Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
-
Nor’Izzah Zainuddin
, Ahmad Anas Nagoor Gunny
Abstract
This study concerns with the production and partial characterization of alkaline cellulase from alkaliphilic cellulolytic (AC) fungi isolated fromsoil in Perlis, Malaysia. The best fungi strain was selected on the basis of producing the highest cellulase at high pH conditions. Cellulase from the selected fungi strain was further characterized under saccharification but varies in operating parameters. Finally, the kinetic model describing the growth of the AC fungi strain was studied by employing the logistic model. Among the tested fungi strains, Basidiomycetes strain (BK1) showed high potentiality for the production of maximum alkaline cellulase production at pH 9 after 72 h of incubation at 30°C containing 6 g·L−1 carboxyl methyl cellulose. The saccharification process showed that the enzyme favour high alkaline condition and proves thermotolerant properties, while 15% (v/v) enzyme loading and 1% substrate concentration recorded the highest glucose production at about 1.2–1.3 mg·mL−1. The novelty of the study is to identify and optimize a unique indigenous fungi that emit alkaliphilic cellulase as alternative usage in biotechnology industries due to its capacity to adapt to the extreme conditions of specific industrial processes. There are revolutionary options for use in biotechnological businesses that involve high pH and therefore have substantial biotechnological promise.
1 Introduction
Cellulose has been identified as a rich and limitless supply of raw material for a range of bioproducts. It is the most common organic polymer, making up roughly 1.5 × 1012 tonnes of the entire annual biomass production [1,2]. It is a main component of raw materials such as cotton and wood, as well as the plant cell wall’s principal structural component. Agricultural and forestry wastes, municipal solid waste, herbaceous and woody plants, and unused standing forest are all examples of cellulosic biomass that contain cellulose. Agricultural waste primarily consists of cellulosic matter, which is easily dispatched by a range of physical, chemical, and biological processes [3]. Cellulase is a family of enzymes that can hydrolyze cellulose on 1,4-d-glucan linkages and create glucose, cellobiose, and cello-oligosaccharides as major products. Exoglucanase (EC 3.2.1.91), endoglucanase (EC 3.2.1.4), and β-glucosidase (EC 3.2.1.21) are the three key components of this multicomponent enzyme system [4]. The majority of the cellulases studied and applied in industries are mainly from nonextremophiles, while only a few reports [5,6] deal with the production of the cellulases from extremophiles. Alkaliphiles research has led to the development of a variety of enzymes with unique characteristic. Over the last two decades, the usage of alkaline cellulase has grown significantly in industries such as animal feeds, fabrics, laundry, and biological wood pulping and paper. Alkaliphiles are used to make this alkaline cellulase [7].
In recent years, alkaline cellulases have been used in various industrial and technological applications since the capability and potential of the enzymes become widely attractive due to its ability. Alkaline cellulases from microorganisms have been applied in a wide range of industries such as agriculture, kraft pulping, textile, food and beverage, animal feed, detergent industry, and biorefinery since the enzymes can increase the products quality [8,9]. Indeed, the demand for these enzymes is increasing at a faster rate than that of yeast and bacteria. Cellulases from fungal sources are preferred for commercial enzyme production because the enzymes produced are of high quality [10]. Most of cellulases produced by fungi and bacteria are well known to perform optimally under alkaline and neutral conditions [11]. The study of extremophiles and extremozymes began with alkaliphiles, and Horikoshi [10] is credited with the pioneering work on these enzymes to widen the application of enzyme in industries that deal with the alkaline environment and the condition such as detergent and textile.
There is a need for the enzymes to be stable for a long period during storage [12]. The alkaline conditions created by these industries, on the other hand, frequently impede enzyme activity and stability. Therefore, it is critical to develop cellulase that can withstand alkaline environments. Alkaphilic fungi can synthesize the enzyme because of their ability to secrete enzymes that are active in a high alkaline environment. Novel alkaliphiles and enzyme could be explored from nature and alkaline environment since this condition favours the growth of alkaliphilic fungi [13]. Based on the previous work by Zainuddin et al. [14], the alkaline cellulases had been screened and isolated from alkaliphilic cellulolytic (AC) fungi, and it is expected that the same enzyme would be able to tolerate the alkaline saccharification conditions and will improve the hydrolysis of the lignocellulosic biomass element. The growth of AC fungi has a significant effect on alkaline cellulase capabilities. As a result, models that give consistent growth parameters for AC fungi growth are desired. Often, kinetic modelling enables researchers to identify key aspects such as process output, process efficiency, process control factors and methodologies, specific growth rate, and scale-up concerns for product manufacturing [12,13,14,15,16,17]. The approach of the present study focuses on the production of potential alkaline cellulase enzyme from AC fungi stains and the ability of the cellulase to hydrolyse microcrystalline (MCC) substrate at different concentrations, pH, as well as temperature. The discovery of AC fungi strains that can emit cellulase enzymes and its isolation and identification, as well as the enhancement of cellulase enzyme activity under optimal operating conditions, constitute this research’s uniqueness.
2 Materials and methods
2.1 Sample collection
Sampling points were chosen from three different alkaline areas at UniMAP Agrotech (6.6522°N, 100.2609°E), Institute at Sungai Chuchuh, (6.6493°N, 100.2574°E), Bukit Keteri (6.5174°N, 100.2450°E), and Sungai Jernih (6.5439°N, 100.2684°E). Samples were taken from 10 to 15 cm depth and were sieved through a 2 mm siever. The samples were maintained at 4°C in sterile sampling bags for future use.
2.2 Isolation of AC fungi
Serial dilutions were used to isolate cellulolytic fungi from soil samples on Czapek-Dox agar medium. The medium consisted of (g·L−1) 30 g sucrose, 2 g NaNO3, 1 g K2HPO4, 0.05 g MgSO4, 0.5 g KCl, 0.01 g FeSO4, 15 g agar, and 1% of carboxylmethyl cellulose (CMC). In a sterile centrifuge tube, 1 g of soil sample was added to 10 mL of sterile distilled water and vortex for 2 min on high speed. Dilutions up to 10−6 were prepared, and 100 µL of suspension spreaded on Czapek-Dox agar plates with varying pH values (pH 8, 9, 10, and 11) for 7 days at 30°C. The fungi produced on plates were labelled as BK1 (Bukit Keteri 1), BK2 (Bukit Keteri 2), SC1 (Sg. Chuchuh 1), SC2 (Sg. Chuchuh 2), and BJ1 (Bukit Jernih 1) and inoculated on Potato Dextrose Agar (PDA) plates at 30°C for 7 days to obtain a single colony and stored under 4°C for future use.
2.3 Screening of AC fungi
The hydrolysis capacity (HC) test with Gram’s iodine was used to screen AC fungi [18]. In this approach, a week-old culture fungi was immersed in 15 mL of sterile distilled water. In the middle of Czapek-Dox agar plates (pH 8, 9, 10, and 11), a tiny well was formed, dilutions were made up to 10−6, and 100 µL of spore suspension was injected. The plates were then incubated at 30°C for 48 h. After that, agar plates were flooded for 3–5 min with Gram’s iodine mix, which contained 2.0 g KI and 1.0 g iodine in 300 mL of distilled water. The diameter of the cleared zone was measured and used as an indicator of cellulase activity.
2.4 Optimization of cellulose hydrolysis using one-factor-at-a-time (OFAT) method
Typically, the saccharification process was carried out in 50 mL Erlenmeyer flasks that included microcrystalline cellulose (MCC), enzyme extracts, and certain buffers based on the pH. After that, flasks were incubated with shaking at 180 rpm, and 200 L hydrolysis samples was taken once every 8 h from each flask. Precisely, the OFAT method was used to determine the optimal level of parameters for cellulose hydrolysis. Total enzyme loading, microcrystalline cellulose (MCC) concentration, pH, and temperature were the four key characteristics that could affect cellulase hydrolysis in this study. The optimum total enzyme loading of crude cellulase for the hydrolysis of MCC in 0.05 M glycine–NaOH buffer (pH 9.0) was determined by incubating the mixture of 1% (w/v) MCC with different concentrations of crude enzyme ranging from 0.3 to 1.2 U at 50°C and 180 rpm. To optimize MCC concentration, the study varied from 1% to 4% (w/v). 0.05 M phosphate buffer (pH 7), 0.05 M Tris-HCl buffer (pH 8), and 0.05 M glycine–NaOH buffer (pH 9 and pH 10) were utilised to examine the effects of pH (7–10). The temperature effect was evaluated by varying the saccharification temperature from 30°C to 90°C. All the experimental runs were monitored their sugar production for 48 h (1, 3, 6, 12, 24, 36, and 48 h). The glucose standard curve was constructed according to the study by Miller et al. [19]. Then, cellulase activity was assayed by the DNS method.
2.5 Analytical methods
2.5.1 Determination of fungi biomass
The best four strains resulted from the HC test were grown at pH 9.0, temperature 30°C, and agitated at 150 rpm in the potato dextrose broth (PDB) medium. Each strain was added to broth medium with 10% (v/v) of inoculum concentration with 107 of total spore. The saccharification media was recovered every 24 h and underwent 10 min centrifugal process at 10,000 rpm under 4°C environments. The residual cell (residue) was dried at 70°C for 24 h, and cell’s dry weight was measured to determine the growth activity.
2.5.2 Cellulase assay
The cellulase activity was measured using crude cellulase extracted from clear saccharification supernatant. The cellulase activity was measured using Ghose’s technique [20]. In a test tube, a rolled strip of Whatmen No.1 filter paper measuring 1.0 × 6.0 cm (50 mg) was dipped in 1 mL of glycine–NaOH buffer (0.05 M, pH 9.0). The mixture was then incubated at 50°C for 60 min with 0.5 mL of crude cellulase. After removing the tubes from the water bath, the reaction was halted by adding 3 mL of 3,5-dinitrosalicylic acid reagent (DNS) to each test tube. The tubes were then boiled for 5 min at 95°C for the colour development. After boiling, all the tubes were transferred into a cold-water bath. All reaction mixtures were diluted with distilled water appropriately (0.2 mL of reaction mixture added with 2.5 mL distilled water in a spectrophotometer cuvette). The absorbance of triplicates colour-formed reaction mixtures was measured against spectrometer zero at 540 nm wavelength, and the average value was taken.
2.5.3 Cell phenotypic identification
After 2 days of incubation, the colony was observed under macroscopic characterizations which are the structural of hyphae and basidiospore. One drop of sterile distilled water and a loop of colony were transferred onto a microscope glass slide. The glass slide was heat fixed on Bunsen burner flames several times. Methylene blue was added onto the smear for staining and washed under slow running tap water. The slide was left to air dry; the colony was observed under high-performance microscope for morphological identification. Later, portions of fungi mycelia were taken from the agar dish to a 1.5 mL microcentrifuge tube containing 50 L autoclaved-ultrapure water using a sterile toothpick. The cell suspension was mixed by the vortex method vigorously for 5 min and then incubated at 95°C for 10 min. After that, the microcentrifuge tube was quickly frozen at −20°C for 10 min. For PCR amplification, the suspension was centrifuged at 11,000 rpm for 5 min, and the 5–10 µL of supernatant was used as the DNA template.
2.5.4 Cell molecular and phylogenetic analysis
For PCR amplification in cell molecular and phylogenetic analysis, the test began by designing specific primers for the target gene or region. In a 25 μL reaction, 1 μL of DNA template was combined with forward and reverse primers (0.5 μL each), Taq DNA polymerase (0.25 μL), dNTP mix (1 μL), and 10× PCR buffer (2.5 μL), with MgCl2 adjusted accordingly. Thirty-five cycles of denaturation at 95°C for 30 s, primer-specific annealing for 30 s, and extension at 72°C were carried out for a time based on the expected amplicon size. The process concluded with a final extension at 72°C for 10 min. The PCR products were then verified on an agarose gel, purified using a DNA kit, and sequenced using the ABI 3,730/3,730 × 1 DNA analyzer. Taxonomic identities were confirmed with Basic Local Alignment Search Tool for Nucleotide (BLASTN) against the National Center for Biotechnology Information (NCBI) nonredundant (nr) database, and phylogenetic analysis was conducted using NCBI BLAST Tree View with the Fast Minimum Evolution tree method. TIANquick Mini Purification Kit (TIANGEN, China) was used to purify PCR products as directed by the manufacturer, while ABI 3,730/3,730 × 1 DNA Analyzer was used for DNA sequencing (Life Technologies, USA). The taxonomic identities of the acquired sequences were confirmed against the nr nucleotide database of the NCBI using the BLASTN databases based on nucleotide query 2.2.3 1+. Phylogenetic analysis was conducted using NCBI BLAST Tree View with the fast minimum evolution tree method.
2.6 Kinetic modelling of alkaliphilic celluloytic fungi
2.6.1 Logistic kinetic models
The logistic equation has been used to describe the cell development in several saccharification processes, including the breakdown of cellulose by the AC fungi. Some derivations have been developed to employ the logistic model (Table 1) in the Polymath software, and the model encompasses both the exponential and stationary stages, as shown in Eq. 2 [1,2]:
where X represents the biomass concentration (mg·g−1 substrate) at time t (h), m indicates the maximum specific growth rate (1/h), X o denotes the beginning biomass concentration, and X m marks the maximum biomass concentration. Non-linear regression was used to fit the model to the experimental data. Samsudin and Mat Don et al. [21], Don and Shoparwe [22], Pazouki et al. [23], Elibol and Mavituna [24] also employed these models. The objective is to use mathematical modelling to convert a complex (biological) entity to a simplified (mathematical) version that can be studied more thoroughly with significant properties established. To estimate the initial condition value, linearization was done for the logistic model (Table 1). Experimental result was analyzed using the linearized equations of the model.
Kinetic growth models selected and their linear form
| Logistic model | |
|---|---|
|
|
(3) |
| Integration from t = 0, X = X | |
|
|
(4) |
| Linear form | |
|
|
(5) |
3 Results and discussion
3.1 Isolation and screening of AC fungi
The soil sample was taken from three different identified limestone areas which were Sungai Chucuh, Bukit Keteri, and Bukit Jernih in Perlis. The soil pH of these alkaline areas was tested by the soil pH meter. The pH obtained was above 9.0. The fungi from the soils were grown on the petri dish containing CMC agar. After 7 days of incubation, there were five different species observed. To have a pure strain of the species, the isolation method for the samples was done and cultured on the PDA. The collected samples were named based on areas which were BK – Bukit Keteri, BJ – Bukit Jernih, and SC – Sg. Chucuh. These five sample strains were used for further study. Figure 1 shows the observation of AC cultivation on the petri dish.
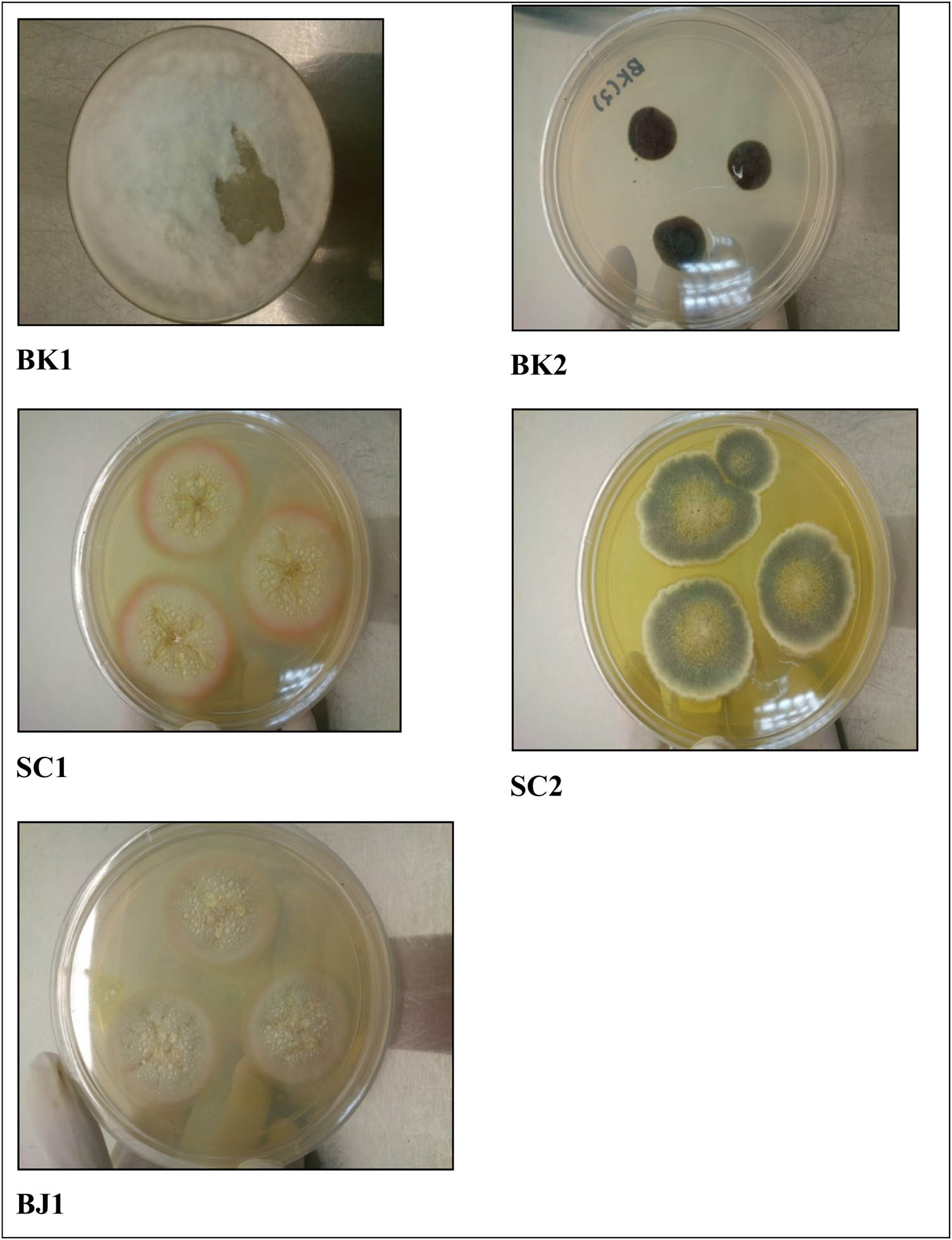
Isolated fungi from CMC agar plates.
3.2 Evaluation of AC fungi in producing alkaline cellulases
Determination of AC strain was done by calculating the HC on different pH of CMC agar plates. The higher value of HC shows the ability of the strain tolerant with a high pH medium. In this study, the collected sample strain was tested, and Aspergillus niger (strain from Bioprocess Laboratory, UniMAP) was used as the comparison factor. These five sample strains were then exposed to plate screening on CMC agar media at pH 8, 9, 10, and 11 using Gram’s iodine as a colouring agent. When Gram’s iodine was added to colonies, they became discoloured, as shown in Figure 2. The ability of the strain to release cellulase enzyme at high pH was demonstrated by the hydrolyzed zone around the colonies. The clearing zone around the colonies, which corresponds to the zone of CMC degradation, can also be utilized to estimate HC as shown in Eq. 1 for further comparative testing between the strains. The HC of isolated fungi is depicted in Table 2.

Clearing zone on CMC agar plate.
HC of isolated fungi in different pH
| Strains | HC | |||
|---|---|---|---|---|
| CMC media pH 8 | CMC media pH 9 | CMC media pH 10 | CMC media pH 11 | |
| BK 1 | 1.6778 ± 0.32 | 1.8074 ± 0.19 | 1.5297 ± 0.25 | 1.3148 ± 0.23 |
| BK 2 | 1.0815 ± 0.21 | 0.6963 ± 0.09 | — | — |
| SC 1 | 1.2735 ± 0.18 | 1.2272 ± .0.30 | 1.2276 ± 0.16 | 1.1218 ± 0.11 |
| SC 3 | 1.2124 ± 0.21 | 1.2284 ± 0.16 | 0.8335 ± 0.27 | 1.2367 ± 0.19 |
| BJ 1 | 1.1484 ± 0.17 | 1.0906 ± 0.22 | 1.1415 ± 0.16 | 1.0682 ± 0.31 |
Based on the results obtained, there are only five sample strains that give positive results. These five strains (BK1, BK2, SC1, SC2, and BJ1) exhibit higher HC values compared to A. niger (commercial strain) within the studied range of pH levels. This exemplifies the alkaliphilic traits of the fungi, which favours high pH conditions for both growth and activity [25]. Furthermore, the breakdown of cellulose at high pH conditions suggests that these fungi have the capacity to release extracellular alkaline-tolerant cellulase. Nevertheless, Ahamed and Vemette claim that this qualitative screening was insufficient to draw a conclusion regarding the success of alkaline cellulase production because only a small number of cellulolytic microorganisms were capable of depolymerizing crystalline cellulose effectively [26]. Furthermore, Liang et al. found no real relationship between cellulolytic indicator and cellulase activity in a variety of liquid media containing CMC and other cellulosic components. Liang and his colleagues also stated that the enzyme generated by these strains was insufficient to detect when cultured in the liquid medium or that the fungi's ability to produce CMC was ineffective when cultivated on agar plates [27]. Agrawal, on the other hand, discovered that the length of the hydrolyzing domain may not correctly reflect the actual cellulase activity [28]. A much more precise quantitative cellulase screening is required to determine the enzymatic degradation of cellulose into fermentable sugar.
3.3 Production of alkaline cellulases bysaccharification
The best AC fungi strains then were further screened by analyzing alkaline cellulases produced under the saccharification process. The sample AC fungi strains were cultivated at 30°C for 7 days in PDB media with a pH of 9 and a mixture of CMC as a carbon source, with varying concentrations of CMC ranging from 1% to 4% employed in the saccharification medium. Figures 3–5 prove that BK1 strain was the best cellulose-forming fungi where with all the tested conditions, BK1 recorded the highest cellulase activity with 0.03 U·mL−1 (second day on 2 g·L−1 CMC concentration), 0.0463 U·mL−1 (fourth day on 4 g·L−1 CMC concentration), and 0.0532 U·mL−1 (third day on 6 g·L−1 CMC concentration). Furthermore, Zahra et al. investigated the optimal CMCase activity and filter paper cellulase activity after 4 days of incubation at 30oC in media containing 5% substrate concentration, 0.05% peptone, and 0.5% KH2PO4 concentration [29]. This also explains the behaviour and the role of CMC as a carbon source in improving the enzyme activity. It was reported that CMC as a substrate induced the production of cellulases by activating cellulose regulatory protein called the cellulase activator molecule [30]. While BJ1 recorded the lowest performance among all the strains with the highest for BJ1 were 0.029 U·mL−1 (2nd day on 2 g·L−1 CMC concentration), 0.0250 U·mL−1 (2nd day on 4 g·L−1 CMC concentration), and 0.02 U·mL−1 (2nd day on 6 g·L−1 CMC concentration). The observed low cellulase production in the study may be attributed to a combination of factors, including inherent variability in cellulase production capabilities among different fungal strains (BK1 and BJ1), suboptimal concentrations of carboxymethyl cellulose (CMC) as a substrate, and potential limitations in the experimental conditions. The strain-specific genetic backgrounds of BK1 and BJ1 could influence cellulase gene expression, impacting enzymatic productivity [31]. In addition, the chosen CMC concentrations may not have been optimal for cellulase induction, and further substrate optimization might be necessary. The relatively short cultivation period of 7 days might not provide sufficient time for some strains to reach peak cellulase production. Nutrient availability in the PDB medium and the absence of essential micronutrients could also play a role.

Average value of cellulase activity (U·mL−1) of strains under 2 g·L−1 of CMC concentration after triplicate.

Average value cellulase activity (U·mL−1) of strains under 4 g·L−1 of CMC concentration after triplicate.

Average value cellulase activity (U·mL−1) of strains under 6 g·L−1 of CMC concentration after triplicate.
3.4 Optimization of cellulase activity (HC) using OFAT method
3.4.1 Effect of different total enzyme loading
The effect of total enzyme loading (5–20%) on the hydrolyzing cellulose by AC BK1 fungi was studied (Figure 6). The lowest glucose concentration was observed (0.2–0.6 mg·mL−1) after 60 h saccharification using 5% and 20% enzyme loading. This might be because the enzyme's ability was insufficient to fully saccharify the cellulose, resulting in a diluted concentration of the enzyme available for saccharification. While saccharification with total enzyme loading at 15% illustrated a high rate of cellulose conversion to glucose around 1.2–1.3 mg·mL−1. A similar trend was observed for parameter 10% enzyme loading, and this was due to the enzyme having the sufficient amount of active site to interact with the substrate. However, the conversion of cellulose using enzyme loading at 10% recorded around 0.8–0.9 mg·mL−1 of glucose. The results showed that alkaline cellulase degraded cellulose, producing oligosaccharides that may enter the cell and induce cellulase expression and glucose production [31]. It was proven that high total enzyme loading affected the glucose production as the more enzymes used was not efficient due to high competition between the enzyme and thus leading to ineffective substrate–enzyme complex.

Glucose production at different of total enzyme loading.
3.4.2 Effect of different microcrystalline cellulose concentration
The effect of microcrystalline cellulose concentration (1–4%) of the characterization on the hydrolyzing cellulase by AC BK1 fungi was studied (Figure 7). Using 4% concentration of microcrystalline cellulose rapidly boosted glucose concentration at 0.9 mg·mL−1 within 4 h of the saccharification process. This may be due to the huge availability of cellulosic material in media, and thus, hydrolyzing the rate becomes effective. However, after 8 h of saccharification, using 4% concentration microcrystalline had decreased to 0.8 mg·mL−1 glucose concentration conversion. This may be due to the saturated active site of the enzyme, and thus, low performance of the conversion occurred [32]. While using 3% MCC concentration produced the least glucose concentration (around 0.6 mg·mL−1) within 4 h of the saccharification process. In addition, the saccharification using 1% microcrystalline cellulose concentration led to the production of glucose once beyond the 8 h characterization. This was most probably because the enzyme still had an empty active site for the substrate to attach and simplify it to become glucose [33].

Glucose production at different microcrystalline cellulose concentrations.
3.4.3 Effect of different pH
The effect of pH (7–10) of the enzymatic saccharification medium on the hydrolyzing cellulose by AC BK1 fungi was studied (Figure 8). At the pH values of 7.0 and 8.0, they recorded the lowest glucose concentration around 1.15–1.2 mg·mL−1, which was observed after 24 h of saccharification at pH of the medium between 7 and 8. As for the saccharification medium at pH 9, it produced a high rate of cellulose conversion to glucose (1.2–1.25 mg·mL−1; 24% increment), after 4–8 h of the saccharification process. But it slightly dropped at 12 h of saccharification. It may be due to the limited active site of enzyme alkaline cellulase. Saccharification with pH 10 obtained highest glucose concentration with 1.35 mg·mL−1 after 24 h. This proves that this AC fungi favours and is active under alkaline condition [29]. Therefore, it is important that the saccharification is operated at the optimum pH condition to obtain the highest production (glucose). A similar observation was reported by other researchers who used alkaline cellulose from fungi strains.

Glucose production at different pH values.
According to Vega et al. [25], the cellulolytic fungi extracted from the Peru tropical rain forest shows the activity performing well at pH 9.4 (Penicillium sp. LM-HP06 (12.9 U·g−1·h−1), Penicillium sp. LM-HP34 (13.8 U·g−1·h−1). It demonstrates how these species have a strong aptitude for cellulose conversion under alkaline conditions. When compared to other alkaline cellulases, the endo-ß-glucanase from the yeast Rhodotorula glutinis was generally stable at a pH range of 2–9, whereas alkaliphilic Bacillus sp No 1139 was stable up to pH 11 [34].
3.4.4 Effect of different temperature
The effect of saccharification temperature (30–60°C) on the hydrolyzing cellulose by AC BK1 fungi was studied (Figure 9). There were gradual increases in the production of glucose concentration for all the runs. The highest generation of glucose concentration was obtained at temperature of 50°C with 1.28 mg·mL−1 after 24 h characterization. This proves the ability of the cellulase to tolerate high temperature [35]. Additionally, when applied to difficult industrial environments, the extremophile's ability to stabilise under extreme conditions adds value. Other than the above, saccharification of alkaline cellulase BK1 at 30°C illustrates low production of glucose concentration (0.73 mg·mL−1). This could be due to the need for temperature to boost the reaction rate and hydrolyzing rate of alkaline cellulase. Too high temperatures do not ensure the increased cellulase conversion activity. Temperature-sensitive enzyme components, such as protein, may suffer irreparable damage. This is described in the saccharification graph at 60°C. According to Kasana et al. [36]. R. glutinis may grow at temperatures ranging from 4°C until slightly below 30°C. The best temperature for growth was 20°C, but its cellulase was active at temperatures ranging from 4°C to 70°C, with a peak at 50°C which was proven in Bai et al.’s research, thermophilic cellulase from Penicillium sp. CR316 has an optimal temperature of 65°C, and the activity is steady at that temperature for 3 h [37]. In comparison to other alkaline cellulases, the optimum temperature of cellulase activity was discovered to vary depending on the organism. While alkaline cellulases from thermophilic species functioned better at higher temperatures, cellulases from Bacillus sp. KSM-635 displayed highest the activity at 40°C, identical to the cellulase in this experiment [38]. Aside from that, alkaline cellulases from thermophilic organisms performed best at high temperatures, such as 60°C for both Bacillus stearothermophilus and Clostridium josui alkaline cellulases [39].

Glucose production at different temperatures.
3.5 Kinetic modelling of akaliphilic celluloytic fungi
3.5.1 Logistic kinetic model
For the kinetic expression, the logistic mathematical model was applied as shown in Figure 10. It was adjusted to the AC fungi biomass experimental results to investigate the BK1 growth. The model accurately illustrated the lag, exponential, and stationary phases. To calculate the EB’s specific growth rate (µ), the highest biomass, X m = 0.4261 mg biomass·g−1 sample from the experimental observations was used, and a plot of linear (Eq. 5) provided µ = 1.053 (day−1). Then, the initial lag period of the current investigation was extended to 6–12 h. The extent of the lag phase can be influenced by a variety of factors, including medium composition (broth), the types and maturities of the AC fungi strain, the number of cells, and physical variables such as temperature and pH [27,28,29]. The AC fungi initiate an exponential phase after a lag phase, which begins between 1 and 5 days. The maximal EB biomass was estimated to be 29.65 mg·g−1 by the logistic model. The AC fungi biomass ceased expanding exponentially once the limiting cellulose ingredient in the broth began to diminish, followed by the stationary phase. The X m–X o of BK1 was 0.399 after 7 days saccharification, and HC proved that it had the highest ability for degradation of cellulose. It could be concluded that the production of enzyme secreted by AC BK1 fungi was very conducive and effective. The POLYMATH software’s data prediction likewise coincided with the experimental output, with R 2 and RMSE values of 0.9963 and 0.32, respectively, as shown in Table 3.

Simulated and experimental data for alkaline cellulase-producing (AC) BK1 fungi growth.
Kinetic parameter obtained from the Logistic models for AC fungi growth
| Kinetic parameters | AC, BK1 |
|---|---|
| X o | 0.027 |
| μ m | 1.053 |
| X m | 0.4261 |
| X m− X o | 0.399 |
| R 2 | 0.9963 |
| RMSE | 0.0032 |
3.5.2 Phylogenetic analysis
Four main samples of isolated AC fungi were sent to Microbiology Company, Genomics BioSci and Tech, Ltd., Taiwan, and the determined species were to be from the genus Basidiomycetes (BK1), Trametes (SC1), Trametes (SC2), and Penicillium (BJ1). Researchers believe that the presence of Basidiomycetes (BK1) species in the saccharification of cellulose can help the hydrolysis mechanism, as the secretion of enzyme cellulolytic enhanced the conversion cellulose to glucose, while for Trametes (SC1) and (SC2), it had proven by the researcher that Trametes secretes laccase which helps in hydrolyzing the cellulose and hemicellulose. Meanwhile, for Penicillium (BJ1) species, research managed to discover their potential for the cellulosic activity by comparing Penicillium with other fungi such as Trichoderma reesei species [40]. The four species’ phylogenetic trees are presented in Figures 11–14. The presence of these four species in this study proved that they had promising abilities that could be explored deeper in the future study.

Phylogenetic trees for BK species (Query_10143).

Phylogenetic trees for BJ species (Query_58523).

Phylogenetic trees for SC1 species (Query_55781).

Phylogenetic trees for SC2 species (Query_49237).
4 Conclusions
The present work attempted to obtain optimum alkaline cellulase conditions using batch saccharification. The objectives of these studies were achieved by combining the experimental work, kinetic studies, and the modelling approach. A pure AC fungi strain with capability to produce the highest alkaline cellulase among the four positive samples was successfully isolated from Bukit Keteri (BK1) area. It consisted of highest amount of HC (pH 8: 1.6778, pH 9: 1.8889, pH 10: 1.5297, pH 11: 1.3148) result compared to Sg Chuchuh SC1 (pH 8: 1.2735, pH 9: 1.2273, pH 10: 1.2276, pH 11: 1.1218), SC2 (pH 8: 1.2124, pH 9: 1.2284, pH 10: 1.2305, pH 11: 1.2367), and Bukit Jernih (pH 8: 1.1484, pH 9: 1.0906, pH 10: 1.1415, pH 11: 1.0682), respectively. Diverse microbial communities of fungi are capable of secreting alkaline cellulase (ability to degrade the cellulose), and phylogenetic analysis proved the presence of genus Basidiomycetes (BK1), Trametes (SC1), Trametes (SC2), and Penicillium (BJ1). They actively secreted cellulase which plays an important role in hydroxylation of cellulose in the saccharification. The one-factor-at-one-time (OFAT) method was used to identify the ideal conditions for cellulase activity in the saccharification flask utilising AC fungi. The cellulase enzyme isolated from the BK1 AC fungus strain favoured and was active at high alkaline conditions (pH 9 and pH 10) according to the research results. In addition, the enzyme was still active at high temperatures, supporting its ability to withstand extreme heat. The greatest glucose generation from the saccharification process was also seen at 15% (v/v) enzyme loading and 1% microcrystalline cellulose concentration. The logistic model accurately represented the growth behaviour of AC fungi in saccharification, with R 2 varying from 0.9923 to 0.9987 and RMSE ranging from 0.0018 to 0.0066. From the findings, alkaline cellulase produced from the newly isolated alkaphilic cellulolytic fungi strain may have considerable potential for industrial application especially when operating in extreme conditions owing to its useful properties.
Acknowledgement
The authors would like to thank the Ministry of Education for financing the research under the Fundamental Research Grant Scheme (FRGS/1/2018/STG01/UNIMAP/03/3), Universiti Malaysia Perlis for the financial support of this study via the Research University Grant, a scholarship (MyMasters) for the first author from the Ministry of Higher Education Malaysia. The authors are grateful to the Researchers Supporting Project Number (RSP2023R326), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: The research was supported by the Fundamental Research Grant Scheme (FRGS/1/2018/STG01/UNIMAP/03/3). The authors are grateful to the Researchers Supporting Project Number (RSP2023R326), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Conceptualization, NIZ, MMZM, AANG, SCBG; writing – original draft preparation, NIZ, MMZM; writing – review and editing, AAA, KP, MRS, MA, MR; supervision, MMZM, AANG, MR; funding acquisition, MMZM and MR. All authors have read and agreed to the submitted version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Mohd Azmi N. The effect of different pretreatment of chicken manure for electricity generation in membrane-less microbial fuel cell. Catalysts. 2022;12(8):810. 10.3390/catal12080810.Search in Google Scholar
[2] Bhardwaj N, Kumar B, Agrawal K, Verma P. Current perspective on production and applications of microbial cellulases: A review. Bioresour Bioprocess. 2021;8(1):10. 10.1186/s40643-021-00447-6.Search in Google Scholar
[3] Saranraj P, Stella D, Reetha D. Microbial cellulases and its applictions: A review. Int J Biochem Biotech Sci. 2012;4(2):1–12. https://www.ijpcbs.com/articles/applications-of-cellulases--review.pdf.Search in Google Scholar
[4] Percival Zhang YH, Himmel ME, Mielenz JR. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol Adv. 2006;24(5):452–81. 10.1016/j.biortech.2013.12.101.Search in Google Scholar PubMed
[5] Gunny AAN, Arbain D, Edwin Gumba R, Jong BC, Jamal P. Potential halophilic cellulases for in situ enzymatic saccharification of ionic liquids pretreated lignocelluloses. Bioresour Technol. 2014;155:177–81. 10.1016/j.biortech.2013.12.101.Search in Google Scholar
[6] Li X, Yu HY. Purification and characterization of an organic-solvent-tolerant cellulase from a halotolerant isolate, Bacillus. sp L1. J Ind Microbiol Biotechnol. 2012;39(8):1117–24. 10.1007/s10295-012-1120-2.Search in Google Scholar PubMed
[7] Ravindran C, Naveenan T, Varatharajan GR. Optimization of alkaline cellulase production by the marine-derived fungi Chaetomium sp. using agricultural and industrial wastes as substrates. Bot Mar. 2010;53(3):275–82. 10.1515/BOT.2010.026.Search in Google Scholar
[8] Ben Hmad I, Gargouri A. Neutral and alkaline cellulases: Production, engineering, and applications. J Basic Microbiol. 2017;57(8):653–8. 10.1002/jobm.201700111.Search in Google Scholar PubMed
[9] Kamarkar M, Ray RR. Current trend in research and application of microbial cellulases. Res J Microbiol. 20116:41–53. 10.3923/jm.2011.4153.Search in Google Scholar
[10] Ejaz U, Sohail M, Ghanemi A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics. 2021;6(3):1–11. 10.3390/biomimetics6030044.Search in Google Scholar PubMed PubMed Central
[11] Fernández-López MG, Batista-García RA, Aréchiga-Carvajal ET. Alkaliphilic/Alkali-Tolerant Fungi: Molecular, Biochemical, and Biotechnological Aspects. J Fungi. 2023;9(6):652. https://doi.org/10.3390/jof906065210.3390/jof9060652Search in Google Scholar PubMed PubMed Central
[12] Horikoshi K. Production of alkaline enzymes by alkaliphilic microorganism part l alkaline protease produced by bacillus no. 221. Agric Biol Chem. 1971;35(9):1407–14. 10.1080/00021369.1971.10860094.Search in Google Scholar
[13] Fujinami S, Fujisawa M. Industrial applications of alkaliphiles and their enzymes Past, present and future. Environ Technol. 2010;31(8–9):845–56. 10.1080/09593331003762807.Search in Google Scholar PubMed
[14] Zainuddin N, Rahman KA, Shaari AR, Yusof SHM, Gunny AAN, Arbain D. The potential of alkaliphiliccellulases in the development of in-situ enzymatic hydrolysis of alkaline pretreated lignocellulosic biomass. Adv Environ Biol. 2014;8(22):90–3.Search in Google Scholar
[15] Wachenheim DE, Patterson JA, Ladisch MR. Analysis of the logistic function model: Derivation and applications specific to batch cultured microorganisms. Bioresour Technol. 2003;86(2):157–64. 10.1016/S0960-8524(02)00149-9.Search in Google Scholar PubMed
[16] Muaz MZM, Abdul R, Vadivelu VM. Recovery of energy and simultaneous treatment of dewatered sludge using membrane-less microbial fuel cell. Environ Prog Sustain Energy. 2019;38(1):208–19. 10.1002/ep.12919.Search in Google Scholar
[17] Shamsuddin NA, Mohd Sabri MNI, Tajarudin HA, Shoparwe NF, Makhtar MMZ. Effect of thermal pre-treatments method on sludge degradation process prior usage in membrane-less microbial fuel cell for electricity generation. IOP Conf Ser Earth Environ Sci. 2021;765(1):012092. 10.1088/1755-1315/765/1/012092.Search in Google Scholar
[18] Goel R, Mino T, Satoh H, Matsuo T. Enzyme activities under anaerobic and aerobic conditions in activated sludge sequencing batch reactor. Water Res. 1998;32(7):2081–8. 10.1016/S0043-1354(97)00425-9.Search in Google Scholar
[19] Miller GL, Blum R, Glennon WE, Burton AL. Measurement of carboxymethylcellulase activity. Anal Biochem. 1960;1(2):127–32. 10.1016/0003-2697(60)90004-X.Search in Google Scholar
[20] Ghose T. Measurement of cellulase activity. Int J Sel Assess. 1987;3(4):245–7. 10.1351/pac198759020257.Search in Google Scholar
[21] Samsudin MDM, Mat Don M. Assessment of bioethanol yield by S. cerevisiae grown on oil palm residues: Monte Carlo simulation and sensitivity analysis. Bioresour Technol. 2015;175:417–23. 10.1016/j.biortech.2014.10.116.Search in Google Scholar PubMed
[22] Don MM, Shoparwe NF. Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochem Eng J. 2010;49(1):95–103. 10.1016/j.bej.2009.12.001.Search in Google Scholar
[23] Pazouki M, Najafpour G, Hosseini MR. Kinetic models of Asp Fumigatus. 2008;7(9):1369–76. 10.5897/AJB2016.15747.Search in Google Scholar
[24] Elibol M, Mavituna F. A kinetic model for actinorhodin production by Streptomyces coelicolor A3(2). Process Biochem. 1999;34(6–7):625–31. 10.1016/S0032-9592(98)00136-8.Search in Google Scholar
[25] Vega K, Villena GK, Sarmiento VH, Ludeña Y, Vera N, Gutiérrez-Correa M. Production of alkaline cellulase by fungi isolated from an undisturbed rain forest of peru. Biotechnol Res Int. 2012;2012:934325. 10.1155/2012/934325.Search in Google Scholar PubMed PubMed Central
[26] Ahamed A, Vermette P. Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem Eng J. 2008;40(3):399–407. 10.1016/j.bej.2007.11.030.Search in Google Scholar
[27] Liang YL, Zhang Z, Wu M, Wu Y, Feng JX. Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27-1. Biomed Res Int. 2014;2014:512497. 10.1155/2014/512497.Search in Google Scholar PubMed PubMed Central
[28] Agrawal S. Cellulases of bacterial origin and their applications: A review. Int J Sci Res. 2014;3(10):1652–5.Search in Google Scholar
[29] Zahra T, Irfan M, Nadeem M, Ghazanfar M, Ahmad Q, Ali S, et al. Cellulase production by trichoderma viride in submerged fermentation using response surface methodology. Punjab University. J Zool. 2020;35(2):223–8. 10.17582/journal.pujz/2020.35.2.223.228.Search in Google Scholar
[30] Gunny AAN, Arbain D, Jamal P, Gumba RE. Improvement of halophilic cellulase production from locally isolated fungi strain. Saudi J Biol Sci. 2015;22(4):476–83. 10.1016/j.sjbs.2014.11.021.Search in Google Scholar PubMed PubMed Central
[31] Hakamada Y, Koike K, Yoshimatsu T, Mori H, Kobayashi T, Ito S. Thermostable alkaline cellulase from an alkaliphilic isolate, Bacillus sp. KSM-S237. Extremophiles. 1997;1(3):151–6. 10.1007/s007920050028.Search in Google Scholar PubMed
[32] Kuhad RC, Gupta R, Singh A. Microbial cellulases and their industrial applications. Enzyme Res. 2011;2011:280696. 10.4061/2011/280696.Search in Google Scholar PubMed PubMed Central
[33] Cunningham L. The Structure and Mechanism of Action of Proteolytic Enzymes. Compr Biochem. 1965;16(8):85–188. 10.1016/j.sjbs.2014.11.021.Search in Google Scholar
[34] Sulyman AO, Igunnu A, Malomo SO. Isolation, purification and characterization of cellulase produced by Aspergillus niger cultured on Arachis hypogaea shells. Heliyon. 2020;6(12):05668. 10.1016/j.heliyon.2020.e05668.Search in Google Scholar PubMed PubMed Central
[35] Nelofer R, Nadeem M, Irfan M, Syed Q, Nawaz S, Tahir A. Conversion of wheat straw into fermentable sugars using carboxymethyl cellulase from Trichoderma Viride through box-behnken design and artificial neural network. J Microbiol Biotechnol Food Sci. 2021;10(4):626–30. 10.15414/jmbfs.2021.10.4.626-630.Search in Google Scholar
[36] Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr Microbiol. 2008;57(5):503–7. 10.1007/s00284-008-9276-8.Search in Google Scholar PubMed
[37] Bai H, Zi H, Huang Y, Han M, Irfan M, Liu N, et al. Catalytic properties of carboxymethyl cellulase produced from newly isolated novel fungus Penicillium ochrochloron ZH1 in submerged fermentation. Catal Lett. 2017;147(8):2013–22. 10.1007/s10562-017-2119-0.Search in Google Scholar
[38] Mohd Zaini Makhtar M, Tajarudin HA, Samsudin MDM, Vadivelu VM, Shoparwe NF, Izzah Zainuddin N. Membrane-less microbial fuel cell: Monte Carlo simulation and sensitivity analysis for COD removal in dewatered sludge. AIP Adv. 2021;11(6):065016. 10.1063/5.003901.Search in Google Scholar
[39] Malik NNA. Mixture of sludge and chicken manure in membrane-less microbial fuel cell for simultaneous waste treatment and energy recovery. Catalysts. 2022;12(776):1–28. 10.3390/catal12070776.Search in Google Scholar
[40] Martins LF, Kolling D, Camassola M, Dillon AJP, Ramos LP. Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresour Technol. 2008;99(5):1417–24. 10.1016/j.biortech.2007.01.060.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

