A selective recovery of zinc and manganese from the spent primary battery black mass as zinc hydroxide and manganese carbonate
-
Cornelius Satria Yudha
, Naufal Atha Winard
Abstract
The major challenge in zinc–carbon battery recycling lies in achieving selective separation of zinc and manganese while producing high-purity materials suitable for advanced applications. This study aims to develop a selective hydrometallurgical approach for recovering high-purity zinc and manganese from spent primary zinc–carbon battery black mass (BM), addressing environmental concerns from improper battery disposal. A three-stage sequential leaching process was employed, consisting of water leaching for impurity removal, alkaline leaching for selective zinc extraction as zincate ions, and glucose-assisted reductive acid leaching for manganese recovery. The BM composition was characterized using scanning electron microscope (SEM)–energy-dispersive X-ray (EDX) analysis, while recovered products were precipitated as Zn(OH)2 and MnCO3 and characterized using X-Ray diffraction (XRD), Fourier transform-infra red (FTIR), SEM–EDX, and elemental mapping techniques. SEM–EDX analysis revealed that BM contains high concentrations of Zn, Mn, C, Na, and K. The sequential leaching achieved excellent metal recovery with 98.2% zinc extraction efficiency and 91.2% manganese recovery, demonstrating high selectivity with minimal cross-contamination. XRD analysis confirmed the formation of crystalline ZnO/Zn3(OH)4(NO3)2 and pure rhodochrosite MnCO3. SEM analysis revealed spherical morphologies for both products, with high purity confirmed by FTIR and EDX mapping. This work presents the first comprehensive study combining glucose-assisted reductive leaching with alkaline selective extraction for simultaneous zinc and manganese recovery from Zn–C batteries, achieving superior separation efficiency compared to conventional single-stage approaches. The as-obtained high-purity products show promising potential for advanced applications in catalysts, Li-ion batteries, and supercapacitors.
1 Introduction
The primary battery is a commercial product that is commonly found in households or offices. It stores chemical energy, which is then converted to electrical energy. This type of battery is non-rechargeable, which allows it to be used once in a cycle, resulting in numerous “dead” cells at the end of its use. The heavy metal content of the spent batteries makes them classified as hazardous waste; thus, the safe disposal of this waste becomes a challenge. Nowadays, alkaline and zinc–carbon batteries are the primary battery market that is commonly found in powering portable devices. These batteries have similarities in structure, such as metallic zinc anode, electrochemical manganese dioxide–carbon cathode, concentrated electrolyte(s), i.e., NaOH/KOH, and metal alloy as the case. The rising demand for these types of batteries results in increasing waste count and increasing demand in metal production. On the other hand, the metal in nature is very limited, and the cost of battery waste landfills becomes concerning. Thus, efficient and safe processing of the spent primary battery is one of the solutions to these problems [1–4].
In the early twentieth century, countries in Europe used pyrometallurgical processing to handle electronic waste. Up until recently, Hydrometallurgical processing has been considered a superior technique compared to the pyrometallurgical approach in metal waste processing due to the recent development in technology. This technique becomes more mature in efficiently converting waste into new products with high purity and less energy consumption. Spent primary batteries have been recycled using the hydrometallurgy method, specifically the leaching process. Acid and base can be used to leach dangerous yet valuable metals from the spent primary batteries with a high recovery rate. However, to improve the sustainability of the process, the final leaching product should be converted into products with high economic value [5].
Usually, the metal recovery from primary battery black mass (BM) is conducted via single-step leaching, single-step reduction leaching, and selective leaching. Single-step leaching, both with and without a reduction step, requires a follow-up separation process of Mn and Zn, which often requires a large amount of solvent or a strict crystallization protocol, which reduces the economic attractiveness due to the increasing operating cost [6]. A selective alkaline leaching and complex leaching are considered simple and in recovering Zn selectively, leaving manganese oxide as residue, which can be leached via reductive leaching. Though studies on metal recovery has been extensively investigated, the product derivation is an interesting topic to be developed [7,8].
Extensive research has been performed to design products derived from alkaline and zinc carbon batteries, focusing on the repurposing of the key components in the batteries, such as zinc, manganese, and carbon. Semiconductors for photocatalysis [9], fertilizers [10], Li-ion batteries [11,12], carbon capture materials [13], pigments, and catalysts [14] have been successfully derived from primary batteries, which increased the economic potential of the overall process. The recovery of valuable metals was performed using hydrometallurgical leaching, followed by a further purification step. Mineral acids, i.e., H2SO4 and HNO3, are a good choice for a rapid recovery of Zn and Mn. However, the purification process often becomes a challenge due to the solubility properties of the elements. In the leachate, Zn is often separated using several techniques, such as electrowinning, selective precipitation, and solvent recovery [15]. On the other hand, the wide variety of products demands a process that is simple, safe, economical, and flexible. Since zinc is an amphoteric species, selective leaching followed by a subsequent precipitation route can result in high-purity zinc and manganese-based products [16].
In this research, the BM from the spent zinc–carbon batteries was processed in three sequential leaching steps to recover zinc (Zn) and manganese (Mn) precipitates. Water leaching of BM can remove the soluble potassium electrolyte; caustic leaching was employed to recover Zn, and reductive acidic leaching was used to recover Mn. The Zn and Mn precipitates were formed via chemical precipitation in the form of Zn(OH)2 and MnCO3, respectively. The novelty of this study lies in the comprehensive characterization-focused approach that combines glucose-assisted reductive leaching with systematic product quality assessment, achieving superior recovery efficiencies while demonstrating the transformation of recovered materials into application-ready derivatives (NCM and ZnO) through X-Ray diffraction (XRD) confirmation. The aim of the study is to analyze the quality of these products using XRD, scanning electron microscope (SEM), and Fourier transform-infra red (FTIR) to ensure that these products have the potential to be used as various precursors for the preparation of advanced materials, distinguishing this work from previous studies by emphasizing material characterization and application potential rather than solely focusing on process optimization.
2 Experimental methods
2.1 Selective leaching of Zn and Mn
The spent primary batteries were collected in the campus area of Universitas Sebelas Maret as a Green Campus Program. Primary batteries from several brands were dismantled and compiled as one. The BM was collected by manually separating the case and graphite rod electrode. The BM was washed using distilled water (DW), filtered, and then dried until a dry BM was obtained. A total of 50 g of dried BM was reacted with 1,000 mL 2 M NaOH solution in a 2,000 mL borosilicate vessel at a temperature of 80°C for 2 h. The solution was cooled to near room temperature, filtered using filter paper, and the residue was washed until the initial volume was reached. The filtrate was labeled as sodium zincate (Na2Zn(OH)4) solution and was stored in a glass bottle. Meanwhile, the residue was dried in an oven at 80°C for a night. About 10 g of residue was reacted with 200 mL of a 1 M H2SO4 solution in a 500 mL beaker at a temperature of 60–80°C. A 10 g of glucose (C6H12O6) was added to the beaker as a reductant. The reaction was maintained at 80°C for 2 h before the solution was cooled to near room temperature. The solution was then filtered, and the residue was washed with filter paper until the initial volume was reached. The filtrate was stored and labeled as MnSO4. The final residue was dried in an oven at 80°C.
The experimental parameters employed in this study were selected based on a comprehensive literature review of the hydrometallurgical processing of spent battery materials. The solid-to-liquid ratio of 0.05 g/mL was chosen to ensure adequate mass transfer while maintaining economic reagent consumption. The alkaline leaching temperature of 80°C was selected based on literature data demonstrating optimal zinc extraction efficiency with minimal manganese co-dissolution. The 2 M NaOH concentration and 2 h reaction time provide a balance between extraction kinetics and selectivity. For the reductive acid leaching stage, the 60–80°C temperature range and glucose addition facilitate controlled reduction of Mn(iv) oxides to extractable Mn(ii) species while minimizing zinc redissolution [7].
2.2 Precipitation of Zn and Mn
The Na2Zn(OH)4 was transferred into a beaker with vigorous stirring and heating at 60°C. A 2 M HNO3 solution was transferred slowly into the beaker to obtain a pH level of 8. After the desired pH was reached, a precipitate was formed, as indicated by a cloudy solution. The mixing was continued for 60 min. The precipitate was filtered and washed several times using DW. The formed cake was dried in an oven at 80°C for a day. The dried Zn precipitate was labeled as Zn(OH)2. Separately but similarly, MnSO4 was also transferred to a beaker with vigorous stirring and heating at 60°C. A 2 M Na2CO3 solution was added to the beaker to obtain a pH level of 8. A white precipitate was formed, and the stirring continued for 2 h. The precipitate was filtered and washed several times. The cake was dried in an oven at 80°C for a day. The dried Mn precipitate was labeled as MnCO3. The dried samples of Zn(OH)2 and MnCO3 were ground and sieved through a 200-mesh screen. The overall process can be seen in Figure 1.

Flow process of Zn(OH)2 and MnCO3 production from spent primary batteries.
2.3 Material characterization
Structural analysis was performed using an X-ray diffractometer by Bruker-D2 Phaser (Bruker, Germany). The functional groups of the prepared powders were analyzed using infrared spectroscopy (FTIR; IR-Spirit, Shimadzu, Japan) at a wavenumber range of 4,000–400 cm−1. The morphological features and chemical composition of the products were examined using a SEM and energy-dispersive X-ray (EDX) by JEOL, Japan. Sample EDX mappings were analyzed to ensure the chemical quality of the as-prepared product. The concentrations of zinc and manganese in the water, alkaline, acid leaching solutions, and recovered solid products were determined using atomic absorption spectroscopy (AAS) with a Shimadzu AA-700 spectrometer. The instrument was operated under flame atomization conditions using an acetylene-air flame, with wavelengths set at 213.9 nm for zinc and 279.5 nm for manganese determination. Calibration standards were prepared from 1,000 mg/L stock solutions to establish linear calibration curves ranging from 0.1 to 5.0 mg/L for both elements.
For liquid samples, the alkaline leaching solution was appropriately diluted with deionized water to bring metal concentrations within the calibration range. Solid samples, including the precipitated Zn(OH)2 and MnCO3 products, were subjected to acid digestion prior to AAS analysis. Approximately 0.1 g of each solid sample was dissolved in 10 mL of concentrated nitric acid (65%) under gentle heating at 80°C for 2 h to ensure complete dissolution. The digested solutions were then cooled, filtered through 0.45 μm membrane filters, and diluted to 100 mL with deionized water. All measurements were performed in triplicate, and the results were expressed as mean values with standard deviations.
3 Results and discussion
3.1 Leaching performance
The leaching efficiency results in Figure 2 demonstrate the effectiveness of the sequential three-stage leaching approach for comprehensive metal recovery from zinc–carbon battery BM. The initial water-leaching step achieved minimal extraction with only 7.8% zinc and 0.8% manganese recovery, serving primarily to remove water-soluble impurities and salts while leaving the majority of metals intact in the solid matrix. The subsequent alkaline leaching stage showed dramatically improved performance with 88.1% zinc extraction while maintaining excellent selectivity with only 1.1% manganese dissolution, confirming the preferential dissolution of zinc as zincate ions [Zn(OH)4]2− under high pH conditions while manganese oxides remained largely unaffected. The final reductive acid leaching using glucose as a reducing agent achieved 91.2% manganese extraction with minimal additional zinc dissolution (2.3%), where glucose effectively reduced Mn(iv) oxides to soluble Mn(ii) species under acidic conditions.

Mn and Zn leaching performance.
The cumulative leaching efficiency of 98.2% for zinc and 91.2% for manganese validates the synergistic effect of the sequential approach, where each stage targets specific metal species based on their distinct chemical behaviors. The water leaching pre-treatment removes interfering ions that could complicate subsequent extraction steps, while the alkaline stage exploits zinc’s amphoteric nature for selective dissolution, leaving manganese-rich residues for the final reductive treatment. The use of glucose in the acid leaching stage is particularly effective as it provides controlled reduction conditions that convert insoluble MnO2 to extractable Mn2+ ions without excessive zinc co-dissolution. The minimal cross-contamination observed throughout the process (1.1% Mn in alkaline leachate and 2.3% Zn in acid leachate) demonstrates the high selectivity achieved, enabling the production of separate metal-rich solutions suitable for recovering high-purity zinc hydroxide and manganese carbonate products from battery waste materials [7,8,17].
3.2 Spent primary batteries characterization
The dismantled spent primary batteries were characterized before being processed in the next step. SEM–EDX was used to evaluate the composition and the topological feature of the BM. Figure 2 shows the SEM image of the BM and the EDX mapping. Meanwhile, the quantitative analysis is listed in Table 1.
Spent primary battery BM quantitative analysis using EDX and AAS
| Element | EDX | AAS | |
|---|---|---|---|
| Mass composition (%) | Atomic composition (%) | Mass composition (%) | |
| Zn | 32.63 ± 2.90 | 17.04 ± 1.52 | 29.2 ± 1.8 |
| Mn | 26.61 ± 1.38 | 16.54 ± 0.86 | 22.5 ± 2.2 |
| K | 16.59 ± 0.60 | 15.98 ± 0.58 | 5 ± 1.8 |
| Cl | 1.32 ± 0.91 | 1.66 ± 0.26 | — |
| O | 22.86 ± 0.91 | 48.78 ± 1.94 | — |
Based on Figure 3 and Table 1, the main elements contained in the BM are Zn, Mn, C, K, and Cl. These materials were formed due to the exhausted chemical reaction during the utilization of the primary battery. The presence of K and Cl indicated that the spent battery used in this research used a conventional alkaline battery and Zn–carbon battery, which often use KOH and chloride-based salt (ZnCl2, NH4Cl) as the electrolyte, respectively. The reaction in exhausted primary batteries can be seen in the following equations [18]:

SEM image (a) and EDX mapping of BM on Zn (b), Mn (c), K (d), and C (e).
Reaction in alkaline batteries
Reaction in zinc–carbon batteries
Based on these reactions, the products that can be processed are either metal oxides (Mn2O3, ZnO) or metal hydroxides (Zn(OH)2). Potassium and chloride ions are expected to be leached during neutral leaching due to their high solubility.
3.3 Zn(OH)2 characterization
The Zn recovery was employed during the second step of the leaching process. It is expected that the Zn-based compounds are leached in a NaOH solution according to Equations (4)–(6). The soluble zincate ions were then precipitated using HNO3 to form a precipitate. The morphology and elemental mapping of the as-prepared precipitate are shown in Figure 4. The Zn(OH)2 secondary particle has a spherical shape (Figure 4a), containing Zn and O based on the elemental mapping. This particle consists of smaller primary particles with a narrow particle distribution that can be seen in Figure 4e and an average particle diameter of 0.146 µm. The small particle size can be advantageous for the formation of nanosized particles [19].
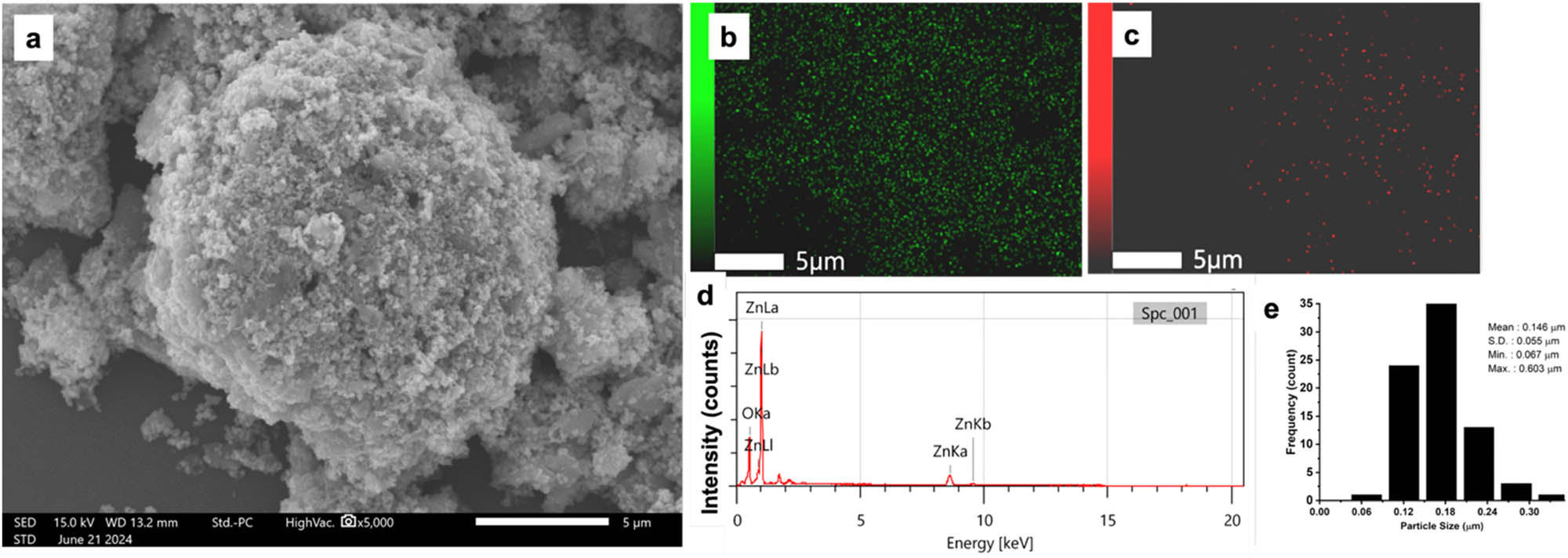
SEM image (a), EDX mapping of Zn(OH)2 on Zn (b), O (c), EDX spectra (d), and particle size histogram (e).
Table 2 shows the quantitative analysis of the samples, which are dominant in Zn and O. It is expected that the majority of the sample is zinc hydroxide compound. Figure 5(a) and (b) shows the X-ray diffraction pattern and FTIR spectra of the zinc hydroxide sample. The XRD analysis reveals a heterogeneous phase assemblage consisting of zinc oxide (ZnO) and zinc hydroxide nitrate (Zn3(OH)4(NO3)2), indicating successful selective leaching as evidenced by the absence of manganese-containing phases in the precipitate. The zinc hydroxide formed due to neutralization of zincate ions [Zn(OH)4]2⁻ with HNO3, by competing equilibria, favors nitrate incorporation into the crystal structure, forming Zn3(OH)4(NO3)2, where
EDX and AAS analysis of Zn(OH)2
| Element | EDX | AAS | |
|---|---|---|---|
| Mass composition (%) | Atomic composition (%) | Mass composition (%) | |
| Zn | 74.02 ± 3.41 | 41.08 ± 1.89 | 49.8 ± 1.3 |
| O | 25.98 ± 0.71 | 58.92 ± 1.61 | — |
| Mn | n.d. | n.d. | 0.4 ± 0.12 |

XRD spectra of Zn(OH)2 (a) and FTIR spectra of Zn(OH)2 (b) from spent primary battery.
The caustic leaching results in a BM residue. The SEM image and EDX spectra of the residue can be seen in Figure 6. As we can see, the residue only contains Mn, O, and a small portion of residual Na. In comparison with Figure 3, the sample contains no Zn, which proves the efficient Zn recovery using such an approach. The evaluation of Zn recovery and kinetic study would be interesting to investigate, especially by employing various other base solutions [16].

SEM image (a), EDX mapping of Mn (b), O (c), Na (d), and EDX spectra of BM caustic leaching residue (e).
3.4 MnCO3 characterization
The last leaching process was employed to recover the manganese oxide material. The leaching of manganese oxide is well discussed in a previous study by Sinha and Purcell [23]. When the battery is exhausted, MnO2 is largely reduced to Mn2O3. Mn2O3 can spontaneously react with dilute sulfuric acid, forming MnSO4 and a residue of insoluble MnO2, as expressed in the following equation:
To further recover Mn from the unreacted MnO2, a reducing agent, glucose, is added. The reductive leaching reaction is expressed in the following equation:
The recovered MnSO4 solution was further processed via carbonate coprecipitation, forming MnCO3. The precipitation reaction can be seen in the following equation:
Figure 6a–f displays the SEM and EDX spectra/mapping of MnCO3, exhibiting a near-perfect spherical particle with an even atomic distribution on the surface. The particle was highly uniform in both shape and size. The distribution histogram is displayed in Figure 7g. This confirms a narrow distribution of particles with submicron size. The average particle size is 0.659 µm. The formation of spherical manganese carbonate was also reported in other studies employing K2CO3 as the precipitation agent [24]. Overall, the utilization of glucose as a reducing agent improves the process’s eco-friendliness compared with other inorganic reducing agents such as SO2 gas, H2O2, and/or FeSO4 [23]. The element compositions of the MnCO3 based on EDX spectroscopy are listed in Table 3. Based on the atomic composition, Mn and O are dominant in the sample. Based on the AAS analysis, a small amount of Zn is detected due to the residual Zn leached using sulfuric acid. The Zn amount is less than 1%. Solvent extraction can be used to separate Zn and Mn; however, it is economically challenging.

(a) SEM image, (b) EDX mapping of MnCO3 on (c) Mn, (d) O, and (e) C, (f) EDX spectra, and (g) particle size histogram.
EDX and AAS analysis of MnCO3
| Element | EDX | AAS | |
|---|---|---|---|
| Mass composition (%) | Atomic composition (%) | Mass composition (%) | |
| Mn | 64.14 ± 2.11 | 33.6 ± 1.1 | 47.77 ± 2.1 |
| C | 3.19 ± 0.25 | 7.64 ± 0.65 | — |
| O | 32.67 ± 0.84 | 58.76 ± 1.5 | — |
| Zn | n.d. | n.d. | 0.78 ± 0.22 |
Figure 8 shows the XRD pattern and FTIR spectra of the MnCO3 sample. The XRD analysis of the recovered manganese carbonate product (Figure 8a) confirms the successful formation of pure rhodochrosite (MnCO3) phase with a well-defined crystalline structure. The diffraction pattern exhibits characteristic peaks at 2θ values of 31.2°, 36.0°, 38.1°, 42.1°, 45.2°, 48.2°, 51.4°, 56.2°, and 58.1°, corresponding to the (104), (006), (110), (113), (202), (024), (018), (116), (221), and (214) crystallographic planes of hexagonal MnCO3, respectively. The sharp, intense peaks with minimal background noise indicate high crystallinity and phase purity of the product, with no detectable impurity phases or residual zinc contamination. The prominence of the (104) reflection as the strongest peak is consistent with the preferred orientation typical of well-crystallized manganese carbonate formed under controlled precipitation conditions. This XRD confirmation validates the effectiveness of the reductive acid leaching followed by carbonate precipitation strategy, demonstrating that the sequential processing approach successfully separates and recovers manganese from the battery BM in a highly pure crystalline form suitable for potential reuse in battery manufacturing or other applications [25,26]. Meanwhile, the FTIR spectra (Figure 8b) confirm a sharp vibration mode of vC═O at the wavenumber range between 1,100 and 1,400, which can be assigned to a carbonate species. A slight and broad peak at wavenumbers ∼3,400 and 1,600 cm−1 indicates the presence of adsorbed H2O molecules as a result of the hydrophilic nature of carbonate.

XRD pattern of MnCO3 (a) and FTIR spectra of MnCO3 (b) from spent primary battery.
3.5 Potential utilization of Zn(OH)2 and MnCO3
Based on the deep investigation of XRD, FTIR, and SEM–EDX results, both Zn(OH)2 and MnCO3 are promising products with good purity. These products can be used as precursors for many products. The submicron size of the samples can be used for the development of nanoparticles. The nanoparticle products can be used as catalysts, sensors, adsorbents, and energy storage applications [27]. Table 4 shows the products that can be derived from Zn(OH)2 and MnCO3.
List of products derived from Zn(OH)2 and MnCO3
| Precursors | Products | Method | Ref. |
|---|---|---|---|
| Zn(OH)2 | Photocatalyst ZnO | Thermal degradation | [28–30] |
| ZnO anode | Thermal degradation | [31–33] | |
| Catalyst | Thermal degradation | [34–36] | |
| Supercapacitor ZnO | Degradation | [37] | |
| Hydrothermal | |||
| MnCO3 | Li-ion cathode material (NCM) | Solid state reaction, sol–gel | [38–40] |
| Supercapacitor | Thermal degradation | [41,42] |
Based on Table 4, the recovered Zn(OH)2 and MnCO3 were used to prepare ZnO material and LiNi0.33Mn0.33Co0.33O2 (NMC111) material. The ZnO was prepared through a simple sintering process as described in our previous study [43]. Meanwhile the NCM111 was prepared through oxalate coprecipitation [44]. The X-ray diffractogram of the materials can be seen in Figure 8.
Based on Figure 9a, the X-ray diffraction pattern displays the successful synthesis of ZnO from Zn(OH)2 recovered from spent primary batteries. The diffractogram exhibits characteristic peaks indexed to (100), (002), (101), (102), (110), and (103) planes, which perfectly match the hexagonal wurtzite structure of ZnO (JCPDS #79-2205) [32,45,46]. The high-intensity, sharp diffraction peaks indicate excellent crystallinity, while the absence of additional peaks confirms phase purity and complete conversion from the Zn(OH)2 precursor. The dominant (101) reflection and well-defined peak positions demonstrate the formation of a well-ordered crystal structure. This result validates the effectiveness of the battery recycling process in producing high-quality ZnO crystals from waste materials. In Figure 9b, the X-ray diffraction pattern shows the successful synthesis of LiNi0.33Mn0.33Co1/3O2 (NMC111) cathode material from BM-derived MnCO3. The diffractogram exhibits characteristic peaks indexed to (003), (101), (006)/(102), (104), (105), (107), and (108)/(110) planes, corresponding to the α-NaFeO2 layered structure (JCPDS #49-0524). The sharp, intense (003) and (104) reflections indicate well-defined layered ordering, while the clear splitting of the (006)/(102) and (108)/(110) doublets suggest good hexagonal structure formation. The relatively high-intensity ratio of I(003)/I(104) suggests minimal cation mixing between lithium and transition metal layers, indicating successful structural development of the NMC cathode material. High background noise can be attributed to the high Co and Mn content, which in the future can be reduced by adding a filter during XRD analysis [47,48]. These preliminary studies provide interesting topics to be developed in future research.
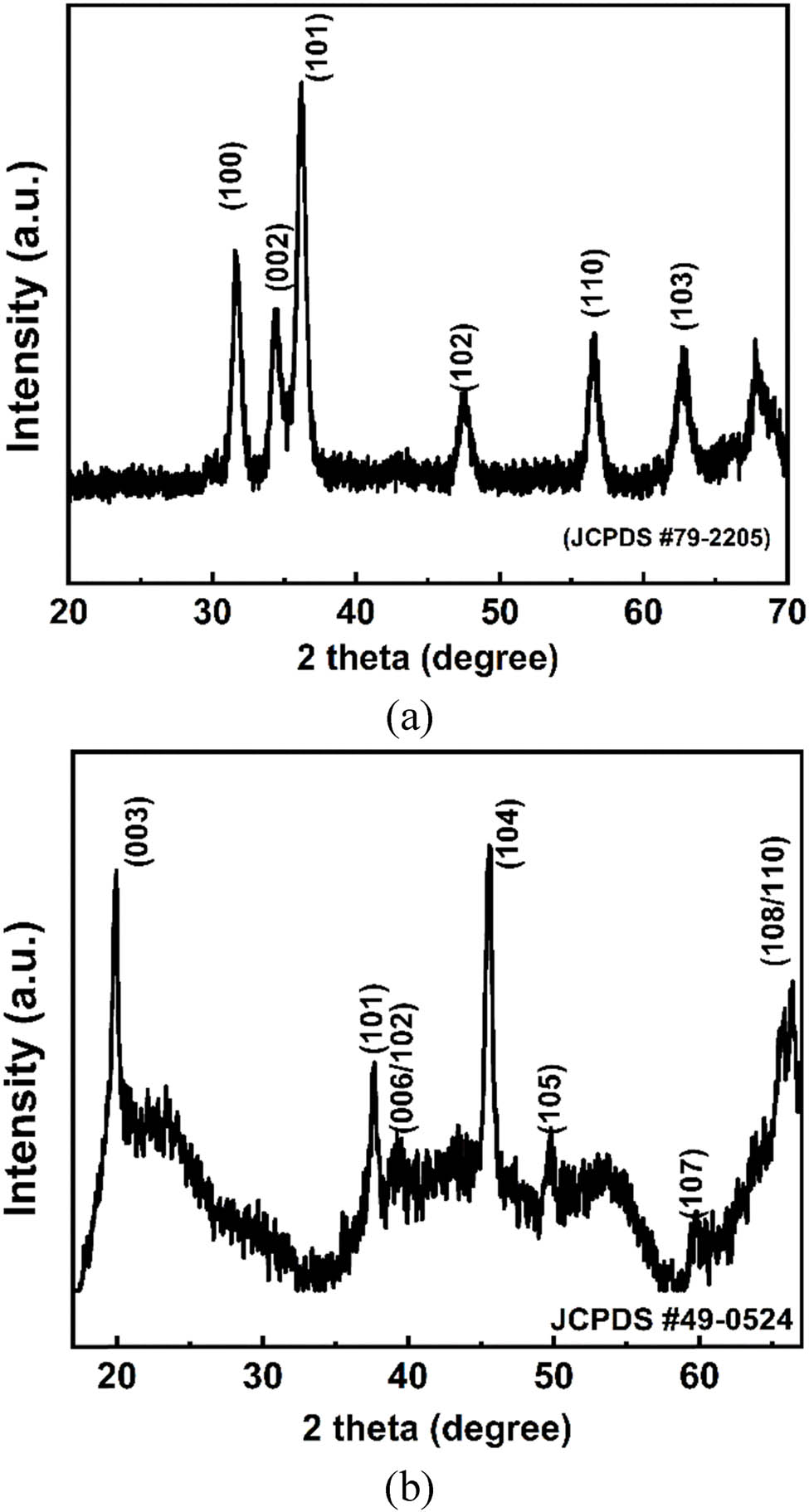
X-ray diffraction pattern of (a) ZnO and (b) NMC111.
4 Conclusion
This study successfully demonstrated a selective three-stage hydrometallurgical approach for recovering high-purity zinc and manganese from spent primary zinc–carbon battery BM. The sequential process comprising water leaching, alkaline leaching, and glucose-assisted reductive acid leaching achieved exceptional metal recovery efficiencies of 98.2% for zinc and 91.2% for manganese with minimal cross-contamination. XRD analysis confirmed the formation of crystalline products, with zinc recovered as a heterogeneous phase mixture containing ZnO and Zn3(OH)4(NO3)2, while manganese was obtained as pure rhodochrosite (MnCO3). The absence of manganese phases in the zinc product XRD pattern validated the selectivity of the alkaline leaching process. SEM characterization revealed submicron-sized spherical particles for both products, while FTIR analysis confirmed the successful formation of target compounds throughout the precipitation processes. The glucose-mediated reductive leaching represents a novel approach for manganese extraction, offering controlled reduction conditions that effectively convert insoluble Mn(iv) oxides to extractable Mn(ii) species. The recovered high-purity zinc and manganese products demonstrate significant potential as precursor materials for advanced applications in sustainable energy storage technologies, catalysts, and environmental remediation. This environmentally sustainable approach provides an effective solution for battery waste management while recovering valuable metals, offering strong potential for industrial scale-up implementation.
Funding information
This research is financially supported by Lembaga Penelitian dan Pengabdian Masyarakat (LPPM) Universitas Sebelas Maret through Research Group Grant (HGR) with contract number 194.2/UN27.22/PT.01.03/2024.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. Cornelius Satria Yudha prepared the conceptualization, developed the methodology, and wrote the final manuscript. Naufal Atha Winard and Anggraini Putri performed the experiments and data curation. Meidiana Arinawati and Enni Apriliyani performed data analysis and project administration.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
[1] Belardi G, Lavecchia R, Medici F, Piga L. Thermal treatment for recovery of manganese and zinc from zinc–carbon and alkaline spent batteries. Waste Manag. 2012 Oct;32(10):1945–51.10.1016/j.wasman.2012.05.008Search in Google Scholar PubMed
[2] García NM, Delgado Cano B, Valverde JL, Heitz M, Avalos Ramirez A. Extraction and separation of potassium, zinc and manganese issued from spent alkaline batteries by a three-unit hydrometallurgical process. J Chem Technol Biotechnol. 2024 Jul;99(7):1553–63.10.1002/jctb.7649Search in Google Scholar
[3] Dobó Z, Dinh T, Kulcsár T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023;9(10):6362–95.10.1016/j.egyr.2023.05.264Search in Google Scholar
[4] Sobianowska-Turek A, Szczepaniak W, Maciejewski P, Gawlik-Kobylińska M. Recovery of zinc and manganese, and other metals (Fe, Cu, Ni, Co, Cd, Cr, Na, K) from Zn–MnO2 and Zn–C waste batteries: hydroxyl and carbonate co-precipitation from solution after reducing acidic leaching with use of oxalic acid. J Power Sources. 2016 Sep;325:220–8.10.1016/j.jpowsour.2016.06.042Search in Google Scholar
[5] Tran LH, Tanong K, Jabir AD, Mercier G, Blais JF. Hydrometallurgical process and economic evaluation for recovery of zinc and manganese from spent alkaline batteries. Metals (Basel). 2020 Sep;10(9):1–16.10.3390/met10091175Search in Google Scholar
[6] Andak B, Özduǧan E, Türdü S, Bulutcu AN. Recovery of zinc and manganese from spent zinc–carbon and alkaline battery mixtures via selective leaching and crystallization processes. J Environ Chem Eng. 2019 Oct;7(5):103372.10.1016/j.jece.2019.103372Search in Google Scholar
[7] Maryam Sadeghi S, Jesus J, Soares HMVM. A critical updated review of the hydrometallurgical routes for recycling zinc and manganese from spent zinc-based batteries. Waste Manag. 2020;113:342–50. Elsevier Ltd.10.1016/j.wasman.2020.05.049Search in Google Scholar PubMed
[8] Sayilgan E, Kukrer T, Ferella F, Akcil A, Veglio F, Kitis M. Reductive leaching of manganese and zinc from spent alkaline and zinc–carbon batteries in acidic media. Hydrometallurgy. 2009 Jun;97(1–2):73–9.10.1016/j.hydromet.2009.01.004Search in Google Scholar
[9] Wang Z, Ma Y, Lin H, Luo L, Zhang H. Zn–Mn bimetallic oxide derived from waste battery to activate peroxymonosulfate for bisphenol A removal under visible light irradiation. J Environ Chem Eng. 2024 Oct;12(5):113351.10.1016/j.jece.2024.113351Search in Google Scholar
[10] Barragán-Mantilla SP, Ortiz R, Almendros P, Sánchez-Martín L, Gascó G, Méndez A. Advances in the sustainable production of fertilizers from spent zinc-based batteries. Sustainability (Switz). 2024 May;16(10):4255.10.3390/su16104255Search in Google Scholar
[11] Pattaweepaiboon S, Hirunpinyopas W, Iamprasertkun P, Pimphor K, Roddecha S, Dirayanti D, et al. Upcycling electrode materials from spent single-use zinc carbon/alkaline batteries into rechargeable lithium-ion battery application. J Energy Storage. 2024 Jan;76:109755.10.1016/j.est.2023.109755Search in Google Scholar
[12] Devi MM, Ankush, Guchhait SK, Sunaina, Suresh SB, Sreekanth M, et al. Energy efficient electrodes for lithium-ion batteries: recovered and processed from spent primary batteries. J Hazard Mater. 2020 Feb;384:12112.10.1016/j.jhazmat.2019.121112Search in Google Scholar PubMed
[13] Ma S, Min J, Yang C, Liao C, Zhu Y, Zhao X, et al. Developing a low-carbon, scalable strategy for the conversion of spent batteries into metal-organic framework-74 for CO2 capture. Resour Conserv Recycl. 2024 Oct;209:107707.10.1016/j.resconrec.2024.107707Search in Google Scholar
[14] Jan J, Chang CL, Chang SM. Preparation of Mn/TiO2 catalysts using recovered manganese from spent alkaline batteries for low-temperature NH3-SCR. J Hazard Mater. 2024 Jul;472:134497.10.1016/j.jhazmat.2024.134497Search in Google Scholar PubMed
[15] Lee JY, Pranolo Y, Cheng CY, Zhang ZW. The recovery of zinc and manganese from synthetic spent-battery leach solutions by solvent extraction. Solvent Extr Ion Exch. 2010 Jan;28(1):73–84.10.1080/07366290903409043Search in Google Scholar
[16] Seyed Ghasemi SM, Azizi A. Alkaline leaching of lead and zinc by sodium hydroxide: kinetics modeling. J Mater Res Technol. 2018 Apr;7(2):118–25.10.1016/j.jmrt.2017.03.005Search in Google Scholar
[17] Buzatu M, Sǎceanu S, Petrescu MI, Ghica GV, Buzatu T. Recovery of zinc and manganese from spent batteries by reductive leaching in acidic media. J Power Sources. 2014;247:612–7.10.1016/j.jpowsour.2013.09.001Search in Google Scholar
[18] Kordesch K, Taucher-Mautner W. Leclanché and zinc–carbon. Amsterdam; 2009. p. 43–54.10.1016/B978-044452745-5.00097-6Search in Google Scholar
[19] Saghatforoush LAL, Mehdizadeh R, Sanati S, Hasanzadeh M. Synthesis of zinc hydroxide nano crystals and application as a new electrochemical sensor for determination of selected sympathomimetic drugs. Acta Chem Slov. 2012;59:863–9.Search in Google Scholar
[20] Mrad M, Chouchene B, Chaabane TB. Effects of zinc precursor, basicity and temperature on the aqueous synthesis of ZnO nanocrystals. S Afr J Chem. 2018;71:103–10.10.17159/0379-4350/2018/v71a13Search in Google Scholar
[21] Ramesh TN, Madhu TL. Thermal decomposition studies of layered metal hydroxynitrates (metal: Cu, Zn, Cu/Co, and Zn/Co). Int J Inorg Chem. 2015 Jan;2015:1–11.10.1155/2015/536470Search in Google Scholar
[22] Khadiran NF, Hussein MZ, Ahmad R, Khadiran T, Zainal Z, Kadir WRWA, et al. Preparation and properties of zinc layered hydroxide with nitrate and phosphate as the counter anion, a novel control release fertilizer formulation. J Porous Mater. 2021 Dec;28(6):1797–811.10.1007/s10934-021-01122-zSearch in Google Scholar
[23] Sinha MK, Purcell W. Reducing agents in the leaching of manganese ores: a comprehensive review. Hydrometallurgy. 2019 Aug;187:168–86.10.1016/j.hydromet.2019.05.021Search in Google Scholar
[24] Lyu L, Xiao L, Lu J, Zhuang L. Manganese carbonate as active material in potassium carbonate electrolyte. Chem Phys Lett. 2020 Jan;738:136899.10.1016/j.cplett.2019.136899Search in Google Scholar
[25] Lu H, Zhang Y, Liu P. Mn2O3 microcubes with three-dimensional porous network structure as electrochemical sensing material for nitrite. J Appl Electrochem. 2016 Oct;46(10):1059–65.10.1007/s10800-016-0985-6Search in Google Scholar
[26] Su J, Liang H, Gong XN, Lv XY, Long YF, Wen YX. Fast preparation of porous MnO/C microspheres as anode materials for lithium-ion batteries. Nanomaterials. 2017 Jun;7(6):121.10.3390/nano7060121Search in Google Scholar PubMed PubMed Central
[27] Oyewola OM, Awonusi AA, Ismail OS. Performance optimization of step-like divergence plenum air-cooled Li-ion battery thermal management system using variable-step-height configuration. Emerg Sci J. 2024 Jun;8(3):795–814.10.28991/ESJ-2024-08-03-01Search in Google Scholar
[28] Joulaee S, Mirzaei M, Hassanpour A, Safardoust-Hojaghan H, Khani A. Efficient removal of anionic and cationic dyes from waste water using green ZnO/NiO/graphene quantum dots nano photocatalyst. Optik (Stuttg). 2023;290(Jan):171324. 10.1016/j.ijleo.2023.171324.Search in Google Scholar
[29] Joni IM, Purwanto A, Iskandar F, Hazata M, Okuyama K. Intense UV-light absorption of ZnO nanoparticles prepared using a pulse combustion-spray pyrolysis method. Chem Eng J. 2009;155(1–2):433–41.10.1016/j.cej.2009.07.011Search in Google Scholar
[30] Bekru AG, Tufa LT, Zelekew OA, Goddati M, Lee J, Sabir FK. Green synthesis of a CuO–ZnO nanocomposite for efficient photodegradation of methylene blue and reduction of 4-nitrophenol. ACS Omega. 2022;7(35):30908–19.10.1021/acsomega.2c02687Search in Google Scholar PubMed PubMed Central
[31] Hsieh CT, Lin CY, Chen YF, Lin JS. Synthesis of ZnO@graphene composites as anode materials for lithium ion batteries. Electrochim Acta. 2013;111:359–65. 10.1016/j.electacta.2013.07.197.Search in Google Scholar
[32] Song J, Kim H, Jae W, Kim T, Futalan CM, Kim J. Porous ZnO/C microspheres prepared with maleopimaric acid as an anode material for lithium-ion batteries. Carbon N Y. 2020;165:55–66. 10.1016/j.carbon.2020.04.035.Search in Google Scholar
[33] Zhang W, Du L, Chen Z, Hong J, Yue L. ZnO nanocrystals as anode electrodes for lithium-ion batteries. J Nanomater. 2016;2016:10–2.10.1155/2016/8056302Search in Google Scholar
[34] Wicaksono D, Kusumaningtyas RD. Synthesis of ZnO/CaO catalyst from eggshell waste for biodiesel production. J Bahan Alam Terbarukan. 2019 Jul;8(1):65–71.10.15294/jbat.v8i1.20185Search in Google Scholar
[35] Adnan, Nisar, Shah R, Zada FM, Khan B, Aziz S, et al. Novel Ni/ZnO nanocomposites for the effective photocatalytic degradation of malachite green dye. Civ Eng J. 2024;10(8):2601–14.10.28991/CEJ-2024-010-08-011Search in Google Scholar
[36] Santis A, Arbeláez O, Cardenas LA, Castellanos J, Velasquez P. Optimizing Cr(VI) reduction in plastic chromium plating wastewater: particle size, irradiation, titanium dose. Emerg Sci J. 2024 Feb;8(1):17–27.10.28991/ESJ-2024-08-01-02Search in Google Scholar
[37] Samuel AJ, Deepi A, Srikesh G, Samson Nesaraj A. Development of two-dimensional Mg doped ZnO nano hybrids as electrode materials for electrochemical supercapacitor applications. Rasayan J Chem. 2020;13(1):562–9.10.31788/RJC.2020.1315528Search in Google Scholar
[38] Zhang S, Deng C, Yang SY, Niu H. An improved carbonate co-precipitation method for the preparation of spherical Li[Ni1/3Co1/3Mn1/3]O2 cathode material. J Alloy Compd. 2009;484(1–2):519–23.10.1016/j.jallcom.2009.04.149Search in Google Scholar
[39] Yin K, Fang W, Zhong B, Guo X, Tang Y, Nie X. The effects of precipitant agent on structure and performance of LiNi1/3Co1/3Mn1/3O2 cathode material via a carbonate co-precipitation method. Electrochim Acta. 2012;85:99–103.10.1016/j.electacta.2012.06.064Search in Google Scholar
[40] Krishna Kumar S, Ghosh S, Martha SK. Synergistic effect of magnesium and fluorine doping on the electrochemical performance of lithium-manganese rich (LMR)-based Ni–Mn–Co-oxide (NMC) cathodes for lithium-ion batteries. Ion (Kiel). 2017;23(7):1655–62.10.1007/s11581-017-2018-9Search in Google Scholar
[41] Cremonezzi JMdeO, Tiba DY, Domingues SH. Fast synthesis of δ-MnO2 for a high-performance supercapacitor electrode. SN Appl Sci. 2020;2(10):1689. 10.1007/s42452-020-03488-2.Search in Google Scholar
[42] Shen L, Peng L, Fu R, Liu Z, Jiang X, Wang D, et al. Synthesis of flower-like MnO2 nanostructure with freshly prepared Cu particles and electrochemical performance in supercapacitors. PLoS One. 2022;17(6 June):1–16. 10.1371/journal.pone.0269086.Search in Google Scholar PubMed PubMed Central
[43] Yudha CS, Rahmawati M, Jumari A, Hutama AP, Purwanto A. Synthesis of zinc oxide (ZnO) from zinc based-fertilizer as potential and low-cost anode material for lithium ion batteries. In ACM International Conference Proceeding Series. Association for Computing Machinery; 2020. p. 1–6.10.1145/3429789.3429860Search in Google Scholar
[44] Nisa SS, Rahmawati M, Yudha CS, Nilasary H, Nursukatmo H, Oktaviano HS, et al. Fast approach to obtain layered transition-metal cathode material for rechargeable batteries. Batteries. 2022;8(1):4.10.3390/batteries8010004Search in Google Scholar
[45] Guo R, Huang X, Wu J, Zhong W, Lin Y, Cao Y. ZnO/C nanocomposite microspheres with capsule structure for anode materials of lithium ion batteries. Ceram Int. 2020;46(12):19966–72. 10.1016/j.ceramint.2020.05.064.Search in Google Scholar
[46] Saadi H, Rhouma FIH, Benzarti Z, Bougrioua Z, Guermazi S, Khirouni K. Electrical conductivity improvement of Fe doped ZnO nanopowders. Mater Res Bull. 2020;129(April):110884. 10.1016/j.materresbull.2020.110884.Search in Google Scholar
[47] Li F, Kong L, Sun Y, Jin Y, Hou P. Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries. J Mater Chem A Mater. 2018;6(26):12344–52.10.1039/C8TA03363CSearch in Google Scholar
[48] Nam KW, Yoon WS, Yang XQ. Structural changes and thermal stability of charged LiNi1/3Co1/3Mn1/3O2 cathode material for Li-ion batteries studied by time-resolved XRD. J Power Sources. 2009;189(1):515–8.10.1016/j.jpowsour.2008.10.130Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Modification of polymers to synthesize thermo-salt-resistant stabilizers of drilling fluids
- Study of the electronic stopping power of proton in different materials according to the Bohr and Bethe theories
- AI-driven UAV system for autonomous vehicle tracking and license plate recognition
- Enhancement of the output power of a small horizontal axis wind turbine based on the optimization approach
- Design of a vertically stacked double Luneburg lens-based beam-scanning antenna at 60 GHz
- Synergistic effect of nano-silica, steel slag, and waste glass on the microstructure, electrical resistivity, and strength of ultra-high-performance concrete
- Expert evaluation of attachments (caps) for orthopaedic equipment dedicated to pedestrian road users
- Performance and rheological characteristics of hot mix asphalt modified with melamine nanopowder polymer
- Second-order design of GNSS networks with different constraints using particle swarm optimization and genetic algorithms
- Impact of including a slab effect into a 2D RC frame on the seismic fragility assessment: A comparative study
- Analytical and numerical analysis of heat transfer from radial extended surface
- Comprehensive investigation of corrosion resistance of magnesium–titanium, aluminum, and aluminum–vanadium alloys in dilute electrolytes under zero-applied potential conditions
- Performance analysis of a novel design of an engine piston for a single cylinder
- Modeling performance of different sustainable self-compacting concrete pavement types utilizing various sample geometries
- The behavior of minors and road safety – case study of Poland
- The role of universities in efforts to increase the added value of recycled bucket tooth products through product design methods
- Adopting activated carbons on the PET depolymerization for purifying r-TPA
- Urban transportation challenges: Analysis and the mitigation strategies for road accidents, noise pollution and environmental impacts
- Enhancing the wear resistance and coefficient of friction of composite marine journal bearings utilizing nano-WC particles
- Sustainable bio-nanocomposite from lignocellulose nanofibers and HDPE for knee biomechanics: A tribological and mechanical properties study
- Effects of staggered transverse zigzag baffles and Al2O3–Cu hybrid nanofluid flow in a channel on thermofluid flow characteristics
- Mathematical modelling of Darcy–Forchheimer MHD Williamson nanofluid flow above a stretching/shrinking surface with slip conditions
- Energy efficiency and length modification of stilling basins with variable Baffle and chute block designs: A case study of the Fewa hydroelectric project
- Renewable-integrated power conversion architecture for urban heavy rail systems using bidirectional VSC and MPPT-controlled PV arrays as an auxiliary power source
- Exploitation of landfill gas vs refuse-derived fuel with landfill gas for electrical power generation in Basrah City/South of Iraq
- Two-phase numerical simulations of motile microorganisms in a 3D non-Newtonian nanofluid flow induced by chemical processes
- Sustainable cocoon waste epoxy composite solutions: Novel approach based on the deformation model using finite element analysis to determine Poisson’s ratio
- Impact and abrasion behavior of roller compacted concrete reinforced with different types of fibers
- Architectural design and its impact on daylighting in Gayo highland traditional mosques
- Structural and functional enhancement of Ni–Ti–Cu shape memory alloys via combined powder metallurgy techniques
- Design of an operational matrix method based on Haar wavelets and evolutionary algorithm for time-fractional advection–diffusion equations
- Design and optimization of a modified straight-tapered Vivaldi antenna using ANN for GPR system
- Analysis of operations of the antiresonance vibration mill of a circular trajectory of chamber vibrations
- Functions of changes in the mechanical properties of reinforcing steel under corrosive conditions
- 10.1515/eng-2025-0153
- Hybrid mechanics-informed machine learning models for predicting mechanical failure in graphene sponge: a low-data strategy for mechanical engineering applications
- Review Articles
- A modified adhesion evaluation method between asphalt and aggregate based on a pull off test and image processing
- Architectural practice process and artificial intelligence – an evolving practice
- Enhanced RRT motion planning for autonomous vehicles: a review on safety testing applications
- Special Issue: 51st KKBN - Part II
- The influence of storing mineral wool on its thermal conductivity in an open space
- Use of nondestructive test methods to determine the thickness and compressive strength of unilaterally accessible concrete components of building
- Use of modeling, BIM technology, and virtual reality in nondestructive testing and inventory, using the example of the Trzonolinowiec
- Tunable terahertz metasurface based on a modified Jerusalem cross for thin dielectric film evaluation
- Integration of SEM and acoustic emission methods in non-destructive evaluation of fiber–cement boards exposed to high temperatures
- Non-destructive method of characterizing nitrided layers in the 42CrMo4 steel using the amplitude-frequency technique of eddy currents
- Evaluation of braze welded joints using the ultrasonic method
- Analysis of the potential use of the passive magnetic method for detecting defects in welded joints made of X2CrNiMo17-12-2 steel
- Analysis of the possibility of applying a residual magnetic field for lack of fusion detection in welded joints of S235JR steel
- Eddy current methodology in the non-direct measurement of martensite during plastic deformation of SS316L
- Methodology for diagnosing hydraulic oil in production machines with the additional use of microfiltration
- Special Issue: IETAS 2024 - Part II
- Enhancing communication with elderly and stroke patients based on sign-gesture translation via audio-visual avatars
- Optimizing wireless charging for electric vehicles via a novel coil design and artificial intelligence techniques
- Evaluation of moisture damage for warm mix asphalt (WMA) containing reclaimed asphalt pavement (RAP)
- Comparative CFD case study on forced convection: Analysis of constant vs variable air properties in channel flow
- Evaluating sustainable indicators for urban street network: Al-Najaf network as a case study
- Node failure in self-organized sensor networks
- Comprehensive assessment of side friction impacts on urban traffic flow: A case study of Hilla City, Iraq
- Design a system to transfer alternating electric current using six channels of laser as an embedding and transmitting source
- Security and surveillance application in 3D modeling of a smart city: Kirkuk city as a case study
- Modified biochar derived from sewage sludge for purification of lead-contaminated water
- The future of space colonisation: Architectural considerations
- Design of a Tri-band Reconfigurable Antenna Using Metamaterials for IoT Applications
- Special Issue: AESMT-7 - Part II
- Experimental study on behavior of hybrid columns by using SIFCON under eccentric load
- Special Issue: ICESTA-2024 and ICCEEAS-2024
- A selective recovery of zinc and manganese from the spent primary battery black mass as zinc hydroxide and manganese carbonate
- Special Issue: REMO 2025 and BUDIN 2025
- Predictive modeling coupled with wireless sensor networks for sustainable marine ecosystem management using real-time remote monitoring of water quality
- Management strategies for refurbishment projects: A case study of an industrial heritage building
- Structural evaluation of historical masonry walls utilizing non-destructive techniques – Comprehensive analysis
Articles in the same Issue
- Research Articles
- Modification of polymers to synthesize thermo-salt-resistant stabilizers of drilling fluids
- Study of the electronic stopping power of proton in different materials according to the Bohr and Bethe theories
- AI-driven UAV system for autonomous vehicle tracking and license plate recognition
- Enhancement of the output power of a small horizontal axis wind turbine based on the optimization approach
- Design of a vertically stacked double Luneburg lens-based beam-scanning antenna at 60 GHz
- Synergistic effect of nano-silica, steel slag, and waste glass on the microstructure, electrical resistivity, and strength of ultra-high-performance concrete
- Expert evaluation of attachments (caps) for orthopaedic equipment dedicated to pedestrian road users
- Performance and rheological characteristics of hot mix asphalt modified with melamine nanopowder polymer
- Second-order design of GNSS networks with different constraints using particle swarm optimization and genetic algorithms
- Impact of including a slab effect into a 2D RC frame on the seismic fragility assessment: A comparative study
- Analytical and numerical analysis of heat transfer from radial extended surface
- Comprehensive investigation of corrosion resistance of magnesium–titanium, aluminum, and aluminum–vanadium alloys in dilute electrolytes under zero-applied potential conditions
- Performance analysis of a novel design of an engine piston for a single cylinder
- Modeling performance of different sustainable self-compacting concrete pavement types utilizing various sample geometries
- The behavior of minors and road safety – case study of Poland
- The role of universities in efforts to increase the added value of recycled bucket tooth products through product design methods
- Adopting activated carbons on the PET depolymerization for purifying r-TPA
- Urban transportation challenges: Analysis and the mitigation strategies for road accidents, noise pollution and environmental impacts
- Enhancing the wear resistance and coefficient of friction of composite marine journal bearings utilizing nano-WC particles
- Sustainable bio-nanocomposite from lignocellulose nanofibers and HDPE for knee biomechanics: A tribological and mechanical properties study
- Effects of staggered transverse zigzag baffles and Al2O3–Cu hybrid nanofluid flow in a channel on thermofluid flow characteristics
- Mathematical modelling of Darcy–Forchheimer MHD Williamson nanofluid flow above a stretching/shrinking surface with slip conditions
- Energy efficiency and length modification of stilling basins with variable Baffle and chute block designs: A case study of the Fewa hydroelectric project
- Renewable-integrated power conversion architecture for urban heavy rail systems using bidirectional VSC and MPPT-controlled PV arrays as an auxiliary power source
- Exploitation of landfill gas vs refuse-derived fuel with landfill gas for electrical power generation in Basrah City/South of Iraq
- Two-phase numerical simulations of motile microorganisms in a 3D non-Newtonian nanofluid flow induced by chemical processes
- Sustainable cocoon waste epoxy composite solutions: Novel approach based on the deformation model using finite element analysis to determine Poisson’s ratio
- Impact and abrasion behavior of roller compacted concrete reinforced with different types of fibers
- Architectural design and its impact on daylighting in Gayo highland traditional mosques
- Structural and functional enhancement of Ni–Ti–Cu shape memory alloys via combined powder metallurgy techniques
- Design of an operational matrix method based on Haar wavelets and evolutionary algorithm for time-fractional advection–diffusion equations
- Design and optimization of a modified straight-tapered Vivaldi antenna using ANN for GPR system
- Analysis of operations of the antiresonance vibration mill of a circular trajectory of chamber vibrations
- Functions of changes in the mechanical properties of reinforcing steel under corrosive conditions
- 10.1515/eng-2025-0153
- Hybrid mechanics-informed machine learning models for predicting mechanical failure in graphene sponge: a low-data strategy for mechanical engineering applications
- Review Articles
- A modified adhesion evaluation method between asphalt and aggregate based on a pull off test and image processing
- Architectural practice process and artificial intelligence – an evolving practice
- Enhanced RRT motion planning for autonomous vehicles: a review on safety testing applications
- Special Issue: 51st KKBN - Part II
- The influence of storing mineral wool on its thermal conductivity in an open space
- Use of nondestructive test methods to determine the thickness and compressive strength of unilaterally accessible concrete components of building
- Use of modeling, BIM technology, and virtual reality in nondestructive testing and inventory, using the example of the Trzonolinowiec
- Tunable terahertz metasurface based on a modified Jerusalem cross for thin dielectric film evaluation
- Integration of SEM and acoustic emission methods in non-destructive evaluation of fiber–cement boards exposed to high temperatures
- Non-destructive method of characterizing nitrided layers in the 42CrMo4 steel using the amplitude-frequency technique of eddy currents
- Evaluation of braze welded joints using the ultrasonic method
- Analysis of the potential use of the passive magnetic method for detecting defects in welded joints made of X2CrNiMo17-12-2 steel
- Analysis of the possibility of applying a residual magnetic field for lack of fusion detection in welded joints of S235JR steel
- Eddy current methodology in the non-direct measurement of martensite during plastic deformation of SS316L
- Methodology for diagnosing hydraulic oil in production machines with the additional use of microfiltration
- Special Issue: IETAS 2024 - Part II
- Enhancing communication with elderly and stroke patients based on sign-gesture translation via audio-visual avatars
- Optimizing wireless charging for electric vehicles via a novel coil design and artificial intelligence techniques
- Evaluation of moisture damage for warm mix asphalt (WMA) containing reclaimed asphalt pavement (RAP)
- Comparative CFD case study on forced convection: Analysis of constant vs variable air properties in channel flow
- Evaluating sustainable indicators for urban street network: Al-Najaf network as a case study
- Node failure in self-organized sensor networks
- Comprehensive assessment of side friction impacts on urban traffic flow: A case study of Hilla City, Iraq
- Design a system to transfer alternating electric current using six channels of laser as an embedding and transmitting source
- Security and surveillance application in 3D modeling of a smart city: Kirkuk city as a case study
- Modified biochar derived from sewage sludge for purification of lead-contaminated water
- The future of space colonisation: Architectural considerations
- Design of a Tri-band Reconfigurable Antenna Using Metamaterials for IoT Applications
- Special Issue: AESMT-7 - Part II
- Experimental study on behavior of hybrid columns by using SIFCON under eccentric load
- Special Issue: ICESTA-2024 and ICCEEAS-2024
- A selective recovery of zinc and manganese from the spent primary battery black mass as zinc hydroxide and manganese carbonate
- Special Issue: REMO 2025 and BUDIN 2025
- Predictive modeling coupled with wireless sensor networks for sustainable marine ecosystem management using real-time remote monitoring of water quality
- Management strategies for refurbishment projects: A case study of an industrial heritage building
- Structural evaluation of historical masonry walls utilizing non-destructive techniques – Comprehensive analysis

