Abstract
In response to significant demand for evaluating the presence of heavy elements in diverse industrial areas of Saudi Arabia, the study sought to analyze the concentration ratios of 16 elements across 11 different artificial soil types in the industrial zones situated in Riyadh, Sudair, and Al-Ahsa. To achieve this objective, the research utilized the wet digestion technique and employed an ICPE-9000 spectrophotometer for quantifying element concentrations. The study had a dual focus: initially, it sought to gauge the pollution attributed to heavy metals arising from enrichment processes, and second, it evaluated the geographical accumulation of pollutants in the soil. The results revealed varying concentration levels of heavy metals across the industrial areas under study. Specifically, the soil in the Yanbu region exhibited the highest concentration values for iron, manganese, zinc, chromium, nickel, copper, cobalt, cadmium, and beryllium. In contrast, the soil in the industrial area of Riyadh had the highest concentration values for lead, mercury (Hg), molybdenum (Mo), selenium (Se), and arsenic (As). Furthermore, the highest concentration of Cd was recorded in the soil of the Jubail region. Conversely, the soil in the Al-Ahsa region displayed the lowest concentration levels for these heavy metals. The conductivity of the synthetic soil ranged from 0.47 to 6.07 μS·cm−1, accompanied by a pH range of 6.6–8.6. The results emphasized the fluctuations in element concentrations, indicating significant implications for both environmental and human health. Notably, around 20% of the gathered samples showed concentrations of heavy elements like Mo, As, Hg, and Se that surpassed the allowable limits.
1 Introduction
Soil contamination with heavy elements represents a severe and enduring environmental challenge that has profound implications for human society [1,2,3,4,5,6]. These heavy elements, which are inorganic pollutants and include heavy metals, are naturally occurring but are also generated as a result of various human works [6]. The soil element ratio has been scientifically established to be a consequence of agricultural and industrial processes [7]. Furthermore, the escalation in the levels of trace metals in the environment due to human activities is performed, recently [8]. While these elements are essential for cellular functions, they can become toxic when present in excessive concentrations. In fact, some heavy metals exhibit toxicity even at exceedingly low levels [9].
Industrial processes contribute significantly to environmental pollution, which, in turn, poses significant problems for human populations [10,11]. High concentrations of these metals can enter the human body through various means, such as suspended dust and direct contact with contaminated materials [12]. Contaminated soils not only jeopardize the ecosystem but also become more problematic as environmental conditions undergo alterations [13].
Among the heavy metals, certain ones are particularly harmful, including arsenic (As), asbestos, cadmium (Cd), uranium, lead (Pb), thallium, mercury (Hg), antimony, manganese (Mn), barium, chlorine (Cl), zinc (Zn), beryllium (Be), bromine, and bismuth [14]. Significantly, certain heavy elements have garnered acknowledgment as extremely toxic substances in contemporary contexts. The mining and utilization of these minerals in diverse industrial procedures have led to previously overlooked environmental and health concerns. The increased presence of these pollutants in the soil contributes to water pollution, particularly as environmental conditions undergo alterations [15,16,17,18].
The presence of heavy metals in coastal environments has a profoundly detrimental impact on their environmental quality [19,20,21,22]. The coastal city of Jeddah, located along the Red Sea in Saudi Arabia, has experienced a significant increase in human activities over the past three decades, resulting in heightened pollution levels. The Red Sea coast, particularly in Yanbu, serves as a vital source of seafood and marine transportation within the Kingdom, making it closely linked to public health concerns. To assess element contaminations in the marine ecosystem, scientists often analyze minerals present in sediments as indicators of toxic elements [23].
Recently, significant industrial progress has taken place in Saudi Arabia, particularly in sectors like petrochemicals, oil, and gas production. Precise and dependable measurement of potentially harmful element ratios in soil or sediment requires the adoption of contemporary methodologies, such as microwave-assisted digestion of organic components. These approaches have been designed to break down metals present in the samples effectively. Many studies have utilized sophisticated technical instruments capable of applying high pressure to the samples for this specific purpose [15–17].
Herein, the research objective is to evaluate the concentration ratios of 16 different elements across 11 types of artificial soils located within industrial zones in Riyadh, Sudair, and Al-Ahsa regions of Saudi Arabia. To achieve this, the study utilized the wet digestion technique and employed an ICPE-9000 spectrophotometer to determine the concentrations of these elements. The study had a dual focus: first, it aimed to assess the presence of pollution due to heavy metals resulting from enrichment processes, and second, it investigated the geographical distribution of pollutants within the soil.
The results unveiled varying levels of concentrations for heavy metals within the industrial areas under investigation. Specifically, the soil in the Yanbu region exhibited the highest concentration values for elements such as Fe, Mn, Ni, Zn, Cu, Cr, Co, Cd, and Be. In contrast, the industrial soil in Riyadh had the highest concentration values for elements such as Pb, Hg, Mo, selenium (Se), and As. Moreover, the highest Cd concentration was detected in the soil of the Jubail region. Conversely, the soil in the Al-Ahsa region displayed the lowest concentration levels for these heavy metals.
This study offers a comprehensive examination of potentially heavy element pollution in the soil of diverse industrial regions in Saudi Arabia. The findings underscore the disparities in heavy metal concentrations, which can have significant implications for the environment. The development of effective strategies for monitoring and mitigating soil pollution in industrial areas is imperative for the preservation of ecosystems and the well-being of the public.
2 Practical part
2.1 Materials and methods
2.1.1 Soil materials
The 11 soil samples were gathered from various locations, encompassing three industrial areas located in Riyadh, Sudayr, and Al-Ahsa, Kingdom of Saudi Arabia. Additionally, soil samples were collected from two coastal areas situated in Jubail and Yanbu, each at varying distances from specific points of interest. Among the samples taken in Yanbu, some were obtained from marine environments, while others were collected from desert regions. This comprehensive sampling approach aimed to capture a diverse range of soil conditions and locations for further analysis and study. The soil samples gathered are manually ground for 15 min using a ceramic mortar to produce fine particles. This procedure is conducted at standard room temperature.
2.1.2 Methods and analyses of the data
Numerous analytical techniques exist for the detection and quantification of heavy elements. However, in the context of this research study, the chosen method was inductively coupled plasma (ICP) technology. This selection was made due to several distinct advantages that ICP offers over alternative methods.
One of the primary advantages of utilizing ICP technology is its ability to measure a wide array of elements efficiently and swiftly. This capability is particularly valuable when dealing with a research study that requires the analysis of a large number of elements within a relatively short time frame. The speed and versatility of ICP technology enable researchers to obtain comprehensive data sets promptly, facilitating more efficient data collection and analysis.
Additionally, ICP technology has a high level of sensitivity, particularly detecting trace amounts of elements. This increased sensitivity allows for the precise measurement of elements even when they are present in minuscule concentrations. This accuracy is of paramount importance in scientific research, where the detection of subtle variations in element concentrations can yield critical insights into various phenomena. Furthermore, ICP technology offers cost-effectiveness than some alternative methods. The efficient use of resources, coupled with the ability to analyze multiple elements simultaneously, makes ICP an economically advantageous choice for both quantitative and qualitative analyses.
2.2 ICP spectrometry conditions and parameters
This study relied on the application of ICP technology for both quantitative and qualitative analyses. This cutting-edge technology featured high-efficiency emission sources along with a charge-coupled device detector, ensuring precision and accuracy in the results obtained. To maintain the integrity of the analysis, high-purity inert argon gas was employed to prevent any potential spectral interference issues.
The detector’s temperature was carefully set at a specific −14.89°C to optimize its performance. The gas played a multifaceted role in the analytical process. First, there was a continuous flow of plasma gas at a rate of 10 L·min−1. Second, an additional 0.6 L·min−1 of gas was introduced into the system. Third, a sample carrier gas was used at a rate of 0.7 L·min−1. These controlled gas flows were essential to create the ideal conditions for the ICP. Furthermore, various key parameters were fine-tuned to ensure the optimal functioning of the ICP technology. The radio frequency power was set at precisely 1.2 kW, and atmospheric pressure was maintained at 450 ± 10 kPa. In terms of orientation, the system operated in the axial direction, with a rotation speed of 20 rpm.
2.3 Setting solutions standards
A standard multi-component solution was utilized. This solution of 1,000 ppm is synthesized through a 5% nitric acid (HNO3) solution.
The soil samples were prepared usinga wet digestion method. To begin, 1 g of the sample and 4 mL of royal water, consisting of 3 mL of HNO3 and 1 mL of hydrochloric acid, were added to the sample. The mixture was allowed to settle for 1 day. It was then cooked on a hot plate to 200°C until it vaporized completely. Following this, the sample was filtered and collected into a volumetric flask with a predetermined volume. To complete the process, deionized water was poured to the flask to reach the desired volume.
2.4 Heavy element analysis of samples
2.4.1 Chemical and physical characteristics of the samples
The pH level was measured, and the element concentration was calculated in mg·kg−1. Additionally, electrical conductivity (EC) and total dissolved solid (TDS) values were measured in S·cm−1 and g·L−1, respectively. Furthermore, the concentration of chlorine anions (Cl–1) was measured in g·L−1.
2.4.2 Analysis of metals at distance or proximity to the source of pollution
Point pollution originates from stationary and fixed sources of pollution, often exemplified by factories and industrial facilities. In contrast, non-point pollution is associated with mobile and diffuse sources of contamination, such as fertilizers in agriculture. In this study, the objective was to assess the impact of proximity to pollution sources on the concentration of elements in the soil. To achieve this, soil samples were obtained from three distinct industrial areas located in Sudayr.
In particular, the research aimed to investigate how varying distances from these industrial sites, specifically distances of 50 and 100 m away from the factories, influenced the heavy metals ratios in the soil. This spatial analysis sought to understand the extent to which point pollution, generated by these industrial complexes, affected the surrounding soil.
By measuring and comparing potentially harmful element concentrations at different distances from the pollution sources, the study aimed to provide valuable insights into the spatial distribution of soil pollution. The choice of Sudayr, known for its industrial activities, served as an ideal location to examine the dynamics of point pollution and its impact on soil quality.
2.5 Statistical analyses and contamination assessment methods
Significant differences were detected through the utilization of analysis of variance (ANOVA). To evaluate the extent of mineral-induced soil contamination, it is essential to assess parameters such as the Pollution Load Index (PLI), fertilization factors (EF), and the Geographical Accumulation Index (I geo).
2.6 Measurement of the concentrations of the elements
The method suggested below for pollution investigations involves the calculation of element concentrations through the PLI. The determination is carried out as follows [24]:
where PLI represents the pollution level, P i stands for the position of the pollution index for an individual element I, and n represents all elements considered.
The Pi is determined as follows: P i = C i /S i , and P i represents the contamination index for a specific element I, C i (element in the sample), and S i is the background concentration of the sample.
When the PLI value exceeds 1, it signifies a polluted site, while a PLI value less than 1 indicates a site without pollution.
In addition to that, the Geographical Index of Accumulation (I geo) is investigated; the calculation for I geo [25] is as follows:
where C n represents the measurement of all elements n in the soil, B n stands for the average element concentration n, which serves as a parameter for the geochemical background, and the constant 1.5 is employed to mitigate the potential impact of fluctuations in the soil background values.
This index, I geo, provides insight into the accumulation of elements in relation to their background levels, helping to assess the degree of geographic accumulation in the soil.
2.7 Soil fertilization assessment
To evaluate the extent of human-induced pollutant deposition on the surface soil, enrichment factor (EF) indicators were estimated using the concentration of iron [26–28] in the topsoil as a reference element. The calculation for EF [29] is as follows:
where EF stands for the enrichment factor, C x represents the target element being assessed, and C reference is the reference element, which in this case is iron (Fe), used for normalization purposes.
The EF values obtained are used to categorize the level of element enrichment in the soil, with different ranges indicating various degrees of enrichment. These categories are as follows:
EF < 2 suggests minimal element deficiency.
2 < EF < 5 notify moderate enrichment.
5 < EF < 20 notify significant enrichment.
20 < EF < 40 notify strong enrichment.
EF > 40 notifies of very high enrichment.
By calculating EF values based on the iron concentration in the topsoil, this method helps assess the element enrichment degree and provides insights into the potential impact of human-related pollutants on the soil.
3 Results and discussion
3.1 Heavy element analysis of samples
3.1.1 Chemical and physical properties of the samples
The physiochemical behavior and the mineral concentrations of soil samples are presented in Tables 1 and 2 and Figure 1. The pH values and TDS values in the five regions vary greatly ranging from 220 to 1,647 mg·L−1 with an average of 436 mg·L−1. Chloride anion concentration ranges from 21 to 400 mg·L−1. Additionally, the EC value ranges from 0.47 to 6.07 μS·cm−1, with an average conductivity of 1.6 μS.
Physicochemical behavior of soil in some industrial areas in Saudi Arabia
| City | pH | EC (S·cm−1) | TDS (mg·L−1) | C1− (mg·L−1) |
|---|---|---|---|---|
| Riyadh | 6.6 | 6.07 | 1647.0 | 273.5 |
| Sudayr | 7.00 | 0.84 | 220.0 | 37.5 |
| Al-Ahsa | 7.00 | 0.87 | 236.0 | 39 |
| Jubail | 7.9 | 0.54 | 146.5 | 24 |
| Yanbu on the sea side | 8.6 | 0.89 | 241.5 | 400 |
| Yanbu on the desert side | 7.1 | 0.47 | 127.5 | 21 |
Average and common range of heavy element concentrations in soil (ppm) [33]
| Common range in sample (ppm) | |||
|---|---|---|---|
| Element | Max. | Min. | Average |
| Be | 40 | 0.1 | 6 |
| Cd | 0.7 | 0.01 | 0.06 |
| Cr | 1.000 | 1 | 100 |
| Mn | 3,000 | 20 | 600 |
| Fe | 55,000 | 7,000 | 38,000 |
| Co | 40 | 1 | 8 |
| Ni | 500 | 5 | 40 |
| Cu | 100 | 2 | 30 |
| Zn | 300 | 10 | 50 |
| As | 50 | 1 | 5 |
| Se | 2 | 0.1 | 0.3 |
| Mo | 5.00 | 0.2 | 2 |
| Hg | 0.3 | 0.01 | 0.03 |
| Pb | 200 | 2 | 10 |
| V | 500 | 20 | 100 |
| Ti | 10,000 | 1,000 | 4,000 |
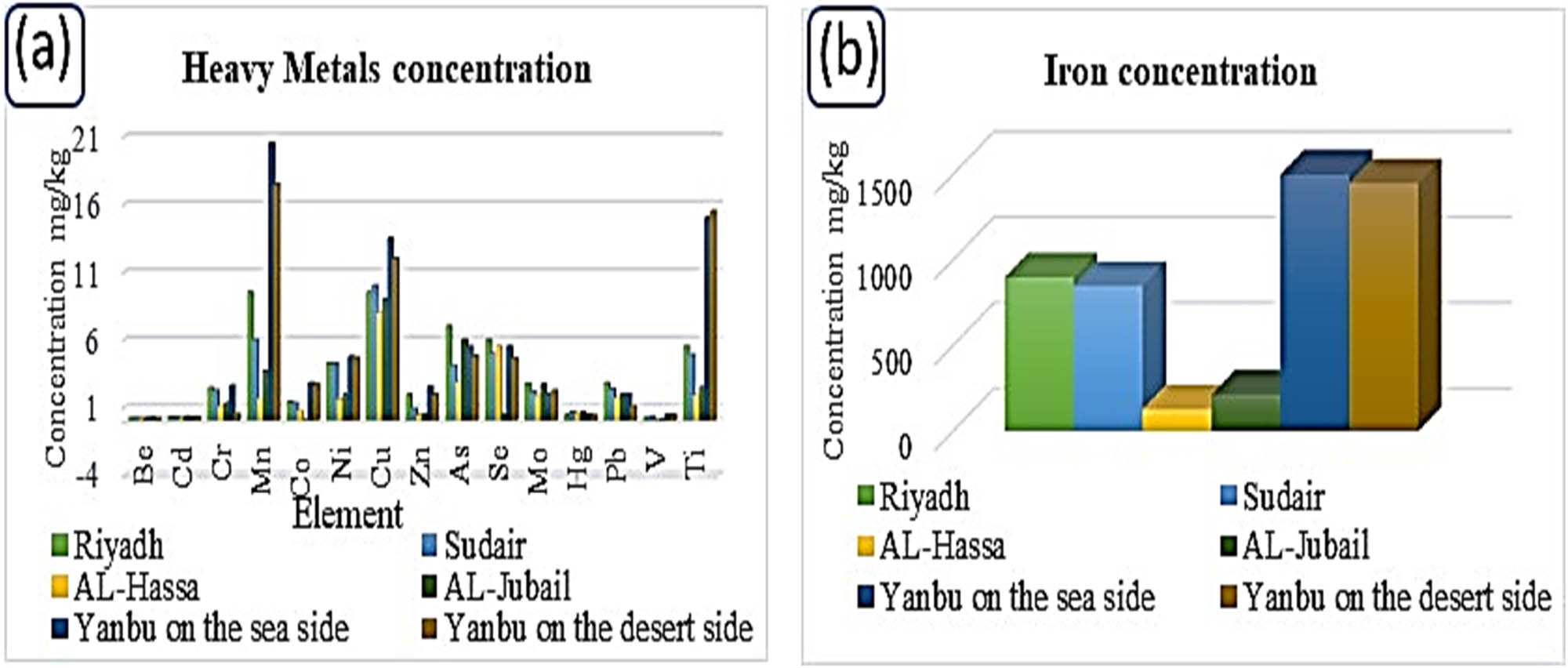
(a) Heavy element concentrations in soil and (b) the iron concentration in the soil of the five industrial cities of the Kingdom of Saudi Arabia (very small standard deviation approximately <1.0%).
According to the European Commission classification, soils are categorized as non-saline when the EC value is below 2, salty within the range of 2–8, and brine within the range of 8–16. Soils with an EC value exceeding 16 are considered highly saline [30–32]. The study results indicate the prevalence of non-saline soil in all regions except for the Riyadh region.
3.1.2 Mineral in the soil in the industrial area of Riyadh
The data presented in Table 3 investigate the potentially harmful element concentrations found in the soil of the first industrial city. This soil contains a range of mineral concentrations ranging from 0.16 to 5.5 ppm, as outlined in Table 4. It is noteworthy that Cu and Cr show a notably high concentration, reaching 9.5 ppm, while the Fe content is recorded at 900 ppm, a level that adheres to established safety and suitability standards [32]. It is crucial to highlight that the average concentrations of As and Mo in this soil exceed the global averages for these elements in soil. Conversely, the average other metal ratios studied in this soil fall below the global average levels.
Heavy metal concentrations (soil) for five industrial cities in Saudi Arabia
| Element concentration (mg·kg −1 ) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Be | Cd | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Mo | Hg | Pb | V | Ti | |
| Industrial city | ||||||||||||||||
| Riyadh | 0.16 | 0.27 | 2.4 | 9.5 | 900 | 1.4 | 4.2 | 9.5 | 1.9 | 7.0 | 6.0 | 2.7 | 0.47 | 2.8 | 0.18 | 5.5 |
| Sudayr | 0.17 | 0.23 | 2.2 | 6.0 | 850 | 1.3 | 4.2 | 10. | 0.9 | 4.1 | 4.9 | 2.1 | 0.65 | 2.3 | 0.30 | 4.9 |
| Al-Ahsa | 0.17 | 0.19 | 1.1 | 1.6 | 130 | 0.7 | 1.6 | 8.0 | 0.4 | 2.8 | 5.5 | 1.8 | 0.55 | 1.7 | 0.06 | 1.9 |
| Al-Jubail | 0.16 | 0.32 | 1.3 | 3.7 | 215 | — | 1.9 | 9.0 | 0.5 | 6.0 | 0.5 | 2.7 | 0.63 | 1.9 | 0.09 | 2.5 |
| Yanbu* | 0.20 | 0.27 | 2.6 | 20. | 150 | 2.8 | 4.7 | 13. | 2.5 | 5.5 | 5.5 | 2.0 | 0.46 | 2.0 | 0.46 | 15. |
| Yanbu** | 0.15 | 0.27 | 0.5 | 17. | 145 | 2.7 | 4.7 | 12. | 2.0 | 4.8 | 4.6 | 2.2 | 0.39 | 1.1 | 0.45 | 15. |
*On the seaside. **On the desert side.
Heavy mineral concentrations in the soil of three industrial areas in Riyadh city
| Country | Element concentration (mg·kg−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Riyadh | Be | Cd | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Mo | Hg | Pb | V | Ti |
| First industrial city | 0.16 | 0.270 | 2.45 | 9.50 | 900 | 1.40 | 4.25 | 9.5 | 1.95 | 7.00 | 6.0 | 2.75 | 0.47 | 2.8 | 0.185 | 5.50 |
| Second industrial city | 0.16 | 0.225 | 1.55 | 4.95 | 500 | 1.05 | 2.85 | 10.0 | 4.20 | 3.65 | 4.7 | 2.25 | 0.37 | 2.1 | 0.130 | 4.05 |
| Third industrial city | 0.16 | 0.245 | 2.05 | 9.50 | 1,100 | 1.55 | 3.25 | 11.0 | 3.40 | 60.0 | 6.0 | 2.40 | 0.50 | 3.0 | 0.175 | 13.5 |
Moving on to the soil of the second industrial city, Table 4 provides an overview of the varying concentrations of elements detected. Among these elements, Fe stands out with the highest concentration, reaching 500 ppm, while vanadium (V) exhibits the lowest concentration at 0.13 ppm. Importantly, the average concentrations of these elements in the soil are all within globally permissible limits, except for Cd, Se, Mo, and Hg, which are recorded at 0.225, 4.7, 2.25, and 0.37 ppm, respectively.
The soil of the third industrial city shows significant variance in possibly element concentrations ranging from 0.165 to 13.5 mg·kg−1. Notably, As and Fe values are 1,100 and 60 ppm, respectively. It is crucial to emphasize that all the measured potentially harmful element concentrations in this soil fall within globally accepted and specified limits.
However, there are notable exceptions to this pattern, particularly concerning the concentrations of Mo, Cd, As, Se, and Hg. These elements stand out due to their concentrations, which significantly exceed global averages. In fact, the values for these elements are 4, 4, 12, 20, and 17 times higher than the global averages, respectively. This raises concerns about potential environmental and health impacts associated with the elevated levels of these heavy metals in the soil.
The findings highlight the importance of continued assessment of the soil quality and potentially heavy elements contamination in industrial areas, particularly in regions where certain heavy metals exceed permissible levels. Such data are crucial for implementing effective pollution control measures and safeguarding both the environment and human health (Figure 2).

(a) Heavy mineral concentrations in soil and (b) iron element concentration in the soil of the three industrial areas in Riyadh city (very small standard deviation approximately <1.2%).
3.1.3 Minerals in the soil in the industrial area of Sudayr
Table 3 presents the outcomes regarding mineral concentrations in the topsoil of the Sudayr region. It is noteworthy that all potentially heavy element concentrations in the soil of this region fall within the acceptable average concentration levels [33]. However, exceptions arise in the case of Cd, Se, and Hg, where their concentrations surpass the global average by factors of 4, 16, and 12, respectively. This highlights potential concerns regarding elevated levels of these specific heavy metals in the soil, necessitating further attention and monitoring to mitigate potential environmental and health risks.
3.1.4 Minerals in the soil in the industrial area of the Al-Ahsa region
The findings presented in Table 3 demonstrate that the concentrations of all elements remain within safe limits, except cadmium, which exceeds the permissible limit by threefold. In addition to that, selenium and mercury concentrations have elevated with 18 allowable values. This notable increase in Se and Hg concentrations raises concerns about potential environmental and health implications, necessitating further investigation and potential remediation measures.
3.1.5 Minerals in the soil at the industrial area of Jubail
The diversity of mineral concentrations in the soil of the industrial area in Jubail is evident from the data presented in Table 3. Here, the concentrations of various elements range from 0.09 to 3.7 mg·kg−1. Interestingly, it is worth noting that Co was not detected in this particular study area, setting it apart from the other regions under investigation.
3.1.6 Minerals in the soil at the industrial area of Yanbu
The results presented in Table 2 offer a comprehensive comparison of element concentrations obtained from various soil environments, including both desert and coastal areas. It is evident that the concentrations of Se, Mo, Hg, and Cd in the soil of the Yanbu desert site exceed the permissible limits set for soil quality. Similarly, the same observation holds for Cd, As, Hg, and Se in the soils at the Yanbu site along the Red Sea. Notably, the concentration levels of cadmium, selenium, and mercury in both desert and marine locations are significantly elevated, with values reaching 4, 16, and 12 times higher than the globally accepted average, respectively. Moreover, the concentration of Mo surpasses the permissible limit in the desert soil, whereas it remains below the safe threshold in the soil near the coastline. As concentration is also notably higher than the upper safe limit for minerals in the soil at the Yanbu Bahri site, while its concentration in the desert area does not exceed the permissible limit. Statistical significance was established at a threshold level of p > 0.05 and was calculated for both desert and coastal areas, highlighting the disparities in element concentrations between these environments.
3.1.7 Comparison of the concentration of elements in the soil at the coast sides of Yanbu and Jubail
Table 7 indicates that the levels of these metals are greater in the stems on the coastal side compared to the coastal area of Jubail. Additionally, it is worth noting that Co was not detected in the soil within the industrial zone of the Jubail region.
3.1.8 Analysis of metals at distance or proximity to the source of pollution
The findings presented in Table 5 and Figure 3 shed light on the concentrations of various elements within plants, particularly in relation to their proximity to the pollution source (the factory). Notably, it appears that the concentrations of these elements are generally higher in plants located closer to the pollution source, specifically at distances of 50 and 100 m. However, a critical observation reveals no statistically t difference in the increase of concentrations for all elements, except for silicon. Silicon, in particular, stands out as its concentration in plants located at a closer distance to the pollution source is notably elevated. It is worth noting that the concentration of silicon is approximately eight times higher than that observed at 50 m from the pollution source and an impressive ten times higher than what is recorded at a distance of 100 m from the source. To ascertain the statistical significance of these differences, a one-way ANOVA was conducted. The outcome of this analysis yielded a significance level value of 0.692, which is greater than the conventional significance threshold of 0.05. In practical terms, this implies that there are no substantial differences in the concentrations of the elements based on their proximity or distance from the pollution source. In other words, the increase in element concentrations in plants near the source of pollution is not statistically significant.
Heavy element concentrations in Sudayr soil and at various distances from the factory
| Country | Element concentration (mg·kg−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sudayr | Be | Cd | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Mo | Hg | Pb | V | Ti |
| At the factory | 0.170 | 0.235 | 2.25 | 6.00 | 850 | 1.3 | 4.25 | 10 | 0.9 | 4.1 | 4.95 | 2.15 | 0.65 | 2.35 | 0.305 | 4.90 |
| 50 m from the factory | 0.160 | 0.240 | 2.20 | 6.50 | 850 | 1.3 | 3.75 | 9.5 | 0.7 | 6.5 | 0.60 | 2.25 | 0.455 | 2.45 | 0.225 | 4.80 |
| 100 m from the factory | 0.175 | 0.240 | 1.75 | 4.25 | 650 | 1.2 | 3.65 | 9.5 | 0.75 | 6.5 | 0.50 | 2.45 | 0.39 | 2.35 | 0.230 | 3.85 |

(a) The Sudayr region metal concentrations at various distances from the factory. (b) Fe concentrations in the soil of the Sudayr region and at different distances from the factory (very small standard deviation approximately <1.1%).
3.1.9 Comparison of mineral concentrations in different regions
Table 2 and Figure 1a provide an overview of the data obtained from the analysis of mineral concentrations in the soil across five industrial cities in the Kingdom. Notably, in the first industrial area of Riyadh, the soil concentrations for certain elements were observed to be higher compared to other industrial cities in different regions. For instance, the soil in the Jubail region recorded the highest concentration of cadmium, reaching 0.32 ppm. Conversely, the Al-Ahsa region exhibited the lowest concentration levels for most elements. This is related to its geographical location, which places it at a considerable distance from the primary sources of oil refining concentrated in the Jubail and Yanbu regions.
Furthermore, the soil in the Yanbu region displayed the highest concentrations of nickel and copper, measuring 13.5 and 4.75 mg·kg−1, respectively. These heightened concentrations could be attributed to oil refining and extensive industrial activities taking place in this region [33,34].
When assessing the concentrations of certain soil elements across all the studied industrial regions, it becomes evident that, for the most part, they fall within the global average concentrations for soil. However, there are significant exceptions to this pattern, particularly for Cd, Se, and Hg. These three elements stand out as their concentrations in the soil are notably higher than the global average. Cd, in particular, is recorded at levels five times greater than the world average for soil elements [35]. Se and Hg, on the contrast, exhibit concentrations that are 20 and 21 times higher than the global average for soil elements, respectively. It is noteworthy that Riyadh and Yanbu are reported to have the highest concentration of the highly toxic Cd metal, with levels reaching 0.27 mg·kg−1. Additionally, Riyadh’s soil records the highest Se concentration at 6 mg·kg−1, while the Sudayr area’s soil exhibits the highest Hg concentration, at 0.65 ppm. Furthermore, the soil in Riyadh and Jubail has a Se concentration of 6.7 mg·kg−1, surpassing the permissible safe limit [37,38].
3.2 Statistical analysis
Table 5 provides a succinct overview of the standard deviation values derived from the analysis of 11 soil samples. When delving deeper into Table 6, it becomes evident that the variance observed in the range of potentially heavy element distributions, in relation to their respective rates, serves as an indicator of potential sample contamination with Cd, As, Se, Mo, and Hg [39,40].
Metal descriptive statistics studied industrial areas
| Element | Min. | Area | Max. | Area | Mean | SD | SE |
|---|---|---|---|---|---|---|---|
| Be | 0.1650 | Riyadh | 0.2050 | Yanbu | 0.1750 | 0.0170 | 0.0076 |
| Cd | 0.1900 | Al-Ahsa | 0.3200 | Al Jubail | 0.2530 | 0.0483 | 0.0216 |
| Cr | 1.1000 | Al-Ahsa | 2.6000 | Yanbu | 1.8600 | 0.6378 | 0.2852 |
| Mn | 1.6500 | Al-Ahsa | 20.500 | Yanbu | 8.2700 | 7.4322 | 3.3238 |
| Co | 0.0000 | Al Jubail | 2.8000 | Yanbu | 1.2800 | 1.0372 | 0.4638 |
| Ni | 1.6000 | Al-Ahsa | 4.7500 | Yanbu | 3.1600 | 1.3804 | 0.6173 |
| Cu | 8.0000 | Al-Ahsa | 13.500 | Yanbu | 10.3000 | 2.1095 | 0.9434 |
| Zn | 0.4300 | Al-Ahsa | 3.4000 | Riyadh | 1.5460 | 1.3327 | 0.5960 |
| As | 2.8500 | Al-Ahsa | 60.000 | Riyadh | 15.6900 | 24.8007 | 11.0912 |
| Se | 0.5000 | Al Jubail | 6.0000 | Riyadh | 4.4900 | 2.2612 | 1.0112 |
| Mo | 1.8500 | Al-Ahsa | 2.7000 | Al Jubail | 2.2200 | 0.3365 | 0.1505 |
| Hg | 0.3600 | Al Jubail | 0.6500 | Sudayr | 0.5050 | 0.1069 | 0.0478 |
| Pb | 1.7000 | Al-Ahsa | 3.0000 | Riyadh | 2.2000 | 0.5037 | 0.2253 |
| V | 0.0650 | Al-Ahsa | 0.4600 | Yanbu | 0.2190 | 0.1641 | 0.0734 |
| Ti | 1.9000 | Al-Ahsa | 15.0000 | Yanbu | 7.5600 | 6.2320 | 2.7870 |
3.2.1 Comparison of the concentrations of metals studied with studies around the world
The concentrations of metals in soil samples gathered from the five selected regions were juxtaposed with those identified in soil from industrial areas in diverse countries across the globe, as detailed in Table 7.
Comparison of the concentrations of heavy metals in the soil of the five industrial cities in the Kingdom of Saudi Arabia with the industrial soil of previous studies around the world
| Be | Cd | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Mo | Hg | Pb | V | Ti | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saudi Arabia (Riyadh) | 0.160 | 0.270 | 2.45 | 9.5 | 900 | 1.40 | 4.25 | 9.5 | 1.95 | 7.00 | 6.00 | 2.75 | 0.47 | 2.80 | 0.185 | 5.5 | Present study |
| Saudi Arabia (Sudayr) | 0.170 | 0.235 | 2.25 | 6.00 | 850 | 1.30 | 4.25 | 10 | 0.9 | 4.10 | 4.95 | 2.15 | 0.65 | 2.35 | 0.305 | 4.9 | |
| Saudi Arabia (Al-Ahsa) | 0.170 | 0.19 | 1.10 | 1.65 | 130 | 0.75 | 1.60 | 8.0 | 0.43 | 2.85 | 5.50 | 1.85 | 0.55 | 1.70 | 0.065 | 1.9 | |
| Saudi Arabia (Jubail) | 0.165 | 0.320 | 1.30 | 3.70 | 215 | — | 1.95 | 9.0 | 0.50 | 6.00 | 0.50 | 2.70 | 0.36 | 1.95 | 0.09 | 2.5 | |
| Saudi Arabia (Yanbu) | 0.215 | 0.270 | 2.55 | 17.5 | 1,450 | 2.70 | 4.70 | 12 | 2.00 | 4.80 | 4.65 | 2.25 | 0.39 | 1.10 | 0.455 | 15.5 | |
| Saudi Arabia (Riyadh) | — | 0.110 | 14.88 | 77.31 | — | — | 15.97 | 9.91 | 20.40 | — | — | — | — | 6.50 | — | — | [41,42] |
| Saudi Arabia (Jubail) | — | 18.60 | 146.0 | 56.60 | — | 41.4 | 42.70 | 94.0 | 54.90 | — | — | — | — | 71.1 | — | — | [12] |
| Saudi Arabia (Ras Tanura) | — | 39.90 | 2.70 | — | — | 0.52 | 2.67 | — | 1.14 | — | — | 13.2 | — | 2.27 | — | — | [43,44] |
| Saudi Arabia (Qassim) | — | 1.65 | 17.4 | 0.26 | — | 1.34 | 2.61 | 0.15 | 21.10 | — | — | — | — | 26.9 | — | — | [27] |
| Iraq | — | 13.30 | 71.2 | — | — | — | 52.50 | 13.1 | — | — | — | — | — | 9.0 | — | — | [36] |
| Azerbaijan | — | 0.18 | 19.9 | 410 | — | — | — | 37.1 | 47.90 | — | — | — | — | 29.2 | — | — | [45] |
| Turkey | — | 7.42 | 86.2 | 1,397 | — | 12.7 | 40.80 | 94.1 | 1,957 | — | — | — | — | 437 | — | — | |
| Turkey | — | 1.34 | 51.21 | — | — | 16.62 | 209.22 | — | 56.62 | — | — | 4.78 | 17.68 | — | — | ||
| Egypt | — | — | 167.0 | — | — | 13 | 35.78 | — | 98 | — | — | — | — | 130.97 | — | — | [46] |
| Greece | — | 0.20 | 193.2 | — | — | — | 58.2 | 123.9 | 137.8 | — | — | — | — | 359.4 | — | — | |
| Mongolia | — | 2.10 | 32.2–77.5 | 539–646 | — | 62–11 | 12.1–21.8 | 21.7–36.5 | 148 | — | — | — | — | 54.7 | — | — | [47] |
| India | — | — | 371.98 | — | — | 17.60 | 55.86 | 371.98 | 811.8 | — | — | — | — | 900.89 | — | — | |
| India | — | — | 8.29 | — | — | 4.84 | 18.78 | — | — | 12.52 | — | — | [48] | ||||
| Nepal | — | 0.12 | 38,383 | — | — | 7.92 | 17.31 | 19.51 | 66.9 | 21.2 | — | — | [49] | ||||
| Italy | — | 0.40 | 90.54 | — | — | 17.05 | 34.67 | 44.36 | 166.7 | 63.67 | — | — | [50] | ||||
| China | — | 0.13 | 68.28 | 598.7 | — | 13.26 | 29.36 | 22.97 | 65.8 | 28.4 | — | — | [51] | ||||
| Poland | — | 0.08 | 3.00 | 180 | — | — | 1.10 | 1.00 | 4.9 | 11 | — | — | [52] | ||||
| Australia | — | 0.40 | 19.00 | — | — | 6 | 13.0 | 23.0 | 187 | 194 | — | — |
3.3 Contamination assessment methods of soil enrichment
Table 8 and Figure 4 offer a synthesis of the minimum, maximum, mean, and standard deviation of various heavy metals identified in 30 soil samples collected from Riyadh Industrial Area, Sudayr, Al-Ahsa, Jubail, and Yanbu in the Kingdom of Saudi Arabia. Notably, Co exhibited the highest EF across all regions, with a median value of 937.3608, indicating severe contamination. This trend was also evident for Mo and Hg in all five areas, underscoring significant levels of contamination and fertilization. Cu exhibited the highest EF in the soils of Al-Ahsa and Jubail, indicating strong fertilization. In contrast, the soils of Riyadh, Sudayr, and Yanbu showed severe pollution and high fertilization for these elements. Lead (Pb) exhibited moderate fertilization in Yanbu soil, while Co was not detected in Jubail soil, showing moderate fertilization in Riyadh, Sudayr, and Yanbu and significant fertilization in Al-Ahsa soil. The level of vineyard soil fertilization was moderate in Al-Ahsa and Jubail, while it was classified as fertilized in Riyadh, Sudayr, and Yanbu. The degree of fertilization increased significantly in the soil of Al-Ahsa and Jubail. These results indicate that soil samples reflect anthropogenic contributions of these elements in the five regions, particularly in Al-Ahsa, suggesting the possibility of environmental mineral pollution from industrial activities. Industrial waste emissions in the study areas could potentially serve as sources of these elements [53].
Mean and range of EF in soil samples of study areas
| Element | Mean of EF | Range of EF | Classification range of pollution level |
|---|---|---|---|
| Be | 8.0611 | 2.1502–20.5744 | Moderate-strong |
| Cd | 114.3008 | 28.8444–234.1705 | Strong-extreme |
| Cr | 2.1767 | 0.9090–4.4376 | Light-moderate |
| Mn | 0.6582 | 0.3920–0.9556 | Light |
| Co | 5.2538 | 0.0000–14.3320 | Light-significant |
| Ni | 4.5116 | 2.0508–8.5430 | Moderate-significant |
| Cu | 28.1445 | 9.4400–64.5470 | Significant-extreme |
| Zn | 1.1377 | 0.5261–1.6434 | Light |
| As | 81.9579 | 13.3128–198.0420 | Significant-extreme |
| Se | 937.3608 | 182.9457–3328.2051 | Extreme |
| Mo | 119.2108 | 24.2051–258.3432 | Strong-extreme |
| Hg | 175.4528 | 36.5800–499.2308 | Strong-extreme |
| Pb | 13.6748 | 3.1467–30.8615 | Moderate-strong |
| V | 0.1266 | 0.0578–0.1815 | Light |
| Ti | 0.1114 | 0.0592–0.1500 | Light |

Percentage of EF for heavy elements in the soil samples of study areas.
3.3.1 Assessment of heavy element pollution
Table 9 and Figure 5 display the PI values for heavy minerals in the industrial zones of Riyadh, Sudayr, and Al-Ahsa. Table 10 provides a summary of the minimum, maximum, and average values for all heavy minerals detected in the cities under study.
PI value for metal in soils
| PI value | Level of pollution |
|---|---|
| PI ≤ 1 | Unpolluted |
| 1 < PI ≤ 2 | Slightly polluted |
| 2 < PI ≤ 3 | Moderately polluted |
| 3 < PI ≤ 5 | Strongly polluted |
| PI > 5 | Very strongly polluted |

Differences in PI values in soil from the study areas.
Mean and range of PI in samples of study areas
| Element | Mean of PI | Range of PI | Classification range of contamination level |
|---|---|---|---|
| Be | 0.0583 | 0.0220–0.0683 | Unpolluted |
| Cd | 0.8433 | 0.6333–1.0667 | Unpolluted–slightly polluted |
| Cr | 0.0207 | 0.0122–0.0289 | Unpolluted |
| Mn | 0.0097 | 0.0019–0.0241 | Unpolluted |
| Co | 0.0674 | 0.0000–0.1474 | Unpolluted |
| Ni | 0.0465 | 0.0235–0.0699 | Unpolluted |
| Cu | 0.2289 | 0.1778–0.3000 | Unpolluted |
| Zn | 0.0163 | 0.0045–0.0358 | Unpolluted |
| As | 1.2069 | 0.2192–4.6154 | Unpolluted–strongly polluted |
| Se | 7.4833 | 0.8333–10.0000 | Unpolluted–very strongly polluted |
| Mo | 0.8538 | 0.7115–1.0385 | Unpolluted–slightly polluted |
| Hg | 1.2625 | 0.9000–1.6250 | Unpolluted–slightly polluted |
| Pb | 0.1100 | 0.0850–0.1500 | Unpolluted |
| V | 0.0017 | 0.0005–0.0035 | Unpolluted |
| Ti | 0.0016 | 0.0004–0.0033 | Unpolluted |
3.3.2 Quantitative scale of the extent of mineral contamination
Tables 11 and 12, along with Figure 6, present a numerical scale that quantifies the degree of mineral contamination in the soil under investigation. The I geo values exhibit a range of variations.
Sort of heavy metal contamination levels in soils based on I geo
| I geo | I geo value | Level of pollution |
|---|---|---|
| 0 | I geo ≤ 0 | Unpolluted |
| 1 | 0 < I geo ≤ 1 | Unpolluted to moderately polluted |
| 2 | 1 < I geo ≤ 2 | Moderately to polluted |
| 3 | 2 < I geo ≤ 3 | Moderately to strongly polluted |
| 4 | 3 < I geo ≤ 4 | Strongly polluted |
| 5 | 4 < I geo ≤ 5 | Strongly to extremely polluted |
| 6 | 5 < I geo | Extremely polluted |
Results of (I geo) at industrial areas of Riyadh, Sudayr, Al-Ahsa, and coastal region of Jubail and Yanbu
| Element | Mean of I geo | Range of I geo | Rating range of contamination level |
|---|---|---|---|
| Be | –4.6895 | –4.7694–(–4.4562) | Unpolluted |
| Cd | –0.8521 | –1.24396–(−0.4919) | Unpolluted |
| Cr | –5.4171 | –6.9393–(−1.4997) | Unpolluted |
| Mn | –7.7562 | –9.5938–(−5.9587) | Unpolluted |
| Co | –4.3126 | –5.2479–(−3.3475) | Unpolluted |
| Ni | –5.1369 | –5.9944–(−4.4245) | Unpolluted |
| Cu | –2.7356 | –3.0768–(−2.3219) | Unpolluted |
| Zn | –7.0112 | –8.3724–(−5.3893) | Unpolluted |
| As | –1.3858 | –2.7744–(1.6215) | Unpolluted |
| Se | 1.9143 | –0.8480–(2.7370) | Unpolluted–Moderately polluted |
| Mo | –0.8259 | –1.0759–(−0.5305) | Unpolluted |
| Hg | –0.2756 | –0.7370–(0.1155) | Unpolluted–Moderately polluted |
| Pb | –3.7976 | –4.1414–(−3.3219) | Unpolluted |
| V | –10.1604 | –11.5507–(−8.7276) | Unpolluted |
| Ti | –10.3119 | –11.8264–(8−.8455) | Unpolluted |

Differences of I geo values in samples of study areas.
4 Conclusions
In this research investigation, a highly sensitive and easily reproducible method was employed, which, despite its simplicity, offers the advantage of facilitating international comparisons to assess the presence of heavy elements responsible for soil contamination. Samples were collected and subjected to analysis in areas lacking prior information regarding pollution levels. Interestingly, among the collected samples, approximately 20% exhibited concentrations of heavy elements such as Mo, As, and Se that exceeded the permissible limits. One notable finding from the study is the stark contrast in the concentration of these elements between the soil samples collected along the Red Sea coast and those from the Arabian Gulf region. Specifically, the concentration of these elements in the soil along the Red Sea coast was significantly higher than what was observed in the Arabian Gulf soil. These findings shed light on the critical role played by soil components in the enrichment process of heavy elements. The research highlights the significance of understanding the distribution and levels of these elements in soil, particularly in areas where such information was previously lacking. The use of a sensitive and easily replicable analytical method allows for the assessment of soil quality and pollution levels on a global scale, facilitating cross-border comparisons and enabling a better grasp of environmental conditions.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R186), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Funding information: Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R186), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Author contributions: Maha Abdallah Alnuwaiser is the only author of this research paper.
-
Conflict of interest: The author states no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Alharbi OML, Basheer AA, Khattab RA, Ali I. Health and environmental effects of persistent organic pollutants. J Mol Liq. 2018a;263:442–53. 10.1016/j.molliq.2018.05.029.Search in Google Scholar
[2] Alharbi OML, Khattab RA, Ali I, Binnaser YS, Aqeel A. Evaluation of the heavy metals threat to the Yanbu shoreline, red sea, Saud Arabia. Mar Freshw Res. 2018b;69(10):1557–68. 10.1071/MF18079.Search in Google Scholar
[3] Ali I, Alothman ZA, Alwarthan A. Supra molecular mechanism of the removal of 17-β-estradiol endocrine disturbing pollutant from water on functionalized iron iron particles. J Mol Liq. 2017;441:123–9. 10.1016/j.molliq.2017.06.005.Search in Google Scholar
[4] Ali I, Gupta VK, Aboul-Enerin HY. Metal ion speciation and capillary electrophoresis: application in the new millennium. Electrophoresis. 2005;26(21):3988–4002. 10.1002/elps.200500216.Search in Google Scholar PubMed
[5] Ali I, Wani WA, Saleem K, Hseih MF. Design and synthesis of thalidomide. Based dithiocarbamate Cu(II), Ni(II) and Ru(III) complexes as anticancer agents. Polyhedron. 2013;56:134–43. 10.1016/j.poly.2013.03.056.Search in Google Scholar
[6] Wang C, Zou X, Feng Z, Hao Z, Cao J. Distribution and transport of heavy metals in estuarine-inner shelf regions of the East China Sea. Sci Total Env. 2018;644:298–305. 10.1016/j.scitotenv.2018.06.383.Search in Google Scholar PubMed
[7] Uluturhan E, Kontas A, Can E. Sediment concentrations of heavy metals in the Home lagoon (Eastern Aegean Sea): assessment of contamination and ecological risks. Mar Poll Bull. 2011;62(9):1989–97. 10.1016/j.marpolbul.2011.06.019.Search in Google Scholar PubMed
[8] Al-Bakheet SA, Attafi JM, Maayah ZH, Abd-Allah AR, Asiri YA, Korashy HM. Effect of long-term human exposure to environmental heavy metals on the expression of detoxification and DNA repair genes. Environ Pollut. 2013;181:226–32. 10.1016/j.envpol.2013.06.014.Search in Google Scholar PubMed
[9] Alloway BJ. Heavy metals in soils. Vol. 2. 3rd edn. Dordrecht, Heidelberg, London New York: Springer; 2012.10.1007/978-94-007-4470-7_1Search in Google Scholar
[10] Meuser H. Contaminated urban soils. Environmental Pollution. Vol. 18: Springer; 2010. p. 318. 10.1007/978-90-481-9328-8.Search in Google Scholar
[11] Achotegui-Castells A, Sardans J, Ribas A, Penuelas J. Identifying the origin of atmospheric inputs of trace elements in the Prades Mountains (Catalonia) with bryopytes, lichens and soil monitoring. Environ Monit Assess. 2013;185:615–29. 10.1007/s10661-012-2579-z.Search in Google Scholar PubMed
[12] Alshahri F, El-Taher A. Assessment of heavy and trace metals in surface soil nearby an oil refinery, Saudi Arabia, using geoaccumulation and pollution indices. Arch Environ Contam Toxicol. 2018;75:390–401. 10.1007/s00244-018-0531-0.Search in Google Scholar PubMed
[13] Cai C, Xiong B, Zhang Y, Li X, Nunes LM. Critical comparison of soil pollution indices for assessing contamination with toxic metals. Water Air Soil Pollut. 2015;226(10):352. 10.1007/s11270-015-2620-2.Search in Google Scholar
[14] Benhaddya ML, Hadjel M. Spatial distribution and contamination assessment of heavy metals in surface soils of Hassi Messaoud”, Algeria. Environ Earth Sci. 2014;71:1473–86. 10.1007/s12665-013-2552-3.Search in Google Scholar
[15] Mokarram M, Saber A, Sheykhi V. Effects of heavy metal contamination on river water quality due to release of industrial effluents. J Clean Prod. 2020;277:123380.10.1016/j.jclepro.2020.123380Search in Google Scholar
[16] Rajendran S, Priya TAK, Khoo KS, Hoang TKA, Ng HS, Munawaroh HSH, et al. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere. 2022;287:132369.10.1016/j.chemosphere.2021.132369Search in Google Scholar PubMed
[17] Rehman Z, Junaid MF, Ijaz N, Khalid U, Ijaz Z. Remediation methods of heavy metal contaminated soils from environmental and geotechnical standpoints. Sci Total Environ. 2023;867:161468.10.1016/j.scitotenv.2023.161468Search in Google Scholar PubMed
[18] Alnuwaiser MA, Rabia M. Hollow mushroom nanomaterials for potentiometric sensing of Pb2+ ions in water via the intercalation of iodide ions into the polypyrrole matrix. Open Chem. 2024;22:20240217.10.1515/chem-2024-0217Search in Google Scholar
[19] Nayek S, Cupta S, Saha RN. Heavy metal distribution and chemical fractionation in water, suspended solids and bed sediments of industrial discharge channel: an implication to ecological risk. Res J Chem Env. 2013;17:26–33.Search in Google Scholar
[20] Jara-Marini ME, Soto-Jiménez MF, Páez-Osuna F. Bulk and bioavailable heavy metals (Cd, Cu, Pb, and Zn) in surface sediments from Mazatlán Harbor (SE Gulf of California). Bull Environ Contam Toxicol. 2008;80:150–3. 10.1007/s00128-007-9334-0.Search in Google Scholar PubMed
[21] Fernandes L, Nayak GN, Ilangovan D. Geochemical assessment of metal concentrations in mangrove sediments along Mumbai coast, India. World Acad Sci Eng Technol. 2012;61:258–63. 10.5281/zenodo.1327841.Search in Google Scholar
[22] Bodin N, N’Gom-Kâ R, Kâ S, Thiaw OT, Tito de Morais L, Le Loc’h F, et al. Assessment of trace metal contamination in mangrove ecosystems from Senegal, West Africa. Chemosphere. 2013;90(2):150–7. 10.1016/j.chemosphere.2012.06.019.Search in Google Scholar PubMed
[23] Attia OEA, Abu Khadra AM, Nawwar AH, Radwan GE. Impacts of human activities on the sedimentological and geochemical characteristics of Mabahhiss Bay, North Hurghada, Red Sea”, Egypt. Arab J Geosci. 2012;5:481–99. 10.1007/s12517-010-0193-3.Search in Google Scholar
[24] Ramanathan T, Ting Y-P. Selection of wet digestion methods for metal quantification in hazardous solid wastes. J Environ Chem Eng. 2015;3(3):1459–67. 10.1016/j.jece.2015.05.006.Search in Google Scholar
[25] Tomlinson D, Wilson J, Harris C, Jeffrey D. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen. 1980;33:566–75.10.1007/BF02414780Search in Google Scholar
[26] Müller G. Index of geo-accumulation in sediments of the Rhine River. Geo J. 1969;2:108–18.Search in Google Scholar
[27] Khalilova H, Mammadov V. Assessing the anthropogenic impact on heavy metal pollution of soils and sediments in urban areas of Azerbaijan’s oil industrial region. Pol J Environ Stud. 2016;25(1):159–66. 10.15244/pjoes/60723.Search in Google Scholar
[28] Çevik F, Göksu MZL, Derici OB, Findik O. An assessment of metal pollution in surface sediments of Seyhan dam by using enrichment factor, geo accumulation index and statistical analyses. Environ Monit Assess. 2009;152:309–17. 10.1007/s10661-008-0317-3.Search in Google Scholar PubMed
[29] Bhuiyan MAH, Surivi NI, Dampare SB, Islam MA, Quraishi SB, Ganyaglo S, et al. Investigation of the possible sources of heavy metal contamination in lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environ Monit Assess. 2009;175:633–49. 10.1007/s10661-010-1557-6.Search in Google Scholar PubMed
[30] Ergin M, Saydam C, Baştürk O, Erdem E, Yörük R. Heavy metal concentrations in surface sediments from the two coastal inlets (Golden Horn Estuary and Izmit Bay) of the northeastern Sea of Marmara. Chem Geol. 1991;91(3):269–85. 10.1016/0009-2541(91)90004-B.Search in Google Scholar
[31] Smith SR, Giller KE. Effective rhizobium leguminosarum biovar Trifolii present in five soils contaminated with heavy metals from long-term applications of sewage sludge or metal mine spoil. Soil Biol Biochem. 1992;24(8):781–8. 10.1016/0038-0717(92)90253-T.Search in Google Scholar
[32] Boulding R. Description and sampling of contaminated soils: A field guide. Boca Raton, USA: CRC Press; 1994.Search in Google Scholar
[33] Mukherjee AB. Chromium in the environment of Finland. Sci Total Env. 1998;217(1–2):9–19. 10.1016/S0048-9697(98)00163-6.Search in Google Scholar
[34] Lindsay W. Chemical equilibria in Soils. 1st edn. New York: A Wiley-Interscience Publication. John Wiley and Sons; 1979.Search in Google Scholar
[35] Mechender G, Dhakate R, Prasanna L, Govil PK. Assessment of heavy metal contamination in soils around Balanagar industrial area, Hyderabad, India. Environ Earth Sci. 2011;63:945–53. 10.1007/s12665-010-0763-4.Search in Google Scholar
[36] Kara M, Dumanoğlu Y, Altıok H, Elbir T, Odabasi M, Bayram A. Spatial distribution and source identification of trace elements in topsoil from heavily industrialized region, Aliaga, Turkey. Environ Monit Assess. 2014;186(10):6017–38. 10.1007/s10661-014-3837-z.Search in Google Scholar PubMed
[37] Nwaichi EO, Wegwu MO, Nwosu UL. Distribution of selected carcinogenic hydrocarbon and heavy metals in an oil-polluted agriculture zone. Environ Monit Assess. 2014;186:8697–706. 10.1007/s10661-014-4037-6.Search in Google Scholar PubMed PubMed Central
[38] Khalilova HK. The impact of oil contamination on soil ecosystem. J B Biol Chem Res. 2015;2(3):133–9.Search in Google Scholar
[39] Zevenhoven R, Kilpinene P. Control of pollutants in flue gases and fuel gases. Report TKK-ENY-4. 1st edn. Espoo: June 2001.Search in Google Scholar
[40] Alshahri F. Heavy metal contamination in sand and sediments near to disposal site of reject brine from desalination plant, Arabian Gulf: assessment of environmental pollution. Environ Sci Pollut Res. 2017;24:1821–31. 10.1007/s11356-016-7961-x.Search in Google Scholar PubMed
[41] Hasayen KA, Al-Osaimi BH, Aljohany AM, Al-Jawdah HM. Spatial distribution of heavy metals in water, soil and anurans’ livers from Al-Hayr area-Riyadh, Saudi Arabia. J Environ Biol. 2017;38(2):231–6. 10.22438/jeb/38/2/MRN-305.Search in Google Scholar
[42] Alshahri F. Uranium and trace metals contamination in topsoil from different Zones around Industrial city, Aljubail, Saudi Arabia. Environ Contam Toxicol. 2019;77:308–19. 10.1007/s00244-019-00642-9.Search in Google Scholar PubMed
[43] Al-Wabel MI, Sallam AS, Usman ARA, Ahmad M, El-Naggar AH, El-Saeid MH, et al. Trace metal levels, sources, and ecological risk assessment in a densely agricultural area from Saudi Arabia. Environ Monit Assess. 2017;189:252. 10.1007/s10661-017-5919-1.Search in Google Scholar PubMed
[44] Al-Dabbas MA, Ali LA, Afaj AH. Determination of heavy metals and polycyclic aromatic hydrocarbon concentrations in soil and in the leaves of plant (Eucalyptus) of selected locations at Kirkuk-Iraq. Arab J Geosci. 2015;8:3743–53. 10.1007/s12517-014-1454-3.Search in Google Scholar
[45] Guler C, Alpaslan M, Kurt MA, Temel A. Deciphering factors controlling trace element distribution in the soils of Karaduvar industrial-agricultural area (Mersin, SE Turkey). Environ Earth Sci. 2010;60:203–18.10.1007/s12665-009-0180-8Search in Google Scholar
[46] Christoforidis A, Stamatis N. Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region Greece. Geoderma. 2009;151:257–63. 10.1016/j.geoderma.2009.04.016.Search in Google Scholar
[47] Tserenpil S, Sapkota A, Liu C-Q, Peng J-H, Liu B, Chr P. Lead isotope and trace element composition of urban soils in Mongolia. Eurasian Soil Sci. 2016;49:879–89. 10.1134/S1064229316080147.Search in Google Scholar
[48] Tiwari JN, Chaturvedi P, Ansari NG, Patel DK, Jain SK, Murthy RC. Assessment of polycyclic aromatic hydrocarbons (PAH) and heavy metals in the vicinity of an oil refinery in India. Soil Sedum Contam. 2011;20:315–28. 10.1080/15320383.2011.560984.Search in Google Scholar
[49] Tripathee L, Kang S, Rupakheti D, Zhang Q, Bajracharya RM, Sharma CM, et al. Spatial distribution, sources and risk assessment of potentially toxic trace elements and rare earth elements in soils of the Lang tang Himalaya, Nepal. Environ Earth Sci. 2016;75:1332. 10.1007/s12665-016-6140-1.Search in Google Scholar
[50] Guagliardi I, Cicchella D, De R, Rosa. A Geostatistical approach to assess concentration and spatial distribution of heavy metals in urban soils. Water Air Soil Pollut. 2012;223:5983–98. 10.1007/s11270-012-1333-z.Search in Google Scholar
[51] Lv J, Lin Y, Zhang Z, Dai J, Dai B, Zhu Y. Identifying the origins and spatial distributions of heavy metals in soils of Jug country (Eastern China) using multivariate and geostatistical approach. J Solids Sediment. 2015;15:163–78. 10.1007/s11368-014-0937-x.Search in Google Scholar
[52] Jarzynska G, Falandysz J. Trace elements profile of Slate Bolete (Leccinumduriusculum) mushroom and associated upper soil horizon. J Geochem Explor. 2012;121:69–75. 10.1016/j.gexplo.2012.07.001.Search in Google Scholar
[53] Turekian KK, Wedepohl KH. Distribution of the elements in some major units of the Earth’s crust. Geol Soc Am. 1961;72:175–92. 10.1130/0016-7606(1961)72[175:DOTEIS]2.0.CO;2.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”
Articles in the same Issue
- Research Articles
- Green polymer electrolyte and activated charcoal-based supercapacitor for energy harvesting application: Electrochemical characteristics
- Research on the adsorption of Co2+ ions using halloysite clay and the ability to recover them by electrodeposition method
- Simultaneous estimation of ibuprofen, caffeine, and paracetamol in commercial products using a green reverse-phase HPTLC method
- Isolation, screening and optimization of alkaliphilic cellulolytic fungi for production of cellulase
- Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities
- Comparative analysis of bio-based amino acid surfactants obtained via Diels–Alder reaction of cyclic anhydrides
- Biosynthesis of silver nanoparticles on yellow phosphorus slag and its application in organic coatings
- Exploring antioxidant potential and phenolic compound extraction from Vitis vinifera L. using ultrasound-assisted extraction
- Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53
- Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro- and nano-drug carriers
- Silicotungstic acid supported on Bi-based MOF-derived metal oxide for photodegradation of organic dyes
- Synthesis and characterization of capsaicin nanoparticles: An attempt to enhance its bioavailability and pharmacological actions
- Synthesis of Lawsonia inermis-encased silver–copper bimetallic nanoparticles with antioxidant, antibacterial, and cytotoxic activity
- Facile, polyherbal drug-mediated green synthesis of CuO nanoparticles and their potent biological applications
- Zinc oxide-manganese oxide/carboxymethyl cellulose-folic acid-sesamol hybrid nanomaterials: A molecularly targeted strategy for advanced triple-negative breast cancer therapy
- Exploring the antimicrobial potential of biogenically synthesized graphene oxide nanoparticles against targeted bacterial and fungal pathogens
- Biofabrication of silver nanoparticles using Uncaria tomentosa L.: Insight into characterization, antibacterial activities combined with antibiotics, and effect on Triticum aestivum germination
- Membrane distillation of synthetic urine for use in space structural habitat systems
- Investigation on mechanical properties of the green synthesis bamboo fiber/eggshell/coconut shell powder-based hybrid biocomposites under NaOH conditions
- Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L.
- Estimation of greenhouse gas emissions from rice and annual upland crops in Red River Delta of Vietnam using the denitrification–decomposition model
- Synthesis of humic acid with the obtaining of potassium humate based on coal waste from the Lenger deposit, Kazakhstan
- Ascorbic acid-mediated selenium nanoparticles as potential antihyperuricemic, antioxidant, anticoagulant, and thrombolytic agents
- Green synthesis of silver nanoparticles using Illicium verum extract: Optimization and characterization for biomedical applications
- Antibacterial and dynamical behaviour of silicon nanoparticles influenced sustainable waste flax fibre-reinforced epoxy composite for biomedical application
- Optimising coagulation/flocculation using response surface methodology and application of floc in biofertilisation
- Green synthesis and multifaceted characterization of iron oxide nanoparticles derived from Senna bicapsularis for enhanced in vitro and in vivo biological investigation
- Potent antibacterial nanocomposites from okra mucilage/chitosan/silver nanoparticles for multidrug-resistant Salmonella Typhimurium eradication
- Trachyspermum copticum aqueous seed extract-derived silver nanoparticles: Exploration of their structural characterization and comparative antibacterial performance against gram-positive and gram-negative bacteria
- Microwave-assisted ultrafine silver nanoparticle synthesis using Mitragyna speciosa for antimalarial applications
- Green synthesis and characterisation of spherical structure Ag/Fe2O3/TiO2 nanocomposite using acacia in the presence of neem and tulsi oils
- Green quantitative methods for linagliptin and empagliflozin in dosage forms
- Enhancement efficacy of omeprazole by conjugation with silver nanoparticles as a urease inhibitor
- Residual, sequential extraction, and ecological risk assessment of some metals in ash from municipal solid waste incineration, Vietnam
- Green synthesis of ZnO nanoparticles using the mangosteen (Garcinia mangostana L.) leaf extract: Comparative preliminary in vitro antibacterial study
- Simultaneous determination of lesinurad and febuxostat in commercial fixed-dose combinations using a greener normal-phase HPTLC method
- A greener RP-HPLC method for quaternary estimation of caffeine, paracetamol, levocetirizine, and phenylephrine acquiring AQbD with stability studies
- Optimization of biomass durian peel as a heterogeneous catalyst in biodiesel production using microwave irradiation
- Thermal treatment impact on the evolution of active phases in layered double hydroxide-based ZnCr photocatalysts: Photodegradation and antibacterial performance
- Preparation of silymarin-loaded zein polysaccharide core–shell nanostructures and evaluation of their biological potentials
- Preparation and characterization of composite-modified PA6 fiber for spectral heating and heat storage applications
- Preparation and electrocatalytic oxygen evolution of bimetallic phosphates (NiFe)2P/NF
- Rod-shaped Mo(vi) trichalcogenide–Mo(vi) oxide decorated on poly(1-H pyrrole) as a promising nanocomposite photoelectrode for green hydrogen generation from sewage water with high efficiency
- Green synthesis and studies on citrus medica leaf extract-mediated Au–ZnO nanocomposites: A sustainable approach for efficient photocatalytic degradation of rhodamine B dye in aqueous media
- Cellulosic materials for the removal of ciprofloxacin from aqueous environments
- The analytical assessment of metal contamination in industrial soils of Saudi Arabia using the inductively coupled plasma technology
- The effect of modified oily sludge on the slurry ability and combustion performance of coal water slurry
- Eggshell waste transformation to calcium chloride anhydride as food-grade additive and eggshell membranes as enzyme immobilization carrier
- Synthesis of EPAN and applications in the encapsulation of potassium humate
- Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential
- Enhancing mechanical and rheological properties of HDPE films through annealing for eco-friendly agricultural applications
- Immobilisation of catalase purified from mushroom (Hydnum repandum) onto glutaraldehyde-activated chitosan and characterisation: Its application for the removal of hydrogen peroxide from artificial wastewater
- Sodium titanium oxide/zinc oxide (STO/ZnO) photocomposites for efficient dye degradation applications
- Effect of ex situ, eco-friendly ZnONPs incorporating green synthesised Moringa oleifera leaf extract in enhancing biochemical and molecular aspects of Vicia faba L. under salt stress
- Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens
- Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)
- Assessment of antiproliferative activity of green-synthesized nickel oxide nanoparticles against glioblastoma cells using Terminalia chebula
- Chlorine-free synthesis of phosphinic derivatives by change in the P-function
- Anticancer, antioxidant, and antimicrobial activities of nanoemulsions based on water-in-olive oil and loaded on biogenic silver nanoparticles
- Study and mechanism of formation of phosphorus production waste in Kazakhstan
- Synthesis and stabilization of anatase form of biomimetic TiO2 nanoparticles for enhancing anti-tumor potential
- Microwave-supported one-pot reaction for the synthesis of 5-alkyl/arylidene-2-(morpholin/thiomorpholin-4-yl)-1,3-thiazol-4(5H)-one derivatives over MgO solid base
- Screening the phytochemicals in Perilla leaves and phytosynthesis of bioactive silver nanoparticles for potential antioxidant and wound-healing application
- Graphene oxide/chitosan/manganese/folic acid-brucine functionalized nanocomposites show anticancer activity against liver cancer cells
- Nature of serpentinite interactions with low-concentration sulfuric acid solutions
- Multi-objective statistical optimisation utilising response surface methodology to predict engine performance using biofuels from waste plastic oil in CRDi engines
- Microwave-assisted extraction of acetosolv lignin from sugarcane bagasse and electrospinning of lignin/PEO nanofibres for carbon fibre production
- Biosynthesis, characterization, and investigation of cytotoxic activities of selenium nanoparticles utilizing Limosilactobacillus fermentum
- Highly photocatalytic materials based on the decoration of poly(O-chloroaniline) with molybdenum trichalcogenide oxide for green hydrogen generation from Red Sea water
- Highly efficient oil–water separation using superhydrophobic cellulose aerogels derived from corn straw
- Beta-cyclodextrin–Phyllanthus emblica emulsion for zinc oxide nanoparticles: Characteristics and photocatalysis
- Assessment of antimicrobial activity and methyl orange dye removal by Klebsiella pneumoniae-mediated silver nanoparticles
- Influential eradication of resistant Salmonella Typhimurium using bioactive nanocomposites from chitosan and radish seed-synthesized nanoselenium
- Antimicrobial activities and neuroprotective potential for Alzheimer’s disease of pure, Mn, Co, and Al-doped ZnO ultra-small nanoparticles
- Green synthesis of silver nanoparticles from Bauhinia variegata and their biological applications
- Synthesis and optimization of long-chain fatty acids via the oxidation of long-chain fatty alcohols
- Eminent Red Sea water hydrogen generation via a Pb(ii)-iodide/poly(1H-pyrrole) nanocomposite photocathode
- Green synthesis and effective genistein production by fungal β-glucosidase immobilized on Al2O3 nanocrystals synthesized in Cajanus cajan L. (Millsp.) leaf extracts
- Green stability-indicating RP-HPTLC technique for determining croconazole hydrochloride
- Green synthesis of La2O3–LaPO4 nanocomposites using Charybdis natator for DNA binding, cytotoxic, catalytic, and luminescence applications
- Eco-friendly drugs induce cellular changes in colistin-resistant bacteria
- Tangerine fruit peel extract mediated biogenic synthesized silver nanoparticles and their potential antimicrobial, antioxidant, and cytotoxic assessments
- Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil
- A highly sensitive β-AKBA-Ag-based fluorescent “turn off” chemosensor for rapid detection of abamectin in tomatoes
- Green synthesis and physical characterization of zinc oxide nanoparticles (ZnO NPs) derived from the methanol extract of Euphorbia dracunculoides Lam. (Euphorbiaceae) with enhanced biosafe applications
- Detection of morphine and data processing using surface plasmon resonance imaging sensor
- Effects of nanoparticles on the anaerobic digestion properties of sulfamethoxazole-containing chicken manure and analysis of bio-enzymes
- Bromic acid-thiourea synergistic leaching of sulfide gold ore
- Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies
- Green synthesis and effective utilization of biogenic Al2O3-nanocoupled fungal lipase in the resolution of active homochiral 2-octanol and its immobilization via aluminium oxide nanoparticles
- Eco-friendly RP-HPLC approach for simultaneously estimating the promising combination of pentoxifylline and simvastatin in therapeutic potential for breast cancer: Appraisal of greenness, whiteness, and Box–Behnken design
- Use of a humidity adsorbent derived from cockleshell waste in Thai fried fish crackers (Keropok)
- One-pot green synthesis, biological evaluation, and in silico study of pyrazole derivatives obtained from chalcones
- Bio-sorption of methylene blue and production of biofuel by brown alga Cystoseira sp. collected from Neom region, Kingdom of Saudi Arabia
- Synthesis of motexafin gadolinium: A promising radiosensitizer and imaging agent for cancer therapy
- The impact of varying sizes of silver nanoparticles on the induction of cellular damage in Klebsiella pneumoniae involving diverse mechanisms
- Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract
- Rhus microphylla-mediated biosynthesis of copper oxide nanoparticles for enhanced antibacterial and antibiofilm efficacy
- Harnessing trichalcogenide–molybdenum(vi) sulfide and molybdenum(vi) oxide within poly(1-amino-2-mercaptobenzene) frameworks as a photocathode for sustainable green hydrogen production from seawater without sacrificial agents
- Magnetically recyclable Fe3O4@SiO2 supported phosphonium ionic liquids for efficient and sustainable transformation of CO2 into oxazolidinones
- A comparative study of Fagonia arabica fabricated silver sulfide nanoparticles (Ag2S) and silver nanoparticles (AgNPs) with distinct antimicrobial, anticancer, and antioxidant properties
- Visible light photocatalytic degradation and biological activities of Aegle marmelos-mediated cerium oxide nanoparticles
- Physical intrinsic characteristics of spheroidal particles in coal gasification fine slag
- Exploring the effect of tea dust magnetic biochar on agricultural crops grown in polycyclic aromatic hydrocarbon contaminated soil
- Crosslinked chitosan-modified ultrafiltration membranes for efficient surface water treatment and enhanced anti-fouling performances
- Study on adsorption characteristics of biochars and their modified biochars for removal of organic dyes from aqueous solution
- Zein polymer nanocarrier for Ocimum basilicum var. purpurascens extract: Potential biomedical use
- Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii
- Novel microwave-based green approach for the synthesis of dual-loaded cyclodextrin nanosponges: Characterization, pharmacodynamics, and pharmacokinetics evaluation
- Bi2O3–BiOCl/poly-m-methyl aniline nanocomposite thin film for broad-spectrum light-sensing
- Green synthesis and characterization of CuO/ZnO nanocomposite using Musa acuminata leaf extract for cytotoxic studies on colorectal cancer cells (HCC2998)
- Review Articles
- Materials-based drug delivery approaches: Recent advances and future perspectives
- A review of thermal treatment for bamboo and its composites
- An overview of the role of nanoherbicides in tackling challenges of weed management in wheat: A novel approach
- An updated review on carbon nanomaterials: Types, synthesis, functionalization and applications, degradation and toxicity
- Special Issue: Emerging green nanomaterials for sustainable waste management and biomedical applications
- Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities
- Special Issue: New insights into nanopythotechnology: current trends and future prospects
- Green synthesis of FeO nanoparticles from coffee and its application for antibacterial, antifungal, and anti-oxidation activity
- Dye degradation activity of biogenically synthesized Cu/Fe/Ag trimetallic nanoparticles
- Special Issue: Composites and green composites
- Recent trends and advancements in the utilization of green composites and polymeric nanocarriers for enhancing food quality and sustainable processing
- Retraction
- Retraction of “Biosynthesis and characterization of silver nanoparticles from Cedrela toona leaf extracts: An exploration into their antibacterial, anticancer, and antioxidant potential”
- Retraction of “Photocatalytic degradation of organic dyes and biological potentials of biogenic zinc oxide nanoparticles synthesized using the polar extract of Cyperus scariosus R.Br. (Cyperaceae)”
- Retraction to “Green synthesis on performance characteristics of a direct injection diesel engine using sandbox seed oil”

