An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
-
Bilal Ahmad Khan
, Muhammad Ather Nadeem
and Nehal Elnaggar
Abstract
One of the most significant biotic constraints that wheat production faces is weed infestation. Wheat is infested with different weeds that cause yield losses (up to 100%) that vary based on the type of weed, their density, and the environmental conditions. Chemical weed control is the most common method to control weeds in wheat. However, widespread herbicide resistance (>365 cases worldwide) has challenged the sustainability of this method. Use of nanoherbicides is a promising strategy to cope with the issue of herbicide resistance. To achieve weed control conditions during the whole growing season, nanoformulations of herbicides are a delivery strategy that involves covering an active component with various materials that vary in size from nano to nanoscale and then releasing the substance in a controlled manner. Nanoherbicides prevent leaching and volatilization of active components and premature degradation through photolysis, hydrolysis, and biodegradation. According to studies, nanoencapsulation of herbicides produces more targeted and less hazardous agricultural formulations. Using nanoherbicides in lower concentrations is beneficial. It lessens the long-term impacts of herbicide residues in wheat fields and the toxicity of these herbicides to the environment. It is also beneficial in eliminating the weeds without ever interacting with the crop plants, which eventually results in a greater wheat yield. This review provides a comprehensive overview of the emerging field of utilizing nanoparticles (NPs) in herbicides for effective weed management in wheat crops. This article explores the novel approach of integrating NPs with herbicidal agents, highlighting their potential benefits and challenges. The review also addresses the current state of research, recent advancements, and potential future directions in this evolving area of agricultural science.
1 Introduction
The biological disadvantages of weeds involve allelopathy (they stop or hinder growth, germination, development of plants that grow nearby, and releasing toxic substances via leaves and roots), promoting invasion by easy dispersal of seeds, proliferating plagues by creating a favorable habitat like acting as a host for ascarides and arthropods, and in cultivated zones promoting persistence and stopping the contamination of products obtained through the normal harvest process [1,2]. The crop yield reproductive output and growth are affected by stress originating through the weeds grown proximity also increases the risk of mortality for whole plant even for its parts [3].
The factors that affect the magnitude of stress depending upon the seedling growth and germination are weed density, weed species, crop sowing methods and time, weed infestation duration, and environmental and fertilizer applications of weed seeds. Agricultural production that includes profits requires wise management decisions for bioeconomic weed control. The weed control is effective for regaining potential yield losses based on the choices that involve crop yield losses and the size of weed population to be caused by that population [4]. Preventive measures, cultural control, biological control, and chemical control are some of the approaches used to manage weeds and boost the production of agricultural plants. In comparison to other ways of weed management, using a chemical weed killer is the most effective weed control. Chemical substances used to control the weed (partially or specifically) are named “herbicides.” Herbicides kill plants through a biochemical or physical method that involves absorbing into plants, transferring them to the site of action while simultaneously altering or interrupting one or more of their metabolic activities [5,6]. They promote agricultural yield by controlling pests from crops. Environmental stuff like natural soil, water, and food are seriously affected by the offensive use of chemical products (herbicides) in agricultural practices [7]. Secondly, resistance in weeds against herbicides is increasing [8], making use of herbicides objectionable and thus increasing pressure to reduce herbicide use [9]. Consequently, it is necessary to decrease the herbicide dosage without diminishing the effectiveness of the treatment. The dose of herbicides for the management of weeds can be reduced by using adjuvants and nanotechnology. Use of nanotechnology is an effective tool for controlling weeds at lower doses of herbicides without sacrificing its efficacy. According to Preisler et al. [10], after a tenfold dilution, non-nano-atrazine (ATZ) and NC + ATZ treatment eliminated Bidens pilosa seedlings, suggesting that ATZ nano-encapsulation increased the herbicidal effectiveness. Compared to their bulk active components, nanoscale herbicides protect plants against weeds and diseases [11]. Sustainable agriculture aims to utilize fewer treatments and lower pesticide doses to decrease environmental contamination and toxin exposure to non-target species [12].
The utilization of matter on a minor scale includes the use of nanotechnology. Various surprising and interesting uses are seen by atoms and molecules when examined at the nanolevel. It is a field of science, that is producing, manipulating, and using composites and materials that range in nanometers. The particles that exhibit a size of 0.1 μm (100 nm) or less, in addition to possessing certain other specified qualities, are referred to as nanoparticles (NPs) [13]. In the environment, NPs react in various ways of entry and transport pathways. They differ significantly from other bulk materials in their properties [14]. The particles derived from the mass material exhibit distinct characteristics, such as size, distribution, and morphology, which differ from those of larger particles. Nanotechnology focuses on the production and stabilization of diverse NPs. It serves as an effective platform for delivering active materials to specific sites without compromising their activity [15]. Nanoformulations could enhance the solubility of poorly soluble active components, allow regulated release, and prevent degradation. Herbicide administration in the form of a nanostructure improves the bioavailability, targeting, and active ingredient release [16].
Nanoherbicides have slow degradation and controlled release under natural conditions that may prevent herbicide toxicity and boost bioactive compound effectiveness [17]. A study by Pontes et al. [18] studied the use of chitosan and tripolyphosphate NPs as carrier systems for paraquat herbicides on crops. The results of experiments revealed that the NPs of parquet cause no herbicide toxicity to crop as compared to non-nano-paraquat. The use of nanocapsulated herbicides for the control of parasitic weeds resulted in a reduction in the phytotoxicity of herbicides on crop plants [19]. Nanocapsules that are meticulously designed usually offer improved penetration through plant cuticles and facilitate a gradual and precise discharge of active components/constituents when they reach the intended weed target [19]. This approach necessitates reduced quantities of herbicides, as they remain unaffected by the crop, resulting in their selective accumulation within the parasitic weed through an effect called the sink effect [20]. Hussein et al. [21] presented information on creating an organic–inorganic nanohybrid material to achieve a controlled release of 2,4-dichlorophenoxy acetate. They used a self-assembly approach to host the herbicide active component that included zinc–aluminum-layered double hydroxide. The controlled release action of nanoherbicides resulting in persistence of herbicides in soil and cause damage to weeds when moisture is available. The “surfactant derived from nanotechnology” NPs in crop production are of utmost significance for eliminating herbicides [22]. Satvekar et al. [23] discovered that the nanoformulations of herbicides, which include nanodispersions and nanoemulsions, are intended to target the seed covering of weeds and hinder the germination of weed-related seeds. As compared to conventional herbicides nanoherbicides have a control release mechanism under natural conditions, less toxic to the environment, more effective even at a tenfold lower dose of recommended herbicides [16].
The excessive use of agrochemicals in the contemporary agricultural landscape has led to the emissions of the topsoil, groundwater, food, and the border ecosystem. This contaminating effect has occurred due to the increasing use of agrochemicals to boost agricultural productivity. It is equally important to consider the negative consequences on the environment, even though increasing crop production is the highest priority. As a result, alternative approaches must be explored. Nanotechnology is gaining significance in the agricultural sector due to its potential to revolutionize various aspects of farming. Nanotechnology can scientifically increase the agricultural productivity and reduce waste using monitoring systems, intelligent chemical and gene delivery mechanisms in crops, nanoherbicides, encapsulation methods, nanoformulations, and other applications. Consequently, this approach indirectly reduces the environmental pollution. Wheat stands as the most widely grown grain crop worldwide. However, weeds pose a significant threat to sustainable wheat production, causing significant yield losses. Chemical weed control is the most applicable method to control weeds in wheat crop. However, continuous use of herbicides has caused severe herbicide resistance in weeds and environmental pollution [8,9]. Alternatively, nanoherbicides offer opportunity to control weeds with the application of very less herbicidal dose as compared to traditional herbicide application [17]. This review article provides a comprehensive overview of the potential application of nanoherbicides for efficient control of weeds in wheat with minimal herbicidal use.
2 NPs and herbicides
2.1 Nanotechnology
Currently, nanotechnology is the sixth revolutionary technology that has emerged. Designing, characterizing, producing, and using a structure, device, or system by regulating its form and size at the nanoscale is the art and science referred to as nanotechnology. NPs enter and transport in the environment in a variety of ways. These particles have enhanced properties such as size, morphology, and distribution that are entirely new compared to larger particles of mass [24]. The remarkable characteristics of NPs are immense due to the confinement of electrons inside a region, just a nanometer in size. These materials are stacked atomically layer by layer and are unique [25]. Nanotechnology deals with various types of NP production and stabilization. NPs act as a delivery vehicle for active materials to targeted sites. Nanoformulations enhance the solubility of active substances with limited solubility and provide a regulated release that efficiently protects against premature degradation [26]. Thus, nanoformulated delivery systems increase the bioavailability. They are able to protect plants and promote their growth. They also detect plant and animal diseases, improve the food quality, and reduce waste to increase sustainability in agriculture [8]. Nanotechnology can transform the agriculture and food industries with innovative gears for rapid detection and molecular management of diseases and refine the ability of plants to absorb nutrients [27]. In plant biotechnology and agriculture, nanodevices and materials are opening up new applications. There is more targeted use of inputs and formulation of new toxins for the management of weeds and pests. There are also new plant and animal characteristics and a diversity of agricultural practices and products within unified production systems [28]. This technology is quickly establishing itself as a matchless meaningful tool in modern agriculture and is foreseen to soon be suited as a major economic power. Agricultural nanotechnology uses include agrochemical and pesticide distribution, nanoscale transporters, and smart packaging [29].
The development of nanoherbicides is being pursued to address the challenges associated with weed management and the depletion of the seed bank of weeds. They control weeds, reduce the amount of chemicals sprayed, and increase the production by minimizing losses of herbicides and pesticides (Figure 1). Nanopesticides and nanoherbicides are used to test new products and herbicides to boost output without causing any contamination to the land or water. One of the primary concerns of using nanotechnology in agricultural settings is the possibility of protection against a wide range of biotic and abiotic stressors [30]. If we can drastically cut herbicide usage, by combining the ideas of allelopathy with nanotechnology, we may be able to reverse the situation by mimicking nature without sacrificing productivity. Nanotechnology not only increases crop yields but also can reduce the use of bulk agrochemicals, controlling weeds with even ten times lower doses compared to commercial herbicides [31]. It also has the potential to provide more effective solutions to current problems facing the agricultural industry, such as the herbicide resistance in weeds developed with the overuse of herbicides with the equivalent action modes [32].
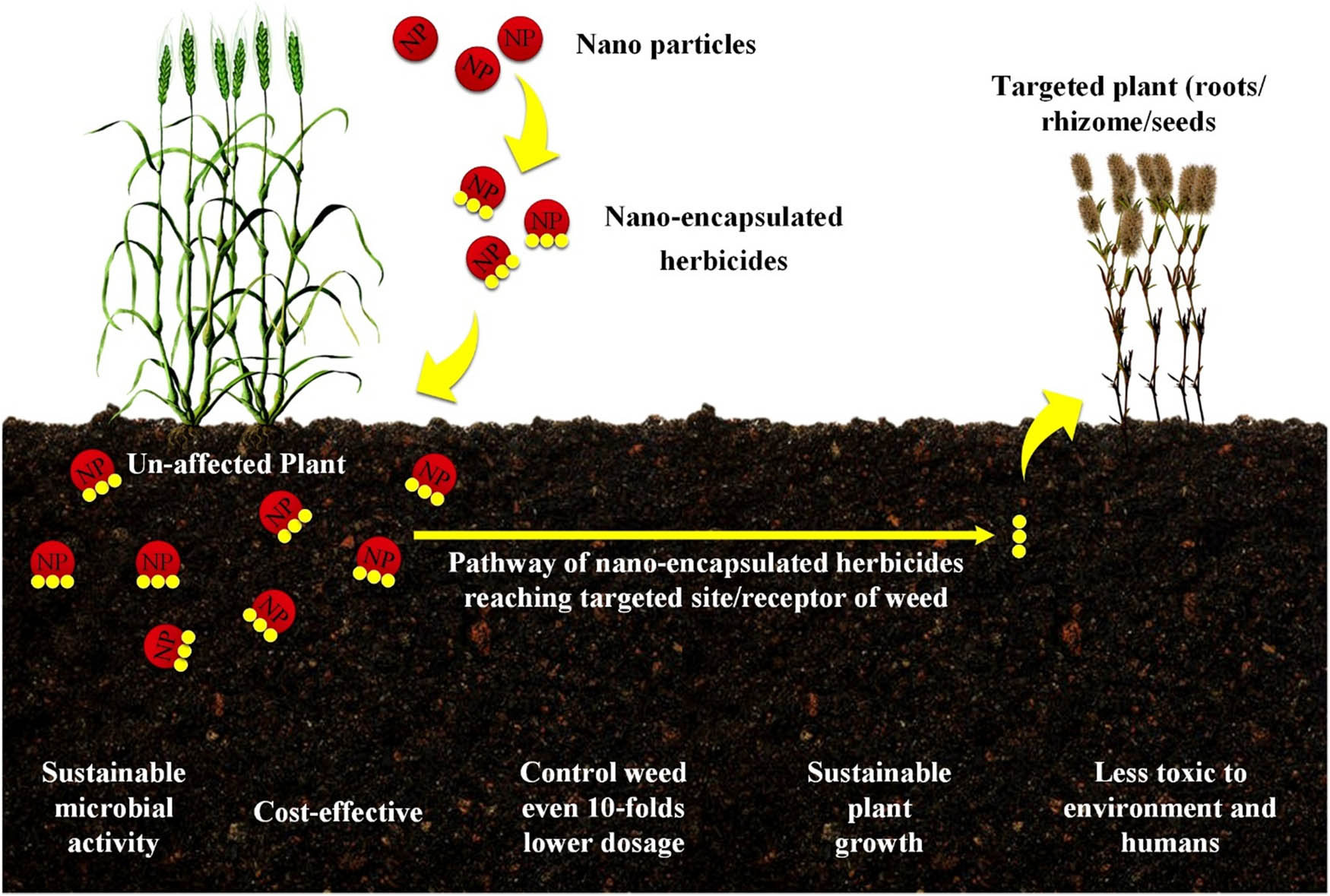
Beneficial aspects of nanoherbicides on targeted wheat weed elimination.
2.2 NPs as a herbicide carrier
Weeds compete with crops for nutrients, light, water, and growing space, leading to challenges in agriculture. Furthermore, to address this issue, herbicides have been extensively used with an estimated usage of 0.79 million tons in various crops like soyabean (Glycine max) [10], wheat (Triticum aestivum) [33], and maize (Zea mays) [34]. However, concerns about the environmental, animal, human, and microbial toxicity of herbicides have led to debate. These substances due to their persistence, mobility, and chemical composition may cause genotoxic effects on exposed organisms, including the risk of cancer, mutations, and even death after prolonged exposure [35]. To mitigate these concerns, the use of nanoherbicides has emerged as a potential solution. Nanoherbicides offer several advantages, including chemical stability, solubility, bioaccessibility, photodecomposition, and soil sorption [36]. According to a recent research by Dhiman et al. [37], cross-linking the disulfide bonds in the herbicide Diuron displayed 85% encapsulation efficiency with chitosan resulting in the development of nanoherbicides. The developed Diuron as a nanoherbicide provided sustained release of this herbicide. The regulated release of nanoherbicides, which were dependent on the concentration of glutathione, improved plant development while having a lower toxicity level. Similarly, the combination of chitosan and sodium triphosphate with ATZ as a herbicide resulted in reduced toxicity against soil microorganisms compared to the application of ATZ alone, thereby minimizing environmental risks [38]. Another study found that NPs of epsilon-caprolactone increased the activity of ATZ [39]. Furthermore, nanoclay-based formulations have been explored for the stabilization of herbs. For instance, Shattar et al. [40] discovered that montmorillonite may function as a surfactant, which allows them to control the release of herbicides and increase their effectiveness. Marimuthu et al. [41] examined the pre-emergence herbicide impact of ATZ nanoencapsulation on B. pilosa without affecting G. max. Nanoencapsulating ATZ increased its herbicidal effectiveness, killing B. pilosa seedlings even after tenfold dilution. However, a short-term assay revealed intense toxicity to soybean when treated with all ATZ-containing products. In a long-term assay, soybean plants gradually recovered from ATZ phototoxicity. A comparison of the effects of nano- and non-nano-ATZ in polynanocapsules did not enhance the herbicides’ long term residual effects on soybean [39]. In another study by Khan et al. [42], the use of fenoxaprop-p-ethyl and clodinofop propargyl NPs reduced the plant height, fresh biomass, and dry biomass of Phalaris minor. Even at a tenfold lower dosage of nanoformulation than nanoherbicides, the exact impact was demonstrated. Khan et al. [43] found that P. minor was ultimately injured by chitosan-based NPs of clodinofop propargyl and fenoxaprop-p-ethyl at the indicated dosage of commercial herbicides. Compared to commercial herbicides, herbicide-loaded NPs were more effective.
2.3 Controlled release formulations and mechanism of nanoherbicides
Controlled release formulations, often known as CRFs, are formulations intended to distribute active substances, such as herbicides, in a controlled way over different periods. The mechanism of release upon response to stimuli is pore diffusion, surface desorption, capsule bulging, and degradation [44,45]. These formulations are typically used to eliminate the possibility of off-target effects and environmental pollution and enhance the effectiveness and durability of herbicide treatments (Figure 1).
CRFs can be used with chitosan-based herbicides to improve their efficacy and reduce their potential adverse effects on nontarget organisms. Chitosan may be released gradually over a period of time, according to the design of CRFs, because the outer chitosan layer has the ability to degrade gradually over time. Mattos et al. [46] conducted a study where they found that employing controlled release techniques for herbicides leads to enhanced availability of active ingredients at a specific location for extended durations compared to traditional methods. Consequently, this approach facilitates a gradual discharge of active ingredients into the soil resulting in comprehensive coverage and prolonged suppression of weed growth. The use of biodegradable chitosan NPs that degrade over time and release the herbicide gradually is a promising approach for controlled release of herbicides [47]. Several factors may be used to limit the rate at which herbicides are released from chitosan NPs. These include the degree of deacetylation, the size and form of the NPs, and the use of stabilizers or crosslinking agents. The degradation rate of chitosan NPs can be controlled by adjusting the degree of deacetylation. Chitin, the natural polymer from which chitosan is derived, has undergone deacetylation to varying degrees [48]. Herbicides are released quicker when chitosan is degraded at a rate corresponding to the degree of deacetylation. The size and appearance of chitosan NPs may also influence herbicide release rates. The surface area-to-volume ratio of smaller NPs is more excellent, which leads to faster degradation and release of herbicides [49]. The shape of the chitosan NPs, such as spherical or rod-shaped NPs, can also affect the release rate. The use of stabilizing agents or crosslinking agents can further control the degradation and release herbicides from chitosan NPs. As an example, crosslinking substances like glutaraldehyde or genipin can improve the stability and structural integrity of chitosan NPs, resulting in slower release of herbicides [50]. The gradual release of soil-applied herbicides from chitosan NPs can provide sustainable weed control and reduce the frequency of herbicide application, resulting in a reduction of herbicide residues in the environment. The use of biodegradable chitosan NPs may also minimize the potential for non-target effects associated with herbicide application.
Surface modification techniques can also be used to control the release of herbicides from chitosan NPs. These techniques involve the addition of functional groups or other materials to the surface of the NPs that can alter their physical and chemical properties [51]. A common technique for surface modification of chitosan NPs is the incorporation of mucoadhesive or lipophilic groups. Mucoadhesive groups, such as thiol or carboxyl groups, can improve the adhesion of the NPs to the plant surface, resulting in more controlled and targeted delivery of herbicides [52]. Lipophilic groups, such as alkyl chains or fatty acids, can increase the affinity of NPs for lipophilic herbicides, resulting in a more gradual release of the herbicide [44]. In addition to these methods, surface modification of chitosan NPs may also be accomplished by the use of cationic surfactants, such as cetyltrimethylammonium bromide (CTAB), which can improve the electrostatic interactions between the NPs and the plant surface [53]. This may improve the adhesion of the NPs to the plant surface and increase their effectiveness as herbicides. The release rate of herbicides from surface-modified chitosan NPs can be controlled by adjusting the properties of the surface-modifying agents, such as their charge, size, and hydrophobicity [54]. The type and concentration of herbicide used can also affect the release rate. Overall, surface modification techniques offer a versatile approach to controlling the release of herbicides from chitosan NPs. These techniques can be used to improve the efficacy and specificity of chitosan-based herbicides and can be adapted for a variety of applications and crop species.
2.4 Chitosan as a matrix for controlled release formulation
Chitosan is extensively utilized in agriculture for various purposes. It serves as a plant defensive mechanism, promotes plant growth, is used for seed coatings, facilitates the regulated and gradual release of insecticides, fertilizers, and nutrients into the soil, and provides protection from frost [55]. Agrochemicals and genetic material in a chitosan matrix act as a reservoir for active ingredients, controlling their release by protecting them from the environment. This property makes chitosan an efficient carrier system for gene delivery in plant transformation or the controlled release of pesticides [47,56]. According to Maleki et al. [57], chitosan is commonly employed as an encapsulating agent, particularly for the preparation of microparticles and NPs. Its cationic charge and low toxicity make it an ideal choice for delivering genes, proteins, and various types of drugs. Chitosan’s biocompatibility, biodegradability, nontoxicity, and adsorption capabilities have also rendered it highly beneficial in terms of the transportation of genetic materials and agrochemicals in a regulated manner [47]. As a result of the existence of free amino acid groups within its structure, chitosan exhibits superior chelating agent properties compared to chitin.
Chitosan has been utilized as a stabilizer and capping agent in the development of a controlled-release herbicide. Nnamonu et al. [58] conducted research where chitosan and starch, reinforced with alginate, were utilized to make slow-release herbicides. To control the release of ATZ and imidacloprid in water and inhibit their leaching in soil, they created a composite gel consisting of carboxymethyl-chitosan (CM-chit) and bentonite (H-bent) as a carrier for encapsulating these two pesticides. According to the research findings, the composite carrier successfully decreased the potential leaching of pesticides, which ultimately helps to reduce the negative impact of pesticides on the environment. In another study conducted by Rashidipour et al. [59], it was noticed that the interaction of paraquat with alginate/chitosan NPs changed the release profile of the herbicides as well as their behavior in the soil. This suggests that such a system could be a promising approach for mitigating the negative effects caused by paraquat. In addition to resolving concerns about environmental toxicity, this strategy has several advantages, one of which is a prolonged period of action for the active component of the herbicide on specific targets [60]. Chitosan has various applications in agriculture, including its use in plant defense mechanisms, plant growth stimulation, seed coating, protection from frost, and regulated and delayed release of insecticides, fertilizers, and nutrients into the soil [61]. One of the most significant benefits of encapsulating agrochemicals and genetic material inside the chitosan matrix is that it can operate as a reservoir protecting active substances. This protective role shelters the components from the environment that surrounds them. At the same time, they are contained inside the chitosan domain, which enables the elements to be released in a regulated manner [62]. Because of this feature, chitosan is an efficient solution for the delivery of genes in plant transformation or for the application of insecticides in a regulated manner. Consequently, the application of herbicides in the form of nanoherbicides is recommended to mitigate environmental hazards and enhance the herbicide efficacy.
3 Significance of herbicide NPs in wheat management for wheat crop
Weeds pose a significant challenge to wheat production, leading to decreased productivity through competition for resources, negative effects on crop plants through allelopathy, and acting as a host for pests and pathogens, thus increasing harvest coats (Figure 2). Global studies have shown that weed competition causes significant wheat yield losses (even up to 100% in severe weed infestation) (Table 1) than the combined impact of insect pests and diseases [92,93]. Wheat yield can be reduced on average by 10–65% due to weed infestation [94]. Non-chemical weed control methods are not much effective in controlling the major weeds (P. minor and Avena fatua) of weed crop due to mimicry of weed plant with wheat [95]. Consequently, the use of herbicides becomes necessary; however, selecting the most suitable herbicide, determining the appropriate timing of application, and using the perfect dosage are the crucial factors to ensure profitable outcomes [96].
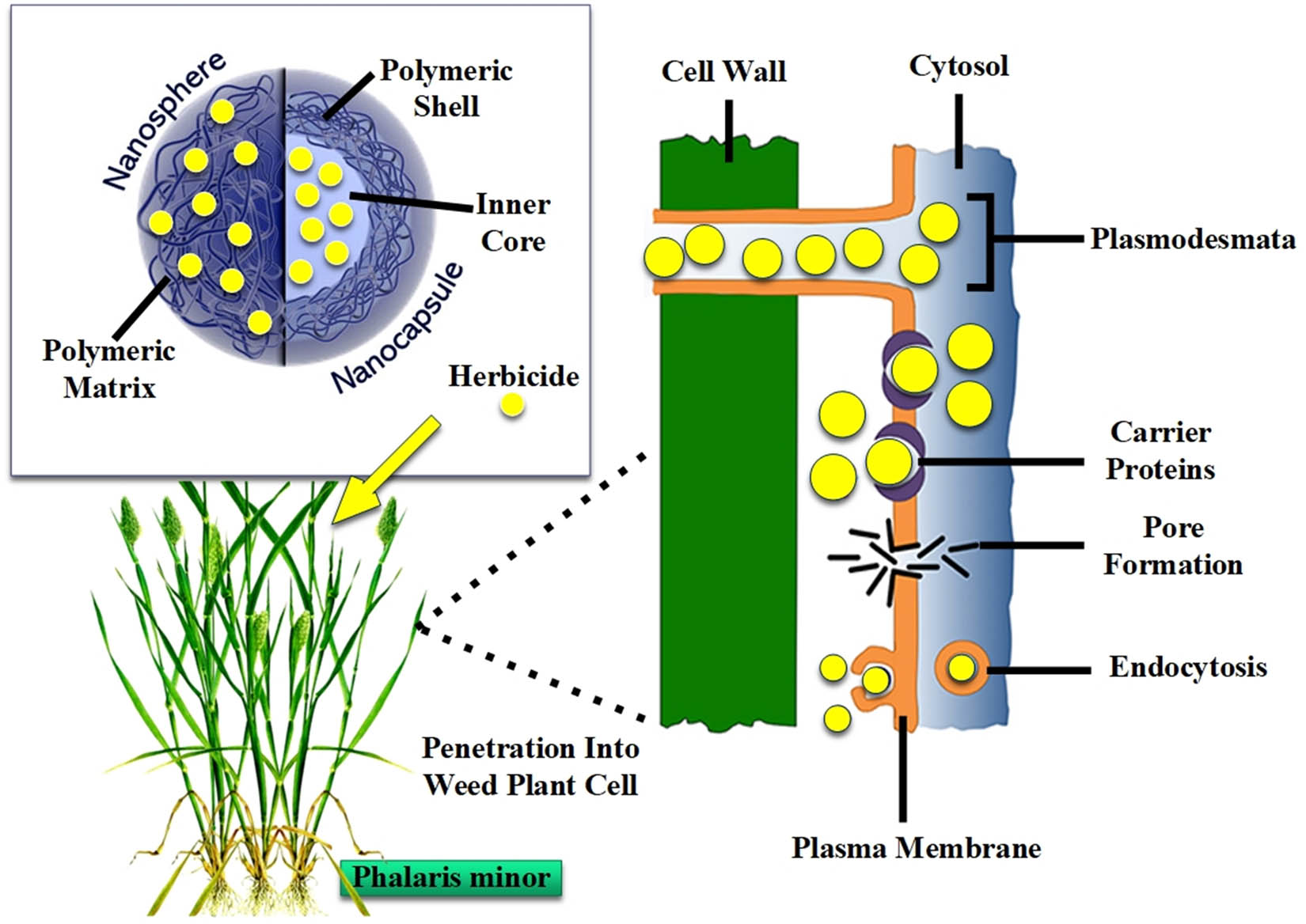
Morphology of a polymeric nanoherbicide and schematic illustration of its penetration into weed’s plant cell.
Wheat yield losses in various countries due to weeds
| Countries | Weed species | Common names | Family | Grain yield reduction | References |
|---|---|---|---|---|---|
| Argentina | Lolium multiflorum | Ryegrass | Poaceae | 20–30% | [63] |
| Argentina | Avena fatua | Wild oats | Poaceae | 20% | [64] |
| Australia | Raphanus raphanistrum | Wild radish | Brassicaceae | 3–18% | [65] |
| Chile | Avena fatua | Wild oats | Poaceae | 3.5–4.5% | [66] |
| Chile | Lolium multiflorum | Ryegrass | Poaceae | 1.3–1.6% | [67] |
| Germany | Stellaria media, Veronica persica, Capsella bursapastoris, and Lamium purpureum | Chickweed, bird’s-eye, shepherd’s purse, and red deadnettle | Caryophyllaceae, Plantaginaceae, Brassicaceae, and Lamiaceae | 1.2 kg·ha−1 plant−1 m2 | [68,69] |
| India | Phalaris minor | Canary grass | Poaceae | 80–100% | [70] |
| India | Rumex spinosus | Devil’s thorn | Polygonaceae | 46% | [71] |
| Iran | Sinapis arvensis L. | Wild mustard | Brassicaceae | 22.1–43.1% | [72] |
| Iran | Avena ludoviciana L. | Wild oats | Poaceae | 26.3–30.3% | [73] |
| Iran | Phalaris minor | Canary grass | Poaceae | 12.2–23% | [74] |
| Iran | Sinapis arvensis | Wild mustard | Brassicaceae | 18% | [72] |
| Jordan | Avena ludoviciana L. | Wild oats | Poaceae | 11% | [75] |
| Pakistan | Phalaris minor | Canary grass | Poaceae | 28–34% | [76] |
| Pakistan | Avena fatua | Wild oats | Poaceae | 78% | [77] |
| Pakistan | Rumex dentatus | Aegean dock | Polygonaceae | 60% | [77] |
| Pakistan | Emex australis | Double gee | Polygonaceae | 20.5% | [78] |
| Pakistan | Emex spinosa | Spiny dock | Polygonaceae | 56–70% | [79] |
| Pakistan | Silybum marianum | Milk thistle | Asteraceae | 30% | [80] |
| Pakistan | Galium aparine L. | Catchweed | Rubiaceae | 24–32% | [81] |
| Turkey | Alopecurus myosuroides | Black-grass | Poaceae | 19–25% | [82] |
| Turkey | Avena spp. | Wild oats | Poaceae | 13–19% | [83] |
| Turkey | Sinapis arvensis | Wild mustard | Brassicaceae | 36.90% | [84] |
| United Kingdom | Alopecurus myosuroides | Black-grass | Poaceae | 10% | [85] |
| United States of America | Avena fatua | Wild oats | Poaceae | ∼50% | [86] |
| United States of America | Sinapis alba | White mustard | Brassicaceae | 28% | [87] |
| United States of America | Lamium amplexicaule | Purple deadnettle | Lamiaceae | 13–38% | [88] |
| Wales | Phalaris minor | Canary grass | Poaceae | 29% | [89] |
| Western Australia | Lolium rigidum | Ryegrass | Poaceae | 11% | [90] |
| Western Australia | Bromus diandrus | Great brome | Poaceae | 36% | [91] |
3.1 Case studies of effective control of weeds by nanoherbicides at a reduced rate
Herbicides are an essential crop protection tool to maximize crop yields and reduce losses caused by weed. However, excessive and repetitive use of herbicides can lead to weed resistance and environmental issues, including water pollution [97]. Nanoherbicides offer a promising solution by effectively controlling weeds even at significantly lower doses while simultaneously reducing the possibility of adverse effects on the environment and toxicity to agricultural plants through controlled herbicide release. For the purpose of determining whether or not poly(epsilon-caprolactone) nanocapsules containing ATZ may have any adverse impact on maize plants, de Albuquerque et al. [98] conducted an experiment. The study compared the effects of commercial ATZ (200 g·ha−1). According to the findings, no adverse effects were reported on maize plants when they were supplied with a dosage of nano-ATZ that was ten times lower than the authorized dosage for commercial ATZ (Table 2).
Common weed flora of wheat crop
| Common name | Botanical name | Family name | Life cycle |
|---|---|---|---|
| Jawdar | Avena fatua | Poaceae | Annual |
| Bang | Cannabis sativa | Cannabaceae | Annual |
| Sarmay | Chenopodium album | Chenopodaceae | Annual |
| Skhabotay | Coronopus didymus | Brassicaceae | Biennial |
| Mandaroo | Euphorbia helioscopia | Euphorbiaceae | Annual |
| Krachay | Fumaria indica | Fumariaceae | Annual |
| Bashka | Malcolmia africana | Brassicaceae | Annual |
| Shpeshtary | Medicago minima | Fabaceae | Annual |
| L. peshtary | Medicago truncatula | Fabaceae | Annual |
| Gwansokay | Phalaris minor | Myrsinaceae | Annual |
| Wakha | Poa annua | Poaceae | Annual |
| Sarbawale | Ranunculus muricatus | Ranunculaceae | Annual/Biennial |
| Spenstargay | Stellaria media | Caryophyllaceae | Annual |
| Margaykhpa | Vicia sativa | Fabaceae | Annual |
According to Sousa et al. [99], nano-ATZ can effectively control weeds (Alternanthera tenella Colla., Brassica juncea, Amaranthus viridis, B. pilosa, and Raphanus raphanistrum) without causing additional harm to susceptible crops when compared to commercial ATZ, as long as a safe interval is maintained between ATZ application and sowing. Khan et al. [100] revealed that the application of chitosan-based herbicide NPs of clodinofop propargyl and fenoxaprop-p-ethyl on wheat exhibited a comparable impact on the visual damage of P. minor (83.00% and 81.50%, respectively). The doses of these herbicides were tenfold lower than the recommended dosage for field application. Zhu et al. [101] reported that clodinofop propargyl and fenoxaprop-p-ethyl NPs affected the survived plant height when used at the prescribed dosage in contrast to the standard herbicides. Comparable effects on the height of Lolium multiflorum L. were observed when applying standard herbicides at their recommended dosage and also when using a tenfold lower dosage of nanocapsules containing both herbicides. Ghosh et al. [102] found that employing ATZ-loaded nanocapsules prepared from poly(ε-caprolactone) (PCL) at the tenfold dilution level produced similar inhibitory impacts on Amaranthus viridis and B. pilosa growth.
In a study conducted by Oliveira et al. [103], the post-emergence herbicidal activity of nanoencapsulated ATZ against mustard was examined. Different chemicals were applied to 30-day-old mustard plants: distilled water (control), nanocapsules without ATZ (NC), commercially formulated ATZ at a dosage of 2,000 g·ha−1, noncapsules containing ATZ (NC + ATZ), and a lower dose of nanoformulation diluted in water at 200 g ATZ ha−1 to determine whether or not a lower dose is beneficial. A dose of 200 g·ha−1, tenfold lower than that of encapsulated ATZ, demonstrated strong post-emergence herbicidal efficacy. Importantly, nanoencapsulated ATZ permits lower herbicide doses without sacrificing effectiveness, which could have environmental benefits. Furthermore, the use of the Allium cepa chromosomal aberration test demonstrated that the NP technology has the potential to mitigate the genotoxic effects of the herbicide [104]. Overall, these developed formulations provide a valuable approach for weed control in agriculture while also mitigating potential risks regarding environment and human health.
A study was conducted to assess the effectiveness and release kinetics of herbicides combined with NPs that are chitosan-based, specifically imazapic and imazapyr, on soil microorganisms [47]. For the purpose of analyzing the impacts, they made use of real-time polymerase chain resistance. The findings of Liu et al. [105] demonstrated that the NPs had an average size of 400 nm and that they were stable for a period of 30 days within the ambient temperature range. Encapsulation efficiencies of 50–70% were achieved, demonstrating satisfactory results. Cytotoxicity assays indicated that they were less toxic than their free counterparts, resulting in reduced genotoxicity. A soil microbiota analysis showed alterations in treated soil microbes. These findings suggest that encapsulating herbicides increase their efficacy and minimize their toxicity. In another study, Farooq et al. [106] focused on the chemical synthesis, characterization, and dose optimization of chitosan-based NPs with clodinofop propargyl and fenoxaprop-p-ethylene to manage the wheat field weed P. minor. The researchers employed the ionic gelation technique to prepare the NPs, which were then sprayed onto P. minor at the 3–4 leaf stage [42]. At the permitted herbicide dose, chitosan-based particles of clodinofop propargyl and fenoxaprop-p-ethyl killed all weeds and caused noticeable damage. The nanopesticides (herbicides) not only help in controlling weeds but also help us in protecting our environment from hazardous effects of toxic herbicides. In comparison to the use of standard herbicides, the application of herbicide-loaded NPs is able to suppress weeds at a lower dosage.
The reduction of the selection pressure exerted by herbicides is the fundamental goal of the management determines for herbicide resistance development, thus reducing the development of resistance. Various strategies can be employed in different situations, including the crop rotation, herbicide rotation, use of herbicide mixtures, adherence to labelled directions, incorporating nonselective herbicides in rotation, considering economic threshold levels for herbicide applications, implementing culture weed methods, and utilizing nanoherbicides to combat resistance [107–109]. The selection of appropriate strategies depends upon factors, such as specific resistance, weed type, and herbicide being used, with the aim of delaying and protecting the development of resistance. To reduce weed resistance, herbicide combination and non-chemical weed management are essential. Additionally, the effectiveness of nonherbicidal methods makes them valuable in managing resistance weeds as well.
4 Herbicide NPs to mitigate chemical control challenges
The chemical weed control method faces significant challenges, including herbicide resistance and the harmful environmental crop-related side effects caused by herbicides. In recent decades, environmental protection authorities have prohibited several herbicides, and resistance has been documented against all main herbicide modes of action. No new herbicides with a novel mode of action have been produced in recent 30 years [110]. Currently, 272 weed species worldwide are resistant to 168 herbicides [97]. Consequently, the options for selecting herbicides for sustainable weed control are limited and diminishing due to a rapid increase in herbicide resistance. In the future, herbicide-resistant weeds in arable fields could cause a significant problem for agricultural sustainability, emphasizing herbicide resistance management to meet global food security concerns. To continue chemical weed control, herbicide-resistant weeds must be detected early and managed to avoid resistance.
4.1 Current status of herbicide-resistant weeds in wheat
Many weed species associated with wheat have developed resistance due to reliance on herbicide control and strong selection pressure of intensive wheat cultivation. Currently, 365 cases of herbicide-resistant weeds in wheat crop have been reported, widespread in 34 countries [97]. These have acquired herbicide resistance to numerous chemical classes and mechanisms [97]. Several agriculturally troublesome weed species have shown cross-resistance and multiple herbicide resistance. More than 76 weeds of wheat widespread globally have developed multiple resistance with two or more (up to 6) site of actions [97]. Resistance may be due to target-site changes or non-target-site processes [111]. Weeds may develop target-site herbicide resistance by gene regulatory alterations (in gene promoters) or point mutations that cause amino acid substitutions, overexpression, or amplification of target genes. Target-site point mutations reduce herbicide binding. Herbicide target gene amplification enhances protein synthesis without changing plant functions. Target-site-based herbicide resistance mechanisms are more common in specific herbicide modes of action. These pathways include ACCase, ALS, and PS II-inhibitors. This is because the protein structures of these herbicides are flexible enough to function normally while inhibiting herbicide binding [112]. In the case of non-target-site herbicide resistance, the amount of herbicide that can reach the target site is restricted by one or more mechanisms or by a combination of these mechanisms. Herbicides undergo metabolism more efficiently, their absorption is diminished, and their translocation is decreased. Weeds and herbicides used in wheat farming systems are examples of herbicide resistance and their processes [113].
4.1.1 Herbicide-resistant grassy weeds in wheat
Various grassy weeds of wheat have developed resistance to different herbicide site of actions [97]. P. minor weed control in wheat relies heavily on the use of selective herbicides during the early or postemergence stage [114]. In P. minor, herbicide resistance has developed due to the continuous and exclusive use of the same mode of action without herbicide rotation or crop rotation. The first case of resistance in this weed was reported in India in 1991 according to Soni et al. [115]. P. minor is resistant to ACCase inhibitors and ALS inhibitors in India, Iran, South Africa, Mexico, USA, and Australia [116,117]. In Pakistan, South Africa, and India, P. minor has shown multiple resistance to ACCase, ALS, and PS II inhibitors [118,119]. A significant number of herbicide-resistant cases in P. minor have been shown in the literature causing the failure of wheat crop. A winter annual grass weed that adapts to soil and climate, downy brome, is also known as cheat grass. An infestation of this plant may result in a loss of up to 92% of wheat production. Winter wheat is susceptible to its destructive effects. In winter wheat, ALS inhibitors are often used to control downy brome infestations selectively. However, downy brome that is resistant to amyotrophic lateral sclerosis (ALS) which was found in Montana’s Clearfield wheat was cross-resistant to other ALS inhibitors. Mutations in the target site caused Ser653Asn substitution in ALS-resistant downy brome. Feral rye, another problematic winter annual grassy weeding wheat, reduces the grain quality and output by 69% at 50–200 plants per square meter. Tank-mixing imazamox with MCPA ester improves herbicide absorption and feral rye control [120].
The western region of the United States is afflicted by grass known as jointed goatgrass, an invasive plant that originated in Europe [121]. Nonselective herbicides like glyphosate, paraquat, or glufosinate may be sprayed during the fallow period or before planting to suppress jointed goatgrass. It is also possible to utilize selective herbicides; however, this depends on the production system. Imazamox selectively controls feral rye, downy brome, jointed goatgrass, and other grass weeds in Clearfield wheat systems. Due to herbicide absorption, transport, and metabolic differences, the three species respond differently to imazamox. Depending on the adjuvant, the feral rye, downy brome, and jointed goatgrass absorb 14C imazamox differently [122]. Wild rye had more herbicide translocation and metabolism than jointed goatgrass. Due to cross-compatibility, genetic similarities, and similar growth behaviors, wheat and jointed goatgrass may exchange genes to acquire imazamox resistance. Originating from the Indian subcontinent, the Mediterranean, and the Arabian Gulf, rigid ryegrass is scientifically referred to as Lolium rigidum Gaudin. Nonetheless, it has become an invasive species in South Africa, Australia, North and South America, and South America. Several populations, nevertheless, have developed substantial levels of resistance to herbicides that inhibit ALS and ACCase, including chlorsulfuron, sulfometuron, diclofop-methyl, and clethodim [123]. According to available evidence, it has developed resistance to numerous herbicides with distinct mechanisms of action, including those that inhibit microtubules and PS II, ALS, and ACCase. Furthermore, resistance to a variety of herbicides has been developed. Rigid ryegrass has acquired resistance to seven distinct herbicides, each possessing a unique mode of action due to the Australian wheat cultivation method [124].
Western Australian populations of rigid ryegrass are resistant to herbicides used to control ALS due to target-site or non-target-site resistance mechanisms. A correlation has been established between alterations in the proline position of the ALS protein and resistance to sulfometuron-methyl. Furthermore, resistance to chlorsulfuron at non-target sites was identified as a consequence of the herbicide’s metabolism, according to the same investigation. Research studies have demonstrated that rigid ryegrass, among other plants, can develop polygenic metabolic resistance to herbicides when applied in inadequate or suboptimal concentrations. A sensitive rigid ryegrass biotype (VLR1) was selectively sprayed with a range of diclofop-methyl rates below the recommended amount (37.5 g·ha−1) to assess the potential for herbicide resistance and phenotypic variation in the degree of resistance to suboptimal concentrations of the herbicide. The development of resistant phenotypes occurred through gene recombination and selection throughout three generations due to the low rates of diclofop-methyl. The metabolic herbicide resistance of rigid ryegrass was investigated by scientists utilizing transcriptomic RNA-Seq analysis [125]. Certain species exhibit a close genetic relationship with rigid ryegrass, including Italian ryegrass (L. multiflorum Lam.) and perennial ryegrass (L. perenne L.) [126].
Several countries, including Australia, have implemented glyphosate resistance-building treatments during dormant periods due to the wheat rotations. Across the globe, wheat production systems are afflicted by wild oats (A. fatua), which is another economically detrimental plant. ACCase-resistant wild oats have been found in 17 countries, with most populations having multiple resistances. The ACCase gene of four wild oat populations resistant to ACCase inhibitors in Western Australia was sequenced and shown to have one, two, or three amino acid changes. Without target-site mutations, some resistant plants showed non-target-site resistance mediated by improved diclofop-methyl metabolism, proving that a plant may have two separate herbicide resistance pathways [127]. The abovementioned literature unveiled the development of severe resistance in grassy weeds. Nanoherbicides offer great potential to deal with the severe issue of herbicide resistance development in grassy weeds of wheat crop, as discussed in Section 3.1.
4.1.2 Herbicide-resistant broadleaf weeds in wheat
Several broadleaf weeds exert an economic impact on wheat cultivation. Various weed species, including weeds from Brassica spp., Amaranthus spp., common lambsquarters (Chenopodium album), oriental mustard (Sisymbrium orientale), wild radish (Raphanus raphanistrum), annual sowthistle (Sonchus oleraceus), prickly lettuce (Lactuca serriola), common chickweed (Stellaria media), shepherd’s purse (Capsella bursa-pastoris), and kochia (Basia scoparia (L.) Scott), have developed widespread resistance against herbicides with different sites of actions [97]. Kochia (Basia scoparia (L.) Scott), field bindweed (Convolvulus arvensis L.), and wild buckwheat (Polygonum convolvulus L.) are three of the most commonly encountered weeds of wheat during the summer season [120]. Field pennycress (Thlaspi arvense L.), common chickweed (Stellaria medium (L.) Vill), prickly lettuce (Lactuca serriola L.), Russian thistle (Salsola tragus L.), and shepherd’s purse (Capsella bursa-pastoris (L.) Medik) are all remarkable winter broadleaf weeds. In addition to effectively controlling annual broadleaf weeds throughout the summer, herbicide treatments made in the spring are also helpful in controlling winter weeds. For effective management, however, the climatic conditions and timing (the stage of development of the crop and the weed) are of the highest priority [128].
For weed control, herbicides containing ALS and synthetic auxin are frequently used. Triasulfuron, prosulfuron, dicamba, 2,4-D, and metsulfuron are sulfonylurea herbicides that exhibit efficacy against most winter annual broadleaf plants [129]. Wheat is affected by the summer annual weed Kochia (Kochia scoparia L. Schrad), which blooms between late August and early September. Early-season ALS herbicides target late-emerging wheat kochia rather than kochia, which has developed resistance to ALS. Specific populations of kochia resistant to ALS in the United States and Canada exhibit resistance to synthetic auxins, PS II, and EPSPS. Increased EPSPS gene copy number is the cause of kochia resistance to phosphatases in wheat-fallow fields; artificial auxin resistance remains unknown [130].
Another weed that causes a significant amount of difficulty in the management of summer fallow, spring wheat, and other crops in the United States, such as mustard, canola, and pulses, is Russian thistle, also known as Sollola tragus L. Wheat production might be reduced by as much as 50% when Russian thistle is present throughout the growing season. In 1987, the first observation of ALS-inhibitor-resistant Russian thistle was made in wheat fields in Washington and Montana. More than 75% of wheat fields is contaminated with this weed [131]. Glyphosate is widely used, both before planting wheat and after harvest, as a burndown treatment. There has been a rise in the number of Russian thistle populations resistant to glyphosate due to repeated treatments; however, the mechanism of glyphosate resistance has not yet been examined. It is necessary to employ proper weed management measures to reduce the development of resistant weeds [132]. Other broadleaf weeds have also resisted ALS inhibitors, routinely used in wheat cropping systems. Pollen movement, seed production, and propagule dissemination are all management measures that should be used to decrease and eradicate the development of resistance.
However, with integrated management approaches, weed-free crops are possible. Thus, cultivators should choose competitive cultivars. The survival of weeds in the presence of herbicides may be better understood by gaining an understanding of the physiological, genetic, pharmacological, and molecular mechanisms that lead to the development of herbicide resistance. Unravelling herbicide resistance processes will delay resistance development and lead to unique herbicides, better herbicide usage, and more sustainable weed management approaches [133]. The current resistance status unveiled the development of severe resistance in broadleaf weeds of wheat. Nanoherbicides can be used as a potential alternative to deal with the severe issue of herbicide-resistant weeds in wheat to ensure sustainable wheat production.
5 Future prospects
Recent nanoenabled herbicides utilize inorganic, organic, and hybrid components to control weeds. According to studies, nanoherbicides may make more targeted and less hazardous agricultural formulations. Smart nanoherbicides and a better understanding of their mechanisms of action are crucial for target and non-target species. In addition, nanoformulation systems may have different biological variables that restrict nanomaterial–cargo complexes and nanomaterials crossing plant barriers. In addition, it is advised that additional research should be carried out to contribute to the improvement of understanding the mechanism of action of nanoherbicides. According to Forini et al. [134], new delivery systems, such as herbicides co-loaded with CRISPR-based genome editing enabling simultaneous action on non-target plant tissues, may minimize the number of agrochemicals needed. Sun et al. [135] found that nanostructured delivery systems cannot precisely target subcellular compartments, mainly based on biorecognition patterns, another critical aspect of nanoherbicide development. Molecular target identification must be improved to produce new nanoformulations. Strategic design, artificial intelligence (AI), and machine learning may accomplish this [136]. Thus, more study is needed to produce nanopesticides for environmentally friendly and sustainable agriculture.
As stated in the research recently released in 2016, the entire market for crop protection chemicals is anticipated to be worth $54.88 billion in 2016 (www.marketandmarket.com). Between 2016 and 2021, the market is expected to rise at a compound annual growth rate (CAGR) of 5.15%, reaching $70.57 billion [137]. Herbicides are expected to have the most significant market share, accounting for 44.2% of the total, followed by insecticides and fungicides. It is anticipated that biopesticides will become the category of products with the highest growth rate [137]. According to the report titled “Herbicides Market by Type (Glyphosate, 2, 4-D, Diquat), Crop Type (Cereals & Grains, Oilseeds & Pulses, Fruits & Vegetables), Mode of Action (Non-selective, Selective), and Region – Global Forecast to 2022,” the global market for herbicides has reached approximately USD 27.21 billion in 2016. It is anticipated to reach about USD 39.15 billion by 2022 while expanding at a compound annual growth rate (CAGR) of 6.25% during the forecasting period. Several variables, including the market for nanoherbicides and prospects, are the primary drivers of the market. Because nanoherbicides are a relatively new technology that is still in the process of development, there is no reliable data available about their market share [138]. Nevertheless, a few reports have recently been published on crop protection agents.
6 Conclusions
The use of herbicides is essential for efficient weed control and has a central role in ensuring sustained crop production. However, there are still issues, such as herbicide persistence in soil, that have been deteriorating the quality of soil. Herbicides have helped to alleviate the problem of weed management, but there are still complications. Besides that, the fact that weeds are increasingly becoming resistant to herbicides has been a significant problem in recent years. The novel method that nanotechnology is employed to release pesticides has the potential to provide encouraging outcomes. Nanoencapsulation of herbicides has been demonstrated to produce formulations that are more focused and less harmful for use in agricultural activities, according to studies. Using nanoencapsulated herbicides would allow for administering lower doses of the herbicide than would be possible with a commercial formulation. The herbicidal activity is far higher than those of commercial formulations. Because it lessens the long-term impacts of herbicide residues in agricultural regions and the toxicity of these herbicides to the environment, the use of herbicides at lower dosages should be encouraged. Encapsulated herbicides may facilitate the delivery of herbicides to weed plants, hence minimizing the amount of herbicide residue accumulated in the soil. It is also beneficial in destroying the weeds without ever interacting with the crop plants, eventually resulting in a greater crop yield. The use of the target-specific release also accomplishes this. Because of this, nanotechnology is a benefit that has the potential to be further improved in terms of target site suppression of the biochemical responses of weed.
-
Funding information: The authors state no funding is involved.
-
Author contributions: Bilal Ahmad Khan, Muhammad Ather Nadeem, Tasawer Abbas, and Nehal Elnaggar: writing – original draft, writing – review and editing, and visualization; Hussam F. Najeeb Alawadi, Muhammad Ashar Ayub, FNU Abdullah, Athar Mahmood, Aneela Nijabat, Muaz Ameen, Bilal Ahmad Khan, Muhammad Ather Nadeem, Nehal Elnaggar, and Hesham Oraby: writing – review and editing.
-
Conflict of interest: The authors state there is no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Hasan M, Ahmad-Hamdani MS, Rosli AM, Hamdan H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants. 2021;10(6):1212. 10.3390/plants10061212.Search in Google Scholar
[2] Valiño A, Pardo-Muras M, Puig CG, López-Periago JE, Pedrol N. Biomass from allelopathic agroforestry and invasive plant species as soil amendments for weed control—A review. Agron. 2023;13(12):2880. 10.3390/agronomy13122880.Search in Google Scholar
[3] Korres NE, Burgos NR, Travlos I, Vurro M, Gitsopoulos TK, Varanasi VK, et al. New directions for integrated weed management: Modern technologies, tools and knowledge discovery. In: Advances in Agronomy. Academic Press; 2019. p. 243–319. 10.1016/bs.agron.2019.01.006.Search in Google Scholar
[4] Lamichhane JR, Debaeke P, Steinberg C, You MP, Barbetti MJ, Aubertot J-N. Abiotic and biotic factors affecting crop seed germination and seedling emergence: a conceptual framework. Plant Soil. 2018;432(1):1–28. 10.1007/s11104-018-3780-9.Search in Google Scholar
[5] Chaudhary N, Choudhary KK, Agrawal SB, Agrawal M. Pesticides usage, uptake and mode of action in plants with special emphasis on photosynthetic characteristics. In: Pesticides in crop production: Physiological and biochemical action. 2020. p. 159–80. 10.1002/9781119432241.ch9.Search in Google Scholar
[6] Hasanuzzaman M, Mohsin SM, Bhuyan MHMB, Bhuiyan TF, Anee TI, Masud AAC, et al. Chapter 3 - Phytotoxicity, environmental and health hazards of herbicides: challenges and ways forward. Butterworth-Heinemann; 2020. 10.1016/B978-0-08-103017-2.00003-9.Search in Google Scholar
[7] Pathak VM, Verma VK, Rawat BS, Kaur B, Babu N, Sharma A, et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front Microbiol. 2022;13:1–29. 10.3389/fmicb.2022.962619.Search in Google Scholar
[8] Nath CP, Singh RG, Choudhary VK, Datta D, Nandan R, Singh SS. Challenges and alternatives of herbicide-based weed management. Agron. 2024;14(1):126. 10.3390/agronomy14010126.Search in Google Scholar
[9] Colbach N, Petit S, Chauvel B, Deytieux V, Lechenet M, Munier-Jolain N, et al. The pitfalls of relating weeds, herbicide use, and crop yield: Don’t Fall Into the Trap! A critical review. Front Agron. 2020;2:1–14. 10.3389/fagro.2020.615470.Search in Google Scholar
[10] Preisler AC, Pereira AE, Campos EV, Dalazen G, Fraceto LF, Oliveira HC. Atrazine nanoencapsulation improves pre-emergence herbicidal activity against Bidens pilosa without enhancing long-term residual effect on Glycine max. Pest Manag Sci. 2020;76(1):141–9. 10.1002/ps.5482.Search in Google Scholar
[11] Jalil SU, Ansari MI. Role of nanomaterials in weed control and plant diseases management. In: Nanomaterials for agriculture and forestry applications. Elsevier; 2020. p. 421–34. 10.1016/B978-0-12-817852-2.00017-2.Search in Google Scholar
[12] Datta S, Singh J, Singh S, Singh J. Earthworms, pesticides and sustainable agriculture: A review. Env Sci Pollut Res. 2016;23(9):8227–43. 10.1007/s11356-016-6375-0.Search in Google Scholar
[13] Mourdikoudis S, Pallares RM, Thanh NTK. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018;10(27):12871–934. 10.1039/C8NR02278J.Search in Google Scholar
[14] Abbas Q, Yousaf B, Amina, Ali MU, Munir MAM, El-Naggar A, et al. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Env Int. 2020;138:105646. 10.1016/j.envint.2020.105646.Search in Google Scholar
[15] Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022;5(6):1593–615. 10.1007/s42247-021-00335-x.Search in Google Scholar
[16] Nsairat H, Lafi Z, Al-Sulaibi M, Gharaibeh L, Alshaer W. Impact of nanotechnology on the oral delivery of phyto-bioactive compounds. Food Chem. 2023;424:136438. 10.1016/j.foodchem.2023.136438.Search in Google Scholar
[17] Bhaskar M, Kumar A, Rani R. Application of nano formulations in agriculture. Biocatal Agric Biotechnol. 2023;54:102934. 10.1016/j.bcab.2023.102934.Search in Google Scholar
[18] Pontes MS, Antunes DR, Oliveira IP, Forini MML, Santos JS, Arruda GJ, et al. Chitosan/tripolyphosphate nanoformulation carrying paraquat: insights on its enhanced herbicidal activity. Env Sci Nano. 2021;8(5):1336–51. 10.1039/D0EN01128B.Search in Google Scholar
[19] Rodríguez-Mejías FJ, Scavo A, Chinchilla N, Molinillo JMG, Schwaiger S, Mauromicale G, et al. Perspectives and advances in organic formulations for agriculture: Encapsulation of herbicides for weed control. Agron. 2023;13(7):1898. 10.3390/agronomy13071898.Search in Google Scholar
[20] Sabry A-KH. Role of nanotechnology applications in plant-parasitic nematode control. Cham: Springer International Publishing; 2019. 10.1007/978-3-030-13296-5_12.Search in Google Scholar
[21] Hussein MZ, Jaafar AM, Yahaya AH, Zainal Z. The effect of single, binary and ternary anions of chloride, carbonate and phosphate on the release of 2,4-dichlorophenoxyacetate intercalated into the Zn–Al-layered double hydroxide nanohybrid. Nanoscale Res Lett. 2009;4(11):1351. 10.1007/s11671-009-9404-9.Search in Google Scholar
[22] Konappa N, Krishnamurthy S, Arakere UC, Chowdappa S, Akbarbasha R, Ramachandrappa NS. Nanofertilizers and nanopesticides: Recent trends, future prospects in agriculture. In: Advances in nano-fertilizers and nano-pesticides in agriculture. Woodhead Publishing; 2021. p. 281–330. 10.1016/B978-0-12-820092-6.00012-4.Search in Google Scholar
[23] Satvekar R, Chavan Y, Sahoo A, Nandre VS. Nanoformulations of natural compounds for herbicide and agri-food application. In: New horizons in natural compound research. Academic Press; 2023. p. 427–43. 10.1016/B978-0-443-15232-0.00014-X.Search in Google Scholar
[24] Goswami L, Kim K-H, Deep A, Das P, Bhattacharya SS, Kumar S, et al. Engineered nano particles: Nature, behavior, and effect on the environment. J Env Manag. 2017;196:297–315. 10.1016/j.jenvman.2017.01.011.Search in Google Scholar
[25] Yuan Y, Hu T, Zhong X, Zhu M, Chai Y, Yuan R. Highly sensitive photoelectrochemical biosensor based on quantum dots sensitizing Bi2Te3 nanosheets and DNA-amplifying strategies. ACS Appl Mater Interfaces. 2020;12(20):22624–9. 10.1021/acsami.0c04536.Search in Google Scholar
[26] Gayathri K, Bhaskaran M, Selvam C, Thilagavathi R. Nano formulation approaches for curcumin delivery- A review. J Drug Deliv Sci Technol. 2023;82:104326. 10.1016/j.jddst.2023.104326.Search in Google Scholar
[27] Iavicoli I, Leso V, Beezhold DH, Shvedova AA. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol Appl Pharmacol. 2017;329:96–111. 10.1016/j.taap.2017.05.025.Search in Google Scholar
[28] Sekaran U, Lai L, Ussiri DAN, Kumar S, Clay S. Role of integrated crop-livestock systems in improving agriculture production and addressing food security – A review. J Agric Food Res. 2021;5:100190. 10.1016/j.jafr.2021.100190.Search in Google Scholar
[29] Handy RD, Clark NJ, Vassallo J. 9 - Nanotechnology for aquaculture and fisheries. In: Nano-enabled sustainable and precision agriculture. Academic Press; 2023. 10.1016/B978-0-323-91233-4.00006-5.Search in Google Scholar
[30] Tortella G, Rubilar O, Pieretti JC, Fincheira P, de Melo Santana B, Fernández-Baldo MA, et al. Nanoparticles as a promising strategy to mitigate biotic stress in agriculture. Antibiotic. 2023;12(2):338. 10.3390/antibiotics12020338.Search in Google Scholar
[31] Mishra D, Khare P. Emerging nano-agrochemicals for sustainable agriculture: Benefits, challenges and risk mitigation. Cham: Springer International Publishing; 2021. 10.1007/978-3-030-63249-6_9.Search in Google Scholar
[32] Duke SO, Dayan FE. The search for new herbicide mechanisms of action: Is there a ‘holy grail’? Pest Manag Sci. 2022;78(4):1303–13. 10.1002/ps.6726.Search in Google Scholar
[33] Sarkar A, Singh T, Mondal A, Kumar S, Das TK, Kaur R, et al. Effect of nano-urea and herbicides on yield and yield attributes of wheat (Triticum aestivum). Indian J Agron. 2023;68(1):97–100. 10.59797/ija.v68i1.211.Search in Google Scholar
[34] Carvalho LB, Godoy IS, Preisler AC, de Freitas Proença PL, Saraiva-Santos T, Verri WA, et al. Pre-emergence herbicidal efficiency and uptake of atrazine-loaded zein nanoparticles: a sustainable alternative to weed control. Env Sci Nano. 2023;10(6):1629–43. 10.1039/D2EN01064J.Search in Google Scholar
[35] Székács A. 3 - Herbicide mode of action. In: Herbicides. Elsevier; 2021. 10.1016/B978-0-12-823674-1.00008-0.Search in Google Scholar
[36] Kumar S, Nehra M, Dilbaghi N, Marrazza G, Hassan AA, Kim K-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J Control Rel. 2019;294:131–53. 10.1016/j.jconrel.2018.12.012.Search in Google Scholar
[37] Dhiman A, Sharma AK, Bhardwaj D, Agrawal G. Biodegradable dual stimuli responsive alginate based microgels for controlled agrochemicals release and soil remediation. Int J Biol Macromol. 2023;228:323–32. 10.1016/j.ijbiomac.2022.12.225.Search in Google Scholar
[38] Maruyama CR, Guilger M, Pascoli M, Bileshy-José N, Abhilash PC, Fraceto LF, et al. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci Rep. 2016;6(1):19768. 10.1038/srep19768.Search in Google Scholar
[39] Zargar M, Bayat M, Saquee FS, Diakite S, Ramzanovich NM, Akhmadovich KAS. New advances in nano-enabled weed management using poly(Epsilon-Caprolactone)-based nanoherbicides: A review. Agric. 2023;13(10):2031. 10.3390/agriculture13102031.Search in Google Scholar
[40] Shattar SFA, Zakaria NA, Foo KY. Preparation of a montmorillonite-derived adsorbent for the practical treatment of ionic and nonionic pesticides. J Mater Res Technol. 2019;8(5):4713–24. 10.1016/j.jmrt.2019.08.017.Search in Google Scholar
[41] Marimuthu S, Pavithran P, Gowtham G. Polymeric systems for the delivery of herbicides to improve weed control efficiency. Rijeka: IntechOpen; 2022. 10.5772/intechopen.104629.Search in Google Scholar
[42] Khan BA, Nadeem MA, Javaid MM, Maqbool R, Ikram M, Oraby H. Chemical synthesis, characterization, and dose optimization of chitosan-based nanoparticles of clodinofop propargyl and fenoxaprop-p-ethyl for management of Phalaris minor (little seed canary grass): First report. Green Process Synth. 2022;11(1):1118–27. 10.1515/gps-2022-0096.Search in Google Scholar
[43] Khan BA, Nadeem MA, Iqbal M, Yaqoob N, Javaid MM, Maqbool R, et al. Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.). Green Process Synth. 2023;12(1):1–11. 10.1515/gps-2022-8152.Search in Google Scholar
[44] Singh A, Dhiman N, Kar AK, Singh D, Purohit MP, Ghosh D, et al. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J Hazard Mater. 2020;385:121525. 10.1016/j.jhazmat.2019.121525.Search in Google Scholar
[45] Kruk T, Chojnacka-Górka K, Kolasińska-Sojka M, Zapotoczny S. Stimuli-responsive polyelectrolyte multilayer films and microcapsules. Adv Colloid Interface Sci. 2022;310:102773. 10.1016/j.cis.2022.102773.Search in Google Scholar
[46] Mattos BD, Tardy BL, Magalhães WLE, Rojas OJ. Controlled release for crop and wood protection: Recent progress toward sustainable and safe nanostructured biocidal systems. J Control Rel. 2017;262:139–50. 10.1016/j.jconrel.2017.07.025.Search in Google Scholar
[47] Kandasamy G, Manisekaran R, Arthikala M-K. Chitosan nanoplatforms in agriculture for multi-potential applications - Adsorption/removal, sustained release, sensing of pollutants & delivering their alternatives – A comprehensive review. Env Res. 2024;240:117447. 10.1016/j.envres.2023.117447.Search in Google Scholar
[48] Sivashankari PR, Prabaharan M. 5 - Deacetylation modification techniques of chitin and chitosan. Woodhead Publishing; 2017. 10.1016/B978-0-08-100230-8.00005-4.Search in Google Scholar
[49] Veerakumar P, Sangili A, Saranya K, Pandikumar A, Lin K-C. Palladium and silver nanoparticles embedded on zinc oxide nanostars for photocatalytic degradation of pesticides and herbicides. J Chem Eng. 2021;410:128434. 10.1016/j.cej.2021.128434.Search in Google Scholar
[50] Thambiliyagodage C, Jayanetti M, Mendis A, Ekanayake G, Liyanaarachchi H, Vigneswaran S. Recent advances in Chitosan-based applications—A review. Mater. 2023;16(5):2073. 10.3390/ma16052073.Search in Google Scholar
[51] Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, et al. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. J Chem Eng. 2019;366:608–21. 10.1016/j.cej.2019.02.119.Search in Google Scholar
[52] Biondo F, Baldassarre F, Vergaro V, Ciccarella G. Controlled biocide release from smart delivery systems: materials engineering to tune release rate, biointeractions, and responsiveness. In: Nanotechnology-based sustainable alternatives for the management of plant diseases. Elsevier; 2022. p. 31–147. 10.1016/B978-0-12-823394-8.00010-X.Search in Google Scholar
[53] Zaheer Z, Bawazir WA, Basaleh AS, Alhogbi BG, Albukhari SM. Effects of anionic and cationic surfactants on the surface Plasmon resonance intensity of biogenic silver nanoparticles: Stability, and position of optical band. J Mol Liq. 2023;385:122363. 10.1016/j.molliq.2023.122363.Search in Google Scholar
[54] Jamshaid H, Din FU, Khan GM. Nanotechnology based solutions for anti-leishmanial impediments: A detailed insight. J Nanobiotechnology. 2021;19(1):106. 10.1186/s12951-021-00853-0.Search in Google Scholar
[55] Chakraborty M, Hasanuzzaman M, Rahman M, Khan MAR, Bhowmik P, Mahmud NU, et al. Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agric. 2020;10(12):624. 10.3390/agriculture10120624.Search in Google Scholar
[56] Mujtaba M, Khawar KM, Camara MC, Carvalho LB, Fraceto LF, Morsi RE, et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int J Biol Macromol. 2020;154:683–97. 10.1016/j.ijbiomac.2020.03.128.Search in Google Scholar
[57] Maleki G, Woltering EJ, Mozafari MR. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends Food Sci Technol. 2022;120:88–99. 10.1016/j.tifs.2022.01.001.Search in Google Scholar
[58] Nnamonu LA, Sha’Ato R, Onyido I. Alginate reinforced chitosan and starch beads in slow release formulation of imazaquin herbicide—Preparation and characterization. Mater Sci Appl. 2012;3(8):1–9. 10.4236/msa.2012.38081.Search in Google Scholar
[59] Rashidipour M, Maleki A, Kordi S, Birjandi M, Pajouhi N, Mohammadi E, et al. Pectin/chitosan/tripolyphosphate nanoparticles: Efficient carriers for reducing soil sorption, cytotoxicity, and mutagenicity of paraquat and enhancing its herbicide activity. J Agric Food Chem. 2019;67(20):5736–45. 10.1021/acs.jafc.9b01106.Search in Google Scholar
[60] Subbiah R, Tiwari RR. The herbicide paraquat-induced molecular mechanisms in the development of acute lung injury and lung fibrosis. Crit Rev Toxicol. 2021;51(1):36–64. 10.1080/10408444.2020.1864721.Search in Google Scholar
[61] Zhang M, Zhang F, Li C, An H, Wan T, Zhang P. Application of chitosan and its derivative polymers in clinical medicine and agriculture. Polym. 2022;14(5):958. 10.3390/polym14050958.Search in Google Scholar
[62] Kashyap PL, Xiang X, Heiden P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int J Biol Macromol. 2015;77:36–51. 10.1016/j.ijbiomac.2015.02.039.Search in Google Scholar
[63] Scursoni JA, Palmano M, De Notta A, Delfino D. Italian ryegrass (Lolium multiflorum Lam.) density and N fertilization on wheat (Triticum aestivum L.) yield in Argentina. Crop Prot. 2012;32:36–40. 10.1016/j.cropro.2011.11.002.Search in Google Scholar
[64] Molinari FA, Blanco AM, Vigna MR, Chantre GR. A simulation model as the core for integrated weed management decision support systems: The case of Avena fatua-Winter Wheat in the Semiarid Pampean Region of Argentina. Cham: Springer International Publishing; 2020. 10.1007/978-3-030-44402-0_15.Search in Google Scholar
[65] Bellotti B, Eslami SV, Gill GS, McDonald G. Wild radish (Raphanus raphanistrum) interference in wheat. Weed Sci. 2006;54(4):749–56. 10.1614/WS-05-180R2.1.Search in Google Scholar
[66] Far MD, Saffari M, Mohammadi-Nejad G, Golkar P. An investigation of the competitive effects of weeds on wheat “Triticum aestivum L.” yield in field condition. Adv Env Biol. 2011;5(8):2475–80.Search in Google Scholar
[67] Pedreros L. Wild oat (Avena fatua L.) and Italian ryegrass (Lolium multiflorum Lam.) effect on wheat yield at two locations. Agricultura Tecnica. 2001;61(3):294–305, https://www.cabidigitallibrary.org/doi/full/10.5555/20013107995.Search in Google Scholar
[68] Keller M, Gutjahr C, Möhring J, Weis M, Sökefeld M, Gerhards R. Estimating economic thresholds for site-specific weed control using manual weed counts and sensor technology: An example based on three winter wheat trials. Pest Manag Sci. 2014;70(2):200–11. 10.1002/ps.3545.Search in Google Scholar
[69] Wang L, Gruber S, Claupein W. Effects of woodchip mulch and barley intercropping on weeds in lentil crops. Weed Res. 2012;52(2):161–8. 10.1111/j.1365-3180.2012.00905.x.Search in Google Scholar
[70] Chhokar RS, Singh S, Sharma RK. Herbicides for control of isoproturon-resistant Littleseed Canarygrass (Phalaris minor) in wheat. Crop Prot. 2008;27(3):719–26. 10.1016/j.cropro.2007.10.004.Search in Google Scholar
[71] Chaudhary A, Chhokar RS, Dhanda S, Kaushik P, Kaur S, Poonia TM, et al. Herbicide resistance to metsulfuron-methyl in Rumex dentatus L. in North-West India and its management perspectives for sustainable wheat production. Sustain. 2021;13(12):6947. 10.3390/su13126947.Search in Google Scholar
[72] Behdarvand P, Chinchanikar GS, Dhumal KN, Baghestani MA. Effects of wild mustard (Sinapis arvensis L.) and wild oat (Avena ludoviciana L.) densities on grain yield and yield components of wheat in response to various levels of nitrogen. Adv Env Biol. 2013;1082–8. https://link.gale.com/apps/doc/A346926569/AONE?u=anon∼3a73784c&sid=googleScholar&xid=59f833a7.Search in Google Scholar
[73] Papapanagiotou AP, Damalas CA, Menexes GC, Eleftherohorinos IG. Resistance levels and chemical control options of sterile oat (Avena sterilis L.) in Northern Greece. Int J Pest Manag. 2020;66(2):106–15. 10.1080/09670874.2019.1569285.Search in Google Scholar
[74] Genedy MS, Sharshar AAH, Sharshar AM, Mahmoud MA, Ibrahim HEA. Effect of deficit irrigation and weed control treatments on grain yield and water productivity for three bread wheat genotypes. J Soil Sci Agric Eng. 2020;11(11):667–75. 10.21608/jssae.2020.136088.Search in Google Scholar
[75] Qasem JR. Chemical control of wild-oat (Avena sterilis L.) and other weeds in wheat (Triticum durum Desf.) in Jordan. Crop Prot. 2007;26(8):1315–24. 10.1016/j.cropro.2006.11.006.Search in Google Scholar
[76] Abbas T, Nadeem MA, Tanveer A, Ali HH, Farooq N. Role of allelopathic crop mulches and reduced doses of tank-mixed herbicides in managing herbicide-resistant Phalaris minor in wheat. Crop Prot. 2018;110:245–50. 10.1016/j.cropro.2017.06.012.Search in Google Scholar
[77] Gul J, Bakht T, Kanwal S. Increasing densities of Avena fatua and Rumex dentatus reduce the yield of wheat under field conditions. Pak J Weed Sci Res. 2018;24(2):155–64. 10.28941/24-2(2018)-9.Search in Google Scholar
[78] Abbas RN, Tanveer A, Ali A, Zaheer ZA. Effects of Emex australis Steinh on germination and early seedling growth of wheat (Triticum aestivum L.). Allelopathy J. 2010;25(2):513–20, https://www.allelopathyjournal.com/archives/?Year=2010&Vol=25&Issue=2&Month=4.Search in Google Scholar
[79] Javaid MM, Tanveer A, Ali HH, Shahid MA, Balal RM, Aqeel MA. Wheat yield loss in a two species competition with Emex australis and Emex spinosa. Planta Daninha. 2016;34(1):35–46. 10.1590/S0100-83582016340100004.Search in Google Scholar
[80] Abbas T, Javeed HMR, Maqbool R, Qamar R, Rehman A, Rehman A-U, et al. Influence of competitive duration of blessed milkthistle (Silybum marianum) with wheat. Weed Technol. 2019;33(2):280–6. 10.1017/wet.2018.77.Search in Google Scholar
[81] Aziz A, Tanveer A, Ali A, Yaseen M. Density dependent interactions between cleavers (Galium aparine) and wheat (Triticum aestivum) planted at different times. Pak J Agric Sci. 2009;46:258–65, https://www.academia.edu/download/80382921/77.pdf.Search in Google Scholar
[82] Ahmad T, Jabran K, Moss SR. Alopecurus myosuroides. In: Biology and management of problematic crop weed species. Academic Press; 2021. p. 1–19. 10.1016/B978-0-12-822917-0.00003-3.Search in Google Scholar
[83] Torun H, Uygur FN. Effect of crop rotations on winter wild oat (Avena sterilis L.) populations in Osmaniye Province Wheat Sown Areas. J Res Weed Sci. 2019;2(4):345–57. 10.26655/jrweedsci.2019.4.5.Search in Google Scholar
[84] Alcántara-de la Cruz R, De Prado R, Gherekhloo J, Hatami ZM, Sadeghipour HR. Continuous use of tribenuron-methyl selected for cross-resistance to acetolactate synthase–inhibiting herbicides in wild mustard (Sinapis arvensis). Weed Sci. 2018;66(4):424–32. 10.1017/wsc.2018.23.Search in Google Scholar
[85] Ahodo K, Freckleton RP, Hicks HL, Oglethorpe D. Estimating the farm-level economic costs of spring cropping to manage Alopecurus myosuroides (black-grass) in UK agriculture. J Agric Sci. 2019;157(4):318–32. 10.1017/S0021859619000650.Search in Google Scholar
[86] Chauhan BS, Mahajan G. Interference of wild oat (Avena fatua) and sterile oat (Avena sterilis ssp. ludoviciana) in wheat. Weed Sci. 2021;69(4):485–91. 10.1017/wsc.2021.25.Search in Google Scholar
[87] Zargar M, Kavhiza NJ, Bayat M, Pakina E. Wild mustard (Sinapis arvensis) competition and control in rain-fed spring wheat (Triticum aestivum L.). Agron. 2021;11(11):2306. 10.3390/agronomy11112306.Search in Google Scholar
[88] Godar AS, Jugulam M, Peterson DE, Shoup D, Varanasi VK. A target-site point mutation in henbit (Lamium amplexicaule) confers high-level resistance to ALS-inhibitors. Weed Sci. 2016;64(2):231–9. 10.1614/WS-D-15-00152.1.Search in Google Scholar
[89] Singh V, Singh H, Raghubanshi AS. Competitive interactions of wheat with Phalaris minor or Rumex dentatus: A replacement series study. Int J Pest Manag. 2013;59(4):245–58. 10.1080/09670874.2013.845320.Search in Google Scholar
[90] Bajwa AA, Latif S, Borger C, Iqbal N, Asaduzzaman M, Wu H, et al. The remarkable journey of a weed: Biology and management of annual ryegrass (Lolium rigidum) in conservation cropping systems of Australia. Plants. 2021;10(8):1505. 10.3390/plants10081505.Search in Google Scholar
[91] Borger CPD, Hashem A, van Burgel A, Gill GS. Invasiveness of agronomic weed species in wheat in Western Australia. Weed Res. 2020;60(4):251–8. 10.1111/wre.12419.Search in Google Scholar
[92] Sardana V, Mahajan G, Jabran K, Chauhan BS. Role of competition in managing weeds: An introduction to the special issue. Crop Prot. 2017;95:1–7. 10.1016/j.cropro.2016.09.011.Search in Google Scholar
[93] Dass A, Shekhawat K, Choudhary AK, Sepat S, Rathore SS, Mahajan G, et al. Weed management in rice using crop competition-a review. Crop Prot. 2017;95:45–52. 10.1016/j.cropro.2016.08.005.Search in Google Scholar
[94] Ayana B. Wheat production as affected by weed diversity and other crop management practices in Ethiopia. Int J Res Stud Agric Sci. 2020;6(9):14–21. 10.20431/2454-6224.0609003.Search in Google Scholar
[95] Abbas T, Zahir ZA, Naveed M, Kremer RJ. Limitations of existing weed control practices necessitate development of alternative techniques based on biological approaches. In: Advances in Agronomy. Academic Press; 2018. p. 239–280. 10.1016/bs.agron.2017.10.005.Search in Google Scholar
[96] Strehlow B, de Mol F, Gerowitt B. Herbicide intensity depends on cropping system and weed control target: Unraveling the effects in field experiments. Crop Prot. 2020;129:105011. 10.1016/j.cropro.2019.105011.Search in Google Scholar
[97] Heap I. The International Herbicide-Resistant Weed Database. Online. Thursday, January 25, 2024. Available www.weedscience.org.Search in Google Scholar
[98] de Albuquerque FP, de Oliveira JL, Moschini-Carlos V, Fraceto LF. An overview of the potential impacts of atrazine in aquatic environments: Perspectives for tailored solutions based on nanotechnology. Sci Total Env. 2020;700:134868. 10.1016/j.scitotenv.2019.134868.Search in Google Scholar
[99] Sousa BTd, Pereira AdES, Fraceto LF, Oliveira HC, Dalazen G. Post-emergence herbicidal activity of nanoatrazine against Alternanthera tenella Colla plants compared to other weed species. Heliyon. 2022;8(7):e09902. 10.1016/j.heliyon.2022.e09902.Search in Google Scholar
[100] Khan BA, Nadeem MA, Alawadi HFN, Javaid MM, Mahmood A, Qamar R, et al. Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.). Green Process Synth. 2023;12(1):1–10. 10.1515/gps-2023-0105.Search in Google Scholar
[101] Zhu G, Wang H, Gao H, Liu Y, Li J, Feng Z, et al. Multiple resistance to three modes of action of herbicides in a single Italian ryegrass (Lolium multiflorum L.) population in China. Agron. 2023;13(1):216. 10.3390/agronomy13010216.Search in Google Scholar
[102] Ghosh S, Sarkar B, Thongmee S. Nanoherbicides for field applications. In: Agricultural nanobiotechnology. Woodhead Publishing; 2022. 10.1016/B978-0-323-91908-1.00010-9.Search in Google Scholar
[103] Oliveira HC, Stolf-Moreira R, Martinez CBR, Sousa GFM, Grillo R, de Jesus MB, et al. Evaluation of the side effects of poly(epsilon-caprolactone) nanocapsules containing atrazine toward maize plants. Front Chem. 2015;3:1–9. 10.3389/fchem.2015.00061.Search in Google Scholar
[104] Liman R, Ciğerci İH, Öztürk NS. Determination of genotoxic effects of Imazethapyr herbicide in Allium cepa root cells by mitotic activity, chromosome aberration, and comet assay. Pestic Biochem Physiol. 2015;118:38–42. 10.1016/j.pestbp.2014.11.007.Search in Google Scholar
[105] Liu Q, Cui H, Muhoza B, Duhoranimana E, Xia S, Hayat K, et al. Fabrication of low environment-sensitive nanoparticles for cinnamaldehyde encapsulation by heat-induced gelation method. Food Hydrocoll. 2020;105:105789. 10.1016/j.foodhyd.2020.105789.Search in Google Scholar
[106] Farooq N, Abbas T, Tanveer A, Javaid MM, Ali HH, Safdar ME, et al. Differential hormetic response of fenoxaprop-p-ethyl resistant and susceptible phalaris minor populations: A potential factor in resistance evolution. Planta Daninha. 2019;37:e019187554. 10.1590/S0100-83582019370100045.Search in Google Scholar
[107] Lamichhane JR, Devos Y, Beckie HJ, Owen MDK, Tillie P, Messéan A, et al. Integrated weed management systems with herbicide-tolerant crops in the European Union: Lessons learnt from home and abroad. Crit Rev Biotechnol. 2017;37(4):459–75. 10.1080/07388551.2016.1180588.Search in Google Scholar
[108] Tataridas A, Kanatas P, Chatzigeorgiou A, Zannopoulos S, Travlos I. Sustainable crop and weed management in the era of the EU green deal: A survival guide. Agron. 2022;12(3):589. 10.3390/agronomy12030589.Search in Google Scholar
[109] Wilcut JW, York AC, Jordan DL. Weed management systems for oil seed crops. New York: Routledge, New York; 2017. 10.1201/9780203752470.Search in Google Scholar
[110] He B, Hu Y, Wang W, Yan W, Ye Y. The progress towards novel herbicide modes of action and targeted herbicide development. Agron. 2022;12(11):2792. 10.3390/agronomy12112792.Search in Google Scholar
[111] Ghanizadeh H, Harrington KC. Non-target site mechanisms of resistance to herbicides. Crit Rev Plant Sci. 2017;36(1):24–34. 10.1080/07352689.2017.1316134.Search in Google Scholar
[112] Bo AB, Won OJ, Sin HT, Lee JJ, Park KW. Mechanisms of herbicide resistance in weeds. Korean J Agric Sci. 2017;44(1):1–15. 10.7744/KJOAS.20170001.Search in Google Scholar
[113] Gage KL, Krausz RF, Walters SA. Emerging challenges for weed management in herbicide-resistant crops. Agric. 2019;9(8):180. 10.3390/agriculture9080180.Search in Google Scholar
[114] Veisi M, Moeini MM, Jahedi A, Mansoori MS, Sabeti P. Weed control efficacy: Response of chickpea to pre- and post-emergence herbicides. Gesunde Pflanz. 2022;74(2):447–56. 10.1007/s10343-022-00621-6.Search in Google Scholar
[115] Soni JK, Nibhoria A, Punia SS, Yadav DB, Choudhary VK, Lalramhlimi B, et al. Herbicide resistant Phalaris minor in India—history of evolution, present status and its management. Phytoparasitica. 2023;51(2):353–78. 10.1007/s12600-022-01039-6.Search in Google Scholar
[116] Gherekhloo J, Hassanpour-bourkheili S, Hejazirad P, Golmohammadzadeh S, Vazquez-Garcia JG, De Prado R. Herbicide resistance in Phalaris species: A review. Plants. 2021;10(11):2248. 10.3390/plants10112248.Search in Google Scholar
[117] Hashim S, Jan A, Fahad S, Ali HH, Mushtaq MN, Laghari KB, et al. Weed management and herbicide resistant weeds: A case study from wheat growing areas of Pakistan. Pak J Bot. 2019;51(5):1761–7. 10.30848/PJB2019-5(28).Search in Google Scholar
[118] Abbas T, Nadeem MA, Tanveer A, Ali HH, Matloob A. Evaluation and management of acetyl-CoA carboxylase inhibitor resistant littleseed canarygrass (Phalaris minor) in Pakistan. Arch Agron Soil Sci. 2017;63(11):1613–22. 10.1080/03650340.2017.1296135.Search in Google Scholar
[119] Peterson MA, Collavo A, Ovejero R, Shivrain V, Walsh MJ. The challenge of herbicide resistance around the world: A current summary. Pest Manag Sci. 2018;74(10):2246–59. 10.1002/ps.4821.Search in Google Scholar
[120] Nakka S, Jugulam M, Peterson D, Asif M. Herbicide resistance: Development of wheat production systems and current status of resistant weeds in wheat cropping systems. J Crop. 2019;7(6):750–60. 10.1016/j.cj.2019.09.004.Search in Google Scholar
[121] Gaskin JF, Espeland E, Johnson CD, Larson DL, Mangold JM, McGee RA, et al. Managing invasive plants on Great Plains grasslands: A discussion of current challenges. Rangel Ecol Manag. 2021;78:235–49. 10.1016/j.rama.2020.04.003.Search in Google Scholar
[122] Soni N, Westra EP, Allegretta G, Araujo ALS, de Pinho CF, Morran S, et al. Survey of ACCase and ALS resistance in winter annual grasses identifies target-site and nontarget-site imazamox resistance in Secale cereale. Pest Manag Sci. 2022;78(12):5080–9. 10.1002/ps.7154.Search in Google Scholar
[123] Barrett M, Brazier-Hicks M, Dayan FE, Duke SO, Edwards R, Ferhatoglu Y, et al. Herbicide metabolism: Crop selectivity, bioactivation, weed resistance, and regulation. Weed Sci. 2019;67(2):149–75. 10.1017/wsc.2018.88.Search in Google Scholar
[124] Traxler C, Gaines TA, Küpper A, Luemmen P, Dayan FE. The nexus between reactive oxygen species and the mechanism of action of herbicides. J Biol Chem. 2023;299(11):105267. 10.1016/j.jbc.2023.105267.Search in Google Scholar
[125] Jugulam M, Shyam C. Non-target-site resistance to herbicides: Recent developments. Plants. 2019;8(10):417. 10.3390/plants8100417.Search in Google Scholar
[126] Brunharo CA, Hanson BD. Multiple herbicide–resistant Italian ryegrass [Lolium perenne L. spp. multiflorum (Lam.) Husnot] in California perennial crops: characterization, mechanism of resistance, and chemical management. Weed Sci. 2018;66(6):696–701. 10.1017/wsc.2018.50.Search in Google Scholar
[127] Keshtkar E, Abdolshahi R, Sasanfar H, Zand E, Beffa R, Dayan FE, et al. Assessing fitness costs from a herbicide-resistance management perspective: A review and insight. Weed Sci. 2019;67(2):137–48. 10.1017/wsc.2018.63.Search in Google Scholar
[128] San Martín C, Long DS, Gourlie JA, Barroso J. Spring crops in three year rotations reduce weed pressure in winter wheat. Field Crop Res. 2019;233:12–20. 10.1016/j.fcr.2018.12.017.Search in Google Scholar
[129] Sarabi V, Ghanbari A, Mohassel MHR, Mahallati MN, Rastgoo M. Broadleaf Weed Control in Corn (Zea mays L.) with Sulfonylurea Herbicides Tank‐mixed with 2, 4‐D + MCPA. J Agron. 2018;110(2):638–45. 10.2134/agronj2017.07.0419.Search in Google Scholar
[130] Kumar V, Jha P. Influence of herbicides applied postharvest in wheat stubble on control, fecundity, and progeny fitness of Kochia scoparia in the US Great Plains. Crop Prot. 2015;71:144–9. 10.1016/j.cropro.2015.02.016.Search in Google Scholar
[131] Kumar V, Spring JF, Jha P, Lyon DJ, Burke IC. Glyphosate-resistant Russian-thistle (Salsola tragus) identified in Montana and Washington. Weed Technol. 2017;31(2):238–51. 10.1017/wet.2016.32.Search in Google Scholar
[132] Soltani N, Brown LR, Shropshire C, Sikkema PH. Weed control in winter wheat (Triticum aestivum L.) with preplant applications of glyphosate plus mesotrione or saflufenacil. Agric Sci. 2015;6(6):594. 10.4236/as.2015.66058.Search in Google Scholar
[133] Jhala AJ, Norsworthy JK, Ganie ZA, Sosnoskie LM, Beckie HJ, Mallory-Smith CA, et al. Pollen-mediated gene flow and transfer of resistance alleles from herbicide-resistant broadleaf weeds. Weed Technol. 2021;35(2):173–87. 10.1017/wet.2020.101.Search in Google Scholar
[134] Forini MM, Pontes MS, Antunes DR, de Lima PH, Santos JS, Santiago EF, et al. Nano-enabled weed management in agriculture: From strategic design to enhanced herbicidal activity. Plant Nano Biol. 2022;1:100008. 10.1016/j.plana.2022.100008.Search in Google Scholar
[135] Sun H, Cao Y, Kim D, Marelli B. Biomaterials technology for agrofood resilience. Adv Funct Mater. 2022;32(30):2201930. 10.1002/adfm.202201930.Search in Google Scholar
[136] Zhang P, Guo Z, Ullah S, Melagraki G, Afantitis A, Lynch I. Nanotechnology and artificial intelligence to enable sustainable and precision agriculture. Nat Plants. 2021;7(7):864–76. 10.1038/s41477-021-00946-6.Search in Google Scholar
[137] Amna, Alharby HF, Hakeem KR, Qureshi MI. Weed control through herbicide-loaded nanoparticles. In: Nanomaterials and plant potential. Cham: Springer; 2019. p. 507–27. 10.1007/978-3-030-05569-1_20.Search in Google Scholar
[138] Mesnage R, Székács A, Zaller JG. Herbicides: Brief history, agricultural use, and potential alternatives for weed control. In: Herbicides. Elsevier; 2021. p. 1–20. 10.1016/B978-0-12-823674-1.00002-X.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

