Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
-
Ming Wu
und Yongwen Feng
Abstract
MicroRNA-132 (miR-132) is correlated with inflammatory response regulation, although its effect on acute kidney injury to provide protection against hemorrhagic shock remains currently unknown. AChE in macrophages of the kidney subjected under hemorrhagic shock is presumed to be regulated by miR-132 after its transcription to alleviate the inflammatory response accordingly. Antagonists such as acetylcholine (Ach) (concentration 10−4mol/L) and galanthamine (Gal) (concentration 10μmol/L) were added into separate groups 1 hour after the macrophages in the kidney were isolated and cultured to induce injury under oxygen and glucose deprivation (OGD) and then cultured for 24 hours. To analyze the effect of miR-132, we placed the renal epithelial cells transfected with miR-132 plasmids with stable expression over the renal macrophages to create a double cell culture system. The expression levels of inflammatory factors and apoptosis under OGD were significantly higher in renal macrophages than in other experimental groups. Moreover, the expression of miR-132 in macrophages of the double cell culture system showing stable expression of miR-132 increased, whereas that of several inflammatory factors was significantly inhibited. The expression levels of AChE mRNA and protein in the macrophages significantly decreased. The cholinergic antiinflammatory pathway in renal macrophages is regulated by miR-132 via inhibition of the hydrolytic activity of cholinesterase to alleviate inflammatory response, which may play a role in the prevention and treatment of kidney injury caused by hemorrhagic shock.
Graphical Abstract
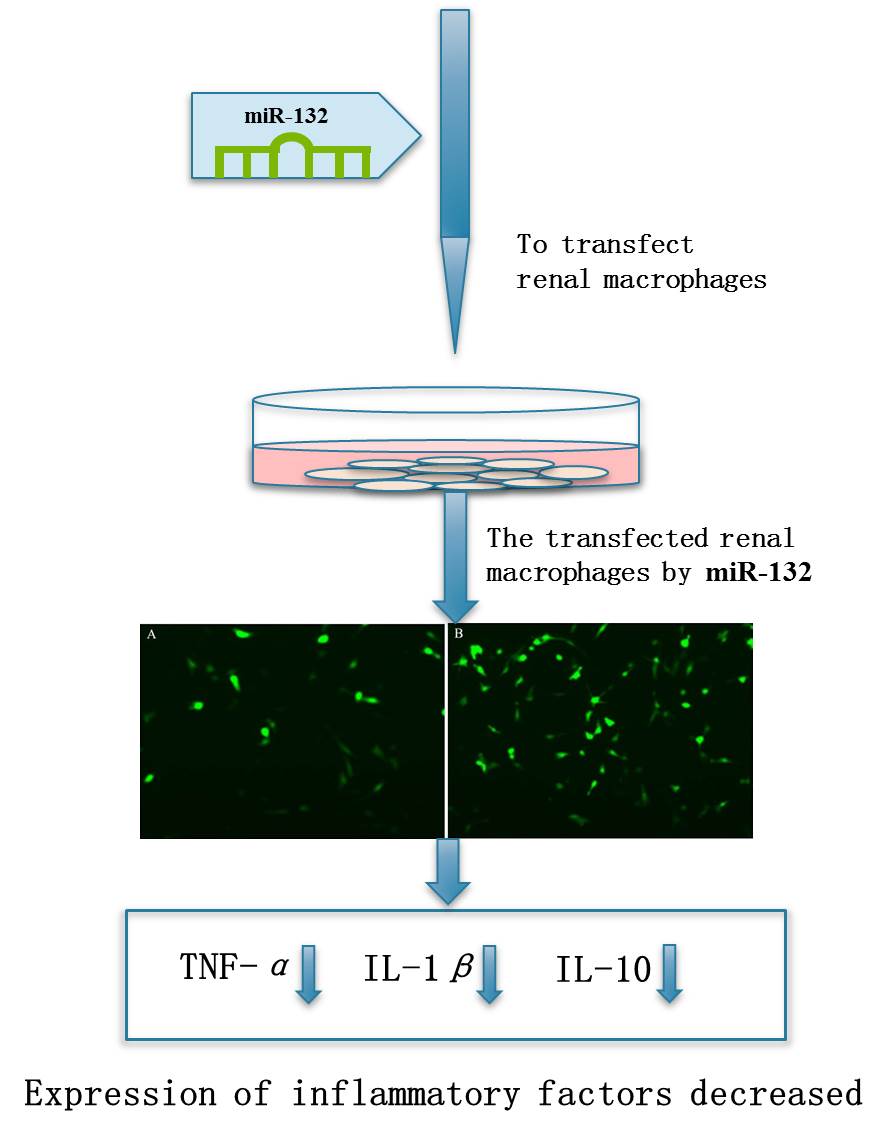
1 Introduction
Hemorrhagic shock is a common complication following trauma and surgery, as well as one of the common issues in emergency cases and critical illnesses [1,2]. Hemorrhagic shock can cause extensive organ dysfunction and metabolic disorders. The death rate yielded by trauma-associated hemorrhagic shock is relatively high, and the primary cause of death in this condition is multiple organ dysfunction syndrome (MODS) induced by sharp reduction in the effective circulating volume, tissue hypoperfusion, reperfusion injury, and endotoxin translocation, among others [3]. Additionally, acute kidney injury (AKI) is one of the primary causes of death among patients with MODS. AKI incidence constantly increases, and irreversible reduction in renal function may be subsequently found in some patients who have suffered from AKI, potentially resulting in chronic kidney disease (CKD) or end-stage renal disease (ESRD) and ultimately in reduced survival of patients [4]. AKI is an independent risk factor for CKD and ESRD, as well as death and other diseases related to kidney disorders.
The microenvironment of macrophages in the kidney changes resulting from the release of large amount of inflammatory mediators when ischemic injury occurs in the kidney [5]. Therefore, in addition to active fluid resuscitation therapies at the early stage of hemorrhagic shock, anti-inflammatory treatment measures that block or reduce the production and release of inflammatory mediators can significantly prevent the progress of hemorrhagic shock [6]. The cholinergic antiinflammatory pathway is a vagal-mediated pathway for real-time regulation of inflammatory response. When an inflammatory response occurs in the human body, vagal afferent neurofibers are stimulated by local inflammatory factors to transfer the inflammatory signals to the hypothalamus [7, 8]. Acetylcholine is then released into the inflammatory site through vagal efferent neurofibers to activate nicotinic ACh receptor 7 (nAChRα7), thereby inhibiting the activation of monocyte macrophages and reducing the release of proinflammatory factors, such as TNF-α, and ultimately regulating the inflammatory response [9, 10, 11]. This study performed isolation and culture of macrophages in the kidney to explore the regulatory function of miR-132 in the cholinergic pathway and its effect on the macrophages.
2 Materials and methods
2.1 Culture of macrophages in the kidney
All procedures in the animal experiments were conducted in accordance with the guidelines developed by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Shenzhen Peking University - The Hong Kong University of Science and Technology Medical Center (Permit SYXK 2015-2016). 10 male eight-week-old SD rats (220g to 250g) were sacrificed by cervical dislocation and disinfected by soaking in 75% alcohol for 5 minutes. The rats were retrieved and positioned upside down, and the bilateral kidneys were surgically removed under sterile conditions. The kidneys were placed in a flat plate filled with precooled RPMI-1640 (Gibco, NY, USA) culture solution (containing 1% calf serum), and the connective tissues and fats were removed. The kidney tissue was squeezed through steel wire gauze (200 mesh) to obtain single cells, which were centrifuged at 200 ×g for 10 minutes and the supernatant was then extracted. A cell solution containing approximately 1 × 108 cells/ml was prepared using RPMI-1640 culture solution containing 1% calf serum. The single cells obtained were inoculated with DMEM culture solution containing 10% FBS (Gibco, NY, USA) in a cell culture flask and placed in CO2 incubator with 5% volume fraction at 37°C under saturated humidity. The macrophages were purified using an adherent method.
2.1.1 Construction of oxygen–glucose deprivation (OGD) model of renal macrophages
The renal macrophages were inoculated on a 24-well plate at a density of 3.0 × 104 cells/ml with 1 ml of culture medium per well. After 24 hours, the culture solution was replaced with serum-free medium and then cultured for another 24 hours; the original culture solution was discarded. For cells in the normal group, Earle’s fluid was added; for cells in the hypoglycemia and hypoxia groups (10%XB+OGD), Na2S2O4 sugar-free Earle’s fluid (4 mmol/ml) was added after the cells were washed once with sugar-free Earle’s fluid. The duration for induction of injury under hypoglycemia and hypoxia was 1 hour. Earle’s fluid was used to wash the cells once after 1 hour. Complete culture solution (10% FBS+1640) was added into the culture, and then the cells under hypoglycemia and hypoxia were divided into two groups; ACh (10×10−5 mol/L, 10%XB+OGD+ACh) was added in one group, whereas the mixture of ACh (10 ×10−5 mol/l) and galanthamine (Gal) (10 μmol/l) was added into the other group and then cultured for 24 hours (10%XB+OGD+ACh+Gal). All of the s were repeated three times.
2.2 Cell transfection
Renal epithelial cells were inoculated in a 12-well culture plate, resulting in 50–70% cell confluency when transfection was performed. miRNA-132 (RiboBio Co., LTD, Guangzhou, China) was overexpressed, and the control vector (6 μg) was transfected into the cells using Lipofectamine 2000 (Invitrogen, USA). The mixed liquor was removed after 8 hours, and then 2 ml of DMEM was added into the complete culture solution. The cells were observed under a fluorescent microscope at 3–5 days after transfection. After miRNA-132 was stably expressed in renal epithelial cells, the abovementioned renal macrophages under hypoglycemia and hypoxia were inoculated in a 96-well culture plate at a density of 5 × 103 cells/cm2. When the cells were overgrown by 50–60%, the DMEM culture solution containing 10% FBS was placed in a double cell culture system containing renal tubular epithelial cells; this culture system was used for transfection and stable expression of miRNA-132 for 24 hours.
2.3 Enzyme-Linked Immunosorbent Assay (ELISA)
The expression levels of TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6 in cultured cell supernatant were determined using ELISA kit (Senxiong Technology Industrial Co., Ltd., Shanghai, China). The absorbance at 450 nm was detected by a microplate reader, and all the results were standardized using a standard curve.
2.4 Cell viability evaluation (MTT assay)
Approximately 1 × 104 cells were inoculated in a 96-well plate, which was subsequently placed in a 5% CO2 cell incubator at 37°C to culture for 24 hours. Appropriate concentrations of the tested compounds were added into the culture solution under suitable conditions and then cultured for 24 hours. Afterwards 1× MTT (50 μL) was added into each well and incubated at 37°C for 4 hours. The supernatant was removed, 150 μL of DMSO was added into each well, and the optical density at 570 nm in each well was determined by a microplate reader.
2.5 Real-time quantitative PCR (RT-qPCR)
A randomized selection of 6 rat kidney macrophages were performed the relative expression levels of miR-132, AChE, AChE-R and AChE-S mRNA were determined by RT-qPCR. TRIzol kit (Invitrogen, USA) was used to extract the total RNA of the renal macrophages. The absorbance of RNA was tested at 260 and 280 nm, and its expression level and purity were determined using an ultraviolet spectrophotometer. Reverse transcription was performed using PrimeScript RT reagent Kit (TaKaRa Biotechnology Co. Ltd., Shanghai, China), total system 10 μL per reaction. RT-qPCR was completed using SYBR Premix Ex Taq II kit (Applied Biosystems, Foster City, CA, USA), total system 20 μL per reaction. PCR amplification was completed using a one-step quantitative PCR system, and U6 snRNA and β-actin were used as standard internal references for the expression levels of miR-132, AChE, AChE-R and AChE-S mRNA. The 2-ΔΔCt method was used for graphical representation of data, and the difference in the samples was evaluated. The kits mentioned above were used in accordance with the manufacturer’s instructions. The primers for miR-132, AChE, AChE-R and AChE-S U6 snRNA, and β-actin are listed in table 1. All of the experiments were repeated three times.
Sequences of primers for reverse transcription and real-time quantitative. PCR
| Gene | Primer sequences |
|---|---|
| miR-132 | RT primer: 5’-GTC GTA TCC AGT GCA GGGTCC GAG GTA |
| TTC GCA CTG GAT ACGACC GAC CA-3’ | |
| PCR primer F: 5’-GCC GCT AAC AGT CTA CAGCCA T-3’ | |
| PCR primer R: 5’-GTG CAG GGT CCG AGG T-3’ | |
| U6 | RT primer: 5’-AAC GCT TCA CGA ATT TGCGT-3’ |
| PCR primer F: 5’-CTC GCT TCG GCA GCACA-3’ | |
| PCR primer R: 5’-AAC GCT TCA CGA ATT TGCGT-3 | |
| AChE | PCR primer F: 5’-AAA CAT GCA GAA GAT GAGGAT-3’ |
| PCR primer R: 5’-GAC CAC TAT AGC AAG CAGGAA C-3’ | |
| AChE-R | PCR primer F: 5’-CCCCTGGACCCCTCTCGAAAC-3’ |
| PCR primer R: 5’-ACCTGGCGGGCTCCCACTC-3 | |
| AChE-S | PCR primer F: 5’-CGGGTCTACGCCTACGTCTTTGAACACCGTG-CTTC-3’ |
| PCR primer R: 5’-CACAGGTCTGAGCAGCGATCCTGCTTGCTG-3’ | |
| β-actin | PCR primer F: 5’-TAC TGC CCT GGC TCC TAGCA-3’ |
| PCR primer R: 5’-TGG ACA GTG AGG CCA GGATAG-3’ | |
2.6 Western blot analysis
To test the AChE and control GAPDH protein, we extracted the total protein of renal macrophages using RIPA, and the same amount of protein sample was used for loading. The total proteins were incubated with blocking buffer for 2 hours after electrophoresis, decolorized with TBST at room temperature, and then washed twice on a shaking table for 10 minutes each time after the AChE antibody (Abcam, Cambridge, MA, USA, ab188358) and GAPDH antibody (Santa Cruz, USA, sc-365062) primary antibody was diluted with TBST to 1:500 and 1:1000 prior to incubation at room temperature for 1–2 hours. The total protein was washed again with TBS for 10 minutes, incubated with secondary antibody dilution buffer (1:800) labeled with HRP at room temperature for 1 hour, decolorized with TBST at room temperature, washed three times on a shaking table for 10 minutes each time, and finally tested with ECL luminescence kit. The results were quantitatively analyzed using Gel pro4.0 gel optical density analysis software. In addition, the integrated optical density was determined.
2.7 Statistical analysis
All analyses were performed using SPSS13.0 software. The results are presented as mean ± SD. Comparison between two groups was performed using one-way ANOVA. P values of <0.05 were regarded as statistically significant.
3 Results
3.1 Effect of ACh and Gal on OGD model of renal macrophages
The OGD model of renal macrophages was constructed to test the effect of miRNA-132 in renal macrophages under AKI caused by hemorrhagic shock. Macrophages subjected under hypoglycemia and hypoxia were processed for 24 hours with ACh and Gal as antagonists, and the expression levels of TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6 were tested in cell culture suspension. The results demonstrated that the expression levels of TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6 after treatment with ACh and Gal were all significantly lower than those in the group of macrophages subjected under hypoglycemia and hypoxia (P < 0.05 or P < 0.01) (Figures 1A–F).
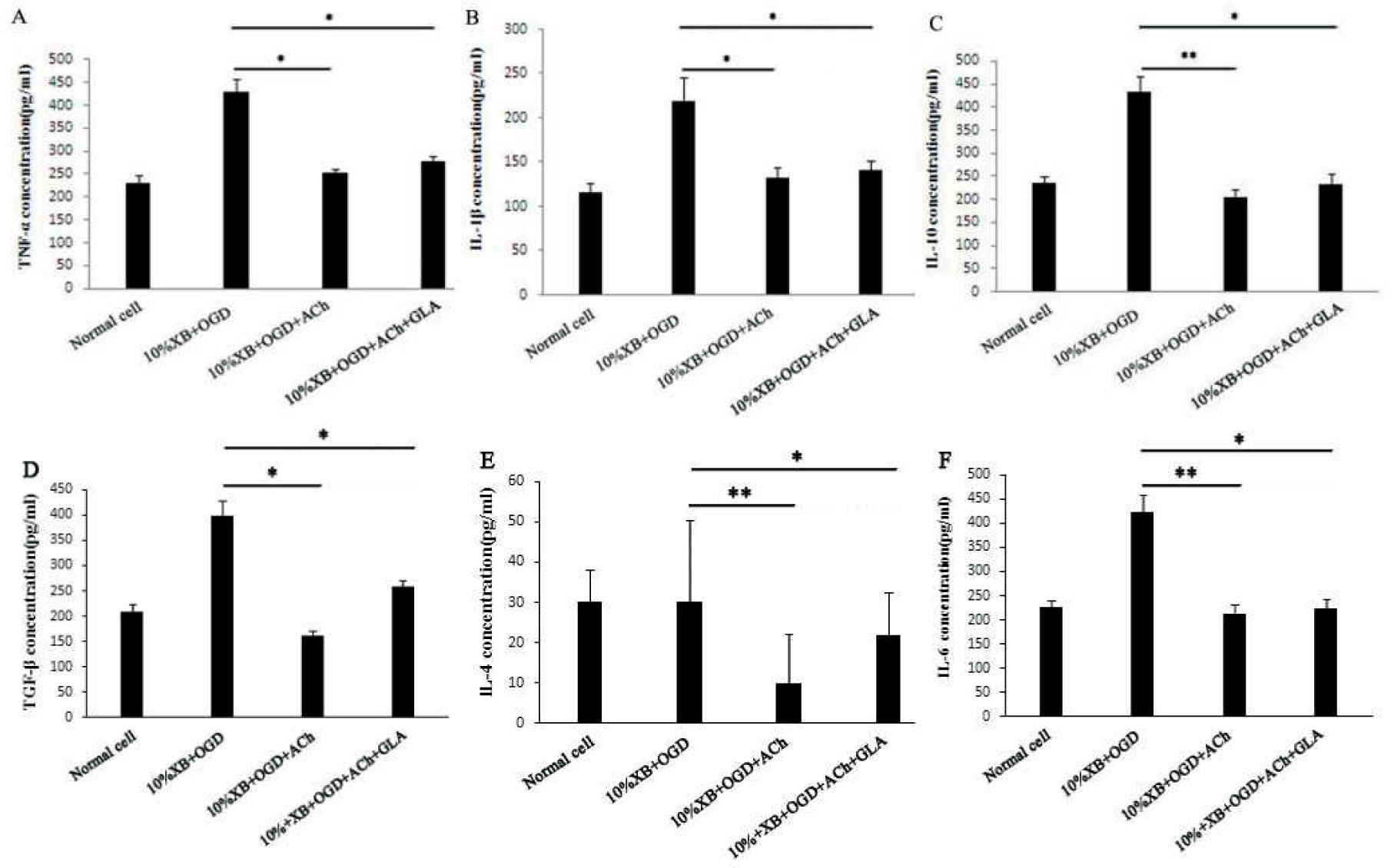
Expression of inflammatory factors in renal macrophages of rats after being processed using different methods: (A) TNF-α, (B) IL-1β, (C) IL-10, (D) TGF-β, (E) IL-4 and (F) IL-6. The results are presented as mean ± SD, * P < 0.05, ** P < 0.01.
3.2 Expression of miRNA-132 in renal epithelial cells
To further verify the effect of miRNA-132, we constructed its expression plasmid and then transfected in renal epithelial cells. We found that 3 days after its transfection, miRNA-132 was stably expressed and the fluorescence intensity was higher than that in the control group (Plasmids were not inserted into the miRNA-132 group) (Figures 2A, B)
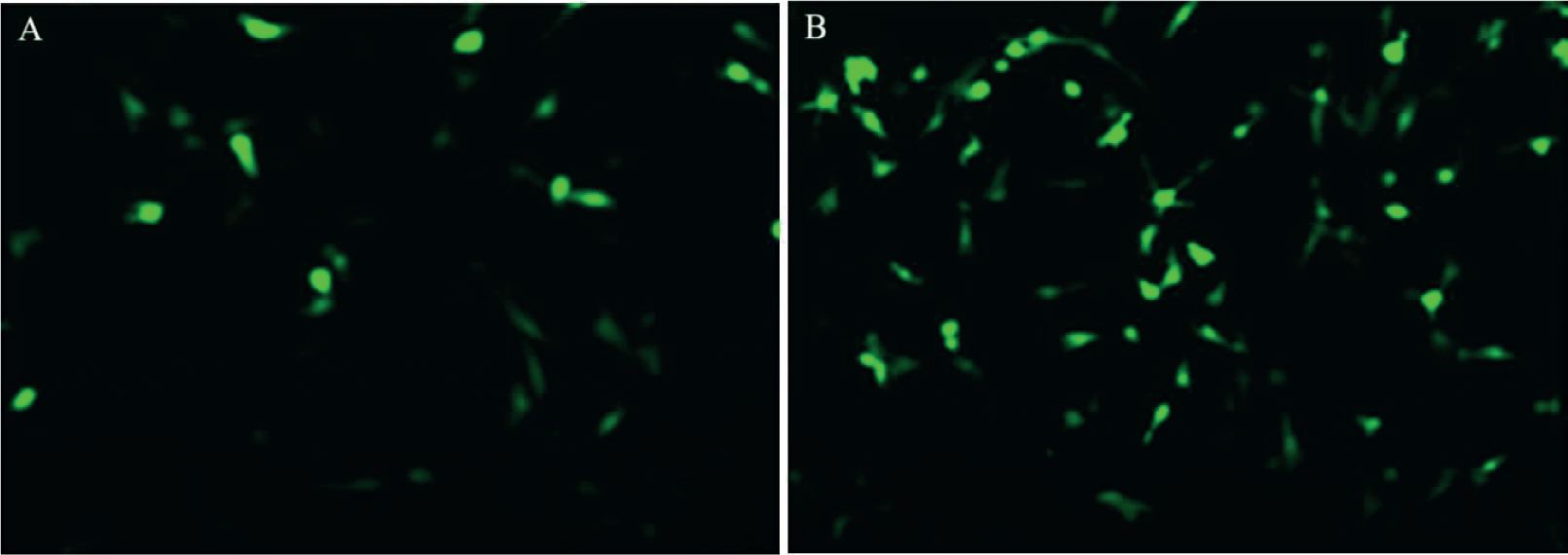
MiRNA-132 expression after transfection of renal epithelial cells in rats through miRNA-132 plasmids; (A) miRNA-132 nc and (B) miRNA-132 . Scale bar = 50 μm.
3.3 Analysis of cell viability
Analysis of cell viability of each experimental group, using the MTT method showed that the cell viability of renal macrophages was significantly reduced in the 10%XB+OGD+miRNA-132 group compared to the 10%XB+OGD group (P < 0.01). Moreover, the cell viability of macrophages in double cell culture system with expression of miRNA-132 was similar to that of normal cells (Figure 3).
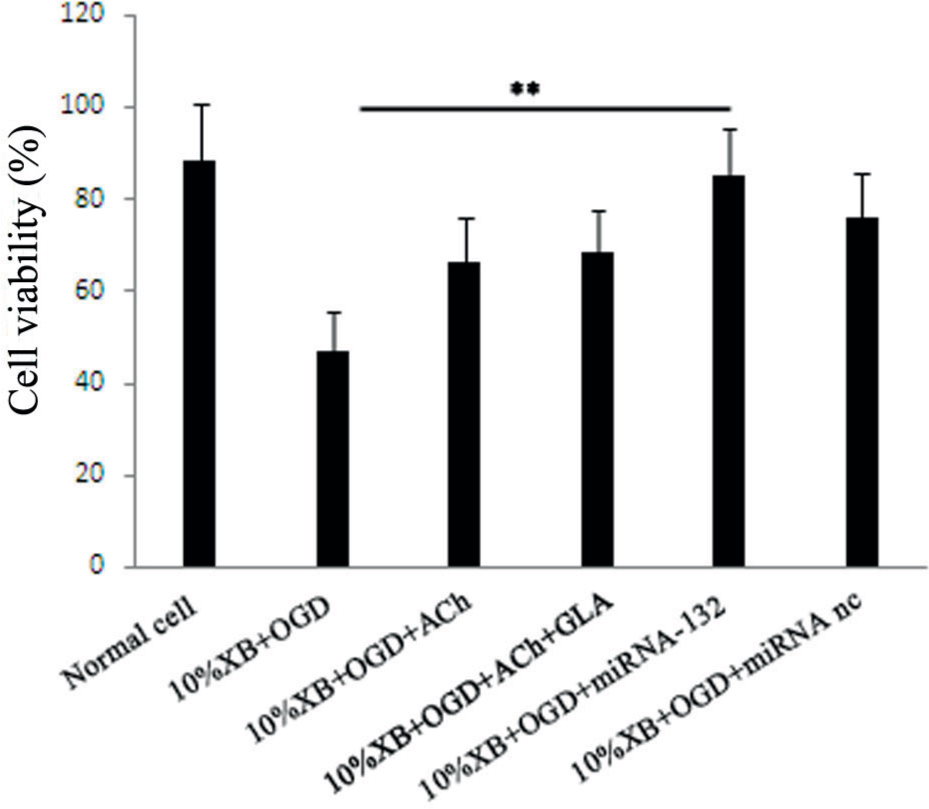
Analysis of cell viability of renal macrophages in rats using the MTT method after processing through different methods. The results are presented as mean ± SD, ** P < 0.01.
3.4 Effect of miRNA-132 on expression of TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6
The effect of miRNA-132 was further verified in the double cell culture system of renal macrophage–renal epithelial cell, and the expression levels of TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6 were analyzed in the double cell culture system, in which miRNA-132 was transfected and stably expressed. Analysis showed that the expression levels of TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6 in the cell suspension with transfected and stably expressed miRNA-132 were significantly lower than that in the macrophages of the hypoglycemia and hypoxia group (P < 0.05 or P < 0.01), demonstrating that miRNA-132 inhibits inflammation (Figures 4A–F).
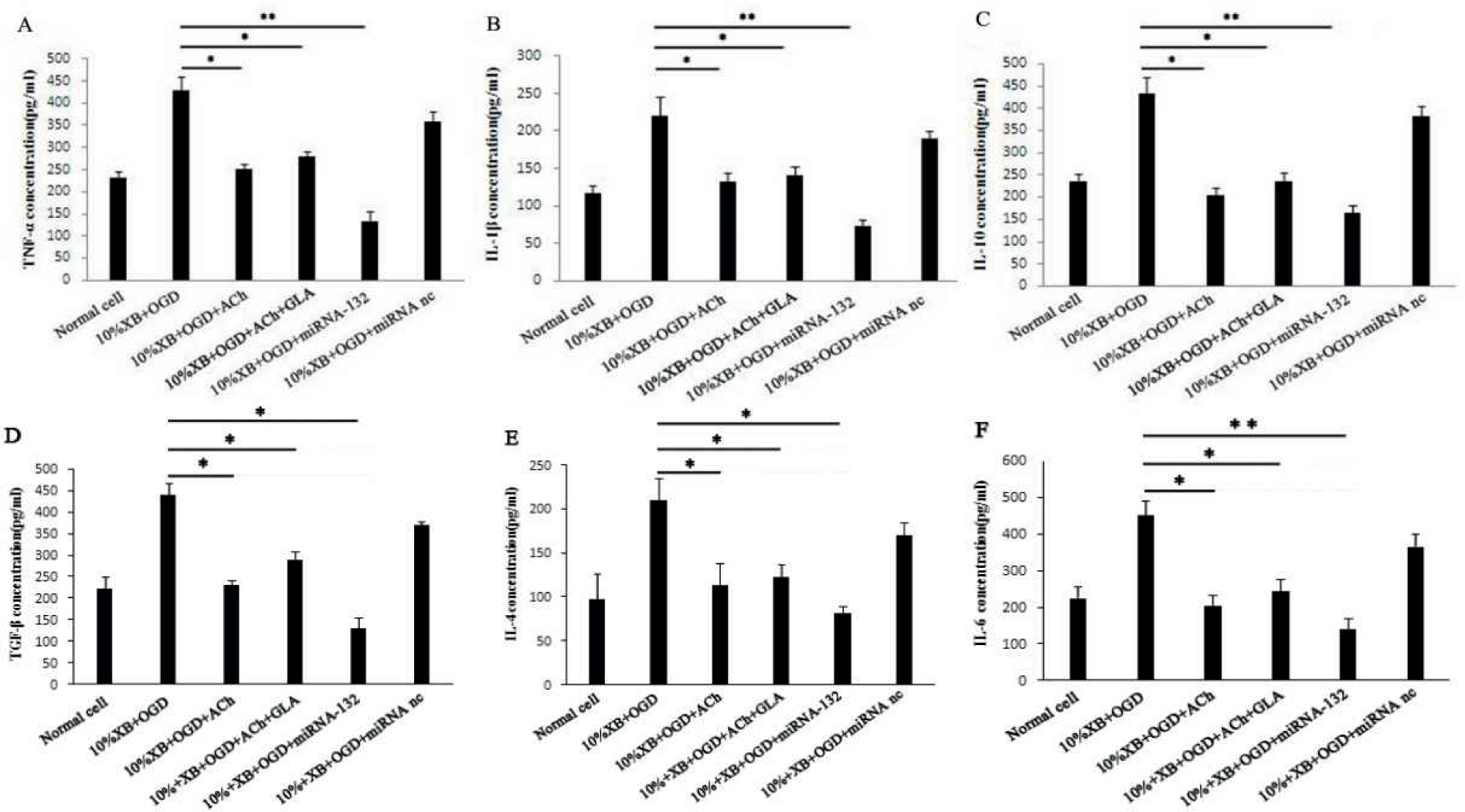
Testing of inflammatory factors in the double cell culture system 24 h after miRNA-132 was transfected and stably expressed: (A) TNF-α, (B) IL-1β, (C) IL-10, (D) TGF-β, (E) IL-4, and (F) IL-6. The results are presented as mean ± SD, * P < 0.05, ** P < 0.01.
3.5 Effect of miRNA-132 on expression levels of AChE mRNA and protein in renal macrophages
The effect of transfected miRNA-132 was investigated, and the results demonstrated that miRNA-132 expression in rat renal macrophages of double cell culture system with transfected expression plasmids was significantly higher than that in other experimental groups (Figure 5A). Moreover, the effects of transfection and expression of miRNA-132 on AChE mRNA and protein and their activities were investigated in the double cell culture system. The results demonstrated that expression of AChE mRNA was significantly inhibited by overexpression of miRNA-132 in renal macrophages (Figure 5B, ** P < 0.01). The results also showed that the expression of AChE protein in the cells was significantly inhibited (Figure 5C). Expression of AChE protein was then quantitatively analyzed (Figure 5D). The effects of transfection and expression of miRNA-132 on AChE-R mRNA in the double cell culture system. We found the expression of AChE-R mRNA was significantly inhibited by overexpression of miRNA-132 (Figure 5F, ** P < 0.01), and there was no obvious change in the expression of AChE-S. The results show that miRNA-132 regulates inflammation by inhibiting the expression of AChE-R protein in renal macrophages. This has some protective effect on renal injury (Figure 6).
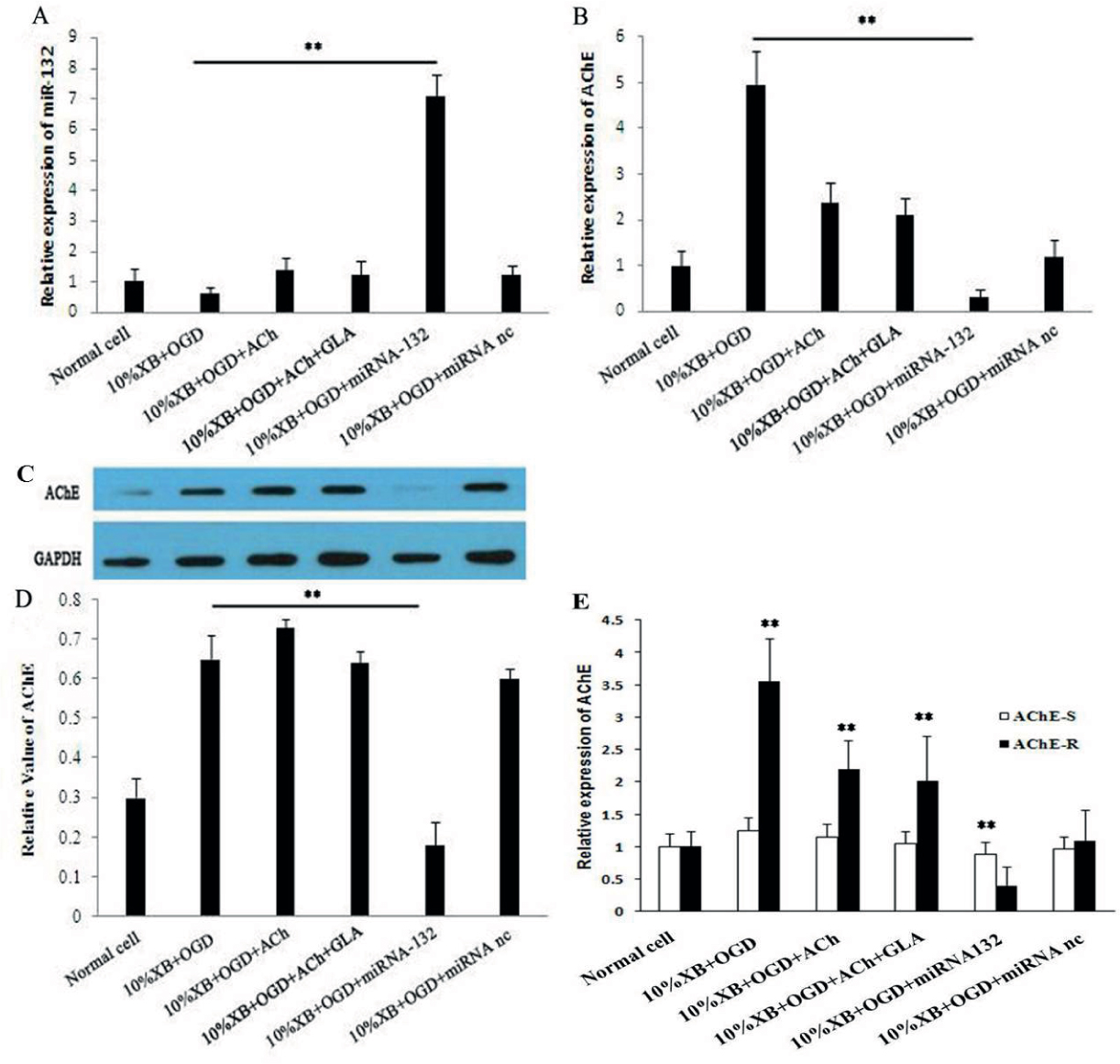
Investigation on the regulatory mechanism of miRNA-132 on the inflammatory pathway in rat renal macrophages of the hypoglycemia and hypoxia groups by testing (A) the relative expression of miRNA-132 in macrophages by using quantitative PCR, (B) the relative expression of AChE mRNA in renal macrophages, (C) the relative expression of AChE protein in renal macrophages, (D) The expression level of AChE protein was quantitatively analyzed, and the results are presented as mean ± SD, * P < 0.05, ** P < 0.01.
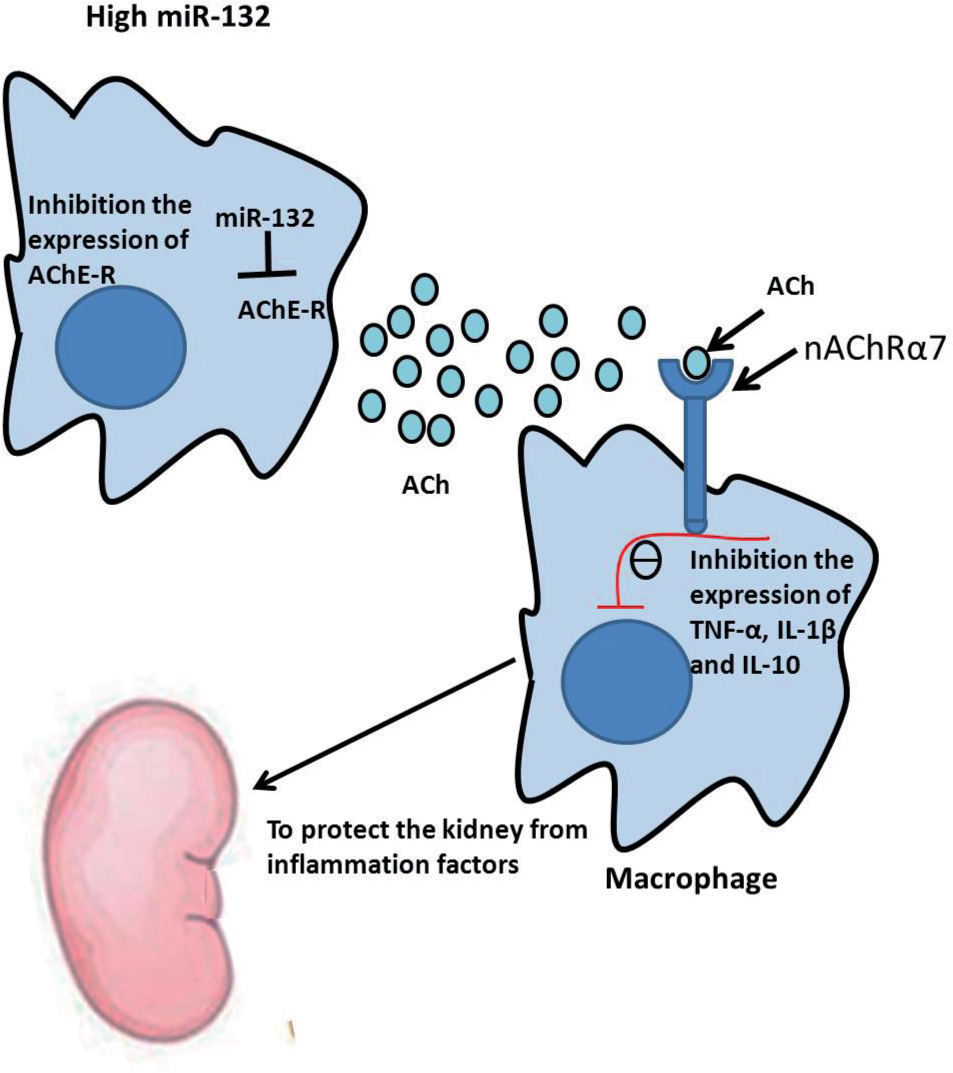
A schematic of the cholinergic anti-inflammatory impact. ACh-mediated anti-inflammatory response was increased by miRNA-132 in the renal macrophage. MiRNA-132 can target the 3’-UTR of AChE mRNA to inhibit AChE expression and decrease the hydrolysis of ACh. nAChRα7 in monocyte– macrophages is activated by ACh to subsequently inhibit the activation of monocyte– macrophage and reduce the release of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-10. MiRNA-132 can inhibit macrophage activation through the role of the above mentioned and protect the kidney.
4 Discussion
It has become evident that microRNAs (miRNAs) are pivotal regulators of many biological processes, including inflammation [12]. Our study has proven that the ACh-mediated anti-inflammatory response was increased by miRNA-132 in renal macrophage–renal epithelial cell system of AKI caused by hemorrhagic shock in rats. Alleviation of inflammatory response, neovascularization, and tissue repair, were promoted by macrophages in the specific microenvironment [6]. The microenvironment of renal macrophages was altered resulting from the release of large number of inflammatory mediators when the kidney is chemically injured. A large nuber of inflammatory mediators, particularly some proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6) were all activated and released, resulting in self-destructive systemic inflammation that eventually developed into MODS [13,14]. Our study found that the expression level of inflammatory factors, such as TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6 was significantly higher in transfected cells than those in normal cells and cells treated with antagonists, such as ACh and Gal.
The cholinergic anti-inflammatory pathway is a neural regulatory pathway, whereas synthesis and release of inflammatory factors is effectively reduced, resulting in significant inhibition of systemic and local inflammatory responses when this pathway is activated [15,16]. nAChRα7 in monocyte–macrophages is activated to subsequently inhibit the activation of monocyte–macrophage and reduce the release of pro-inflammatory cytokines, such as TNF-α. Acetylcholinesterase is not only present in neuronal cells but also in immunocytes [17]; the highest expression was observed in macrophages [18]. Whenever an inflammatory response occurs, ACh is rapidly hydrolyzed by AChE secreted from macrophages to prevent the inhibitory effect of ACh on macrophage activation. MiRNA-132 expression is upregulated in the human monocyte–macrophage cell line THP-1 processed by LPS, whereas miRNA-132 acts as the ligand of immune response involved in regulation of TLR signal molecules and activation of NF-KB pathway, demonstrating the close relationship between miRNA-132 and inflammatory response [19]. MiRNA-132 can target the 3’-UTR of AChE mRNA to inhibit AChE expression and regulate cholinergic anti-inflammatory pathway accordingly [20].
The hydrolytic activity of AChE is presumed to be reduced by miRNA-132 via the inhibition of AChE expression in renal macrophages, improving the microenvironment in the kidney, inducing the conversion of renal macrophages into type M2, promoting repair of renal tubule and renal interstitium and improving renal function [20,21]. Our investigation found that after the renal epithelial cells with transfection and stable expression of miRNA-132 are placed over the macrophages of a kidney injured by hypoglycemia and hypoxia, the expression levels of TNF-α, IL-1β, IL-10, TGF-β, IL-4 and IL-6 in cell culture supernatant are significantly inhibited. Moreover, the expression level of miRNA-132 in the double cell culture system is significantly higher than that in other experimental groups. The cholinergic anti-inflammatory effect of miRNA-132 results from targeted inhibition of the hydrolysis of AChE on ACh, whereas our results demonstrated that the expression level of AChE mRNA and protein in renal macrophages that stably expresses miRNA-132 is significantly reduced. Several splice variants of AChE are known (e.g., AChE-S, -R), which result in forms that differ in solubility and subcellular localization. AChE-R furthermore exhibits a unique C-terminal sequence, which is responsible for nonenzymatic actions of this variant [22]. It is involved in proliferation, apoptosis and development of various cell types in different organs, for example brain and hematopoietic cells [23]. In our study, we found increases in miRNA-132 can suppress AChE-R, whereas AChE-R suppression would elevate synaptic ACh, increasing the cholinergic anti-inflammatory effect.
Our study has proven that potential regulation of the inflammatory response by miRNA-132 in renal macrophages possibly plays a preventive, therapeutic, and protective role toward kidney injury caused by hemorrhagic shock.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant No. U1204810), the Guangdong Natural Science Foundation (grant No. 2014A030313709), Shenzhen Science and Technology Planning Project (grant No. JCYJ20130401112313541, No. JCYJ20140415162338855, No.JCYJ20150330102401099, No.JCYJ 20160425103130218, No.JCYJ20170306091335008), and Clinical and Foundation Bridge Project of Shengzhen Second Hospital (2015-16)
Conflict of interest: Authors state no conflict of interest.
References
[1] Cocchi M.N., Kimlin E., Walsh M., Donnino M.W., Identification and resuscitation of the trauma patient in shock, Emerg Med. Clin. North Am., 2007, 25(3), 623-642.10.1016/j.emc.2007.06.001Suche in Google Scholar PubMed
[2] Zhou B., Wang G., Peng N., He X., Guan X., Liu Y., Pre-Hospital Induced Hypothermia Improves Outcomes in a Pig Model of Traumatic Hemorrhagic Shock, Adv. Clin. Exp. Med., 2015, 24(4), 571-578.10.17219/acem/29044Suche in Google Scholar PubMed
[3] Coca S.G., Yusuf B., Shlipak M.G., Garg A.X., Parikh C.R., Longterm risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis, Am. J. Kidney Dis., 2009, 53(6), 961-973.10.1053/j.ajkd.2008.11.034Suche in Google Scholar PubMed PubMed Central
[4] Coca S.G., Singanamala S., Parikh C.R., Chronic kidney disease after acute kidney injury: a systematic review and metaanalysis, Kidney Int., 2012, 81(5), 442-448.10.1038/ki.2011.379Suche in Google Scholar PubMed PubMed Central
[5] Molitoris B.A., Levin A., Warnock D.G., Joannidis M., Mehta R.L., Kellum J.A., et al., Improving outcomes of acute kidney injury: report of an initiative Nature clinical practice, Nephrology., 2007, 3(8), 439-442.10.1038/ncpneph0551Suche in Google Scholar PubMed
[6] Van Ginderachter J.A., Movahedi K., Hassanzadeh Ghassabeh G., Meerschaut S., Beschin A., Raes G., et al., Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion, Immunobiology., 2006, 211(6-80), 487-501.10.1016/j.imbio.2006.06.002Suche in Google Scholar PubMed
[7] Tracey K.J., Physiology and immunology of the cholinergic antiinflammatory pathway, J. Clin. Invest., 2007, 117(20), 289-296.10.1172/JCI30555Suche in Google Scholar PubMed PubMed Central
[8] Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., et al., Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation, Nature, 2003,421(6921), 384-388.10.1038/nature01339Suche in Google Scholar PubMed
[9] Tracey K.J, The inflammatory reflex, Nature., 2002, 420(6917), 853-859.10.1038/nature01321Suche in Google Scholar PubMed
[10] Metz C.N., and Tracey K.J, It takes nerve to dampen inflammation, Nat. Immunol., 2005, 6(8), 756-757.10.1038/ni0805-756Suche in Google Scholar PubMed
[11] Yoshikawa H., Kurokawa M., Ozaki N., Nara K., Atou K., Takada E., et al., Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7, Clin. Exp. Immunol., 2006, 146(1), 116-123.10.1111/j.1365-2249.2006.03169.xSuche in Google Scholar PubMed PubMed Central
[12] Mann M., Mehta A., Zhao J.L., Lee K., Marinov G.K., Garcia-Flores Y., et al., An NF-kappaB-microRNA regulatory network tunes macrophage inflammatory responses, Nat. Commun., 2017, 8, 851. 10.1038/s41467-017-00972-zSuche in Google Scholar PubMed PubMed Central
[13] Ricardo S.D., van Goor H., Eddy A.A., Macrophage diversity in renal injury and repair, J. Clin. Invest., 2008, 118(11), 3522-3530.10.1172/JCI36150Suche in Google Scholar PubMed PubMed Central
[14] Wang Y., Wang Y., Cao Q., Zheng G., Lee V.W., Zheng D., et al., By homing to the kidney, activated macrophages potently exacerbate renal injury, Am. J. Pathol., 2008, 172(6), 1491-1499.10.2353/ajpath.2008.070825Suche in Google Scholar PubMed PubMed Central
[15] van Maanen M.A., Vervoordeldonk M.J., Tak P.P., The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis, Nat. Rev. Rheumatol., 2009, 5(4), 229-232.10.1038/nrrheum.2009.31Suche in Google Scholar PubMed
[16] Hilderman M., Qureshi A.R., Al-Abed Y., Abtahi F., Lindecrantz K., Anderstam B., et al., Cholinergic antiinflammatory pathway activity in dialysis patients: a role for neuroimmunomodulation? Clin. Kidney. J., 2015, 8(5), 599-605.10.1093/ckj/sfv074Suche in Google Scholar PubMed PubMed Central
[17] Han Z., Li L., Wang L., Degos V., Maze M., Su H., et al., Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture, J. Neurochem., 2014, 131(4), 498-508.10.1111/jnc.12817Suche in Google Scholar PubMed PubMed Central
[18] Klegeris A., Budd T.C., Greenfield S.A., Acetylcholinesterase-induced respiratory burst in macrophages: evidence for the involvement of the macrophage mannose-fucose receptor, Biochim. Biophys. Acta, 1996., 1289(1), 159-167.10.1016/0304-4165(95)00105-0Suche in Google Scholar PubMed
[19] Taganov K.D., Boldin M.P., Chang K.J., Baltimore D., NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses, Proc. Natl. Acad. Sci. U S A., 2006, 103(3), 12481-12486.10.1073/pnas.0605298103Suche in Google Scholar PubMed PubMed Central
[20] Shaked I., Meerson A., Wolf Y., Avni R., Greenberg D., Gilboa-Geffen A., et al., MicroRNA-132 potentiates cholinergic antiinflammatory signaling by targeting acetylcholinesterase, Immunity., 2009, 31(6), 965-973.10.1016/j.immuni.2009.09.019Suche in Google Scholar PubMed
[21] Soreq H., and Wolf Y., NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol. Med., 2011, 17(10), 548-555.10.1016/j.molmed.2011.06.009Suche in Google Scholar PubMed
[22] Mishra N., Friedson L., Hanin G., Bekenstein U., Volovich M., Bennett E.R., et al., Antisense miR-132 blockade via the AChE-R splice variant mitigates cortical inflammation, Sci. Rep., 2017, 7, 42755. 10.1038/srep42755Suche in Google Scholar PubMed PubMed Central
[23] Blohberger J., Kunz L., Einwang D., Berg U., Berg D., Ojeda S.R., et al., Readthrough acetylcholinesterase (AChE-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis., 2015, 6(10), e1685. 10.1038/cddis.2015.51.Suche in Google Scholar PubMed PubMed Central
© 2018 Ming Wu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Artikel in diesem Heft
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
Artikel in diesem Heft
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution

