Abstract
In this article, ZnO quantum dots (QDs)-g-C3N4 complexes were prepared by a combined sol–gel method and ultrasound-assisted chemical method, and ZnO-g-C3N4 composites with different doping ratios were also prepared for photocatalytic degradation of dye wastewater. The composites were characterized by scanning electron microscopy, transmission electron microscopy, X-ray diffractometry, Fourier transform infrared, X-ray photoelectron spectroscopy, diffuse reflectance spectroscopy, and photoluminescence. The photocatalytic performance of the best ZnO QDs-g-C3N4 complexes with different g-C3N4 doping amounts was investigated, and the kinetics of their photocatalytic reactions were analyzed, and it was found that the best effect of ZnO-g-C3N4 10% could reach 89.08% and ZnO QDs-g-C3N4 could reach 91.53%. It was also demonstrated that ZnO-g-C3N4 10%, ZnO QDs-g-C3N4 cyclic stability is better, and the reaction mechanism of ZnO QDs-g-C3N4 was investigated. It can be used for the degradation of dyes in environmental wastewater and the removal of harmful substances from the natural environment.
1 Introduction
Rapid economic development from the past century to this century and the use of drugs and chemicals in agriculture have made water pollution more and more serious [1]. The deterioration of water quality in rivers and lakes and the scarcity of freshwater resources have seriously threatened the health of the water environment [2]. Especially in the era of rapid industrial development, printing and dyeing wastewater treatment has been a key issue. Dye wastewater with large amount, high organic content and high chroma is widely recognized as intractable industrial wastewater [3,4,5,6]. To solve these problems, numerous researchers have used various methods to treat dye wastewater [7].
Among the many water pollution treatment options that have been developed, photocatalysis is an effective method for solving water pollution problems because of its low operating costs, its ability to completely mineralize pollutants, and its ability to degrade organic pollutants to less toxic or non-toxic components [8]. Photocatalysis can use solar energy to degrade pollutants by exciting electron transfer in semiconductor photocatalysts [9]. The most important factor in this technology is the photocatalyst, which converts solar energy into chemical energy that destroys the pollutant. Currently, metals, metal oxides, nonmetals, semiconductors, metal–organic frameworks, and other materials have been extensively studied for the degradation of dye molecules in wastewater [10,11,12].
As a representative of photocatalytic materials, zinc oxide (ZnO) stands out in water treatment because of its unique attributes, such as low price, good stability, and obvious surface and interface effects [13,14,15]. However, ZnO photocatalyst itself also has some defects: its own light absorption capacity is very poor and electron-hole pair separation rate is very low [16,17,18,19]. Therefore, it has become a hot topic of research to improve the degradation efficiency under visible light through research on its own modification: doping modification, crystal appearance control, thin film loading, composite of different materials, etc. [20,21,22,23]. Also known as semiconductor nanoparticles or semiconductor nanocrystals, quantum dots (QDs) have a size-dependent electronic structure and excellent optoelectronic properties.
QDs have been regarded as a new generation of fluorescent biomarker probes that can replace traditional fluorescent organic molecules by researchers worldwide and have been widely used in various disciplines such as cell labeling [24,25], nano-drug delivery [26,27,28,29], and biosensors [30]. The main methods commonly used for the synthesis of ZnO QDs are hydrothermal method [31], sol–gel method [32], and microemulsion method [33].
The advantages of g-C3N4, such as high stability of physicochemical properties, diverse structures, simple preparation, and abundant and cheap preparation materials, make it widely used in photocatalytic carbon dioxide conversion [34,35,36,37,38], photocatalytic pollutant treatment [39,40,41], photocatalytic organic synthesis [42,43,44], and especially in photocatalytic decomposition of aquatic hydrogen.
Rhodamine B (RhB) is one of the main pollutants in dye wastewater. RhB is a bright-red synthetic onion quinone dye. Because of its bright color, it is widely used in dyeing and textile industry.
Commonly used water treatment methods have high treatment costs, low removal rate, secondary pollution, and other disadvantages, so it needs a water treatment technology with high efficiency, environmental protection, complete reaction, and no secondary pollution. Semiconductor photocatalysis technology has thus emerged. It is favoured by researchers in environmental wastewater treatment due to its conditions of high efficiency, non-pollution, low energy consumption, simple operation and easy control. It can also directly convert organic matter in water into non-toxic materials without causing secondary pollution [45,46,47,48].
Waseem et al. [49] greatly improved the degradation rate by doping neodymium and erbium on ZnO, where the neodymium-doped ZnO was essentially completely degraded for methylene blue within 10 min. Suwiwat et al. [50] used highly mesoporous carbon-ZnO nanocomposites and found that the adsorption of methylene blue and Congo red were 399 and 410 mg g−1, respectively, and the synergistic effect of adsorption and photocatalytic degradation resulted in a maximum removal of methylene blue of 614 mg g−1 and Congo red of 2,628 mg g−1. Huang et al. [51] prepared thin layers of porous amino-rich g-C3N4 by a precursor modification strategy using different acid-reformed dicyandiamide and applied them to the degradation of RhB.
In this article, ZnO-g-C3N4, ZnO QDs-g-C3N4 complexes with different doping ratios were synthesized, and a series of characterizations were performed to demonstrate their successful preparation. The photocatalytic degradation experiments were carried out using RhB solution as the simulated dye wastewater in the degradation experiments under visible light. The stability of the catalyst was investigated after five cycles using catalyst cycling stability experiments. The principle of photocatalytic degradation of ZnO QDs and its complexes was investigated using free radical capture experiments.
2 Material preparation
2.1 Preparation of ZnO-g-C3N4
One gram of ZnO was added to a beaker containing 60 mL of anhydrous ethanol, and the corresponding mass of g-C3N4 weighed in different ratios was added to the beaker and sonicated for 1 h. It was poured into the hydrothermal reactor and put into the high temperature drying oven at 140°C for 10 h. The purpose of fully combining the two powders under high temperature and pressure was achieved. The material was removed and centrifuged, the supernatant was poured off, and the remaining powder was placed in a vacuum drying oven at 60°C for 24 h to dry the sample sufficiently. Then the samples were put into a high temperature drying oven at 200°C and kept for 120 min, which can improve the crystallinity and remove the water from the photocatalyst at the same time. The products were labeled as ZC-3, ZC-5, ZC-7, ZC-10, and ZC-20 by adding g-C3N4 with different mass fractions of 3%, 5%, 7%, 10%, and 20%, respectively.
2.2 Preparation method of ZnO QDs-g-C3N4
One gram of ZnO QDs is weighed and added into a beaker containing 60 mL of anhydrous ethanol; then 0.01 g of g-C3N4 is added into the beaker and ultrasonication is performed for 1 h so that the mixed solution can be fully mixed. After the ultrasound is finished, the material in the beaker is poured into the hydrothermal reaction kettle and put into the high-temperature drying oven. The temperature of the high-temperature drying oven is set at 140℃ and the time is set at 10 h, to achieve the purpose of fully combining the two powders under high temperature and high pressure.
The material is taken out and centrifuged. The supernatant of the centrifuged material is poured out and the remaining powder was put into a vacuum drying oven at 60℃ for 24 h to dry the sample. To improve the crystallinity of the samples, the samples were then placed in a high-temperature drying oven set at 200°C for 120 min. At the same time, the water in the photocatalyst was removed and the product was labeled ZC QDs-10.
3 Results and discussion
3.1 Effect of different pH solutions on the degradation performance of RhB
A measure of 0.02 g of photocatalyst ZnO-g-C3N4 10% composite was taken, and 50 mL of 10 mg·L−1 of RhB solution with pH values of 1, 3, 5, 7, and 11 were prepared. The effect of pH of the solution on the degradation performance was discussed by dark reaction for 60 min and light reaction for 6 h at room temperature. The experimental results are shown in Figure 1.
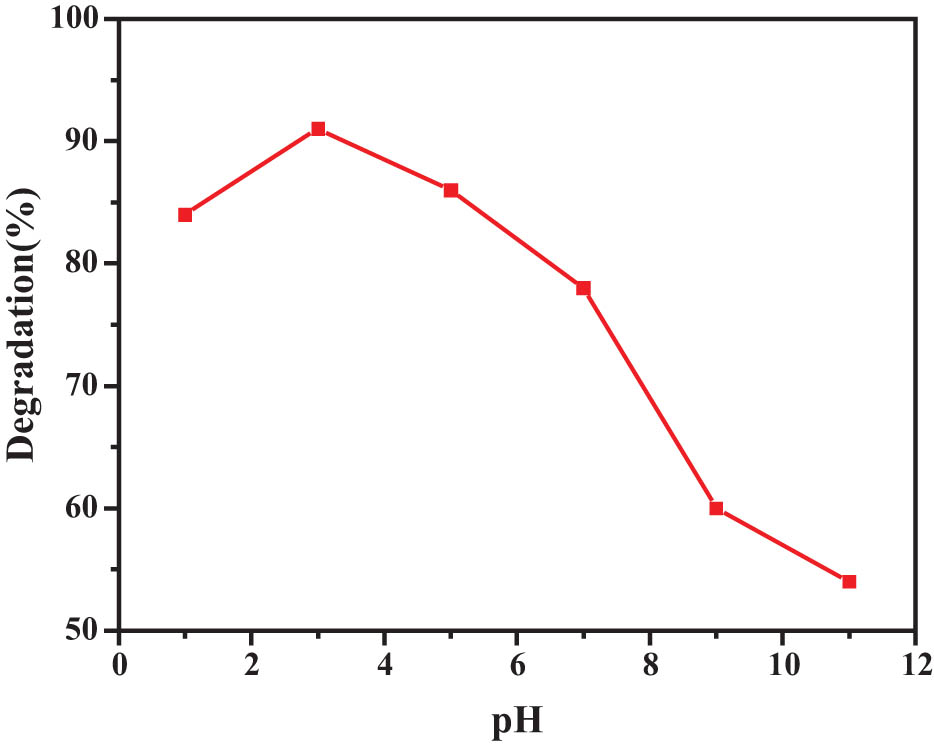
Effect of different pH solutions on the degradation performance of RhB.
As can be seen from Figure 1, the degradation of catalyst is relatively good when the pH value is in the acidic range. When pH = 3, its degradation rate reaches the maximum, about 91%. The reason for this phenomenon is that the OH− or H+ added to the solution changes the charge characteristics and interface properties of the surface of the photocatalyst of ZnO-g-C3N4 10% composite material, as well as the existence form of degradation substances, which further affects the catalytic activity of the photocatalyst. When there is a large amount of OH− in the solution, the catalyst itself is negatively charged, while the analog dye RhB is positively charged. At this time, it will trap the resulting photogenic holes, thus reducing the hydroxyl radical. Therefore, in alkaline environment, its degradation ability is greatly inhibited. When a large amount of H+ exists in the solution, the situation is just the opposite. It can transfer the photogenerated electrons to itself, which is further conducive to the generation of oxidizing hydroxyl radical and improve the degradation performance of the photocatalyst. Therefore, in the acidic range, the degradation performance of the catalyst is very good, and the degradation rate of the simulated dye RhB is also high.
3.2 Field emission scanning electron microscopy (FESEM) (ZG)
The morphology of the nanoparticles can be directly observed by FESEM. Figure 2a and b shows that the morphology of ZnO nanoparticles shows a relatively regular granular structure and all particles have a relatively uniform size, so the preparation of the material is successful. As can be seen in Figure 2c, the prepared samples have a lamellar structure that is mostly broken, with a highly irregular surface and the appearance of holes, probably because of the unsatisfactory melamine condensation effect during firing. The structure of the lamellae was thin and relatively uniformly distributed. Figure 2d–f presents the SEM scans of ZnO-g-C3N4 with different mass percentages of doped g-C3N4; it can be seen that the surface morphology of the binary composite ZnO-g-C3N4 is also small particles and shows a uniform distribution, in which g-C3N4 is not clearly visible in the figure, indicating that ZnO is dispersed more uniformly on the surface of the flake g-C3N4, which also indicates that the two materials have good affinity when they are compounded. Moreover, the microscopic morphology of g-C3N4-ZnO did not change significantly with the increase of the mass percentage of g-C3N4.
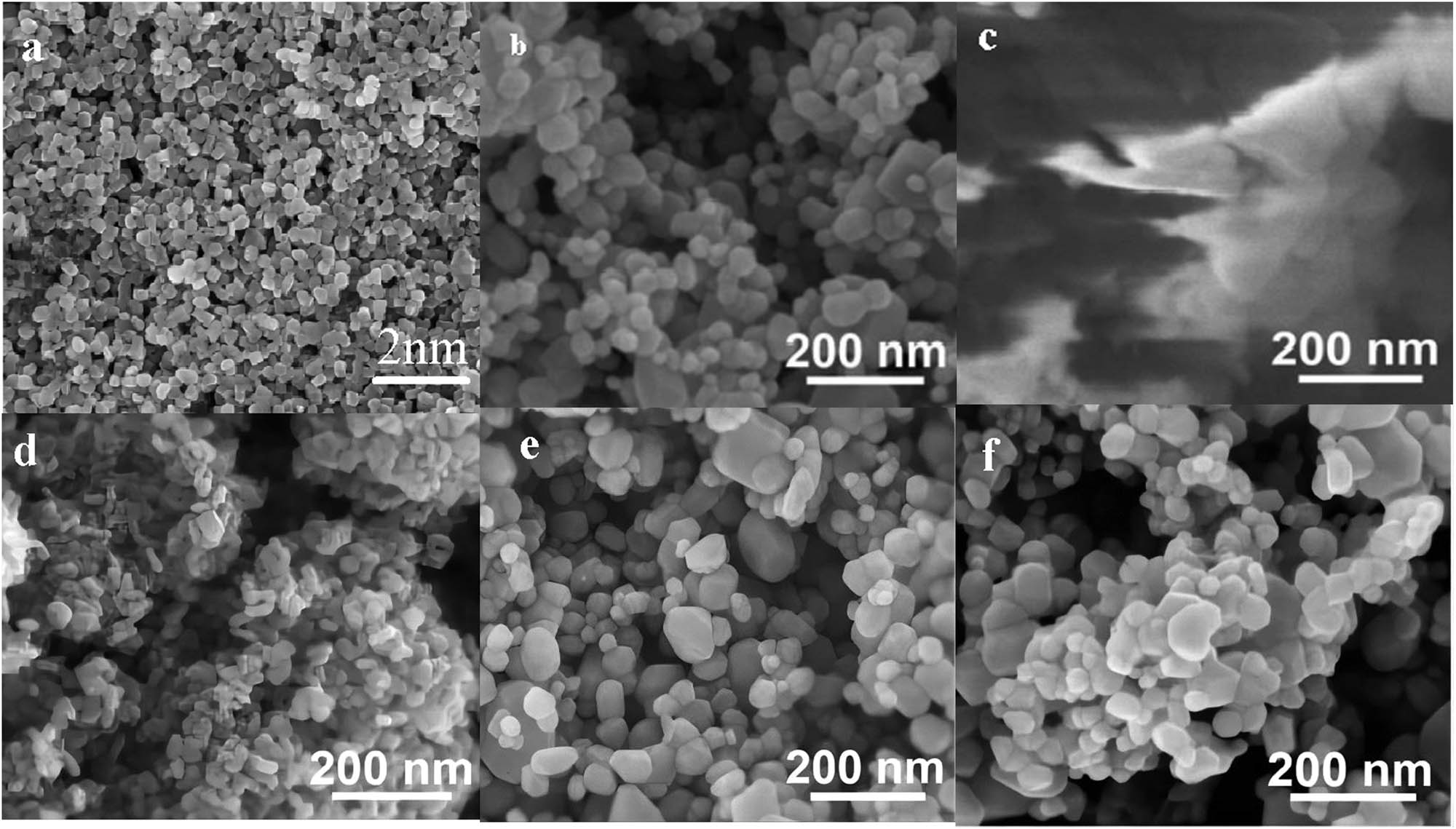
FESEM images of (a,b) ZnO, (c) g-C3N4, (d) ZnO-g-C3N4 3%, (e) ZnO-g-C3N4 10%, and (f) ZnO-g-C3N4 20% nanoparticles.
3.3 Transmission electron microscopy (TEM) (ZG)
To further observe the morphological characteristics inside the ZnO-g-C3N4 10% composite and the distribution of ZnO in g-C3N4, TEM characterization was performed. It can be seen from Figure 3a that ZnO is well dispersed, one by one in granular form with relatively uniform size. The spherical ZnO is attached to the g-C3N4 sheet in Figure 3b, verifying that the composite consists of ZnO and g-C3N4.
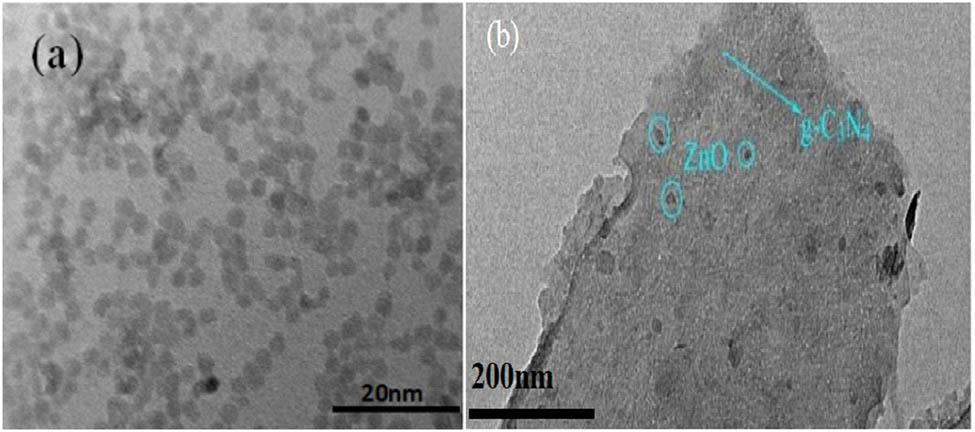
TEM diagram of (a) ZnO and (b) ZnO-g-C3N4 10% nanoparticles.
3.4 High-resolution transmission electron microscopy (HRTEM) (ZG QDs)
In order to obtain the morphological characteristics of ZnO QDs, HRTEM analysis was performed using the ZnO QDs. It can be seen that the produced samples are well dispersed and have a relatively uniform particle size scale distribution. The dark-colored part with lattice display is the core of ZnO QDs. Figure 4a and b is the TEM image of ZnO QDs. As can be seen from the TEM image in Figure 4c, the sample prepared is the ZnO QDs-g-C3N4 composite structure.
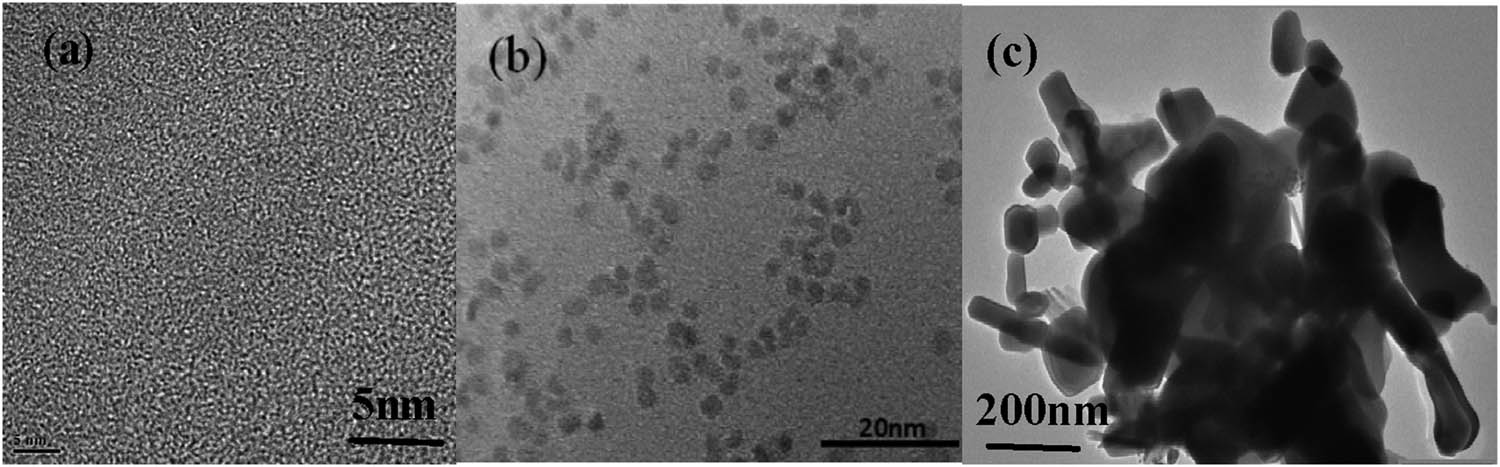
HRTEM images of (a,b) ZnO QDS and (c) ZnO QDS-g-C3N4 10% nanoparticles.
3.5 X-ray diffractometry (XRD)
Figure 5 shows the XRD plots of ZnO-g-C3N4 binary composites with different g-C3N4 contents. And it can be seen that the XRD plots of ZC binary composites with different g-C3N4 contents correspond well with the XRD peak positions of single ZnO. The peak of g-C3N4 appeared at 2θ of 27.8° in the binary composites 20% ZC and 10% ZC, indicating that g-C3N4 was successfully compounded with ZnO by hydrothermal method. g-C3N4 peaks did not appear in the XRD plots of 3% ZC, 5% ZC, and 7% ZC, probably due to the relatively low content of g-C3N4. 10% ZC had the sharpest peak pattern with more prominent and better recognizability.
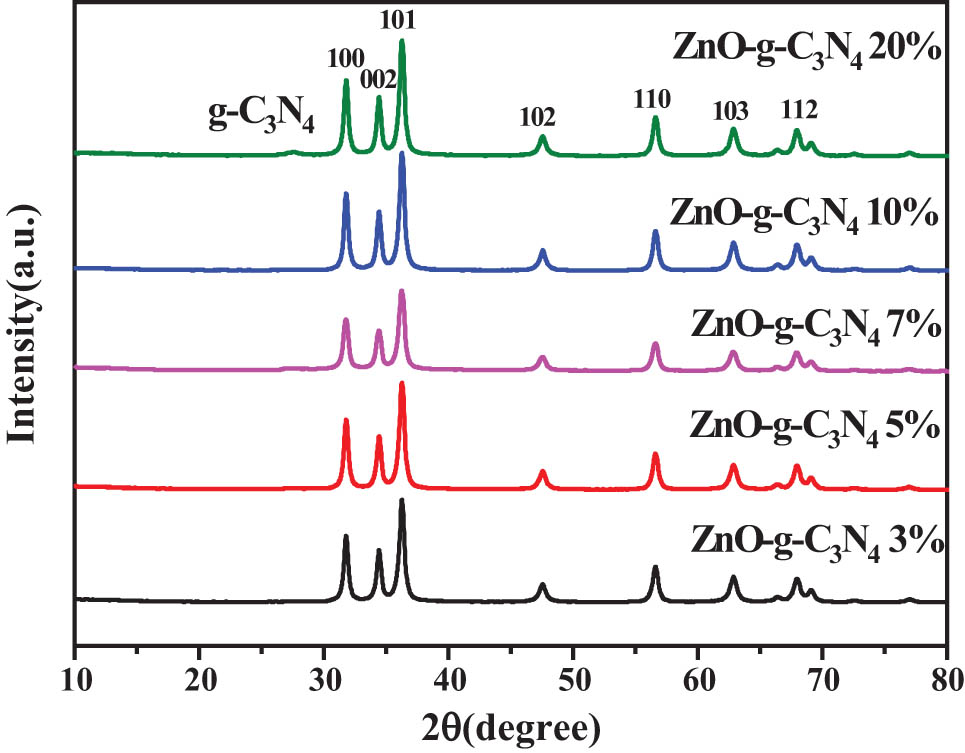
XRD diagram of composite samples with different g-C3N4 contents of ZnO-g-C3N4.
The data of the XRD patterns of ZnO QDs-g-C3N4 10%, ZnO-g-C3N4 10%, ZnO, and ZnO QD composite samples are displayed in Figure 6. It can be seen from the figure that compound ZnO QDs-g-C3N4 10% was compared with ZnO-g-C3N4 10%, and the peak area widened, which could improve the photocatalytic performance.
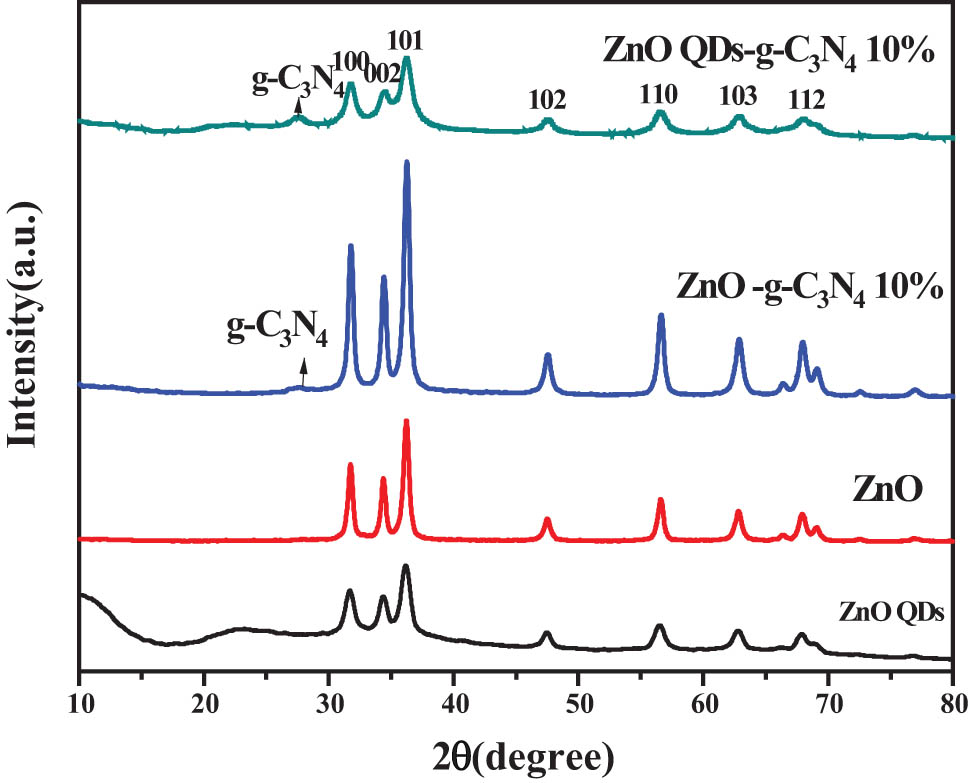
XRD plots of ZnOQDs-g-C3N4 10%, ZnO-g-C3N4 10%, ZnO, and ZnO QD composite samples.
3.6 Fourier transform infrared (FT-IR)
The complexes also have typical stretching vibrational absorption peaks of g-C3N4 around 1240–1640 cm−1 and 810 cm−1. This also indicates the successful complexation of ZnO, ZnO QDs with g-C3N4 in the complexes ZnO-g-C3N4 10%, ZnO QDs-g-C3N4 10%. The absorption peaks near 1,240–1,640 cm−1 and 810 cm−1 are attributed to the typical stretching vibration of g-C3N4, which also indicates the successful compounding of ZnO with g-C3N4 in the complex ZnO-g-C3N4.
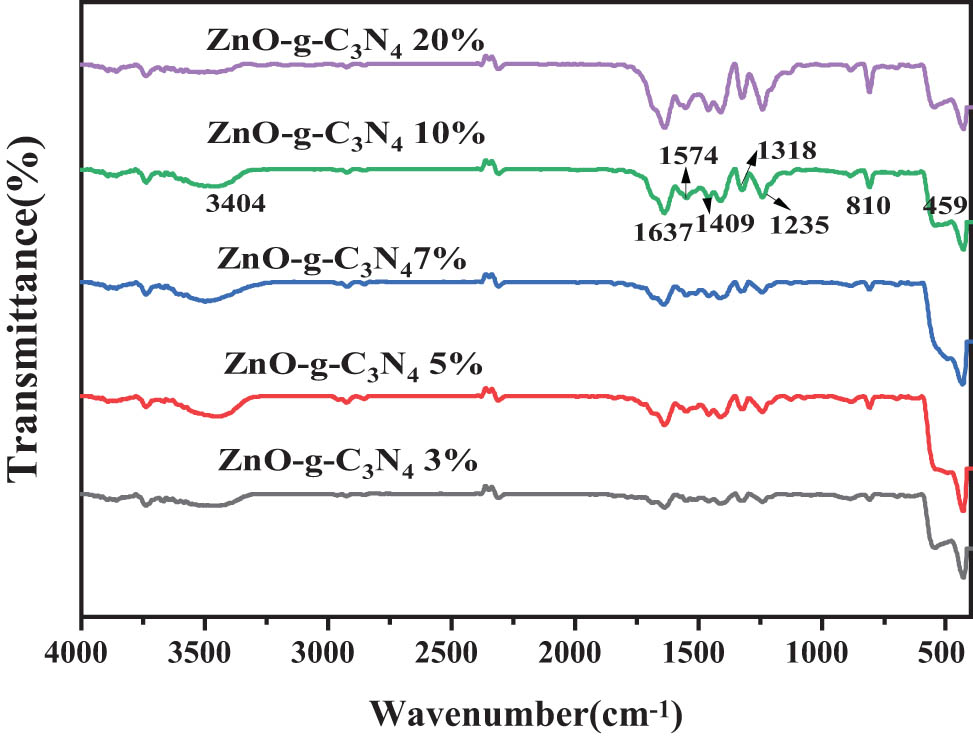
FT-IR spectrum map of the composite samples with different g-C3N4 contents of ZnO-g-C3N4.
For the composites ZnO-g-C3N4 10%, ZnO QDs-g-C3N4 10%, it can be seen from Figure 8 that both have a wider typical absorption peak of ZnO in the range of 500 cm−1 vibrated by Zn-O stretching. The complexes also have typical stretching vibrational absorption peaks of g-C3N4 around 1240–1640 cm−1 and 810 cm−1. This also indicates the successful complexation of ZnO, ZnO QDs with g-C3N4 in the complexes ZnO-g-C3N4 10%, ZnO QDs-g-C3N4 10% in the successful complexation of ZnO, ZnO QDs, and g-C3N4.
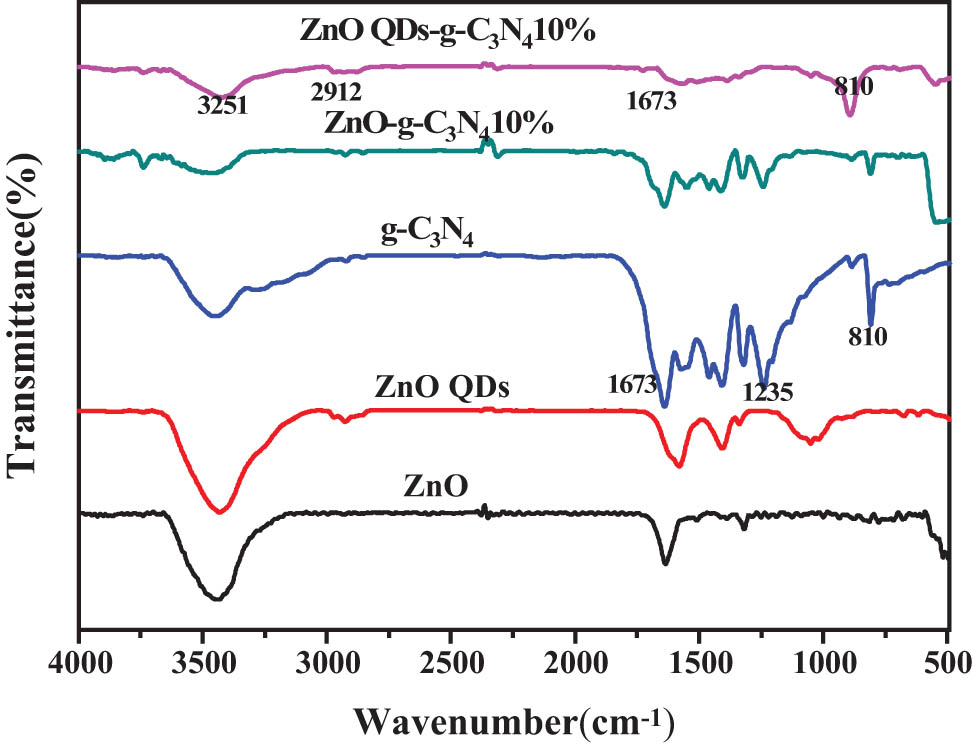
The FT-IR spectral map of ZnO-g-C3N4 10% and ZnO QDs-g-C3N4 10%.
3.7 X-ray photoelectron spectroscopy (XPS)
Figure 9a–f presents the full spectrum analysis diagram of ZC QD composite sample and the XPS diagram of four elements. The presence of the four elements can be seen in the spectrogram of the XPS of the ZnOC QD composite in Figure 9a. The peak positions of C, N, O, and Zn in the binary composite can be determined from the figure, and there are no miscellaneous peaks in the figure, indicating the successful synthesis of ZC binary composite.
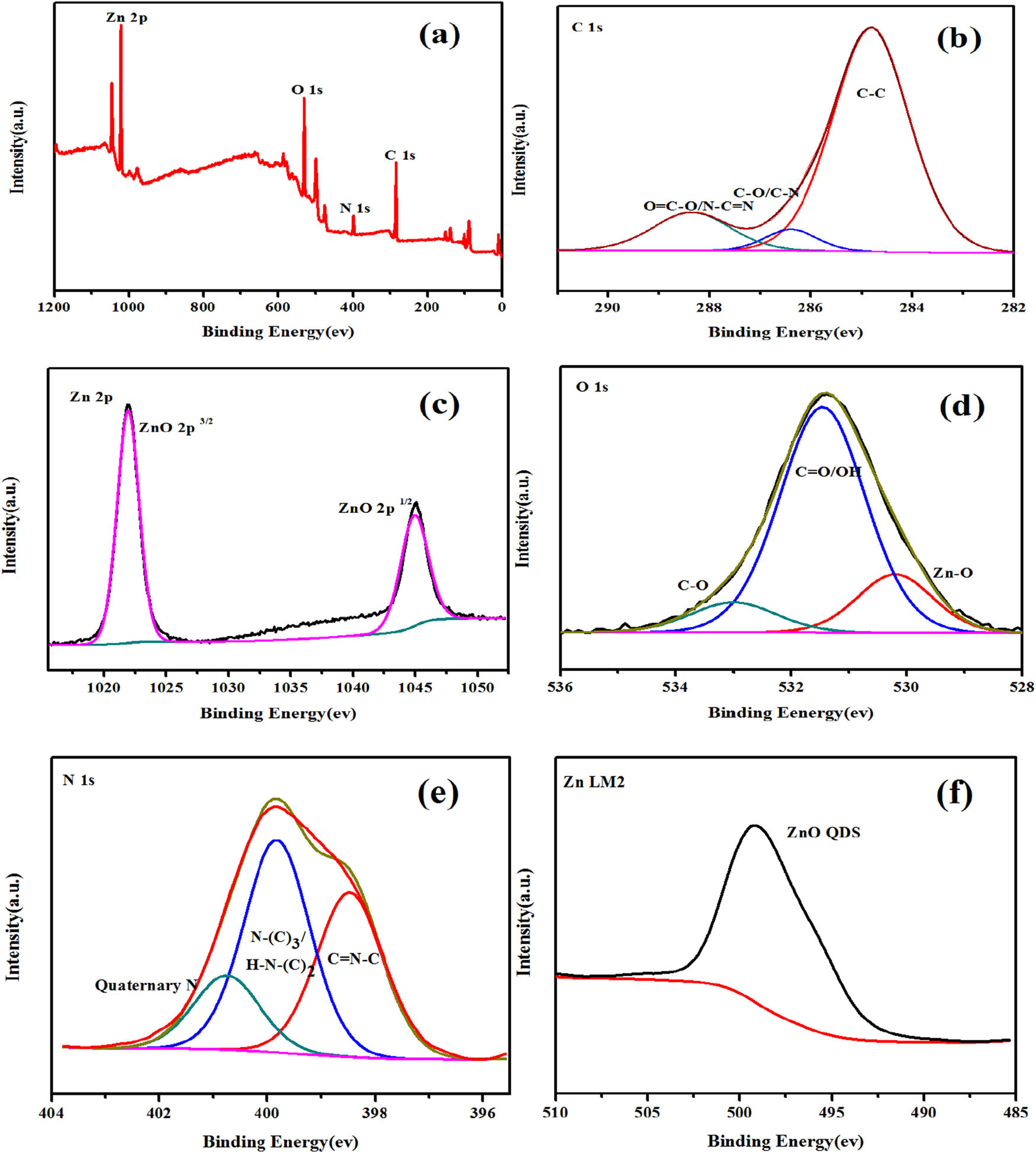
The full-spectrum analysis diagram of the ZC composite samples and the XPS diagram of the four elements. (a) Full spectrum of ZC composite sample, (b) C spectrum, (c) 2p spectrum of Zn, (d) O spectrum, (e) N spectrum, (f) LM2 spectrum of Zn.
Figure 9b shows that C1s has three feature peaks. C1s peak with peak position of 284.6 eV is used in the experiment to achieve calibration of peak position error. The C1s orbital in ZC sample can be divided into C–C, C–O, and N–C═N components, whose binding energies are located at 284.8, 286.4, and 288.7 eV, respectively [52,53]. There are two peaks at 284.8 and 288.7 eV, which correspond to C–C and N–C═N groups in g-C3N4.
Figure 9c shows that O1s has three feature peaks, namely Zn–O, C═O/–OH, and C–O, with binding energies at 530.3, 531.8, and 533.0 eV, respectively. The XPS spectra of O1s of ZC were obtained at high resolution. Through Gaussian fitting, O with two components of binding energies of 530.3 and 531.4 eV was found. The peak of 531.4 eV binding energy is generally considered to be OH− adsorbed on the surface of the material, and the peak of 530.3 eV is O2− in ZnO [54,55,56].
As shown in Figure 9d and e, it can be seen that the energy difference of orbital spin splitting peaks (2p3/2 and 2p1/2) in Zn2p spectrum is about 23 eV, and two peaks (Zn2p3/2 and 2p1/2) appear at 1,021.7 and 1,044.8 eV, respectively, which is consistent with the XPS results of Zn2+ [57].
In Figure 9f, the 398.5 eV peak in N1s corresponds to the SP2 hybrid aromatic bonded to carbon atoms in g-C3N4, and the corresponding 399.8 eV peak tertiary N(N–(C)3/H–N–(C)2); the third peak with high binding energy at 400.9 eV is attributed to quaternary N in an aromatic ring [54,58]. Through element analysis, this is consistent with the data in the existing literature. It further proves that the ZC QD composite sample is successfully prepared.
3.8 Diffuse reflectance spectroscopy
The pure ZnO and ZnO QDs are white, and when combined with g-C3N4, the color of the sample changes from white to light yellow. Pure ZnO and ZnO QDs show strong intrinsic absorption in UV region. At the same time, due to the presence of surface defects (such as Zni, VZn, and VO), ZnO and ZnO QDs also show a certain light response in the visible region. Figure 10 shows the UV spectra of g-C3N4 with different contents of ZnO-g-C3N4. g-C3N4 has a band edge positioned at about 460 nm, corresponding to a band gap of 2.70 eV. However, the visible light absorption of bulk g-C3N4 is weak in the visible region from 500 to 800 nm. Compared with pure ZnO, the absorption edge of the ZnO-g-C3N4 nanocomposite photocatalyst is red-shifted with the increase of g-C3N4 content in the feedstock and the visible light absorption becomes stronger. It is noteworthy that the best visible light absorption is achieved when the mass proportion of g-C3N4 is 10%. The visible light absorption of the composite photocatalyst decreased as the amount of g-C3N4 continued to increase, probably because the excess coverage of the amorphous g-C3N4 layer on the ZnO surface affected the light absorption rate.
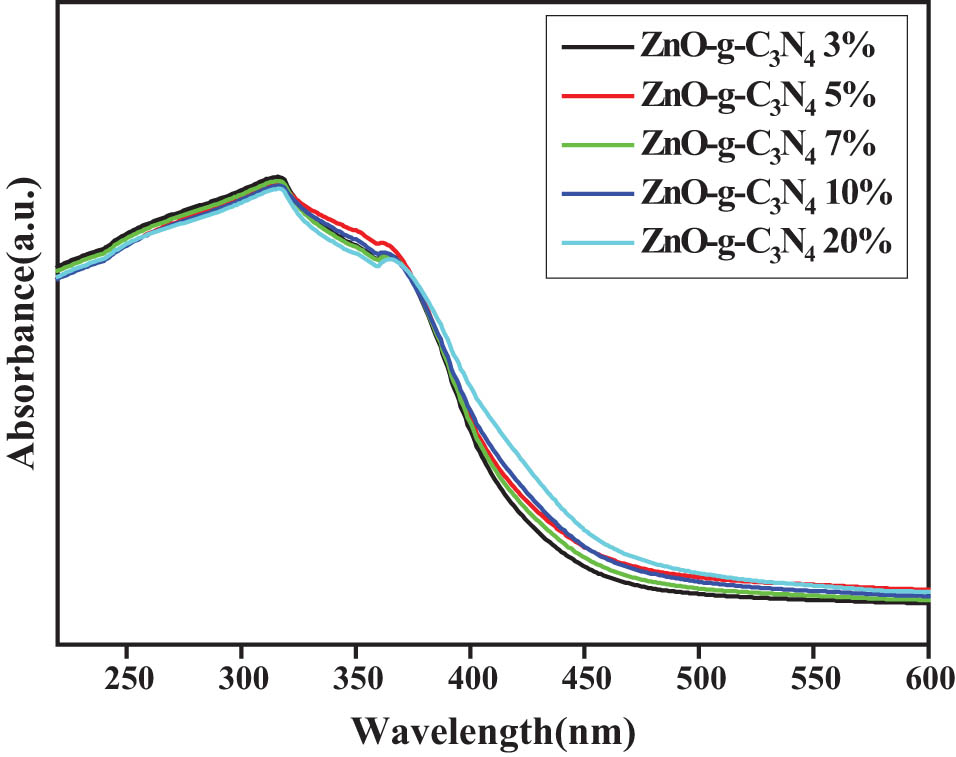
UV spectrogram of g-C3N4 with different contents of ZnO-g-C3N4.
According to Figure 11, the non-complex radiation centers are reduced, and the exciton absorption capacity is improved. ZnO QDs-g-C3N4 10% exciton absorption peak position and half peak width did not change significantly, indicating that the main body of the sample optical absorption is still ZnO core. The position of the 10% exciton absorption region of ZnO QDs-g-C3N4 has a slight red shift, because the particle size of QDs is changed due to the reaction conditions. In summary, ZnO QDs-g-C3N4 10% nanocomposite has the best photocatalytic performance.
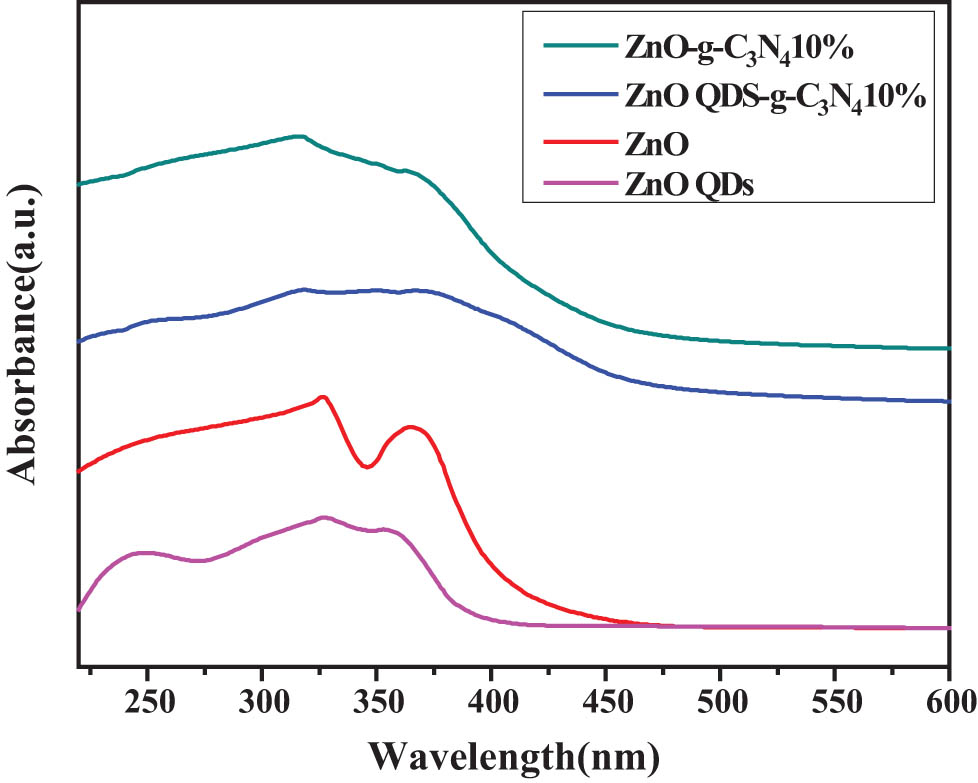
UV spectroscopy of ZnOQDs-g-C3N4 10%, ZnO-g-C3N4 10%, ZnOQDs, and ZnO.
3.9 Photoluminescence (PL)
The recombination of photogenerated electron-hole pairs causes luminescence (PL); therefore, measuring the intensity of PL spectra helps us further understand the transport and compounding processes of photogenerated carriers in semiconductors. The emission of PL is caused by the recombination of photogenerated carriers [59]. In general, the weaker the fluorescence intensity, the better the photocatalytic activity. It can be seen from Figure 12 that ZnO-g-C3N4 10% photocatalytic effect is the best. The PL intensity is significantly reduced due to the carrier transfer between valence band (VB) of g-C3N4 and conduction band (CB) of ZnO, which inhibits, and limits, the recombination of photogenerated carriers [60,61].
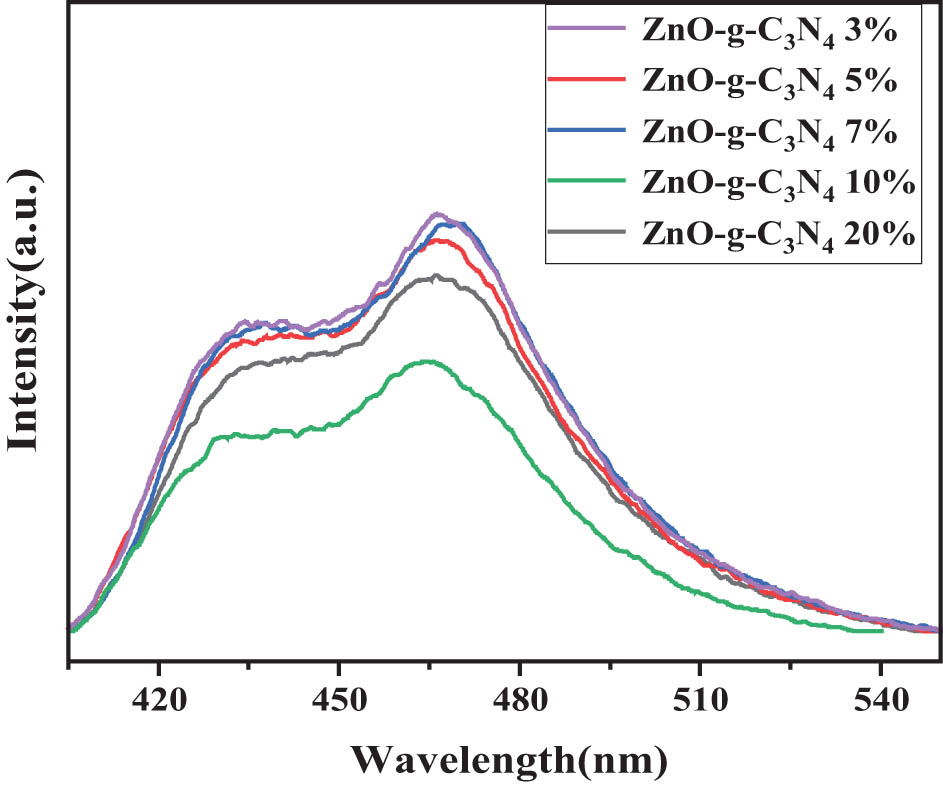
PL spectra of ZnO-g-C3N4 with different g-C3N4 contents.
3.10 Photocatalytic performance of different g-C3N4 doping amount catalysts
The experimental conditions are as follows: 10 mg·L−1 RhB solution 50 mL, photocatalyst ZnO-g-C3N4 dosage 0.02 g, pH = 3, room temperature, dark adsorption for 60 min, and light reaction time 6 h.
Figure 13 shows the degradation curves of catalysts with different g-C3N4 doping levels. The photocatalytic efficiency of RhB solution was investigated by using ZnO and g-C3N4 ZC binary composite materials with different doping mass ratios (3%, 5%, 7%, 10%, 20%) under visible light. Efficiency of photocatalytic degradation of composite materials with different g-C3N4 doping mass ratios was investigated by comparing with single ZnO.
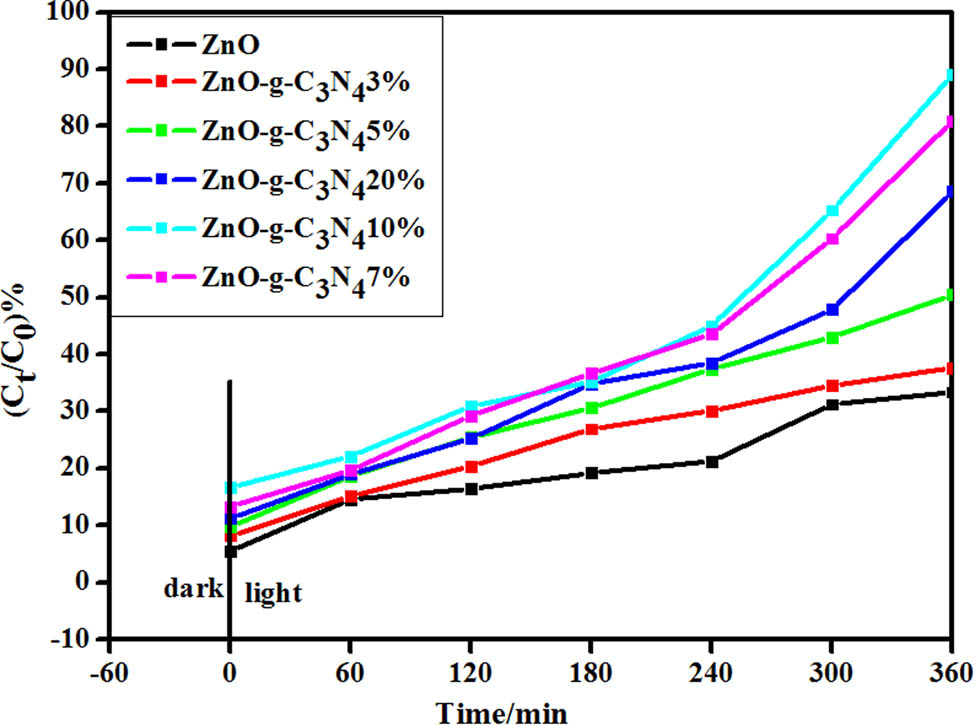
Degradation plots of different g-C3N4 doping catalysts.
As shown in Figure 13, the degradation rates of ZnO, ZC-3%, ZC-5%, ZC-7%, ZC-10%, and ZC-20% on RhB solution were 37.69%, 50.49%, 68.57%, 89.08%, 89.08%, and 80.96%, respectively, when visible light was irradiated for 360 min. It can be seen that in binary composite ZC, the higher the amount of g-C3N4 doping, the stronger the photocatalytic efficiency, and the visible light catalytic efficiency of ZC-10% is the highest. The main mechanism involves electron excitation from the VB of g-C3N4 to the CB under visible light irradiation, and then further transfer to the CB of ZnO. According to the aforementioned data, visible light catalytic efficiency is the best when the percentage of g-C3N4 is 10% in a certain range.
3.11 Photocatalytic activity of the optimum doping amount ZnO QDs-g-C3N4 catalyst
In the experiment, the photocatalytic ZnO-g-C3N4 10% degradation rate is the highest. Using ZnO-g-C3N4 10% as a reference to prepare the same proportion of ZnO QDs-g-C3N4 10% catalyst, its photocatalytic performance was explored. The experimental conditions are as follows: 50 mL, 10 mg·L−1 RhB solution, photocatalyst ZnO QDs-g-C3N4 0.02 g, pH = 3, room temperature, dark adsorption for 6 min, and light reaction time for 6 h.
Figure 14 is the degradation curve of ZnO QDs-g-C3N4 catalyst with the optimal doping amount. The graph shows that the adsorption energy of the photocatalyst doped with g-C3N4 was significantly enhanced during the dark adsorption process for the first 60 min. After 60 min, the degradation ability of the ZnO QDs-g-C3N4 composite photocatalyst doped with 10% g-C3N4 was significantly stronger than that of the pure ZnO-g-C3N4 powder catalyst.
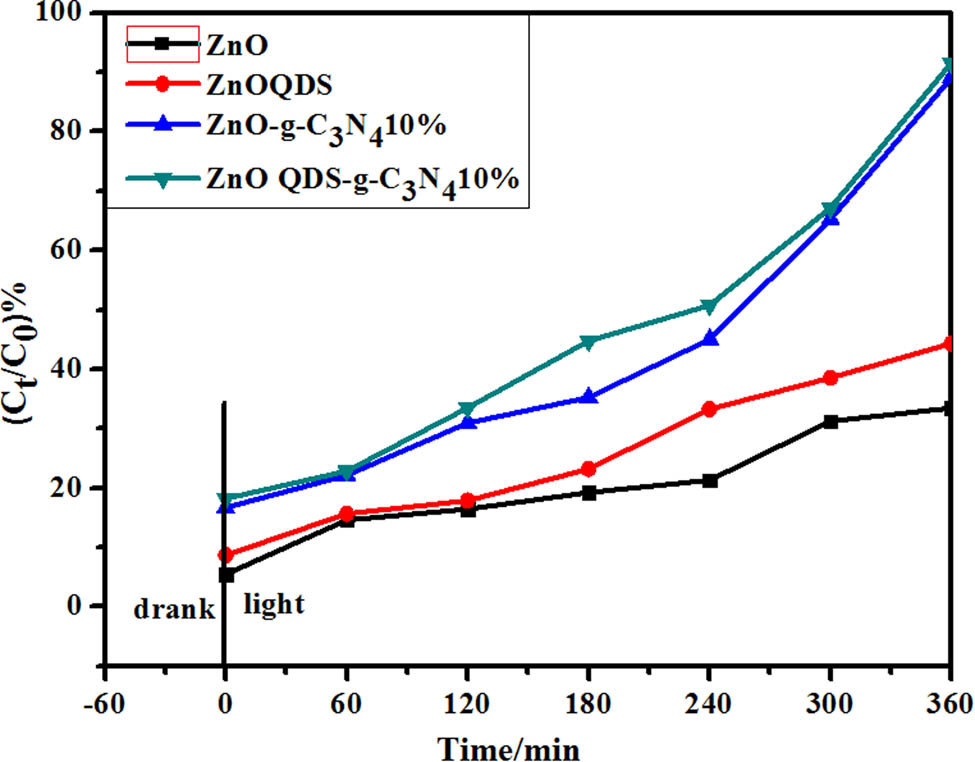
Degradation graph of optimal ZnOQDs-g-C3N4 catalyst.
When the light was turned on for 6 h, the degradation rate of pure ZnO was only about 33%, while that of the ZnO-g-C3N4 composite photocatalyst achieved almost 89% degradation. Compared with ZnO-g-C3N4 10%, ZnO QDs-g-C3N4 10% composite has smaller particle size, which increases the visible light absorption contact area and improves the photocatalytic efficiency. The photocatalytic efficiency of ZnO QDs-g-C3N4 10% composite was about 91%; it is obvious that the photo-catalytic efficiency of ZnO QDs-g-C3N4 10% was higher.
3.12 Photocatalytic reaction kinetics analysis of different g-C3N4 doping amount catalysts
To reach the adsorption–desorption equilibrium, the composite samples were subjected to a dark reaction in RhB for 1 h. Subsequent photodegradation was performed, and it can be seen from Figure 15 that the addition of g-C3N4 to form a binary hetero-junction improved the degradation efficiency of RhB.
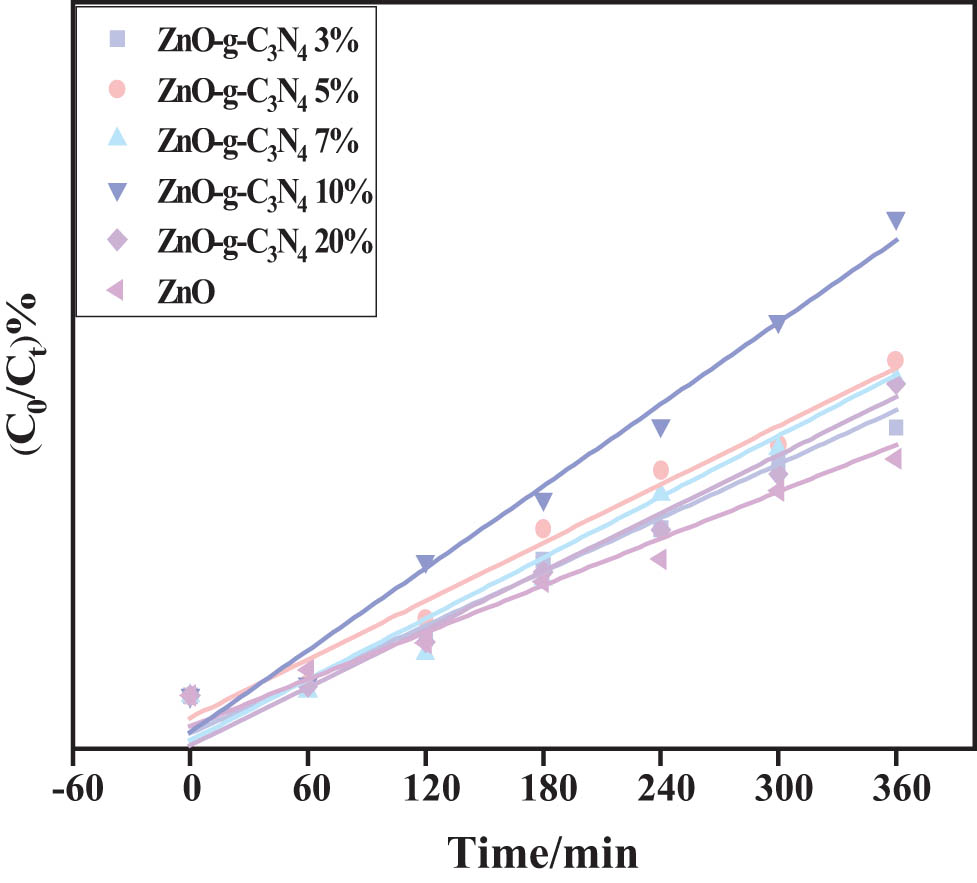
ZC photocatalytic degradation of RhB primary reaction kinetic constants.
The highest degradation efficiency was observed when the g-C3N4 doping was 10%, which may be related to the low efficiency of photo-generated electron-hole pair separation in the binary composite. It can be seen from Figure 15 that the photodegradation efficiency conforms to the first-order kinetic equation ln(C t /C o) = −kt, where t and k represent the time constant and the rate constant, respectively.
As shown in Table 1, ZC-10% has a higher k value, k = 0.002965, which is about twice that of ZnO. R 2 of each ZC sample is more than 0.9, and it is concluded that each sample conforms to the first-order reaction kinetic equation for D RhB.
ZC photocatalytic degradation of RhB primary kinetic data
| Sample name | Regression equation | K | R 2 |
|---|---|---|---|
| ZnO | y = 0.0995x − 0.1077 | 0.00147 | 0.928 |
| ZnO-g-C3N4 3% | y = 0.1151x − 0.1327 | 0.00168 | 0.9746 |
| ZnO-g-C3N4 5% | y = 0.1177x − 0.0781 | 0.00182 | 0.9821 |
| ZnO-g-C3N4 7% | y = 0.1311x − 0.1575 | 0.00190 | 0.9843 |
| ZnO-g-C3N4 10% | y = 0.1998x − 0.2098 | 0.00296 | 0.9169 |
| ZnO-g-C3N4 20% | y = 0.1295x − 0.2182 | 0.00182 | 0.8533 |
3.13 Photocatalytic reaction kinetic analysis of optimum doping amount of ZnO-g-C3N4 and ZnO QDs-g-C3N4 catalysts
The starting time of the photoreaction was set to zero, and the ln(C 0/C t ) versus time t was analyzed for the ZC and ZC QD samples; as shown in Figure 16, a linear relationship exists for both samples, which is consistent with the primary reaction kinetics.
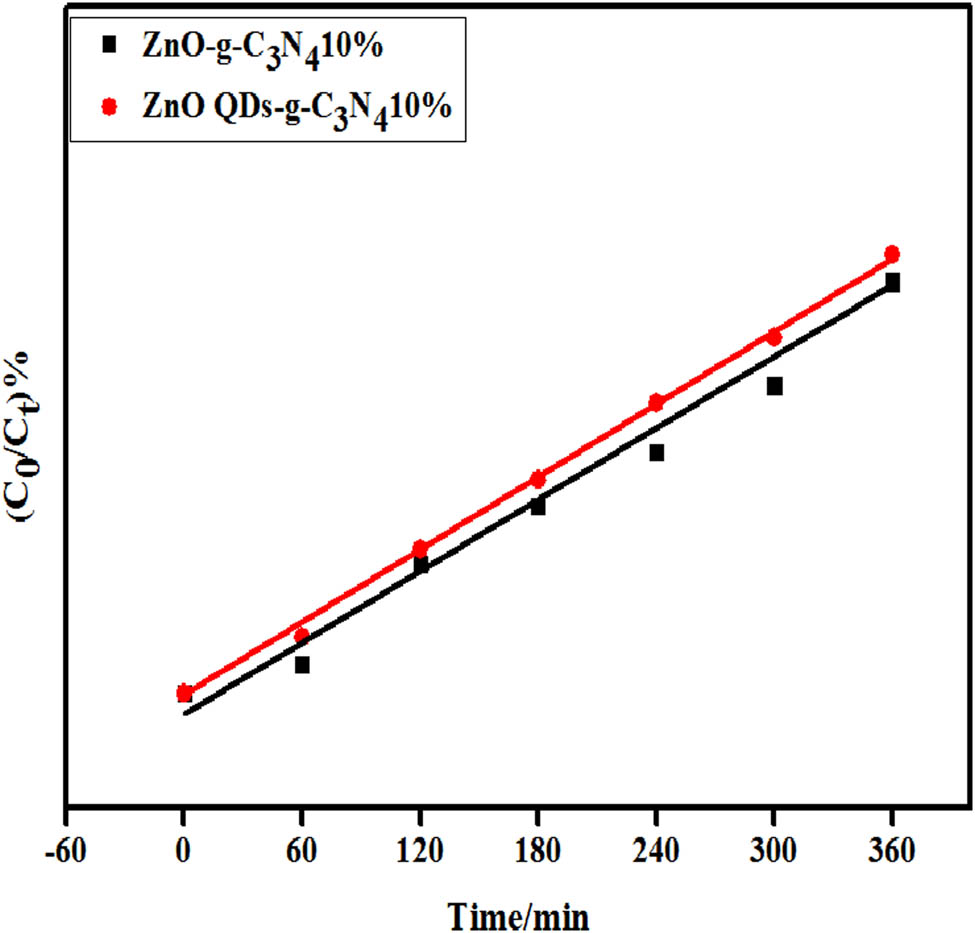
Primary reaction constant for the photocatalyzed degradation of RhB in composite samples with optimal doped amounts of g-C3N4.
As shown in Table 2, ZC QDs-10% has the highest catalytic efficiency compared to other material, k = 0.00323 min−1, about 2.2 times that of ZnO nanotubes. The correlation coefficient R 2 of ZC QD composite sample is more than 0.9, and it is concluded that the sample conforms to the first-order reaction kinetic equation for D RhB.
Linear fit data for g-C3N4 optimal amount of composite material
| Sample name | Regression equation | K | R 2 |
|---|---|---|---|
| ZnO-g-C3N4 10% | y = 0.1998x – 0.2098 | 0.00296 | 0.9169 |
| ZnO QDs-g-C3N4 10% | y = 0.2174x – 0.2182 | 0.00323 | 0.9525 |
3.14 Cycle stability analysis
The stability and reusability of the catalysts were evaluated by cycling experiments of photocatalytic degradation of RhB. The ZG-10% sample with the best photocatalytic performance was selected to be washed with water and alcohol four times, dried, sonicated, and dried, respectively, for recycling. The control light time was at 180 min, and five replicate experiments were conducted, and the results are shown in Figure 17.
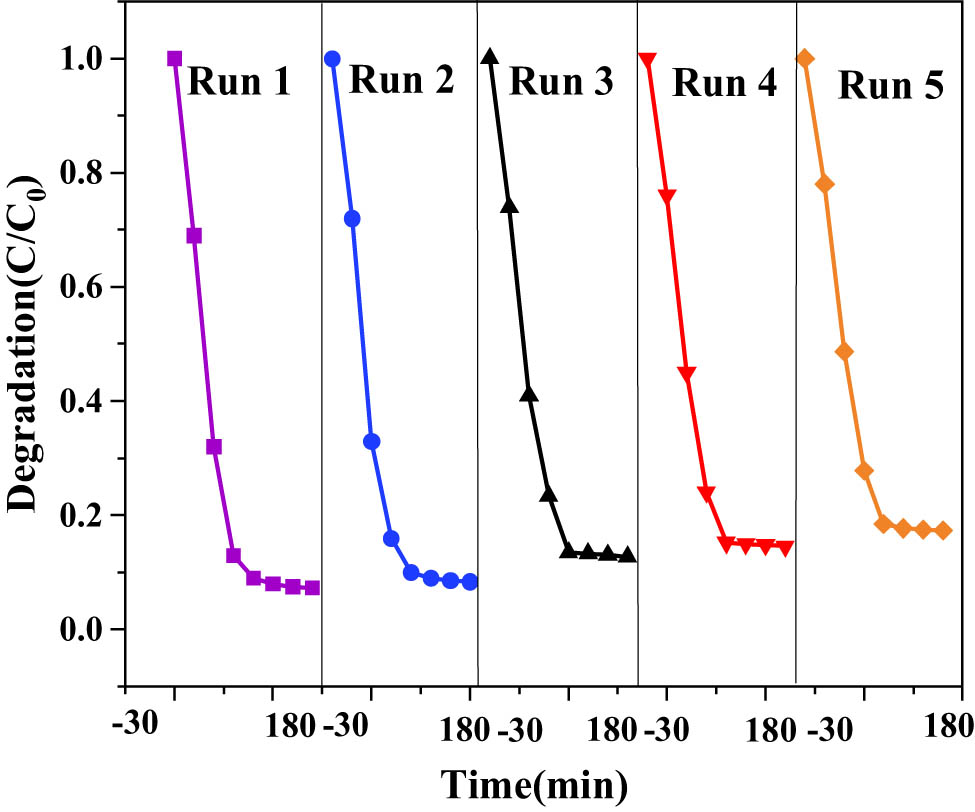
Stability of ZnO-g-C3N4 10% photocatalytic degradation of RhB.
It can be found that after repeating the experiment five times, the first degradation effect is the best, and after that the degradation rate decreases each time, but the change is not significant, and the catalyst still has a high degradation rate of about 83%. It showed that the catalysts were stable, regenerable, and reusable.
The 10% sample of ZnO QDs-g-C3N4 with the best photocatalytic performance was selected, filtered, sonicated, and dried, and then recovered for reuse. After repeating the experiment four times, the best degradation effect was achieved in the first time, and the degradation rate decreased in each subsequent time, but the change was not significant, and the catalyst still had a high degradation rate of about 85%. It showed that the catalysts were stable, regenerable, and reusable.
As can be seen from Figure 18, the photocatalytic degradation rate of the ZC QDs-10% photocatalyst decreased somewhat after four uses, but the decrease was not significant, which may be due to the photocorrosion phenomenon of ZnO QDs. Overall, the prepared photocatalysts have good photocatalytic stability and repeated use performance.
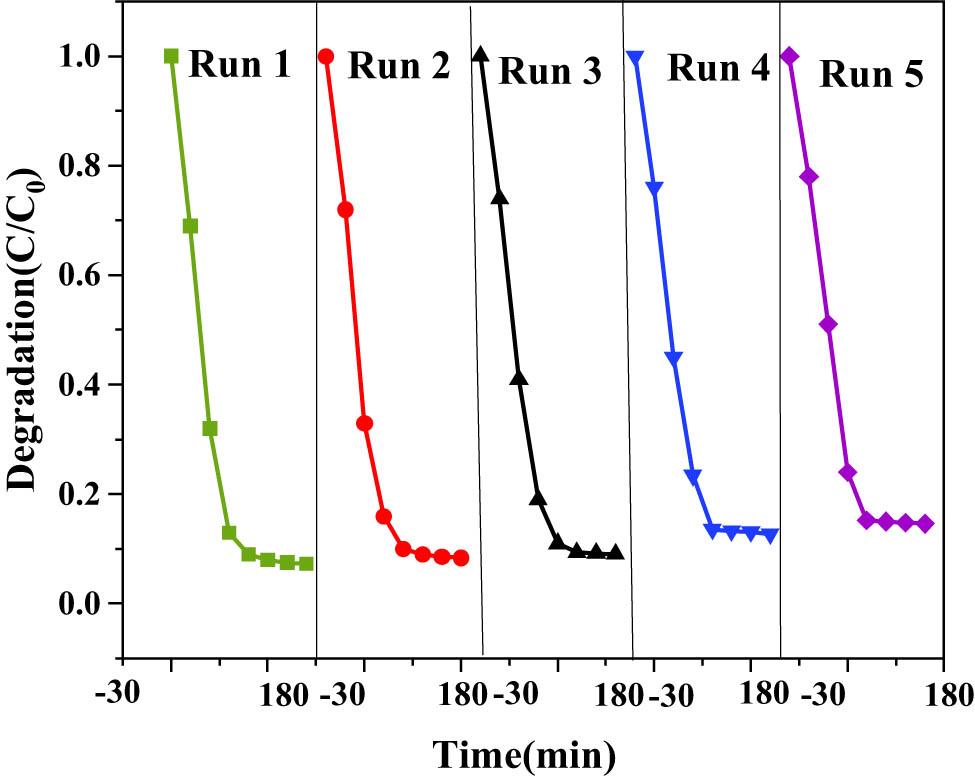
Stability of ZnO QDs-g-C3N4 10% photocatalytic degradation of RhB.
3.15 Analysis of free radical capture experiment results
In order to elucidate the photocatalytic mechanism of ZC QD composite samples in more depth, different trapping agents were added in the photocatalytic process to identify the main active species. The photocatalytic activity was significantly reduced after the addition of the trapping agents, indicating that the active species is the main active species under this photocatalytic system. ZC-10% was selected as a control for RhB degradation, and the changes in photocatalytic degradation of RhB after the addition of the three trapping agents and the results are shown in Figure 19.
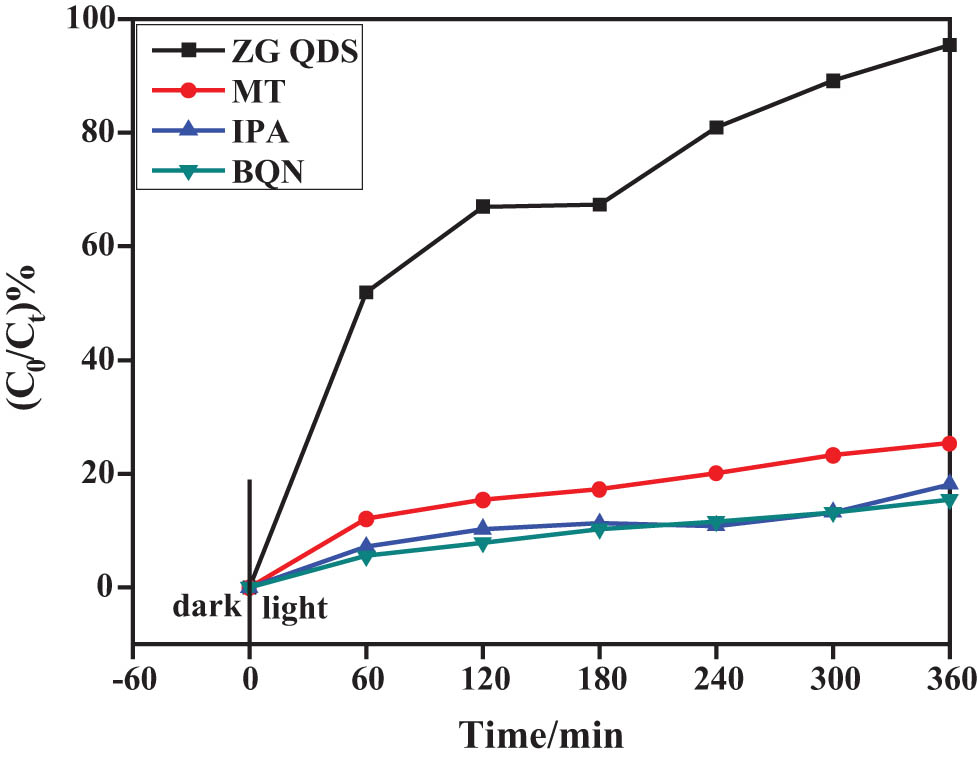
Photocatalytic performance of RhB by ZCQDs with BQN, MT, and IPA.
As shown in Figure 19, the ZC QD sample D RhB = 91.53%. To reveal the main active species in the photocatalytic degradation of RhB in ZC QD composites, p-benzoquinone (BQN), isopropyl alcohol (IPA), and methanol (MT) were added as superoxide radical (˙O2−), hydroxyl radical (˙OH), and hole (h+) trapping agents. The photocatalytic degradation efficiency of the samples showed a significant decrease when all three trapping agents were added, which indicated that ˙O2−, ·˙OH, and h+ were the main active species in the photocatalytic process of the samples under visible light irradiation, where the effect size was ˙O2− > ˙OH > h+ in order.
3.16 ZnO QDs-g-C3N4 10% catalyst mechanistic analysis
Under UV irradiation, g-C3N4 is excited to produce photogenerated electrons and holes. Because the CB of g-C3N4 is higher than that of ZnO, the generated photogenerated electrons are rapidly transferred from CB of g-C3N4 to CB of ZnO, which effectively prevents the electron-hole pair recombination. At the same time, the electrons in ZnO CB can reduce O2 to form ˙O2−, while the holes in g-C3N4 VB can combine with water to form ˙OH. RhB can also be directly oxidized to CO2 and H2O, thus degrading organic pollutants efficiently. The photocatalytic mechanism diagram is shown in Figure 20.
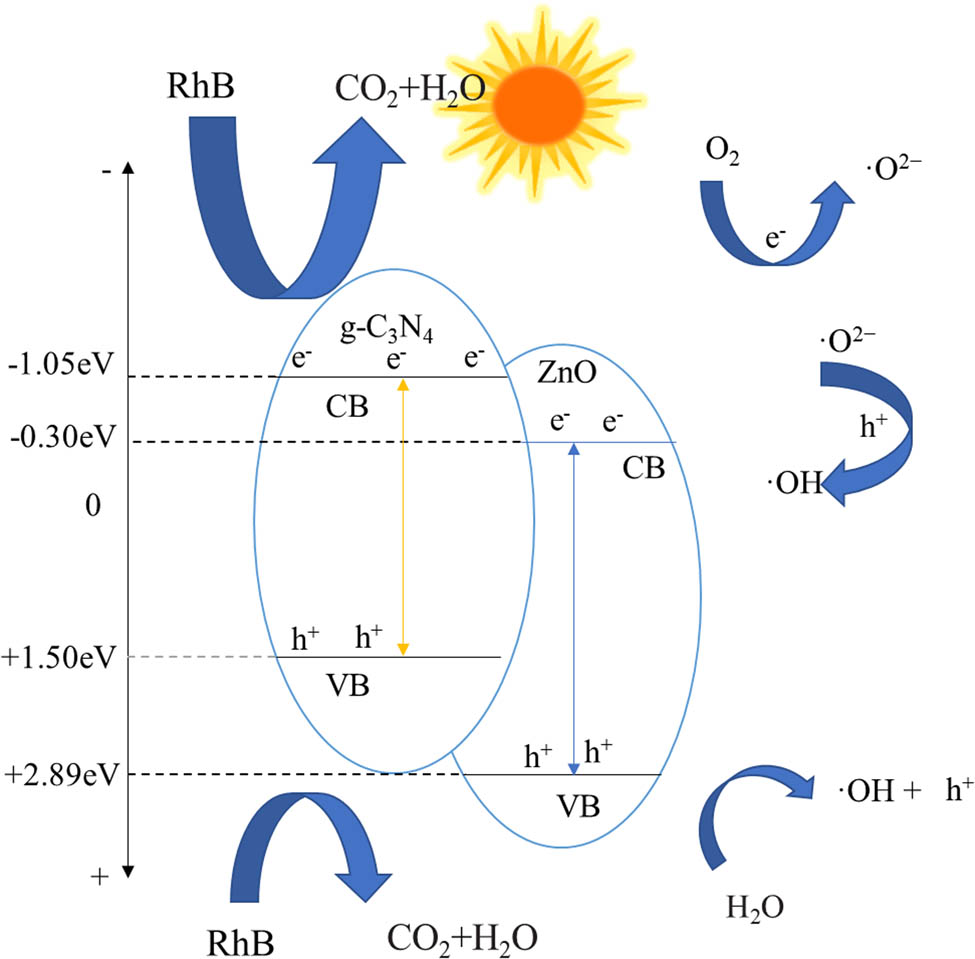
Schematic diagram of the photocatalytic reaction mechanism.
4 Conclusion
In this article, a series of ZnO-g-C3N4 composites, ZnO QDs-g-C3N4 composite photocatalysts, were prepared. The degradation ability of the catalysts for RhB pollutants was tested, and it was found that ZnO-g-C3N4 10% worked best. ZnO-g-C3N4 10% and ZnO QDs-g-C3N4 degradation rates under visible light conditions were 89.08% and 91.53%, respectively. The ability of the catalysts to degrade RhB pollutants was tested, and the cyclic stability of the catalysts and the photocatalytic mechanism of the active components of the composite samples were analyzed. Therefore, this work is helpful to enrich the preparation method of heterojunction and promote the further development of environmental wastewater purification research.
-
Funding information: This work was supported by the Natural Science Foundation of Heilongjiang Province (Grants No. LH2019H055), 2019 Heilongjiang University of Chinese Medicine “Outstanding Young Backbone Teachers Support Program” project, and Science and Technology Plan of Heilongjiang Provincial Health Commission (Grant No. 20222121 020963).
-
Author contributions: Haiyang Liu and Zhe Wang: writing – original draft preparation, writing – review and editing, methodology, formal analysis; Heng Zhang and Lixia Jin: writing – original draft preparation, formal analysis, visualization, project administration; Haiyang Liu: data analysis; Lixia Jin: resources. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Zhang C, Li Y, Shuai DM, Shen Y, Xiong W, Wang LQ. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere. 2019;214:462–79.10.1016/j.chemosphere.2018.09.137Search in Google Scholar PubMed
[2] Vattikuti SVP, Reddy PAK, Shim J, Byon C. Visible-light-driven photocatalytic activity of SnO2–ZnO quantum dots anchored on g-C3N4 nanosheets for photocatalytic pollutant degradation and H2 production. ACS Omega. 2018;3(7):7587–602.10.1021/acsomega.8b00471Search in Google Scholar PubMed PubMed Central
[3] Jo WK, Selvam NCS. Enhanced visible light-driven photocatalytic performance of ZnO-g-C3N4 coupled with graphene oxide as a novel ternary nanocomposite. J Haz Mater. 2015;299:462–70.10.1016/j.jhazmat.2015.07.042Search in Google Scholar PubMed
[4] Amani-Ghadim AR, Arefi-Oskoui S, Mahmoudi R, Sareshkeh AT, Khataee A, Khodam F, et al. Improving photocatalytic activity of the ZnS QDs via lanthanide doping and photosensitizing with GO and g-C3N4 for degradation of an azo dye and bisphenol-A under visible light irradiation. Chemosphere. 2022;295:133917.10.1016/j.chemosphere.2022.133917Search in Google Scholar PubMed
[5] Liu Y. Preparation method of g-C3N4 composite photocatalyst and its photo- catalytic performance. North China University of Science and Technology; 2021.Search in Google Scholar
[6] Hashem EM, Hamza MA, Elshazly A, Elrahman SA, Elanany EM, Mohamed RT, et al. Novel Z-Scheme/Type-II CdS@ZnO/g-C3N4 ternary nanocomposites for the durable photodegradation of organics: Kinetic and mechanistic insights. Chemosphere. 2020;277:128730.10.1016/j.chemosphere.2020.128730Search in Google Scholar PubMed
[7] Liu XL, Ma R, Zhuang L, Hu BW, Chen JR, Liu XY, et al. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit Rev Env Sci Tec. 2021;51(8):751–90.10.1080/10643389.2020.1734433Search in Google Scholar
[8] Raaja Rajeshwari M, Kokilavani S, Sudheer, Khan S. Recent developments inarchitecturing the g-C3N4 based nanostructured photocatalysts: Synthesis, modifications and applications in water treatment. Chemosphere. 2022;291:132735.10.1016/j.chemosphere.2021.132735Search in Google Scholar PubMed
[9] Zuarez-Chamba M, Rajendran S, Herrera-Robledo M, Priya AK, Navas-Cárdenas C. Bi-based photocatalysts for bacterial inactivation in water: Inactivation mechanisms, challenges, and strategies to improve the photocatalytic activity. Environ Res. 2022;209:112834.10.1016/j.envres.2022.112834Search in Google Scholar PubMed
[10] Cheng J, Yan X, Mo Q, Liu B, Wang J, Yang X, et al. Facile synthesis of g-C3N4/BiVO4 heterojunctions with enhanced visible light photocatalytic performance. Ceram Int. 2017;43(1):301–7.10.1016/j.ceramint.2016.09.156Search in Google Scholar
[11] Li J, Li Y, Zhang W, Naraginti S, Sivakumar A, Zhang C. Fabrication of novel tetrahedral Ag3PO4/g-C3N4/BiVO4 ternary composite for efficient detoxification of sulfamethoxazole. Process Saf Environ. 2020;143:340–7.10.1016/j.psep.2020.07.009Search in Google Scholar
[12] Hu J, Chen C, Zheng Y, Zhang G, Guo C, Li C. Spatially separating redox centers on Z‐Scheme ZnIn2S4/BiVO4 hierarchical heterostructure for highly efficient photocatalytic hydrogen evolution. Small. 2020;16(37):2002988.10.1002/smll.202002988Search in Google Scholar PubMed
[13] Ismael M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J Alloy Compd. 2020;846:156446.10.1016/j.jallcom.2020.156446Search in Google Scholar
[14] Saha A, Chakraborti S. Effect of ZnO quantum dots on Escherichia coli global transcription regulator: A molecular investigation. Int J Biol Macromol. 2018;117:1280–8.10.1016/j.ijbiomac.2018.06.001Search in Google Scholar PubMed
[15] Wu JM, Tsay LY. ZnO quantum dots-decorated ZnO nanowires for the enhancement of antibacterial and photocatalytic performances. Nanotechnology. 2015;26(39):395704.10.1088/0957-4484/26/39/395704Search in Google Scholar PubMed
[16] Sharma Y, Shubhangani S, Vineet S. Classification and impact of synthetic textile dyes on aquatic flora: a review. Reg Stud Mar Sci. 2021;45:101802.10.1016/j.rsma.2021.101802Search in Google Scholar
[17] Benson NU, Agboola OD, Fred-Ahmadu OH, De-la-Torre GE, Oluwalana A, Williams AB. Micro(nano)plastics prevalence, food web interactions, and toxicity assessment in aquatic organisms: a review. Front in Mar Sci. 2022;9:851281.10.3389/fmars.2022.851281Search in Google Scholar
[18] He Y, Wang Y, Hu J, Wang K, Zhai Y, Chen Y, et al. Photocatalytic property correlated with microstructural evolution of the biochar/ZnO composites. J Mater Res Technol. 2021;11:1308–21.10.1016/j.jmrt.2021.01.077Search in Google Scholar
[19] Xue H, Wang X, Xu Q, Dhaouadi F, Sellaoui L, Seliem M, et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem Eng J. 2022;430:132801.10.1016/j.cej.2021.132801Search in Google Scholar
[20] Sumanta S, Souman P, Sujata T, Satish K, Abhijit B, Uttam K, et al. Adsorption of methylene blue on chemically modified lychee seed biochar: dynamic, equilibrium, and thermodynamic study. J Mol Liq. 2020;315:113743.10.1016/j.molliq.2020.113743Search in Google Scholar
[21] Hien TD, Nam TT, Dai XT. Investigation into the adsorption of methylene blue and methyl orange by UiO-66-NO2 nanoparticles. J Anal Methods Chem. 2021;2021:5512174.10.1155/2021/5512174Search in Google Scholar PubMed PubMed Central
[22] Guo X, Han S, Yang J, Wang X, Chen S, Quan S. Effect of synergistic interplay between surface charge, crystalline defects, and pore volume of MIL-100(Fe) on adsorption of aqueous organic dyes. Ind Eng Chem Res. 2020;59:2113–22.10.1021/acs.iecr.9b05715Search in Google Scholar
[23] Zhang Y, Han H, Wang X, Zhang M, Chen Y, Zhai C, et al. Utilization of NaP zeolite synthesized with different silicon species and NaAlO2 from coal fly ash for the adsorption of rhodamine B. J Haz Mat. 2021;415:125627.10.1016/j.jhazmat.2021.125627Search in Google Scholar PubMed
[24] Collins MC, Gunst PR, Cascio WE, Kypson AP, Muller-Borer BJ. Labeling and imaging mesenchymal stem cells with quantum dots. Methods Mol Biol. 2012;906:199–210.10.1007/978-1-61779-953-2_15Search in Google Scholar PubMed
[25] Xu J, Teslaa T, Wu TH, Chiou PY, Teitell MA, Teitell S. Nanoblade delivery and incorporation of quantum dot conjugates into tubulin networks in live cells. Nano Lett. 2012;12(11):5669–72.10.1021/nl302821gSearch in Google Scholar PubMed PubMed Central
[26] Chen G, Zhu JY, Zhang ZL, Zhang W, Ren JG, Wu M, et al. Transformation of cell-derived microparticles into quantum- dot-labeled nanovectors for antitumor siRNA deliveryAngew. Chem Int (Edition in English). 2015;54(3):1036–40.10.1002/anie.201410223Search in Google Scholar PubMed
[27] Probst CE, Zrazhevskiy P, Bagalkot V, Gao X. Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv Drug Deliver Rev. 2013;65(5):703–18.10.1016/j.addr.2012.09.036Search in Google Scholar PubMed PubMed Central
[28] Yong KT, Wang Y, Roy I, Rui H, Swihart MT, Law WC, et al. Preparation of quantum dot/drug nanoparticle formulations for traceable targeted delivery and therapy. Theranostics. 2012;2(7):681–94.10.7150/thno.3692Search in Google Scholar PubMed PubMed Central
[29] Isherwood B, Timpson P, McGhee EJ, Anderson KI, Canel M, Serrels A, et al. Live cell in vitro and in vivo imaging applications: accelerating drug discovery. Pharmaceutics. 2011;3(2):141–70.10.3390/pharmaceutics3020141Search in Google Scholar PubMed PubMed Central
[30] Zamaleeva AI, Despras G, Luccardini C, Collot M, Waard M, Oheim M, et al. FRET-based nanobiosensors for imaging intracellular Ca2+ and H+ Microdomains. Sensors (Basel). 2015;15(9):24662–80.10.3390/s150924662Search in Google Scholar PubMed PubMed Central
[31] Lan CX, Liu W, Cheng ZJ, Pan QS, Huang DS, Dong LX. Hydrothermal-based synthesis of CdS/ZnO quantum dots. Adv Mater Res. 2014;875–877:362–5.10.4028/www.scientific.net/AMR.875-877.362Search in Google Scholar
[32] Liu KK, Shan CX, Zhou R, Zhao Q, Shen DZ. Large-scale synthesis of ZnO nanoparticles and their application as phosphors in light-emitting devices. Opt Mater Express. 2017;7(7):2682.10.1364/OME.7.002682Search in Google Scholar
[33] Wang Y, Zhang X, Wang A, Li X, Wang G. Zhao. Synthesis of ZnO nanoparticles from microemulsions in a flow type microreactor. Chem Eng J. 2014;235:191–7.10.1016/j.cej.2013.09.020Search in Google Scholar
[34] Nguyen HL. Reticular materials for artificial photoreduction of CO2. Adv Energy Mater. 2020;46(10):200209.10.1002/aenm.202002091Search in Google Scholar
[35] Song X, Li X, Zhang X. Fabricating C and O Co-doped carbon nitride with intramolecular donor-acceptor systems for efficient photoreduction of CO2 to CO. Appl Catal B-Environ. 2020;268:118736.10.1016/j.apcatb.2020.118736Search in Google Scholar
[36] Mahvelati-Shamsabadi T, Lee BK. Photocatalytic H2 evolution and CO2 reduction over phosphorus-doped g-C3N4 nanostructures: Electronic, optical, and surface properties. Renew Sust Energ Rev. 2020;130:109957.10.1016/j.rser.2020.109957Search in Google Scholar
[37] Jiang Z, Wan W, Li H, Yuan S, Zhao H, Wong P. A Hierarchical Z-scheme α-Fe2O3/g-C3N4 hybrid for enhanced photocatalytic CO2 reduction. Adv Mater. 2018;30:1706108.10.1002/adma.201706108Search in Google Scholar PubMed
[38] Akhundi A, Habibi-Yangjeh A, Abitorabi M, Pouran S. Review on photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal Rev. 2019;61:595–628.10.1080/01614940.2019.1654224Search in Google Scholar
[39] Sahua RS, Shiha Y, Chen WL. New insights of metal free 2D graphitic carbon nitride for photocatalytic degradation of bisphenol A. J Haz Mater. 2021;402:123509.10.1016/j.jhazmat.2020.123509Search in Google Scholar PubMed
[40] Yang Y, Zeng G, Huang D, Zhang C, He D, Zhou C, et al. Molecular engineering of polymeric carbon nitride for highly efficient photocatalytic oxytetracycline degradation and H2O2 production. Appl Catal B-Environ. 2020;272:118970.10.1016/j.apcatb.2020.118970Search in Google Scholar
[41] Zhang Q, Peng Y, Lin Y, Wu S, Yu X, Yang C. Bisphenol S-doped g-C3N4 nanosheets modified by boron nitride quantum dots as efficient visible-light-driven photocatalysts for degradation of sulfamethazine. Chem Eng J. 2021;405:126661.10.1016/j.cej.2020.126661Search in Google Scholar
[42] Kurpil B, Markushyna Y, Savateev A. Visible-light-driven reductive (Cyclo) dimerization of chalcones over heterogeneous carbon nitride photocatalyst. ACS Catal. 2019;9:1531–8.10.1021/acscatal.8b04182Search in Google Scholar
[43] Geng P, Tang Y, Pan G. A g-C3N4-based heterogeneous photocatalyst for visible light mediated aerobic benzylic C-H oxygenations. Green Chem. 2019;21:6116–22.10.1039/C9GC02870FSearch in Google Scholar
[44] Zhou Y, Zhang L, Wang W. Direct functionalization of methane into ethanol over copper modified polymeric carbon nitride via photocatalysis. Nat Commun. 2019;10:506.10.1038/s41467-019-08454-0Search in Google Scholar PubMed PubMed Central
[45] Hsu SH, Lin YY, Huang S, Lem KW, Nguyen DH, Lee DS. Synthesis of water-dispersible zinc oxide quantum dots with antibacterial activity and low cytotoxicity for cell labeling. Nanotechnology. 2013;24(47):475102.10.1088/0957-4484/24/47/475102Search in Google Scholar PubMed
[46] Thurston JH, Clifford AJ, Henderson BS, Smith TR, Quintana D, Cudworth KF, et al. Development of photoactive g-C3N4/Poly(vinyl alcohol) composite hydrogel films with antimicrobial and antibiofilm activity. ACS Appl Bio Mater. 2020;3(3):1681–9.10.1021/acsabm.9b01240Search in Google Scholar
[47] Bellanger X, Billard P, Schneider R, Balan L, Merlin C. Stability and toxicity of ZnO quantum dots: Interplay between nanoparticles and bacteria. J Haz Mater. 2015;283:110–6.10.1016/j.jhazmat.2014.09.017Search in Google Scholar PubMed
[48] Mahmoodi NM, Karimi B, Mazarji M, Moghtaderi H. Cadmium selenide quantum dot-zinc oxide composite: Synthesis, characterization, dye removal ability with UV irradiation, and antibacterial activity as a safe and high-performance photocatalyst. J Photoch Photobio B. 2018;188:19–27.10.1016/j.jphotobiol.2018.08.023Search in Google Scholar PubMed
[49] Waseem R, Syed MF, Mohammad O, Bahnemann D, Munee M. Facile fabrication of highly efficient modified ZnO photocatalyst with enhanced photocatalytic, antibacterial and anticancer activity. RSC Adv. 2016;6:78335–50.10.1039/C6RA06774CSearch in Google Scholar
[50] Suwiwat S, Andrew JH, Yuvarat N, Sujittra Y, Nontipa S. Rice straw-derived highly mesoporous carbon-zinc oxide nanocomposites as high performance photocatalytic adsorbents for toxic dyes. J Clean Prod. 2021;318:128583.10.1016/j.jclepro.2021.128583Search in Google Scholar
[51] Huang T, Chen J, Zhang L, Khataee A, Han Q, Liu X, et al. Precursor-modified strategy to synthesize thin porous amino-rich graphitic carbon nitride with enhanced photocatalytic de gradation of RhB and hydrogen evolution performances. Chin J Catal. 2022;43(22):497–506.10.1016/S1872-2067(21)63873-1Search in Google Scholar
[52] Mishra DK, Mohapatra J, Sharma MK, Chattarjee R, Singh SK, Varma S, et al. Carbon doped ZnO: Synthesis, characterization and interpretation. J Magn Magn Mater. 2013;329:146–52.10.1016/j.jmmm.2012.09.058Search in Google Scholar
[53] György E, Pérez Del Pino A, Logofatu C, Duta A, Isac L. Effect of nitrogen doping on wetting and photoactive properties of laser processed zinc oxide-graphene oxide nanocomposite layers. J Appl Phys. 2014;116:024906.10.1063/1.4890015Search in Google Scholar
[54] Kumaresan N, Sinthiya MMA, Praveen Kumar M, Ravichandran S, Ramesh Babu R, Sethurman K, et al. Investigation on the g-C3N4 encapsulated ZnO nanorods heterojunction coupled with GO for effective photocatalytic activity under visible light irradiation. Arab J Chem. 2020;13:2826–43.10.1016/j.arabjc.2018.07.013Search in Google Scholar
[55] Zhang X, Qin J, Hao R, Wang L, Shen X, Yu R, et al. Carbon-doped ZnO nanostructures: Facile synthesis and visible light photocatalytic applications. J Phys Chem C. 2015;119:20544–54.10.1021/acs.jpcc.5b07116Search in Google Scholar
[56] Dai K, Lu L, Liang C, Dai J, Zhu G, Liu Z, et al. Graphene oxide modified ZnO nanorods hybrid with high reusable photocatalytic activity under UV-LED irradiation. Mater Chem Phys. 2014;143:1410–6.10.1016/j.matchemphys.2013.11.055Search in Google Scholar
[57] de Moraes NP, Bacetto LA, dos Santos GS, Pinto da Silva MLC, Machado JPB, Campos TMB, et al. Synthesis of novel ZnO/carbon xerogel composites: Effect of carbon content and calcination temperature on their structural and photocatalytic properties. Ceram Int. 2019;45:3657–67.10.1016/j.ceramint.2018.11.027Search in Google Scholar
[58] Zhu B, Xia P, Li Y, Ho W, Yu J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl Surf Sci. 2017;391:175–83.10.1016/j.apsusc.2016.07.104Search in Google Scholar
[59] Srinivas M. Preparation, characterization and photocatalytic activity of nickel-substituted CoFe2O4: exploration of changes in the micro structural parameters and distribution of cations in the lattice. Mater Res Express. 2019;6(11):1150f9.10.1088/2053-1591/ab51afSearch in Google Scholar
[60] Prabhu S, Pudukudy M, Harish S, Navaneethan M, Sohila S, Murugesan K, et al. Facile construction of djembe-like ZnO and its composite with g-C3N4 as a visible-light-driven heterojunction photocatalyst for the degradation of organic dyes. Mater Sci Semicon Proc. 2020;106:104754.10.1016/j.mssp.2019.104754Search in Google Scholar
[61] Guo X, Duan J, Li C, Zhang Z, Wang W. Highly efficient Z-scheme g-C3N4/ZnO photocatalysts constructed by co-melting-recrystallizing mixed precursors for wastewater treatment. J Mater Sci. 2020;55(5):2018–31.10.1007/s10853-019-04097-0Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

