Abstract
An improved, greener synthesis of motexafin gadolinium (MGd), a promising radiosensitizer and imaging agent that has the potential to treat or diagnose cancer, has been developed. Notably, this study addresses critical difficulties related to MGd synthesis and analysis of possible approaches, minimizing hazardous reagents/solvents, without column chromatography. The synthesis has been improved to enhance the overall yield and purity of MGd. The process changes outlined in this study are intended to lower production costs and time while maintaining the compound’s characteristics. This synthesis of MGd is an important step toward attaining its full potential in the diagnosis and treatment of cancer.
Graphical abstract

1 Introduction
Cancer remains a severe threat to human health around the world, demanding the development of novel treatment strategies that not only improve therapeutic efficacy but also reduce related side effects. The motexafin gadolinium (MGd) {4,5-diethyl-10,23-dimethyl-9,24-bis(3-hydroxypropyl)-16,-17-bis(3,hydroxypropyl)oxy-13,20,25,26,27-pentaazapentacyclo-[20.2.1.13,6.19,11.014,19]heptacosa-3,5,8,10,12,14,16,18,20,22,24-undecaene gadolinium} is a molecule in the texaphyrin class known as the Xcytrin as shown in Figure 1. MGd has emerged as a feasible candidate for improving the outcome of cancer radiotherapy. MGd is a small molecule that accumulates exclusively in tumor cells, making it a suitable radiosensitizer with the potential to boost the tumor-killing effects of radiation therapy while sparing healthy surrounding tissues. This one-of-a-kind property of MGd has drawn much interest in its ability to improve cancer treatment since it has the potential to improve cancer treatment efficacy [1].

Chemical structure of MGd.
These compounds have shown tremendous promise in terms of sensitizing tumor cells to ionizing radiation. The mechanism underlying radiosensitization involves the production of reactive oxygen species in the presence of oxygen, which can induce DNA damage and cell death when integrated with radiation therapy. Furthermore, the capacity of MGd to preferentially concentrate in tumor tissues by binding to biologically vital targets such as tumor vasculature and cell membranes contributes to its appeal as a radiosensitizer [2].
The MGd is a water-soluble gadolinium complex and clinical derivative, that exhibits notable selectivity for uptake and retention within tumors. This discernible selectivity is supported by extensive preclinical research and findings from early-stage clinical trials [3]. Extensively researched for its magnetic resonance imaging (MRI) detectability, MGd has been the subject of comprehensive studies involving both X-ray radiation therapy and, more recently, direct use as a chemotherapeutic agent, either independently or in combination with other anticancer agents [4]. This strategy offers an additional benefit, as it enables the creation of conjugates featuring both cleavable and non-cleavable linkages. This versatility allows for a more comprehensive evaluation of the significance of drug release. Furthermore, these conjugates present an added advantage by potentially facilitating tumor-localized imaging and therapeutic activities specifically at targeted tumor sites [1].
MGd can selectively localize to cancerous lesions, as confirmed through various methods, including MRI. Recently, this core has been employed in the development of functionalized magnetic nanoparticles as dual-mode MRI contrast agents and concurrent hyperthermia agents [5]. Hence, MGd will be an ideal partner for novel hybrids to be effective multifunctional agents for cancer imaging and treatment, as shown in Figure 2.

Hybridization approach using MGd for dual effect.
To date, various studies have been conducted to investigate the potential of MGd, mostly in preclinical models and early-phase clinical trials. However, the literature only reports small-scale synthesis, which involves a complex and lengthy process with multiple steps, stringent reaction conditions, and column purifications. This results in lower overall yield due to intermediate purification steps and the use of hazardous chemicals that generate waste. Therefore, we attempted to alleviate safety concerns and mitigate isolation issues for lab scale-up by simplifying the synthesis process, reducing time and cost, and minimizing the need for intermediate purification by avoiding column purification. Our focus included using environmentally benign solvents and reagents, optimizing one-pot methods, exploring novel catalysts and reaction conditions, developing advanced purification methods to efficiently remove impurities, and utilizing techniques like crystallization and distillation. This article attempts to address this gap by focusing on the initial process development of MGd and contributes to the increasing knowledge of this promising radiosensitizer. Moreover, the successful development of MGd as a radiosensitizer is of paramount importance in the field of cancer therapy, as it can significantly improve the prognosis and quality of life of cancer patients.
2 Experimental section
2.1 Preparation of compound 8
To a stirred mixture of compound 3 (131.5 g, 1.16 mol, 1 eq) and compound 6 (220 g, 1.16 mol, 1 eq) that were dissolved in dry toluene (700 ml) under an N2 atmosphere, DBU (300 ml, 2.32 mol, 2 eq) was added slowly and the solution was stirred for 16 h. The precipitation of white solid was observed. TLC shows completion of the reaction, R f = 0.4 (10% EtOAc in Pet ether). The reaction mixture was neutralized by 1 N HCl until the pH of the solution became 7. The organic layer was separated and washed with water. To the toluene layer containing compound 7, 10% aq NaOH (500 ml) was added and refluxed for 2 h. TLC shows completion of the reaction, R f = 0.25 (50% EtOAc in Pet ether). The reaction mass was allowed to cool to room temperature and the organic layer was separated. To the aqueous layer, conc. HCl was added at 0°C to form a white solid. The obtained solid was collected by filtration followed by water wash. The obtained solid dried in a vacuum to furnish compound 8 (91 g, 47%) as a white solid (which turns brown color on standing for a long time). 1H NMR (DMSO-d 6, 400 MHz): δ 1.03 (t, 3H), 1.08 (t, 3H), 2.32 (q, 2H), 2.61 (q, 2H), 6.61 (d, 1H), 11.0 (1H, br), 11.9 (1H, br).
2.2 Preparation of compound 9
Compound 8 (90 g, 0.538 mol, 1 eq) was sublimed at 160°C for 2 h. TLC shows completion of the reaction, R f = 0.8 (50% EtOAc in Pet ether). Compound 9 (53 g, 80%) was isolated by fractional distillation as a light brown liquid (moderately unstable, stored in the freezer, solidified at −20°C). 1H NMR (CDCl3, 400 MHz): δ 1.25 (t, 6H), 2.49 (q, 4H), 6.58 (d, 2H), 7.92 (s, 1H).
2.3 Preparation of compound 16
To a suspension of compound 15 (400 g, 1.26 mol, 1 eq) in glacial acetic acid (5 l), lead tetra acetate (562.4 g, 1.26 mol, 1 eq) was added portion wise and stirred for 5 h at 25–30°C. TLC shows completion of the reaction, R f = 0.25 (30% EtOAc in Pet ether). The reaction mixture was quenched in ice-cold water to get precipitation, filtered, and washed with water. Crude was recrystallized from aqueous acetone and dried under high vacuum to get compound 16 (336 g, 71%) as white powder. 1H NMR (CDCl3, 400 MHz): δ 2.06 (s, 3H), 2.29 (s, 3H), 2.46 (t, 2H), 2.77 (t, 2H), 4.12 (s, 3H), 5.05 (s, 2H), 5.31 (s, 2H), 7.34–7.42 (m, 5H), 9.00 (br s, 1H).
2.4 Preparation of compound 18
To a solution of compound 17 (30 g, 182.88 mmol, 1 eq) in CH2Cl2 (300 ml), TEA (46.20 g, 457.20 mmol, 2.5 eq), Tosyl chloride (53.50 g, 274.32 mmol, 1.5 eq), and a catalytic amount of DMAP (300 mg) were added at 0°C. The reaction mixture was stirred for 3 h at 27°C. TLC shows completion of the reaction, R f = 0.6 (25% EtOAc in Pet ether). The reaction mixture was washed with water (300 ml) followed by brine (300 ml), dried over anhydrous Na2SO4, and concentrated to obtain 40 g crude compound 18 oily liquid, used as it is without further purification.
2.5 Preparation of compound 19
To the solution of catechol (5.5 g, 49.99 mmol, 1 eq) in DMF (50 ml), K2CO3 (27.61 g, 199.98 mmol, 4 eq) followed by a solution of compound 18 (39.79 g, 124.97 mmol, 2.5 eq) in DMF (30 ml) was added at RT under N2. The reaction mixture was allowed to 80°C for 3 days. TLC shows completion of the reaction. The mixture was allowed to cool down to ambient temperature before pouring into deionized water (500 ml). Methyl tert-butyl ether (2 × 300 ml) was used to extract the product and the combined organic phases were dried over MgSO4 and removed in vacuo. A yellow oil was obtained and dried under a reduced vacuum to obtain 19 (38.5 g, 74%). 1H NMR (CDCl3, 400 MHz) δ 6.92 (m, 4H, ArH), 4.16 (t, J = 6.6 Hz, 4H, O–CH2), 3.85 (t, J = 6.9 Hz, 4H), 3.4–3.7 (m, 16H), 3.37 (s, 6H); MS-EI m/z 403.2 (M+H)+.
2.6 Preparation of compound 20
Compound 19 (35 g, 87.08 mmol, 1 eq) was dissolved in AcOH (35 ml) and cooled to 0°C by using ice–salt mixture. Fuming HNO3 (35 ml) [Caution! nitric acid is highly corrosive liquid and overcharging may generate exotherm and release significant of toxic NO x gas, must be handled carefully with all necessary safety precaution and PPE in a certified chemical fume hood] was added drop wise by maintaining temperature between 0 and 5°C and stirred for 3 h at same temperature. TLC shows completion of the reaction, R f = 0.6 (5% MeOH in CHCl3). The reaction mixture was quenched with ice water. Extracted with DCM (3 × 100 ml), the combined organic layer was washed with 5% aq KOH solution repeatedly followed by brine (100 ml), dried over anhydrous Na2SO4, and concentrated. The obtained crude was dissolved in a mixture of acetone and n-hexane under constant stirring at −5°C. The resulting precipitate was collected by filtration to obtain compound 20 (35 g, 82%) as a yellow solid. 1H NMR (CDCl3, 400 MHz): δ 3.36 (s, 6H), 3.52–3.75 (m, 16H), 3.90 (t, 4H), 4.33 (t, 4H), 7.48 (s, 2H); MS-EI m/z 493.2 (M+H)+.
2.7 Preparation of compound 21
To a solution of compound 20 (10 g, 20.32 mmol, 1 eq) in absolute EtOH (200 ml), NiCl2·6H2O (0.97 g, 4.06 mmol, 0.2 eq) and (Boc)2O (35.43 g, 162.56 mmol, 8 eq) were added. The reaction mixture was cooled to 0°C, NaBH4 (4.8 g, 142.27 mmol, 7 eq) was added slowly portion wise and stirred for 30 min. To this, TEA (8.5 ml, 60.96 mmol, 3 eq) was added and stirred for 1 h. TLC shows completion of the reaction, R f = 0.6 (5% MeOH in CHCl3). The reaction mixture was concentrated and the crude was purified by using silica gel column chromatography (230–400 mesh, 5–10% MeOH in DCM as mobile phase). Product fractions were collected and concentrated to obtain the desired compound 21 (6.3 g, 49%) as light-yellow gummy liquid. 1H NMR (DMSO d 6, 400 MHz): δ 1.48 (s, 18H), 3.21 (s, 6H), 3.29 (t, 4H), 3.42 (t, 4H), 3.50–3.59 (t, 8H), 3.73 (t, 4H), 4.00 (t, 4H), 7.02 (s, 2H), 8.26 (s, 2H); MS-EI m/z 631.4 (M−H)+.
2.8 Preparation of compound 22 (method A)
To a solution of compound 21 (6.3 g, 9.968 mmol, 1 eq) in anhydrous 1,4-dioxane (60 ml), 4 M HCl in 1,4-dioxane (50 ml) was added slowly at 0°C and stirred for 4 h at 27°C. TLC shows completion of the reaction, R f = 0.05 (10% MeOH in CHCl3). The reaction mixture was concentrated. The crude compound was triturated with ether (3 × 50 ml), decanted, and dried under a high vacuum to obtain compound 22 (4.4 g, 87%) as a brown gummy solid.
2.9 Preparation of compound 22 (method B)
To a solution of compound 20 (24 g, 48.73 mmol, 1 eq) in methanol (240 ml), 10% palladium on carbon was added carefully (2.4 g, ∼50% wet) (Caution! pyrophoric! should be used patiently with PPE and safety precautions) and stirred in an autoclave under hydrogen pressure. TLC shows completion of the reaction, R f = 0.05 (10% MeOH in CHCl3). The catalyst was filtered carefully (Caution! expected to cause a fire while filtration of Pd/C in methanol in open air) and washed twice swith methanol. The filtrate was evaporated to half and cooled to 0°C. 4 M HCl in 1,4-dioxane (75 ml) was added slowly at 0–5°C and stirred for 1 h at room temperature. Reaction mass concentrated under reduced pressure and triturated with methyl tert-butyl ether, dried under high vacuum to afford compound 22 (19.7 g, 80%) as a brown gummy solid. 1H NMR (DMSO d 6, 400 MHz): δ 3.32 (s, 6H), 3.35–3.60 (m, 16H), 3.72 (t, 4H), 3.98 (t, 4H), 5.60–6.45 (s, br, 4H), 6.72 (s, 2H); MS-EI m/z 433.3 (M+H)+.
2.10 Preparation of compound 23
To a stirred mixture of compound 16 (300 g, 775.5 mmol, 1 eq) and compound 9 (48 g, 387.7 mmol, 0.5 eq) in methanol (3 l), a catalytic amount of PTSA (13.5 g, 77.5 mmol, 0.1 eq) was added at 28°C under N2 atmosphere. The reaction mixture was heated to 60°C and maintained for 2 h. TLC shows completion of the reaction, R f = 0.7 (50% EtOAc in pet ether). The reaction mixture was cooled to 0°C, stirred for 30 min, and filtered. The solid was washed with cold methanol (500 ml). The solid was dried under a high vacuum to get compound 23 (180 g, 30%) as white powder. mp 161–162°C; 1H NMR (CDCl3, 400 MHz): δ 1.05 (t, 6H), 1.89 (s, 6H), 2.30–2.50 (m, 9H), 2.64 (t, 4H), 4.06 (t, 4H), 3.78 (t, 4H), 3.55 (s, 1H), 5.00 (s, 4H), 7.20 (s, 1H), 7.22–7.35 (m, 10H), 8.25 (s, 1H), 10.88 (s, 1H).
2.11 Preparation of compound 24
To cold solution of compound 23 (175 g, 224.87 mmol, 1 eq) in toluene (1.75 l), dimethyl sulfide complex (64.08 ml, 674.61 mmol, 3 eq) [Caution! wear the appropriate PPE and safety measures] was added drop wise at 0°C–5°C, heated to 50°C and stirred for 5 h. TLC shows completion of the reaction, R f = 0.2 (5% MeOH in CH2Cl2). The reaction mixture was cooled to 0°C, quenched with water (1.0 l), and stirred for 1.0 h. To the organic layer, 5% aq sodium hydroxide solution (1.0 l) was added and stirred for 1 h, separated, and washed with 1.0 N HCl (300 ml) followed by 10% aq sodium chloride solution (300 ml), dried over anhydrous Na2SO4, and concentrated. The crude compound was suspended in cold methanol (1 l) and stirred for 10 min. Filtered, washed with ice cold methanol (100 ml) and recrystallized from DCM/ethanol. The solid was collected and dried under a high vacuum to obtain compound 24 (70 g, 43%) as off-white solid (which turns to pink on long standing). mp 172–173°C; 1H NMR (DMSO d 6, 400 MHz): δ 0.95 (t, 6H), 1.39 (t, 4H), 2.13 (s, 6H), 2.30–2.40 (m, 9H), 3.37 (s, 4H), 3.67 (s, 4H), 4.36 (t, 2H), 5.23 (s, 4H), 7.28–7.49 (m, 11H), 9.42 (s, 1H).
2.12 Preparation of compound 25
To a solution of compound 24 (67.5 g, 97.35 mmol, 1 eq) in dry THF (675 ml), wet 10% Pd/C (21 g, 30% w/w) was added in an autoclave under hydrogen pressure (3 kg·cm−3) and stirred for 5 h. TLC shows completion of the reaction, R f = 0.15 (10% MeOH in CH2Cl2). The reaction mixture was filtered through a celite bed and washed with THF (300 ml). The combined filtrate was concentrated and suspended in DCM (300 ml). Stirred for 10 min, filtered and washed with ice cold DCM (150 ml). The solid was collected and dried under a high vacuum to obtain compound 25 (37.5 g, 75%) as white solid (which turns to pink on long standing). 1H NMR (DMSO d 6, 400 MHz): δ 0.98 (t, 6H), 1.08 (t, 1H), 1.38 (t, 4H), 2.13 (t, 6H), 2.20–2.32 (m, 8H), 3.57 (m, 2H), 3.69 (s, 4H), 4.36 (s, 2H), 9.50 (s, 1H), 10.69 (s, 2H), 11.78 (s, 2H).
2.13 Preparation of compound 26
To a suspension of compound 25 (35 g, 66.40 mmol, 1 eq) in trimethyl orthoformate (210 ml), trifluoroacetic acid (210 ml) was added drop wise at −20°C and stirred for 1 h at 27°C. TLC shows completion of the reaction, R f = 0.25 (10% MeOH in CH2Cl2). The reaction mixture was poured into crushed ice and extracted with DCM (3 × 200 ml). The combined organic layer was washed with brine (200 ml), dried over anhydrous Na2SO4, filtered, and concentrated to obtain compound 26 (17.5 g, 53%) as dark brown solid. The crude compound was used in the next step without any purification.
2.14 Preparation of compound 27
To stirred mixture of compound 26 (17.5 g, 36.37 mmol, 1 eq) and compound 22 (18.32 g, 40 mmol, 1.1 eq) in dry methanol (300 ml), 4 M dry HCl in 1,4-dioxane (25 ml) slowly at 27°C was added under an argon atmosphere. The reaction mixture was heated to 50°C and maintained for 4 h. TLC shows completion of the reaction, R f = 0.25 (10% MeOH in CH2Cl2). The reaction was cooled to 27°C, and 50 g of activated charcoal was added and stirred for 30 min. The dark suspension was filtered through celite to remove the carbon and the filtrate was concentrated. The crude was dried under a high vacuum to obtain 32.5 g of compound 27 as a dark red solid. The crude compound was crystallized from iso-propanal and heptane to obtain compound 27 (20 g, 63%) as a scarlet red solid. 1H NMR (400 MHz, CD3OD): δ 1.10 (t, 6H), 1.76 (p, 4H), 2.36 (s, 6H), 2.46 (q, 4H), 2.64 (t, 4H), 3.29 s, 6H), 3.31 (t, 4H), 3.43–3.85 (m, 20H), 4.10 (s, 4H), 4.22 (t, 4H), 7.45 (s, 2H), 8.36 (s, 2H); 13C NMR (100 MHz, CD3OD): δ 9.18, 9.41, 16.79, 17.08, 18.41, 20.83, 21.00, 30.73, 34.09, 59.04, 62.18, 70.66, 70.83, 71.34, 71.43, 71.80, 71.86, 72.96, 103.69, 122.34, 123.22, 125.45, 125.65, 130.92, 141.75, 150.26; UV/vis: nm 481, 370; MS-EI m/z 878.5 (M+H)+; HPLC purity: ∼87.58%.
2.15 Preparation of compound 1
The Gd(OAc)3 (18 g, 53.36 mmol, 1.5 eq) was dissolved in dry MeOH (250 ml) and TEA (56 ml, 391.2 mmol, 11 eq) was added at 27°C. The reaction mixture was bubbled with air for 10 min, and a solution of compound 27 (31.2 g, 35.56 mmol, 1 eq) in dry MeOH (250 ml) drop wise was added. The reaction mixture was heated to reflux and maintained for 3 h with continuous air bubbling through the balloon. TLC shows completion of the reaction, R f: 0.3 (15% MeOH in DCM). The reaction mixture was cooled to room temperature, filtered through celite and thoroughly washed with MeOH (150 ml). The filtrate was concentrated in a vacuum. The crude was suspended in acetone (300 ml), stirred for 15 min, and filtered. The solid was washed with 100 ml of cold acetone. The solid was dissolved in 500 ml of 1:9 mixture of water in MeOH and stirred with acetic acid-washed zeolite (2 × 100 g). Filtered and concentrated. The crude compound was dissolved in Millipore water (300 ml) and passed through acetic acid-washed Ambersep resin (Ambersep 900 OH, flow rate 5 ml·min−1). Collected fractions were concentrated in a vacuum and the solid obtained was triturated with acetone (3 × 100 ml), filtered, and dried under a high vacuum for several hours to obtain compound 1 (13.2 g, 27%) as a dark green microcrystalline solid. UV/vis: nm 318, 342, 418, 478, 739; MS-EI m/z 1089.5 [M-(mPEG3-OH-Ac)]+; HPLC purity: ∼95.86%.
3 Results and discussion
This study addresses one of the most important aspects of the use of MGd, such as its use in efficient synthetic approaches for gram scale preparation. The goal is to understand each chemical step to develop a better process, subsequently translate for large-scale production, and pave the way for successful clinical translation. However, multiple critical issues persist in the reported protocols, including multi-step process, column chromatography, hazardous reagents, low overall yields, extended reaction times, and significant preparation costs. We focused primarily on improving the process for the preparation of penultimate intermediates of MGd, as shown in Figure 3.
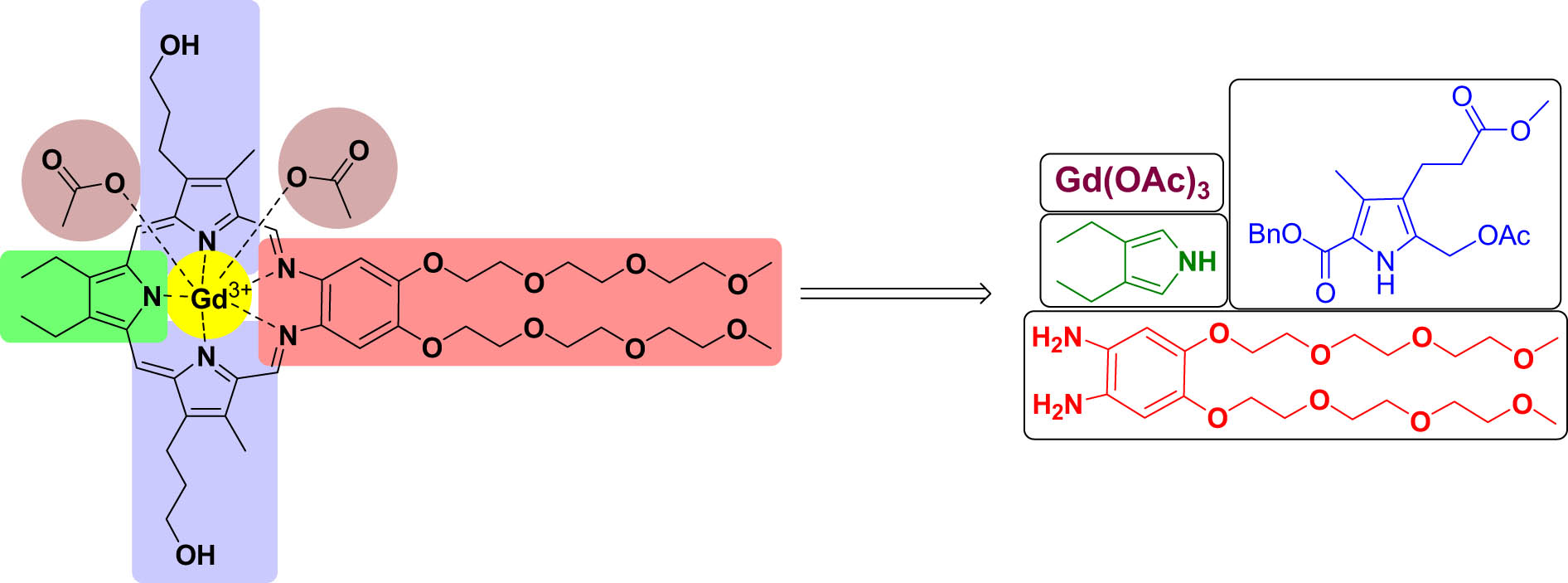
Retrosynthesis of MGd.
Ethyl isocyanoacetate (3) was synthesized from the commercially available glycine ethyl ester [6]. Synthesis of compound 7 (3,4-diethylpyrrole-2-carboxylate) was achieved by one-pot synthesis as reported in the literature [7]. The economical and commercially available DMAP [6] was used to achieve the Henry reaction (using 1‐nitropropane and propionaldehyde) and acetylation (with acetic anhydride) simultaneously instead of DBU and sulfuric acid to get compound 6. The Magnus–Schöllkopf–Barton–Zard cyclization was achieved in dry toluene using compound 3 and compound 7 instead of dry THF. The toluene layer was directly subjected to hydrolysis with NaOH to avoid extraction, separation, and distillation of organic solvents such as ethyl acetate or diethyl ether [8]. By following an acid–base purification technique, excellent purity of compound 8 was achieved. Compound 8 was sublimed at 160°C for 2 h and subsequently isolated by fractional distillation [6,9]. Hence, compound 9 was successfully optimized without column chromatography purification as shown in Scheme 1.

Telescopic synthesis of compound 9.
Benzyl acetoacetate (10) was treated with a solution of sodium nitrite to get oxime 11. The methyl 4-acetyl-5-oxohexanoate (14) was prepared via a sodium ethoxide-catalyzed Michael addition reaction of acetylacetone (13) to methyl acrylate (12). The synthesis of pyrrole 15 was achieved by a one-pot zinc metal-catalyzed reduction of oxime 11 to corresponding amine followed by in situ condensation with 14. Compound 15 was treated with lead tetraacetate in acetic acid [10] without acetic anhydride [2] to furnish acetoxyl derivative 16 as shown in Scheme 2.

Synthesis of compound 16.
The synthesis of 1-bromo-2-(2-(2-methoxyethoxy)ethoxy)ethane [11] from alcohol 17 by the Appel reaction was avoided due to the hazardous bromine and column purification to purge by-product (TPPO) [12]. The (2-[2-(2-methoxyethoxy)ethoxy]ethanol 17 was transformed to tosyl derivative 18 by treatment with tosyl chloride followed by a nucleophilic substitution reaction with the phenoxide ion of catechol to afford compound 19. Dinitro derivative 20 was obtained by nitration with nitric acid in acetic acid followed by recrystallization by isopropyl alcohol [13]. Considering its toxicity, safety, and environmental risk, hydrazine hydrate was avoided as a hydrogen source under reflux [14] for the reduction of nitro to amine [15]. Initially, compound 20 was converted to Boc-protected amine 21 by one-pot reduction of nitro to amine by sodium borohydride (7 eq) and a catalytic amount of nickel chloride (0.1 eq) followed by Boc protection using Boc anhydride. Subsequently, Boc deprotection was achieved by 4 N HCl in 1,4 dioxane, which was isolated as HCl salt of diamine 22 to avoid further oxidation/decomposition of the diamine. In the additional protection/deprotection step, an excess catalyst was used, and the column was easily removed by switching the catalyst to Pd/C under a hydrogen atmosphere [16] as shown in Scheme 3.

Synthesis of compound 22.
Scheme 4 shows that p-toluene sulfonic acid catalyzed the addition of electron-rich pyrrole (9) to compound 16 at the C2 and C5 positions of 9, effectively displacing acetate moiety 16 to afford compound 23. Considering the tedious and time-consuming workup and hazards associated with extremely reactive lithium aluminum hydride [17], commonly used reducing agents for converting esters to alcohols were avoided. Despite being more reactive than BH3·DMS, BH3·THF is available commercially as a 1 M THF solution only. However, the more stable BH3·DMS is a less expensive and readily available neat reagent [18]. Moreover, economical and easily recyclable/reusable toluene is used as a solvent with BH3. DMS was used instead of THF [19] to obtain the alcohol derivative 24. Debenzylation was achieved by traditional hydrogenolysis of carboxylic esters using 10% Pd/C to afford diacid derivative 25.

Synthesis of compound 25.
Compound 25 was transformed to the corresponding aldehyde derivative 26 via a decarboxylation–formylation sequence similar to a Clezy formylation by treatment with trimethyl orthoformate in trifluoroacetic acid [2]. Acid-catalyzed imine condensation of dialdehyde 26 with the aromatic diamine 21 resulted in a macrocyclic core of Texaphyrin 27, which was subsequently treated with Gd(OAc)3 to obtain the desired final product, as shown in Scheme 5.

Synthesis of the MGd.
The process development of MGd represents a critical advancement in the quest to harness its potential as a promising radiosensitizer for cancer therapy. This research endeavor has addressed several key aspects related to the synthesis and optimization of MGd, shedding light on the path toward its successful lab scale-up. The improved synthesis method described herein offers increased efficiency and enhanced yield, mitigating some of the challenges associated with the gram-scale synthesis of MGd. These modifications not only make MGd more accessible for research purposes but also have the potential to reduce synthesis time and cost. This process reduces overall waste generation and obeys the majority of green chemistry principles, as hazardous substances are replaced with less hazardous alternatives, and greener solvents, avoiding the excessive use of solvents [20,21].
In conclusion, the process development of MGd represents a vital step in revealing the full potential of this compound for further fight against cancer. The synthesis described in this report overcomes numerous challenges commonly encountered in current preparative methods. This approach not only demonstrates the feasibility of producing MGd efficiently and safely but also underscores its significance as a promising adjunct to radiation therapy.
Acknowledgments
The authors (I. Islam, B. Al Somaie, and Y. Tamboli) are thankful to King Abdullah International Medical Research Center (KAIMRC), Riyadh, Saudi Arabia, for funding and facilities to carry out research work.
-
Funding information: The work was funded by King Abdullah International Medical Research Center (KAIMRC) through project NRC23R/680/10.
-
Author contributions: Y. Kalisha Vali: performed the experiments, writing – original draft; Imadul Islam, Barrak Al Somaie, and Abdulrahman Abdullah Al Sayyari: formal analysis, contributed to scientific discussions and literature support, resources, writing – review and editing; Rambabu Gundla: conceptualization, supervision, writing – review and editing; and Yasinalli Tamboli: project administration, performed experiments and analysis (partially), writing – original draft, review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Lee MH, Kim EJ, Lee H, Park SY, Hong KS, Kim JS, et al. Acid-triggered release of doxorubicin from a hydrazone-linked Gd3+ -texaphyrin conjugate. Chem Commun. 2016;52:10551–4.10.1039/C6CC05673CSearch in Google Scholar
[2] Yang Y, Chen S, Liu L, Li S, Zeng Q, Zhao X, et al. Increasing cancer therapy efficiency through targeting and localized light activation. ACS Appl Mater Interfaces. 2017;9:23400–8.10.1021/acsami.7b05463Search in Google Scholar PubMed
[3] Mehta MP, Rodrigus P, Terhaard CHJ, Rao A, Suh J, Roa W, et al. J Clin Oncol. 2003;21:2529–36.10.1200/JCO.2003.12.122Search in Google Scholar PubMed
[4] Wei WH, Fountain M, Magda D, Wang Z, Lecane P, Mesfin M, et al. Gadolinium texaphyrin–methotrexate conjugates. Towards improved cancer chemotherapeutic agents. Org Biomol Chem. 2005;3:3290–6.10.1039/b503664jSearch in Google Scholar PubMed
[5] Preihs C, Arambula JF, Magda D, Jeong H, Yoo D, Cheon J, et al. Recent developments in texaphyrin chemistry and drug discovery. Inorg Chem. 2013;52:12184–92.10.1021/ic400226gSearch in Google Scholar PubMed PubMed Central
[6] Matsuo T, Hayashi A, Abe M, Matsuda T, Hisaeda Y, Hayashi T. Meso-unsubstituted iron corrole in hemoproteins: remarkable differences in effects on peroxidase activities between myoglobin and horseradish peroxidase. J Am Chem Soc. 2009;131(42):15124–5.10.1021/ja907428eSearch in Google Scholar PubMed
[7] Steinbrueck A, Brewster II JT, Zhao MY, Sedgwick AC, Sessler JL. Convenient decagram scale preparation of ethyl 3,4-diethylpyrrole-2-carboxylate, a versatile precursor for pyrrole-based macrocycles and chromophores. Results Chem. 2020;2:100075.10.1016/j.rechem.2020.100075Search in Google Scholar
[8] Tamboli Y, Kashid B, Yadav RP, Rafeeq M, Merwade AY. Large-scale practical synthesis of boc-protected 4-fluoro-l-proline. Org Process Res Dev. 2020;24:1609–13.10.1021/acs.oprd.0c00080Search in Google Scholar
[9] Sessler JL, Mozaffari A, Johnson MR. 3,4-Diethylpyrrole and 2,3,7,8,12,13,17,18-Octaethylporphyrin. Org Synth. 2003;70:68.10.1002/0471264180.os070.09Search in Google Scholar
[10] Pereira N, Serra AC, Pineiro M, Gonsalves AMDAR, Abrantes M, Laranjo M, et al. Synthetic porphyrins bearing β-propionate chains as photosensitizers for photodynamic therapy. J Porphyr Phthalocyanines. 2010;14:438–45.10.1142/S1088424610002227Search in Google Scholar
[11] Pefkianakis EK, Manthou VS, Paraskevopoulou P, Sakellariou G, Vougioukalakis GC. A new family of fullerene derivatives bearing long alkyl and triethyleneglycol moieties. ChemistrySelect. 2016;1(6):1232–8.10.1002/slct.201600405Search in Google Scholar
[12] Tamboli Y, Kashid BB, Yadav RP, Rafeeq M, Yeole R, Merwade AY. Triphenylphosphine oxide removal from reactions: the role of solvent and temperature. ACS Omega. 2021;6:13940–5.10.1021/acsomega.1c01996Search in Google Scholar PubMed PubMed Central
[13] Madden H, Hemmi G, Mody T. High-purity texaphyrin metal complexes. US Patent; 2007. p. US20070072838.Search in Google Scholar
[14] Niemeier JK, Kjell DP. Hydrazine and aqueous hydrazine solutions: evaluating safety in chemical processes. Org Process Res Dev. 2013;17(12):1580–90.10.1021/op400120gSearch in Google Scholar
[15] Mehta MP, Rodrigus P, Terhaard CHJ, Rao A, Suh J, Roa W, et al. Radiation sensitization using texaphyrins. US Patent; 1999. US5888997A.Search in Google Scholar
[16] de Hatten X, Asil D, Friend RH, Nitschke JR. Aqueous self-assembly of an electroluminescent double-helical metallopolymer. J Am Chem Soc. 2012;134:19170–8.10.1021/ja308055sSearch in Google Scholar PubMed
[17] Takayanagi H. Preparation of pyrrole derivative as intermediate for texaphyrins. JP Patent; 1995. JP07316131A.Search in Google Scholar
[18] Ötvös SB, Kappe CO. Continuous-flow amide and ester reductions using neat borane dimethylsulfide complex. ChemSusChem. 2020;13(7):1800–7.10.1002/cssc.201903459Search in Google Scholar PubMed PubMed Central
[19] Kilbile JT, Tamboli Y, Shukla JD, Merwade AY. Short and scalable synthesis of enantiopure n-boc-trans-4-methyl-l-prolinol. Org Process Res Dev. 2023;27(5):938–44.10.1021/acs.oprd.3c00053Search in Google Scholar
[20] Cue BW, Zhang J. Green process chemistry in the pharmaceutical industry. Green Chem Lett Rev. 2009;2(4):193–211.10.1080/17518250903258150Search in Google Scholar
[21] Tickner JA, Simon RV, Jacobs M, Pollard LD, van Bergen SK. The nexus between alternatives assessment and green chemistry: supporting the development and adoption of safer chemicals. Green Chem Lett Rev. 2021;14(1):23–44.10.1080/17518253.2020.1856427Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

