Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
Abstract
In this study, the aqueous seed extract of Trachyspermum copticum was employed to fabricate silver nanoparticles (AgNPs) for their antibacterial performance. The structural characteristics of the phytofabricated AgNPs were investigated using ultraviolet–visible, energy dispersive X-ray, dynamic light scattering, zeta potential, field emission scanning electron microscope, and Fourier transform infrared spectroscopy. Further, the antibacterial effects of AgNPs were evaluated against two standard gram-positive strains of Staphylococcus aureus and Streptococcus pyogenes and two standard gram-negative strains of Escherichia coli and Klebsiella pneumoniae. The findings showed the phytofabrication of spherical-shaped AgNPs with a Z-average diameter of 119.7 nm and the polydispersity index of 0.56. The minimum inhibitory concentration of AgNPs against S. aureus, S. pyogenes, E. coli, and K. pneumoniae strains was 0.25, 1, 0.5, and 0.5 μg·mL−1, respectively. In addition, the minimum bactericidal concentration of these nanoparticles (NPs) against the mentioned strains was 8, 16, 4, and 4 μg·mL−1, respectively. Furthermore, the AgNPs enhanced the generation of reactive oxygen species significantly in all studied bacteria compared to the control (P < 0.05) suggesting an oxidative-associated antibacterial mechanism of AgNPs. Moreover, a dose-dependent cell death was found in all bacterial samples based on flow cytometry analysis. Overall, this study showed a biological approach for the biosynthesis of AgNPs with significant antibacterial effects.
1 Introduction
As of late, green-synthesized silver nanoparticles (AgNPs) have been delivering great attention in biomedical applications, and because of their eco-friendly nature, they have been the target of research. The green approach makes the process of the culture easier to purify, increases the potential for large-scale synthesis, and reduces overall costs. Accordingly, the production of plant-mediated AgNPs requires combining the aqueous extract with a solution of silver nitrate [1]. To produce metal-based nanoparticles (MNPs) in an environmentally friendly manner, various types of plants, plant extracts, plant tissue, fruits, microbes, and marine algae have been used thus far. Plants have a diverse collection of bioactive secondary metabolites in their systems [2]. Plant extracts are utilized in a reduction process alongside a variety of metals and metal oxides, including zinc, silver, gold, iron, copper, and platinum for biosynthesis [3,4]. Significantly, medicinal plants are not only heavily involved in the research and development of bio-scaffolding, but they are also extensively used for medicinal applications against pathogenic bacteria [5,6]. In the process of the biosynthesis of nanoparticles (NPs), plant extracts may serve both as reducing agents and as stabilizing agents [7]. In comparison to other approaches, this process is both quick and suitable for the large-scale synthesis of MNPs [8]. Further, among different biological resources, mentioned plants have typically been sought after because they offer a diverse selection of functional phytochemical groups that contain very little harmful material [9]. The plant Trachyspermum copticum is a member of the family Apiaceae possessing antibacterial characteristics and is the biofactory used in the present study to fabricate AgNPs [10,11,12]. In India, Eastern Asia, Iran, and Egypt, the seeds of T. copticum are commonly used as an aromatic herb and spice in culinary preparation as well as in traditional medical practices. Hence, due to the safety and extensive use of the seeds of T. copticum as food additives, we decided to investigate them for the green synthesis of AgNPs. Besides, this plant has more benefits. For instance, previous research has demonstrated that the essential oil derived from T. copticum fruit had antibacterial as well as antioxidant properties [13,14]. The plant reduces clinical symptoms of functional dyspepsia and has restorative benefits on stomach ulcers in animal models. It also has anti-inflammatory and antioxidant activities [10]. Various scholars have focused on the biofabricatoin of AgNPs using herbal resources with different diameters and morphologies and examined their ability to tackle bacterial strains. For instance, Tehri et al. reported the biofabrication of spherical AgNPs from Litchi chinensis leaf extracts. The size of the AgNPs ranged from 5 to 15 nm and had a spherical form. In this particular research project, it was shown that biosynthesized NPs possessed antibacterial characteristics that had an excellent effect on bacteria in comparison to those of the medicines tetracycline and gentamicin [15]. Similarly, an aqueous flower extract of Morinega oleifera was used in the plant-mediated synthesis of spherical AgNPs by Bindhu et al. They reported that the average size of the AgNPs was 8 nm. Staphylococcus aureus and Klebsiella pneumonia were two examples of potentially dangerous bacteria that could be combatted effectively by biosynthesized AgNPs with an antibacterial action [16]. Alternatively, Singh et al. discovered that the leaf extract of Carissa carandas L. was used to phytofabricate AgNPs. The ability of these NPs to kill bacteria was tested against a variety of bacterial strains, and the results suggested that these AgNPs could have strong bactericidal impact [17]. Likewise, extracts of Hyssopus officinalis and Calendula officinalis were used in the plant-mediated synthesis of almost spherical AgNPs by Balciunaitiene et al. These AgNPs had an average size of 16.8 ± 5.8 and 35.7 ± 4.8 nm, respectively. Both of these naturally occurring AgNPs were able to exert a bactericidal effect against various microorganisms [18]. Although the antibacterial activity of biogenic AgNPs was reported in the literature, further research is required to explore their antibacterial mechanisms. The novelty of this study was not only the phytofabrication and structural characterization of colloidal silver particles but also exploring a mechanistic approach for antibacterial performance these NPs against gram-positive and gram-negative bacteria.
2 Materials and methods
2.1 Materials and reagents
All chemicals and reagents utilized in this study were prepared from Sigma-Aldrich, USA.
2.2 Plant specimen and microbial strain
The seeds of the plant T. copticum were purchased from a local market located in Tehran, Iran. Then, the plant seeds were properly identified and deposited in the Department of Pharmacognosy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran, with the allocation of voucher specimen number sbmu-8033. The bacterial strains including two gram-positive bacteria S. aureus (ATCC 25923), Streptococcus pyogenes (ATCC 19615), and two gram-negative bacteria Escherichia coli (ATCC 25922) and Klebsiella pneumoniae (ATCC 10031) were prepared from Department of Microbiology, Central Research Laboratories, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2.3 Plant-mediated synthesis of AgNPs
Dried seeds of T. capticum were pulverized followed by sieving by a 20-mesh-sized strainer as the primary steps of preparation of the herbal extract. Afterward, 1,000 mL of deionized sterilized water and 100 g of the herbal seeds powder were transferred into an Erlenmeyer flask. After remaining at room temperature for 1 day and filtration using the Whatman Number-1 filter, a rotary evaporator was employed for evaporation of the extracted water. Then, the concentrated extract was put in a hot air oven at 40°C for 72 h to dry the extract, and then until further use, it was put at the temperature of 4°C. Subsequently, an Erlenmeyer flask containing 100 mL of an aqueous solution of AgNO3 at the concentration of 1 mM was poured with 100 mL of the 0.1% filtered herbal extract. After separately adjusting the pH of the mixture to 6, 7, 8, and 9, they were transferred onto a rotary shaker (JAL TAJHIZ®, JTSL 40, Iran) for incubation at 28°C and 150 rpm for 2 days. Eventually, observation of the Tyndall effect and color change in the mixture confirmed the formation of the herbal-mediated AgNPs.
2.4 Structural characterization of AgNPs
Ultraviolet–visible (UV–vis) spectroscopy (Shimadzu, UV-1280, Japan) was performed to determine the formation of the AgNPs. The surface morphology of the biogenic AgNPs was determined via the field emission scanning electron microscope (FE-SEM) (Sigma VP, ZEISS, Germany) with an acceleration voltage of 15 kV. For identification of the chemical compounds binding to the surface of the NPs, a mixture of the AgNPs and potassium bromide at the ratio of 1:100 was prepared followed by compression to a 2-mm semitransparent disk for 2 min. Afterward, the disk was analyzed using Fourier transform infrared (FT-IR) spectroscopy (Agilent, Cary 630 model, US) over the wavelength range of 400–4,000 cm−1. The energy dispersive X-ray (EDX) technique was also applied to chemically characterize the biosynthesized NPs. In addition, characterization of the polydispersity index (PdI) and hydrodynamic size of the NPs was performed by Zetasizer with a scattering angle of 90° at 25°C (Malvern Instruments Ltd, Malvern, United Kingdom) operating dynamic light scattering (DLS) technique.
2.5 Antibacterial properties of AgNPs
2.5.1 Well diffusion assay
According to the method described previously [19], after the preparation of the bacterial inoculums aseptically the cell density was modified to 0.5 McFarland standard (108 CFU·mL−1) coupled with covering the Mueller Hinton Agar (MHA) in the plates with the inoculums. After dispersing the AgNPs in deionized water at the concentration of 1 mg·mL−1, 50 and 100 μL of the stock were separately poured into the wells with a diameter of 6 mm created in the contents of the plates. Moreover, 100 μL per well of 0.5 mM aqueous AgNO3 solution and 100 μL per well of the aqueous extract of T. capticum (0.1% stock) were considered for comparison. Eventually, the plates were incubated for 1 day at 37°C.
2.5.2 Broth microdilution assay
2.5.2.1 Minimum inhibitory concentration (MIC) test
The broth microdilution assay was conducted based on the method described elsewhere [20]. Briefly, according to the guidelines of Clinical and Laboratory Standards Institute culturing of the bacteria S. aureus (ATCC 25923), S. pyogenes (ATCC 19615), E. coli (ATCC 25922), and K. pneumoniae (ATCC 10031) in a 96-well microtiter plate containing Mueller Hinton Broth (MHB) was carried out to estimate the MIC values of the AgNPs [21]. Then, after adding 100 µL of MHB medium to twelve wells of the 96-well plate, 100 µL of the AgNPs was transferred to the first well. After that, the second well was filled with 100 µL of the first well content, and the other wells were similarly filled except for the twelfth well, which remained devoid of NPs and was regarded as negative control. Finally, all the wells were poured with 10 µL of the bacterial suspension with a cell density of 108 CFU·mL−1, which was equivalent to 0.5 McFarland. The antibiotic tetracycline was also assayed as a positive control utilizing the same method. After wrapping the plates with paraffin and mixing the components thoroughly, the plates were incubated at 37°C for 1 day. Accordingly, the MIC value was considered the sample concentration of a well in which no growth was visible [20].
2.5.2.2 Minimum bactericidal concentration (MBC) test
To determine the MBC value, 50 µL of broth medium was extracted from wells of the MIC micro-plate. These samples were then evenly distributed onto sterile MHA plates and left to incubate at 37°C for a period of 24 h. Upon completion of the incubation period, the lowest concentration of the AgNPs at which almost no bacterial growth (99.9%) was observed on the agar plates was identified as the MBC value.
2.5.3 Reactive oxygen species (ROS) assay
To evaluate the amount of ROS, known as unstable molecules damaging the biological compositions in cells, the TPR-ROS Assay Kit (Teb Pazhouhan Razi, Iran) was employed using 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) which is a ROS-sensitive probe. This fluorometric assay involved the hydrolysis of DCFH-DA to produce a non-fluorescent molecule (DCFH). This hydrolysis is facilitated by an ester hydrolysis enzyme. Subsequently, DCFH is rapidly oxidized to form strong green fluorescence dichlorofluorescein. The intensity of this fluorescence at λ ex = 485 and λ em = 535 is directly proportional to the amount of ROS present in the cells. For this study, bacterial strains were seeded in a 96-well plate at a density of 1.5 × 108 cells per well and incubated for 24 h. The culture medium was then replaced, and the cells were treated with biofabricated AgNPs for an additional day. Following the treatment, the TPR kit protocol was followed, and the fluorescence intensity was measured using a Hybrid Multimode Reader Cytation 3 (BioTek Company, USA).
2.5.4 Flow cytometry-based quantitative assessment of cell viability
Initially, different concentrations of biofabricated AgNPs were added to reference strains of S. aureus (ATCC 25923), S. pyogenes (ATCC 19615), E. coli (ATCC 25922), and K. pneumoniae (ATCC 10031) at the concentration of 0.5 McFarland (108 CFU·mL−1) in the Sabouraud Dextrose Broth (SDB) medium. After incubating the components at 28°C and 125 rpm for 24 h, the samples were centrifuged at 8,000 rpm for 1 min followed by separating and discarding the supernatant. Then, the phosphate-buffered saline buffer was used to wash the plates. This step was repeated one more time and after adding 2 µg·mL−1 of propidium iodide dye, the plates were incubated for 20 min. Finally, a flow cytometer (BD FACSCalibur™, USA) and FlowJo software (Tree Star, Inc., Ashland, OR, USA) were employed to assess the samples and analyze the data, respectively.
3 Results and discussion
3.1 T. copticum aqueous seed extract-derived AgNPs: preparation and characterization
A schematic procedure for herbal-mediated fabrication of AgNPs is shown in Figure 1. The reaction of T. copticum aqueous seed extract and aqueous silver nitrate solution led to the appearance of a brown color from pale yellow after 48 h of incubation suggesting the fabrication of AgNPs. Previous studies also reported similar color changes [22,23,24]. Figure 2a shows the color change in different pH values of the reacting matrix representing the darker brown color in higher pH values. Besides, the Tyndall effect, which is light scattering by particles in a colloidal system, was observed in the prepared phytofabricated colloidal AgNPs, while no Tyndall effect was seen in the aqueous seed extract and aqueous AgNO3 solution. The color change and the Tyndall effect are two macroscopic signs for the biofabrication of AgNPs. Previous studies also employed the Tyndall effect to confirm the colloidal characteristics of biofabricated NPs [19,25,26]. In the next step, several analytical techniques confirmed the phytofabrication of AgNPs.
![Figure 1
A schematic illustration for phytosynthesis of colloidal silver particles by employing aqueous seed extract of T. copticum [The Figure was developed in part using Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).].](/document/doi/10.1515/gps-2023-0242/asset/graphic/j_gps-2023-0242_fig_001.jpg)
A schematic illustration for phytosynthesis of colloidal silver particles by employing aqueous seed extract of T. copticum [The Figure was developed in part using Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).].
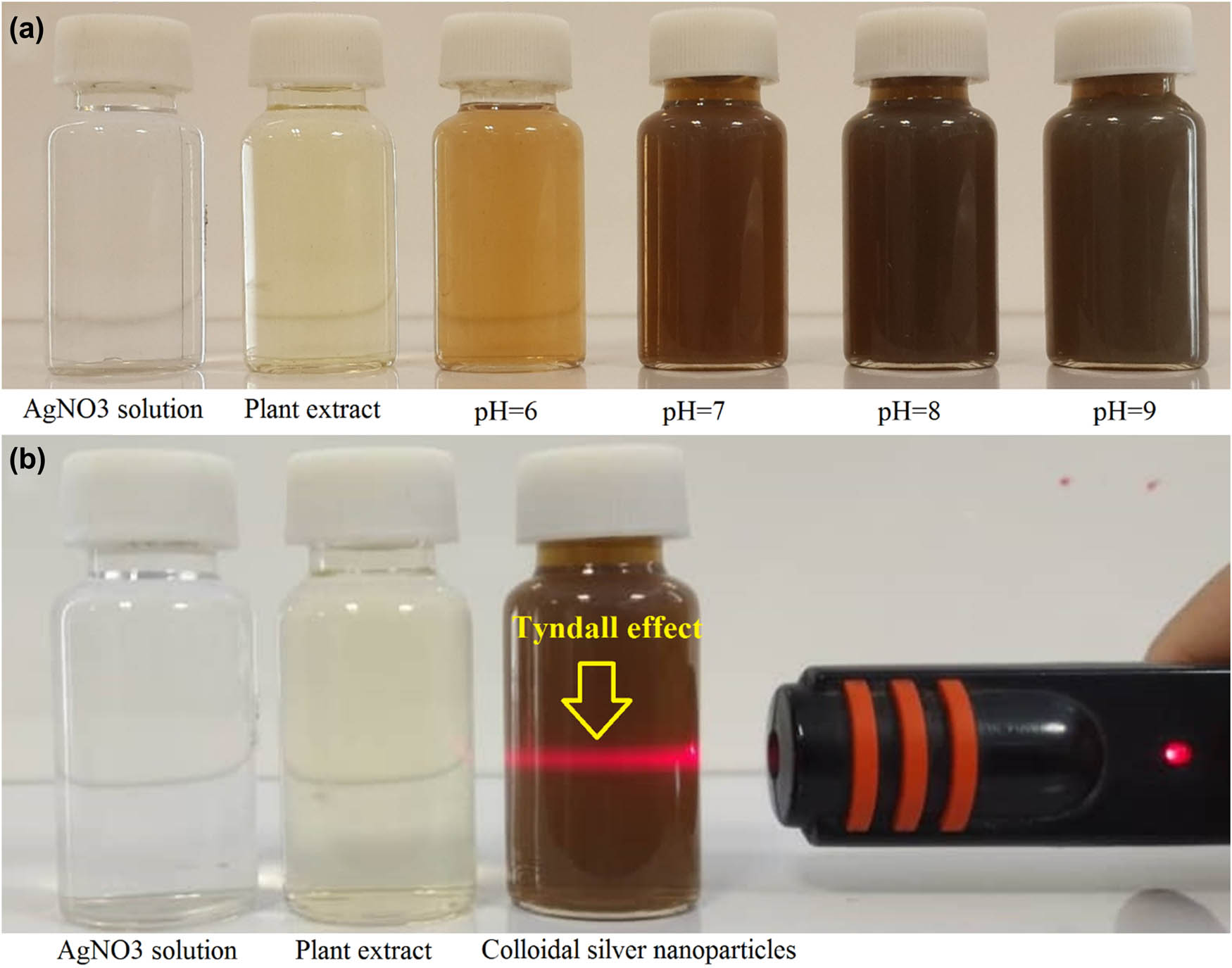
(a) The color alteration from pale yellow to dark brown following the phytofabrication of AgNPs in different pH values. (b) The Tyndall effect was observed in the prepared phytofabricated colloidal AgNPs, while no Tyndall effect was seen in the aqueous seed extract and aqueous AgNO3 solution.
The UV–vis spectroscopy scanned the samples from the wavelength of 200–800 nm. No wavelength of maximum absorption (λmax) was observed for the aqueous silver nitrate solution (Figure 3a) and T. copticum aqueous seed extract (Figure 3b) at the wavelength of around 400 –450 nm. However, as shown in Figure 4a, AgNPs with different pH values showed a wavelength of maximum transmittance at around 420 nm indicating the fabrication of AgNPs. Previous studies also reported a strong λ max between 400 and 450 nm for fabrication of AgNPs [26,27,28]. The higher the absorbance peak, the more the biofabrication of AgNPs. Hence, the maximum phytofabrication of AgNPs referred to the sample of AgNPs with a pH value of 9. This peak refers to a phenomenon named surface plasmon resonance (SPR) that occurs at metal surfaces, such as silver and gold when a light beam interacts with the surface of conductive NPs at a particular angle [29,30]. In a similar study, it was also reported that the SPR peak of phytofabricated AgNPs in UV–vis spectroscopy was higher in the pH value of 9 compared to acidic pH values representing the higher fabrication of AgNPs in alkaline conditions [26]. This could be attributed to the difference between the ionizing process of functional groups in alkaline and acidic conditions. The elemental composition of herbal-mediated fabricated AgNPs was confirmed by EDX analysis representing a distinct signal from silver (Ag) at 3.0 keV (Figure 4b). This finding was in agreement with the previous studies [26,27,28].

(a) Aqueous AgNO3 solution UV–vis spectroscopy and (b) T. copticum aqueous seed extract UV–vis spectroscopy.

(a) T. copticum aqueous seed extract-derived AgNPs UV–vis spectroscopy at pH values of 6, 7, 8, and 9. (b) EDX spectrum of phytofabricated AgNPs confirming a distinct signal from silver (Ag) at 3.0 keV.
Figure 5a shows a Z-average size of 119.7 nm with a PdI of 0.56 for the colloidal AgNPs at a pH value of 9. PdI is an indicator in the range of 0.01 to 1 estimating the average uniformity of a colloidal system. The PdI values of less than 0.7 indicate uniform particle size samples and the PdI values of above 0.7 indicate a broad particle size distribution [31]. Besides, Figure 5b shows a zeta potential of −15 mV for phytofabricated silver particles. Colloidal samples with a zeta potential of positive than 30 mV or negative than −30 mV are considered electrostatically stable owing to the large repulsive forces between the particles that prevent particle aggregation [32]. In the current study, the phytofabricated AgNPs were stable due to a combined electrostatic and steric stabilization. Steric stabilization can be defined as the adsorption of large molecules, such as polymers or surfactants on the surface of NPs to avoid aggregation [33]. In the current study, the phytocompounds that exist in the seed extract play the dual role of reducing and capping agents. Hence, steric stabilization refers to the conjugated phytochemicals on the surface of AgNPs. In addition, the FE-SEM image confirmed the fabrication of spherical and well-dispersed AgNPs (Figure 5c). Moreover, Figure 5d shows the FT-IR spectrum of AgNPs with absorption bands at 3,324.79, 2,929.69, 11,595.30, 1,394.02, 1,073.47, and 670.92 cm−1 in the region of 450–4,000 cm−1 that may be assigned to the stretching vibrations of O–H, C–H, N–H, O–H, C–O, and C═C, respectively. Besides, Figure 5e shows the FT-IR spectrum of T. copticum aqueous seed extract with absorption bands at 3,302.42, 2,929.69, 2,855.14, 1,707.12, 1,587.85, 1,513.30, 1,416.39, 1,259.84, 1,155.47, 1,036.00, 805.10, and 663.47 cm−1 in the region of 450–4,000 cm−1 that may be assigned to the stretching vibrations of O–H, C–H, C–H, C═O, N–H, N–O, O–H, C–O, C–O, C–O, C–H, and C═C, respectively. The FTIR analysis confirmed the presence of phytocompound surface functional groups responsible for the bioreduction and stability of AgNPs. This finding was in agreement with the previous studies that stated the role of phytocompounds as reducing and capping agents [26,27,28]. However, future studies are required to explore the exact phytocompounds that surround the surface of AgNPs.
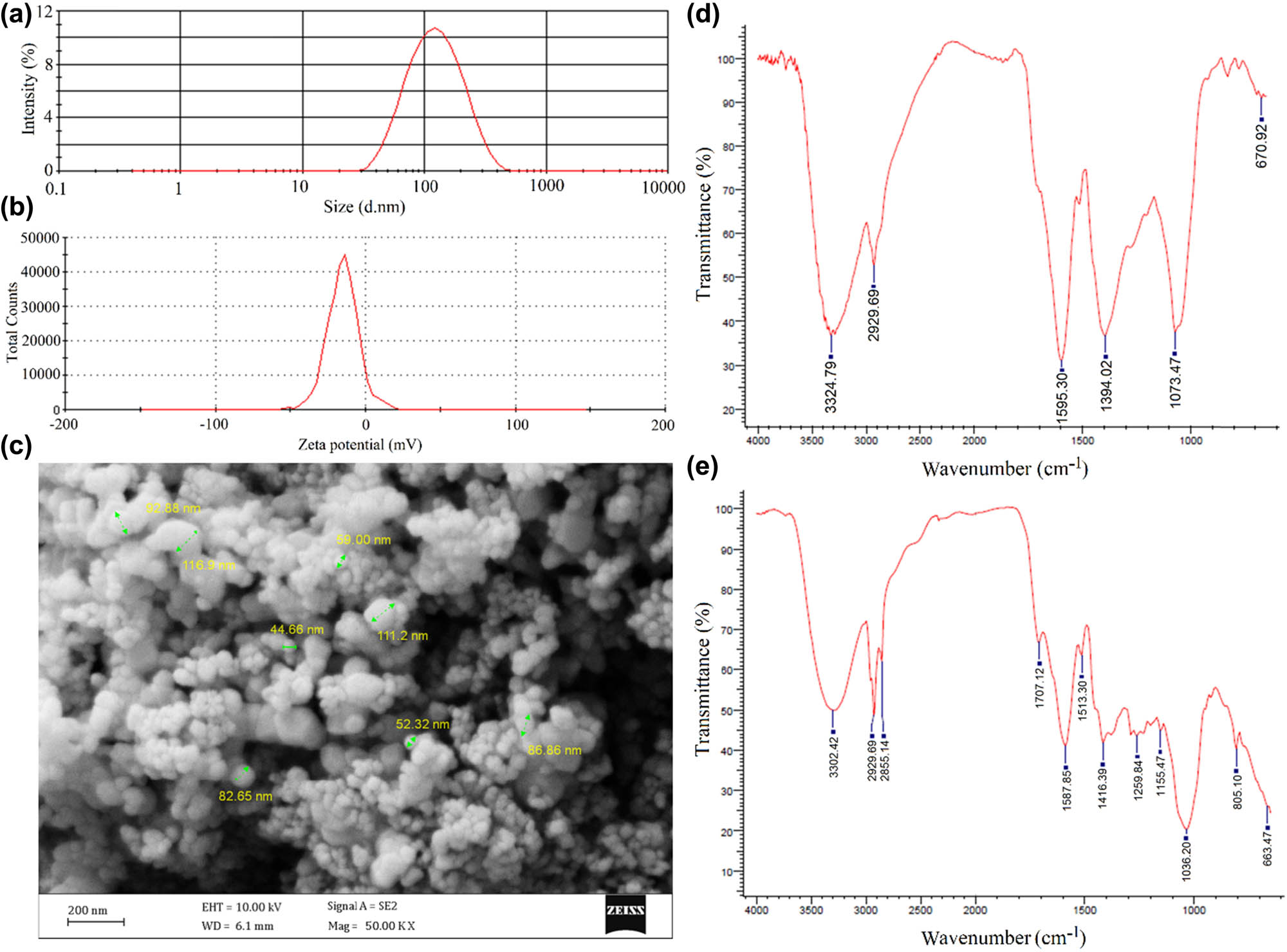
(a) Hydrodynamic size distribution of AgNPs with an average diameter of 119.7 nm, and PdI of 0.56 at a pH value of 9. (b) Zeta potential of −15 mV for colloidal AgNPs at a pH value of 9; (c) FE-SEM image of spherical shaped AgNPs. (d) FT-IR spectrum of AgNPs representing the presence of AgNPs surface surrounding functional groups. (e) FT-IR spectrum of T. copticum aqueous seed extract.
3.2 Antibacterial properties of AgNPs
3.2.1 Well diffusion assay
Figure 6 shows the results of the well diffusion assay of AgNPs, aqueous silver nitrate solution, and T. copticum aqueous seed extract against all tested bacteria. The inhibition zone (IZ) of 50 and 100 µL of AgNPs with a concentration of 1 mg·mL−1 and 100 µL of 1 mM AgNO3 solution was found to be 23 ± 1, 23.33 ± 0.58, and 18.33 ± 0.58 mm, respectively against S. aureus (ATCC 25923). Notably, no IZ was found for 100 µL of 0.1% aqueous seed extract of T. copticum against S. aureus. Besides, the IZ of 50 and 100 µL of 1 mg·mL−1 AgNPs and 100 µL of 1 mM AgNO3 solution was found to be 24.33 ± 0.58, 24.33 ± 0.58, and 15 ± 1 mm, respectively, against S. pyogenes (ATCC 19615). Notably, no IZ was found for 100 µL of 0.1% aqueous seed extract of T. copticum against S. pyogenes. In addition, the IZ of 50 and 100 µL of 1 mg·mL−1 AgNPs and 100 µL of 1 mM AgNO3 solution was found to be 17 ± 1, 16.33 ± 1.15, and 15.67 ± 1.53 mm, respectively against E. coli (ATCC 25922). Besides, no IZ was found for 100 µL of 0.1% aqueous seed extract of T. copticum against E. coli. Moreover, the IZ of 50 and 100 µL of 1 mg·mL−1 AgNPs and 100 µL of 1 mM AgNO3 solution was found to be 14.67 ± 0.58, 14.33 ± 0.58, and 10.67 ± 1.53 mm, respectively against K. pneumoniae (ATCC 10031). Notably, no IZ was found for 100 µL of 0.1% aqueous seed extract of T. copticum against K. pneumoniae. Notably, no significant difference was found between the IZ of 50 and 100 µL of 1 mg·mL−1 AgNPs in all four tested bacteria (P > 0.05). Besides, the difference between the IZ of 50 and 100 µL of 1 mg·mL−1 AgNPs and 100 µL of 1 mM AgNO3 solution was significant in S. aureus, S. pyogenes, and K. pneumoniae (P < 0.05). However, no significant difference was found between the IZ of 50 and 100 µL of 1 mg·mL−1 AgNPs and 100 µL of 1 mM AgNO3 solution in E. coli (P > 0.05).

Well diffusion assay of 100 µL aqueous AgNO3 solution (0.5 mM), 100 µL aqueous seed extract of T. copticum (0.1%), 50 µL AgNPs (1 mg·mL−1), and 100 µL AgNPs (1 mg·mL−1) against S. aureus, S. pyogenes, E. coli, and K. pneumoniae. Data were presented as mean ± standard deviation (SD). The difference between the groups with no common letter was deemed statistically significant at a 95% confidence level (P < 0.05).
In agreement with our findings, Kelkawi et al. biosynthesized AgNPs using 1 mL of Mentha pulegium aqueous extract. It was revealed that the biological NPs were anisotropic morphologically and their diameter was recorded as 5–50 nm. The antibacterial activity of biogenic NPs was also assessed against S. aureus, S. pyogenes, and E. coli with mean IZ of 9.65 ± 0.64, 10.63 ± 0.67, and 10.20 ± 1.20 mm, respectively. The suggested mechanisms regarding the antibacterial activity of the AgNPs were a surge in permeability and DNA damage as a result of disturbance of the bacterial cell membrane and over generation of ROS. Notably, it was indicated that the existence of a high concentration of biological extract encapsulating the particles could inhibit the proper release of AgNPs. Therefore, AgNPs biosynthesized using low concentrations of M. pulegium were found to be more efficient antibacterial agents [27]. Similarly, Rawat and coworkers reported that the Polygonatum graminifolium-mediated fabricated AgNPs with spherical shape and size between 3 and 15 nm could inhibit the growth of S. aureus and E. coli with IZ of 27 and 16 mm, respectively. Further, the scanning electron microscope (SEM) results exhibited irregular shapes and significant damage to the cell wall in AgNP-treated bacterial cells compared to the control confirming the probable attribution of antibacterial potential to the release of AgNPs through the disrupted bacterial cell membrane [28]. Likewise, Csakvari et al. evaluated the antibacterial potential of the AgNPs, biosynthesized using leaf extract of Cannabis sativa, against gram-positive and negative bacteria. It was reported that sphere-shaped AgNPs with a diameter of <69 nm exhibited the IZ of 10–14 mm for K. pneumonia, 12–13 mm for S. aureus, and 10–12 mm for E. coli. It was hypnotized that due to the thicker layer of peptidoglycan in gram-positive bacteria, the reduction of Ag ions to AgNPs was more effective in gram-negative bacteria. Further, the authors suggested that the mechanisms underlying the antibacterial activity of the NPs were similar disruption of the cell membrane in bacteria, affecting the enzymes in the respiratory chain, and their destructive influence on the bacterial genetic material [34]. The strong inhibitory effect of Abelmoschus esculentus-derived AgNPs against bacterial growth was also confirmed by Devanesan and colleagues using the agar well diffusion method. It was indicated that spherical AgNPs with an average diameter of 16.19 nm and at a dose of 100 µL were able to form IZ of 14 ± 0.5 mm for K. pneumonia, 13.0 ± 1.2 mm for S. aureus and S. pyogenes, and 11.0 ± 1.0 mm for E. coli. It was suggested that Ag ions released after attachment of AgNPs to the bacterial cell wall could lead to the reaction with carboxyl, thiol, and phosphate groups of the amino acids causing the destruction of cell wall and intracellular organelles. Further, production of free radicals, especially ROS by biosynthesized AgNPs, could induce intracellular toxicity and eventually cell death [35]. According to Garibo et al., 5 nm-sized AgNPs biosynthesized utilizing Lysiloma acapulcensis extract could actively combat strains of different pathogenic bacteria. The results of this study revealed that the spherical and quasi-spherical AgNPs were effective against S. aureus, P. aeruginosa, and E. coli with IZ of 16.0 ± 1.0, 15.0 ± 0.5, and 18.0 ± 1.3 mm, respectively. The authors hypothesized that the bacteriostatic effect of the biofabricated AgNPs was attributed to damages induced to the cell membrane and the inability of bacterial DNA to replicate [36]. Moreover, Golabiazar et al. examined the efficacy of the phytofabricated AgNPs against four different strains of bacteria. The mentioned NPs were found to be cubic morphological and their average size was reported as 17–18 nm. The results exhibited that the growth of S. aureus and S. pyogenes as gram-positive bacteria and E. coli and K. pneumonia as gram-negative bacteria were hindered by the AgNPs with IZ ranging from 0.6 to 3 cm. Attachment of the biological AgNPs to the cell membrane of bacteria followed by undermining the membrane function and increased permeability was regarded as the probable role of AgNPs as antibacterial agents [37]. In a similar study, Mariadoss and coworkers reported the herbal-mediated biosynthesis of AgNPs using Malus domestica with a nearly spherical structure and an average size of 50–107.3 nm. These green AgNPs at the concentration of 500 μg·mL−1 revealed IZ of 23 and 8 mm against E. coli and K. pneumonia, respectively [38]. Rautela et al. assessed the antimicrobial activity of the biogenic AgNPs against pathogenic bacteria as well. The seeds extract of Tectona grandis was employed to form AgNPs mostly spherical with a diameter of less than 100 nm confirmed by SEM analyses. It was indicated that strains of S. aureus (17 mm) and E. coli (20 mm) treated with the AgNPs exhibited IZs in the plates. The mechanism by which the NPs hindered the bacterial growth was thought to be the leakage of sugar and proteins from the damaged bacterial membrane resulting in a decrease in the bacterial function [39]. Alternatively, Mahmoodi Esfanddarani and coworkers biofabricated AgNPs by methanol extract of Malva sylvestris and indicated their spherical and hexagonal shape along with a size of between 20 and 40 nm according to TEM results. The acceptable inhibitory effect of the biologically formed AgNPs was recorded against E. coli and S. aureus with IZ of 10.2 ± 0.5 and 10.4 ± 0.5 mm, respectively [40]. In addition, the antibacterial activity of the A. esculentus-derived AgNPs was measured against different bacterial strains. It was reported that the phytofabricated AgNPs were spherical in shape with an average diameter of 20–30 nm and the IZ diameters obtained were 16.27 ± 0.51 mm corresponding to E. coli and 12.47 ± 0.31 mm corresponding to S. aureus. The authors considered oxidative stress caused by over generation of ROS responsible for the antibacterial effect. The disruption of bacterial membrane, DNA, lipids, and proteins was believed to be the main mechanism underlying this process [41]. Moreover, Lateef et al. introduced a similar green route for the production of AgNPs utilizing the extract of cocoa pod husk. The size distribution of the AgNPs was found to be between 4 and 32 nm. It was confirmed that cocoa pod husk-mediated AgNPs possessed a significant antibacterial effect with IZ of 10–14 mm against E. coli and K. pneumonia [42]. Further, Ajah et al. used Haemophilus influenza for the bioreduction of Ag ions and biosynthesized AgNPs with predominantly spherical morphology and an average diameter of 80.05–101.15 nm. The antibacterial effect of the NPs was studied against Streptococcus spp., Klebsiella spp., S. aureus, and E. coli, and the results demonstrated that the environmentally safe AgNPs could form IZ of 15, 24, 31, and 18 mm, correspondingly when the brain heart infusion broth incubated in light used. Although the exact mechanism of inhibition of bacterial growth is yet to be fully addressed, the authors suggested that production of free radicals and ROS, an increase in penetration by binding AgNPs to the external proteins and forming pores, and disruption of DNA replication could play a role in the death of the bacteria [43]. Alternatively, Baghayeri and coworkers reported another approach for the biological manufacturing of AgNPs utilizing an aqueous extract of Salvia leriifolia. The diameter recorded for the NPs was 27 nm on average and they were mostly spherical morphologically. In this study, the antibacterial activity of the NPs was confirmed through well diffusion assay and it was reported that the green AgNPs were able to inhibit the growth of K. pneumonia, Staphylococcus coagulase, S. pneumonia, and E. coli with IZ of 16.33 ± 0.9, 18.67 ± 0.4, 20 ± 0.0, and 14.0 ± 0.7 mm, respectively. The possible antibacterial mechanisms included cellular leakage as a result of more permeability, interference with the proper function of enzymes, proteins, or DNA, and formation of a toxic environment by the generation of free radicals [44]. In a similar experiment, Samuggam et al. reported the biological synthesis of AgNPs with a mean particle size of 17 nm and spherical shape using the extract of Spondias mombin. The AgNPs were found to be suitable antibacterial tools forming IZ with diameters of 20.65 ± 0.35, 23.65 ± 0.35, 22, and 20.65 ± 0.35 mm in the plates consisting of strains of Staphylococcus haemolyticus, Staphylococcus epidermidis, S. aureus, and S. pyogens, respectively. In the case of gram-negative bacteria, the zones of 21 mm corresponding to E. coli and 0.33 mm corresponding to K. pneumonia were observed. The authors hypothesized that ROS formed by the AgNPs were responsible for the conversions in the structure of the bacterial cells leading to inactivation of the bacteria [45]. In a study, Alzubaidi et al. prepared AgNPs using ethanolic extract of flaxseed and reported the structure of the NPs as spherical and their diameter as 46.98 ± 12.45 nm. This study confirmed that the NPs could actively inhibit the growth of four different bacterial species in an environmentally sound manner. The IZ of 11.0 ± 0.42, 10.8 ± 1.02, 8.9 ± 1.08, and 8.7 ± 1.04 mm was observed in the plates for E. coli, K. pneumonia, S. aureus, and S. pyogenes, respectively. Although there was no reliable evidence for any exact mechanism regarding the antibacterial effect of AgNPs, better penetration of NPs into the bacterial cells because of their nano-sized diameter and production of oxidative stress damaging the bacterial function were considered the probable mechanisms [46]. Likewise, Alkhulaifi et al. reported the biosynthesis of AgNPs with spherical shape and an average diameter of 59.74 nm using peels of Citrus limon. These NPs exhibited notable antibacterial activity against E. coli and S. aureus with IZ of both 35 mm [47]. In another study, Citradewi et al. employed an environment-friendly process to prepare the AgNPs and examine their toxic effect on pathogenic bacteria. Cockle shell was utilized to produce AgNPs from Ag ions with irregular spherical morphology and a diameter ranging from 4 to 12 nm. The bactericidal activity of the biogenic NPs was assessed against S. aureus, S. pyogenes, E. coli, and K. pneumonia and it was concluded that the AgNPs were more effective against gram-negative bacteria (i.e., E. coli and K. pneumonia) with wider IZs comparing to gram-positive bacteria naming S. aureus and S. pyogenes [48].
3.2.2 Broth microdilution assay
3.2.2.1 MIC and MBC assessment
As shown in Figure 7a, the MIC values of the AgNPs were found to be 0.25, 1, 0.5, and 0.5 µg·mL−1 against S. aureus, S. pyogenes, E. coli, and K. pneumoniae, respectively. Besides, the MIC values of the standard antibiotic (tetracycline) were found to be 0.25, 0.25, 2, and 0.5 µg·mL−1 against S. aureus, S. pyogenes, E. coli, and K. pneumoniae, respectively. According to the MIC values, E. coli was more sensitive to AgNPs than tetracycline. In contrast, S. pyogenes was more sensitive to tetracycline than AgNPs. The MBC values also confirmed these findings. As shown in Figure 7b, the MBC values of the AgNPs were found to be 8, 16, 4, and 0.5 µg·mL−1 against S. aureus, S. pyogenes, E. coli, and K. pneumoniae, respectively. Besides, the MBC values of the standard antibiotic (tetracycline) were found to be 0.5, 0.5, 8, and 8 µg·mL−1 against S. aureus, S. pyogenes, E. coli, and K. pneumoniae, respectively. As shown in Figure 8, several mechanisms were proposed for the antibacterial activity of AgNPs in the literature. In a study, Barabadi et al. reported the preparation of AgNPs using Zataria multiflora as the biofactory. The NPs were spherical and their hydrodynamic size was recorded as 25.5 nm. The antibacterial activity of biosynthesized AgNPs was measured against S. aureus with MIC of 4 µg·mL−1 compared to commercial AgNPs with MIC of 8 µg·mL−1. The probable mechanisms were found to be the bactericidal influence of Ag ions released after penetration of AgNPs and disturbance of the respiratory chain and bacterial cell wall [26]. Likewise, de Aragão et al. assessed the antibacterial effect of biogenic AgNPs. The polysaccharides of the seaweed Gracilaria birdiae were employed as stabilizing and reducing agents for the formation of spherical AgNPs with a diameter ranging from 20.2 to 94.9 nm. It was exhibited that at the polysaccharide concentration of 0.05% (pH 11), the AgNPs inhibited the growth of S. aureus and E. coli with MIC of 81.2 and 40.6, respectively [49]. Eren and Baran also evaluated the ability of biologically synthesized AgNPs to tackle bacterial growth. It was reported that using leaves of maize (Zea mays L.) could reduce Ag ions to stable AgNPs with a mean size of 12.63 nm and spherical structure. The results regarding the antibacterial potential of the biogenic AgNPs revealed the MIC of 0.337 µg·mL−1 for S. aureus and 0.084 µg·mL−1 for E. coli [50]. Alternatively, Biswas and coworkers conducted research regarding the role of biosynthesized AgNPs as antibacterial agents. It was exhibited that the Solanum viarum-derived AgNPs were mainly spherical and oval morphologically and their particle diameter was between 2 and 40 nm. The green AgNPs were effective against E. coli with MIC of 0.41 ± 0.00 mM, S. aureus susp. aureus with MIC of 0.13 ± 0.01 mM, Streptococcus pneumoniae and K. pneumonia with MIC of more than 1 mM [51]. Similarly, Das Mahapatra et al. investigated the potential of biologically manufactured AgNPs for confronting some pathogenic bacteria. According to this study Oxalis corniculata aqueous extract was able to reduce Ag ions to AgNPs with spherical shape and an average diameter of 40 nm. These ecofriendly NPs at the concentration of 40 µg·mL−1 were tested against S. pyogenes, E. coli, K. pneumonia, and S. aureus with MIC of 0.84, 0.72, 0.78, and 0.76 mM, respectively [52]. Furthermore, in a study AgNPs were biofabricated using Murraya koenigii (L.) with spheroidal morphology and a size ranging from 5 to 20 nm. These biosynthesized AgNPs effectively hindered the growth of extended-spectrum β-lactamase-producing E.coli, control strain of E. coli, methicillin-resistant S. aureus, and methicillin-sensitive S. aureus with MIC of 64, 16, 32, and 32 μg·mL−1, respectively. The possible mechanism by which the green AgNPs tackled the bacterial strains was the release of Ag ions into the bacterial cells followed by disturbance of cell division and respiration function by NPs leading to the destruction of the microorganism [53]. Likewise, Soliman and colleagues used Rhodotorula sp. strain ATL72 as the green source for the synthesis of AgNPs biologically. The results revealed that the size range of the NPs was 8.8–21.4 nm and they were oval and spherical shaped according to TEM results. The efficacy of the biogenic AgNPs was evaluated against strains of E. coli and it was reported that the growth of E. coli was completely inhibited by the AgNPs at the concentration of 1 µg·mL−1. In the case of the mechanism underlying the antibacterial activity, it was thought that the interaction of AgNPs with the thiol groups present in the proteins could cause the unfolding and degradation of the proteins and eventually malfunctioning of different bacterial vital processes, especially translation [54]. Similarly, Altinsoy et al. proposed a green method for the production of AgNPs using E. coli, Saccharomyces cerevisiae, and Bacillus subtilis as reducing agents. The results of characteristic analyses revealed that the size of the NPs ranged from 126 to 323 nm and they possessed various structures. Further, different bacterial strains including S. pneumonia and K. pneumonia were tested and AgNPs were found significantly effective at the concentration of 500 µg·mL−1 by 85% against the mentioned bacteria according to MIC results [55]. Hemmati and colleagues as well examined the antibacterial potential of green-synthesized AgNPs. In this study, they used the aqueous flower extract of Fritillaria for the formation of AgNPs with an average size of 10 nm and mostly spherical morphology. MIC tests exhibited that AgNPs at the concentration of 4, 2, and 1 µg·mL−1 affected the bacterial strains of Staphylococcus saprophyticus, S. pyogenes/S. pneumonia, and K. pneumonia/ E. coli/S. epidermidis/S. aureus, respectively [56]. Accordingly, Mohanta and coworkers biosynthesized AgNPs using three different biofactories including Bridelia retusa, Glochidion lanceolarium, and Semecarpus anacardium. The NPs were characterized as spherical in a size ranging from 52 to 96 nm according to SEM analyses. The toxic effect of biogenic NPs was evaluated against S. aureus and E. coli and in the case of B. retusa MIC values of 64.13 ± 0.3 and 43.94 ± 0.2 µg·mL−1 were recorded, respectively. The AgNPs biosynthesized by S. anacardium inhibited the growth of against S. aureus (33.77 ± 0.2 µg·mL−1) and E. coli (23.49 ± 0.2 µg·mL−1) effectively as well. Further, the MIC values of 43.94 ± 0.2 µg·mL−1 corresponding to S. aureus and 44.02 ± 0.3 µg·mL−1 corresponding to E. coli were recorded for the G. lanceolarium-mediated synthesis of AgNPs. The suggestion regarding the antibacterial activity of the AgNPs was similarly damaging the cell membrane and oxidizing the proteins of plasma membrane. However, it was possible that NPs could block the pores on the membrane leading to a decrease in cell respiration [21]. Similarly, Muthulakshmi and coworkers reported an ecofriendly approach for the preparation of AgNPs and assessed their antibacterial potential against different microorganisms including S. aureus, S. pyogenes, Streptococcus mutans, E. coli, and K. pneumonia. The culture filtrate from Enterobacter cloacae was the biological source of the synthesis to form the AgNPs with a size range of 12–30 nm and spherical structure. MIC values of 6.3, 3.1, 12.5, 3.1, and 6.3 µg·mL−1 were obtained for S. aureus, S. pyogenes, K. pneumonia, S. mutans, and E. coli, respectively. It was suggested that the attachment of Ag ions to DNA and disruption of replication could be responsible for the antibacterial effect of the NPs [57]. Likewise, AgNPs were produced utilizing Wild Ginger (Zingiber zerumbet) extract, and their toxic effect on different bacterial strains was tested. These NPs were spherical according to SEM results and most of the NPs were in a diameter of 153.2 nm. The antibacterial assessments revealed that AgNPs biosynthesized by the aqueous extract of Z. zerumbet at the concentration of 50 µg·mL−1 could tackle S. aureus and S. mutans with MIC values of both 25 µg·mL−1 [58]. Alternatively, Youssef et al. reported the green fabrication of AgNPs using honey as the reducing factor. The biologically formed AgNPs were found to be sphere-shaped and their diameter was between 25 and 70 nm. In this study, the role of the NPs as alternative antibacterial agents was assessed and it was exhibited that E. coli with MIC of 6.3 µg·mL−1 was highly sensitive to the biogenic AgNPs compared to K. pneumonia strains with MIC of 12.5 µg·mL−1. the authors suggested that the formation of reactive Ag ions and the massive surface area could attributed to the antibacterial activity of the NPs [59]. Wypij et al. also focused on the biological synthesis of AgNPs using Streptomyces xinghaiensis OF1 strain as the biofactory. According to TEM analyses the NPs were spherical in shape and 5–20 nm in size. This study confirmed that the green synthesized AgNPs could inhibit the growth of different microorganisms including S. aureus with MIC of 256 µg·mL−1 and E. coli with MIC of 64 µg·mL−1 [60].

(a) MIC and (b) MBC assessment of AgNPs compared to tetracycline as standard antibiotic against S. aureus, S. pyogenes, E. coli, and K. pneumoniae.
![Figure 8
Proposed antibacterial mechanisms of colloidal silver particles [The Figure was developed in part using Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).].](/document/doi/10.1515/gps-2023-0242/asset/graphic/j_gps-2023-0242_fig_008.jpg)
Proposed antibacterial mechanisms of colloidal silver particles [The Figure was developed in part using Servier Medical Art, which is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).].
3.2.3 ROS assay
The oxidative-associated antibacterial mechanism of AgNPs was measured by evaluating the ROS level of bacterial supernatant after one-night incubation with AgNPs at the concentration of MIC and 20*MIC in comparison to standard antibiotic (tetracycline) with the same concentrations. The supernatant of nontreated bacteria with the same conditions was considered as negative control. As shown in Figure 9, the results showed a significant dose-dependent increase in the ROS concentration of AgNP-treated samples with the concentrations of MIC and 20*MIC against S. aureus, E. coli, and K. pneumoniae (P < 0.05). However, no significant difference was found between the ROS concentration of AgNP-treated samples with the concentrations of MIC and 20*MIC against S. pyogenes. In addition, a significant dose-dependent increase was found in the ROS concentration of tetracycline-treated samples with the concentrations of MIC and 20*MIC against S. aureus, S. pyogenes, and K. pneumoniae (P < 0.05). However, no significant difference was found between the ROS concentration of tetracycline-treated samples with the concentrations of MIC and 20*MIC against E. coli. Overall, both AgNPs and tetracycline enhanced the generation of ROS significantly in all studied bacteria compared to the control (P < 0.05) suggesting an oxidative-associated antibacterial mechanism. According to the literature, the intracellular oxidative stress causes damages to the bacterial cell wall and intracellular moieties containing DNA, proteins, and lipids leading to cell death [61]. In a similar study, the colloidal silver particles were phytosynthesized by employing leaf extract from Ocimum gratissimum with a hydrodynamic particle size of 31 nm and a zeta potential of −15 mV. The O. gratissimum-derived AgNPs showed significant antibacterial activity against resistant strains of E. coli and S. aureus and enhanced their intracellular ROS generation by 85.68% and 89.36%, respectively. The authors reported that the AgNP-induced oxidative damage is one of the main antibacterial mechanisms of AgNPs [62]. Likewise, the phytofabricated spherical-shaped AgNPs by using the aqueous extract of aerial parts of Astragalus spinosus in the range of 30–40 nm showed significant antibacterial activity against Actinomyces viscosus and Streptococcus mutans. The ROS assay revealed that the green-synthesized AgNPs increased the ROS levels in both tested bacteria compared to the control group [63]. Alternatively, plant-mediated synthesis of uniformly spherical-shaped AgNPs was reported by using the leaf extract of Allophylus cobbe with an average hydrodynamic particle size of 5 ± 4 nm. The phytosynthesized AgNPs showed a great bactericidal performance and increased the intracellular overgeneration of ROS alone and in combination with standard antibiotics in both tested gram-negative and gram-positive bacteria [64].

ROS assessment of AgNPs compared to tetracycline as a standard antibiotic at the concentrations of MIC and 20*MIC against S. aureus, S. pyogenes, E. coli, and K. pneumoniae. Data was presented as mean ± standard deviation (SD). The difference between the groups with no common letter was deemed statistically significant at a 95% confidence level (P < 0.05).
3.2.4 Flow cytometry-based quantitative assessment of cell viability
Flow cytometric analysis represented a dose-dependent bacterial cell death in the AgNP-treated samples against S. aureus (Figure 10a), S. pyogenes (Figure 10b), E. coli (Figure 10c), and K. pneumoniae (Figure 10d). As shown in Figure 10a, bacterial cell death of 0.985% (a), 20.3% (b), 47.1% (c), and 55.4% (d) was found in the control group (nontreated bacterial sample) and in the AgNP-treated cells at the concentrations of MIC (0.25 µg·mL−1), 10*MIC (2.5 µg·mL−1), and 20*MIC (5 µg·mL−1), respectively, against S. aureus. Besides, as shown in Figure 10b, bacterial cell death of 1.07% (a), 17.4% (b), 39.3% (c), and 52.8% (d) was found in the control group (nontreated bacterial sample) and the AgNP-treated cells at the concentrations of MIC (1 µg·mL−1), 10*MIC (10 µg·mL−1), and 20*MIC (20 µg·mL−1), respectively, against S. pyogenes. Moreover, as shown in Figure 10c, bacterial cell death of 0.813% (a), 16.1% (b), 47.1% (c), and 64.6% (d) was found in the control group (nontreated bacterial sample) and the AgNP-treated cells at the concentrations of MIC (0.5 µg·mL−1), 10*MIC (5 µg·mL−1), and 20*MIC (10 µg·mL−1), respectively, against E. coli. Furthermore, as shown in Figure 10d, bacterial cell death of 0.453% (a), 17% (b), 38.5% (c), and 61.9% (d) was found in the control group (nontreated bacterial sample), and the AgNP-treated cells at the concentrations of MIC (0.5 µg·mL−1), 10*MIC (5 µg·mL−1), and 20*MIC (10 µg·mL−1), respectively, against K. pneumoniae. In a similar study, the plant-mediated synthesized AgNPs from Aloe arborescens with an average size of 38 ± 2 nm showed significant antibacterial activity against Pseudomonas aeruginosa and S. aureus with MIC values of 8 and 12 µg·mL−1, respectively. Then, the flow cytometry analysis was performed to compare the percentage of dead cells in AgNP-treated samples at the concentration of MIC. The findings showed 66.8% and 25.5% cell death in AgNP-treated P. aeruginosa and S. aureus at the concentration of 8 and 12 µg·mL−1 over 12 h incubation at 37°C, respectively [65]. Alternatively, flow cytometry analysis was conducted to evaluate the cell viability of the phytofabricated AgNP-treated E. coli and S. aureus. The findings showed 89.24% and 93.62% cell death against AgNP-treated E. coli and S. aureus at the concentration of 4 and 8 µg·mL−1 over 12 h incubation at 37°C, respectively [62]. Similarly, flow cytometry analysis was conducted to evaluate the cell viability of the phytofabricated AgNP-treated P. aeruginosa. The flow cytometric susceptibility assay showed 94.68% cell death against AgNP-treated P. aeruginosa at the concentration of 32 µg·mL−1 over 12 h incubation at 37°C [66].

Flow cytometry-based quantitative assessment to compare cell viability in the bacterial samples that were treated with AgNPs against ((a) S. aureus, (b) S. pyogenes, (c) E. coli, and (d) K. pneumoniae. (a) Nontreated sample (control), (b) AgNP-treated sample at MIC concentration, (c) AgNP-treated sample at 10*MIC concentration, and (d) AgNP-treated sample at 20*MIC concentration).
Significantly, flow cytometric analysis represented a dose-dependent bacterial cell death in the tetracycline-treated samples against S. aureus (Figure 11a), S. pyogenes (Figure 11b), E. coli (Figure 11c), and K. pneumoniae (Figure 11d). As shown in Figure 11a, bacterial cell death of 0.985% (a), 18% (b), 41.2% (c), and 51.6% (d) was found in the control group (nontreated bacterial sample) and the tetracycline-treated cells at the concentrations of MIC (0.25 µg·mL−1), 10*MIC (2.5 µg·mL−1), and 20*MIC (5 µg·mL−1), respectively, against S. aureus. Besides, as shown in Figure 11b, bacterial cell death of 1.07% (a), 11.9% (b), 29% (c), and 46.5% (d) was found in the control group (nontreated bacterial sample) and the tetracycline-treated cells at the concentrations of MIC (0.25 µg·mL−1), 10*MIC (2.5 µg·mL−1), and 20*MIC (5 µg·mL−1), respectively against S. pyogenes. Moreover, as shown in Figure 11c, bacterial cell death of 0.813% (a), 12.7% (b), 38% (c), and 58.8% (d) was found in the control group (nontreated bacterial sample) and the tetracycline-treated cells at the concentrations of MIC (2 µg·mL−1), 10*MIC (20 µg·mL−1), and 20*MIC (40 µg·mL−1), respectively, against E. coli. Furthermore, as shown in Figure 11d, bacterial cell death of 0.453% (a), 13.1% (b), 30.6% (c), and 57.6% (d) was found in the control group (nontreated bacterial sample) and the tetracycline-treated cells at the concentrations of MIC (0.5 µg·mL−1), 10*MIC (5 µg·mL−1), and 20*MIC (10 µg·mL−1), respectively, against K. pneumoniae.

Flow cytometry-based quantitative assessment to compare cell viability in the bacterial samples that were treated with tetracycline against ((a) S. aureus, (b) S. pyogenes, (c) E. coli, and (d) K. pneumoniae. (a) Nontreated sample (control), (b) tetracycline-treated sample at MIC concentration, (c) tetracycline-treated sample at 10*MIC concentration, and (d) tetracycline-treated sample at 20*MIC concentration).
4 Conclusion
The current investigation springs a new approach for the fabrication of AgNPs by employing the aqueous seed extract of T. copticum which is found to be a potent antibacterial agent against both gram-positive and gram-negative bacteria that can be a significant achievement to combat microbial infections. The analytical characterizing techniques (UV–vis, DLS, FE-SEM, EDX, and FT-IR) represented the phytofabrication of colloidal silver particles with a hydrodynamic size of 119.7 nm and spherical morphology. Interestingly, our findings showed a dose-dependent cell death in all AgNP-treated bacteria based on the data obtained from flow cytometry analysis. More interestingly, we found an oxidative-associated antibacterial mechanism of AgNPs owing to the significant overgeneration of ROS in all AgNP-treated bacteria. Overall, the T. copticum aqueous seed extract-derived AgNPs could be further exploited as a considerable candidate to combat microbial infections. However, further investigations are required to evaluate the in vivo performance of the biogenic AgNPs. Furthermore, the synergetic interaction of these nanostructures with standard commercial antibiotic drugs should be investigated in future studies.
-
Funding information: This work was supported by a grant from Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant Number 43007299).
-
Author contributions: Hamed Barabadi: project administration, conceptualization, supervision, formal analysis, methodology, validation, writing – original draft, writing – review and editing, visualization; Faraz Mojab: conceptualization, formal analysis, methodology; writing – original draft, writing – review and editing; Fatemeh Ashouri: formal analysis, methodology; writing – original draft, writing – review and editing; Kamyar Jounaki: formal analysis, methodology, writing – review and editing; Reza Jahani: formal analysis, methodology, writing – review and editing; Ali Ramezani: formal analysis, methodology, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Sathishkumar G, Gobinath C, Karpagam K, Hemamalini V, Premkumar K, Sivaramakrishnan S. Phyto-synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf B. 2012;95:235–40. 10.1016/j.colsurfb.2012.03.001.Suche in Google Scholar PubMed
[2] Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–56. 10.1016/j.biotechadv.2013.01.003.Suche in Google Scholar PubMed
[3] Dauthal P, Mukhopadhyay M. Biosynthesis of palladium nanoparticles using Delonix regia leaf extract and its catalytic activity for nitro-aromatics hydrogenation. Ind Eng Chem Res. 2013;52:18131–9. 10.1021/ie403410z.Suche in Google Scholar
[4] Abomuti MA, Danish EY, Firoz A, Hasan N, Malik MA. Green synthesis of zinc oxide nanoparticles using Salvia officinalis leaf extract and their photocatalytic and antifungal activities. Biology. 2021;10(11):1075. 10.3390/biology10111075.Suche in Google Scholar PubMed PubMed Central
[5] Beyene HD, Werkneh AA, Bezabh HK, Ambaye TG. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain Mater Technol. 2017;13:18–23. 10.1016/j.susmat.2017.08.001.Suche in Google Scholar
[6] Ohtaki M, Toshima N. Photoreduction of rhodium(iii) ions in water with ultraviolet light aiming to prepare the dispersions of ultrafine particles. Chem Lett. 1990;19(4):489–92. org/10.1246/cl.1990.489.Suche in Google Scholar
[7] Kumar V, Yadav SK. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol. 2009;84(2):151–7. 10.1002/jctb.2023.Suche in Google Scholar
[8] Malik P, Shankar R, Malik V, Sharma N, Mukherjee TK. Green chemistry based benign routes for nanoparticle synthesis. J Nanopart. 2014;2014:302429. 10.1155/2014/302429.Suche in Google Scholar
[9] Modarres M, Taghavizadeh Yazdi ME. Elicitation improves phenolic acid content and antioxidant enzymes activity in Salvia leriifolia cell cultures. Iran J Sci Technol Trans A Sci. 2021;45:849–55. 10.1007/s40995-021-01070-y.Suche in Google Scholar
[10] Azimi M, Zahedi MJ, Mehrabani M, Tajadini H, Zolala F, Baneshi MR, et al. Effect of Apium graveolens and Trachyspermum copticom on clinical symptoms of patients with functional dyspepsia. Avicenna J Phytomed. 2017;7(6):554–64.10.31661/gmj.v6i2.772Suche in Google Scholar
[11] Kardan Yamchi J, Mahboubi M, Kazemian H, Hamzelou G, Mehdi F. The chemical composition and anti-mycobacterial activities of Trachyspermum copticum and Pelargonium graveolens essential oils. Recent Pat Anti-Infect Drug Discovery. 2020;15:68–74. 10.2174/1574891X14666191028113321.Suche in Google Scholar PubMed PubMed Central
[12] Malekinejad H, Bazargani-Gilani B, Tukmechi A, Ebrahimi H. A cytotoxicity and comparative antibacterial study on the effect of Zataria multiflora Boiss, Trachyspermum copticum essential oils, and Enrofloxacin on Aeromonas hydrophila. Avicenna J Phytomed. 2012;2(4):188–95. 10.22038/AJP.2012.108.Suche in Google Scholar
[13] Khosravi AR, Shokri H, Sohrabi N. Potential effects of Trachyspermum copticum essential oil and propolis alcoholic extract on Mep3 gene expression of Microsporum canis isolates. J Mycol Med. 2014;24(3):e101–7. 10.1016/j.mycmed.2014.03.003.Suche in Google Scholar PubMed
[14] Rasooli I, Fakoor MH, Yadegarinia D, Gachkar L, Allameh A, Rezaei MB. Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int J Food Microbiol. 2008;122(1–2):135–9. 10.1016/j.ijfoodmicro.2007.11.048.Suche in Google Scholar PubMed
[15] Tehri N, Kaur R, Maity M, Chauhan A, Hooda V, Vashishth A, et al. Biosynthesis, characterization, bactericidal and sporicidal activity of silver nanoparticles using the leaves extract of Litchi chinensis. Prep Biochem Biotechnol. 2020;50(9):865–73. 10.1080/10826068.2020.1762212.Suche in Google Scholar PubMed
[16] Bindhu MR, Umadevi M, Esmail GA, Al-Dhabi NA, Arasu MV. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J Photochem Photobiol B. 2020;205:111836. 10.1016/j.jphotobiol.2020.111836.Suche in Google Scholar PubMed
[17] Singh R, Hano C, Nath G, Sharma B. Green biosynthesis of silver nanoparticles using leaf extract of Carissa carandas L. and their antioxidant and antimicrobial activity against human pathogenic bacteria. Biomolecules. 2021;11(2):299. 10.3390/biom11020299.Suche in Google Scholar PubMed PubMed Central
[18] Balciunaitiene A, Puzeryte V, Radenkovs V, Krasnova I, Memvanga PB, Viskelis P, et al. Sustainable–green synthesis of silver nanoparticles using aqueous Hyssopus officinalis and Calendula officinalis extracts and their antioxidant and antibacterial activities. Molecules. 2022;27(22):7700. 10.3390/molecules27227700.Suche in Google Scholar PubMed PubMed Central
[19] Vahidi H, Kobarfard F, Kosar Z, Mahjoub MA, Saravanan M, Barabadi H. Mycosynthesis and characterization of selenium nanoparticles using standard Penicillium chrysogenum PTCC 5031 and their antibacterial activity: A novel approach in microbial nanotechnology. Nanomed J. 2020;7(4):315–23. 10.22038/NMJ.2020.07.00008.Suche in Google Scholar
[20] Serri A, Mahboubi A, Zarghi A, Moghimi HR. PAMAM-dendrimer enhanced antibacterial effect of vancomycin hydrochloride against gram-negative bacteria. J Pharm Pharm Sci. 2019;22:10–21. 10.18433/jpps29659.Suche in Google Scholar PubMed
[21] Mohanta YK, Biswas K, Jena SK, Hashem A, Abd Allah EF, Mohanta TK. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front Microbiol. 2020;11:1143. 10.3389/fmicb.2020.01143.Suche in Google Scholar PubMed PubMed Central
[22] Tesfaye M, Gonfa Y, Tadesse G, Temesgen T, Periyasamy S. Green synthesis of silver nanoparticles using Vernonia amygdalina plant extract and its antimicrobial activities. Heliyon. 2023;9(6):e17356. 10.1016/j.heliyon.2023.e17356.Suche in Google Scholar PubMed PubMed Central
[23] Nguyen LAT, Van Mai B, Van Nguyen D, Nguyen NQT, Van Pham V, Pham TLM, et al. Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines. Green Process Synth. 2023;12(1):20230024. 10.1515/gps-2023-0024.Suche in Google Scholar
[24] Ajaykumar AP, Mathew A, Chandni AP, Varma SR, Jayaraj KN, Sabira O, et al. Green synthesis of silver nanoparticles using the leaf extract of the medicinal plant, Uvaria narum and its antibacterial, antiangiogenic, anticancer and catalytic properties. Antibiotics. 2023;12(3):564. 10.3390/antibiotics12030564.Suche in Google Scholar PubMed PubMed Central
[25] Thanayutsiri T, Patrojanasophon P, Opanasopit P, Ngawhirunpat T, Laiwattanapaisal W, Rojanarata T. Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles. Green Process Synth. 2023;12(1):20228109. 10.1515/gps-2022-8109.Suche in Google Scholar
[26] Barabadi H, Mojab F, Vahidi H, Marashi B, Talank N, Hosseini O, et al. Green synthesis, characterization, antibacterial and biofilm inhibitory activity of silver nanoparticles compared to commercial silver nanoparticles. Inorg Chem Commun. 2021;129:108647. 10.1016/j.inoche.2021.108647.Suche in Google Scholar
[27] Kelkawi AHA, Abbasi Kajani A, Bordbar A-K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017;11(4):370–6. 10.1049/iet-nbt.2016.0103.Suche in Google Scholar PubMed PubMed Central
[28] Rawat V, Sharma A, Bhatt VP, Pratap Singh R, Maurya IK. Sunlight mediated green synthesis of silver nanoparticles using Polygonatum graminifolium leaf extract and their antibacterial activity. Mater Today Proc. 2020;29:911–6. 10.1016/j.matpr.2020.05.274.Suche in Google Scholar
[29] Jana J, Ganguly M, Pal T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 2016;6(89):86174–211. 10.1039/C6RA14173K.Suche in Google Scholar
[30] Dubey SP, Lahtinen M, Sillanpää M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A. 2010;364(1):34–41. 10.1016/j.colsurfa.2010.04.023.Suche in Google Scholar
[31] Noor NS, Kaus NH, Szewczuk MR, Hamid SB. Formulation, characterization and cytotoxicity effects of novel thymoquinone-PLGA-PF68 nanoparticles. Int J Mol Sci. 2021;22(17):9420. 10.3390/ijms22179420.Suche in Google Scholar PubMed PubMed Central
[32] Gupta V, Trivedi P. Chapter 15 - In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In: Grumezescu AM, editor. Lipid Nanocarriers for Drug Targeting. William Andrew Publishing; 2018. p. 563–627. 10.1016/B978-0-12-813687-4.00015-3.Suche in Google Scholar
[33] Selvamani V. Chapter 15 – Stability studies on nanomaterials used in drugs. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, editors. Characterization and biology of nanomaterials for drug delivery. Elsevier; 2019. p. 425–44. 10.1016/B978-0-12-814031-4.00015-5.Suche in Google Scholar
[34] Csakvari AC, Moisa C, Radu DG, Olariu LM, Lupitu AI, Panda AO, et al. Green synthesis, characterization, and antibacterial properties of silver nanoparticles obtained by using diverse varieties of Cannabis sativa leaf extracts. Molecules. 2021;26(13):4041. 10.3390/molecules26134041.Suche in Google Scholar PubMed PubMed Central
[35] Devanesan S, AlSalhi MS. Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int J Nanomed. 2021;16:3343–56. 10.2147/IJN.S307676.Suche in Google Scholar PubMed PubMed Central
[36] Garibo D, Borbón-Nuñez HA, de León JND, García Mendoza E, Estrada I, Toledano-Magaña Y, et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci Rep. 2020;10:12805. 10.1038/s41598-020-69606-7.Suche in Google Scholar PubMed PubMed Central
[37] Golabiazar R, Othman KI, Khalid KM, Maruf DH, Aulla SM, Yusif PA. Green synthesis, characterization, and investigation antibacterial activity of silver nanoparticles using Pistacia atlantica leaf extract. BioNanoScience. 2019;9(2):323–33. 10.1007/s12668-019-0606-z.Suche in Google Scholar
[38] Mariadoss AVA, Ramachandran V, Shalini V, Agilan B, Franklin JH, Sanjay K, et al. Green synthesis, characterization and antibacterial activity of silver nanoparticles by Malus domestica and its cytotoxic effect on (MCF-7) cell line. Microb Pathog. 2019;135:103609. 10.1016/j.micpath.2019.103609.Suche in Google Scholar PubMed
[39] Rautela A, Rani J, Debnath M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J Anal Sci Technol. 2019;10(1):5. 10.1186/s40543-018-0163-z.Suche in Google Scholar
[40] Mahmoodi Esfanddarani H, Abbasi Kajani A, Bordbar A-K. Green synthesis of silver nanoparticles using flower extract of Malva sylvestris and investigation of their antibacterial activity. IET Nanobiotechnol. 2018;12(4):412–6. 10.1049/iet-nbt.2017.0166.Suche in Google Scholar PubMed PubMed Central
[41] Senthil B, Devasena T, Prakash B, Rajasekar A. Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J Photochem Photobiol, B. 2017;177:1–7. 10.1016/j.jphotobiol.2017.10.010.Suche in Google Scholar PubMed
[42] Lateef A, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, et al. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: its antimicrobial, antioxidant and larvicidal activities. J Nanostruct Chem. 2016;6(2):159–69. 10.1007/s40097-016-0191-4.Suche in Google Scholar
[43] Ajah HA, Khalaf KJ, Hassan AS, Aja HA. Extracellular biosynthesis of silver nanoparticles by Haemophilus influenzae and their antimicrobial activity. J Pharm Sci Res. 2018;10(1):175–9. https://www.jpsr.pharmainfo.in/Documents/Volumes/vol10Issue01/jpsr10011837.pdf.Suche in Google Scholar
[44] Baghayeri M, Mahdavi B, Hosseinpor-Mohsen Abadi Z, Farhadi S. Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl Organomet Chem. 2018;32(2):e4057. 10.1002/aoc.4057.Suche in Google Scholar
[45] Samuggam S, Chinni SV, Mutusamy P, Gopinath SCB, Anbu P, Venugopal V, et al. Green synthesis and characterization of silver nanoparticles using Spondias mombin extract and their antimicrobial activity against biofilm-producing bacteria. Molecules. 2021;26(9):2681. 10.3390/molecules26092681.Suche in Google Scholar PubMed PubMed Central
[46] Alzubaidi AK, Al-Kaabi WJ, Ali AA, Albukhaty S, Al-Karagoly H, Sulaiman GM, et al. Green synthesis and characterization of silver nanoparticles using flaxseed extract and evaluation of their antibacterial and antioxidant activities. Appl Sci. 2023;13(4):2182. 10.3390/app13042182.Suche in Google Scholar
[47] Alkhulaifi MM, Alshehri JH, Alwehaibi MA, Awad MA, Al-Enazi NM, Aldosari NS, et al. Green synthesis of silver nanoparticles using Citrus limon peels and evaluation of their antibacterial and cytotoxic properties. Saudi J Biol Sci. 2020;27(12):3434–41. 10.1016/j.sjbs.2020.09.031.Suche in Google Scholar PubMed PubMed Central
[48] Citradewi PW, Hidayat H, Purwiandono G, Fatimah I, Sagadevan S. Clitorea ternatea-mediated silver nanoparticle-doped hydroxyapatite derived from cockle shell as antibacterial material. Chem Phys Lett. 2021;769:138412. 10.1016/j.cplett.2021.138412.Suche in Google Scholar
[49] de Aragão AP, de Oliveira TM, Quelemes PV, Perfeito MLG, Araújo MC. Santiago JdAS, et al. Green synthesis of silver nanoparticles using the seaweed Gracilaria birdiae and their antibacterial activity. Arab J Chem. 2019;12(8):4182–8. 10.1016/j.arabjc.2016.04.014.Suche in Google Scholar
[50] Eren A, Baran M. Green synthesis, characterization and antimicrobial activity of silver nanoparticles (AgNPs) from maize (Zea mays L.). Appl Ecol Env Res. 2019;17(2):4097–105. 10.15666/aeer/1702_40974105.Suche in Google Scholar
[51] Biswas A, Vanlalveni C, Adhikari PP, Lalfakzuala R, Rokhum L. Green biosynthesis, characterisation and antimicrobial activities of silver nanoparticles using fruit extract of Solanum viarum. IET Nanobiotechnol. 2018;12(7):933–8. 10.1049/iet-nbt.2018.0050.Suche in Google Scholar PubMed PubMed Central
[52] Das Mahapatra A, Patra C, Pal K, Mondal J, Sinha C, Chattopadhyay D. Green synthesis of AgNPs from aqueous extract of Oxalis corniculata and its antibiofilm and antimicrobial activity. J Indian Chem Soc. 2022;99(7):100529. 10.1016/j.jics.2022.100529.Suche in Google Scholar
[53] Qais FA, Shafiq A, Khan HM, Husain FM, Khan RA, Alenazi B, et al. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg Chem Appl. 2019;2019:4649506.10.1155/2019/4649506Suche in Google Scholar PubMed PubMed Central
[54] Soliman H, Elsayed A, Dyaa A. Antimicrobial activity of silver nanoparticles biosynthesised by Rhodotorula sp. strain ATL72. Egypt J Basic Appl Sci. 2018;5(3):228–33. 10.1016/j.ejbas.2018.05.005.Suche in Google Scholar
[55] Altinsoy BD, Şeker Karatoprak G, Ocsoy I. Extracellular directed ag NPs formation and investigation of their antimicrobial and cytotoxic properties. Saudi Pharm J. 2019;27(1):9–16. 10.1016/j.jsps.2018.07.013.Suche in Google Scholar PubMed PubMed Central
[56] Hemmati S, Rashtiani A, Zangeneh MM, Mohammadi P, Zangeneh A, Veisi H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019;158:8–14. 10.1016/j.poly.2018.10.049.Suche in Google Scholar
[57] Muthulakshmi K, Uma C, Sivagurunathan P, Kumar SS, Yoganathan K. Evaluation of antimicrobial activity of silver nanoparticles from silver resistant isolate Enterobacter cloacae (MK163462) against certain clinical. Int J Pharm Bio Sci. 2018;8(4):558–64.Suche in Google Scholar
[58] Ramzan M, Karobari MI, Heboyan A, Mohamed RN, Mustafa M, Basheer SN, et al. Synthesis of silver nanoparticles from extracts of wild ginger (Zingiber zerumbet) with antibacterial activity against selective multidrug resistant oral bacteria. Molecules. 2022;27(6):2007. 10.3390/molecules27062007.Suche in Google Scholar PubMed PubMed Central
[59] Youssef GA, El-Boraey AM, Abdel-Tawab MM. Eco-friendly green synthesis of silver nanoparticles from Egyptian honey: evaluating its antibacterial activities. Egypt J Bot. 2019;59(3):709–21. 10.21608/ejbo.2019.6597.1261.Suche in Google Scholar
[60] Wypij M, Czarnecka J, Świecimska M, Dahm H, Rai M. Golinska P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J Microbiol Biotechnol. 2018;34(2):23. 10.1007/s11274-017-2406-3.Suche in Google Scholar PubMed PubMed Central
[61] Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD. Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9(5):2673–702. 10.1039/C8RA08982E.Suche in Google Scholar PubMed PubMed Central
[62] Das B, Dash SK, Mandal D, Ghosh T, Chattopadhyay S, Tripathy S, et al. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab J Chem. 2017;10(6):862–76. 10.1016/j.arabjc.2015.08.008.Suche in Google Scholar
[63] Ghabban H, Alnomasy SF, Almohammed H, Al Idriss OM, Rabea S, Eltahir Y. Antibacterial, cytotoxic, and cellular mechanisms of green synthesized silver nanoparticles against some cariogenic bacteria (Streptococcus mutans and Actinomyces viscosus). J Nanomater. 2022;2022:9721736. 10.1155/2022/9721736.Suche in Google Scholar
[64] Gurunathan S, Han JW, Kwon D-N, Kim J-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett. 2014;9(1):373. 10.1186/1556-276X-9-373.Suche in Google Scholar PubMed PubMed Central
[65] Kumar SSD, Houreld NN, Kroukamp EM, Abrahamse H. Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria. J Photochem Photobiol, B. 2018;178:259–69. 10.1016/j.jphotobiol.2017.11.001.Suche in Google Scholar PubMed
[66] Das B, Dash SK, Mandal D, Adhikary J, Chattopadhyay S, Tripathy S, et al. Green-synthesized silver nanoparticles kill virulent multidrug-resistant Pseudomonas aeruginosa strains: A mechanistic study. BLDE Univ J Health Sci. 2016;1(2):89–101. 10.4103/2468-838X.196087.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Artikel in diesem Heft
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

