Abstract
Zr-metal-organic frameworks (Zr-MOFs) were prepared by a solvothermal method and characterized by X-ray diffraction, scanning electron microscopy, and thermogravimetry. Zr-MOFs were used to remove doxycycline hydrochloride (DOC) from wastewater. According to the experimental results, the maximum adsorption capacity of DOC by Zr-MOFs within 5 h was 148.7 mg·g−1. From the pseudo-second-order kinetics model, all R 2 values were greater than 0.99, which proved that the adsorption of DOC by Zr-MOFs was consistent with practice. According to the Freundlich isotherm model, the adsorption of DOC by Zr-MOFs proceeded via multilayer adsorption. The aforementioned results show that Zr-MOFs have good application prospects for removing DOC from wastewater.
1 Introduction
Antibiotics have been widely used in human and veterinary medicine to prevent and treat infectious diseases. However, due to poor metabolism in animals and humans, about 30–90% of administered antibiotics are excreted into the aquatic environment after treatment. Many antibiotics cannot be fully used and therefore enter nature, which may produce drug-resistant bacteria [1]. Doxycycline hydrochloride (DOC) readily dissolves in water [2]. Sewage treatment methods do not adequately remove antibiotics from water and sometimes produce secondary pollution. Therefore, many different technologies have emerged to remove DOC, including microbial degradation, phytoremediation, constructed wetland, microbial fuel cell enhanced biodegradation, constructed wetland-coupled microbial fuel cell degradation, and adsorption methods [3,4,5,6]. Among them, the degradation rate of the microbial degradation method and phytoremediation method is low. The constructed wetland method requires a large land area and long operation times. The enhanced biodegradation method of microbial fuel cells is restricted by many factors, such as solution concentration, electrode material, aeration rate, ionic strength, and external resistance and temperature, so the accuracy of the obtained data is low [7,8,9]. The removal rate using the constructed wetland-coupled microbial fuel cell degradation method is greatly affected by influent co-matrix and antibiotic concentration. Its development is also constrained by immature technology and high costs [10]. The adsorption method in this article has a simple procedure, is safe, uses simple equipment, has a narrow pH change, and uses less organic solvent to remove pollutants. However, it suffers from poor selectivity and unstable adsorbent performance.
Metal-organic frameworks (MOFs) have ultra-high specific surface areas, high and adjustable porosities, high adsorption capacities, easy synthesis and recycling [11], and show broad application prospects in many fields such as separation [12,13], energy storage [14,15], catalysis [16,17,18,19,20,21,22], adsorption [23,24,25], advanced oxidation [26], and devices [27,28,29,30]. Since MOFs have so many advantages, in this article, 2-amino-terephthalic acid was used as the organic chain and zirconium acetate as the metal ion to prepare Zr-MOFs, which were then used to remove DOC from an aqueous solution.
2 Experimental
2.1 Experimental reagents and instruments
The ligand 2-amino-terephthalic acid, metal-derived zirconium acetate, and DOC were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.
An X-ray diffraction (XRD) diffractometer (Td-3300; Dandong Tongda Technology Co., Ltd.), a JSM-6700F field emission scanning electron microscope (Japan Electronics Co., Ltd.), an IRAffinity-1 infrared spectrometer, and a DTG-60 differential thermo resynchronization analyzer (Shimadzu, Japan) were used to analyze the Zr-MOFs. The structure and morphology of the materials were characterized.
2.2 Preparation of Zr-MOFs
The compound was synthesized according to the method previously described [16,20]. 2-Amino-terephthalic acid (0.3623 g) was dissolved in 15 mL of N,N-dimethylformamide (DMF). Then, 3.305 mL of zirconium acetate was mixed in a reaction kettle and then placed in a constant-temperature drying box set to 150°C. After reacting for 14 h, it was taken out and cooled to room temperature. The products were transferred to a centrifugal pipe and then centrifuged. Then, crystals were transferred to a beaker, magnetically stirred, and then washed three times with DMF and water. Centrifugation was repeated after each washing to remove unreacted starting matter, and the sample was put into a drying oven.
2.3 Removal of antibiotics by Zr-MOFs
Different masses of Zr-MOF samples obtained by the hot solvent method (20, 30, 40, and 50 mg) were added to 200 mL of DOC solutions with different concentrations (20, 30, 40, and 50 mg·L−1). Then, they were stirred under natural light. Samples were taken every 30 min, and changes in antibiotic concentration in the solution at 269 nm were analyzed using a UV spectrometer. Then, by analyzing the relationship between time and concentration, the adsorption capacity and removal rate of DOC by Zr-MOFs were calculated. The adsorption capacity and removal rate of DOC were calculated using the following two formulas:
where C 0 is the initial concentration of DOC, C e is the concentration at adsorption equilibrium, C t is the concentration at time t, V is the volume of solution, and m is the mass of Zr-MOFs.
3 Results and discussion
3.1 Structure of Zr-MOFs
The infrared spectrum in Figure 1 has strong absorption peaks at 1,581 and 1,375 cm−1. Strong absorption peaks also appeared between 1,620–1,550 and 1,420–1,300 cm−1, which indicate that carboxylic acids reacted with the metal salt [31,32]. In Figure 2, the XRD patterns of the materials show that Zr-MOF had a poor crystallinity and small particle size. Figure 3 shows that Zr-MOFs had a better morphology and dispersion. This was mainly because intermolecular interactions between organic ligands were weakened, and the deprotonation of organic ligands was enhanced, which promoted the growth of crystals in the solvent.
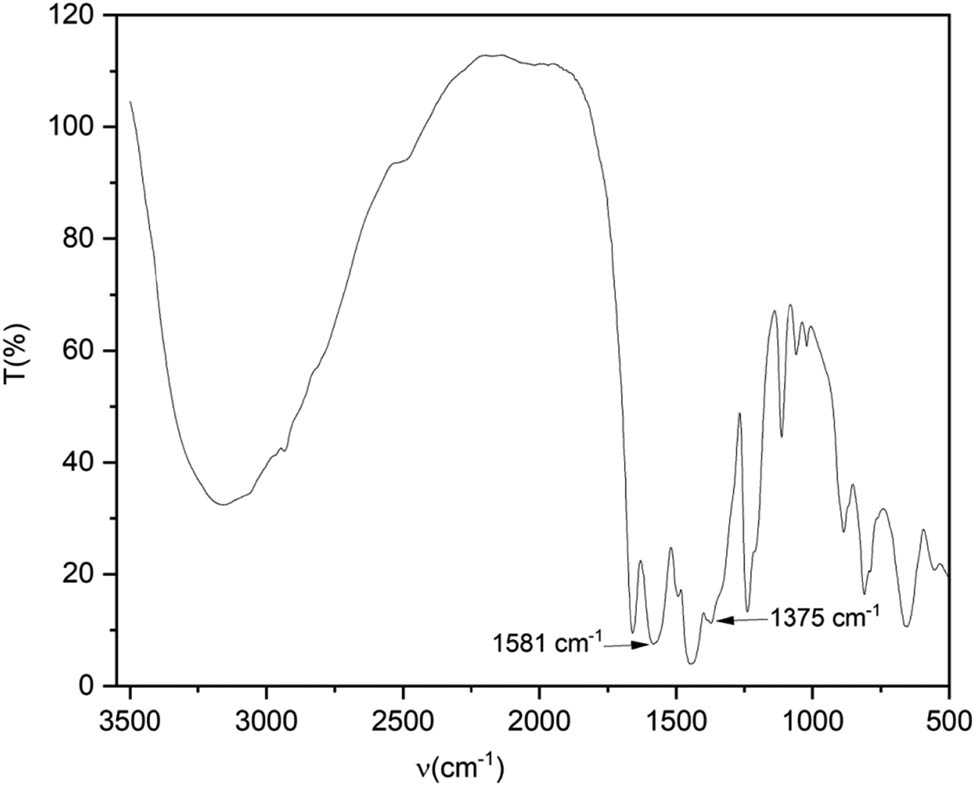
FTIR spectrum of Zr-MOF.

XRD pattern of Zr-MOF.

SEM images of Zr-MOF.
Figure 4 shows that the Brunauer–Emmett–Teller surface area was 405.7776 m2·g−1. The adsorption average pore diameter was 3.61059 nm, indicating the mesoporous nature of the material. As can be seen from Figure 3, the Zr-MOFs displayed type IV of isotherms with H3 hysteresis loops, indicating the mesoporous properties of the sample.

N2 adsorption–desorption isotherms of Zr-MOF.
As shown in Figure 5, the thermogravimetric curves of the Zr-MOFs were characterized by three different stages: (1) a mass loss of 12.3% at about 90°C, which was dominated by residual solvent molecules; (2) a mass loss of 13% between 91°C and 275°C, which was mainly due to the oxidation of metal ions; and (3) a mass loss that began at 275°C and ended at 500°C, leaving a residual amount of 16% [33]. This corresponded to the destruction of the framework, indicating that the material was stable below 275°C. In most cases, Zr-MOFs generated via reactions between metal ions and organic ligands have more stable structures [34].

Thermogravimetric analysis of Zr-MOFs.
3.2 DOC removal using Zr-MOFs
To study the ability of Zr-MOFs to remove DOC, this experiment controlled the amount of Zr-MOFs, DOC-HCl, and reaction time. As shown in Figure 6, when the concentration of DOC was 30 ppm and the concentration of Zr-MOF was 50 mg, the removal rate reached 84.4% after 5 h. After three cycles, the removal rate of Zr-MOFs was 19.7%, indicating reasonable reusability (Figure 7). It can also be seen from the figure that when the concentration of DOC was unchanged, the removal rate gradually increased after adding Zr-MOF. When the dosage of Zr-MOF was constant, the removal rate increased upon decreasing the DOC concentration. When the mass of Zr-MOF was 30 mg, and the concentration of DOC was 50 mg·L−1, the maximum adsorption capacity was 148.7 mg·g−1.

Removal rate of DOC by Zr-MOF: (a) 50 ppm, (b) 40 ppm, (c) 30 ppm, and (d) 20 ppm.

Reusability of Zr-MOFs for the removal of DOC.
To verify the consistency between theory and experimental practice, the first-order and second-order kinetics were simulated to study the removal of DOC by Zr-MOF. The results are shown in Figures 8 and 9 and Table 1. The formulas are as follows [35,36,37]:

Pseudo-first-order kinetic model for the adsorption DOC over Zr-MOF: (a) 50 ppm, (b) 40 ppm, (c) 30 ppm, and (d) 20 ppm.

Pseudo-second-order (PSO) kinetic model for the adsorption DOC over Zr-MOF: (a) 50 ppm, (b) 40 ppm, (c) 30 ppm, and (d) 20 ppm.
Kinetic parameters for the adsorption of DOC over Zr-MOF
| Concentration | Mass | PSO kinetics | Pseudo-first-order kinetics | ||
|---|---|---|---|---|---|
| K (g·mg−1·min−1) | R 2 | K (L·min−1) | R 2 | ||
| 20 | 20 | 0.00776 | 0.9961 | 0.00139 | 0.94461 |
| 30 | 0.00899 | 0.99768 | 0.00211 | 0.84618 | |
| 40 | 0.01023 | 0.99601 | 0.00317 | 0.97492 | |
| 50 | 0.01185 | 0.99949 | 0.00385 | 0.85806 | |
| 30 | 20 | 0.00703 | 0.99804 | 0.0008454 | 0.78386 |
| 30 | 0.00839 | 0.99436 | 0.00132 | 0.94285 | |
| 40 | 0.00835 | 0.99648 | 0.00218 | 0.95092 | |
| 50 | 0.00927 | 0.99878 | 0.00284 | 0.92905 | |
| 40 | 20 | 0.00691 | 0.99818 | 0.0005822 | 0.82319 |
| 30 | 0.00759 | 0.99889 | 0.009629 | 0.88152 | |
| 40 | 0.00786 | 0.99832 | 0.00139 | 0.9147 | |
| 50 | 0.00807 | 0.99164 | 0.00213 | 0.92649 | |
| 50 | 20 | 0.00604 | 0.99884 | 0.0005586 | 0.88356 |
| 30 | 0.00599 | 0.99838 | 0.0009758 | 0.89983 | |
| 40 | 0.00608 | 0.99922 | 0.00162 | 0.88962 | |
| 50 | 0.00638 | 0.99808 | 0.0022 | 0.92532 | |
As shown in Figures 8 and 9 and Table 1, the R 2 value of the second-order kinetics model was better than that of the first-order model. The simulation results were in agreement with the experimental results. The adsorption of DOC by Zr-MOFs occurred mainly via chemisorption.
The experimental results were also analyzed by the Langmuir and Freundlich isotherm models [38,39]. When the concentration of DOC adsorbed by Zr-MOFs was 20 ppm, the parameters obtained by Langmuir and Freundlich models are shown in Figure 10 and Table 2. The R 2 values were 0.99162 and 0.99787, respectively. The data showed that the Freundlich model was more consistent with the experimental adsorption data of DOC, indicating that the adsorption of DOC by Zr-MOFs proceeded via multilayer adsorption.

Adsorption isotherms of DOC adsorption onto Zr-MOFs: (a) Freundlich isotherm and (b) Langmuir isotherm.
Adsorption isotherm parameters of DOC onto MOFs at room temperature
| T (K) | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| k | R 2 | q m (mg·g−1) | K f (mg·g−1·(L·mg−1)1/n ) | n | R 2 | |
| 293 | 0.00801 | 0.99932 | 124.8 | 61.3925 | 4.9017 | 0.85564 |
To explore the effect of pH on DOC adsorption, 30 mg of Zr-MOF was added to 50 ppm DOC solutions with different pH values. As shown in Figure 11, the adsorption capacity of DOC increased with the pH. The zeta potential of Zr-MOFs was −9.98 mV, which might be attributed to the strong electrostatic interactions between DOC molecules and Zr-MOF adsorbent surfaces. The maximum adsorption capacity was obtained at pH 6 and 10.
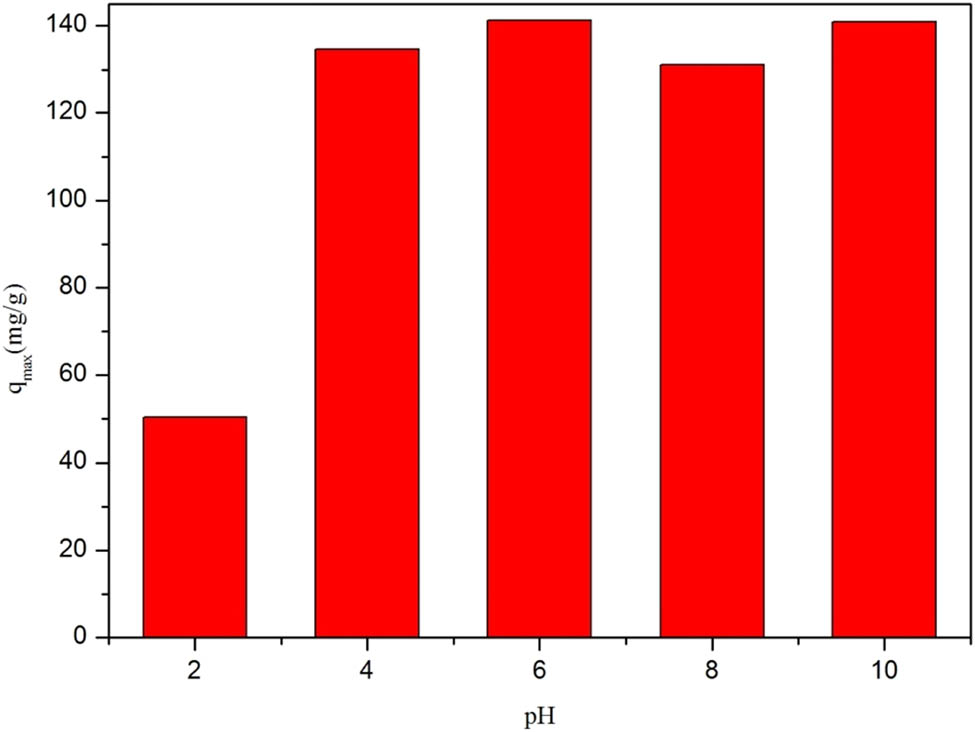
Effect of pH on the adsorption amount of DOC.
To verify the influence of temperature, 30 mg of Zr-MOFs was added to 50 ppm DOC solutions at different temperatures and compared with ambient normal temperature. The adsorption capacity slightly decreased upon increasing the temperature, and the adsorption effect was the best at ambient temperature.
To gain further insight into the mechanism of DOC adsorption, the thermodynamic equilibrium constant (K 0) and Gibbs free energy change (ΔG 0), enthalpy change (ΔH 0), and entropy change (ΔS 0) were determined using the following equations:
where K 0 is the Langmuir adsorption constant (L·mol−1) and R is the gas constant (8.314 J·mol−1·K−1). A linear plot of ln K 0 versus 1/T was obtained as shown in Figure 8. ΔH 0 and ΔS 0 were calculated from (−slope × R) and (intercept × R) of the van’t Hoff plot as shown in Figure 9 and Table 3.
Thermodynamic parameters of DOC adsorption onto MOFs
| T (K) | ΔG 0 (kJ·mol−1) | ΔH 0 (−slope × R) (kJ·mol−1) | S° (intercept × R) (J·mol−1·K−1) |
|---|---|---|---|
| 303 | −10.5 | −25.6 | −49.7 |
As shown in Figure 12 and Table 3, the negative ΔG 0 and ΔH 0 values indicated that the adsorption of DOC over MOFs was spontaneous and exothermic. Enthalpy changes due to chemisorption fall in the range of 84 and 420 kJ·mol−1, while physical absorption tends to occur below 84 kJ·mol−1 [40]. Thus, the adsorption of DOC over MOFs may have occurred by physisorption. Moreover, the entropy change ΔS 0 was positive, which revealed that the process increased the randomness because the number of desorbed water molecules was larger than that of adsorbed DOC molecules [41].
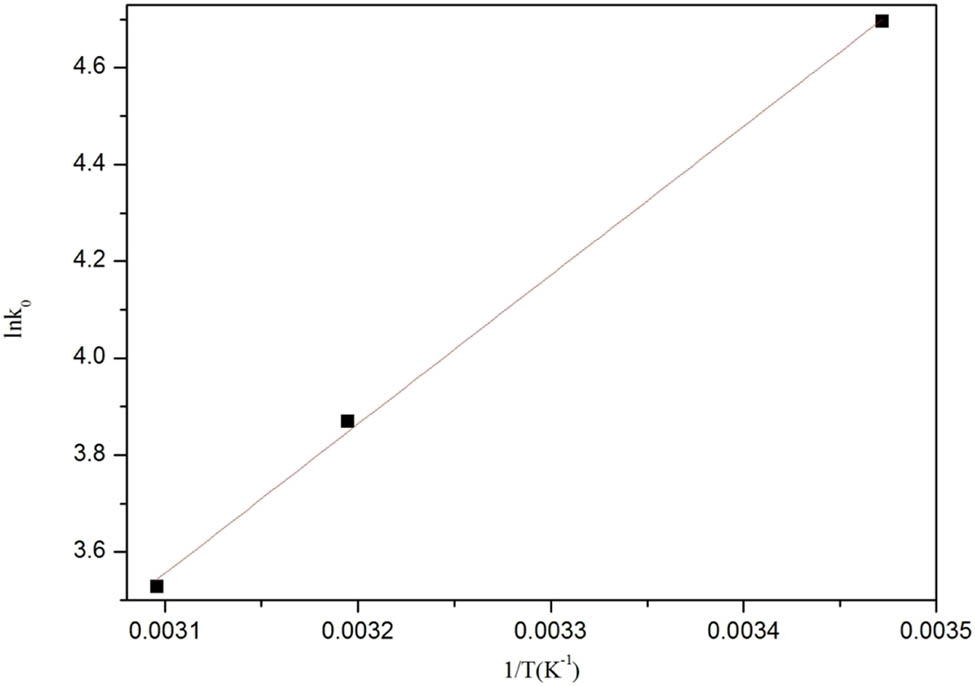
Van’t Hoff plots used to determine the ΔH and ΔS of DOC adsorption over Zr-MOFs.
To study whether the removal of DOC by Zr-MOFs was affected by light, 30 mg of Zr-MOFs was added to 50 ppm DOC, and the results were compared under natural lighting and catalysis by a xenon lamp, as shown in Figure 13. After comparison and sampling within the same time period, the amount of DOC adsorbed under xenon lamp irradiation was 34.5 mg·g−1 less than that under natural light.
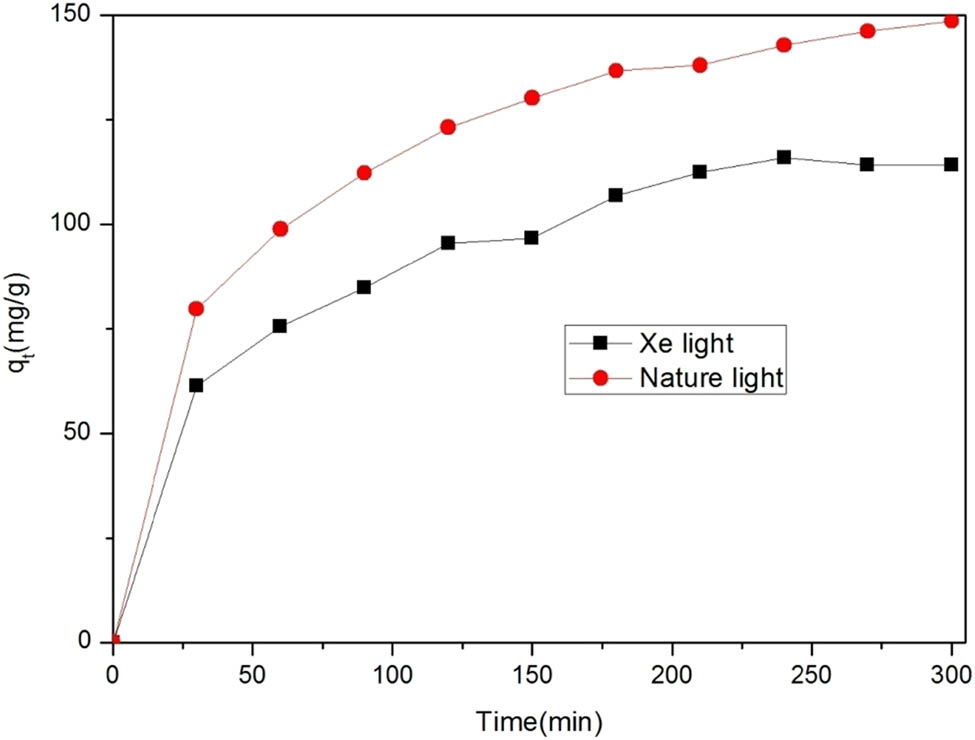
Comparison of DOC removal by Zr-MOFs.
A comparison of the adsorption capacity of DOC by other materials is shown in Table 4. Zr-MOF had the best adsorption capacity for DOC. The adsorption of DOC by Zr-MOFs occurred via a combination of chemisorption and physical adsorption, which led to a better removal effect. Zr-MOFs and DOC could form hydrogen bonds. Zr-MOFs are porous materials, which allow them to absorb DOC. Zr-MOFs may contain unreacted groups that could adsorb DOC via electrostatic adsorption. Because both Zr-MOFs and DOC have benzene rings, the two molecules may be bound together by π–π stacking. Due to the aforementioned effects, Zr-MOFs could remove DOC [46,47,48,49,50].
4 Conclusion
A hot solvent method was used to prepare Zr-MOFs, which were characterized using XRD, scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), and differential thermal-thermogravimetric analysis. Zr-MOFs were used to remove the antibiotic DOC, and the results showed that the best removal effect was with pH = 6 and pH = 10; simulated kinetics showed that DOC removal by Zr-MOFs followed the second-order kinetics model, with an R 2 > 0.99. The Langmuir and Freundlich isotherm model analysis showed that the adsorption mechanism was consistent with multilayer adsorption. It can be seen that DOC can be removed by Zr-MOFs and may have practical applications.
-
Funding information: This work was supported by Doctoral Fund of Anshun University (asxybsjj202103), Guizhou Education Department Youth Science and Technology Talents Growth Project (KY[2019]149), and Undergraduate Innovation and Entrepreneurship Project number [S202210667150].
-
Author contributions: Qinhui Ren writing – original draft; Fuhua Wei: writing – review and editing; Yufu Ma: methodology; Lan Qin, Hongliang Chen: formal analysis; Zhao Liang, Siyuan Wang: visualization.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Cao B, Yu XL, Wang C, Lu S, Xing D, Hu X. Rational collaborative ablation of bacterial biofilms ignited by physical cavitation and concurrent deep antibiotic release. Biomaterials. 2020;262:120341.10.1016/j.biomaterials.2020.120341Search in Google Scholar PubMed
[2] Verma SK, Jha E, Panda PK, Thirumurugan A, Patro S, Parashar SKS, et al. Molecular insights to alkaline based bio-fabrication of silver nanoparticles for inverse cytotoxicity and enhanced antibacterial activity. Mat Sci Eng C-Mater. 2018;92:807–18.10.1016/j.msec.2018.07.037Search in Google Scholar PubMed
[3] Liu SG, Ding YQ, Li PF, Diao KS, Tan XC, Lei FH, et al. Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N, N-dimethyl dehydroabietylamine oxide. Chem Eng J. 2014;248:135–44.10.1016/j.cej.2014.03.026Search in Google Scholar
[4] Chen F, Yang Q, Li X, Zeng G, Wang D, Niu C, et al. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl Catal B. 2017;200:330–42.10.1016/j.apcatb.2016.07.021Search in Google Scholar
[5] Saleha TA, Sarıb A, Tuzen M. Optimization of parameters with experimental design for the adsorption of mercury using polyethylenimine modified-activated carbon. J Env Chem Eng. 2017;5:1079–88.10.1016/j.jece.2017.01.032Search in Google Scholar
[6] Hernández-Montoya V, Pérez-Cruz MA, Mendoza-Castillo DI, Moreno-Virgen MR, Bonilla-Petriciolet A. Competitive adsorption of dyes and heavy metals on zeolitic structures. J Env Manage. 2013;116:213–21.10.1016/j.jenvman.2012.12.010Search in Google Scholar PubMed
[7] Wang H, Yuan X, Wu Y. Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl Catal B-Environ. 2015;174–175:445–54.10.1016/j.apcatb.2015.03.037Search in Google Scholar
[8] Huo SH, Yan XP. Metal–organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J Mater Chem. 2012;22:7449–55.10.1039/c2jm16513aSearch in Google Scholar
[9] Dias EM, Petit JC. Towards the use of metal–organic frameworks for water reuse: a review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. Mater Chem A. 2015;3:22484–506.10.1039/C5TA05440KSearch in Google Scholar
[10] Bajpai VK, Shukla S, Khan I, Kang SM, Haldorai Y, Tripathi KM, et al. A sustainable graphene aerogel capable of the adsorptive elimination of biogenic amines and bacteria from soy sauce and highly efficient cell proliferation. ACS Appl Mater Inter. 2019;11:43949–63.10.1021/acsami.9b16989Search in Google Scholar PubMed
[11] Wei FH, Xiao L, Ren QH, Wang K, Qin L, Chen HL, et al. The application of Bimetallic metal-organic frameworks for antibiotics adsorption. J Saudi Chem Soc. 2022;26:101562.10.1016/j.jscs.2022.101562Search in Google Scholar
[12] Jaramillo DE, Reed DA, Jiang HZH, Oktawiec J, Mara MW, Forse AC, et al. Selective nitrogen adsorption via backbonding in a metal-organic framework with exposed vanadium sites. Nat Mater. 2020;19(5):517–21.10.1038/s41563-019-0597-8Search in Google Scholar PubMed
[13] Zhang L, Jee S, Park J, Jung M, Wallacher D, Franz A, et al. Exploiting dynamic opening of aperturesin partially fluorinated MOF for enhancing H-2 desorption temperature and isotope separation. J Am Chem Soc. 2019;141(50):19850–8.10.1021/jacs.9b10268Search in Google Scholar PubMed PubMed Central
[14] Bi S, Banda H, Chen M, Niu L, Chen MY, Wu TZ, et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat Mater. 2020;19:552–8.10.1038/s41563-019-0598-7Search in Google Scholar PubMed
[15] Boyd PG, Chidambaram A, Garcia-Diez E, Ireland CP, Daff TD, Bounds R, et al. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture. Nature. 2019;576(7786):253–6.10.1038/s41586-019-1798-7Search in Google Scholar PubMed
[16] Bi FK, Zhao ZY, Yang Y, Liu Q, Huang WY, Huang YD, et al. Efficient degradation of toluene over ultra-low Pd supported on UiO-66 and its functional materials: Reaction mechanism, water-resistance, and influence of SO2. Environ Funct Mater. 2022;1:166–81.10.1016/j.efmat.2022.07.002Search in Google Scholar
[17] Zhang XD, Zhao ZY, Zhao SH, Xiang S, Gao WK, Wang L, et al. The promoting effect of alkali metal and H2O on Mn-MOF derivatives for toluene oxidation: A combined experimental and theoretical investigation. J Catal. 2022;415:218–35.10.1016/j.jcat.2022.10.005Search in Google Scholar
[18] Zhao SH, Yang Y, Bi FK, Chen YF, Wu MH, Zhang XD, et al. Oxygen vacancies in the catalyst: Efficient degradation of gaseous pollutants. Chem Eng J. 2023;454:140376.10.1016/j.cej.2022.140376Search in Google Scholar
[19] Rao RZ, Ma ST, Gao B, Bi FK, Chen YF, Yang Y, et al. Recent advances of metal-organic framework-based and derivative materials in the heterogeneous catalytic removal of volatile organic compounds. J Colloid Interface Sci. 2023;636:55–72.10.1016/j.jcis.2022.12.167Search in Google Scholar PubMed
[20] Jie BR, Lin HD, Zhai YX, Ye JY, Zhang DY, Xie YF, et al. Mechanism, design and application of fluorescent recognition based on metal organic frameworks in pollutant detection. Chem Eng J. 2023;454:139931.10.1016/j.cej.2022.139931Search in Google Scholar
[21] Li HH, Yu JY, Gong YS, Lin NP, Yang QL, Zhang XD, et al. Perovskite catalysts with different dimensionalities for environmental and energy applications: A review. Sep Purif Technol. 2023;307:122716.10.1016/j.seppur.2022.122716Search in Google Scholar
[22] Yu JY, Li HH, Lin NP, Gong YS, Jiang H, Chen JJ, et al. Oxygen-deficient engineering for perovskite oxides in the application of AOPs: Regulation, detection, and reduction mechanism. Catalysts. 2023;13(1):148.10.3390/catal13010148Search in Google Scholar
[23] Xiong G, Wang BB, You LX, Ren BY, He YK, Ding F, et al. Hypervalent silicon-based, anionic porous organic polymers with solid microsphere or hollow nanotube morphologies and exceptional capacity for selective adsorption of cationic dyes. Mater Chem. 2019;7(1):393–404.10.1039/C8TA07109HSearch in Google Scholar
[24] Zhao QY, Zhao ZY, Rao RZ, Yang Y, Ling SY, Bi FK, et al. Universitetet iOslo-67 (UiO-67)/graphite oxide composites with high capacities of toluene: Synthesis strategy and adsorption mechanism insight. J Colloid Interface Sci. 2022;627:385–97.10.1016/j.jcis.2022.07.059Search in Google Scholar PubMed
[25] Wang Y, Gong YS, Lin NP, Yu L, Du BB, Zhang XD. Enhanced removal of Cr(VI) from aqueous solution by stabilized nanoscale zero valent iron and copper bimetal intercalated montmorillonite. J Colloid Interface Sci. 2022;606:941–52.10.1016/j.jcis.2021.08.075Search in Google Scholar PubMed
[26] Lin HD, Jie BR, Ye JY, Zhai YX, Luo ZJ, Shao GJ, et al. Recent advance of macroscopic metal-organic frameworks for water treatment: A review. Surf Interfaces. 2023;36:102564.10.1016/j.surfin.2022.102564Search in Google Scholar
[27] Ren QH, Nie M, Yang LL, Wei FH, Ding B, Chen HL, et al. Synthesis of MOFs for RhB adsorption from wastewater. Inorganics. 2022;10(3):27.10.3390/inorganics10030027Search in Google Scholar
[28] Li JN, Han X, Zhang XR, Sheveleva AM, Cheng YQ, Tuna F, et al. Capture of nitrogen dioxide and conversion to Nitric acid in a porous metal-organic framework. Nat Chem. 2019;11:1085–90.10.1038/s41557-019-0356-0Search in Google Scholar PubMed
[29] Mohammad-Pour GS, Hatfield KO, Fairchild DC. A solid-solution approach for redox active metal-organic frameworks with tunable redox conductivity. J Am Chem Soc. 2019;141(51):19978–82.10.1021/jacs.9b10639Search in Google Scholar PubMed
[30] Liang RR, Ru-Han A, Xu SQ, Qi QY, Zhao X. Fabricating organic nanotubes through selective disassembly of two-dimensional covalent organic frameworks. J Am Chem Soc. 2019;142:70–4.10.1021/jacs.9b11401Search in Google Scholar PubMed
[31] Wei FH, Zheng T, Ren QH, Chen HL, Peng JH, Ma Y, et al. Preparation of metal-organic frameworks by microwave-assisted ball milling for the removal of CR from wastewater. Green Process Synth. 2022;11(1):595–603.10.1515/gps-2022-0060Search in Google Scholar
[32] Ren QH, Wei FH, Chen HL, Chen D, Ding B. Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater. Green Process Synth. 2021;10(1):125–33.10.1515/gps-2021-0020Search in Google Scholar
[33] Crabtree RH. The organometallic chemistry of the transition metals. 5th edn. East China University of Science and Technology Publishing Co. Ltd.; 2012. p. 148.Search in Google Scholar
[34] Nguyen VT, Nguyen TB, Chen CW, Hung CM, Chang JH, Dong CD. Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Bioresour Technol. 2019;284:197–203.10.1016/j.biortech.2019.03.096Search in Google Scholar PubMed
[35] Wei FH, Zhang H, Ren QH, Chen HL, Yang LL, Ding B, et al. Removal of organic contaminants from wastewater with GO/MOFs composites. PLoS One. 2021;16(7):e0253500.10.1371/journal.pone.0253500Search in Google Scholar PubMed PubMed Central
[36] Chen JF, Yang Y, Zhao SH, Bi FK, Song L, Liu N, et al. Stable black phosphorus encapsulation in porous mesh-like UiO-66 promoted charge transfer for photocatalytic oxidation of toluene and o-dichlorobenzene: Performance, degradation pathway, and mechanism. ACS Catal. 2022;12:8069–81.10.1021/acscatal.2c01375Search in Google Scholar
[37] Wei FH, Ren QH, Liang Z, Chen D. Synthesis of graphene oxide/metal-organic frameworks composite materials for removal of Congo red from wastewater. Chemistryselect. 2019;4(19):5755–62.10.1002/slct.201900363Search in Google Scholar
[38] Zhang XD, Bi FK, Zhao ZY, Yang Y, Li YT, Song L, et al. Boosting toluene oxidation by the regulation of Pd species on UiO-66: Synergistic effect of Pd species. J Catal. 2022;413:59–73.10.1016/j.jcat.2022.06.015Search in Google Scholar
[39] Du QX, Rao RZ, Bi FK, Yang Y, Zhang WM, Yang YQ, et al. Preparation of modified zirconium-based metal-organic frameworks (Zr-MOFs) supported metals and recent application in environment: A review and perspectives. Surf Interfaces. 2022;28:101647.10.1016/j.surfin.2021.101647Search in Google Scholar
[40] Hu J, Yu H, Dai W, Yan X, Hu X, Huang H. Enhanced adsorptive removal ofhazardous anionic dye “Congo red” by a Ni/Cu mixed-component metal–organic porous material. RSC Adv. 2014;4:35124.10.1039/C4RA05772DSearch in Google Scholar
[41] Yılmaz E, Sert E, Atalay FS. Synthesis, characterization of a metal organic framework:MIL-53 (Fe) and adsorption mechanisms of methyl red onto MIL-53 (Fe). J Taiwan Inst Chem Eng. 2016;65:323–30.10.1016/j.jtice.2016.05.028Search in Google Scholar
[42] Rostamian R, Behnejad H. Insights into doxycycline adsorption onto grapheme nanosheet: a combined quantum mechanics, thermodynamics, and kinetic study. Env Sci Pollut Res. 2018;25:2528–37.10.1007/s11356-017-0687-6Search in Google Scholar PubMed
[43] Liu S, Xu WH, Liu YG, Tan XF, Zeng GM, Li X, et al. Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Sci Total Env. 2017;592:546–53.10.1016/j.scitotenv.2017.03.087Search in Google Scholar PubMed
[44] Ghaemi M, Absalan G. Fast removal and determination of doxycycline in water samples and honey by Fe3O4 magnetic nanoparticles. J Iran Chem Soc. 2015;12:1–7.10.1007/s13738-014-0450-6Search in Google Scholar
[45] Zaidi S, Sivasankar V, Chaabane T, Alonzo V, Omine K, Maachi R, et al. Separate and simultaneous removal of doxycycline and oxytetracycline antibiotics by electro-generated adsorbents (EGAs). J Env Chem Eng. 2019;7:102876.10.1016/j.jece.2018.102876Search in Google Scholar
[46] Rego RM, Kurkuri MD, Kigga MA. Comprehensive review on water remediation using UiO-66 MOFs and their derivatives. Chemosphere. 2022;302:134845.10.1016/j.chemosphere.2022.134845Search in Google Scholar PubMed
[47] Chen J, Ouyang JB, Chen WQ, Zheng ZP, Yang Z, Liu ZR, et al. Fabrication and adsorption mechanism of chitosan/Zr-MOF (UiO-66) composite foams for efficient removal of ketoprofen from aqueous solution. Chem Eng J. 2022;431:134045.10.1016/j.cej.2021.134045Search in Google Scholar
[48] Sriram G, Bendre A, Mariappan E, Altalhi T, Kigga M, Ching YC, et al. Recent trends in the application of metal-organic frameworks (MOFs) for the removal of toxic dyes and their removal mechanism-a review. Sustain Mater Techno. 2022;31:e00378.10.1016/j.susmat.2021.e00378Search in Google Scholar
[49] Du CY, Zhang Y, Zhang Z, Zhou L, Yu GL, Wen XF, et al. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: A review. Chem Eng J. 2022;431:133932.10.1016/j.cej.2021.133932Search in Google Scholar
[50] Song FJ, Cao SR, Liu ZH, Su HT, Chen ZQ. Different decorated ZIF-67 adsorption performance towards methamphetamine revealed by theoretical and experimental investigations. J Mol Liq. 2022;364:119950.10.1016/j.molliq.2022.119950Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

