Abstract
UV absorbers developed for finishing of textile materials play a significant role in protection against UV radiations but their discharge in wastewater during processing and laundry action also retain serious concern to living species due to their recalcitrant nature. The current study examined the mineralization and degradation of two vinylsulfone and nitrogen (N-) containing UV absorber compounds (1a, 2a) via two effective Fenton and UV/H2O2 oxidation. The results showed that both the Fenton and UV/H2O2 processes mineralized the synthesized UV absorbers effectively; however the mineralization process with Fenton oxidation was more effective than the UV/H2O2. The mineralization of synthesized UV absorbers was affected by process parameters (dosage of Fe2+ and H2O2 pH and reaction time). Under attained optimum conditions of Fenton oxidation, dose of Fe2+ (15 mg/L), H2O2 (500 mg/L), pH (3.0) and contact time (120 minutes), 75.43 and 77.54% of Chemical Oxygen Demand removal was achieved for 1a and 2a, respectively. Whereas, the optimum conditions of UV/H2O2 process were H2O2 (700 mg/L), pH(3.0) and irradiation time (200 minutes) that brought 54.33 and 57.65% COD removal in case of 1a and 2a, respectively. The results indicated that the Fenton oxidation can be successfully employed for the mineralization of triazine based UV absorbers.
1 Introduction
A number of different synthetic dyes, finishes and detergents have been employed frequently in several industries including textile [1, 2], leather [3], automobiles [4], food and beverage [5], pharmaceutical [6], furniture [7], paper industry [8] and many more. They are preferred over naturally existing dyes and finishes due to their availability in various attractive shades, good wash and light fastness [9] and superior properties too [10, 11, 12]. However, considerable health issues are connected with them due to their recalcitrant nature [13, 14], high molecular weight and complex structures [2].
Consequently, a huge amount of these chemicals can be found in industrial effluents, changing the taste, odor and color of water [15], and reacting with many heavy metals to form stable complexes [16]. Therefore, industrial waste comprises of huge quantities of organic pollutants. They represent high chemical oxygen demand (COD)/total organic carbon (TOC) values [13] along with low biodegradability of industrial effluents [1]. Hence, their discharge into water bodies without any prior treatment causes serious health issues for aquatic and terrestrial life [17] due to their toxic and carcinogenic properties [18].
UV absorbers are excessively utilized in sunscreens [19] and on clothing [10] to protect us from the damages of UV radiations. Many organic UV absorbers incorporated in sunscreens, such as 2-phenylbenzimdazole-5-sulfonic acid (PBSA) [20] and monochlorotriazine based UV absorbers [10], to combat with UV light. The presence of SO3H enhanced the photostablity of PBSA due to extended conjugation, but due to their high molecular weight and complex structure, their presence in effluents also generates high toxicity to biosphere [20].
Many physical, biological and chemical treatment methods are reported in literature, dealing with pollutants in industrial wastewater, such as precipitation, adsorption [21], membrane separation [22], treatment with bacteria [23] and oxidation [24]. All these methods convert pollutants in industrial effluents to smaller toxic molecules, resulting in incomplete mineralization [25]. Oxidation of organic pollutants by advance oxidation processes (AOPs) have been utilized to degrade a large variety of organic contaminates like dyes [26], pesticides [27] and pharmaceuticals [28].
The above processes produce hydroxyl radicals that are highly reactive with electrical potential 2.8 V and the organic substances are completely mineralized [13]. The combination of H2O2 with Fe2+ and UV radiation is characterized by a reduced cost and a high efficiency for removing non-biodegradable organic contaminants from water [24, 29]. The decomposition of hydrogen peroxide with Fe2+ and UV light generate reactive species such as OH- and •OH [30, 31, 32] (equation 1-3).
In the current investigation, two new reactive hetrofunctional vinyl sulfone and nitrogen (N-) containing organic UV absorber compounds (1a, 2a) that were designed and synthesized in our previous studies [33] were selected for mineralization. The degradation study of these UV absorbers has been not reported yet. The comparative degradation study was performed by Fenton and UV/H2O2 processes. The influencing parameters of processes were optimized to improve the degradation process.
2 Experimental
2.1 Chemicals and reagents
Both UV absorbers (1a-2a) were synthesized in the laboratory and their structures were confirmed with spectroscopic techniques. Structures of these UV absorbers are shown in Figure 1. H2O2 (30% solution w/w), FeSO4.7H2O (99%), Na2SO3, H2SO4 (1M) and NaOH (1M) were of analytical grade and purchased from Sigma Aldrich. All of the solutions were made in distilled water.
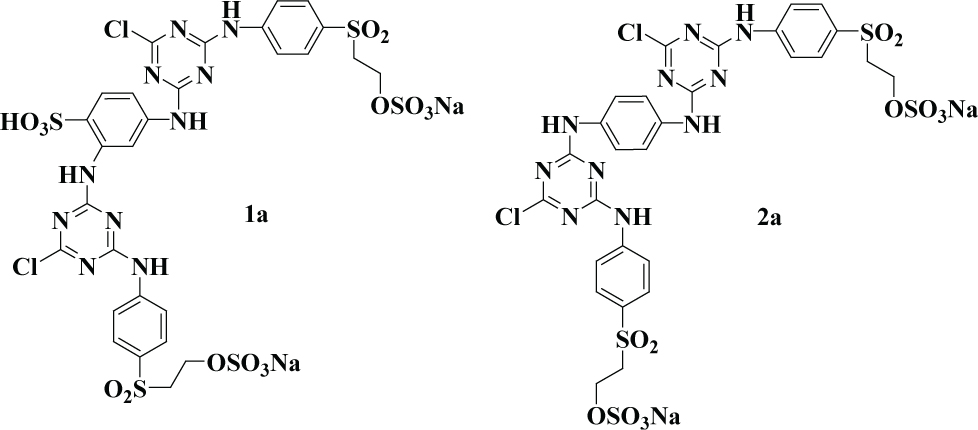
Structures of UV absorbers (1a-2a) selected for degradation.
2.2 Fenton procedure
UV absorber solution (500 ppm) was prepared in distilled water and pH adjusted using a digital pH meter with the help of NaOH (1 M) and H2SO4 (1M). The experiments were conducted in a conical flask (500 mL) containing 100 mL of UV absorber solution. The desired amount of FeSO4.7H2O and H2O2 was added into the flask at the start of reaction. The conical flasks were covered by aluminum foil and placed on a water bath shaker (100 rpm) at room temperature (25±1°C) in Fenton process. To evaluate the effect of time, the sample (2 mL) was withdrawn periodically and the reactive species were quenched by Na2SO3 (0.5 mL) and the COD removal (%) of UV absorber solution was analyzed. To examine the possible maximum removal of UV absorber, the effect of different experimental parameters such as pH (2.0, 3.0, 5.0, 7.0 and 9.0), contact time (40, 80, 120, 160 and 200 min), H2O2 dose (100, 300, 500, 700, 900 mg/L) and FeSO4 concentration (5, 10, 15, 20, 25 mg/L) were also assessed [30].
2.3 UV/H2O2
A UV absorber solution (500 ppm) was prepared and adjusted to the pH of solution using a digital pH meter. A photo-reactor emitting at 254 nm was utilized for this experiment. An aqueous solution of UV absorber (100 mL) was placed under UV lamp and the desired amount of hydrogen peroxide (30%) was added at the start of the reaction and stirred at 100 rpm speed. To evaluate the effect of time, the sample was withdrawn periodically and the COD removal (%) of UV absorber was analyzed. To examine the possible maximum removal of UV absorber by UV/H2O2, the effect of different experimental parameters such as pH (2.0, 3.0, 5.0, 7.0 and 9.0), contact time (40, 80, 120, 160 and 200 min) and H2O2 dose (100, 300, 500, 700, 900 mg/L) were also measured [24].
2.4 Analytical methods
The concentration of UV absorbers (1a and 2a) was measured by calculating their absorbance at their λmax 292 nm and 282 nm, respectively using Perkin Elmer Lambda (CE-7200)UV/Vis Spectrophotometer [20]. The pH of the solution was measured with the use of a digital pH meter (Hanna Instruments, Europe) [32]. The COD of solution was determined via the colorimetric method using equation 4 [30]
Where, COD0 and CODt are the chemical oxygen demand of UV absorber before and after degradation respectively.
Ethical approval: The conducted research is not related to either human or animals use.
3 Results and discussion
3.1 Generation of reactive species by Fe2+/UV irradiation
The generation of reactive oxygen species (OH-, •OH) by the decomposition of hydrogen peroxide by Fe (II) or UV irradiations plays a central role in degradation of organic contaminants in water [34, 35]. As the generation of reactive oxygen species is enhanced, it ultimately improves the degradation process [36]. For the optimization of influencing factors of the Fenton and UV/H2O2 processes, a number of experiments were conducted and results are discussed here.
3.2 Fenton Oxidation
3.2.1 Effect of pH on COD removal
The efficiency of the oxidative process of organic toxins has been highly affected by pH change in Fenton oxidation [24, 37]. But, there is no such consistency reported regarding the optimum pH, and it varies from one case to another. In the current study, the pH of aqueous solutions of UV absorbers were set at pH 2.0, 3.0, 5.0, 7.0 and 9.0 under constant H2O2 dosage of 100 mg/L, Fe2+ dosage of 5 mg/L and contact time of 120 minutes. Figure 2 (A) demonstrated that the maximum COD removal efficiency (%) was attained at pH of 3.0 in case of both the UV absorbers (1a-2a). With an increase in pH above 3.0, the COD removal efficiency of Fenton process was reduced. When the pH value increased from 2.0 to 3.0, the mineralization of absorbers proportionally increased, and then inversely decreased after pH 3.0. Such behavior of the Fenton process can be explained by a decline in the amount of free ions in solution due to precipitation of iron or reaction of •OH with one another to reduce the quantity of free hydroxyl radical at pH > 4.0. The pH < 3.0 causes the creation of [Fe2+(H2O)]2+ in medium, which slowly reacts with hydrogen peroxide and generates fewer hydroxyl radicals. Kinetically, H2O2 decomposition follows pseudo-first order kinetics [38].
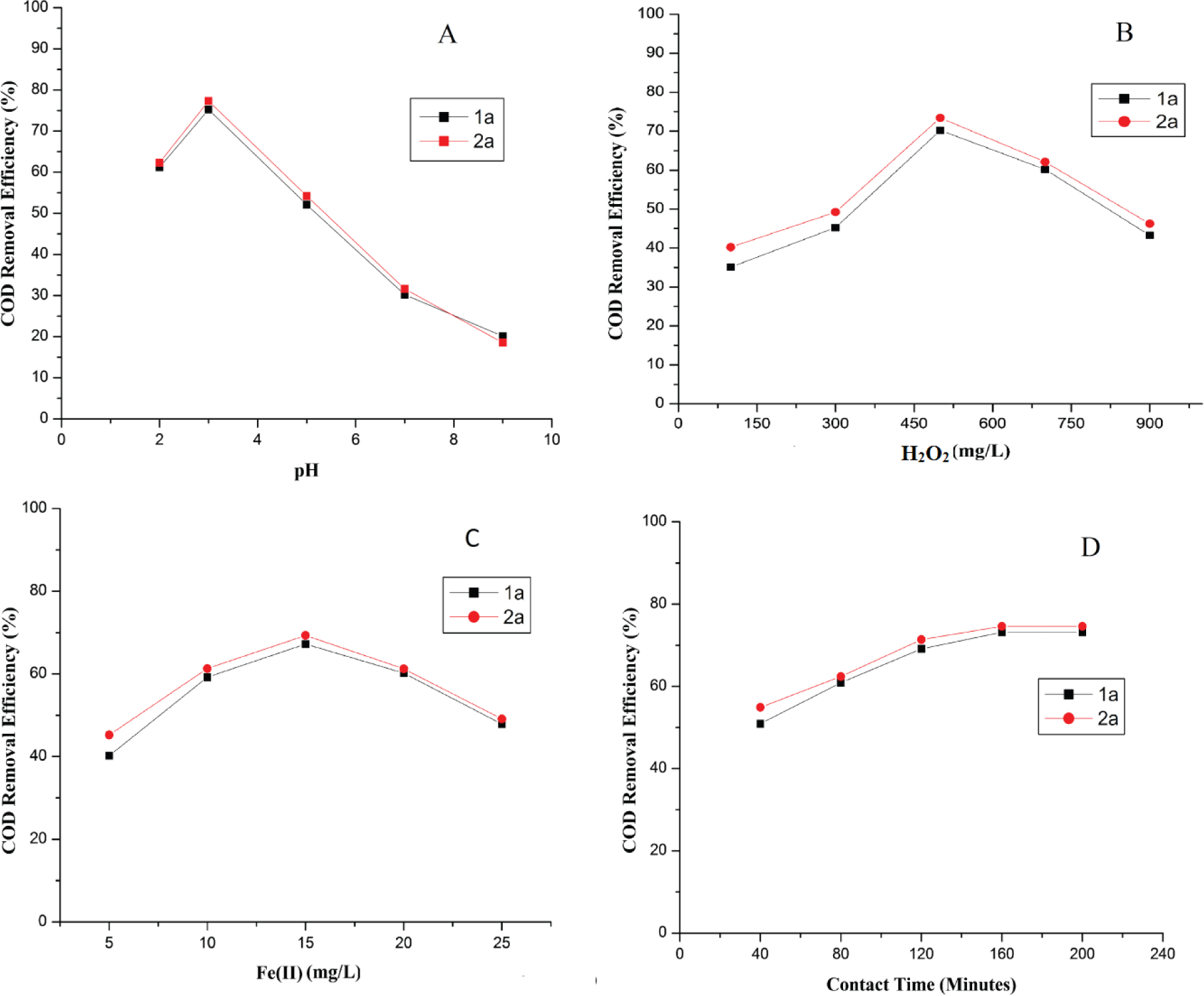
Effect of pH (A), H2O2 (B), Fe2+(C) and contact time (D) on COD removal efficiency of UV absorbers (1a-2a) in Fenton oxidation.
3.2.2 Effect of H2O2 concentration COD removal
For the optimization of H2O2 dosage, the experiments were performed by varying the concentration of hydrogen peroxide (100, 300, 500, 700 and 900 mg/L) under constant pH of 3.0, Fe2+ dosage 5 mg/L and contact time 120 minutes. Figure 2(B) explained the effect of hydrogen peroxide concentration on the COD removal efficiency (%) of UV absorbers (1a-2a). The results confirmed that maximum COD removal efficiency (%) was achieved with 500 mg/L of hydrogen peroxide in Fenton oxidation. After the optimum level had been reached, no further improvement in COD removal efficiency (%) was achieved. Excess H2O2 produced per-hydroxy radical; having less oxidation potential and starts working as hydroxyl radical scavenger [39].
3.2.3 Effect of Fe2+ dosage on the COD removal
Figure 2(C) demonstrated that the maximum COD removal efficiency (%) was attained with 15 mg/L of iron dosage for UV absorbers (1a-2a). It was found that the COD removal efficiency of absorbers have a similar changing pattern with an increase of Fe2+ dose. The COD removal was increased considerably as the concentration of iron increased but after a certain level, the COD removal was decreased. As the dose of iron rises, the COD removal efficiency improves. After 15 mg/L, addition of Fe2+ is unproductive. Higher dose of Fe(II) after optimum level would generate other side and competitive reactions, decreases the concentration of free radicals [(reaction (6), (7), (8) and (9)]. Figure 3 shows the absorbance of aqueous solutions of UV absorbers prior and following the degradation via Fenton oxidation.
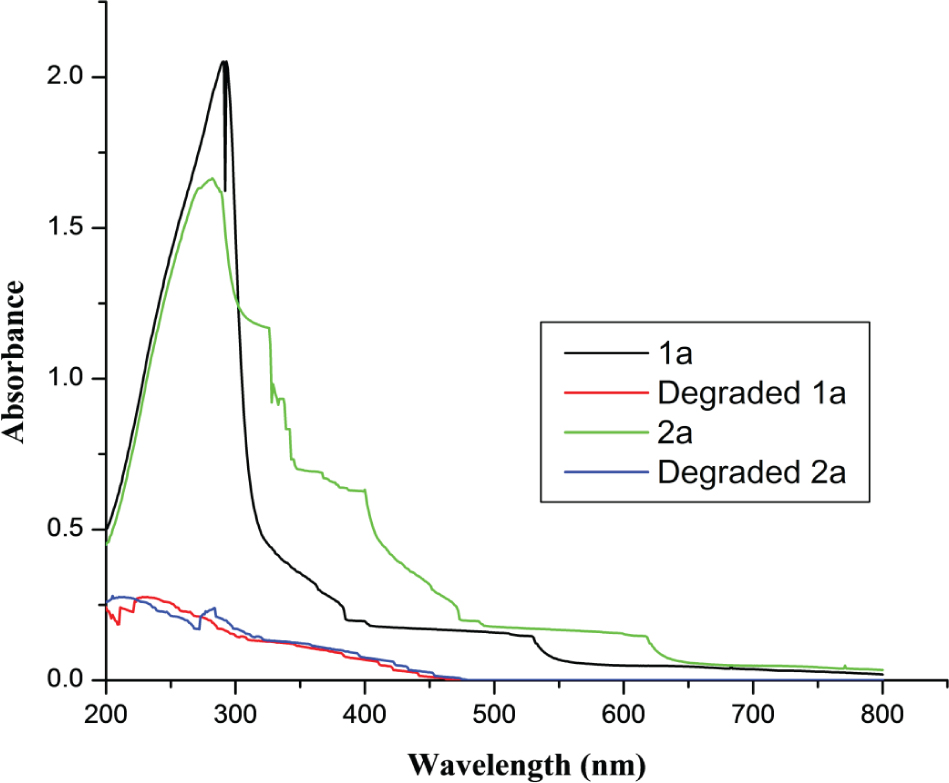
UV-Vis spectra of absorbers in aqueous solution (500 ppm) before and after degradation in Fenton oxidation.
3.2.4 Effect of reaction time on the COD removal
Figure 2(D) demonstrated that maximum COD removal efficiency (%) was attained at 80 minutes in Fenton process. After the optimum level of time duration, no further improvement in COD removal efficiency (%) was achieved. As the reaction time increased, the improvement in COD removal (%) increased linearly, and then it became constant. This can be explained by the fact that most of the hydrogen peroxide reacted with Fe (II) in the beginning of the reaction. The rate of the reaction was high at start of the reaction than it increased slowly and became constant. A study on the effect of time for the treatment of landfill leachate in terms of COD removal efficiency (%) revealed that organic pollutants were speedily degraded in the first 20 minutes, after which any further increase in time duration became insignificant. Generation of a layer of foam on the surface of solution was observed as the oxidation proceeded due to formation of carbon dioxide [40].
3.3 UV/H2O2 Oxidation
3.3.1 Effect of pH on COD removal
With the UV irradiation time of 200 minutes and concentration of H2O2 100 mg/L, the effect of pH including 2.0, 3.0, 5.0, 7.0 and 9.0 on the mineralization of synthesized UV absorbers is shown in Figure 4(A). When the pH value increased from 2.0 to 3.0, the mineralization of UV absorbers increased and then linearly decreased. A pH of 3.0 achieved the maximum mineralization of UV absorbers 1a and 2a which were 55.34 and 57.68 respectively. At a pH of 9.0, the COD removal percentage of 1a and 2a were 21.23 and 18.54 respectively.
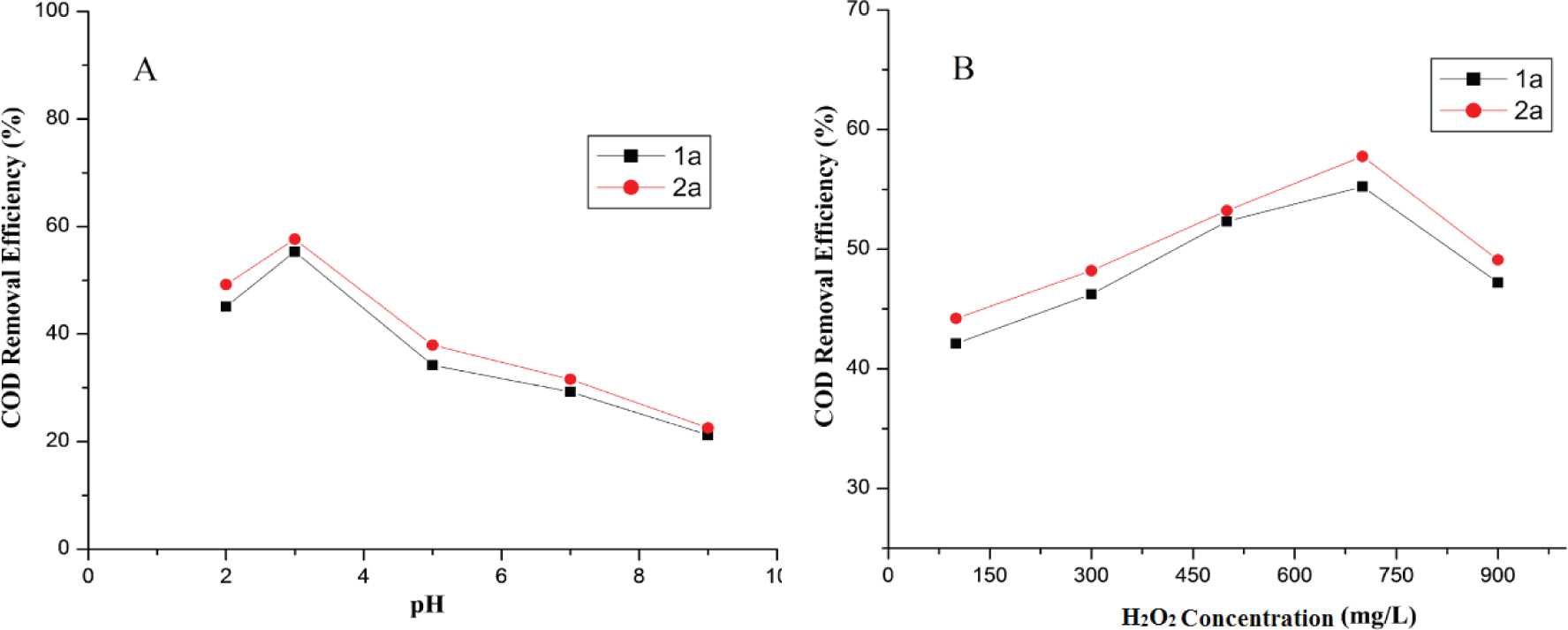
Effect of pH (A) and concentration of H2O2 (B) COD removal efficiency of UV absorbers (1a-2a) in UV/H2O2.
3.3.2 Effect of H2O2 concentration
In the experiments using a constant irradiation time of 200 minutes and pH of 3.0, hydrogen peroxide dosage were set at 100, 300, 500, 700 and 900 mg/L. The effect of H2O2 concentration on mineralization of UV absorbers is shown in Figure 4(B). The maximum COD removal of both the UV absorbers achieved at a 700 mg/L of H2O2. Particularly, the removals were 55.23 and 57.67 % for UV absorber 1a and 2a respectively.
3.3.3 Effect of irradiation time on the COD removal
Preliminary experiments were performed by varying UV irradiation times to 40, 80, 120, 160 and 200 minutes under constant dosage of H2O2 (100 mg/L) and pH of 3.0. Figure 5 shows the effect of UV irradiation time on mineralization of target UV absorbers (1a and 2a). It is evident from the figure that mineralization increases gradually with time. The COD removal (%) of UV absorbers (1a and 2a) was 54.2 and 57.32 respectively at the irradiation time of 200 minutes. Abdelraheem et al. (2015) mineralized the UV absorber and found that due to strong photostablity of UV absorber, its degradation appeared after 285 minutes of irradiation time. This result showed that degradation of these compounds was due to attack of hydroxyl radicals that were produce through decomposition of H2O2 by UV radiations.
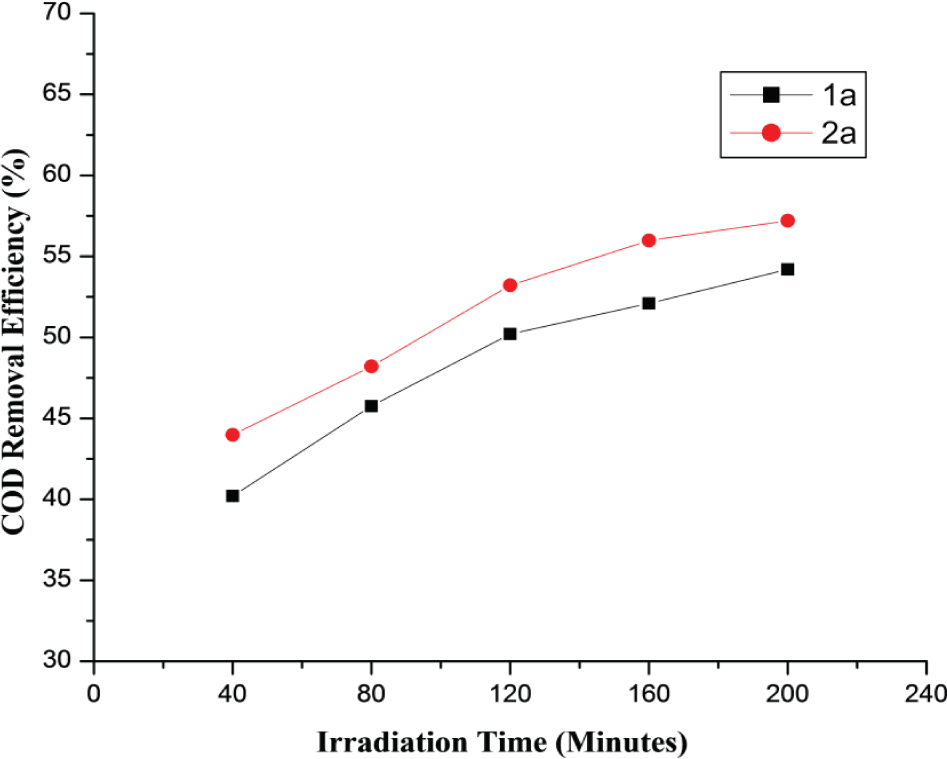
Effect of irradiation time on COD removal efficiency of UV absorbers (1a-2a) in UV/H2O2 process.
4 Comparison of Fenton and UV/H2O2 oxidation
Comparison of Fenton and UV/H2O2 oxidation based on COD removal percentage (%) shows that, ferrous ions catalyzed the production of •OH from hydrogen peroxide during Fenton oxidation. The generation of hydroxyl free radicals increases as the concentration of ferrous ions increases up to a optimum level, which propagated the oxidation of UV absorbers. However, the generations of hydroxyl free radicals in UV/H2O2 is less due to the presence of photostable organic UV absorbers, ultimately it reduces the efficiency of UV/H2O2 oxidation. Table 1 shows that economically Fenton oxidation is better for mineralization of selected triazine based UV absorbers.
optimum condition for Fenton and UV/H2O2 oxidation for mineralization of selected UV absorbers (1a-2a).
| Sr. No. | Selected parameters | Fenton Oxidation | UV/H2O2 Oxidation |
|---|---|---|---|
| 1 | pH | 3.0 | 3.0 |
| 2 | H2O2 | 500 mg/L | 700 mg/L |
| 3 | Fe2+ | 15 mg/L | - |
| 4 | Reaction Time | 120 minutes | 200 minutes |
| 5 | COD Removal (%) | 75-77% | 54-57% |
5 Conclusion
In this study, two new triazine based UV absorbers designed for cotton fabric were degraded by applying two advance oxidation processes (Fenton oxidation and UV/H2O2). The results indicated that Fenton oxidation was more efficient towards mineralization of both the UV absorbers than UV/ H2O2 and results were in agreement with previous literature [20, 30, 35, 40]. Under attained optimum conditions of Fenton oxidation, dose of Fe2+ (15 mg/L), H2O2 (500 mg/L), pH (3.0) and contact time (120 minutes), 75.43% and 77.54% of COD removal were achieved for 1a and 2a, respectively. Whereas, the optimum conditions of UV/H2O2 process were H2O2 (700 mg/L), pH (3.0) and irradiation time (200 minutes) which brought 54.33 and 57.65%. It is concluded that synthesized organic triazine based UV absorbers can efficiently be mineralized and degraded via AOPs.
Acknowledgment
This study was supported by the Department of Chemistry, University of Agriculture, Faisalabad.
Conflict of interest: There is no conflict of interest.
References
[1] GilPavas, E., Dobrosz-Gómez, I., Gómez-García, M.Á., Coagulation-flocculation sequential with Fenton or Photo-Fenton processes as an alternative for the industrial textile wastewater treatment. J. Environ. Manage., 2017, 191, 189-197.10.1016/j.jenvman.2017.01.015Search in Google Scholar PubMed
[2] Mokhtari, J., Phillips, D.A., Taylor, J.A., Synthesis and evaluation of a series of trisazo, monochloro-s-triazinyl (MCT) reactive dyes for cotton. Dyes Pigments., 2004, 63, 51-63.10.1016/j.dyepig.2004.02.001Search in Google Scholar
[3] Piccin, J., Gomes, C., Feris, L., Gutterres, M., Kinetics and isotherms of leather dye adsorption by tannery solid waste. Chem. Eng. J., 2012, 183, 30-38.10.1016/j.cej.2011.12.013Search in Google Scholar
[4] Pang, C., Neubauer, N., Boyles, M., Brown, D., Kanase, N., Hristozov, D., Fernandes, T., Stone, V., Wohlleben, W., Marcomini, A., Releases from transparent blue automobile coatings containing nanoscale copper phthalocyanine and their effects on J774 A1 macrophages. NanoImpact., 2017, 7, 75-8310.1016/j.impact.2017.06.002Search in Google Scholar
[5] Garcia-Falcón, M., Simal-Gándara, J., Determination of food dyes in soft drinks containing natural pigments by liquid chromatography with minimal clean-up. Food Control, 2005, 16, 293-297.10.1016/j.foodcont.2004.03.009Search in Google Scholar
[6] Wainwright, M., Dyes in the development of drugs and pharmaceuticals. Dyes Pigments, 2008, 76, 582-589.10.1016/j.dyepig.2007.01.015Search in Google Scholar
[7] Jewitt, J., Great wood finishes: A step-by-step guide to beautiful results. Taunton Press., 2000, 78-102.Search in Google Scholar
[8] Hunger, K., Industrial dyes: chemistry, properties, applications. John Wiley & Sons., 2007, 339-425.Search in Google Scholar
[9] Siddiqua, U.H., Ali, S., Iqbal, M., Hussain, T., Relationship between structures and dyeing properties of reactive dyes for cotton dyeing. J. Mol. Liq., 2017, 241, 839-276.10.1016/j.molliq.2017.04.057Search in Google Scholar
[10] Czajkowski, W., Paluszkiewicz, J., Stolarski, R., Kaźmierska, M., Grzesiak, E., Synthesis of reactive UV absorbers, derivatives of monochlorotriazine, for improvement in protecting properties of cellulose fabrics. Dyes Pigments, 2006, 71, 224-230.10.1016/j.dyepig.2005.07.004Search in Google Scholar
[11] Patel, D.R., Patel, K.C., Synthesis, antimicrobial activity and application of some novel quinazolinone based monoazo reactive dyes on various fibres. Dyes Pigments, 2011, 90, 1-10.10.1016/j.dyepig.2010.10.013Search in Google Scholar
[12] Waring, D.R., Hallas, G., The chemistry and application of dyes. Springer Science & Business Media., 2013, 49-106.Search in Google Scholar
[13] Mahamallik, P., Pal, A., Degradation of Textile Wastewater by Modified Photo-Fenton Process: Application of Co (II) Adsorbed Surfactant-modified Alumina as Heterogeneous Catalyst. J. Environ. Chem. Eng., 2017, 2886-2893.10.1016/j.jece.2017.05.044Search in Google Scholar
[14] Vautier, M., Guillard, C., Herrmann, J.-M., Photocatalytic degradation of dyes in water: case study of indigo and of indigo carmine. J. Catal., 2001, 201, 46-59.10.1006/jcat.2001.3232Search in Google Scholar
[15] Chequer, F.M.D., de Oliveira, G.A.R., Ferraz, E.R.A., Cardoso, J.C., Zanoni, M.V.B., de Oliveira, D.P., Textile dyes: dyeing process and environmental impact, Ecofriendly textile dyeing and finishing. InTech., 2013, Ch. 06.Search in Google Scholar
[16] Ngah, W.W., Teong, L., Hanafiah, M., Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohyd. Polym., 2011, 83, 1446-1456.10.1016/j.carbpol.2010.11.004Search in Google Scholar
[17] Diao, Z.-H., Liu, J.-J., Hu, Y.-X., Kong, L.-J., Jiang, D., Xu, X.-R., Comparative study of Rhodamine B degradation by the systems pyrite/H2O2 and pyrite/persulfate: Reactivity, stability, products and mechanism. Sep. Purif. Technol., 2017, 184, 374-383.10.1016/j.seppur.2017.05.016Search in Google Scholar
[18] Khan, S., Malik, A., Environmental and health effects of textile industry wastewater, Environmental Deterioration and Human Health. Springer, 2007, 55-71.10.1007/978-94-007-7890-0_4Search in Google Scholar
[19] Lapidot, N., Gans, O., Biagini, F., Sosonkin, L., Rottman, C., Advanced sunscreens: UV absorbers encapsulated in sol-gel glass microcapsules. J. Sol-Gel Sci. Techn., 2003, 26, 67-72.10.1023/A:1020785217895Search in Google Scholar
[20] Abdelraheem, W.H., He, X., Duan, X., Dionysiou, D.D., Degradation and mineralization of organic UV absorber compound 2-phenylbenzimidazole-5-sulfonic acid (PBSA) using UV-254nm/H2O2. J. Hazard. Mater., 2015, 282, 233-240.10.1016/j.jhazmat.2014.07.041Search in Google Scholar
[21] Li, J., Jin, P., Dai, W., Wang, C., Li, R., Wu, T., Tang, C., Excellent performance for water purification achieved by activated porous boron nitride nanosheets. Mater. Chem. Phys., 2017, 196, 186-193.10.1016/j.matchemphys.2017.02.049Search in Google Scholar
[22] Pal, P., Chapter 5 - Water Treatment by Membrane-Separation Technology, Industrial Water Treatment Process Technology. Butterworth-Heinemann, 2017, pp. 173- 242.10.1016/B978-0-12-810391-3.00005-9Search in Google Scholar
[23] Pearce, C., Lloyd, J., Guthrie, J., The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigments, 2003, 58, 179-196.10.1016/S0143-7208(03)00064-0Search in Google Scholar
[24] Bensalah, N., Chair, K., Bedoui, A., Efficient degradation of tannic acid in water by UV/H2O2 process. Sustainable Environ. Res., 2018, 28, 1-11.10.1016/j.serj.2017.04.004Search in Google Scholar
[25] Módenes, A., Espinoza-Quiñones, F., Manenti, D., Borba, F., Palácio, S., Colombo, A., Performance evaluation of a photo-Fenton process applied to pollutant removal from textile effluents in a batch system. J. Environ. Manage., 2012, 104, 1-8.10.1016/j.jenvman.2012.03.032Search in Google Scholar
[26] Akpan, U., Hameed, B., Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: a review. J. Hazard. Mater., 2009, 170, 520-529.10.1016/j.jhazmat.2009.05.039Search in Google Scholar
[27] Ikehata, K., Gamal El-Din, M., Snyder, S.A., Ozonation and advanced oxidation treatment of emerging organic pollutants in water and wastewater. Ozone: Sci. Eng., 2008, 30, 21-26.10.1080/01919510701728970Search in Google Scholar
[28] Klavarioti, M., Mantzavinos, D., Kassinos, D., Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int., 2009, 35, 402-417.10.1016/j.envint.2008.07.009Search in Google Scholar
[29] Song, W., Ravindran, V., Pirbazari, M., Process optimization using a kinetic model for the ultraviolet radiation-hydrogen peroxide decomposition of natural and synthetic organic compounds in groundwater. Chem. Eng. Sci., 2008, 63, 3249-3270.10.1016/j.ces.2008.03.024Search in Google Scholar
[30] Ertugay, N., Acar, F.N., Removal of COD and color from Direct Blue 71 azo dye wastewater by Fenton’s oxidation: Kinetic study. Arab. J. Chem., 2017, 10, 1158- 1163.10.1016/j.arabjc.2013.02.009Search in Google Scholar
[31] Hermosilla, D., Cortijo, M., Huang, C.P., Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ., 2009, 407, 3473-3481.10.1016/j.scitotenv.2009.02.009Search in Google Scholar
[32] Kausley, S.B., Desai, K.S., Shrivastava, S., Shah, P.R., Patil, B.R., Pandit, A.B., Mineralization of alkyd resin wastewater: Feasibility of different advanced oxidation processes. J. Environ. Chem. Eng., 2017, https://doi.org/10.1016/j.jece.2017.04.00110.1016/j.jece.2017.04.001Search in Google Scholar
[33] Sahar, A., Ali, S., Hussain, T., Irfan, M., Eliasson, B., Iqbal, J., UV absorbers for cellulosic apparels: A computational and experimental study. Spectrochim. Acta A., 2018, 188, 355–361.10.1016/j.saa.2017.07.037Search in Google Scholar
[34] Kim, S.M., Vogelpohl, A., Degradation of organic pollutants by the photo-Fenton-process. Chem. Eng. Technol., 1998, 21, 187-191.10.1002/(SICI)1521-4125(199802)21:2<187::AID-CEAT187>3.0.CO;2-HSearch in Google Scholar
[35] Muruganandham, M., Swaminathan, M., Decolourisation of Reactive Orange 4 by Fenton and photo-Fenton oxidation technology. Dyes Pigments., 2004, 63, 315-321.10.1016/j.dyepig.2004.03.004Search in Google Scholar
[36] Huston, P.L., Pignatello, J.J., Degradation of selected pesticide active ingredients and commercial formulations in water by the photo-assisted Fenton reaction. Water Res., 1999, 33, 1238-1246.10.1016/S0043-1354(98)00330-3Search in Google Scholar
[37] Shemer, H., Kunukcu, Y.K., Linden, K.G., Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere., 2006, 63, 269-276.10.1016/j.chemosphere.2005.07.029Search in Google Scholar PubMed
[38] Lucas, M.S., Peres, J.A., Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dyes Pigments, 2006, 71, 236-244.10.1016/j.dyepig.2005.07.007Search in Google Scholar
[39] Zhang, Y., Zhuang, Y., Geng, J., Ren, H., Xu, K., Ding, L., Reduction of antibiotic resistance genes in municipal wastewater effluent by advanced oxidation processes. Sci. Total Environ., 2016, 550, 184-191.10.1016/j.scitotenv.2016.01.078Search in Google Scholar PubMed
[40] Zhang, H., Choi, H.J., Huang, C.-P., Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater., 2005, 125, 166-174.10.1016/j.jhazmat.2005.05.025Search in Google Scholar PubMed
© 2018 Anum Sahar et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution
Articles in the same Issue
- Regular Articles
- The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane
- The preparation and antibacterial activity of cellulose/ZnO composite: a review
- Linde Type A and nano magnetite/NaA zeolites: cytotoxicity and doxorubicin loading efficiency
- Performance and thermal decomposition analysis of foaming agent NPL-10 for use in heavy oil recovery by steam injection
- Spectroscopic (FT-IR, FT-Raman, UV, 1H and 13C NMR) insights, electronic profiling and DFT computations on ({(E)-[3-(1H-imidazol-1-yl)-1-phenylpropylidene] amino}oxy)(4-nitrophenyl)methanone, an imidazole-bearing anti-Candida agent
- A Simplistic Preliminary Assessment of Ginstling-Brounstein Model for Solid Spherical Particles in the Context of a Diffusion-Controlled Synthesis
- M-Polynomials And Topological Indices Of Zigzag And Rhombic Benzenoid Systems
- Photochemical Transformation of some 3-benzyloxy-2-(benzo[b]thiophen-2-yl)-4Hchromen-4-ones: A Remote Substituent Effect
- Dynamic Changes of Secondary Metabolites and Antioxidant Activity of Ligustrum lucidum During Fruit Growth
- Studies on the flammability of polypropylene/ammonium polyphosphate and montmorillonite by using the cone calorimeter test
- DSC, FT-IR, NIR, NIR-PCA and NIR-ANOVA for determination of chemical stability of diuretic drugs: impact of excipients
- Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachlorideinduced Hepatotoxicity in Rats
- Prunus cerasifera Ehrh. fabricated ZnO nano falcates and its photocatalytic and dose dependent in vitro bio-activity
- Organic biocides hosted in layered double hydroxides: enhancing antimicrobial activity
- Experimental study on the regulation of the cholinergic pathway in renal macrophages by microRNA-132 to alleviate inflammatory response
- Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl} naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes
- M-Polynomials and Topological Indices of Dominating David Derived Networks
- Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria
- Analysis of Bowel Diseases from Blood Serum by Autofluorescence and Atomic Force Microscopy Techniques
- Hydrographic parameters and distribution of dissolved Cu, Ni, Zn and nutrients near Jeddah desalination plant
- Relationships between diatoms and environmental variables in industrial water biotopes of Trzuskawica S.A. (Poland)
- Optimum Conversion of Major Ginsenoside Rb1 to Minor Ginsenoside Rg3(S) by Pulsed Electric Field-Assisted Acid Hydrolysis Treatment
- Antioxidant, Anti-microbial Properties and Chemical Composition of Cumin Essential Oils Extracted by Three Methods
- Regulatory mechanism of ulinastatin on autophagy of macrophages and renal tubular epithelial cells
- Investigation of the sustained-release mechanism of hydroxypropyl methyl cellulose skeleton type Acipimox tablets
- Bio-accumulation of Polycyclic Aromatic Hydrocarbons in the Grey Mangrove (Avicennia marina) along Arabian Gulf, Saudi Coast
- Dynamic Change of Secondary Metabolites and spectrum-effect relationship of Malus halliana Koehne flowers during blooming
- Lipids constituents from Gardenia aqualla Stapf & Hutch
- Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives
- Effect of Humic Acid on the Degradation of Methylene Blue by Peroxymonosulfate
- Serum containing drugs of Gua Lou Xie Bai decoction (GLXB-D) can inhibit TGF-β1-Induced Epithelial to Mesenchymal Transition (EMT) in A549 Cells
- Antiulcer Activity of Different Extracts of Anvillea garcinii and Isolation of Two New Secondary Metabolites
- Analysis of Metabolites in Cabernet Sauvignon and Shiraz Dry Red Wines from Shanxi by 1H NMR Spectroscopy Combined with Pattern Recognition Analysis
- Can water temperature impact litter decomposition under pollution of copper and zinc mixture
- Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC)
- Validated thin-layer chromatographic method for alternative and simultaneous determination of two anti-gout agents in their fixed dose combinations
- Fast removal of pollutants from vehicle emissions during cold-start stage
- Review Article
- Catalytic activities of heterogeneous catalysts obtained by copolymerization of metal-containing 2-(acetoacetoxy)ethyl methacrylate
- Antibiotic Residue in the Aquatic Environment: Status in Africa
- Regular Articles
- Mercury fractionation in gypsum using temperature desorption and mass spectrometric detection
- Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators
- Epithelial–Mesenchymal Transition Induced by SMAD4 Activation in Invasive Growth Hormone-Secreting Adenomas
- Physicochemical properties of stabilized sewage sludge admixtures by modified steel slag
- In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2
- Synthesis and Characterization of Pd exchanged MMT Clay for Mizoroki-Heck Reaction
- A new selective, and sensitive method for the determination of lixivaptan, a vasopressin 2 (V2)-receptor antagonist, in mouse plasma and its application in a pharmacokinetic study
- Anti-EGFL7 antibodies inhibit rat prolactinoma MMQ cells proliferation and PRL secretion
- Density functional theory calculations, vibration spectral analysis and molecular docking of the antimicrobial agent 6-(1,3-benzodioxol-5-ylmethyl)-5-ethyl-2-{[2-(morpholin-4-yl)ethyl] sulfanyl}pyrimidin-4(3H)-one
- Effect of Nano Zeolite on the Transformation of Cadmium Speciation and Its Uptake by Tobacco in Cadmium-contaminated Soil
- Effects and Mechanisms of Jinniu Capsule on Methamphetamine-Induced Conditioned Place Preference in Rats
- Calculating the Degree-based Topological Indices of Dendrimers
- Efficient optimization and mineralization of UV absorbers: A comparative investigation with Fenton and UV/H2O2
- Metabolites of Tryptophane and Phenylalanine as Markers of Small Bowel Ischemia-Reperfusion Injury
- Adsorption and determination of polycyclic aromatic hydrocarbons in water through the aggregation of graphene oxide
- The role of NR2C2 in the prolactinomas
- Chromium removal from industrial wastewater using Phyllostachys pubescens biomass loaded Cu-S nanospheres
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Preparation of Calcium Fluoride using Phosphogypsum by Orthogonal Experiment
- The mechanism of antibacterial activity of corylifolinin against three clinical bacteria from Psoralen corylifolia L
- 2-formyl-3,6-bis(hydroxymethyl)phenyl benzoate in Electrochemical Dry Cell
- Electro-photocatalytic degradation of amoxicillin using calcium titanate
- Effect of Malus halliana Koehne Polysaccharides on Functional Constipation
- Structural Properties and Nonlinear Optical Responses of Halogenated Compounds: A DFT Investigation on Molecular Modelling
- DMFDMA catalyzed synthesis of 2-((Dimethylamino)methylene)-3,4-dihydro-9-arylacridin-1(2H)-ones and their derivatives: in-vitro antifungal, antibacterial and antioxidant evaluations
- Production of Methanol as a Fuel Energy from CO2 Present in Polluted Seawater - A Photocatalytic Outlook
- Study of different extraction methods on finger print and fatty acid of raw beef fat using fourier transform infrared and gas chromatography-mass spectrometry
- Determination of trace fluoroquinolones in water solutions and in medicinal preparations by conventional and synchronous fluorescence spectrometry
- Extraction and determination of flavonoids in Carthamus tinctorius
- Therapeutic Application of Zinc and Vanadium Complexes against Diabetes Mellitus a Coronary Disease: A review
- Study of calcined eggshell as potential catalyst for biodiesel formation using used cooking oil
- Manganese oxalates - structure-based Insights
- Topological Indices of H-Naphtalenic Nanosheet
- Long-Term Dissolution of Glass Fibers in Water Described by Dissolving Cylinder Zero-Order Kinetic Model: Mass Loss and Radius Reduction
- Topological study of the para-line graphs of certain pentacene via topological indices
- A brief insight into the prediction of water vapor transmissibility in highly impermeable hybrid nanocomposites based on bromobutyl/epichlorohydrin rubber blends
- Comparative sulfite assay by voltammetry using Pt electrodes, photometry and titrimetry: Application to cider, vinegar and sugar analysis
- MicroRNA delivery mediated by PEGylated polyethylenimine for prostate cancer therapy
- Reversible Fluorescent Turn-on Sensors for Fe3+ based on a Receptor Composed of Tri-oxygen Atoms of Amide Groups in Water
- Sonocatalytic degradation of methyl orange in aqueous solution using Fe-doped TiO2 nanoparticles under mechanical agitation
- Hydrotalcite Anchored Ruthenium Catalyst for CO2 Hydrogenation Reaction
- Production and Analysis of Recycled Ammonium Perrhenate from CMSX-4 superalloys
- Topical Issue on Agriculture
- New phosphorus biofertilizers from renewable raw materials in the aspect of cadmium and lead contents in soil and plants
- Survey of content of cadmium, calcium, chromium, copper, iron, lead, magnesium, manganese, mercury, sodium and zinc in chamomile and green tea leaves by electrothermal or flame atomizer atomic absorption spectrometry
- Biogas digestate – benefits and risks for soil fertility and crop quality – an evaluation of grain maize response
- A numerical analysis of heat transfer in a cross-current heat exchanger with controlled and newly designed air flows
- Freshwater green macroalgae as a biosorbent of Cr(III) ions
- The main influencing factors of soil mechanical characteristics of the gravity erosion environment in the dry-hot valley of Jinsha river
- Free amino acids in Viola tricolor in relation to different habitat conditions
- The influence of filler amount on selected properties of new experimental resin dental composite
- Effect of poultry wastewater irrigation on nitrogen, phosphorus and carbon contents in farmland soil
- Response of spring wheat to NPK and S fertilization. The content and uptake of macronutrients and the value of ionic ratios
- The Effect of Macroalgal Extracts and Near Infrared Radiation on Germination of Soybean Seedlings: Preliminary Research Results
- Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill
- Topical Issue on Research for Natural Bioactive Products
- Synthesis of (±)-3,4-dimethoxybenzyl-4-methyloctanoate as a novel internal standard for capsinoid determination by HPLC-ESI-MS/MS(QTOF)
- Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, Aedes aegypti
- Effect of Flammulina velutipes (golden needle mushroom, eno-kitake) polysaccharides on constipation
- Bioassay-directed fractionation of a blood coagulation factor Xa inhibitor, betulinic acid from Lycopus lucidus
- Antifungal and repellent activities of the essential oils from three aromatic herbs from western Himalaya
- Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers
- Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum
- α-Terpineol, a natural monoterpene: A review of its biological properties
- Utility of essential oils for development of host-based lures for Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), vector of laurel wilt
- Phenolic composition and antioxidant potential of different organs of Kazakh Crataegus almaatensis Pojark: A comparison with the European Crataegus oxyacantha L. flowers
- Isolation of eudesmane type sesquiterpene ketone from Prangos heyniae H.Duman & M.F.Watson essential oil and mosquitocidal activity of the essential oils
- Comparative analysis of the polyphenols profiles and the antioxidant and cytotoxicity properties of various blue honeysuckle varieties
- Special Issue on ICCESEN 2017
- Modelling world energy security data from multinomial distribution by generalized linear model under different cumulative link functions
- Pine Cone and Boron Compounds Effect as Reinforcement on Mechanical and Flammability Properties of Polyester Composites
- Artificial Neural Network Modelling for Prediction of SNR Effected by Probe Properties on Ultrasonic Inspection of Austenitic Stainless Steel Weldments
- Calculation and 3D analyses of ERR in the band crack front contained in a rectangular plate made of multilayered material
- Improvement of fuel properties of biodiesel with bioadditive ethyl levulinate
- Properties of AlSi9Cu3 metal matrix micro and nano composites produced via stir casting
- Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process
- Modeling of Total Phenolic contents in Various Tea samples by Experimental Design Methods
- Nickel doping effect on the structural and optical properties of indium sulfide thin films by SILAR
- The effect mechanism of Ginnalin A as a homeopathic agent on various cancer cell lines
- Excitation functions of proton induced reactions of some radioisotopes used in medicine
- Oxide ionic conductivity and microstructures of Pr and Sm co-doped CeO2-based systems
- Rapid Synthesis of Metallic Reinforced in Situ Intermetallic Composites in Ti-Al-Nb System via Resistive Sintering
- Oxidation Behavior of NiCr/YSZ Thermal Barrier Coatings (TBCs)
- Clustering Analysis of Normal Strength Concretes Produced with Different Aggregate Types
- Magnetic Nano-Sized Solid Acid Catalyst Bearing Sulfonic Acid Groups for Biodiesel Synthesis
- The biological activities of Arabis alpina L. subsp. brevifolia (DC.) Cullen against food pathogens
- Humidity properties of Schiff base polymers
- Free Vibration Analysis of Fiber Metal Laminated Straight Beam
- Comparative study of in vitro antioxidant, acetylcholinesterase and butyrylcholinesterase activity of alfalfa (Medicago sativa L.) collected during different growth stages
- Isothermal Oxidation Behavior of Gadolinium Zirconate (Gd2Zr2O7) Thermal Barrier Coatings (TBCs) produced by Electron Beam Physical Vapor Deposition (EB-PVD) technique
- Optimization of Adsorption Parameters for Ultra-Fine Calcite Using a Box-Behnken Experimental Design
- The Microstructural Investigation of Vermiculite-Infiltrated Electron Beam Physical Vapor Deposition Thermal Barrier Coatings
- Modelling Porosity Permeability of Ceramic Tiles using Fuzzy Taguchi Method
- Experimental and theoretical study of a novel naphthoquinone Schiff base
- Physicochemical properties of heat treated sille stone for ceramic industry
- Sand Dune Characterization for Preparing Metallurgical Grade Silicon
- Catalytic Applications of Large Pore Sulfonic Acid-Functionalized SBA-15 Mesoporous Silica for Esterification
- One-photon Absorption Characterizations, Dipole Polarizabilities and Second Hyperpolarizabilities of Chlorophyll a and Crocin
- The Optical and Crystallite Characterization of Bilayer TiO2 Films Coated on Different ITO layers
- Topical Issue on Bond Activation
- Metal-mediated reactions towards the synthesis of a novel deaminolysed bisurea, dicarbamolyamine
- The structure of ortho-(trifluoromethyl)phenol in comparison to its homologues – A combined experimental and theoretical study
- Heterogeneous catalysis with encapsulated haem and other synthetic porphyrins: Harnessing the power of porphyrins for oxidation reactions
- Recent Advances on Mechanistic Studies on C–H Activation Catalyzed by Base Metals
- Reactions of the organoplatinum complex [Pt(cod) (neoSi)Cl] (neoSi = trimethylsilylmethyl) with the non-coordinating anions SbF6– and BPh4–
- Erratum
- Investigation on Two Compounds of O, O’-dithiophosphate Derivatives as Corrosion Inhibitors for Q235 Steel in Hydrochloric Acid Solution

