Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
-
Agnieszka Malinowska-Gniewosz
, Andrzej Massalski
Abstract
The heterogeneous nature and widespread anthropogenic impacts on industrial water biotopes in the Trzuskawica S.A., pose challenges to biomonitoring of this habitat. Generally, the concentration of trace elements in the industrial water biotopes reflects the anthropogenic impacts. With X-ray fluorescence method (TXRF) in waters 17 elements:P,S,Cl,K,Ca,Ti,Cr, Mn,Fe,Ni,Cu,Zn,Br,Rb, Sr, Ba, Pb were revealed. High amounts of Ca, from 300 ppm to 198 ppm in May and from 999 ppm to 231 ppm in September 2015, was determined. A total of 36 diatoms were found in two reservoirs and drainage ditch, but only three taxa: Cymatopleura radiosa, Navicula upsaliensis and Nitzschia angustata were present in all 7 sampling sites. These species are known to be tolerant to organic pollution, eutrophication, and also characteristic for limestone waters. The results of CVA showed that dintoms in the water reservoir stocked with fish were distinguished by highest species richness. The relationships between diatoms and environmental variables confirm the positive correlation with the currently functioning industrial plant (despite the increased water temperature and large content of trace elements). Our results suggest that, though heterogeneity in both diatoms and selected elements in industrial waters, diatoms can be useful indicators of habitat conditions.
1 Introduction
Recently the number and importance of activities and exploitation areas increased in many countries. Artificial water biotopes have been created in places where there were opencast mines [l], abandoned granite or limestone quarries [2]. The hydrochemical properties of their waters are mainly determined by regional geochemical background. The knowledge of the microorganisms diversity present in industrial water biotopes of operating plants is generally poor, in spite of their important role in the assessment of the state, dynamics and monitoring of such environment. Anthropogenic activities such as urban, industrial and agricultural changes, increasing consumption of water result in degradation of environment in a wide sense of the term. Diatoms are being used to assess ecological conditions in water biotopes around the world [3]. They respond directly and sensitively to many physical, chemical and biological changes in water ecosystems, such as temperature, nutrient concentrations and others [4]. The heterogeneous nature of industrial environments poses challenges to monitoring industrial waters. Bioassessment of human impacts on industrial water biotopes might be improved by accounting for the natural heterogeneity of these systems, which may require more frequent sampling and multiple environmental criteria. For instance, diatom species in habitats with excessive and periodic fine sediment deposition are often dominated by the species with relatively high motility e.g. Gyrosigma, Nitzschia, Surirella.It is difficult to determine which environmental factors are most significant in defining species distribution. Several authors [5,6] indicated that substrate is a critical limiting factor. Diatoms are ecologically diverse and flourish in virtually every industrial water habitat. There is no accurate count of the number of diatom species; however, estimates on the order of 104 are often given [7]. Well-developed epiphytic, epipelic, and planktonic diatom assemblages could all potentially be used for bioassessment [8]. Diatoms were used as indictors of various environmental parameters, including salinity, conductivity, phosphorus and water level, and also are involved in the purification of water by oxygenation (photosynthesis) and absorption of heavy metal ions: nickel, lead, zinc and titanium [9].
Beginnings of the limestone industrial production in what is presently Trzuskawica S.A., date back to the year 1910, and it is now one of the largest an industrial plant in Europe producing cement, lime and asphalt mass. According to our knowledge the present studies are one of the first, if not the first of this type. The relationships between diatom species and environmental variables in water biotopes of the functioning industrial plant were studied.
2 Materials and methods
2.1 Samples
Seven water sites for algological, physical and chemical materials were sampled from March till September 2015 in Trzuskawica S.A. located in the southern Poland (Figure 1). Four sites (1-4) from reservoir stocked with fish (A), two (5-6) from reservoir of technological water (B) and one (7) from drainage ditch (C) were selected (Figure 2). Diatoms and water samples were collected once every month for seven months, but the limestone rocks were gathered only once. Technological waters used for lime and cement production were discharged into the two reservoirs and mixed with sludge. The limestone rocks were crushed and flooded with water which was subsequently drained by a drainage ditch into the reservoir stocked with fish. Water reservoirs are connected by a drainage ditch to the Bobrza River, a tributary of the Black Nida River. Analysis of water quality and physical parameters from 7 sampling sites is presented in Table 1.

Location of sampling sites: water reservoir stocked with fish (A), reservoir of technological water (B), the drainage ditch of mining area (C).

Study area: water reservoir stocked with fish (A), reservoir of technological water (B), the drainage ditch of mining area (C).
Physico-chemical water parameters in two reservoirs (A-B) and drainage ditch (C) in 2015.
| Water biotopes | Temperature [°C] | pH | Conductivity [μS cm–1] | Secchi depth [cm] |
|---|---|---|---|---|
| A | 7.4 | 7.8 | 767.6 | 66.3 |
| B | 8.7 | 7.8 | 711.2 | 12.8 |
| C | 11.2 | 8.2 | 865.2 | 36 |
All algological samples containing diatoms had a volume of 100 ml and were transported to the laboratory where they were analyzed using a light microscope Nikon Eclipse 600 equipped with a digital camera Nikon Coolpix 4500. Diatoms were identified according to [5,10,11,12,13].
Water samples for chemical analysis were collected from flowing water (drainage ditch) and from stagnant water (two reservoirs). Temperature, pH and electrolytic conductivity were measured with the multi measurement device HANNA pH-meter Instruments HI9126 (Table 1). Water transparency was measured using the Secchi disk [14]. All these measurements were done in situ. The elemental analysis of waters was carried out using Total reflection X-Ray Fluorescence analysis (TXRF) in X-Ray Methods Laboratory (Institute of Physics, Jan Kochanowski University in Kielce, Poland) in May and September 2015.
2.1.1 TXRF – Total Reflection X-Ray Fluorescence analysis (spectrometer S2 PICOFOX)
Analysis was performed using S2 Picofox spectrometer (Bruker AXS Microanalysis GmbH) equipped with air cooled, 30 W X-ray tube with Mo anode working with parameters: high voltage 50 kV and electron current 600 μA. The measurement system includes a Peltier-cooled XFlash® Silicon Drift Detector with area 10mm2 and energy resolution < 160 eV at 100 kcps (for Mn Kα line). This device allows to measure the characteristic X-rays of elements from Al (aluminium) to U (uranium), with exception of Zr (zirconium) to Ru (ruthenium). The internal standard containing 100 μL solution with a concentration of selenium equal to 1,961 ppm was added to the analyzed water. Liquid samples were measured directly after adding Se internal standard (500 μL water + 100 μL Se + 1 mL HNO3) while diatom samples in the amount of 0.2 – 0.3 g were mineralized with 4 mL of high purity HNO3 and 0.1 mL of 100 μg/g Se standard (or 6 ml of HNO3 and 0.2 ml Se (100μg/g)). The sample was further mineralized in microwave digestion system. Next, 5 μL of solution was pipetted into Synsil backing, and this drop was dried in infrared.
2.2 Data analysis
Diatoms data were presented in three ways: a) relative abundance within a sample (species abundance/total abundance × 100), b) mean species relative abundance across all sites where present, and c) species frequency across all studied water biotopes. Two approaches to examine the relationship between water chemistry of the sampling sites and sampling time of water, diatoms and limestone rocks in the three industrial water biotopes (A-C) in the Trzuskawica S.A. were used. The first approach was an indirect analysis of changes in water chemistry while collecting materials from sampling sites. The principal component analysis (PCA) was performed. The second approach was a direct analysis on the relationship between the element concentrations and the changes in months (May, June, September) and sampling sites (A, B, C). Canonical variate analysis (CVA) was applied as the discriminant analysis [15]. The importance of these variables in relation to months and sampling sites was tested using a Monte Carlo permutation test and CVA was done with Canoco v. 5.0 statistic software test [16].
Ethical approval
The conducted research is not related to either human or animals use.
3 Results
We identified a total of 36 diatoms from 24 genera, the vast majority of which were typically epilithic or epiphytic forms. The most species belong to genera Amphora, Cymatopleura, Eunotia, Gomphonema, Navicula and Nitzschia which are characteristic for waters rich in calcium carbonate (Table 4). Only 16 species were common (i.e., present at < 10% relative abundance in at least one sample) and 10 species were reached > 20% relative abundance in at least one sample.
The pH range was 7.4–11.2 for the analyzed sites. In the reservoirs A and B the waters pH was slightly alkaline, whereas the high pH 11.2 was recorded in the drainage ditch (C) due to large amounts crashed limestone (Table 1). There was relatively high variation of conductivity in the industrial water biotopes, ranging from 711 μS cm–1(reservoir of technological water B) to 865 μS cm-1 (drainage ditch C). The diatoms were dominated by neutrophilous and alkaliphilous, mesotraphentic and eutraphentic, ß-mesosaprobous (Table 4).
The highest average annual temperature of water was recorded in the drainage ditch (C), while the lowest in the water reservoir stocked with fish (A). The Secchi depth was the highest (66.3 cm) in reservoir A, and the lowest (12.8 cm) in reservoir B.
Using TXRF method 17 elements: P, S, Cl, K, Ca, Ti, Cr, Mn, Fe, Ni, Cu, Zn, Br, Rb, Sr, Ba, and Pb in two reservoirs (A and B) and drainage ditch (C) were determined in May 2015 (Table 2). For diatoms development main organic elements (C, N, P, S, Si) and microelements (Cu, Mn, Se, Zn, Mo, B) are important. However, in studied industrial waters not all the elements mentioned above were detected. Among organic elements only P and S were found. The highest P concentration (13.9 ppm) was in site 7 (drainage ditch C), while in site 6 (reservoir B) was the lowest (7.2 ppm). Whereas the highest S concentration (42.1 ppm) was in site 3 (reservoir A), while in site 7 (drainage ditch C) was the lowest (38.2 ppm). Among microelements only Cu, Mn and Zn were found. The highest Cu concentration (0.01 ppm) was in site 4 (reservoir A), while in site 7 (drainage ditch C) was the lowest (0.003 ppm). Whereas the highest Mn concentration (0.02 ppm) was in site 3 (reservoir A), while in site 7 (drainage ditch C) was the lowest (0.008 ppm). The highest Zn concentration (0.4 ppm) was in site 5 (reservoir B), while in site 3 (reservoir A) was the lowest (0.04 ppm). Generally, the highest elements concentration was in water reservoirs A and B. The average values of elements concentration were detected in the drainage ditch water (C). The nine elements namely: Ca, Fe, K, Mn, Ni, P, S, Sr, Zn, were identified in the limestone rocks and in the reservoirs water (A, B, C).
Element concentrations in two water reservoirs A (sites 2-4) and B (sites 5-6), and drainage ditch C (site 7) determined by TXRF method in ppm units with total experimental uncertainty (in %) (May 18th of 2015).
| Element TXRF | Concentration (ppm) ± total experimental uncertainty (%) *LLD (lower limits of detection) | |||||
|---|---|---|---|---|---|---|
| site 2 | site 3 | site 4 | site 5 | site 6 | site 7 | |
| P | 10.5 ± 0.3 | 8.4 ± 0.3 | 11.9 ± 0.41 | 11.4 ± 0.4 | 7.2 ± 0.3 | 13.9 ± 0.4 |
| S | 41.9 ± 0.2 | 42.1 ± 0.2 | 43.8 ± 0.3 | 39.1 ± 0.2 | 39.8 ± 0.2 | 38.2 ± 0.2 |
| Cl | 97.6 ± 0.3 | 99.2 ± 0.3 | 113.9 ± 0.4 | 65.5 ± 0.3 | 68.9 ± 0.2 | 46.8 ± 0.2 |
| K | 6.8 ± 0.06 | 7.1 ± 0.05 | 8.5 ± 0.07 | 6.5 ± 0.06 | 6.5 ± 0.05 | 2.5 ± 0.05 |
| Ca | 249.5 ± 0.3 | 198.2 ± 0.2 | 262.4 ± 0.4 | 288.7 ± 0.3 | 211.4 ± 0.3 | 300.03 ± 0.3 |
| Ti | <0.01* | 0.03 ± 0.006 | 0.02 ±0.008 | 0.2 ± 0.008 | 0.2 ± 0.009 | <0.02* |
| Cr | <0.008* | <0.009* | <0.01* | <0.01* | <0.008* | 0.02 ± 0.005 |
| Mn | 0.1 ± 0.002 | 0.2 ± 0.006 | 0.1 ± 0.005 | 0.1 ± 0.005 | 0.06 ± 0.005 | <0.008* |
| Fe | 0.6 ± 0.005 | 0.3 ± 0.005 | 0.9 ± 0.005 | 2.2 ± 0.01 | 1.7 ± 0.01 | 0.05 ± 0.002 |
| Ni | 0.003 ±0.003 | 0.009 ±0.002 | 0.01 ±0.002 | 0.005 ±0.002 | 0.01 ± 0.002 | <0.005* |
| Cu | 0.006 ±0.003 | 0.009 ±0.002 | 0.01 ±0.002 | 0.005 ±0.002 | 0.008 ± 0.002 | <0.003* |
| Zn | 0.1 ± 0.002 | 0.04 ± 0.002 | 0.2 ± 0.002 | 0.4 ± 0.002 | 0.1 ± 0.002 | 0.2 ± 0.002 |
| Br | 0.1 ± 0.003 | 0.1 ± 0.003 | 0.1 ± 0.003 | 0.1 ± 0.003 | 0.1 ± 0.003 | 0.08 ± 0.002 |
| Rb | <0.003* | <0,003* | <0.003* | 0.009 ±0.003 | 0.006 ± 0.003 | <0.003 |
| Sr | 0.3 ± 0.003 | 0.3 ± 0.003 | 0.3 ± 0.03 | 0.3 ± 0.003 | 0.3 ± 0.003 | 0.3 ± 0.003 |
| Ba | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.07 ±0.02 | <0.04* | <0.03 | 0.04 ±0.02 |
| Pb | 0.006 ±0.003 | 0.006 ±0.003 | 0.01 ±0.003 | 0.04 ±0.003 | 0.03 ±0.003 | 0.003 ±0.003 |
The uncertainty values are the expanded uncertainty with an expansion of level about 95% with factor of k = 2. The value of extended uncertainty is given as a range (± U95%).
Using TXRF method 17 elements: P, S, Cl, K, Ca, Ti, Cr, Mn, Fe, Ni, Cu, Zn, Br, Rb, Sr, Ba, and Pb in two reservoirs and drainage ditch were determined in September 2015 (Table 3). The highest P concentration (44.9 ppm) was in site 5 (reservoir B), while in site 4 (reservoir A) was the lowest (7.2 ppm). Whereas the highest S concentration (45.1 ppm) was in site 3 (reservoir A), while in site 1 (reservoir A) was the lowest (32.9 ppm). Among microelements only Cu, Mn and Zn were found. The highest Cu concentration (0.1 ppm) was in site 1 (reservoir A), while in site 4 (reservoir A) was the lowest (0.008 ppm). Whereas the highest Mn concentration (1 ppm) was in site 5 (reservoir B), while in site 7 (drainage ditch C) was the lowest (<0.01 ppm). The highest Zn concentration (1 ppm) was in site 5 (reservoir B), while in site 1 (reservoir A) was the lowest (0.08 ppm). Generally, the highest elements concentration was in water reservoirs A and B. The average values of elements concentration were detected in water of the drainage ditch (C) water of mining area.
Element concentrations in two water reservoirs A (sites 1-4) and B (site 5), and drainage ditch C (site 7) determined by TXRF method in ppm units with total experimental uncertainty (in %) (September 22nd 2015).
| Element TXRF | Concentration (ppm) ± total experimental uncertainty (%) *LLD (lower limits of detection) | |||||
|---|---|---|---|---|---|---|
| site 1 | site 2 | site 3 | site 4 | site 5 | site 7 | |
| P | 12.3 ±0.4 | 11.8 ±0.4 | 7.4 ±0.5 | 7.2 ±0.4 | 44.9 ±0.8 | 10.6 ±0.4 |
| S | 32.9 ±0.3 | 43.9 ±0.3 | 45.1 ±0.36 | 42.8 ±0.3 | 44.5 ±0.4 | 40.08 ±0.3 |
| Cl | 4.4 ±0.1 | 0.6 ±0.1 | < 0.26* | 0.4 ±0.1 | <0.4* | <0.2* |
| K | 6.7 ±0.08 | 6.8 ±0.08 | 8.5 ±0.08 | 8.01 ±0.08 | 21.8 ±0.1 | 4.8 ±0.07 |
| Ca | 239.6±0.4 | 272.4 ±0.4 | 231.9 ±0.4 | 226.8 ±0.4 | 999.4 ±1.3 | 285.3 ±0.4 |
| Ti | 0.07 ±0.01 | 0.04±0.01 | 0.1 ±0.01 | 0.2 ±0.01 | 3.5 ±0.03 | <0.02* |
| Cr | <0.01* | <0.01* | <0.01* | <0.01* | 0.1 ±0.01 | <0.01* |
| Mn | 0.01 ±0.005 | 0.02±0.006 | 0.01 ±0.005 | 0.01 ±0.006 | 1 ±0.01 | <0.01* |
| Fe | 0.2 ±0.006 | 0.6±0.009 | 0.3 ±0.009 | 0.08 ±0.006 | 32.4 ±0.07 | 0.02 ±0.003 |
| Ni | 0.009 ±0.003 | <0.006* | <0.006* | <0.006* | 0.04 ±0.006 | <0,006* |
| Cu | 0.1 ±0.003 | 0.02 ±0.003 | 0.01±0.003 | 0.008 ±0.003 | 0.06 ±0.003 | 0.03 ±0.003 |
| Zn | 0.08 ±0.003 | 0.05 ±0.003 | 0.04±0.003 | 0.04 ±0.003 | 1 ±0.009 | 0.1 ±0.003 |
| Br | 0.1 ±0.003 | 0.1 ±0.003 | 0.1 ±0.003 | 0.08 ±0.003 | 0.09 ±0.003 | 0.08 ±0.003 |
| Rb | 0.02 ±0.003 | 0.01 ±0.003 | 0.01±0.003 | 0.01 ±0.003 | 0.1 ±0.003 | <0.003* |
| Sr | 0.3 ±0.003 | 0.3 ±0.003 | 0.3 ±0.003 | 0.3 ±0.003 | 0.7 ±0.006 | 0.3 ±0.003 |
| Ba | <0.05* | <0.05* | <0.06* | 0.09 ±0.02 | <0.08* | <0.05* |
| Pb | 0.08 ±0.002 | <0.006* | <0.006* | 0.006 ±0.003 | 0.6 ±0.006 | <0.006* |
The uncertainty values are the expanded uncertainty with an expansion of level about 95% with factor of k = 2. The value of extended uncertainty is given as a range (± U95%).
Ecological characteristic of diatoms acc. [5] most often found in Trzuskawica S.A in 2015. Explanation: sampling i.e.: water biotops 1-7 (see Figs 1-2). pH (R): acidophilous (2), circumneutral (3), alkaliphilous (4), alkalibiontic (5). Salinity (H): fresh (1), fresh brackish (2), brackish fresh (3). Nitrogen uptake metabolism (N): nitrogen-autotrophic taxa (1), nitrogen-autotrophic taxa (2). Oxygen requirements (O): continuously high (1), fairly high (2), moderate (3). Saprobity (S): oligosaprobous (1), p-mesosaprobous (2), α-mesosaprobous (3). Trophic state (T): oligotraphentic (1), oligo-mesotraphentic (2), mesotraphentic (3), meso-eutraphentic (4), eutraphentic (5), oligo-to eutraphentic (7).
| Species | Sampling | R | H | N | O | S | T |
|---|---|---|---|---|---|---|---|
| Amphora copulata (Kutzing) Schoeman & Archibald | 2, 3, 4, 5 | 4 | 2 | 2 | 2 | 2 | 5 |
| Caloneis schumanniana (Grunow) Cl. | 5, 6 | 5 | 2 | 1 | 2 | 1 | 3 |
| Cocconeis placentula Ehr. | 1, 6 | 4 | 2 | 2 | 3 | 2 | 5 |
| Craticula cuspidata (Kutzing) Mann in Round | 5, 6, 7 | 4 | 2 | 2 | 3 | 3 | 5 |
| Cyclotella radiosa (Grunow) Lem. | 2, 3, 4 | 4 | 2 | 1 | 2 | 2 | 5 |
| Cymatopleura solea (Brêb.) W. Smith | 1,2,3,4,5,6,7 | 4 | 2 | 2 | 3 | 2 | 5 |
| Cymbella cymbiformis Agardh | 4, 5, 6 | 3 | 2 | 1 | 1 | 1 | 2 |
| Cymbella lanceolata var. lanceolata (Agardh) Agardh | 4, 5 | 4 | 2 | 1 | 1 | 2 | 7 |
| Cymbopleura inaequalis (Ehr.) Krammer | 4, 6 | 5 | 2 | 1 | 1 | 1 | 3 |
| Ellerbeckia arenaria (Moore ex Ralfs) Crawford | 2, 3 | 4 | 1 | 1 | 1 | 1 | 1 |
| Eunotia arcubus Norpel & Lange-Bertalot | 3, 4, 5, 6 | 3 | 1 | 1 | - | 1 | 2 |
| Eunotia glacialifalsa Lange-Bertalot in Krammer & Lange-Bertalot | 3, 4, 6 | 2 | 1 | 1 | 1 | 1 | 2 |
| Fragillaria parasitica var. parasitica (W. Smith) Grun. in Van Heurck | 4 | 4 | 2 | 1 | 1 | 2 | 4 |
| Gomphonema acuminatum var. acuminatum Ehr. | 4, 5, 6 | 4 | 2 | 1 | 2 | 2 | 5 |
| Gomphonema pala Reichardt | 4, 5, 6, 7 | 4 | 2 | 1 | 2 | 2 | 4 |
| Gyrosigma obtusatum (Sullivant & Wormley) Boyer | 6, 7 | 4 | 2 | 2 | 1 | 2 | 5 |
| Navicula radiosa Kutzing | 4, | 3 | 2 | 2 | 2 | 2 | 4 |
| Navicula upsaliensis (Grun.) Peragallo | 1,2,3,4,5,6,7 | 4 | 2 | - | - | 2 | - |
| Nitzschia angustata (W. Smith) Grun. in Cl. & Grun. | 1,2,3,4,5,6,7 | 3 | 2 | 1 | 1 | 1 | 3 |
| Nitzschia brevissima Grunow in Van Heurck | 5, 6, 7 | 3 | 3 | - | 3 | 2 | 5 |
| Nitzschia denticula Grunow in Cl. & Grun. | 5, 6, 7 | 4 | 2 | 1 | 1 | 2 | 3 |
| Pinnularia viridis (Nitzsch) Ehr. | 4, 5, 6 | 3 | 2 | 2 | 3 | 2 | 7 |
| Ulnaria biceps (Ehrenberg) Compere | 4 | 4 | 2 | - | - | - | 5 |
Element concentrations in two water reservoirs and drainage ditch (in ppm units) using TXRF method were measured in 6 sites (site 2-7) in May and in 6 sites (site 1-5, 7) in September 2015. In May for a technical reason it impossible to carry on the collection from the site 1, whereas in September for the same reason there was no collection from the site 6.
The elements Ca and S in water are used to build the diatoms silica cell walls. The highest Ca concentration (300 ppm) was in site 7 (drainage ditch C) in May, whereas in September the highest (999,4 ppm) was in site 5 (reservoir B), while in site 3 (reservoir A) was the lowest (198 ppm) in May, but in September in site 4 (reservoir A) was the lowest (226.8 ppm). The highest S concentration (43.8 ppm) was in site 4 (reservoir A) in May, whereas in September the highest (45.1 ppm) was in site 3 (reservoir A), while in site 7 (drainage ditch C) was the lowest (38.2 ppm) in May, but in September in site 1 (reservoir A) was the lowest (32.9 ppm).
The P element plays a major role in the structural framework of DNA, RNA, ATP, and the phospholipids, which form cell membranes. Low phosphate levels are an important limit for growth in aquatic systems. However, Sr and alkaline earth metals, are highly reactive chemicals. In groundwater Sr combines the calcium ions, and forms co-precipitates with calcium minerals such as calcite and anhydrite at an increased pH.
Figure 3 presents striking differences for all sites in the elemental concentration for the May, June and September 2015. Samples collected in May are characterized by the lowest level of all 17 elements in two reservoirs and drainage ditch. In June in drainage ditch we observed higher level of Cl, and in remaining sites higher level of S, Br and Sr. In September, in drainage ditch and in reservoir A we did not find such high levels of elements concentration, however, in drainage ditch were large amount of Mn, Fe, Cr, Ti, Pb, Rb, Cu and P.

Principal components analysis (PCA), changes in the content of elements in studied water biotopes in the month (May, June and September).
The discrimination analysis (DA) shows that S, Br, K, Cl and Fe differentiate statistically water biotopes studied (Figure 4). The variables explain 70.5% of the total variance of these elements concentration. In June contents of these elements were highest in reservoir with technological water (B) and in drainage ditch (C), while in September the lowest in reservoir stocked with fish (A) were recorded. Two discriminatory analysis, which separately considered the time of testing and the sampling sites were performed. First analysis considering the sampling sites showed the significance of differentiation in Cr, P and Rb content. These elements together account for 45.9% of the total variability. Higher content of these elements were found in the reservoir with technological water (B), while the Cr (the second ordination axis) was also higher in a drainage ditch (C). The elevated levels of S and Cl in reservoir stocked with fish (A) was not considered significant (Figure 4). The second discriminant analysis present that in May, June and September the collected samples contained different amounts of elements concentration. The S and Cl were significant elements in June sampling, whereas in September were significant amounts of P and Pb (Figs 5-6). None of the elements was significant for May sampling. Elements significantly differentiating studied data explain 75.5% of the total variability.
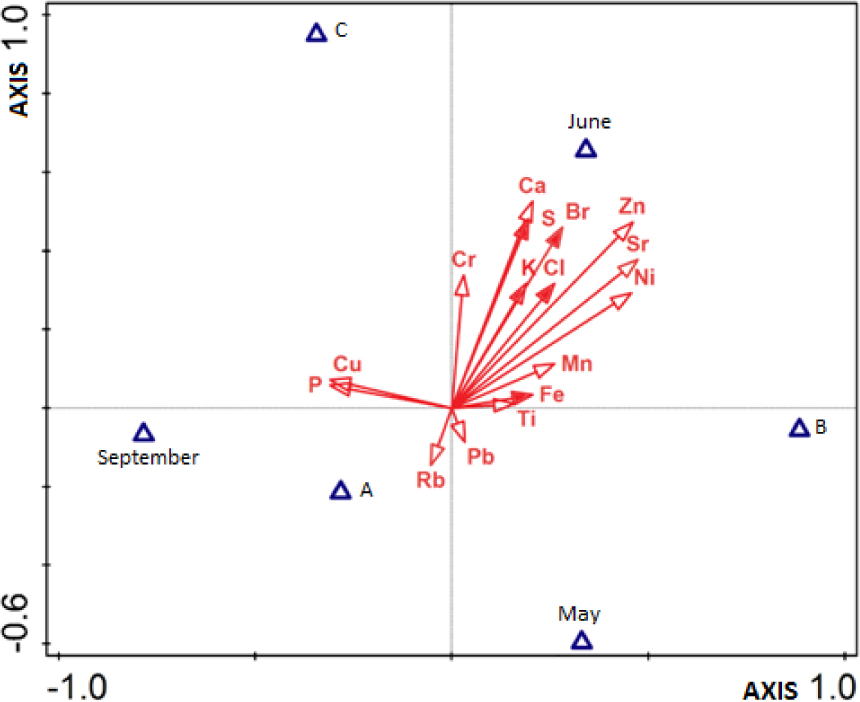
Discriminant analysis (CVA, Canonical Variate Analysis) of data on changes in the content of elements in the studied water biotopes and in the studied months (May, June, September). Elements, which significantly differentiate the test set indicates full returns vectors. It explain 70.5 % of the total variability of harvest.

Discriminant analysis (CVA) of data on changes in the content of elements in the studied water biotopes: water reservoir stocked with fish (A), reservoir of technological water (B), and the drainage ditch of mining area (C). Elements, which significantly differentiate the test set indicates full returns vectors. It explain together 45.9 % of the total variability of harvest.

Discriminant analysis (CVA) of data on changes in the content of elements in the studied months (May, June and September). Elements, which significantly differentiate the test set indicates full returns vectors. It explain together 75.5 % of the total variability of harvest.
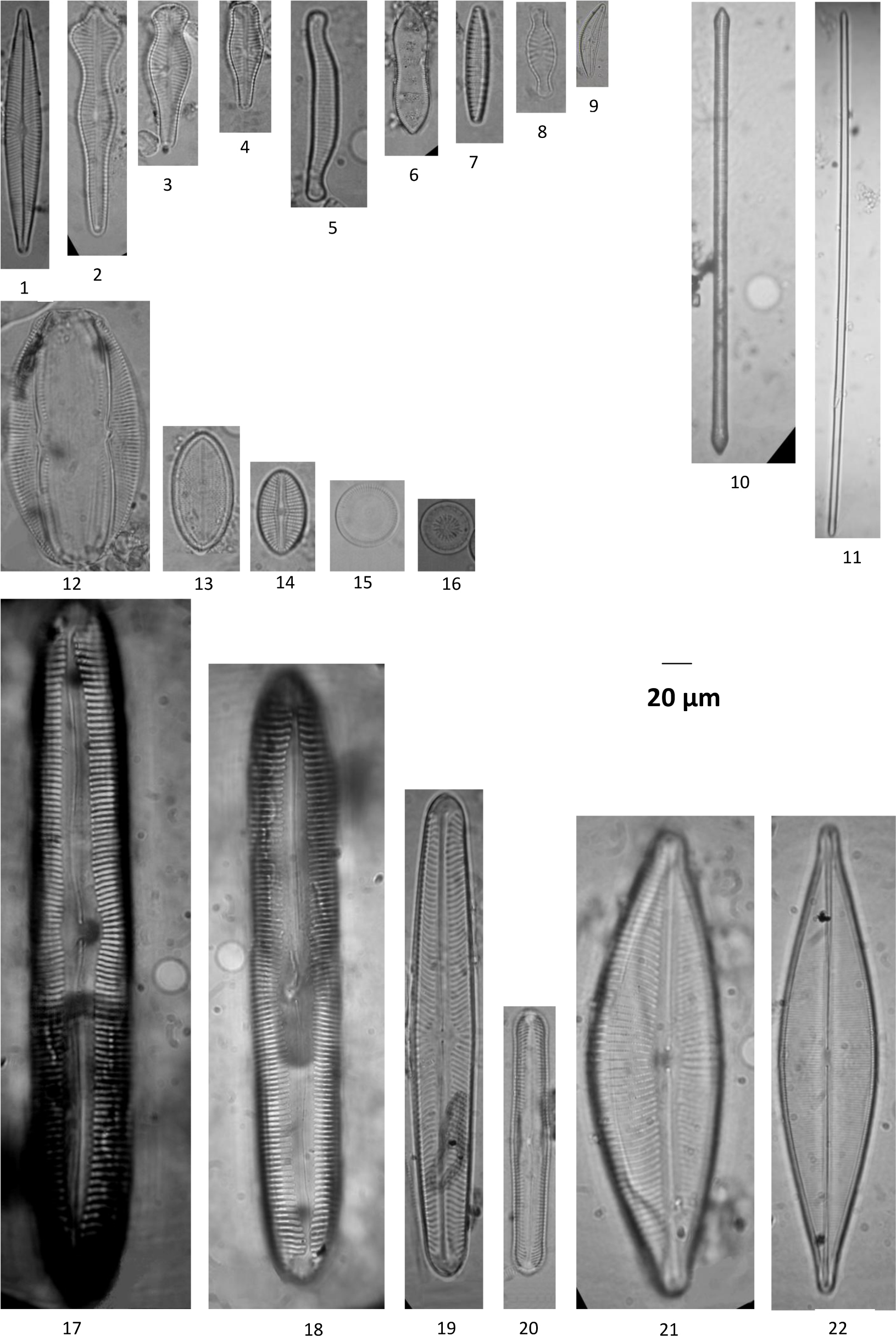
Diatoms in the industrial water biotopes: Navicula angusta (1), Gomphonema acuminatum (2), Gomphonema capitatum (3), Gomphonema pala (4), Eunotia arcubus (5), Cymatopleura solea (6), Nitzschia denticula (7), Planothidium daui (8), Cymbopleura ineaqualis (9), Ulnaria biceps (10), Flagilaria capitata (11), Amphora copulata (12), Cocconeis placentula (13), Cocconeis sp. (14), Ellerbackia arenaria (15), Cyclotella radiosa (16), Pinnularia viridis (17), Pinnularia nobilis (18), Pinnularia divergens (19), Pinnularia gibba (20), Cymbopleura inaequalis (21), Craticula cuspidata (22). All LM micrographs, scale bars = 20 μm.
4 Discussion
Philips [17] and Philips and Rainbow [18] described diatoms as bioindicators of pollution in aquatic environments. These organisms should reflect only contaminants specific to a particular site and found in many different locations ensuring wide geographic relevance [19]. Bioindicators should also be sensitive to specific pollutants and tolerate large concentration of the pollutants in the environment [5]. Mehta and Gaur [20] stated that algae can effectively remove metals from multi-metal solutions and dead cells absorb more metal than live cells. Various pretreatments enhance metal sorption capacity of algae. Algal periphyton has great potential for removing metals from wastewaters. The algae have ability to absorb high concentrations of heavy metals making them a suitable candidate for removing these ions from the wastewaters. Mehta and Gaur [21] suggested that there is a possibility to build an efficient and commercially viable algal technology based upon filling the gap of knowledge concerning metal sorption by algae. The toxic level of heavy metal ions in various algal species can be highly strain specific, which consequently determines the potential remediation capacity using a specific algal strain [22]. However, a heavy metal ion may exhibit a selective interaction with one specific algal strain, in addition to differences between similar species. All heavy metals, including those that are essential micronutrients (e.g., copper, zinc, etc.), are toxic to algae at high concentrations [23].
The concentration of different elements such as: K, Ca, Ti, Mn, Fe, Co, Ni, Br, Sr, Zn, Cu, Pb, and As in Fucus vesiculosus samples using energy-dispersive X-Ray fluorescence (EDXRF) were described by Carvalho et al. [24]. The base of this red alga showed higher concentrations for most elements than other parts, except in the case of As. Whereas in our study using X-ray fluorescence method (TXRF) we found 17 elements, but only 11 elements (K, Ca, Ti, Mn, Fe, Ni, Br, Sr, Zn, Cu, Pb) were the same. While in our samples we identified additionally P, S, Cl, Cr, Rb and Ba, but As and Co were only present in Carvalho et al. [24].
Hernández-Àvila et al. [25] concluded that Si element (the main component of diatom frustule) is not capable of effecting anionic exchange, but with an adequate activation can be used as anionic exchangers, hovewer, the diatoms are used in the treatment of wastewater contaminated with heavy metals.
Despite the toxic influence of high concentrations of heavy metals (e.g., Cd, Cu, Zn, Pb, Hg, Cr, As) on diatoms, some of them are able to inhabit degraded and polluted freshwater of marine and terrestrial environments. The effects of metals on diatom communities have been studied in many polluted watersheds as well as in laboratory experiments [26]. However, Panday et al. [27] suggested that to understand responses of algal communities to metals it is necessary to create application of algal criteria for biomonitoring of these pollutants. Diatoms species richness in metal polluted sites is very low in comparison with unpolluted ones. Usually, in freshwaters and soils common are diatoms, however some species of other taxonomic groups (e.g., coccoid green algae) also occur. Our results suggest that the studied two reservoirs and drainage ditch are specific biotopes of increased heavy metals resistance. Mechanisms of their heavy metal resistance or even tolerance are still poorly known. This resistance of diatoms may be a result of limited metal bioavailability and/or intrinsic morphological and biochemical features of organism Two basic mechanisms may be involved: metal avoidance and intracellular metal detoxification [18]. External cell structures or regulated ion transport system may prevent only some diatom/algae from excessive internal metal accumulation.
It is of interest that electrolytic conductivity optima for diatoms ranged from 25.96 μS cm–1 to 324.76 μS cm–1[28,29]. Most of the diatoms that exhibited highest affinity towards Ca(HCO3)2 water type had low (Diatoma mesodon)to moderate (Nitzschia sinuata) conductivity optima. Diatoms that had highest optima for proportion of Na+ and Cl– either had very low (Eunotia flexuosa) or relatively high conductivity optima. Many diatoms known as acidophilous taxa (Eunotia rhomboidea, E. paludosa) had relatively high optima for concentration of Cl– and K+ and very low conductivity optima [30].
Our studies indicate the importance from both taxonomical and ecological points of view, since our results showed that there may be new possibilities for using the phenotypic plasticity of diatoms to assess water quality and as potential bioindicators of nutrient availability in industrial ecosystems.
5 Conclusions
Diatoms are an important part of industrial water biotopes, playing diverse roles as powerful and reliable environmental indicators. A total of 36 species of diatoms were found in industrial water biotopes (in two reservoirs and drainage ditch) from Trzuskawica S.A. and 17 elements: P, S, Cl, K, Ca, Ti, Cr, Mn, Fe, Ni, Cu, Zn, Br, Rb, Sr, Ba, Pb using X-ray fluorescence method (TXRF) were revealed. An algological study in Trzuskawica S.A. reported in the present paper was,to our knowledge, the first of its type. The presence or absence and distribution of diatom species in industrial water biotopes is dependent on the water chemistry. It was found that total elemental content occurring in waters was positively correlated with diatom species occurrence. Ecological characteristic of diatoms indicate significant number of species prefering alkaline waters (with a large amount of calcium ions). However, some diatom species which have individual chemical and ecological preferences contrary to accepted standards were observed.
Acknowledgements
We would like to thank Łukasz Lechowicz, Jan Kochanowski University, for help in collecting samples. We are also grateful to Anna Wojciechowska, Mikołaj Kopernik University, for her review of the statistical methods and to Ilona Stabrawa, Jan Kochanowski University, for particle size analysis by TXRF.
Conflict of interest: Authors state no conflict of interest.
References
[1] Abel A., Michael A., Zartl A., Werner F., Impact of erosion-transported overburden dump materials on water quality in Lake Cospuden evolved from a former open cast lignite mine south of Leipzig, Germany, Environ. Geol., 2000, 39, 683-688.10.1007/s002540050482Search in Google Scholar
[2] Galas J., Limnological study on a lake formed in a limestone quarry (Kraków, Poland) I. Water Chemistry, Pol. J. Env. St., 2003, 12(3), 297-300.Search in Google Scholar
[3] Stevenson R.J., Pan Y., Van Dam H., Assessing ecological conditions in rivers and streams with diatoms, In: E.F. Stoermer, J.P. Smol (Eds), The Diatoms: Applications to the Environmental and Earth Sciences, 2nd, Cambridge University Press, Cambridge, 2010, 57-85.10.1017/CBO9780511763175.005Search in Google Scholar
[4] Kelly M.G., Use of the trophic diatom index to monitor eutrophication in rivers, Wat. Res., 1998, 32, 236-242.10.1016/S0043-1354(97)00157-7Search in Google Scholar
[5] Van Dam H., Martens A., Sinkeldam J., A coded checklist and ecological indicators values of freshwater diatoms from the Netherlands, Neth. J. Aqua. Ecol. 1994, 28(1), 117-133.10.1007/BF02334251Search in Google Scholar
[6] Johansen J.R., Diatoms of aerial habitats, In: J.P. Smol, E.F. Stoermer (Eds), The Diatoms: Applications for Environmental and Earth Sciences, Cambridge University Press, Cambridge, UK, 2010, 287-308.Search in Google Scholar
[7] Guillard R.P.L., Kilham P., The ecology of marine planktonic diatoms, In Werner D. (Ed), The Biology of Diatoms, Blackwell Sci. Publ., Oxford, 1977, 372-469.Search in Google Scholar
[8] Slate J., Stevenson R.J., Recent and abrupt environmental change in the Florida Everglades indicated from siliceous microfossils, Wetlands 2000, 20, 346-56.10.1672/0277-5212(2000)020[0346:RAAECI]2.0.CO;2Search in Google Scholar
[9] Round F.E., Crawford R.M., Mann D.G., The diatoms: biology and morphology of the Genera, Cambridge, New York, Port Chester, Melbourne, Sydney, Cambridge University Press.,1990, 520-747.Search in Google Scholar
[10] Krammer K., Lange-Bertalot H., Bacillariophyceae 3. Centrales, Fragilariaceae, Eunotiaceae, In: H. Ettl, H. Gerloff, H. Heyning, D. Mollenhauer (Eds), Süßwasserflora von Mitteleuropa, Gustav Fischer Verlag, Stuttgart, Jena, 1991, 2(3), 1–576.Search in Google Scholar
[11] Lange-Bertalot H., Metzeltin D., OligotrophieIndikatoren. 800 Taxa in drei ökologisch diversen Seen-Typen, In: H. Lange-Bertalot (Ed), Iconographia Diatomologica. Annotated Diatom Micrographs 2, Koeltz Scientific Books, Koenigstein, 1996, 1-390.Search in Google Scholar
[12] John D.M., Whitton B.A., Brook A.J., The Freshwater Algal Flora of the British Isles: An identification guide to freshwater and terrestrial algae. Cambridge University Press, Cambridge, 2011, 1-896.Search in Google Scholar
[13] Whitton B.A., Ecology of Cyanobacteria II. Their Diversity in Space and Time: Springer Science+Business Media B.V., 2012, 1-706.10.1007/978-94-007-3855-3Search in Google Scholar
[14] Cole G.A., Textbook of Limnology. Waveland Press, Incorporated, 1994, 1-231.Search in Google Scholar
[15] Teer Braak C.J.F., Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis, Ecol., 1986, 67, 1167-1179.10.2307/1938672Search in Google Scholar
[16] Ter Braak C.J.F., Šmilauer P., CANOCO reference manual and User‘s guide to Canoco for windows: software for canonical community ordination (version 4.5), Microcomputer Power, Ithaca, NY, USA, 2002, 1-500.Search in Google Scholar
[17] Phillips D.J.H., Arsenic in aquatic organisms: A review, emphasizing chemical speciation. Aquatic Toxicology, 1990, 151-186.10.1016/0166-445X(90)90036-OSearch in Google Scholar
[18] Phillips D.J.H., Rainbow P.S., Biomonitoring of trace aquatic contaminants. Barking, New York, Elsevier Applied Science, 1993, 1-371.10.1007/978-94-011-2122-4_1Search in Google Scholar
[19] Melville F., Pulkownik A., Investigation of mangrove macroalgae as biomonitors of estuarine metal contamination. Science of Total Environment, 2007, 387, 301-309.10.1016/j.scitotenv.2007.06.036Search in Google Scholar
[20] Mehta S.K., Gaur J.P., Use of algae for removing heavy metal ions from wastewater: progress and prospects. Critical Reviews in Biotechnology, 2005, 25, 113-152.10.1080/07388550500248571Search in Google Scholar PubMed
[21] Mehta S.K., Gaur J.P., Heavy-metal-induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. The New Phytologist, 1999, 143, 253-259.10.1046/j.1469-8137.1999.00447.xSearch in Google Scholar
[22] Zeraatkar A.K., Ahmadzadeh H., Talebi A.F., Moheimani N.R., McHenry M.P., Potential use of algae for heavy metal bioremediation, a critical review. Journal of Environmental Management, 2016, 181, 817-831.10.1016/j.jenvman.2016.06.059Search in Google Scholar PubMed
[23] Rai L.C., Gaur J.P., Kumar H.D., Phycology and heavy-metal pollution. Biological Reviews, 1981, 56, 2, 99-151.10.1111/j.1469-185X.1981.tb00345.xSearch in Google Scholar
[24] Carvalho M.L., Ferreira J.G., Amorim P., Marques M.I.M., Ramos M.T., Study of heavy metals and other elements in macrophyte algae using energy-dispersive X-Ray fluorescence. 2015, 1-31.Search in Google Scholar
[25] Hernández-Ávila J., Salinas-Rodríguez E., Cerecedo-Sáenz E., Reyes-Valderrama M.I., Arenas-Flores A., Román-Gutiérrez A, Rodríguez-Lugo V. Diatoms and their capability for heavy metals removal by cationic exchange. Metals, 2017, 2-13.10.3390/met7050169Search in Google Scholar
[26] Morin S., Cordonier A., Lavoie I., Arini A., Blanco S., Duong T., Tomés E., Bonet B., Corcoll N., Faggiano L., Laviale M., Pérès F., Becares E., Coste M., Feurtet-Mazel A., Fortin C., Guasch H., Sabater S., Consistency in diatom response to metal-contaminated environments emerging and priority pollutants in rivers, In: H. Guasch, A. Ginebreda, A. Geiszinger (Eds), The Handbook of Environmental Chemistry, Berlin, Heidelberg, Springer, 2012, 117-146.10.1007/978-3-642-25722-3_5Search in Google Scholar
[27] Pandey L.K., Kumar D., Yadav A., Rai J., Gaur J.P., Morphological abnormalities in periphytic diatoms as a tool for biomonitoring of heavy metal pollution in a river. Ecological Indicators, 2014, 36, 272-279.10.1016/j.ecolind.2013.08.002Search in Google Scholar
[28] Bere T., Tundisi J.G., Biological monitoring of lotic ecosystems: the role of diatoms. Brazilian Journal of Biology, 2010, 70, 3, 493-502.10.1590/S1519-69842010005000009Search in Google Scholar PubMed
[29] Remy F., Darchambeau F., Melchior A., Lepoint G., Impact of food type on respiration, fractionation and turnover of carbon and nitrogen stable isotopes in the marine amphipod Gammarus aequicauda (Martynov, 1931). Journal of Experimental Marine Biology and Ecology, 2017, 486, 358-367.10.1016/j.jembe.2016.10.031Search in Google Scholar
[30] Rémy M., Berthon V., Castets V., Rimet F., Thiers A., Labat F., Fontan B., Modelling diatom life forms and ecological guilds for river biomonitoring. Knowledge & Management of Aquatic Ecosystems, 2017, 418, 1-15.10.1051/kmae/2016033Search in Google Scholar
© 2018 Agnieszka Malinowska-Gniewosz et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
Articles in the same Issue
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution

