Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
-
Shujahadeen B. Aziz
, Muhamad H. Hamsan
, Ahmed F. Abdulrahman
Abstract
The aim of this study is to address the growing concern about microplastics in the ocean and their potential harm to human health through ingestion. The MPs issue is largely a result of the increasing demand for electronic devices and their components. To tackle this challenge, the research aimed to develop a green polymer electrolyte that used glycerol as a plasticizing agent to improve ionic conductivity. The polymer host included chitosan and polyvinyl alcohol and was composed of sodium acetate. To evaluate the performance of the polymer electrolyte, various analytical techniques were used, including impedance and electrochemical studies. The ionic conductivity of 7.56 × 10−5 S·cm−1 was recorded. The dielectric property study confirmed the ionic conduction process in the system and revealed the existence of non-Debye type relaxation, as indicated by asymmetric peaks of tanδ spectra. The alternating conductivity exhibits three distinguished regions. The polymer electrolyte was discovered to be electrochemically stable up to 2.33 V and capable of storing energy as a non-Faradaic electrochemical double-layer capacitor (EDLC). The cyclic voltammetry pattern is a leaf like shape. The EDLC was able to be charged and discharged up to 1 V, and it showed cyclability and could be used in low-voltage applications.
1 Introduction
Fossil fuel consumption has caused high levels of pollution, prompting efforts to study and create renewable energy materials. Human tricks, specific energy consumption, and production are the main sources of ruthless green issues, e.g., environmental degradation and global warming [1]. Global electrical energy is 44.2% from coal, 38.0% from natural gases, 17.1% from renewable energy, and 0.6% and 5% from fuel oil/diesel and hydro, respectively. The use of coal as an energy source has a significant disadvantage in that it emits CO2 into the atmosphere and contributes to global warming. Natural biodegradable biopolymers, often referred to as the future “green materials,” are showing great promise as ideal hosts for creating biopolymer electrolytes designed for a variety of energy devices [2,3]. These biopolymers come from sources like cellulose, starch, chitosan (CH), and alginate, and they stand out for being environmentally friendly, easy to find, and sustainable [4]. They naturally decompose over time, resulting in minimal environmental impact, representing a substantial advancement in contrast to conventional energy storage approaches; consequently, they are commonly referred to as “green” polymers [2,4]. This innovative approach not only makes energy devices like batteries, fuel cells, and supercapacitors safer and more efficient, but it also aligns with the global trend of using more sustainable resources and reducing waste. Thus, researchers are turning to green polymer membranes for electrochemical devices resembling solar cells, supercapacitors, fuel cells, and batteries to address this. This shift aims to achieve the goal of clean energy that is both sustainable and affordable [5]. Minimizing environmental impact is critical in the pursuit of a high-tech future. Micro-sized energy storage will be required for automation and robotics, which could result in a large amount of plastic waste if not properly planned for.
One of the simplest electrochemical devices is electrochemical double-layer capacitors (EDLCs) due to their simple preparation methods, long life cycles, and large power density [6,7]. In contrast to conventional batteries, EDLCs do not contain hazardous explosive components, thereby enhancing their safety profile. Moreover, the readiness of EDLCs ensures safety for both individuals and the surrounding environment. Compared to lead acid batteries, EDLC is significantly smaller and has a rapid rate of charging and discharging [8]. Carbon has been chosen as the right material to provide a large surface for ion adsorption. Electrostatic interactions between ions and electrons govern the energy storage procedure of an EDLC [9]. Activated carbon (AC) stands out as a prime candidate for EDLC electrodes due to its robust chemical and physical properties, large surface area, and porous structure [10]. Polyvinyl alcohol (PVA) is widely used to reduce plastic waste because it is a versatile polymer with properties similar to those of natural polymers, such as being eco-friendly, biodegradable, biocompatible, and having good film-forming abilities [11]. To achieve the fabrication of polymer electrolyte films with superior performance, an approach known as polymer blending can be employed, wherein additional polymers are incorporated into the polymer host. When two polymers are combined together, their functional groups will act as channels for ionic transportation [12]. It is widely recognized that polymer blends have a high concentration of functional groups, which enhances their performance in assorted fields such as drug delivery, energy storage devices (ESDs), polymer electrolytes, and tissue engineering [13]. PVA has been reported to be biocompatible with various polymers such as hydroxypropylmethylcellulose [14], dextran [15], starch [16], methylcellulose [17], and pectin [18].
CH is another excellent green polymer. CH has a linear structure made up of (1,4)-linked-2-amino-deoxy-glucan units derived via chitin deacetylation. After cellulose, chitin is the most prevalent carbohydrate on the planet [19]. CH is widely used in the green polymer edible film industry due to its film-forming properties [20]. In the food industry, CHs films are employed as bioactive packaging for the preservation of apples, raspberries, cherimoya, kiwi, and loquat [21,22,23,24,25]. The functional groups in the linear CH polymer chains also play a crucial role in ionic conduction, making CH a good choice for a greener future with economic and environmental benefits [26].
Glycerol is important in enhancing the performance of polymer-based electrolytes as it increases salt dissociation and ionic conductivity. This is due to glycerol’s high dielectric property, which weakens the forces between cations and anions [27,28,29]. The addition of the right amount of glycerol boosts the ionic conductivity and mechanical strength of green polymers. In this work, a glycerol-based CH-PVA polymer electrolyte will be tested with sodium acetate (NaOAc) as the ionic source. The best performing films will be used as separator in EDLC fabrication. According to the study, incorporating green polymer electrolytes in an EDLC assembly has resulted in a breakthrough. The assembled EDLC recorded a high specific capacitance (∼74 F·g−1), which is similar to values recorded for liquid and gel-based electrolytes. The electrolyte used in the EDLC assembly also displayed a relatively high energy density, comparable to that of lead-acid batteries, as illustrated in Figure 1.
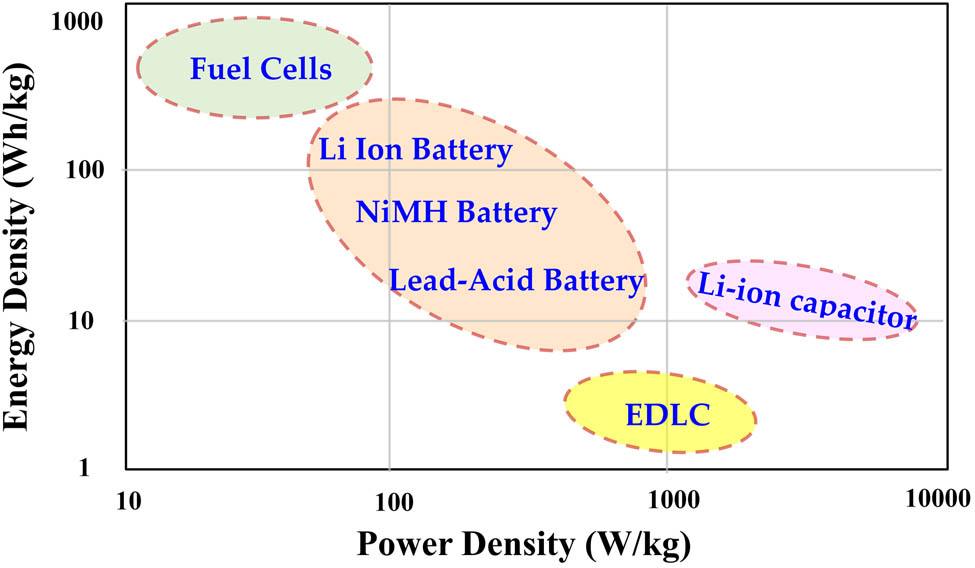
The Ragone plot for various electrochemical devices.
Previous research indicates that EDLC devices, which have energy densities comparable to batteries, could potentially revolutionize energy resources through the use of non-toxic materials. However, the low energy density of EDLC devices has prevented their commercialization. As a result, many research groups and companies are concentrating on developing EDLC devices with exceptionally high energy density. This would allow for novel opportunities in energy storage and expand knowledge regarding standards such as the Ragone plot for electrochemical devices. The current study’s results, involving an EDLC device that delivers high energy density and excellent performance over 500 cycles, could stimulate discussion regarding the future of EDLC devices and their potential to replace battery technology. This research presents promising insights into the development of solid biopolymer electrolytes, offering potential solutions for sustainable energy storage systems.
2 Experimental method
2.1 Electrolyte preparation
CH from
2.2 Transference number measurement (TNM) study
The contribution of each charge carrier in an EDLC was analyzed with TNM to enhance its high performance. The analysis was conducted using a V&A Instrument DP3003 digital DC power supply. The current was recorded using a UT803 True RMS multimeter, which was connected to a computer for data extraction. The ionic (
where
2.3 Linear sweep voltammetry (LSV) study
The ability of the SPE film as an energy storage option was evaluated using LSV. The strength, redox reactions, and behavior of the film within a range of 0–3.5 V at a sweeping rate of 50 mV·s−1 were examined through LSV. The investigation was completed with a Digi-IVY DY2300 Potentiostat. The top-performing CH-PVA electrolyte was positioned among two SS electrodes and enclosed in a Teflon casing for LSV analysis. The electrode and electrolyte configurations used in the LSV study, which relied solely on the most conductive electrolyte, are depicted schematically in Figure 2. This sandwich configuration allows for precise and controlled measurements of the film’s electrochemical behavior and its ion transport properties in which both electrodes were constructed of SS.

Schematic showing the LSV analysis electrolyte and electrode setup.
2.4 Assembly of EDLC
Figure 3 depicts the methods and materials needed to create AC electrodes. A planetary ball miller was used to combine dry AC with carbon black (CB). To prevent excessive air bubble formation, the AC-CB powder was added to a solution of N-methyl pyrrolidone (anhydrous,
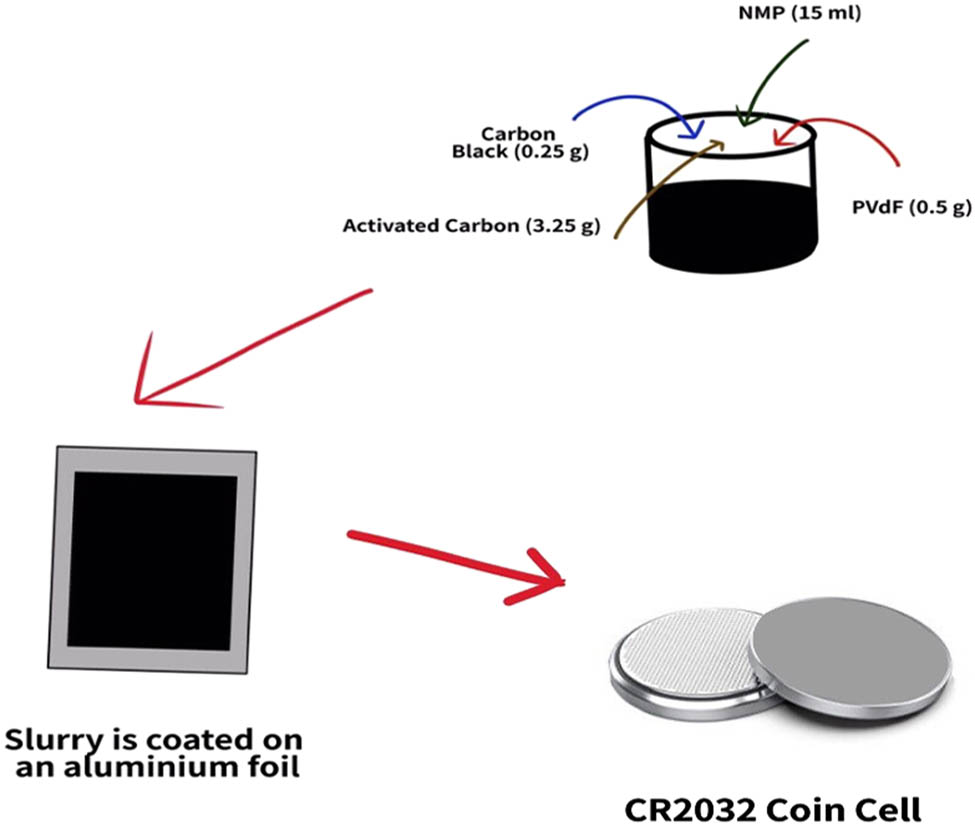
The fabrication process of the EDLC.
2.5 Device characterizations
2.5.1 Cyclic voltammetry (CV) study
The method of charge storage in EDLC is based on capacitive charge separation, as opposed to redox reactions. To affirm this mechanism, the EDLC was subjected to a CV study. The capacitance (
The CV plot area,
2.5.2 Galvanostatic charge–discharge (GCD) of assembled EDLC
The GCD test was conducted using a
where the symbol “
3 Result and discussion
3.1 EIS and AC conductivity study
The characterization of ionic conductivity in polymeric materials can be effectively carried out through the use of electrochemical impedance spectroscopy [30]. This technique allows for the determination of inter-particle interactions, including grain and grain boundary effects, through the analysis of complex impedance data [31]. Figure 4 displays the Cole–Cole plot for the plasticized systems to determine the DC conductivity. Typically, the frequency domain analysis of impedance spectra yields two distinct regions, a half-circle at high frequency and a line at low frequency. It has been suggested that the low-frequency (LFr) line arises from the contribution of blocking electrodes, while the high-frequency (HFr) half-circle reflects the ionic conduction mechanism of the bulk electrolyte [32,33,34]. The interface between electrodes and electrolyte samples can be viewed as a capacitor in the presence of blocking electrodes. When the impedance is ideal, the impedance plot displays a vertical spike. It is notable that an increase in plasticizer concentration is known to result in a decline in the HFr semicircle diameter [35]. Each sample’s bulk resistance (R b) is shown in the insets of the impedance charts. The equation given below indicates that the DC ionic conductivity in polymer-based electrolytes is linked to the ion density and mobility [36]:
where n, q, and µ denote the total number of ions, charge of ions, and ionic mobility, respectively. According to the formula, increasing either ion mobility or charge carrier density results in improved DC ionic conductivity. By following up on impedance plots and locating the real axis value at the semicircle’s lowest point, one may estimate the dc value. Following is an equation that could be used to compute the DC conductivity:
where A and t represent the area and thickness of the sample, respectively [37]. Table 1 lists the DC conductivity of the polymer electrolytes (PEs) in which the conductivity boosts with glycerol incorporation as the glycerol improves more mobile ions into the PE and decreases the R b. The reduction in R b leading to its minimum value corresponds to the attainment of the highest conductivity (7.56 × 10−5 S·cm−1), indicating the successful interaction among the components of the electrolyte. The LFr spike appearance in Figure 4(b–e) indicates dissociation of more salts by glycerol and thus increase in conductivity. This is attributed to the improved flexibility of the polymer chains, which helps in faster migration of ions.

Cole–Cole plot for the plasticized systems (a) CSPVNACT1, (b) CSPVNACT2, (c) CSPVNACT3, (d) CSPVNACT4, and (e) CSPVNACT5 to determine the DC conductivity.
DC ionic conductivity of the CSPVNACT samples
| Sample code | DC conductivity (S·cm−1) |
|---|---|
| CSPVNACT1 | 9.85 × 10−9 |
| CSPVNACT2 | 2.27 × 10−6 |
| CSPVNACT3 | 9.84 × 10−6 |
| CSPVNACT4 | 3.85 × 10−5 |
| CSPVNACT5 | 7.56 × 10−5 |
3.2 AC conductivity
In Figure 5, the frequency dependency of the AC conductivity in the CH-PVA-NaOAc-glycerol systems is demonstrated. Conductance spectra typically exhibit three regions: an LFr zone due to a proper electrode polarization (EP), a plateau region representing the DC conductivity of the system, and an HFr zone due to bulk structural interactions. Determining AC conductivity in disordered materials can be a complex task due to the diverse range of charge states that are present [38,39,40].
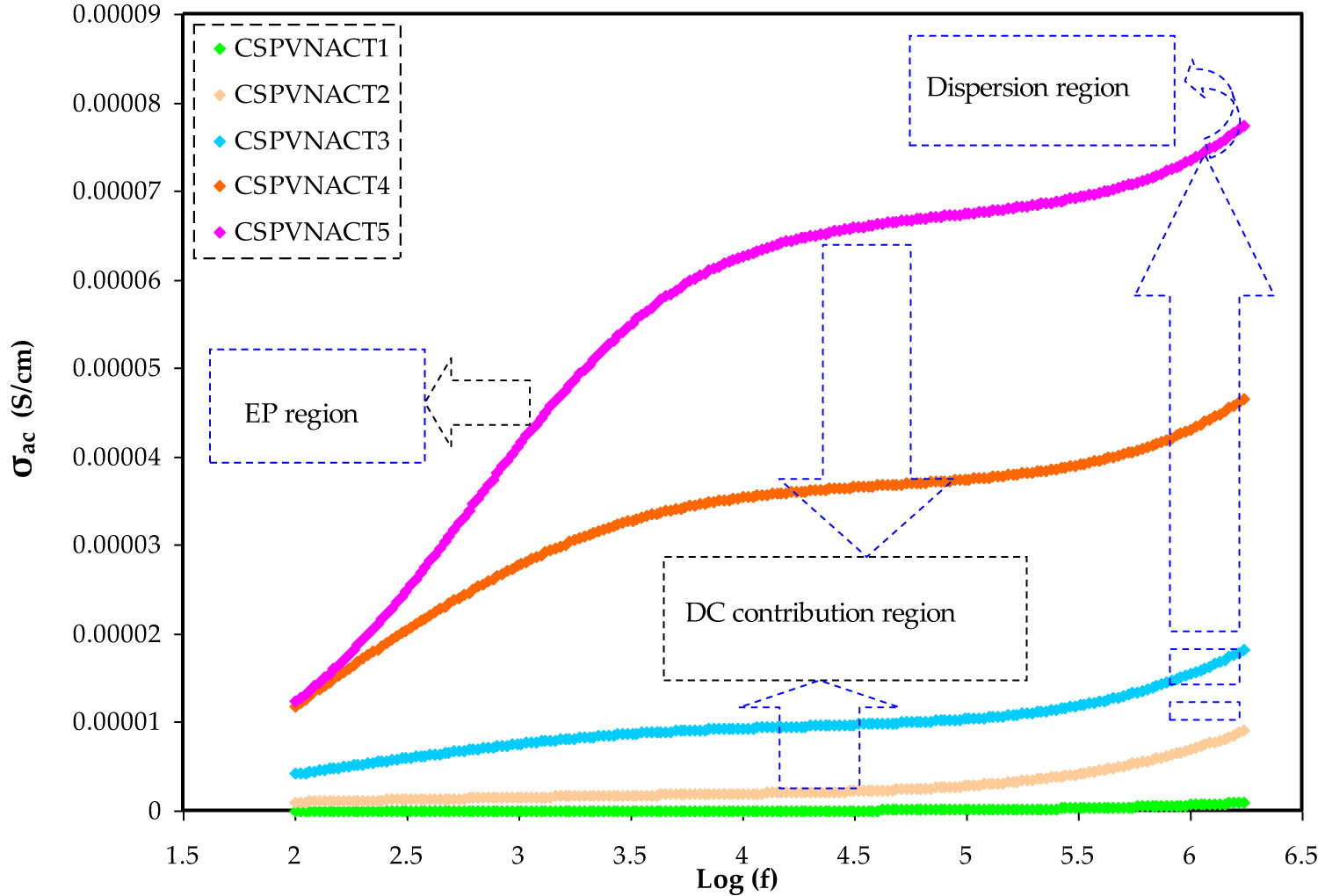
AC conductivity plot for the plasticized systems to distinguish EP, DC, and dispersion regions.
The frequency behavior provides a valuable tool for evaluating the localization of states. A remarkable conductivity dispersion is a hallmark of electrical conduction in disordered materials, often described as a combination of a frequency-independent term and a frequency-dependent contribution [39]. The equation below summarizes this relationship:
where σ(ω) signifies the total conductivity, and σ ac and σ dc denote the frequency-dependent AC conductivity and frequency-independent DC conductivity, respectively. The alternating current conductivity is given by the expression (Aω S), where A is a temperature-dependent parameter, and ω is a power law exponent. This research showed that the doped systems had two distinct regions: an LFr dispersion zone originated by ion-blocking at the electrode–electrolyte interface, and a plateau mid-frequency area that reflects bulk conductivity [39]. The DC conductivity, extrapolated from the plateau area to zero frequency, was found to be in agreement with the Cole–Cole plotted values. At low frequencies, the conductance was observed to be high due to the buildup of charges at the electrodes, while at intermediate frequencies, low applied field duration and a gradual charging procedure led to an AC conductivity value similar to DC conduction [38,39,40].
3.3 Dielectric properties
The complex dielectric permittivity (
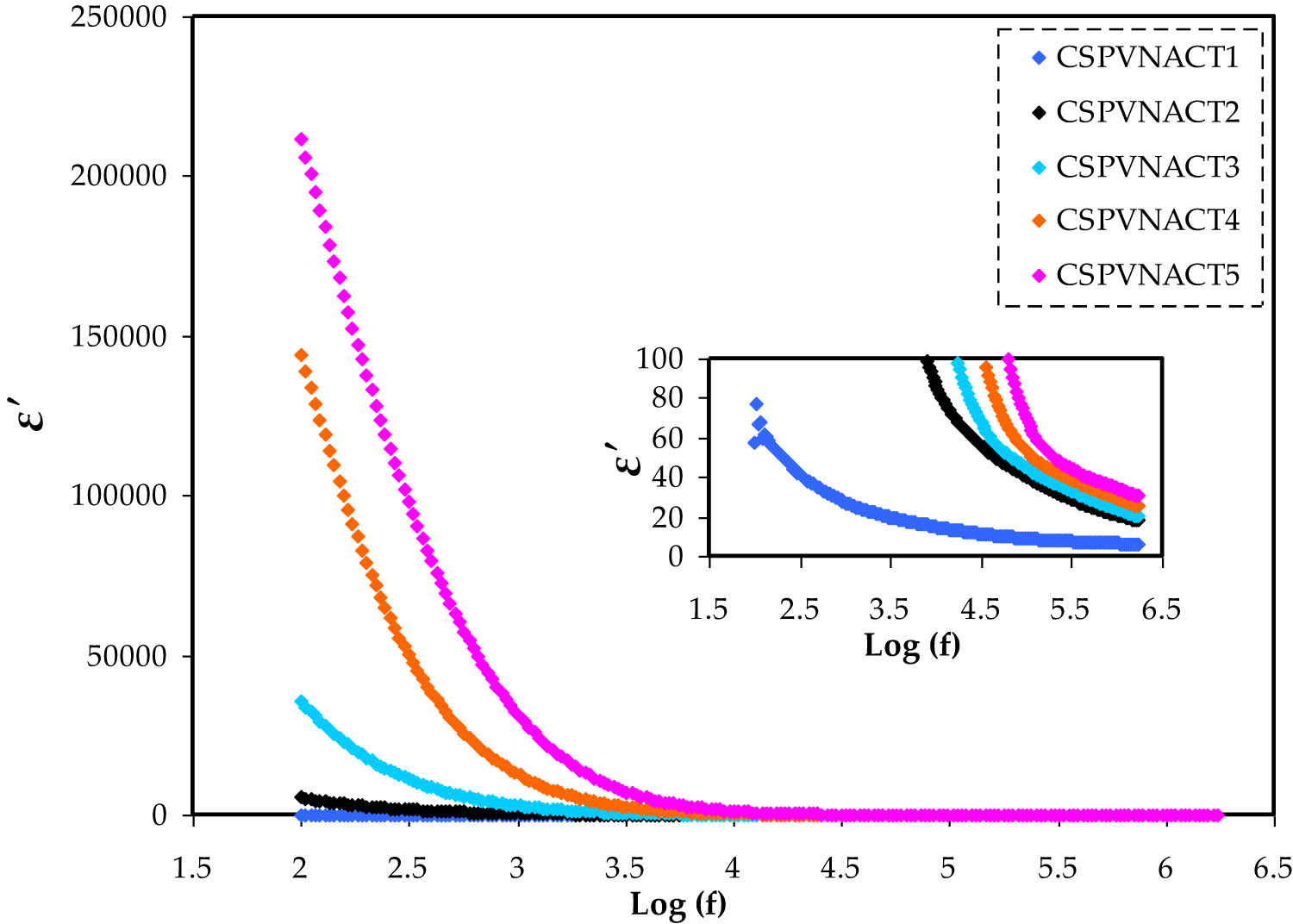
The variation in

The variation in
The symbol C o represents vacuum capacitance, ω = 2πf indicates the angular frequency, and f represents the working frequency. At low frequencies, EP at the electrode surface causes a dispersion region to appear. Meanwhile, high frequencies exhibit a plateau region due to the ionic dipoles’ inability [41,42]. The connection among the dielectric constant and the quantity of free ions in the electrolytes is as follows:
where
Ionic conductivity in polymer electrolytes is a multifaceted process affected by salt concentration and dissociation, polymeric host dielectric characteristics, chain flexibility, and ion aggregation [44,48]. A key feature of ion-conducting polymer electrolytes is their ionic conductivity; the dielectric study of these systems is also of great importance in gaining an understanding of ion transport and ion-molecule interactions in solid polymer electrolytes [49]. Figure 8 shows the variation in loss tangent (tanδ) against frequency for the CSPVNACT electrolytes. It can be seen that the loss tangent peaks of the CSPVNACT samples change in frequency as a function of the plasticizer concentration. The relaxation peaks observed in the plasticized electrolytes could be attributed to almost complete ion relaxation times. The presence of a relaxation process in the CSPVNACT samples signifies that glycerol enhances the segmental motion of the polymer blend chains, consequently promoting increased free volume and improved ion transport [50,51]. Quick segmental motion and ionic mobility both contribute to ionic transport within the CH-PVA blending matrix. Table 2 shows the relaxation time values extracted from frequency peaks. It shows that adding plasticizers via salt dissociation could enhance carrier concentration and segmental motion and thus reduce the required time for DC conduction [52]. The peak shifts to the HFr side, demonstrating the shorter relaxation period. The outcomes of the present investigation are in line with those reported in prior studies [53]. The dielectric field and polarization are in phase at low frequencies, while the polarization lags behind the electric field frequency at high frequencies. The dielectric loses energy and emits heat as a consequence of the phase change. When the relaxation process is equal to the duration of the applied electric field, maximum loss tangent is obtained, resulting in a resonant state [54]. According to Jiang et al. [55], the principal reason of the rise in the loss tangent (tanδ) with increasing frequency of operation is resistive losses. This phenomenon arises due to the challenge faced by the ionic mobility of the electrolyte samples in keeping pace with HFr electric fields. As can be seen in Figure 8, the tanδ value increases with frequency, climbs to its maximum value, and then declines. Moreover, the highest loss tangent value shifts to high frequency along with an increase in glycerol concentration.
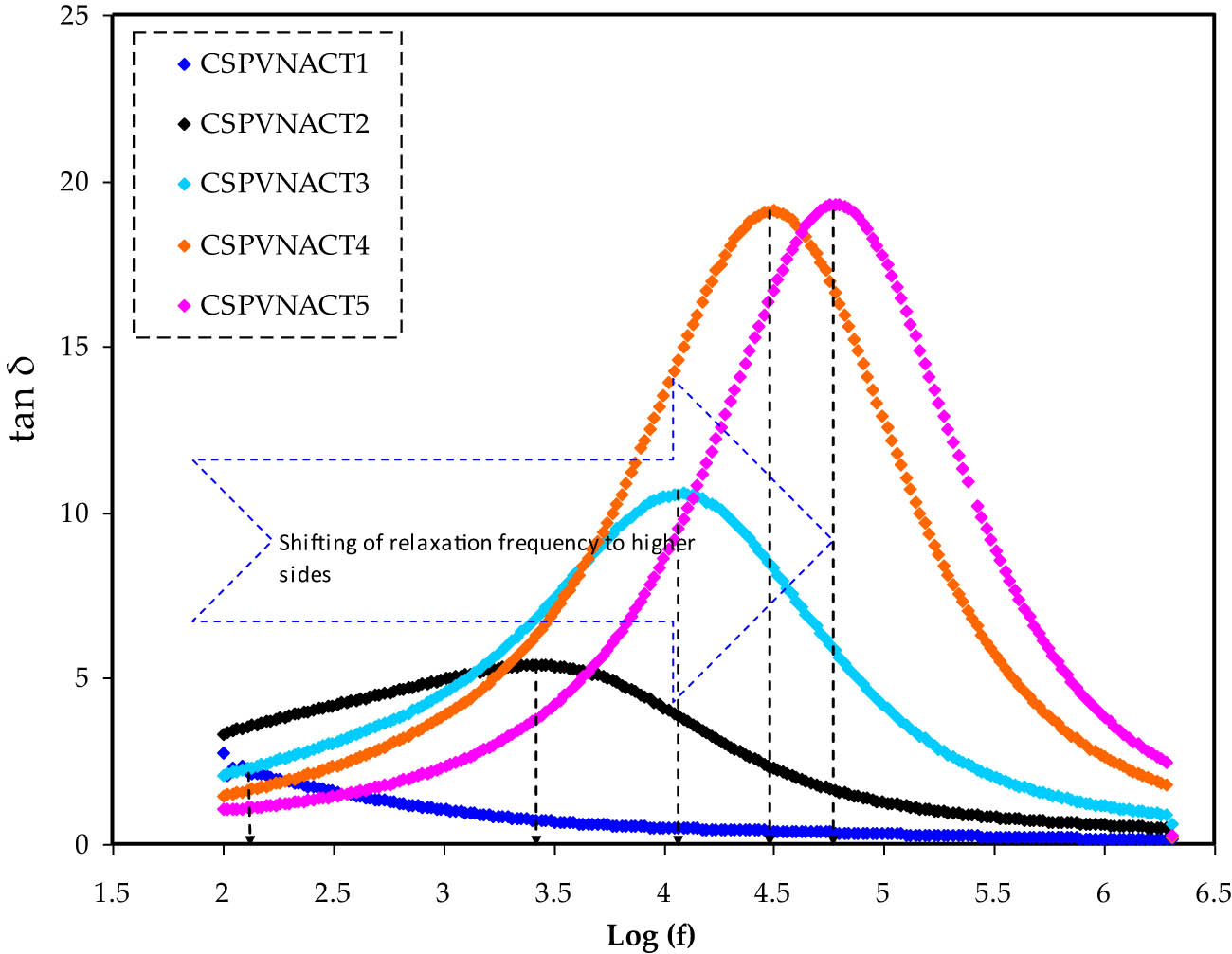
Tanδ vs Log(f) for all CSPVNACT electrolytes.
Relaxation time extracted from peak frequency
| f | T = 1/(2πf) |
|---|---|
| 105 | 1.51 × 10−3 |
| 2,050 | 7.77 × 10−5 |
| 13,460 | 1.18 × 10−5 |
| 26,920 | 5.92 × 10−6 |
| 51,244 | 3.11 × 10−6 |
3.4 Electrochemical studies
3.4.1 LSV study
The LSV is used to determine the breakdown voltage of polymer electrolyte membranes. The application of voltage will reveal any redox reactions within the polymer electrolyte. Figure 9 presents the breakdown voltage profile of the CH-PVA electrolyte film, which demonstrates exceptional stability. No significant current is observed as the film is subjected to a linear potential sweep from 0 to +2.33 V. This represents the creation of a charge double-layer (CDL) at the interface of the polymer electrolyte and SS electrodes. When the potential exceeds 2.33 V, the current value sharply increases. The plot established that the CH-PVA film has decomposed beyond +2.33 V, which indicates the degradation of oxide or oxidation of functional groups [56]. This phenomenon triggers the polarization process to be unstable. This is a general idea that the performance of the EDLC will be unstable at potentials greater than 2.33 V. As reported by Vahini et al. [57], the Na-based polymer electrolyte film exhibited instability in its current response when the applied potential exceeded 2 V. A study indicated that a polymer electrolyte incorporating sodium iodide (NaI) as the ionic source and methylcellulose as the polymer host demonstrated electrochemical stability up to 1.7 V [58]. Hence, the optimized CH-PVA electrolyte in this work can be useful in low voltage energy storage applications.

The LSV plot at 50 mV·s−1 for the best conducting polymer electrolyte.
3.4.2 Transference number study
Most ESDs rely on the ionic species of the polymer electrolyte. Ions are required in an EDLC to ensure that the energy storage process can be done via the CDL. TNM analysis is one of the easiest ways to check the dominance of a charge carrier. Figure 10 presents the TNM plot of the most conducting CH-PVA film at 0.2 V driving voltage. Both electrons and ions were thought to contribute equally to the current, hence 6.3
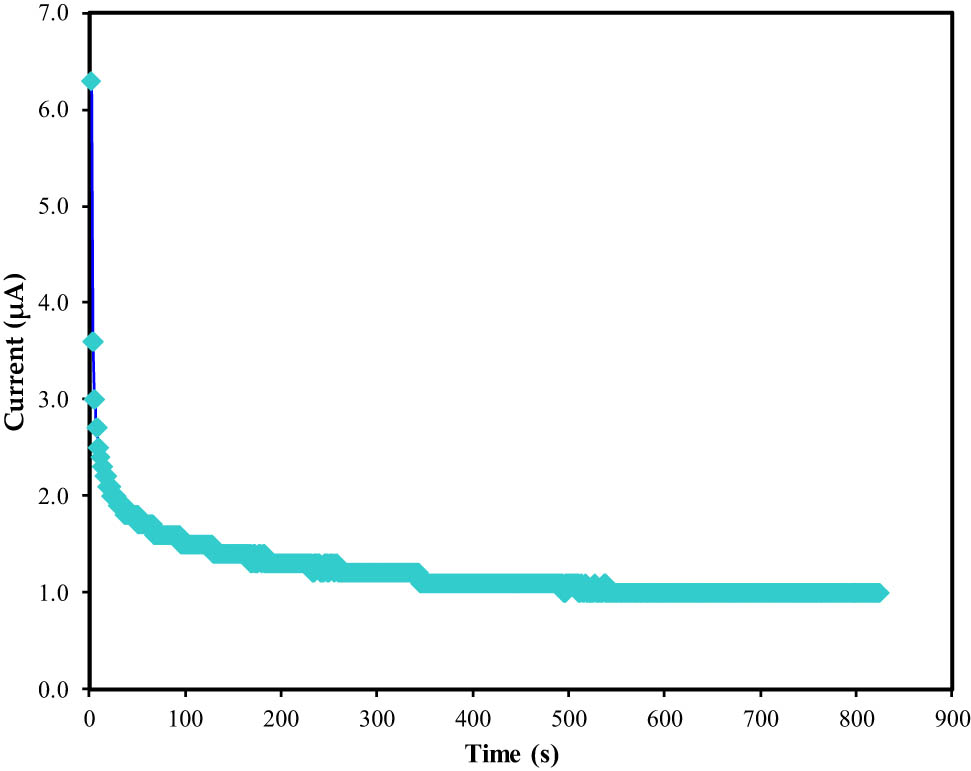
The TNM plot for the optimal conducting polymer electrolyte.
3.4.3 CV of the assembled EDLC
The electrostatic interactions between the electrons of the carbon-based electrodes and the ions of the electrolyte cause the energy storage, which is known as a non-Faradaic capacitor. Through CV analysis, the existence of non-Faradaic processes in the EDLC was verified. The CV curve when the scan rate was lowered from 100 to 20 mV·s−1 is shown in Figure 11(a) and (b) displays the specific capacitance as opposed to scan rate. As observed in Figure 11(a), the CV curve of the EDLC at 100 mV·s−1 looks like the shape of a typical leaf, where the calculated C cyc is 9.4 F·g−1. At a rapid scan rate, the development of the CDL is unstable due to the imbalance conduction of ions [64]. As the scan rate decreased to 50 and 20 mV·s−1, the C cyc is obtained as 20 and 38 F·g−1, respectively. The curve at the edges of the plot of 20 mV·s−1 is smaller than 50 and 100 mV·s−1. Furthermore, the plot has deviated to a rectangular-like shape at 20 mV·s−1. This indicates a low internal resistance. The efficient and stable transport of ions occurs at a low current flow, allowing complete polarization of ions. Figure 12 shows the schematic design of the fabricated EDLC. Obtaining a perfect rectangular shape CV plot is challenging due to the roughness of the carbon electrodes, as their porosity causes a varying distance between ions in the electrolyte and their corresponding active sites in the AC electrode. Figure 13 shows the mechanism of CDL formation in EDLC. The absence of humps or peaks in the plot confirms that the EDLC in this work functions as a non-Faradaic capacitor, exhibiting the scan rate-dependent characteristics typical of capacitor cells.
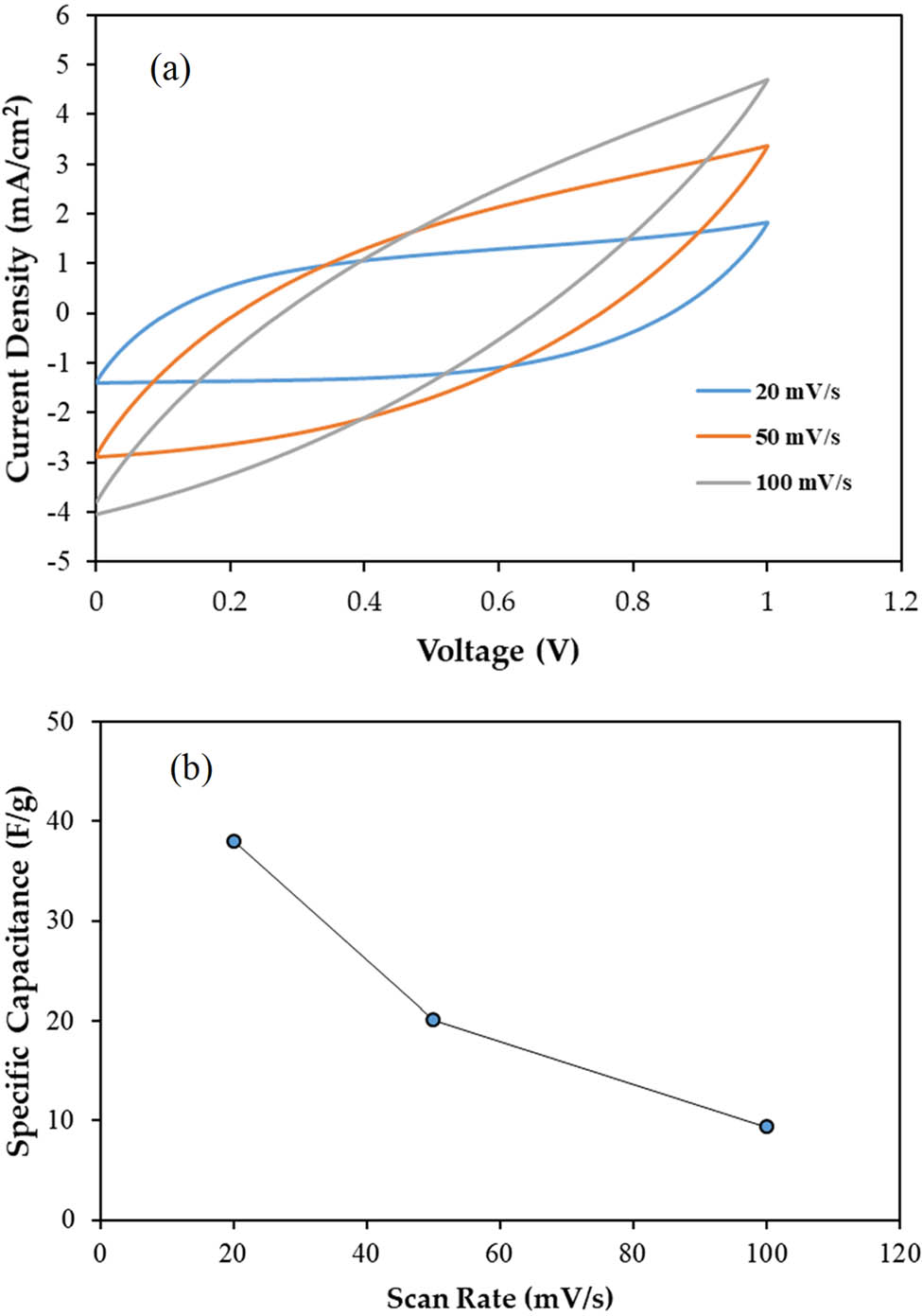
(a) CV curves of the CH-PVA based EDLC at various scan rates and (b) specific capacitance as opposed to scan rate.
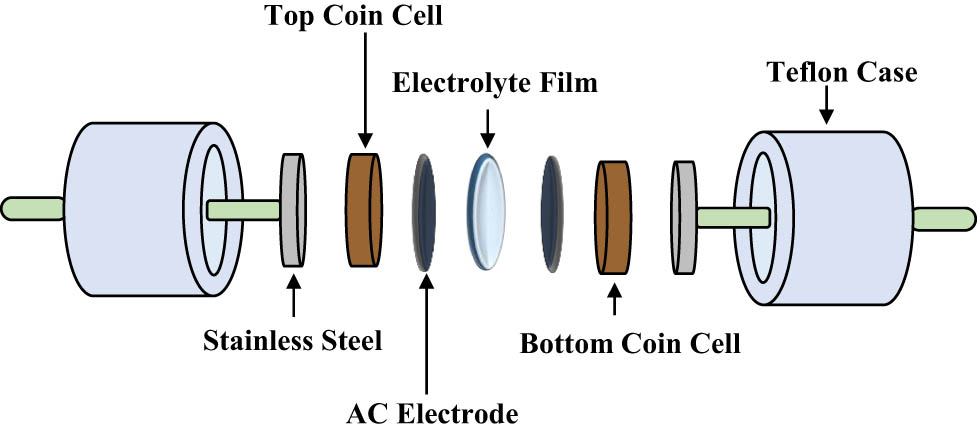
Schematic illustration of EDLC setup.
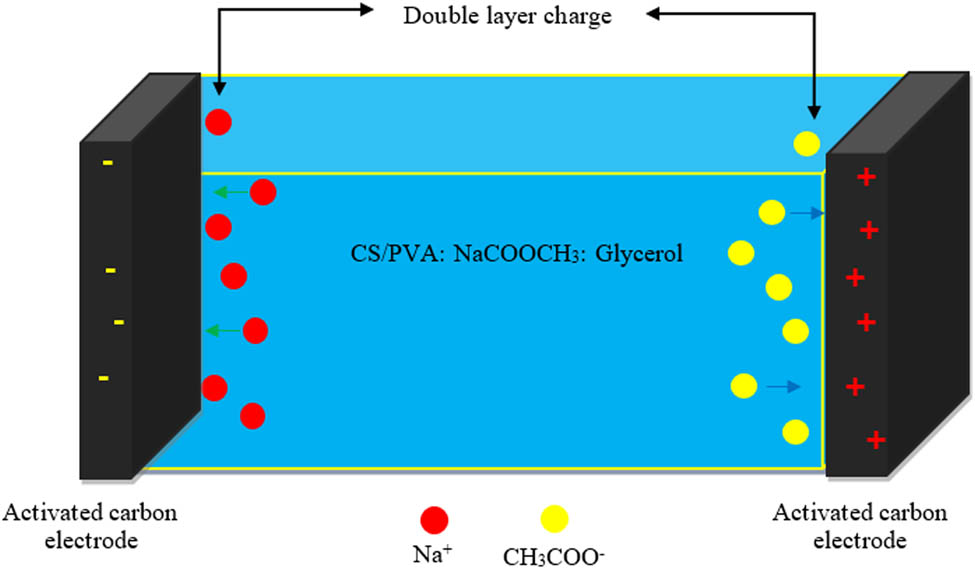
Mechanism of CDL formation in EDLC.
3.4.4 Charge–discharge of the assembled EDLC
The charge–discharge curve for the EDLC is displayed in Figure 14 for a number of cycles. The EDLC is charged to a maximum of 1 V and then discharged down to 0 V. Because of the electrostatic interaction between the ions in the electrolyte and the electrons in the electrodes, the curve should normally have a triangular form. However, because of the intercalation and de-intercalation processes, the discharge curve for ordinary batteries is often non-linear [65].
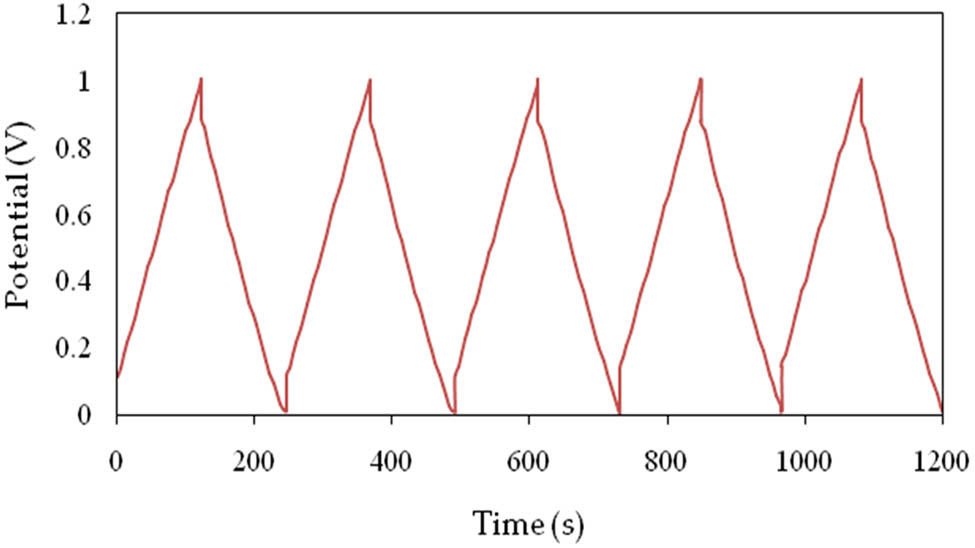
The GCD for the EDLC device.
Figure 15 shows the CH-PVA-based EDLC’s capacitance (C
s) and efficiency when the current density is
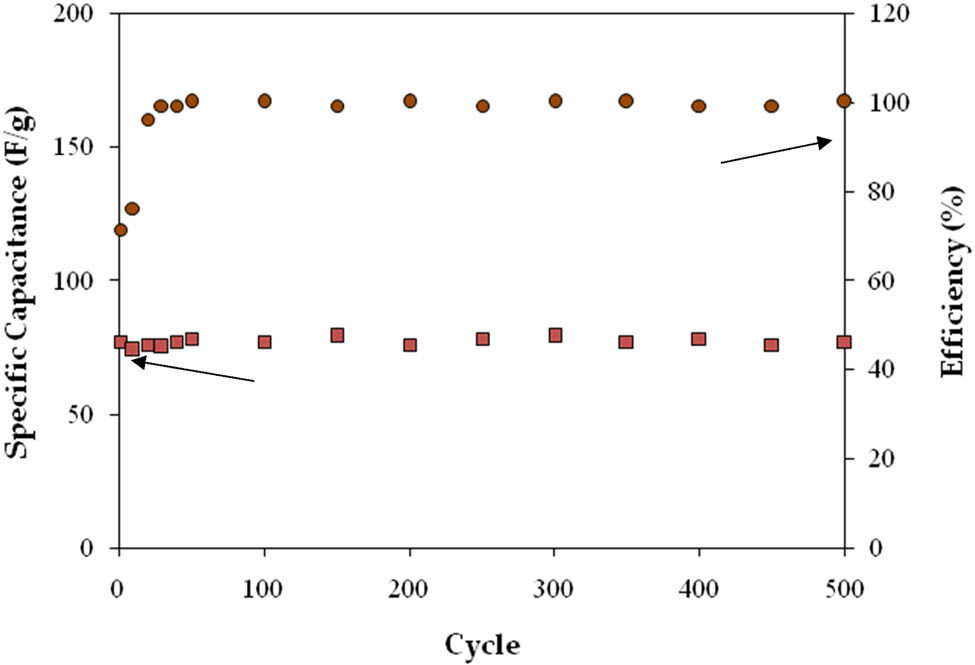
Calculating the EDLC’s efficiency over 500 cycles and its specific capacitance.
The ESR is a crucial factor for determining the quality of the electrolyte–electrode interface and the internal resistance of an EDLC. Figure 14 shows a noticeable voltage drop (V dr) before the discharge process. The EDLC’s average V dr was around 0.12 V. Figure 16 depicts the EDLC’s ESR values across 500 complete charge–discharge cycles with an average value of 87 Ohms. This is due to the polymer electrolyte and the gap between the electrodes and the electrolyte in the EDLC [66]. Lower ESR values indicate a good connection between the electrodes and the electrolyte, allowing for efficient charge carrier conduction to the electrodes and the formation of a CDL through electrostatic interactions [69].
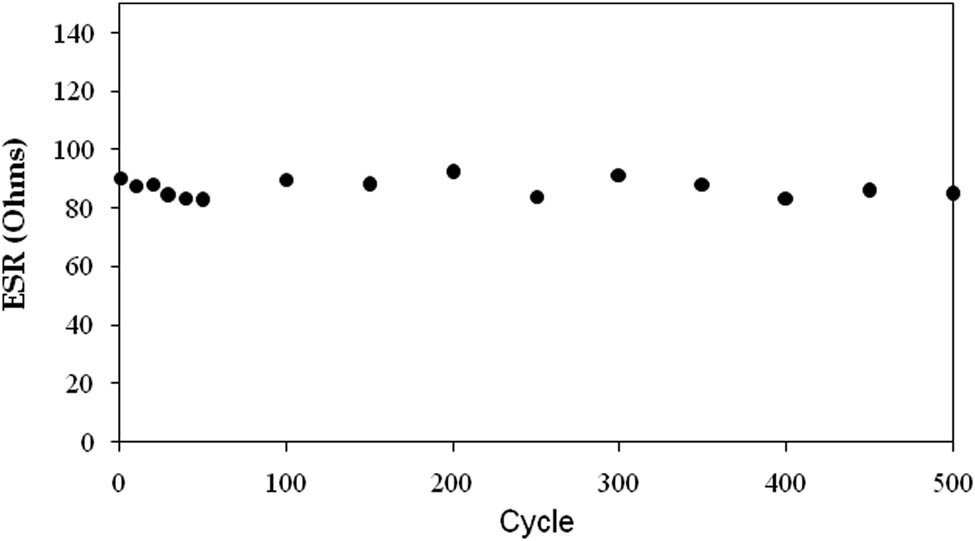
ESR plot of the assembled CH-PVA based EDLC throughout the 500 cycles.
Figure 17 illustrates the changes in
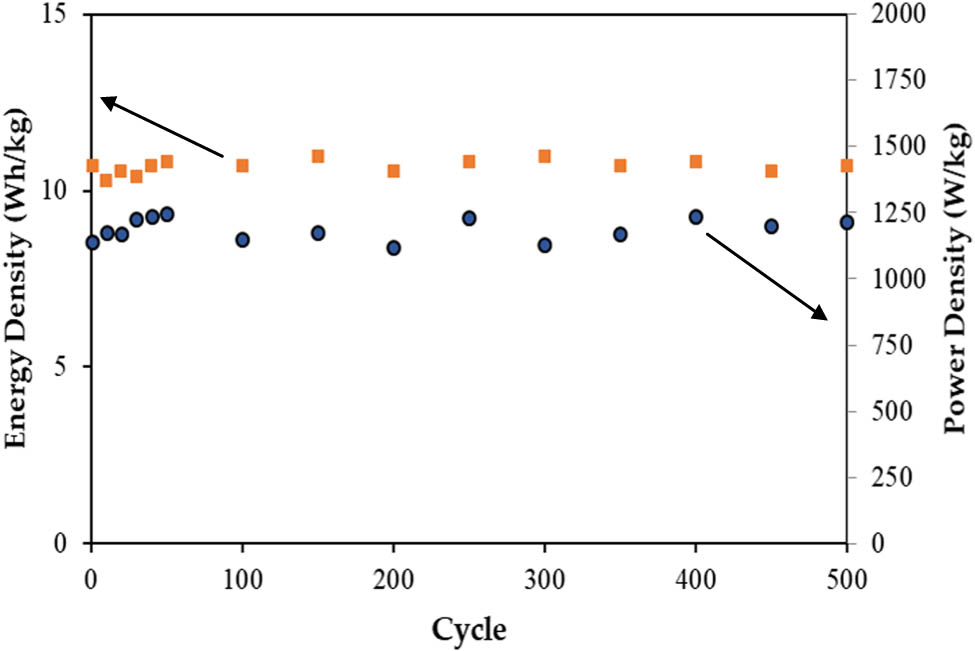
The E and P of the assembled EDLC.
ESDs need to store a lot of energy and provide quick access to electricity when needed. To compare different ESDs, scientists use the Ragone plot, which shows the relationship between energy density (Wh·kg−1) and power density (W·kg−1) on a logarithmic scale. Examples of ESDs include batteries, capacitors, supercapacitors, flywheels, and magnetic ESDs, which occupy different regions on the Ragone plot. When choosing an ESD, power density is an important factor to consider. The study suggests that EDLC devices based on biopolymers are the future of energy storage, but more research is needed to ensure consistent results. To achieve this, researchers need to carefully prepare films, encapsulate the ESDs properly, and analyze the results thoroughly. Our group is working on developing EDLC ESDs that are as good as or better than batteries. To our knowledge, EDLC is the future of ESDs, provided that it can store energy like a battery. The goal for EDLC in the future is shown in Figure 18, and achieving it will help protect us from toxic materials and reduce the effects of temperature changes on our environment.

The obtained energy density position in Ragone plot.
4 Conclusion
A polymer blend electrolyte composed of CH, PVA, NaOAc, and glycerol has been created as a means of reducing plastic waste, particularly micro-plastics, in oceans. This material was found to be suitable for use as an electrode separator in an AC-based EDLC. It was found that at 55% glycerol, the system revealed a high ionic conductivity of 7.56 × 10−5 S·cm−1. The dielectric property study confirmed the ionic conduction process in the system and revealed the existence of non-Debye type relaxation, as indicated by asymmetric peaks of the loss tangent pattern. The alternating conductivity exhibits EP, a plateau (mid frequency region) and dispersion region at high frequency. The majority of the conductivity in the electrolyte was due to ions, with a contribution of 0.84, while electrons contributed only 0.16. The highest conducting CH-PVA-NaOAc-glycerol electrolyte has a potential limit of 2.33 V and can be applied in various electrochemical applications. With no redox peaks in the CV plot and a scan rate-dependent capacitance of 9.4–38 F·g−1, it was clear that the EDLC exhibited capacitive behavior. In the future, the use of other plasticizers such as sorbitol and polyethylene glycol could increase the salt dissociation and improve the specific capacitance of the electrolyte. Additionally, replacing NaOAc with a salt with lower lattice energy may also increase the conductivity value. The charge and discharge measurement of the CH-PVA-NaOAc-glycerol-based EDLC showed an average specific capacitance of 77 F·g−1, an ESR of 87 Ohms, an energy density of 10.7 Wh·kg−1, and a power density of 1,188 W·kg−1. The EDLC also demonstrated high cycling ability by completing 500 charge–discharge cycles. The performance of the EDLC, can be improved by incorporating nano-fillers, polymer grafting, and utilizing different types of carbon, salt, and polymers to enhance both electrodes and electrolytes, which are key components of EDLC. Ensuring tight contact between the electrodes and electrolytes is crucial in achieving this improvement. In conclusion, microbial-based EDLC has proven to be a viable option for low-voltage applications.
Acknowledgements
The authors gratefully acknowledge all supports for this study from the Ministry of Higher Education and Scientific Research-Kurdish National Research Council (KNRC), Kurdistan Regional Government. The financial support from the University of Sulaimani, and University of Human Development and University of Cihan Sulaimaniya are greatly appreciated. This research is partially funded by Khalifa University fund number CIRA-2020-051. The authors express their gratitude to the support of Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R58), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Funding information: Authors state no funding involved.
-
Author contributions: Shujahadeen B. Aziz, Muhamad H. Hamsan, and Rebar T. Abdulwahid: formal analysis, methodology, investigation, and writing original draft; Shujahadeen B. Aziz, Norhana Abdul Halim, Jamal Hassan, Mohd F. Z. Kadir, and Salah R. Saeed: conceptualization, supervision, project administration, and writing – review and editing; Ahmed F. Abdulrahman, Sameerah I. Al-Saeedi, Jihad M. Hadi, and Samir M. Hamad: validation, funding acquisition, and writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Abdulkadir BA, Dennis JO, Bin Abd. Shukur MF, Nasef MME, Usman F, Adam AA, et al. Dielectric study of gel polymer electrolyte based on PVA-K2CO3 -SiO2. IOP Conf Ser Mater Sci Eng. 2021;1092:012066. 10.1088/1757-899x/1092/1/012066.Search in Google Scholar
[2] Mukherjee C, Varghese D, Krishna JS, Boominathan T, Rakeshkumar R, Dineshkumar S, et al. Recent advances in biodegradable polymers – Properties, applications and future prospects. Eur Polym J. 2023;192:112068. 10.1016/j.eurpolymj.2023.112068.Search in Google Scholar
[3] Amalraj J, Lakshmanan P, Amarnath CA, Pyarasani RD, Saravanan C. Biodegradable polymer blend nanocomposites for energy storage application. Polymer blend nanocomposites for energy storage applications. Elsevier Inc; 2023. p. 175–202. 10.1016/B978-0-323-99549-8.00009-1.Search in Google Scholar
[4] Rayung M, Aung MM, Azhar SC, Abdullah LC, Su’ait MS, Ahmad A, et al. Bio-based polymer electrolytes for electrochemical devices: Insight into the ionic conductivity performance. Materials (Basel). 2020;13:838. 10.3390/ma13040838.Search in Google Scholar PubMed PubMed Central
[5] Mohanapriya S, Rambabu G, Bhat SD, Raj V. Pectin based nanocomposite membranes as green electrolytes for direct methanol fuel cells. Arab J Chem. 2020;13:2024–40. 10.1016/j.arabjc.2018.03.001.Search in Google Scholar
[6] Tomi R, Daisuke T, Toshihiko K. Characteristics of electric double-layer capacitors based on solid polymer electrolyte composed of sodium polyacrylate. J Phys Conf Ser. 2022;2368:012002. 10.1088/1742-6596/2368/1/012002.Search in Google Scholar
[7] Hadi JM, Aziz SB, Nofal MM, Hussen SA, Hamsan MH, Brza MA, et al. Electrical, dielectric property and electrochemical performances of plasticized silver ion-conducting chitosan-based polymer nanocomposites. Membranes (Basel). 2020;10:151. 10.3390/membranes10070151.Search in Google Scholar PubMed PubMed Central
[8] Lage-Rivera S, Ares-Pernas A, Abad MJ. Last developments in polymers for wearable energy storage devices. Int J Energy Res. 2022;46:10475–98. 10.1002/er.7934.Search in Google Scholar
[9] Muralee Gopi CVV, Vinodh R, Sambasivam S, Obaidat IM, Kim HJ. Recent progress of advanced energy storage materials for flexible and wearable supercapacitor: From design and development to applications. J Energy Storage. 2020;27:101035. 10.1016/j.est.2019.101035.Search in Google Scholar
[10] Kraiwattanawong K. A review on the development of a porous carbon-based as modeling materials for electric double layer capacitors. Arab J Chem. 2022;15:103625. 10.1016/j.arabjc.2021.103625.Search in Google Scholar
[11] Sadiq M, Raza MMH, Murtaza T, Zulfequar M, Ali J. Sodium ion-conducting polyvinylpyrrolidone (PVP)/polyvinyl alcohol (PVA) blend electrolyte films. J Electron Mater. 2021;50:403–18. 10.1007/s11664-020-08581-1.Search in Google Scholar
[12] Gautam L, Warkar SG, Ahmad SI, Kant R, Jain M. A review on carboxylic acid cross-linked polyvinyl alcohol: Properties and applications. Polym Eng Sci. 2022;62:225–46. 10.1002/pen.25849.Search in Google Scholar
[13] Sharma S, Sudhakara P, Singh J, Ilyas RA, Asyraf MRM, Razman MR. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers (Basel). 2021;13:2623. 10.3390/polym13162623.Search in Google Scholar PubMed PubMed Central
[14] Huang J, Chen M, Zhou Y, Li Y, Hu Y. Functional characteristics improvement by structural modification of hydroxypropyl methylcellulose modified polyvinyl alcohol films incorporating roselle anthocyanins for shrimp freshness monitoring. Int J Biol Macromol. 2020;162:1250–61. 10.1016/j.ijbiomac.2020.06.156.Search in Google Scholar PubMed
[15] Abdulwahid RT, Aziz SB, Kadir MFZ. Insights into ion transport in biodegradable solid polymer blend electrolyte based on FTIR analysis and circuit design. J Phys Chem Solids. 2022;167:110774. 10.1016/j.jpcs.2022.110774.Search in Google Scholar
[16] Abedi-Firoozjah R, Chabook N, Rostami O, Heydari M, Kolahdouz-Nasiri A, Javanmardi F, et al. PVA/starch films: An updated review of their preparation, characterization, and diverse applications in the food industry. Polym Test. 2023;118:107903. 10.1016/j.polymertesting.2022.107903.Search in Google Scholar
[17] Mustafa MF, Ridwan NIM, Hatta FF, Yahya MZA. Effect of dimethyl carbonate plasticizer on ionic conductivity of methyl cellulose-based polymer electrolytes. Malaysian J Anal Sci. 2012;16:283–9.Search in Google Scholar
[18] Teleky B-E, Mitrea L, Plamada D, Nemes SA, Călinoiu, Lavinia-Florina MSP, et al. Development of pectin and poly(vinyl alcohol)-based active packaging enriched with itaconic acid and apple pomace-derived antioxidants. Antioxid Artic. 2022;11:1729. 10.3390/antiox11091729.Search in Google Scholar PubMed PubMed Central
[19] Jiménez-Gómez CP, Cecilia JA. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules. 2020;25:3981. 10.3390/molecules25173981.Search in Google Scholar PubMed PubMed Central
[20] Leceta I, Guerrero P, De La Caba K. Functional properties of chitosan-based films. Carbohydr Polym. 2013;93:339–46. 10.1016/j.carbpol.2012.04.031.Search in Google Scholar PubMed
[21] Tezotto-Uliana JV, Fargoni GP, Geerdink GM, Kluge RA. Chitosan applications pre- or postharvest prolong raspberry shelf-life quality. Postharvest Biol Technol. 2014;91:72–7. 10.1016/j.postharvbio.2013.12.023.Search in Google Scholar
[22] Sahraei Khosh Gardesh A, Badii F, Hashemi M, Ardakani AY, Maftoonazad N, Gorji AM. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT. 2016;70:33–40. 10.1016/j.lwt.2016.02.002.Search in Google Scholar
[23] Duran M, Aday MS, Zorba NND, Temizkan R, Büyükcan MB, Caner C. Potential of antimicrobial active packaging “containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating” to extend shelf life of fresh strawberry. Food Bioprod Process. 2016;98:354–63. 10.1016/j.fbp.2016.01.007.Search in Google Scholar
[24] Liu K, Liu J, Li H, Yuan C, Zhong J, Chen Y. Influence of postharvest citric acid and chitosan coating treatment on ripening attributes and expression of cell wall related genes in cherimoya (Annona cherimola Mill.) fruit. Sci Hortic (Amst). 2016;198:1–11. 10.1016/j.scienta.2015.11.008.Search in Google Scholar
[25] Petriccione M, Pasquariello MS, Mastrobuoni F, Zampella L, Di Patre D, Scortichini M. Influence of a chitosan coating on the quality and nutraceutical traits of loquat fruit during postharvest life. Sci Hortic (Amst). 2015;197:287–96. 10.1016/j.scienta.2015.09.051.Search in Google Scholar
[26] Hadi JM, Aziz SB, Saeed SR, Brza MA, Abdulwahid RT, Hamsan MH, et al. Investigation of ion transport parameters and electrochemical performance of plasticized biocompatible chitosan-based proton conducting polymer composite electrolytes. Membranes (Basel). 2020;10:363. 10.3390/membranes10110363.Search in Google Scholar PubMed PubMed Central
[27] Shukur MF, Ithnin R, Kadir MFZ. Ionic conductivity and dielectric properties of potato starch-magnesium acetate biopolymer electrolytes: the effect of glycerol and 1-butyl-3-methylimidazolium chloride. Ion (Kiel). 2016;22:1113–23. 10.1007/s11581-015-1627-4.Search in Google Scholar
[28] Chai MN, Isa MIN. Novel proton conducting solid bio-polymer electrolytes based on carboxymethyl cellulose doped with oleic acid and plasticized with glycerol. Sci Rep. 2016;6:27328. 10.1038/srep27328.Search in Google Scholar PubMed PubMed Central
[29] Mattos RI, Raphael E, Majid SR, Arof AK, Pawlicka A. Enhancement of electrical conductivity in plasticized chitosan based membranes. Mol Cryst Liq Cryst. 2012;554:150–9. 10.1080/15421406.2012.633862.Search in Google Scholar
[30] Nasef MM, Saidi H, Dahlan KZM. Preparation of composite polymer electrolytes by electron beam-induced grafting: Proton- and lithium ion-conducting membranes. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At. 2007;265:168–72. 10.1016/j.nimb.2007.08.044.Search in Google Scholar
[31] Biskri ZE, Rached H, Bouchear M, Rached D, Aida MS. A comparative study of structural stability and mechanical and optical properties of fluorapatite (Ca5(PO4)3F) and lithium disilicate (Li2Si2O5) components forming dental glass–ceramics: First principles study. J Electron Mater. 2016;45:5082–95. 10.1007/s11664-016-4681-4.Search in Google Scholar
[32] Abdulwahid RT, Aziz SB, Kadir MFZ. Environmentally friendly plasticized electrolyte based on chitosan (CS): Potato starch (PS) polymers for EDLC application: Steps toward the greener energy storage devices derived from biopolymers. J Energy Storage. 2023;67:107636. 10.1016/j.est.2023.107636.Search in Google Scholar
[33] Dennis JO, Adam AA, Ali MKM, Soleimani H, Shukur MFBA, Ibnaouf KH, et al. Substantial proton ion conduction in methylcellulose/pectin/ammonium chloride based solid nanocomposite polymer electrolytes: Effect of ZnO nanofiller. Membranes (Basel). 2022;12:706. 10.3390/membranes12070706.Search in Google Scholar PubMed PubMed Central
[34] Adam AA, Soleimani H, Shukur MFBA, Dennis JO, Abdulkadir BA, Hassan YM, et al. A new approach to understanding the interaction effect of salt and plasticizer on solid polymer electrolytes using statistical model and artificial intelligence algorithm. J Non Cryst Solids. 2022;587:121597. 10.1016/j.jnoncrysol.2022.121597.Search in Google Scholar
[35] Tripathi M, Tripathi SK. Electrical studies on ionic liquid-based gel polymer electrolyte for its application in EDLCs. Ion (Kiel). 2017;23:2735–46. 10.1007/s11581-017-2051-8.Search in Google Scholar
[36] Abdulwahid RT, Aziz SB, Kadir MFZ. Design of proton conducting solid biopolymer blend electrolytes based on chitosan-potato starch biopolymers: Deep approaches to structural and ion relaxation dynamics of H+ ion. J Appl Polym Sci. 2022;139:e52892. 10.1002/app.52892.Search in Google Scholar
[37] Kim KM, Ryu KS, Kang S-G, Chang SH, Chung IJ. The effect of silica addition on the properties of poly((vinylidene fluoride)-co-hexafluoropropylene)-based polymer electrolytes. Macromol Chem Phys. 2001;202:866–72. 10.1002/1521-3935(20010301)202:6<866::aid-macp866>3.3.co;2-3.Search in Google Scholar
[38] Fuzlin AF, Rasali NMJ, Samsudin AS. Effect on ammonium bromide in dielectric behavior based alginate solid biopolymer electrolytes. IOP Conf Ser Mater Sci Eng. 2018;342:012080. 10.1088/1757-899X/342/1/012080.Search in Google Scholar
[39] Aziz SB, Abidin ZHZ. Electrical and morphological analysis of chitosan: AgTf solid electrolyte. Mater Chem Phys. 2014;144:280–6. 10.1016/j.matchemphys.2013.12.029.Search in Google Scholar
[40] Chaurasia SK, Saroj AL, Shalu, Singh VK, Tripathi AK, Gupta AK, et al. Studies on structural, thermal and AC conductivity scaling of PEO-LiPF6 polymer electrolyte with added ionic liquid [BMIMPF6]. AIP Adv. 2015;5:077178. 10.1063/1.4927768.Search in Google Scholar
[41] Tripathi SK, Jain A, Gupta A, Mishra M. Electrical and electrochemical studies on magnesium ion-based polymer gel electrolytes. J Solid State Electrochem. 2012;16:1799–806. 10.1007/s10008-012-1656-0.Search in Google Scholar
[42] Basha SKS, Sundari GS, Kumar KV, Rao MC. Structural and dielectric properties of PVP based composite polymer electrolyte thin films. J Inorg Organomet Polym Mater. 2017;27:455–66. 10.1007/s10904-016-0487-3.Search in Google Scholar
[43] Pradhan DK, Choudhary RNP, Samantaray BK. Studies of dielectric and electrical properties of plasticized polymer nanocomposite electrolytes. Mater Chem Phys. 2009;115:557–61. 10.1016/j.matchemphys.2009.01.008.Search in Google Scholar
[44] Pradhan DK, Choudhary RNP, Samantaray BK. Studies of dielectric relaxation and AC conductivity behavior of plasticized polymer nanocomposite electrolytes. Int J Electrochem Sci. 2008;3:597–608.10.1016/S1452-3981(23)15547-7Search in Google Scholar
[45] Nithya H, Selvasekarapandian S, Arun Kumar D, Sakunthala A, Hema M, Christopherselvin P, et al. Thermal and dielectric studies of polymer electrolyte based on P(ECH-EO). Mater Chem Phys. 2011;126:404–8. 10.1016/j.matchemphys.2010.10.047.Search in Google Scholar
[46] Kulshrestha N, Chatterjee B, Gupta PN. Structural, thermal, electrical, and dielectric properties of synthesized nanocomposite solid polymer electrolytes. High Perform Polym. 2014;26:677–88. 10.1177/0954008314541820.Search in Google Scholar
[47] Agrawal SL, Singh M, Tripathi M, Dwivedi MM, Pandey K. Dielectric relaxation studies on [PEO-SiO2]: NH4SCN nanocomposite polymer electrolyte films. J Mater Sci. 2009;44:6060–8. 10.1007/s10853-009-3833-9.Search in Google Scholar
[48] Manjunatha H, Damle R, Pravin K, Kumaraswamy GN. Modification in the transport and morphological properties of solid polymer electrolyte system by low-energy ion irradiation. Ion (Kiel). 2018;24:3027–37. 10.1007/s11581-018-2518-2.Search in Google Scholar
[49] Hadi JM, Aziz SB, Mustafa MS, Brza MA, Hamsan MH, Kadir MFZ, et al. Electrochemical impedance study of proton conducting polymer electrolytes based on PVC doped with thiocyanate and plasticized with glycerol. Int J Electrochem Sci. 2020;15:4671–83. 10.20964/2020.05.34.Search in Google Scholar
[50] Subba Reddy CV, Han X, Zhu QY, Mai LQ, Chen W. Dielectric spectroscopy studies on (PVP + PVA) polyblend film. Microelectron Eng. 2006;83:281–5. 10.1016/j.mee.2005.08.010.Search in Google Scholar
[51] Hirankumar G, Mehta N. Effect of incorporation of different plasticizers on structural and ion transport properties of PVA-LiClO4 based electrolytes. Heliyon. 2018;4:e00992. 10.1016/j.heliyon.2018.e00992.Search in Google Scholar PubMed PubMed Central
[52] Nicolau A, Nucci AM, Martini EMA, Samios D. Electrical impedance spectroscopy of epoxy systems II: Molar fraction variation, resistivity, capacitance and relaxation processes of 1,4-butanediol diglycidyl ether/succinic anhydride and triethylamine as initiator. Eur Polym J. 2007;43:2708–17. 10.1016/j.eurpolymj.2007.03.018.Search in Google Scholar
[53] Wang W, Alexandridis P. Composite polymer electrolytes: Nanoparticles affect structure and properties. Polymers. 2016;8:387. 10.3390/polym8110387.Search in Google Scholar PubMed PubMed Central
[54] Marzantowicz M, Dygas JR, Krok F, Florjańczyk Z, Zygadło-Monikowska E. Conductivity and dielectric properties of polymer electrolytes PEO:LiN(CF3SO2)2 near glass transition. J Non Cryst Solids. 2007;353:4467–73. 10.1016/j.jnoncrysol.2007.04.046.Search in Google Scholar
[55] Jiang H, Hong L, Venkatasubramanian N, Grant JT, Eyink K, Wiacek K, et al. The relationship between chemical structure and dielectric properties of plasma-enhanced chemical vapor deposited polymer thin films. Thin Solid Films. 2007;515:3513–20. 10.1016/j.tsf.2006.10.126.Search in Google Scholar
[56] Rafi NSM, Abidin SZZ, Majid SR, Zakaria R. Preparation of agarose-based biopolymer electrolytes containing calcium thiocyanate: Electrical and electrochemical properties. Int J Electrochem Sci. 2022;17:220713. 10.20964/2022.07.21.Search in Google Scholar
[57] Vahini M, Muthuvinayagam M, Isa MIN. Preparation and characterization of biopolymer electrolytes based on pectin and NaNO3 for battery applications. Polym Sci - Ser A. 2019;61:823–31. 10.1134/S0965545X19060129.Search in Google Scholar
[58] Aziz SB, Brevik I, Hamsan MH, Brza MA, Nofal MM, Abdullah AM, et al. Compatible solid polymer electrolyte based on methyl cellulose for energy storage application: Structural, electrical, and electrochemical properties. Polymers (Basel). 2020;12:2257. 10.3390/polym12102257.Search in Google Scholar PubMed PubMed Central
[59] Abdulwahid RT, Aziz SB, Kadir MFZ. Replacing synthetic polymer electrolytes in energy storage with flexible biodegradable alternatives: sustainable green biopolymer blend electrolyte for supercapacitor device. Mater Today Sustain. 2023;23:100472. 10.1016/j.mtsust.2023.100472.Search in Google Scholar
[60] Abbas Adam A, Soleimani H, Muhammad MF, Ojur Dennis J, Abubakar Abdulkadir B, Mudassir Hassan Y, et al. Structural behavior and ion dynamics of methylcellulose/tri-potassium phosphate-based solid biopolymeric electrolytes. Mol Cryst Liq Cryst. 2022;759:29–34.10.1080/15421406.2022.2160901Search in Google Scholar
[61] Teo LP, Buraidah MH, Arof AK. Development on solid polymer electrolytes for electrochemical devices. Molecules. 2021;26:6499. 10.3390/molecules26216499.Search in Google Scholar PubMed PubMed Central
[62] Ibrahim S, Ahmad A, Mohamed NS. Characterization of novel castor oil-based polyurethane polymer electrolytes. Polymers (Basel). 2015;7:747–59. 10.3390/polym7040747.Search in Google Scholar
[63] Irfan M, Manjunath A, Mahesh SS, Somashekar R, Demappa T. Influence of NaF salt doping on electrical and optical properties of PVA/PVP polymer blend electrolyte films for battery application. J Mater Sci Mater Electron. 2021;32:5520–37. 10.1007/s10854-021-05274-1.Search in Google Scholar
[64] Muchakayala R, Song S, Wang J, Fan Y, Bengeppagari M, Chen J, et al. Development and supercapacitor application of ionic liquid-incorporated gel polymer electrolyte films. J Ind Eng Chem. 2018;59:79–89. 10.1016/j.jiec.2017.10.009.Search in Google Scholar
[65] Petnikota S, Chua R, Zhou Y, Edison E, Srinivasan M. Amorphous vanadium oxide thin films as stable performing cathodes of lithium and sodium-ion batteries. Nanoscale Res Lett. 2018;13:363. 10.1186/s11671-018-2766-0.Search in Google Scholar PubMed PubMed Central
[66] Pal B, Yang S, Ramesh S, Thangadurai V, Jose R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019;1:3807–35. 10.1039/c9na00374f.Search in Google Scholar PubMed PubMed Central
[67] Nadiah NS, Omar FS, Numan A, Mahipal YK, Ramesh S, Ramesh K. Influence of acrylic acid on ethylene carbonate/dimethyl carbonate based liquid electrolyte and its supercapacitor application. Int J Hydrog Energy. 2017;42:30683–90. 10.1016/j.ijhydene.2017.10.140.Search in Google Scholar
[68] Ngai KS, Ramesh S, Ramesh K, Juan JC. A review of polymer electrolytes: fundamental, approaches and applications. Ion (Kiel). 2016;22:1259–79. 10.1007/s11581-016-1756-4.Search in Google Scholar
[69] Wojciechowski J, Kolanowski Ł, Bund A, Lota G. The influence of current collector corrosion on the performance of electrochemical capacitors. J Power Sources. 2017;368:18–29. 10.1016/j.jpowsour.2017.09.069.Search in Google Scholar
[70] Hamsan MH, Shukur MF, Kadir MFZ. NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ion (Kiel). 2017;23:3429–53. 10.1007/s11581-017-2155-1.Search in Google Scholar
[71] Winie T, Jamal A, Saaid FI, Tseng TY. Hexanoyl chitosan/ENR25 blend polymer electrolyte system for electrical double layer capacitor. Polym Adv Technol. 2019;30:726–35. 10.1002/pat.4510.Search in Google Scholar
[72] Yassine M, Fabris D. Performance of commercially available supercapacitors. Energies. 2017;10:1340. 10.3390/en10091340.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

