Abstract
Cellulose-based aerogels have become promising adsorbents for organic pollutants and spilled oil due to their high selectivity and excellent sorption capacity. However, the high costs of preparation limit their practicality. In this study, cellulose fibers were fabricated from corn straw via simple alkaline pulping and bleaching. A cellulose aerogel (CA) was then prepared by the freeze-drying method with polyvinyl alcohol as the binder. After treatment with methyltrimethoxysilane by facile chemical vapor deposition, a modified cellulose aerogel (MCA) with water contact angles up to 154.8° was obtained, which exhibited superhydrophobicity. Importantly, the MCA has both high porosity (98.35–98.94%) and low density (16.33–23.95 mg·cm−3). In addition, the MCA can separate oil–water mixtures by gravity filtration with separation efficiency up to 97.3% and flux as high as 9827 L·m−2·h−1. This conversion of agricultural wastes into an efficient and high value-added adsorbing material is a promising development in the field of oil–water separation that offers a green and efficient strategy to combat the leakage of organic solvents and oil into the environment.
1 Introduction
With the continuous expansion of industrialization in recent years, the problem of oily wastewater discharge has become increasingly serious. The frequent occurrence of marine oil spills has brought about serious environmental and ecological problems that have seriously threatened human health and economic development. In addition, oily wastewater is widely derived from manufacturing, petroleum, food, pharmaceutical and other industries [1]. To date, oily wastewater treatment methods fall into three categories: physical methods (such as gravitational sedimentation and adsorption) [2,3], chemical methods (such as flocculants) [4], and biological methods (such as microbial degradation and bioremediation) [3,5]. The use of flocculants can lead to secondary contamination, and biological treatment takes a long time to work [6,7]. Among the currently available methods, physical adsorption is therefore the most desirable for the oily wastewater treatment because of its high efficiency and low cost [8].
As a type of lightweight material with high porosity, aerogels are generally obtained by freeze-drying or critical point drying cryogels [9] and have attracted widespread attention as adsorbent materials. In the freeze-drying process, the solvent in the frozen aquagel is replaced with air to form a three-dimensional porous material [10]. Carbon-based aerogels, such as graphene oxide/carbon nanotube-based foam [11], chemically modified graphene [12,13], carbon nanotube sponges [14], and carbon nanofiber aerogels [15] that have shown low density, high compressibility, high porosity, and superhydrophobicity/superoleophilicity, have already been widely studied and used in the field of oil–water separation. However, the preparation of these aerogels involves the use of harmful or costly precursors, which hinder their large-scale usage as adsorbents [16]. Furthermore, although commercial organic aerogels such as polyimide aerogels [17], polyurethane [18], and melamine aerogels [19] have good reusability, their scope of application is limited due to secondary contamination and poor environmental compatibility. Recently, bio-based aerogels have attracted much attention due to the availability of the key source materials, as well as their renewability, degradability, and biocompatibility [20]. As the most abundant resource in nature, cellulose has the advantages of being renewable, offering environmental protection and of low cost, and is considered to be an ideal alternative material for the preparation of aerogel [21]. Cellulose can be applied in the field of oil/water separation through simple deployment methods and even reused, thus offering both environmental protection and economic benefits [22].
As a large agricultural country, the planting area of corn in China reached 4.33 × 107 hectare in 2021 [23]. However, a large amount of corn straw is hitherto crushed, buried, or burned after corn harvesting, resulting in both a waste of resources and serious environmental problem [24]. Corn straw has three main components similar to trees, but corn straw has an advantage over trees because of its shorter growth cycle [25]. Corn stover is an ideal biomass resource with high cellulose content. Therefore, it has already been demonstrated that corn straw can be used as a green and low-cost carbonaceous precursor to synthesize porous cellulose aerogels (CAs) for applications of adsorption and separation [26]. Moreover, hydrophobic groups with a low surface energy can be introduced into the CA by physical or chemical methods to improve its hydrophobicity and oil adsorption capacity [27]. In the present study, chemical vapor deposition (CVD) modification was used to reduce the dosage of the modifier needed compared to the impregnation process.
In this study, based on the utilization of agricultural waste and the advantages of CAs, we chose corn straw as the raw material to fabricate a modified cellulose aerogel (MCA). Crucially, the low-cost methyltrimethoxysilane (MTMS) was selected to modify the corn straw fiber aerogel to achieve hydrophobic performance compared with other studies. The influence of different mass ratios of corn straw cellulose (CSC)/PVA on its structure, morphology, hydrophobicity, and oil adsorption capacity was compared and analyzed. A comparative study was conducted on the performance and recyclability of MCA in adsorbing various organic solvents and oils. MCA was used to efficiently and continuously remove organic solvents and oils from water. This study can provide an environment-protecting and economical method to fabricate the biomass aerogels that could be easily used to deal with oil spill and environmental pollution.
2 Experimental methods
2.1 Materials and reagents
The corn straw was collected from a farm in Jiangsu province, China. Sodium hydroxide, sodium chlorite, glacial acetic acid, Sudan red III, methylene blue, poly(vinyl alcohol) (molecular weight: 75,000; hydrolysis rate: over 99%), and MTMS (98% were all of analytical grade and obtained from Shanghai Macklin Biochemical Co., Ltd. n-Hexane, petroleum ether, cyclohexane, dichloromethane, chloroform, carbon tetrachloride, and other chemicals were all purchased from Aladdin (Shanghai). Engine oil (5w40), dimethyl silicone oil (PMX-200), and castor oil were purchased commercially (Guangdong, China). All reagents were used as obtained without additional processing.
2.2 Pre-treatment of the corn straw
First, the dried corn straw was cut into 3 cm segments, then cleaned with deionized water, and dried. Next, the straw was ground with a high-speed grinder, passed through a 60-mesh screen, and stored for subsequent experiments. The ground material (20 g) was placed into a beaker containing sodium hydroxide solution (1.0 L, 4 wt%) and heated to 80°C for 2 h under mechanical agitation. The resulting crude straw cellulose was filtered and washed with deionized water (DI) until the pH value was constant. Subsequently, the crude straw cellulose was mixed with a 5% (w/v) bleaching solution that consisted of an equal amount of sodium chlorite solution (2%, w/v), an acetic acid buffer solution (2N), and deionized water and stirred at 80°C for 2 h. Finally, the residue was filtered and washed with DI until neutral pH, and CSC was obtained after drying the residue at 60°C for 36 h.
2.3 Preparation of hydrophobic CA
Dried cellulose fibers (1 g) obtained from the previous pre-treatments were mixed with water (50 mL). After that, the mixture was homogenized using a blender for 10 min. Approximately 50 mL of PVA solution (0.4, 0.8, 1.2, 1.6, and 2.0 wt%) was added to the above cellulose fiber suspension, and the mixture was heated at 80°C for 2 h. Then, the mixture was sonicated for 5 min, and the uniformly dispersed system was frozen below 0°C for 24 h before being lyophilized at −50°C for 48 h to obtain the CA.
The preparation process of MCA and the corresponding reaction mechanism are illustrated in Figure 1. In order to enhance the hydrophobicity of the aerogel, the CVD method was used to silanize the CA. A vial containing MTMS (5 mL) and CA was fixed in a sealed glass chamber and heated at 80°C for 6 h to get the MCA [28]. The above-obtained materials were labeled CA-X and MCA-X, as indicated in Table 1.
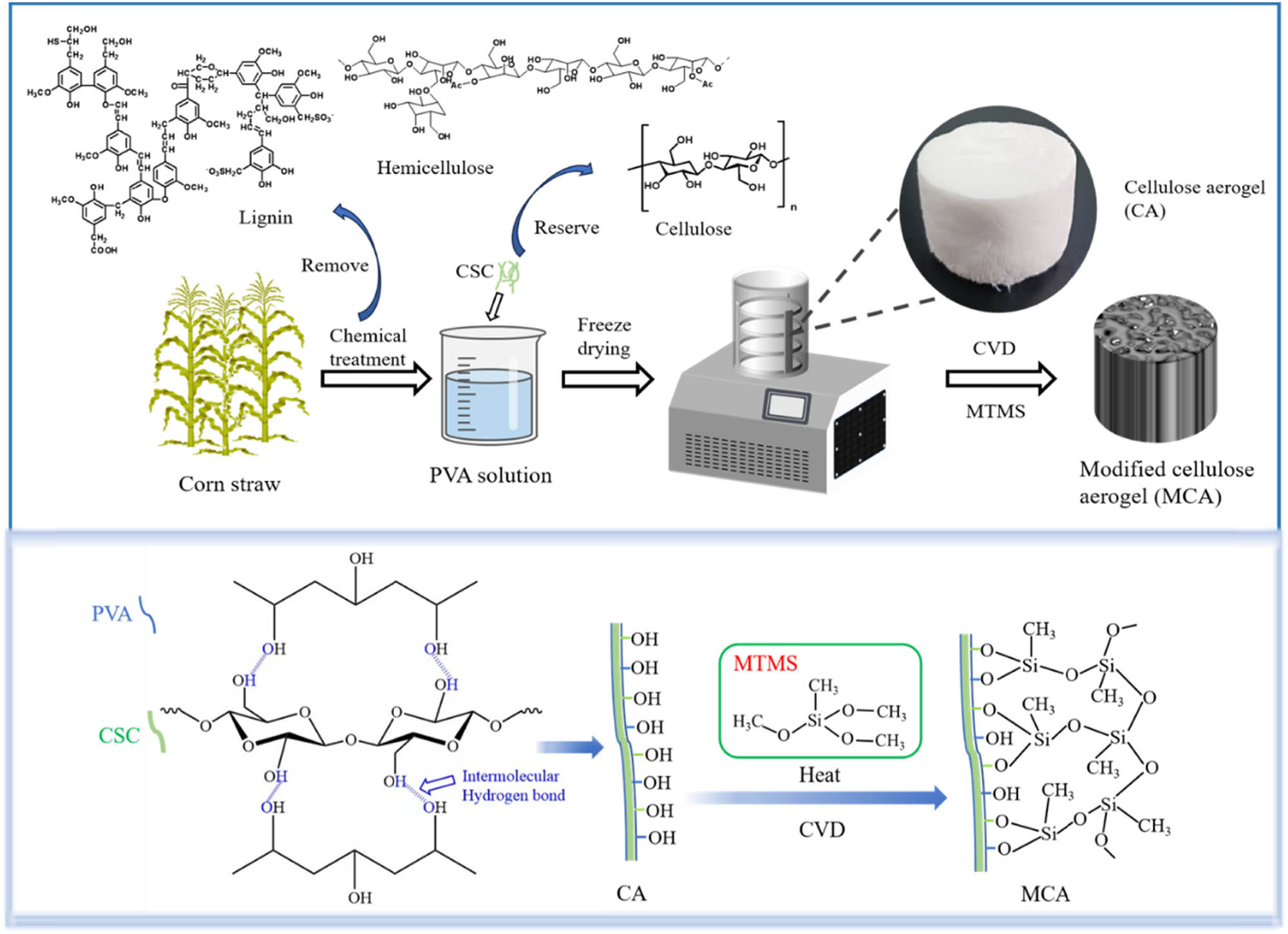
Schematic diagram of the preparation of MCA from corn straw.
Ratio of cellulose to PVA
| Samples | Cellulose/PVA (g:g) | ||||
|---|---|---|---|---|---|
| 5:1 | 5:2 | 5:3 | 5:4 | 5:5 | |
| CA | CA-1 | CA-2 | CA-3 | CA-4 | CA-5 |
| MTMS modified | MCA-1 | MCA-2 | MCA-3 | MCA-4 | MCA-5 |
2.4 Characterization
The SEM images of the samples were obtained using a field-emission scanning electron microscope (SEM, SU8010) to investigate the microscopic morphologies. The water contact angle (WCA) values were measured using a DataPhysics OCA 100 optical system with a water droplet of 4 μL. The surface area and pore structure of MCA were determined by an Autosorb iQ-MP-MP device (Quantachrome Instruments). A Fourier transform infrared (FT-IR) spectrometer (Nicolet 5700) was used to determine the change in the groups in the modified samples. X-ray diffraction (XRD) patterns of the aerogels were obtained on a Rigaku SmartLab X-ray powder diffractometer (Cu-Kα radiation). X-ray photoelectron spectroscopy (XPS) measurements of the aerogels were carried out with a Thermo Scientific K-Alpha spectrometer.
2.5 Density and porosity measurements
The density of the MCA was calculated from Eq. 1 [29].
where a stands for the aerogel and m (mg) and V (cm3) represent the mass and volume of the aerogels, respectively.
The porosity of the aerogel was calculated using Eq. 2 [30].
where ω
c and ω
PVA are the weight percentages of cellulose and PVA, respectively.
2.6 Oil adsorption property and reusability measurements
To test the adsorption capacity of the material, the mass of MCA was weighed on an electronic balance, then removed and immersed in various oils or organic solvents until saturation. The saturated MCA was removed and placed in air for 20 s until the weight did not change. The saturated aerogel was then weighed to obtain M1. For some organic solvents, the saturated MCA was placed in a closed container and weighed quickly to avoid volatilization. The adsorption capacity of various oils and the organic solvent was calculated as follows:
where Q is the adsorption capacity of the MCA, and M 0 and M 1 are the MCA’s weight before and after adsorption, respectively. Each adsorption datum is the average value obtained from three repeated experiments.
By squeezing and centrifugation, oils or organic solvents adsorbed by MCA could be directly removed. Then, the compressed sample was immersed in oil again, and its adsorption capacity was measured. In the centrifugation test, the same method was used as before. Carbon tetrachloride was used in the test. The contaminant removal process was cycled ten times to test its reusability.
2.7 Continuous oil–water separation experiment
Continuous oil–water separation was conducted by a peristaltic pump assisted MCA. The oil–water mixture was obtained by directly mixing oil (Sudan red III, dyed red) and water (dyed blue). Specifically, the peristaltic pump suction pipe was connected to a cylindrical MCA (R = 2 cm; H = 2 cm), and the other end of the pipe was fixed to the collection beaker. n-Hexane was sucked into the container using the peristaltic pump to achieve continuous separation. The MCA was placed in an 8 mm inner diameter funnel as a filter element, and the CH2Cl2/water mixture was injected into the funnel. CH2Cl2 was then collected in the beaker below, and its volume measured with a measuring cylinder. The flux (F) could be evaluated according to the following equation:
where V (L) is the volume of the collected liquid, S (m2) is the filter area of the separation layer, and t (h) is the filtration time.
2.8 Water-in-oil emulsion separation
To prepare the water-in-n-hexane emulsions, Span 80 was first dissolved in 49 mL of water (0.1 mg·mL−1), and then 1 mL of n-hexane was added under stirring at 800 rpm for 2 h. The MCA was fixed as a filter layer in the device to separate the emulsions. The entire separation process was only carried out under the action of gravity.
3 Results and discussion
3.1 Structural and chemical properties of aerogels
As shown in Figure 2a, the prepared MCA had a spongy appearance. It possessed operability as to be fashioned into a variety of shapes and sizes by molding. From the SEM images of MCA (Figure 2b–d), it can be seen that the MCA consisted of a 3D network structure. During the preparation process, the cellulose fibers were stirred and cut at a high speed by the blender. After stirring and ultrasonic treatment, cellulose dispersion was formed. During the lyophilization process, highly porous structures were gradually formed as the ice sublimates, and the aerogel structure did not shrink and collapse [32]. These porous structures provide storage space for oil adsorption, which was beneficial for the filtration of oil or emulsions. Moreover, nitrogen adsorption–desorption isotherms of MCA-3 (Figure S1a) exhibited a type-IV isotherm with a hysteresis loop, indicating the average pore diameter exceeds 10 nm. It can be concluded from the Barrett–Joyner–Halenda pore size distributions (Figure S1b) that the corresponding pore diameters were mainly distributed in the range of 1.8–25 nm, which indicated the presence of mesoporous structures [33]. Notably, the contribution of micropores and mesopores to the total pore volume was low, as seen from the SEM images. Macroscopic and microscopic pores of MCA were interconnected to form numerous liquid transport and storage channels. In Figure 2e, the energy-dispersive X-ray spectrum of MCA shows the presence Si element and a uniform distribution of silicon on the aerogel, proving that the CA was successfully modified by MTMS. The CA was successfully modified by MTMS. Considering the effect of the porosity of aerogel on its adsorption capacity, the effects of different ratios of CSC/PVA on the density and porosity of MCA were subsequently investigated. The average density of aerogel increased and the porosity decreased with the increase of PVA content, which in turn may affect the adsorption properties of MCA. The porosity of the aerogel decreased from 98.94% to 98.35%, and the density increased from 16.33 to 23.95 mg·cm−3 (Figure 2f). Therefore, MCA-3 with moderate porosity and density was selected for subsequent experiments.
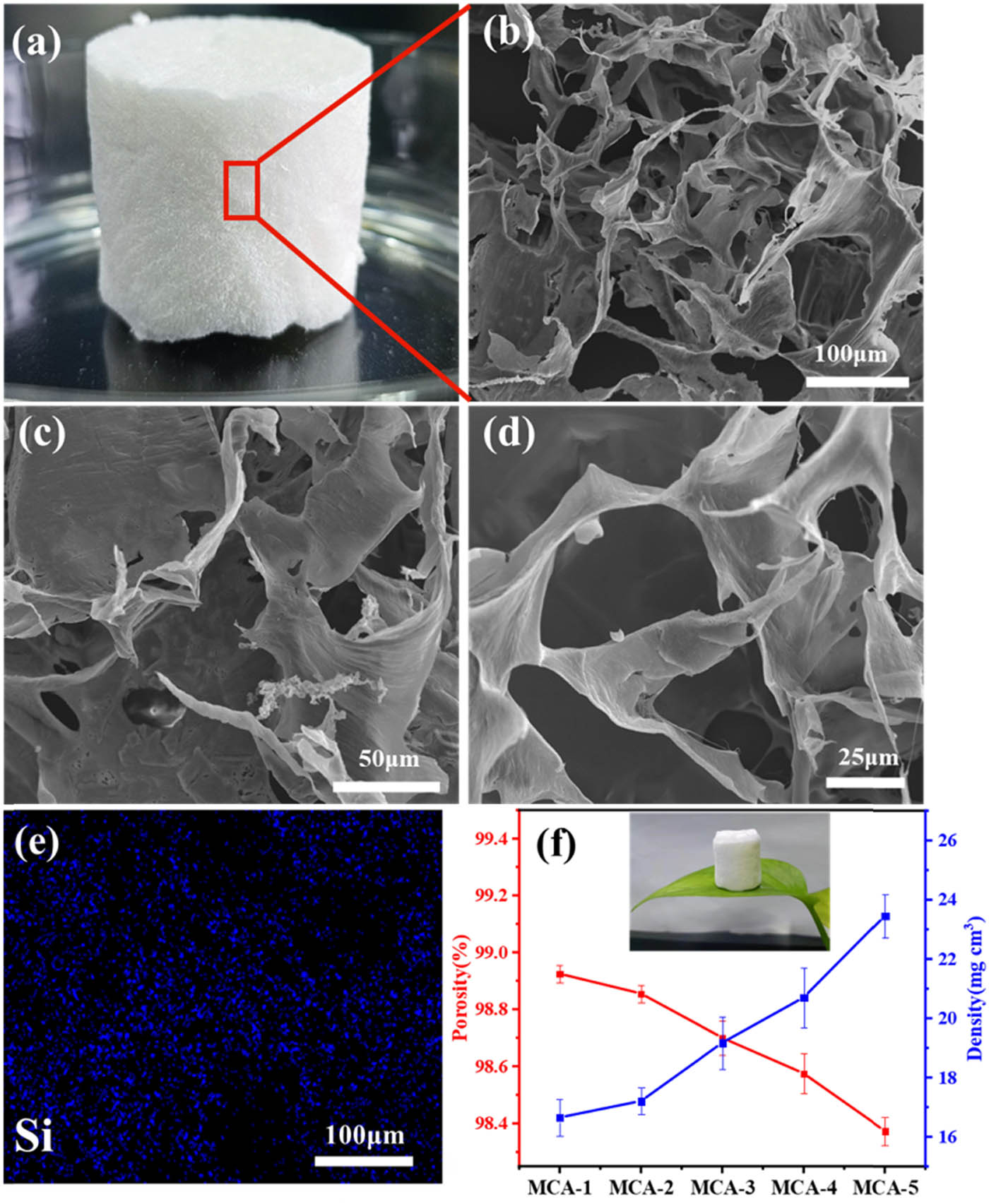
(a) Photograph of the MCA, (b)–(d) SEM images of the MCA in different magnification times, (e) EDX mapping of Si on MCA, and (f) density and porosity of MCA.
The effect of functional groups on the modification process was investigated by comparing and analyzing the changes in chemical groups of aerogels before and after modification. FT-IR spectra (Figure 3a) show that both CA-3 and MCA-3 have a wide band at 3,380 cm−1, caused by the stretching vibration of hydroxyl (–OH) groups. The reason is that the reaction between the hydroxyl groups of cellulose and MTMS led to the reduction of the hydroxyl groups in the CA. The characteristic peaks of CH3, CH2, and C–O groups appear at 2,920, 1,450, and 1,090 cm−1, respectively, and correspond to the functional groups of the cellulose composites [34]. Among them, the in-plane bending vibration of CH2 at 1,450 cm−1 indicates the presence of long carbon chains. In addition, MCA-3 shows new vibration bands at 1,270 cm−1 (Si–CH3) and 771 cm−1 (Si–O–Si) which confirm that Si–CH3 groups were successfully grafted onto CA [35]. These Si–CH3 groups on the surface of the CA have a lower surface energy, which is conducive to improving the hydrophobicity of the aerogel. To investigate the structural changes of aerogels, CA-3 and MCA-3 were characterized by XRD (Figure 3b). CA-3 shows two apparent diffraction peaks at 2θ = 16° and 22.4°, which are the characteristic peaks of natural cellulose [36]. Compared with CA-3, the XRD peaks of MCA-3 disappear at 16° and weaken at 22.4°, which was caused by the decrease in cellulose mass fraction after aerogel modification.
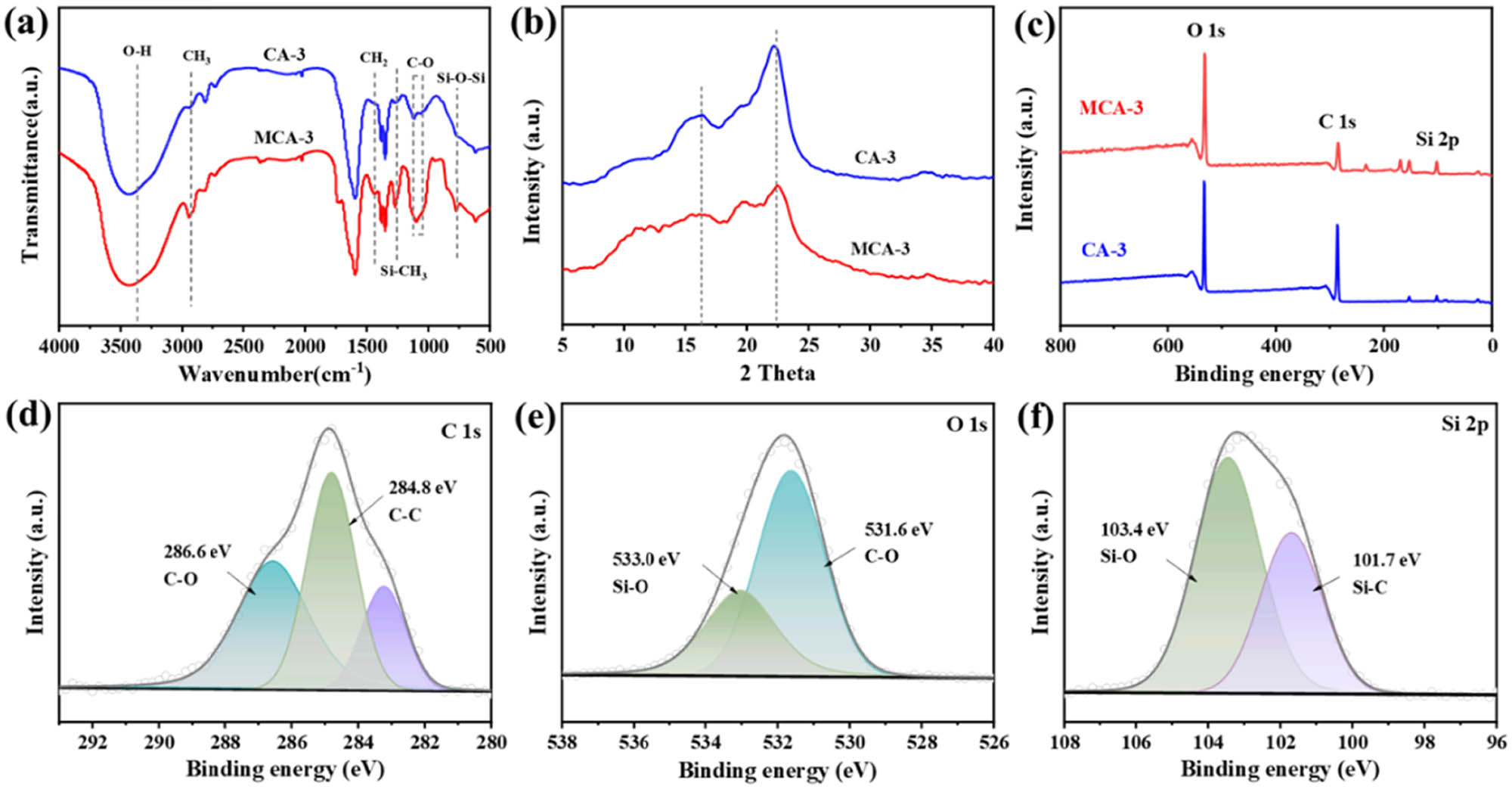
(a) FTIR spectra, (b) XRD patterns, and (c) XPS survey spectra of the original aerogel CA-3 and the modified aerogel MCA-3. (d) C 1s spectrum, (e) O 1s spectrum, and (f) Si 2p spectrum of MCA-3.
XPS was employed to investigate the changes in the surface chemical structure of MCA-3 compared to CA-3 and to confirm the effective silylation (Figure 3c–f). The CA-3 contains elements C and O, and the MCA-3 is composed of elements C, O, and Si (Figure 4c). In the case of MCA-3, the O 1s spectrum shows two peaks at 531.6 and 533.0 eV (Figure 3d) that correspond to C–O and Si–O components, respectively. Furthermore, the Si–C bond at 101.7 eV and the Si–O bond at 103.4 eV are observed in the Si 2p spectrum of the MCA-3 (Figure 3f). These results further confirm the silanization reaction between the hydroxyl groups of cellulose and MTMS.
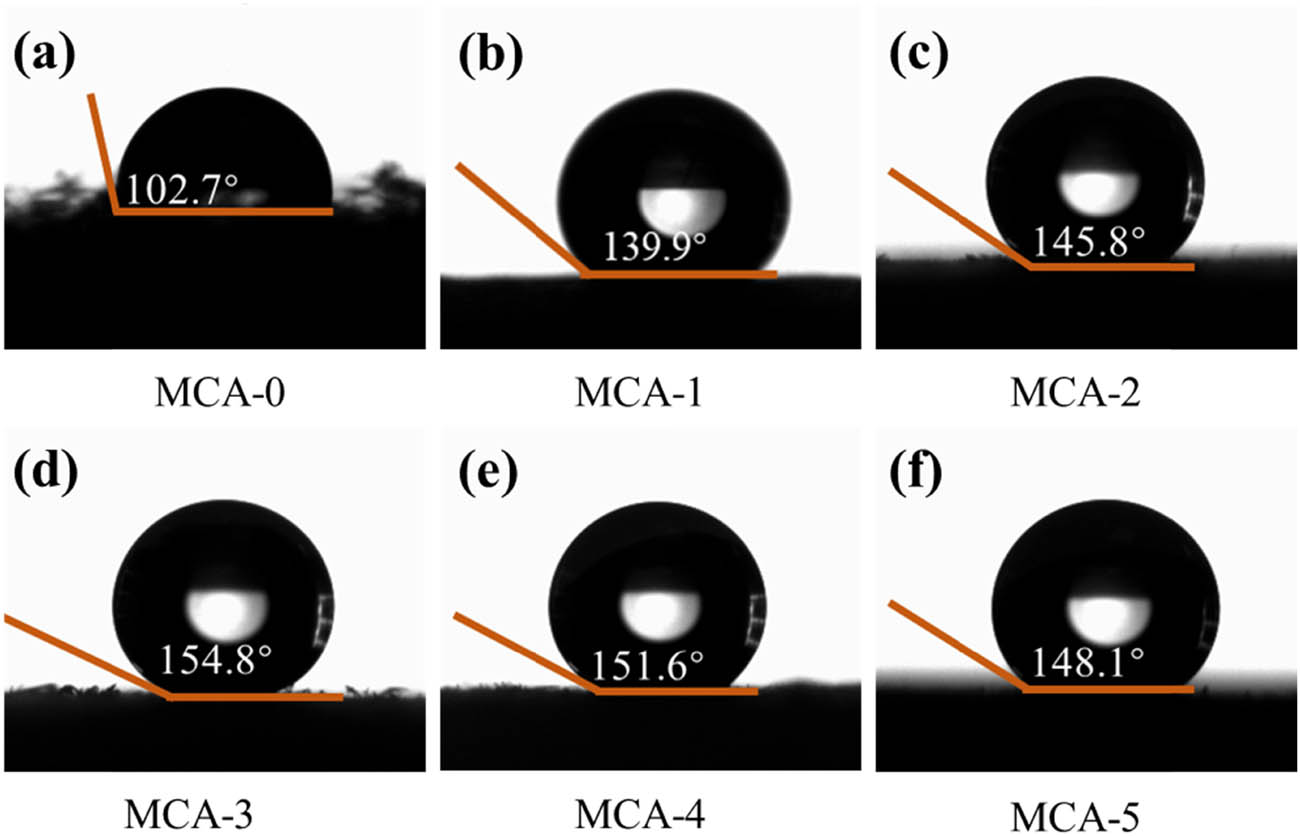
The photographs of water contact angle (WCA) of (a) MCA-0, (b) MCA-1, (c) MCA-2, (d) MCA-3, (e) MCA-4, and (f) MCA-5.
The CA prepared from pure cellulose aqueous dispersion exhibit an obvious collapse phenomenon, and its structure is easy to destroy. The addition of PVA prevented the significant contraction of CA and added structural support. The hydrophobic properties of MCA modified by MTMS of aerogels prepared with different proportions of CSC/PVA were also different. In the contact angle measurement, it was found that the MCA containing PVA modified by MTMS showed better hydrophobic properties (Figure 4), meaning that the functional groups on the surface of MCA also affected the hydrophobic property [37]. When the PVA solution concentration increased from 0 to 1.2 wt%, the contact angle increased from 102.4° to 154.8°, as presented in Figure 4a–d. However, as the PVA solution continued to increase, the MCA contact angle decreased, as shown in Figure 4e–f. The excellent hydrophobic properties of the MCA were the basis of its separation of oil and water by the aerogel adsorbent [38]. In summary, from the combined results of SEM, FTIR spectroscopy, XPS, and WCA, the low surface energy functional groups, and porous structure of the MCA sample determine its superhydrophobicity [39].
3.2 Oil sorption capacity and reusability
The aerogel showed different wettability behaviors before and after modification. With a large number of hydroxyl groups, water and oil droplets were rapidly adsorbed when placed on the surface of CA, indicating that CA has high affinity for oil and water (Figure S2a). When water and oil droplets dripped onto the MCA, it was observed that the droplets remained spherical on the MCA, and the oil was rapidly adsorbed (Figure S2b). To further investigate its hydrophobicity, MCA was immersed in water (Figure S2c), which formed an air gap on the surface due to its hydrophobicity and lipophilicity. When MCA was placed in a petroleum ether/water mixture, saturated MCA can float stably on water for a long time because of its low density and hydrophobicity (Figure S2d).
Due to its porous structure and superhydrophobicity, the MCA could be an excellent candidate for oil/water separation [40]. As shown in Figure 5a, the MCA quickly adsorbed n-hexane floating on water when it came into contact with it (dyed with Sudan red III). Similarly, when MCA was pressed into a beaker containing tetrachloromethane at the bottom, the organic solvent was also completely captured by the hydrophobic MCA (Figure 5b). By virtue of its excellent water resistance and adsorption capacity for surface and underwater solvents, MCA has the potential to adsorb both floating and underwater pollutants.
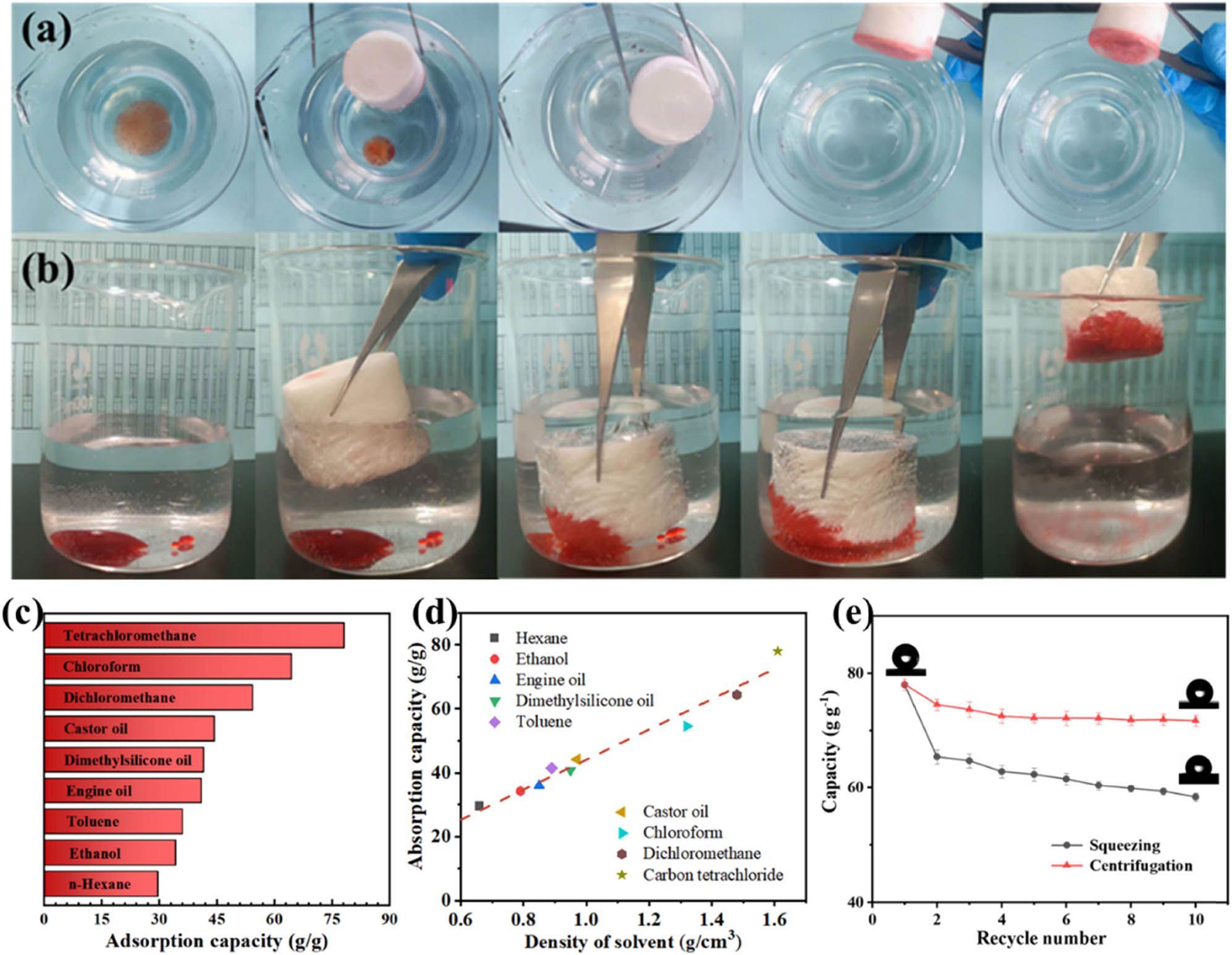
Removal performance for (a) cyclohexane and (b) tetrachloromethane by MCA. (c) Adsorption performance of MCA-3 for various oils and organic solvents. (d) Adsorption capacity as a function of solvent density. (e) Adsorption performance of MCA-3 at multiple centrifugation and squeezing cycles.
Due to its porous structure and superoleophilicity, MCA-3 displayed a large adsorption capacity for various oils and organic solvents (29.5–78.2 g·g−1) (Figure 5c). Furthermore, the adsorption capacities for high-density tetrachloromethane and chloroform were higher than those for low-density hexane, alcohol, and engine oil, which is positively related to their density (Figure 5d). Generally, as the volume and porosity of the aerogel are constant, we can say that the adsorption capacity is dependent on the density of solvent [41].
In the next part of the investigation, two methods, compression and centrifugation, were used to recover the adsorbed tetrachloromethane to evaluate the recoverability and reusability of the MCA [42]. From Figure 5e, it is evident that as the number of cycles increased, the oil adsorption capacity of MCA decreased. However, the adsorption performance tended to stabilize after the MCA was recycled ten times. Compared to other typical adsorption materials (Table S1), the synthesized MCA has the advantage that the raw material came from agricultural wastes generated from farming production. In particular, the MCA-3 had a contact angle of up to 154.8°, which was significantly better than most aerogel materials currently used to separate oil from water.
3.3 Continuous oil/water separation capability
As illustrated in Figure 6a, MCA-3 could be used as a filter element to separate the CH2Cl2/H2O mixed solution. CH2Cl2 (stained with Sudan red III) quickly passed through the porous structure of MCA-3 into the conical flask placed below it under the effect of gravity, while the water (stained with methylene blue) was retained in the funnel due to the excellent hydrophobicity of MCA-3. During the process, the MCA achieved both high filtration efficiency (97.3%) and high flux (9,827.1 L·m−2·h−1). In conclusion, the MCA effectively separated wastewater containing heavy oil from water without requiring additional driving pressure. During practical applications, the MCA can also be combined with a peristaltic pump to achieve high-efficiency continuous oil/water separation (Figure 6b and c) (Supporting information video S1). The catheter of the peristaltic pump was inserted into the MCA and then the MCA was placed in the n-hexane/water mixture solution. When the peristaltic pump power was turned on, n-hexane was continuously adsorbed by the MCA and collected into a container through the pipeline. Afterward, the MCA adsorbed the remaining organic solvent while repelling water simultaneously to maintain a continuous solvent flow. Ultimately, the light solvent can be successfully and efficiently separated from water, leaving only water in the container.
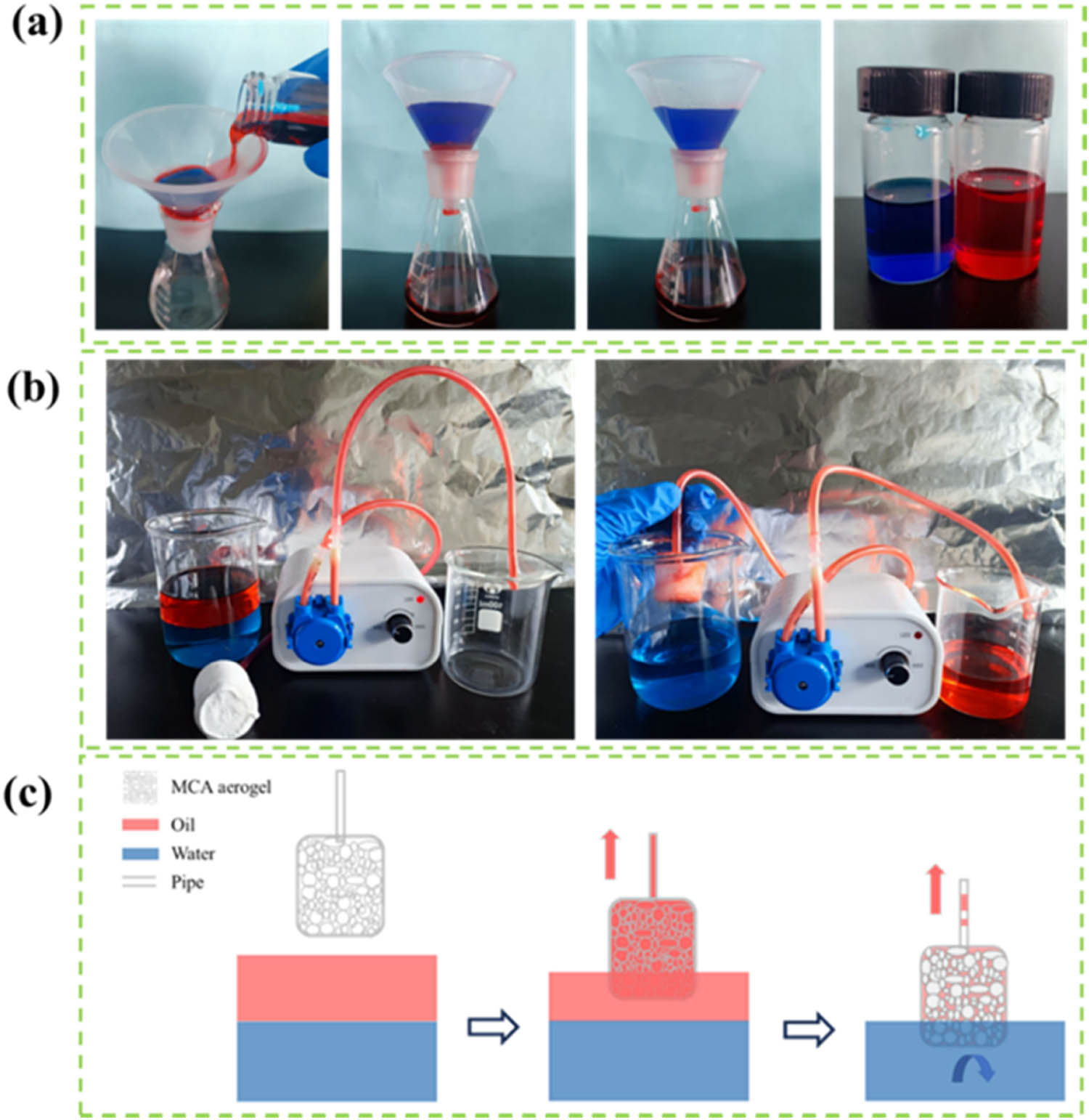
(a) Oil (dyed red)–water (dyed blue) mixture filtration test. (b) Continuous oil/water separation by a peristaltic pump. (c) Schematic diagram of the oil/water separation mechanism of the MCA.
The above results demonstrated that MCA has potential for practical application in large-scale oil spill collection. The process of continuous oil/water separation could be divided into three steps. First, the solvent diffused into the abundant pores inside the MCA. Second, the adsorbed liquid filled the entire adsorbent. Finally, the solvent was sucked out of the MCA under additional driving pressure.
Moreover, the MCA could separate n-hexane from stabilized water-in-oil emulsion due to its superhydrophobic/superoleophilic character [43]. The MCA was made into a suitable shape and placed in the filtering device. After pouring the stable n-hexane aqueous emulsion into the device, it can be seen that n-hexane flowed through the MCA, while water was kept in the device (Figure 7a). After filtration, obvious droplets were not observed in the collected filtrate when viewed under a microscope (Figure 7b), which proved that n-hexane was effectively removed from the emulsion. The separation mechanism of water-in-oil emulsions is shown in Figure S3. Due to the good lipophilicity of MCA, it was captured from the emulsion when the outer oil droplets came into contact with the surface of MCA and then diffused and permeated through the MCA. At the same time, the water droplets were intercepted by the hydrophobic layer owing to the hydrophobicity of MCA, while the oil passed through the aerogel under the action of gravity and capillary force. Subsequently, the water droplets gradually left and floated off the surface of the MCA.
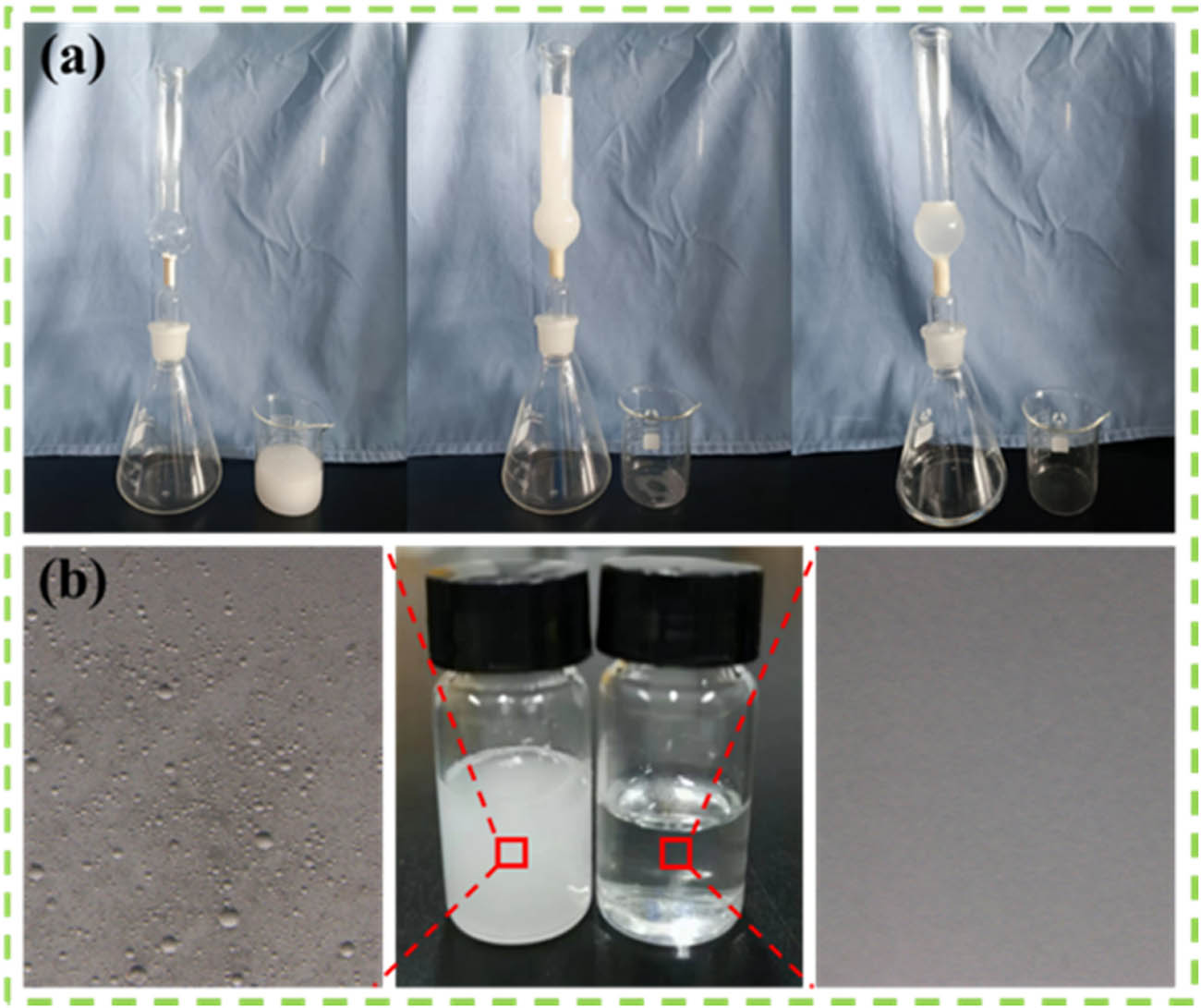
(a) Process of water-in-oil emulsion separation. (b) Light micrographs of emulsion before and after filtration.
4 Conclusions
In summary, a handy and low-cost method was successfully applied to synthesize CAs from corn straw. The obtained CA was modified with MTMS to obtain more hydrophobic MCA with a WCA of up to 154.8°, which is higher than most cellulose-based superhydrophobic aerogels reported to date. The stable three-dimensional network structure coupled with high porosity (98.35–98.94%) and low density (16.33–23.95 mg·cm−3) promoted the adsorption of oils and organic solvents via the internal channels of the MCA, leading to a high adsorption capacity (29.5–78.2 g·g−1). In addition, the MCA recovered oils from the oil/water systems within seconds, demonstrating high separation efficiency. After ten cycles of oil adsorption, it continued to exhibit good adsorption performance and reusability. With the above advantages of high adsorption capacity, excellent oil/water sorption selectivity, and renewable source material, the MCA is an ideal adsorbent to combat water pollution caused by oil spills and chemical leakage.
Acknowledgements
This work was supported by the Innovation Team Project in the Universities of Guangdong province, China [grant number 2017GKCXTD006]. The authors thank AiMi Academic Services for English language editing.
-
Funding information: This work was supported by the Innovation Team Project in the Universities of Guangdong province, China [grant number 2017GKCXTD006].
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results, and approved the final version of the manuscript. Li Chen: investigation, data curation, and writing – original draft. Gengyuan Zhang: software and methodology. Baofeng Xu: methodology and supervision. Juanwei Guo: writing – review & editing, supervision, and funding acquisition.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
[1] Gupta K, Joshi P, Khatri OP. h-BN and graphene-based ultralight hybrid aerogels: Highly efficient sorbent for recovery of hydrocarbon oils and organic solvents. J Environ Chem Eng. 2021;9(6):106788. 10.1016/j.jece.2021.106788.Search in Google Scholar
[2] Liu L, Kong G, Zhu Y, Lai D, Zhang S, Che C. Ultralight, compressive and superhydrophobic methyltriethoxysilane-modified graphene aerogels for recyclable and selective organic pollutants adsorption from water. Appl Surf Sci. 2022;598:153694. 10.1016/j.apsusc.2022.153694.Search in Google Scholar
[3] Dhaka A, Chattopadhyay P. A review on physical remediation techniques for treatment of marine oil spills. J Environ Manage. 2021;288:112428. 10.1016/j.jenvman.2021.112428.Search in Google Scholar PubMed
[4] Wang T, Yang WL, Hong Y, Hou YL. Magnetic nanoparticles grafted with amino-riched dendrimer as magnetic flocculant for efficient harvesting of oleaginous microalgae. Chem Eng J. 2016;297:304–14. 10.1016/j.cej.2016.03.038.Search in Google Scholar
[5] Ren X, Zeng G, Tang L, Wang J, Wan J, Liu Y, et al. Sorption, transport and biodegradation – An insight into bioavailability of persistent organic pollutants in soil. Sci Total Environ. 2018;610–611:1154–63. 10.1016/j.scitotenv.2017.08.089.Search in Google Scholar PubMed
[6] Ge J, Zhao HY, Zhu HW, Huang J, Shi LA, Yu SH. Advanced sorbents for oil‐spill cleanup: recent advances and future perspectives. Adv Mater. 2016;28(47):10459–90. 10.1002/adma.201601812.Search in Google Scholar PubMed
[7] Jjagwe J, Olupot PW, Menya E, Kalibbala HM. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: a review. J Bioresour Bioprod. 2021;6(4):292–322. 10.1016/j.jobab.2021.03.003.Search in Google Scholar
[8] Jamsaz A, Goharshadi EK. An environmentally friendly superhydrophobic modified polyurethane sponge by seashell for the efficient oil/water separation. Process Saf Environ. 2020;139:297–304. 10.1016/j.psep.2020.04.042.Search in Google Scholar
[9] Ganesamoorthy R, Vadivel VK, Kumar R, Kushwaha OS, Mamane H. Aerogels for water treatment: A review. J Clean Prod. 2021;329:129713. 10.1016/j.jclepro.2021.129713.Search in Google Scholar
[10] Long LY, Weng YX, Wang YZ. Cellulose aerogels: synthesis, applications, and prospects. Polymers. 2018;10(6):623. 10.3390/polym10060623.Search in Google Scholar PubMed PubMed Central
[11] Sun H, Xu Z, Gao C. Multifunctional, ultra‐flyweight, synergistically assembled carbon aerogels. Adv Mater. 2013;25(18):2554–60. 10.1002/adma.201204576.Search in Google Scholar PubMed
[12] Jacquemin L, Song Z, Le Breton N, Nishina Y, Choua S, Reina G, et al. Mechanisms of radical formation on chemically modified graphene oxide under near infrared irradiation. Small. 2023;19(16):2207229. 10.1002/smll.202207229.Search in Google Scholar PubMed
[13] Ji K, Gao Y, Zhang L, Wang S, Yue Q, Xu X, et al. A tunable amphiphilic Enteromorpha-modified graphene aerogel for oil/water separation. Sci Total Environ. 2021;763:142958. 10.1016/j.scitotenv.2020.142958.Search in Google Scholar PubMed
[14] Ye Z, Zhao B, Wang Q, Chen K, Su M, Xia Z, et al. Crack‐induced superelastic, strength‐tunable carbon nanotube sponges. Adv Funct Mater. 2023;33(44):2303475. 10.1002/adfm.202303475.Search in Google Scholar
[15] Hu G, Wang J, Liu D, Zhang X, Yu B, Huang T, et al. A Joule-heated carbon nanofiber aerogel-supported catalyst for hydrogen production via methanol steam reforming. Carbon. 2023;214:118311. 10.1016/j.carbon.2023.118311.Search in Google Scholar
[16] Wang S, Peng X, Zhong L, Tan J, Jing S, Cao X, et al. An ultralight, elastic, cost-effective, and highly recyclable superabsorbent from microfibrillated cellulose fibers for oil spillage cleanup. J Mater Chem A. 2015;3(16):8772–81. 10.1039/c4ta07057g.Search in Google Scholar
[17] Shen Y, Li D, Wang L, Zhou Y, Liu F, Wu H, et al. Superelastic Polyimide Nanofiber-Based Aerogels Modified with Silicone Nanofilaments for Ultrafast Oil/Water Separation. Acs Appl Mater Inter. 2021;13(17):20489–500. 10.1021/acsami.1c01136.Search in Google Scholar PubMed
[18] Yu T, Halouane F, Mathias D, Barras A, Wang Z, Lv A, et al. Preparation of magnetic, superhydrophobic/superoleophilic polyurethane sponge: Separation of oil/water mixture and demulsification. Chem Eng J. 2020;384:123339. 10.1016/j.cej.2019.123339.Search in Google Scholar
[19] Zhang H, Ma H, Ma Y, Lou Y, Jiao Y, Xu J. Hierarchical boric acid/melamine aerogel based on reversible hydrogen bonds with robust fire resistance, thermal insulation and recycling properties. Compos Part B-Eng. 2023;252:110507. 10.1016/j.compositesb.2023.110507.Search in Google Scholar
[20] Sun Y, Ye W, Xi J, Chu Y, Xiao H, Wu W. Ultralight and shape recovery bio-based aerogel for oil-water separation. J Environ Chem Eng. 2022;10(6):108822. 10.1016/j.jece.2022.108822.Search in Google Scholar
[21] Zhang M, Jiang S, Li M, Wang N, Liu L, Liu L, et al. Superior stable, hydrophobic and multifunctional nanocellulose hybrid aerogel via rapid UV induced in-situ polymerization. Carbohyd Polym. 2022;288:119370. 10.1016/j.carbpol.2022.119370.Search in Google Scholar PubMed
[22] Leng W, He S, Zhang X, Wang X, Navarathna CM. Biobased aerogels for oil spill remediation. supramolecular gels: materials and emerging applications. 2021;169–213. 10.1002/9783527817009.ch7.Search in Google Scholar
[23] Li Y, Chen X, Liu A, Li X, Xu W, Duan X, et al. Preparation of aerogels from corn stalks and research on their properties and gelation behavior. Ind Crop Prod. 2023;203:117211. 10.1016/j.indcrop.2023.117211.Search in Google Scholar
[24] Koul B, Yakoob M, Shah MP. Agricultural waste management strategies for environmental sustainability. Environ Res. 2022;206:112285. 10.1016/j.envres.2021.112285.Search in Google Scholar PubMed
[25] Linh NTT, Nam NTH, Dat NM, Cong CQ, Viet ND, Trinh DN, et al. Fabrication and antibacterial activity of polyester fabric modified by nanocellulose and graphene oxide-based silver nanoparticles. J Mater Sci. 2022;57(41):19513–31. 10.1007/s10853-022-07864-8.Search in Google Scholar
[26] Xiao P, Cao L, Wang H, Yan G, Chen Q. Rational design of three-dimensional metal-organic framework-derived active material/graphene aerogel composite electrodes for alkaline battery-supercapacitor hybrid device. Surf Interfaces. 2022;33:102266. 10.1016/j.surfin.2022.102266.Search in Google Scholar
[27] Wang W, Yang D, Mou L, Wu M, Wang Y, Tan F, et al. Remodeling of waste corn stalks into renewable, compressible and hydrophobic biomass-based aerogel for efficient and selective oil/organic solvent absorption. Colloid Surface A. 2022;645:128940. 10.1016/j.colsurfa.2022.128940.Search in Google Scholar
[28] Chen Z, Zhan B, Li S, Wei D, Zhou W, Liu Y. Facile fabrication of corn stover-based aerogel for oil/water separation. Sep Purif Technol. 2022;298:121642. 10.1016/j.seppur.2022.121642.Search in Google Scholar
[29] Gong L, Zhu H, Wu W, Lin D, Yang K. A durable superhydrophobic porous polymer coated sponge for efficient separation of immiscible oil/water mixtures and oil-in-water emulsions. J Hazard Mater. 2022;425:127980. 10.1016/j.jhazmat.2021.127980.Search in Google Scholar PubMed
[30] Pawcenis D, Twardowska E, Leśniak M, Jędrzejczyk RJ, Sitarz M, Profic-Paczkowska J. TEMPO-oxidized cellulose for in situ synthesis of Pt nanoparticles. Study of catalytic and antimicrobial properties. Int J Biol Macromol. 2022;213:738–50. 10.1016/j.ijbiomac.2022.06.020.Search in Google Scholar PubMed
[31] Geng H. A facile approach to light weight, high porosity cellulose aerogels. Int J Biol Macromol. 2018;118:921–31. 10.1016/j.ijbiomac.2018.06.167.Search in Google Scholar PubMed
[32] Wang Y, Zeng X, Wang W, Zhou P, Zhang R, Chen H, et al. Superhydrophobic polyimide/cattail-derived active carbon composite aerogels for effective oil/water separation. Sep Purif Technol. 2023;308:122994. 10.1016/j.seppur.2022.122994.Search in Google Scholar
[33] Yan Z, Zhu K, Li X, Wu X. Recyclable bacterial cellulose aerogel for oil and water separation. J Polym Environ. 2022;30(7):2774–84. 10.1007/s10924-021-02369-y.Search in Google Scholar
[34] Peng D, Zhao J, Liang X, Guo X, Li H. Corn stalk pith-based hydrophobic aerogel for efficient oil sorption. J Hazard Mater. 2023;448:130954. 10.1016/j.jhazmat.2023.130954.Search in Google Scholar PubMed
[35] Dilamian M, Noroozi B. Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohyd Polym. 2021;251:117016. 10.1016/j.carbpol.2020.117016.Search in Google Scholar PubMed
[36] Zhao H, Kwak JH, Zhang ZC, Brown HM, Arey BW, Holladay JE. Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis. Carbohyd Polym. 2007;68(2):235–41. 10.1016/j.carbpol.2006.12.013.Search in Google Scholar
[37] Zhang X, Lei Y, Li C, Sun G, You B. Superhydrophobic and multifunctional aerogel enabled by bioinspired salvinia leaf‐like structure. Adv Funct Mater. 2022;32(14):2110830. 10.1002/adfm.202110830.Search in Google Scholar
[38] Gao J, Wang J, Cai M, Xu Q, Zhang J, Cao X, et al. Advanced superhydrophobic and multifunctional nanocellulose aerogels for oil/water separation: A review. Carbohyd Polym. 2023;300:120242. 10.1016/j.carbpol.2022.120242.Search in Google Scholar PubMed
[39] Ye R, Long J, Peng D, Wang Y, Zhang G, Xiao G, et al. Oil/water separation using elastic bio-aerogels derived from bagasse: Role of fabrication steps. J Hazard Mater. 2022;438:129529. 10.1016/j.jhazmat.2022.129529.Search in Google Scholar PubMed
[40] Qu YX, Guo KY, Pan HT, Wu ZH, Guo BF, Feng XL, et al. Facile synthesis of mechanically flexible and super-hydrophobic silicone aerogels with tunable pore structure for efficient oil-water separation. Mater Today Chem. 2022;26:101068. 10.1016/j.mtchem.2022.101068.Search in Google Scholar
[41] Wang Q, Qin Y, Xue C, Yu H, Li Y. Facile fabrication of bubbles-enhanced flexible bioaerogels for efficient and recyclable oil adsorption. Chem Eng J. 2020;402:126240. 10.1016/j.cej.2020.126240.Search in Google Scholar
[42] Song P, Cui J, Di J, Liu D, Xu M, Tang B, et al. Carbon microtube aerogel derived from kapok fiber: an efficient and recyclable sorbent for oils and organic solvents. ACS Nano. 2019;14(1):595–602. 10.1021/acsnano.9b07063.Search in Google Scholar PubMed
[43] Ma X, Zhou S, Li J, Xie F, Yang H, Wang C, et al. Natural microfibrils/regenerated cellulose-based carbon aerogel for highly efficient oil/water separation. J Hazard Mater. 2023;454:131397. 10.1016/j.jhazmat.2023.131397.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

