Abstract
Foaming agents, despite holding potential in steam injection technology for heavy oil recovery, are still poorly investigated. In this work, we analyzed the performance of the foaming agent NPL-10 in terms of foam height and half-life under various conditions of temperature, pH, salinity, and oil content by orthogonal experiments. The best conditions of use for NPL-10 among those tested are T=220°C, pH 7, salinity 10000 mg·L–1 and oil content 10 g·L–1. Thermal decomposition of NPL-10 was also studied by thermogravimetric and differential thermal analyses. NPL-10 decomposes above 220°C, and decomposition is a two-step process. The kinetic triplet (activation energy, kinetic function and pre-exponential factor) and the corresponding rate law were calculated for each step. Steps 1 and 2 follow kinetics of different order (n = 2 and ½, respectively). These findings provide some criteria for the selection of foaming agents for oil recovery by steam injection.
1 Introduction
The extraction of heavy crude oil from reservoirs is usually carried out by steam injection. There are several forms of the technology, with the major ones being steam stimulation, steam flooding and steam-assisted gravity drainage [1,2]. The main challenge in the use of steam for heavy oil recovery is steam channeling, i.e., the ability to make steam flow in a single direction (from the injector to the extractor) in such a way as to maximize the extraction yield. Another problem posed by steam injection is that steam can seriously damage the underground well structure. This phenomenon can endanger the lives of workers while resulting into thermal dispersion and poor mining effect [3, 4, 5]. Heavy oil reservoirs in China are quite heterogeneous in terms of layer permeability, thus requiring highly different conditions for optimal steam channeling [4, 5]. Foams can improve steam channeling and enhance oil recovery by reducing the surface tension and viscosity of fluids [6, 7, 8, 9]. However, the use of foaming agents in heavy oil recovery is still limited due to the small number of studies on the behavior of such agents at high temperature and salinity, which prevents the selection of the most appropriate agent.
In this work, we analyzed the performance of a high temperature foaming agent (NPL-10) in terms of foam height and half-life in order to establish the optimal conditions of its use. The thermal decomposition process of NPL-10 was analyzed by thermogravimetric and differential thermal analyses, and the kinetics of non-isothermal decomposition was studied to quantify the effect of temperature on decomposition rate.
2 Experimental
2.1 Chemicals
Test solutions for orthogonal experiments were prepared using deionized water. Three solutions with different total concentrations (5000, 10000 and 15000 mg·L–1) were used. Solutions had the following composition: 5000 mg·L–1 solution:NaHCO33000mg·L–1, NaCl700mg·L–1;10000mg·L–1 solution: NaHCO3 6000 mg·L–1, NaCl 1400 mg·L–1; 15000 mg·L–1 solution: NaHCO3 9000 mg·L–1, NaCl 2100 mg·L–1. Concentrations of other ions were simulated by a water preparation and salinity calculation software. Heavy oil was collected from Du-66 block area of Liaohe Oilfield and dehydrated before use. Foaming agent NPL-10 (long-chain alpha olefin sulfonate) was prepared according to literature [10, 11], and had purity > 97%.
2.2 Methods
Reactions were performed in a high temperature, high pressure reaction kettle WYF-1 supplied by Haian Jiangsu oil factory. Foamability and foam stability were determined by a 2151 Roche foam instrument (standard form) using a customized GB/T 7462-94 Ross-Miles method. Thermogravimetric and differential thermal analyses were carried out by a thermal analyzer DuPont 2100 (Perkin Elmer, USA) under nitrogen.
Ethical approval
This research is not related to human or animal use.
3 Results and discussion
3.1 Performance analysis of NPL-10
We studied the effect of four factors (temperature, salinity, pH, and oil content) on the foam height and half-life of NPL-10 by orthogonal experiments [12, 13, 14]. The height and half-life of NPL-10 foam were measured under various combinations of T (220, 260, and 300°C), pH (5, 7, and 9), salinity (5000, 10000, and 15000 mg·L–1) and oil content (0, 5, and 10 g·L–1; Table 1). The sensitivity of foam height and half-life to each factor was examined by a trend chart (Figure 1). Results showed that foam height and half-life depend on temperature (A), pH (B), salinity (C) and oil content (D) to a different extent (Figure 1). At 220°C, the order of factors observed for foam height is A>B>C>D; in other words, foam height mainly depends on temperature. Foam height decreases sharply on increasing temperature, and the most marked decrease is observed when temperature rises from 260°C to 300°C. Conversely, an increase in the other factors (pH, salinity and oil content) has almost no effect on foam height.
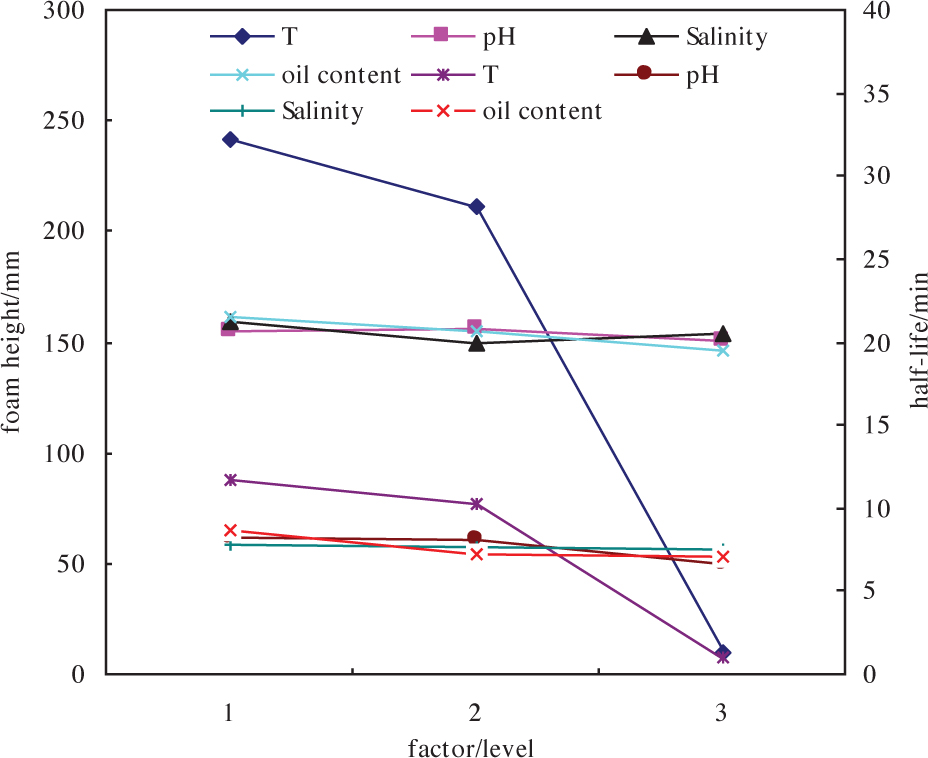
NPL-10 trend chart of factors at three different levels. Level 1 = 220°C, level 2 = 260°C; level 3 = 300°C. Top lines (dark blue, light blue, black and pink): effect on foam height. Bottom lines (violet, red, brown, green): effect on foam half-life.
Orthogonal experimental data for foam height and half-life.
| factors A | B | C | D | height/half-life | |
|---|---|---|---|---|---|
| entry | T (°C) | pH | salinity (mg·L–1) | oil density (g.L–1) | (mm/min) |
| 1 | 220 | 5 | 5000 | 0 | 255/13.5 |
| 2 | 220 | 7 | 10000 | 5 | 240/11.67 |
| 3 | 220 | 9 | 15000 | 10 | 230/10 |
| 4 | 260 | 5 | 10000 | 10 | 200/10.2 |
| 5 | 260 | 7 | 15000 | 0 | 220/11.5 |
| 6 | 260 | 9 | 5000 | 5 | 213/9 |
| 7 | 300 | 5 | 15000 | 5 | 11/1.01 |
| 8 | 300 | 7 | 5000 | 10 | 8/0.9 |
| 9 | 300 | 9 | 10000 | 0 | 9/0.93 |
| K1 | 725/35.17 | 466/24.17 | 476/23.4 | 484/25.93 | |
| K2 | 633/30.7 | 468/24.07 | 449/22.8 | 464/21.68 | |
| K3 | 28/2.84 | 452/19.39 | 461/22.8 | 438/21.1 | |
| k1 | 241.67/11.72 | 155.33/8.23 | 158.67/7.8 | 161.33/8.64 | |
| k2 | 211/10.23 | 156/8.02 | 149.67/7.6 | 154.67/7.23 | |
| k3 | 9.33/0.95 | 150.67/6.64 | 153.67/7.5 | 146/7.03 | |
| Range R | 232.33/10.78 | 5.34/1059 | 9/0.3 | 15.33/1.61 | |
| Secondary sequence | A>B>C>D / A>D>B>C | ||||
| Optimized combination | A1B2C2D3 | ||||
K1, K2, K3 are the sum of the level 1, 2, and 3 data on the corresponding factors, respectively. k1, k2, k3 are the comprehensive average of 1, 2, and 3 level data, respectively.
The order of factors observed for foam half-life at 220°C is A>D>C>B. The most important factor for foam half-life is still temperature, but the second one is the oil content, not pH. Analogously to foam height, foam halflife decreases significantly on increasing temperature but is not significantly influenced by other factors.
3.2 Thermal decomposition of NPL-10
After determining the best conditions of use for NPL-10, we studied its thermal decomposition process by non-isothermal thermogravimetric analysis (TGA) and differential thermal analysis (DTA) [15, 16, 17, 18].
3.2.1 Non-isothermal thermogravimetric analysis (TGA) and differential thermal analysis (DTA)
Non-isothermal thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were carried out at three different heating rates (5, 15, and 20 K·min–1). TGA indicated that, regardless of heating rate, NPL-10 starts decomposing at about 250°C, and decomposition is complete at about 500°C (Figure 2). DTA highlighted that thermal decomposition is a two-step process: the first decomposition step occurs at 200–300°C, with a mass loss of 4–26%; the second step occurs at 300–400°C, with 15–59% mass loss (Figure 3). Both steps are endothermic. Furthermore, both the rate of mass loss and the peak temperature of thermal decomposition gradually increase on increasing the heating rate.
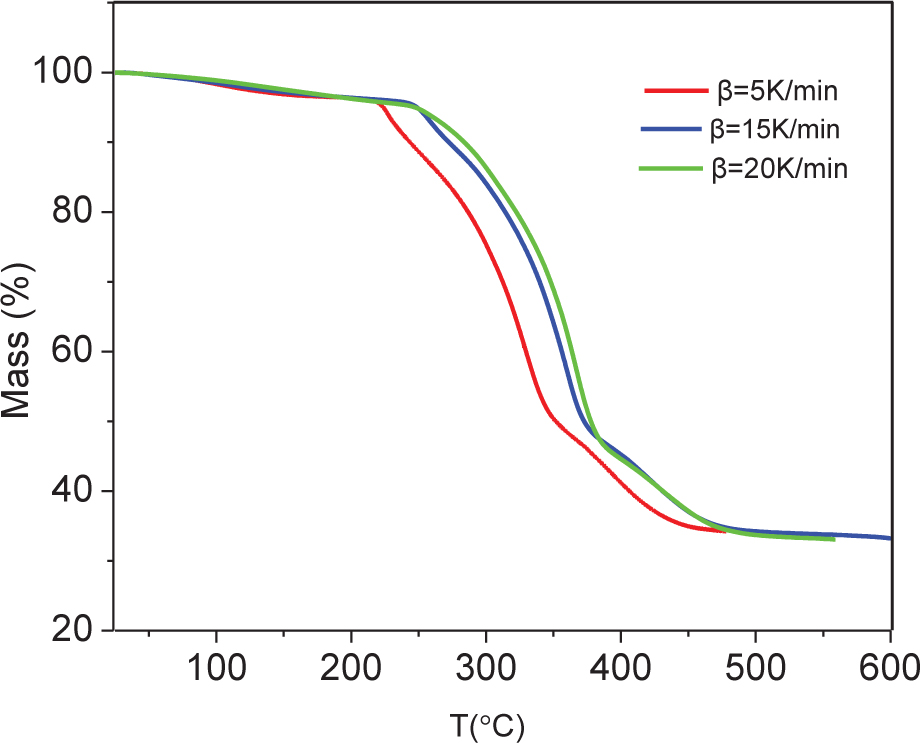
Thermogravimetric analysis (TGA) curve of NPL-1.
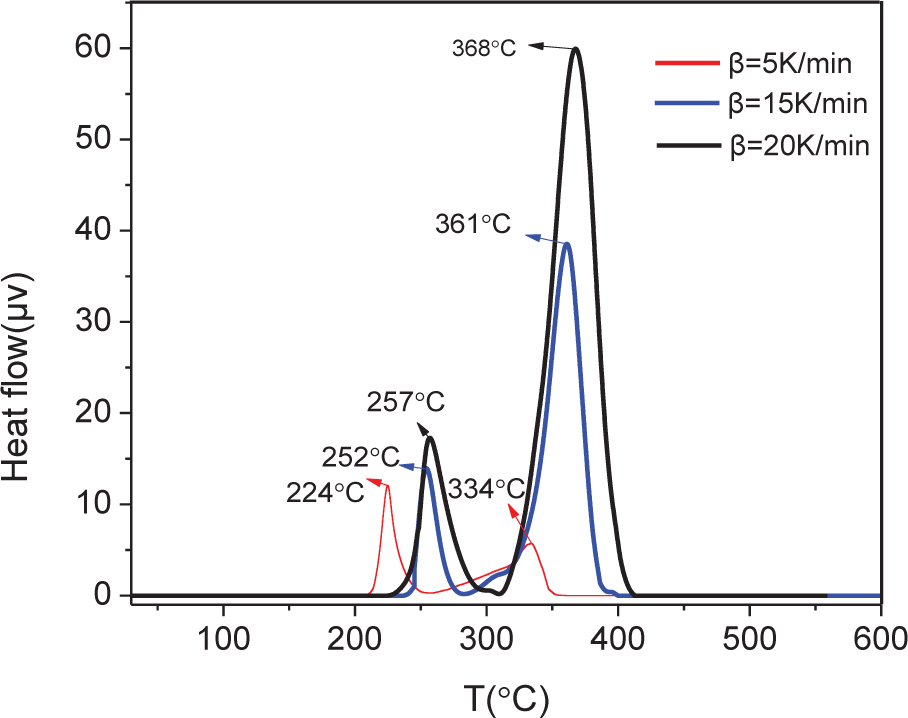
Differential thermal analysis (DTA) curve of NPL-10.
3.2.2 Kinetics of thermal decomposition reaction
The thermal decomposition rate can be expressed by Eq. (1) as a function of two independent variables: the transformation rate α and temperature T [19]:
where T (K) is the absolute temperature of sample, α (%) is the decomposition rate, and k is the rate constant for the decomposition reaction. The heating rate β is constant in GTA, and DTA, and is expressed by Eq. (2):
The rate constant k depends on temperature T according to the Arrhenius equation [20]:
where A is the pre-exponential factor (s–1), Ea is the activation energy (J·mol–1), and R is the gas constant (8.314 J·mol–1·K–1).
Combining Eqs. (1), (2) and (3) provides Eq. (4), which is the rate law for the decomposition reaction:
We calculated the kinetic triplet (the activation energy Ea, the pre-exponential factor A, and the mechanism function g(α)) for both steps of NPL-10 decomposition process.
The activation energy Ea was obtained by Eq. (5) using KAS iterative method:
where
and
KAS iterative method is divided into three steps: ① Assume h(x)=1 to estimate the initial activation energy E1 for given β and g(α) values by Eq. (5). ② Bring E1 and the corresponding T into Eq. (7), then use the calculated x value to derive h(x) by Eq. (6); enter x and h(x) values into Eq. (5), and use the linear regression of ln[β/h(x)T2] vs 1/T to calculate the line slope (–E2/R) and hence E2. ③ Repeat step ② by replacing E1 by E2. When |Ei–EM| < 0.01 kJ·mol–1, Ei can be considered a real value.
The activation energy Ea was calculated for α = 0.2–0.8 at 0.05-unit intervals (Table 2). A plot of the activation energy Ea as a function of a for steps 1 and 2 (Figure 4) shows two curves of different shape, indicating that decomposition follows a different mechanism in steps 1 and 2. The non-linearity of curves suggests a complex mechanism for both steps.
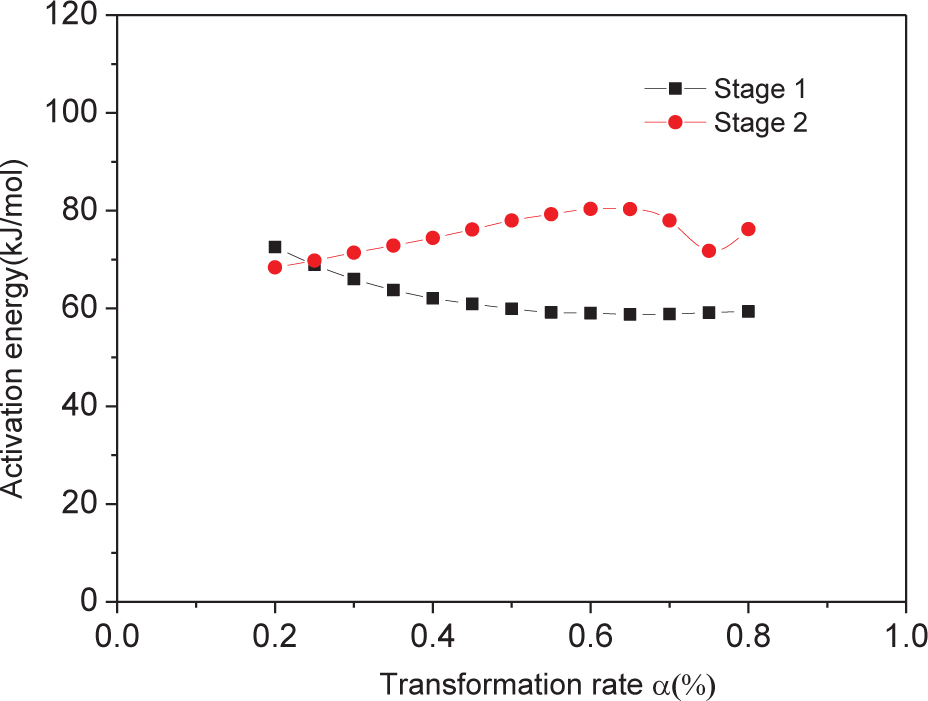
Line chart for thermal decomposition of NPL-10.
Activation energies calculated by KAS iterative method.
| Ea (kJ·mol–1) | ||
|---|---|---|
| α (%) | step 1 | step 2 |
| 0.2 | 72.56 | 68.41 |
| 0.25 | 68.94 | 69.79 |
| 0.3 | 66.02 | 71.41 |
| 0.35 | 63.78 | 72.87 |
| 0.4 | 62.07 | 74.44 |
| 0.45 | 60.95 | 76.16 |
| 0.5 | 59.95 | 78.00 |
| 0.55 | 59.21 | 79.28 |
| 0.6 | 59.05 | 80.37 |
| 0.65 | 58.78 | 80.34 |
| 0.7 | 58.86 | 78.00 |
| 0.75 | 59.16 | 71.79 |
| 0.8 | 59.40 | 76.24 |
| average value | 62.21 | 75.16 |
To determine the most probable kinetic functior g(α) for the thermal decomposition of NPL-10, we performed the linear regressen anaiyais of kinetic functions (Table 3) [18]. Eq. (5) was modified into Eq. (6):
Linear regression analysis of 41 kinetic functions for steps 1 and 2.
| entry | step 1 | step 2 | |||
|---|---|---|---|---|---|
| g(α) | slope | R2 | slope | R2 | |
| 1 | α2 | –3.0968 | 0.9339 | –1.8111 | 0.8884 |
| 2 | α+(1–α)ln(1–α) | –3.507 | 0.9522 | –2.1661 | 0.918 |
| 3 | [1–(1–α)1/2]1/2 | –0.4148 | 0.9984 | –2.1661 | 0.918 |
| 4 | [1–(1–α)1/2]2 | –0.4148 | 0.9984 | –0.3096 | 0.9576 |
| 5 | [1–(1–α)1/3]1/2 | –0.2479 | 0.9997 | –0.1897 | 0.9669 |
| 6 | [1–(1–α)1/3]2 | –0.4959 | 0.9997 | –0.3794 | 0.9669 |
| 7 | 1–2/3α–(1–α)2/3 | 0.4612 | 0.9765 | 0.4244 | 0.9981 |
| 8 | [(1+α)1/3–1]2 | 0.8862 | 0.9969 | 0.7346 | 0.9927 |
| 9 | [(1–α)1/3–1]2 | –3.6837 | 0.9395 | –3.9547 | 0.8843 |
| 10 | [–ln(1–α)]–1/4 | –2.4013 | 0.9836 | –1.6665 | 0.9699 |
| 11 | [–ln(1–α)]–1/3 | –2.4013 | 0.9836 | –1.6665 | 0.9699 |
| 12 | [–ln(1–α)]–2/3 | –4.8026 | 0.9836 | –3.3331 | 0.9699 |
| 13 | [–ln(1–α)]1/2 | –2.4013 | 0.9836 | –1.6665 | 0.9699 |
| 14 | [–ln(1–α)]2/3 | –4.8026 | 0.9836 | –3.3331 | 0.9699 |
| 15 | [–ln(1–α)]3/4 | –7.2039 | 0.9836 | –4.9996 | 0.9698 |
| 16 | –ln(1–α) | –2.4013 | 0.9836 | –1.6665 | 0.9699 |
| 17 | [–ln(1–α)]3/2 | –7.2039 | 0.9836 | –4.9996 | 0.9699 |
| 18 | [–ln(1–α)]2 | –4.8026 | 0.9836 | –3.3331 | 0.9699 |
| 19 | [–ln(1–α)]3 | –7.2039 | 0.9836 | –4.9996 | 0.9699 |
| 20 | [–ln(1–α)]4 | –9.6053 | 0.9836 | –6.6661 | 0.9699 |
| 21 | ln[α/(1–α)] | – | – | – | – |
| 22 | α1/4 | –1.5484 | 0.9339 | –0.9056 | 0.5324 |
| 23 | α1/3 | –1.5484 | 0.9339 | –0.9056 | 0.6583 |
| 24 | α1/2 | –1.5484 | 0.9339 | –0.9056 | 0.9984 |
| 25 | 1–(1–α)1/1=α | – | – | – | – |
| 26 | α3/2 | –4.6452 | 0.9339 | –2.7167 | 0.8884 |
| 27 | α2 | –3.0968 | 0.9339 | –1.8111 | 0.8884 |
| 28 | 1–(1–α)1/4 | –0.1773 | 1 | –0.137 | 0.9705 |
| 29 | 1–(1–α)1/3 | –0.2479 | 0.9997 | –0.1897 | 0.9669 |
| 30 | 3[1–(1–α)1/3] | –0.2479 | 0.9997 | –0.1897 | 0.9669 |
| 31 | 1–(1–α)1/2 | –0.4148 | 0.9984 | –0.3096 | 0.9576 |
| 32 | 2[1–(1–α)1/2] | –0.4148 | 0.9984 | –0.3096 | 0.9576 |
| 33 | 1–(1–α)2 | –1.1053 | 0.8649 | –0.5383 | 0.7757 |
| 34 | 1–(1–α)3 | –0.8504 | 0.7976 | –0.3491 | 0.6769 |
| 35 | 1–(1–α)4 | –0.6831 | 0.738 | –0.239 | 0.6033 |
| 36 | (1–α)–1 | –2.2862 | 0.9812 | –2.08 | 0.9951 |
| 37 | (1–α)–1–1 | –3.8346 | 0.9994 | –2.9855 | 0.9966 |
| 38 | (1–α)–1/2 | –2.2862 | 0.9812 | –2.08 | 0.9951 |
| 39 | Inα | – | – | – | – |
| 40 | lnα2 | – | – | – | – |
| 41 | (1–α)–2 | –4.5724 | 0.9812 | –4.16 | 0.9951 |
where
Linear regression analysis did not provide a straight line for four functions, namely 21 (dependent on pressure in nddhion to temperature, 25 (tew of Mampel Pewer), 39 and 40 (both functions of index law). The kinetic functions with the slope closest to –1 and best R2 values were g(α)= 1–(1–α)2 (Table 3, entry 33) for step 1 and g(α)= α1/2 for step 2 of thermal decomposition (Table 3, entry 31). These results indicate that the two steps of thermal decomposition follow a different order of reaction (n=2 for step 1 and n=1/2 for step 2).
Pre-exponential factors A were calculated by entering Ea values (Table 2) and g(α) values (Table 3) into Eq. (5). The pre-exponential factor of step 1 is A1=3.86·1011s–1, the pre-exponential factor of step 2 is A2=5.60·1013 s–1. Calculation of Ea, g(α), and A allowed to derive the rate law of the decomposition process. The rate law for the first step of thermal decomposition is:
the rate law for the second step is:
4 Conclusions
The optimal conditions (in terms of both foam height and half-life) for the use of foaming agent NPL-10 in heavy oil recovery by steam injection are the following: T=220°C, pH 7, 10000 mg·L–1 salinity, and 10 g·L–1 oil content;
NPL-10 starts decomposing above 220°C;
Decomposition of NPL-10 occurs in two steps that follow a different kinetic law, with the rate of the first step being more sensitive to temperature than the rate of the second step.
The derived kinetic model provides a guidance for the selection of the optimal quantity of NPL-10 in heavy oil recovery by steam injection.
Acknowledgment
this work was supported by the Natural Science Foundation of Heilongjiang Province of China (Project No. E2015036), the National Science and Technology Major Projects of China for Oil and Gas (Projects No. 2016ZX05055-006 and 2016ZX05012- 001), and the Cultivation Fund of Northeast Petroleum University of China.
Conflict of interest: Authors state no conflict of interest.
References
[1] Koottungal L., Special Report 2010 worldwide EOR survey. Oil & Gas Journal, 2010, 108(14), 41-53.Search in Google Scholar
[2] Kamari A., Nikookar M., Sahranavard L., et al., Efficient screening of enhanced oil recovery methods and predictive economic analysis. Neural Computing and Applications, 2014, 25(3-4), 815-824.10.1007/s00521-014-1553-9Search in Google Scholar
[3] Hiraski G.J., The steam-foam process. Journal of Petroleum Technology, 1989, 41(05), 449-456.10.2118/19505-PASearch in Google Scholar
[4] Ma K., Lopez-Salinas J. L., Puerto M. C., et al., Estimation of parameters for the simulation of foam flow through porous media. Part 1: the dry-out effect. Energy & Fuels, 2013, 27(5), 2363-2375.10.1021/ef302036sSearch in Google Scholar
[5] Farajzadeh R., Krastev R., Zitha P.L.J., Foam films stabilized with alpha olefin sulfonate (AOS). Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 324(1), 35-40.10.1016/j.colsurfa.2008.03.024Search in Google Scholar
[6] Farajzadeh R., Andrianov A., Krastev R., et al., Foam–oil interaction in porous media: Implications for foam assisted enhanced oil recovery. Advances in colloid and interface science, 2012, 183, 1-13.10.2118/154197-MSSearch in Google Scholar
[7] Ma K., Lopez-Salinas J.L., Puerto M.C., et al., Estimation of parameters for the simulation of foam flow through porous media. Part 1: the dry-out effect. Energy & Fuels, 2013, 27(5), 2363-2375.10.1021/ef302036sSearch in Google Scholar
[8] Worthen A.J., Bryant S.L., Huh C., et al., Carbon dioxide-in-water foams stabilized with nanoparticles and surfactant acting in synergy. AlChE Journal, 2013, 59(9), 3490-3501.10.1002/aic.14124Search in Google Scholar
[9] El-Amin M.F., Salama A., Sun S., Numerical and dimensional analysis of nanoparticles transport with two-phase flow in porous media. Journal of Petroleum Science and Engineering, 2015, 128, 53-64.10.1016/j.petrol.2015.02.025Search in Google Scholar
[10] Keijzer P.P.M., Muijs H.M., Janssen-van Rosmalen R., et al., Application of Steam Foam in the Tia Juana Field, Venezuela:Laboratory Tests and Field Results. SPE Enhanced Oil Recovery Symposium, 20-23 April, Tulsa, Oklahoma, 1986, SPE-14905.10.2118/14905-MSSearch in Google Scholar
[11] Maini B.B., Ma V., Thermal Stability of Surfactants for Steamflood Applications. SPE Oilfield and Geothermal Chemistry Symposium, 9-11 March, Phoenix, Arizona,1985, SPE-13572.10.2118/13572-MSSearch in Google Scholar
[12] Kleijnen J.P.C., Sensitivity analysis and related analyses: a review of some statistical techniques. Journal of Statistical Computation and Simulation, 1997, 57(1-4), 111-142.10.1080/00949659708811805Search in Google Scholar
[13] Tang B., Orthogonal array-based Latin hypercubes. Journal of the American statistical association, 1993, 88(424), 1392-1397.10.1080/01621459.1993.10476423Search in Google Scholar
[14] Kleijnen J.P.C., An overview of the design and analysis of simulation experiments for sensitivity analysis. European Journal of Operational Research, 2005, 164(2), 287-300.10.1016/j.ejor.2004.02.005Search in Google Scholar
[15] Einaga H., Futamura S., Ibusuki T., Heterogeneous photocatalytic oxidation of benzene, toluene, cyclohexene and cyclohexane in humidified air: comparison of decomposition behavior on photoirradiated TiO2 catalyst. Applied Catalysis B: Environmental, 2002, 38(3), 215-225.10.1016/S0926-3373(02)00056-5Search in Google Scholar
[16] Patel P., Hull T.R., McCabe R.W., et al., Mechanism of thermal decomposition of poly(ether ether ketone)(PEEK) from a review of decomposition studies. Polymer Decomposition and Stability, 2010, 95(5), 709-718.10.1016/j.polymdegradstab.2010.01.024Search in Google Scholar
[17] Zhao Z., Chaos M., Kazakov A., et al., Thermal decomposition reaction and a comprehensive kinetic model of dimethyl ether. International Journal of Chemical Kinetics, 2008, 40(1), 1-18.10.1002/kin.20285Search in Google Scholar
[18] Robledo-Ortiz J.R., Zepeda C., Gomez C., et al., Non-isothermal decomposition kinetics of azodicarbonamide in high density polyethylene using a capillary rheometer. Polymer Testing, 2008, 27(6), 730-735.10.1016/j.polymertesting.2008.05.004Search in Google Scholar
[19] Kandelbauer A., Wuzella G., Mahendran A., et al., Model-free kinetic analysis of melamine–formaldehyde resin cure. Chemical Engineering Journal, 2009, 152(2), 556-565.10.1016/j.cej.2009.05.027Search in Google Scholar
[20] Ptáček P., Bartoníčková E., Švec J., et al., The kinetics and mechanism of thermal decomposition of SrCO3 polymorphs. Ceramics International, 2015, 41(1), 115-126.10.1016/j.ceramint.2014.08.043Search in Google Scholar
© 2018 Fa-Jun Zhao et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
Articles in the same Issue
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution

