholar PubMed PubMed Central
[27] Oyelami O, Maxwell S, Adelusola K, Aladekoma T, Oyelese A. Aflatoxins in the autopsy brain tissue of children in Nigeria. Mycopathologia. 1995;132(1):35–8.10.1007/BF01138602Search in Google Scholar
[28] Casteels-van Daele M, Eggermont E. Reye’s syndrome. BMJ. 1994;308(6933):919–20. 10.1136/bmj.308.6933.919b.Search in Google Scholar PubMed PubMed Central
[29] Maxwell S, Apeagyei F, De Vries H, Mwanmut D, Hendrickse R. Aflatoxins in breast milk, neonatal cord blood and sera of pregnant women. J Toxicol: Toxin Rev. 1989;8(1–2):19–29.10.3109/15569548909059735Search in Google Scholar
[30] Olson LC, Bourgeois CH Jr, Cotton RB, Harikul S, Grossman RA, Smith TJ. Encephalopathy and fatty degeneratiof on the viscera in northeastern Thailand. Clinical syndrome and epidemiology. Pediatrics. 1971;47(4):707–16.10.1542/peds.47.4.707Search in Google Scholar
[31] Hatem NL, Hassab HM, Abd Al-Rahman EM, El-Deeb SA, El-Sayed Ahmed RL. Prevalence of aflatoxins in blood and urine of Egyptian infants with protein-energy malnutrition. Food Nutr Bull. 2005;26(1):49–56. 10.1177/156482650502600106.Search in Google Scholar PubMed
[32] de Vries HR, Maxwell SM, Hendrickse RG. Aflatoxin excretion in children with kwashiorkor or marasmic kwashiorkor–a clinical investigation. Mycopathologia. 1990;110(1):1–9. 10.1007/bf00442763.Search in Google Scholar
[33] Oyelami OA, Maxwell SM, Adelusola KA, Aladekoma TA, Oyelese AO. Aflatoxins in the lungs of children with kwashiorkor and children with miscellaneous diseases in Nigeria. J Toxicol Env Health. 1997;51(6):623–8. 10.1080/00984109708984048.Search in Google Scholar PubMed
[34] Denning DW, Quiepo SC, Altman DG, Makarananda K, Neal GE, Camallere EL, et al. Aflatoxin and outcome from acute lower respiratory infection in children in The Philippines. Ann Trop Paediatr. 1995;15(3):209–16. 10.1080/02724936.1995.11747774.Search in Google Scholar PubMed
[35] Dvorácková I, Píchová V. Pulmonary interstitial fibrosis with evidence of aflatoxin B1 in lung tissue. J Toxicol Env Health. 1986;18(1):153–7. 10.1080/15287398609530856.Search in Google Scholar PubMed
[36] Deng Z-L, Ma Y. Aflatoxin sufferer and p53 gene mutation in hepatocellular carcinoma. World J Gastroenterol. 1998;4(1):28.10.3748/wjg.v4.i1.28Search in Google Scholar PubMed PubMed Central
[37] Bbosa GS, Kitya D, Lubega A, Ogwal-Okeng J, Anokbonggo WW, Kyegombe DB. Review of the biological and health effects of aflatoxins on body organs and body systems. Aflatoxins-recent Adv future Prospect. 2013;12:239–65.Search in Google Scholar
[38] Chen C, Mitchell NJ, Gratz J, Houpt ER, Gong Y, Egner PA, et al. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Env Int. 2018;115:29–37. 10.1016/j.envint.2018.03.001.Search in Google Scholar PubMed PubMed Central
[39] Abdel-Hamid AA, Firgany Ael D. Vitamin E supplementation ameliorates aflatoxin B1-induced nephrotoxicity in rats. Acta Histochem. 2015;117(8):767–79. 10.1016/j.acthis.2015.08.002.Search in Google Scholar PubMed
[40] Naaz F, Abdin MZ, Javed S. Protective effect of esculin against prooxidant aflatoxin B1-induced nephrotoxicity in mice. Mycotoxin Res. 2014;30(1):25–32. 10.1007/s12550-013-0185-8.Search in Google Scholar PubMed
[41] Shi D, Liao S, Guo S, Li H, Yang M, Tang Z. Protective effects of selenium on aflatoxin B1-induced mitochondrial permeability transition, DNA damage, and histological alterations in duckling liver. Biol Trace Elem Res. 2015;163(1–2):162–8. 10.1007/s12011-014-0189-z.Search in Google Scholar PubMed
[42] Wang H, Dick R, Yin H, Licad-Coles E, Kroetz DL, Szklarz G, et al. Structure− function relationships of human liver cytochromes P450 3A: aflatoxin B1 metabolism as a probe. Biochemistry. 1998;37(36):12536–45.10.1021/bi980895gSearch in Google Scholar PubMed
[43] Uetsuka K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci. 2011;12(8):5213–37.10.3390/ijms12085213Search in Google Scholar PubMed PubMed Central
[44] Kourousekos GD, Theodosiadou EK, Lymberopoulos AG, Belibasaki S, Boscos C. Effect of aflatoxin B(1) on blood serum oestradiol-17β and progesterone concentrations during the luteal phase and the synchronized oestrus of goats. Anim Reprod. 2018;15(1):75–83. 10.21451/1984-3143-2017-ar939.Search in Google Scholar PubMed PubMed Central
[45] Adedara IA, Nanjappa MK, Farombi EO, Akingbemi BT. Aflatoxin B1 disrupts the androgen biosynthetic pathway in rat Leydig cells. Food Chem Toxicol. 2014;65:252–9. 10.1016/j.fct.2013.12.027.Search in Google Scholar PubMed
[46] Agnes VF, Akbarsha MA. Spermatotoxic effect of aflatoxin B(1) in the albino mouse. Food Chem Toxicol. 2003;41(1):119–30. 10.1016/s0278-6915(02)00171-0.Search in Google Scholar PubMed
[47] Uriah N, Ibeh IN, Oluwafemi F. A study on the impact of aflatoxin on human reproduction. Afr J Reprod Health. 2001;5(1):106–10.10.2307/3583204Search in Google Scholar
[48] Agnes VF, Akbarsha MA. Pale vacuolated epithelial cells in epididymis of aflatoxin-treated mice. Reproduction. 2001;122(4):629–41. 10.1530/rep.0.1220629.Search in Google Scholar PubMed
[49] Althnaian T, Albokhadaim I, El-Bahr S. Effect of aflatoxin B1 on histopathology and oxidative stress biomarkers in testis of rats with special references to gene expression of anti-oxidant enzymes. Int J Pharmacology. 2016;12(4):408–14.10.3923/ijp.2016.408.414Search in Google Scholar
[50] Wu K, Liu M, Wang H, Rajput SA, Al Zoubi OM, Wang S, et al. Effect of zearalenone on aflatoxin B1-induced intestinal and ovarian toxicity in pregnant and lactating rats. Ecotoxicol Env Saf. 2023;258:114976. 10.1016/j.ecoenv.2023.114976.Search in Google Scholar PubMed
[51] Hasanzadeh S, Amani S. Aflatoxin B1 effects on ovarian follicular growth and atresia in the rat. Comp Clin Pathol. 2013;22:563–72.10.1007/s00580-012-1446-1Search in Google Scholar
[52] Ibeh INSD. AFB1 and reproduction. I. Reproductive performance in female rats. Afr J Reprod Health. 1997;1:79–84.10.2307/3583378Search in Google Scholar
[53] Partanen HA, El-Nezami HS, Leppänen JM, Myllynen PK, Woodhouse HJ, Vähäkangas KH. Aflatoxin B1 transfer and metabolism in human placenta. Toxicol Sci. 2010;113(1):216–25. 10.1093/toxsci/kfp257.Search in Google Scholar PubMed
[54] Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41(9):740–55. 10.3109/10408444.2011.575766.Search in Google Scholar PubMed
[55] Abdulrazzaq Y, Padmanabhan R, Bastaki S, Kochyil J, Shafiullah M. Teratogenic effects of aflatoxin B1 in mice exposed in early and late gestation. Pediatric Res. 2011;70(5):405.10.1038/pr.2011.630Search in Google Scholar
[56] Bbosa GS, Kitya D, Odda J, Ogwal-Okeng J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health. 2013;5:14–34. 10.4236/health.2013.510A1003Search in Google Scholar
[57] Monosson E. Biotransformation. National Library of Medicine (NLM): The Encyclopeadia of earth; 2012. http://www.eoearth.org/view/article/150674.Search in Google Scholar
[58] Eaton DL, Gallagher EP, Bammler TK, Kunze KL. Role of cytochrome P4501A2 in chemical carcinogenesis: implications for human variability in expression and enzyme activity. Pharmacogenetics. 1995;5(5):259–74. 10.1097/00008571-199510000-00001.Search in Google Scholar
[59] Dhanasekaran D, Shanmugapriya S, Thajuddin N, Panneerselvam A. Aflatoxins and aflatoxicosis in human and animals. Aflatoxins-Biochem Mol Biol. 2011;10(22717):221–54.10.5772/22717Search in Google Scholar
[60] Zhang BC, Zhu YR, Wang JB, Wu Y, Zhang QN, Qian GS, et al. Oltipraz chemoprevention trial in Qidong, Jiangsu Province, People’s Republic of China. J Cell Biochem Suppl. 1997;28-29:166–73.10.1002/(SICI)1097-4644(1997)28/29+<166::AID-JCB20>3.0.CO;2-ESearch in Google Scholar
[61] Amin Y, Mohamed R, Zakaria A, Wehrend A, Hussein HA. Effects of aflatoxins on some reproductive hormones and composition of buffalo’s milk. Comp Clin Pathol. 2019;28(4):1191–6.10.1007/s00580-019-03006-wSearch in Google Scholar
[62] Bedard LL, Massey TE. Aflatoxin B1-induced DNA damage and its repair. Cancer Lett. 2006;241(2):174–83. 10.1016/j.canlet.2005.11.018.Search in Google Scholar
[63] Donnelly PJ, Stewart RK, Ali SL, Conlan AA, Reid KR, Petsikas D, et al. Biotransformation of aflatoxin B1 in human lung. Carcinogenesis. 1996;17(11):2487–94. 10.1093/carcin/17.11.2487.Search in Google Scholar
[64] Essigmann JM, Croy RG, Nadzan AM, Busby WF Jr, Reinhold VN, Büchi G, et al. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977;74(5):1870–4. 10.1073/pnas.74.5.1870.Search in Google Scholar
[65] Baertschi SW, Raney KD, Shimada T, Harris TM, Guengerich FP. Comparison of rates of enzymatic oxidation of aflatoxin B1, aflatoxin G1, and sterigmatocystin and activities of the epoxides in forming guanyl-N7 adducts and inducing different genetic responses. Chem Res Toxicol. 1989;2(2):114–2. 10.1021/tx00008a008.Search in Google Scholar
[66] Baertschi SW, Raney KD, Stone MP, Harris TM. Preparation of the 8, 9-epoxide of the mycotoxin aflatoxin B1: the ultimate carcinogenic species. J Am Chem Soc. 1988;110(23):7929–31.10.1021/ja00231a083Search in Google Scholar
[67] Johnson WW, Guengerich FP. Reaction of aflatoxin B1 exo-8,9-epoxide with DNA: kinetic analysis of covalent binding and DNA-induced hydrolysis. Proc Natl Acad Sci U S A. 1997;94(12):6121–5. 10.1073/pnas.94.12.6121.Search in Google Scholar PubMed PubMed Central
[68] Shen HM, Ong CN, Lee BL, Shi CY. Aflatoxin B1-induced 8-hydroxydeoxyguanosine formation in rat hepatic DNA. Carcinogenesis. 1995;16(2):419–22. 10.1093/carcin/16.2.419.Search in Google Scholar PubMed
[69] Yarborough A, Zhang YJ, Hsu TM, Santella RM. Immunoperoxidase detection of 8-hydroxydeoxyguanosine in aflatoxin B1-treated rat liver and human oral mucosal cells. Cancer Res. 1996;56(4):683–8.Search in Google Scholar
[70] Barraud L, Douki T, Guerret S, Chevallier M, Jamard C, Trepo C, et al. The role of duck hepatitis B virus and aflatoxin B1 in the induction of oxidative stress in the liver. Cancer Detect Prev. 2001;25(2):192–201.Search in Google Scholar
[71] Towner RA, Qian SY, Kadiiska MB, Mason RP. In vivo identification of aflatoxin-induced free radicals in rat bile. Free Radic Biol Med. 2003;35(10):1330–40. 10.1016/j.freeradbiomed.2003.08.002.Search in Google Scholar PubMed
[72] Shen HM, Shi CY, Shen Y, Ong CN. Detection of elevated reactive oxygen species level in cultured rat hepatocytes treated with aflatoxin B1. Free Radic Biol Med. 1996;21(2):139–46. 10.1016/0891-5849(96)00019-6.Search in Google Scholar PubMed
[73] Liao S, Shi D, Clemons-Chevis CL, Guo S, Su R, Qiang P, et al. Protective role of selenium on aflatoxin b1-induced hepatic dysfunction and apoptosis of liver in ducklings. Biol Trace Elem Res. 2014;162(1–3):296–301. 10.1007/s12011-014-0131-4.Search in Google Scholar PubMed
[74] Lei M, Zhang N, Qi D. In vitro investigation of individual and combined cytotoxic effects of aflatoxin B1 and other selected mycotoxins on the cell line porcine kidney 15. Exp Toxicol Pathol. 2013;65(7–8):1149–57. 10.1016/j.etp.2013.05.007.Search in Google Scholar PubMed
[75] Peng X, Zhang S, Fang J, Cui H, Zuo Z, Deng J. Protective roles of sodium selenite against aflatoxin B1-induced apoptosis of jejunum in broilers. Int J Environ Res Public Health. 2014;11(12):13130–43. 10.3390/ijerph111213130.Search in Google Scholar PubMed PubMed Central
[76] Chen K, Fang J, Peng X, Cui H, Chen J, Wang F, et al. Effect of selenium supplementation on aflatoxin B₁-induced histopathological lesions and apoptosis in bursa of Fabricius in broilers. Food Chem Toxicol. 2014;74:91–7. 10.1016/j.fct.2014.09.003.Search in Google Scholar PubMed
[77] Wang F, Shu G, Peng X, Fang J, Chen K, Cui H, et al. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int J Environ Res Public Health. 2013;10(7):2834–44. 10.3390/ijerph10072834.Search in Google Scholar PubMed PubMed Central
[78] Liu S, Li C, Dai D. Effect of ligustrazine on MDA, SOD and T-AOC in erythrocyte membrane in patients with hemoglobin H disease. J Clin Exp Med. 2010;11.Search in Google Scholar
[79] Yi X, Gang C, Shengjun W, Yongjun F, Ruijun C, Qinkai D. The effect of various pulse amplitudes on the amount of SOD, MDA, and T-AOC in the diaphagm of rabbit atter diaphragm pacing. Zhonghua Wuli Yixue Yu Kangfu Zazhi. 2004;26(5):269–71.Search in Google Scholar
[80] Souto NS, Claudia Monteiro Braga A, Lutchemeyer de Freitas M, Rechia Fighera M, Royes LFF, Schneider Oliveira M, et al. Aflatoxin B1 reduces nonenzymatic anti-oxidant defenses and increases protein kinase C activation in the cerebral cortex of young rats. Nutr Neurosci. 2018;21(4):268–75. 10.1080/1028415x.2017.1278837.Search in Google Scholar PubMed
[81] Kimbrough TD, Llewellyn GC, Weekley LB. The effect of aflatoxin B1 exposure on serotonin metabolism: response to a tryptophan load. Metab Brain Dis. 1992;7(4):175–82. 10.1007/bf01000244.Search in Google Scholar PubMed
[82] Coulombe RA Jr, Sharma RP. Clearance and excretion of intratracheally and orally administered aflatoxin B1 in the rat. Food Chem Toxicol. 1985;23(9):827–30. 10.1016/0278-6915(85)90283-2.Search in Google Scholar PubMed
[83] Egbunike GN, Ikegwuonu FI. Effect of aflatoxicosis on acetylcholinesterase activity in the brain and adenohypophysis of the male rat. Neurosci Lett. 1984;52(1–2):171–4. 10.1016/0304-3940(84)90369-0.Search in Google Scholar PubMed
[84] Lakkawar AW, Chattopadhyay SK, Johri TS. Experimental aflatoxin B1 toxicosis in young rabbits-a clinical and patho-anatomical study. Slovenian Veterinary Res. 2004;41(2):73–81.Search in Google Scholar
[85] Goerttler K, Löhrke H, Schweizer HJ, Hesse B. Effects of aflatoxin B1 on pregnant inbred Sprague-Dawley rats and their F1 generation. A contribution to transplacental carcinogenesis. J Natl Cancer Inst. 1980;64(6):1349–54. 10.1093/jnci/64.6.1349.Search in Google Scholar PubMed
[86] Kourousekos G, Lymberopoulos A. Occurrence of aflatoxins in milk and their effects on reproduction. J Hellenic Veterinary Med Soc. 2007;58(4):306–12.10.12681/jhvms.14994Search in Google Scholar
[87] Hasanzadeh S, Hosseini E, Rezazadeh L. Effects of aflatoxin B1 on profiles of gonadotropic (FSH and LH), steroid (testosterone and 17β-estradiol) and prolactin hormones in adult male rat. Iran J Vet Res. 2011;12(4):332–6.Search in Google Scholar
[88] Supriya C, Girish BP, Reddy PS. Aflatoxin B1-induced reproductive toxicity in male rats: Possible mechanism of action. Int J Toxicol. 2014;33(3):155–61. 10.1177/1091581814530764.Search in Google Scholar PubMed
[89] Stocco DM. The role of the StAR protein in steroidogenesis: challenges for the future. J Endocrinol. 2000;164(3):247–53. 10.1677/joe.0.1640247.Search in Google Scholar PubMed
[90] Faridha A, Faisal K, Akbarsha M. Duration-dependent histopathological and histometric changes in the testis of aflatoxin B1-treated mice. J Endocrinol Reprod. 2006;10(2):117–33.Search in Google Scholar
[91] Verma RJ, Nair A. Effect of aflatoxins on testicular steroidogenesis and amelioration by vitamin E. Food Chem Toxicol. 2002;40(5):669–72. 10.1016/s0278-6915(01)00131-4.Search in Google Scholar PubMed
[92] Egbunike GN, Emerole GO, Aire TA, Ikegwuonu FI. Sperm production rates, sperm physiology and fertility in rats chronically treated with sublethal doses of aflatoxin B1. Andrologia. 1980;12(5):467–75. 10.1111/j.1439-0272.1980.tb01702.x.Search in Google Scholar PubMed
[93] Ibrahim M, Salim M. Effect of aflatoxin B1 on steroid hormones in young male rats. J Env Sci. 1994;7(2):125–40.Search in Google Scholar
[94] Skinner MK. Cell-cell interactions in the testis. Endocr Rev. 1991;12(1):45–77.10.1210/edrv-12-1-45Search in Google Scholar PubMed
[95] Skinner MK. Cell-cell interactions in the testis. Ann N Y Acad Sci. 1987;513:158–71.10.1111/j.1749-6632.1987.tb25006.xSearch in Google Scholar PubMed
[96] Van Thiel DH, Sherins RJ, Myers Jr GH, De Vita VT Jr. Evidence for a specific seminiferous tubular factor affecting follicle-stimulating hormone secretion in man. J Clin Invest. 1972;51(4):1009–19. 10.1172/jci106861.Search in Google Scholar
[97] Storvik M, Huuskonen P, Kyllönen T, Lehtonen S, El-Nezami H, Auriola S, et al. Aflatoxin B1--a potential endocrine disruptor--up-regulates CYP19A1 in JEG-3 cells. Toxicol Lett. 2011;202(3):161–7. 10.1016/j.toxlet.2011.01.028.Search in Google Scholar PubMed
[98] Huuskonen P, Myllynen P, Storvik M, Pasanen M. The effects of aflatoxin B1 on transporters and steroid metabolizing enzymes in JEG-3 cells. Toxicol Lett. 2013;218(3):200–6. 10.1016/j.toxlet.2013.01.015.Search in Google Scholar PubMed
[99] Castelli D, Seralini GE, Lafaurie M, Krebs B, Stora C. Ovarian function during aflatoxin B1-induced hepatocarcinogenesis in the rat. Res Commun Chem Pathol Pharmacol. 1986;53(2):183–94.Search in Google Scholar
[100] Hawksworth DL, Lücking R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectr. 2017;5(4). 10.1128/microbiolspec.FUNK-0052-2016.Search in Google Scholar PubMed
[101] Selim MI, Popendorf W, Ibrahim MS, el Sharkawy S, el Kashory ES. Aflatoxin B1 in common Egyptian foods. J AOAC Int. 1996;79(5):1124–9.10.1093/jaoac/79.5.1124Search in Google Scholar
[102] Topham JC. Chemically-induced transmissible abnormalities in sperm-head shape. Mutat Res. 1980;70(1):109–14. 10.1016/0027-5107(80)90063-9.Search in Google Scholar PubMed
[103] Yamasaki T, Teel RW, Lau BH. Effect of allixin, a phytoalexin produced by garlic, on mutagenesis, DNA-binding and metabolism of aflatoxin B1. Cancer Lett. 1991;59(2):89–94. 10.1016/0304-3835(91)90171-d.Search in Google Scholar PubMed
[104] Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med. 2011;4:99–104. 10.2147/ijgm.S16275.Search in Google Scholar PubMed PubMed Central
[105] El-Azab SM, Abdelhamid A, Shalaby HA, Mehrim A, Ibrahim AH. Study of aflatoxin B1 as a risk factor that impairs the reproductive performance in females–Egypt. Toxicological & Env Chem. 2010;92(2):383–9.10.1080/02772240902927510Search in Google Scholar
[106] Tajik P, Mirshokraee P, Khosravi A. Effects of different concentrations of aflatoxin B on ram epididymal and ejaculatory sperm viability and motility in vitro. Pak J Biol Sci. 2007;10(24):4500–4. 10.3923/pjbs.2007.4500.4504.Search in Google Scholar PubMed
[107] Hasanzadeh S, Rezazadeh L. Effects of aflatoxin B1 on the growth processes of spermatogenic cell series in adult male rats. Comp Clin Pathol. 2013;22(4):555–62.10.1007/s00580-012-1445-2Search in Google Scholar
[108] Fapohunda SO, Ezekiel CN, Alabi OA, Omole A, Chioma SO. Aflatoxin-mediated sperm and blood cell abnormalities in mice fed with contaminated corn. Mycobiology. 2008;36(4):255–9. 10.4489/myco.2008.36.4.255.Search in Google Scholar PubMed PubMed Central
[109] Clarke RN, Doerr JA, Ottinger MA. Age-related changes in testicular development and reproductive endocrinology associated with aflatoxicosis in the male chicken. Biol Reprod. 1987;36(1):117–24. 10.1095/biolreprod36.1.117.Search in Google Scholar PubMed
[110] Murad AF, Ahmed S, Abead S. Toxicity effect of aflatoxin B1 on reproductive system of albino male rats. Pak J Biol Sci. 2015;18(3):107.10.3923/pjbs.2015.107.114Search in Google Scholar
[111] Coulombe RA Jr. Nonhepatic disposition and effects of aflatoxin B1. The toxicology of aflatoxins: Human health, veterinary and agricultural significance. Elsevier; 1994. p. 89–101.10.1016/B978-0-12-228255-3.50010-6Search in Google Scholar
[112] Maryamma K, Sivadas C. Aflatoxicosis in goats (an experimental study). Indian Vet J. 1975;52(2):385–92.Search in Google Scholar
[113] Doerr JA, Ottinger MA. Delayed reproductive development resulting from aflatoxicosis in juvenile Japanese quail. Poult Sci. 1980;59(9):1995–2001. 10.3382/ps.0591995.Search in Google Scholar PubMed
[114] Sharlin JS, Howarth B, Jr, Wyatt RD. Effect of dietary aflatoxin on reproductive performance of mature White Leghorn males. Poult Sci. 1980;59(6):1311–5. 10.3382/ps.0591311.Search in Google Scholar PubMed
[115] Faridha A, Faisal K, Akbarsha MA. Aflatoxin treatment brings about generation of multinucleate giant spermatids (symplasts) through opening of cytoplasmic bridges: light and transmission electron microscopic study in Swiss mouse. Reprod Toxicol. 2007;24(3–4):403–8. 10.1016/j.reprotox.2007.04.071.Search in Google Scholar PubMed
[116] El-Shewy EA, Ebrahem MF, editors. Ameliorative effect of vitamin E against the toxicity of aflatoxin B1 on rats with special reference to its effect on male fertility. Proceedings of the 1st Annual Conference FVM; 2004.Search in Google Scholar
[117] Supriya C, Akhila B, Pratap Reddy K, Girish BP, Sreenivasula Reddy P. Effects of maternal exposure to aflatoxin B1 during pregnancy on fertility output of dams and developmental, behavioral and reproductive consequences in female offspring using a rat model. Toxicol Mech Methods. 2016;26(3):202–10. 10.3109/15376516.2016.1151967.Search in Google Scholar PubMed
[118] Wangikar PB, Dwivedi P, Sinha N, Sharma AK, Telang AG. Effects of aflatoxin B1 on embryo fetal development in rabbits. Food Chem Toxicol. 2005;43(4):607–15. 10.1016/j.fct.2005.01.004.Search in Google Scholar PubMed
[119] Fetaih HA, Dessouki AA, Hassanin AA, Tahan AS. Toxopathological and cytogenetic effects of aflatoxin B1 (AFB1) on pregnant rats. Pathol Res Pract. 2014;210(12):1079–89. 10.1016/j.prp.2014.06.001.Search in Google Scholar PubMed
[120] Yang X, Ai X, Richard SA, Xu J. A silent congenital ethmoidal encephalocele progressing into frontoethmoidal meningoencephalocele with episodic seizures in adulthood: A case report and literature review. Adv Biosci ClMed. 2018;6(3):20–4.10.7575/aiac.abcmed.v.6n.3p.20Search in Google Scholar
[121] Richards CG. Frontoethmoidal meningoencephalocele: a common and severe congenital abnormality in South East Asia. Arch Dis Child. 1992;67(6):717–9. 10.1136/adc.67.6.717.Search in Google Scholar PubMed PubMed Central
[122] Aung T, Hta K. Epidemiology of frontoethmoidal encephalomeningocoele in Burma. J Epidemiol Community Health. 1984;38(2):89–98. 10.1136/jech.38.2.89.Search in Google Scholar PubMed PubMed Central
[123] da Silva JVB, de Oliveira CAF, Ramalho LNZ. Effects of prenatal exposure to aflatoxin B1: A review. Molecules. 2021;26(23). 10.3390/molecules26237312.Search in Google Scholar PubMed PubMed Central
[124] Orsi RB, Oliveira CA, Dilkin P, Xavier JG, Direito GM, Corrêa B. Effects of oral administration of aflatoxin B1 and fumonisin B1 in rabbits (Oryctolagus cuniculus). Chem Biol Interact. 2007;170(3):201–8. 10.1016/j.cbi.2007.08.002.Search in Google Scholar PubMed
[125] Supriya C, Reddy PS. Prenatal exposure to aflatoxin B1: developmental, behavioral, and reproductive alterations in male rats. Naturwissenschaften. 2015;102(5–6):26. 10.1007/s00114-015-1274-7.Search in Google Scholar PubMed
[126] Błaszczyk A, Osiecka R, Skolimowski J. Induction of chromosome aberrations in cultured human lymphocytes treated with ethoxyquin. Mutat Research/Genetic Toxicol Environ Mutagenesis. 2003;542(1–2):117–28.10.1016/j.mrgentox.2003.09.004Search in Google Scholar PubMed
[127] Ito Y, Ohnishi S, Fujie K. Chromosome aberrations induced by aflatoxin B1 in rat bone marrow cells in vivo and their suppression by green tea. Mutat Res. 1989;222(3):253–61. 10.1016/0165-1218(89)90141-9.Search in Google Scholar
[128] Abdel-Wahhab MA, Nada SA, Farag IM, Abbas NF, Amra HA. Potential protective effect of HSCAS and bentonite against dietary aflatoxicosis in rat: with special reference to chromosomal aberrations. Nat Toxins. 1998;6(5):211–8. 10.1002/(sici)1522-7189(199809/10)6:5<211: aid-nt31>3.0.co;2-8.Search in Google Scholar
[129] Bonnett M, Taylor ER. The structure of the aflatoxin B1-DNA adduct at N7 of guanine. Theoretical intercalation and covalent adduct models. J Biomolecular Structure Dyn. 1989;7(1):127–49.10.1080/07391102.1989.10507756Search in Google Scholar
[130] Sinha S, Prasad V. Effect of dietary concentration of crude aflatoxin on meiotic chromosomes, sperm morphology and sperm count in mice, Mus musculus. Proc Indian Natl Sci Acad Part B, Biol Sci. 1990;56(3):269–76.Search in Google Scholar
[131] Anwar WA, Khalil MM, Wild CP. Micronuclei, chromosomal aberrations and aflatoxin-albumin adducts in experimental animals after exposure to aflatoxin B1. Mutat Res. 1994;322(1):61–7. 10.1016/0165-1218(94)90033-7.Search in Google Scholar
[132] Ito Y, Ito M. Suppressive effect of (-)-epigallocatechin gallate on aflatoxin B1-induced chromosome aberrations in rat bone marrow cells. J Health Sci. 2001;47(3):248–57.10.1248/jhs.47.248Search in Google Scholar
[133] Abdel-Wahhab MA, Nada SA, Amra HA. Effect of aluminosilicates and bentonite on aflatoxin-induced developmental toxicity in rat. J Appl Toxicol. 1999;19(3):199–204. 10.1002/(sici)1099-1263(199905/06)19:3<199: aid-jat558>3.0.co;2-d.Search in Google Scholar
[134] Wangikar PB, Dwivedi P, Sharma AK, Sinha N. Effect in rats of simultaneous prenatal exposure to ochratoxin A and aflatoxin B1. II. Histopathological features of teratological anomalies induced in fetuses. Birth Defects Res B Dev Reprod Toxicol. 2004;71(6):352–8. 10.1002/bdrb.20022.Search in Google Scholar
[135] Wallace RA, King JL, Sanders GP. Biology, the science of life. Pearson Scott Foresman; 1986, 1217 pp.Search in Google Scholar
[136] El Darawany A. Nutritional and biological studies on mycotoxins [Egypt]. Egypt; 1985.Search in Google Scholar
[137] Nowar M, Sherif R, Gbreel G, El-Darawany A. Teratogenicity of aflatoxins (B1 + G1) mixture in rats and chick embryos. Egypt J Environ Mutagenesis, Teratogenesis Carcinogensis. 1986;2:6–12.Search in Google Scholar
[138] El-Nahla S, Imam H, Moussa E, Ibrahim A, Ghanam A. Teratogenic effects of aflatoxin in rabbits (Oryctolagus cuniculus). J Veterinary Anat. 2013;6(2):67–85.10.21608/jva.2013.45024Search in Google Scholar
[139] Arora RG, Frölén H, Nilsson A. Interference of mycotoxins with prenatal development of the mouse. I. Influence of aflatoxin B1, ochratoxin A and zearalenone. Acta Vet Scand. 1981;22(3-4):524–34. 10.1186/bf03548677.Search in Google Scholar
[140] Wangikar PB, Dwivedi P, Sinha N. Effect in rats of simultaneous prenatal exposure to ochratoxin A and aflatoxin B1. I. Maternal toxicity and fetal malformations. Birth Defects Res B Dev Reprod Toxicol. 2004;71(6):343–51. 10.1002/bdrb.20021.Search in Google Scholar
[141] Arora RG, Appelgren LE, Bergman A. Distribution of [14C]-labelled aflatoxin B1 in mice. Acta Pharmacol Toxicol (Copenh). 1978;43(4):273–9. 10.1111/j.1600-0773.1978.tb02265.x.Search in Google Scholar PubMed
[142] Su Q-Y. The toxification and detoxification mechanisms of aflatoxin b1 in human: an update:. In Xi-Dai long, aflatoxin B1 occurrence, detection and toxicological effects. IntechOpen; 2019.10.5772/intechopen.89221Search in Google Scholar
[143] Abdulmajeed NA. Therapeutic ability of some plant extracts on aflatoxin B1 induced renal and cardiac damage. Arab J Chem. 2011;4(1):1–10.10.1016/j.arabjc.2010.06.005Search in Google Scholar
[144] Naaz F, Javed S, Abdin MZ. Hepatoprotective effect of ethanolic extract of Phyllanthus amarus Schum. et Thonn. on aflatoxin B1-induced liver damage in mice. J Ethnopharmacol. 2007;113(3):503–9. 10.1016/j.jep.2007.07.017.Search in Google Scholar PubMed
[145] Choudhary A, Verma RJ. Ameliorative effects of black tea extract on aflatoxin-induced lipid peroxidation in the liver of mice. Food Chem Toxicol. 2005;43(1):99–104. 10.1016/j.fct.2004.08.016.Search in Google Scholar PubMed
[146] Sivanesan D, Begum VH. Preventive role of Gynandropsis gynandra L., against aflatoxin B1 induced lipid peroxidation and anti-oxidant defense mechanism in rat. Indian J Exp Biol. 2007;45(3):299–303.Search in Google Scholar
[147] Yousef MI, Salem MH, Kamel KI, Hassan GA, El-Nouty FD. Influence of ascorbic acid supplementation on the haematological and clinical biochemistry parameters of male rabbits exposed to aflatoxin B1. J Env Sci Health B. 2003;38(2):193–209. 10.1081/pfc-120018449.Search in Google Scholar PubMed
[148] Traber MG, Atkinson J. Vitamin E, anti-oxidant and nothing more. Free Radic Biol Med. 2007;43(1):4–15. 10.1016/j.freeradbiomed.2007.03.024.Search in Google Scholar PubMed PubMed Central
[149] Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. Faseb J. 1999;13(10):1145–55.10.1096/fasebj.13.10.1145Search in Google Scholar
[150] Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res. 2000;39(3):231–55. 10.1016/s0163-7827(00)00006-0.Search in Google Scholar PubMed
[151] Johnson NM, Egner PA, Baxter VK, Sporn MB, Wible RS, Sutter TR, et al. Complete protection against aflatoxin B(1)-induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer Prev Res (Phila). 2014;7(7):658–65. 10.1158/1940-6207.Capr-13-0430.Search in Google Scholar
[152] Roebuck BD, Liu YL, Rogers AE, Groopman JD, Kensler TW. Protection against aflatoxin B1-induced hepatocarcinogenesis in F344 rats by 5-(2-pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz): predictive role for short-term molecular dosimetry. Cancer Res. 1991;51(20):5501–6.Search in Google Scholar
[153] Kensler TW, Gange SJ, Egner PA, Dolan PM, Muñoz A, Groopman JD, et al. Predictive value of molecular dosimetry: individual versus group effects of oltipraz on aflatoxin-albumin adducts and risk of liver cancer. Cancer Epidemiol Biomarkers Prev. 1997;6(8):603–10.Search in Google Scholar
[154] Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci. 2011;120(Suppl 1):S28–48. 10.1093/toxsci/kfq283.Search in Google Scholar PubMed PubMed Central
[155] Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Cancer Inst. 1999;91(4):347–54. 10.1093/jnci/91.4.347.Search in Google Scholar PubMed
[156] Yates MS, Kwak M-K, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-, 12-dioxooleana-1, 9 (11)-dien-28-oyl] imidazole. Cancer Res. 2006;66(4):2488–94.10.1158/0008-5472.CAN-05-3823Search in Google Scholar PubMed
[157] Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102(12):4584–9. 10.1073/pnas.0500815102.Search in Google Scholar PubMed PubMed Central
[158] Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, et al. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6(1):154–62. 10.1158/1535-7163.Mct-06-0516.Search in Google Scholar PubMed
[159] Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5(12):3232–9. 10.1158/1535-7163.Mct-06-0444.Search in Google Scholar
[160] Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, et al. The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice. Cancer Res. 2007;67(6):2414–9. 10.1158/0008-5472.Can-06-4534.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- EDNRB inhibits the growth and migration of prostate cancer cells by activating the cGMP-PKG pathway
- STK11 (LKB1) mutation suppresses ferroptosis in lung adenocarcinoma by facilitating monounsaturated fatty acid synthesis
- Association of SOX6 gene polymorphisms with Kashin-Beck disease risk in the Chinese Han population
- The pyroptosis-related signature predicts prognosis and influences the tumor immune microenvironment in dedifferentiated liposarcoma
- METTL3 attenuates ferroptosis sensitivity in lung cancer via modulating TFRC
- Identification and validation of molecular subtypes and prognostic signature for stage I and stage II gastric cancer based on neutrophil extracellular traps
- Novel lumbar plexus block versus femoral nerve block for analgesia and motor recovery after total knee arthroplasty
- Correlation between ABCB1 and OLIG2 polymorphisms and the severity and prognosis of patients with cerebral infarction
- Study on the radiotherapy effect and serum neutral granulocyte lymphocyte ratio and inflammatory factor expression of nasopharyngeal carcinoma
- Transcriptome analysis of effects of Tecrl deficiency on cardiometabolic and calcium regulation in cardiac tissue
- Aflatoxin B1 induces infertility, fetal deformities, and potential therapies
- Serum levels of HMW adiponectin and its receptors are associated with cytokine levels and clinical characteristics in chronic obstructive pulmonary disease
- METTL3-mediated methylation of CYP2C19 mRNA may aggravate clopidogrel resistance in ischemic stroke patients
- Understand how machine learning impact lung cancer research from 2010 to 2021: A bibliometric analysis
- Pressure ulcers in German hospitals: Analysis of reimbursement and length of stay
- Metformin plus L-carnitine enhances brown/beige adipose tissue activity via Nrf2/HO-1 signaling to reduce lipid accumulation and inflammation in murine obesity
- Downregulation of carbonic anhydrase IX expression in mouse xenograft nasopharyngeal carcinoma model via doxorubicin nanobubble combined with ultrasound
- Feasibility of 3-dimensional printed models in simulated training and teaching of transcatheter aortic valve replacement
- miR-335-3p improves type II diabetes mellitus by IGF-1 regulating macrophage polarization
- The analyses of human MCPH1 DNA repair machinery and genetic variations
- Activation of Piezo1 increases the sensitivity of breast cancer to hyperthermia therapy
- Comprehensive analysis based on the disulfidptosis-related genes identifies hub genes and immune infiltration for pancreatic adenocarcinoma
- Changes of serum CA125 and PGE2 before and after high-intensity focused ultrasound combined with GnRH-a in treatment of patients with adenomyosis
- The clinical value of the hepatic venous pressure gradient in patients undergoing hepatic resection for hepatocellular carcinoma with or without liver cirrhosis
- Development and validation of a novel model to predict pulmonary embolism in cardiology suspected patients: A 10-year retrospective analysis
- Downregulation of lncRNA XLOC_032768 in diabetic patients predicts the occurrence of diabetic nephropathy
- Circ_0051428 targeting miR-885-3p/MMP2 axis enhances the malignancy of cervical cancer
- Effectiveness of ginkgo diterpene lactone meglumine on cognitive function in patients with acute ischemic stroke
- The construction of a novel prognostic prediction model for glioma based on GWAS-identified prognostic-related risk loci
- Evaluating the impact of childhood BMI on the risk of coronavirus disease 2019: A Mendelian randomization study
- Lactate dehydrogenase to albumin ratio is associated with in-hospital mortality in patients with acute heart failure: Data from the MIMIC-III database
- CD36-mediated podocyte lipotoxicity promotes foot process effacement
- Efficacy of etonogestrel subcutaneous implants versus the levonorgestrel-releasing intrauterine system in the conservative treatment of adenomyosis
- FLRT2 mediates chondrogenesis of nasal septal cartilage and mandibular condyle cartilage
- Challenges in treating primary immune thrombocytopenia patients undergoing COVID-19 vaccination: A retrospective study
- Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway
- Phospholipid transfer protein ameliorates sepsis-induced cardiac dysfunction through NLRP3 inflammasome inhibition
- Postoperative cognitive dysfunction in elderly patients with colorectal cancer: A randomized controlled study comparing goal-directed and conventional fluid therapy
- Long-pulsed ultrasound-mediated microbubble thrombolysis in a rat model of microvascular obstruction
- High SEC61A1 expression predicts poor outcome of acute myeloid leukemia
- Comparison of polymerase chain reaction and next-generation sequencing with conventional urine culture for the diagnosis of urinary tract infections: A meta-analysis
- Secreted frizzled-related protein 5 protects against renal fibrosis by inhibiting Wnt/β-catenin pathway
- Pan-cancer and single-cell analysis of actin cytoskeleton genes related to disulfidptosis
- Overexpression of miR-532-5p restrains oxidative stress response of chondrocytes in nontraumatic osteonecrosis of the femoral head by inhibiting ABL1
- Autologous liver transplantation for unresectable hepatobiliary malignancies in enhanced recovery after surgery model
- Clinical analysis of incomplete rupture of the uterus secondary to previous cesarean section
- Abnormal sleep duration is associated with sarcopenia in older Chinese people: A large retrospective cross-sectional study
- No genetic causality between obesity and benign paroxysmal vertigo: A two-sample Mendelian randomization study
- Identification and validation of autophagy-related genes in SSc
- Long non-coding RNA SRA1 suppresses radiotherapy resistance in esophageal squamous cell carcinoma by modulating glycolytic reprogramming
- Evaluation of quality of life in patients with schizophrenia: An inpatient social welfare institution-based cross-sectional study
- The possible role of oxidative stress marker glutathione in the assessment of cognitive impairment in multiple sclerosis
- Compilation of a self-management assessment scale for postoperative patients with aortic dissection
- Left atrial appendage closure in conjunction with radiofrequency ablation: Effects on left atrial functioning in patients with paroxysmal atrial fibrillation
- Effect of anterior femoral cortical notch grade on postoperative function and complications during TKA surgery: A multicenter, retrospective study
- Clinical characteristics and assessment of risk factors in patients with influenza A-induced severe pneumonia after the prevalence of SARS-CoV-2
- Analgesia nociception index is an indicator of laparoscopic trocar insertion-induced transient nociceptive stimuli
- High STAT4 expression correlates with poor prognosis in acute myeloid leukemia and facilitates disease progression by upregulating VEGFA expression
- Factors influencing cardiovascular system-related post-COVID-19 sequelae: A single-center cohort study
- HOXD10 regulates intestinal permeability and inhibits inflammation of dextran sulfate sodium-induced ulcerative colitis through the inactivation of the Rho/ROCK/MMPs axis
- Mesenchymal stem cell-derived exosomal miR-26a induces ferroptosis, suppresses hepatic stellate cell activation, and ameliorates liver fibrosis by modulating SLC7A11
- Endovascular thrombectomy versus intravenous thrombolysis for primary distal, medium vessel occlusion in acute ischemic stroke
- ANO6 (TMEM16F) inhibits gastrointestinal stromal tumor growth and induces ferroptosis
- Prognostic value of EIF5A2 in solid tumors: A meta-analysis and bioinformatics analysis
- The role of enhanced expression of Cx43 in patients with ulcerative colitis
- Choosing a COVID-19 vaccination site might be driven by anxiety and body vigilance
- Role of ICAM-1 in triple-negative breast cancer
- Cost-effectiveness of ambroxol in the treatment of Gaucher disease type 2
- HLA-DRB5 promotes immune thrombocytopenia via activating CD8+ T cells
- Efficacy and factors of myofascial release therapy combined with electrical and magnetic stimulation in the treatment of chronic pelvic pain syndrome
- Efficacy of tacrolimus monotherapy in primary membranous nephropathy
- Mechanisms of Tripterygium wilfordii Hook F on treating rheumatoid arthritis explored by network pharmacology analysis and molecular docking
- FBXO45 levels regulated ferroptosis renal tubular epithelial cells in a model of diabetic nephropathy by PLK1
- Optimizing anesthesia strategies to NSCLC patients in VATS procedures: Insights from drug requirements and patient recovery patterns
- Alpha-lipoic acid upregulates the PPARγ/NRF2/GPX4 signal pathway to inhibit ferroptosis in the pathogenesis of unexplained recurrent pregnancy loss
- Correlation between fat-soluble vitamin levels and inflammatory factors in paediatric community-acquired pneumonia: A prospective study
- CD1d affects the proliferation, migration, and apoptosis of human papillary thyroid carcinoma TPC-1 cells via regulating MAPK/NF-κB signaling pathway
- miR-let-7a inhibits sympathetic nerve remodeling after myocardial infarction by downregulating the expression of nerve growth factor
- Immune response analysis of solid organ transplantation recipients inoculated with inactivated COVID-19 vaccine: A retrospective analysis
- The H2Valdien derivatives regulate the epithelial–mesenchymal transition of hepatoma carcinoma cells through the Hedgehog signaling pathway
- Clinical efficacy of dexamethasone combined with isoniazid in the treatment of tuberculous meningitis and its effect on peripheral blood T cell subsets
- Comparison of short-segment and long-segment fixation in treatment of degenerative scoliosis and analysis of factors associated with adjacent spondylolisthesis
- Lycopene inhibits pyroptosis of endothelial progenitor cells induced by ox-LDL through the AMPK/mTOR/NLRP3 pathway
- Methylation regulation for FUNDC1 stability in childhood leukemia was up-regulated and facilitates metastasis and reduces ferroptosis of leukemia through mitochondrial damage by FBXL2
- Correlation of single-fiber electromyography studies and functional status in patients with amyotrophic lateral sclerosis
- Risk factors of postoperative airway obstruction complications in children with oral floor mass
- Expression levels and clinical significance of serum miR-19a/CCL20 in patients with acute cerebral infarction
- Physical activity and mental health trends in Korean adolescents: Analyzing the impact of the COVID-19 pandemic from 2018 to 2022
- Evaluating anemia in HIV-infected patients using chest CT
- Ponticulus posticus and skeletal malocclusion: A pilot study in a Southern Italian pre-orthodontic court
- Causal association of circulating immune cells and lymphoma: A Mendelian randomization study
- Assessment of the renal function and fibrosis indexes of conventional western medicine with Chinese medicine for dredging collaterals on treating renal fibrosis: A systematic review and meta-analysis
- Comprehensive landscape of integrator complex subunits and their association with prognosis and tumor microenvironment in gastric cancer
- New target-HMGCR inhibitors for the treatment of primary sclerosing cholangitis: A drug Mendelian randomization study
- Population pharmacokinetics of meropenem in critically ill patients
- Comparison of the ability of newly inflammatory markers to predict complicated appendicitis
- Comparative morphology of the cruciate ligaments: A radiological study
- Immune landscape of hepatocellular carcinoma: The central role of TP53-inducible glycolysis and apoptosis regulator
- Serum SIRT3 levels in epilepsy patients and its association with clinical outcomes and severity: A prospective observational study
- SHP-1 mediates cigarette smoke extract-induced epithelial–mesenchymal transformation and inflammation in 16HBE cells
- Acute hyper-hypoxia accelerates the development of depression in mice via the IL-6/PGC1α/MFN2 signaling pathway
- The GJB3 correlates with the prognosis, immune cell infiltration, and therapeutic responses in lung adenocarcinoma
- Physical fitness and blood parameters outcomes of breast cancer survivor in a low-intensity circuit resistance exercise program
- Exploring anesthetic-induced gene expression changes and immune cell dynamics in atrial tissue post-coronary artery bypass graft surgery
- Empagliflozin improves aortic injury in obese mice by regulating fatty acid metabolism
- Analysis of the risk factors of the radiation-induced encephalopathy in nasopharyngeal carcinoma: A retrospective cohort study
- Reproductive outcomes in women with BRCA 1/2 germline mutations: A retrospective observational study and literature review
- Evaluation of upper airway ultrasonographic measurements in predicting difficult intubation: A cross-section of the Turkish population
- Prognostic and diagnostic value of circulating IGFBP2 in pancreatic cancer
- Postural stability after operative reconstruction of the AFTL in chronic ankle instability comparing three different surgical techniques
- Research trends related to emergence agitation in the post-anaesthesia care unit from 2001 to 2023: A bibliometric analysis
- Frequency and clinicopathological correlation of gastrointestinal polyps: A six-year single center experience
- ACSL4 mediates inflammatory bowel disease and contributes to LPS-induced intestinal epithelial cell dysfunction by activating ferroptosis and inflammation
- Affibody-based molecular probe 99mTc-(HE)3ZHER2:V2 for non-invasive HER2 detection in ovarian and breast cancer xenografts
- Effectiveness of nutritional support for clinical outcomes in gastric cancer patients: A meta-analysis of randomized controlled trials
- The relationship between IFN-γ, IL-10, IL-6 cytokines, and severity of the condition with serum zinc and Fe in children infected with Mycoplasma pneumoniae
- Paraquat disrupts the blood–brain barrier by increasing IL-6 expression and oxidative stress through the activation of PI3K/AKT signaling pathway
- Sleep quality associate with the increased prevalence of cognitive impairment in coronary artery disease patients: A retrospective case–control study
- Dioscin protects against chronic prostatitis through the TLR4/NF-κB pathway
- Association of polymorphisms in FBN1, MYH11, and TGF-β signaling-related genes with susceptibility of sporadic thoracic aortic aneurysm and dissection in the Zhejiang Han population
- Application value of multi-parameter magnetic resonance image-transrectal ultrasound cognitive fusion in prostate biopsy
- Laboratory variables‐based artificial neural network models for predicting fatty liver disease: A retrospective study
- Decreased BIRC5-206 promotes epithelial–mesenchymal transition in nasopharyngeal carcinoma through sponging miR-145-5p
- Sepsis induces the cardiomyocyte apoptosis and cardiac dysfunction through activation of YAP1/Serpine1/caspase-3 pathway
- Assessment of iron metabolism and iron deficiency in incident patients on incident continuous ambulatory peritoneal dialysis
- Tibial periosteum flap combined with autologous bone grafting in the treatment of Gustilo-IIIB/IIIC open tibial fractures
- The application of intravenous general anesthesia under nasopharyngeal airway assisted ventilation undergoing ureteroscopic holmium laser lithotripsy: A prospective, single-center, controlled trial
- Long intergenic noncoding RNA for IGF2BP2 stability suppresses gastric cancer cell apoptosis by inhibiting the maturation of microRNA-34a
- Role of FOXM1 and AURKB in regulating keratinocyte function in psoriasis
- Parental control attitudes over their pre-school children’s diet
- The role of auto-HSCT in extranodal natural killer/T cell lymphoma
- Significance of negative cervical cytology and positive HPV in the diagnosis of cervical lesions by colposcopy
- Echinacoside inhibits PASMCs calcium overload to prevent hypoxic pulmonary artery remodeling by regulating TRPC1/4/6 and calmodulin
- ADAR1 plays a protective role in proximal tubular cells under high glucose conditions by attenuating the PI3K/AKT/mTOR signaling pathway
- The risk of cancer among insulin glargine users in Lithuania: A retrospective population-based study
- The unusual location of primary hydatid cyst: A case series study
- Intraoperative changes in electrophysiological monitoring can be used to predict clinical outcomes in patients with spinal cavernous malformation
- Obesity and risk of placenta accreta spectrum: A meta-analysis
- Shikonin alleviates asthma phenotypes in mice via an airway epithelial STAT3-dependent mechanism
- NSUN6 and HTR7 disturbed the stability of carotid atherosclerotic plaques by regulating the immune responses of macrophages
- The effect of COVID-19 lockdown on admission rates in Maternity Hospital
- Temporal muscle thickness is not a prognostic predictor in patients with high-grade glioma, an experience at two centers in China
- Luteolin alleviates cerebral ischemia/reperfusion injury by regulating cell pyroptosis
- Therapeutic role of respiratory exercise in patients with tuberculous pleurisy
- Effects of CFTR-ENaC on spinal cord edema after spinal cord injury
- Irisin-regulated lncRNAs and their potential regulatory functions in chondrogenic differentiation of human mesenchymal stem cells
- DMD mutations in pediatric patients with phenotypes of Duchenne/Becker muscular dystrophy
- Combination of C-reactive protein and fibrinogen-to-albumin ratio as a novel predictor of all-cause mortality in heart failure patients
- Significant role and the underly mechanism of cullin-1 in chronic obstructive pulmonary disease
- Ferroptosis-related prognostic model of mantle cell lymphoma
- Observation of choking reaction and other related indexes in elderly painless fiberoptic bronchoscopy with transnasal high-flow humidification oxygen therapy
- A bibliometric analysis of Prader-Willi syndrome from 2002 to 2022
- The causal effects of childhood sunburn occasions on melanoma: A univariable and multivariable Mendelian randomization study
- Oxidative stress regulates glycogen synthase kinase-3 in lymphocytes of diabetes mellitus patients complicated with cerebral infarction
- Role of COX6C and NDUFB3 in septic shock and stroke
- Trends in disease burden of type 2 diabetes, stroke, and hypertensive heart disease attributable to high BMI in China: 1990–2019
- Purinergic P2X7 receptor mediates hyperoxia-induced injury in pulmonary microvascular endothelial cells via NLRP3-mediated pyroptotic pathway
- Investigating the role of oviductal mucosa–endometrial co-culture in modulating factors relevant to embryo implantation
- Analgesic effect of external oblique intercostal block in laparoscopic cholecystectomy: A retrospective study
- Elevated serum miR-142-5p correlates with ischemic lesions and both NSE and S100β in ischemic stroke patients
- Correlation between the mechanism of arteriopathy in IgA nephropathy and blood stasis syndrome: A cohort study
- Risk factors for progressive kyphosis after percutaneous kyphoplasty in osteoporotic vertebral compression fracture
- Predictive role of neuron-specific enolase and S100-β in early neurological deterioration and unfavorable prognosis in patients with ischemic stroke
- The potential risk factors of postoperative cognitive dysfunction for endovascular therapy in acute ischemic stroke with general anesthesia
- Fluoxetine inhibited RANKL-induced osteoclastic differentiation in vitro
- Detection of serum FOXM1 and IGF2 in patients with ARDS and their correlation with disease and prognosis
- Rhein promotes skin wound healing by activating the PI3K/AKT signaling pathway
- Differences in mortality risk by levels of physical activity among persons with disabilities in South Korea
- Review Articles
- Cutaneous signs of selected cardiovascular disorders: A narrative review
- XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis
- A narrative review on adverse drug reactions of COVID-19 treatments on the kidney
- Emerging role and function of SPDL1 in human health and diseases
- Adverse reactions of piperacillin: A literature review of case reports
- Molecular mechanism and intervention measures of microvascular complications in diabetes
- Regulation of mesenchymal stem cell differentiation by autophagy
- Molecular landscape of borderline ovarian tumours: A systematic review
- Advances in synthetic lethality modalities for glioblastoma multiforme
- Investigating hormesis, aging, and neurodegeneration: From bench to clinics
- Frankincense: A neuronutrient to approach Parkinson’s disease treatment
- Sox9: A potential regulator of cancer stem cells in osteosarcoma
- Early detection of cardiovascular risk markers through non-invasive ultrasound methodologies in periodontitis patients
- Advanced neuroimaging and criminal interrogation in lie detection
- Maternal factors for neural tube defects in offspring: An umbrella review
- The chemoprotective hormetic effects of rosmarinic acid
- CBD’s potential impact on Parkinson’s disease: An updated overview
- Progress in cytokine research for ARDS: A comprehensive review
- Utilizing reactive oxygen species-scavenging nanoparticles for targeting oxidative stress in the treatment of ischemic stroke: A review
- NRXN1-related disorders, attempt to better define clinical assessment
- Lidocaine infusion for the treatment of complex regional pain syndrome: Case series and literature review
- Trends and future directions of autophagy in osteosarcoma: A bibliometric analysis
- Iron in ventricular remodeling and aneurysms post-myocardial infarction
- Case Reports
- Sirolimus potentiated angioedema: A case report and review of the literature
- Identification of mixed anaerobic infections after inguinal hernia repair based on metagenomic next-generation sequencing: A case report
- Successful treatment with bortezomib in combination with dexamethasone in a middle-aged male with idiopathic multicentric Castleman’s disease: A case report
- Complete heart block associated with hepatitis A infection in a female child with fatal outcome
- Elevation of D-dimer in eosinophilic gastrointestinal diseases in the absence of venous thrombosis: A case series and literature review
- Four years of natural progressive course: A rare case report of juvenile Xp11.2 translocations renal cell carcinoma with TFE3 gene fusion
- Advancing prenatal diagnosis: Echocardiographic detection of Scimitar syndrome in China – A case series
- Outcomes and complications of hemodialysis in patients with renal cancer following bilateral nephrectomy
- Anti-HMGCR myopathy mimicking facioscapulohumeral muscular dystrophy
- Recurrent opportunistic infections in a HIV-negative patient with combined C6 and NFKB1 mutations: A case report, pedigree analysis, and literature review
- Letter to the Editor
- Letter to the Editor: Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Erratum
- Erratum to “Bladder-embedded ectopic intrauterine device with calculus”
- Retraction
- Retraction of “XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis”
- Corrigendum
- Corrigendum to “Investigating hormesis, aging, and neurodegeneration: From bench to clinics”
- Corrigendum to “Frankincense: A neuronutrient to approach Parkinson’s disease treatment”
- Special Issue The evolving saga of RNAs from bench to bedside - Part II
- Machine-learning-based prediction of a diagnostic model using autophagy-related genes based on RNA sequencing for patients with papillary thyroid carcinoma
- Unlocking the future of hepatocellular carcinoma treatment: A comprehensive analysis of disulfidptosis-related lncRNAs for prognosis and drug screening
- Elevated mRNA level indicates FSIP1 promotes EMT and gastric cancer progression by regulating fibroblasts in tumor microenvironment
- Special Issue Advancements in oncology: bridging clinical and experimental research - Part I
- Ultrasound-guided transperineal vs transrectal prostate biopsy: A meta-analysis of diagnostic accuracy and complication rates
- Assessment of diagnostic value of unilateral systematic biopsy combined with targeted biopsy in detecting clinically significant prostate cancer
- SENP7 inhibits glioblastoma metastasis and invasion by dissociating SUMO2/3 binding to specific target proteins
- MARK1 suppress malignant progression of hepatocellular carcinoma and improves sorafenib resistance through negatively regulating POTEE
- Analysis of postoperative complications in bladder cancer patients
- Carboplatin combined with arsenic trioxide versus carboplatin combined with docetaxel treatment for LACC: A randomized, open-label, phase II clinical study
- Special Issue Exploring the biological mechanism of human diseases based on MultiOmics Technology - Part I
- Comprehensive pan-cancer investigation of carnosine dipeptidase 1 and its prospective prognostic significance in hepatocellular carcinoma
- Identification of signatures associated with microsatellite instability and immune characteristics to predict the prognostic risk of colon cancer
- Single-cell analysis identified key macrophage subpopulations associated with atherosclerosis
Articles in the same Issue
- Research Articles
- EDNRB inhibits the growth and migration of prostate cancer cells by activating the cGMP-PKG pathway
- STK11 (LKB1) mutation suppresses ferroptosis in lung adenocarcinoma by facilitating monounsaturated fatty acid synthesis
- Association of SOX6 gene polymorphisms with Kashin-Beck disease risk in the Chinese Han population
- The pyroptosis-related signature predicts prognosis and influences the tumor immune microenvironment in dedifferentiated liposarcoma
- METTL3 attenuates ferroptosis sensitivity in lung cancer via modulating TFRC
- Identification and validation of molecular subtypes and prognostic signature for stage I and stage II gastric cancer based on neutrophil extracellular traps
- Novel lumbar plexus block versus femoral nerve block for analgesia and motor recovery after total knee arthroplasty
- Correlation between ABCB1 and OLIG2 polymorphisms and the severity and prognosis of patients with cerebral infarction
- Study on the radiotherapy effect and serum neutral granulocyte lymphocyte ratio and inflammatory factor expression of nasopharyngeal carcinoma
- Transcriptome analysis of effects of Tecrl deficiency on cardiometabolic and calcium regulation in cardiac tissue
- Aflatoxin B1 induces infertility, fetal deformities, and potential therapies
- Serum levels of HMW adiponectin and its receptors are associated with cytokine levels and clinical characteristics in chronic obstructive pulmonary disease
- METTL3-mediated methylation of CYP2C19 mRNA may aggravate clopidogrel resistance in ischemic stroke patients
- Understand how machine learning impact lung cancer research from 2010 to 2021: A bibliometric analysis
- Pressure ulcers in German hospitals: Analysis of reimbursement and length of stay
- Metformin plus L-carnitine enhances brown/beige adipose tissue activity via Nrf2/HO-1 signaling to reduce lipid accumulation and inflammation in murine obesity
- Downregulation of carbonic anhydrase IX expression in mouse xenograft nasopharyngeal carcinoma model via doxorubicin nanobubble combined with ultrasound
- Feasibility of 3-dimensional printed models in simulated training and teaching of transcatheter aortic valve replacement
- miR-335-3p improves type II diabetes mellitus by IGF-1 regulating macrophage polarization
- The analyses of human MCPH1 DNA repair machinery and genetic variations
- Activation of Piezo1 increases the sensitivity of breast cancer to hyperthermia therapy
- Comprehensive analysis based on the disulfidptosis-related genes identifies hub genes and immune infiltration for pancreatic adenocarcinoma
- Changes of serum CA125 and PGE2 before and after high-intensity focused ultrasound combined with GnRH-a in treatment of patients with adenomyosis
- The clinical value of the hepatic venous pressure gradient in patients undergoing hepatic resection for hepatocellular carcinoma with or without liver cirrhosis
- Development and validation of a novel model to predict pulmonary embolism in cardiology suspected patients: A 10-year retrospective analysis
- Downregulation of lncRNA XLOC_032768 in diabetic patients predicts the occurrence of diabetic nephropathy
- Circ_0051428 targeting miR-885-3p/MMP2 axis enhances the malignancy of cervical cancer
- Effectiveness of ginkgo diterpene lactone meglumine on cognitive function in patients with acute ischemic stroke
- The construction of a novel prognostic prediction model for glioma based on GWAS-identified prognostic-related risk loci
- Evaluating the impact of childhood BMI on the risk of coronavirus disease 2019: A Mendelian randomization study
- Lactate dehydrogenase to albumin ratio is associated with in-hospital mortality in patients with acute heart failure: Data from the MIMIC-III database
- CD36-mediated podocyte lipotoxicity promotes foot process effacement
- Efficacy of etonogestrel subcutaneous implants versus the levonorgestrel-releasing intrauterine system in the conservative treatment of adenomyosis
- FLRT2 mediates chondrogenesis of nasal septal cartilage and mandibular condyle cartilage
- Challenges in treating primary immune thrombocytopenia patients undergoing COVID-19 vaccination: A retrospective study
- Let-7 family regulates HaCaT cell proliferation and apoptosis via the ΔNp63/PI3K/AKT pathway
- Phospholipid transfer protein ameliorates sepsis-induced cardiac dysfunction through NLRP3 inflammasome inhibition
- Postoperative cognitive dysfunction in elderly patients with colorectal cancer: A randomized controlled study comparing goal-directed and conventional fluid therapy
- Long-pulsed ultrasound-mediated microbubble thrombolysis in a rat model of microvascular obstruction
- High SEC61A1 expression predicts poor outcome of acute myeloid leukemia
- Comparison of polymerase chain reaction and next-generation sequencing with conventional urine culture for the diagnosis of urinary tract infections: A meta-analysis
- Secreted frizzled-related protein 5 protects against renal fibrosis by inhibiting Wnt/β-catenin pathway
- Pan-cancer and single-cell analysis of actin cytoskeleton genes related to disulfidptosis
- Overexpression of miR-532-5p restrains oxidative stress response of chondrocytes in nontraumatic osteonecrosis of the femoral head by inhibiting ABL1
- Autologous liver transplantation for unresectable hepatobiliary malignancies in enhanced recovery after surgery model
- Clinical analysis of incomplete rupture of the uterus secondary to previous cesarean section
- Abnormal sleep duration is associated with sarcopenia in older Chinese people: A large retrospective cross-sectional study
- No genetic causality between obesity and benign paroxysmal vertigo: A two-sample Mendelian randomization study
- Identification and validation of autophagy-related genes in SSc
- Long non-coding RNA SRA1 suppresses radiotherapy resistance in esophageal squamous cell carcinoma by modulating glycolytic reprogramming
- Evaluation of quality of life in patients with schizophrenia: An inpatient social welfare institution-based cross-sectional study
- The possible role of oxidative stress marker glutathione in the assessment of cognitive impairment in multiple sclerosis
- Compilation of a self-management assessment scale for postoperative patients with aortic dissection
- Left atrial appendage closure in conjunction with radiofrequency ablation: Effects on left atrial functioning in patients with paroxysmal atrial fibrillation
- Effect of anterior femoral cortical notch grade on postoperative function and complications during TKA surgery: A multicenter, retrospective study
- Clinical characteristics and assessment of risk factors in patients with influenza A-induced severe pneumonia after the prevalence of SARS-CoV-2
- Analgesia nociception index is an indicator of laparoscopic trocar insertion-induced transient nociceptive stimuli
- High STAT4 expression correlates with poor prognosis in acute myeloid leukemia and facilitates disease progression by upregulating VEGFA expression
- Factors influencing cardiovascular system-related post-COVID-19 sequelae: A single-center cohort study
- HOXD10 regulates intestinal permeability and inhibits inflammation of dextran sulfate sodium-induced ulcerative colitis through the inactivation of the Rho/ROCK/MMPs axis
- Mesenchymal stem cell-derived exosomal miR-26a induces ferroptosis, suppresses hepatic stellate cell activation, and ameliorates liver fibrosis by modulating SLC7A11
- Endovascular thrombectomy versus intravenous thrombolysis for primary distal, medium vessel occlusion in acute ischemic stroke
- ANO6 (TMEM16F) inhibits gastrointestinal stromal tumor growth and induces ferroptosis
- Prognostic value of EIF5A2 in solid tumors: A meta-analysis and bioinformatics analysis
- The role of enhanced expression of Cx43 in patients with ulcerative colitis
- Choosing a COVID-19 vaccination site might be driven by anxiety and body vigilance
- Role of ICAM-1 in triple-negative breast cancer
- Cost-effectiveness of ambroxol in the treatment of Gaucher disease type 2
- HLA-DRB5 promotes immune thrombocytopenia via activating CD8+ T cells
- Efficacy and factors of myofascial release therapy combined with electrical and magnetic stimulation in the treatment of chronic pelvic pain syndrome
- Efficacy of tacrolimus monotherapy in primary membranous nephropathy
- Mechanisms of Tripterygium wilfordii Hook F on treating rheumatoid arthritis explored by network pharmacology analysis and molecular docking
- FBXO45 levels regulated ferroptosis renal tubular epithelial cells in a model of diabetic nephropathy by PLK1
- Optimizing anesthesia strategies to NSCLC patients in VATS procedures: Insights from drug requirements and patient recovery patterns
- Alpha-lipoic acid upregulates the PPARγ/NRF2/GPX4 signal pathway to inhibit ferroptosis in the pathogenesis of unexplained recurrent pregnancy loss
- Correlation between fat-soluble vitamin levels and inflammatory factors in paediatric community-acquired pneumonia: A prospective study
- CD1d affects the proliferation, migration, and apoptosis of human papillary thyroid carcinoma TPC-1 cells via regulating MAPK/NF-κB signaling pathway
- miR-let-7a inhibits sympathetic nerve remodeling after myocardial infarction by downregulating the expression of nerve growth factor
- Immune response analysis of solid organ transplantation recipients inoculated with inactivated COVID-19 vaccine: A retrospective analysis
- The H2Valdien derivatives regulate the epithelial–mesenchymal transition of hepatoma carcinoma cells through the Hedgehog signaling pathway
- Clinical efficacy of dexamethasone combined with isoniazid in the treatment of tuberculous meningitis and its effect on peripheral blood T cell subsets
- Comparison of short-segment and long-segment fixation in treatment of degenerative scoliosis and analysis of factors associated with adjacent spondylolisthesis
- Lycopene inhibits pyroptosis of endothelial progenitor cells induced by ox-LDL through the AMPK/mTOR/NLRP3 pathway
- Methylation regulation for FUNDC1 stability in childhood leukemia was up-regulated and facilitates metastasis and reduces ferroptosis of leukemia through mitochondrial damage by FBXL2
- Correlation of single-fiber electromyography studies and functional status in patients with amyotrophic lateral sclerosis
- Risk factors of postoperative airway obstruction complications in children with oral floor mass
- Expression levels and clinical significance of serum miR-19a/CCL20 in patients with acute cerebral infarction
- Physical activity and mental health trends in Korean adolescents: Analyzing the impact of the COVID-19 pandemic from 2018 to 2022
- Evaluating anemia in HIV-infected patients using chest CT
- Ponticulus posticus and skeletal malocclusion: A pilot study in a Southern Italian pre-orthodontic court
- Causal association of circulating immune cells and lymphoma: A Mendelian randomization study
- Assessment of the renal function and fibrosis indexes of conventional western medicine with Chinese medicine for dredging collaterals on treating renal fibrosis: A systematic review and meta-analysis
- Comprehensive landscape of integrator complex subunits and their association with prognosis and tumor microenvironment in gastric cancer
- New target-HMGCR inhibitors for the treatment of primary sclerosing cholangitis: A drug Mendelian randomization study
- Population pharmacokinetics of meropenem in critically ill patients
- Comparison of the ability of newly inflammatory markers to predict complicated appendicitis
- Comparative morphology of the cruciate ligaments: A radiological study
- Immune landscape of hepatocellular carcinoma: The central role of TP53-inducible glycolysis and apoptosis regulator
- Serum SIRT3 levels in epilepsy patients and its association with clinical outcomes and severity: A prospective observational study
- SHP-1 mediates cigarette smoke extract-induced epithelial–mesenchymal transformation and inflammation in 16HBE cells
- Acute hyper-hypoxia accelerates the development of depression in mice via the IL-6/PGC1α/MFN2 signaling pathway
- The GJB3 correlates with the prognosis, immune cell infiltration, and therapeutic responses in lung adenocarcinoma
- Physical fitness and blood parameters outcomes of breast cancer survivor in a low-intensity circuit resistance exercise program
- Exploring anesthetic-induced gene expression changes and immune cell dynamics in atrial tissue post-coronary artery bypass graft surgery
- Empagliflozin improves aortic injury in obese mice by regulating fatty acid metabolism
- Analysis of the risk factors of the radiation-induced encephalopathy in nasopharyngeal carcinoma: A retrospective cohort study
- Reproductive outcomes in women with BRCA 1/2 germline mutations: A retrospective observational study and literature review
- Evaluation of upper airway ultrasonographic measurements in predicting difficult intubation: A cross-section of the Turkish population
- Prognostic and diagnostic value of circulating IGFBP2 in pancreatic cancer
- Postural stability after operative reconstruction of the AFTL in chronic ankle instability comparing three different surgical techniques
- Research trends related to emergence agitation in the post-anaesthesia care unit from 2001 to 2023: A bibliometric analysis
- Frequency and clinicopathological correlation of gastrointestinal polyps: A six-year single center experience
- ACSL4 mediates inflammatory bowel disease and contributes to LPS-induced intestinal epithelial cell dysfunction by activating ferroptosis and inflammation
- Affibody-based molecular probe 99mTc-(HE)3ZHER2:V2 for non-invasive HER2 detection in ovarian and breast cancer xenografts
- Effectiveness of nutritional support for clinical outcomes in gastric cancer patients: A meta-analysis of randomized controlled trials
- The relationship between IFN-γ, IL-10, IL-6 cytokines, and severity of the condition with serum zinc and Fe in children infected with Mycoplasma pneumoniae
- Paraquat disrupts the blood–brain barrier by increasing IL-6 expression and oxidative stress through the activation of PI3K/AKT signaling pathway
- Sleep quality associate with the increased prevalence of cognitive impairment in coronary artery disease patients: A retrospective case–control study
- Dioscin protects against chronic prostatitis through the TLR4/NF-κB pathway
- Association of polymorphisms in FBN1, MYH11, and TGF-β signaling-related genes with susceptibility of sporadic thoracic aortic aneurysm and dissection in the Zhejiang Han population
- Application value of multi-parameter magnetic resonance image-transrectal ultrasound cognitive fusion in prostate biopsy
- Laboratory variables‐based artificial neural network models for predicting fatty liver disease: A retrospective study
- Decreased BIRC5-206 promotes epithelial–mesenchymal transition in nasopharyngeal carcinoma through sponging miR-145-5p
- Sepsis induces the cardiomyocyte apoptosis and cardiac dysfunction through activation of YAP1/Serpine1/caspase-3 pathway
- Assessment of iron metabolism and iron deficiency in incident patients on incident continuous ambulatory peritoneal dialysis
- Tibial periosteum flap combined with autologous bone grafting in the treatment of Gustilo-IIIB/IIIC open tibial fractures
- The application of intravenous general anesthesia under nasopharyngeal airway assisted ventilation undergoing ureteroscopic holmium laser lithotripsy: A prospective, single-center, controlled trial
- Long intergenic noncoding RNA for IGF2BP2 stability suppresses gastric cancer cell apoptosis by inhibiting the maturation of microRNA-34a
- Role of FOXM1 and AURKB in regulating keratinocyte function in psoriasis
- Parental control attitudes over their pre-school children’s diet
- The role of auto-HSCT in extranodal natural killer/T cell lymphoma
- Significance of negative cervical cytology and positive HPV in the diagnosis of cervical lesions by colposcopy
- Echinacoside inhibits PASMCs calcium overload to prevent hypoxic pulmonary artery remodeling by regulating TRPC1/4/6 and calmodulin
- ADAR1 plays a protective role in proximal tubular cells under high glucose conditions by attenuating the PI3K/AKT/mTOR signaling pathway
- The risk of cancer among insulin glargine users in Lithuania: A retrospective population-based study
- The unusual location of primary hydatid cyst: A case series study
- Intraoperative changes in electrophysiological monitoring can be used to predict clinical outcomes in patients with spinal cavernous malformation
- Obesity and risk of placenta accreta spectrum: A meta-analysis
- Shikonin alleviates asthma phenotypes in mice via an airway epithelial STAT3-dependent mechanism
- NSUN6 and HTR7 disturbed the stability of carotid atherosclerotic plaques by regulating the immune responses of macrophages
- The effect of COVID-19 lockdown on admission rates in Maternity Hospital
- Temporal muscle thickness is not a prognostic predictor in patients with high-grade glioma, an experience at two centers in China
- Luteolin alleviates cerebral ischemia/reperfusion injury by regulating cell pyroptosis
- Therapeutic role of respiratory exercise in patients with tuberculous pleurisy
- Effects of CFTR-ENaC on spinal cord edema after spinal cord injury
- Irisin-regulated lncRNAs and their potential regulatory functions in chondrogenic differentiation of human mesenchymal stem cells
- DMD mutations in pediatric patients with phenotypes of Duchenne/Becker muscular dystrophy
- Combination of C-reactive protein and fibrinogen-to-albumin ratio as a novel predictor of all-cause mortality in heart failure patients
- Significant role and the underly mechanism of cullin-1 in chronic obstructive pulmonary disease
- Ferroptosis-related prognostic model of mantle cell lymphoma
- Observation of choking reaction and other related indexes in elderly painless fiberoptic bronchoscopy with transnasal high-flow humidification oxygen therapy
- A bibliometric analysis of Prader-Willi syndrome from 2002 to 2022
- The causal effects of childhood sunburn occasions on melanoma: A univariable and multivariable Mendelian randomization study
- Oxidative stress regulates glycogen synthase kinase-3 in lymphocytes of diabetes mellitus patients complicated with cerebral infarction
- Role of COX6C and NDUFB3 in septic shock and stroke
- Trends in disease burden of type 2 diabetes, stroke, and hypertensive heart disease attributable to high BMI in China: 1990–2019
- Purinergic P2X7 receptor mediates hyperoxia-induced injury in pulmonary microvascular endothelial cells via NLRP3-mediated pyroptotic pathway
- Investigating the role of oviductal mucosa–endometrial co-culture in modulating factors relevant to embryo implantation
- Analgesic effect of external oblique intercostal block in laparoscopic cholecystectomy: A retrospective study
- Elevated serum miR-142-5p correlates with ischemic lesions and both NSE and S100β in ischemic stroke patients
- Correlation between the mechanism of arteriopathy in IgA nephropathy and blood stasis syndrome: A cohort study
- Risk factors for progressive kyphosis after percutaneous kyphoplasty in osteoporotic vertebral compression fracture
- Predictive role of neuron-specific enolase and S100-β in early neurological deterioration and unfavorable prognosis in patients with ischemic stroke
- The potential risk factors of postoperative cognitive dysfunction for endovascular therapy in acute ischemic stroke with general anesthesia
- Fluoxetine inhibited RANKL-induced osteoclastic differentiation in vitro
- Detection of serum FOXM1 and IGF2 in patients with ARDS and their correlation with disease and prognosis
- Rhein promotes skin wound healing by activating the PI3K/AKT signaling pathway
- Differences in mortality risk by levels of physical activity among persons with disabilities in South Korea
- Review Articles
- Cutaneous signs of selected cardiovascular disorders: A narrative review
- XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis
- A narrative review on adverse drug reactions of COVID-19 treatments on the kidney
- Emerging role and function of SPDL1 in human health and diseases
- Adverse reactions of piperacillin: A literature review of case reports
- Molecular mechanism and intervention measures of microvascular complications in diabetes
- Regulation of mesenchymal stem cell differentiation by autophagy
- Molecular landscape of borderline ovarian tumours: A systematic review
- Advances in synthetic lethality modalities for glioblastoma multiforme
- Investigating hormesis, aging, and neurodegeneration: From bench to clinics
- Frankincense: A neuronutrient to approach Parkinson’s disease treatment
- Sox9: A potential regulator of cancer stem cells in osteosarcoma
- Early detection of cardiovascular risk markers through non-invasive ultrasound methodologies in periodontitis patients
- Advanced neuroimaging and criminal interrogation in lie detection
- Maternal factors for neural tube defects in offspring: An umbrella review
- The chemoprotective hormetic effects of rosmarinic acid
- CBD’s potential impact on Parkinson’s disease: An updated overview
- Progress in cytokine research for ARDS: A comprehensive review
- Utilizing reactive oxygen species-scavenging nanoparticles for targeting oxidative stress in the treatment of ischemic stroke: A review
- NRXN1-related disorders, attempt to better define clinical assessment
- Lidocaine infusion for the treatment of complex regional pain syndrome: Case series and literature review
- Trends and future directions of autophagy in osteosarcoma: A bibliometric analysis
- Iron in ventricular remodeling and aneurysms post-myocardial infarction
- Case Reports
- Sirolimus potentiated angioedema: A case report and review of the literature
- Identification of mixed anaerobic infections after inguinal hernia repair based on metagenomic next-generation sequencing: A case report
- Successful treatment with bortezomib in combination with dexamethasone in a middle-aged male with idiopathic multicentric Castleman’s disease: A case report
- Complete heart block associated with hepatitis A infection in a female child with fatal outcome
- Elevation of D-dimer in eosinophilic gastrointestinal diseases in the absence of venous thrombosis: A case series and literature review
- Four years of natural progressive course: A rare case report of juvenile Xp11.2 translocations renal cell carcinoma with TFE3 gene fusion
- Advancing prenatal diagnosis: Echocardiographic detection of Scimitar syndrome in China – A case series
- Outcomes and complications of hemodialysis in patients with renal cancer following bilateral nephrectomy
- Anti-HMGCR myopathy mimicking facioscapulohumeral muscular dystrophy
- Recurrent opportunistic infections in a HIV-negative patient with combined C6 and NFKB1 mutations: A case report, pedigree analysis, and literature review
- Letter to the Editor
- Letter to the Editor: Total parenteral nutrition-induced Wernicke’s encephalopathy after oncologic gastrointestinal surgery
- Erratum
- Erratum to “Bladder-embedded ectopic intrauterine device with calculus”
- Retraction
- Retraction of “XRCC1 and hOGG1 polymorphisms and endometrial carcinoma: A meta-analysis”
- Corrigendum
- Corrigendum to “Investigating hormesis, aging, and neurodegeneration: From bench to clinics”
- Corrigendum to “Frankincense: A neuronutrient to approach Parkinson’s disease treatment”
- Special Issue The evolving saga of RNAs from bench to bedside - Part II
- Machine-learning-based prediction of a diagnostic model using autophagy-related genes based on RNA sequencing for patients with papillary thyroid carcinoma
- Unlocking the future of hepatocellular carcinoma treatment: A comprehensive analysis of disulfidptosis-related lncRNAs for prognosis and drug screening
- Elevated mRNA level indicates FSIP1 promotes EMT and gastric cancer progression by regulating fibroblasts in tumor microenvironment
- Special Issue Advancements in oncology: bridging clinical and experimental research - Part I
- Ultrasound-guided transperineal vs transrectal prostate biopsy: A meta-analysis of diagnostic accuracy and complication rates
- Assessment of diagnostic value of unilateral systematic biopsy combined with targeted biopsy in detecting clinically significant prostate cancer
- SENP7 inhibits glioblastoma metastasis and invasion by dissociating SUMO2/3 binding to specific target proteins
- MARK1 suppress malignant progression of hepatocellular carcinoma and improves sorafenib resistance through negatively regulating POTEE
- Analysis of postoperative complications in bladder cancer patients
- Carboplatin combined with arsenic trioxide versus carboplatin combined with docetaxel treatment for LACC: A randomized, open-label, phase II clinical study
- Special Issue Exploring the biological mechanism of human diseases based on MultiOmics Technology - Part I
- Comprehensive pan-cancer investigation of carnosine dipeptidase 1 and its prospective prognostic significance in hepatocellular carcinoma
- Identification of signatures associated with microsatellite instability and immune characteristics to predict the prognostic risk of colon cancer
- Single-cell analysis identified key macrophage subpopulations associated with atherosclerosis

