Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
-
Amenah S. Alotaibi
, Abrar M. Alhumairi
Abstract
The risks and challenges of the NEOM project on water bodies can be somehow resolved by using Cystoseria sp., a brown-green macroalga, and natural renewable resource species, which are appealing due to their sustainability, cost-effectiveness, and eco-friendliness. Lipid was extracted from Cystoseria sp. collected from Sharma beach, Neom, Tabuk, Kingdom of Saudi Arabia. It was treated with different solvents, petroleum ether, methanol, and petroleum ether:methanol (1:1), to obtain biofuel. Petroleum ether and methanol were the most significant solvents for extractions of six different hydrocarbon compounds, followed by methanol. Tetrahydradecane 5-methyl 60.03% in petroleum ether, 59.51% in methanol, and 51.39% in petroleum ether:methanol is obtained. Removal of 10 mg·L−1 methylene blue (MB) by alga using 0.2 g·L−1 of Cystoseria sp. and its residues after methanol extract was achieved in 120 min. Zeta potential analysis of alga confirms that different negative charges on adsorbent surfaces undergo conformational change with different solvents and adsorb the positively charged MB via electrostatic interaction force. The production of bioethanol efficiency percentage from Cystoseria sp. ranges from 5% to 68.97%. Hence, Cystoseria sp. can be a renewable resource to yield biodiesel and bioethanol and eliminate MB from wastewater, maintaining environmental sustenance and economic development.
1 Introduction
The mega NEOM project contributed to continuing as a sustainable city, facilitating social and economic transformation planned across the northern Red Sea coast of Tabuk region in Saudi Arabia. Besides providing a wide range of benefits, such as promoting an economically sustainable, feasible planned city, these mega projects are always associated with numerous challenging factors and risks. Environmental reliefs like water, air, and soil during the development of large cities always remain under major threat [1].
Research provides an opportunity to evaluate and focus on risks associated with water bodies that are affected mainly by unmanaged pollutants discharged into sea and river streams, which can otherwise lead to water scarcity. Nature has hitherto created bioremediation to remove various contaminants from wastewater with the help of several biological communities such as alga, yeast, fungi, and bacteria that have proved cheap and easy treatment for wastewater purification. Globally, researchers explore consistently to evaluate biological organisms for better results to improve a healthy environment out of waste. Various types of contaminants, either organic material or inorganic substances and some non-biodegradable xenobiotic pollutants such as halogenated compounds, azo dyes, polycyclic aromatic hydrocarbons, nitroaromatic compounds, triazenes, and dichloro diphenyl trichloroethane accumulate and result in prolonged sustenance which is tremendously increasing in the aquatic ecosystem not only affecting the aquatic life but also has its adverse impact on higher tropic level organisms entering through the food chain [2,3]. Moreover, these pollutants have toxic, carcinogenic, teratogenic, and mutagenic effects. Hence, it is necessary to eliminate toxic pollutants frequently from water bodies. Studies have revealed that among diverse biological organisms, algal species possess the excellent property that offers a suitable biological plate form to detoxify and destroy a wide range of noxious environmental pollutants [4]. The efficacy of phyco-remediation has been demonstrated in treating wastewater, sequestering carbon, eliminating nutrients, and remediation of contaminants, including heavy metals, dyes, organic compounds, and surplus nitrogen, phosphorus continuously discharged from industrial effluents has been extensively studied [5].
Algal candidates are the dominant group of organisms of plant kingdom grown on the surface of water or suspended in the water, involved in the reclamation of wastewater treatment, which is cost-effective, environment friendly, and socially acceptable. Generally, the cell wall structure of macroalgae organisms consists of lipids, heteropolysaccharides containing functional groups like hydroxyl, carboxyl, amino, and phosphate, and charged substituents that make algae form an interactive force between the cell wall of the alga and substrates such as dye and other toxic elements. There are three major types of algae: brown algae (Chromophyta), red algae (Rhodophyta), and green algae (Chlorophyta), varying in cell wall constituents commonly found in seaweeds of coastal marine habitats. The cell wall of brown and blue-green alga is composed of cellulose, the structural support; alginic acid, a polymer of mannuronic and beta-glucuronic acids and the corresponding salts of sodium, potassium, magnesium, and calcium; and sulfated polysaccharides, which are bound to several functional groups facilitates efficient bio-sorption mechanism [6].
Toxic and hazardous pollutants such as dye frequently accumulate in the wastewater and need urgent removal from aquatic ecosystems. Studies on some macroalgae species, such as Sargassum sp., and Ulva sp., have depicted properties to adhere the dye to the cell walls via mechanism of bio-sorption [7]. Some dyes, such as crystal violet dye (CVD) C25H30N3Cl, one of the most mitotic poisons, have mutagenic and carcinogenic effects on aquatic and human life. CVD leads to potent clastogenic tumor growth in some species of fish and also respiratory, renal failure, and permanent blindness in humans. Thus, CVD is considered a critical biohazard substance and needs to be urgently removed from wastewater [8]. Methylene blue (MB) dye (C16H18ClN3S) is a toxic pollutant at very low concentrations in water bodies and causes serious health problems such as vomiting, mental disorders, and difficulty in breathing. For the removal of MB and CVD, various methods are applied, such as flocculation, coagulation, precipitation, and photodegradation, despite these methods not completely removing the dye from wastewater and being expensive. Studies have revealed that the biological removal of dyes from wastewater using marine macroalga Sargassum muticum brown alga is appealing because of widespread growth in nature and is renewable, sustainable that is cost-effective, and ecofriendly which involves adsorption, bio-sorption, and ion exchange and chelation mechanism for degrading and removing toxic dyes from the environment [9]. Several macroalgae species such as Laminaria japonica, Nizamuddinia zanardinii, and Sargassum epiphyllum have been employed as low-cost efficient bio-sorbents for the removal of dye at a high rate under favorable conditions [10]. Microalga such as Chlorella vulgaris is gaining importance in removing MB dye via electrostatic interaction by surface bio-sorption, enhancing the efficient elimination or degradation of pollutants from wastewater [11]. According to studies, the presence of alginates in the cell walls of brown algae and carrageenan in those of red algae is responsible for metal binding to biomass. Alginate has a strong affinity for bivalent metals. Therefore, maximum bio-sorption uptake occurs [12]. Brown algae have been found to be of most valuable importance because they detoxify large quantities of toxic element ions such as Co, Mo, Ca, Mg, Cu, Zn, Cr, Pb, and Se from aqueous solutions that are intracellularly accumulated by active biological transport bio-sorption technique [13,14]. The maximum bio-sorption of cadmium (Cd2+) from aqueous solution was obtained using immobilized Turbinaria ornata biomasses [15]. Green algae species like Cladophora glomerata and brown algae like Cystoseria indica, N. zanardinii, Sargassum glaucescens, and Padina australis that were studied showed excellent accumulators of oxidized metals like Cu2+, Pb2+, and Cr3+ as well as Zn2+, Ni2+, Fe2+, Cd2+, Mn2+, and Co2+ [16]. Various factors affect the efficiency and rate of bio-sorption dyes from aqueous solutions, including dye concentration, pH, temperature, contact time, and static agitation [17]. Macroalgae are sunlight-driven cell factories that convert carbon dioxide into potential biofuels and foods and also produce high-value bioactive products. Biofuels derived from algae have emerged as a significant energy source touted for its potential as a sustainable and cleaner alternative to fossil fuels. Therefore, renewable and alternative energy sources have attracted growing attention to the search for sustainable development [18].
Studies have evaluated that microalga cultivated in open wastewater can be a potential source for biodiesel production. Macroalga produces different macromolecules like lipids, proteins, and carbohydrates that can be converted into biofuel such as biodiesel, bio-crude oil, bio-jet fuel, pyrolysis oil, bioethanol, biomethane, biohydrogen, etc. [19]. Not only biodiesel, but some microalgae can also generate heat and electricity by burning algal biomass to produce methane gas like wood. Studies have reported unique properties of alga to produce hydrogen that can be used as a sustainable resource for tomorrow [20].
Algae biomass can be applied for bio-diesel production using a transesterification pathway. To overcome this critical issue, several studies performed on the renewable natural resource of algae biomass with the properties of high lipid content, rapid growth rate, and ability to capture and sequester carbon have been explored scientifically as a feedstock for bio-diesel production [21]. Oil of alga species Tetraselmis indica was converted into diesel by using Ca(OCH3)2 as a nano-catalyst, increasing the transesterification mechanism rate [22]. Extensive studies on different micro-algal strains, such as Chlorella protothecoides, Chlorella sorokiniana, and Nannochloropsis sp., with 15–45% lipid, have been conventionally worked out to convert crude oil of specific strains into biodiesel at a temperature of 60°C by ex situ transesterification process which satisfied the need of bio-diesel fuel [23]. Recent research on wet microalgal transesterification to biodiesel production using phosphonium carboxylate ionic liquid catalysts reduced energy requirements and proved economically viable and cost-effective [24,25].
Marine macroalgae for bioethanol production are a growing motivation in the research field, wastewater macroalgae is required to be thoroughly evaluated for bioethanol production, which will be a concept of waste into wealth. Bioethanol is produced by enzymatic saccharification and fermentation anaerobically. A complex sugar starch is converted into simple sugar by saccharification/hydrolysis by alcoholic fermentation with the help of yeast or bacteria. Various microalgae species such as Chlamydomonas reinhardtii, Scenedesmus obliquus, C. vulgaris, Dunaliella sp., and Scenedesmus dimorphus produce up to a 69.7% carbohydrate content, which then produces ethanol via alcoholic fermentation is ideal. Further studies have improved the yield of bioethanol from algal strains of Saccharomyces cerevisiae, Tetraselmis sp. after enzymatic and organic solvent pretreatment techniques and maintaining various parameters (i.e., temperature, time duration, and pH) [26]. Red and brown species of algae such as Laurencia obtusa, Cystoseira compressa, Colpomenia sinuosa, and Sargassum sp., a brown macroalga, widely distributed in tropical and subtropical seas have been evaluated for sugar content and on pretreatment with chemical like H2SO4, HCl, and H3PO4 with different concentrations at an optimum temperature of 21°C for 20 min. The hydrolysate obtained with higher sugar content efficiently produced a high ethanol concentration, i.e., 0.146 g·g−1 of dry biomass of C. compressa and L. obtusa [27].
This study aims to discover the recent advancements in the production of biodiesel and bioethanol from defatted macroalga (Cystoseria sp.). Different solvents will be applied to extract the lipids and convert them into biofuels. Additionally, the study will explore the use of defatted Cystoseria sp. to remove MB and determine the water’s toxicity after dye elimination for possible reuse.
2 Materials and methods
2.1 Alga collection
The biomass of the brown alga Cystoseira sp. was collected in December 2022 from the beach of Sharma, Tabuk, Saudi Arabia (28°01′53.4″N 35°13'08.6″ E). Morphological features were used to identify the alga [28]. The alga was washed with running tap water followed by distilled water, air dried, and kept overnight at 50°C in the oven until a constant weight was achieved and grounded.
2.2 Extraction of lipids using various solvents
The lipids extraction was conducted using three different solvent systems: methanol, petroleum ether, and methanol:petroleum ether (1:1 v/v) to compare the results. A mixture of 10 g of dried Cystoseira sp. biomass and 100 mL of each solvent was stirred using a magnetic stirrer for 2 h. The algal residue was then separated by filtration.
The filtrates were evaporated and weighed. The extracted lipids were determined as a percentage using the following equation: Lipid % = (S1/S2) × 100, where S1 is the lipids weight and S2 is the dry weight.
2.2.1 Gas chromatography–mass spectrometry (GC/MS)
The lipid contents were determined using GC/MS analysis according to the previous work [29].
2.3 Decolorization study
After the lipid extraction, the defatted alga was used in the bioremediation of MB from an aqueous solution. Stock solutions of MB were prepared by dissolving MB dye in 1 L of distilled water to a concentration of 5 and 10 mg·L−1. Treatments (control, methanol, petroleum ether, and methanol:petroleum ether), Cystoseira sp. biomass concentration (0.2 and 0.4 g), and contact time (30, 60, and 120 min) were tested to investigate the effect of these parameters on MB removal. The bio-sorption experiments were conducted in 250 mL Erlenmeyer flasks with 100 mL of MB volume. The suspensions were incubated and agitated in a shaker incubator at 600 rpm and 30°C during each contact time. The absorbance of MB concentrations was initially measured. After the conduction of all experiments, about 10 mL of algal suspensions was centrifuged, measuring the absorbance of the supernatant of the sample at 665 nm. The decolorization percentage was calculated using the decolorization percentage equation [30].
2.4 Ethanol production
2.4.1 Chemical saccharification
The residue biomass was used after defatting to produce bioethanol. Sulfuric acid (2%) was used in hydrolysis of 0.1 g Cystoseira sp. biomass by autoclaving at 121°C for 15 min. The mixture was then cooled and filtrated. The total reducing sugars were determined according to Krishnaveni et al. [31]. The calibration curve of glucose (µg·mL−1) was obtained from our previous work [29].
2.4.2 Ethanol production from extracted sugar
S. cerevisiae was inoculated with extracted sugar (1 g of sugar/100 mL of nutrient medium). The nutrient medium consists of the following nutrients: 0.2 g of (NH4)2SO4, 0.5 g of yeast extract, 0.04 g of MgSO4, and 0.1 g KH2PO4/100 mL. The pH was then adjusted to 5.5 and the flasks were incubated at 30°C. After 2 days of fermentation, ethanol concentration was determined by spectrophotometer at wavelength 660 nm.
The ethanol production method was measured using the potassium dichromate method. A mixture of 1.0 mL potassium dichromate (0.1 M) and 6.0 mL of concentrated sulfuric acid was prepared. About 1 mL of the mixture was added to 1 mL of the fermentation cultures [27,32]. The ethanol standard curve was obtained from our previous work [29]. Figure 1 shows the schematic diagram of the experimental procedure.
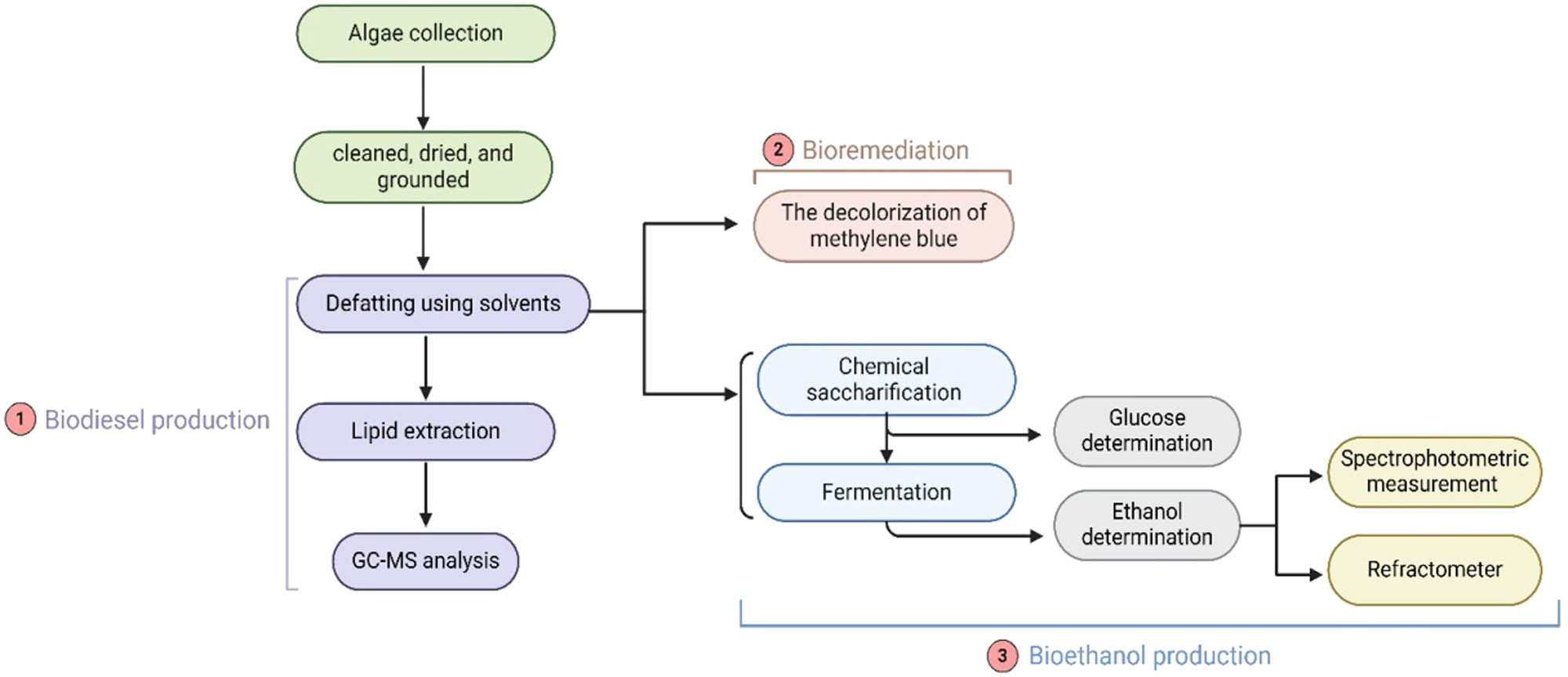
Schematic diagram of the experimental procedure.
2.5 Fourier transform infrared spectrometer (FT-IR) analysis of Cystoseira sp.
The dehydrated Cystoseira sp. biomass samples were subjected to FT-IR analyses ranging from 500 to 4,000 cm−1 (Thermo Fisher Nicolet IS10, Waltham, MA, USA) to determine the functional groups on the algal surface that may be important to the binding of MB dye to the algal biomass.
2.6 Zeta potential
Zeta potential analysis (Malvern Zeta size Nano-Zs90, Malvern, PA, USA) was used to determine the charge of alga and algal residue after defatted by different solvents.
2.7 Toxicity measurements before and after dye removal using onion bulbs (Allium cepa)
Healthy onion bulbs of the same size were acquired from a local market; the bulbs had dry skin. The dead roots were removed, and the bulb was suspended in a 50 mL tiny beaker filled with 40 mL of the solution under consideration. The bottom of the onion bulb was entirely in touch with the solution being tested. The test solution consisted of a single 10 mg·L−1 dye solution, a dye removal solution, and tap water. All treatment groups were incubated at room temperature in the dark. To test the toxicity, root length was measured every 3 days during cultivation.
2.8 Statistical analysis
The experiments were performed in triplicate (n = 3). The experimental error was determined and expressed as standard errors of means, and SPSS 16 was used for the statistics, with one-way ANOVA.
3 Results and discussion
3.1 Lipid yields
Figure 2 shows lipid yield extracted from brown alga, Cystoseira sp., by different solvents. The results depict that petroleum ether:methanol (1:1) is the most effective solvent for extractions of lipids followed by methanol. The lowest lipid yields were with alga treated by petroleum ether. These results agree with Jeong and Park [33] that the best solvent among the 13 solvents for lipid extractions from Enteromorpha intestinalis was a combination of chloroform:methanol (2:1), followed by butanol, and the lowest lipid yield was obtained when applied with hexane and petroleum ether. The best solvent for extraction of lipids from macroalgae Rhizoclonium sp. was hexane:ether (1:1) [34]. Polar lipids in the cellular membrane have effective hydrogenic or electrostatic bonds with protein molecules, and by treating with a polar solvent (alcohol) the link between lipid and protein is split, proving that chloroform–methanol is the best solvent for extractions of the lipids from macro-green alga Cladophora [35]. The combined solvents of methanol and cyclohexane at 1:2 achieved well and supported the maximum recovery of crude lipids from the macro-brown alga Padina tetrastromatica biomass due to methanol being highly polar solvent, which was consumed to extract the polar lipids. Meanwhile, cyclohexane is a nonpolar solvent that is highly stable and suitable for extracting natural lipids [36].
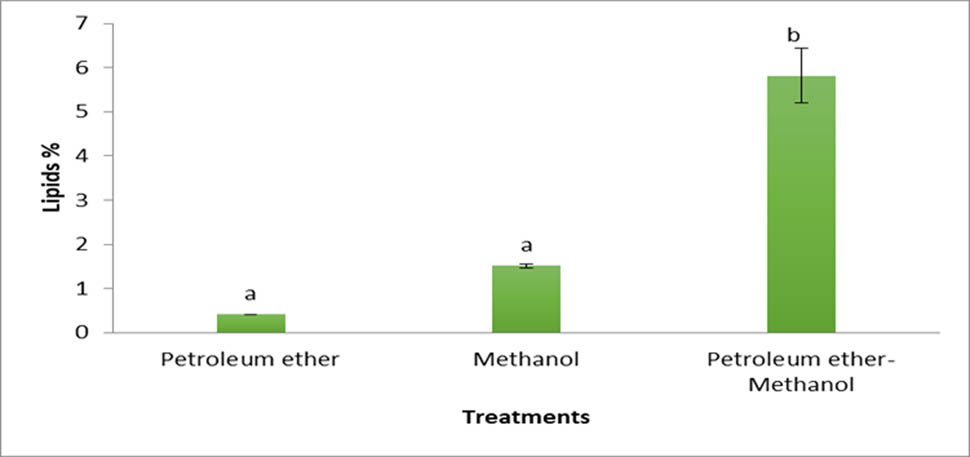
Cystoseira sp. lipid yields (percentage) with different solvents.
3.2 GC–MS analysis of lipids profile of Cystoseira sp. macroalga
Figure 3 and Table 1 display the GC–MS analysis of lipids extracted from Cystoseira sp. macroalga by different solvents. The results demonstrated there are six compounds found in petroleum ether extract, three compounds in methanol extract, and four compounds in methanol:petroleum extracts. The most predominant hydrocarbon is tetradecane, 5-methyl, which represents 60.03% in petroleum ether extract, 59.51% in methanol extract, and 51.39% in methanol:petroleum ether extracts (1:1). Similar results were also revealed by studies of lipids extraction from Turbinaria turbinata with hexane:methanol extract which contains major amounts of petroleum hydrocarbon tetradecane, 5-methyl [29]. Tridecane, tetradecane, heptadecane, and pentadecane were the most abundant hydrocarbon compounds in biofuel liquid products [37]. The biojet formed by mixing the biomolecule (1-pentanol or 2-pentanol) with n-paraffin (n-tetradecane) can improve the energetic efficacy and reduce the emissions of carbon dioxide [38]. The proportion of n-tetradecane in the new fuel blend affects combustion properties such as maximum burning rate and duration, which impact engine noise emissions and efficiency [39].
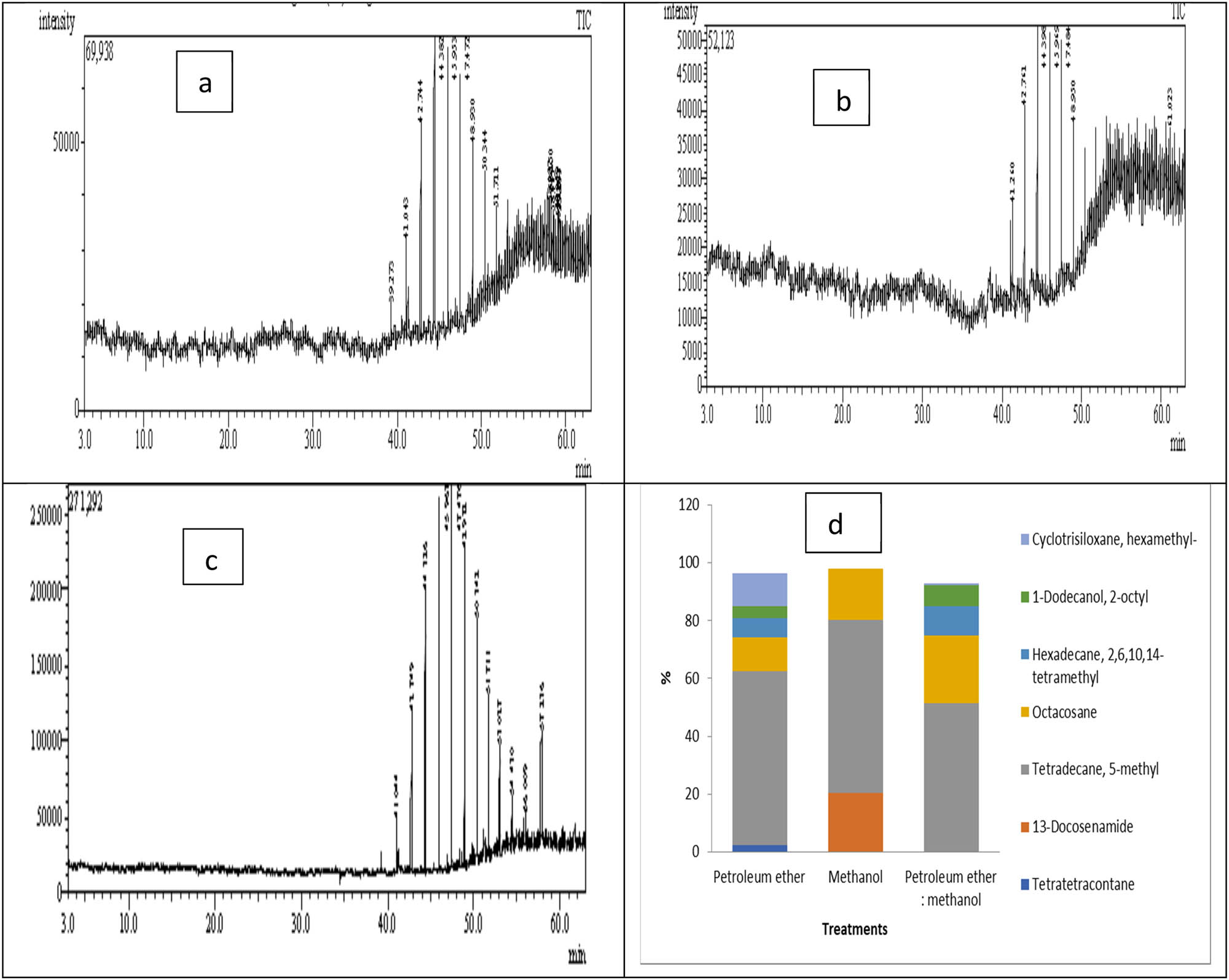
GC–MS analysis of Cystoseira sp. lipids extraction using different solvents: petroleum ether (a), methanol (b), petroleum ether:methanol (1:1) (c), comparison among lipid contents (d).
GC–MS profile of lipid contents of Cystoseira sp. extracted by different solvents
| Rt | Compounds | Petroleum ether | Methanol | Petroleum ether:methanol | Chemical structures |
|---|---|---|---|---|---|
| 39.273 | Tetratetracontane | 2.33 | — | — | C44H90 |
| 41.045 | Tetradecane, 5-methyl | 10.76 | — | 2.20 | C15H32 |
| 41.260 | 13-Docosenamide | — | 20.56 | — | C22H43NO |
| 42.745 | Tetradecane, 5-methyl | 11.14 | — | 6.72 | C15H32 |
| 42.761 | Tetradecane, 5-methyl | — | 12.50 | — | C15H32 |
| 44.382 | Tetradecane, 5-methyl | 15.06 | 17.64 | 12.39 | C15H32 |
| 45.953 | Tetradecane, 5-methyl | 14.10 | 18.06 | 16.10 | C15H32 |
| 47.472 | Octacosane | 11.78 | 17.86 | 16.60 | C44H90 |
| 48.930 | Tetradecane, 5-methyl | 8.97 | 11.31 | 13.98 | C15H32 |
| 50.344 | Hexadecane, 2,6,10,14-tetramethyl | 6.62 | — | 10.01 | C20H42 |
| 51.711 | 1-Dodecanol, 2-octyl | 4.14 | — | 7.29 | C20H42O |
| 53.027 | Octacosane | — | — | 4.00 | C44H90 |
| 54.430 | Octacosane | — | — | 2.96 | C44H90 |
| 56.009 | Cyclotrisiloxane, hexamethyl | — | — | 0.65 | C6H18O3Si3 |
| 58.465 | Cyclotrisiloxane, hexamethyl | 3.04 | — | — | C6H18O3Si3 |
| 59.019 | Cyclotrisiloxane, hexamethyl | 1.15 | — | — | C6H18O3Si3 |
| 59.100 | Cyclotrisiloxane, hexamethyl | 2.25 | — | — | C6H18O3Si3 |
| 59.133 | Cyclotrisiloxane, hexamethyl | 1.13 | — | — | C6H18O3Si3 |
| 59.187 | Cyclotrisiloxane, hexamethyl | 3.97 | — | — | C6H18O3Si3 |
3.3 Phycoremediation of MB
The results in Figure 4 show the percentage removal of MB (5 mg·L−1) by 0.2 g·L−1 Cystoseira sp. alga biomass and its residue after treatment by different solvents. The results cleared the significant values among alga biomass and alga biomass after extraction by different solvents about the removal of MB dye at various times. The algal biomass after extraction by methanol was the best treatment in the removal of MB after agitation for 120 min, followed by petroleum ether residue at the same time. The results show that the removal percentage was increased when the contact time increased. Chemical modification of S. muticum algal biomass increases the sorption capacity of methyl blue, especially if the lipid fraction is removed from the alga [40]. Defatting brown alga L. japonica biomass significantly diminished the organic leaching and boosted the adsorption capacity of MB [41].
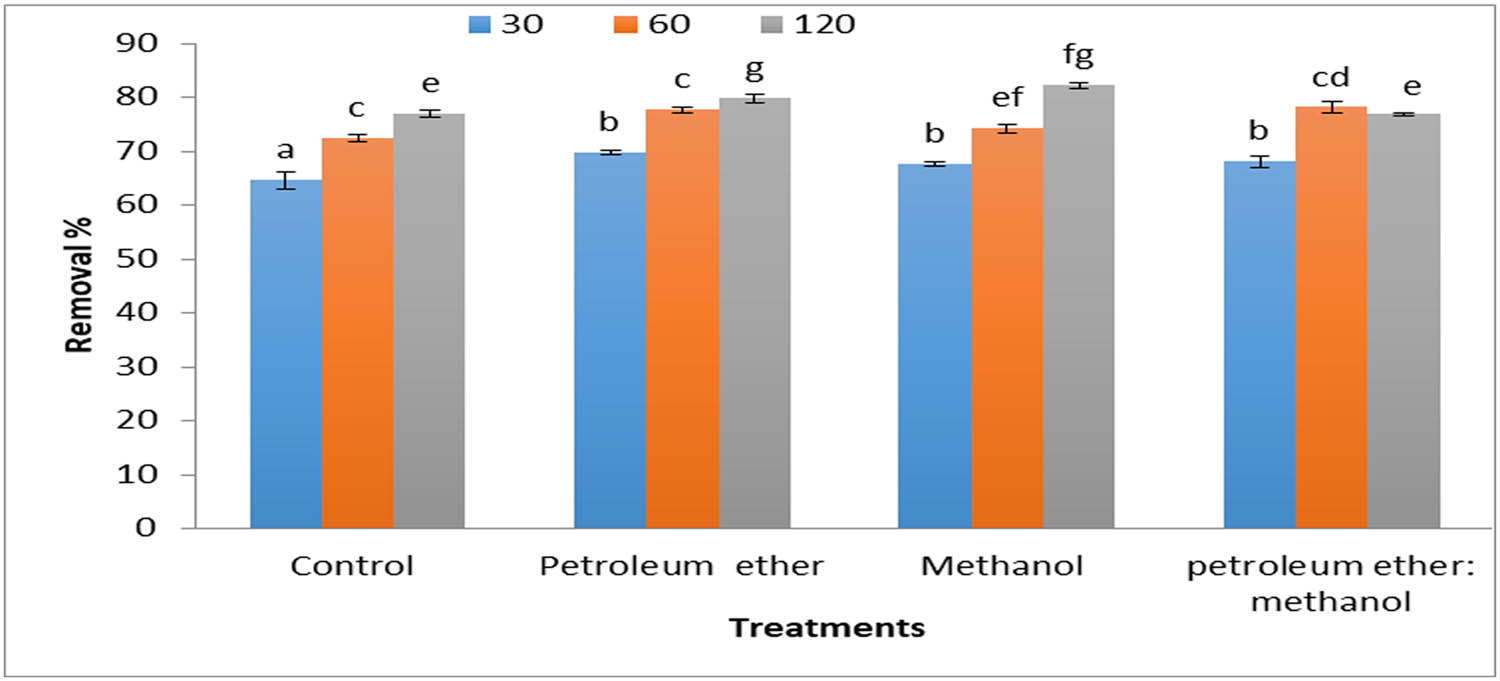
Removal percentages of MB (5 mg·L−1) by 0.2 g·L−1 Cystoseira sp. alga biomass and its residue after treatment by different solvents.
Figure 5 demonstrates removal percentages of MB (10 mg·L−1) by 0.2 g·L−1 Cystoseira sp. alga biomass and its residue after treatment by different solvents. The results proved the defatted alga by petroleum ether and methanol was the best biomass for removing MB at contact times 30, 60, and 120 min.
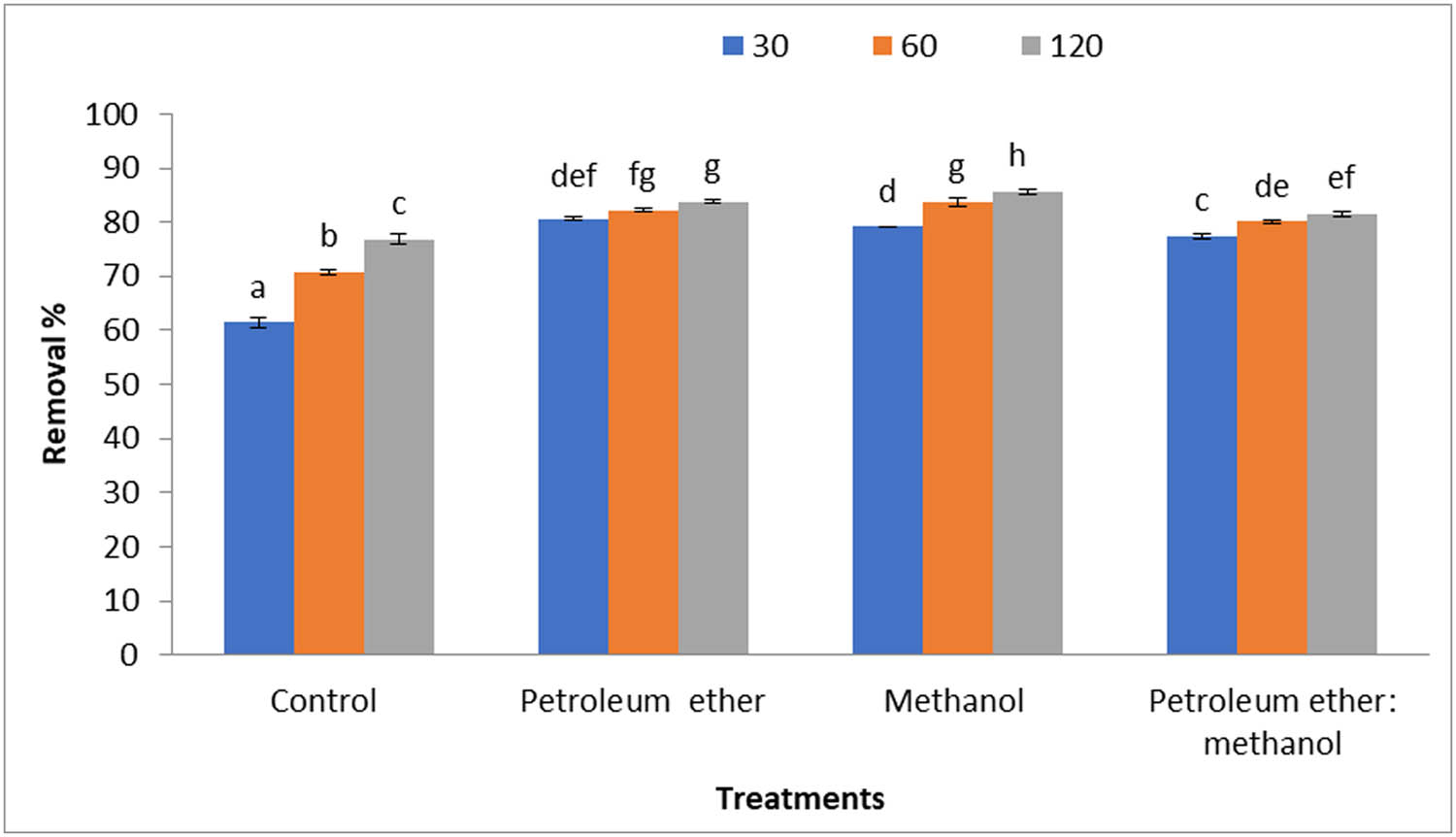
Removal percentages of MB (10 mg·L−1) by 0.2 g·L−1 Cystoseira sp. alga biomass and its residue after treatment by different solvents.
Figure 6 depicts that the removal percentage of MB dye decreased with the low concentrations of dye and high concentrations of algal biomass. Meanwhile, when the dye concentrations increased at the same concentrations of algal and defatted algal biomass increased, the dye removal percentage increased (Figure 7). The heat map reveals the effect of different parameters such as time (30, 60, and 120 min), biomass concentrations (0.2 and 0.4 g·L−1), and dye concentrations (5 and 10 mg·L−1) on the dye removal percentage (Figure 8). The results demonstrated that algal biomass of 0.4 g·L−1 after extraction with petroleum ether was the most effective in MB removal with a contact time of 120 min, with dye concentrations of 0.4 mg·L−1. Also, algal biomass after methanol extraction (0.2 mg·L−1) removes 10 mg·L−1 dye with a contact time of 120 min. After extraction by methanol, the alga residue is the best treatment, which led to a high concentration of MB dye removal at low concentrations of alga used (0.2 mg·L−1), whereas in petroleum ether residue extract, the alga concentration is 0.4 g·L−1 and dye removal is 0.4 mg·L−1. Similar studies also reported that biomass of the brown alga S. muticum has a good potential capacity to remove MB dye, and it offers a promising opportunity as a low-cost bio-adsorbent for the removal of cationic dye from industrial wastewaters [42]. Brown macroalga N. zanardinii is a promising adsorbent for eliminating MB dye from aqueous solutions [43]. The best conditions for removing MB dye by Sargassum hemiphyllum were a contact time of 120 min, 0.5 g·L−1 algal biomass, and pH 5 as described by Liang et al. [44]. It is noteworthy that when all the available binding sites of the bio-sorbent are almost completely filled by dye molecules, the bio-sorption activity reaches the equilibrium state [45].
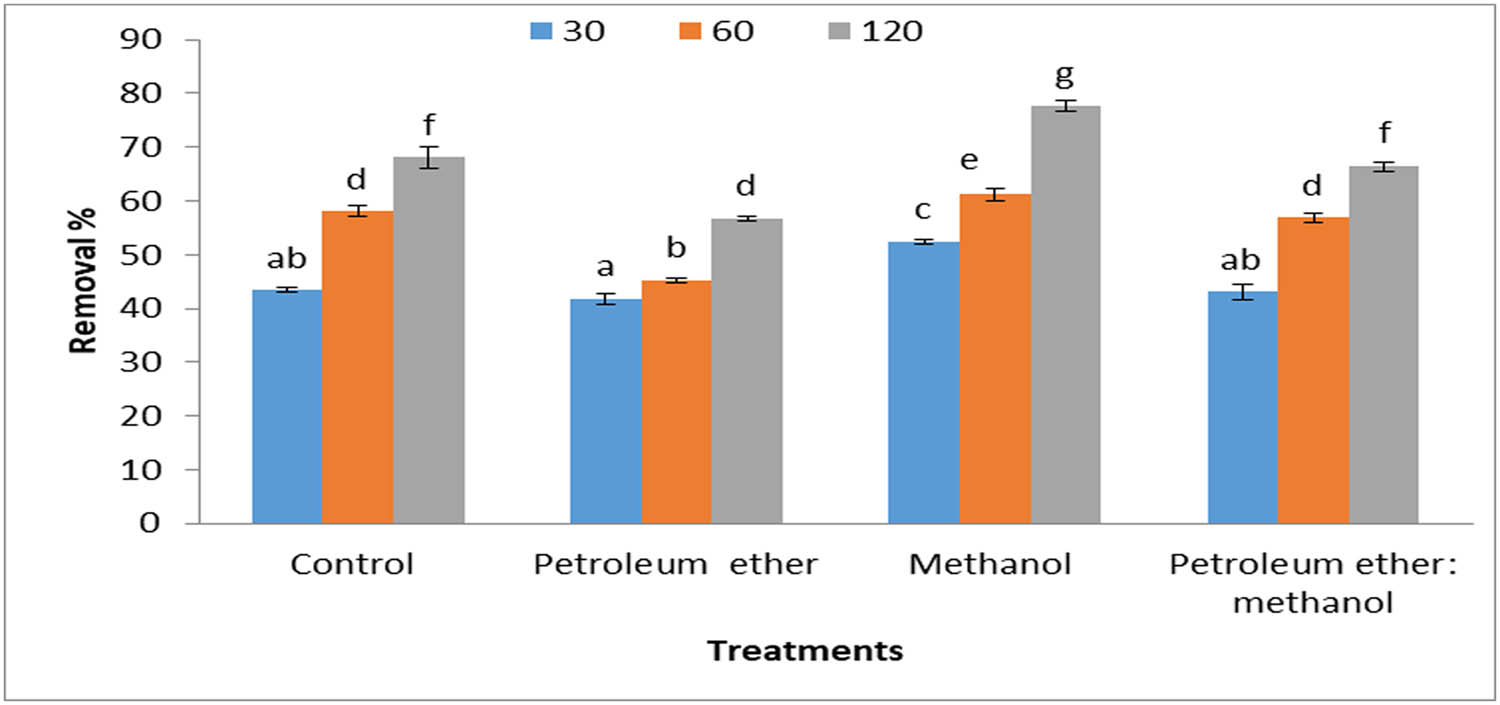
Removal percentages of methyl blue (5 mg·L−1) by 0.4 g·L−1 Cystoseira sp. alga biomass and its residue after treatment by different solvents.
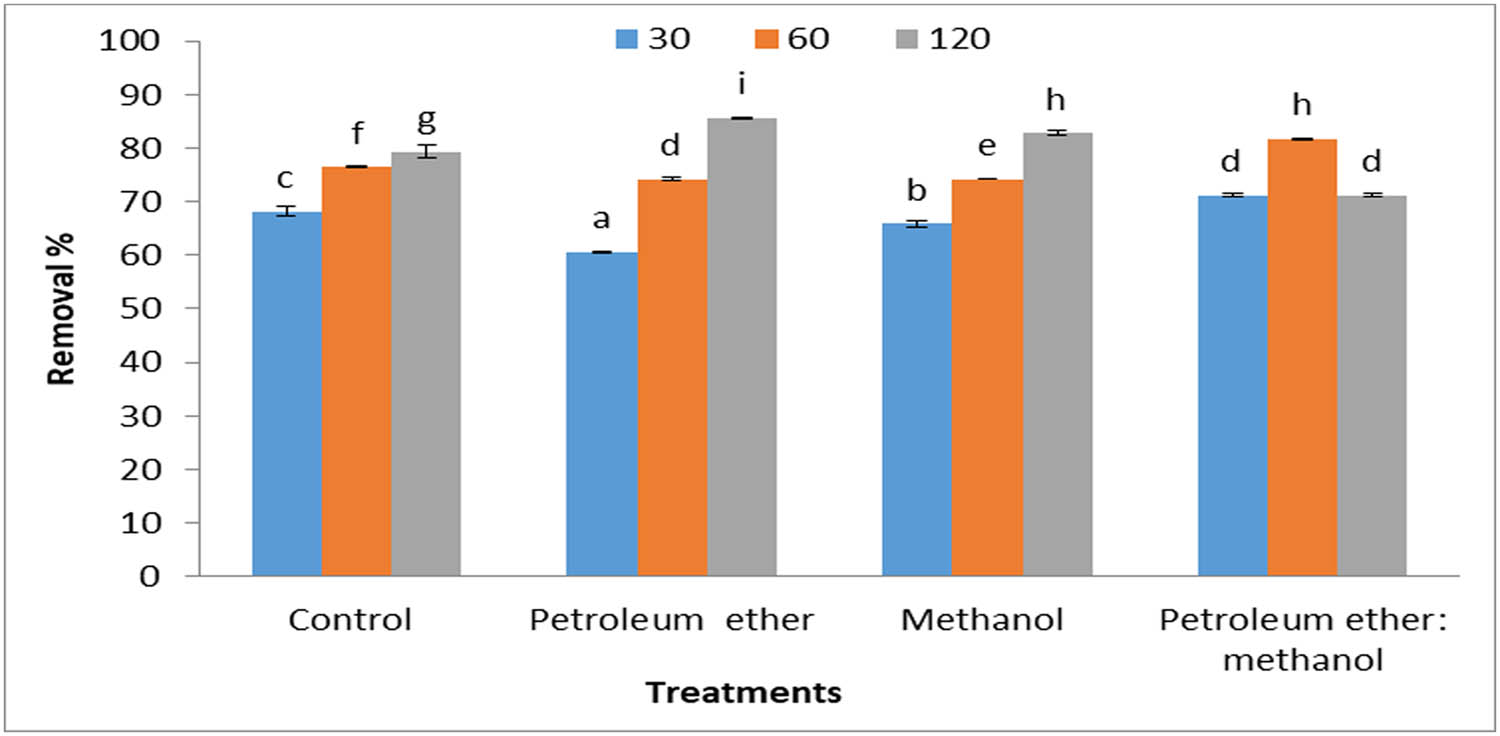
Removal percentages of MB (10 mg·L−1), by 0.4 g·L−1 Cystoseira sp. alga biomass and its residue after treatment by different solvents.
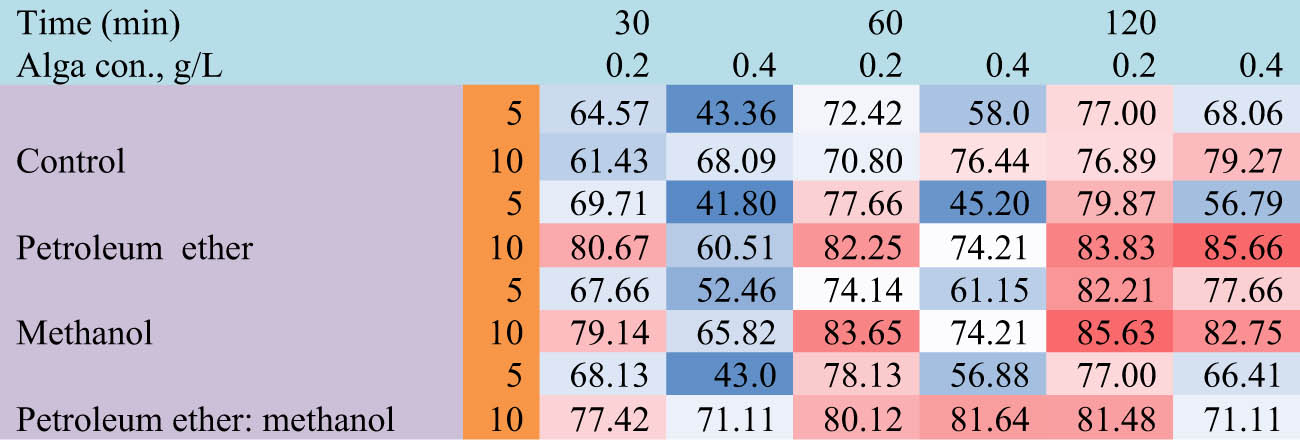
Heat map of MB percentage removal by Cystoseira sp. alga biomass and its residue after treatment by different solvents (alga conc., 0.2 and 0.4 g·L−1; dye conc., 5 and 10 mg·L−1; contact time 30, 60, and 120 min). Red color is high removal percentage, and blue color, low removal percentage.
3.4 FT-IR analysis
The results in Table 2 and Figure 9 demonstrate the FT-IR analysis of alga and its residue after extraction by petroleum ether, methanol, and petroleum ether:methanol (1:1), after and before MB dye adsorption. The results denoted five functional groups at wavenumbers 3,339, 1,647, 1,417, 1,032, and 872 cm−1, assigned to the functional groups –OH, N–H, CH bonds, O–CH3, and C–C stretching, respectively. The hydroxyl groups were not observed in the alga, defatted alga by methanol, and petroleum ether:methanol (1:1). Meanwhile, it was observed in alga and defatted alga that absorbed MB with some modification according to the solvents was extracted. All other groups were noticed in alga and its residue after extractions by different solvents and after adsorption of MB dye. The change in the functional group positions denoted the structure change by adsorbed dye and by defatting alga with various solvents. A previous study by Daneshvar et al. [46] revealed the involvement of hydroxyl and carboxylic aliphatic groups during the bio-sorption process of MB dye by macroalgae.
FT-IR analysis of alga and its residue after extraction by petroleum ether, methanol, and petroleum ether:methanol (1:1), after and before MB dye adsorption
| Wavenumbers (cm−1) | Functional groups | Control | Petroleum ether | Methanol | Petroleum ether:methanol (1:1) | Refs | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dye absorption | Before | After | Before | After | Before | After | Before | After | ||
| 3,339 | Hydroxyl group | Nd | D | +25 | −4 | Nd | −51 | Nd | −145 | [49] |
| 1,647 | N–H | D | +5 | −33 | −33 | −5 | −21 | −16 | −35 | [50] |
| 1,417 | CH bonds | −15 | D | −13 | −18 | −15 | +2 | +10 | −14 | [31] |
| 1,032 | O–CH3 | −21 | D | −26 | −16 | −3 | −29 | −19 | D | [51] |
| 872 | C–C stretching | −8 | D | +5 | +4 | −9 | +4 | −6 | +4 | [52] |
D: detected; Nd: not detected; −, +: shifted.
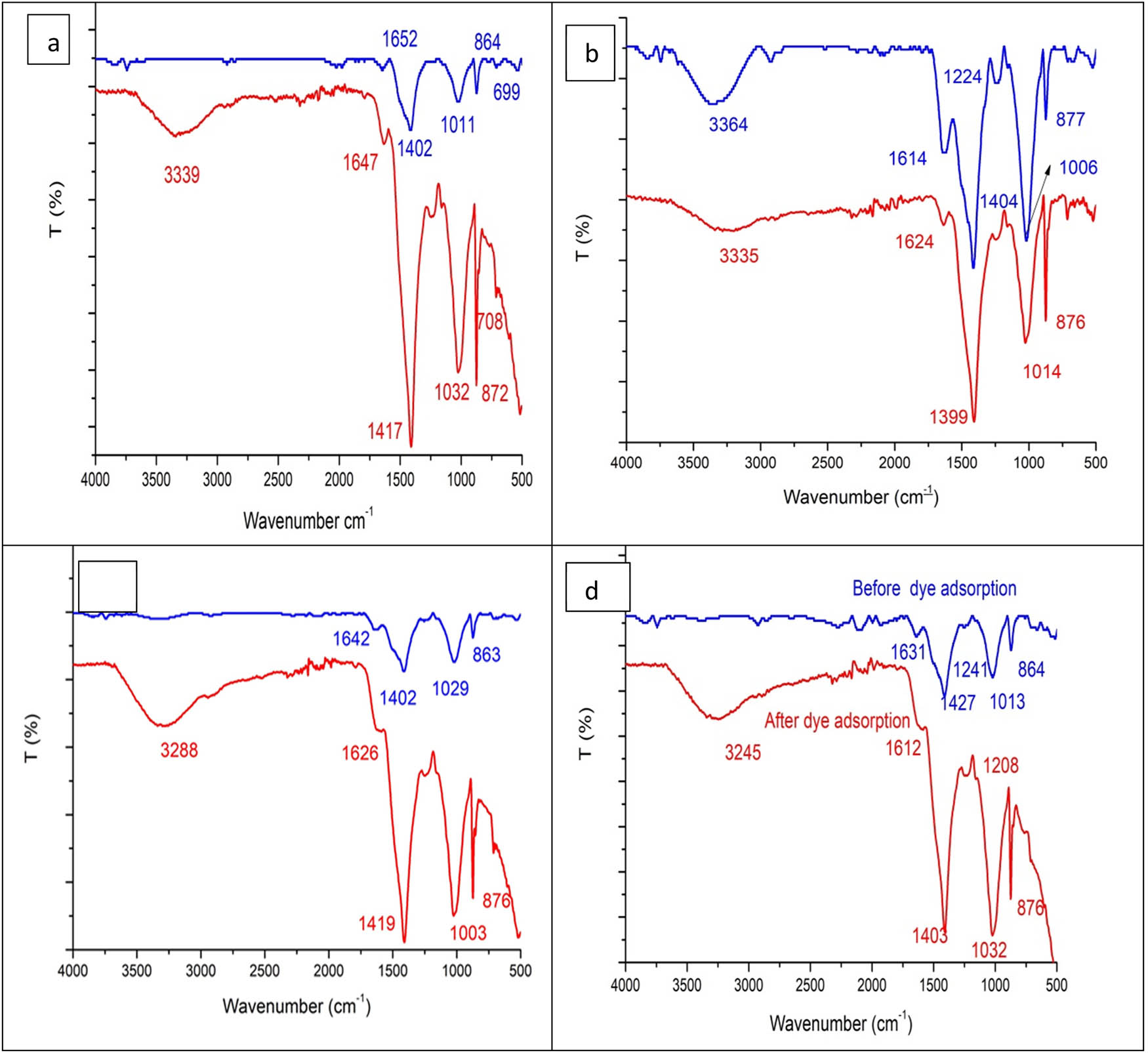
FT-IR analysis of alga (a) and its residue after extraction by petroleum ether (b), methanol (c), and petroleum ether:methanol (1:1) (d), after and before MB dye adsorption (blue color before dye adsorption; red color after dye adsorption).
The change of functional groups and position contributes to the different results of MB removal [29]. The FT-IR is beneficial even for small sample quantities and can be used to observe the dyes’ adsorption rate [47]. Studies on FT-IR analysis exhibited hydroxyl and carbonyl groups on the surface of the peanut husk that adsorbed MB [48].
3.5 Zeta potential
The number of electric charges on the alga surface and defatted alga by different solvents was measured by zeta potential. Figures 10 and 11 indicate the surface charges of alga and alga residue after defatting by different solvents. The results showed the alga biomass had zeta potential (−3 mV), defatted alga by petroleum ether (−3.57 mv), by methanol (−12 mV), and by petroleum ether:methanol (1:1) (−7.32 mV). The results indicate the best dye removal was obtained by defatted alga with methanol extract. It is expected that the defatted residue by methanol has more negative charges which increases the electrostatic force of attraction with the positive charge of MB [53,54]. The mechanisms of absorption of dye may be the presence of negatively charge on the adsorbent’s surface that led to the adsorption of MB dye at neutral pH due to electrostatic interactions [55]. The increase in electrostatic attraction takes place between the negatively charged surface and the positively charged MB dye [56]. The bio-sorption process is due to the presence of various functional groups of bio-macromolecules such as lipids, polysaccharides, and proteins on the algal cell wall surface, these functional groups (e.g., carboxyl, thiol, sulfydryl, phosphate, and amino groups) act as adsorption sites [57].
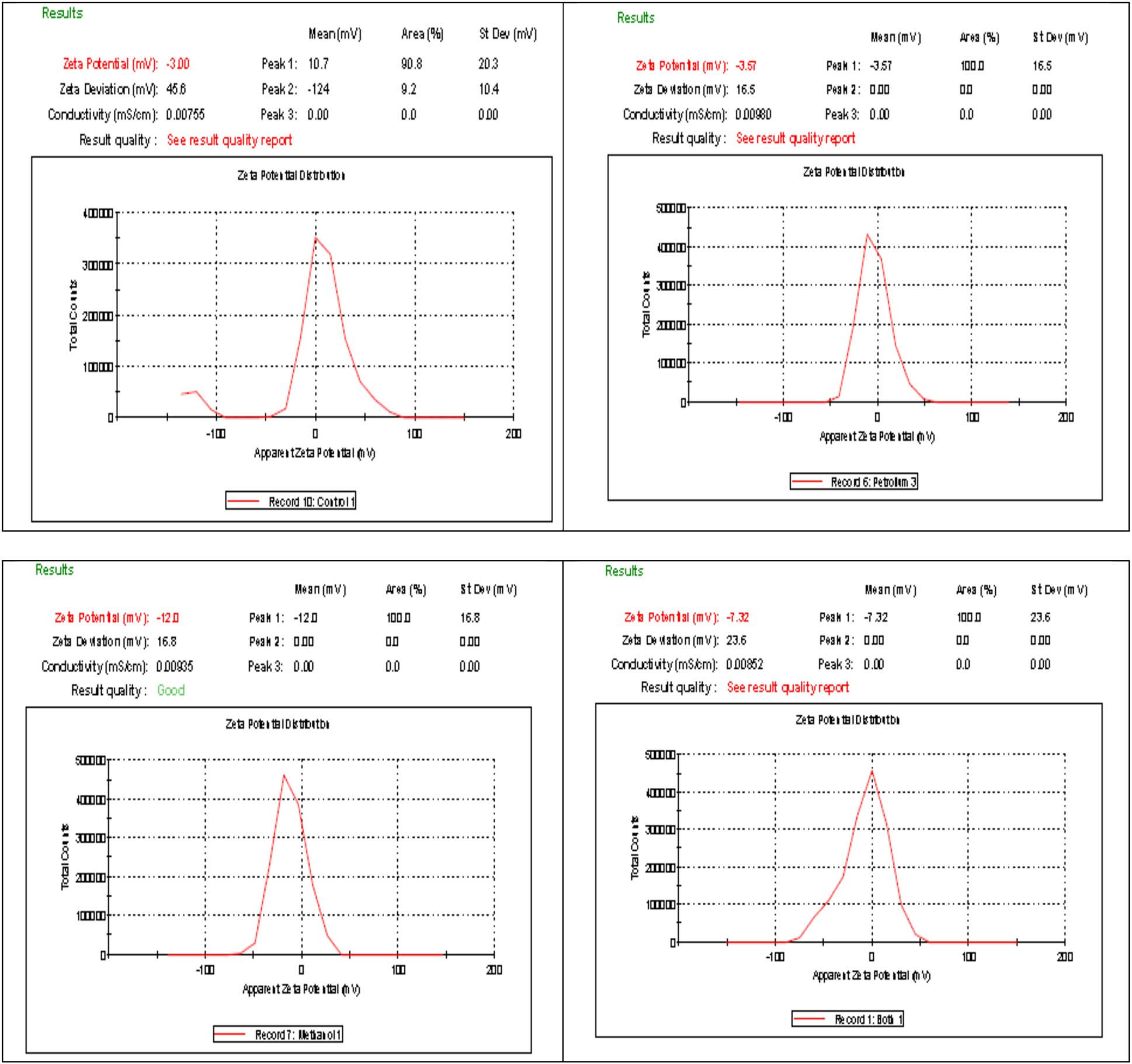
Zeta potential of alga (a) and its residue after extraction by petroleum ether (b), methanol (c), and petroleum ether:methanol (1:1) (d).
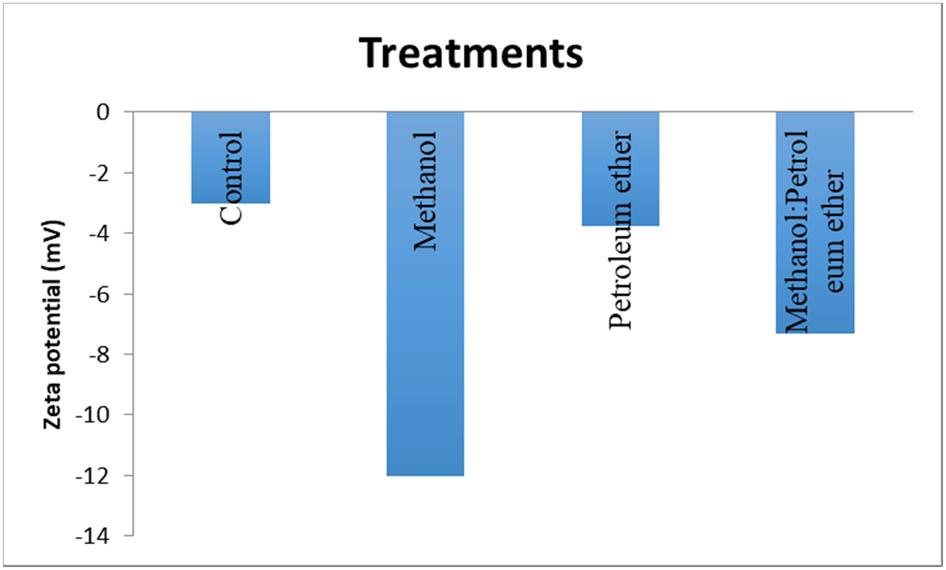
Histogram of zeta potential and its residue after extraction by petroleum ether, methanol, and petroleum ether:methanol (1:1).
3.6 Reducing sugars
The results in Figure 12 elucidate the total reducing sugar from Cystoseria sp., and its residues after defatting in different solvents. The results show no significant change among control (algal biomass, defatted in methanol, and petroleum ether). Meanwhile, the sugar content was increased to 45.7% from brown algae in methanol:petroleum ether extract (1:1). The total sugar content of Cystoseria, brown algae hydrolyzed by sulfuric acid, was 39.53%. The lowest sugar content, 35.9%, was obtained by alga defatted with methanol. Studies report that the total sugar obtained from brown algae ranged from 16.88% to 86.88% [58]. The sugar content of macroalgae ranged between 8.9% and 66.7% [59], and 12.25 ± 1.60 and 47.92 ± 66 mg·g−1 [60]. The sugar content reported from the extracted Tunisian brown seaweed Cystoseria barbata is 50.79% [61].
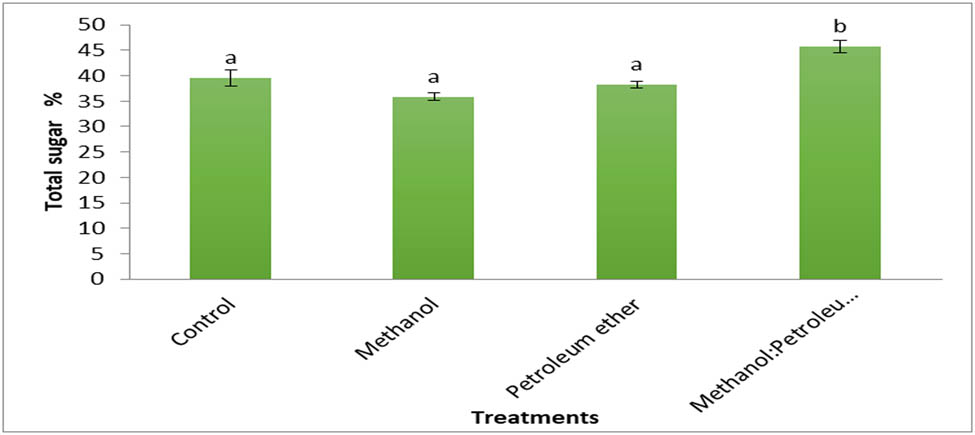
Total reducing sugar (percentage) of Cystoseira sp. and its residue after defatting by different solvents.
3.7 Bioethanol yields
The results in Figure 13 show the bioethanol yields after fermentation by yeast after 24 and 48 h of fermentation processes. The results indicated the fermentation efficiency ranged between 5% and 68.97%. The bioethanol efficiency increased with the increase in fermentation periods. The best bioethanol efficiency was noticed with defatted alga by petroleum ether for 48 h. These results are within the range of the literature, as following, bioethanol levels obtained from green alga Spirogyra hyalina ranged from 2.12% to 3.18% in 24 h, 4.13% to 5.93% in 48 h, and 8.18% to 9.60% in the 72 h of fermentation [62]. The bioethanol obtained from Sargassum sp. using acid hydrolysis was 24.67% after 6 days of fermentation and decreased to 19.81% on the seventh day [63]. Bioethanol is fabricated by the fermentation of algal carbohydrates and serves as a co-product after lipid removal from macroalgae for diesel production [64]. The highest bioethanol yields of Gelidium amansii and Kappaphycus alvarezii algae were 0.32 and 0.34 g·g−1, respectively [65]. Fermentation of the concentrated sugar fractions extracted from Ulva ohnoi with S. cerevisiae at 30°C for 24 h resulted in outstanding ethanol yields of 0.39 and 0.43 g·g−1 sugar. These yields approached their theoretical maximums of 76.1% and 84.58%, respectively [66]. The optimal conditions for fermenting the obtained marine macroalgae waste hydrolysates imply a biomass:acid ratio of 10% (w/v), resulting in a bioethanol concentration of 2.2 g·L−1 and a yield of 21 mg bioethanol/g biomass [67].
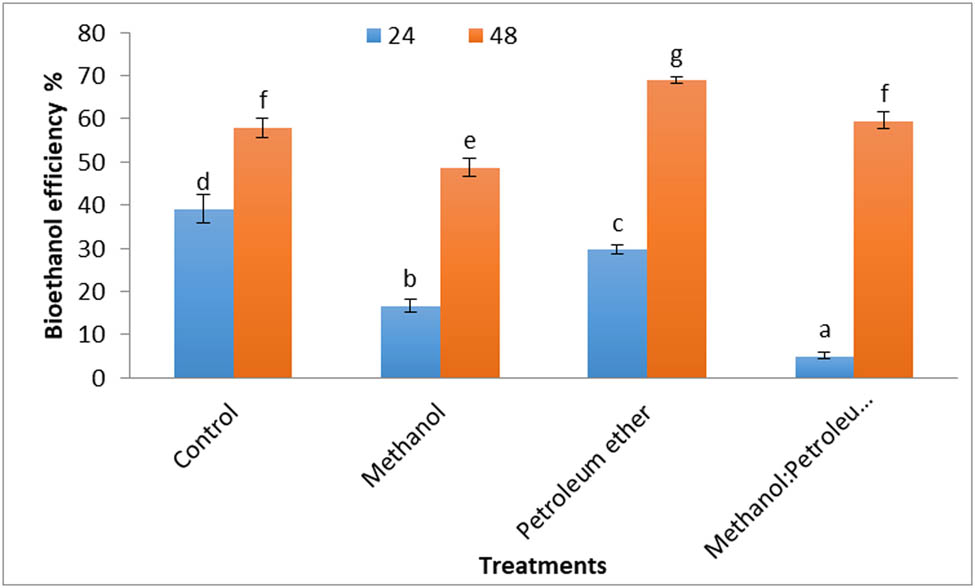
Bioethanol efficiency (percentage) of Cystoseira sp. and its residue after defatting by different solvents.
3.8 Cytotoxicity
Figure 14a and b depicts the growth lengths of onion bulb roots under various treatments. The data demonstrate the hazardous effects of MB before and after decolorization by defatting algae with methanol, as determined by the root length of an onion bulb. The results show that bulbs cultured in 10 mg·L−1 MB produced little growth, indicating that MB is a highly hazardous dye. When the dye was treated with defatted alga extracted with methanol, toxicity was reduced (85.36% dye removal). Figure 14a and b shows that after the dye is eliminated by the alga, the root length increases, but not as much as the control (tap water). The frequency of mitotic abnormalities in onion bulbs positively correlated with the dye concentrations [68]. Contact time and dye concentration affect the decolorization process. Contact time is an important parameter in determining the optimum time for the decolorization of dyes. The results showed that the removal of MB increases rapidly over hours.
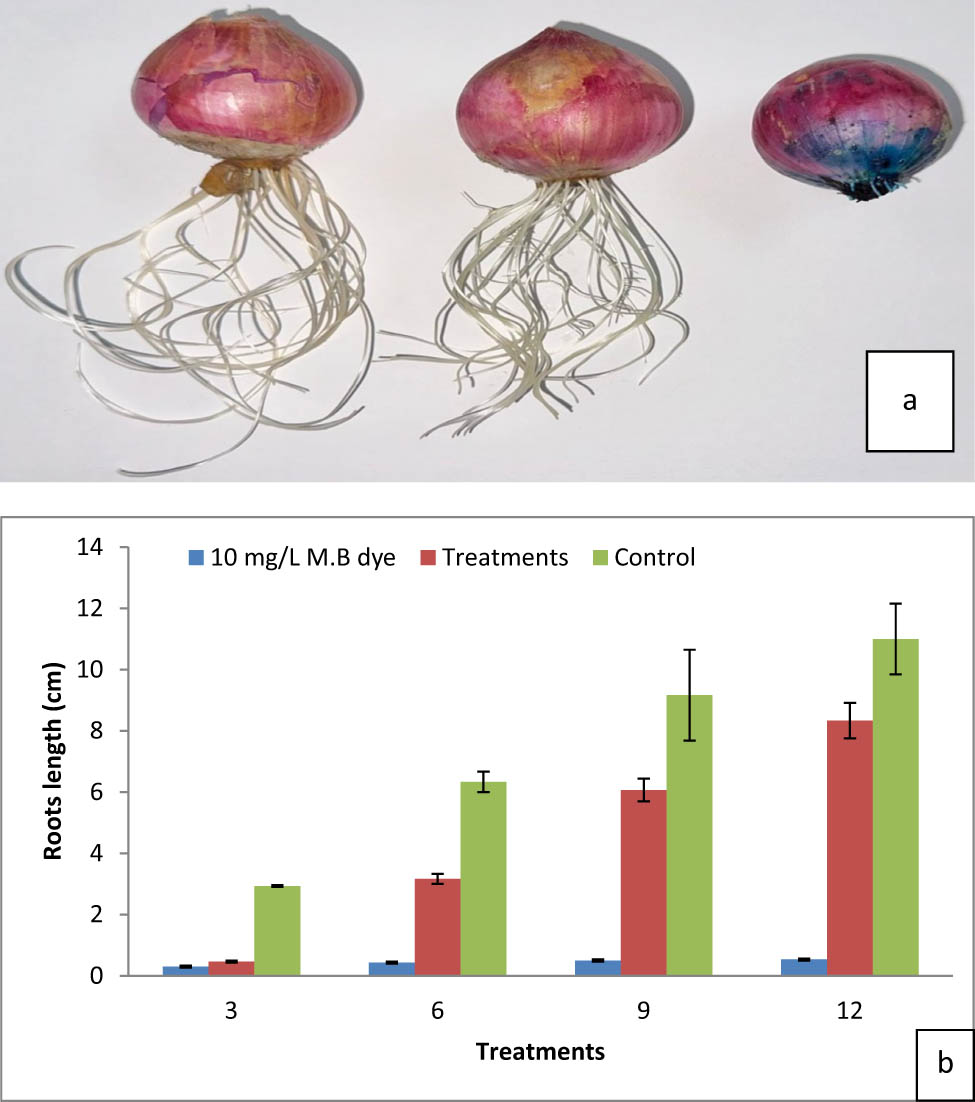
(a) and (b) Effect of dye and decolorization on onion root length (cm).
4 Conclusion
In this study, Cystoseira sp., marine brown alga, was used as a feedstock for the production of biodiesel and bioethanol, and studies demonstrate the removal of toxic pollutant MB dye. Different solvents (petroleum ether, methanol, and petroleum ether:methanol (1:1)) were used to extract lipids from alga. The best solvent identified was petroleum ether, which led to 60.03% of tetradecane, 5-methyl hydrocarbon from Cystoseria. The alga and its residue, after treatment with methanol, efficiently removed MB 10 mg·L−1 using a low concentration of 0.2 g·L−1 of algae biomass for 120 min. The alga Cystoseria showed efficient bioethanol production, approximately 12–13%, after the fermentation of algal biomass by yeast for 48 h. The studies, therefore, suggest that Cystoseira sp., a brown macroalgae in wastewater and seawater, must be encouraged and developed for cultivation so that its metabolites (such as lipids and sugar) can be employed for biofuel and bioethanol production and also can be used for removal of toxic dyes such as MB under the influence of different parameters such as time of exposure, concentration of algae and biomass. The algae Cystoseira sp., which is a natural, renewable, and sustainable resource, can prove an appealing organism for maintaining the aquatic ecosystem and a healthy environment and provide opportunities for the economic growth and development of our nation.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number (0120-1443-S).
-
Funding information: This article is derived from a research grant funded by the Research, Development, and Innovation Authority (RDIA) – Kingdom of Saudi Arabia with grant number (0120-1443-S).
-
Author contributions: A.S.A., H.G., A.M.A., M.A., D A.A., and Y.F.A.: resources, investigation, and wrote the introduction; A.M.A.: writing – original draft, visualization, and methodology; R.A.A.: writing – review & editing, writing – original draft, methodology, formal analysis, and data curation.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article. Data that have been used are confidential.
References
[1] Koirala MP. Urban infrastructure projects and challenges, risk identifying for emerging new cities of Nepal. Int J Res – Granthaalayah. 2018;6:97–108.10.29121/granthaalayah.v6.i12.2018.1086Suche in Google Scholar
[2] Godheja J, Shekhar SK, Siddiqui SA, Modi DR. Xenobiotic compounds present in soil and water: a review on remediation strategies. J Environ Anal Toxicol. 2016;6(392):2161–0525.10.4172/2161-0525.1000392Suche in Google Scholar
[3] Dhakal K, Gadupudi GS, Lehmler HJ, Ludewig G, Duffel MW, Robertson LW. Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs). Environ Sci Pollut Res. 2018 Jun;25:16277–90.10.1007/s11356-017-9694-xSuche in Google Scholar PubMed PubMed Central
[4] Liu L, Bilal M, Duan X, Iqbal HM. Mitigation of environmental pollution by genetically engineered bacteria—current challenges and future perspectives. Sci Total Environ. 2019 Jun;667:444–54.10.1016/j.scitotenv.2019.02.390Suche in Google Scholar PubMed
[5] Znad H, Awual MR, Martini S. The utilization of algae and seaweed biomass for bioremediation of heavy metal-contaminated wastewater. Molecules. 2022 Feb;27(4):1275.10.3390/molecules27041275Suche in Google Scholar PubMed PubMed Central
[6] Popper ZA, Michel G, Hervé C, Domozych DS, Willats WG, Tuohy MG, et al. Evolution and diversity of plant cell walls: from algae to flowering plants. Annu Rev Plant Biol. 2011 Jun;62(1):567–90.10.1146/annurev-arplant-042110-103809Suche in Google Scholar PubMed
[7] Bonyadi Z, Nasoudari E, Ameri M, Ghavami V, Shams M, Sillanpää M. Biosorption of malachite green dye over Spirulina platensis mass: process modeling, factors optimization, kinetic, and isotherm studies. Appl Water Sci. 2022 Jul;12(7):167.10.1007/s13201-022-01690-8Suche in Google Scholar
[8] Jana S, Ray J, Mondal B, Pradhan SS, Tripathy T. pH responsive adsorption/desorption studies of organic dyes from their aqueous solutions by katira gum-cl-poly (acrylic acid-co-N-vinyl imidazole) hydrogel. Colloids Surf, A. 2018 Sep;553:472–86.10.1016/j.colsurfa.2018.06.001Suche in Google Scholar
[9] Cengiz S, Cavas L. Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Bioresour Technol. 2008 May;99(7):2357–63.10.1016/j.biortech.2007.05.011Suche in Google Scholar PubMed
[10] Fawzy MA, Aloufi AS, Hassan SH, Alessa AH, Alsaigh AA, Koutb M, et al. Sustainable use of marine macroalga Sargassum muticum as a biosorbent for hazardous crystal violet dye: isotherm, kinetic and thermodynamic modeling. Sustainability. 2023 Oct;15(20):15064.10.3390/su152015064Suche in Google Scholar
[11] Chin JY, Chng LM, Leong SS, Yeap SP, Yasin NH, Toh PY. Removal of synthetic dye by Chlorella vulgaris microalgae as natural adsorbent. Arab J Sci Eng. 2020 Sep;45:7385–95.10.1007/s13369-020-04557-9Suche in Google Scholar
[12] Ordóñez JI, Cortés S, Maluenda P, Soto I. Biosorption of heavy metals with algae: critical review of its application in real effluents. Sustainability. 2023 Mar;15(6):55217385-95.10.3390/su15065521Suche in Google Scholar
[13] Raize O, Argaman Y, Yannai S. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol Bioeng. 2004 Aug;87(4):451–8.10.1002/bit.20136Suche in Google Scholar PubMed
[14] Romera E, González F, Ballester A, Blázquez ML, Munoz JA. Comparative study of biosorption of heavy metals using different types of algae. Bioresour Technol. 2007 Dec;98(17):3344–53.10.1016/j.biortech.2006.09.026Suche in Google Scholar PubMed
[15] Fawzy MA, Darwish H, Alharthi S, Al-Zaban MI, Noureldeen A, Hassan SH. Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box–Behnken experimental design. Sci Rep. 2022 Feb;12(1):3256.10.1038/s41598-022-07288-zSuche in Google Scholar PubMed PubMed Central
[16] Pahlavanzadeh H, Keshtkar AR, Safdari J, Abadi Z. Biosorption of nickel (II) from aqueous solution by brown algae: equilibrium, dynamic and thermodynamic studies. J Hazard Mater. 2010 Mar;175(1-3):304–10.10.1016/j.jhazmat.2009.10.004Suche in Google Scholar PubMed
[17] Borham A, Haroun M, Saleh IA, Zomot N, Okla MK, Askar M, et al. A statistical optimization for almost-complete methylene blue biosorption by Gracilaria bursa-pastoris. Heliyon. 2024 Aug;10(15):1–19.10.1016/j.heliyon.2024.e34972Suche in Google Scholar PubMed PubMed Central
[18] Ma J, Wang Z, Zhang J, Waite TD, Wu Z. Cost-effective Chlorella biomass production from dilute wastewater using a novel photosynthetic microbial fuel cell (PMFC). Water Res. 2017 Jan;108:356–64.10.1016/j.watres.2016.11.016Suche in Google Scholar PubMed
[19] Viswanath B, Mutanda T, White S, Bux F. The microalgae – a future source of biodiesel. Dyn Biochem Process Biotechnol Mol Biol. 2010;4(1):37–47.Suche in Google Scholar
[20] Aljabri H, Das P, Khan S, AbdulQuadir M, Thaher M, Hawari AH, et al. A study to investigate the energy recovery potential from different macromolecules of a low-lipid marine Tetraselmis sp. biomass through HTL process. Renewable Energy. 2022 Apr;189:78–89.10.1016/j.renene.2022.02.100Suche in Google Scholar
[21] Yahya M, Dutta A, Bouri E, Wadström C, Uddin GS. Dependence structure between the international crude oil market and the European markets of biodiesel and rapeseed oil. Renewable Energy. 2022 Sep;197:594–605.10.1016/j.renene.2022.07.112Suche in Google Scholar
[22] Muscat A, De Olde EM, de Boer IJ, Ripoll-Bosch R. The battle for biomass: a systematic review of food-feed-fuel competition. Glob Food Secur. 2020 Jun;25:100330.10.1016/j.gfs.2019.100330Suche in Google Scholar
[23] Mittal V, Ghosh UK. Comparative analysis of two different nanocatalysts for producing biodiesel from microalgae. Mater Today: Proc. 2022 Jan;63:515–9.10.1016/j.matpr.2022.03.652Suche in Google Scholar
[24] Chen YH, Huang BY, Chiang TH, Tang TC. Fuel properties of microalgae (Chlorella protothecoides) oil biodiesel and its blends with petroleum diesel. Fuel. 2012 Apr;94:270–3.10.1016/j.fuel.2011.11.031Suche in Google Scholar
[25] Malekghasemi S, Kariminia HR, Plechkova NK, Ward VC. Direct transesterification of wet microalgae to biodiesel using phosphonium carboxylate ionic liquid catalysts. Biomass Bioenergy. 2021 Jul;150:106126.10.1016/j.biombioe.2021.106126Suche in Google Scholar
[26] Reyimu Z, Özçimen D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J Clean Prod. 2017 May;150:40–6.10.1016/j.jclepro.2017.02.189Suche in Google Scholar
[27] Hamouda RA, Hussein MH, El-Naggar NE. Potential value of red and brown seaweed for sustainable bioethanol production. Bangladesh J Botany. 2015;44(4):565–70.10.3329/bjb.v44i4.38571Suche in Google Scholar
[28] Papanfuss GF, Jensen JB. The morphology, taxonomy, and nomenclature of Cystophyllum trinode (Forsskål) J. Agardh and Cystoseira myrica (SG Gmelin) C. Agardh (Fucales: Cystoseiraceae). Blumea: Biodivers Evol Biogeogr Plants. 1967 Jan;15(1):17–24.Suche in Google Scholar
[29] Alotaibi AS, Alhumairi AM, Ghabban H, Alenzi AM, Hamouda RA. Simultaneous production of biofuel, and removal of heavy metals using marine alga Turbinaria turbinata as a feedstock in NEOM Region, Tabuk. Ecotoxicol Environ Saf. 2024 Apr;275:116224.10.1016/j.ecoenv.2024.116224Suche in Google Scholar PubMed
[30] Telke AA, Joshi SM, Jadhav SU, Tamboli DP, Govindwar SP. Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT. Biodegradation. 2010 Apr;21:283–96.10.1007/s10532-009-9300-0Suche in Google Scholar PubMed
[31] Krishnaveni S, Balasubramanian T, Sadasivam S. Sugar distribution in sweet stalk sorghum. Food Chem. 1984 Jan;15(3):229–32.10.1016/0308-8146(84)90007-4Suche in Google Scholar
[32] Hamouda RA, Sherif SA, Dawoud GT, Ghareeb MM. Enhancement of bioethanol production from Ulva fasciata by biological and chemical saccharification. Rendiconti Lincei. 2016 Dec;27:665–72.10.1007/s12210-016-0546-2Suche in Google Scholar
[33] Jeong GT, Park DH. Optimization of lipid extraction from marine green macro-algae as biofuel resources. Korean J Chem Eng. 2015 Dec;32:2463–7.10.1007/s11814-015-0083-1Suche in Google Scholar
[34] Saengsawang B, Bhuyar P, Manmai N, Ponnusamy VK, Ramaraj R, Unpaprom Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel. 2020 Aug;274:117841.10.1016/j.fuel.2020.117841Suche in Google Scholar
[35] Yuvarani M, Kubendran D, Salma Aathika AR, Karthik P, Premkumar MP, Karthikeyan V, et al. Extraction and characterization of oil from macroalgae Cladophora glomerata. Energy Sources, Part A: Recovery, Utilization, Environ Eff. 2017 Dec;39(23):2133–9.10.1080/15567036.2017.1400608Suche in Google Scholar
[36] Ashokkumar V, Salim MR, Salam Z, Sivakumar P, Chong CT, Elumalai S, et al. Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina tetrastromatica. Energy Convers Manag. 2017 Mar;135:351–61.10.1016/j.enconman.2016.12.054Suche in Google Scholar
[37] Al-Muttaqii M, Kurniawansyah F, Prajitno DH, Roesyadi A. Hydrocarbon biofuel production by hydrocracking process with nickel–iron supported on HZSM-5 catalyst. IOP Conf Ser: Mater Sci Eng. Vol. 543, No. 1, IOP Publishing; 2019 Jun. p. 012055.10.1088/1757-899X/543/1/012055Suche in Google Scholar
[38] Vidyashankar S, VenuGopal KS, Swarnalatha GV, Kavitha MD, Chauhan VS, Ravi R, et al. Characterization of fatty acids and hydrocarbons of chlorophycean microalgae towards their use as biofuel source. Biomass Bioenergy. 2015 Jun;77:75–91.10.1016/j.biombioe.2015.03.001Suche in Google Scholar
[39] Janssen A, Pischinger S, Muether M. Potential of cellulose-derived biofuels for soot free diesel combustion. SAE Int J Fuels Lubr. 2010 Jan;3(1):70–84.10.4271/2010-01-0335Suche in Google Scholar
[40] Rubin E, Rodriguez P, Herrero R, Sastre de Vicente ME. Adsorption of methylene blue on chemically modified algal biomass: equilibrium, dynamic, and surface data. J Chem Eng Data. 2010 Dec;55(12):5707–14.10.1021/je100666vSuche in Google Scholar
[41] Shao H, Li Y, Zheng L, Chen T, Liu J. Removal of methylene blue by chemically modified defatted brown algae Laminaria japonica. J Taiwan Inst Chem Eng. 2017 Nov;80:525–32.10.1016/j.jtice.2017.08.023Suche in Google Scholar
[42] El Atouani S, Belattmania Z, Reani A, Tahiri S, Aarfane A, Bentiss F, et al. Brown seaweed Sargassum muticum as low-cost biosorbent of methylene blue. Int J Environ Res. 2019 Feb;13:131–42.10.1007/s41742-018-0161-4Suche in Google Scholar
[43] Daneshvar E, Vazirzadeh A, Niazi A, Kousha M, Naushad M, Bhatnagar A. Desorption of methylene blue dye from brown macroalga: effects of operating parameters, isotherm study and kinetic modeling. J Clean Prod. 2017 May;152:443–53.10.1016/j.jclepro.2017.03.119Suche in Google Scholar
[44] Liang J, Xia J, Long J. Biosorption of methylene blue by nonliving biomass of the brown macroalga Sargassum hemiphyllum. Water Sci Technol. 2017 Sep;76(6):1574–83.10.2166/wst.2017.343Suche in Google Scholar PubMed
[45] Deniz F. Cost-efficient and sustainable treatment of malachite green, a model micropollutant with a wide range of uses, from wastewater with Pyracantha coccinea MJ Roemer plant, an effective and eco-friendly biosorbent. J Taibah Univ Sci. 2024 Dec;18(1):2253592.10.1080/16583655.2023.2253592Suche in Google Scholar
[46] Daneshvar E, Vazirzadeh A, Niazi A, Sillanpää M, Bhatnagar A. A comparative study of methylene blue biosorption using different modified brown, red and green macroalgae – effect of pretreatment. Chem Eng J. 2017 Jan;307:435–46.10.1016/j.cej.2016.08.093Suche in Google Scholar
[47] Bartošová A, Blinová L, Sirotiak M, Michalíková A. Usage of FTIR-ATR as non-destructive analysis of selected toxic dyes. Res Pap Faculty Mater Sci Technol Slovak Univ Technol. 2017 Jun;25(40):103–11.10.1515/rput-2017-0012Suche in Google Scholar
[48] Song J, Zou W, Bian Y, Su F, Han R. Adsorption characteristics of methylene blue by peanut husk in batch and column modes. Desalination. 2011 Jan;265(1–3):119–25.10.1016/j.desal.2010.07.041Suche in Google Scholar
[49] Devi TR, Gayathri SFTIR. and FT-Raman spectral analysis of paclitaxel drugs. Int J Pharm Sci Rev Res. 2010;2(2):106–10.Suche in Google Scholar
[50] El-Naggar NE, Hamouda RA, Saddiq AA, Alkinani MH. Simultaneous bioremediation of cationic copper ions and anionic methyl orange azo dye by brown marine alga Fucus vesiculosus. Sci Rep. 2021 Feb;11(1):3555.10.1038/s41598-021-82827-8Suche in Google Scholar PubMed PubMed Central
[51] El-Naggar NE, Hamouda RA, Rabei NH, Mousa IE, Abdel-Hamid MS. Phycoremediation of lithium ions from aqueous solutions using free and immobilized freshwater green alga Oocystis solitaria: mathematical modeling for bioprocess optimization. Environ Sci Pollut Res. 2019 Jul;26(19):19335–51.10.1007/s11356-019-05214-xSuche in Google Scholar PubMed
[52] Asyana V, Haryanto F, Fitri LA, Ridwan T, Anwary F, Soekersi H. Analysis of urinary stone based on a spectrum absorption FTIR-ATR. J Phys Conf Ser. 2016 Mar;694(1):012051IOP Publishing.10.1088/1742-6596/694/1/012051Suche in Google Scholar
[53] Wijayanti TA, Ansori M. Application of modified green algae Nannochloropsis sp. as adsorbent in the simultaneous adsorption of methylene blue and Cu (II) cations in solution. Sustainable Environ Res. 2021 Dec;31(1):1–2.10.1186/s42834-021-00090-ySuche in Google Scholar
[54] Najim AA, Mohammed AA. Biosorption of methylene blue from aqueous solution using mixed algae. Iraqi J Chem Pet Eng. 2018 Dec;19(4):1–1.10.31699/IJCPE.2018.4.1Suche in Google Scholar
[55] Chandra TS, Mudliar SN, Vidyashankar S, Mukherji S, Sarada R, Krishnamurthi K, et al. Defatted algal biomass as a non-conventional low-cost adsorbent: surface characterization and methylene blue adsorption characteristics. Bioresour Technol. 2015 May;184:395–404.10.1016/j.biortech.2014.10.018Suche in Google Scholar PubMed
[56] El Nemr A, Shoaib AG, El Sikaily A, Mohamed AE, Hassan AF. Evaluation of cationic methylene blue dye removal by high surface area mesoporous activated carbon derived from Ulva lactuca. Environ Process. 2021 Mar;8:311–32.10.1007/s40710-020-00487-8Suche in Google Scholar
[57] Hamouda RA, El-Naggar NE, Doleib NM, Saddiq AA. Bioprocessing strategies for cost-effective simultaneous removal of chromium and malachite green by marine alga Enteromorpha intestinalis. Sci Rep. 2020 Aug;10(1):13479.10.1038/s41598-020-70251-3Suche in Google Scholar PubMed PubMed Central
[58] Demirel Z, Yildirim ZD, Tuney I, Kesici K, Sukatar A. Biochemical analysis of some brown seaweeds from the Aegean Sea. Botanica Serbica. 2012;36(2):91–5.Suche in Google Scholar
[59] Dobrinčić A, Pedisić S, Zorić Z, Jurin M, Roje M, Čož-Rakovac R, et al. Microwave assisted extraction and pressurized liquid extraction of sulfated polysaccharides from Fucus virsoides and Cystoseira barbata. Foods. 2021 Jun;10(7):1481.10.3390/foods10071481Suche in Google Scholar PubMed PubMed Central
[60] Ozgun S, Turan F. Biochemical composition of some brown algae from Iskenderun Bay, the northeastern Mediterranean coast of Turkey. J Black Sea/Mediterranean Environ. 2015 Aug;21(2):125–34.Suche in Google Scholar
[61] Sellimi S, Kadri N, Barragan-Montero V, Laouer H, Hajji M, Nasri M. Fucans from a Tunisian brown seaweed Cystoseira barbata: structural characteristics and antioxidant activity. Int J Biol Macromol. 2014 May;66:281–8.10.1016/j.ijbiomac.2014.02.041Suche in Google Scholar PubMed
[62] SulfahriAmin, M, Sumitro SB, Saptasari M. Bioethanol production from algae Spirogyra hyalina using Zymomonas mobilis. Biofuels. 2016 Nov;7(6):621–6.10.1080/17597269.2016.1168028Suche in Google Scholar
[63] Wardani AK, Herrani R. Bioethanol from sargassum sp using acid hydrolysis and fermentation method using microbial association. J Phys Conf Ser. 2019 Jun;1241(1):012008. IOP Publishing.10.1088/1742-6596/1241/1/012008Suche in Google Scholar
[64] John RP, Anisha GS, Nampoothiri KM, Pandey A. Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour Technol. 2011 Jan;102(1):186–93.10.1016/j.biortech.2010.06.139Suche in Google Scholar PubMed
[65] Mushlihah S, Husain DR, Langford A, Tassakka AC. Fungal pretreatment as a sustainable and low cost option for bioethanol production from marine algae. J Clean Prod. 2020 Aug;265:121763.10.1016/j.jclepro.2020.121763Suche in Google Scholar
[66] Perumal PK, Patel AK, Singhania RR, Saini R, Chen CW, Dong CD. Harnessing Ulva ohnoi for eco-friendly bioethanol production via hydrothermal pretreatment. J Taiwan Inst Chem Eng. 2024 Nov;164:105662.10.1016/j.jtice.2024.105662Suche in Google Scholar
[67] Pardilhó S, Oliveira J, Pires JC, Maia Dias J. Bioethanol production from marine macroalgae waste: optimisation of thermal acid hydrolysis. Waste Biomass Valoriz. 2024 Feb;1.10.1007/s12649-023-02320-3Suche in Google Scholar
[68] Tripathy SK, Rao DA Mitotic aberrations induced by orange red (a food additive dye) as a potential genotoxicant on root tip cells of onion (Allium cepa L.). Int Food Res J. 2015;22(1):383.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

