Abstract
Much effort was taken to elucidate how organic precursors appeared in early Earth, and attention was paid to two impact experiments: hypervelocity impacts by a propellant gun which simulated meteorite collides to Earth forming fatty acids and amino acids from inorganics, and micro-impacts by a planetary ball-mill which formed ammonium and acetic acid from inorganics. Our extended study on micro-impacts showed the formation of carboxylic acids, amines, and amino acids from Fe3C/Fe4N, carbon, and carbonates/nitrates by milling up to 30 h at 40 G. Fe(CO2)2·2H2O accelerated the formation a step further. Cu addition caused superior capability to form amines and amino acids. Two reaction fields were disclosed. In the impact field, the hydration of ferrous materials generated hydrogen which hydrogenated inorganic carbons to organics and ferrous transient materials and, in the maturing field, hydrogenated materials were then transformed into complex organics. Iron and CO2 were presumably the key components in the Hadean Ocean. Discussions on the mechano-chemical reaction were extended to serpentinization coupled with diastrophism of oceanic crusts and further led to a depiction that organic precursors were formed by micro-impacts and frictions of rocks and sands (like milling-balls) due to tremors in crusts. It provides a new path on how organic precursors were formed on the aqua-planet Earth.
1 Introduction
Much effort has been paid to elucidate how organic precursors, the origin of life, appeared in the inorganic world of early Earth. Simple gears like Miller’s synthesis [1,2] were used in the early days. Recently, sophisticated means like the asteroid Ryugu mission in space [3] and hypervelocity impacts by a propellant gun simulating meteorite collides to Earth [4,5,6] were deployed. The most recent challenge by a ball-milling, an old-fashioned technology, also showed the formation of ammonium and acetic acid from Fe3C and Fe4N in water [7]. Based on these findings, new challenges were encountered to form more complex organic molecules and further to clarify effects of the mechano-chemical (MeChem hereafter) reaction, which formed organics from inorganic materials.
As illustrated in Figure 1, there are mainly two reaction fields. One is an impact field where collisions of small milling balls (ϕ = 2 mm) produce a temperature exceeding 1,000 K and a high pressure [8,9] and transform the starting inorganic materials to new materials, stable or unstable, which are then quenched. The produced energies at the field are moderate and far less than hypervelocity impacts, which generate a high temperature (>2,900 K) and a pressure (20 GPa) [6]. The other is a maturing field where newly formed materials react with each other to form stabler materials, probably enhanced by the friction of balls. It should be noticed that, in both fields, materials are built up to more complex organic materials and at the same time broken down to simpler organic materials. Since there is no organic material provided in the beginning, built-up reactions are taking place as a whole.
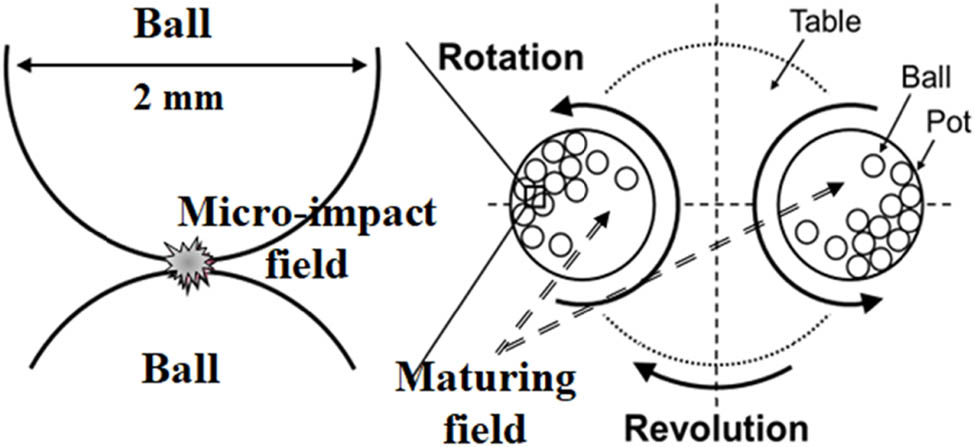
A schematic illustration of micro-impact and maturing fields.
In the previous experiments, it was frequently observed that pots were too tight to open after milling. It was simply due to the abnormal pressure built up. The excess hydrogen formation was caused in part by a reaction of eroded metal pots with water, and an extremely high hydrogen pressure was seemingly very harmful to obtain organics effectively. Thus, ZrO2-coated pots were introduced, and amounts of starting materials were also reduced to prevent excessive hydrogen pressure in this study. As starting materials, solid C, FeS, FeSO4, NaNO3, and Cu were used in addition to Fe4C, Fe3N, Fe(NO3)3, CaCO3, and MnCO3 which were used in the previous experiment. These compounds were assumed to be activated by the versatile MeChem reaction. Fe(CO2)2 was also introduced as a good carbon source since it decomposes to CO, CO2, and Fe3O4 at about 600 K. The introduction was also expected to prevent pressure build-up and thus larger starting amounts could be applied. Furthermore, it might easily produce HCOOH and enhance the formation of organics at a step higher. Cu was introduced to observe its affinity with NH3. The clay, montmorillonite, was expected to enhance reactions, first by increasing viscosities of muddy liquids, second by adsorbing organics as Fuller’s earth, and third by catalytic actions like Lewis acidic centers.
Finally, discussions are developed on how starting inorganic materials transformed to organics by the MeChem reaction and extended further; how organic precursors were formed 4 billion years ago with regard to serpentinization, diastrophism of terrestrial crusts, and global carbon/nitrogen cycles on early Earth.
2 Materials and methods
To produce micro-impacts, a planetary ball mill (“High-G” X382; Kurimoto, Ltd., Japan) was used. The mill accommodated two pots at a time and created a centrifugal acceleration of up to 150 G (rotating in the same planetary direction) continuously for days. Pot temperatures were kept below about 330 K by forced cooling. Zirconia-coated pots were used to avoid undesirable metal erosion causing high hydrogen pressures. Zirconia balls with a diameter of 2 mm were also used as milling balls. The centrifugal acceleration G was estimated using mechanical dimensions such as revolution and diameters based on the equation by Hashishin et al. [10].
As starting materials, Fe4N, Cu powder (Kojundo Chemical Laboratory, Japan), Fe3C (Rare Metallic, Japan), Fe(NO3)3·9H2O, Fe(CO2)2·2H2O, CaCO3, MnCO3, FeSO4·7H2O, FeS (Kanto Chemical, Japan), montmorillonite (Alfa Aesar, UK), and Ketjen black (Nippon Ketjen, Japan) for solid carbon were used. To eliminate possible organic contamination, Ketjen black and Cu powders were fired at 1,073 K and 873 K for 2 h in nitrogen flow, respectively.
Experimental conditions and starting materials are summarized in Table 1. Notice that the total amount of starting materials in this experiment was about 10 g or more; in most cases, it was far greater than the amount of about 100 mg in propellant gun experiments [5,6]. Larger starting amounts resulted in larger amounts of final products, which made chemical analyses much easier. Milling operations were carried out at 40 G (rotating in the reverse planetary direction) for 30 h, except #12–14 (10, 6.5, and 3.5 h, respectively) and #15 and 16 (20 h). After milling, muddy products were separated into liquids and solids by centrifugation. For organic analyses, the liquids were only used although the solids might contain some organics. The solids were dried in a cool box for XRD analyses. Some were dried at 373 K for further XRD analyses.
Starting materials (weight in g) and milling time
| No. | Fe4N | NO3 | C | Fe3C | FeCO2 | M* | H2O | Others | Milling time (h) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 2.701 | 2.057 | 0.503 | 162.0 | FeS 0.105 MnCO3 5.245 | 30 | |||
| #2 | 2.480 | 2.501 | 5.020 | 0.507 | 161.4 | FeS 0.101 | 30 | ||
| #3 | 1.958 | 1.410 | 0.510 | 160.0 | FeSO4 12.873 CaCO3 3.768 | 30 | |||
| #4 | 0.502 | 180.2 | FeS 3.018 | 30 | |||||
| #8 | 9.953 | 0.643 | 11.303 | 1.070 | 170.1 | 30 | |||
| #9 | 10.005 | 1.008 | 11.358 | 170.0 | *1 NH4 C 2.067 | 30 | |||
| #10 | 2.415 | 0.480 | 1.000 | 189.1 | 30 | ||||
| #11 | 1.001 | 0.194 | 192.0 | *2 NH4C 1.029 | 30 | ||||
| #12 | 8.339 | 0.700 | 9.010 | 0.301 | 170.2 | *3 FeS 0.498 | 10 | ||
| #13 | 8.340 | 0.702 | 8.998 | 0.300 | 170.4 | *3 FeS 0.501 | 6.5 | ||
| #14 | 8.341 | 0.699 | 9.006 | 0.299 | 175.9 | *3 FeS 0.508 | 3.5 | ||
| #15 | Na 1.700 | 7.198 | 170.0 | Cu 11.713 | 20 | ||||
| #16 | Fe 2.699 | 7.200 | 170.0 | Cu 11.138 | 20 |
M*: montmorillonite, FeNO3: Fe(NO3)3·9H2O, Na: NaNO3, NH4C: (NH4)2CO3, FeCO2: Fe(CO2)2·2H2O; *1 CeO2 0.119 La2O3 0.102 YCl3·6H2O 0.177; *2 CeO2 0.101 La2O3 0.093 YCl3·6H2O 0.128 ErCl3·6H2O 0.091; *3 AgNO3 0.105 ZnCl2 0.107 ZnO 0.104 CuCO3Cu(OH)·2H2O 0.105 all within ±0.005.
The amount of organic carbon and nitrogen in liquids was evaluated using a total organic carbon analyzer (TOC; TOC-LCPH; Shimadzu, Japan). The organics were analyzed at the Analytical Institute (Qualtec Co., Ltd., Japan) using a gas chromatograph mass spectrometer (GC-MS; GC-MS-QP2010Ultra, column: DB-5 at MS Scan: 35-300, MS SIM: 26, 27, 28, 29, 30, sphere 31, 32, 42, 43, 44, 45, 46, 58, 60, 73, 76; Shimadzu, Japan) following the standard procedure as described before [7]. Some specimens were evaluated at the Tohoku University using an ultra-high-performance liquid chromatograph tandem mass spectrometry (LC-MSMS; LCMS-8040; UHPLC/MSMS, Shimadzu, Japan) after derivatization with the AccQ-Tag reagent (Waters, USA). The details of the UHPLC/MSMS conditions are described elsewhere [11,12]. Crystalline phases of the solids were identified by powder X-ray diffraction (XRD; D2 PHASER; Bruker AXS, Germany with its XRD software) using Cu Kα radiation. A few liquids were evaluated by X-ray fluorescence analysis (ED-XRF; Rayny EDX-800; Shimadzu, Japan) to check metal contents.
3 Results and discussion
It was evident that no gas blew out from pots when opened in the course of this experiment as expected. Zirconia coatings of the pots were clearly effective in reducing excessive gas built-up (hydrogen and presumably CH4). It was also noticed that specimens were generally dark brown or black muds when pots were opened and that some specimens showed light brown or yellow color and turned to black when exposed to air. This was simply due to the fact that the atmosphere in pots was strongly reductive and ferrous Fe was oxidized to ferric. When montmorillonite was present, muddy specimens were very viscous and occasionally bubbles were observed for a while after opening pots. Bubbles probably indicated degassing of CO2 from muds. Sewage-like odors indicated the formation of some organics. Most liquids were transparent but few showed slight bluish-green color, which was an indication of Fe ions in liquids. The specimen #15 (#15 hereafter) showed a fine blue color. ED-XRF analyses revealed that it contained Cu ions of 860 ppm and Fe of 120 ppm, and #16 showed very slight green color and contained only Cu of 270 ppm.
Typical organics observed by GC-MS measurements were ethanol, acetone, formic acid, and acetic acid as shown in Figure 2. These results are summarized in Table 2 along with TOC values, magnetization, and pH changes. High TOC values obviously showed the formation of some organics. An increase in pH values was simply due to NH3 formation from Fe4N, Fe(NO3)3, or NaNO3 [7,13,14]. Magnetization was a sensible indicator of magnetite formation and most specimens showed its presence, even if its presence was very small.
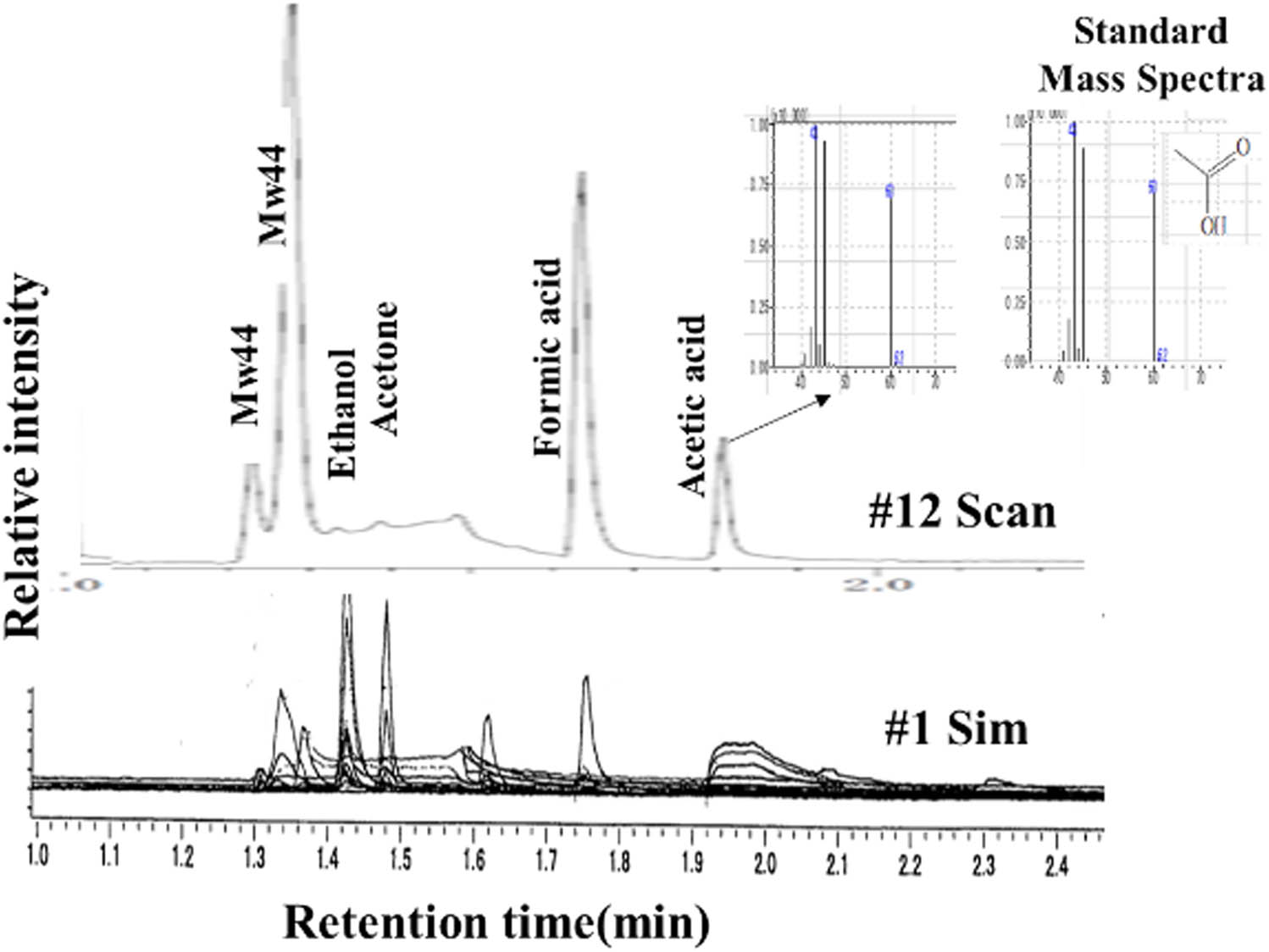
GC-MS chromatograms and spectra of specimens #1 and #12.
Summary of experimental results of TOC (mg·L−1), GC-MS (ppm), and pH
| No. | TOC | Ethanol | Acetone | Formic acid | Acetic acid | Magnetism | pH change | Group |
|---|---|---|---|---|---|---|---|---|
| #1 | 97 | 6 | 7 | TR | TR | Y | 7 → 9 | G2 |
| #2 | 492 | 4 | 7 | 196 | 187 | Y | 5 → 9 | G3 |
| #3 | 114 | 6 | 8 | BD | TR | Y | 4 → 7 | G2 |
| #4 | 21 | BD | BD | BD | BD | No | 7 → 7 | #4 |
| #8 | 947 | BD | 21 | 850 | 86 | Y | 5 → 12 | G3 |
| #9 | 648 | BD | 26 | 581 | 101 | Y | 10 → 11 | G3 |
| #10 | 32 | 30 | 20 | BD | BD | Y | 7 → 12 | G1 |
| #11 | 49 | BD | 19 | 57 | 67 | No | 10 → 11 | G1 |
| #12 | 402 | TR | BD | 378 | 93 | Y | 10 → 12 | G3 |
| #13 | 334 | BD | BD | 245 | 86 | Y | 10 → 12 | G3 |
| #14 | 255 | BD | BD | 172 | 71 | Y | 10 → 12 | G3 |
| #15 | 1,046 | TR | TR | TR | 20 | No | 6 → 7 | G4 |
| #16 | 332 | TR | TR | TR | TR | No | 4 → 7 | G4 |
TR: trace amount; BD: below detection.
3.1 Organic analyses of the liquids
Based on the characteristics of starting materials, specimens are assembled into five groups in the following discussion, namely #4 which contained no carbon source, Group1 (#10 and #11) which contained solid C, Group2 (#1 and #3) which contained Fe4C but no Fe2(CO2)2, Group3 (#2, #8, #9, #12, #13, and #14) which contained Fe(CO2)2, and Group4 (#15 and #16) which contained Cu. The assembling clearly visualized features of the observed results in Table 2 as shown in Figure 3 (amount of formic acid and acetic acid vs TOC values). The observed features are as follows:
The TOC value of #4, 21 mg‧L−1, was the lowest among the specimens. Since the starting materials of #4 (blank specimen) contained no carbon sources, the TOC value of 21 mg‧L−1 was mostly due to some organic contaminants. Considering that no nitrogen was detected (Table 4), the organic contaminants were most likely botanical fabrics, cellulose, and not surfactants or proteins which generally contain nitrogen. The TOC values exceeding 21 mg‧L−1 were thus proofs of some organic formations. All specimens in Groups 1–4 showed higher TOC values and formed some organics doubtlessly.
TOC values of any specimens were far greater than total amounts of detected organics by GC-MS measurements. The differences were clearly due to numerous organics which were below the detection limits of GC-MS measurements.
Three zones (#4 ignored) are observed in Figure 3. It was apparent that TOC values of Group1 < Group2 < Group3 and that amounts of two acids also showed the same behavior; Group1 < Group2 < Group3 as a whole. Comparing these behaviors with characteristics of Group1 (contained only solid C), Group2 (contained Fe3C), and Group3 (contained Fe(CO2)2), it was deduced that activities of carbon were in order of solid carbon < Fe3C < Fe(CO2)2 to produce organics under these experimental conditions.
#11 showed remarkably higher amounts of carboxylic acids than #10, although their TOC values did not show such a big difference. This irregularity of #11 is discussed with respect to yields of carbon and mass balances later.
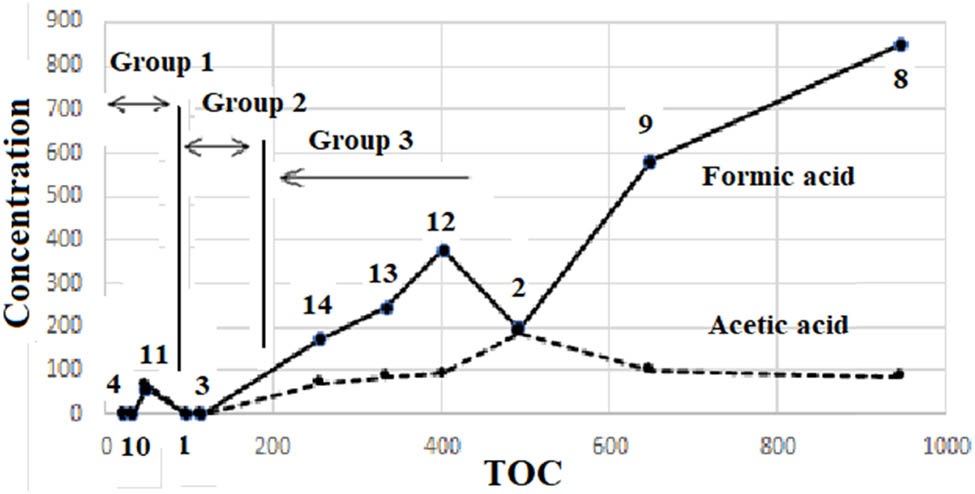
TOC values vs carboxylic acid concentrations of specimens #1 to #14.
An example of LC-MSMS measurements is shown in Figure 4, and measurement results are summarized in Table 3. Simple amino acids like glycine and alanine were mainly observed, and serine, sarcosine, and valine were rarely observed. When amounts of glycine and alanine were plotted against TOC values (Figure 5), the same zone behavior as that in Figure 3 was observed. Implications by LC-MSMS measurements are as follows:
Three zones (Group1, Group2, and Group4) showed that amounts of amines and amino acids trailed the same behavior as discussed above. It showed again that the activities of carbon were in order of solid carbon < Fe3C < Fe(CO2)2 as discussed above.
Group4 showed higher amounts of amines and amino acids than other groups. Since Cu was contained only in Group4, Cu might have an unusual effect different from Fe.
#4 (blank specimen) showed small amounts of contaminant amines and amino acids. These amounts were so small to be reflected in TOC values.
B-alanine showed abnormally large values which were not related to TOC values at all. It was assumed that there was some systematic contamination associated with the experimental setup. Although the cause of the contamination was unclear, the formation of amines and other amino acids was certain in all specimens of Groups 2–4 as stated just above.
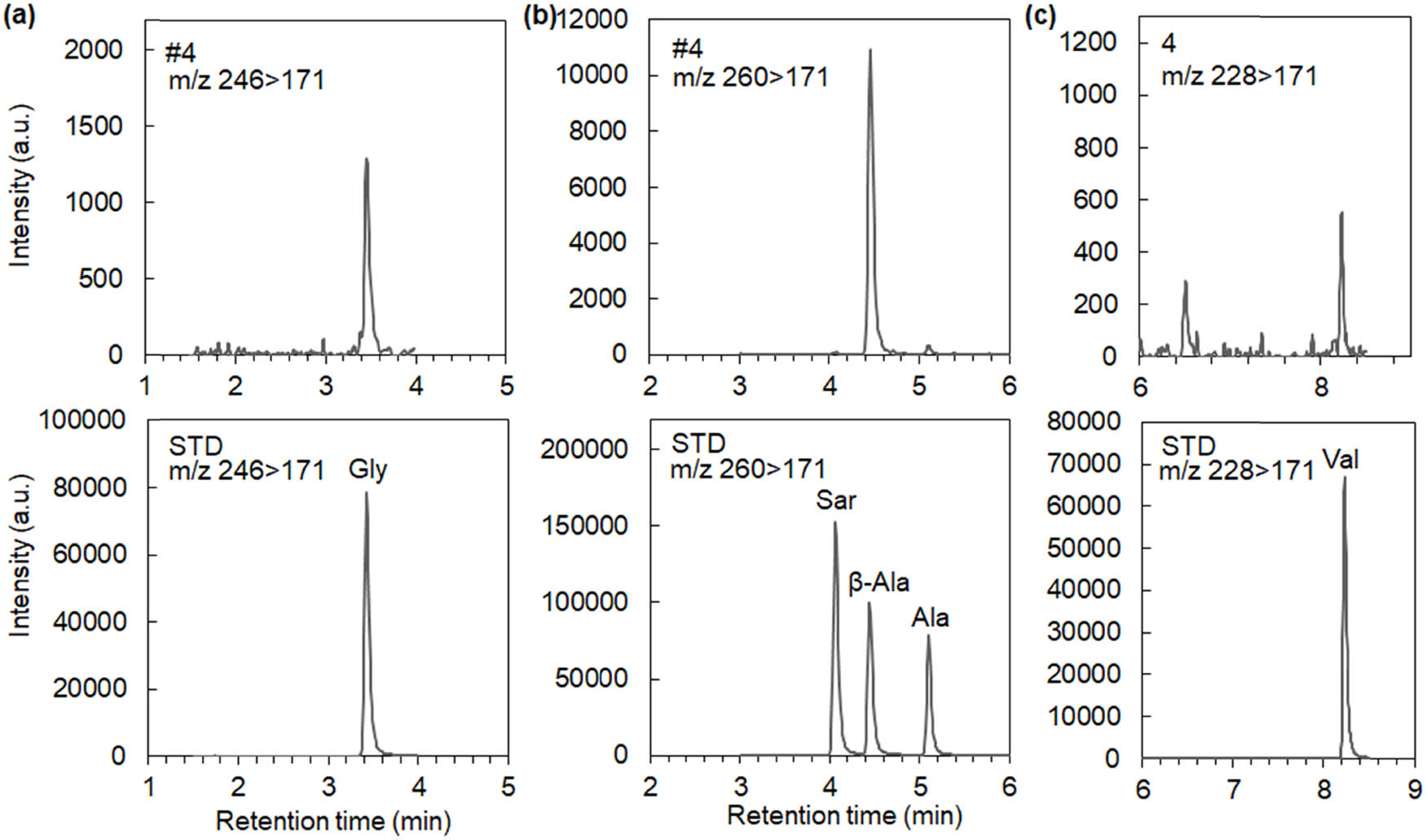
LC-MSMS spectra of specimen #4. MRM chromatograms of AccQ-derivatized (a) glycine, (b) C3 amino acids, and (c) valine and their referential standards.
Summary of experimental results of LC-MSMS (in μM)
| No. | TOC | Methylamine | Ethylamine | Gly | Ala | B-Ala | Ser | Sar | Val |
|---|---|---|---|---|---|---|---|---|---|
| #4 | 21 | 0.06 | 0.07 | 0.04 | 0.01 | 0.2 | BD | BD | TR |
| #11 | 49 | 0.07 | 0.03 | 0.03 | 0.01 | 1.5 | BD | TR | TR |
| #1 | 97 | 0.3 | 0.1 | 0.1 | 0.10 | 0.2 | 0.03 | BD | 0.03 |
| #16 | 332 | 1.0 | 0.2 | 0.3 | 0.05 | 0.2 | TR | BD | 0.01 |
| #15 | 1,046 | 0.3 | 0.3 | 0.1 | 0.04 | 0.2 | TR | TR | TR |
TR: trace amount; BD: below detection.
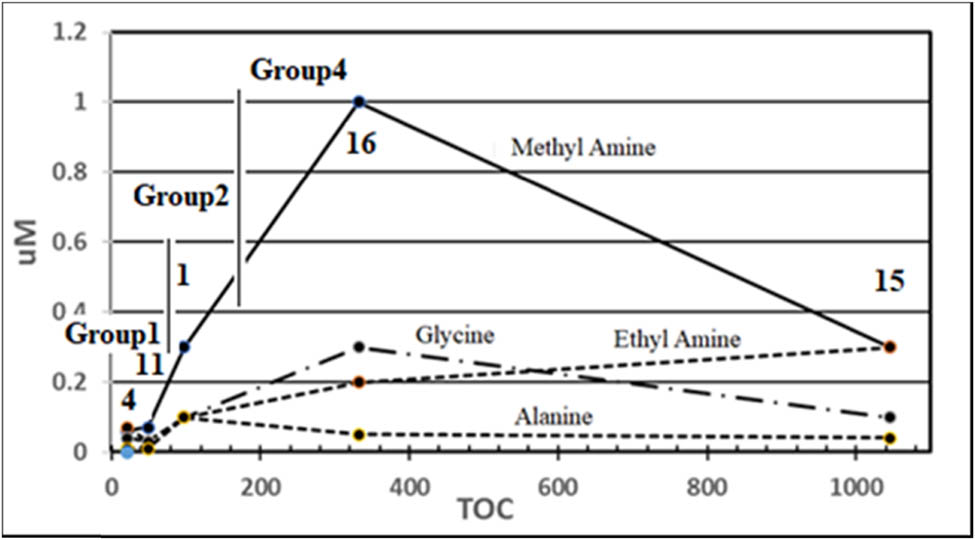
Amounts of amines and amino acids vs TOC values of #4, #11, #1, #16, and #15.
All of the above discussions based on TOC, GC-MS, and LC-MSMS measurements clearly proved that the MeChem reaction was effective to form organic materials like alcohols, carboxylic acids, amines, and amino acids from inorganic materials like Fe4C, Fe3N, and carbonate/nitrates.
3.2 Mass balances of carbon, nitrogen, and inorganic materials
Yields of inorganic carbon in starting materials to organics (Yield.C) were calculated based on the data listed in Tables 1 and 2, and summarized in Table 4 along with TOC, Calc.C, Obs.N, Calc.N, and Yield.N. From Table 4, some aspects of the MeChem reaction were elucidated as follows:
All Yield.C values except #4 were in a range of 0.01–0.18 which were extremely higher than those reported by Furukawa et al. [6]. This was simply due to numerous micro-impacts, millions of million repetitions of the MeChem reaction.
As pointed out above, #11 of Group1 showed remarkably higher contents of carboxylic acids than #10 of the same Group1. #11 also showed a higher Yield.C of 0.03 than 0.01 of #10. Since NH4CO3 was only the difference between #10 and #11 (Table 2), NH4CO3 was provably more active carbon source than solid C to form carboxylic acids.
Among Group2 (containing Fe3C), #3 showed the second highest Yield.C value of 0.16, while #1 showed a moderate value of 0.09. The second highest value was probably due to the reaction of CaCO3 and FeSO4 forming CaSO4 and Fe3O4 as observed in #3 (Figure 6). In the reaction, hydrogen was generated by the hydrothermal oxidation of ferrous Fe to ferric and, at the same time, CO2 was released from CaCO3 when CaSO4 formed. Newly generated hydrogen reduced CO2 to CH4 and further to simple organics, while CaCO3 and MnCO3 without FeSO4 were rather stable as also shown in Figure 6. The series of reactions is just like the serpentinization reaction that ultramafic rocks react with carbonated sea water forming H2, CH4, and Fe3O4 [15,16].
A Yield.C of 0.18 and also the highest TOC of 1,046 mg‧L−1 was realized by #15 in which the liquid uniquely showed a clear blue color. Since the liquid contained high Cu ions of 860 ppm, Fe of 120 ppm (no Na ions observed), and since the solid contained CuCN as shown in Figure 6, the liquid most probably contained some Cu/Fe/CN/NH3 complexes and NH4CN. CN ions in the liquid contributed to high values.
Group4 (#15 and #16) showed the highest TOC values and higher contents of amines and amino acids. This was apparently due to either Cu or NO3 (Table 1). More studies are necessary in combination with the CN formation.
The presence of CN ions reminds us of the well-known reactions, HCN chemistry [17] and Strecker amino acid synthesis [18]. The formation of nucleobases from HCN [19] and HCN with NH3 [20] and also from formamide in the presence of goethite [21] was reported. Furthermore, extensive discussions have been made whether the traditional HCN-based concept or the formamide-based scenario [22,23] is the most probable hypothesis for the origin of life. These reports strongly suggest that nucleobases like adenine could be formed in #15 and #16, which contained CN− and NH3. Nucleobase formations by hypervelocity impacts [11] also support this hypothesis.
XRD patterns (Figure 6) reveal that Fe4C, Fe3N, FeS, and Fe(CO2)2 in water transformed to mainly magnetite, goethite, and transient-Fe and/or green rust and that, during the transformation, the generated hydrogen hydrogenated carbon and CO2 to organics as discussed above. XRD diffraction lines marked as “g” correspond to Fe(HCO3)2:PDF00-007-0043, the source of which was not reliable. However, considering the unintentional coincidence of patterns and ferrous experimental conditions rich in CO3, it is not unreasonable to assume compound g as the remains of Fe(HCO3)2. It is, thus, expressed as transient-Fe. Nitrogen transformed mostly to NH3. When Cu was present (#15 and #16), CuCN and Cu2O were formed as solids, and CN and NH4 ions were in liquids as stated above. The different effects of Fe and Cu suggest that some other metals might show superior effects and efficiencies to form organics.
The nitrogen in Fe3N, NaNO3, and Fe(NO3)3 was transformed to mostly NH4NO3, some solids, and probably N2 [7] as well as recovered in liquids by 50–90%. Exceptions were #13, #14, and #16. #13 and #14 were only milled by short milling at 3.5 and 6.5 h, respectively, and thus unreacted components remained in solids. In the case of #16, CuCN formation was the cause of the low nitrogen in its liquid.
Chemical analyses of solids, which were beyond our capabilities, should have yielded organic formation and might reveal some effects of montmorillonite although any clear effect of montmorillonite was observed in this study.
Amount of carbon and nitrogen before and after milling and their yields
| No. | TOC | Calc.C | Yield.C | Obs.N | Calc.N | Yield.N | Group |
|---|---|---|---|---|---|---|---|
| #1 | 97 | 849 | 0.09 | 750 | 984 | 0.76 | G2 |
| #2 | 492 | 5,189 | 0.09 | 513 | 907 | 0.57 | G3 |
| #3 | 114 | 589 | 0.16 | 571 | 722 | 0.79 | G2 |
| #4 | 21 | 0 | — | 0 | 0 | — | #4 |
| #8 | 947 | 9,125 | 0.1 | 1,724 | 3,453 | 0.5 | G3 |
| #9 | 648 | 9,317 | 0.07 | 3,443 | 7,018 | 0.49 | G3 |
| #10 | 32 | 2,538 | 0.01 | 568 | 754 | 0.75 | G1 |
| #11 | 49 | 1,010 | 0.03 | 1,670 | 1,870 | 0.89 | G1 |
| #12 | 402 | 7,067 | 0.05 | 2,078 | 4,090 | 0.51 | G3 |
| #13 | 334 | 7,051 | 0.04 | 1,513 | 4,090 | 0.37 | G3 |
| #14 | 255 | 6,837 | 0.03 | 1,048 | 3,958 | 0.26 | G3 |
| #15 | 1,046 | 5,653 | 0.18 | 911 | 1,648 | 0.55 | G4 |
| #16 | 332 | 5,655 | 0.06 | 622 | 1,651 | 0.38 | G4 |
Calc.C: all carbon content of starting materials excluding CO3, in mg‧L−1; calc.N: all nitrogen content of starting materials, in mg‧L−1; obs.N: nitrogen content in liquid after milling observed by the TOC measurement, in mg‧L−1; Yield.C: yield of inorganic carbon to organic carbon in liquid, (TOC-21)/Calc.C; Yield.N: yield of nitrogen, Obs.N/Calc.N: In this calculation, CO3 was excluded since strong tendency of C to form CO2 was assumed.
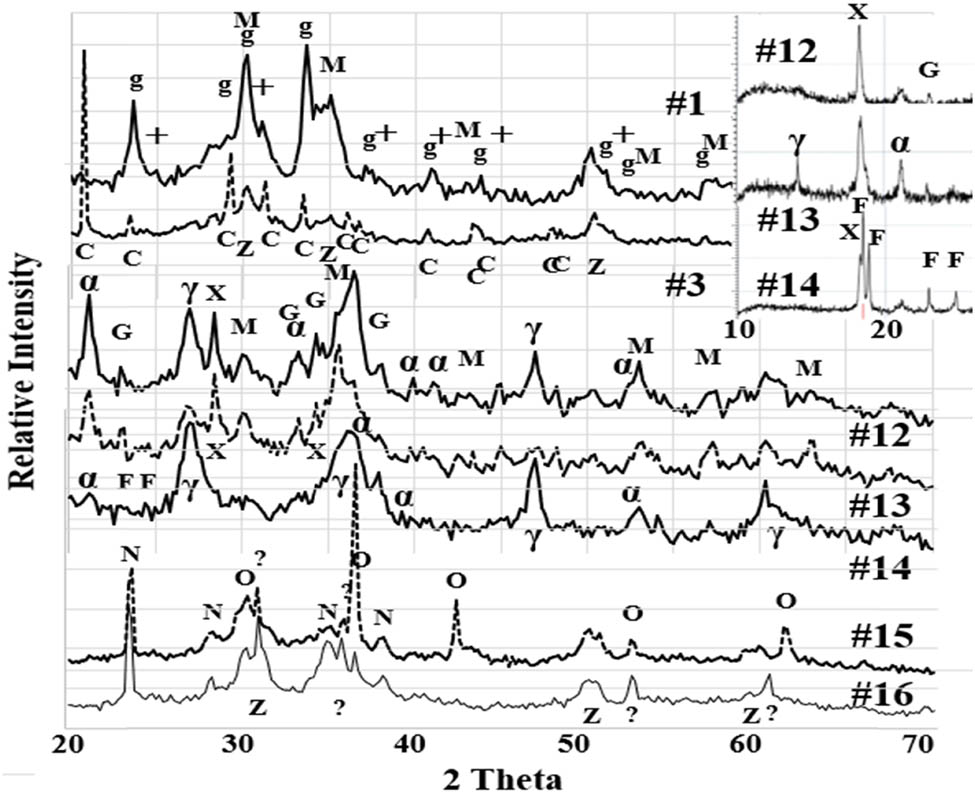
Some XRD patterns of solid products after high-energy ball millings. Note that #1 and #3 were in Group2, #12, #13, and #14 in Group3, and #15 and #16 in Group4. Insets: XRD patterns of #12, #13, and #14 after heat dried at 373 K. M: magnetite, G: green rust, g: pseud-Fe(HCO3)2, α: α-FeOOH, γ: γ-FeOOH, F: Fe(CO2)2, C: CaSO4, +: MnCO3, Z: ZrO2, N: CuCN, O: Cu2O, X: Fe glucose.
Phase changes observed in #12, #13, and #14 (Figure 6) disclosed some dynamic aspects of the MeChem reaction. After 3.5 h of milling (#14), Fe(CO2)2 almost disappeared. All other starting materials also disappeared and α- and γ-FeOOH were dominant. After 6.5 h (#13), Fe3O4, green rust, and/or transient-Fe appeared adding to α- and γ-FeOOH while Fe(CO2)2 disappeared. However, the XRD pattern of #13 after heat treatment (Insets) still showed a small amount of Fe(CO2)2, which was in amorphous or nanoparticle states before heat treatments.
At this point of 10 h, all starting materials were mostly consumed and the secondary chain reactions with newly formed products were assumed to be formed. In the primary reaction, it was observed that Yield.C and Yield.N of #14, #13, #2, and #8 (corresponding to 3.5, 6.5, 10, and 30 h of milling) increased from 0.04 to gradually 0.06 and from 0.26 to 0.50, respectively. These yields appeared to be saturating and indicated that the primary reaction was saturating. There are still many ambiguities left as follows:
A phase X in the inset shows a sharp diffraction line. It is actually the highest diffraction line in #12. Although it is indexed as Fe gluconate; PDF00-005-0257, it is not conclusive. There are still many unindexed diffraction lines which may correspond to complex organic or inorganic materials.
What is the lowest limit of G to form organics? At lower revolutions of mills, the effects of impact fields get weaker and far longer milling times are necessary to achieve enough number of impacts. Considering the effect of serpentinization, milling at above 200°C would be plausible to enhance the reaction and to reduce milling times.
Trials with materials that were available on early Earth, CaCO3, CuFeS2, and Fe(NO3)3/NH3 in water would be very interesting. Olivine and carbonated water as a carbon source would also be an interesting combination.
3.3 A link; hypervelocity/micro-impacts, serpentinization, and shocks at faults in crusts
There are common features, e.g., starting materials including Fe and CO3 produce H2, CH4, magnetite, and organics. Serpentinization is a hydrothermal reaction of ultramafic ferrous rocks with carbonated sea water forming H2, CH4, and magnetite at faults in crusts [15,26] as shown in Figure 7. Hypervelocity impacts [6,11] create extremely high temperatures at contact edges of Fe/Ni powders, a kind of micro-plasma, and activate nitrogen and other ingredients. Activated gaseous ingredients are quenched and form organics during a cooling process, presumably at about 300–100°C for a few minutes. The formation of organics in this short time is astonishing since 10–30 h milling times are required in micro-impact experiments. This is probably because activated ingredients are well mixed to realize fast reactions at the atomic scale, of 0.1 nm, which is far shorter than the mixing level of 10 nm in ball-milling experiments.
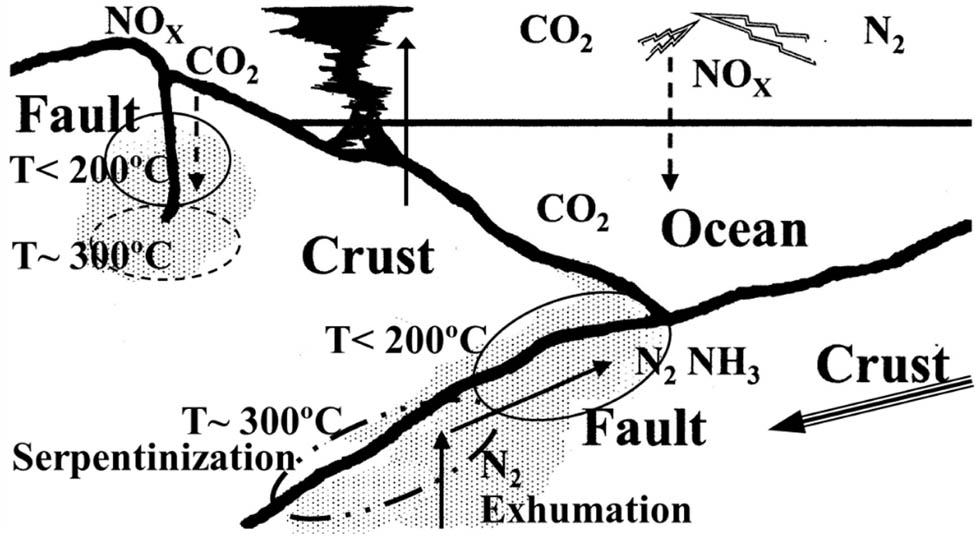
An illustration of carbon and nitrogen transfer at faults where the MeChem reaction should work most effectively.
It is quite natural to imagine that the impacts of rocks and sand create the MeChem reaction. Impacts of asteroids, meteorites, and interplanetary dust doubtlessly have great energies and create the MeChem reaction. While vibrations of rocks and sands created by earthquakes, for instance, behave just like milling balls and create the MeChem reaction, big or small. Although impact energies of rocks and sands produced by earthquakes are mostly very small at land surfaces, the energies would be tremendous at faults where stresses are accumulated. Near or at hypocenters, centers of shocks with highest G located at faults in deep crusts, pressures are tremendously high as well as temperatures. Then, the MeChem reactions take place very efficiently there. It is also expected that the impacts more effectively cause the MeChem reaction combined with serpentinization to form organics at faults in deep oceanic crusts. These events occur frequently and organics will be accumulated for a long period of time.
Moreover, faults are ideally positioned in ocean beds as inorganic ingredients are abundant here. More than 1 teramole of carbon per year are subducted as carbonate or carbonaceous materials [26], which are far greater than meteoritic materials of 37,000–78,000 tons every year falling on Earth (http://curious.astro.cornell.edu/about-us/75-our-solar-system/comets-meteors-and-steroids/meteorites/313-how-many-meteorites-hit-earth-each-year-intermediate). A great amount of Fe nitrate is also subducted into crusts [27]. Furthermore, it is noted that the MeChem reaction occurs even in the area of temperature below 200°C where serpentinization is assumed to be inactive and should enhance the reaction further in areas of temperature 200–350°C where serpentinization is assumed to work effectively [15].
This vision is further extended to the following hypothesis. When the CO2 content in atmosphere was thousand times higher on early Earth [28], subducted amount of carbon was even greater and then the MeChem reaction and serpentinization were probably far more active in the Hadean Ocean. As nitrogen sources, NH3 and N2 were built up along with upward movements of mantles [15,29,30] in faults where crusts collided, and the NO x produced through lightning and photochemical processes dropped down to Hadean Ocean [31]. All of these carbonates and nitrates are deposited as sediments with rocks and sands at sea floors in an anoxic world rich with CO2 and ferrous irons [32]. The transient materials discussed above, green rust and/or transient-Fe, might have played a big role. At events of seismic tremors due to any diastrophism of crusts, rocks, and sands produced micro-impacts and frictions just like milling balls. Organic precursors were thus formed in sediments. Even small earthquakes might cause violent tremors or impact well enough to form organics in the vicinity of hypocenters located in faults where the MeChem reaction was accelerated by high temperatures and pressures. In Hadean Eon, crusts were unstable, and diastrophism was so vigorous that the MeChem reaction was assumed to be extremely active. Because diastrophism was a universally occurring event on Earth, organic precursors were kept accumulating for billions of years and kept feeding primordial soup [33].
Hypervelocity/micro-impacts and serpentinization are phenomenologically described in short as follows. Hypervelocity impacts by propellant gun experiments (micro-plasma formation and quenching) [6,11] reproduce intense meteorite impacts forming organic precursors and imply further that organics are contained in coronal mass ejections of solar plasma flares. Micro-impacts of balls (fairly low energy and just enough to hydrogenate carbon components) also formed precursors and imply further that the micro-impacts of rocks and sands caused by any seismic activity in the lithosphere work efficiently to form precursors at hypocenters along faults. Serpentinization assisted by the MeChem reaction also forms precursors.
4 Conclusions
Micro-impacts induced by a high-energy ball milling at 40 G and up to 30 h formed organic precursors including amino acids from inorganic materials like Fe3C, Fe4N, carbonates, nitrates, and water. Unusually high yields were apparently due to frequent collisions of numerous balls, although the MeChem reaction due to each collision produced only a fraction of organics. The observed results and some remarks are as follows:
Yields of inorganic carbons to organic ones (Yield.C) were very high, up to 0.18. As carbon sources, Fe3C, solid carbon, and (NH4)2CO3 were effective. CaCO3 was activated by the FeSO4 addition. Fe(CO2)2 was a very effective carbon source and greatly enhanced organic formations.
Starting materials, Fe4C, and Fe(CO2)2 transferred to organics, magnetite, FeOOH, green rust, and/or transient-Fe in a short milling time of a few hours, and then gradually transformed to organics like alcohols, carboxylic acid, amines, and amino acids. Primary transformations appeared to be saturated at about 10 h under these experimental conditions.
Fe3N, NaNO3, and Fe(NO3)3 were effective nitrogen sources. The activity of (NH4)2CO3 as a nitrogen source was unclear. Nitrogen transformed mainly to NH3. When Cu was present, CN ions were dominant. Other metals and combinations might offer different results.
The formation of CN ions and NH3 strongly indicate the formation of nucleobases according to the traditional HCN chemistry or the formamide-based model.
The concept of mechanically enhanced serpentinization suggests that millings at lower G and a higher temperature of about 200°C may work to form organics.
It is expected that combinations of CaCO3, CuFeS2, Fe(NO3) x , and/or NH3 which were available on early Earth produce organic precursors including nucleobases.
It is suggested that CO2 and Fe were key components to form organic precursors in the Hadean Ocean and that the micro-impact-induced MeChem synthesis was a key process.
Our experiments demonstrated that the micro-impact-induced MeChem synthesis can be regarded as the dynamic crustal hydrothermal reaction or the mechanically enhanced serpentinization and offer a new route to produce organic precursors, the origin of life in early Earth. Over a billion years, great amounts of precursors have been accumulated in crusts as the mechano-chemical cradle on the aqua-planet.
Acknowledgements
The authors wish to thank Mr. N. Tsuchida (Kaneka Techno Research Corporation, Japan) for elaborated works by GC-MS and IC measurements and Ms. K. Fukuyama (Osaka University, Japan) for XRD and ED-XRF measurements. They also thank Dr. T. Kozawa (Osaka University, Japan) and Mr. S. Fujimoto (Kurimoto, Ltd., Japan) for their valuable suggestions and continued support in the course of this study.
-
Funding information: The authors state no funding involved. Most work has been carried out by expertise, skills, labors, and resources of all members.
-
Author contributions: Koichi Kugimiya: writing – original draft, methodology, formal analysis, conceptualization; Kenji Asai: formal analysis, resources; Takashi Harada: writing – review, formal analysis; Yoshihiro Furukawa: writing – review and editing, formal analysis; Makio Naito: supervision, resources.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Miller SL. A production of amino acids under possible primitive earth conditions. Science. 1953;117:528–9.10.1126/science.117.3046.528Search in Google Scholar PubMed
[2] Miller SL, Urey HC. Organic compound synthesis on the primitive earth: Several questions about the origin of life have been answered, but much remains to be studied. Science. 1959;130:245–51.10.1126/science.130.3370.245Search in Google Scholar PubMed
[3] Naraoka H, Takano Y, Dworkin JP, Oba Y, Hamase K, Furusho A, et al. Soluble organic molecules in samples of the carbonaceous asteroid (162173) Ryugu. Science. 2023;379:eabn9033. 10.1126/science.abn9033.Search in Google Scholar PubMed
[4] Sekine T. Shock wave chemical synthesis. Eur J Solid State Inorg Chem. 1997;34:823–33.Search in Google Scholar
[5] Nakazawa H, Sekine T, Kakegawa T, Nakazawa S. High yield shock synthesis of ammonia from iron, water and nitrogen available on the early Earth. Earth Planet Sci Lett. 2005;235:356–60.10.1016/j.epsl.2005.03.024Search in Google Scholar
[6] Furukawa Y, Sekine T, Oba M, Kakegawa T, Nakazawa H. Biomolecule formation by oceanic impacts on early Earth. Nat Geosci. 2009;2:62–6.10.1038/ngeo383Search in Google Scholar
[7] Kugimiya K, Kozawa T, Harada T, Naito M. Mechano-chemical synthesis of ammonia and acetic acid from inorganic materials in water. Green Process Synth. 2019;8(1):223–9.10.1515/gps-2018-0073Search in Google Scholar
[8] Dachille F, Roy R. High-pressure phase transformations in laboratory mechanical mixers and mortars. Nature. 1960;186(34):71.10.1038/186034a0Search in Google Scholar
[9] Nogi K, Naito M, Kondo A, Nakahira A, Niihara K, Yokoyama T. New method for elucidation of temperature at the interface between particles under mechanical stirring. Funtai Oyobi Funmatsu Yakin. 1996;43(3):396–401.10.2497/jjspm.43.396Search in Google Scholar
[10] Hashishin T, Tan Z, Yamamoto K, Qiu N, Kim J, Numako C, et al. Quenching ilmenite with a high-temperature and high-pressure phase using super-high-energy ball milling. Sci Rep. 2014;4:4700-1-7.10.1038/srep04700Search in Google Scholar PubMed PubMed Central
[11] Furukawa Y, Nakazawa H, Sekine T, Kobayashi T, Kakegawa T. Nucleobase and amino acid formation through impacts of meteorites on the early ocean. Earth Planet Sci Lett. 2015;429:216–22.10.1016/j.epsl.2015.07.049Search in Google Scholar
[12] Takeuchi Y, Furukawa Y, Kobayashi T, Sekine T, Terada N, Kakegawa T. Impact-induced amino acid formation on Hadean Earth and Noachian Mars. Sci Rep. 2020;10(1):9220.10.1038/s41598-020-66112-8Search in Google Scholar PubMed PubMed Central
[13] Summers DP, Chang S. Prebiotic ammonia from reduction of nitrite by iron (II) on the early Earth. Nature. 1993;365:630–3.10.1038/365630a0Search in Google Scholar PubMed
[14] Brandes JA, Boctor NZ, Cody GD, Cooper BA, Hazen RM, Yoder Jr HS. Abiotic nitrogen reduction on the early Earth. Nature. 1998;395:365–7.10.1038/26450Search in Google Scholar PubMed
[15] Cannat M, Fontaine F, Escartin J. Serpentinization and associated hydrogen and methane fluxes at slow spreading ridges. Geophysical Monograph Series. (Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges. American Geophysical Union) 2010;188:241–64.10.1029/2008GM000760Search in Google Scholar
[16] Holm NG, Oze C, Mousis O, Waite JH, Guilbert-Lepoutre A. Serpentinization and the formation of H2 and CH4 on celestial bodies (Planets, Moons, Comets). Astrobiology. 2015;15(7):587–600.10.1089/ast.2014.1188Search in Google Scholar PubMed PubMed Central
[17] Berndt ME, Allen DE, Seyfried WE. Reduction of CO2 during serpentinization of olivine at 300°C and 500. Geology. 1996;24(4):351–4.10.1130/0091-7613(1996)024<0351:ROCDSO>2.3.CO;2Search in Google Scholar
[18] Strecker A. Ueber einen neuen aus Aldehyd - Ammoniak und Blausäure entstehenden Körper. Ann Chem. 1854;91(3):349–51.10.1002/jlac.18540910309Search in Google Scholar
[19] Oro J. Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive Earth conditions. Nature. 1961;191:1193–4.10.1038/1911193a0Search in Google Scholar
[20] Oro J, Kimball AP. Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide. Arch Biochem Biophys. 1961;94:217–27.10.1016/0003-9861(61)90033-9Search in Google Scholar
[21] Shanker U, Bhushan B, Bhattacharjee G, Kamaluddin. Formation of nucleobases from formamide in the presence of iron oxides: implication in chemical evolution and origin of life. Astrobiology. 2011;11(3):225–33.10.1089/ast.2010.0530Search in Google Scholar
[22] Saladino R, Crestini C, Costanzo G, Negri R, Mauro ED. A possible prebiotic synthesis of purine, adenine, cytosine, and 4(3H)-pyrimidinone from formamide: implications for the origin of life. Bioorg Med Chem. 2001;9(5):1249–53.10.1016/S0968-0896(00)00340-0Search in Google Scholar
[23] Šponer JE, Šponer J, Nováková O, Brabec V, Šedo O, Zdráhal Z, et al. Emergence of the first catalytic oligonucleotides in a formamide-based origin scenario. Chemistry. 2016;22(11):3572–86.10.1002/chem.201503906Search in Google Scholar PubMed
[24] Fan Z, Weng W, Zhou J, Gu D, Xiao W. Catalytic decomposition of methane to produce hydrogen: A review. J Energy Chem. 2021;58:415–30.10.1016/j.jechem.2020.10.049Search in Google Scholar
[25] Kawase S, Kawai K, Matsui T. Effects for SDGs by direct methane reforming reaction with iron-based catalysts. The 54th Symposium on Powder Technology (Hosokawa Powder Technology Foundation); 2022. p. 39–46.Search in Google Scholar
[26] Okamoto A, Oyanagi R, Yoshida K, Uno M, Shimizu H, Satish-Kumar M. Rupture of wet mantle wedge by self-promoting carbonation. Comm Earth Environ. 2021;2:151. 10.1038/s43247-021-00224-5.Search in Google Scholar
[27] Hatakeyama K, Katayama I, Hirauchi K, Michibayashi K. Mantle hydration along outer-rise faults inferred from serpentinite permeability. Sci Rep. 2017;7:1387.10.1038/s41598-017-14309-9Search in Google Scholar PubMed PubMed Central
[28] Kasting JF, Howard MT. Atmospheric composition and climate on the early Earth. Philos Trans R Soc Lond B. 2006;361:1733–42.10.1098/rstb.2006.1902Search in Google Scholar PubMed PubMed Central
[29] Yoshioka T, Wiedenbeck M, Shcheka S, Kepplera H. Nitrogen solubility in the deep mantle and the origin of Earth’s primordial nitrogen budget. Earth Planet Sci Lett. 2018;488:134–43.10.1016/j.epsl.2018.02.021Search in Google Scholar
[30] Mysen B. Nitrogen in the Earth: abundance and transport. Prog Earth Planet Sci. 2019;6:38. 10.1186/s40645-019-0286-x.Search in Google Scholar
[31] Wong ML, Charnay BD, Gao P, Yung YL, Russell MJ. Nitrogen oxides in early earth’s atmosphere as electron acceptors for life’s emergence. Astrobiology. 2017;10:975–83. 10.1089/ast.2016.1473.Search in Google Scholar PubMed
[32] Tosca NJ, Jiang CZ, Rasmussen B, Muhling J. Products of the iron cycle on the early Earth. Free Radic Biol Med. 2019;140:138–53. 10.1016/j.freeradbiomed.2019.05.005.Search in Google Scholar PubMed
[33] Kitadai N, Aono M, Oono Y. Origin of metabolism: A perspective. ChikyuKagaku (Geochemistry). 2016;50:155–76.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”


