Abstract
The increase in wastewater collection and treatment systems has shown the need for adequate treatment and final disposal of the resulting sludge. Heat treatment through pyrolysis is an alternative, as it promotes the valorization of these wastes by converting them into biochar. Biochar is a multifunctional material that can serve as an adsorbent for many types of pollutants, including removing heavy metals. This study’s main aim is to produce, characterize, and evaluate the remedial potential of biochar resulting from the pyrolysis of sewage sludge (SS) in water polluted with lead. Pre-prepared sludge-derived biochar without any activation and biochar derived from SS activated with phosphoric acid (H3PO4) was prepared using a pre-chemical modification method by mixing a specified amount of H3PO4 with SS. The batch adsorption tests were used to verify the parameters of the adsorbent concentration, solution pH, contact time, and initial concentration. The adsorbent was characterized using X-ray diffraction, scanning electron microscopy, Brunauer-Emmett-Teller, Fourier transform infrared spectroscopy, and energy dispersive spectrometer methods. The adsorption capacity was determined using the Langmuir and Freundlich models. The surface area of biochar increased from 35.769 m2/g for non-activated biochar to 136.04 m2/g for activated biochar with H3PO4. Biochar removed 87.36% of the lead. The activated biochar’s granular shape improved lead removal from the solution. This research has significant implications for removing lead from water using inexpensive sludge-based adsorbents.
1 Introduction
Sewage sludge (SS), which is a solid by-product, is acquired from the oxidation trench, aeration tank, or secondary sedimentation tank after the biological treatment of wastewater treatment plants [1–3]. The improper disposal of SS has emerged as a significant concern due to its potential to cause secondary contamination and substantial harm to human health. Developed countries generate the most significant quantity of SS annually, varying between 100,000 and 500,000 tons [4]. If not effectively managed, this substance can pose a substantial burden [5]. Kacprzak et al. [6] have created numerous environmentally beneficial and cutting-edge alternatives for the disposal of SS. The production of biochar from sludge is an exciting way to treat the problem because it would make treating sludge easier and give us products worth a lot more. Biochar made from sludge that is produced through pyrolysis is being used more and more for adsorption [7].
Over the past century, increased industrialization has placed significant stress on the environment [8]. Activities such as automobile maintenance, tetraethyl production [9], and the process of refining and manufacturing batteries [10] have released a significant quantity of harmful lead (Pb2+) into the water supply. The most abundant heavy metal in the world is lead, a hazardous and poisonous substance with a high molecular weight [11–13]. Furthermore, lead can accumulate in the human body and cause cancer, anemia, kidney failure, long-lasting brain damage [14], and severe mutations [15]. Pollution from lead has recently emerged as one of the most pressing issues with water contamination in emerging nations [16]. For this reason, eliminating Pb2+ from wastewater is a critical and immediate need for protecting human health and safety [17].
Electrocoagulation, ion exchange, chemical precipitation, membrane processes, coagulation, filtration, and adsorption are several methods that have been developed in the past several decades to remove lead from wastewater. Due to its low cost, simple process, and high removal efficiency, adsorption has received widespread attention and is one of the most popular removal techniques among these methods [18–20]. Heavy metals have been removed from aqueous solutions using a variety of sorbents. The high renewal and production costs of conventional sorbents may make them unattractive [21], while biochar, a cheaper material that is good at removing lead from water, has received much attention [22].
Biochar, a byproduct of the pyrolysis of SS in an anoxic environment at temperatures ranging from 350 to 900°C, abundantly contains organic carbon, mineral components, and functional groups that include oxygen [23]. Pyrolysis, which converts sludge into biochar, offers a potential solution for waste recycling and sludge reduction [24].
The surface modification technique is widely used because it is easy to modify and has high adsorption effectiveness. Many biochar-based products are made by impregnating adsorbents onto biochar surfaces. This is especially common with surfaces that have distinctive physical and chemical characteristics and a high ability to adsorb substances [25–27]. To enhance biochar’s capacity to absorb heavy metals and other pollutants, surface modification might increase the number of functional groups, adsorption sites, or other specific adsorption structures present in the substance [28].
Chemical modification techniques include activation by KOH, NaOH, ZnCl2, and phosphoric acid (H3PO4). Activated biochar treated with H3PO4 acid reacts with carbon atoms to form pores in the solid structure, leading to a varied pore size distribution and a higher specific surface area. This process is cost-effective, less corrosive, and shows excellent dispersibility [29]. The modification process adds phosphate bonds to enhance the crosslinking density. Pyrolysis then breaks down these bonds, leading to the formation of larger pores [30].
Several studies have found that activation can produce biochar with an improved porous structure, which leads to enhanced Pb2+ adsorption capacities [31]. According to one study, the application of KMnO4 modification to pine wood biochar resulted in a 20-fold increase in Pb2+ removal capacity, raising it from 2.35 to 47.05 mg/g [32]. Depci et al. [33] used ZnCl2 as an activator to produce activated carbon from apple pulp. These activated carbons showed an adsorption capacity of 15.96 mg/g for Pb2+.
Multiple researchers [34,35] have demonstrated the significance of the pyrolysis process in determining the sorption capacity of biochar generated from sludge. This study used the pyrolysis of SS to produce biochar, which was then pre-chemically modified with H3PO4 as an impregnating agent. The non-activated biochar and activated biochar were analyzed using various techniques to assess the impact of chemical activation. Subsequently, batch adsorption was conducted under various conditions to investigate the process of eliminating lead ions (Pb2+) from aqueous solutions. Adsorption isothermal models were also discussed in this work.
2 Novel contribution
The novel contribution of this research is the use of H3PO4 as an activator of sewage sludge-derived biochar (SDBC) for lead ion removal, which has not been previously documented in the scientific literature. Previous studies have used other materials to activate biochar sludge for lead ion removal. This study focuses on the effect of H3PO4 as an activator of biochar.
3 Materials and methods
3.1 Chemicals and reagents
All chemicals and reagents used in this study (standard solution lead 1,000 ppm, NaOH, H3PO4, and HCl) are from a company (Thomas Baker Ltd) of high analytical grade. All the water used is deionized.
3.2 Sample collection
In this study, sludge collected from the Al-Rustamiya Southern project in Baghdad from the drying bed was used. Sludge is the final product of wastewater treatment processes, prepared for final disposal or alternative uses, such as biochar conversion into valuable materials. The material was sun-dried for 48 h to remove any residual moisture, and it was then ground and passed through a 2 mm sieve.
3.3 SDBC
Preparation was performed following the method of Fan et al. [36]. The dried and powdered sludge was placed in a crucible and then introduced into a pyrolysis furnace at a temperature of 550°C for 2 h. The heating rate was adjusted to 10°C/min until the target temperature was achieved. Nitrogen gas flows at a rate of 2 L/min to create an oxygen-free environment. The sample was kept in the pyrolysis oven for 3 h for cooling. Next, the biochar was rinsed with deionized water and left to dry in the oven for 6 h at 105°C. The resulting sludge biochar was crushed to a size of 75 μm and stored for future adsorption tests.
3.4 Active-sludge-derived biochar (A-SDBC)
The preparation process followed the instructions provided by Huang et al. [37] with minimal alterations. A solution of H3PO4 (45% by weight) impregnated SS in a glass flask at a ratio of 1:2.4 (g SS/g H3PO4) for 24 h. Then, they were dried in an oven for 2 h at 105°C. Next, we gently decomposed the dried mixture within an electric muffle furnace, maintaining a heating rate of 10°C/min at 550°C for 2 h while maintaining a nitrogen gas flow of 2 L/min. To achieve a pH of neutral, the product was rinsed with a solution of 0.1 M NaOH and deionized water. After that, they were dried in an oven at 105°C for 6 h, then sieved and crushed to produce particles with a size of 75 μm. The biochar was placed in an airtight container and labeled for experiments (A-SDBC).
3.5 Morphological characterization of adsorbents
Various methods have been employed to analyze the surface chemistry, crystalline characteristics, and content of sludge biochar.
Using X-ray diffraction patterns (XRD; Shizuzu-6000, Japan), scanning electron microscopy (SEM; Thermo Fisher, Inspect F50, USA), energy dispersive spectrometer (EDS; Bruker, Germany), Fourier transform infrared spectroscopy (FTIR; Shimadzu-1800 with a resolution of 4/cm, Japan), and Brunauer-Emmett-Teller (BET; Q-surf 9600, USA).
3.6 Experiments of Pb2+ ions adsorption onto biochar
The first test to find out how well biochar absorbs lead ions from the water was carried out by stirring 60 mg of biochar, a contact time of 120 min at 250 rpm, and an initial Pb2+ concentration of 5 mg/L. The volume of the beaker glass was 100 mL, with a pH of 7 at 25°C. The biochar with the highest percentage of lead removed was selected for further testing.
Several factors affecting lead adsorption were studied, including solution pH (2–9), initial Pb2+ concentration (1–25 mg/L), amount of biochar (10–100 mg), and contact period (0–180 min). About 10 mg/L of the initial concentration was stirred at 250 rpm on a shaker in a 100 mL beaker glass until it reached equilibrium at 25°C. pH was adjusted with 0.1 M NaOH and HCl solutions. The biochar was extracted from the aqueous solution using a centrifuge for 10 min at 5,000 rpm. The final Pb2+ concentration in mg/L was evaluated using an atomic absorption spectrometer (Shimadzuaa-7000f, Japan).
Equation (1) was utilized to determine the quantity of metal ions that were removed [38]:
In Equation (2), qe (mg/g) was utilized to determine the absorption capacity of the absorbent in terms of the amount of metal absorbed per unit mass:
where C 0 is the initial concentration (mg/L), C e is the final concentration (mg/L), V is the volume of solution (L), and M is the mass of the adsorbent (g).
3.7 Isotherm study
To comprehend the process of heavy metal-biochar adsorption, it is necessary to use isotherm models to ascertain the manner in which heavy metal molecules are dispersed on the surface of biochar. Two isotherm models, namely Langmuir and Freundlich, were utilized in order to investigate the adsorption mechanisms of Pb2+ ions onto biochar.
The Langmuir model in Equation (3) proposes a monolayer adsorption process where all active binding sites have uniform energy and absorb only one heavy metal molecule [39]. The model utilized is stated in a nonlinear form:
where q m is the maximum adsorption capacity of biochar in a single layer (mg/g) and K L is the affinity constant in the Langmuir model in (L/mg).
Freundlich model demonstrates that the surface heterogeneity of sorbents is influenced by multilayer adsorption and the uneven distribution of energy among active sorption sites. Equation (4) represents the nonlinear mathematical form of the Freundlich model, denoted by Harja et al. [39]:
where K F is the affinity constant in the Freundlich model in (mg/g) (L/mg), 1/n the dimensionless intensity parameter. Values of 1/n less than 1, equal to 0, equal to 1, and more than 1 indicate favorable, irreversible, linear, and unfavorable adsorption processes, respectively.
4 Results and discussion
4.1 Characterization of the biochar
4.1.1 XRD
XRD patterns in Figure 1 showed that quartz (SiO2) was the primary material in both SS and A-SDBC. For SS, the peaks were at 26.6°, 50°, and 68°, and for A-SDBC, they were at 26.6°. SiO2 is typically the most common mineral in SS and A-SDBC samples. This is because clay and sand are still in the sludge from treating sewage [40,41].
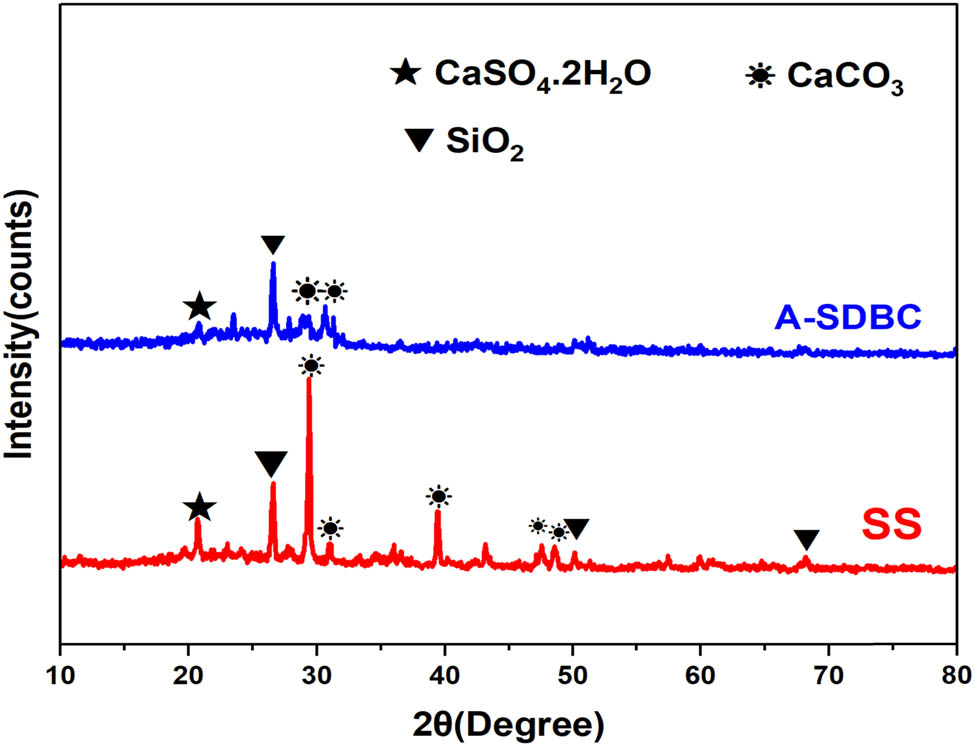
XRD spectra for SS and A-SDBC.
Both samples displayed gypsum peak (CaSO4·2H2O) at 2θ 20.8° in their spectra. Gypsum found in SS may be due to the application of aluminum sulfate in the final sewage treatment phase. The peak intensity was low in A-SDBC, perhaps because sulfur was lost in gaseous form during pyrolysis [42].
There were calcite (CaCO3) diffraction peaks on the SS surface that matched the ones found by Yin et al. [41] and Li et al. [43]. These peaks were at 29.5°, 31.6°, 39.6°, 47.5°, and 48.5°. The diffraction peaks of CaCO3 vanish on the surface of A-SDBC. The disappearance could be due to the high-temperature pyrolysis of inorganic chemicals present in the sludge, which causes the machine components to transform into gas and ash [44].
4.2 SEM and BET
Field-emission scanning electron microscopy (FE-SEM) methods were employed to examine the shapes and structures of SS, SDBC, and A-SDBC. Figure 2(a) shows that the raw SS structure is relatively smooth. SDBC exhibits a wrinkled structure with pores that are unevenly scattered, as shown in Figure 2(b). Furthermore, after H3PO4 activation, the A-SDBC sponge-like structure exhibited enhanced micropores, as shown in Figure 2(c). Table 1 indicates that the specific surface area of SS was 2.48 m2/g. The specific surface area increased to 35.769 and 136.04 m2/g for SDBC and A-SDBC, respectively, when activated with H3PO4, demonstrating the effectiveness of this approach in enhancing the base material’s surface area. Impregnation in H3PO4 may make the biochar surface coarser and increase the number of holes due to chemical reactions and the volatilization of organic substances during pyrolysis, thereby increasing the surface area. The many mesopores are essential for adsorbing lead [37].
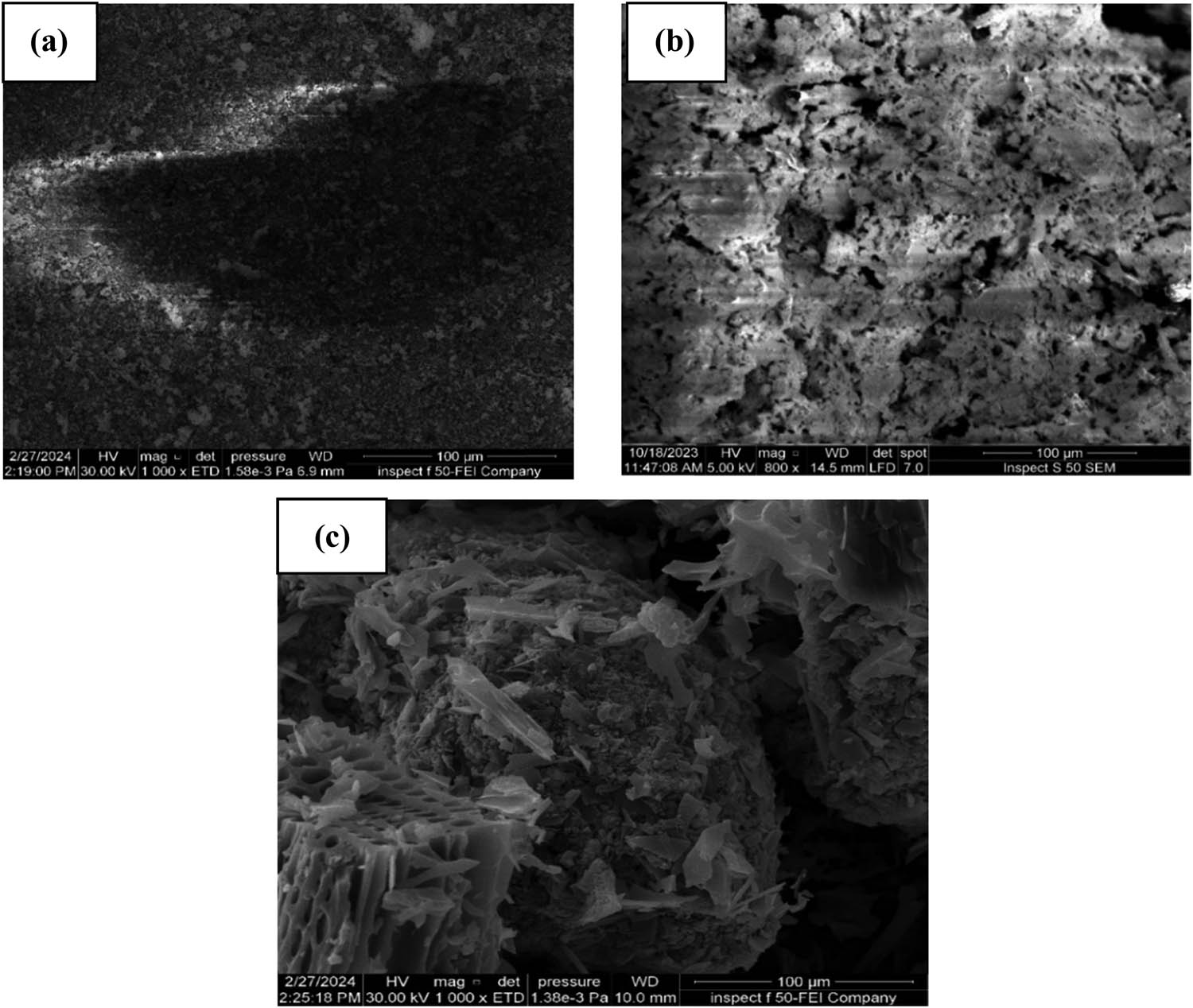
FE-SEM images for (a) SS, (b) SDBC, and (c) A-SDBC.
Pore volume, surface area, and average pore size for SS, SDBC, and A-SDBC
| Pore volume (cm3/g) | Surface area (m2/g) | Average pore size (nm) | |
|---|---|---|---|
| SS | 0.0035 | 2.48 | 5.64 |
| SDBC | 0.042 | 35.769 | 4.696 |
| A-SDBC | 0.21 | 136.04 | 6.17 |
4.2.1 FTIR and EDS
FTIR spectroscopy was used to investigate the surface functional groups of the biochar: SS and SDBC (Figure 3(a)) and A-SDBC (Figure 3(b)). Most of the components of biochar are carbon and oxygen, so the functional groups observed are also associated with these elements. The absorption of hydroxyl groups O–H is at about 3,424/cm. This indicates alcohols and phenols. The absorption peaks in the wave region (2,600–3,000/cm) indicate the presence of an aliphatic structure for vibrations of C–H in SS. After pyrolysis, the C–H peak intensity decreased and disappeared in A-SDBC, indicating that the majority of aliphatic hydrocarbons were transformed into gases such as methane and carbon dioxide or converted into aromatic structures [45]. A-SDBC showed C═C stretching vibrations (1,612/cm), indicating the aromatic properties of biochar [46]. C–O (1,418/cm) vibrations indicate ether and C–O–C (1,078/cm) vibrations indicate lactone structures. Surface groups on biochar can react with metals via adsorption [47,48].
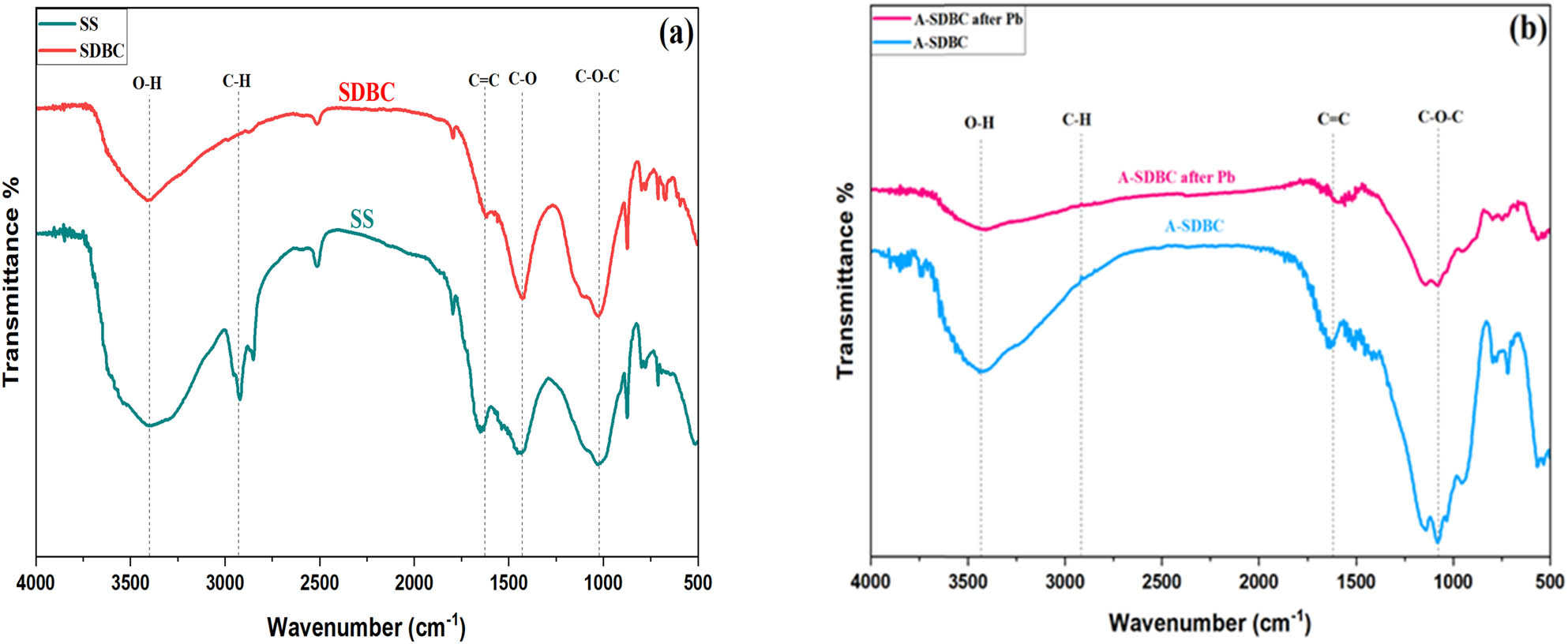
(a) FTIR spectrum for raw SS and SDBC and (b) FTIR spectrum for A-SDBC and A-SDBC after Pb2+ adsorption.
By examining the EDS, as shown in Figure 4, the appearance of the phosphorus (P) peak on the surface of the biochar (A-SDBC) confirms the adequate preparation of the material.
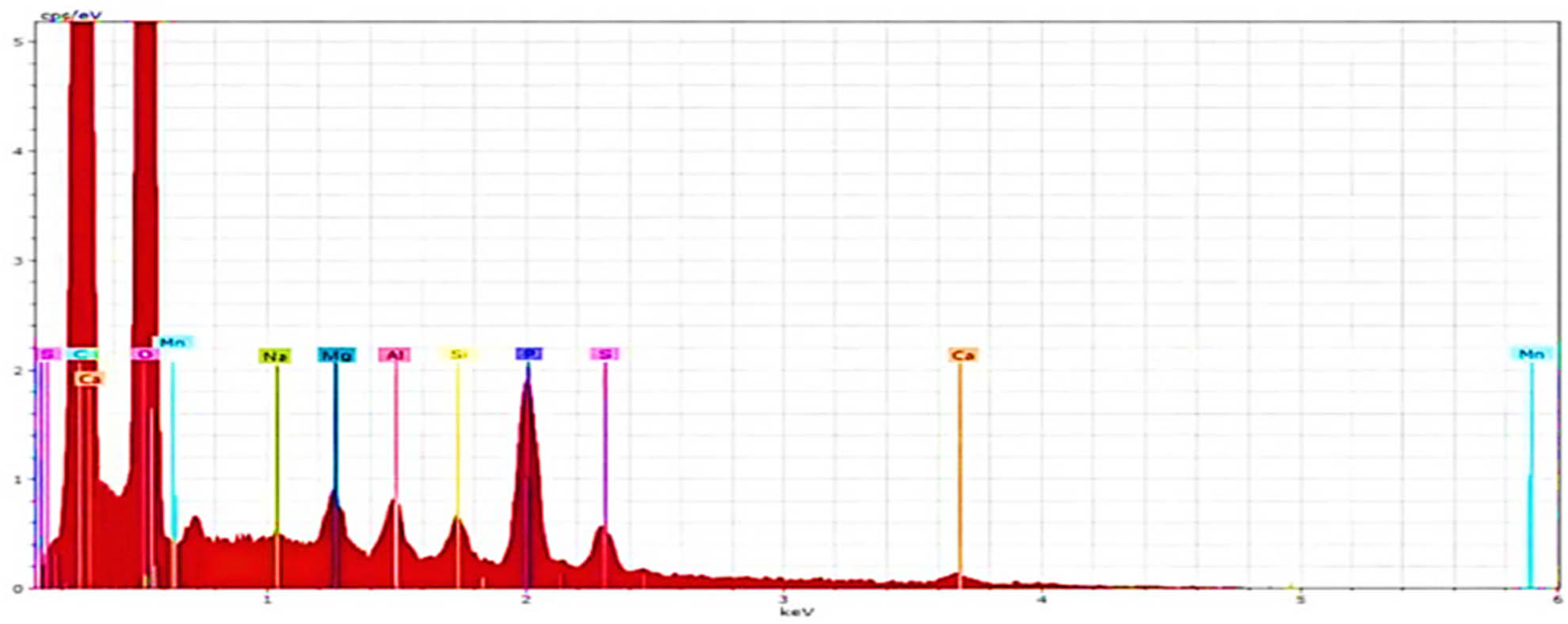
EDS images for A-SDBC.
4.3 Exploring biochar’s adsorption potential
The outcomes of a monitoring experiment that assessed the ability of the biochar to adsorb Pb2+ ions. The H3PO4-activated biochar has a significantly higher R% for Pb2+ ion removal (86.4% for A-SDBC) compared to the non-activated biochar (41.29% for SDBC). Adsorption tests were conducted to remove lead ions under different operating conditions, and A-SDBC was selected based on the initial results and characterization of the biochar.
4.4 Optimal parameters for the adsorption of Pb2+ on the A-SDBC
A-SDBC test was used to evaluate biochar’s potential as an adsorbent for lead. This includes the dose of adsorbent, pH, contact time, and the initial concentration.
4.5 Effect of dosage on pb2+ adsorption
The effect of the A-SDBC adsorbent on the Pb2+ removal efficiency was studied at different adsorption doses (10, 30, 60, 80, and 100 mg). The study maintained constant adsorption conditions, including a pH of 7, an initial concentration of 10 mg/L, a contact time of 3 h at 250 rpm, and a temperature of 25°C. Figure 5(a) demonstrates an increase in the removal ratio of Pb2+ with increasing adsorbent doses until it reaches 100 mg of adsorbent A-SDBC. With 100 mg of biochar, the removal rate reached 81.27%. This result is consistent with previous research [49,50]. Higher doses of adsorbent lead to more binding sites for interaction with Pb2+, leading to improved removal efficiency [51]. Hence, a dose of 100 mg is chosen to perform the adsorption for the rest of the experiments.

Parameter effect on Pb2+ adsorption: (a) effect of dosage, (b) effect of pH, (c) effect of contact time, and (d) effect of initial Pb2+ ion concentration.
4.6 Effect of pH on pb2+ adsorption
The surface mechanism of the adsorbent mainly influences the pH of the solution. The study maintained constant adsorption conditions, including a dose of 100 mg, a contact time of 3 h at 250 rpm, an initial concentration of 10 mg/L, and a temperature of 25°C. Figure 5(b) illustrates that the removal percentage of the Pb2+ ion increases as the pH of the solution increases from 2 to 6. This outcome can be compared with the findings of Rojas-Mayorga et al. [52]. An increase in adsorbent pH causes deprotonation of functional groups on A-SDBC adsorbents, resulting in a negatively charged surface that promotes cation adsorption through electrostatic contact [53]. Hence, a pH of 6 is chosen to perform the adsorption for the rest of the experiments.
4.7 Effect of contact time on Pb2+ adsorption
Among the many factors to consider when choosing a wastewater treatment system is the equilibration time. With a time of 0–180 min, the experiment was conducted for lead adsorption by A-SDBC under constant conditions (a dose of 100 mg, a pH of 6, an initial concentration of 10 mg/L, and a temperature of 25°C). Figure 5(c) illustrates that an increase in adsorption capacity was seen with increasing contact time. At first, lead absorption was obviously faster, but then it increased slowly. The absorption capacity is 8.048 mg/g at a time of 60 min. After 60 min, the absorption of lead ions reached equilibrium, and there was no further increase due to metal ions previously absorbed from the solution. Similar results were found by Shafiq et al. [54]. The increase in contact time enhanced the absorption of Pb2+, most likely due to the abundance of vacant sites, allowing a larger surface area to absorb more ions at the active sites of the biochar [55,56]. The decrease in the absorption of metal ions over time was caused by the saturation of active biochar sites [57]. Hence, a time of 60 min is chosen to perform the adsorption for the rest of the experiments.
4.8 Effect of initial concentrations on Pb2+ adsorption
The initial metal concentration primarily influences the absorption process under constant conditions (a dose of 100 mg, a pH of 6, a contact time of 60 min at 250 rpm, a temperature of 25°C, an initial concentration of 1–25 mg/L). Figure 5(d) illustrates that the absorption capacity of Pb2+ on the adsorbent A-SDBC increased from 0.84 to 18.01 mg/g when the concentration of primary metals increased. The same outcome was reported by Huda et al. [58]. Biochar’s ability to absorb more metal ions at high concentrations is due to its empty active sites, which make it easier for metal ions to interact with biochar. Low Pb2+ concentrations have lower adsorption capacity because the adsorption sites on biochar are fully occupied [59–61].
4.9 Adsorption isotherm
The Langmuir and Freundlich isotherm parameters of the model are shown in Table 2, along with the correlation coefficient (R 2). Nonlinear graphs are shown in Figure 6. The Langmuir isotherm model described the absorption of Pb2+ ions onto A-SDBC more precisely, as evidenced by the higher R 2 value of 0.994 compared to Freundlich’s R 2 value of 0.991. The Langmuir model value indicates that the maximal adsorption capacity of Pb2+ ions (q max) is 28.556 mg/g. A monolayer adsorption mechanism controlled the adsorption of lead ions on biochar, ensuring consistent binding energy at the active regions of the biochar through chemical adsorption [62]. Cheng et al. [63] also confirmed this outcome while studying the removal of Pb2+ using biochar made from sludge.
Isotherm model (Langmuir and Freundlich) parameters for Pb2+ ion adsorption
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| R 2 | K L | q max | R 2 | K F | 1/n | |
| A-SDBC | 0.994 | 0.232 | 28.556 | 0.991 | 5.694 | 0.601 |

Isotherm models for Pb2+ adsorption on A-SDBC.
The exceptional heavy metal adsorption ability of A-SDBC is attributed to its high pore volume, extensive surface area, and functional surface oxygen groups [37].
4.10 Characterization of remaining biochar after adsorption of Pb2+
Figure 7 shows images of EDS after A-SDBC treatment of lead. A Pb2+ element appeared in the EDS, showing that A-SDBC was stabilized in lead.

EDS image for A-SDBC after Pb2+ adsorption.
From Figure 3(b), the FTIR position of the peak of biochar remained relatively stable before and after the adsorption of Pb2+. Changes in absorbed energy have led to significant alterations in infrared transmittance following the adsorption of Pb2+ on biochar. The O–H stretching frequency at 3,466 cm−1 was reduced following the adsorption of Pb2+, indicating the cleavage of O–H bonds post-Pb2+ adsorption. Functional groupings containing oxygen atoms, including C–O–C and O–H, were present on the biochar surface, resulting in a negative charge that attracted Pb2+ for adsorption [61]. The reason behind this is that when Pb2+ ions adsorb to these functional groups, a smaller number of peaks are seen when comparing the two FTIR patterns [64–66].
5 Conclusion
This research established that SS could be used as a component of an adsorbent to remove lead ions from contaminated water. Converting sludge into biochar is an economically and environmentally beneficial process, as the organic materials in the sludge can be exploited and converted into valuable material instead of being disposed of in traditional ways.
Commercial activated carbon and other popular adsorbent materials can be expensive; therefore, using this material as a possible low-cost heavy metal adsorbent is a prospective alternative.
The addition of H3PO4 enhanced the biochar’s ability to adsorb lead ions. The activated biochar exhibited a BET surface area of 136.04 m2/g, four times greater than the non-active biochar. It was found that the Langmuir model accurately represents the adsorption of Pb2+, with a maximum of 28.556 mg/g at a dose of 100 mg, pH 6, 60 min, and 25°C.
In conclusion, H3PO4-activated SS biochar is an effective solution for the purification of water contaminated with lead, so biochar sludge is a promising sorbent.
-
Funding information: The authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. Aya L. Khalil conducted the experiments, developed the theory, and performed the computations. Faris H. Al-Ani and Abdul Hameed M.J. Al-Obaidy verified the analytical methods, conceived the original idea, and supervised the findings of this work. All authors provided critical feedback and helped shape the research, analysis, and final manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Most datasets generated and analyzed in this study are comprised in this submitted manuscript. The other datasets are available on reasonable request from the corresponding author with the attached information.
References
[1] Ho SH, Yang ZK, Nagarajan D, Chang JS, Ren NQ. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour Technol. 2017;246:142–9. 10.1016/j.biortech.2017.08.025.Suche in Google Scholar PubMed
[2] Tahiri V, Denaj A, Prenga D. Assessment of the presence of pharmaceutical compounds in wastewaters and in aquatic environment. JHEF. 2023;4(3):290–302. 10.28991/HEF-2023-04-03-03.Suche in Google Scholar
[3] Ali HQ, Üçüncü O. Modeling and optimizing wastewater stabilization ponds for domestic wastewater treatment. Civ Eng J. 2023;9(11):2834–46. 10.28991/CEJ-2023-09-11-014.Suche in Google Scholar
[4] Grobelak A, Czerwinska K, Murta´s A. General considerations on sludge disposal, industrial and municipal sludge. In: Industrial and Municipal Sludge. Butterworth-Heinemann; 2019. p. 135–53. 10.1016/B978-0-12-815907-1.00007-6.Suche in Google Scholar
[5] Samolada MC, Zabaniotou AA. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014;34:411–20. 10.1016/j.wasman.2013.11.003.Suche in Google Scholar PubMed
[6] Kacprzak M, Neczaj E, Fijałkowski K, Grobelak A, Grosser A, Worwag M, et al. Sewage sludge disposal strategies for sustainable development. Environ Res. 2017;156:39–46. 10.1016/j.envres.2017.03.010.Suche in Google Scholar PubMed
[7] Devi P, Saroha AK. Utilization of sludge-based adsorbents for the removal of various pollutants: a review. Sci Total Environ. 2017;578:16–33. 10.1016/j.scitotenv.2016.10.220.Suche in Google Scholar PubMed
[8] Zhang J, Wang Q. Sustainable mechanisms of biochar derived from brewers’ spent grain and sewage sludge for ammonia–nitrogen capture. J Clean Prod. 2016;112:3927–34. 10.1016/j.jclepro.2015.07.096.Suche in Google Scholar
[9] Bogusz A, Oleszczuk P, Dobrowolski R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour Technol. 2015;196:540–9. 10.1016/j.biortech.2015.08.006.Suche in Google Scholar PubMed
[10] Loría KC, Emiliani J, Bergara CD, Herrero MS, Salvatierra LM, Pérez LM. Effect of daily exposure to Pb-contaminated water on Salvinia biloba physiology and phytoremediation performance. Aquat Toxicol. 2019;210:158–66. 10.1016/j.aquatox.2019.02.019.Suche in Google Scholar PubMed
[11] Al-Edani TY, Al-Tameemi HJ, Jasim ZF. Phytoremediation of heavy metals (Cd, Cu, Fe, and Pb) by using aquatic plants in Shatt Al-Arab River. Eng Technol J. 2019;37(3C):365–9. 10.30684/etj.37.3C.10.Suche in Google Scholar
[12] Salman GK, Bohan AJ, Jaed GM. Use of nano-magnetic material for removal of heavy metals from wastewater. Eng Technol J. 2017;35(9A):903–8. 10.30684/etj.35.9A.6.Suche in Google Scholar
[13] Haamoodi AL, Fatlawi AHA, Taher GA. Removal lead and cadmium ions in industrial wastewater using graphene nanosheets. Eng Technol J. 2016;34(9):1903–14.10.30684/etj.34.9A.16Suche in Google Scholar
[14] Qu J, Meng Q, Lin X, Han W, Jiang Q, Wang L, et al. Microwave-assisted synthesis of β-cyclodextrin functionalized celluloses for enhanced removal of Pb (II) from water: adsorptive performance and mechanism exploration. Sci Total Environ. 2021;752:141854. 10.1016/j.scitotenv.2020.141854.Suche in Google Scholar PubMed
[15] Wang L, Lei T, Ren Z, Jiang X, Yang X, Bai H, et al. Fe3O4@ PDA@ MnO2 core-shell nanocomposites for sensitive electrochemical detection of trace Pb (II) in water. J Electroanal Chem. 2020;864:114065. 10.1016/j.jelechem.2020.114065.Suche in Google Scholar
[16] Aneke F, Adu J. Adsorption of heavy metals from contaminated water using leachate modular tower. Civ Eng J. 2023;9(6):1522–41. 10.28991/CEJ-2023-09-06-017.Suche in Google Scholar
[17] Awual MR, Hasan MM, Islam A, Rahman MM, Asiri AM, Khaleque MA, et al. Offering an innovative composited material for effective lead (II) monitoring and removal from polluted water. J Clean Prod. 2019;231:214–23. 10.1016/j.jclepro.2019.05.125.Suche in Google Scholar
[18] Al Rawi MF, Al Kindi GY, Al Refaae JK, Hussain TA, Al-Haidri HA. Adsorption, isotherms, and kinetics for phenol removal on biochar prepared from wheat husk. IOP Conf Ser: Earth Environ Sci. 2023;1222(1):012012. 10.1088/1755-1315/1222/1/012012.Suche in Google Scholar
[19] Nadeem R, Zafar MN, Afzal A, Hanif MA, Saeed R. Potential of NaOH pretreated Mangifera indica waste biomass for the mitigation of Ni (II) and Co (II) from aqueous solutions. J Taiwan Inst Chem Eng. 2014;45(3):967–72. 10.1016/j.jtice.2013.09.012.Suche in Google Scholar
[20] Li X, Wang C, Zhang J, Liu J, Liu B, Chen G. Preparation and application of magnetic biochar in water treatment: a critical review. Sci Total Environ. 2020;711:134847. 10.1016/j.scitotenv.2019.134847.Suche in Google Scholar PubMed
[21] Ahmed W, Mehmood S, Núñez-Delgado A, Ali S, Qaswar M, Shakoor A, et al. Enhanced adsorption of aqueous Pb (II) by modified biochar produced through pyrolysis of watermelon seeds. Sci Total Environ. 2021;784:147136. 10.1016/j.scitotenv.2021.147136.Suche in Google Scholar PubMed
[22] Li Y, Yu H, Liu L, Yu H. Application of co-pyrolysis biochar for the adsorption and immobilization of heavy metals in contaminated environmental substrates. J Hazard Mater. 2021;420:126655. 10.1016/j.jhazmat.2021.126655.Suche in Google Scholar PubMed
[23] Liu X, Wang Y, Gui C, Li P, Zhang J, Zhong H, et al. Chemical forms and risk assessment of heavy metals in sludge-biochar produced by microwave-induced low-temperature pyrolysis. RSC Adv. 2016;6(104):101960–7. 10.1039/C6RA22511J.Suche in Google Scholar
[24] Min X, Ge T, Li H, Shi Y, Fang T, Sheng B, et al. Combining impregnation and co-pyrolysis to reduce the environmental risk of biochar derived from sewage sludge. Chemosphere. 2022;290:133371. 10.1016/j.chemosphere.2021.133371.Suche in Google Scholar PubMed
[25] Qiu M, Hu B, Chen Z, Yang H, Zhuang L, Wang X. Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar. 2021;3:117–23. 10.1007/s42773-021-00098-y.Suche in Google Scholar
[26] Tahir AHF, Al-Obaidy AHMJ, Mohammed FH. Biochar from date palm waste, production, characteristics and use in the treatment of pollutants: a review. IOP Conf Ser: Mater Sci Eng. 2020;737(1):12171.10.1088/1757-899X/737/1/012171Suche in Google Scholar
[27] Wu Z, Chen X, Yuan B, Fu M-L. A facile foaming-polymerization strategy to prepare 3D MnO2 modified biochar-based porous hydrogels for efficient removal of Cd(II) and Pb(II). Chemosphere. 2020;239:124745. 10.1016/j.chemosphere.2019.124745.Suche in Google Scholar PubMed
[28] Liu R, Wang H, Han L, Hu B, Qiu M. Reductive and adsorptive elimination of U(VI) ions in aqueous solution by SFeS@ Biochar composites. Environ Sci Pollut Res. 2021;28:55176–85. 10.1007/s11356-021-14835-0.Suche in Google Scholar PubMed
[29] Guo F, Peng K, Liang S, Jia X, Jiang X, Qian L. Evaluation of the catalytic performance of different activated biochar catalysts for removal of tar from biomass pyrolysis. Fuel. 2019;258:116204. 10.1016/j.fuel.2019.116204.Suche in Google Scholar
[30] Sajjadi B, Zubatiuk T, Leszczynska D, Leszczynski J, Chen WY. Chemical activation of biochar for energy and environmental applications: a comprehensive review. Rev Chem Eng. 2019;35(7):777–815. 10.1515/revce-2018-0003.Suche in Google Scholar
[31] Zhang J, Shao J, Jin Q, Zhang X, Yang H, Chen Y, et al. Effect of deashing on activation process and lead adsorption capacities of sludge-based biochar. Sci Total Environ. 2020;716:137016. 10.1016/j.scitotenv.2020.137016.Suche in Google Scholar PubMed
[32] Zhang A, Li X, Xing J, Xu G. Adsorption of potentially toxic elements in water by modified biochar: a review. J Environ Chem Eng. 2020;8(4):104196. 10.3390/biomass4020012.Suche in Google Scholar
[33] Depci T, Kul AR, Önal Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: study in single- and multi-solute systems. Chem Eng J. 2012;200(202):224–36. 10.1016/j.cej.2012.06.077.Suche in Google Scholar
[34] Chen T, Zhang Y, Wang H, Lu W, Zhou Z, Zhang Y, et al. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour Technol. 2014;164:47–54. 10.1016/j.biortech.2014.04.048.Suche in Google Scholar PubMed
[35] Zhang W, Mao S, Chen H, Huang L, Qiu R. Pb (II) and Cr (VI) sorption by biochars pyrolyzed from the municipal wastewater sludge under different heating conditions. Bioresour Technol. 2013;147:545–52. 10.1016/j.biortech.2013.08.082.Suche in Google Scholar PubMed
[36] Fan S, Wang Y, Wang Z, Tang J, Tang J, Li X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: adsorption kinetics, equilibrium, thermodynamics and mechanism. J Environ Chem Eng. 2017;5(1):601–11. 10.1016/j.jece.2016.12.019.Suche in Google Scholar
[37] Huang Y, Li S, Chen J, Zhang X, Chen Y. Adsorption of Pb (II) on mesoporous activated carbons fabricated from water hyacinth using H3PO4 activation: adsorption capacity, kinetic and isotherm studies. Appl Surf Sci. 2014;293:160–8. 10.1016/j.apsusc.2013.12.123.Suche in Google Scholar
[38] Al-Rubaye MF, Mohammed FH, AL-Obaidy AHM. Modified biochar derived from date palm trunks for removing cation dyes from aqueous solution. AIP Conf Proc. 2023;2977:1. 10.1063/5.0182308.Suche in Google Scholar
[39] Harja M, Buema G, Lupu N, Chiriac H, Herea DD, Ciobanu G. Fly ash coated with magnetic materials: improved adsorbent for Cu (II) removal from wastewater. Materials. 2020;14:63. 10.3390/ma14010063.Suche in Google Scholar PubMed PubMed Central
[40] Fachini J, Figueiredo CCD. Pyrolysis of sewage sludge: physical, chemical, morphological and mineralogical transformations. Braz Arch Biol Technol. 2022;65:22210592. 10.1590/1678-4324-2022210592.Suche in Google Scholar
[41] Yin Q, Liu M, Ren H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J Environ Manage. 2019;249:109410. 10.1016/j.jenvman.2019.109410.Suche in Google Scholar PubMed
[42] de Figueiredo CC, Reis ADSJ, de Araujo AS, Blum LE, Shah K, Paz-Ferreiro J. Assessing the potential of sewage sludge-derived biochar as a novel phosphorus fertilizer: influence of extractant solutions and pyrolysis temperatures. Waste Manage. 2021;124:144–53. 10.1016/j.wasman.2021.01.044.Suche in Google Scholar PubMed
[43] Li C, Huang Q, Zhang H, Wang Q, Xue R, Guo G, et al. Characterization of biochars produced by co-pyrolysis of hami melon (Cantaloupes) straw mixed with polypropylene and their adsorption properties of cadmium. Int J Environ Res Public Health. 2021;18(21):11413. 10.3390/ijerph182111413.Suche in Google Scholar PubMed PubMed Central
[44] Chen Y, Du L, Li S, Song W, Jensen PA, Lin W. Pyrolysis of antibiotic mycelial dreg and characterization of obtained gas, liquid and biochar. J Hazard Mater. 2021;402:123826. 10.1016/j.jhazmat.2020.123826.Suche in Google Scholar PubMed
[45] Li B, Ding S, Fan H, Ren Y. Experimental investigation into the effect of pyrolysis on chemical forms of heavy metals in sewage sludge biochar (SSB), with brief ecological risk assessment. Materials. 2021;14(2):447. 10.3390/ma14020447.Suche in Google Scholar PubMed PubMed Central
[46] Zhang L, Ren Y, Xue Y, Cui Z, Wei Q, Han C, et al. Preparation of biochar by mango peel and its adsorption characteristics of Cd (II) in solution. RSC Adv. 2020;10(59):35878–88. 10.1039/D0RA06586B.Suche in Google Scholar PubMed PubMed Central
[47] Chen X, Chen G, Chen L, Chen Y, Lehmann J, McBride MB, et al. Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution. Bioresour Technol. 2011;102:8877–84. 10.1016/j.biortech.2011.06.078.Suche in Google Scholar PubMed
[48] Pituello C, Francioso O, Simonetti G, Pisi A, Torreggiani A, Berti A, et al. Characterization of chemical–physical, structural and morphological properties of biochars from biowastes produced at different temperatures. J Soils Sediment. 2014;15:792–804. 10.1007/s11368-014-0964-7.Suche in Google Scholar
[49] Rashed MN, Gad AAAE, Fathy NM. Adsorption of Cd (II) and Pb (II) using physically pretreated camel bone biochar. Adv J Chem-Sect A. 2019;2(4):347–64. 10.33945/SAMI/AJCA.2019.4.8.Suche in Google Scholar
[50] Jung KW, Lee SY, Choi JW, Lee YJ. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites. Adsorption behavior and mechanisms for the removal of copper (II) from aqueous media. Chem Eng J. 2019;369:29–541. 10.1016/j.cej.2019.03.102.Suche in Google Scholar
[51] Hokkanen S, Bhatnagar A, Repo E, Lou S, Sillanpää M. Calcium hydroxyapatite micro fibrillated cellulose composite as a potential adsorbent for the removal of Cr (VI) from aqueous solution. Chem Eng J. 2016;283:445–52. 10.1016/j.cej.2015.07.035.Suche in Google Scholar
[52] Rojas-Mayorga CK, Silvestre-Albero J, Aguayo-Villarreal IA, Mendoza-Castillo DI, Bonilla-Petriciolet A. A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous-Mesoporous Mater. 2015;209:38–44. 10.1016/j.micromeso.2014.09.002.Suche in Google Scholar
[53] Cechinel MAP, Selene MAGU, Antônio AUS. Study of lead (II) adsorption onto activated carbon originating from cow bone. J Clean Prod. 2014;65:342–9. 10.1016/j.jclepro.2013.08.020.Suche in Google Scholar
[54] Shafiq M, Alazba AA, Amin MT. Preparation of Zn Mg Al-layered double hydroxide and rice husk biochar composites for Cu (II) and Pb (II) ions removal from synthetic wastewater. Water. 2023;15(12):2207. 10.3390/w15122207.Suche in Google Scholar
[55] Fu Y, Zhang N, Shen Y, Ge X, Chen M. Micro-mesoporous carbons from original and pelletized rice husk via one-step catalytic pyrolysis. Bioresour Technol. 2018;269:67–73. 10.1016/j.biortech.2018.08.083.Suche in Google Scholar PubMed
[56] Foroutan R, Peighambardoust SJ, Amarzadeh M, Korri AK, Peighambardoust NS, Ahmad A, et al. Nickel ions abatement from aqueous solutions and shipbuilding industry wastewater using ZIF-8-chicken beak hydroxyapatite. J Mol Liq. 2022;356:119003. 10.1016/j.molliq.2022.119003.Suche in Google Scholar
[57] Cechinel MAP, de Souza AAU. Study of lead (II) adsorption onto activated carbon originating from cow bone. J Clean Prod. 2014;65(15):342–9. 10.1016/j.jclepro.2013.08.020.Suche in Google Scholar
[58] Huda BN, Wahyuni ET, Mudasir M. Simultaneous adsorption of Pb(II) and Cd(II) in the presence of Mg (II) ion using eco-friendly immobilized dithizone on coal bottom ash. S Afr J Chem Eng. 2023;45(1):315–27. 10.1016/j.sajce.2023.06.007.Suche in Google Scholar
[59] Lim HK, Teng TT, Ibrahim MH, Ahmad A, Chee HT. Adsorption and removal of zinc (II) from aqueous solution using powdered fsh bones. APCBEE Proc. 2012;1:96–102. 10.1016/j.apcbee.2012.03.017.Suche in Google Scholar
[60] Sabrina M, Jahan SA, Saha B, Sharmin N, Ahmed S. Kinetic and thermodynamic investigation on adsorption of lead onto apatite extracted from mixed fsh bone. Environ Nanotechnol Monit Manag. 2022;18:100738. 10.1016/j.enmm.2022.100738.Suche in Google Scholar
[61] Mohan D, Pittman CU, Jr, Bricka M, Smith F, Yancey B, Mohammad J, et al. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci. 2007;310(1):57–73. 10.1016/j.jcis.2007.01.020.Suche in Google Scholar PubMed
[62] Wang T, Zhang D, Fang K, Zhu W, Peng Q, Xie Z. Enhanced nitrate removal by physical activation and Mg/Al layered double hydroxide modified biochar derived from wood waste: adsorption characteristics and mechanisms. J Environ Chem Eng. 2021;9(4):105184. 10.1016/j.jece.2021.105184.Suche in Google Scholar
[63] Cheng X, Deng J, Li X, Wei X, Shao Y, Zhao Y. Layered double hydroxides loaded sludge biochar composite for adsorptive removal of benzotriazole and Pb(II) from aqueous solution. Chemosphere. 2022;287:131966. 10.1016/j.chemosphere.2021.131966.Suche in Google Scholar PubMed
[64] Yang W, Lu C, Liang B, Yin C, Lei G, Wang B, et al. Removal of Pb (II) from aqueous solution and adsorption kinetics of corn stalk biochar. Separations. 2023;10(8):438. 10.3390/separations10080438.Suche in Google Scholar
[65] Abdulrahman MS, Alsarayreh AA, Barno SK, Abd Elkawi MA, Abbas AS. Activated carbon from sugarcane as an efficient adsorbent for phenol from petroleum refinery wastewater: equilibrium, kinetic, and thermodynamic study. Open Eng. 2023;13(1):20220442. 10.1515/eng-2022-0442.Suche in Google Scholar
[66] Obeid AF, Nile BK, Farouk M. Sulfate removal from wastewater by using waste material as an adsorbent. Open Eng. 2024;14(1):20220532. 10.1515/eng-2022-0532.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Modification of polymers to synthesize thermo-salt-resistant stabilizers of drilling fluids
- Study of the electronic stopping power of proton in different materials according to the Bohr and Bethe theories
- AI-driven UAV system for autonomous vehicle tracking and license plate recognition
- Enhancement of the output power of a small horizontal axis wind turbine based on the optimization approach
- Design of a vertically stacked double Luneburg lens-based beam-scanning antenna at 60 GHz
- Synergistic effect of nano-silica, steel slag, and waste glass on the microstructure, electrical resistivity, and strength of ultra-high-performance concrete
- Expert evaluation of attachments (caps) for orthopaedic equipment dedicated to pedestrian road users
- Performance and rheological characteristics of hot mix asphalt modified with melamine nanopowder polymer
- Second-order design of GNSS networks with different constraints using particle swarm optimization and genetic algorithms
- Impact of including a slab effect into a 2D RC frame on the seismic fragility assessment: A comparative study
- Analytical and numerical analysis of heat transfer from radial extended surface
- Comprehensive investigation of corrosion resistance of magnesium–titanium, aluminum, and aluminum–vanadium alloys in dilute electrolytes under zero-applied potential conditions
- Performance analysis of a novel design of an engine piston for a single cylinder
- Modeling performance of different sustainable self-compacting concrete pavement types utilizing various sample geometries
- The behavior of minors and road safety – case study of Poland
- The role of universities in efforts to increase the added value of recycled bucket tooth products through product design methods
- Adopting activated carbons on the PET depolymerization for purifying r-TPA
- Urban transportation challenges: Analysis and the mitigation strategies for road accidents, noise pollution and environmental impacts
- Enhancing the wear resistance and coefficient of friction of composite marine journal bearings utilizing nano-WC particles
- Sustainable bio-nanocomposite from lignocellulose nanofibers and HDPE for knee biomechanics: A tribological and mechanical properties study
- Effects of staggered transverse zigzag baffles and Al2O3–Cu hybrid nanofluid flow in a channel on thermofluid flow characteristics
- Mathematical modelling of Darcy–Forchheimer MHD Williamson nanofluid flow above a stretching/shrinking surface with slip conditions
- Energy efficiency and length modification of stilling basins with variable Baffle and chute block designs: A case study of the Fewa hydroelectric project
- Renewable-integrated power conversion architecture for urban heavy rail systems using bidirectional VSC and MPPT-controlled PV arrays as an auxiliary power source
- Exploitation of landfill gas vs refuse-derived fuel with landfill gas for electrical power generation in Basrah City/South of Iraq
- Two-phase numerical simulations of motile microorganisms in a 3D non-Newtonian nanofluid flow induced by chemical processes
- Sustainable cocoon waste epoxy composite solutions: Novel approach based on the deformation model using finite element analysis to determine Poisson’s ratio
- Impact and abrasion behavior of roller compacted concrete reinforced with different types of fibers
- Architectural design and its impact on daylighting in Gayo highland traditional mosques
- Structural and functional enhancement of Ni–Ti–Cu shape memory alloys via combined powder metallurgy techniques
- Design of an operational matrix method based on Haar wavelets and evolutionary algorithm for time-fractional advection–diffusion equations
- Design and optimization of a modified straight-tapered Vivaldi antenna using ANN for GPR system
- Analysis of operations of the antiresonance vibration mill of a circular trajectory of chamber vibrations
- Functions of changes in the mechanical properties of reinforcing steel under corrosive conditions
- Enhanced PAPR reduction in NOMA systems using modified SLM and PTS techniques for power-efficient 5G and beyond networks
- Hybrid mechanics-informed machine learning models for predicting mechanical failure in graphene sponge: a low-data strategy for mechanical engineering applications
- Design of shafts of a two-piece chain conveyor as a part of a modification of a mobile working machine
- Review Articles
- A modified adhesion evaluation method between asphalt and aggregate based on a pull off test and image processing
- Architectural practice process and artificial intelligence – an evolving practice
- 10.1515/eng-2025-0148
- Special Issue: 51st KKBN - Part II
- The influence of storing mineral wool on its thermal conductivity in an open space
- Use of nondestructive test methods to determine the thickness and compressive strength of unilaterally accessible concrete components of building
- Use of modeling, BIM technology, and virtual reality in nondestructive testing and inventory, using the example of the Trzonolinowiec
- Tunable terahertz metasurface based on a modified Jerusalem cross for thin dielectric film evaluation
- Integration of SEM and acoustic emission methods in non-destructive evaluation of fiber–cement boards exposed to high temperatures
- Non-destructive method of characterizing nitrided layers in the 42CrMo4 steel using the amplitude-frequency technique of eddy currents
- Evaluation of braze welded joints using the ultrasonic method
- Analysis of the potential use of the passive magnetic method for detecting defects in welded joints made of X2CrNiMo17-12-2 steel
- Analysis of the possibility of applying a residual magnetic field for lack of fusion detection in welded joints of S235JR steel
- Eddy current methodology in the non-direct measurement of martensite during plastic deformation of SS316L
- Methodology for diagnosing hydraulic oil in production machines with the additional use of microfiltration
- Special Issue: IETAS 2024 - Part II
- Enhancing communication with elderly and stroke patients based on sign-gesture translation via audio-visual avatars
- Optimizing wireless charging for electric vehicles via a novel coil design and artificial intelligence techniques
- Evaluation of moisture damage for warm mix asphalt (WMA) containing reclaimed asphalt pavement (RAP)
- Comparative CFD case study on forced convection: Analysis of constant vs variable air properties in channel flow
- Evaluating sustainable indicators for urban street network: Al-Najaf network as a case study
- Node failure in self-organized sensor networks
- Comprehensive assessment of side friction impacts on urban traffic flow: A case study of Hilla City, Iraq
- Design a system to transfer alternating electric current using six channels of laser as an embedding and transmitting source
- Security and surveillance application in 3D modeling of a smart city: Kirkuk city as a case study
- Modified biochar derived from sewage sludge for purification of lead-contaminated water
- The future of space colonisation: Architectural considerations
- Design of a Tri-band Reconfigurable Antenna Using Metamaterials for IoT Applications
- Special Issue: AESMT-7 - Part II
- Experimental study on behavior of hybrid columns by using SIFCON under eccentric load
- Special Issue: ICESTA-2024 and ICCEEAS-2024
- A selective recovery of zinc and manganese from the spent primary battery black mass as zinc hydroxide and manganese carbonate
- Special Issue: REMO 2025 and BUDIN 2025
- Predictive modeling coupled with wireless sensor networks for sustainable marine ecosystem management using real-time remote monitoring of water quality
- Management strategies for refurbishment projects: A case study of an industrial heritage building
- Structural evaluation of historical masonry walls utilizing non-destructive techniques – Comprehensive analysis
Artikel in diesem Heft
- Research Articles
- Modification of polymers to synthesize thermo-salt-resistant stabilizers of drilling fluids
- Study of the electronic stopping power of proton in different materials according to the Bohr and Bethe theories
- AI-driven UAV system for autonomous vehicle tracking and license plate recognition
- Enhancement of the output power of a small horizontal axis wind turbine based on the optimization approach
- Design of a vertically stacked double Luneburg lens-based beam-scanning antenna at 60 GHz
- Synergistic effect of nano-silica, steel slag, and waste glass on the microstructure, electrical resistivity, and strength of ultra-high-performance concrete
- Expert evaluation of attachments (caps) for orthopaedic equipment dedicated to pedestrian road users
- Performance and rheological characteristics of hot mix asphalt modified with melamine nanopowder polymer
- Second-order design of GNSS networks with different constraints using particle swarm optimization and genetic algorithms
- Impact of including a slab effect into a 2D RC frame on the seismic fragility assessment: A comparative study
- Analytical and numerical analysis of heat transfer from radial extended surface
- Comprehensive investigation of corrosion resistance of magnesium–titanium, aluminum, and aluminum–vanadium alloys in dilute electrolytes under zero-applied potential conditions
- Performance analysis of a novel design of an engine piston for a single cylinder
- Modeling performance of different sustainable self-compacting concrete pavement types utilizing various sample geometries
- The behavior of minors and road safety – case study of Poland
- The role of universities in efforts to increase the added value of recycled bucket tooth products through product design methods
- Adopting activated carbons on the PET depolymerization for purifying r-TPA
- Urban transportation challenges: Analysis and the mitigation strategies for road accidents, noise pollution and environmental impacts
- Enhancing the wear resistance and coefficient of friction of composite marine journal bearings utilizing nano-WC particles
- Sustainable bio-nanocomposite from lignocellulose nanofibers and HDPE for knee biomechanics: A tribological and mechanical properties study
- Effects of staggered transverse zigzag baffles and Al2O3–Cu hybrid nanofluid flow in a channel on thermofluid flow characteristics
- Mathematical modelling of Darcy–Forchheimer MHD Williamson nanofluid flow above a stretching/shrinking surface with slip conditions
- Energy efficiency and length modification of stilling basins with variable Baffle and chute block designs: A case study of the Fewa hydroelectric project
- Renewable-integrated power conversion architecture for urban heavy rail systems using bidirectional VSC and MPPT-controlled PV arrays as an auxiliary power source
- Exploitation of landfill gas vs refuse-derived fuel with landfill gas for electrical power generation in Basrah City/South of Iraq
- Two-phase numerical simulations of motile microorganisms in a 3D non-Newtonian nanofluid flow induced by chemical processes
- Sustainable cocoon waste epoxy composite solutions: Novel approach based on the deformation model using finite element analysis to determine Poisson’s ratio
- Impact and abrasion behavior of roller compacted concrete reinforced with different types of fibers
- Architectural design and its impact on daylighting in Gayo highland traditional mosques
- Structural and functional enhancement of Ni–Ti–Cu shape memory alloys via combined powder metallurgy techniques
- Design of an operational matrix method based on Haar wavelets and evolutionary algorithm for time-fractional advection–diffusion equations
- Design and optimization of a modified straight-tapered Vivaldi antenna using ANN for GPR system
- Analysis of operations of the antiresonance vibration mill of a circular trajectory of chamber vibrations
- Functions of changes in the mechanical properties of reinforcing steel under corrosive conditions
- Enhanced PAPR reduction in NOMA systems using modified SLM and PTS techniques for power-efficient 5G and beyond networks
- Hybrid mechanics-informed machine learning models for predicting mechanical failure in graphene sponge: a low-data strategy for mechanical engineering applications
- Design of shafts of a two-piece chain conveyor as a part of a modification of a mobile working machine
- Review Articles
- A modified adhesion evaluation method between asphalt and aggregate based on a pull off test and image processing
- Architectural practice process and artificial intelligence – an evolving practice
- 10.1515/eng-2025-0148
- Special Issue: 51st KKBN - Part II
- The influence of storing mineral wool on its thermal conductivity in an open space
- Use of nondestructive test methods to determine the thickness and compressive strength of unilaterally accessible concrete components of building
- Use of modeling, BIM technology, and virtual reality in nondestructive testing and inventory, using the example of the Trzonolinowiec
- Tunable terahertz metasurface based on a modified Jerusalem cross for thin dielectric film evaluation
- Integration of SEM and acoustic emission methods in non-destructive evaluation of fiber–cement boards exposed to high temperatures
- Non-destructive method of characterizing nitrided layers in the 42CrMo4 steel using the amplitude-frequency technique of eddy currents
- Evaluation of braze welded joints using the ultrasonic method
- Analysis of the potential use of the passive magnetic method for detecting defects in welded joints made of X2CrNiMo17-12-2 steel
- Analysis of the possibility of applying a residual magnetic field for lack of fusion detection in welded joints of S235JR steel
- Eddy current methodology in the non-direct measurement of martensite during plastic deformation of SS316L
- Methodology for diagnosing hydraulic oil in production machines with the additional use of microfiltration
- Special Issue: IETAS 2024 - Part II
- Enhancing communication with elderly and stroke patients based on sign-gesture translation via audio-visual avatars
- Optimizing wireless charging for electric vehicles via a novel coil design and artificial intelligence techniques
- Evaluation of moisture damage for warm mix asphalt (WMA) containing reclaimed asphalt pavement (RAP)
- Comparative CFD case study on forced convection: Analysis of constant vs variable air properties in channel flow
- Evaluating sustainable indicators for urban street network: Al-Najaf network as a case study
- Node failure in self-organized sensor networks
- Comprehensive assessment of side friction impacts on urban traffic flow: A case study of Hilla City, Iraq
- Design a system to transfer alternating electric current using six channels of laser as an embedding and transmitting source
- Security and surveillance application in 3D modeling of a smart city: Kirkuk city as a case study
- Modified biochar derived from sewage sludge for purification of lead-contaminated water
- The future of space colonisation: Architectural considerations
- Design of a Tri-band Reconfigurable Antenna Using Metamaterials for IoT Applications
- Special Issue: AESMT-7 - Part II
- Experimental study on behavior of hybrid columns by using SIFCON under eccentric load
- Special Issue: ICESTA-2024 and ICCEEAS-2024
- A selective recovery of zinc and manganese from the spent primary battery black mass as zinc hydroxide and manganese carbonate
- Special Issue: REMO 2025 and BUDIN 2025
- Predictive modeling coupled with wireless sensor networks for sustainable marine ecosystem management using real-time remote monitoring of water quality
- Management strategies for refurbishment projects: A case study of an industrial heritage building
- Structural evaluation of historical masonry walls utilizing non-destructive techniques – Comprehensive analysis

