Abstract
Spheroidal particles constitute the primary particulate signature within coal gasification fine slag (FS) residues. This research endeavor is centered on elucidating the physical configuration and intrinsic attributes of these spheroidal particles and deducing their primary genesis mechanisms through the application of optical microscopy (OM), scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX), transmission electron microscopy, and field-emission scanning electron microscopy. Notably, the spheroidal particles prevalent in FS predominantly comprise inorganic constituents like aluminum, silicon, and calcium. These particles exhibit a relatively uniform morphology with smooth exteriors and a spectrum of sizes. They predominantly manifest in encapsulated, adhered, and dispersed structural forms. The formation trajectory of these spheroidal particles encompasses multiple phases, encompassing the melting of the ash matrix derived from coal, the coalescence of the liquid phase, internal nucleation processes, layer-by-layer deposition, the bridging and aggregation of minute particles, and growth via precursor-mediated pathways.
1 Introduction
Coal gasification technology stands as a prominent component within the contemporary coal chemical industry chain, with its advancements and stability serving as pivotal factors in ensuring the seamless progression of gasification projects [1,2,3]. Gasification fine slag (FS), a by-product of the gasification process, holds immense significance in understanding FS’s formation mechanisms and potential applications. Undergoing intricate physical and chemical transformations, FS demonstrates characteristic patterns in particle distribution, morphology, elemental composition, and microstructure, manifesting consistently and predictably.
Figure 1 illustrates the production process of FS within a Shell gasifier. Raw coal particles are introduced into the gasifier and undergo rapid pyrolysis, leading to the expansion and fragmentation of the coal particles, the consumption of carbonaceous matrix components, and the formation of minute ash particles. A fraction of these particles accumulate on the gasifier wall and are collected from the descending molten slag stream along the furnace perimeter, ultimately depositing into the slag removal system. Within this system, the molten slag solidifies into particles of diverse sizes, commonly termed coarse slag in industrial terminology. Another segment of the particulate matter migrates from the gasifier’s apex into the raw syngas stream. A ceramic filter intercepts fly ash generated during washing or captured by dust removal mechanisms. The remaining uncaptured particles blend with black water in the cooling chamber and are subsequently conveyed to a filter press, undergoing a flash evacuation process to form FS, ultimately [4,5,6].

Schematic of Shell coal gasification.
Due to the high temperatures and pressures encountered during the production of FS, its composition and particle morphology exhibit consistent and predictable characteristics. Several researchers have scrutinized the particle morphology of FS, considering the multifaceted processes that slag undergoes within gasification furnaces, including particle fragmentation, ash agglomeration, and slag deposition phenomena [7]. The inorganic constituents of FS primarily comprise crystalline phases such as silicates, aluminosilicates, calcium–iron compounds, and iron oxides, alongside glassy phases like lime–iron–aluminosilicate glass. Furthermore, FS is recognized for its elevated carbon content [8].
Comparatively, fly ash represents the fine particulate matter captured from flue gases during coal combustion in coal-fired power stations. During the combustion process, minerals present within the coal, such as quartz, feldspar, and clay minerals, melt at elevated temperatures, followed by rapid quenching, forming minute vitreous particles. Fly ash particles typically exhibit spherical or porous spherical morphologies characterized by smooth surfaces and a predominantly vitreous nature, often enriched with high oxide compositions. While the macroscopic mechanisms underlying the formation of both spherical particles share broad similarities, distinct differences in reaction temperature, reaction atmosphere, and reactor configuration affect the generation pathways of FS and fly ash [9,10,11].
Due to the intricate nature of the constituents within FS, the transformation process from raw coal to FS entails the evolution of individual components and the intricate interplay between carbon and inorganic components. The molten phase, composed of abundant mineral particles within the gasifier, envelops the carbonaceous material present within the particles, thereby facilitating a complex interaction and transformation [12]. Some scholars have observed that the ore-rich particles within FS consist of low-porosity solid particles with a minimal presence of large pores. These large pores are isolated within the particles, accompanied by cracks and pores on their surfaces. Some ore-rich particles exhibit a relatively smooth surface morphology, whereas others display small particles embedded within the surface. Additionally, some aspects within these particles are uniformly distributed. The primary composition of the ore-rich particles in FS is aluminum silicate. Besides discrete residual carbon particles, two other forms of residual carbon are present within the ore-rich particles. The first form is embedded within the matrix of the ore-rich particles, and the second form is chemically and physically associated with inorganic compounds within the matrix. The main functional groups of the residual carbon within the matrix of ore-rich particles include C–C, C–H, and C–O, with the C–O groups chemically bonding with inorganic elements to form C–O–M groups (where M denotes the inorganic elements present in the ore-rich particles) [13,14,15]. There are several kinds of ash in FS: (A) some ash is wholly separated from the residual carbon, (B) some ash is on the surface of the residual carbon, (C) some ash is filled in the pores of the residual carbon matrix, and (D) some of the ash and residual carbon are melted together [16].
FS comprises numerous spherical particles [17], manifesting as minute, closed spheres encapsulated by fine flocculent material [18]. Various scholars have extensively studied the occurrence state of these spherical particles. Xu et al. [19] observed that the inorganic matter primarily adopts a spherical morphology, whereas the carbon residue exists in dispersed flocculent structures. Inorganic components tend to coalesce into larger, carbon-free spheres upon melting and integrating into the carbon matrix. The catalyst developed by Dong et al. [20] through FS primarily consists of SiO2 and silicate, with its surface adorned with numerous flocculent carbon structures derived from molten metal compounds exhibiting a spherical configuration. Melting experiments conducted on fine carbon ash mixtures substantiated that inorganic components tend to coalesce into larger, carbon-free spheres upon melting within a carbon powder matrix [21]. Acid leaching treatment of FS results in a rough surface, with the carbon film completely disappearing post-combustion, leaving the surface of spherical particles with a rugged texture. A fraction of the pellets fractured, adopting irregular shapes. Numerous smaller particles are arranged in a mosaic pattern within the larger spheres, with some small spheres detaching to create spherical pits on the surface [22]. The elemental distribution within most spheres exhibits uniformity, with organic carbon being integrated into the interior of the inorganic matrix. This structural characteristic arises due to the gasification process, where the gasification temperature surpasses the ash flow temperature in the raw coal. Consequently, a more significant proportion of ash is exposed to the ambient environment, leading to softening as carbon is consumed. Subsequently, the slag envelops the coke particles, resulting in a tight encapsulation of the carbon particles by the slag [23].
Despite numerous scholars offering detailed descriptions of FS and acknowledging the presence of spherical particles, their discussions on these particles’ physical attributes and microscopic states remain cursory. The primary contributions of this paper are as follows:
Various morphological characterization techniques were employed to delineate the intrinsic properties of the gasification FS produced in an operational Shell gasification plant.
An in-depth investigation was conducted into spherical particles’ morphological characteristics and formation mechanisms, with a particular emphasis on their unique morphologies.
The formation pathway of spherical particles is elucidated as follows: melting of the ash matrix within coal, liquid phase condensation, internal nucleation, layer-by-layer deposition, “bridging” and aggregation among smaller particles, and subsequent growth.
2 Experimental
The flowchart of the whole procedure is shown in Figure 2.

Flowchart of the whole investigation procedure.
Step 1: Determination of the basic properties of FS, including “industrial analysis, elemental analysis, chemical composition, and particle size distribution interval.”
Step 2: Various morphological characterization methods were used to describe the intrinsic characteristics of the gasification FS produced in the actual Shell gasification plant.
2.1 Sample preparation
Table 1 shows the proximate and ultimate analysis of FS. FS is obtained from a Shell gasifier in China. The gasification unit is in regular operation. Pre-determined quantities of FS are loaded into a high-temperature muffle furnace and subjected to heating at 815 ± 15°C until complete ashing is achieved, as determined by a constant residual mass (FSA). Various components of FSA are present as oxides, as shown in Table 2. The highest content is SiO2, followed by Al2O3, CaO, and Fe2O3. MgO, Na2O, K2O, and P2O5 are present in small amounts.
Proximate analysis and ultimate analysis of FS (d*, wt%)
| Sample | Proximate analysis | Ultimate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| A** ± 0.50% | V*** ± 0.30% | FC**** | C ± 0.50% | H ± 0.15% | O***** | N ± 0.08% | S ± 0.10% | |
| FS | 42.95 | 2.72 | 54.33 | 52.69 | 0.38 | 1.18 | 0.54 | 2.25 |
*Dry basic; **A – ash; ***V – volatile component; ****FC – fixed carbon; *****Minusing.
Chemical composition analysis of ash in FS (wt%)
| Sample | SiO2 | Al2O3 | CaO | Fe2O3 | SO3 | MgO | K2O | TiO2 | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| FSA | 49.23 | 24.47 | 10.02 | 8.52 | 3.54 | 1.01 | 0.98 | 1.53 | 0.39 | 0.31 |
The particle size of the pretreated FS is analyzed, and the results are shown in Figure 3. FS exhibits the characteristic “three-peak distribution.” This indicates a certain coherence for FS formation. The first, second, and third peaks range from 0.5 to 5 μm, 5 to 30 μm, and >30 μm, respectively, with the particle sizes of the samples overlapping.

Particle size distribution curves.
2.2 Experimental instruments and methods
The particle size of FS was analyzed using a laser particle size analyzer (Mastersizer 2000, Malvern, England). The chemical composition analysis of FSA was analyzed by an X-ray fluorescence spectrometer (XRF, Axios mAX, PANalytical, Netherlands).
Various morphological characterization methods were used to describe the intrinsic characteristics of the FS produced in the actual Shell gasification plant.
Transreflectance optical microscopy (OM, DM2700, Leica, Germany): sequential drying, sampling, placing onto a slide, immersing in cedar oil, and observation for FS were performed.
Scanning electron microscopy-X-ray energy dispersive analysis (SEM-EDX, Tescan VEGA 3 SBH-BRUKER XFlash 6|30, Germany): sequential drying, sampling, fixing, conductive treatment, and observation for FS were performed.
Transmission electron microscopy (TEM, Tecnai G2 F20, United States): This sequential drying, sampling, uniform dispersion, particle loading, and observation for FS were performed.
Field emission scanning electron microscopy (FESEM, Zeiss G500, Germany): sequential drying, sampling, dispersion, fixing, conductive treatment, and observation for FS were performed.
3 Results and discussion
3.1 Macroscopic morphology analysis of particles in FS
The coexistence of carbon and inorganic components in FS results in diverse optical refractive indices within the pine tar. The optical properties of the various fine constituents can be discerned by analyzing the transmitted and reflected light under a microscope, mainly focusing on the inorganic components. The microscopic examination of the oil mirror of the fine particles, as depicted in Figure 4, reveals that the macroscopic morphology of the oil mirror primarily exhibits two distinct colors. One color corresponds to a dark component, primarily comprising irregular particles of residual carbon. The other color represents a high-reflectivity component, exhibiting a more pronounced reflection and predominantly consisting of varying-sized spherical particles. These spherical particles occur in various physical forms, including encapsulation (Figure 4a), attachment (Figure 4b and c), and scattering (Figure 4d).

Optical microscopic characteristics of oil impregnation in FS.
In Figure 5a and b, localized regions are randomly sampled to derive comprehensive particle statistics. The particle sizes within these regions are observed to exhibit variability and dispersion. The macrostructure of the produced FS can be broadly classified into two primary categories, encompassing individual spherical particles of varying sizes. Specifically, the maximum diameters of the spherical particles observed in Figure 5a and b are approximately 45 and 63 μm, respectively.

Overall particle size distribution in FS.
Further analysis of the FS particles was conducted through SEM-EDX morphology characteristics and microchemical composition distribution, as shown in Figure 6. Figure 6a1 and a2 predominantly exhibits spherical particles, while Figure 6a3–a9 shows irregular particles. Based on each region’s elemental composition, the FS’s main constituents are Si, Al, Ca, Fe, and C. The average mass proportion of the spheroidal particles (Figure 6a1, a2) is m C:m O:m Si:m Al:m Fe:m Ca ≈ 11:39:25:13:6:6. Upon comparison of the element composition of irregular particles, they can be categorized into three types: (1) high C type (Figure 6a5, a6, and a7), (2) high Fe type (Figure 6a3, a8, and a9), and (3) other types (Figure 6a4).

Microscopic chemical composition of FS.
Furthermore, it is hypothesized that spheroidal particle formation occurs subsequent to coal ash’s reaction within the gasifier. The primary constituents of these spheroidal particles are silicon aluminum oxide, potentially encapsulating or incorporating a minor quantity of carbonaceous materials during their formation. The mass fraction of carbon elements in high C-type particles can reach up to 77.86% (Figure 6a7), potentially stemming from unreacted or incompletely reacted carbon matrices in the raw coal. In high Fe-type particles, the mass ratio of Fe to C exceeds 65%, suggesting an enrichment of these elements in the FS. This enrichment may be attributed to (1) the partial reaction of raw coal particles and (2) the encapsulation of a certain quantity of carbon matrix by inorganic components within coal particles. The elemental composition of other coal particles does not significantly differ from that of other coal particles or the raw coal itself, implying that the enrichment phenomenon may be linked to these factors.
3.2 Microscopical characteristics of spheroidal particles
Based on the morphological characteristics of FS particles, the proportion of spherical particles is notably high, and their morphology and elemental composition exhibit a substantial degree of uniformity and predictability. Further investigation of the morphological features of these particles using advanced, high-precision magnification instruments and techniques can offer specific insights into their genesis. Figure 7 illustrates the TEM morphological features, whereas Figures 8 and 9 depict the elemental mapping diagrams of spherical particles obtained through FESEM.

Morphological characteristics of spheroidal particles.
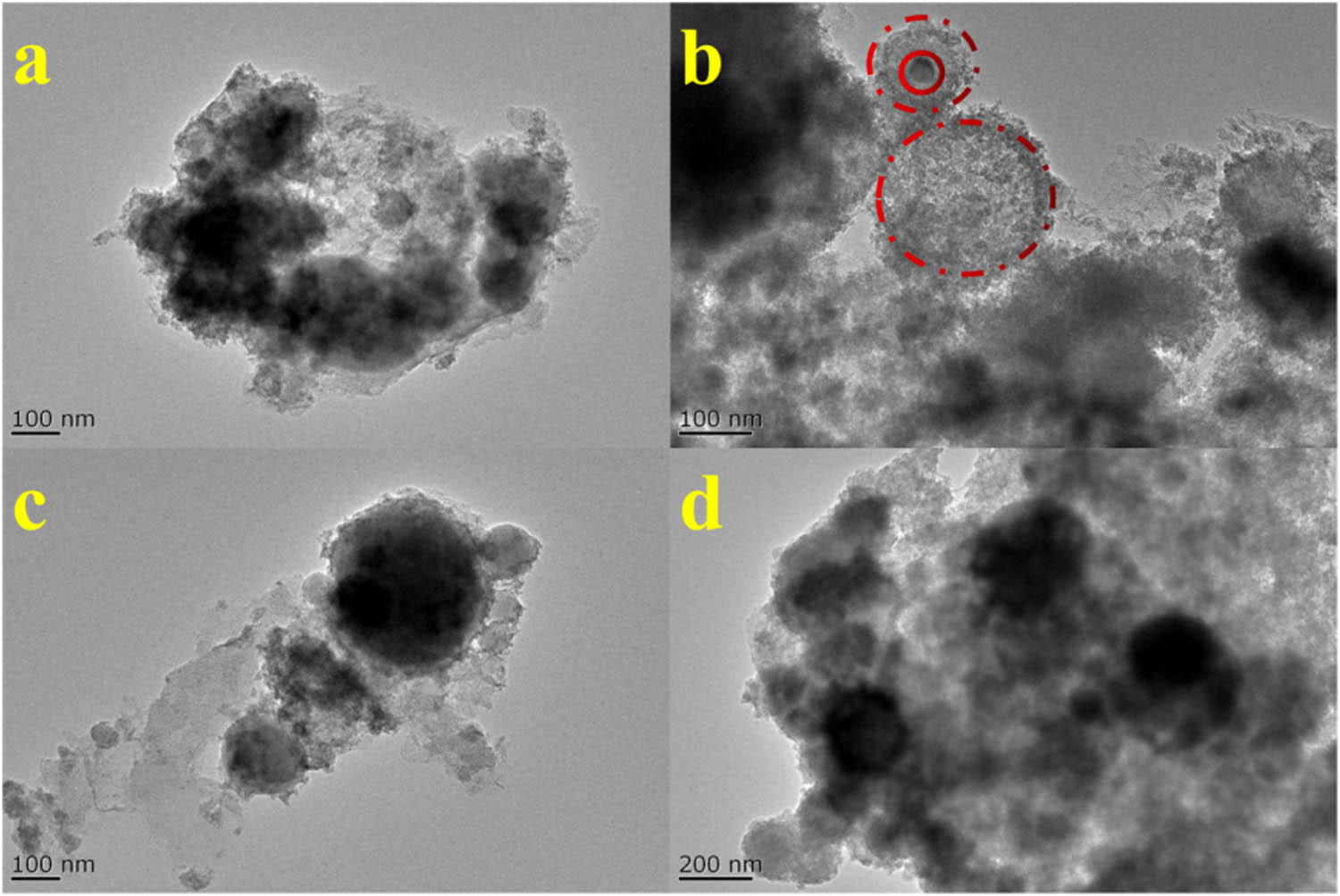
TEM image of spheroidal particles.

TEM image of spheroidal particles.
In Figure 7a–i, the morphology of the particles is predominantly spherical, with both adhered and isolated spherical particles being observed. The diameters of these spherical particles exhibit variation, with the surfaces of the larger particles appearing rough and non-smooth (Figure 7a, b, and d). Some smoother surfaces display scattered small and large particles at various locations (Figure 7c), accompanied by varying protrusions. Additionally, there are regions where molten material covers another part but is not fully fused, leading to scattered white areas with varying sizes and protrusions. Partial depressions can be discerned on the surface of some spherical particles (Figure 7c), or a pattern resembling the precursor of spherical particles is evident (Figure 7e). These observations indicate that the formation of spherical particles is not direct but rather the result of specific physical and chemical processes.
Upon further magnification to observe the smaller-sized spherical particles (Figure 7f), numerous pits and smaller particles are revealed on their surfaces (Figure 7h and i) while maintaining their spherical shape. This suggests that the formation of large-sized spherical particles is not solely a result of direct ash reaction. Instead, it is plausible that smaller particles accumulate and enrich during the reaction process, progressively growing into larger particles. The pits on the surface have undergone a specific degree of reaction, adopting a shape reminiscent of a “precursor.” Considering the ambient environment and elemental composition, the spherical particles within the FS consist primarily of inorganic components, specifically silicon-aluminum minerals, which undergo high temperatures and gradually melt, enhancing the liquid phase content. Under the influence of microscopic surface tension, the formation of smaller particles accumulates and enriches, ultimately culminating in the generation of large-sized spherical particles.
To gain deeper insights into the characteristics of spherical particles within FS, TEM was utilized to examine the ultrastructure of particles smaller than 0.2 μm, as depicted in Figure 8. TEM provides a means to visualize FS at the ultrastructural level, offering high-resolution images. Figure 8a illustrates that spherical particles appear as darker regions, surrounded by smaller flocs of varying sizes and thicknesses. These particles undergo a stage where a distinct core forms and is progressively enveloped by additional flocs, facilitating continued growth.
Figure 8b uncovers two distinct modes of spherical particle formation: (1) precursor formation initiated by smaller spherical particles possessing an internal core, characterized by nucleation and growth as the primary mechanisms, and (2) the accumulation of numerous floccules under the influence of microscopic forces, such as the van der Waals force, which prevails between particles in solid, liquid, or gaseous states. The boundaries of small flocculent particles adhere and accumulate due to these microscopic forces.
Figure 8c and d demonstrate that during a specific formation stage, interactions between spheroidal particles persist, with flocculent particles aggregating at the edges. This leads to the adhesion of multiple spheroidal particles, ultimately resulting in the formation of larger-sized particles. These observations suggest a complex interplay between nucleation, growth, and aggregation processes in forming spherical particles within FS.
Additional analysis of relatively autonomous spherical particles is presented in Figure 9. Regions 1, 2, and 3 of Figure 9a–c, respectively, exhibit the presence of needle-like flocculations interspersed between two or more spherical particles. These flocculations are primarily governed by intermolecular forces, leading to the formation of “microscopic bridging” phenomena among multiple particles.
Figure 9d–f demonstrate that finer particles adhere to the surface of larger particles sequentially, layer-by-layer, thereby facilitating the progressive growth of spherical particles. Concurrently, as illustrated in Figure 9g–i, spherical particles exhibit a “shell” phenomenon characterized by layer thicknesses of approximately 1 nanometer (nm), which resides at the atomic-scale microscopic level. This observation further substantiates the existence of a core–shell structure within the spherical particles.
Under the influence of intermolecular forces, the core–shell structure progressively adheres to smaller particles in a layered fashion, promoting their growth. Additionally, it displays a “bridging” effect between particles of relatively comparable sizes, thereby enhancing interparticle interactions and fostering their continued growth.
The utilization of FESEM provides a clear and comprehensive understanding of the elemental distribution on the sample’s surface. Figures 10 and 11 illustrate the elemental composition of the surface of spherical particles within FS. The primary constituents of these particles are O, Si, Al, C, Ca, and Fe, with O, Si, and Al collectively comprising approximately 90% of the total mass. The elemental distribution exhibits a uniform pattern, suggesting that the spherical particles are homogeneous and share a similar formation pathway. However, upon closer inspection of the individual elemental distributions, notable differences emerge, particularly in the proportion of carbon elements. This observation implies that during the thermal conversion process of raw coal ash, the O, Si, Al, Ca, and Fe elements undergo fragmentation and melting, creating a distinct carbon matrix that encapsulates the particles. Consequently, variations in the carbon content among the spherical particles are evident, underscoring the unique nature of their formation process.

Surface elemental distribution of spheroidal particle 1.

Surface elemental distribution of spheroidal particle 2.
3.3 Analysis of formation mechanism
Under high-temperature conditions in the gasification process, the inorganic components exhibit a propensity for phase transitions and aggregation, influenced by hydrodynamic and reaction kinetic factors. Specific constituents within coal ash possess glass-forming capabilities, melting at elevated temperatures to form a vitreous substance that encapsulates and immobilizes other components, ultimately forming spherical particles. The surface tension effect during the melting of minerals in coal ash facilitates the formation of spherical particles. These particles undergo fusion, solidification, and growth processes to achieve spherical shapes. The genesis of spherical particles in FS is a consequence of the combined effects of melting and polymerization. Consequently, the growth mechanism of these spherical particles involves intricate chemical reactions within a multi-component medium. Relevant researchers have emphasized that the smooth-surfaced spherical particles observed in FS are a product of rapid cooling of SiO2, Al2O3, CaO, and other slag components at high temperatures. Notably, the spherical particles in FS are predominantly formed from fully melted particles, as cited in previous studies [8,24,25].
The spherical particles in FS exhibit a high degree of regularity, prompting a detailed examination of their growth process, as depicted in Figure 12. The ash matrix within FS undergoes crushing, heating, and melting procedures, condensing the liquid phase into spherical shapes due to surface tension effects. Through intermolecular forces, smaller particles adhere to the surfaces of larger particles, forming a core–shell structure. This structure serves as the precursor for internal nucleation, where formed precursors, such as small molecules or ion clusters, provide the essential materials and conditions for crystal nucleation. A single layer of molecules or ions deposits onto the particle surface through multiple cycles, forming a submicron or nanometer-scale layered geometric structure.

The growth process of spheroidal particles in FS.
Additional flocculent materials encapsulate the particle boundaries, with needle-like flocculent particles accumulating at the edges, causing multiple spherical particles to adhere to each other. This layered adhesion continues, contributing to the formation of larger particles. Meanwhile, a “bridging” effect occurs among small particles between spherical particles of relatively similar sizes. The interaction between two or more small molecules or ions forms a connected structure, facilitating the aggregation of the dispersed system and enhancing inter-particle interactions.
The formation pathway of spherical particles in FS can be summarized as follows: melting of the ash matrix in coal, liquid phase condensation, internal nucleation, layered stacking, “bridging” and aggregation among small particles, and subsequent growth.
3.4 Application scenario analysis
To advance the development of efficient coal gasification technologies, the research and removal of FS represent a pivotal direction for future exploration. The insights garnered from this study hold promise across multiple facets. The utilization strategy for FS resources will necessarily vary according to their composition and inherent properties. A more nuanced and qualitative utilization of FS can be achieved by first elucidating the characteristics of its constituent components. FS’s physicochemical attributes and mineralogy constitute the cornerstone for its resourceful and high-value applications. FS demonstrates considerable potential for deployment in various domains, including building materials, fillers, and catalyst supports, as depicted in Figure 13. By leveraging its diverse physicochemical properties and mineral endowments, FS can be harnessed for innovative and high-impact applications, thereby contributing to the sustainable utilization of coal-derived by-products.
FS boasts particle sizes within the micron–nanoscale range. As particle size diminishes, alterations in the surface structure and reactivity of the particles occur, resulting in an augmentation of the specific surface area. Micron–nanopowdered particles exhibit distinctive surface effects, minor side effects, quantum effects, and macroscopic quantum tunneling effects, collectively imparting them exceptional magnetic, mechanical, chemical, and surface and interface properties.
FS contains many spherical particles, which are advantageous in material synthesis and building material production. Specifically: (a) In these contexts, the spherical particles in FS effectively fill pores, enhancing material compactness and ultimately bolstering product strength and durability. (b) The spherical particles in FS can be more uniformly dispersed within composites, mitigating stress concentration points and improving overall material properties, such as compressive strength and toughness. For instance, they can serve as fillers in polypropylene and polyethylene with beneficial effects. (c) The uniformity and stability of spherical particles lend themselves to excellent controllability during processing, including powder compaction molding, mixing uniformity, and sintering density.
FS is abundant in elements such as silicon, aluminum, and calcium, with SiO2 and Al2O3 being prominent chemical constituents akin to those in Portland cement clinker. It exhibits robust hydration activity and pozzolanic activity. Furthermore, its particle size distribution demonstrates a specific gradation, rendering it well-suited as an aggregate and admixture in building materials, construction engineering, and concrete production [26,27,28,29,30].

Resource utilization of FS.
Potential industrial applications include: (a) enhancing resource utilization efficiency by facilitating the extraction of valuable elements and compounds, such as carbon and silicon, from gasification FS through advanced separation and recovery techniques. (b) Leveraging the distinctive characteristics of spherical particles within FS to innovate and develop new materials exhibiting exceptional performance metrics, such as mechanical strength, durability, and reactivity. (c) Utilizing comprehensive insights from research on FS morphology to optimize and streamline industrial production processes, enhancing productivity and cost-effectiveness. (d) Expanding the scope and diversity of resourceful utilization pathways for FS while mitigating environmental pollution and promoting sustainable industrial practices.
4 Conclusion
FS comprises numerous spheroidal particles, predominantly consisting of inorganic constituents such as aluminum, silicon, and calcium. These particles exhibit a relatively uniform shape, with smooth surfaces and various sizes. Encapsulation, attachment, and dispersion patterns encapsulate their primary morphological features.
During the crushing and thermal melting of the ash matrix within coal, the liquid phase coalesces into spherical shapes due to surface tension and intermolecular forces, initially manifesting as precursor forms of internal nucleation. Many needle-like and flocculent particles aggregate at the periphery of the spheroidal particles, resulting in mutual adhesion among multiple particles and progressive surface layering. This leads to the formation of larger particles. Additionally, spheroidal particles of similar sizes are interconnected by smaller particles, forming a cohesive structure that enhances the aggregation of the dispersed system and facilitates further growth of the spheroidal particles.
FS’s physicochemical attributes and mineralogy constitute the cornerstone for its resourceful and high-value applications. FS demonstrates considerable potential for deployment in various domains, including building materials, fillers, and catalyst supports.
-
Funding information: The completion of this work and related results received support from the Anhui University of Science and Technology (Grant No. 2023yjrc90), the Postgraduate Innovation Fund of Anhui University of Science and Technology (Grant No. 2021CX1002), and the National Natural Science Foundation of China (Grant No. 22408004).
-
Author contributions: Yijin Li: methodology and writing – original draft; Lirui Mao: conceptualization, methodology, and data curation.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Mao L, Li H, Zhang Y, Wu C. Preparing coal water slurry from BDO tar to achieve resource utilization: combustion process of BDO tar-coal water slurry. Energy Fuels. 2019;10(33):10297–10306. 10.1021/acs.energyfuels.9b02479.Search in Google Scholar
[2] Baoliang X, Chengli W, Xingzhao Z, Hanxu L, Zhiguo L, Facun J. Regulation of ash-fusion behaviors for high ash-fusion-temperature coals in the Huainan & Huaibei mining area by flux addition. Solid Fuel Chem. 2022;56(4):304–13. 10.3103/S0361521922040036.Search in Google Scholar
[3] Mao L, Zheng M, Li H. Acceleration effect of BDO tar on coal water slurry during co-gasification. Energy. 2022;262:125432. 10.1016/j.energy.2022.125432.Search in Google Scholar
[4] Cao J, Zhang W, Li Y, Zhao C, Zheng Y, Yu B. Current status of hydrogen production in China. Prog Chem. 2021;33(12):2215–44. 10.7536/PC201128.Search in Google Scholar
[5] Wang H, Cheng L, Pu J, Zhao J. Melting characteristics of coal ash and properties of fly ash to understand the slag formation in the Shell gasifier. ACS Omega. 2021;6(24):16066–75. 10.1021/acsomega.1c01949.Search in Google Scholar PubMed PubMed Central
[6] Shen Z, Nikolic H, Caudill LS, Liu K. A deep insight on the coal ash-to-slag transformation behavior during the entrained flow gasification process. Fuel. 2021;289:119953. 10.1016/j.fuel.2020.119953.Search in Google Scholar
[7] Pan C, Liang Q, Guo X, Dai Z, Liu H, Gong X. Characteristics of different sized slag particles from entrained-flow coal gasification. Energy Fuels. 2016;30(2):1487–95. 10.1021/acs.energyfuels.5b01326.Search in Google Scholar
[8] Wu S, Huang S, Wu Y, Gao J. Characteristics and catalytic actions of inorganic constituents from entrained-flow coal gasification slag. J Energy Inst. 2015;88(1):93–103. 10.1016/j.joei.2014.04.001.Search in Google Scholar
[9] Yadav VK, Yadav KK, Tirth V, Jangid A, Gnanamoorthy G, Choudhary N, et al. Recent advances in methods for recovery of cenospheres from fly ash and their emerging applications in ceramics, composites, polymers and environmental cleanup. Crystals. 2021;11(9):1067. 10.3390/cryst11091067.Search in Google Scholar
[10] Mor S, Vig N, Mehta SK, Ravindra K. Physico‑chemical characterization of coal combustion by‑products derived from thermoelectric power plants. Environ Dev Sustain. 2024. 10.1007/s10668-024-05317-7.Search in Google Scholar
[11] Zhang X, Wu G, Yao T, Zhang C, Yue Y. Characterization of individual fly ash particles in surface snow at Urumqi Glacier No. 1, Eastern Tianshan. Chin Sci Bull. 2011;56(32):3464–73. 10.1007/s11434-011-4684-8.Search in Google Scholar
[12] Ding L, Zhou Z, Guo Q, Wang Y, Yu G. In situ analysis and mechanism study of char-ash/slag transition in pulverized coal gasification. Energy Fuels. 2015;29(6):3532–44. 10.1021/acs.energyfuels.5b00322.Search in Google Scholar
[13] Miao Z, Chen L, Chen K, Zhang X, Zhang Y, Wu J. Physical properties and microstructures of residual carbon and slag particles present in fine slag from entrained-flow coal gasification. Adv Powder Technol. 2020;31(9):3781–9. 10.1016/j.apt.2020.07.019.Search in Google Scholar
[14] Miao Z, Guo F, Zhao X, Guo Z, Guo Y, Zhang Y, et al. Effects of acid treatment on physicochemical properties and gasification reactivity of fine slag from Texaco gasifier. Chem Eng Res Des. 2021;169:1–8. 10.1016/j.cherd.2021.01.020.Search in Google Scholar
[15] Miao Z, Wu J, Zhang Y, Zhao X, Guo F, Guo Z, et al. Chemical characterizations of different sized mineral-rich particles in fine slag from Entrained-flow gasification. Adv Powder Technol. 2020;31(9):3715–23. 10.1016/j.apt.2020.07.010.Search in Google Scholar
[16] Guo F, Zhao X, Guo Y, Zhang Y, Wu J. Fractal analysis and pore structure of gasification fine slag and its flotation residual carbon. Colloids Surf A: Physicochem Eng Asp. 2020;585:124148. 10.1016/j.colsurfa.2019.124148.Search in Google Scholar
[17] Dai G, Zheng S, Wang X, Bai Y, Dong Y, Du J, et al. Combustibility analysis of high-carbon fine slags from an entrained flow gasifier. J Environ Manag. 2020;271:111009. 10.1016/j.jenvman.2020.111009.Search in Google Scholar PubMed
[18] Jiang P, Xie C, Luo C, Meng W, Yang G, Yu G, et al. Distribution and modes of occurrence of heavy metals in opposed multi-burner coal-water-slurry gasification plants. Fuel. 2021;303(3):121163. 10.1016/j.fuel.2021.121163.Search in Google Scholar
[19] Xu S, Zhou Z, Gao X, Yu G, Gong X. The gasification reactivity of unburned carbon present in gasification slag from entrained-flow gasifier. Fuel Process Technol. 2009;90(9):1062–70. 10.1016/j.fuproc.2009.04.006.Search in Google Scholar
[20] Dong Y, Mao S, Guo F, Shu R, Bai J, Qian L, et al. Coal gasification fine slags: Investigation of the potential as both microwave adsorbers and catalysts in microwave-induced biomass pyrolysis applications. Energy. 2021;238(9):121867. 10.1016/j.energy.2021.121867.Search in Google Scholar
[21] Krishnamoorthy V, Tchapda A, Pisupati S. A study on fragmentation behavior, inorganic melt phase formation, and carbon loss during high temperature gasification of mineral matter rich fraction of Pittsburgh No. 8 coal. Fuel. 2017;208:247–59. 10.1016/j.fuel.2017.06.134.Search in Google Scholar
[22] Zhang J, Zuo J, Jiang Y, Zhu D, Zhang J, Wei C. Kinetic analysis on the mesoporous formation of coal gasification slag by acid leaching and its thermal stability. Solid State Sci. 2019;100:106084. 10.1016/j.solidstatesciences.2019.106084.Search in Google Scholar
[23] Miao Z, Wu J, Zhang Y, Zhao X, Guo F, Guo Z, et al. Physicochemical characteristics of mineral-rich particles present in fine slag from entrained-flow gasifiers. Energy Fuels. 2020;34(1):616–23. 10.1021/acs.energyfuels.9b02732.Search in Google Scholar
[24] Li L, Liu J, Li X, Peng Z, Han C, Lian W, et al. Physicochemical characteristics of residual carbon and inorganic minerals in coal gasification fine slag. Molecules. 2024;29(16):3956. 10.3390/molecules29163956.Search in Google Scholar PubMed PubMed Central
[25] Kukushkin S, Osipov AV, Redkovav A. Morphological stability criterion for a spherical crystallization front in a multicomponent system with chemical reactions. Phys Solid State. 2014;56(12):2530–6. 10.1134/S106378341412018X.Search in Google Scholar
[26] Guo F, Guo Y, Chen L, Jia W, Zhu Y, Li Y, et al. Multitudinous components recovery, heavy metals evolution and environmental impact of coal gasification slag: A review. Chemosphere. 2023;338:139473. 10.1016/j.chemosphere.2023.139473.Search in Google Scholar PubMed
[27] Yan S, Xuan W, Cao C, Zhang J. A review of sustainable utilization and prospect of coal gasification slag. Environ Res. 2023;238(2):117186. 10.1016/j.envres.2023.117186.Search in Google Scholar PubMed
[28] Li Y, Wei C, Liu X, Zhang Z, Wan J, He X. Application of gasification slag in construction materials and high value-added materials:a review. Constr Bulid Mater. 2023;402:133013. 10.1016/j.conbuildmat.2023.133013.Search in Google Scholar
[29] Ren L, Ding L, Guo Q, Gong Y, Yu G, Wang F. Characterization, carbon-ash separation and resource utilization of coal gasification fine slag: A comprehensive review. J Clean Prod. 2023;398:136554. 10.1016/j.jclepro.2023.136554.Search in Google Scholar
[30] Su S, Tahir MH, Cheng X, Zhang J. Modification and resource utilization of coal gasification slag-based material: A review. J Environ Chem Eng. 2024;12(2):112112. 10.1016/j.jece.2024.112112.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

