Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
-
Sikander Ali
, Afra Ejaz
, Thamer H. Albekairi
Abstract
The research deals with the isoflavone genistein production, followed by the β-glucosidase production from Aspergillus oryzae. The Cajanus cajan leaf extract was prepared and the optimized extraction parameters were leaf powder weight (1 g), agitation time (75 min), and temperature (60°C). The optimal conditions for β-glucosidase production by submerged fermentation were 0.4% (w/v) (NH4)2SO4 as nitrogen source, 0.05% (w/v) MgSO4 as magnesium source, 2 ml (v/v) size of inoculum, and 60 min incubation time. The Al2O3 nanocrystals (NCs) were synthesized by optimal volume of leaf extract (25 ml) and procurement period (50 min) along with Al2NO3 and NaOH. The β-glucosidase immobilization on Al2O3 NCs improved the specific activity from 2.38 ± 0.002 to 5.64 ± 0.07 U·mg−1. The maximum genistein production was achieved with the rate of biotransformation (48 h) and enzyme concentration (1% (v/v)) along with the substrate level. In fourier transform infrared spectroscopy analysis, the difference between both β-glucosidases free and Al2O3 immobilized was obtained with peaks at 1,120 and 2,150 cm−1. The X-ray diffraction analysis for the NCs was obtained from 10° to 80° with several intensities. and zeta potential size distribution was recorded at 16.2% of intensity with 206.4 d nm. After immobilization, the stability of the β-glucosidase was increased, thereby increasing its potential in the pharmaceutical, biofuel, food, and beverage industries.
1 Introduction
Enzymes are involved in several current industries because of their proficiency and their particular characteristics [1]. β-Glucosidase enzyme is a diverse of several proteins, broadly distributed among animals, plants, fungi, as well as bacteria [2,3]. The enzyme β-d-glucoside glucohydrolase is the systematic name of β-glucosidase; the name indicates involvement in the hydrolysis of glycosidic bonds and hydrolysis of isoflavones [4]. The β-glucosidase is the group of enzymes disturbed into other subgroups depending on the basis of origin for substrate specificity. There are probable chances for the utilization of enzymes in a diversity of applications as in biotechnology processes, biofuel production, hydrolysis of isoflavones, production of oligosaccharides, as well as taste enhancement [1,2]. The efficiency for β-glucosidase production presented at a low rate by Aspergillus carbonarius. The heterologous nature of β-glucosidase has been investigated by intracellular (Escherichia coli, Tillandsioideae plastome) and extracellular (Saccharomyces cerevisiae, Pichia pastoris) by Modrackova et al. and Khadye et al. [5,6]. In Aspergillus oryzae, the structure developed by the conidiospores attached sturdily with one another and shaped as a chain of streptococcus, and A. oryzae is a filamentous fungus that is extensively functional in conventional fermentation [7,8]. Mandels and Weber’s media was used for enzyme production as an important constituent [9]. Bacterial and fungal strains are well recognized for extraction of β-glucosidase [3].
For the production of enzyme, submerged fermentation was carried out with media containing different ingredients in an Erlenmeyer flask and soybean flour, and wheat bran was used in the media for solid-state fermentation [10,11]. The pH of the media was attuneded from 5.0 to 10.0 [12]. Penicillium verruculosum was also used for the production of enzyme by response surface methodology to increase the production rate by fermentation. The effect of metallic ions was calculated with the other compounds to check the β-glucosidase activity [13,10]. Nanoparticles (NPs) have a lot of remarkable properties like as biocompatibility to control the enzyme [14]. NPs are utilized in the administration of diverse cancers throughout vigorous growth for in vivo treatment for cancers, exposure of tumor cell biomarkers, and practice in embattled drug release and catalyzers, adsorbents, ceramic manufacture, cosmetics, textile, and microelectronics [15,16]. The Al2O3 nanocrystals (NCs) have been used for antimicrobial and treat other diseases as well as have therapeutic effects for cancer and immunotherapy. The Al2O3 NC is also used for drug delivery and has higher thermal conductivity to water [17,18]. The metal salt when combined with oxide has great adsorption effects so can be used for the immobilization of enzymes [19]. The technique of particle swarm optimization was employed on Al2O3 NCs [20]. β-Glucosidase produced from Aspergillus flavus has an optimal pH of 4.5, and the reusability of the immobilized enzyme was checked under optimized conditions [21,22]. The enzyme β-glucosidase converts glycoside to isoflavone (sophoricoside to genistein) at optimum temperature [23]. The biotechnological process increases by incubating “pigeon pea” extract through immobilized β-glucosidase for the conversion of glycoside to isoflavone (genistin to genistein) [24,25,9]. Genistein has pre-emptive medicine and physiological functions, such as antioxidant, anti-inflammatory, antiosteoclastic, anti-cancer, and anti-obesity actions [26,27]. The produced isoflavones from diverse sources have influential estrogenic effects and lessening of menopausal symptoms [28,29].
The main objectives of the present investigation were an assortment of leaves from Cajanus cajan, the extracellular β-glucosidase enzyme production through wild-type A. oryzae (strain Isl-9), then the immobilization process of this β-glucosidase by Al2O3 along with their characterization, as well as biotransformation from natural sophoricoside to genistein by both free β-glucosidase and Al2O3-immobilized fungal β-glucosidase with their optimization. These objectives are highly important for the genistein high yield when enzymes get immobilized on NCs which are metal oxide and have chemical stability. Then, the lower amount of genistein can be very effective for the treatment of several diseases as for biomedical and biotechnological purposes. The products from the research β-glucosidase, Al2O3 NCs, and genistein can be reusable.
2 Materials and methods
2.1 Chemicals
The chemicals used to transfigure this study were different purveyors of Acros Organics, Fisher Scientific, Daejung Chemicals, and Sigma-Aldrich from Belgium, the United States, South Korea, and Germany, respectively. The premium-grade chemicals were also employed by Dr. Ikram Ul Haq Institute of Industrial Biotechnology at GCUL.
2.2 Pretreatment and extraction of C. cajan L. Millsp. leaves
The cleaned C. cajan leaves were attained from the Botany Department of Government College University, Lahore. The leaves were suitably cleansed, air-dried for 3 h, and shade-dried for 4 days. About 25 ml of distilled water was added in 0.5 g of dried leaf powder and the resulting solution was agitated at 30°C and 80–120 rpm for 30 min. After agitation, the extract was centrifuged at 3,500 rpm for 20 min and the absorbance of leaf extract was measured at 225 and 250 nm using a UV–VIS spectrophotometer (Shimadzu UV–VIS spectrophotometer PharmaSpec-1700, China).
2.3 Optimization of the extraction process
For the optimal extraction process, various parameters were monitored employing the one-factor-at-a-time (OFAT) method. Using a UV–VIS spectrophotometer, optimal extraction conditions were determined by varying the concentration of leaf powder (from 0.25 to 1.5 g), time of incubation (from 15 to 90 min), and incubation temperature (from 40 to 70°C). All the experiments were run in triplicates and the absorbance was measured at 225 and 250 nm.
2.4 Production and optimization of extracellular β-glucosidase from A. oryzae Isl-9 (A. oryzae Isl-9)
A. oryzae Isl-9 stock culture was procured from the culture collection center of the Dr. Ikram-ul-Haq Institute of Industrial Biotechnology (IIIB), GCUL, and maintained on potato dextrose agar slants. A homogeneous suspension containing conidial spores was prepared by adding 10 ml of sterilized 0.05% (w/v) Monoxal OT in 72 h old culture of A. oryzae Isl-09 for enzyme production.
Fungal β-glucosidase was produced aseptically in a 250 ml Erlenmeyer flask containing sucrose mineral salt medium using the submerged fermentation technique. The fermentation medium containing g·L−1 composition of sucrose (0.3), NH4Cl (0.4), NaCl (0.5), KH2PO4 (0.1), and MgSO4 (0.05), was autoclaved for 15 min at 15 psi (121°C). The sterile flasks were seeded with 1 ml of conidial spores for incubation in a shaking incubator at 30°C and 160 rpm for 3 days [30,31]. Following the incubation process, the broth was filtered and centrifuged to collect the fungal mycelia and enzyme supernatant, respectively. The fungal biomass was washed 2–3 times with ice-cold water. After washing, the biomass was oven-dried at 95°C for 1–2 h to make it 90% moisture-free. The dried fungal mycelium and enzyme-containing supernatant were stored at 4°C till further use.
The β-glucosidase production parameters were monitored by following the OFAT method. The optimal conditions were determined by varying the nitrogen source (NH4Cl and (NH4)2SO4) concentrations (from 0.1 to 0.6% w/v), magnesium source (MgO, MgSO4, and MgCl2) concentrations (from 0.025 to 0.15), and inoculum size (from 0.5–3 ml). Moreover, enzyme activity assay was performed after 30 and 60 min incubation period and the absorbance was measured at 400–460 nm to calculate the specific activity of the enzyme.
2.5 In situ biotransformation reaction for genistein production from natural sophoricoside
The aerobic biotransformation reactions for the production of genistein from natural sophoricoside were carried out using enzyme broth and the yield (%) of genistein was calculated. The enzyme supernatant (2.5 ml) was mixed with an equivalent volume of natural sophoricoside containing leaf extract in a 1:1 ratio at slightly basic conditions and incubated at 35°C and 160 rpm for 3 days:
2.6 Optimization of genistein production process
Using UV–VIS spectrophotometry, genistein production parameters were monitored by the OFAT method. The optimal rate of biotransformation was determined by varying the time of incubation (from 24 to 96 h) and the volume of enzyme supernatant (from 0.5 to 3.0 ml).
2.7 Green synthesis of Al2O3 NCs
The green synthesis of NCs was achieved by using leaf extract of C. cajan to make the synthesis process toxicants free and less expensive [32]. For this purpose, 50 ml of Al2NO3 (5 mM) concentration was first heated for 10 min at room temperature. The dried leaf extract (10 ml) was added gradually at room temperature, while constantly stirring. The pH of the reaction mixture was adjusted to 11 by gradually adding 1 M NaOH. After 30 min, the color of the reaction mix transitioned from being translucent to a bright yellow, and then to a dark yellow shade, indicating the formation of Al2O3 NCs. The NCs obtained were subjected to centrifugation at 6,000 rev·min−1 for 20 min to separate them from the reaction mix. The collected NCs were dried at 37°C overnight [33].
2.8 Immobilization of fungal β-glucosidase
For enzyme immobilization, the β-glucosidase (0.14 U·ml−1) was added to 0.1 g of Al2O3 NCs in phosphate-buffered saline (PBS) under neutral conditions and kept at 4°C for 20 h. After incubation, Al2O3-bound β-glucosidase was washed and centrifuged for the successful removal of the unbound enzyme. The immobilized β-glucosidase was stored in PBS of 50 mM (pH 7.0) at 4°C. The total yield (Ya) of immobilized β-glucosidase was measured by:
2.9 Characterization of Al2O3 NCs
Different techniques were used for the characterization analysis of free and immobilized Al2O3 NCs. The UV/VIS spectroscopic analysis was achieved by diluting the sample and the spectra were recorded from 200 to 800 nm [34]. The fourier transform infrared spectroscopy (FTIR) chemical analysis was carried out to examine the functional groups of the compounds plus to determine their nature. A small amount of diluted sample was placed on the sample holder on an FTIR spectroscope (Spectrum-100, Perkin-Elmer, USA), and the transmittance (%) was recorded from 4,000 to 400 cm−1 [35]. The X-ray diffraction (XRD) analysis was carried out for the confirmation of the crystalline nature of NCs. The sample was prepared in powdered form by lyophilization. Different peaks were obtained in the range of 10°–80° at angle 2θ. The diffractometer by means of copper anode (CuKα) is used for the detection of the surface of the NCs [36]. The scanning electron microscopy (SEM) analysis was employed for the confirmation of the size and surface of the NCs. The beam of electrons was passed through the powdered sample, and the SEM micrographs were obtained at different magnifications [37]. The zeta potential of NCs was measured to evaluate the stability and size of the NCs [38]. The sample was sonicated and then examined by Zetasizer Nanoinstruments (Malvern Instruments Ltd, Ver. 7.10). The total count rate for the size distribution of Al2O3 NCs was 104.9 kcps (kilo counts per second) at 25°C.
2.10 Analytical techniques for the estimation of enzyme activity and protein content
The β-glucosidase enzyme activity was examined following the procedure of Watanabe et al., with some modifications [39]. The β-glucosidase activity was measured in a reaction mixture containing 0.2 ml of p-NPG substrate (0.1%), 0.2 ml of phosphate buffer (1 M; pH 6.0), and 0.4 ml of enzyme broth. The reaction mix was incubated at 30°C for 1 h. Following the incubation, the reaction was stopped by adding 2.5 ml of Na2CO3 (1 M), and the release of pNP was estimated by measuring the absorbance (A 400) using a UV–VIS spectrophotometer [40]. Under specified reaction conditions, 1 U of β-glucosidase was expressed as the amount of enzyme required to produce 1 μmol·min−1 pNP.
The total protein in the enzyme supernatant was determined quantitatively by the Bradford method, using bovine serum albumin as a standard protein [41].
3 Results and discussion
3.1 Preparation of C. cajan leaf extract
3.1.1 Effect of different time intervals
The effects of different time intervals to prepare the C. cajan leaf extract were evaluated as shown in Figure 1. The dry weight of leaf powder of C. cajan was boiled with peptone saline for several time intervals from 15, 30, 45, 60, and 75−90 min for the preparation of leaf extract. The optimized value of extract was achieved as 0.55 ± 0.013 mg·ml−1 as well as 0.66 ± 0.18 mg·ml−1 after agitation (75 min). By analyzing, the proper pellets with optical density were acquired as 0.612 and 0.824 with 225 and 250 nm of wavelength correspondingly. The red line in the graph at 250 nm showed the maximum peak with 75 min of time interval whereas the minimum peak with 30 min of time interval. On the other hand, the black line in the graph showed that 225 nm with the minimum peak with 30 min of time interval also had the highest peak with 60 min of time interval. The smallest yield of leaf extract was attained with 15 min of time interval in the readings equally. The most excellent environment for leaf extract preparation was with 40 min of time interval. Leaf extract has anti-oxidant and anti-aging properties, when used with aqueous solvent [42]. The Soxhlet method was utilized when ethyl-acetate and chloroform were taken as aqueous solvents for a time interval of 8 h of C. cajan leaf extract preparation [43].
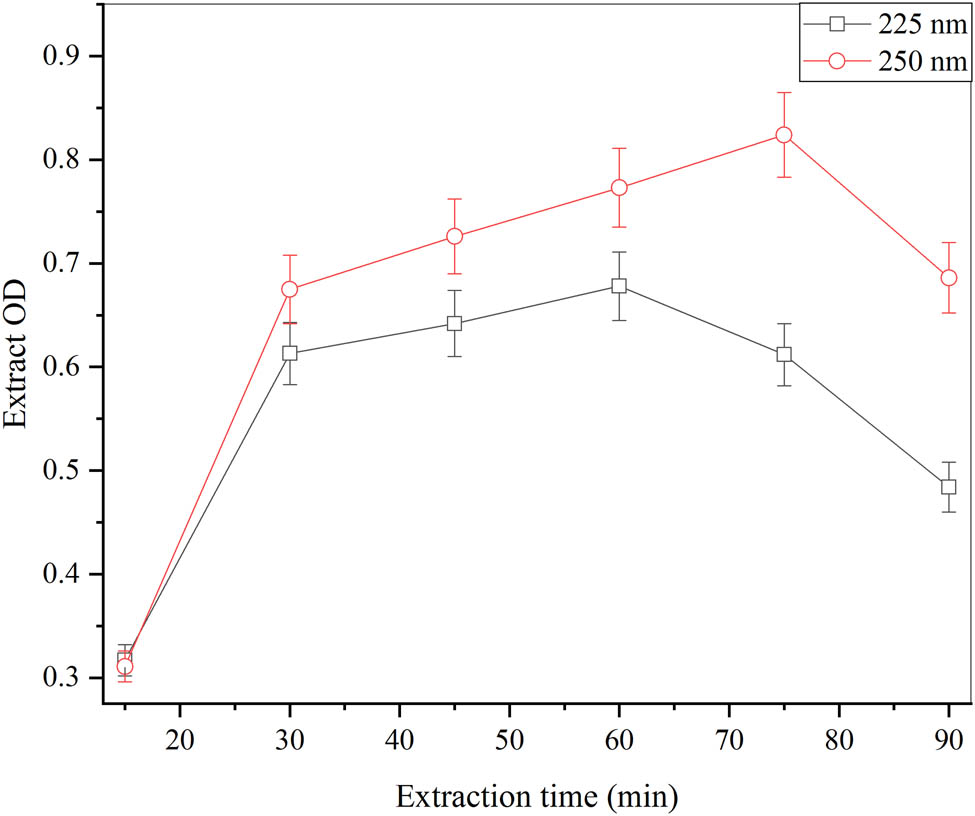
Effect of different time intervals for the preparation of C. cajan leaf extracts at (pH 4.5, volume of peptone-saline 18.75 ml, incubation temperature 30°C, weight of leaf powder 1 g) and error bars at ρ ≤ 0.05 indicating standard deviation among the values.
The competence of extracts increases when the effectiveness of the time increases and it never causes any loss of solute. The higher extraction yield was obtained when the solvent-to-solid ratio increased and required a long-time concentration mentioned by Raghuwanshi et al. [44]. The time interval of 75 min has a great impact on extraction. It gives maximum yield by prolonged exposure in diluent for sufficient desired products and their highest activity.
3.1.2 Effect of different temperature
The temperature effects for C. cajan leaf extract preparation were evaluated. The different temperatures were utilized for the evaluation between 50 and 60°C for optimal analysis as shown in Figure 2(a). The spectra for the effect of two different temperatures are shown in Figure 2(b). Then, the UV–visible spectroscopy was done for optimal check with 200–800 nm of wavelength. The pink line shown in the spectrum at 50°C presented the lowest peak at a wavelength of 275 nm with 0.35 of absorbance and the maximum peak at a wavelength of 225 nm with 0.48 of absorbance. Whereas the blue line at 60°C presented the lowest peak with a wavelength of 275 nm with 0.45 of absorbance and the highest peak at 225 nm of wavelength with 0.68 of absorbance. These results corresponded to the investigation of Nehra et al. and Nagati et al. [43,45].
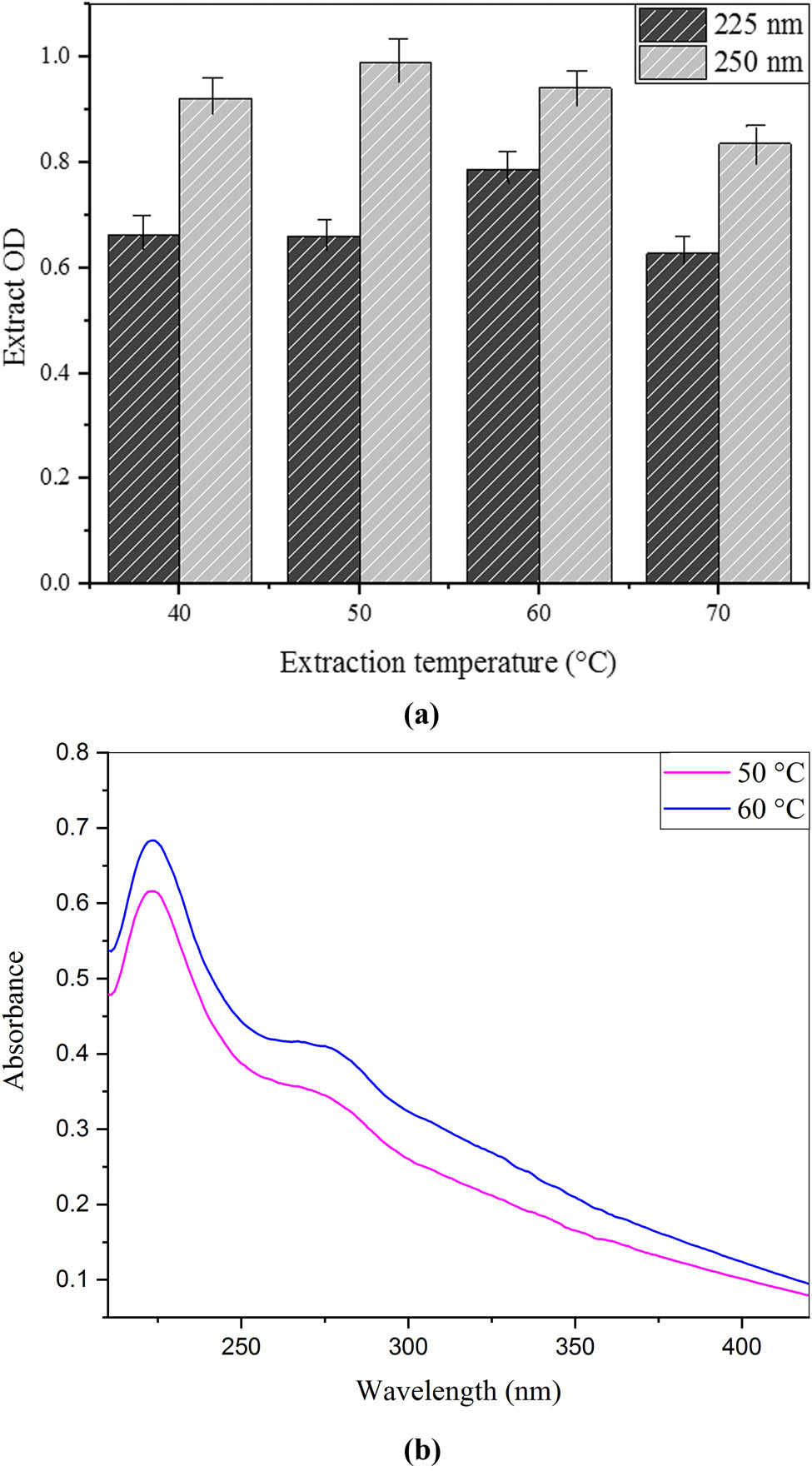
(a) Effect of different temperatures on the preparation of C. cajan leaf extracts. (b) UV/VIS spectroscopy for the effect of different temperatures on the preparation of C. cajan leaf extracts.
On the other hand, 40°C of temperature was found by Raghuwanshi et al. [44]. At high temperatures, the extract’s solubility and the reaction yield also increase [24]. This parameter has a great influence on the extraction optimization process as phenol compounds can be easily separated. Temperature above this can cause degradation and oxidation of preferred compounds.
3.2 Production of β-glucosidase from A. oryzae Isl-9 strain under submerged fermentation
3.2.1 Effect of different nitrogen sources
The different sources of nitrogen were used for the β-glucosidase production from A. oryzae via submerged fermentation and their effects were evaluated. The result is given in Figure 3. The two different nitrogen sources NH4Cl and (NH4)2SO4 were evaluated. The enhanced cell mass production along with the greater microbial activity of the enzyme was achieved. The several concentrations used were 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6% (w/v). After the comparison, 0.4% (w/v) (NH4)2SO4 was attained as an optimal concentration of nitrogen source. The highest β-glucosidase production was achieved having a large pellet size than that of other concentrations used. The maximum activity of the enzyme as 3.6 ± 0.7 U·mg−1 was obtained with 0.4% (w/v) of (NH4)2SO4 and the activity of the enzyme acquired as 3.2 ± 0.9 U·mg−1 with 0.4% (w/v) of NH4Cl. The nitrogen source (NH4)2SO4 showed the highest peak of 3.07 ± 0.45 mg·ml−1 and NH4Cl with the smallest peak of 2.5 ± 0.47 mg·ml−1.
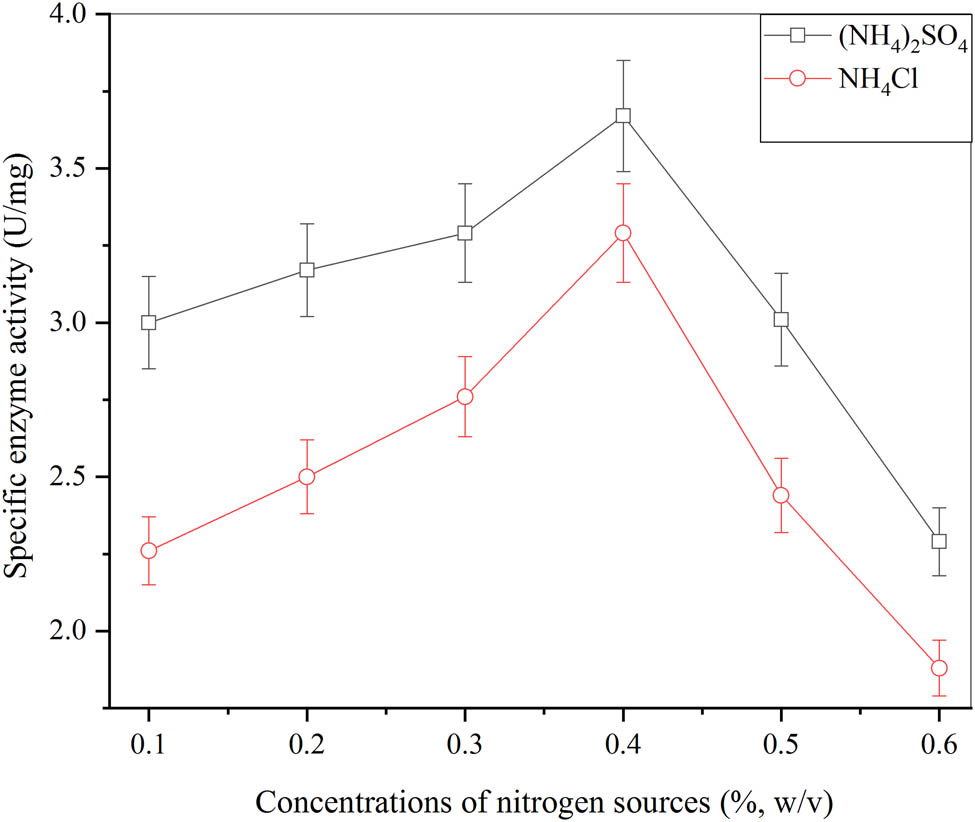
Effect of different nitrogen sources for the production of β-glucosidase from A. oryzae Isl-9 under submerged culture (cane sugar 4.5% (w/v), K2HPO4 0.1% (w/v), MgSO4 0.05% (w/v), inoculum size 1 ml (v/v), incubation time 60 min, incubation temperature 30°C) and error bars at ρ ≤ 0.05 indicating standard deviation among the values.
Abdullah et al. achieved a similar result with 0.4% (w/v) of (NH4)2SO4 to produce β-glucosidase by A. oryzae Isl-9 strain via submerged fermentation [31]. It stated that the cell mass was enhanced by this concentration of nitrogen source. For a microbe to grow in fermentation media, the nitrogen source was a high requirement as it synthesizes amino acids, nitrogenous compounds, vitamins, and bioactive compounds. It gave high production of extracellular β-glucosidase when used in fermentation media as presented by Ahmed et al. [2].
3.2.2 Effect of different magnesium sources
The different sources of magnesium were used for β-glucosidase production and their effects were evaluated. The results are given in Figure 4. The three magnesium sources MgSO4, MgO, and MgCl2 were employed for enhanced cell mass having the greatest activity of enzyme. Several concentrations were used (0.025, 0.05, 0.075, 0.1, 0.125, and 0.15% (w/v)) for different sources. Then, 0.05% (w/v) MgSO4 was achieved for the optimal concentration source of magnesium. The maximum β-glucosidase production was achieved with a larger pellet size than that of the pellets produced by other sources. Then, the greatest activity of the enzyme as 4.08 ± 0.55 U·mg−1 was acquired with 0.05% (w/v) of MgSO4. Whereas the higher activity of the enzyme obtained was 3.85 ± 0.96 and 3.44 ± 0.32 U·mg−1 with 0.05% (w/v) of MgO as well as MgCl2. After analysis, MgSO4 showed the highest peak as 2.65 ± 0.89 mg·ml−1 and MgO and MgCl2 showed the lowest peaks as 2.25 ± 0.90 and 1.97 ± 0.31 mg·ml−1, respectively. This optimum concentration of MgSO4 was necessary for prolonged exponential growth of microbe and results in increased cell mass. It provided magnesium ions to the media for the increase in enzymatic activity. The concentration of 0.03% (w/v) of MgSO4 was utilized for the production of β-glucosidase through A. oryzae Isl-9 strain by Olajuyigbe et al. [46]. The results are given in Figure 4. These are relative with a concentration of MgSO4 as 0.05% (w/v) employed by Liao et al. [47].
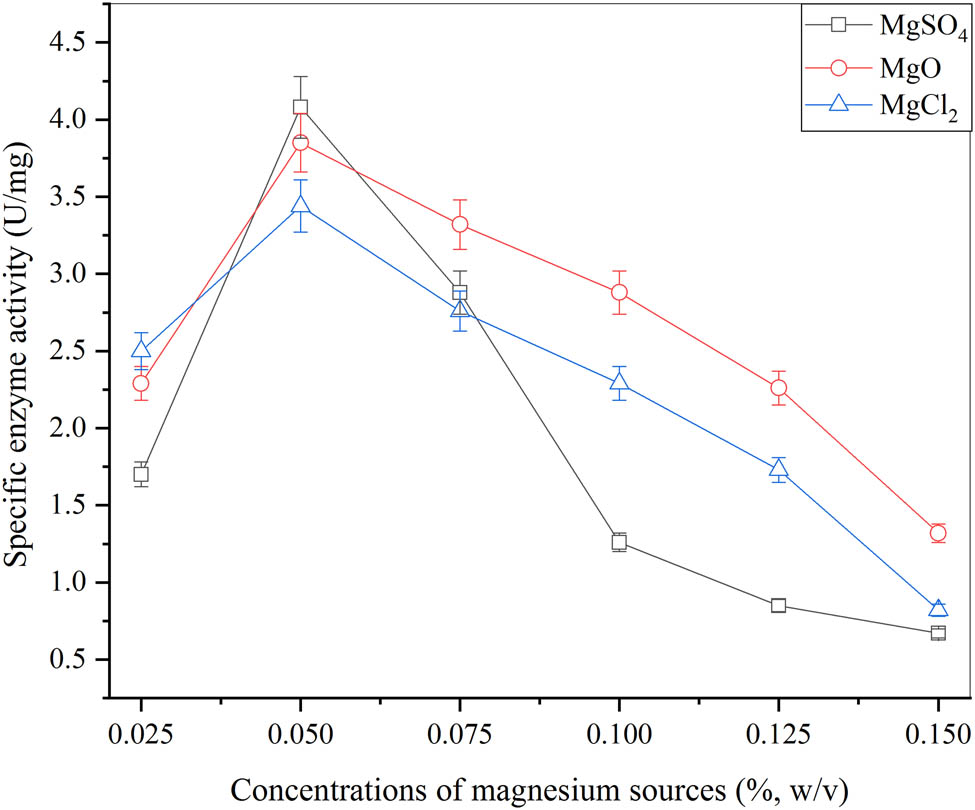
Effect of different magnesium sources for the production of β-glucosidase from A. oryzae Isl-9 under submerged culture (cane sugar 4.5% (w/v), (NH4)2SO4 0.4%(w/v), KH2PO4 0.075% (w/v), inoculum size 1 ml (v/v), incubation time 60 min, incubation temperature 30°C), and error bars at ρ ≤ 0.05 indicating standard deviation among the values.
3.2.3 Effect of size of inoculum
Several sizes of inoculum were utilized to produce β-glucosidase through A. oryzae Isl-9 wild-type strain via submerged fermentation. Their effects were noted and shown in Figure 5. These six altered inoculum sizes were compared to achieve maximum cell mass production along with the largest activities of the enzyme. The different inoculum volumes were used (0.5, 1, 1.5, 2, 2.5, and 3% v/v) to obtain the optimization parameter. Then, the optimal volume was achieved as 2% (v/v) for further optimization. The maximum β-glucosidase production was attained by having the large pellet with this optimized size of inoculum than that of the other volumes. The highest specific activity of the enzyme as 5.64 ± 0.07 U·mg−1 was acquired at 250 nm of wavelength and the specific activity of the enzyme as 5.32 ± 0.09 U·mg−1 was obtained at 240 nm of wavelength. The size of inoculum with 2% (v/v) showed the highest peak as 4.98 ± 0.72 mg·ml−1 at a wavelength of 250 nm and the lowest peak as 4.36 ± 0.02 mg·ml−1 at 240 nm of wavelength. The production of β-glucosidase stopped with the huge size of inoculum utilized with high intensity of activity of the enzyme. But at a low level of conidial suspension, β-glucosidase production increased with a decrease in enzyme activity. So, the 2% (v/v) conidial inoculum was optimized as the ideal size. The size of inoculum (1.5)% (v/v) used by Khalid et al. [48]. The volume of inoculum as 0.01% (v/v) was used for investigation in Ahmed et al. studies to enhance the production of β-glucosidase [2]. On the other hand, 5% (v/v) of conidial suspension was attained by Molina et al. that were as reported high production of β-glucosidase under submerged fermentation [49].
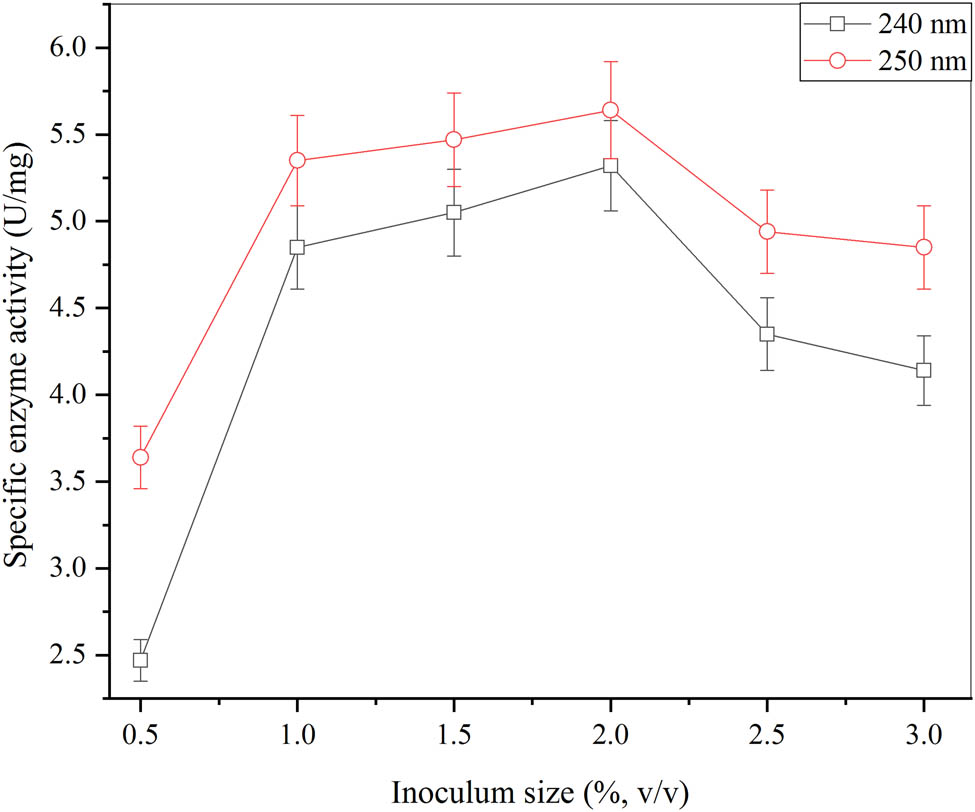
Effect of different sizes of inoculum for the production of β-glucosidase from A. oryzae Isl-9 under submerged culture (cane sugar 4.5% (w/v), (NH4)2SO4 0.4% (w/v), KH2PO4 0.075% (w/v), MgSO4 0.05% (w/v), incubation time 60 min, temperature incubation 30°C) and error bars at ρ ≤ 0.05 indicating standard deviation among the values.
3.2.4 Effect of different time intervals
Two different incubation time intervals were compared for the basic enzyme activity as shown in Figure 6(a). The highest enzyme activity was obtained at 60 min. In the spectra, it is demonstrated that the samples were utilized for optimization of the time period taken in enzyme assay; the black line presented the sample with 30 min of time interval while the red line presented the sample with 60 min of time interval as given in Figure 6(b). The black line showed its first two peaks at 275 and 290 nm with an absorbance of 0.25. However, its highest peak was at 235 nm with an absorbance of 0.74. On the other hand, 60 min incubation time for enzyme assay also presented the peaks at the same wavelength as 30 min but two lower peaks at absorbance of 0.5 while the highest peak with absorbance of 1.5. The results were in correspondence with the findings of Almeida et al. [13]. However, 10 min of the incubation period for enzyme assay was utilized by Abdullah et al. [31]. A longer incubation period helped the microbe to grow at a high rate so that the production of enzymes would be high. It is stated that if the enzyme remains with the substrate for a longer period, the greater the amount of product formed hence the greater the enzyme activity.
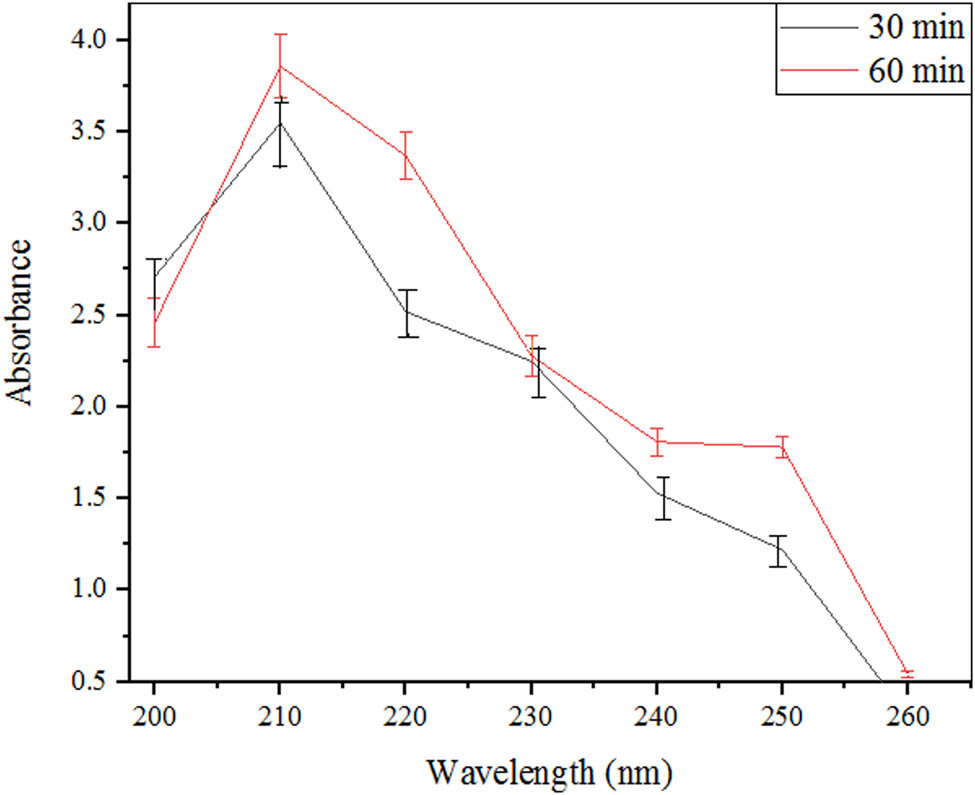
Effect of different time intervals for the production of β-glucosidase from A. oryzae Isl-9 under submerged culture.
3.3 Characterization
3.3.1 UV/VIS spectroscopy
Various levels of extract of C. cajan leaves were employed and the result is given in Figure 7a. The spectrum illustrated various leaf extract levels which presented some peaks with the use of several absorbances and wavelengths. The optimized leaf extract level was 25 ml which showed the line with maximum peak at the wavelength of 286 nm along with 0.88 of absorbance. The highest leaf extract level of 30 ml presented that the line has the maximum peak at a wavelength of 283 nm along with 0.8 absorbance. The level of leaf extract as 15 ml of line has a large peak at a wavelength of 280 nm along with 0.6 absorbance. The several procurement periods and their effects were utilized for the optimization parameters to produce Al2O3 NCs via green synthesis. The results are given in Figure 7b. The spectrum showed that the optimized procurement period (10 min−1·h) was evaluated for green synthesis of Al2O3 NCs. The maximum peak line shown at 300 nm along with 1.35 of absorbance was the optimized value. The other line having a procurement period of 60 min presented the peak at a wavelength of 300 nm along with 1.0 of absorbance. The minimum peak line was obtained by a procurement period of 40 min along with an absorbance of 1.25 at a wavelength of 300 nm. The procurement period of 50 min was utilized as the optimized value. Sabah et al. stated that when the beam of illuminating light increases, the absorbance becomes short, and spectrum peaks shift to a wavelength below 300 nm [50]. Ismail et al. investigated the maximum peak of green synthesis of Al2O3 at a wavelength of 245 nm along with all other optimal states [51]. The results shown in the above spectra are in accordance that showed the highest peak at 210 nm when Al2NO3 was used [52]. The same results were given by Nassar and Zainab 2015 showed high peaks at 210 and 225 nm. When the short wavelength appears for the high peaks, it determines the short size of NCs [53].
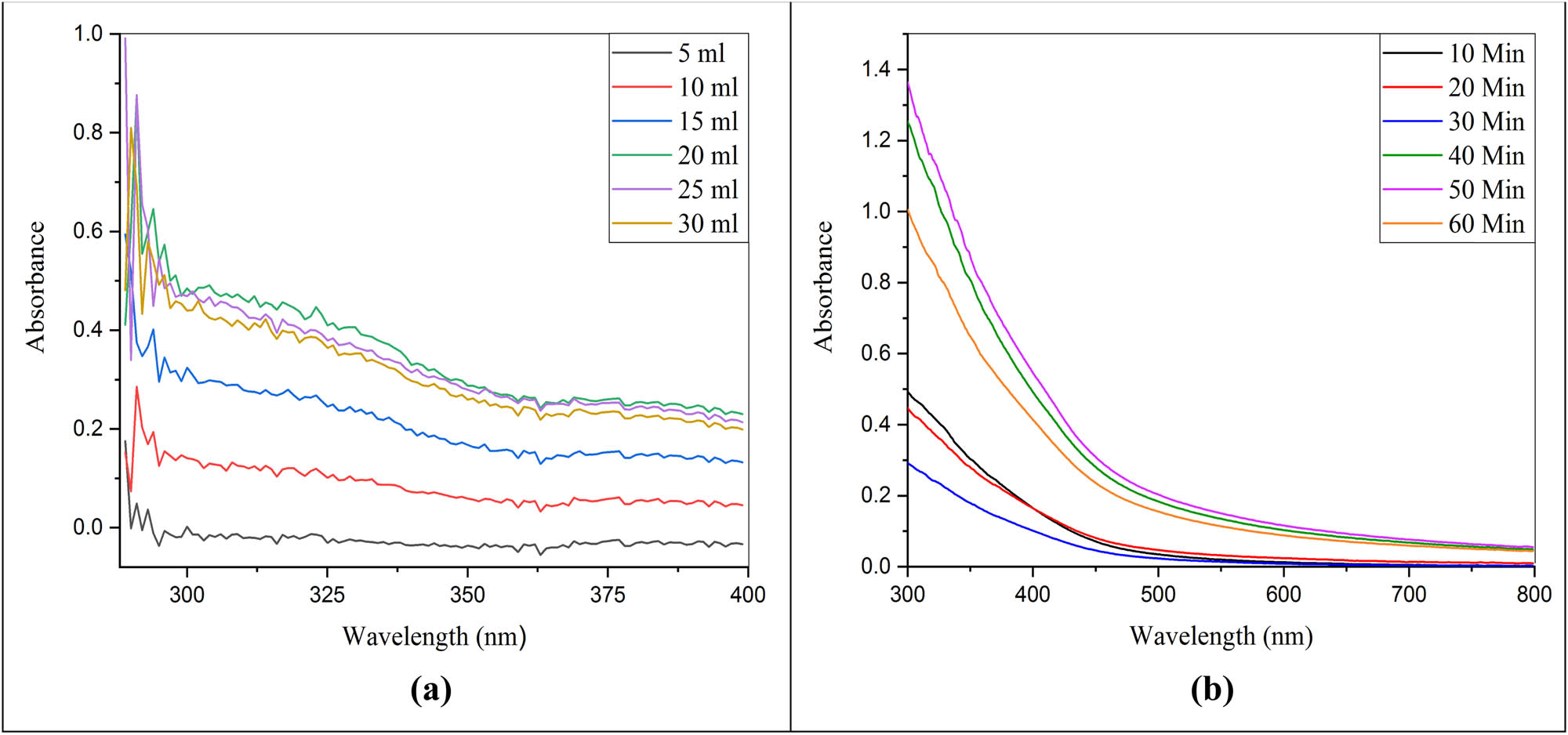
UV–VIS absorption spectrum of Al2O3 NCs. (a) Effect of different levels of C. cajan leaf extracts (Al2NO3 30 mM). (b) Effect of different procurement periods of C. cajan leaf extract (25 ml) for green synthesis of Al2O3 NCs.
3.3.2 FTIR
The change in functional groups was observed at a range from 400 to 4,000 cm−1 [35]. The total scans utilized were 120 for every sample. Throughout the infrared (IR) spectroscopy, the samples were oxidized at diverse temperatures. The other peaks at 1,633 cm−1 showed OH deformation vibrations which indicated the presence of Al2O3. There is high bending vibration at 1,120 cm−1 which also showed the change in hydroxyl group differentiating free enzyme from immobilized enzyme. There was a minor modification in both peaks of free β-glucosidase and immobilized β-glucosidase as 3,305 and 3,346 cm−1 but a high change at 2,150 cm−1. The result is given in Figure 8. The correspondence result was obtained by Goldstein et al. having Al–O groups with vibration at 1,000 cm−1, CH stretches to 3,000 cm−1, and OH at 3,500 cm−1 [54]. The peaks obtained were utilized for the evolution of oxides and also for the comparison between β-glucosidase with and without Al2O3 NCs.
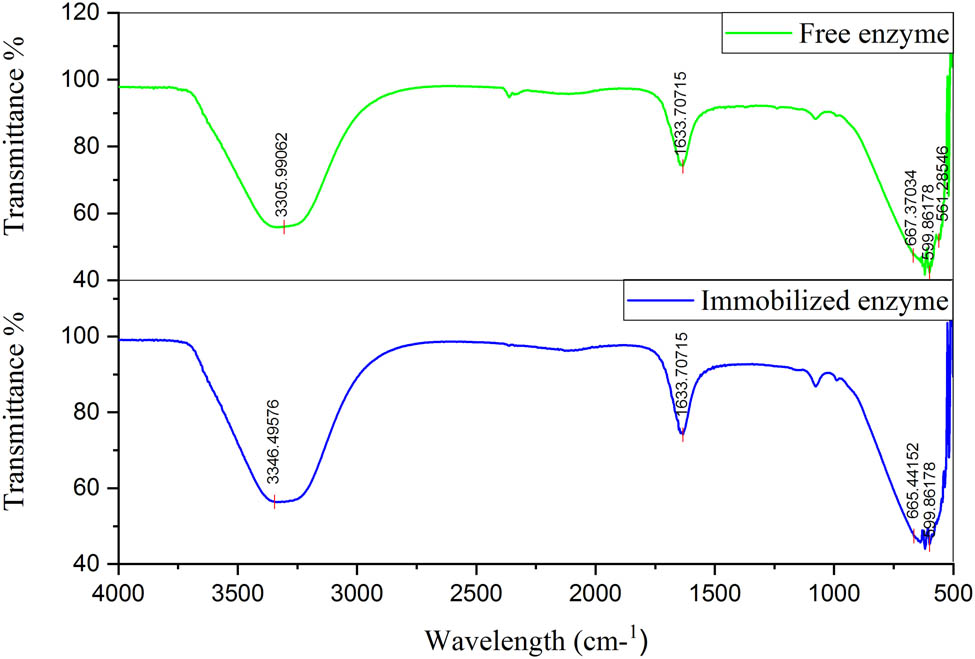
Fourier transform IR spectra for comparison between free and Al2O3-immobilized enzyme.
3.3.3 XRD
The crystalline nature of the NCs was observed with the different peaks. It was obtained by software X’Pert high score by JCPDS recorded at 311, 400, 422, 511, and 440, which confirmed the nature of Al2O3 NCs. The resultant peaks proved the presence of Al2O3 NCs. Figure 9 displays the intensity of the diffracted x-rays at diffraction angle (2-theta), along with the Miller indices. Sharpness of the peaks indicate the crystalline nature of the NCs. The results correspond to Piriyawong et al. and intensity peaks by Ansari and Husain [55,56].
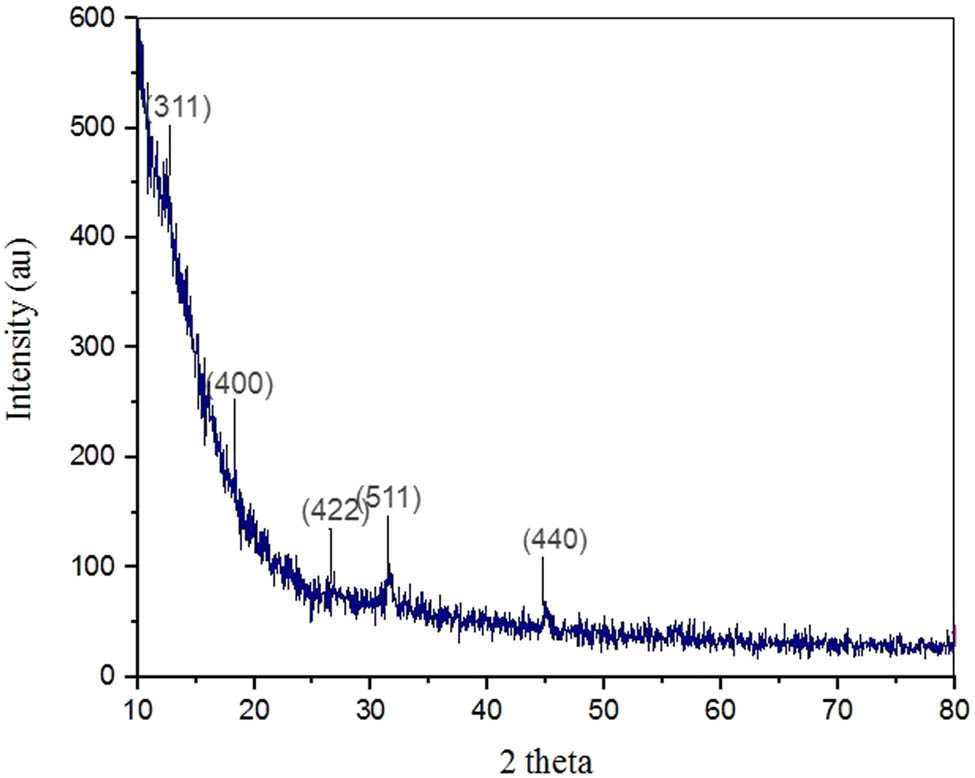
XRD patterns of Al2O3 NCs.
3.3.4 SEM
The beam of electrons was used for the demonstration of surface topography. The beam of electrons is specifically employed in scanning electron microscopy. This is utilized to determine the NC size along with the sample composition. When the beam of electrons passes through the sample, it creates an image with accurate measurement. The images presented several aggregates showing different small sizes of NCs joined together. The results are shown in Figure 10a as 1 µm at 7.02 KX of magnification and in Figure 10b–d as 200 and 300 at 10.0 KX of magnification. These images were obtained after several examinations of the structure and crystalline nature of Al2O3 NCs. These results correspond with the Guo et al. studies [14]. However, there were contradictory results of micrographs by SEM at a magnification of 200 nm by Kubica et al. [57]. NC size and distribution were also attained with the SEM at 1 µm investigated by Majeed et al. [58].
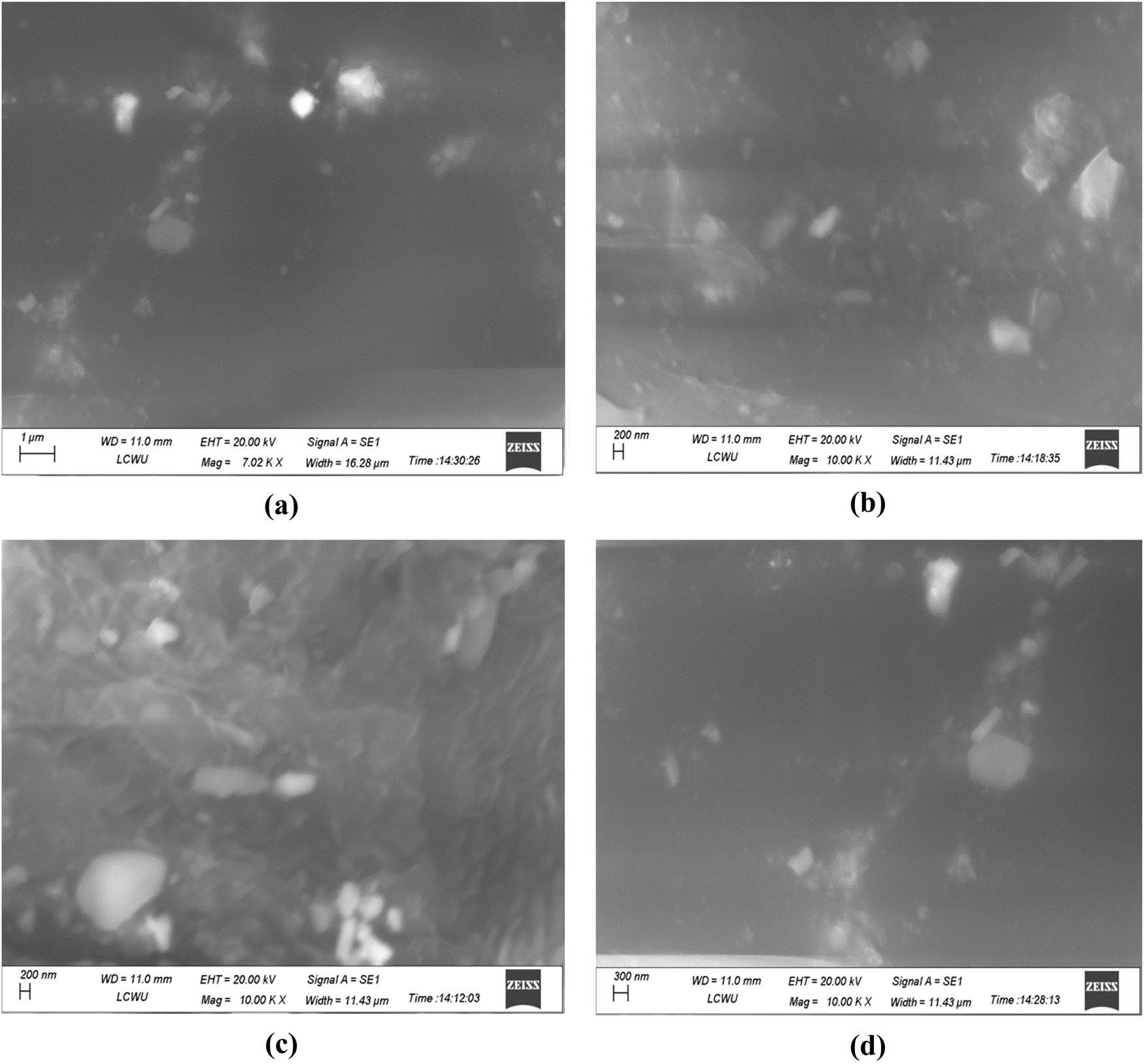
SEM micrographs of Al2O3 NCs immobilized β-glucosidase resolved at different magnifications: (a) 1 µm at 7.02 KX, (b) 200 nm at 10.00 KX, (c) 200 nm at 10.00 KX, and (d) 300 nm at 10.00 KX.
3.3.5 Zeta size analyzer
The zeta potential can influence the NPs’ propensity to infuse membranes with cationic particles. The majority of cellular membranes were charged negatively, so a zeta analyzer can be easily employed by the disruption of the cell wall. The exact size of the particles was obtained by Rukhma et al. [59]. The inspection was achieved at the count rate of 104.9 kcps investigated by Clogston and Patri [38]. The results are shown in Figure 11. The size obtained by the zetasizer was 206.4 d·nm at an intensity of 16.2%. Zeta potential is an interpretation of potential contributions from differently charged particles that could have contributed to the results. The zeta potential measurement is a tremendously significant parameter and is used in a broad range of industries including pharmaceuticals, medicine, brewing, ceramics, mineral processing, and water treatment. Dissociation of any acidic groups on a particle surface gave a negatively charged surface. The measurement of zeta potential was reliably connected to the stability phase of nanofluids, the stability time increases with the increase of zeta potential absolute value Khoshnevisan and Barkhi [60]. These results were in correspondence as described by Choudhary et al. [61].
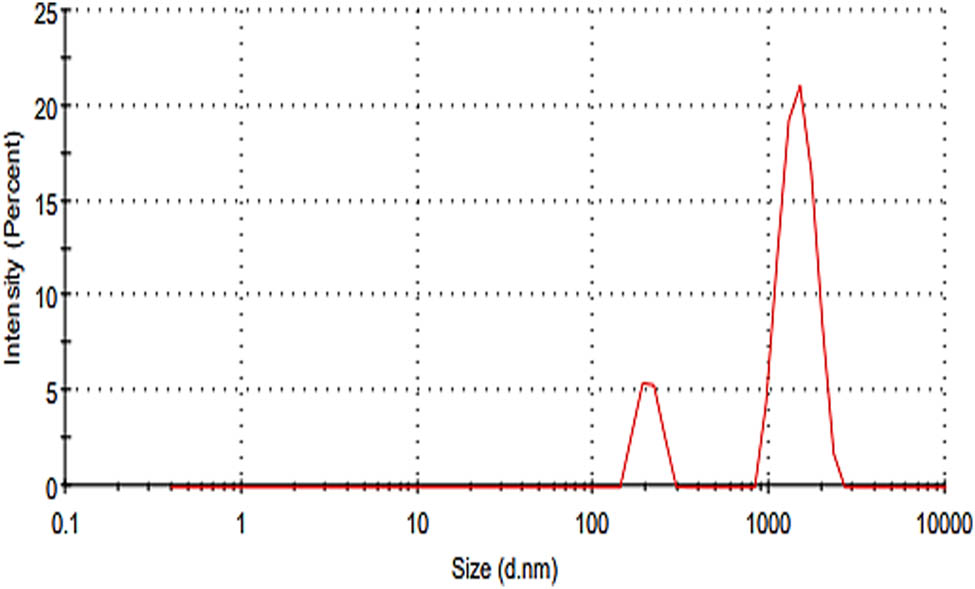
Zeta size distribution.
3.4 Optimization of biotransformation of sophoricoside to genistein
3.4.1 Effect of different rates of biotransformation and yield of genistein production
The several rates for the biotransformation were used to produce genistein from natural sophoricosidase by free β-glucosidase and immobilized β-glucosidase. Their effects on the optimizing value were evaluated. Various phenolic compounds from natural sources were converted from glycosides by hydrolysis. 2 ml of both free β-glucosidase and immobilized β-glucosidase and 3 ml of distilled water were used to increase the overall volume to 5 ml in four tubes. Both samples were then compared at 300 nm of wavelength for the determination of the optimized parameter. The diverse rates of biotransformation (24, 48, 72, 96 h) were used along with the substrate level as 2.5% (v/v), and their result is given in Figure 12. The process of biotransformation was held for 72 h for the appropriate results at 35°C in a shaking incubator (160 rpm). Both the free and immobilized β-glucosidase generated isoflavones as 0.43 ± 0.16 and 0.90 ± 0.46 mg·ml−1 at a wavelength of 300 nm. The maximum peak was developed by the Al2O3 immobilized β-glucosidase rather than that of the free β-glucosidase.
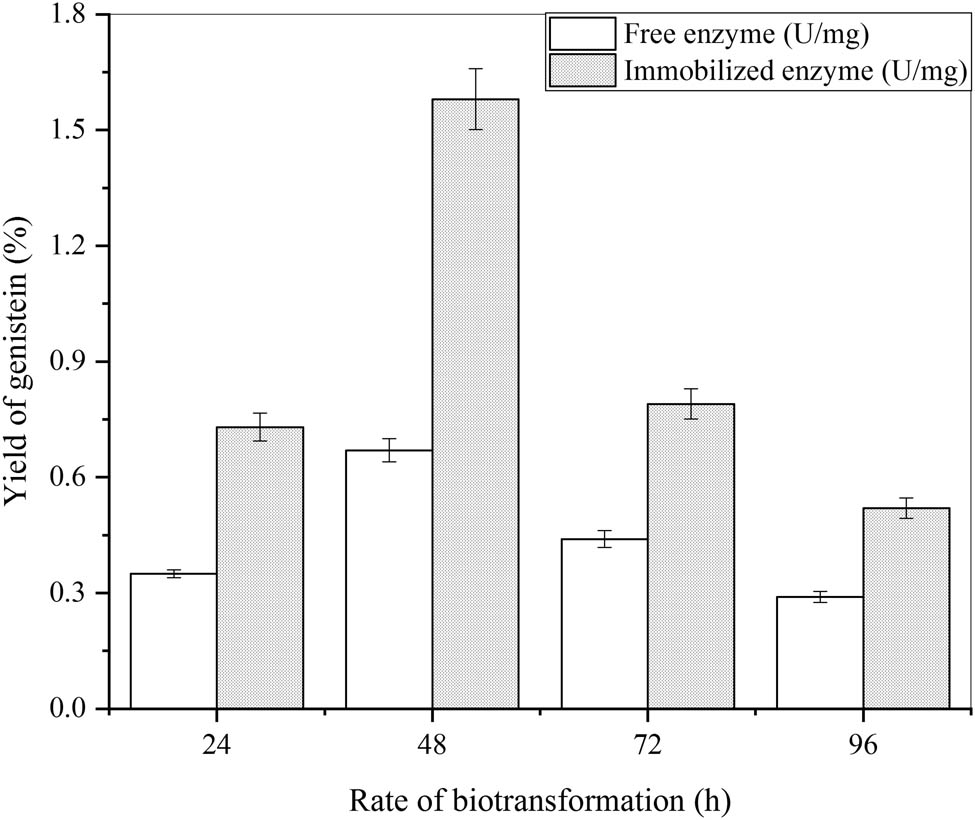
Effect of different rates of biotransformation of natural sophoricoside (in C. cajan leaf extract) to genistein by free and immobilized β-glucosidase (substrate level 2.5 ml (v/v), enzyme concentration 2 ml (v/v)) and error bars at ρ ≤ 0.05 indicating standard deviation among the values.
For the optimal value, the rate of biotransformation was used for 48 h at a wavelength of 300 nm. Al2O3 immobilized β-glucosidase demonstrates improved optimization outcomes rather than that of the free β-glucosidase. If the biotransformation is prolonged, the yield of genistein decreases which is the reason for 48 h as the rate of biotransformation is in correspondence to Mei et al. [62]. The results were similar to Kim where 48 h is the optimized parameter for biotransformation [63]. Wang et al. results were contradictory as different rates of biotransformation were utilized and 24 h was obtained as the optimized rate for genistein production [64]. This the optimal as basic compounds remain in the solvent without any loss so the yield can be increased.
3.4.2 Effect of different enzyme concentrations
The phenolic compounds from natural sources are converted from glycosides. An optimal enzyme volume was obtained after hydrolysis. The free β-glucosidase and Al2O3 immobilized β-glucosidase activities were 1.47 and 2.35 U·mg−1. The results were in correspondence with the Pandjaitan et al. investigation [65]. These results were relative to the studies of Abdella et al. since 1% (v/v) of enzyme concentration was taken as the optimal parameter [66]. This concentration of enzyme produced a high yield of genistein. If the concentration increases or decreases in the solvent, the yield of genistein is affected as it may lose its antioxidant activity. The appropriate wavelength for the biotransformation of genistein was taken as 300 nm as shown in Figure 13. In this spectrum, the free enzyme showed the smallest amount along with 0.1 absorbance and that of the immobilized enzyme at 1.0. The result was opposite to the Cesar et al. studies where 382 nm was the wavelength to show the high genistein yield [67].
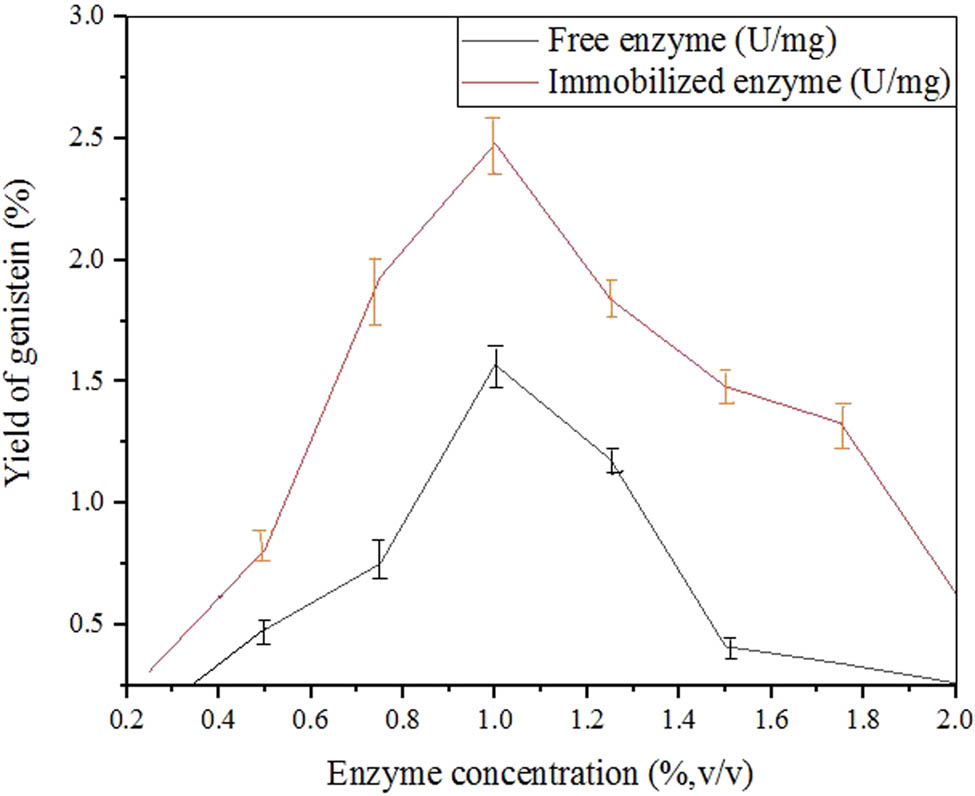
Effect of different enzyme concentrations for biotransformation.
4 Conclusion
In current years, there is a dire need for the production of bioactive compounds from medicinal plants that could be used for drug discovery and development. This work aimed at the synthesis of A. oryzae Isl-9 β-glucosidase under submerged fermentation and its immobilization on Al2O3 NCs produced from C. cajan leaf extracts. After optimizing fermentation parameters, in situ biotransformation was carried out for the eco-friendly and cost-effective production of genistein from natural sophoricoside (C. cajan leaf extract). Al2O3 NCs fabricated by dried leaf extract were crystalline in nature. Various other analytical techniques were also employed for the characterization of free and immobilized NCs. After immobilization, the stability of the β-glucosidase was increased, thereby increasing its potential in the pharmaceutical, biofuel, food, and beverage industries.
Acknowledgements
The authors are thankful to the Researchers Supporting Project number (RSPD2024R568), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: Authors state no funding involved.
-
Author contributions: Conceptualization, Sikander Ali; methodology, Afra Ejaz; software, Tariq Aziz; validation, Rukhma; formal analysis, M. Usman Ahmad, investigation, Najeeb Ullah; resources, Abid Sarwar; data curation, Abdulrahman Alshammari; writing – original draft preparation, Sikander Ali; writing – review and editing, Abid Sarwar; visualization, Thamer H. Albekairi; supervision, Tariq Aziz; and project administration, Sikander Ali.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article.
References
[1] Makkliang F, Juengsanguanpornsuk W, Phaisan S, Sakdamas A, Putalun W, Sakamoto S, et al. Transformation of Pueraria candollei var. mirifica phytoestrogens using immobilized and free β-glucosidase, a technique for enhancing estrogenic activity. RSC Adv. 2021;11(51):32067–76. 10.1039/d1ra05109a.Suche in Google Scholar PubMed PubMed Central
[2] Ahmed A, Nasim FH, Batool K, Bibi A. Microbial β-glucosidase: sources, production and applications. J Appl Environ Microbiol. 2017;5(1):31–46. 10.12691/jaem-5-1-4.Suche in Google Scholar
[3] Srivastava N, Rathour R, Jha S, Pandey K, Srivastava M, Thakur VK, et al. Microbial β- glucosidase enzymes: recent advances in biomass conversation for biofuels application. Biomolecules. 2019;9:220. 10.3390/biom9060220.Suche in Google Scholar PubMed PubMed Central
[4] Ariaeenejad S, Nooshi-Nedamani S, Rahban M, Kavousi K, Pirbalooti AG, Mirghaderi SS, et al. A novel high glucose-tolerant β-glucosidase: targeted computational approach for metagenomic screening. Front Bioeng Biotechnol. 2020;8:1–14. 10.3389/fbioe.2020.00813.Suche in Google Scholar PubMed PubMed Central
[5] Modrackova N, Vlkova E, Tejnecky V, Schwab C, Bunesova VN. Bifidobacterium β-Glucosidase activity and fermentation of dietary plant glucosides is species and strain specific. Microorganisms. 2020;8:839. 10.3390/microorganisms8060839.Suche in Google Scholar PubMed PubMed Central
[6] Khadye VS, Sawant S, Shaikh K, Srivastava R, Chandrayan S, Odaneth AA. Optimal secretion of thermostable β-glucosidase in Bacillus subtilis by signal peptide optimization. Pro Exp Purif. 2021;182:105–43. 10.1016/j.pep.2021.105843.Suche in Google Scholar PubMed
[7] Suleiman WB. A multi-aspect analysis of two analogous aspergillus spp. belonging to section Flavi: Aspergillus flavus and Aspergillus oryzae. BMC Microbiol. 2023;23(1):1–9. 10.1186/s12866-023-02813-0.Suche in Google Scholar PubMed PubMed Central
[8] Jin FJ, Hu S, Wang BT, Jin L. Advances in genetic engineering technology and its application in the industrial fungus Aspergillus oryzae. Front Microbiol. 2021;12:1–14. 10.3389/fmicb.2021.644404.Suche in Google Scholar PubMed PubMed Central
[9] Karami F, Ghorbani M, Mahoonak AS, Khodarahmi R. Fast, inexpensive purification of β-glucosidase from Aspergillus niger and improved catalytic/physicochemical properties upon the enzyme immobilization: Possible broad prospects for industrial applications. LWT Food Sci Technol. 2019;118(108770):0023–6438. 10.1016/j.lwt.2019.108770.Suche in Google Scholar
[10] Singhvi MS, Zinjarde SS. Production of pharmaceutically important genistein and daidzein from soybean flour extract by using β-glucosidase derived from Penicillium janthinellum NCIM 1171. Procss Biochem. 2020;12(10):1107. 10.1016/j.procbio.2020.07.014.Suche in Google Scholar
[11] Shin KC, Kang SH, Oh DK, Kim DW, Kim SH, Na CS, et al. Production of daidzein and genistein from seed and root extracts of korean wild soybean (glycine soja) by thermostable β-galactosidase from thermoproteus uzoniensis. Appl Sci. 2022;12:3481. 10.3390/app12073481.Suche in Google Scholar
[12] Neesa L, Islam R, Jahan N, Zohora US, Rahman MS. Optimization of culture conditions and reaction parameters of β-glucosidase from a new isolate of Bacillus subtilis (B1). J App Biotechnol Rep. 2020;7(3):152–8. 10.30491/jabr.2020.109990.Suche in Google Scholar
[13] Almeida JM, Lima VA, Giloni-Lima PC, Knob A. Passion fruit peel as novel substrate for enhanced β-glucosidases production by Penicillium verruculosum: potential of the crude extract for biomass hydrolysis. Biomass Bioeng. 2014;72:216–26. 10.1016/j.biombioe.2014.11.002.Suche in Google Scholar
[14] Guo Y, Chen X, Zhang X, Pu S, Zhang X, Yang C, et al. Comparative studies on ZIF-8 and SiO nanoparticles as carrier for immobilized β-glucosidase. Mol Catal. 2018;459:1–7. 10.1016/j.mcat.2018.08.004.Suche in Google Scholar
[15] Goorbandi RG, Mohammadi MR, Malekzadeh K. Synthesizing efficacious genistein in conjugation with superparamagnetic Fe3O4 decorated with bio-compatible carboxymethylated chitosan against acute leukemia lymphoma. Biomater Res. 2020;24:9. 10.1186/s40824-020-00187-2.Suche in Google Scholar PubMed PubMed Central
[16] Gudkov SV, Burmistrov DE, Smirnova VV, Semenova AA, Lisitsyn AB. A mini review of antibacterial properties of Al₂O₃ nanoparticles. J Nanomater. 2022;12:2635. 10.3390/nano12152635.Suche in Google Scholar PubMed PubMed Central
[17] Hassanpour P, Panahi Y, Kalan AE, Akbarzadeh A, Davaran S, Nasibova AN, et al. Biomedical applications of aluminium oxide nanoparticles. Micro Nano Lett. 2018;13(9):1227–31. 10.1049/mnl.2018.5070.Suche in Google Scholar
[18] Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–31. 10.1016/j.arabjc.2017.05.011.Suche in Google Scholar
[19] Pugniere M, Juan CS, Coletti-Previero MA, Previero A. Immobilization of enzymes on alumina by means of pyridoxal 5′-phosphate. Biosci Rep. 1988;8(3):263–9. 10.1007/BF01115043.Suche in Google Scholar PubMed
[20] Song TT, Yang M, Chai JW, Callsen M, Zhou J, Yang T, et al. The stability of aluminium oxide monolayer and its interface with two-dimensional materials. Sci Rep. 2016;6:1–9. 10.1038/srep29221.Suche in Google Scholar PubMed PubMed Central
[21] Chen Z, Liu Y, Liu L, Chen Y, Li S, Jia Y. Purification and characterization of a novel β-glucosidase from Aspergillus flavus and its application in saccharification of soybean meal. Prep Biochem Biotechnol. 2019;49(7):671–8. 10.1080/10826068.2019.1599397.Suche in Google Scholar PubMed
[22] Moreira AA, Ferreira ACV, Silva JB, Ribeiro MLL. Treatment of sugarcane bagasse for the immobilization of soybean β-glucosidase and application in soymilk isoflavones. Brazilian J Food Technol. 2019;22:201–43. 10.1590/1981-6723.24318.Suche in Google Scholar
[23] Feng C, Jin S, Xia XX, Guan Y, Luo M, Zu YG, et al. Effective bioconversion of sophoricoside to genistein from Fructus sophorae using immobilized Aspergillus niger and yeast. World J Microbiol Biotechnol. 2015;31:187–97. 10.1007/s11274-014-1777-y.Suche in Google Scholar PubMed
[24] Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13:20. 10.1186/s13020-018-0177-x.Suche in Google Scholar PubMed PubMed Central
[25] Phadungcharoen N, Winotapun W, Khomniyawanit A, Krataichan F, Rojanarata T. Facile and green fabrication of biocatalytic chitosan beads by one-step genipin-mediated β-glucosidase immobilization for production of bioactive genistein. Sustain Chem Pharm. 2019;14:2352–5541. 10.1016/j.scp.2019.100187.Suche in Google Scholar
[26] Behloul N, Wu G. Genistein: a promising therapeuticagent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698(1–3):31–8. 10.1016/j.ejphar.2012.11.013.Suche in Google Scholar PubMed
[27] Bhattarai G, Poudel SB, Kook SH, Lee JC. Anti-inflammatory, anti-osteoclastic, and antioxidant activities of genistein protect against alveolar bone loss and periodontal tissue degradation in a mouse model of periodontitis. J Biomed Mater Res. 2017;105(9):2510–21. 10.1002/jbm.a.36109.Suche in Google Scholar PubMed
[28] Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Meta Pharm. 2013;38:15–25. 10.1007/s13318-012-0112-y.Suche in Google Scholar PubMed
[29] Miller KA, Frankel F, Takahashr H, Vance N, Stiegerwald C, Edelstein S. Collected literature on isoflavones and chronic diseases. Cogent Food Agr. 2016;2(1):1135861. 10.1080/23311932.2015.1135861.Suche in Google Scholar
[30] Ali S, Khalid SW. Kinetic and parametric optimization for the enhanced production of a novel fungal exo-inulinase under liquid culture. Pak J Zool. 2020;52(5):1657–64.10.17582/journal.pjz/20180225180228Suche in Google Scholar
[31] Abdullah R, Chudhary SN, Kaleem A, Iqtedari M, Nisari K, Iftikhar T, et al. Enhanced production of β-glucosidase by locally isolated fungal strain employing submerged fermentation. Biosci J Uberlandia. 2019;35(5):1552–9. 10.14393/BJ-v35n5a2019-47943.Suche in Google Scholar
[32] Manikandan V, Jayanthi P, Priyadharsan A, Vijayaprathap E, Anbarasan PM, Velmurugan P. Green synthesis of pH-responsive Al2O3 nanoparticles: application to rapid removal of nitrate ions with enhanced antibacterial activity. J Photochem Photobiol A: Chem. 2019;371:205–15. 10.1016/j.jphotochem.2018.11.009.Suche in Google Scholar
[33] Dutt A, Updhyay LSB. Synthesis of cysteine-functionalized silver nanoparticles using green tea extract with application for lipase immobilization. Anal Lett. 2018;51(7):1071–86. 10.1080/00032719.2017.1367399.Suche in Google Scholar
[34] De Caro C, Claudia H. UV/VIS spectrophotometry - fundamentals and applications. Mettler Toledo, Switzerland. 2015;9(30):1–48.Suche in Google Scholar
[35] Djebaili K, Mekhalif Z, Boumaza A, Djelloul A. XPS, FTIR, EDX, and XRD analysis of Al2O3 scales grown on PM2000 alloy. J Spectrosc. 2015;16:868. 10.1155/2015/868109.Suche in Google Scholar
[36] Tsybulya SV, Kryukovaa GN. New X-ray powder diffraction data on δ-Al2O3. Inter Cent Diff Data. 2003;18:4. 10.1154/1.1604128.Suche in Google Scholar
[37] Bejar L, Huape E, Medina A, Abraham A, Mejia C, Aguilar CP, et al. Study by SEM of carbon nanotubes deposited by CVD using Al2O3 and TiO2 as catalysts. Microsc Microanal. 2019;25:2. 10.1017/S1431927619012650.Suche in Google Scholar
[38] Clogston JD, Patri AK. Zeta potential measurement. Met Mol Biol. 2011;697:63–70. 10.1007/978-1-60327-198-1_6.Suche in Google Scholar PubMed
[39] Watanabe A, Suzuki M, Ujiie S, Gomi K. Purification and enzymatic characterization of a novel β-1, 6-glucosidase from Aspergillus oryzae. J Biosci Bioeng. 2016;121:259–64. 10.1016/j.jbiosc.2015.07.011.Suche in Google Scholar PubMed
[40] Cheng Y, Wei H, Sun R, Tian Z, Zheng X. Rapid method for protein quantitation by Bradford assay after elimination of the interference of polysorbate 80. Anal Biochem. 2016;494:37–9. 10.1016/j.ab.2015.10.013.Suche in Google Scholar PubMed
[41] Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. 10.1016/0003-2697(76)90527-3.Suche in Google Scholar
[42] Tungmunnithum D, Drouet S, Lorenzo JM, Hano C. Green extraction of antioxidant flavonoids from Pigeon Pea (Cajanuscajan (L.) Millsp.)seeds and its antioxidant potentials using ultrasound-assisted methodology. Molecules. 2021;26:7557. 10.3390/molecules26247557.Suche in Google Scholar PubMed PubMed Central
[43] Nehra S, Singh S, Sangwan S, Rani S. Antioxident activity of Pod coat extracts of Pigeon Pea (C. cajan L.) and their efficacy in stabilization of soybean oil. J Antiox Act. 2021;2(2):29–41. 10.14302/issn.2471-2140.jaa-21-3960.Suche in Google Scholar
[44] Raghuwanshi A, Dudeja SS, Khurana AL. Effect of temperature on flavonoid production in pigeon pea [Cajanuscajan (L) Millsp] in relation to nodulation. Biol Fert Soils. 1994;17(4):314–6. 10.1007/bf00383988.Suche in Google Scholar
[45] Nagati VB, Koyyati R, Donda MR, Alwala J, Padigya KRKPRM. Green synthesis and characterization of silver nanoparticles from Cajanuscajan leaf extract and its antibacterial activity. Int J Nanomater Biostruct. 2012;2(3):39–43.Suche in Google Scholar
[46] Olajuyigbe FM, Nlekerem CM, Ogunyewo OA. Production and characterization of highly thermostable β-glucosidase during biodegradation of methyl cellulose by Fusarium oxysporum. Biochem Res Int. 2015;8:397–424. 10.1155/2016/3978124.Suche in Google Scholar PubMed PubMed Central
[47] Liao J, Zhang S, Zhang X. Effects of mixed adding crude extracts of β-glucosidases from three different non-saccharomyces yeast strains on the quality of cabernet sauvignon wines. J Fungi. 2022;8:710. 10.3390/jof8070710.Suche in Google Scholar PubMed PubMed Central
[48] Khalid A, Ali S, Rukhma M, Jahangeer M, Sarwar A, Nelofer R, et al. Immobiliztion of Aoryzae tyrosine hydrolase on ZnO nanocrystals for improved stability and catalytic efficiency towards L-dopa production. Sci Rep. 2023;13(1):22882.10.1038/s41598-023-50198-xSuche in Google Scholar PubMed PubMed Central
[49] Molina G, Lima EAD, Borin GP, Barcelos MCSD, Pastore GM. Beta-glucosidase from penicillium. N Fut Develop Micro Biotechnol Bioeng. 2018;7:37–4.10.1016/B978-0-444-63501-3.00007-7Suche in Google Scholar
[50] Sabah T, Kareem HJ, Al-attar N. Synthesis and biomedical activity of aluminium oxide nanoparticles by laser ablation technique. Res J Pharm Technol. 2023;16(3):1267–3.10.52711/0974-360X.2023.00209Suche in Google Scholar
[51] Ismail A, Kabary H, Samy A. Synthesis of α-Al2O3 nanoparticles from pepsi cans wastes and its fungicidal effect on some mycotoxins producing fungal isolates. Res Square. 2021;1:1–16.10.21203/rs.3.rs-774484/v1Suche in Google Scholar
[52] Mahmoud AK. Production of alumina nanoparticles (Al2O3) using pulsed laser ablation technique in distilled water solution. Int J Curr Eng Technol. 2014;2:314–6.10.14741/ijcet/spl.2.2014.57Suche in Google Scholar
[53] Nassar S, Zainab B. Study the effect of different liquid media on the synthesis of alumina (Al2O3) nanoparticle by pulsed laser ablation technique. Manuf Sci Technol. 2015;3:77–81.10.13189/mst.2015.030401Suche in Google Scholar
[54] Goldstein DN, McCormick JA, George SM. Al2O3 atomic layer deposition with trimethylaluminum and ozone studied by in situ transmission FTIR spectroscopy and quadrupole mass spectrometry. J Phys Chem C. 2008;112(49):19530–9.10.1021/jp804296aSuche in Google Scholar
[55] Piriyawong V, Thongpool V, Asanithi P, Limsuwan P. Preparation and characterization of alumina nanoparticles in deionized water using laser ablation technique. J Nanomater. 2012;1(810403):1–6. 10.1155/2012/819403.Suche in Google Scholar
[56] Ansari S, Husain Q. Immobilization of Kluyveromyces lactis β galactosidase on concanavalin: A layered aluminium oxide nanoparticles—Its future aspects in biosensor applications. J Mol Catal B-Enzym. 2011;70:119–26.10.1016/j.molcatb.2011.02.016Suche in Google Scholar
[57] Kubica M, Skoneczny W, Bara M. Analysis of Al2O3 nanostructure using scanning microscopy. Scanning. 2018;14:845.10.1155/2018/8459768Suche in Google Scholar PubMed PubMed Central
[58] Majeed N, Abdulmajeed B, Yaseen A. The Influence of the preparation and stability of nanofluids for heat transfer. J Eng. 2019;25:45–54.10.31026/j.eng.2019.04.04Suche in Google Scholar
[59] Rukhma M, Ghazi A, Mujahid H, Mukhtar A, Sarwar N, Ullah, et al. Fabrication of polydopamine-functionalized nanobioconjugates for improved stability and catalytic efficiency towards industrial application. Biomass Convers Biorefin. 2023;14:1–14.10.1007/s13399-023-05114-8Suche in Google Scholar
[60] Khoshnevisan K, Barkhi M. Information about zeta potential. Zeta Potential. 2015;1:1–9. 10.13140/RG.2.1.4554.3844.Suche in Google Scholar
[61] Choudhary R, Khurana D, Kumar A, Subudhi S. Stability analysis of Al2O3/water nanofluids. J Exp Nanosci. 2017;12:1–12. 10.1080/17458080.2017.1285445.Suche in Google Scholar
[62] Mei J, Chen X, Liu J, Yi Y, Zhang Y, Ying G. A biotransformation process for production of genistein from sophoricoside by a strain of Rhizopus oryza. Sci Rep. 2019;9(1):6564. 10.1038/s41598-019-42996-z.Suche in Google Scholar PubMed PubMed Central
[63] Kim BG. Biological synthesis of genistein in E. coli. J Microbiol Biotechnol. 2020;30(5):770–6.10.4014/jmb.1911.11009Suche in Google Scholar PubMed PubMed Central
[64] Wang D, Khan MS, Cui L, Song X, Zhu H, Ma T, et al. A novel method for the highly efficient biotransformation of genistein from genistin using a high-speed counter-current chromatography bioreactor. RSC Adv. 2019;9:4892–9.10.1039/C8RA10629KSuche in Google Scholar PubMed PubMed Central
[65] Pandjaitan N, Hettiarachchy N, Ju ZY. Enrichment of genistein in soy protein concentrate with b‐glucosidase. J Food Sci. 2000;65(3):403–7.10.1111/j.1365-2621.2000.tb16016.xSuche in Google Scholar
[66] Abdella A, Baz AFE, Mahrous EE, Maksoud AAAE, Ibrahim IA. Response surface methodology for optimization of genistein content in soy flour and its effect on the antioxidant activity. Iran J Pharm Res. 2018;17(3):124.Suche in Google Scholar
[67] Cesar IDC, Braga FC, Soares CDV, Nunan EDA, Antonio G, Moreira-Campos PELM. Quantification of genistein and genistin in soy dry extracts by UV-Visible spectrpohotometric methods. Quim Nova. 2008;31(8):1933–6. 10.1590/S0100-40422008000800003.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

