Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
-
Yee Jin Wong
Abstract
Green synthesis has become an alternative to replace chemical synthesis in nanotechnology because of its low cost and toxicity. The synthesis of nanocomposite (NC) has attracted the attention of researchers, as its synergistic effect might enhance its effectiveness in anticancer to overcome multidrug resistance. In this study, copper oxide/zinc oxide (CuO/ZnO) NC was green synthesized from the crude extract of Musa acuminata leaves with Zn(NO3)2·6H2O and Cu(NO3)2·3H2O as precursors. CuO/ZnO NC was characterized via UV-Vis analysis with a peak detected at 365 nm with a bandgap energy of 3.43 eV. Field emission scanning electron microscopy analysis showed an agglomerated, irregular shape with a size ranging from 31.8 to 85.7 nm. X-ray diffraction analysis depicted the crystallite size of 24.78 nm with hexagonal wurtzite of ZnO and monoclinic of CuO. Fourier transform infrared spectroscopy analysis showed the presence of functional groups, including hydroxyl groups, carbonyl groups, amines, alkanes, aromatic amines, Cu–O, and Zn–O. The cytotoxic effect of CuO/ZnO NC toward the colorectal cancer cells (HCC2998) was evaluated by the MTT assay and found to be dose-dependent. The results showed a significant cell mortality at 100 µg·mL−1 CuO/ZnO NC with 45.79% cell death at 24 h. The findings of the present study suggest the potential of CuO/ZnO NC to be utilized as an anticancer agent.
1 Introduction
Nowadays, nanotechnology is used in many fields, such as chemical, electronic, and agricultural, to mention a few [1]. Nanotechnology is also focused on research studies in either the environmental or medical field [2]. Nanomedicine is the word used to describe nanotechnology applied in clinical settings. Nanomedicine utilizes the nanoparticles for diagnosing, treating, and controlling the diseases [3]. In other words, nanotechnology is currently used to enhance human health, especially in the treatment of cancer, a non-communicable disease that contributes to the high global death rate because it causes abnormal cell growth, leading to organ malfunction [4]. According to WHO [5], cancer is the main factor that contributes to the mortality rate around the world, with the five most prevalent cancers including lung, colon, breast, prostate, and rectal cancers. The present common treatment regimens for cancer, including chemotherapy, surgery, immunotherapy, and radiotherapy, are effective in killing the cancerous cells; however, at the same time, the normal healthy cells would be killed, indicating that these treatments would weaken the human’s body immune system in fighting against cancer [6].
Zinc oxide nanoparticles (ZnO NPs) are shown to more specifically kill malignant cells as opposed to normal cells, and it was discovered that they may be connected to the apoptotic activity of the cells [7]. This finding is similar to that reported by Saravanan et al. [8] that ZnO NPs are highly toxic to bacteria cells but have little or no impact on human cells, which indicate that ZnO NPs are potential candidates to be applied in the medical field as anticancer or antibacterial agents. The mild toxic effect is the reason that researchers applied ZnO NPs in cancer therapy as compared to other metal oxide nanoparticles. It is undeniable that ZnO NPs also exert antibacterial, fungicidal, and antiviral activity other than cytotoxic effects toward cancer cells [9].
Researchers are also interested in copper oxide nanoparticles (CuO NPs) because of their low band gap energy, antibacterial and anti-inflammatory qualities as well as their chemical stability [10]. CuO NPs were found to promote a more cytotoxic effect toward the breast malignant cells (MDA-MB-231) compared with the normal cells (HBL 100) in a dose-dependent manner [11].
The copper ions released from CuO NPs can interfere with the G2/M phase of the cell cycle in non-small cell lung cancer [12,13]. Furthermore, the synthesized CuO NPs exhibit cytotoxicity by generating reactive oxygen species (ROS) or triggering a cascade of events leading to DNA damage and apoptosis, ultimately resulting in the death of malignant cells. In addition to their anticancer properties, CuO NPs have been utilized in antibacterial applications, the cosmetics industry, photothermal therapy, and drug delivery systems [14].
The combination of metal oxide nanocomposites (NCs) has emerged as a novel area of research, offering improved efficiency for medical applications. CuO and ZnO NPs are recognized for their antibacterial and anticancer properties. However, treatments utilizing only one type of metal oxide often fall short of achieving effective biological activities. As a result, the synthesis of CuO/ZnO NC, integrating two types of metal oxide nanoparticles, holds significant potential in addressing the limitations and enhancing therapeutic outcomes [15].
The potential of CuO/ZnO NC was highlighted in a study by Adeyemi et al. [16], which demonstrated that the NC was smaller in size compared to individual nanoparticles. The researchers employed a green synthesis method to produce CuO NPs, ZnO NPs, and CuO/ZnO NCs. The findings revealed that the CuO/ZnO NCs ranged in size from 20 to 32 nm, smaller than the CuO NPs, which measured between 30 and 50 nm. This suggests that the smaller size of NCs, which provides a larger surface area, is a key factor driving research into NCs over individual nanoparticles. The increasing ratio of ZnO to CuO NPs will reduce the size of CuO formed in the NC. It is possible to modify the characteristics of materials for the production of products for certain applications by integrating the desirable features of several nanoscale building blocks in NCs [17]. Researchers have primarily focused on green-synthesized CuO/ZnO NCs for water treatment, particularly in degrading dyes released from industrial processes [18,19,20]. However, the application of CuO/ZnO NCs in the medical field remains limited and underexplored. Among the cancers, colorectal cancer would be the concern in this research. As reported by the Malaysia National Cancer Registry from 2012 to 2016, colon cancer ranked first in males with a percentage of 16.9, whereas, among females, it ranked second with a percentage of 10.7 in terms of cancer prevalence. The risk for males to have colorectal cancer is higher than that of females with the increment in age. The statistics showed that colon cancer in Malaysia increased for both males and females from 2012 to 2016 [21].
Green synthesis of nanoparticles has gained significant attention from researchers due to its advantages over chemical synthesis. It offers a safer, cost-effective, and environmentally friendly alternative, as it involves a single-step process that minimizes the production of harmful by-products. In contrast, chemical synthesis methods often generate toxic by-products that pose risks to both human health and the environment, making green synthesis a more sustainable approach for nanoparticle production [22]. For instance, a study by Dowlath and team compared chemically synthesized iron oxide NPs versus green synthesized iron oxide NPs derived from the source of the reducing and capping agent, Cardiospermum halicacabum. From the findings, it was found that the cell viability of the human peripheral blood mononuclear cells was 84.04 ± 0.94% when treated with 100 μg·mL−1 NPs while the cell viability was 53.68% ± 1.50 for the chemical alternative, indicating the lower toxicity of green-synthesized NPs [23]. It is undeniable that the NPs synthesized using the green method are more stable as compared to the chemically synthesized NPs [24]. Besides, the unique characteristic of utilizing green synthesis alternatives in producing NPs is that no toxic by-products are produced during the whole process, which indicates that no further purification is needed to remove the toxic by-products. Plants and microorganisms gather and detoxify the inorganic metal ions [25,26]. In comparison to preparing the NPs produced from algae, bacteria, and fungi, green synthesizing the metallic or metal oxide NPs using plants will be a better choice owing to quick, easy, and straightforward alternatives, and plants act as the supply of carbon, necessary for the NC formation and stabilization. Therefore, synthesizing NPs using plants as a source of reducing agent is more common to be introduced in the field of medications for delivery of drugs [1]. Apart from that, the green-synthesized NPs were found to exert better antibacterial and anticancer activities over chemically produced ones [27].
The green synthesis of CuO/ZnO NCs has been vastly applied to the water treatment to degrade the dyes disposed from the factories to reduce water pollution. Moreover, the synthesis of CuO/ZnO NCs is able to facilitate the photocatalytic ability of the NCs via a combination of the different metal oxide NPs with varying band gap energies [28]. However, there are only a few studies reported on the anticancer effect of CuO/ZnO NCs [29].
Since cancer drugs are expensive and require long treatment periods, the development of NCs has produced a more cost-effective option for treating cancer that is more targeted and does not destroy healthy cells, making it a better option for patients with cancer. Thus, it is important to know whether the CuO/ZnO NC is effective as an alternative cancer agent to treat colorectal cancer. Besides, the green synthesis alternative applied in the synthesis of the NC would probably bring less toxic effects as well as fewer side effects to the cancer patients who receive the treatment.
In this research, Musa acuminata was selected as the plant source for green synthesis due to its wide availability; more importantly, no previous studies have used M. acuminata to synthesize CuO/ZnO NC for testing their cytotoxic effects on colorectal cancer cells. Another reason for choosing this plant is based on the research that demonstrated the cytotoxicity of the extract from M. acuminata flowers increased with higher concentrations when applied to HeLa cells, showing promising results for cancer treatment [30]. Bananas contain bioactive compounds, including phenolic compounds such as flavonoids, anthocyanins, biogenic amines, and tannins. Among the phenolic compounds, flavonoids support the integrity of connective tissue proteoglycans by blocking hyaluronidase activity, which would stop the metastasis of cancer cells [29]. The phenolic compounds exert anti-inflammation and antibacterial effects, while beta carotene, ascorbic acid, cinnamic acid, and ferulic acid can be found in the leaves of the banana tree [31,32]. The lectin from the leaves of M. acuminata is reported to activate caspases, at the same time impeding the growth of the cancerous cells as well as reducing the VEGF production. Besides, the activation of the Bax gene and downregulation of Bcl-2 gene expression contribute to cell death via apoptosis, which showed significant results on anticancer effects on both HeLa and EAC cell growth, indicating that M. acuminata contains the bioactive compounds that combat the cancer cells [33].
Additionally, M. acuminata was utilized as a capping agent in the synthesis of silver and gold NPs for anti-cancer studies on MCF-7 breast cancer cells and normal Vero cells. The results showed that the nanoparticles exhibited both antibacterial and anticancer activities, with an IC50 of 30 μg·mL−1 for MCF-7 cells and 55 μg·mL−1 for Vero cells, highlighting the more lethal effects on cancer cells and less toxic effects on normal cells [34]. Another study employed banana leaves as reducing and capping agents to synthesize silver nanoparticles (Ag NPs) via a microwave-assisted method and revealed that Ag NPs induced significant cytotoxic effects on breast cancer (MCF7) and lung cancer (A549) cells [35].
Therefore, the present research aimed to green synthesize CuO/ZnO NCs using M. acuminata as capping and reducing agents to investigate the anticancer effect on colorectal cancer cells (HCC2998).
2 Materials and methods
M. acuminata leaves were obtained from UTAR Agriculture Park in Kampar, Perak. Zinc nitrate hexahydrate, Zn(NO3)2·6H2O, and copper nitrate trihydrate, Cu(NO3)2·3H2O, were obtained from GENE Chemicals. The colorectal cancer cells (HCC2998) used in this study were procured from the Hamon Cancer Center (USA). The MTT powder, thiazolyl blue tetrazolium bromide, 98.00%, were bought from Merck (Germany). All glassware and pipette tips used in this study were autoclaved before use. All chemicals were used without further purification.
2.1 Preparation of M. acuminata leaf extract
The fresh leaves of M. acuminata were obtained from UTAR Agriculture Park. The leaves were washed, pat dried with tissue, cut, and weighed. The leaves were placed on a tray and incubated at 50°C for 72 h until the leaves became dry and crunchy. The weight of the dried leaves was measured. The dried leaves were blended into powder. Then, 50 g of the powder was mixed with 1 L of distilled water and left overnight. The mixture was filtered using filter paper, placed into 50 mL centrifuge tubes, and left for overnight freezing at −20°C. It took 3 weeks for the freeze-dried process to obtain the crude extract. A brownish-green sticky crude extract was observed on the wall of the centrifuge tube. The crude extract was stored in the chest freezer for further use [36].
2.2 Green synthesis of CuO/ZnO NCs
About 4 g of 0.1 M Zn(NO3)2·6H2O and 2 g of 0.01 M Cu(NO3)2·3H2O were weighed without further purification. About 2.5 g of the crude extract was mixed well with 50 mL of distilled water using a hot plate magnetic stirrer. Zn(NO3)2·6H2O and Cu(NO3)2·3H2O were added to the extract with constant stirring between 70°C and 80°C until saturation. The net weight of the crucible was weighed. The dark green paste was transferred to a crucible. The dark green paste was calcinated in Furnace large Nabertherm at 400°C for about 2 h. Brown CuO/ZnO NCs were formed. CuO/ZnO NCs were kept in the tightly closed glass vial at room temperature [37]. Figure 1 shows the workflow for the preparation of M. acuminata leaf extract and green synthesis of CuO/ZnO NCs.
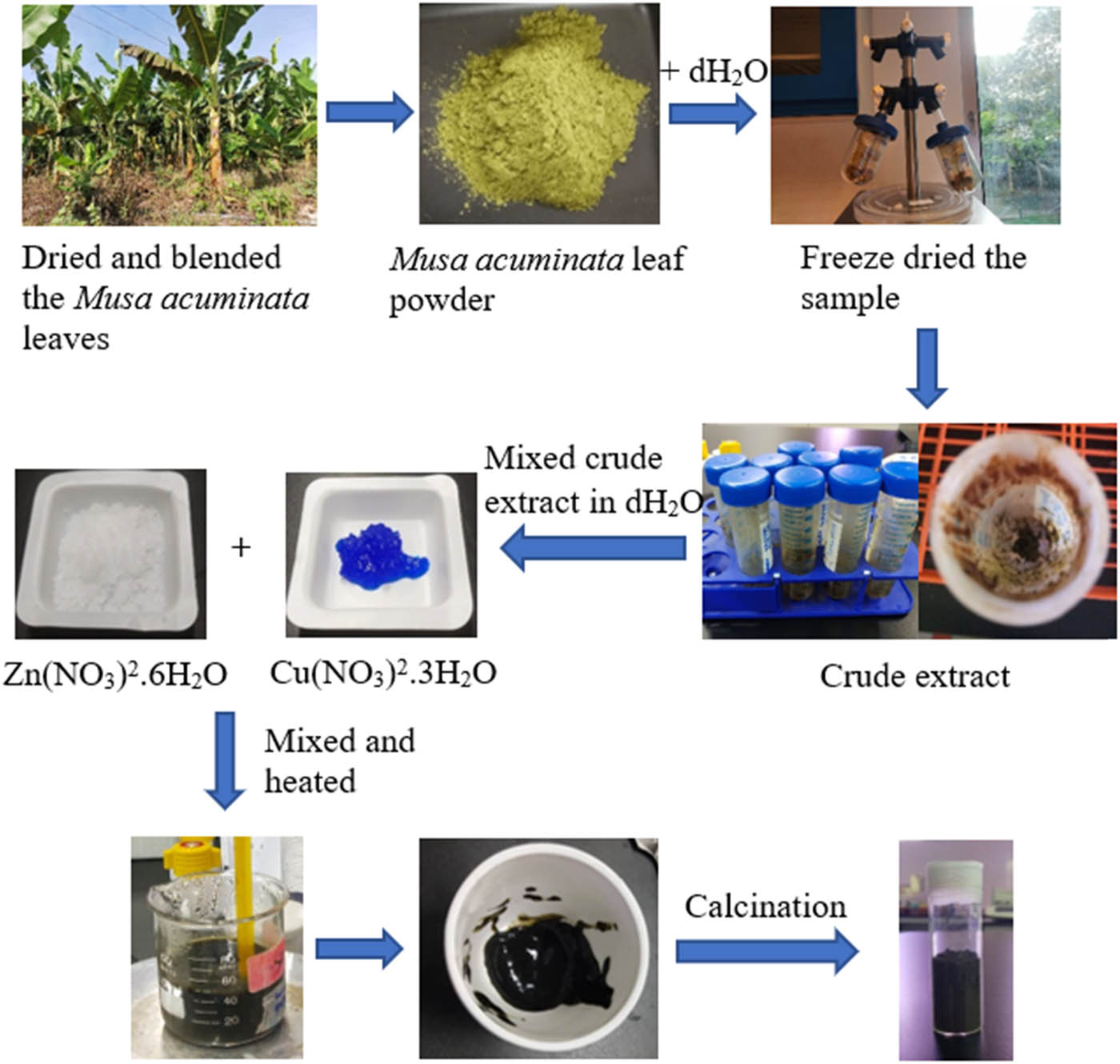
Workflow for green synthesis of CuO/ZnO NCs using aqueous extract of M. acuminata (banana) leaves.
2.3 Characterization of NCs
2.3.1 UV-vis spectroscopy analysis
The synthesized CuO/ZnO NCs from the reduction of metal ions (zinc and copper ions) from Zn(NO3)2·6H2O and 2 g of Cu(NO3)2·3H2O were mixed with deionized water. The mixture was sonicated to obtain the colloid form before UV-Vis spectroscopy analysis at room temperature using a UV-Vis spectrophotometer (Thermo Fisher Scientific G 10S UV-Vis, USA) in the wavelength range of 300–800 nm [6,7].
2.3.2 Field emission scanning electron microscopy (FE-SEM) analysis
The surface morphology of the CuO/ZnO NC was confirmed by the FE-SEM analysis (JOEL JSM 6710F, Japan) [6,7].
2.3.3 Fourier transform infrared spectroscopy (FT-IR) analysis
The green synthesized CuO/ZnO NCs were analyzed by FT-IR spectroscopy (Perkin Elmer Spectrum RX-1 FT-IR Spectrometer, USA) to identify the functional groups present in the CuO/ZnO NCs using the KBr pellet approach in which the CuO/ZnO NC and KBr were mixed in a ratio of 1:10 to form the pellet. The analysis was performed and recorded at a scan range of 4,000–400 cm−1 with a resolution of 4 cm−1 [6,7].
2.3.4 X-ray diffraction (XRD) analysis
The synthesized CuO/ZnO NC was completely dried and characterized using an X-ray diffractometer (Shimadzu XRD 6000, Japan) with Cu-Kα radiation in the 2θ range of 30°–70° at room temperature (25°C), 40 kV, and 20 mA.
2.4 Cytotoxicity test
2.4.1 Subculture of HCC2998 colorectal carcinoma cells
The cells were washed with 5 mL of PBS. About 3 mL of trypsin was added to a T25 flask and incubated for about 10 min. Then, 3 mL of complete growth medium (CGM) was added to the T25 flask, and the content in the flask was transferred to a 15 mL centrifuge tube for centrifugation for about 10 min at 25°C, 1,000 rpm. The pellet was mixed with the CGM and was transferred to new T25 flasks. The cells were incubated in a CO2 incubator at 37°C [38].
2.4.2 Preparation for MTT assay – cell counting
Cells were washed with 5 mL of PBS twice. About 3 mL of trypsin was added to the flask and incubated for about 10 min. About 3 mL of CGM was added to the flask to stop the trypsinization reaction. The suspension was transferred to a 15 mL centrifuge tube and centrifuged at 1,000 rpm for 10 min. The cells were resuspended in 5 mL of Dulbecco’s modified Eagle’s medium (DMEM). About 10 µL of the colorectal cancer cells was diluted with about 10 µL of the Trypan blue dye in an Eppendorf tube. About 20 µL of the suspension was pipetted into the chambers under a coverslip. The hemocytometer was viewed under an inverted microscope. The living cells, clearly seen under an inverted microscope, were counted for the four corner squares. The concentration of the viable cells per milliliter was calculated using Eqs. 1 and 2:
where DF is the dilution factor (cell·mL−1), V final is the final volume (µL), and V cells is the volume of the cells (µL).
The volume of the suspension required for seeding was calculated using Eq. 3 and was used for centrifugation:
where V seeding is the volume of the suspension required for seeding, N cells is the number of cells required, and C suspension is the concentration of the suspension. The supernatant medium was removed.
2.4.3 MTT assay
The HCC2998 colorectal carcinoma cells were seeded into the 96-well plate with 10,000 cells/well with DMEM. The cells were incubated overnight at 37°C, 5.00% CO2, 95.00% air, and 100.00% humidity. The cells were exposed to different concentrations of NC and cisplatin up to 100 µg·mL−1 for 24 h. About 20 µL of MTT was added to all wells and was incubated in the dark for 4 h. The sample with only DMEM acted as a blank, the sample with only colorectal cancer cells was considered a negative control, and colorectal cancer cells treated with cisplatin acted as a positive control. The MTT was removed from each well. About 100 µL of DMSO was added to all wells and incubated for 10–15 min in the dark. The absorbance was measured at 570 nm using a microplate reader or ELISA reader (Bio-Rad Laboratories: Model 680, USA). The cell viability after 24 h was determined using Eq. 4.
where OD is the optical density. The results were analyzed to evaluate the cytotoxic effect.
2.4.4 Statistical analysis
Statistical analysis was performed to investigate the variance for various concentrations of green-synthesized CuO/ZnO NCs and colorectal carcinoma cells (HCCC2998) treated with cisplatin. The mean and standard deviation in triplicate were obtained. The analysis for the significant cytotoxic effect of the synthesized CuO/ZnO NCs was performed using one-way ANOVA (SPSS version 26) at a significance level of p < 0.05.
3 Results
3.1 Characterization of the green-synthesized CuO/ZnO NC
The green-synthesized CuO/ZnO NC was characterized by e UV-Vis, FE-SEM, XRD, and FT-IR analysis, respectively, to identify and confirm the energy bandgap, morphology, crystallite nature, size of the synthesized CuO/ZnO NC, and the presence of functional groups in the M. acuminata leaf extract-mediated synthesized CuO/ZnO NC.
3.1.1 UV-visible (UV-Vis) spectroscopy analysis
UV-Vis analysis was conducted to confirm the formation of the green-synthesized CuO/ZnO NC. Figure 2 shows the UV-Vis absorption spectrum for the CuO/ZnO NC. A peak was observed at a wavelength of 365 nm for the M. acuminata leaf extract-mediated synthesized CuO/ZnO NC. Figure 3 shows (αhv)² vs Hv, which is also known as a Tauc plot, where hv is the photon energy and α is the absorption coefficient of CuO/ZnO NCs. The energy bandgap of CuO/ZnO NCs determined from Figure 3 was 3.43 eV.
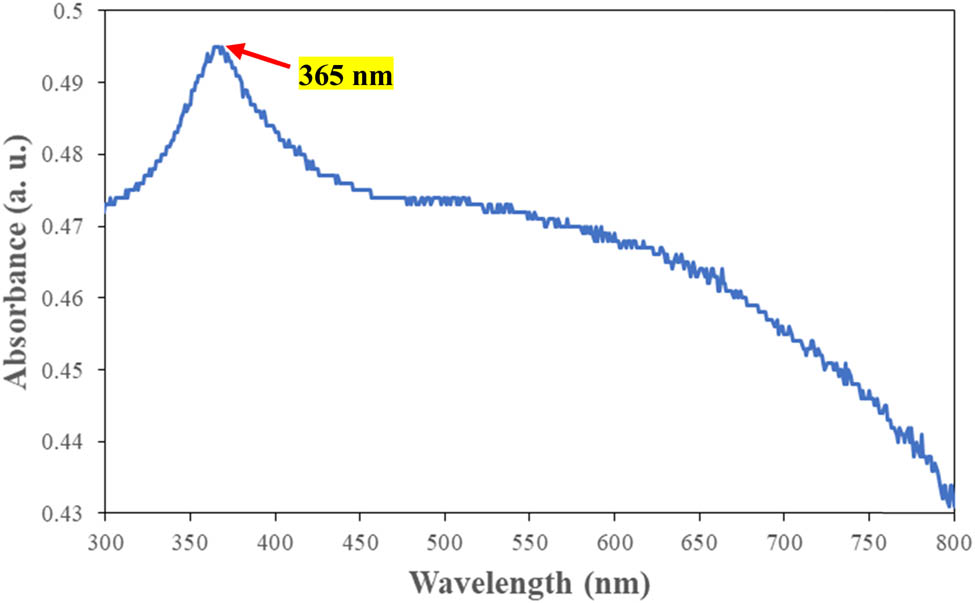
UV-Vis absorption spectrum of CuO/ZnO NCs.
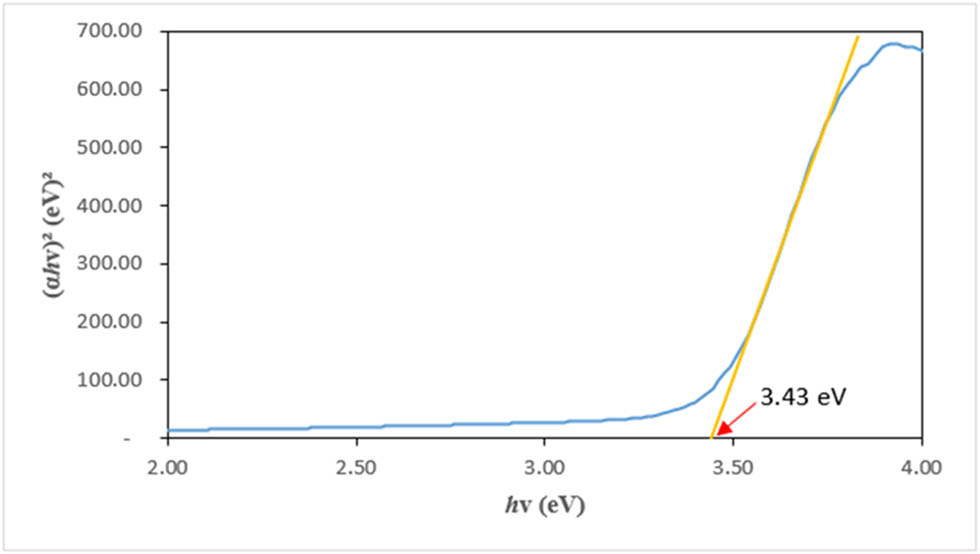
Energy bandgap for the green-synthesized CuO/ZnO NCs.
3.1.2 FE-SEM
FE-SEM was performed to observe the surface morphology of the green-synthesized CuO/ZnO NC. Figure 4(a) and (b) shows the FE-SEM for M. acuminata leaf extract-mediated synthesized CuO/ZnO NC at different magnifications. Both images show an irregularly shaped CuO/ZnO NC with agglomeration. The size of the green-synthesized CuO/ZnO NCs ranged from 31.8 to 85.7 nm.
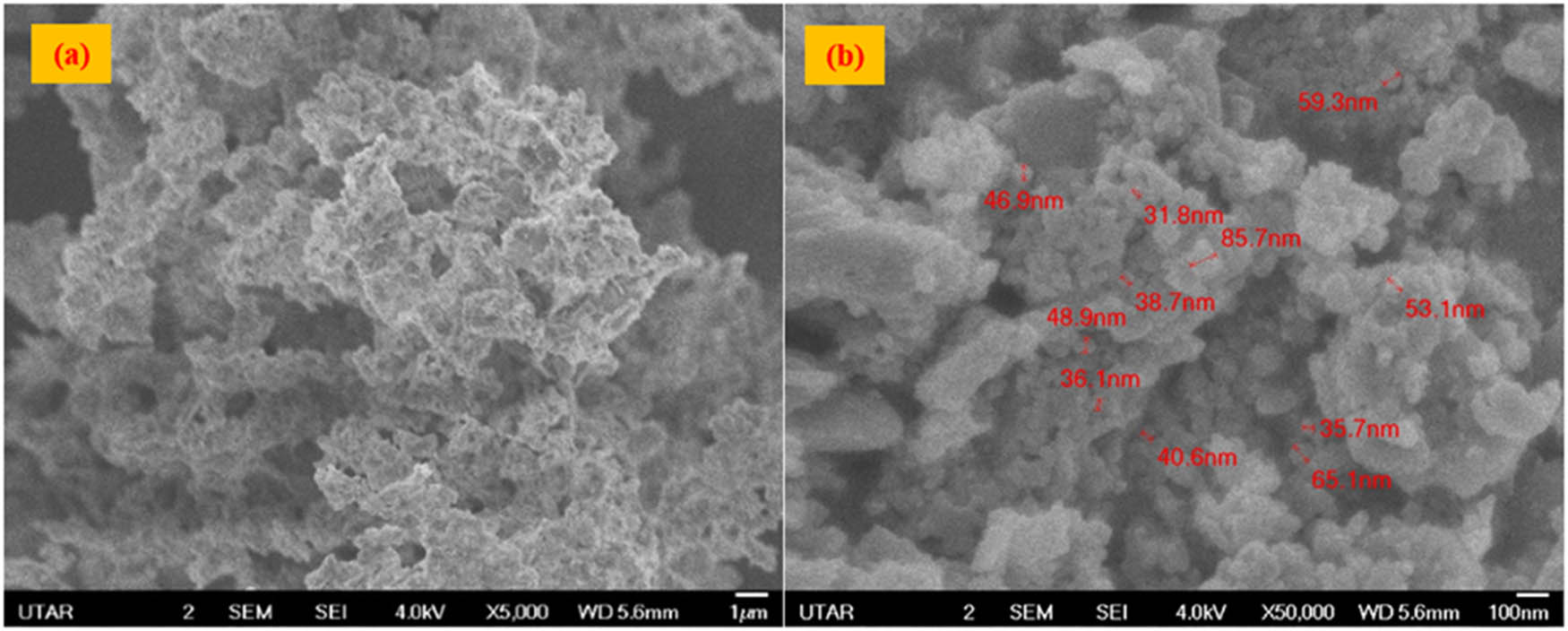
Morphology of the synthesized CuO/ZnO NC by FE-SEM: (a) 5,000× and (b) 50,000× magnifications.
3.1.3 XRD analysis
XRD analysis was performed to determine the crystalline structure and size of the green-synthesized CuO/ZnO NC. Figure 5 displays the XRD patterns of the green-synthesized CuO/ZnO NC, which was calcinated at 400°C from 2θ = 30° to 70°. The XRD analysis demonstrated nine sharp diffraction peaks at 2θ of 31.84°, 34.54°, 36.34°, 47.55°, 56.67°, 62.91°, 66.38°, and 67.99°, which are the peaks for ZnO with corresponding Miller indices of (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0) (1 0 3), (2 0 0), (1 1 2), and (2 0 1), while the less prominent diffraction peaks at 2θ of 32.69°, 35.60°, 38.86°, 48.71°, 50.69°, 56.85°, 60.75°, and 61.67° are the peaks for CuO with corresponding Miller indices of (1 1 0), (0 0 2), (1 1 1), (−1 1 2), (0 2 0), (2 0 2), (1 0 2), and (−1 1 3). From the XRD analysis, the crystalline structure of ZnO was hexagonal wurtzite, while that of CuO was a monoclinic structure. These results proved the presence of CuO and ZnO in the green-synthesized CuO/ZnO NC. The detected peaks for the CuO/ZnO NC that corresponds to the Miller index are labeled in Figure 5. The crystalline size of the CuO/ZnO NC was 24.78 nm from XRD analysis.
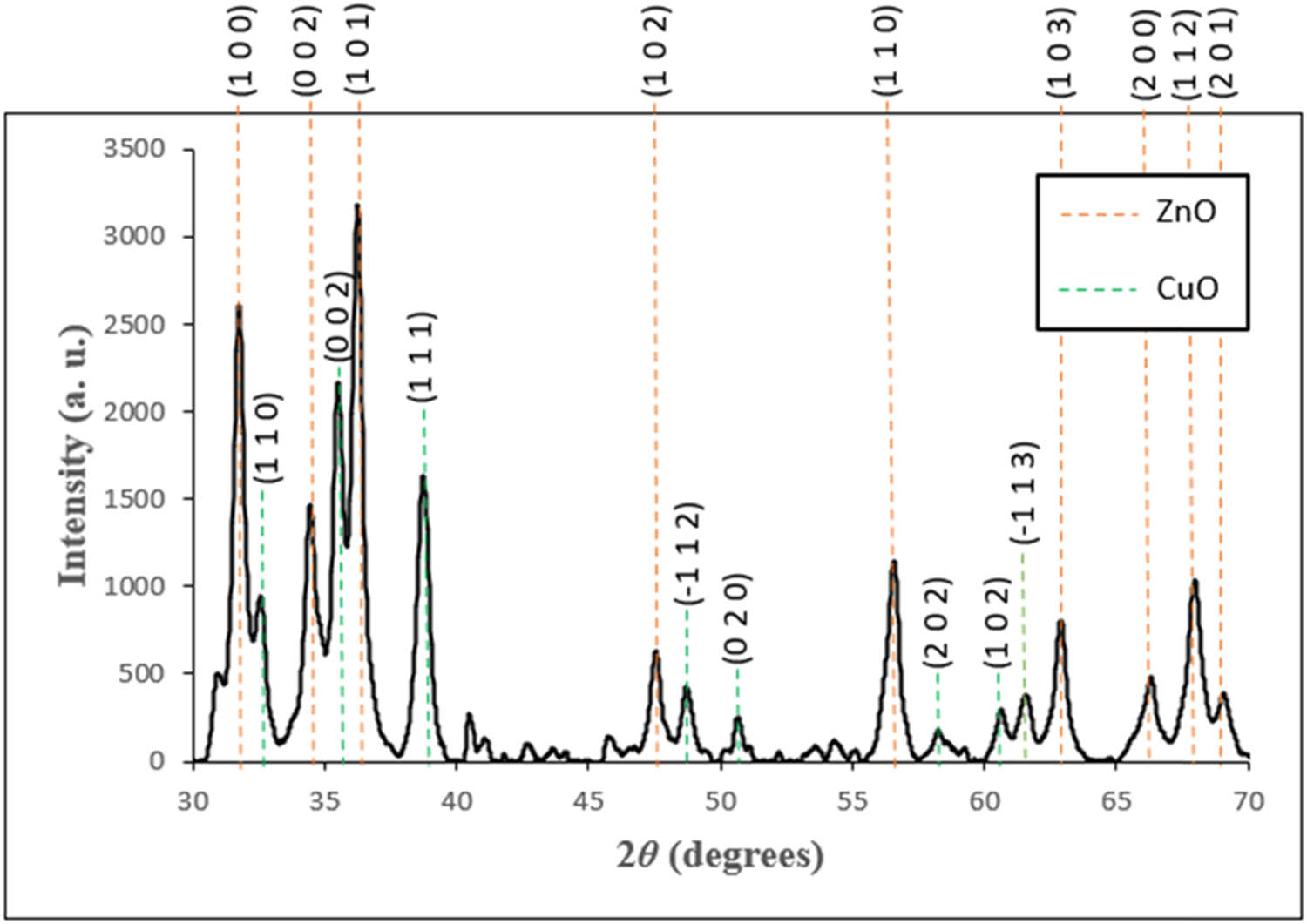
XRD patterns of M. acuminata-mediated CuO/ZnO NCs from 30° to 70°.
3.1.4 FT-IR analysis
FT-IR analysis was performed to determine the presence of functional groups in the green-synthesized CuO/ZnO NCs. Figure 6 depicts the FT-IR graph with nine bands detected at 478, 841, 1,041, 1,114, 1,384, 1,439, 1,637, 2,363, and 3,413 cm−1. Molecular motions of the detected peaks were identified: Zn–O stretching at 478 cm−1, Cu–O stretching at 841 cm−1, C–O and C═O stretching vibrations at 1,114 and 1,041 cm−1, C–N stretching at 1,384 cm−1, C–C aromatic stretching at 1,439 cm−1, N–H bending at 1,637 cm−1, C═O stretching at 2,363 cm−1, and O–H stretching at 3,413 cm−1. The possible functional groups detected and the possible presence of biomolecules corresponding to the wavenumber in the M. acuminata leaf extract-mediated synthesized CuO/ZnO NC are listed in Table 1.
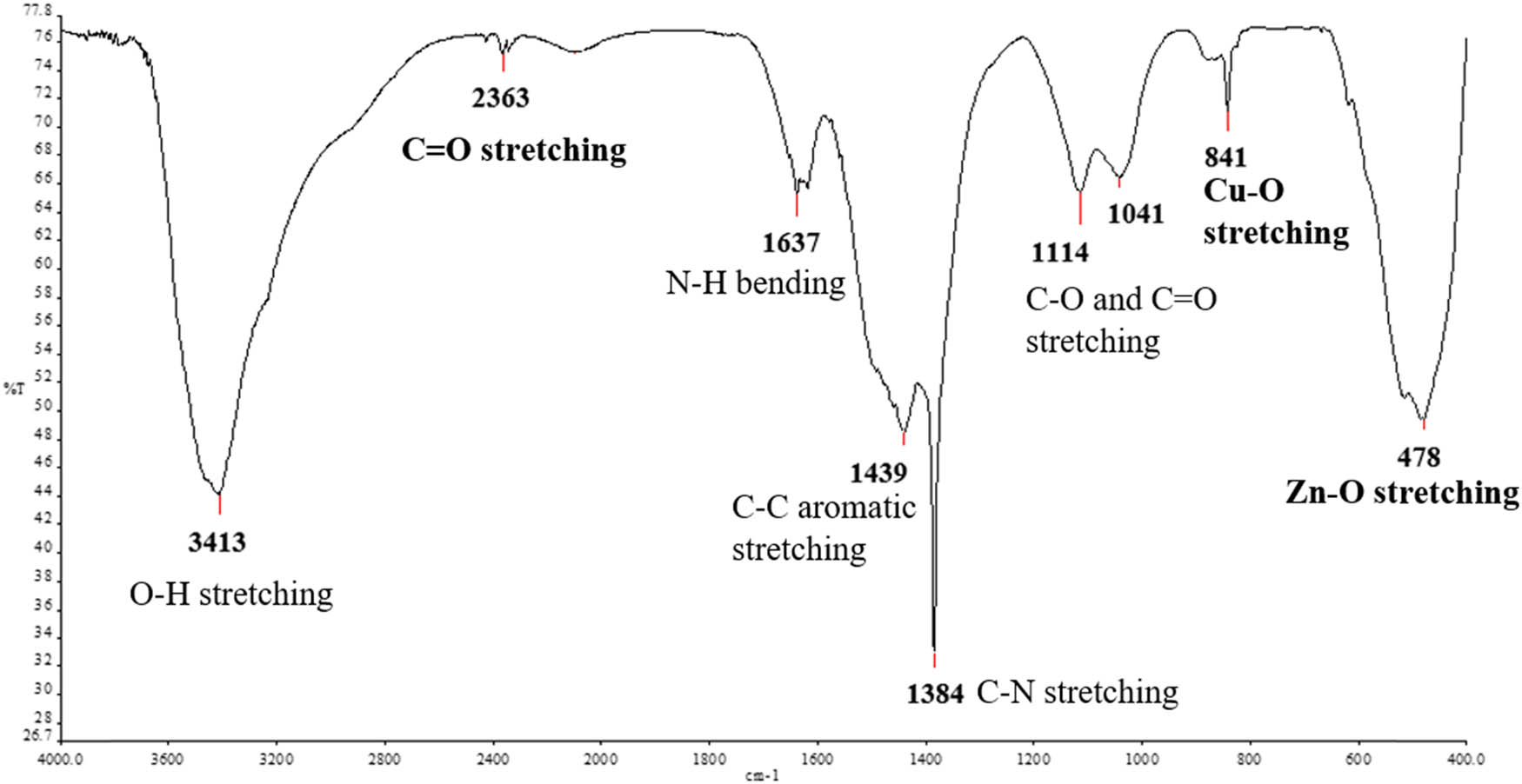
FT-IR spectrum of M. acuminata-mediated synthesized CuO/ZnO NC.
FT-IR analysis on the possible functional groups and biomolecules present in the M. acuminata-mediated synthesized CuO/ZnO NC
| Wavenumber (cm−1) | Molecular motion | Functional group | Biomolecules |
|---|---|---|---|
| 3,413 | O–H stretching | Hydroxyl group | Phenolic compound |
| 2,363 | C═O stretching | Carbonyl group | Carbon dioxide |
| 1,637 | N–H bending | Amines | Alkaloid |
| 1,439 | C–C aromatic stretching | Alkane | Carotenoid wax |
| 1,384 | C–N stretching | Aromatic amine | Biogenic amines |
| 1,114, 1,041 | C–O and C═O stretching vibration | Carbonyl group | Saponin flavonoid |
| 841 | Cu–O stretching | Cu–O | Inorganic compound |
| 478 | Zn–O stretching | Zn–O | Inorganic compound |
3.2 Cytotoxicity testing using the MTT assay
The cytotoxic effect of the green-synthesized CuO/ZnO NC was tested for the colorectal cancer cells (HCC2998) using the MTT assay. The cell morphology without treatment and after treatment with the CuO/ZnO NC and cisplatin drug at 100 μg·mL−1 was observed under an inverted microscope at 10× magnification, as shown in Figure 7.
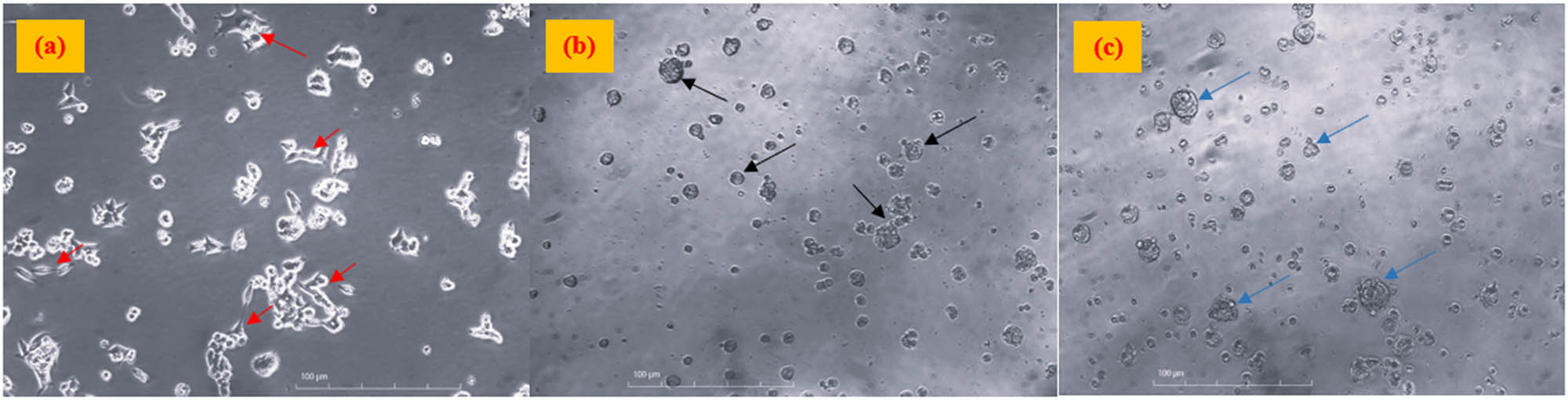
Colorectal cancer cells (a) without treatment (negative control), (b) treated with cisplatin (positive control), and (c) treated with CuO/ZnO NCs at 100 μg·mL−1 with 10× magnification. Note: The images were taken before the addition of MTT reagents. Red arrows show the cells with elongated shapes, whereas black and blue arrows show irregular to round shape cells after treatment.
As shown in Figure 7(a), the red arrows point to the non-treated colorectal cancer cells, which have an elongated shape with a shiny appearance. However, the morphological change from elongated shape to irregular and rounded shape was observed after treating the HCC2998 cells with both CuO/ZnO NC and cisplatin (positive control) at 100 μg·mL−1, as shown in Figure 7(b) and (c), respectively.
Besides, the percentage of cell viability was calculated. Figure 8 shows that when the concentration of CuO/ZnO NC and cisplatin increased, the percentage of cell viability decreased. The cell viability for HCC2998 treated with 100 µg·mL−1 CuO/ZnO NC was 54.21%, and that for HCC2998 treated with cisplatin was 27.71% after 24 h treatment; in other words, the NC and cisplatin showed around 46.00 and 72.00% cell death on HCC2998, respectively. This result indicates that the green-synthesized CuO/ZnO NC exerted a cytotoxic effect on the cancer cells. However, cisplatin showed a better cytotoxic effect compared to CuO/ZnO NCs. The one-way ANOVA was done to identify the significant cytotoxic effect on the cancer cells after receiving treatments. From the analysis, the significant result with p < 0.05 was found at 100 µg·mL−1 for CuO/ZnO NC (Figure 8), whereas the positive control cisplatin showed significant results with p < 0.05 at 25, 50 and 100 µg·mL−1.
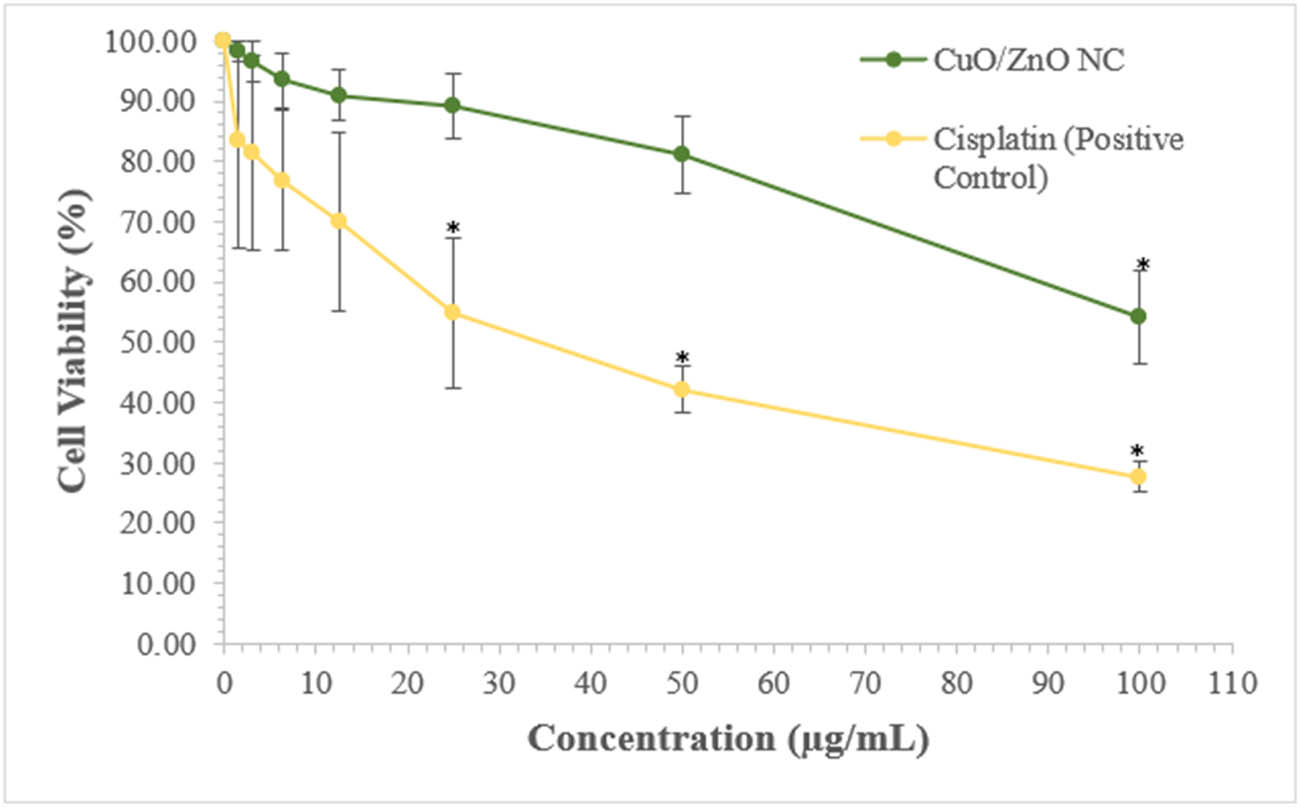
Cell viability curve for various concentrations of CuO/ZnO NC and cisplatin on colorectal cancer cells (HCC2998) after 24 h treatment with mean ± standard deviation (SD). *Significant difference of the colorectal cancer cells when treated with various concentrations of CuO/ZnO NC and cisplatin.
Figure 9 shows the IC50 for cisplatin toward the colorectal cancer cells to be 34 µg·mL−1, whereas the IC50 for CuO/ZnO NC was not determined since the maximum cell cytotoxicity was only 45.79%.
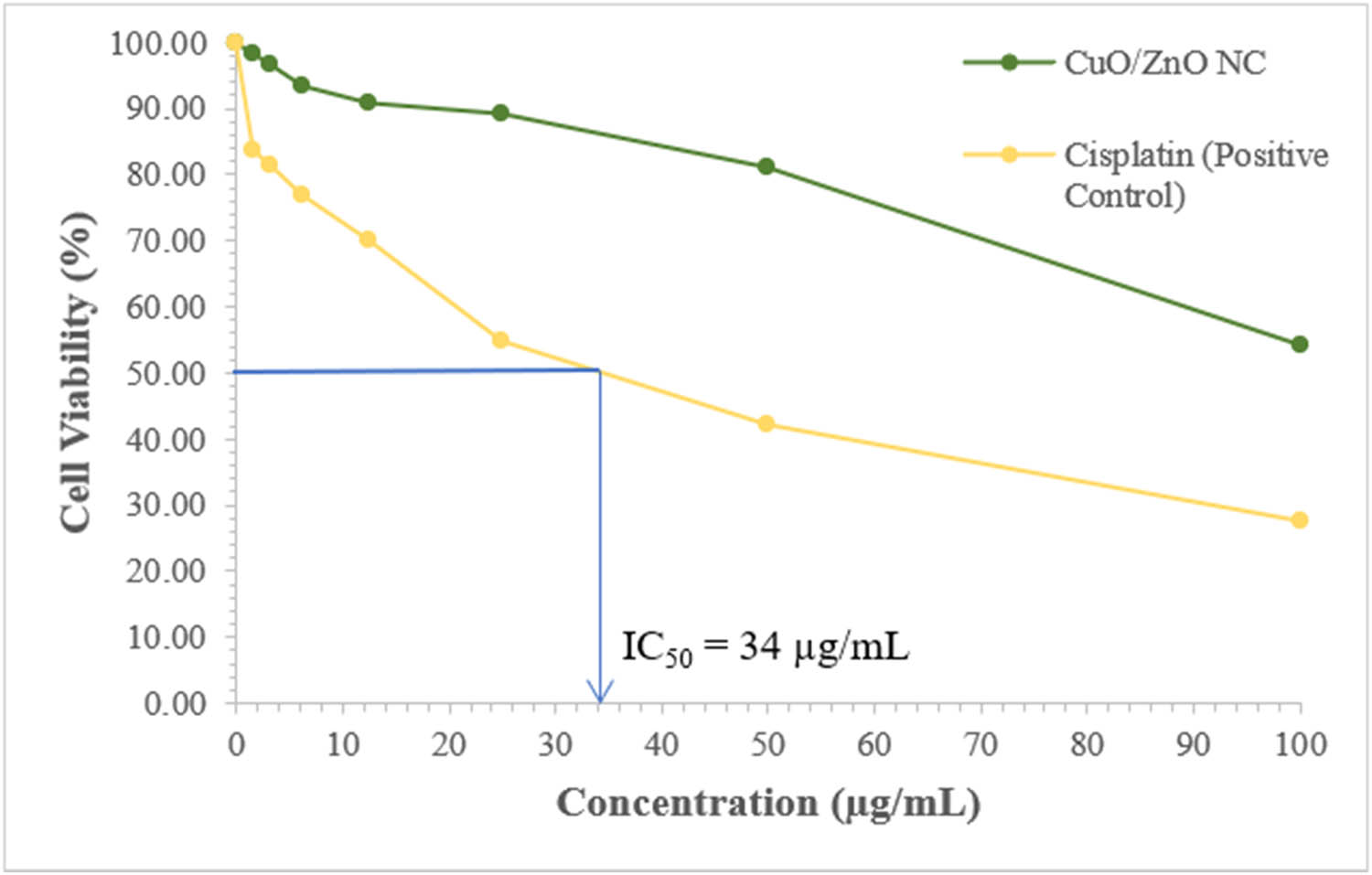
Determination of the IC50 for the cisplatin toward the colorectal cancer cells from cell viability against concentration.
4 Discussion
4.1 Characterization of the green-synthesized CuO/ZnO NC
4.1.1 Absorbance and bandgap energy of green-synthesized CuO/ZnO NCs
The absorbance of the green-synthesized CuO/ZnO NC was determined by UV-Vis spectroscopy analysis, which showed the peak at 365 nm. The formation of a peak in that specific wavelength was due to the surface plasmon resonance in which the vibration was detected as a result of the reaction between the emitted light photons and the conductivity of the electrons of the NC that depends on the morphology and the size of the NC [39]. This result was coherent with the previous studies in which the synthesized CuO/ZnO NC was in the wavelength range between 300 and 400 nm [40–42]. Therefore, this indicated that the synthesized NCs were CuO/ZnO.
Besides, the optical bandgap energy for the CuO/ZnO NC was determined using the Tauc plot with a bandgap energy of 3.43 eV. Tauc plot is used to determine the direct bandgap energy of the synthesized NC by drawing a straight line on the steepest part of the slope of the graph plotted and the extrapolated line that touched the x-axis, which is the value of the bandgap energy of that particular NCs [43]. Since CuO/ZnO NC is made of semiconductor components, the determination of bandgap energy is important. The bandgap energy that was reported in this study is similar to the finding that reported a bandgap energy of 3.25 eV for the synthesized CuO/ZnO NC [44]. The higher bandgap energy reported in the present study might be due to the smaller size of the synthesized CuO/ZnO NC. As reported by one of the research findings, the bandgap energy of the NC is interrelated to its structure and size [45]. The higher the bandgap energy, the smaller the size of the NC. The larger surface area of the NC contributes to better effectiveness in cytotoxic effect toward the cancerous cells.
4.1.2 Surface morphology of the M. acuminata-synthesized CuO/ZnO NCs
The morphology of the green-synthesized CuO/ZnO NC was determined by FE-SEM analysis, which showed that the NC had a size smaller than 100 nm, confirming the successful synthesis of CuO/ZnO NC as the size of the synthesized NC ranged from 31.8 to 85.7 nm with irregular shape (spherical and rod shapes). The irregular shape of the CuO/ZnO NC was because the surface energy of the synthesized NC was high [46]. Besides, agglomeration of NC was also observed. This observation was consistent with the findings from previous research that reported that the green synthesized CuO/ZnO NC by using the Calotropis gigantea leaves showed agglomeration by SEM analysis [47]. Since the condition for the crude extract of M. acuminata leaves, which act as the reducing and capping agents, was sticky after freeze-dried and, thus, this would contribute to the agglomeration of the synthesized CuO/ZnO NC. This statement was consistent with another study, which reported that the unique property of the plant extract was sticky [37]. Agglomeration of NC might also be driven by the forces of attraction between the synthesized nanocrystals [48].
4.1.3 Crystallite structure and size of the M. acuminata-synthesized CuO/ZnO NC
The crystallite structure of the synthesized CuO/ZnO NC was hexagonal wurtzite for ZnO while monoclinic for CuO, which is consistent with the previous findings [49,50]. The intensity of the peaks in the XRD spectrum represents the crystallinity of the tested sample in which the higher the intensity, the higher the crystallinity. As shown in Figure 5, high-intensity peaks were detected for ZnO, while low-intensity peaks were detected for CuO. This indicated that ZnO showed high crystallinity while the crystallinity of CuO was poor. Another reason that contributes to the low intensity of CuO is its small size [51]. Besides, the lower intensity of the CuO peaks might be due to a lower amount of the precursor, Cu(NO3)2·3H2O, which was used in synthesizing CuO/ZnO NC [29]. The finding that is consistent with the results of this study reported that the low intensity and crystallinity of CuO in CuO/ZnO NC correlated with the ZnO’s coating effect on the synthesized CuO, which subsequently prevented subsequent oxidation of the copper surface [52].
The crystallite structure of the synthesized CuO/ZnO NC was determined based on the 2θ from 30° to 70° and was found to be hexagonal shaped for ZnO. The crystallite size of the green synthesized CuO/ZnO NC was determined using the Scherrer equation (Eq. 5):
where D represents the crystallite size of NC, 0.9 represents the Scherrer’s constant, λ represents the wavelength of XRD radiation, β denotes the full width at half-maximum (FWHM) of peaks, and θ denotes the Bragg angle [53]. The average for the crystallite size of CuO/ZnO NC was calculated to be 24.78 nm. This result is similar to that reported in previous research, that the crystallite size was 23.21 nm for the CuO/ZnO NC [53]. Since the crystallite size for the synthesized CuO/ZnO NC was smaller than 100 nm, it revealed that the CuO/ZnO NC was nanocrystalline.
4.1.4 Presence of functional groups and the possible biomolecules in the M. acuminata-synthesized CuO/ZnO NC
The functional groups as well as the possible biomolecules that are present in the CuO/ZnO NC were determined from Figure 6. The FT-IR spectrum with absorption of 478 cm−1 indicated the Zn–O stretching with its corresponding biomolecules named inorganic compounds. It was reported previously that the FT-IR spectrum for Zn–O was at 469 cm−1, which is similar to the finding in this study [54]. The finding was further validated by other research findings that the Zn–O stretching vibration for the green-synthesized ZnO NPs by using leaves of Cassia auriculata was between 432 and 656 cm−1 [55].
The second band at 841 cm−1 belonged to the stretching of Cu–O, which confirmed that the biomolecule was an inorganic compound. A few studies reported that the FT-IR spectrum for the detection of Cu–O stretching was found in the range between 444 and 1,021.1 cm−1 [56–59]. From the findings of the study, 841 cm−1 was in the range for the Cu–O stretching, which confirmed the presence of CuO in the CuO/ZnO NCs. The peak at 2,363 cm−1 corresponded to the carbon dioxide in the atmosphere [60,61].
The remaining six bands at 1,041, 1,114, 1,384, 1,439, 1,637, and 3,413 cm−1 with possible functional groups of carbonyl group, carboxylic group, or aromatic amines, alkanes, amines, and hydroxyl groups, respectively, were related to the bioactive components that were present in the banana leaves. Examples of the bioactive compounds that could be found in banana leaves are flavonoids, alkaloids, and saponins [62,63].
The bands found at 1,041 and 1,114 cm−1 belonged to C–O or C═O stretching vibrations with carbonyl groups or ketone groups and with possible biomolecules of saponin and flavonoid. This is consistent with another finding that the peak at 1,041 cm−1 was for C–O stretching [64]. The FT-IR band at 1,044 cm−1, which is similar to the finding in this study with a band at 1,041 cm−1, was found to be the ketone group with C═O stretching [65]. For the absorption band at 1,114 cm−1, the detected molecular motion was the C–O stretching with possible biomolecules of saponin [66]. The band at an absorption peak at 1,384 cm−1 with possible molecular motion of C–N stretching under aromatic amine (biogenic amines) as in previous research, which reported that the FT-IR spectrum with a band at 1,384 cm−1 represented the C–N stretching, which was due to the presence of amine groups in the compound [67]. The sharp peak intensity for the band at 1,384 cm−1 indicates the high abundance of saponin and aromatic amine in the synthesized CuO/ZnO NC.
Furthermore, the FT-IR spectrum showed a band at 1,439 cm−1 representing the C–C aromatic stretching belonging to the alkane group with aromatic rings that were possible biomolecules of carotenoids and wax. As reported by previous research, the bands found at 1,444 and 1,422 cm−1 belonged to the aromatic hydrocarbon [37]. The finding was in the range of the wavenumber of the research, and thus, the possible functional group would be the aromatic hydrocarbon. In addition, the band at 1,637 cm−1 belonged to the N–H bending that corresponded to amine groups and the possible functional group of alkaloids, which is consistent with a previous study [68]. The last band was shown in the FT-IR spectrum at 3,413 cm−1 with possible hydroxyl functional groups with molecular motion of O–H stretching. This result is similar to the research from another study that the band detected at 3,416 cm−1 indicated the O–H stretching [69]. Another researcher’s finding was also consistent with this study in that the detected band in the FT-IR spectrum at 3,413 cm−1 revealed the hydroxyl functional group with possible biomolecules of phenolic compounds [70].
4.2 Cytotoxic effect of the M. acuminata-synthesized CuO/ZnO NC
Figure 7(a) displays the elongated, shiny colorectal cancer cells, indicating their viability and secure attachment to the 96-well plate after 24 h of incubation without any treatment. This served as the negative control, ensuring the absence of contamination during the assay. The CuO/ZnO NC possess unique properties that allow them to selectively target cancerous cells, sparing normal cells through ligand-receptor binding followed by endocytosis. Once inside, the NC penetrates the cancer cell membrane either directly or via ion transporters, accumulating in the cytoplasm. This accumulation leads to the production of ROS, which induces apoptosis in the cancer cells [71,72].
As shown in Figure 7(b) and (c), both treated cells with cisplatin and CuO/ZnO NC showed the morphology of the irregular or round shape of cells where the dying cells slowly detached from the 96-well plate [73]. Besides, cell shrinkage could be observed, which was the hallmark of cell death as the apoptotic bodies were observed with smaller round sizes around the dying cells. The black dots inside the cells can be either the granules of the cell components or the CuO/ZnO NC, as the NCs could attack the cancerous cells via the route of entry, either endocytosis or ion channel to induce ROS that promote apoptosis [9]. Compared to the colorectal cancer cells treated with cisplatin, the cells treated with CuO/ZnO NC showed more dying cells with apoptotic bodies that were still attached to the 96-well plate. In the microscopic view, the colorectal cancer cells treated with cisplatin showed more small spots as compared to that of CuO/ZnO NC, where the cell debris from the apoptosis of the colorectal cancer cells indicated that more cells actually died after treatment with cisplatin as compared to that of CuO/ZnO NC [74].
The percentage of cell viability with various concentrations of CuO/ZnO NC and cisplatin are depicted in Table 2 and Figure 8. As shown in Figure 8, the overall trend for the line graph was that the cell viability decreased with increasing concentrations of CuO/ZnO NC and cisplatin, indicating that the survival of the HCC2998 cancer cells depends on the dose or concentration of the CuO/ZnO NCs. The trend was listed clearly for evidence. The cell viability decreased with increasing concentration of CuO/ZnO NC and cisplatin up to 54.21 and 27.71%, respectively, for 100.00 µg·mL−1 at 24 h.
Percentage of cell viability (%) of colorectal cancer cells (HCC2998) treated with various concentrations of CuO/ZnO NC and cisplatin (positive control) for 24 h
| Concentration (µg·mL−1) | Cell viability (%) | |
|---|---|---|
| CuO/ZnO NC | Cisplatin (positive control) | |
| 0 | 100 ± 0 | 100 ± 0 |
| 1.5625 | 98.32 ± 1.68 | 83.57 ± 17.99 |
| 3.125 | 96.67 ± 3.33 | 81.46 ± 16.15 |
| 6.25 | 93.42 ± 4.54 | 76.84 ± 11.62 |
| 12.5 | 91.02 ± 4.25 | 70.02 ± 14.84 |
| 25 | 89.23 ± 5.46 | 54.91 ± 12.36* |
| 50 | 81.19 ± 6.44 | 42.19 ± 3.95* |
| 100 | 54.21 ± 7.76* | 27.71 ± 2.55* |
*Significant difference of the colorectal cancer cells treated with various concentrations of CuO/ZnO NC and cisplatin, respectively.
The cytotoxic effect for the cells treated with CuO/ZnO NC was found to be statistically significant at p < 0.05 and 100.00 µg·mL−1, whereas for that of the positive control cisplatin, it was found to be statistically significant at p < 0.05 from 25.00 µg·mL−1.
A previous study utilized Dovyalis caffra as a source of green synthesis to synthesize CuO/ZnO NC toward human breast cancer cells (MCF-7) with IC50 of 3.84 µg·mL−1 [16]. However, with the same tested cell lines with different sources of green synthesis, Lonicera caprifolium from another study showed a higher IC50 of 54.00 µg·mL−1 [75]. These findings demonstrated that different sources of green synthesis would affect the cytotoxicity of the NC toward cancer cells. Similar to our study, the effect of CuO/ZnO NC on the breast carcinoma cells (MCF7) was reported to increase as the concentration of the CuO/ZnO NC increased, indicating the dose-dependent relation between the concentration and the percentage of cell viability [16]. From their findings, it was demonstrated that the CuO/ZnO NC exerted a better effect on the MCF7 compared to the present study. This might be due to the unique property of the CuO/ZnO NC in selectively targeting the cells. This was proven by a study that the effectiveness of CuO/ZnO NC varies among different types of cancer cells. In that study, CuO/ZnO NC was synthesized using the extract of Sambucus nigra and tested against the melanoma cancer cells (A375) and lung carcinoma (A549), and it was found that the CuO/ZnO NC exerted high cytotoxicity toward the A375 cells due to a smaller IC50 (41.00 µg·mL−1) compared to that of A549 (785.00 µg·mL−1) [29].
Cisplatin is one of the most commonly used chemotherapeutic drugs for treating cancer patients, along with 5-fluorouracil. It is effective against a wide range of malignancies, including lung, neck, colorectal, and ovarian cancers, among others [76]. Given its frequent use in the treatment of colorectal cancer, cisplatin was employed as the positive control in this study. Cisplatin exerts its anticancer effects by promoting apoptosis through DNA disruption, leading to DNA fragmentation and impairment, which ultimately inhibits the growth of cancerous cells [77].
The entry of the nanoparticles toward the cancer cells is either via endocytosis or ion channels. The possible mechanism for nanoparticles to exert a cytotoxic effect on the cancer cells is the generation of ROS, which promotes oxidative stress and, as a result, induces apoptosis of the cancer cells. Upon the entry of nanoparticles via endocytosis, the generation of the ROS would contribute to DNA damage, which in turn causes cell cycle arrest, leading to apoptosis of cancer cells [9,78]. Besides, the nanoparticle uptake via the endocytosis pathway would fuse with lysosomes, the nanoparticles would be digested by the low pH of the enzyme, and the toxic metal ions would be removed via autophagy [9,79]. The induction of the ROS can improve the enhanced permeability and retention effect, which causes the accumulation of nanoparticles on the cancer cells to exert cytotoxic effects [80]. For the nanoparticles that undergo dissolution, the metal ions will be released and enter the cancer cells through the ion channel to suppress the function of the Bcl-2 markers and prevent apoptosis. This suppression action would indirectly lead to the release of cytochrome c from the mitochondria, which initiates the caspase pathway and apoptosis of cancer cells [9,81,82].
5 Conclusion
In the present study, CuO/ZnO NC was successfully green synthesized by using the leaves of M. acuminata and characterized using various techniques. The UV-Vis spectroscopy analysis showed that the synthesized CuO/ZnO NC was detected at a wavelength 365 nm with a bandgap energy of 3.43 eV. FE-SEM analysis depicted that the synthesized CuO/ZnO NC was in nanoscale ranging from 31.8 to 85.7 nm, and irregular shape of NCs was observed with agglomeration. The synthesized CuO/ZnO NC was determined with a crystallite size of 24.78 nm from the XRD analysis in which e ZnO was detected with hexagonal wurtzite while CuO was of a monoclinic structure. ZnO in the NC showed higher crystallinity compared to CuO due to higher intensity peaks detected for ZnO. From the FT-IR analysis, the possible functional groups present in the CuO/ZnO NC were hydroxyl groups, carbonyl groups, amines, alkanes, aromatic amines, Cu–O and Zn–O with corresponding possible biomolecules including phenolic compound, carbon dioxide, alkaloid, carotenoids or wax, biogenic amines, saponin or flavonoid, and inorganic compounds. The treatment of CuO/ZnO NC showed a dose-dependent cell death with a significant anticancer effect at 100.00 µg·mL−1 with 45.79% cell death in 24 h.
The findings of this study provide valuable insights into the potential of CuO/ZnO NCs as a cost-effective anticancer agent, offering a financially viable alternative to expensive long-term cancer treatments. The results suggest that CuO/ZnO NC could serve as a promising option for cancer therapy, particularly for patients seeking more affordable treatment. Future research is required on the in vivo testing of the synthesized NC to investigate the anti-cancer effects through animal studies to report the efficacy of this NC.
The use of M. acuminata leaf extract as the reducing agent in this study posed no issue as the leaves are readily available in Malaysia and Asian countries, and the preparation of the leaf extract to synthesize the NCs was straightforward and efficient. Furthermore, from a commercial perspective, the low cost of production of green NCs compared to conventional chemotherapeutic drugs presents a promising opportunity for large-scale production. Since NC synthesized using the green method is more stable, cheaper, eco-friendly, and has no toxic by-products compared to the chemically synthesized one, no further purification is needed to remove the toxic by-products.
Acknowledgements
The authors would like to express sincere gratitude and appreciation to UTAR for providing the equipment and facilities for this research project.
-
Funding information: This research was funded by Universiti Tunku Abdul Rahman Research Funding (UTARRF 6200/SB7).
-
Author contributions: Yee Jin Wong: investigation, formal analysis, and writing – original draft; Hemaroopini Subramaniam: methodology and formal analysis; Ling Shing Wong: methodology and writing – review and editing; Anto Cordelia Tanislaus Antony Dhanapal: methodology and writing – review and editing; Yu Bin Chan: methodology, investigation, and writing – review and editing; Mohammod Aminuzzaman: methodology and writing – review and editing; Lai-Hock Tey: methodology and writing – review and editing; Ashok Kumar Janakiraman: methodology and writing – review and editing; Saminathan Kayarohanam: methodology, formal analysis, and writing – review and editing; Sinouvassane Djearamane: supervision, project administration, formal analysis, and writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Bukhari A, Ijaz I, Gilani E, Nazir A, Zain H, Saeed R, et al. Green synthesis of metal and metal oxide nanoparticles using different plants’ parts for antimicrobial activity and anticancer activity: A review article. Coatings. 2021;11(11):1374.10.3390/coatings11111374Suche in Google Scholar
[2] Mani VM, Nivetha S, Sabarathinam S, Barath S, Das MP, Basha S, et al. Multifunctionalities of mycosynthesized zinc oxide nanoparticles (ZnONPs) from Cladosporium tenuissimum FCBGR: Antimicrobial additives for paints coating, functionalized fabrics and biomedical properties. Prog Org Coat. 2022;163:106650. 10.1016/j.porgcoat.2021.106650.Suche in Google Scholar
[3] Soares S, Sousa J, Pais A, Vitorino C. Nanomedicine: Principles, properties, and regulatory issues. Front Chem. 2018;6:360. 10.3389/fchem.2018.00360.Suche in Google Scholar PubMed PubMed Central
[4] Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules. 2019;25(1):112. 10.3390/molecules25010112.Suche in Google Scholar PubMed PubMed Central
[5] World Health Organization. Cancer. World Health Organization; 2022 (24 December 2022). https://www.who.int/news-room/fact-sheets/detail/cancer.Suche in Google Scholar
[6] Shyamalagowri S, Charles P, Manjunathan J, Kamaraj M, Anitha R, Pugazhendhi A. In vitro anticancer activity of silver nanoparticles phyto-fabricated by hylocereus undatus peel extracts on human liver carcinoma (HEPG2) cell lines. Process Biochem. 2022;116:17–25. 10.1016/j.procbio.2022.02.022.Suche in Google Scholar
[7] Rishikesan S, Basha MAM. Synthesis, characterization and evaluation of antimicrobial, antioxidant & anticancer activities of copper doped zinc oxide nanoparticles. Acta Chim Slov. 2020;67(1):235–45. 10.17344/acsi.2019.5379.Suche in Google Scholar
[8] Saravanan M, Gopinath V, Chaurasia MK, Syed A, Ameen F, Purushothaman N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathog. 2018;115:57–63.10.1016/j.micpath.2017.12.039Suche in Google Scholar PubMed
[9] Anjum S, Hashim M, Malik SA, Khan M, Lorenzo JM, Abbasi BH, et al. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers. 2021;13(18):4570. 10.3390/cancers13184570.Suche in Google Scholar PubMed PubMed Central
[10] Mohammadi-Aloucheh R, Habibi-Yangjeh A, Bayrami A, Latifi-Navid S, Asadi A. Enhanced anti-bacterial activities of zno nanoparticles and zno/cuo nanocomposites synthesized using vaccinium arctostaphylos L. fruit extract. Artif Cells Nanomed Biotechnol. 2018;46(sup1):1200–9. 10.1080/21691401.2018.1448988.Suche in Google Scholar PubMed
[11] Nagaraj E, Karuppannan K, Shanmugam P, Venugopal S. Exploration of bio-synthesized copper oxide nanoparticles using Pterolobium hexapetalum leaf extract by photocatalytic activity and biological evaluations. J Clust Sci. 2019;30(4):1157–68.10.1007/s10876-019-01579-8Suche in Google Scholar
[12] Bhavana S, Kusuma CG, Gubbiveeranna V, Sumachirayu CK, Ravikumar H, Nagaraju S. Green route synthesis of copper oxide nanoparticles using vitex altissima [l] leaves extract and their potential anticancer activity against A549 cell lines and its apoptosis induction. Inorg Nano-Metal Chem. 2022;54(10):1012–25.10.1080/24701556.2022.2081195Suche in Google Scholar
[13] Kalaiarasi A, Sankar R, Anusha C, Saravanan K, Aarthy K, Karthic S, et al. Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol Lett. 2017;40(2):249–56.10.1007/s10529-017-2463-6Suche in Google Scholar PubMed
[14] Akintelu SA, Folorunso AS, Folorunso FA, Oyebamiji AK. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon. 2020;6(7):04508.10.1016/j.heliyon.2020.e04508Suche in Google Scholar PubMed PubMed Central
[15] He X, Yang DP, Zhang X, Liu M, Kang Z, Lin C, et al. Waste eggshell membrane-templated cuo-zno nanocomposites with enhanced adsorption, catalysis and antibacterial properties for water purification. Chem Eng J. 2019;369:621–33.10.1016/j.cej.2019.03.047Suche in Google Scholar
[16] Adeyemi JO, Onwudiwe DC, Oyedeji AO. Biogenic synthesis of CuO, ZnO, and CuO–ZnO nanoparticles using leaf extracts of Dovyalis caffra and their biological properties. Molecules. 2022;27(10):3206. 10.3390/molecules27103206.Suche in Google Scholar PubMed PubMed Central
[17] Anu A, Abdul Khadar M. Cuo–zno nanocomposite films with efficient interfacial charge transfer characteristics for optoelectronic applications. SN Appl Sci. 2019;1(9):1057. 10.1007/s42452-019-1002-6.Suche in Google Scholar
[18] Sakib A, Masum S, Hoinkis J, Islam R, Molla M. Synthesis of CuO/ZnO NCs and their application in photodegradation of toxic textile dye. J Compos Sci. 2019;3(3):91. 10.3390/jcs3030091.Suche in Google Scholar
[19] Bordbar M, Negahdar N, Nasrollahzadeh M. Melissa officinalis L. leaf extract assisted green synthesis of Cuo/ZnO nanocomposites for the reduction of 4-nitrophenol and rhodamine B. Sep Purif Technol. 2018;191:295–300. 10.1016/j.seppur.2017.09.044.Suche in Google Scholar
[20] Fang H, Guo Y, Wu T, Liu Y. Biomimetic synthesis of urchin-like CuO/ZnO nanocomposites with excellent photocatalytic activity. New J Chem. 2018;42(15):12779–86. 10.1039/c8nj02052c.Suche in Google Scholar
[21] National Cancer Registry. Malaysia national cancer registry report (mncr) 2012–2016; 2019. (30 December 2022). https://www2.moh.gov.my/moh/resources/Penerbitan/Laporan/Umum/2012-2016%20(MNCRR)/MNCR_2012-2016_FINAL_(PUBLISHED_2019).pdf.Suche in Google Scholar
[22] Fakhari S, Jamzad M, Fard HK. Green synthesis of zinc oxide nanoparticles: A comparison. GCLR. 2019;12(1):19–24. 10.1080/17518253.2018.1547925.Suche in Google Scholar
[23] Dowlath MJ, Musthafa SA, Mohamed Khalith SB, Varjani S, Karuppannan SK, Ramanujam GM, et al. Comparison of characteristics and biocompatibility of green synthesized iron oxide nanoparticles with chemical synthesized nanoparticles. Environ Res. 2021;201:111585. 10.1016/j.envres.2021.111585.Suche in Google Scholar PubMed
[24] El Bialy BE, Hamouda RA, Abd Eldaim MA, El Ballal SS, Heikal HS, Khalifa HK, et al. Comparative toxicological effects of biologically and chemically synthesized copper oxide nanoparticles on mice. Int J Nanomed. 2020;15:3827–42.10.2147/IJN.S241922Suche in Google Scholar PubMed PubMed Central
[25] Vinayagam R, Pai S, Murugesan G, Varadavenkatesan T, Selvaraj R. Synthesis of photocatalytic zinc oxide nanoflowers using peltophorum pterocarpum pod extract and their characterization. Appl Nanosci. 2021;13(1):847–57. 10.1007/s13204-021-01919-z.Suche in Google Scholar
[26] Raklami A, Meddich A, Oufdou K, Baslam M. Plants – microorganisms-based bioremediation for heavy metal cleanup: recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int J Mol Sci. 2022;23(9):5031.10.3390/ijms23095031Suche in Google Scholar PubMed PubMed Central
[27] Fakhar-e-Alam M, Shafiq Z, Mahmood A, Atif M, Anwar H, Hanif A, et al. Assessment of green and chemically synthesized copper oxide nanoparticles against hepatocellular carcinoma. J King Saud Univ Sci. 2021;33(8):101669. 10.1016/j.jksus.2021.101669.Suche in Google Scholar
[28] Lavín A, Sivasamy R, Mosquera E, Morel MJ. High proportion ZnO/CuO nanocomposites: Synthesis, structural and optical properties, and their photocatalytic behavior. Surf Interfaces. 2019;17:100367. 10.1016/j.surfin.2019.100367.Suche in Google Scholar
[29] Cao Y, Dhahad HA, El-Shorbagy MA, Alijani HQ, Zakeri M, Heydari A, et al. Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sci Rep. 2021;11(1):23479. 10.1038/s41598-021-02937-1.Suche in Google Scholar PubMed PubMed Central
[30] Das A, Bindhu J, Deepesh P, Shanmuga Priya G, Soundariya S. In vitro anticancer study of bioactive compound isolated from Musa extract (musa acuminata). Indian J Public Health Res Dev. 2020;11(1):340–6. 10.37506/v11/i1/2020/ijphrd/193841.Suche in Google Scholar
[31] Sidhu JS, Zafar TA. Bioactive compounds in banana fruits and their health benefits. Food Qual Saf. 2018;2(4):183–8.10.1093/fqsafe/fyy019Suche in Google Scholar
[32] Vijay N, Shashikant D, Mohini P. Assessment of antidiabetic potential of Musa acuminata peel extract and its fractions in experimental animals and characterisation of its bioactive compounds by HPTLC. Arch Physiol Biochem. 2019;128(2):360–72.10.1080/13813455.2019.1683585Suche in Google Scholar PubMed
[33] Srinivas BK, Shivamadhu MC, Jayarama S. Musa acuminata lectin exerts anti-cancer effects on Hela and EAC cells via activation of caspase and inhibitions of AKT, ERK, and JNK pathway expression and suppresses the neoangiogenesis in in-vivo models. Int J Biol Macromol. 2021;166:1173–87.10.1016/j.ijbiomac.2020.10.272Suche in Google Scholar PubMed
[34] Valsalam S, Agastian P, Esmail GA, Ghilan AK, Al-Dhabi NA, Arasu MV. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J Photochem Photobiol B: Biol. 2019;201:111670.10.1016/j.jphotobiol.2019.111670Suche in Google Scholar PubMed
[35] Raghavendra N, Hublikar LV, Patil SM, Bhat P. Microwave assisted biosynthesis of silver nanoparticles using banana leaves extract: Phytochemical, spectral characterization, and anticancer activity studies. J Water Environ Nanotechnol. 2018;6(1):49–61.Suche in Google Scholar
[36] Abdullah FH, Abu Bakar NHH, Abu Bakar M. Low temperature biosynthesis of crystalline zinc oxide nanoparticles from Musa acuminata peel extract for visible-light degradation of methylene blue. Optik. 2020;206:164279. 10.1016/j.ijleo.2020.164279.Suche in Google Scholar
[37] Chan YB, Selvanathan V, Tey L-H, Akhtaruzzaman M, Anur FH, Djearamane S, et al. Effect of calcination temperature on structural, morphological and optical properties of copper oxide nanostructures derived from Garcinia mangostana L. leaf extract. Nanomaterials. 2022;12(20):589–608. 10.3390/nano12203589.Suche in Google Scholar PubMed PubMed Central
[38] Segeritz CP, Vallier L. Basic science methods for clinical researchers. Academic Press; 2017. p. 151–72. 10.1016/b978-0-12-803077-6.00009-6.Suche in Google Scholar
[39] Jana J, Ganguly M, Pal T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016;6(89):86174–211. 10.1039/C6RA14173K.Suche in Google Scholar
[40] Bekru AG, Tufa LT, Zelekew OA, Goddati M, Lee J, Sabir FK. Green synthesis of a CuO–ZnO NCs for efficient photodegradation of methylene blue and reduction of 4-nitrophenol. ACS Omega. 2022;7(35):30908–19. 10.1021/acsomega.2c02687.Suche in Google Scholar PubMed PubMed Central
[41] Das S, Srivastava VC. Synthesis and characterization of ZnO/CuO nanocomposite by electrochemical method. Mater Sci Semicond Process. 2017;57:173–7. 10.1016/j.mssp.2016.10.031.Suche in Google Scholar
[42] Subha PP, Jayaraj MK. Enhanced room temperature gas sensing properties of low temperature solution processed ZnO/CuO heterojunction. BMC Chem. 2019;13(1):4. 10.1186/s13065-019-0519-5.Suche in Google Scholar PubMed PubMed Central
[43] Jubu PR, Yam FK, Igba VM, Beh KP. Tauc-plot scale and extrapolation effect on bandgap estimation from UV–VIS–NIR Data – A case study of β-GA2O3. J Solid State Chem. 2020;290:121576. 10.1016/j.jssc.2020.121576.Suche in Google Scholar
[44] Noothongkaew S, Thumthan O, An K-S. UV-photodetectors based on CuO/ZnO nanocomposites. Mater Lett. 2018;233:318–23. 10.1016/j.matlet.2018.09.024.Suche in Google Scholar
[45] Singh M, Goyal M, Devlal K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J Taibah Univ Sci. 2018;12(4):470–5. 10.1016/j.jtusci.2017.06.007.Suche in Google Scholar
[46] Hussein S, Mahmoud AM, Elgebaly HA, Hendawy OM, Hassanein EH, Moustafa SM, et al. Green synthesis of trimetallic NCs (Ru/Ag/PD)-NP and its in vitro antimicrobial and anticancer activities. J Chem. 2022;2022:1–14. 10.1155/2022/4593086.Suche in Google Scholar
[47] Rajith Kumar CR, Betageri VS, Nagaraju G, Pujar GH, Onkarappa HS, Latha MS. One-pot green synthesis of ZnO–CuO nanocomposites and their enhanced photocatalytic and antibacterial activity. ANSN. 2020;11(1):015009. 10.1088/2043-6254/ab6c60.Suche in Google Scholar
[48] Ashraf MA, Peng W, Zare Y, Rhee KY. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res Lett. 2018;13(1):214. 10.1186/s11671-018-2624-0.Suche in Google Scholar PubMed PubMed Central
[49] Chauhan A, Verma R, Batoo KM, Kumari S, Kalia R, Kumar R, et al. Structural and optical properties of copper oxide nanoparticles: A study of variation in structure and antibiotic activity. JMR. 2021;36(7):1496–509. 10.1557/s43578-021-00193-7.Suche in Google Scholar
[50] Shinde RS, Khairnar SD, Patil MR, Adole VA, Koli PB, Deshmane VV, et al. Synthesis and characterization of ZnO/CuO NCs as an effective photocatalyst and gas sensor for environmental remediation. J Inorg Organomet Polym Mater. 2022;32(3):1045–66. 10.1007/s10904-021-02178-9.Suche in Google Scholar
[51] Zhu L, Li H, Liu Z, Xia P, Xie Y, Xiong D. Synthesis of the 0D/3d Cuo/ZnO heterojunction with enhanced photocatalytic activity. J Phys Chem C. 2018;122(17):9531–9. 10.1021/acs.jpcc.8b01933.Suche in Google Scholar
[52] Chan YB, Aminuzzaman M, Tey L-H, Win YF, Watanabe A, Djearamame S, et al. Impact of diverse parameters on the physicochemical characteristics of green-synthesized zinc oxide-copper oxide nanocomposites derived from an aqueous extract of Garcinia mangostana L. leaf. Materials. 2023;16(15):5421–39. 10.3390/ma16155421.Suche in Google Scholar PubMed PubMed Central
[53] Rani P, Kaur G, Rao KV, Singh J, Rawat M. Impact of green synthesized metal oxide nanoparticles on seed germination and seedling growth of Vigna radiata (Mung Bean) and Cajanus cajan (red gram). J Inorg Organomet Polym Mater. 2020;30(10):4053–62. 10.1007/s10904-020-01551-4.Suche in Google Scholar
[54] Faisal S, Jan H, Shah SA, Shah S, Khan A, Akbar MT, et al. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega. 2021;6(14):9709–22. 10.1021/acsomega.1c00310.Suche in Google Scholar PubMed PubMed Central
[55] Ramesh P, Saravanan K, Manogar P, Johnson J, Vinoth E, Mayakannan M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens Bio-Sens Res. 2021;31:100399. 10.1016/j.sbsr.2021.100399.Suche in Google Scholar
[56] Amin F, Fozia, Khattak B, Alotaibi A, Qasim M, Ahmad I, et al. Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. eCAM. 2021;2021:1–12. 10.1155/2021/5589703.Suche in Google Scholar PubMed PubMed Central
[57] Araya-Castro K, Chao T-C, Durán-Vinet B, Cisternas C, Ciudad G, Rubilar O. Green synthesis of copper oxide nanoparticles using protein fractions from an aqueous extract of brown algae Macrocystis pyrifera. Processes. 2020;9(1):78. 10.3390/pr9010078.Suche in Google Scholar
[58] Keabadile OP, Aremu AO, Elugoke SE, Fayemi OE. Green and traditional synthesis of copper oxide nanoparticles – comparative study. Nanomaterials. 2020;10(12):2502. 10.3390/nano10122502.Suche in Google Scholar PubMed PubMed Central
[59] Sukumar S, Rudrasenan A, Padmanabhan Nambiar D. Green-synthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extract and their applications. ACS Omega. 2020;5(2):1040–51. 10.1021/acsomega.9b02857.Suche in Google Scholar PubMed PubMed Central
[60] Georgieva V, Retoux R, Ruaux V, Valtchev V, Mintova S. Detection of CO2 and O2 by iron loaded LTL zeolite films. Front Chem Eng. 2018;12(1):94–102. 10.1007/s11705-017-1692-5.Suche in Google Scholar
[61] Li X, Wei Y, Xu J, Xu N, He Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FT-IR macro- and micro-spectroscopy coupled with chemometrics. Biotechnol Biofuels Bioprod. 2018;11(1):263. 10.1186/s13068-018-1251-4.Suche in Google Scholar PubMed PubMed Central
[62] Harith SS, Yasim NHM, Harun A, Wan Omar WSA, Musa MS. Phytochemical screening, antifungal and antibacterial activities of Musa acuminata plant. MJAS. 2018;22(3):452–7. 10.17576/mjas-2018-2203-11.Suche in Google Scholar
[63] Mostafa HS. Banana plant as a source of valuable antimicrobial compounds and its current applications in the food sector. J Food Sci. 2021;86(9):3778–97. 10.1111/1750-3841.15854.Suche in Google Scholar PubMed
[64] Ibrahim MM, Fahmy TYA, Salaheldin EI, Mobarak F, Youssef MA, Mabrook MR. Role of tosyl cellulose acetate as potential carrier for controlled drug release. Life Sci. 2021;12(10):127–33. 10.7537/marslsj121015.16.Suche in Google Scholar
[65] Trifunschi S, Munteanu MF, Agotici V, Pintea S, Gligor R. Determination of flavonoid and polyphenol compounds in Viscum album and Allium sativum extracts. Int Curr Pharm J. 2015;4(5):382–5. 10.3329/icpj.v4i5.22861.Suche in Google Scholar
[66] Zakiyaturrodliyah L, Brotosudarmo TH. Saponin from purple eggplant (Solanum melongena L.) and their activity as pancreatic lipase inhibitor. IOP Conf Ser: Mater Sci Eng. 2019;509:012139. 10.1088/1757-899x/509/1/012139.Suche in Google Scholar
[67] Yang L, May PW, Yin L, Smith JA, Rosser KN. Ultra fine carbon nitride nanocrystals synthesized by laser ablation in liquid solution. J Nanopart Res. 2007;9(6):1181–5. 10.1007/s11051-006-9192-4.Suche in Google Scholar
[68] Kumar S, Boro JC, Ray D, Mukherjee A, Dutta J. Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon. 2019;5(6):e01867. 10.1016/j.heliyon.2019.e01867.Suche in Google Scholar PubMed PubMed Central
[69] Jeyachitra R, Senthilnathan V, Senthil TS. Studies on electrical behavior of Fe doped ZnO nanoparticles prepared via co-precipitation approach for photo-catalytic application. J Mater Sci: Mater Electron. 2017;29(2):1189–97. 10.1007/s10854-017-8021-0.Suche in Google Scholar
[70] Selvanathan V, Aminuzzaman M, Tey L-H, Razali SA, Althubeiti K, Alkhammash HI, et al. Muntingia calabura leaves mediated green synthesis of CuO nanorods: Exploiting phytochemicals for unique morphology. Materials. 2021;14(21):6379. 10.3390/ma14216379.Suche in Google Scholar PubMed PubMed Central
[71] Gavas S, Quazi S, Karpiński TM. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res Lett. 2021;16(1):173. 10.1186/s11671-021-03628-6.Suche in Google Scholar PubMed PubMed Central
[72] Szewczyk OK, Roszczenko P, Czarnomysy R, Bielawska A, Bielawski K. An overview of the importance of transition-metal nanoparticles in cancer research. Int J Mol Sci. 2022;23(12):6688. 10.3390/ijms23126688.Suche in Google Scholar PubMed PubMed Central
[73] Dietz C, Infanger M, Romswinkel A, Strube F, Kraus A. Apoptosis induction and alteration of cell adherence in human lung cancer cells under simulated microgravity. Int J Mol Sci. 2019;20(14):3601. 10.3390/ijms20143601.Suche in Google Scholar PubMed PubMed Central
[74] Ölander M, Handin N, Artursson P. Image-based quantification of cell debris as a measure of apoptosis. ACS. 2019;91(9):5548–52. 10.1021/acs.analchem.9b01243.Suche in Google Scholar PubMed
[75] Zadeh FA, Bokov DO, Salahdin OD, Abdelbasset WK, Jawad MA, Kadhim MM, et al. Cytotoxicity evaluation of environmentally friendly synthesis copper/zinc bimetallic nanoparticles on MCF-7 cancer cells. Rend Lincei Sci Fis Nat. 2022;33(2):441–7. 10.1007/s12210-022-01064-x.Suche in Google Scholar PubMed PubMed Central
[76] Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F. Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway. J Transl Med. 2018;16(1):96. 10.1186/s12967-018-1471-1.Suche in Google Scholar PubMed PubMed Central
[77] Jiang W, Yan Y, Chen M, Luo G, Hao J, Pan J, et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-KB to the COX-2 promoter. Aging. 2020;12(1):611–27. 10.18632/aging.102644.Suche in Google Scholar PubMed PubMed Central
[78] Chaturvedi VK, Yadav N, Rai NK, Ellah NH, Bohara RA, Rehan IF, et al. Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: A new era of Herbonanoceutics. Molecules. 2020;25(13):3091. 10.3390/molecules25133091.Suche in Google Scholar PubMed PubMed Central
[79] Huang Y, Li X, Cao J, Wei X, Li Y, Wang Z, et al. Use of dissociation degree in lysosomes to predict metal oxide nanoparticle toxicity in immune cells: Machine learning boosts nano-safety assessment. Environ Int. 2022;164:107258. 10.1016/j.envint.2022.107258.Suche in Google Scholar PubMed
[80] Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193. 10.3389/fmolb.2020.00193.Suche in Google Scholar PubMed PubMed Central
[81] Chen Q, Fang C, Xia F, Wang Q, Li F, Ling D. Metal nanoparticles for cancer therapy: Precision targeting of DNA damage. Acta Pharm Sin B. 2024;14(3):1132–49. 10.1016/j.apsb.2023.08.031.Suche in Google Scholar PubMed PubMed Central
[82] Perumal P, Sathakkathulla NA, Kumaran K, Ravikumar R, Selvaraj JJ, Nagendran V, et al. Green synthesis of zinc oxide nanoparticles using aqueous extract of shilajit and their anticancer activity against Hela cells. Sci Rep. 2024;14(1):2204. 10.1038/s41598-024-52217-x.Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

