Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
-
Son Nguyen Thi
Abstract
In this study, we synthesized 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one through a one-pot reaction using all starting materials in a microwave oven. The presence of the solid base MgO catalyzed the reaction in the eco-friendly solvent of ethanol as a green chemistry approach owing to the noticeable advantages of short reaction times to save energy and less toxic starting materials for environmental friendliness. This indicates that the one-pot reaction makes the process simpler, in which the reaction time (1 h) is shorter than that of conventional methods (10 h). The yield of the reactions reached 55–76% for 18 final products consisting of 17 derivatives of morpholine or thiomorpholine with various aldehydes and one extended moiety of the primary amine, of which 13/18 final compounds were new. The purification procedure was performed without using polluting solvents. The structures were confirmed using IR, 1H-NMR, 13C-NMR, and MS analyses.
1 Introduction
Over the past decade, sulfur–nitrogen heterocyclic compounds have played a significant role in the development of pharmacology owing to their wide range of biological activities. Among the available sulfur–nitrogen heterocycle structures, the thiazole fragment is of great interest in medicinal chemistry because of its number of reactive carbon sites for addition, condensation, oxidation–reduction, and substitution reactions [1]. The thiazolidin-4-one framework (Figure 1) is noticeable, appearing in the structure of more than 15,000 compounds, including several anti-cancer and anti-inflammatory drugs, such as darbufelone [2] and thiazolidomycin, which is active against antibiotics [3], and etozolin-novel diuretics [4,5], ralitoline, which is used as anticonvulsant [6]. In addition, these compounds also show diversity in biological activities such as antiparasitic [7], anti-HIV [8], and COX inhibitory [9]. In recent years, various studies have been conducted on thiazolidin-4-one, which exhibits anti-colon [10], breast [11], ovarian cancer activity, and tumor growth [12,13]. Therefore, the discovery of new compounds or methods for synthesizing compounds containing this structural framework has become a promising field. Among thiazolidin-4-one derivatives, compounds containing 5-arylidene-2-(morpholin-4-yl) groups are of interest because of their potential application as non-steroidal anti-inflammatory drugs [14]. Some compounds in this series have shown the ability to inhibit the DYRK1A enzyme at nanomolar concentrations for application in β-cell stabilization and insulin homeostasis regulation.

Structures of thiazolidin-4-one derivatives.
The usual method (Table 1) for synthesizing thiazolidin-4-one starts with a primary alkyl/aryl amine reacting with isothiocyanate to yield the corresponding thiourea. Cyclization was performed with haloacetic acid to obtain 2-imino-1,3-thiazolidin-4-one. However, the disadvantage of this method is obtaining two 2-imino-1,3-thiazolidin-4-one isomers, which are challenging to separate [15,16]. Another method uses alpha-chloroamide derivatives that undergo a cyclization reaction with isothiocyanate in the presence of a weak base [17,18], followed by a Knoevenagel condensation reaction with aldehyde derivatives in the presence of a base catalyst for 5–12 h to obtain 5-arylidene-2-imino-1,3-thiazolidin-4-one derivatives [18,19]. It is possible to use rhodanine as the starting material and solid phase in two steps: replacing the sulfur in rhodanine with amine and performing Knoevenagel condensation. This method overcomes the formation of a mixture of two isomers but requires expensive solids and long reaction times [20]. In recent years, multi-agent one-pot methods and microwave techniques have significantly shortened reaction times. However, the reaction proceeds in two stages, forming poisonous gas with low yields [21]. In general, all conventional methods have certain limitations in research and application of these compounds in daily life, such as many-stage reactions, long reaction times, and isomer products being difficult to separate from each other, toxic, flammable explosive, and expensive raw materials.
Various synthesis of 5-arylidene-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one
| Methodology | Catalyst | Solvent | Reaction time | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 2 steps condensation/reflux | Piperidine | EtOH | 7 h | 45–85 | [22] |
| One-pot/room temperature | Silica/pyridine | EtOH | 100 h | 76 | [23] |
| 2 steps condensation/microwave/80°C/elimination of hydrogen sulfide | — | — | 1 h | 49–52 | [21] |
| One-pot/180°C | Acid acetic | EtOH | 45 min | 61–83 | [24] |
| One-pot/80°C | MgO | EtOH | 1 h | 73 | This work |
New approaches using a combination of microwave techniques and metal oxide catalysis have achieved excellent results in organic synthesis, including the synthesis of thiazolidin-4-one heterocycles. Among these metal oxides, MgO is used because of its low price and high activity in many organic reactions [25]. As a solid base, MgO can catalyze many condensation and addition reactions, in which MgO has many advantages, such as easy recovery and reduced separation workload, reduction in the number of compounds participating in the reaction, and high selectivity, which is considered an approach to green chemistry [26,27,28].
In this work, we focus on synthesizing the compounds 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one (III) by a one-pot reaction with the help of microwaves from completely new ingredients including morpholine-4-carbothioamide, aldehyde and chloroacetyl chloride as a new point of research. The use of a MgO solid base catalyst with an environmentally friendly solvent, ethanol, is considered a highlight. The scope of this study was expanded to include morpholine and thiomorpholine.
2 Experimental methods
The following materials were used in this study: chloroacetyl chloride, morpholine, thiomorpholine (Merck, 98%), ethanol 96%, 1,4-dioxane, DMF, acetic acid, magnesium metal, magnesium nitrate, sodium hydroxide, polyvinyl alcohol (PVA), hydrotalcite, Al2O3 (China, 98%), SBA15-SO3H, and SBA15-SH (self-prepared) [29].
2.1 Characterization
Reactions were performed in a microwave oven (Qpro-M). The melting point was recorded on As One ATM01 apparatus; IR spectra were recorded on FTIR Affinity-IS apparatus; NMR spectra were recorded on a Bruker 500 Mv instrument, 500 MHz apparatus at Faculty of Chemistry, VNU University of Science; HRMS was recorded on a high resolution LC-MS LTQ ORBITAP XL instrument at the Institute of Chemistry, VAST; thin layer chromatography-TLC was performed with a silica gel-coated plastic plate, visualized by UV-VIS at Faculty of Chemistry, VNU University of Science. An Empyrean (PANalytical) X-ray diffraction system was used to determine the crystal structure. The shapes were captured using a Nova NanoSEM 450 (FEI) with a scanning electron microscope at the Faculty of Physics, VNU University of Science.
3 Magnesium oxide preparation
MgO was prepared using two methods. In the first method, magnesium metal was burned in the air and labeled MgO–B. In the second method, magnesium oxide, named MgO–S, was synthesized by calcinating Mg(OH)2 at 350°C, and a precipitate was obtained by mixing Mg(NO3)2 and NaOH in the presence of PVA [30].
3.1 General procedure for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one (III.a,b)
A mixture of morpholine/thiomorpholine-4-carbothioamide (I.a,b) (0.3 mmol, 1 equiv.), chloroacetyl chloride (0.45 mmol, 1.5 equiv.), the corresponding aldehyde (II.1–12) (0.3 mmol, 1 equiv.); base MgO–B (0.3 mmol), and EtOH solvent (5.0 mL) was refluxed in the microwave with stirring, at 80°C for 1 h, and checked by TCL. At the end of the reaction, the mixture was allowed to cool to room temperature. Then, the reaction mixture was extracted with ethyl acetate (20.0 mL). The organic layer was washed twice with water and dried with Na2SO4. The product was obtained after flash chromatography on a silica gel column with n-hexane/ethyl acetate to yield 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one (III.a,b) (Scheme 1).
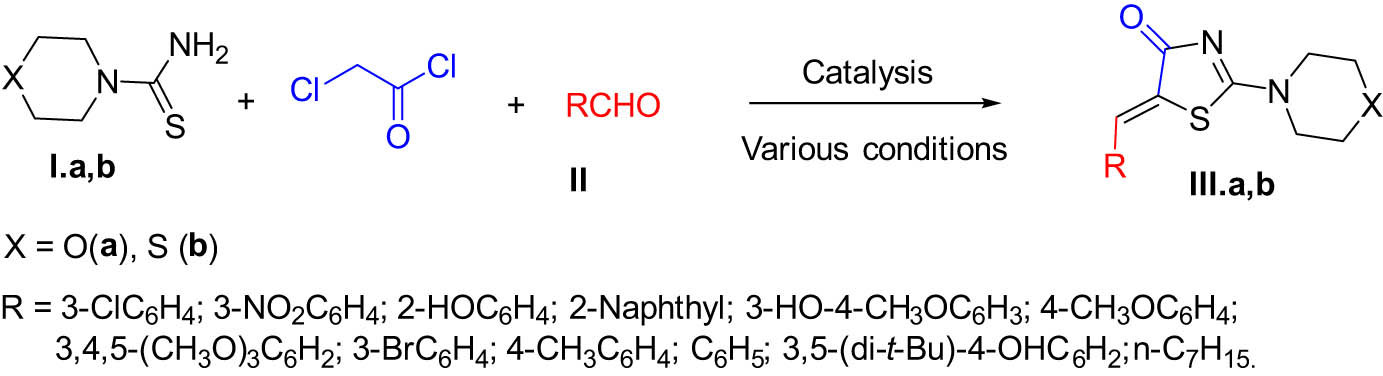
One-pot reaction of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives.
4 Results and discussion
The MgO–B and MgO–S samples synthesized by the dry method and in solution showed slight differences in composition, as indicated by the XRD patterns in Figure 2. Specifically, when burning Mg in air, the MgO–B sample shows a high single phase with characteristic diffraction peaks at position 2θ = 36.93, 42.87, and 62.09 (ICCD#01-089-7746). Meanwhile, the MgO–S sample synthesized through calcinating the precipitation from the solution shows the presence of Mg(OH)2, corresponding to diffraction peaks at 18.72, 37.97, 50.78, and 58.71 (ICCD#01-083-0114). The surface morphology and alkalinity of the materials may be the core effects of the one-pot reaction.

XRD patterns of MgO–B and MgO–S.
The SEM and TEM images (Figure 3) show apparent differences in the surface morphology of the MgO materials. The MgO–B sample, owing to the lack of interaction of OH groups in the solid, and MgO particles tend to be uniformly distributed and clearly distinguished from each other with a size of 100 nm. However, in the solid state, they remain attached to form many clusters of materials. Simultaneously, MgO–S tends to form thin layers that pile on each other to form solid blocks with diverse shapes and sizes ranging from a few tens of nanometers to 500nm. The resulting morphology can be explained by the pyrolysis of Mg(OH)2 at high temperatures, which causes sintering between the MgO particles and water evaporation combined with burning PVA to form MgO layers.

SEM images of MgO–S (a and b) and MgO–B (c and d), and TEM images of MgO–B (e and f).
5 Structure of III
In the first step of the experiment, 5-(3-nitrobenzylidene)-2-(morpholin-4-yl)-1,3-thiazol-4(5H)-one (III.a-2), named III.a-2, was chosen for further optimization. For compound III.a-2, morpholine-4-carbothioamide (I.a) (1 equiv.), chloroacetyl chloride (1.5 equiv.), 3-nitrobenzaldehyde (1 equiv.), MgO–B (30 wt% to I.a), and ethanol (5.0 mL) were mixed. The reaction was refluxed for 30 min in a microwave with a power of 200 W to obtain III.a-2 in isolated yields of 53%. The structure of compound III.a-2 was confirmed using IR, NMR, and MS, as shown in Figures 4–6. In the IR spectrum of compound III.a-2 (Figure 4), there are signals characteristic for valence vibrations of ν(C═O) bonds at 1699.29 cm−1 and ν(Ar–H) 3084.18 cm−1. The appearance of absorption bands in the region of 2800–3000 cm−1 presents ν(C–H) of CH2-morpholine, ν(NO2): 1348.24; 1517.98 cm−1, ν(C═C) in the region of 1552.70 cm−1, ν(C–O–C) at 1109.07 cm−1 and the deformation dynamics of the benzene ring δ (1.3-disubstituted-Ar) at 881.47 cm−1.

IR spectra of compound III.a-2.
In the 1H-NMR spectrum (Figure 5), the number of protons recorded is equal to the number of protons in the molecular formula. The proton of H2’ gives a singlet signal at 8.40 ppm, that of the proton H4’ at 8.23–8.25 ppm, and the multiplet signal of proton H6’ at 7.89–7.75 ppm and ═CHAr; the signal at 7.62–7.65 ppm is the proton in the H5’. Previous reports have confirmed that the compounds of III remain in the isomer (Z) due to their dominant kinetic stability compared with isomers (E). The proton signals from the methylene group (CH2–) in the isomer (Z) usually have a lower chemical shift than those of the (E) isomers [31]. The photons H2” and H6” for morpholine via thiomorpholine moieties demonstrate the triplet signal assigned to proton CHaxHeq–O–CHaxHeq at 4.13 ppm and 3.69 ppm, while 3.83–3.88 ppm responds to the signal of CHaxHeq–O–CHaxHeq of the photons H3” and H5”.

1H-NMR spectra of compound III.a-2.
The appearance of the following signals from the 13C-NMR spectrum (Figure 6) are at 179.9 (C4), 174.9 (C2), 148.8 (C3′), 136.0 (C1′), 135.8 (C2′), 131.0 (C5), 130,1 (C4′), 128.9 (C5′), 124,0 (═CH–Ar), 123.2 (C6′), 66.4, 66.2 (CH2–O–CH2), and 49.1, 29.7 (CH2–N–CH2). On HRMS (ESI): m/z calcd for C14H13N3O4S [M + H]+ 320.6699, found: 320.0675.

13C-NMR spectra of compound III.a-2.
5.1 Optimization of the one-pot reaction for III.a-2 over MgO
To study the influence of the OH group on the surface of the material on its ability to catalyze the one-pot reaction to form morpholine-thiazole compounds, two types of materials, MgO–B and MgO–S, were studied under the same catalytic reaction conditions. On the other hand, solvents with different polarities were also used to evaluate the interaction of catalysts and solvents. In addition, several other solid catalysts, such as modified SBA-15 (–SO3H or –SH), hydrotalcite, aluminum oxide (Al2O3), and homogeneous catalysts containing CH3COONa in CH3COOH acid as a reference, were also observed.
From the results in Table 2, MgO shows the ability to promote the one-pot reaction to synthesize 5-(3-nitrobenzylidene)-2-(morpholin-4-yl)-1,3-thiazol-4(5H)-one (III.a-2) in ethanol reaching 53% and 47%, respectively, for MgO–B and MgO–S. The presence of the OH group in MgO–S may affect the conversion of the morpholine-4-carbothioamide and chloroacetyl chloride mixture because of the electrophilic substitution of the OH group on the MgO sample and the Cl group of chloroacetyl chloride. On the other hand, MgO can, in addition to acting as a base catalyst for the reaction, also be a water adsorbent that prevents the hydrolysis of chloroacetyl chloride, leading to an improvement in reaction performance. However, in other solvents, such as DMF and dioxane, the yields of the whole process were not significant. When evaluating other factors related to the acidic or basic states of the materials, materials containing SBA15 modified with SO3H and SH groups, which were used as solid acids, and Al2O3 and hydrotalcite, which had both acidic and basic functions, were used as catalysts. Unfortunately, the yield of the one-pot reactions using the above catalysts may have been more favorable. In summary, the heterogeneous catalytic ability of MgO in the one-pot reaction is acceptably equivalent to that of homogeneous AcONa in AcOH. The reaction was performed for a shorter time (only 30 min), without any intermediate purification, and in a green solvent of ethanol for reaction and final purification with the best yield as a key point of the research.
Optimization of reaction conditions with various catalysts
 |
||
|---|---|---|
| No. | Catalyst | Isolated yield (%) |
| 1. | MgO–B | 53 |
| 2. | MgO–S | 47 |
| 3. | Hydrotalcite | 16 |
| 4. | SBA15-SO3H | — |
| 5. | SBA15-SH | 7 |
| 6. | Al2O3 | 9 |
| 7. | CH3COONa | Trace |
| 8. | Et3N | Trace |
| 9. | — | — |
Reaction conditions: I.a (0.3 mmol); chloroacetyl chloride (0.45 mmol); II-2 (0.3 mmol); different catalysts (30 wt% to I.a); ethanol (5.0 mL), stirred at 80°C in an oven at 200 W for 30 min.
From research with different types of catalysts, MgO–B was selected for further optimization experiments. The amount of catalyst loading in the reaction system varied from 30 to 200 wt%, corresponding to compound I.a, as shown in Figure 7. The reaction yields between I.a and 3-nitrobenzaldehyde increased from 53 to 64% when the catalyst loading was increased from 30 to 100 wt%. However, when the MgO loading exceeded 100 wt%, the yield changed insignificantly.

Optimization of the reaction conditions with various catalyst loadings. Reaction conditions: I.a (0.3 mmol); chloroacetyl chloride (0.45 mmol); II-2 (0.3 mmol); catalysts MgO–B with various rates; ethanol (5.0 mL), stirred at 80°C in an oven at 200 W, for 30 min.
Different solvents were used with the MgO–B catalyst (Figure 8) to optimize the reaction conditions, showing that EtOH gave the best yield of 64%, while other solvents showed no improvement. Ethyl alcohol is also an environmentally friendly green solvent, which is a crucial point exploited in this study (Table 3).

Yields of condensation reactions with various solvents. Reaction conditions: I.a (0.3 mmol); chloroacetyl chloride (0.45 mmol); II-2 (0.3 mmol); catalysts MgO–B (100 wt%); various solvents (5.0 mL), stirred at 80°C in an oven at 200 W, for 30 min.
Effect of reaction times and microwave power
| No. | Time (min) | Isolated yield (%) | |
|---|---|---|---|
| With microwavea | Without microwaveb | ||
| 1. | 15 | 39 | — |
| 2. | 30 | 64 | — |
| 3. | 45 | 69 | — |
| 4. | 60 | 73 | 11 |
| 5. | 120 | 52 | 18 |
| 6. | 180 | — | 30 |
| 7. | 240 | — | 52 |
| 8. | 360 | — | 63 |
| 9. | 600 | — | 65 |
Reaction conditions: I.a (0.3 mmol); chloroacetyl chloride (0.45 mmol); II-2 (0.3 mmol); MgO–B (100 wt%); EtOH (5.0 mL), stirred at 80°C. Stirred at 80°C (a) in an oven at 200 W or (b) without a microwave oven.
In order to determine the advantage of microwave energy for the reaction, the catalytic ability of MgO in reactions supported by microwave energy and conventional heat (from a magnetic stirring heating block) at 80°C was investigated for about 15 min to 10 h. MgO–B is a good catalyst for the condensation of aldehydes and morpholine derivatives (similar to the Knoevenagel condensation reaction). The one-pot reaction yield was maximized with the help of a microwave oven, reaching 73% after 1 h of working. After that time, at a reaction time of 120 min, the reaction yields decreased, and a darkening of the solution was observed, possibly because the microwave heating process caused overheating and charring. The decreasing trend of the reaction yields after 45 min with the observation of the darkening of the solution was the reason for the reaction stopping after 2 h of working. Meanwhile, the rate of the one-pot reaction heated from a conventional heating block was much slower. After 2 h, only 11% of the products were identified, and after 10 h, the yield of the whole process only reached 65%. About 10 h of working in conventional heating is significant for time and energy waste when compared with 1 h working with the microwave oven. Further time to operate with conventional energy may not be necessary. Therefore, using microwaves for 1 hour of working time is an optimized condition to save time and energy, which can be an advantage for later industrial applications.
The microwave power was studied to optimize the performance of the one-pot reaction with three power values at 100, 200, and 300 W. The results in Figure 9 show that the furnace power of 200 W gives the highest reaction efficiency of 73% and the lowest of 44% at 100 W. At the higher power of 300 W, the reaction efficiency decreased slightly, and the appearance of a black solid from charring due to overheating was observed. The optimal condition selected was a microwave oven power at 200 W.

Effects of reaction time and microwave power. Reaction conditions: I.a (0.3 mmol); chloroacetyl chloride (0.45 mmol); II-2 (0.3 mmol); MgO–B (0.3 mmol); EtOH (5.0 mL), stirred at 80°C for 60 min.
To demonstrate the “green chemistry” aspect of the method, the reusability of the material was evaluated to determine the reaction efficiency and material structure after each reuse. After each reuse, the catalytic material was filtered with filter paper, washed with ethanol, and calcined at 500°C for 3 h to remove all organic compounds that may have been adsorbed on the catalyst surface.
As shown in Figure 10, the efficiency of the one-pot condensation reaction to create 2-(morpholin-4-yl)-1,3-thiazol-4(5H)-one on the MgO catalyst system tends to decrease slightly after each reuse. This is consistent with the structural state and phase composition of the material, which changed somewhat during the reuse study, specifically after three reuses. The surface morphological structure of the material lost its original 100 nm granules (Figure 3), but instead, they tended to clump with overlapping layers (Figure 10b). The phase composition is the MgO crystalline phase, and no Mg(OH)2 crystalline phase can be observed. However, a small unidentified diffraction peak appears at 2

Reusability of MgO–B: yields after recycling (a), SEM image (b), and XRD (c) of MgO after third recycle. Reaction conditions: I.a (0.3 mmol); chloroacetyl chloride (0.45 mmol); II-2 (0.3 mmol); MgO–B (0.3 mmol); EtOH (5.0 mL), stirred at 80°C for 60 min.
However, the popularity and low cost of MgO in the chemical production industry are also advantageous. Direct recycling may not be an energy- or cost-saving strategy. Waste catalysts containing only MgO are environmentally safe and easily incorporated into other industrial processes to obtain pure MgO back into the catalytic process.
To investigate the optimized conditions, the reaction was carried out with 3-nitrobenzaldehyde under various furnace power, time, temperature, acid concentration, and solvent conditions. After treatment, the yield of 5-(3-nitrobenzylidene)-2-(morpholin-4-yl)-1,3-thiazol-4(5H)-one (III.a-2) was 73%.
5.2 Extending the scope of the one-pot reaction over MgO
The microwave-assisted one-pot reaction method, which synthesizes 5-alkyl/arylidene-1,3-thiazolidin-4-one derivatives in the presence of MgO as a solid base catalyst, was entirely suitable for opening extensive research scales with different derivatives of benzaldehyde and morpholine or thiomorpholine for the products of III.a1–9,12; III.b1–4,10–12 and III.c-2, respectively, in Table 4. The obtained yields of the derivatives were acceptable or good, ranging from 55 to 76%. As a positive result, 18 final products have been obtained, of which 13/18 compounds have not been mentioned in any literature before; the summary of known and unknown compounds is listed in the supporting information. The influence of the substituents on the reaction yields was not evident; electrophilic substituents tended to increase the reaction efficiency. In addition, substances with morpholine as the starting material yielded higher yields than those with thiomorpholine. In addition, compound III.c-2 was successfully synthesized with N-propylthiourea, which demonstrates the flexibility of the one-pot methodology.
Synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives
 |
A plausible mechanism for product formation is presented in Scheme 2, in which MgO activates a three-component reaction. Initially, under the influence of MgO, a positive charge appears on the C═O of chloroacetyl chloride, which is favorable for the attack of the electron pair on the NH2 group in thiourea to create intermediate (A). Then, the intramolecular nucleophile of S is attacked into chloromethyl carbon with a ring closure to form thiazolidin-4-one (B) while removing MgCl2, which can dissolve in EtOH. The mutual tautomerization of 1,3-thiazolidin-4-one into 1,3-thiazolidin-4-ol (B′), accompanied by the activation of MgO, facilitates Knoevenagel condensation with the corresponding aldehyde to form the product, followed by the separation of H2O. MgO is insoluble in EtOH; therefore, at the end of the reaction, the catalyst can be filtered and activated for reuse.

Mechanism of the formation of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one.
In the proposed reaction mechanism, the intermediate compound that did not undergo the Knoevenagel condensation step (compound B) was found to be a trace on the GCMS chromatogram, and the product of the direct condensation reaction between substances I.a,b and aldehydes to form N′-alkyl/aryl-N-alkyl/phenylmethylidene thiourea compounds also achieved yields below 10%. Competition exists between the two condensation reactions under the same conditions, but the one-pot reaction combined with the Knoevenagel condensation dominates. This also explains why the efficiency of the one-pot reaction is only moderate.
6 Conclusions
The one-pot reaction in the microwave in the presence of the solid MgO catalyst and new starting compounds showed significant superiority over the traditional heating method by reducing the reaction time from 10 to 1 h. Ethanol is an environmentally friendly, green solvent. Seventeen compounds of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivative were synthesized in a good yield of 55–76% with an extended scope of one derivative with a primary amine, in which there were 13 new final compounds. The structure of the products was confirmed by modern physicochemical methods, such as IR, NMR, and MS.
-
Funding information: This research was performed under the research project QG.21.10 –“A new approach to the synthesis of some 5-arylidene-3-aryl-2-arylimino-1,3-thiazolidin-4-one derivatives” of Vietnam National University, Hanoi.
-
Author contributions: Son Nguyen Thi: writing – original draft, formal analysis, project administration; Duc Nguyen Van: conducting experiments; Linh Nguyen Nhat Thuy: conducting experiments; Anh Pham Nam: conducting experiments; Boi Luu Van: conducting experiments. Hoang Do Huy: writing – review and editing, methodology, and visualization.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
[1] Arshad MF, Alam A, Alshammari AA, Alhazza MB, Alzimam IM, Alam MA, et al. Thiazole: A versatile standalone moiety contributing to the development of various drugs and biologically active agents. Molecules. 2022;27(13):3994.10.3390/molecules27133994Search in Google Scholar PubMed PubMed Central
[2] Yele V, Mohammed AA, Wadhwani AD. Synthesis and evaluation of aryl/heteroaryl benzohydrazide and phenylacetamide derivatives as broad‐spectrum antibacterial agents. ChemistrySelect. 2020;5(34):10581–87.10.1002/slct.202002178Search in Google Scholar
[3] Jain AK, Vaidya A, Ravichandran V, Kashaw SK, Agrawal RK. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorg Med Chem. 2012;20(11):3378–95.10.1016/j.bmc.2012.03.069Search in Google Scholar PubMed
[4] Lesyk R, Zimenkovsky B. 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr Org Chem. 2004;8(16):1547–77.10.2174/1385272043369773Search in Google Scholar
[5] Sharma A, Sharma D, Saini N, Sharma SV, Thakur VK, Goyal RK, et al. Recent advances in synthetic strategies and SAR of thiazolidin-4-one containing molecules in cancer therapeutics. Cancer Metast Rev. 2023;42(3):847–89.10.1007/s10555-023-10106-1Search in Google Scholar PubMed PubMed Central
[6] Löscher W, Von Hodenberg A, Nolting B, Fassbender CP, Taylor C. Ralitoline: A reevaluation of anticonvulsant profile and determination of “active” plasma concentrations in comparison with prototype antiepileptic drugs in mice. Epilepsia. 1991;32(4):560–8.10.1111/j.1528-1157.1991.tb04693.xSearch in Google Scholar PubMed
[7] Zhang X, Li X, Li D, Qu G, Wang J, Loiseau PM, et al. Ionic liquid mediated and promoted eco-friendly preparation of thiazolidinone and pyrimidine nucleoside–thiazolidinone hybrids and their antiparasitic activities. Bioorg Med Chem Lett. 2009;19(22):6280–3.10.1016/j.bmcl.2009.09.101Search in Google Scholar PubMed
[8] Petrou A, Eleftheriou P, Geronikaki A, Akrivou MG, Vizirianakis I. Novel thiazolidin-4-ones as potential non-nucleoside inhibitors of HIV-1 reverse transcriptase. Molecules. 2019;24(21):3821.10.3390/molecules24213821Search in Google Scholar PubMed PubMed Central
[9] Ottaná R, Mazzon E, Dugo L, Monforte F, Maccari R, Sautebin L, et al. Modeling and biological evaluation of 3, 3′-(1, 2-ethanediyl) bis [2-(4-methoxyphenyl)-thiazolidin-4-one], a new synthetic cyclooxygenase-2 inhibitor. Eur J pharmacology. 2002;448(1):71–80.10.1016/S0014-2999(02)01888-5Search in Google Scholar PubMed
[10] Ottanà R, Carotti S, Maccari R, Landini I, Chiricosta G, Caciagli B, et al. In vitro antiproliferative activity against human colon cancer cell lines of representative 4-thiazolidinones. Part I Bioorg Med Chem Lett. 2005;15(17):3930–3.10.1016/j.bmcl.2005.05.093Search in Google Scholar PubMed
[11] Karalı N, Terzioğlu N, Gürsoy A. Synthesis and primary cytotoxicity evaluation of new 5‐bromo‐3‐substituted‐hydrazono‐1H‐2‐indolinones. Arch der Pharm: An Int J Pharm Med Chem. 2002;335(8):374–80.10.1002/1521-4184(200211)335:8<374::AID-ARDP374>3.0.CO;2-KSearch in Google Scholar
[12] Havrylyuk D, Mosula L, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R. Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur J Med Chem. 2010;45(11):5012–21.10.1016/j.ejmech.2010.08.008Search in Google Scholar
[13] Zhou H, Wu S, Zhai S, Liu A, Sun Y, Li R, et al. Design, synthesis, cytoselective toxicity, structure–activity relationships, and pharmacophore of thiazolidinone derivatives targeting drug-resistant lung cancer cells. J Med Chem. 2008;51(5):1242–51.10.1021/jm7012024Search in Google Scholar
[14] Bourahla K, Guihéneuf S, Limanton E, Paquin L, Le Guével R, Charlier T, et al. Design and microwave Synthesis of New (5 Z) 5-Arylidene-2-thioxo-1, 3-thiazolinidin-4-one and (5 Z) 2-Amino-5-arylidene-1, 3-thiazol-4 (5H)-one as New Inhibitors of Protein Kinase DYRK1A. Pharmaceuticals. 2021;14(11):1086.10.3390/ph14111086Search in Google Scholar
[15] Bolli MH, Abele S, Binkert C, Bravo R, Buchmann S, Bur D, et al. Mangold Cl. 2-imino-thiazolidin-4-one derivatives as potent, orally active S1P1 receptor agonists. J Med Chem. 2010;53(10):4198–211.10.1021/jm100181sSearch in Google Scholar
[16] Ottanà R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, et al. 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem. 2005;13(13):4243–52.10.1016/j.bmc.2005.04.058Search in Google Scholar
[17] Moghaddam FM, Hojabri L. A novel synthesis of some 2‐imino‐4‐thiazolidinone derivatives. J Heterocycl Chem. 2007;44(1):35–8.10.1002/jhet.5570440106Search in Google Scholar
[18] Chavan AA, Pai NR. Synthesis and antimicrobial screening of 5-arylidene-2-imino-4-thiazolidinones. Arkivoc. 2007;16:148–55.10.3998/ark.5550190.0008.g16Search in Google Scholar
[19] Kundenapally R, Domala R, Bathula S. Synthesis and Antibacterial Activity of Novel 5-arylidene-2-imino-3-(2-phenyl-1, 8-naphthyridin-3-yl) thiazolidin-4-ones. Asian J Pharm Clin Res. 2019;12:150–3.10.22159/ajpcr.2019.v12i18.34273Search in Google Scholar
[20] Pulici M, Quartieri F. Traceless solid-phase synthesis of 2-amino-5-alkylidene-thiazol-4-ones. Tetrahed Lett. 2005;46(14):2387–91.10.1016/j.tetlet.2005.02.059Search in Google Scholar
[21] Bourahla K, Derdour A, Rahmouni M, Carreaux F, Bazureau JP. A practical access to novel 2-amino-5-arylidene-1, 3-thiazol-4 (5H)-ones via sulfur/nitrogen displacement under solvent-free microwave irradiation. Tetrahedron Lett. 2007;48(33):5785–9.10.1016/j.tetlet.2007.06.078Search in Google Scholar
[22] Insuasty A, Ramírez J, Raimondi M, Echeverry C, Quiroga J, Abonia R, et al. Synthesis, Antifungal and Antitumor Activity of Novel (Z)-5-Hetarylmethylidene-1, 3-thiazol-4-ones and (Z)-5-Ethylidene-1, 3-thiazol-4-ones. Molecules. 2013;18(5):5482–97.10.3390/molecules18055482Search in Google Scholar PubMed PubMed Central
[23] Mukhopadhyay C, Ray S. Rapid and straightforward one-pot expeditious synthesis of 2-amino-5-alkylidene-thiazol-4-ones at room temperature. Tetrahedron Lett. 2011;52(48):6431–8.10.1016/j.tetlet.2011.09.090Search in Google Scholar
[24] Anderluh M, Jukič M. Three-component one-pot synthetic route to 2-amino-5-alkylidene-thiazol-4-ones. Tetrahedron. 2009;65(1):344–50.10.1016/j.tet.2008.10.045Search in Google Scholar
[25] Shariati N, Baharfar R. An efficient one‐pot synthesis of 2‐amino‐5‐arylidenethiazol‐4‐ones catalyzed by MgO nanoparticles. J Chin Chem Soc. 2014;61(3):337–40.10.1002/jccs.201300425Search in Google Scholar
[26] Lalithamba H, Latha H, Narendra N, Mala S. Green synthesis, structural, electrical and catalytic properties of nano-MgO. J Electron Mater. 2024;53(1):30–40.10.1007/s11664-023-10779-ySearch in Google Scholar
[27] Kiyani H, Ghorbani F. Expeditious green synthesis of 3, 4-disubstituted isoxazole-5 (4 H)-ones catalyzed by nano-MgO. Res Chem Intermed. 2016;42:6831–44.10.1007/s11164-016-2498-7Search in Google Scholar
[28] Dabhane H, Ghotekar S, Tambade P, Pansambal S, Oza R, Medhane V. MgO nanoparticles: Synthesis, characterization, and applications as a catalyst for organic transformations. Eur J Chem. 2021;12(1):86–108.10.5155/eurjchem.12.1.86-108.2060Search in Google Scholar
[29] Rostamnia S, Pourhassan F. The SBA-15/SO3H nanoreactor as a highly efficient and reusable catalyst for diketene-based, four-component synthesis of polyhydroquinolines and dihydropyridines under neat conditions. Chin Chem Lett. 2013;24(5):401–3.10.1016/j.cclet.2013.03.020Search in Google Scholar
[30] Singh JP, Singh V, Sharma A, Pandey G, Chae KH, Lee S. Approaches to synthesize MgO nanostructures for diverse applications. Heliyon. 2020;6(9):04882–96.10.1016/j.heliyon.2020.e04882Search in Google Scholar PubMed PubMed Central
[31] Sing WT, Lee CL, Yeo SL, Lim SP, Sim MM. Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor. Bioorg Med Chem Lett. 2001;11(2):91–4.10.1016/S0960-894X(00)00610-7Search in Google Scholar
[32] Kandeel K. The behaviour of E, Z-5-arylmethylidene-2-thioxo-1, 3-thiazolidin-4-one and 3-[(2-oxo-2H-1-benzopyran-3-yl) dithio]-2H-1-benzopyran-2-one derivatives towards some amines. name. Chem Pap. 2004;58(5):334.Search in Google Scholar
[33] Omar M, Sherif F. Studies on 4‐Thiazolidinoenes. VI. Reactions of 5‐arylmethylene‐2‐alkylthio‐2‐thiazolin‐4‐ones with ammonium carbonate and amines. J Praktische Chem. 1980;322(5):835–42.10.1002/prac.19803220520Search in Google Scholar
[34] Lobo P, Boja Poojary BP, Prasad D, Kumari N. Synthesis, spectroscopic characterization and antimicrobial activity of 5-arylidene-2-substituted-1, 3-thiazol-4-one. Der Pharma Chemica. 2014;6(5):14–18.Search in Google Scholar
[35] Raouf A, MT O, El Bayoumy, K. Studies on 4-thiazolidinones. II. reaction of secondary amines with 5-arylidenerhodanines and their salts. Acta Chimica Academiae Scientiarum Hungaricae. 1974;83(3–4):359–65.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

