Abstract
Utilizing an innovative and highly light-absorbing photocathode, the direct conversion of hydrogen gas from Red Sea water is achieved. This involves creating a new nanocomposite thin film through a one-pot synthesis method, combining poly(O-chloroaniline) with trichalcogenides (MoS3) and MoO3 (MoS3–molebednium oxide/poly(o-chloroaniline)). This nanocomposite has remarkable morphological and optical properties with potential as a photocathode for hydrogen generation by harnessing the power of Red Sea water. This nanocomposite exhibits a unique semi-spherical architecture, with an average size of around 150 nm. These semi-spherical particles are surrounded by a dense network of fibers, forming a complex structure that provides ample space for trapping photons when exposed to light. The distinctive morphology significantly influences the optical properties of this nanocomposite, showing strong absorbance across a wide range of optical wavelengths up to ∼700 nm, with a promising bandgap of 1.75 eV. The hydrogen generation is quantified by measuring the generated photocurrent density (J ph) as a function of the light incidence frequency using various optical filters at a fixed potential of −0.8 V. The highest J ph values are recorded at −0.558 and −0.553 mA·cm−2 for wavelengths of 340 and 440 nm, respectively; the value reaches its maximum at the white light with a wavelength of −0.63 mA·cm−2. Elevating the temperature from 30°C to 50°C results in a substantial enhancement of the J ph values, increasing from −0.63 to −0.71 mA·cm−2, respectively. This temperature increase leads to a noteworthy improvement in incidnce photon to current conversion from 1.85% to 2.22%. This photoelectrode demonstrates not only cost-effectiveness but also eco-friendliness, making it an attractive choice for H2 generation by Red Sea water as a natural, environmentally friendly, and economically viable hydrogen source. Consequently, this study holds significant promise for industrial applications due to its economic and eco-friendly characteristics.
1 Introduction
The escalating demand for energy, driven by industrialization and burgeoning populations, is predominantly met through the utilization of fossil fuels. However, this heavy reliance on non-renewable sources is placing an increasing burden on the global environment. The adverse environmental impacts of fossil fuel usage, such as air and water pollution and greenhouse gas emissions, have prompted widespread apprehension among nations. Recognizing the urgency of addressing both pollution concerns and the impending energy crisis, numerous countries are actively engaged in ongoing endeavors to explore alternative methods for establishing clean and sustainable energy systems [1,2].
Efforts are being steadfastly directed toward the development and implementation of renewable energy solutions. The global emphasis is shifting toward harnessing energy from sources such as solar, wind, hydro, and geothermal power [3,4]. This transition aims to mitigate the environmental toll associated with conventional energy production, contributing to a more sustainable and eco-friendly future. Countries worldwide are investing in research, technology, and infrastructure to facilitate the widespread adoption of clean energy practices. The pursuit of innovative solutions is driven by a shared commitment to reduce dependency on finite fossil fuel reserves and curb the ecological repercussions linked to their extraction and combustion [5,6,7].
Hydrogen gas (H2) occupies a significant role as a secondary energy source due to its lightweight nature, high calorific value, and environmentally friendly combustion characteristics, setting it apart from other energy sources [8,9]. Despite its potential as a sustainable energy solution, the current technologies for hydrogen production remain relatively expensive. However, ongoing efforts are consistently focusing on the development of more cost-effective and streamlined methods for hydrogen production.
Various methods have been employed to generate hydrogen (H2), encompassing processes such as the thermal decomposition of water through nuclear energy, steam reforming using methane or propane, solid electrolysis, petrochemical procedures reliant on petroleum, biomass gasification, and water splitting. Notably, water electrolysis and water splitting emerge as superior options, given their lack of secondary pollutant emissions, setting them apart from other contemporary H2 production technologies [10,11]. Among these methods, the water-splitting process stands out as the most innovative and advanced, particularly when facilitated by a photocatalyst in the presence of light. Nanoscale semiconductors, acting as photocatalysts due to their optical properties, are frequently chosen for this purpose. However, despite their advantages, these nanomaterials exhibit a drawback in their inability to function optimally under visible or ambient solar light conditions. The water-splitting process utilizing nanoscale semiconductors is considered cutting-edge due to its capacity to produce H2 without releasing secondary pollutants. This environmentally friendly characteristic positions it as a source and a promising technology for renewable gas. The photocatalytic surface, activated by light, plays a pivotal role in this method, contributing to the efficiency of the water-splitting process. While nanomaterials, particularly semiconductors, hold promise, their limitation in harnessing visible or ambient solar light remains a challenge that researchers are actively working to overcome. The ongoing pursuit of more effective nanomaterials capable of harnessing a broader spectrum of light underscores the commitment to refining water-splitting technology for enhanced and sustainable hydrogen production. Other researchers have conducted experiments aimed at improving polymer materials for hydrogen generation. However, these attempts yielded only modest results in terms of photocurrent values (J ph) [12,13,14]. Additionally, it is worth noting that all these researchers relied on a hydrogen generation system that necessitated the use of an external electrolyte. This reliance on external electrolytes not only imposes cost constraints on hydrogen gas production but also contributes to increased corrosion susceptibility of the photocathode [15,16].
Researchers have indeed made efforts to enhance polymer materials for the purpose of generating hydrogen. Regrettably, the outcomes of these endeavors have shown limited success, particularly with regard to the photocurrent values achieved. Furthermore, it is important to highlight that all of these study initiatives have hinged on hydrogen generation systems that require the incorporation of an external electrolyte. This dependency on external electrolytes has led to cost implications, as it adds to the expenses associated with hydrogen gas production. Moreover, the use of external electrolytes has also raised concerns about the heightened susceptibility of the photocathode to corrosion, further complicating the overall efficiency and practicality of the hydrogen generation process [17].
Herein, a novel nanocomposite thin film, MoS3–molebednium oxide/poly(o-chloroaniline) [MoO3/POCA], is successfully fabricated through a one-pot technique. This nanocomposite exhibits exceptional morphological and optical properties, making it a prospective candidate as a photocathode for hydrogen generation. Importantly, the hydrogen generation experiments are conducted using Red Sea water without the addition of any supplementary electrolytes. Relative to its excellent optical properties and significant bandgap, this photocatalyst electrode can efficiently produce hydrogen gas directly from Red Sea water without the need for additional sacrificial agents or external inputs. The hydrogen generation process is investigated under diverse lighting conditions, including monochromatic light, white light, and the absence of light (darkness). Furthermore, the hydrogen generation tests are conducted at different temperatures, and the incidnce photon to current conversion (IPCE) is determined as a result. What distinguishes this photoelectrode is its notable cost-effectiveness and eco-friendliness, making it an appealing option for hydrogen production, utilizing the abundant and environmentally friendly resource that is Red Sea water. As a result, this research holds significant potential for industrial applications due to its economic feasibility and environmentally sustainable attributes.
2 Materials and methods
2.1 Materials
K2S2O8 and NaOH were provided by the Egyptian company Pio Chem. O-Chloroaniline and HCl were acquired from the German company Merk. Sodium molybdate (Na2MoO4) was sourced from Winlab in the United Kingdom.
2.2 MoS3–MoO3/POCA nanocomposite preparation
The POCA polymer is created through the oxidation of the OCA monomer, utilizing K2S2O8 as the oxidizing agent. This reaction is meticulously conducted with precise control over the concentrations of the monomer, oxidant, and acid, maintaining concentrations of 0.06, 0.15, and 0.4 M, respectively. Subsequently, the resulting polymer goes through a series of treatment steps, including thorough washing, to prepare it for subsequent characterization processes.
The synthesis of the MoS3–MoO3/POCA nanocomposite thin film follows a similar procedure, using Na2MoO4 and K2S2O8 as the oxidants, both at a concentration of 0.07 M for the oxidation reaction. Through this oxidation process, the MoS3–MoO3/POCA nanocomposite is generated as a thin film that coats glass slides. This comprehensive process ensures the production of the desired nanocomposite material for further analysis and application.
2.3 MoS3–MoO3/POCA thin-film photocathode for green hydrogen generation
Assessing the efficiency of the MoS3–MoO3/POCA thin-film photocathode for green hydrogen production involves measuring the generated current density (J ph) using the PowerStation CHI608E. This J ph value is indicative of the photocathode’s activity, representing how quickly electrons are transferred to the surrounding Sea water to drive the process of water splitting.
In the experimental setup, a three-electrode cell is utilized, with the MoS3–MoO3/POCA thin film serving as the main electrode. This setup also includes counter (constructed from graphite) and reference electrodes (calomel). To illuminate the system, a metal halide lamp functions as the light source.
To evaluate the photocathode’s sensitivity in splitting Red Sea water, tests are conducted under various conditions. These conditions encompass complete darkness, white light exposure, and exposure to different monochromatic lights. The control over these lighting conditions is achieved using various wavelength filters. Throughout this experimental process, thermodynamic parameters are deduced by analyzing the J ph values recorded at different temperatures.
3 Results and discussion
3.1 Analyses
The chemical composition analysis of both POCA and the MoS3–MoO3/POCA nanocomposite was conducted using fourier-transform infrared spectroscopy (FTIR) analysis, as illustrated in Figure 1(a). In this analysis, the distinctive functional groups of POCA were identified based on their respective wavenumber positions, which provide insights into the vibrational behavior of electron bonds within these groups. These functional groups encompass N–H, C–N, and C═C, each characterized by its specific position on the FTIR spectrum. Upon examining the MoS3–MoO3/POCA nanocomposite, it was observed that these same functional groups were still present like the pure POCA. However, there were slight alterations in their positions on the spectrum. These modifications can be attributed to the incorporation of the inorganic filler, MoS3–MoO3, into the nanocomposite matrix. A comprehensive summary of these wavenumber positions, both before and after the formation of the nanocomposite, is provided in Table 1.
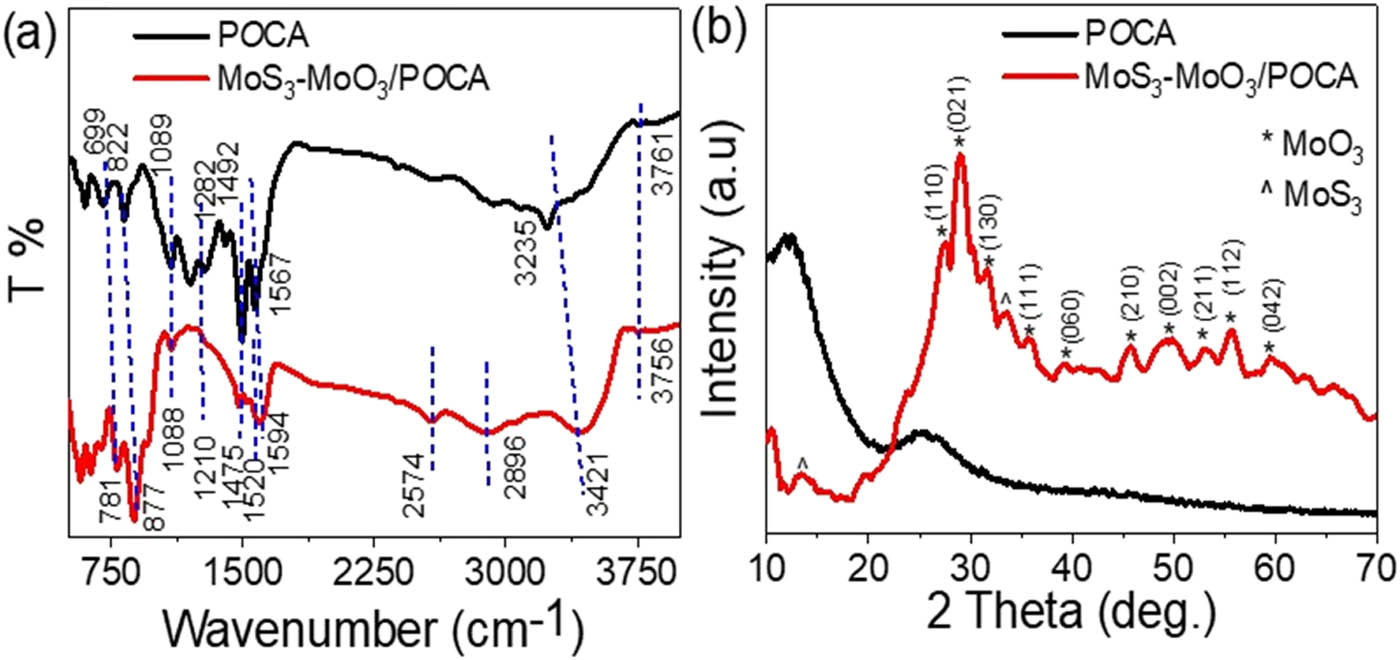
(a) FTIR of the synthesized POCA and MoS3–MoO3/POCA and (b) XRD patterns of these materials.
Condensed representation of the bands’ associated groups found in both POCA and the MoS3–MoO2/POCA nanocomposite
| Function group | Band position (cm−1) | |
|---|---|---|
| MoS3–MoO3/POCA | POCA | |
| N–H | 3,421 | 3,235 |
| Quinoid C═C | 1,594 | 1,567 |
| Benzene C═C | 1,475 | 1,492 |
| C–N | 1,320 | 1,304 |
| C–H | 1,088 | 1,089 |
| Para-disubstituted ring | 877 | 822 |
The X-ray diffraction (XRD) analysis, Figure 1(b), complements the FTIR findings and offers insights into the structural aspects of the materials, including their crystalline nature, growth orientation, and size. Notably, the XRD pattern of the POCA component demonstrates an amorphous nature, indicated by the absence of discernible peaks. This outcome aligns with the typical behavior observed in most polymeric materials, which tend to lack well-defined crystalline structures.
Conversely, the XRD pattern of the MoS3–MoO3/POCA nanocomposite exhibits distinct and sharp peaks, attesting to its significant crystallinity. The presence of MoO3 in the nanocomposite is unmistakably confirmed by the appearance of peaks at specific angles: 27.3°, 29.2°, 31.7°, 35.9°, 39.1°, 45.8°, 49.8°, 53.2°, 55.9°, and 59.5° for the miller indices (110), (021), (130), (111), (060), (210), (002), (211), (112), and (042), respectively (as per JCPDS-05-0508) [18]. Likewise, the presence of MoS3 is established through identifiable week peaks at 13.6° and 33.6°; these very week peaks indicate the amorphous nature of MoS3 [19].
To determine the crystalline size of the MoS3–MoO3/POCA nanocomposite, a calculation was performed utilizing Eq. 1 [20,21]. The outcome of this calculation yielded an estimated size of 30 nm. This particular assessment serves a dual purpose, shedding light on both the crystalline nature of the nanocomposite and offering invaluable insights into its structural attributes. The utilization of Eq. 1, in this context, facilitated the quantification of the nanocomposite’s crystalline size. This parameter is of paramount importance in materials science, as it provides a crucial dimension of understanding concerning the atoms and molecules within the material. In the case of the MoS3–MoO3/POCA nanocomposite, the determined crystalline size of 30 nanometers suggests a well-organized and ordered structure at the nanoscale. Furthermore, the estimation of crystalline size is significant in the context of the nanocomposite’s performance and properties. It has implications for its optical, mechanical, and electronic behaviors. A smaller crystalline size can lead to altered optical properties, increased mechanical strength, and enhanced electrical conductivity. Conversely, a larger crystalline size may result in different optical characteristics and altered mechanical and electronic behaviors. Thus, this characterization not only confirms the crystalline nature of the nanocomposite but also imparts critical knowledge about how its structural properties can impact its overall performance in various applications [22].
The elemental composition of the synthesized MoS3–MoO3/POCA material is determined through X-ray photoelectron spectroscopy (XPS) survey analysis, as depicted in Figure 2(a). This analysis provides insights into the constituents of the material and their respective binding energies. In Figures 2(d)–(f), the binding energies of C, N, and Cl elements within the polymer composite are identified at 285.4, 400.3, and 200.4 electron volts (eV), respectively. The presence of Cl is associated with the acid anion, which intercalates within the polymer chains and establishes electrostatic interactions with the positively charged nitrogen atoms in POCA. This interaction is clearly discerned in the XPS survey [23].

XPS analyses of POCA and the MoS3–MoO3/POCA nanocomposite: (a) survey spectra, along with individual spectra for (b) Mo, (c) O, (d) C, (e) N, and (f) Cl elements.
Furthermore, the inorganic filler, MoS3, is unequivocally detected. The binding energies for MoS3 are found at Mo3d5/2 (231.4 eV) and Mo3d3/2 (233.8 eV), as illustrated in Figure 2(b). These binding energies confirm the existence of MoS3 in the material [24].
Similarly, the XPS survey confirms the formation of Mo(VI) for MoO3 at 232.6 and 235.7 eV that demonstrate the Mo3d5/2 and Mo3d3/2, correspondingly [25], as indicated in Figure 2(b). Additionally, the presence of oxygen (O) is established at a binding energy of 531.6 eV (Figure 2(c)).
So, the XPS survey analysis gives a wide understanding of the elemental composition and charges of the MoS3–MoO3/POCA material. It confirms that the main elements constructed the polymer POCA, such as carbon (C), nitrogen (N), chlorine (Cl), molybdenum (Mo), and oxygen (O), and their respective binding energies. These findings are instrumental in characterizing the material’s chemical composition and offer valuable insights into their potential applications.
Figure 3 provides an insightful visual representation scanning electron microscope (SEM) of the morphological characteristics of the synthesized MoS3–MoO3/POCA nanocomposite (Figure 3(a)) and the pure POCA material (Figure 3(b)). The pure POCA polymer exhibits a stark contrast when compared to the MoS3–MoO3/POCA nanocomposite. The pure polymer displays the formation of long, fibrous materials characterized by a significant level of porosity. In contrast, the nanocomposite showcases a distinct semispherical structure, with an average diameter of approximately 150 nm. These semispherical particles are enveloped by a highly fibrous network that forms an intricate structure, creating a substantial network and cavity for the effective trapping of photons during light exposure. Figure 3(c), which presents a TEM image, further reinforces these features of the nanocomposite. It reveals the presence of conspicuously dark particles embedded within the polymer fiber, which appears with a faint color. This configuration underscores the unique structure of the nanocomposite, where the semi-spherical particles are encapsulated by a finely textured fiber network [26].

(b) SEM, (c) transmission electron microscopy, and (d) simulated morphological of MoS3–MoO3/POCA nanocomposite, while (a) SEM of pure polymer, POCA.
Additionally, Figure 3(d) offers a simulated image of the polymer composite, vividly illustrating the semi-spherical particles coated with the intricate fiber network. This network comprises minute particles that contribute to the composite’s distinct morphology. These structural disparities have significant implications for the material’s properties and performance, particularly in photon trapping during light exposure, making the nanocomposite an intriguing subject for various applications.
Figure 4(a) provides insight into the optical characteristics of the synthesized MoS3–MoO3/POCA nanocomposite and the pure polymer POCA, revealing significant improvements in their optical properties following the formation of the polymer composite. This improvement is especially notable in the nanocomposite’s absorbance spectrum, which exhibits a broad and intense absorbance profile, extending well to the near-infrared segment. This striking absorbance behavior underscores the material’s exceptional light-absorbing capabilities. Conversely, the pure POCA polymer displays a modest absorbance pattern, characterized by strong peak in the ultraviolet region at 300 nm, but with absorbance extending up to 700 nm.

Optical characteristics of both the synthesized pure polymer POCA and the MoS3–MoS3–MoO3/POCA nanocomposite: (a) absorbance and (b) bandgap measurements.
Using the Tauc equation, (Eqs. 2 and 3) [27], to estimate the bandgap, the substantial enhancements in optical properties are further substantiated. The calculated bandgap values demonstrate a decrease from 1.82 to 1.75 eV following the formation of the polymer composite. This reduction in bandgap is indicative of improved light absorbance within the nanocomposite.
These enhancements in optical properties can be attributed to the nanocomposite’s morphological characteristics. The presence of morphological features such as cavities and traps plays a pivotal role in facilitating light absorbance. This morphological behavior contributes to the formation of efficient pathways for photon absorbency and electron transitions. Consequently, the nanocomposite generates substantial hot electron clouds that are readily available to participate in various chemical reactions, particularly those related to hydrogen generation.
So. these enhancements in optical behavior make the nanocomposite well suited for applications requiring efficient light absorption and electron transition capabilities, particularly in the context of hydrogen generation reactions [28,29].
3.2 Photoelectrochemical measurements
The photoelectrochemical evaluation of the synthesized MoS3–MoO3/POCA nanocomposite thin-film photocathode is conducted to facilitate hydrogen generation through the splitting of Red Sea water, and the chemical composition (heavy metals) of which is detailed in Table 2. The utilization of Red Sea water holds promise due to its cost-effectiveness and environmentally friendly nature, offering a sustainable source of green hydrogen gas. Notably, this study is carried out without the need for additional electrolytes, underscoring the potential to harness natural resources for this purpose.
Heavy metal sacrificing agent concentration (µg·L−1) in the Red Sea water
| Heavy metal | Concentration (µg·L−1) |
|---|---|
| Fe | 12 |
| Cu | 100 |
| Cd | 1 |
| B | 132 |
| Mn | 9 |
| Zn | 44 |
| Cr | 5 |
| Pb | 8 |
| Ni | 1 |
The testing procedure is conducted within a three-electrode cell configuration, with the MoS3–MoO3/POCA nanocomposite thin-film photocathode serving as the working electrode. To complement the setup, graphite is employed, and then, the calomel is utilized as the reference electrode. The optical filters are deployed to precisely control the wavelengths of incident light. This rigorous testing approach aims to assess the sensitivity and efficiency of the photocathode in catalyzing the desired photoelectrochemical reactions.
Figure 5(a) displays the impact of white light on the sensitivity of the created MoS3–MoO3/POCA nanocomposite thin-film photocathode. As the incident light intensity increases, there is a corresponding rise in the generated photocurrent, shifting from −0.43 to −0.63 mA·cm−2 at a fixed potential of −0.8 V. This notable variation in photocurrent (J ph) clearly signifies the remarkable sensitivity of the photocathode to incident photons. The diverse components within this photocathode contribute to its absorbance characteristics, amalgamating into a composite material with unified absorbance properties.

Sensitivity of the MoS3–MoO3/POCA nanocomposite thin-film photocathode (a) under light and dark through current–potential relationship and (b) under chopped light.
In Figure 5(b), we observe an enhancement in sensitivity during both light activation and deactivation phases, highlighting the stability and reproducibility of the synthesized MoS3–MoO3/POCA nanocomposite thin-film photocathode. This particular sample exhibits exceptional stability and sensitivity, a quality that has been thoroughly evaluated. The straightforward fabrication techniques employed for this photocathode, as well as its application within the context of a photoelectrochemical cell, are worth noting. Additionally, the study of its performance in Red Sea water, a natural sample, provides valuable insights into its potential for industrial applications [30].
In Figure 6(a), we further investigate the sensitivity of the MoS3–MoO3/POCA thin-film photocathode by subjecting it to various photon wavelengths. As different wavelengths of light interact with the photocathode, specific photocurrents (J ph) are generated, and these values show a clear trend of increasing with the photons. This energy relationship is in accordance with equation, E = hv [31]. Calculations reveal that each photon carries an energy of 3.6 and 2.8 eV for wavelengths of 340 and 440 nm, respectively. Consequently, these two wavelengths result in substantial J ph values, indicating the photocathode’s heightened sensitivity.

Potential–current relationship for the estimation of the responsivity of the synthesized MoS3–MoO3/POCA nanocomposite thin-film photocathode (a) at various light frequency and (b) the J ph values (at −0.8 V).
Figure 6(b) provides the electrochemical estimated current behavior of these various optical photons. The maximum J ph values observed are −0.558 and −0.553 mA·cm−2 for the wavelengths of 340 and 440 nm, respectively. This behavior aligns seamlessly with the optical characteristics presented in Figure 4 for the bandgap under various transfer processes of hot electrons to the upper band. These hot electrons form dense clouds that are readily available for transfer to the adjacent Red Sea water solution. Within this water, the electrons play a pivotal role in a complex mechanism that culminates in the production of OH˙ radicals. These radicals subsequently recombine with water molecules, driving a splitting reaction that leads to the evolution of hydrogen gas [32,33]. Notably, the presence of heavy metals in the water acts as catalysts, promoting and facilitating the splitting reaction. These metal ions exhibit dynamic motion within the water when subjected to the applied potential [34,35]. Conversely, the favorable J ph values observed at 550 and 730 nm are attributed to the interaction between the nanocomposite’s vibrational bonds and incident wavelengths within the infrared region. The broad range of wavelengths that elicit substantial photocurrent underscores the photocathode’s exceptional sensitivity across a wide spectrum of electromagnetic radiation.
Furthermore, the study also examined how J ph values are influenced by different temperatures, (Figure 7(a)). From this figure, the J ph values show a substantial increase, transitioning from −0.63 to −0.71 mA·cm−1 with 30°C to 50°C temperature values, correspondingly. This trend is intimately connected to the heightened movement of ions as temperatures climb, which, in turn, correlates with the kinetic energy of these ions. Consequently, the production of hydrogen gas increases in tandem with these heightened ion movements, driven by amplified rates of diffusion and enhanced overall conductivity of the solution [36].

(a) Evaluated J ph values using the synthesized MoS3–MoO3/POCA photocathode under different temperatures ranging from 30°C to 60°C and (b) the corresponding incident photon-to-current efficiency (IPCE) at these varied temperatures.
Simultaneously, the investigation encompasses the estimation of incident photon to electron IPCE, as governed by Eq. 1, across different temperatures, with results graphically depicted in Figure 7(b). Here, it becomes apparent that as the temperature escalates from 30°C to 60°C, the IPCE exhibits an upward trajectory, elevating from 1.85% to 2.22%, respectively. This intriguing behavior is related to the intricate processes occurring within the photocathode. Initially, incident photons give rise to the formation of electron–hole pairs. Subsequently, efficient charge separation occurs, and hot electrons undergo swift transitions into the neighboring solution.
The IPCE values [17,37] obtained are particularly promising, considering that the photocathode was synthesized using a one-pot technique and was supported on a glass substrate. Moreover, the utilization of eco-friendly natural sources underscores the potential for green hydrogen production and gives additional cost-effective advantages, making this research especially valuable in the context of sustainable energy solutions.
So, these findings are particularly promising, given the environmentally friendly and cost-effective approach employed, further emphasizing the potential for green hydrogen production as part of a sustainable energy strategy.
The synthesized MoS3–MoO3/POCA nanocomposite photocathode exhibits exceptional characteristics, particularly in the context of its small bandgap, calculated to be 1.75 eV, as demonstrated in Figure 4(b). This small bandgap has a crucial role in facilitating the sequence of electron transitions within the nanocomposite [38,39]. MoS3, being a primary material, effectively accumulates all the electrons during this process. Subsequently, these accumulated electrons undergo additional transitions toward the Red Sea water, initiating the hydrogen generation reaction.
Concurrently, POCA serves as the primary receptor for the holes generated within the nanocomposite, facilitating their movement in the opposite direction. This orchestrated movement of both photogenerated electrons and holes sets the stage for the efficient generation of H2 gas, showcasing a remarkable behavior as depicted in Figure 8.

Hydrogen generation under the sequential movements in the holes and electrons in the opposite direction.
The nanocomposite’s ability to harness the synergistic effects of MoS3, MoO3, and POCA in the photocathode structure demonstrates promising potential for advancing clean energy technologies, particularly in the context of hydrogen production. The well-coordinated electron and hole movements within the nanocomposite contribute to its effective performance, making it a noteworthy candidate for sustainable and efficient hydrogen generation (Table 3).
Estimated J ph value of this fabricated MoS3–MoO3/POCA photocathode in comparison with the previous studies
| Photoelectrode | Electrolyte | J ph (mA·cm−2) |
|---|---|---|
| CoS2-CoO/poly-2-aminothiophenol [40] | Red Sea water | 0.13 |
| Ppy/graphene oxide [41] | Sewage water | 0.11 |
| Cr2S3-Cr2O3/poly-2-aminobenzene-1-thiol [42] | Sewage water | 0.017 |
| As2O3/polypyrrole [43] | Red Sea water | 0.24 |
| Poly(3‐aminobenzoic acid) frame [44] | H2SO4 | 0.08 |
| MnS2-MnO2/Poly-2-amino-1-mercaptobenzne [45] | Sewage water | 0.26 |
| ZnO nanowires [31] | Na2SO4 | 0.05 |
| MoS3–MoO3/POCA (this work) | Red Sea water | 0.63 |
4 Conclusions
This study provides a novel synthesized composite and technique for the direct conversion of hydrogen gas from Red Sea water. This process involves the creation of a novel nanocomposite thin film through a one-pot synthesis method, which combines MoS3–MoO3/POCA. This nanocomposite possesses remarkable morphological and optical properties, making it a promising photocathode candidate for hydrogen generation from Red Sea water. With a distinctive semi-spherical architecture and an average size of around 150 nm, the nanocomposite particles are enveloped by a dense network of fibers, creating a complex structure that effectively traps photons when exposed to light. This unique morphology significantly influences the optical behavior of the nanocomposite, demonstrating strong absorbance across a broad range of optical wavelengths up to approximately 700 nm, along with a promising bandgap of 1.75 eV. When subjected to both dark and light at a constant potential of −0.8 V, the photocurrent density experiences a significant increase, shifting from −0.43 to −0.63 mA·cm−2, correspondingly. Notably, the highest recorded J ph values are achieved at −0.558 and −0.553 mA·cm−2 for wavelengths of 340 and 440 nm, underscoring the nanocomposite’s impressive sensitivity to different light wavelengths.
Furthermore, a substantial enhancement in J ph values is observed as the temperature rises from 30°C to 50°C, escalating from −0.63 to −0.71 mA·cm−2, correspondingly. This temperature-dependent improvement is accompanied by a notable increase in the IPCE, surging from 1.85% to 2.22%.
What sets this photoelectrode apart is its remarkable cost-effectiveness and eco-friendliness, rendering it an attractive choice for hydrogen generation using the naturally abundant and environmentally friendly resource, Red Sea water. Consequently, this study holds substantial promise for various industrial applications, owing to its economically viable and environmentally sustainable characteristics.
Acknowledgment
Researchers Supporting Program Number (RSPD2024R845), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: This research was funded by Researchers’ Supporting Program Number (RSPD2024R845), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Mohamed Rabia – experimental and writing; Eman Aldosari – writing, supervision, and project management; Qinfang Zhang – supervision, revision, and management.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Giuntoli F, Menegon L, Siron G, Cognigni F, Leroux H, Compagnoni R, et al. Methane-hydrogen-rich fluid migration may trigger seismic failure in subduction zones at forearc depths. Nat Commun. 2024;15:1–16. 10.1038/s41467-023-44641-w.Search in Google Scholar PubMed PubMed Central
[2] Jovičević-Klug M, Souza Filho IR, Springer H, Adam C, Raabe D. Green steel from red mud through climate-neutral hydrogen plasma reduction. Nature. 2024;625(7996):703–9. 10.1038/s41586-023-06901-z.Search in Google Scholar PubMed PubMed Central
[3] Purvis G, Šiller L, Crosskey A, Vincent J, Wills C, Sheriff J, et al. Generation of long-chain fatty acids by hydrogen-driven bicarbonate reduction in ancient alkaline hydrothermal vents. Commun Earth Environ. 2024;5:1–9. 10.1038/s43247-023-01196-4.Search in Google Scholar
[4] Tsao CW, Narra S, Kao JC, Lin YC, Chen CY, Chin YC, et al. Dual-plasmonic Au@Cu7S4 Yolk@shell nanocrystals for photocatalytic hydrogen production across visible to near infrared spectral region. Nat Commun. 2024;15:1–13. 10.1038/s41467-023-44664-3.Search in Google Scholar PubMed PubMed Central
[5] Petrakopoulou F, García-Tenorio E. Evaluating hydrogen-based electricity generation using the concept of total efficiency. Energy Convers Manag. 2023;293:117438. 10.1016/J.ENCONMAN.2023.117438.Search in Google Scholar
[6] Meda US, Rajyaguru YV, Pandey A. Generation of green hydrogen using self-sustained regenerative fuel cells: opportunities and challenges. Int J Hydrog Energy. 2023;48:28289–314. 10.1016/J.IJHYDENE.2023.03.430.Search in Google Scholar
[7] Sharma R, Almáši M, Punia RC, Chaudhary R, Nehra SP, Dhaka MS, et al. Solar-driven polymer electrolyte membrane fuel cell for photovoltaic hydrogen production. Int J Hydrog Energy. 2023;48:37999–8014. 10.1016/J.IJHYDENE.2022.12.175.Search in Google Scholar
[8] Constantinou P, Stock TJZ, Tseng L-T, Kazazis D, Muntwiler M, Vaz CAF, et al. EUV-induced hydrogen desorption as a step towards large-scale silicon quantum device patterning. Nat Commun. 2024;15:1–13. 10.1038/s41467-024-44790-6.Search in Google Scholar PubMed PubMed Central
[9] Giovanniello MA, Cybulsky AN, Schittekatte T, Mallapragada DS. The Influence of additionality and time-matching requirements on the emissions from grid-connected hydrogen production. Nat Energy. 2024;2024:1–11. 10.1038/s41560-023-01435-0.Search in Google Scholar
[10] Abdelazeez AAA, Ben A, Trabelsi G, Alkallas FH, Alfaify S, Shkir M, et al. Reproducible preparation of thin graphene films using a green and efficient liquid-phase exfoliation method for applications in photovoltaics. 2023;13(9):1628. 10.3390/coatings13091628.Search in Google Scholar
[11] Xie H, Zhao Z, Liu T, Wu Y, Lan C, Jiang W, et al. A Membrane-based seawater electrolyser for hydrogen generation. Nature. 2022;612(7941):673–8. 10.1038/s41586-022-05379-5.Search in Google Scholar PubMed
[12] Alsultan M, Choi J, Jalili R, Wagner P, Swiegers GF. Synergistic amplification of (photo)catalytic oxygen and hydrogen generation from water by thin-film polypyrrole composites. Mol Catal. 2020;490:110955. 10.1016/J.MCAT.2020.110955.Search in Google Scholar
[13] Abdelazeez AAA, Trabelsi ABG, Alkallas FH, Elaissi S, Rabia M. Facile preparation of flexible lateral 2D MoS2 nanosheets for photoelectrochemical hydrogen generation and optoelectronic applications. Photonics. 2022;9:638. 10.3390/PHOTONICS9090638.Search in Google Scholar
[14] El-Rahman AMA, Rabia M, Mohamed SH. Nitrogen doped TiO2 films for hydrogen generation and optoelectronic applications. J Mater Sci: Mater Electron. 2023;34:1–9. 10.1007/S10854-023-10551-2/METRICS.Search in Google Scholar
[15] Hafeez HY, Mohammad J, Suleiman AB, Ndikilar CE, Sa’id RS, Muhammad I. Robust one-pot solvothermal incorporation of InVO4 with polymeric-C3N4 nanosheets with improved charge carrier separation and transfer: a highly efficient and stable photocatalyst for solar fuel (H2) Generation. Mater Sci Eng: B. 2023;297:116682. 10.1016/J.MSEB.2023.116682.Search in Google Scholar
[16] Xu J, Jiao Z, Li Z, Tian Y, Liu B, Yue G, et al. Tuning acceptor content and molecular structure in linear donor–acceptor polymeric photocatalysts for ultrahigh-performance visible-light-driven hydrogen evolution. Chem Eng J. 2023;473:145359. 10.1016/J.CEJ.2023.145359.Search in Google Scholar
[17] Ahmed A, Ahmed AM, Abdel-Khaliek AA, Shaban M, Abdelazeez AAA, Rabia M. CuFeO2/Cu photoelectrode modified with SrTiO3 perovskite for efficient hydrogen generation from sanitation water. Int J Energy Res. 2023;2023:1–10. 10.1155/2023/4440117.Search in Google Scholar
[18] Sen SK, Dutta S, Khan MR, Manir MS, Dutta S, Al Mortuza A, et al. Characterization and antibacterial activity study of hydrothermally synthesized H-MoO3 nanorods and α-MoO3 nanoplates. BioNanoScience. 2019;9:873–82. 10.1007/S12668-019-00671-7/TABLES/3.Search in Google Scholar
[19] Shirota G, Nasu A, Deguchi M, Sakuda A, Tatsumisago M, Hayashi A. Electrode Performance of amorphous MoS3 in all-solid-state sodium secondary batteries. J Power Sources Adv. 2021;10:100061. 10.1016/J.POWERA.2021.100061.Search in Google Scholar
[20] Lim DJ, Marks NA, Rowles MR. Universal scherrer equation for graphene fragments. Carbon. 2020;162:475–80. 10.1016/J.CARBON.2020.02.064.Search in Google Scholar
[21] Burton AW, Ong K, Rea T, Chan IY. On the estimation of average crystallite size of zeolites from the Scherrer equation: a critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mater. 2009;117:75–90. 10.1016/J.MICROMESO.2008.06.010.Search in Google Scholar
[22] Zulqarnain M, Ali SS, Cheng C, Nadeem K, Rizwan M, Anwar T. Structural tuning interlinking various optical, dielectric and magnetic trends in annealed Mn0.5Zn0.5Fe2O4 spinel ferrites nanostructures. J Magn Magn Mater. 2023;565:170252. 10.1016/J.JMMM.2022.170252.Search in Google Scholar
[23] Rabia M, Elsayed AM, Salem AM, Abdallah Alnuwaiser M. Highly uniform multi-layers reduced graphene oxide/poly-2-aminobenzene-1-thiol nanocomposite as a promising two electrode symmetric supercapacitor under the effect of absence and presence of porous-sphere polypyrrole nanomaterial. Micromachines. 2023;14:1424. 10.3390/MI14071424.Search in Google Scholar PubMed PubMed Central
[24] Cheng CK, Lin JY, Huang KC, Yeh TK, Hsieh CK. Enhanced efficiency of dye-sensitized solar counter electrodes consisting of two-dimensional nanostructural molybdenum disulfide nanosheets supported Pt nanoparticles. Coatings. 2017;7:167. 10.3390/COATINGS7100167.Search in Google Scholar
[25] Elsayed AM, Alkallas FH, Trabelsi ABG, Rabia M. Highly uniform spherical MoO2-MoO3/Polypyrrole core-shell nanocomposite as an optoelectronic photodetector in UV, Vis, and IR domains. Micromachines. 2023;14:1694. 10.3390/MI14091694.Search in Google Scholar
[26] Zhang K, Jiang Z, Qiu Z. Effect of different lengths of side groups on the thermal, crystallization and mechanical properties of novel biodegradable poly(ethylene succinate) copolymers. Polym Degrad Stab. 2021;187:109542. 10.1016/J.POLYMDEGRADSTAB.2021.109542.Search in Google Scholar
[27] Haryński Ł, Olejnik A, Grochowska K, Siuzdak K. A facile method for tauc exponent and corresponding electronic transitions determination in semiconductors directly from uv–vis spectroscopy data. Optical Mater. 2022;127:112205. 10.1016/J.OPTMAT.2022.112205.Search in Google Scholar
[28] Ayub S, Siddique A, Khalil A, Shaheen R, Bilal Tahir M, ben hadj hassine S, et al. An extensive investigation of structural, electronic, optical, magnetic, and mechanical properties of YGaO3 for photovoltaic and optoelectronic applications: first-principles approach. Inorg Chem Commun. 2023;153:110754. 10.1016/J.INOCHE.2023.110754.Search in Google Scholar
[29] Khan MJI, Batool HS, Akhtar P, Latif A, Ahmad J, Gull U, et al. Theoretical investigations of electronic structure, magnetic and optical properties of CdS-X (X = Sm, La, Ce) materials for optoelectronic applications. Solid State Commun. 2023;373–374:115332. 10.1016/J.SSC.2023.115332.Search in Google Scholar
[30] Wu Y, Liu X, Pandey A, Zhou P, Dong WJ, Wang P, et al. III-nitride nanostructures: emerging applications for micro-LEDs, ultraviolet photonics, quantum optoelectronics, and artificial photosynthesis. Prog Quantum Electron. 2022;85:100401. 10.1016/J.PQUANTELEC.2022.100401.Search in Google Scholar
[31] Al-saeedi SI. Photoelectrochemical green hydrogen production utilizing ZnO nanostructured photoelectrodes. 2023;14(5):1047. 10.3390/mi14051047.Search in Google Scholar PubMed PubMed Central
[32] Rasras AJ, Al-Far RH, Younes EA, Shakdofa MM, Al-Rifai NM. Synthesis and Crystal Structure, Hirshfeld surface analysis, and DFT calculation of 2-((4-(1-Benzyl-2-Methyl-4-Nitro-1H-Imidazol-5-Yl)Piperazin-1-Yl)Methyl)-5-Phenyl-1,3,4-Oxadiazole. J Mol Struct. 2023;1280:135069. 10.1016/J.MOLSTRUC.2023.135069.Search in Google Scholar
[33] Shi Y, Li L, Xu Z, Guo F, Shi W. Construction of full solar-spectrum available S-scheme heterojunction for boosted photothermal-assisted photocatalytic H2 production. Chem Eng J. 2023;459:141549. 10.1016/J.CEJ.2023.141549.Search in Google Scholar
[34] Wang Y, Meng C, Zhao L, Zhang J, Chen X, Zhou Y. Surface and near-surface engineering design of transition metal catalysts for promoting water splitting. Chem Commun. 2023;59:8644–59. 10.1039/D3CC01593A.Search in Google Scholar PubMed
[35] Mo QL, Hou S, Wei ZQ, Fu XY, Xiao G, Xiao FX. Fine tuning of charge motion over homogeneous transient metal chalcogenides heterostructured photoanodes for photoelectrochemical water splitting. Chem Eng J. 2022;433:133641. 10.1016/J.CEJ.2021.133641.Search in Google Scholar
[36] Saha D, Kruse P. Choice–review–conductive forms of MoS2 and Their applications in energy storage and conversion. J Electrocheml Soc. 2020;167:126517. 10.1149/1945-7111/ABB34B.Search in Google Scholar
[37] Mohamed HSH, Rabia M, Zhou X-G, Qin X-S, Khabiri G, Shaban M, et al. Phase-junction Ag/TiO2 nanocomposite as photocathode for H2 generation. J Mater Sci Technol. 2021;83:179–87. 10.1016/j.jmst.2020.12.052.Search in Google Scholar
[38] Takata T, Jiang J, Sakata Y, Nakabayashi M, Shibata N, Nandal V, et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature. 2020;581(7809):411–4. 10.1038/s41586-020-2278-9.Search in Google Scholar PubMed
[39] Al Angari YM, Ewais HA, Rabia M. Hydrogen generation from red sea water using CsSnI2Cl lead-free perovskite/porous CuO nanomaterials: Coast of Jeddah, Saudi Arabia. J Mater Sci: Mater Electron. 2023;34:1–12. 10.1007/S10854-023-11597-Y/METRICS.Search in Google Scholar
[40] Rabia M, Aldosari E, Zhang Q. Green hydrogen photoelectrochemically produced from red sea water using a photocathode dichalcogenides (CoS2)-CoO/Poly-2-Aminothiophenol nanocomposite with moon-like shape. Chem Pap. 2024;2024:1–13. 10.1007/S11696-024-03478-3.Search in Google Scholar
[41] Hamid MMA, Alruqi M, Elsayed AM, Atta MM, Hanafi HA, Rabia M. Testing the photo-electrocatalytic hydrogen production of polypyrrole quantum dot by combining with graphene oxide sheets on glass slide. J Mater Sci: Mater Electron. 2023;34:1–11. 10.1007/S10854-023-10229-9/METRICS.Search in Google Scholar
[42] Rabia M, Elsayed AM, Alnuwaiser MA. Cr2S3-Cr2O3/Poly-2-aminobenzene-1-thiol as a highly photocatalytic material for green hydrogen generation from sewage water. Micromachines. 2023;14:1567. 10.3390/MI14081567.Search in Google Scholar PubMed PubMed Central
[43] Hadia NMA, Rabia M, Alzaid M, Mohamed WS, Hasaneen M, Ezzeldien M, et al. As2O3-Poly(1H-Pyrrole) nanocomposite for hydrogen generation from red sea water with high efficiency. Phys Scr. 2023;98:085509. 10.1088/1402-4896/ACE391.Search in Google Scholar
[44] Modibane KD, Waleng NJ, Ramohlola KE, Maponya TC, Monama GR, Makgopa K, et al. Poly(3-Aminobenzoic Acid) decorated with cobalt zeolitic benzimidazolate framework for electrochemical production of clean hydrogen. Polymers. 2020;12:1581. 10.3390/polym12071581.Search in Google Scholar PubMed PubMed Central
[45] Rabia M, Elsayed AM, Alnuwaiser MA. Mn (IV) Oxide/Mn (IV) sulfide/Poly-2-amino-1-mercaptobenzene for green hydrogen generation. Surf Innov. 2023;12:282–91. 10.1680/JSUIN.23.00031.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

