Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
-
Noor Ul Huda
, Nadia Mushtaq
Abstract
The synthesis of silver nanoparticles (AgNPs) by the green method is favored as compared to chemical synthesis due to their appreciable properties of less toxicity and simple synthesis. The current study designed the biosynthesis of AgNPs in one step by using the plant Kickxia elatine (KE) extract and then investigated its inhibiting activity against rat’s brain acetylcholinesterase (AChE) ex vivo. Ultraviolet spectrum at 416 nm confirmed the formation of AgNPs. X-ray diffractometer calculated size was reported to be 42.47 nm. The SEM analysis confirmed spherical-shaped AgNPs. FT-IR suggested that the phytochemical groups present in the KE extract and their nanoparticles (NPs) are responsible for the biosynthesized of NPs. EDX analysis presented that Ag was the chief element with 61.67%. Both KE extract and AgNPs showed significant anti-AChE activity at 175 µg·mL−1. Statistical analysis showed that both KE and AgNPs exhibited non-competitive type inhibition against AChE, i.e. V max decreased (34.17–68.64% and 22.29–62.10%), while K m values remained constant. It is concluded that KE and AgNPs can be considered an inhibitor of rats’ brain AChE. Furthermore, the synthesis of AgNP-based drugs can be used as a cheaper and alternative option against diseases such as Alzheimer’s disease.
1 Introduction
Nanotechnology is the generation of solid nanometer-sized nanoparticles (NPs) with restricted shapes and sizes and gaining enormous attention all over the world in the last many years [1]. The importance of Nanoscience is concerned with the outstanding and unique optical, physical, and chemical properties of NPs and their use in the fields of the medicines, agriculture, cosmetics, garment, and food industries [2,3]. Recently, metallic NPs are synthesized using gold (Au), zinc (Zn), copper (Cu), iron (Fe), silver (Ag), magnesium (Mg), etc. [4]. Among NPs, AgNPs are gaining significant attention due to their small size in the range of 1–100 nm and related to the size of human body proteins [5,6]. The nanostructured AgNPs have a vital role as antimicrobial agents [7], water purifiers, surgical, and wound care products, antioxidants [8], acetylcholinesterase (AChE) inhibitors [9], and anti-diabetic agents [10,11]. Nobel AgNPs have attracted enormous attention due to their broad-spectrum application in growing demand goods such as cosmetics, textiles, electronics, biosensors, catalysts, and medicine products. The essential characteristics of biosynthesized NPs such as shape and size are particularly attributed to the type and biochemical’s constituents of selected natural sources. It is worth mentioning that no study has been reported yet examining the effects of collective biomaterials on NP fabrication and their biological activities. Thus, this research focuses on the combinative effect of abundant and low cost of marine-derived materials of biopolymer chitosan and the brown marine algae extract as an extracellular green platform on bio-fabrication of AgNPs [1].
AgNPs have attracted a great deal of interest due to their excellent antimicrobial, antioxidants, anti-Alzheimer’s disease, anticancer, and catalytic properties. The main drawback with the chemical and physical methods of AgNPs formation is that they are extremely costly and also involve the use of toxic, hazardous chemicals and they contain potential environmental and biological stakes. However, the use of harmful substances and the high-energy consumption required for the preparation of AgNPs represent disadvantages that limit their large-scale production. Currently, researchers are using green methods for the biosynthesis of AgNPs where the bio-extracts are used as mediators for the fabrication of AgNPs. These metabolites (e.g., vitamins enzymes, proteins, polysaccharides, amino acids, and organic acids) involve directly in the reduction of Ag ions to produce NPs. The secondary metabolites in the reduction of metal ions into NPs and in supporting their subsequent stability have also been postulated [12]. Recently reported plant extracts that were used for the biosynthesis of AgNPs included Urtica diocia [13], Tropaeolum majus [14], Skimmia laureola [15], Azadirachta indica [16], and Araucaria angastifolia [17].
Alzheimer’s disease (AD) is associated with memory and thinking impairment, behavioral problems, and disturbance in daily living activities [6]. AD is common in old people due to irreversible neuronal loss. The deficiency of acetylcholine (ACh) in synapses of the cerebral cortex is one of the important sufferers of AD [18] and can be treated by the inhibition of AChE that hydrolyzes ACh into choline and acetate [18]. Synthetic AChE inhibitors (tacrine, donepezil, and rivastigmine) cause significant side effects [19]. Therefore, researchers have been focused on herbal inhibitors of AChE (tacrine, donepezil, and galantamine), which can be used in the treatment of AD with very less or no side effects [20].
Kickxia elatine (KE) is an annual plant used in conventional medicine as a wound-healing agent, sedative, and general tonic, and also in case of bleeding and lacrimation. Worldwide only a few species of Kickxia have been analyzed for its phytochemical constituents, which result in the isolation of flavonoids and iridoid glycosides and conferred good antiglycation activity (inhibition of α-glycosidase). [21] extracted four types of flavonoids (flavone, glycosides, pectolinarin, and acetylpectolinarin) from KE, which suggests its strong antioxidant activity and prevents the development of heart ailment, cancer, diabetes, and some other disorders like Alzheimer’s disease and dementia [22]. Linarioside extracted from Kickxia spuria, Kickxia elatine (L.) Dumort, and Kickxia commutata expressed strong anti-diabetic activities [23]. KE is one of the least explored members of Plantaginaceae. In the present project, AgNPs’ synthesis from KE was undertaken that has not been evaluated previously. The present work is based on the hypothesis that KE extract and synthesized K. elatine silver nanoparticles (AgNPs) will bear effective anti-Alzheimer’s disease activity due to the presence of biologically active ingredients in KE.
2 Materials and methods
2.1 Collection and processing of KE
The KE plant was obtained from the Ghoriwala area of district Bannu, KP Pakistan, in June 2021. The plant was recognized by Dr. Tahir Iqbal, an expert taxonomist, and a voucher specimen was deposited (NH II) at the Botany Department, University of Science and Technology, Bannu. The plant was dried and powdered. About 100 g of KE was extracted with 500 mL of ethanol and concentrated with a rotary evaporator to harvest crude extract (KE). The concentrated KE was air-dried at 37°C and then stored at 4°C for further investigation.
2.2 KE-mediated AgNPs
AgNPs were synthesized using a standard procedure [24]. About 1.5 g KE crude extract was dissolved in 100 mL of deionized water. The supernatant was stored for activity. About 1,000 mL of 1 mM AgNO3 solution was prepared and adjusted the pH at 9. Furthermore, 100 mL of supernatant (1 g·100 ddH2O−1) and 1,000 mL of AgNO3 (1 mM) solution were mixed in a 1:10 ratio and incubate the mixture at 40°C. The color change to reddish-brown after 1 h is an indication of chemical reduction, i.e., the formation of AgNPs. The solution was further incubated for 24 h at 40°C. AgNPs were definite by ultraviolet–visible (UV–Vis) spectrometry between 200 and 800 nm. AgNPs were centrifuged at 14,000 rpm. The obtained pellet of NPs was dried incubator at 50°C and used for further characterization.
2.3 Size and shape optimization of AgNPs
AgNPs’ synthesis was optimized by using different intrinsic factors such as KE volume, temperature, reaction time, Ag salt molarity, pH, and stability time. To assess the effect of KE concentration on the fabrication of AgNPs, its concentration varied as 0.25, 0.5, 1, 1.5, and 2 mL. AgNPs were prepared at different temperatures 20°C, 40°C, 60°C, 80°C, and 100°C and at varied time intervals (1, 2, 3, and 24 h) to estimate the effect of temperature and time. To analyze the effect of Ag salt concentration, NPs were prepared at different dilution (0.5, 1, 1.5, and 2 mM) of Ag-salt. To assess the effect of pH, AgNPs were prepared at varied pH from 5 to 12, respectively, because NPs’ synthesis is supported by both acidic and basic conditions. The NPs’ stability was checked after 24 h, 30 days, and 3 months.
2.4 Characterization of AgNP
AgNPs’ synthesis was confirmed by Shimadzu UV Spectrophotometer (UV-1800). KE and NPs were inquired for functional groups by using Nicolet iS50 FT-IR. The crystalline structure of AgNPs was examined by using the JEOL X-ray diffractometer (XRD) model (JDX-3532, Japan). SEM analysis was used to examine the morphology of AgNPs. EDX was done to know the elemental constituents of AgNPs.
2.5 Experimental animals
The study was ethically approved by the Biotechnology Ethical Committee, USTB, Bannu, Pakistan, on March 17, 2020, under reference number USTB-534/2020. The experiment was performed on male Sprague Dawley rats that were obtained from a Veterinary Research Institute, Peshawar, Pakistan. The average age of the animals was 3–3.5 months, and their weight was 310–350 g. The rats were retained in a controlled temperature well-aired room in steel cages with a 12 h dark–light period. The rats had free entree to food. Rats were not subjected to any specific treatment before slaughtering. The animals were sacrificed by cervical dislocation. The brain was excised, weighed, and washed with 50 mM phosphate buffer.
2.6 Preparation of brain homogenate (BH)
About 1 g of brain was minced and homogenized in 10 mL of 50 mM phosphate buffer and followed by centrifugation at 4°C for 10 min at 10,000 rpm in a high-speed cooling centrifuge.
2.7 Protein estimation
The protocol was used to estimate the proteins in BH. Bovine serum albumin was used as standard [25].
2.8 Anti-acetyl cholinesterase activity
AChE inhibition strength was evaluated by the methodology of Ahmed et al. [26]. The 1 mL mixture assay contained DTNB 10 mM, 50 mM phosphate buffer of pH 7.4, 100 μL of BH as an enzyme, 67 µL water, and different volumes of KE and AgNPs (75, 125, and 175 µL). The mixture was subjected to incubation for 5 min at 37°C. With the addition of substrate acetyl thiocholine (0.05–1 mM), the reaction was started. The ACh hydrolysis rate was measured every 15 s for the 90 s by the development of thiolate di-anion that reacts with DTNB. The activity of the enzyme was scrutinized by the extent of the yellow color formation. The activity was repeated in triplicate to eliminate the errors and % inhibition is calculated by the following equation:
2.9 Kinetic determinations
For assessing the type of inhibition, kinetics studies were done. The interaction of KE, AgNPs, and enzyme BH was assessed by the double reciprocal plot [27]. 1/V was assessed at ACh (0.05–1 mM) with and without KE and its NPs. Michaelis constants (K m) were deliberated by V vs V/S [28] and 1/V vs 1/S plots [27]. The inhibition constant (K i) was calculated by Cornish-Bowden plots [34]. KI was calculated by scheming the Dixon plot [29]. IC50 was calculated by inhibition/activity vs concentration of samples.
2.10 Statistical analysis
For data analysis, a two-way analysis of variance was performed. Data were shown as mean ± standard. The graphs were drawn in Origin Pro 8.5 and Slide Write.
3 Results
3.1 UV–Vis spectroscopy of AgNPs
The aqueous extract of KE was green which changed to reddish-brown after the addition of AgNO3 solution and incubation at 40°C for 24 h. The color change was observed due to the bio-reduction of Ag+.
After chromatic observation, the AgNPs were subjected to UV–Vis spectral analysis in the range of 200–800 nm and displayed a peak at 416 nm with the absorption of 1.98 at optimal conditions as 24 h incubation time, 1.5 mL of aqueous extract concentration, 1 mM silver nitrate solution concentration, and 40°C temperature at pH 9, while the plant extract did not exhibited absorption spectrum in 200–800 nm (Figure 1).

The UV–Vis absorption spectrum of AgNPs at optimal conditions.
3.2 Factors affecting biosynthesis of AgNPs
The AgNPs’ stability was checked after 24 h, 30 days, and 3 months. The sharp peaks at 416 and 409 nm were reported after 24 h and 30 days and confirmed the presence of AgNPs, but after 3 months, this peak becomes broader with the low absorbance of 0.50 (Figure 2a).

UV spectra of AgNPs at different stability periods (a), extract volume (b), time intervals (c), temperature (d), silver nitrate solution concentration (e), and pH (f).
To standardize the NPs’ formation route, different volumes of KE extract varied from 0.25, 0.5, 1, 1.5, and 2 mL were tested in this study, which exhibited a spectrum of absorption in the range of 200–800 nm with the increase in the intensity of peaks from 0.76 to 2.22, respectively (Figure 2b).
The UV–Vis spectra outcomes publicized a rise in the absorbance intensity of the AgNPs with time (Figure 2c). An absorption peak (390 nm) of very low intensity (0.79) appeared after 1 h of reaction, which transformed into a sharp and visible peak of 416 nm after 24 h and represented the formation of stable and spherical AgNPs.
Figure 2d shows the temperature stability of AgNPs (20–100°C) and suggests that SPR peaks became sharper by increasing the temperature from 20°C to 40°C. Further increase in temperature (up to 100°C) results in the broadening of peaks.
Assessment of the influence of AgNO3 concentration (0.5–2 mM) on AgNPs’ synthesis showed absorbance at 407, 416, 403, and 411 nm, respectively (Figure 2e).
The pH effect (5–12) on the biogenic synthesis of NPs was also evaluated (Figure 2f). At pH 5 and 6, no absorption peaks appeared. But sharp bands were detected as the pH was increased from 6 to 9. At higher pH beyond 9, broader peaks formed.
3.3 FT-IR spectroscopy of KE and synthesized AgNPs
The FT-IR spectrum of KE and AgNPs is presented in Figure 3. Different peaks were demonstrated that correspond to the different functional groups (Table 1). The various peaks reported by KE and AgNP at 3,272.72 and 3,300.39 cm−1 indicate O–H stretch, 2,916.20 and 2,922.52 cm−1 signify the C–H stretch, 2,854.45 and 2,847.93 cm−1 correspond to the OH group of carbonyl compounds of the protein, 2,174.70 and 2,181.18 cm−1 for C≡C stretch, 2,078.26 and 2,023.71 cm−1 for N═C═S stretch, 1,975.49 and 1,906.71 cm−1 indicate C═C═C stretch of allenes, 1,728.06 and 1,735.17 cm−1 resemble the C═O vibrations of conjugated aldehydes, 1,185.77 and 1,233.99 cm−1 resemble C–O stretching of alcohol, ester, ether, and carboxylic acid, 1,062.45 and 1,024.22 cm−1 represent C–N aliphatic amines, and 643.47 and 630.03 cm−1 attribute to C–Br stretch.

FT-IR analysis of KE and AgNPs.
FT-IR interpretation of KE extract (whole plant) and its silver nanoparticles
| S. no. | KE extract peak potion (cm−1) | AgNPs peak position (cm−1) | Corresponding frequency range (cm−1) | Chemical bond | Functional group |
|---|---|---|---|---|---|
| 1 | 3,272.72 | 3,300.39 | 3,500–3,200 (s) | O–H (stretch) | Alcohol and phenol |
| 2 | 2,916.20 | 2,922.52 | 3,000–2,850 (m) | C–H (stretch) | Alkanes |
| 3 | 2,854.45 | 2,847.93 | 3,300–2,500 (m) | O–H (stretch) | Carboxylic acid |
| 4 | 2,174.70 | 2,181.18 | 22,60–2,100 (w) | C≡C (stretch) | Alkynes |
| 5 | 2,078.26 | 2,023.71 | 2,140–1,990 (s) | N═C═S (stretch) | Metal carbonyl (isothiocynate) |
| 6 | 1,975.49 | 1,906.71 | 2,000–1,900 (m) | C═C═C (stretch) | Allene |
| 7 | 1,728.06 | 1,735.17 | 1,740–1,720 (s) | C═O (stretch) | Conjugated Aldehyde |
| 8 | 1,632.41 | 1,604.74 | 1,650–1,600 (m) | C═C (stretch) | Conjugated ketone and alkene |
| 9 | 1,336.75 | 1,371.54 | 1,390–1,310 (m) | O–H (bend) | Phenols |
| 10 | 1,185.77 | 1,233.99 | 1,320–1,000 (s) | C–O (stretch) | Alcohol, ester, ether, carboxylic acid |
| 11 | 1,062.45 | 1,024.22 | 1,250–1,020 (m) | C–N (stretch) | Aliphatic amines |
| 12 | 877.47 | 863.24 | 890–850 | Aromatic compounds | |
| 13 | 643.47 | 630.03 | 690–515 (s) | C–Br (stretch) | Halo compounds |
3.4 SEM of AgNPs
The nanostructure and surface morphology studies of size and shape were interpreted by SEM (Figure 4a). The outcomes showed that AgNPs are mono-dispersive with the constant arrangement in the matrix. The particle size distribution graph was deliberated by Nano Measurer software (Figure 4b), which demonstrated that 90% of AgNPs are in an average size of 50 nm.

SEM analysis (a) and SEM demonstrated the size (b) of AgNPs.
3.5 XRD analysis of AgNPs
XRD exploration of AgNPs presented four strong Bragg reflections 38°, 44°, 64°, and 77° at 2θ values, which attributes to the plane of (111), (200), (220), and (311) reflections and conferring face-centered cubic crystal (FCC) structure Figure 5. The size (42.47 nm) was calculated by the Debye–Scherrer equation (Eq. 2) and presented in Table 2.

XRDpattern analysis of AgNPs.
The grain size, interplaner spacing, and lattice constant of AgNPs
| S. no. | 2θ Value | Element | hkl | FMHM (β) of intense peak (radians) | Particle size (D) [nm] | d spacing [Å] | Lattice constant (α) [Å] |
|---|---|---|---|---|---|---|---|
| 1 | 38.07 | Ag | 111 | 0.0035 | 40.76 | 2.361 | 4.089 |
| 2 | 44.32 | Ag | 200 | 0.0033 | 44.71 | 2.041 | 4.083 |
| 3 | 64.39 | Ag | 220 | 0.0037 | 37.56 | 1.445 | 4.088 |
| 4 | 77.40 | Ag | 311 | 0.0030 | 46.84 | 1.231 | 4.084 |
3.6 EDX analysis of AgNPs
EDX was conducted for identifying the elemental constituents involved in the biosynthesis of AgNPs (Figure 6a). The EDX spectrometry indicated that AgNPs contain 61.67% of silver, 15.82% carbon, 15.65% oxygen, 4.93% sodium, 0.83% calcium, 0.82% chlorine, and 0.28 silicon by percent weight (Figure 6b). Therefore, the EDX analysis reveals the elemental composition of AgNPs.

EDX spectra (a) and elemental profile (b) of biosynthesized AgNPs.
3.7 Anti-Alzheimer’s disease activity
At ACh concentration (0.5 mM), BH acetylcholinesterase (BH-AChE) inhibition ability of KE, AgNPs, and AgNO3 was evaluated. Figure 7a represents that KE, AgNPs, and AgNO3 salt exhibited the highest 65.02%, 75.25%, and 21.68% inhibition against AChE at 175 µg·mL−1 concentration, respectively.
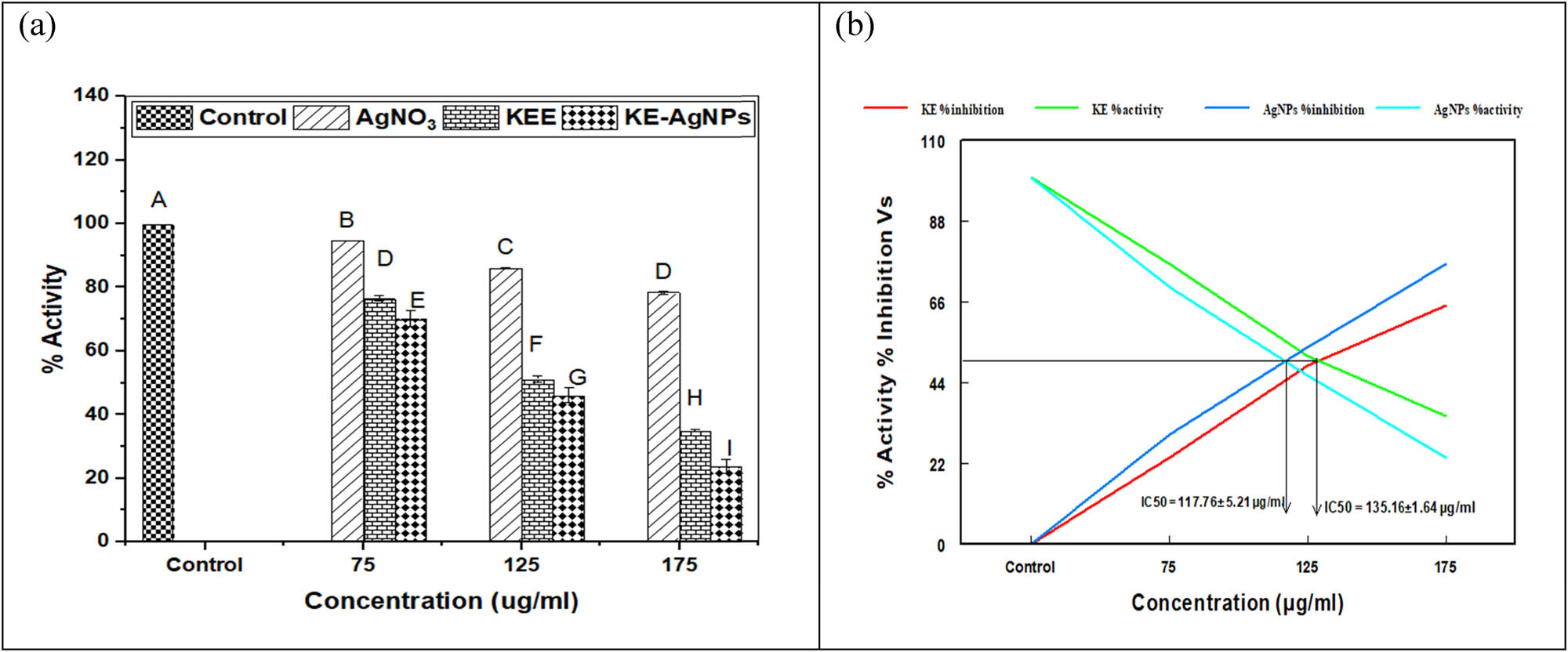
(a) In vitro ache inhibitory capability of silver nanoparticles (AgNPs), KE extract, and AgNO3. (b) IC50 values of AgNPs and KE cause 50% inhibition of BH ache.
3.8 Calculation of IC50
The IC50 values are displayed in Figure 7b and showed IC50 values of KE and AgNPs that are 135.16 ± 1.64 and 117.76 ± 5.21 µg·mL−1, respectively, while AgNO3 salt did not cause 50% inhibition at any concentration due to a lack of phytochemicals from the plant extract.
3.9 Effects of plant and AgNPs on K m and V max
Kinetics studies revealed that both AgNPs and KE caused a non-competitive inhibition of AChE (Figure 8a and b), respectively. In such type of inhibition, V max decreased (34.17–68.64% and 22.29–62.10%) in the concentration-dependent mode for KE and AgNPs, respectively, while K m values remain constant (Table 3).

(a and b) Lineweaver–Burk (reciprocal of enzyme velocity vs reciprocal of ACh) plot showing the non-competitive type of inhibition (K m constant and V max decrease) caused by AgNPs and KE, respectively.
Influence of KE and AgNPs on K m and V max of Sprague Dawley rats brain homogenate (AChE)
| S. no. | AgNPs | KE extract | ||||
|---|---|---|---|---|---|---|
| Concentration (µg) | V max (µmol·min−1·mg−1 protein) | % Decrease | K m (mM) | V max (µmol·min−1·mg−1 protein) | % Decrease in V max | K m (mM) |
| 0 | 0.3137 | 0 | 0.037 | 0.1644 | 0 | 0.018 |
| 75 | 0.2065 | 34.17 | 0.038 | 0.1278 | 22.29 | 0.018 |
| 125 | 0.1657 | 47.17 | 0.037 | 0.0829 | 49.57 | 0.018 |
| 175 | 0.0984 | 68.64 | 0.038 | 0.0623 | 62.10 | 0.018 |
3.10 Influence of AgNPs and KE on K Iapp and V maxiapp
The effect of AgNPs and KE on K Iapp and V maxiapp was studied (Figure 9a and b) and showed that with the increase in substrate concentration (0.05–1 mM), K iapp remains constant while V maxiapp values decreased from 48.43% to 150.12% and 7.66% to 49.52%, respectively, for AgNPs and KE (Table 4).

(a and b) Determination of K Iapp and V maxapp for AgNPs and KE using Dixon plot for BH-AChE.
Effect of KE and AgNPs on K Iapp and V maxiapp of Sprague Dawley rats’ brain homogenate (AChE); the V maxiapp and K Iapp were calculated by Dixon plot of Figure 9a and b
| S. no. | AgNPs | KE extract | ||||
|---|---|---|---|---|---|---|
| ACh concentration (mM) | V maxiapp (µmol·min−1·mg−1 protein) | % Decrease in V maxiapp | K Iapp (mM) | V maxiapp (µmol·min−1·mg−1 protein) | % Decrease in V maxiapp | K Iapp (mM) |
| 0.05 | 0.2563 | 0 | 45.58 | 0.313 | 0 | 31.098 |
| 0.1 | 0.3804 | 48.43 | 46.49 | 0.337 | 7.66 | 31.23 |
| 0.25 | 0.4420 | 72.47 | 47.09 | 0.356 | 13.78 | 31.93 |
| 0.5 | 0.4980 | 94.33 | 48.27 | 0.415 | 32.59 | 30.08 |
| 1 | 0.6410 | 150.12 | 46 | 0.468 | 49.52 | 30.43 |
3.11 Determination of K I, K i, and K m of AChE
The dissociation constant (K I) was calculated for AgNPs and KE as 46.69 and 30.95 µg respectively (Figure 9a and b). While the inhibitory constant (K i) was calculated to be 32 (Figure 10a) and 19 µg (Figure 10b) for AgNPs and KE, respectively. K m (Michaelis–Menten constant) was 0.018 and 0.037 mM for KE and AgNPs, respectively (Table 5).

(a and b) Determination of inhibitory constant (K i) for AgNPs and KE using Cornish–Bowden plots for BH-AChE.
Study of kinetic parameters of rat BH-AChE inhibition by AgNPs and KE
| Sample | K i (µg) | K I (µg) | K m (mM) | IC50 (µg·mL−1) |
|---|---|---|---|---|
| AgNPs | 32 | 46.69 | 0.037 | 117.76 ± 5.21 |
| KE | 19 | 30.95 | 0.018 | 135.16 ± 1.64 |
K i – inhibition constant, K I – dissociation constant of the AChE–ACh–KE complex into the AChE–ACh complex and free KE, K m – Michaelis–Menten constant, and IC50 – 50% inhibitory concentration.
4 Discussion
Engineered nano-medicine is a fast-developing and important field that is related to the fabrication of metallic NPs by using metals like silver, gold, and platinum [30] and exhibits in the field of biomedicines, applied sciences, and material sciences. The rat’s BH-AChE inhibition by KE extract and AgNPs was done in this study. AgNPs’ characterization is an important tool for their stability, collection, bio-dispersity, and biological efficacy in the cells.
Metal-based NPs have free electrons and excitation of electrons with the light of a specific wavelength [31,32]. AgNPs have brown color due to a change in the SPR band detected by the UV–Vis spectrophotometry and appeared at 416 nm with higher absorption of 1.98 showing the reduction and stabilization of Ag+ ions in the aqueous solution. A similar absorbance band at 420 nm was reported by ref. [33] in AgNPs synthesized from M. pulegium leaves.
The size, shape, and fabrication of the AgNPs are affected by extract volume, temperature, time, AgNO3 concentration, and pH [34]. Therefore, in the current study, these parameters were studied to optimize and control the size and shape of the AgNPs and revealed the optimum conditions that produced stable and small-size AgNPs were 1.5 mL (extract concentration), 1 mM (silver nitrate salt concentration), 40°C (temperature), 24 h (reaction time), and pH 9. The current investigation is also supported by the report of [15] on Hippeastrum hybridum-induced silver NPs.
The biological activities of the silver NPs are greatly influenced by their stability. The results are supported by Jabariyan and Zanjanchi [35] who used grape juice for the biosynthesis of AgNPs and found no significant changes in the shape, position, and symmetry of the SPR absorption peak for 30 days. But after 3 months the SPR band became broader with low absorbance of 0.50 and showed that AgNPs lost their stability.
Plant extract consists of various phytochemicals such as tannins, phenols, alkaloids, flavonoids, anthocyanins, phenols, polysaccharides, and polyphenols that attribute to the reduction of Ag+ and the formation of stable and isotropic AgNPs [36]. At an optimal concentration of 1.5 mL, the sharp band (416 nm) attributes to the fabrication of small and isotropic particles. These findings are in agreement with earlier reports [37,38] that have proposed that the rise in M. pulegium leaves extract concentration is linked positively with the stability of AgNPs.
Moreover, the time influence on nanofabrication and size of AgNPs was also testified at different radiation times of 1, 2, 3, and 24 h. After 1 h of reaction, an absorption peak of 390 nm appeared at very low intensity, which transformed into a sharp and visible peak of 416 nm after 24 h suggesting the enhancement of synthesized NPs and complete reduction of Ag ions into stable and spherical AgNPs. Thus, to get maximum- and small-sized NPs, the optimum time was suggested to be 24 h. Awwad and Salem [39] reported 60 min for stable mulberry leaves extract-based AgNPs.
Generally, the NPs are prepared at room temperature, but the reaction is very slow and a long time is required for a complete reaction at room temperature. Therefore, the rate of reaction can be accelerated by elevating the temperature. The SPR peaks became sharper by raising the temperature from 20°C to 40°C suggesting that an increase in temperature up to 40°C led to a complete and rapid reduction of Ag+ and ultimately uniform nucleation of Ag nuclei that result in the formation of small-sized NPs [40]. Furthermore, an increase in temperature up to 100°C resulted in the broadening of peaks and revealed the biosynthesis of large-sized NPs. The AgNPs tend to be poly-dispersed with the increase in temperature beyond 40°C [41].
In the current study, the various concentrations of AgNO3 were tested to get the optimum size of AgNPs (Figure 2e) and revealed that at a concentration of 0.5 mM silver nitrate was deficient for the fabrication of NPs. Interestingly, a 1 mM concentration of AgNO3 supported the rapid formation of minute-sized silver NPs. Thus, a very little amount of AgNO3 salt is required for the biosynthesis of potential and ideal-size AgNPs. With further increase in reactant concentration (1.5–2 mM), the rate of reduction of Ag ion will decrease and the accumulation of AgNO3 makes the mixture solution foggy that subsequently resulting in broad peaks. The broad peak indicates large-size NPs [42]. The broad peak indicates large-size NPs. Kaya et al. [43] reported that small nanometer-sized NPs are biologically more active.
One of the important parameters is pH, which affects the rate of biosynthesis of NPs by changing the electrical load of phytochemicals and alternatively affects the capping and stabilizing ability of Ag ions [44]. Khan et al. [45] reviewed that the size of NPs is probably higher in the acidic medium as compared to the basic media. In the present report, the sharp SPR bands were detected as the pH was increased from 6 to 9 and indicated the biosynthesis of isotropic and stable AgNPs in the basic medium. The alkaline condition assisted in the reduction of biomolecules present in extracts. At higher pH beyond 9, formations of broader peaks indicate large-size NPs. Thus, a pH study showed that the fabrication of AgNPs is enhanced by basic conditions while repressed by acid conditions [46].
FT-IR analysis identifies the existence of phyto-molecules, which account for Ag metal reduction and their collaboration with the stabilization and capping of AgNPs. FT-IR analysis confirmed various bond stretches at their respective peaks of KE and AgNPs, which conferred the presence of polyhydroxy, carboxyl, phenol, lipids, proteins, alkynes, amide, aliphatic amines, halo compounds, alkene, etc. The phenolic and alcoholic compounds are potentially stabilizing mediators involved in the reduction of AgNPs [47]. The carbonyl compounds of proteins attribute to the stabilization of AgNPs by capping them [48]. In the present study, FT-IR confirmed the interaction between biomolecules and different functional groups of KE extract in the reduction and biosynthesis of AgNPs. Thus, from the IR spectrum, it may be presumed that these biomolecules are involved in the stabilization and capping of AgNPs [49].
The morphological studies of size and shape were interpreted by SEM and showed that AgNPs are mono-dispersive with uniform alignment in the matrix. The grain sizes of the NPs valued from the SEM (50 nm) are comparable to XRD data (42.47 nm). The results are consistence with an earlier report in which AgNPs synthesized using Morus alba leaf are spherical with sizes ranging below 50 nm [50].
XRD investigation of AgNPs presented the four strong Bragg reflections that correspond to the pure silver metal with FCC symmetry. These results are in line with earlier reports by [51] where AgNPs synthesized from Euphorbia serpens were FCC crystals of 50 nm size and similar diffraction planes were observed. Hublikar et al. [52] reported that Carissa carandas L. leaf-synthesized AgNPs are crystalline with crystallite sizes 35 and 30 nm at 25°C and 60°C, respectively.
EDX analysis gives information concerning the participation of elements in the reduction of Ag+ both qualitatively and quantitatively [53]. The quantitative profile of elements indicated that AgNPs contain an appreciable and high percentage of silver, followed by carbon, oxygen, sodium, calcium, chlorine, and silicon. The silver showed a high-intensity peak at 3 keV due to SPR and confirmed the complete reduction of Ag+ to AgNPs [53]. The presence of carbon and oxygen corresponds to an aromatic compound that gives stability to reduced Ag [54,55].
Neurodegenerative disorders such as Alzheimer’s disease occur due to a low level of the neurotransmitter ACh or a high level of AChE [56]. The enzyme AChE catalyzes the hydrolysis of ACh after its liberation at the cholinergic synapses to return to its resting state after activation [57] and results in the reduction of contact time between the postsynaptic membrane and neurotransmitter ACh. Thus, delaying the transfer of information that leads to memory loss. Therefore, the main therapeutic approach in the prevention of Alzheimer’s disease involved the increasing level of ACh and controlling the activity of AChE by using reversible inhibitors of AChE that will balance the cholinergic system and allow more contact time for information transformation through neurons [56,58]. The effects of silver ions and AgNPs against AChE were also studied in vivo by [46] in which they used three different doses of the synthesized AgNPs and revealed decreased AChE activity. Currently, we confirmed the inhibition of Sprague rat’s BH-AChE by KE extract, AgNPs, and AgNO3 (Figure 7a) and found that at a fixed 0.5 mM concentration of substrate (ACh), KE, KE-NPs, and AgNO3 exhibited 65.02%, 75.25%, and 21.68% inhibition at 175 μg·mL−1, respectively. The IC50 values of AgNPs (117.76 ± 5.21 μg·mL−1) are significantly higher as compared to KE (117.76 ± 5.21 μg·mL−1) and suggest that reduced AChE activity by AgNPs might be due to quercetin in the extract that is responsible for capping off the nanofabricated NPs. Abdelhafez et al. [59] reported a decrease in AChE activity in rats treated with biosynthesized AgNPs and are in best agreement with our present study.
According to kinetic studies, both KE and AgNPs inhibit AChE in a non-competitive mode (K m remains constant while V max decreased). In such a type of inhibition, the KE and AgNPs bind to an allosteric site and alternatively decline the efficacy of the enzyme [60]. The release of silver ions by AgNPs due to surface oxidation might be the possible mode of action as it increases interaction with AChE and subsequently inhibits its activity [61,62]. AgNPs are more effective compared to plant extract due to their morphology [63–65].
5 Conclusion
KE, an indigenous plant found in abundance in Pakistan, has been successfully utilized for the biosynthesis of simple, quick, colloidal, and stable AgNPs. The NPs were characterized by UV–Vis spectroscopy, SEM, FT-IR, EDX, and XRD analysis. AgNPs were also scrutinized for anti-AChE activities in BH and showed good inhibitory enzymatic properties as compared to their respective KE extract and AgNO3. Thus, it may be concluded that green-synthesized AgNPs can be used as a cheaper and alternative option against AD disease.
-
Funding information: This research was funded through the Researchers Supporting Project (RSPD2023R816), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Noor Ul Huda: formal analysis, investigation, methodology, writing – original draft; Hazem K. Ghneim: data curation, funding acquisition, methodology, writing – review and editing; Fozia Fozia: formal analysis, validation, writing – review and editing; Mushtaq Ahmed: formal analysis, project administration, supervision, writing – review and editing; Nadia Mushtaq: resources, writing – review and editing; Naila Sher: writing – original draft; Rahmat Ali Khan: writing – original draft; Ijaz Ahmad: project administration, writing – review and editing; Yazeed A. Al-Sheikh: writing – original draft; John P. Giesy: writing – original draft; Mourad A. M. Aboul-Soud: funding acquisition, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All the relevant data is provided in the article.
References
[1] Sher N, Ahmed M, Mushtaq N, Khan RA. Synthesis of biogenic silver nanoparticles from the extract of Heliotropium eichwaldi L. and their effect as antioxidant, antidiabetic, and anti-cholinesterase. Appl Organomet Chem. 2022;37:e6950.10.1002/aoc.6950Search in Google Scholar
[2] Melkamu WW, Bitew LTJH. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) JF Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon. 2021;7:e08459.10.1016/j.heliyon.2021.e08459Search in Google Scholar PubMed PubMed Central
[3] Parmar S, Kaur H, Singh J, Matharu AS, Ramakrishna S, Bechelany M. Recent advances in green synthesis of Ag NPs for extenuating antimicrobial resistance. Nanomaterials. 2022;12:1115.10.3390/nano12071115Search in Google Scholar PubMed PubMed Central
[4] Kumar H, Bhardwaj K, Kuča K, Kalia A, Nepovimova E, Verma R, et al. Flower-based green synthesis of metallic nanoparticles: applications beyond fragrance. Nanomaterials (Basel). 2020;10:201–23.10.3390/nano10040766Search in Google Scholar PubMed PubMed Central
[5] Dash SS, Banerjee J, Samanta S, Giri B, Dash SK. Microwave-assisted fabrication of silver nanoparticles utilizing seed extract of areca catechu with antioxidant potency and evaluation of antibacterial efficacy against multidrug resistant pathogenic bacterial strains. J BioNanoScience. 2022;12:210–27.10.1007/s12668-021-00927-1Search in Google Scholar
[6] Sher N, Alkhalifah DHM, Ahmed M, Mushtaq N, Shah F, Fozia F, et al. Comparative study of antimicrobial activity of silver, gold, and silver/gold bimetallic nanoparticles synthesized by green approach. Molecules. 2022;27(22):7895.Search in Google Scholar
[7] Vanin dos Santos Lima M, Beloni de Melo G, Gracher Teixeira L, Grella Miranda C, Hermes de Araújo PH, Sayer C, et al. Reviews Green synthesis of silver nanoparticles using Ilex paraguariensis extracts: antimicrobial activity and acetilcolinesterase modulation in rat brain tissue. J Green Chem Lett. 2022;15:128–38.Search in Google Scholar
[8] Abdellatif AAH, Alhathloul SS, Aljohani ASM, Maswadeh H, Abdallah EM, Hamid Musa K, et al. Green synthesis of silver nanoparticles incorporated aromatherapies utilized for their antioxidant and antimicrobial activities against some clinical bacterial isolates. Bioinorg Chem Appl. 2022;2022:2432758.10.1155/2022/2432758Search in Google Scholar PubMed PubMed Central
[9] Vanin dos Santos Lima M, Beloni de Melo G, Gracher Teixeira, Grella Miranda L, Hermes de Araújo C, Sayer PH, C, et al. Green synthesis of silver nanoparticles using Ilex paraguariensis extracts: antimicrobial activity and acetilcolinesterase modulation in rat brain tissue. J Green Chem Lett. 2022;15:128–38.10.1080/17518253.2021.2024896Search in Google Scholar
[10] Majeed S, Danish M, Zakariya NA, Hashim R, Ansari MT, Alkahtani S, et al. In vitro evaluation of antibacterial, antioxidant, and antidiabetic activities and glucose uptake through 2-NBDG by Hep-2 liver cancer cells treated with green synthesized silver nanoparticles. J Oxid Med Cell Longev. 2022;2022.10.1155/2022/1646687Search in Google Scholar PubMed PubMed Central
[11] Ferdous Z, Nemmar A. Health impact of silver nanoparticles: a review of the biodistribution and toxicity following various routes of exposure. Int J Mol Sci. 2020;21:2375.10.3390/ijms21072375Search in Google Scholar PubMed PubMed Central
[12] Farhadi L, Mohtashami M, Saeidi J, Azimi-nezhad M, Taheri G, Khojasteh-Taheri R, et al. Green synthesis of chitosan-coated silver nanoparticle, characterization, antimicrobial activities, and cytotoxicity analysis in cancerous and normal cell lines. J J Inorg Organomet Polym Mater. 2022;32:1637–49.10.1007/s10904-021-02208-6Search in Google Scholar
[13] Sher N, Ahmed M, Mushtaq N, Khan RA. Cytotoxicity and Genotoxicity of green synthesized silver, gold, and silver/gold bimetallic on BHK‐21 cells and Human Blood Lymphocytes Using MTT and Comet assay. Appl Organomet Chem. 2022;e6968.10.1002/aoc.6968Search in Google Scholar
[14] Bawazeer S, Rauf A, Shah SUA, Shawky AM, Al-Awthan YS, Bahattab OS, et al. Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies. J Green Process. 2021;10:85–94.10.1515/gps-2021-0003Search in Google Scholar
[15] Sher N, Ahmed M, Mushtaq N, Khan RA. Enhancing antioxidant, antidiabetic, and antialzheimer performance of Hippeastrum hybridum (L.) using silver nanoparticles. Appl Organomet Chem. 2022;36:e6724.10.1002/aoc.6724Search in Google Scholar
[16] Verma A, Mehata MS. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J Radiat Res Appl Sci. 2016;9:109–15.10.1016/j.jrras.2015.11.001Search in Google Scholar
[17] Zamarchi F, Vieira IC. Determination of paracetamol using a sensor based on green synthesis of silver nanoparticles in plant extract. J Pharm Biomed Anal. 2021;196:113912.10.1016/j.jpba.2021.113912Search in Google Scholar PubMed
[18] Sher N, Ahmed M, Mushtaq N, Khan RA. Calligonum polygonoides reduced nanosilver: A new generation of nanoproduct for medical applications. Eur Integ Med. 2020;33:101042.10.1016/j.eujim.2019.101042Search in Google Scholar
[19] Ahmed M, Khan SZ, Sher N, Rehman ZU, Mushtaq N, Khan RA. Kinetic and toxicological effects of synthesized palladium(II) complex on snake venom (Bungarus sindanus) acetylcholinesterase. J Venom Anim Toxins Incl Trop Dis. 2021;27:e20200047.10.1590/1678-9199-jvatitd-2020-0047Search in Google Scholar PubMed PubMed Central
[20] Grossberg GT. Cholinesterase inhibitors for the treatment of Alzheimer’s disease: getting on and staying on. J Curr Ther Res. 2003;64:216–35.10.1016/S0011-393X(03)00059-6Search in Google Scholar PubMed PubMed Central
[21] Yuldashev MP, Malikov VM, Batirov ÉK. Flavonoids of the epigeal part of Kickxia elatine. J Chem Nat Compd. 1996;32:30–2.10.1007/BF01373784Search in Google Scholar
[22] Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. J Biochem Pharmacol. 1983;32:1141–8.10.1016/0006-2952(83)90262-9Search in Google Scholar PubMed
[23] Venditti A, Frezza C, Serafini I, Ciccòla A, Sciubba F, Serafini M, et al. Iridoids of chemotaxonomy relevance, a new antirrhinoside ester and other constituents from Kickxia spuria subsp. integrifolia (Brot.) R. Fern. J Chem Biodivers. 2018;15:e1700473.10.1002/cbdv.201700473Search in Google Scholar PubMed
[24] Sher N, Ahmed M, Mushtaq N. Biogenic synthesis of gold nanoparticles using Heliotropium eichwaldi L and neuroprotective potential via anticholinesterase inhibition in rat brain. Appl Organomet Chem. 2022;e7000.10.1002/aoc.7000Search in Google Scholar
[25] Levard C, Hotze EM, Lowry GV, Brown Jr, Gordon E. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Env Sci Technol. 2012;46:6900–14.10.1021/es2037405Search in Google Scholar PubMed
[26] Ahmed M, Batista J, Rocha T, Mazzanti CM, Hassan W, Morsch VM. Comparative study of the inhibitory effect of antidepressants on cholinesterase activity in Bungarus sindanus (krait) venom, human serum and rat striatum. J Enzyme Inhib Med Chem. 2008;23:912–7.10.1080/14756360701809977Search in Google Scholar PubMed
[27] Lineweaver H, Burk D. The Determination of Enzyme Dissociation Constants. J Am Chem Soc. 1934;56:658–66.10.1021/ja01318a036Search in Google Scholar
[28] Hofstee BH. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952;116:329–31.10.1126/science.116.3013.329Search in Google Scholar PubMed
[29] Dixon M, Webb EC. Enzymes. London: Longmans; 1964.Search in Google Scholar
[30] Kumari SC, Padma PN, Anuradha K. Green Silver Nanoparticles Embedded in Cellulosic Network for Fresh Food Packaging. J Pure Appl Microbiology. 2021;15(3):1236–44.10.22207/JPAM.15.3.13Search in Google Scholar
[31] Shrivastava S, Dash D. Applying nanotechnology to human health: revolution in biomedical sciences. J Nanotech. 2009;2009.10.1155/2009/184702Search in Google Scholar
[32] Mousavi B, Tafvizi F, Zaker Bostanabad S. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). J Artif Cells Nanomed Biotechnol. 2018;46:499–510.10.1080/21691401.2018.1430697Search in Google Scholar PubMed
[33] Naghmouchi S, Al-Zaban MI, Al-Zaben M, Alharbi N, Bahatheq A. Generation and characterization of silver nanoparticles in mentha pulegium extract and evaluation of biological activities of the prepared extract. J Nanomater. 2022;2022.10.1155/2022/5410274Search in Google Scholar
[34] Anigol LB, Charantimath JS, Gurubasavaraj PM. Effect of concentration and pH on the size of silver nanoparticles synthesized by green chemistry. J Org Med Chem Int J. 2017;3:1–5.Search in Google Scholar
[35] Jabariyan S, Zanjanchi MA. Colorimetric detection of cadmium ions using modified silver nanoparticles. J Appl Phys A. 2019;125:1–10.10.1007/s00339-019-3167-7Search in Google Scholar
[36] Singh A, Gaud B, Jaybhaye S. Optimization of synthesis parameters of silver nanoparticles and its antimicrobial activity. J Mater Sci Energy Technol. 2020;3:232–6.Search in Google Scholar
[37] Wang Y, Wei S, Wang K, Wang Z, Duan J, Cui L, et al. Evaluation of biosynthesis parameters, stability and biological activities of silver nanoparticles synthesized by Cornus Officinalis extract under 365 nm UV radiation. J RSC Adv. 2020;10:27173–82.10.1039/D0RA04482BSearch in Google Scholar PubMed PubMed Central
[38] Amin M, Anwar F, Janjua MR, Iqbal MA, Rashid U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int J Mol Sci. 2012;13:9923–41.10.3390/ijms13089923Search in Google Scholar PubMed PubMed Central
[39] Awwad AM, Salem NM. Green synthesis of silver nanoparticles byMulberry LeavesExtract. J Nanosci Nanotechnol. 2012;2:125–8.10.5923/j.nn.20120204.06Search in Google Scholar
[40] Singh A, Gaud B, Jaybhaye S. Optimization of synthesis parameters of silver nanoparticles and its antimicrobial activity. Mater Sci Energy Technol. 2020;3:232–6. 10.1016/j.mset.2019.08.004.Search in Google Scholar
[41] Ahmad N, Jabeen M, Haq ZU, Ahmad I, Wahab A, Islam ZU, et al. Green fabrication of silver nanoparticles using Euphorbia serpens Kunth aqueous extract, their characterization, and investigation of its in vitro antioxidative, antimicrobial, insecticidal, and cytotoxic activities. BioMed Res Int. 2022;2022.10.1155/2022/5562849Search in Google Scholar PubMed PubMed Central
[42] Balavijayalakshmi J, Ramalakshmi V. Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. J Appl Res Technol. 2017;15:413–22.10.1016/j.jart.2017.03.010Search in Google Scholar
[43] Kaya H, Aydın F, Gürkan M, Yılmaz S, Ates M, Demir V, et al. A comparative toxicity study between small and large size zinc oxide nanoparticles in tilapia (Oreochromis niloticus): Organ pathologies, osmoregulatory responses and immunological parameters. J Chemosphere. 2016;144:571–82.10.1016/j.chemosphere.2015.09.024Search in Google Scholar PubMed
[44] Dubey SP, Lahtinen M, Sillanpaa M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloid Surf A. 2010;364:34–41.10.1016/j.colsurfa.2010.04.023Search in Google Scholar
[45] Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2017;12:908.10.1016/j.arabjc.2017.05.011Search in Google Scholar
[46] Sher N, Ahmed M, Mushtaq N. Synthesis, optimization, and characterization of silver/gold allied bimetallic from Hippeastrum hybridum (L.) and their ex vivo anti-acetylcholinesterase activity in rat brain. Appl Organomet Chem. 2023;134:e7082.10.1002/aoc.7082Search in Google Scholar
[47] Siakavella IK, Lamari F, Papoulis D, Orkoula M, Gkolfi P, Lykouras M, et al. Effect of plant extracts on the characteristics of silver nanoparticles for topical application. J Pharm. 2020;12:1244.10.3390/pharmaceutics12121244Search in Google Scholar PubMed PubMed Central
[48] Reddy NV, Satyanarayana BM, Sivasankar S, Pragathi D, Subbaiah KV, Vijaya T. Eco-friendly synthesis of silver nanoparticles using leaf extract of Flemingia wightiana: Spectral characterization, antioxidant and anticancer activity studies. J SN Appl Sci. 2020;2:1–10.10.1007/s42452-020-2702-7Search in Google Scholar
[49] Sherin L, Sohail A, Mustafa M, Jabeen R, Ul-Hamid A. Facile green synthesis of silver nanoparticles using Terminalia bellerica kernel extract for catalytic reduction of anthropogenic water pollutants. Coll Inter Sci Communi. 2020;37:100276.10.1016/j.colcom.2020.100276Search in Google Scholar
[50] Aguş O, Abalı Y, Arslan O, Keskin NOS. Facile and controlled production of silver borate nanoparticles. SN Appl Sci. 2019;1:1–8.10.1007/s42452-019-0686-ySearch in Google Scholar
[51] Singh R, Hano C, Nath G, Sharma B. Green biosynthesis of silver nanoparticles using leaf extract of Carissa carandas L. and their antioxidant and antimicrobial activity against human pathogenic bacteria. J Biomolecules. 2021;11:299.10.3390/biom11020299Search in Google Scholar PubMed PubMed Central
[52] Hublikar LV, Ganachari SV, Raghavendra N, Banapurmath NR, Patil VB, Yunus Khan TM, et al. Biogenesis of silver nanoparticles and its multifunctional anti-corrosion and anticancer studies. J Coat. 2021;11:1215.10.3390/coatings11101215Search in Google Scholar
[53] Khan AU, Yuan Q, Khan ZUH, Ahmad A, Khan FU, Tahir K. An eco-benign synthesis of Ag NPs using aqueous extract of Longan fruit peel: Antiproliferative response against human breast cancer cell line MCF-7, antioxidant and photocatalytic deprivation of methylene blue. Photochem Photobio B: Biol. 2018;183:367–73.10.1016/j.jphotobiol.2018.05.007Search in Google Scholar PubMed
[54] Begildayeva T, Lee SJ, Yu Y, Park J, Kim TH, Theerthagiri J, et al. Production of copper nanoparticles exhibiting various morphologies via pulsed laser ablation in different solvents and their catalytic activity for reduction of toxic nitroaromatic compounds. Haz Mater. 2021;409:124412.10.1016/j.jhazmat.2020.124412Search in Google Scholar PubMed
[55] Govindarajan M, Benelli G. One-pot green synthesis of silver nanocrystals using Hymenodictyon orixense: a cheap and effective tool against malaria, chikungunya and Japanese encephalitis mosquito vectors? J RSC Adv. 2016;6:59021–9.10.1039/C6RA10228JSearch in Google Scholar
[56] Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. J Curr neuropharm. 2013;11:315–35.10.2174/1570159X11311030006Search in Google Scholar PubMed PubMed Central
[57] Panchal J, Islam M, DeBoeuf K, Farley J. Signaling mechanisms underlying nicotine-induced upregulation of α7 nicotinic acetylcholine receptor (nAChR). J Biophy. 2020;118:420.10.1016/j.bpj.2019.11.2371Search in Google Scholar
[58] Rezazadeh NH, Buazar F, Matroodi S. Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci Rep. 2020;10:1–13.10.1038/s41598-020-76726-7Search in Google Scholar PubMed PubMed Central
[59] Abdelhafez OH, Ali TFS, Fahim JR, Desoukey SY, Ahmed S, Behery FA, et al. Anti-inflammatory potential of green synthesized silver nanoparticles of the soft coral Nephthea sp. supported by metabolomics analysis and docking studies. Intl Nanomed. 2020;15:5345.10.2147/IJN.S239513Search in Google Scholar PubMed PubMed Central
[60] Froede HC, Wilson IB, Kaufman H. Acetylcholinesterase: theory of noncompetitive inhibition. J Arch Biochem biophy. 1986;247:420–3.10.1016/0003-9861(86)90601-6Search in Google Scholar PubMed
[61] Wang Z, Zhao J, Li F, Gao D, Xing B. Adsorption and inhibition of acetylcholinesterase by different nanoparticles. J Chemosphere. 2009;77:67–73.10.1016/j.chemosphere.2009.05.015Search in Google Scholar PubMed
[62] Shah A, Ahmed A, Sher N, Mushtaq M, Khan RA. Fozia; Midrarullah, Efficacy of Silene arenosa extract on acetylcholinesterase in Bungarus sindanus(krait) venom. J Tradit Chin Med. 2021;41:349–54.Search in Google Scholar
[63] Sepahvand M, Buazar F, Sayahi MH. Novel marine‐based gold nanocatalyst in solvent‐free synthesis of polyhydroquinoline derivatives: Green and sustainable protocol. Appl Organomet Chem. 2020;34:e6000.10.1002/aoc.6000Search in Google Scholar
[64] Sher N, Alkhalifah DHM, Ahmed M, Mushtaq N, Shah F, Fozia F, et al. Comparative study of antimicrobial activity of silver, gold, and silver/gold bimetallic nanoparticles synthesized by green approach. Molecules. 2022;27:7895.10.3390/molecules27227895Search in Google Scholar PubMed PubMed Central
[65] Ahmed M, Sher N, Mushtaq N, Khan RA. Phytochemical analysis and inhibitory effects of Calligonum polygonoides on pancreatic α-amylase and β-glucosidase enzymes. J Tradit Chin Med. 2022;42:426.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”
Articles in the same Issue
- Research Articles
- Value-added utilization of coal fly ash and recycled polyvinyl chloride in door or window sub-frame composites
- High removal efficiency of volatile phenol from coking wastewater using coal gasification slag via optimized adsorption and multi-grade batch process
- Evolution of surface morphology and properties of diamond films by hydrogen plasma etching
- Removal efficiency of dibenzofuran using CuZn-zeolitic imidazole frameworks as a catalyst and adsorbent
- Rapid and efficient microwave-assisted extraction of Caesalpinia sappan Linn. heartwood and subsequent synthesis of gold nanoparticles
- The catalytic characteristics of 2-methylnaphthalene acylation with AlCl3 immobilized on Hβ as Lewis acid catalyst
- Biodegradation of synthetic PVP biofilms using natural materials and nanoparticles
- Rutin-loaded selenium nanoparticles modulated the redox status, inflammatory, and apoptotic pathways associated with pentylenetetrazole-induced epilepsy in mice
- Optimization of apigenin nanoparticles prepared by planetary ball milling: In vitro and in vivo studies
- Synthesis and characterization of silver nanoparticles using Origanum onites leaves: Cytotoxic, apoptotic, and necrotic effects on Capan-1, L929, and Caco-2 cell lines
- Exergy analysis of a conceptual CO2 capture process with an amine-based DES
- Construction of fluorescence system of felodipine–tetracyanovinyl–2,2′-bipyridine complex
- Excellent photocatalytic degradation of rhodamine B over Bi2O3 supported on Zn-MOF nanocomposites under visible light
- Optimization-based control strategy for a large-scale polyhydroxyalkanoates production in a fed-batch bioreactor using a coupled PDE–ODE system
- Effectiveness of pH and amount of Artemia urumiana extract on physical, chemical, and biological attributes of UV-fabricated biogold nanoparticles
- Geranium leaf-mediated synthesis of silver nanoparticles and their transcriptomic effects on Candida albicans
- Synthesis, characterization, anticancer, anti-inflammatory activities, and docking studies of 3,5-disubstituted thiadiazine-2-thiones
- Synthesis and stability of phospholipid-encapsulated nano-selenium
- Putative anti-proliferative effect of Indian mustard (Brassica juncea) seed and its nano-formulation
- Enrichment of low-grade phosphorites by the selective leaching method
- Electrochemical analysis of the dissolution of gold in a copper–ethylenediamine–thiosulfate system
- Characterisation of carbonate lake sediments as a potential filler for polymer composites
- Evaluation of nano-selenium biofortification characteristics of alfalfa (Medicago sativa L.)
- Quality of oil extracted by cold press from Nigella sativa seeds incorporated with rosemary extracts and pretreated by microwaves
- Heteropolyacid-loaded MOF-derived mesoporous zirconia catalyst for chemical degradation of rhodamine B
- Recovery of critical metals from carbonatite-type mineral wastes: Geochemical modeling investigation of (bio)hydrometallurgical leaching of REEs
- Photocatalytic properties of ZnFe-mixed oxides synthesized via a simple route for water remediation
- Attenuation of di(2-ethylhexyl)phthalate-induced hepatic and renal toxicity by naringin nanoparticles in a rat model
- Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production
- Microfluidic steam-based synthesis of luminescent carbon quantum dots as sensing probes for nitrite detection
- Transformation of eggshell waste to egg white protein solution, calcium chloride dihydrate, and eggshell membrane powder
- Preparation of Zr-MOFs for the adsorption of doxycycline hydrochloride from wastewater
- Green nanoarchitectonics of the silver nanocrystal potential for treating malaria and their cytotoxic effects on the kidney Vero cell line
- Carbon emissions analysis of producing modified asphalt with natural asphalt
- An efficient and green synthesis of 2-phenylquinazolin-4(3H)-ones via t-BuONa-mediated oxidative condensation of 2-aminobenzamides and benzyl alcohols under solvent- and transition metal-free conditions
- Chitosan nanoparticles loaded with mesosulfuron methyl and mesosulfuron methyl + florasulam + MCPA isooctyl to manage weeds of wheat (Triticum aestivum L.)
- Synergism between lignite and high-sulfur petroleum coke in CO2 gasification
- Facile aqueous synthesis of ZnCuInS/ZnS–ZnS QDs with enhanced photoluminescence lifetime for selective detection of Cu(ii) ions
- Rapid synthesis of copper nanoparticles using Nepeta cataria leaves: An eco-friendly management of disease-causing vectors and bacterial pathogens
- Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange
- Development of a fuzzy logic model for the prediction of spark-ignition engine performance and emission for gasoline–ethanol blends
- Micro-impact-induced mechano-chemical synthesis of organic precursors from FeC/FeN and carbonates/nitrates in water and its extension to nucleobases
- Green synthesis of strontium-doped tin dioxide (SrSnO2) nanoparticles using the Mahonia bealei leaf extract and evaluation of their anticancer and antimicrobial activities
- A study on the larvicidal and adulticidal potential of Cladostepus spongiosus macroalgae and green-fabricated silver nanoparticles against mosquito vectors
- Catalysts based on nickel salt heteropolytungstates for selective oxidation of diphenyl sulfide
- Powerful antibacterial nanocomposites from Corallina officinalis-mediated nanometals and chitosan nanoparticles against fish-borne pathogens
- Removal behavior of Zn and alkalis from blast furnace dust in pre-reduction sinter process
- Environmentally friendly synthesis and computational studies of novel class of acridinedione integrated spirothiopyrrolizidines/indolizidines
- The mechanisms of inhibition and lubrication of clean fracturing flowback fluids in water-based drilling fluids
- Adsorption/desorption performance of cellulose membrane for Pb(ii)
- A one-pot, multicomponent tandem synthesis of fused polycyclic pyrrolo[3,2-c]quinolinone/pyrrolizino[2,3-c]quinolinone hybrid heterocycles via environmentally benign solid state melt reaction
- Green synthesis of silver nanoparticles using durian rind extract and optical characteristics of surface plasmon resonance-based optical sensor for the detection of hydrogen peroxide
- Electrochemical analysis of copper-EDTA-ammonia-gold thiosulfate dissolution system
- Characterization of bio-oil production by microwave pyrolysis from cashew nut shells and Cassia fistula pods
- Green synthesis methods and characterization of bacterial cellulose/silver nanoparticle composites
- Photocatalytic research performance of zinc oxide/graphite phase carbon nitride catalyst and its application in environment
- Effect of phytogenic iron nanoparticles on the bio-fortification of wheat varieties
- In vitro anti-cancer and antimicrobial effects of manganese oxide nanoparticles synthesized using the Glycyrrhiza uralensis leaf extract on breast cancer cell lines
- Preparation of Pd/Ce(F)-MCM-48 catalysts and their catalytic performance of n-heptane isomerization
- Green “one-pot” fluorescent bis-indolizine synthesis with whole-cell plant biocatalysis
- Silica-titania mesoporous silicas of MCM-41 type as effective catalysts and photocatalysts for selective oxidation of diphenyl sulfide by H2O2
- Biosynthesis of zinc oxide nanoparticles from molted feathers of Pavo cristatus and their antibiofilm and anticancer activities
- Clean preparation of rutile from Ti-containing mixed molten slag by CO2 oxidation
- Synthesis and characterization of Pluronic F-127-coated titanium dioxide nanoparticles synthesized from extracts of Atractylodes macrocephala leaf for antioxidant, antimicrobial, and anticancer properties
- Effect of pretreatment with alkali on the anaerobic digestion characteristics of kitchen waste and analysis of microbial diversity
- Ameliorated antimicrobial, antioxidant, and anticancer properties by Plectranthus vettiveroides root extract-mediated green synthesis of chitosan nanoparticles
- Microwave-accelerated pretreatment technique in green extraction of oil and bioactive compounds from camelina seeds: Effectiveness and characterization
- Studies on the extraction performance of phorate by aptamer-functionalized magnetic nanoparticles in plasma samples
- Investigation of structural properties and antibacterial activity of AgO nanoparticle extract from Solanum nigrum/Mentha leaf extracts by green synthesis method
- Green fabrication of chitosan from marine crustaceans and mushroom waste: Toward sustainable resource utilization
- Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)
- The enhanced adsorption properties of phosphorus from aqueous solutions using lanthanum modified synthetic zeolites
- Separation of graphene oxides of different sizes by multi-layer dialysis and anti-friction and lubrication performance
- Visible-light-assisted base-catalyzed, one-pot synthesis of highly functionalized cinnolines
- The experimental study on the air oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid with Co–Mn–Br system
- Highly efficient removal of tetracycline and methyl violet 2B from aqueous solution using the bimetallic FeZn-ZIFs catalyst
- A thermo-tolerant cellulase enzyme produced by Bacillus amyloliquefaciens M7, an insight into synthesis, optimization, characterization, and bio-polishing activity
- Exploration of ketone derivatives of succinimide for their antidiabetic potential: In vitro and in vivo approaches
- Ultrasound-assisted green synthesis and in silico study of 6-(4-(butylamino)-6-(diethylamino)-1,3,5-triazin-2-yl)oxypyridazine derivatives
- A study of the anticancer potential of Pluronic F-127 encapsulated Fe2O3 nanoparticles derived from Berberis vulgaris extract
- Biogenic synthesis of silver nanoparticles using Consolida orientalis flowers: Identification, catalytic degradation, and biological effect
- Initial assessment of the presence of plastic waste in some coastal mangrove forests in Vietnam
- Adsorption synergy electrocatalytic degradation of phenol by active oxygen-containing species generated in Co-coal based cathode and graphite anode
- Antibacterial, antifungal, antioxidant, and cytotoxicity activities of the aqueous extract of Syzygium aromaticum-mediated synthesized novel silver nanoparticles
- Synthesis of a silica matrix with ZnO nanoparticles for the fabrication of a recyclable photodegradation system to eliminate methylene blue dye
- Natural polymer fillers instead of dye and pigments: Pumice and scoria in PDMS fluid and elastomer composites
- Study on the preparation of glycerylphosphorylcholine by transesterification under supported sodium methoxide
- Wireless network handheld terminal-based green ecological sustainable design evaluation system: Improved data communication and reduced packet loss rate
- The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent
- Green synthesis of silver nanoparticles using Saccharum officinarum leaf extract for antiviral paint
- Study on the reliability of nano-silver-coated tin solder joints for flip chips
- Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique
- Anticancer and antimicrobial potential of zinc/sodium alginate/polyethylene glycol/d-pinitol nanocomposites against osteosarcoma MG-63 cells
- Nanoporous carbon@CoFe2O4 nanocomposite as a green absorbent for the adsorptive removal of Hg(ii) from aqueous solutions
- Characterization of silver sulfide nanoparticles from actinobacterial strain (M10A62) and its toxicity against lepidopteran and dipterans insect species
- Phyto-fabrication and characterization of silver nanoparticles using Withania somnifera: Investigating antioxidant potential
- Effect of e-waste nanofillers on the mechanical, thermal, and wear properties of epoxy-blend sisal woven fiber-reinforced composites
- Magnesium nanohydroxide (2D brucite) as a host matrix for thymol and carvacrol: Synthesis, characterization, and inhibition of foodborne pathogens
- Synergistic inhibitive effect of a hybrid zinc oxide-benzalkonium chloride composite on the corrosion of carbon steel in a sulfuric acidic solution
- Review Articles
- Role and the importance of green approach in biosynthesis of nanopropolis and effectiveness of propolis in the treatment of COVID-19 pandemic
- Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic
- Green-processed nano-biocomposite (ZnO–TiO2): Potential candidates for biomedical applications
- Reaction mechanisms in microwave-assisted lignin depolymerisation in hydrogen-donating solvents
- Recent progress on non-noble metal catalysts for the deoxydehydration of biomass-derived oxygenates
- Rapid Communication
- Phosphorus removal by iron–carbon microelectrolysis: A new way to achieve phosphorus recovery
- Special Issue: Biomolecules-derived synthesis of nanomaterials for environmental and biological applications (Guest Editors: Arpita Roy and Fernanda Maria Policarpo Tonelli)
- Biomolecules-derived synthesis of nanomaterials for environmental and biological applications
- Nano-encapsulated tanshinone IIA in PLGA-PEG-COOH inhibits apoptosis and inflammation in cerebral ischemia/reperfusion injury
- Green fabrication of silver nanoparticles using Melia azedarach ripened fruit extract, their characterization, and biological properties
- Green-synthesized nanoparticles and their therapeutic applications: A review
- Antioxidant, antibacterial, and cytotoxicity potential of synthesized silver nanoparticles from the Cassia alata leaf aqueous extract
- Green synthesis of silver nanoparticles using Callisia fragrans leaf extract and its anticancer activity against MCF-7, HepG2, KB, LU-1, and MKN-7 cell lines
- Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators
- Green synthesis of Kickxia elatine-induced silver nanoparticles and their role as anti-acetylcholinesterase in the treatment of Alzheimer’s disease
- Phytocrystallization of silver nanoparticles using Cassia alata flower extract for effective control of fungal skin pathogens
- Antibacterial wound dressing with hydrogel from chitosan and polyvinyl alcohol from the red cabbage extract loaded with silver nanoparticles
- Leveraging of mycogenic copper oxide nanostructures for disease management of Alternaria blight of Brassica juncea
- Nanoscale molecular reactions in microbiological medicines in modern medical applications
- Synthesis and characterization of ZnO/β-cyclodextrin/nicotinic acid nanocomposite and its biological and environmental application
- Green synthesis of silver nanoparticles via Taxus wallichiana Zucc. plant-derived Taxol: Novel utilization as anticancer, antioxidation, anti-inflammation, and antiurolithic potential
- Recyclability and catalytic characteristics of copper oxide nanoparticles derived from bougainvillea plant flower extract for biomedical application
- Phytofabrication, characterization, and evaluation of novel bioinspired selenium–iron (Se–Fe) nanocomposites using Allium sativum extract for bio-potential applications
- Erratum
- Erratum to “Synthesis, characterization, and evaluation of nanoparticles of clodinofop propargyl and fenoxaprop-P-ethyl on weed control, growth, and yield of wheat (Triticum aestivum L.)”

