Abstract
Both linezolid and vancomycin have good efficacy in the treatment of resistant Gram-positive bacterial infections. This systematic review and meta-analysis aimed to compare the efficacy and safety of linezolid vs vancomycin for the treatment of resistant Gram-positive bacterial infections in children under 12 years.
Five randomly controlled trials involving 638 children that were treated with linezolid and vancomycin for resistant Gram-positive bacterial infections were searched from medical databases. Meta-analysis showed that linezolid and vancomycin had equivalent efficacies in clinical cure rates in the intent-to-treat population (95% confidence interval [CI] 0.88, 2.09) and microbiologically evaluable patients (95% CI: 0.46, 2.47). Linezolid and vancomycin also had equivalent pathogen eradication rates for Staphylococcus aureus (95% CI: 0.31, 4.81), methicillin-resistant S. aureus (95% CI: 0.36, 5.34), Enterococcus faecalis (95% CI: 0.32, 8.76), and coagulase-negative Staphylococci (95% CI: 0.43, 4.01). Vancomycin resulted in a higher incidence of alanine aminotransferase increase (95% CI: 0.37, 0.97), red man syndrome (95% CI: 0.01, 0.28), and rash (95% CI: 0.11, 0.73) than linezolid. Clinically, linezolid had a superior safety to vancomycin for resistant Gram-positive infections.
Linezolid might be prescribed for the treatment of resistant Gram-positive bacterial infections in children under 12 years.
1 Introduction
Gram-positive pathogens are the most common causes of nosocomial infections in infants and children. They cause a high morbidity of hospital-acquired pneumonia, bacteremia, and mortality [1,2]. The pathogenic bacteria causing pneumonia mainly include coagulase-negative Staphylococci (CoNS), Staphylococcus aureus, Streptococcus pneumoniae, and methicillin-resistant S. aureus (MRSA) [1–4]. The emergence and increased frequency of drug-resistant Gram-positive bacteria, including MRSA and vancomycin-resistant Enterococci (VRE), are becoming increasing problems for the treatment of nosocomial infections in pediatrics.
Vancomycin is a well-tolerated and effective glycopeptide antibiotic and is the first choice treatment for late-onset sepsis due to resistant Staphylococci by neonatologists [1,5,6]. Vancomycin-containing regimens are frequently prescribed for infections caused by multidrug-resistant Gram-positive organisms [3]. However, the emergence of VRE and higher incidence of adverse events are challenging its prescription [5,7,8]. For instance, vancomycin causes idiosyncratic drug actions, including red man syndrome, increased liver enzyme activity, and nephrotoxicity in neonates [4,7,8].
Linezolid is a bacterial protein synthesis inhibitor [9,10]. Linezolid, as the first new thiazolidinone antibacterial drug, has a unique mode of action. It binds to the bacterial 50S ribosomal subunit to prevent the formation of the 70S initiation complex and inhibit protein synthesis in bacteria [9,10]. Linezolid was approved by the Food and Drug Administration of the United States for marketing and pediatric use in 2002 and was approved in China in August 2007. It is mainly used for hospital-acquired pneumonia, bacteremia, and infections caused by multidrug-resistant Gram-positive pathogens, including MRSA, methicillin-resistant CoNS, and VRE [1–4,11,12]. Additionally, linezolid is well tolerated and as effective as vancomycin for the treatment of Gram-positive bacterial infections [2,11]. It is effective for infections of MRSA and VRE [2].
A large number of randomized controlled trials (RCTs) and review analyses have shown the efficacy and safety of linezolid vs vancomycin for the treatment of Gram-positive bacterial infections in adults [2,13–17]. Some reports proposed that linezolid had a significantly lower frequency of drug-related adverse events in patients from birth to 12 years of age than vancomycin [2,4]. However, there was no systematic analysis for comparing the efficacy and safety of linezolid vs vancomycin for the treatment of resistant Gram-positive bacterial infections in neonates, infants, and children <12 years. This study aimed to evaluate the efficacy and safety of linezolid vs vancomycin for the treatment of resistant Gram-positive bacterial infections and to provide medical evidence for pediatricians or neonatologists.
2 Materials and methods
2.1 Ethics statement
This study was a systematic review to compare the efficacy and safety of linezolid and vancomycin in treating Gram-positive bacterial infections. Neither animal nor human experiments were performed by any one of the authors, and therefore ethics committee approval was not applicable. This study was designed, conducted, and performed following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses [18].
2.2 Literature source and search strategy
RCTs were searched in comprehensive databases, including PubMed, EMBASE, and Cochrane library using the following words: linezolid, pneumonia, and Gram-positive infections. The search strategy was “Linezolid[MeSH Terms]” AND “newborn[MeSH Terms] OR infant[MeSH Terms] OR children[MeSH Terms] OR child[MeSH Terms] OR pediatrics[MeSH Terms] OR adolescent[MeSH Terms].” Eligible clinical studies that were published up to February 2019 and that compared the efficacy and safety of linezolid and vancomycin in pediatric patients (<12 years) with Gram-positive bacterial infections were included. Additional trials were searched in the reference lists of the review articles and included studies.
2.3 Study selection
Eligible clinical trials were selected independently by two authors. Trials were included if they met the following inclusion criteria: (1) RCTs involving pediatric patients (<12 years) with resistant Gram-positive bacterial infections; and (2) patients in the treatment group were treated with linezolid, and patients in the control group were treated with vancomycin. We put no restrictions on race and publication year. Trials were excluded if they were (1) published in non-English; (2) literature duplications, reviews, and case reports; and (3) trials that treated patients in the treatment group with other antibacterial agents in addition to linezolid or treated patients in the control group with other antibacterial agents in addition to vancomycin.
2.4 Data extraction
The primary outcomes were the clinical cure rate and pathogen eradication rate. Clinical cure was defined as the disappearance or decrease in main clinical symptoms and pulmonary signs at the end of treatment or the test-of-cure follow-up visit. The safety profiles (adverse events) of linezolid and vancomycin in pediatric patients with Gram-positive infections were extracted.
2.5 Assessment of trial quality
Trial quality was assessed using the five-point Jadad scoring tool [19,20], which consists of five items and each item contributes one point to the total score. Trials scoring ≥3 and ≤2 were deemed to be high and low quality, respectively. Two authors assessed quality independently. Discussion or adjudication by a third reviewer was required to resolve disagreements. Publication bias was not assessed because of the small number of included studies.
2.6 Statistical analysis
Meta-analysis was performed using Reviewer Manager 5.1 software (RevMan, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). The statistical heterogeneity of data across included trials was assessed by the Q test and quantified with the I 2 statistic test. Data of P < 0.10 and I 2 > 50% were defined as significantly heterogeneous, while data of P > 0.10 and I 2 < 50% were significantly homogeneous. Meta-analysis was performed with the fixed-effects model due to the significant data homogeneity across the included trials. For meta-analysis of dichotomous outcomes, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the Mantel–Haenszel method. Significant differences in efficacy and safety outcomes between linezolid and vancomycin were indicated as P < 0.05.
3 Results
3.1 Study selection
The search in medical databases generated 667 reports. After removing duplications (n = 180) and screening for title, abstract, and full-text, five trials were included (Figure 1 and Table 1)[1,2,3,4,12].
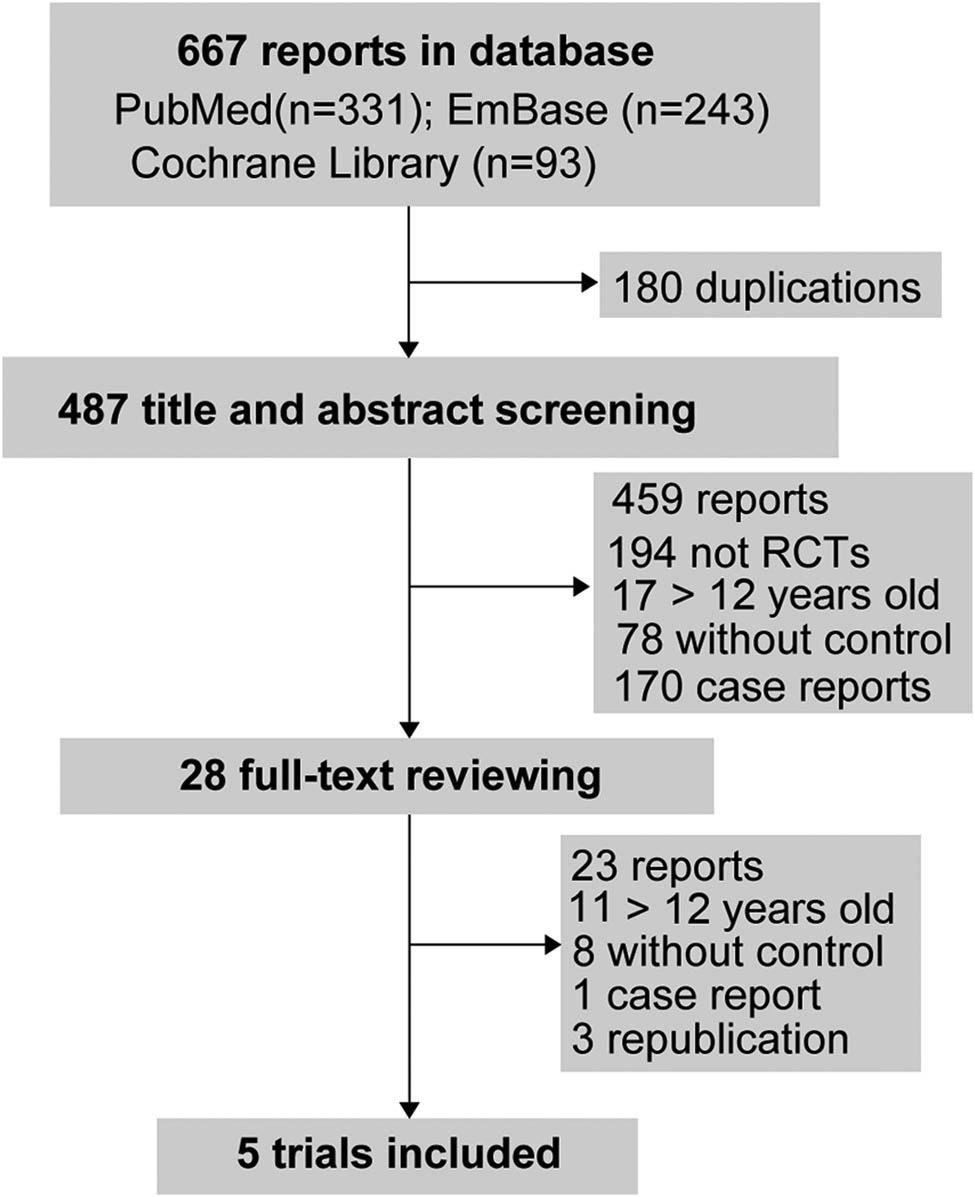
The flow diagram of study selection processing.
Baseline characteristics of the five included studies
| Author (year) | Study type | No of patients (ITT) | Age (median) | Type of infection | Organism(s) | Clinical/microbiological efficacy | Jadad score |
|---|---|---|---|---|---|---|---|
| Kaplan et al., 2003 [2] | RCCT Phase III Open label | 219–102 | 0–11 years (1.8 years) | Nosocomial pneumonia; cSSSIs Bacteremia Systemic infections | MSSA; MRSA; S. pyogenes; S. pyogenes; CoNS; Enterococcus spp. | Clinical success: 89.3% LZD, 84.5% Van microbiological success: MSSA: 95% LZD, 94% Van MRSA: 88% LZD, 90% Van; MR-CoNS: 85% LZD, 83% Van | 3 |
| Jantaush et al., 2003 [1] | RCCT; Phase III Open label (subset analysis) | 104–48 | <12 years (1.15 years); <12 years (1.2 years) | Bacteremia and HAP | S. aureus; CoNS; Enterococcus spp. | Clinical success: Bacteremia: 84.8% LZD, 80% Van Pneumonia: 90% LZD, 100% Van Microbiological eradication: HAP: 100% LZD, 100%, Van Catheter-related bacteremia: CoNS: 81.8% LZD, 75% Van; Bacteremia: CoNS: 90% LZD, 75% Van | 3 |
| Deville et al., 2003 [3] | RCCT; Phase III; Open label (subset analysis) | 43–20 | 0–90 days (18 days); 0–90 days (36 days) | Nosocomial pneumonia; cSSSIs; bacteremia | MSSA; MRSA; CoNS; Enterococcus spp. | Clinical success: 84.4% LZD, 76.9% Van Microbiological eradication: CoNS 88% LZD, 100% Van | 3 |
| Kaplan et al., 2003 [4] | RCT, multinational, multicenter study | 20–14 | <12 years | Pneumonia, bacteremia or complicated SSSI | MRSA | Clinical success: 94.1% LZD, 90.0% Van; Microbiological eradication: CoNS 88.2% LZD, 90.0% Van | 4 |
| Shibata et al., 2018 [12] | RCT, multicenter | 32–36 | 35 days (range: 4–472) | NICU | Gram-positive infections | Microbiological eradication: 90.6% LZD, 72.2% Van | 4 |
ITT, intention to treat; LZD, linezolid; Van, vancomycin; q8h, every 8 h; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; MR-CoNS, methicillin-resistant coagulase-negative Staphylococci; RCCT, randomized comparator controlled trial; HAP, hospital-acquired pneumonia; cSSSI, complicated skin/skin structure infection.
3.2 Trial characteristics
The five intent-to-treat trials involved 638 children with resistant Gram-positive bacterial infections. Four studies were published by the same research team on children (<12 years old) treated with linezolid and vancomycin for 10–28 days [1–4]. All five trials were of high quality (Jadad score: 3–4; Table 1). Four trials reported the clinical cure rate [1–4] and microbiological eradication rate [1–4]. Five trials [1–4,12] reported the safety of linezolid and vancomycin for resistant Gram-positive infections in infants and neonates (4–472 days; Table 2).
Safety assessment for treatment of resistant Gram-positive infections in children
| Adverse events | Study | Linezolid | Vancomycin | I 2 (%) | P | OR (95% CI) | P | ||
|---|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | ||||||
| Diarrhea | [1–3,12] | 14 | 379 | 10 | 178 | 0 | 0.86 | 0.66 (0.18,69.14) | 0.34 |
| Nausea | [1,2] | 5 | 316 | 0 | 145 | 0 | 0.86 | 2.76 (0.34,22.70) | 0.34 |
| Vomiting | [1,2,12] | 18 | 348 | 14 | 181 | 0 | 0.62 | 1.15 (0.62,2.12) | 0.67 |
| Rash | [1,2,4] | 5 | 336 | 10 | 159 | 51 | 0.13 | 0.29 (0.11,0.73) | 0.009 |
| Anemia | [1–4] | 7 | 379 | 2 | 178 | 0 | 0.98 | 1.33 (0.36,4.88) | 0.67 |
| Red man syndrome | [2,4] | 0 | 233 | 13 | 113 | 0 | 0.45 | 0.04 (0.01,0.28) | 0.001 |
| Abnormal hematology | |||||||||
| Hemoglobin | [1–3,12] | 68 | 386 | 27 | 197 | 32 | 0.22 | 1.27 (0.78,2.08) | 0.34 |
| White blood cell count | [1–3,12] | 43 | 386 | 21 | 197 | 0 | 0.52 | 0.92 (0.52,1.60) | 0.76 |
| Neutrophil count | [1–3,12] | 22 | 375 | 9 | 192 | 0 | 0.90 | 1.20 (0.54,2.68) | 0.66 |
| Platelet count | [1–3,12] | 59 | 386 | 34 | 197 | 0 | 0.97 | 0.86 (0.54,1.38) | 0.53 |
| Chemistries | |||||||||
| Alanine aminotransferase increase | [1–3,12] | 34 | 379 | 27 | 194 | 0 | 0.61 | 0.60 (0.37,0.97) | 0.04 |
| Total bilirubin | [1–3,12] | 33 | 376 | 11 | 191 | 0 | 0.82 | 1.50 (0.78,2.87) | 0.22 |
| Creatinine | [1–3,12] | 10 | 387 | 2 | 197 | 0 | 0.50 | 1.90 (0.48,7.45) | 0.36 |
OR, odds ratio; CI, confidential interval.
3.3 Efficacy in the clinical cure rate
The clinical cure rate data across trials were not heterogeneous (I 2 = 0%, P > 0.10). Meta-analysis showed that there was no statistical difference in the overall clinical cure rate between linezolid and vancomycin (OR = 1.36, 95% CI: 0.88, 2.09; Figure 2a) and clinical cure rate in microbiologically evaluable patients (OR = 1.06, 95% CI: 0.46, 2.47; Figure 2b).
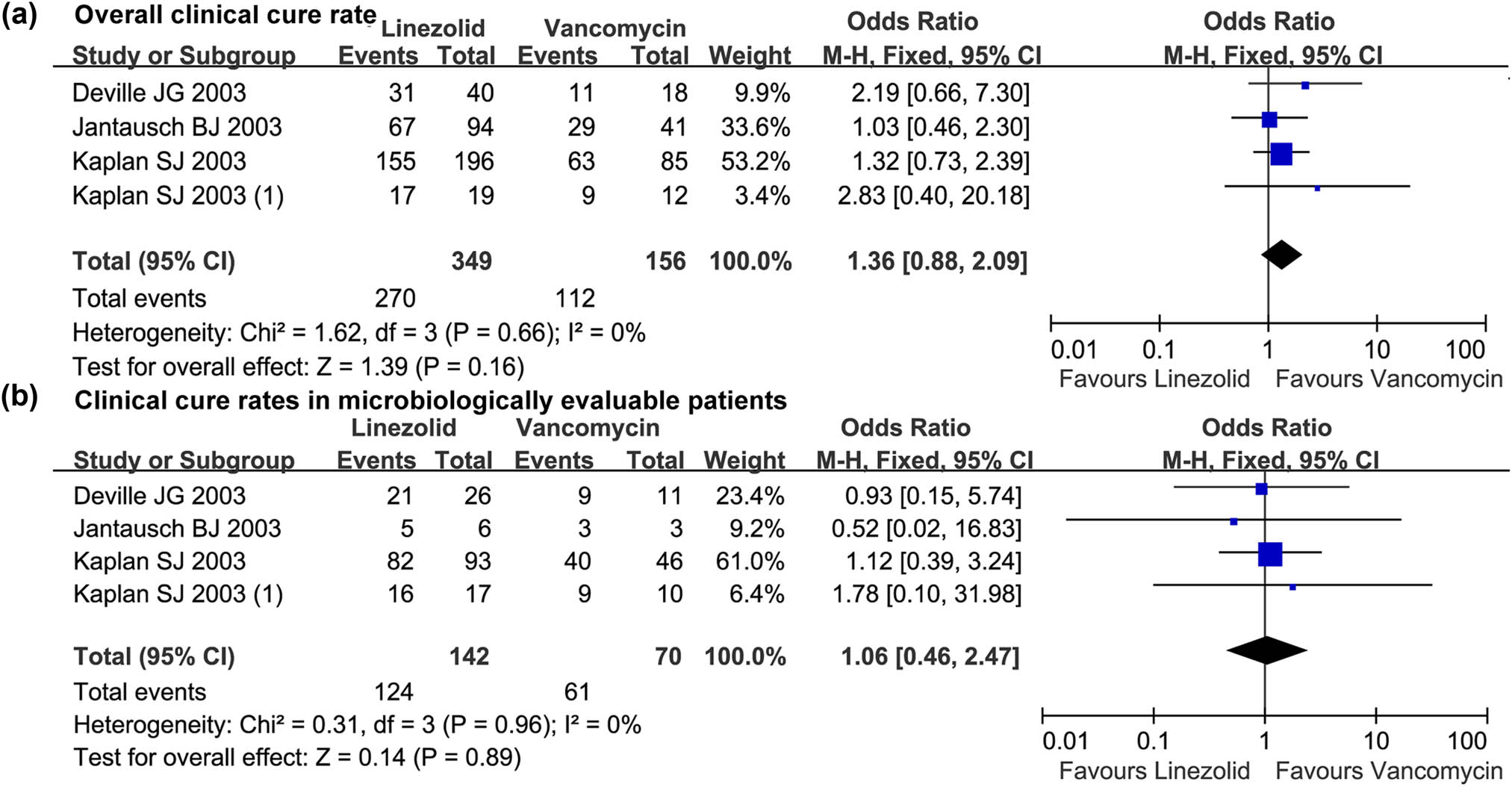
The forest plot of the clinical cure rate of linezolid vs vancomycin in children (<12 years) with resistant Gram-positive bacterial infections. (a) and (b) The comparative overall clinical cure rate and clinical cure rates in microbiologically evaluable patients treated with linezolid vs vancomycin in the treatment of resistant Gram-positive bacterial infections in children under 12 years. M-H, Mantel-Haenszel; CI, confidential interval.
3.4 Efficacy in the pathogen eradication rate
The pathogen eradication rate data were not heterogeneous across four trials (I 2 = 0%, P > 0.10). A meta-analysis showed that linezolid and vancomycin achieved equivalent efficacies in the eradication rate for S. aureus (OR = 1.21, 95% CI: 0.31, 4.81), MRSA (OR = 1.39, 95% CI: 0.36, 5.34), Enterococcus faecalis (OR = 1.66, 95% CI: 0.32, 8.76), and CoNS (OR = 1.31 95% CI: 0.43, 4.01; Figure 3) in microbiologically evaluable patients.
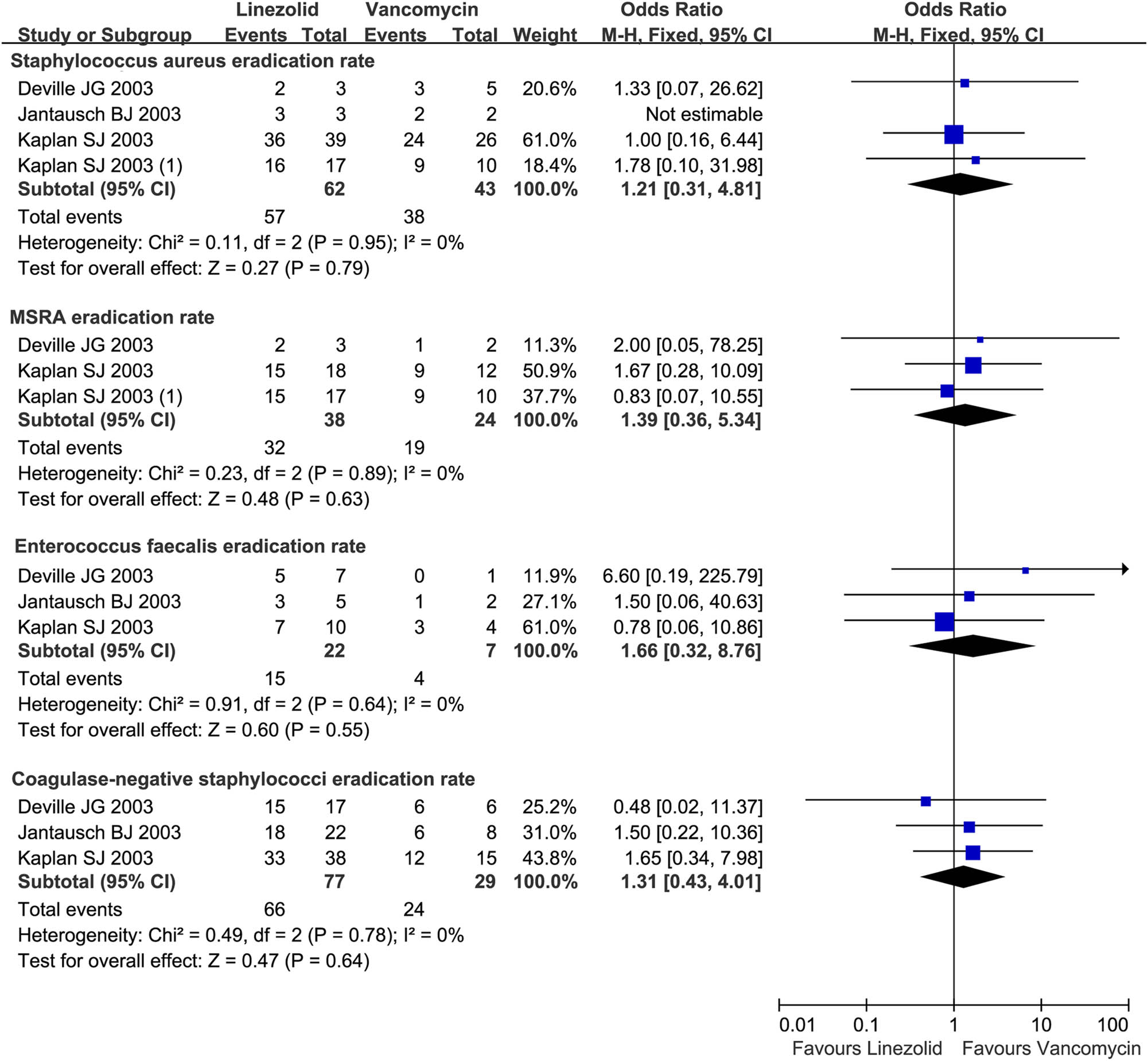
Pathogen eradication rate of linezolid vs. vancomycin in children (<12 years) with resistant Gram-positive bacterial infections. Pathogen eradication rate for S. aureus, MRSA, Enterococcus faecalis, and CoNS in microbiologically evaluable patients treated with linezolid vs vancomycin for the treatment of resistant Gram-positive bacterial infections in children under 12 years. M-H, Mantel-Haenszel; CI, confidential interval.
3.5 Adverse events
Totally, linezolid treatment had a lower frequency of adverse events in children with resistant Gram-positive bacterial infection than vancomycin (90/411 vs 83/214; OR = 0.49, 95% CI: 0.33, 0.72; Figure 4). The subgroup analysis indicated that linezolid and vancomycin achieved equivalent frequencies of diarrhea (95% CI: 0.18, 69.14), nausea (95% CI: 0.34, 22.70), vomiting (95% CI: 0.62, 2.12), anemia (95% CI: 0.36, 4.88), and abnormal laboratory hematology values (including hemoglobin, white blood cell count, neutrophil count, and platelet count), total bilirubin (95% CI: 0.78, 2.87), and creatinine (95% CI: 0.48, 7.45; Table 2). Meta-analysis showed that vancomycin contributed to a higher incidence of alanine aminotransferase increase (95% CI: 0.37, 0.97), red man syndrome (95% CI: 0.01, 0.28), and rash (95% CI: 0.11, 0.73; Table 2) than linezolid.
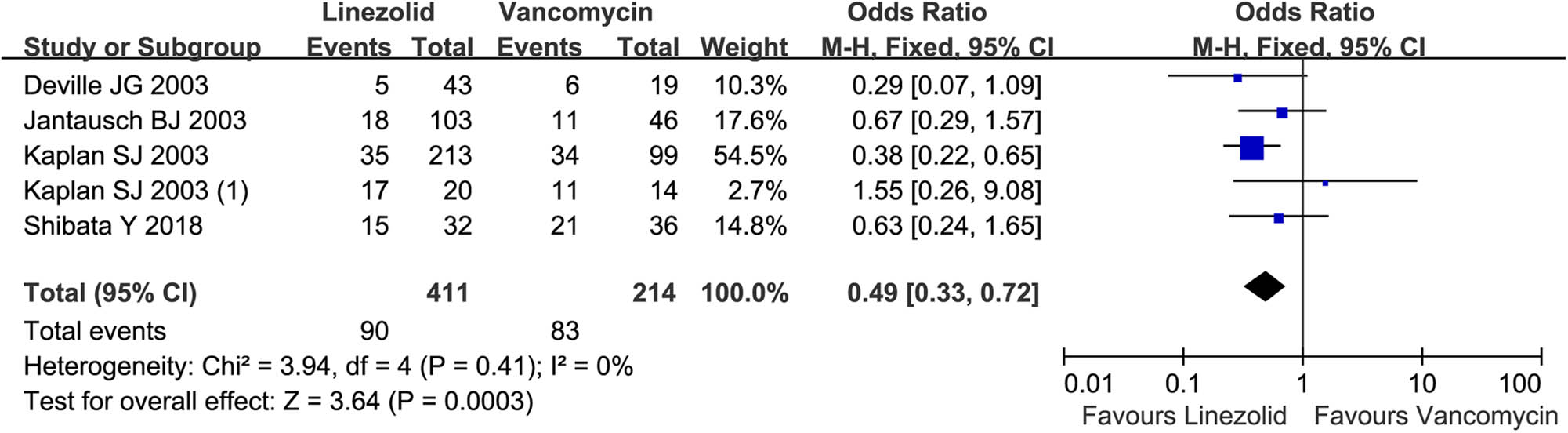
Total adverse event rate by linezolid vs. vancomycin in the treatment of resistant Gram-positive bacterial infections in children <12 years. M-H, Mantel-Haenszel; CI, confidential interval.
4 Discussion
Our present study confirmed that vancomycin and linezolid had equivalent efficacies against resistant Gram-positive bacterial infections in children under 12 years. In view of safety, vancomycin generated a higher frequency of adverse events, including rash, red man syndrome, and an increase in alanine aminotransferase, than linezolid. These results confirmed that linezolid had a high efficacy and safety in the treatment of resistant Gram-positive bacterial infections in children under 12 years.
Linezolid inhibits protein synthesis and the formation of ribosomal subunit in bacteria [9,10]. It has strong antibacterial activity against drug-resistant S. aureus and good permeability in lung tissue [21,22]. Jacqueline et al. [21] showed that linezolid could reduce proinflammatory cytokine tumor necrosis factor α and neutrophil infiltration in a mouse model of MRSA-induced pneumonia. They also showed that linezolid presented a decreased endothelial permeability at 48 h postinfection, while vancomycin resulted in a time-dependent increase of endothelial permeability. This study might indicate that linezolid had superior efficacy against vancomycin in the treatment of MRSA pneumonia [21]. Linezolid also decreased the incidence of nephrotoxicity and adverse events vs vancomycin in the treatment of Gram-positive bacterial infections [4,16,23]. Our present study confirmed that linezolid caused a lower incidence of adverse events than vancomycin particularly in rash, red man syndrome, and abnormal increase in alanine aminotransferase.
Our present study confirmed that linezolid and vancomycin had equivalent efficacies in the treatment of Gram-positive bacterial infections. This finding was in line with the other systematic reviews that were previously reported by Ioannidou et al. [14] and Garazzino and Tovo [24]. A study by Li et al. [23] proposed that the efficacy of linezolid was superior against vancomycin in the treatment of infections caused by MRSA. Liang et al. [16] also revealed that linezolid had a superior clinical and microbiological outcome to vancomycin in skin and soft-tissue infections caused by S. aureus. Both the studies found that linezolid presented a better eradication rate than vancomycin in microbiologically evaluable adult patients [16,23]. The result in our study showed that there were no differences in clinical cure rates in microbiologically evaluable and clinically evaluable patients (<12 years) between linezolid and vancomycin. This result was consistent with that reported by Ioannidou et al. [14]. The sample size and patients’ age in these comparisons might be responsible for the differences between these studies.
There is increasing evidence showing the emergence of linezolid-resistant S. aureus during the treatment of infections, as well as the co-emergence of linezolid-resistant S. aureus and Enterococcus faecium in a patient with MRSA pneumonic sepsis [25–28]. Sánchez-García et al. found a clinical outbreak of linezolid-resistant S. aureus in ventilator-assisted pneumonia and bacteremia [29]. Toh et al. identified that the acquired linezolid resistance in a hospital MRSA strain was associated with the presence of the cfr gene [27]. The cfr gene is linked to the ermB gene, which confers resistance to all the clinically relevant antibiotics that target the large ribosomal subunit in bacteria [27]. Besier et al. [26] also identified a mutation in the 23S rRNA gene in S. aureus that conferred linezolid resistance. The increasing emergence of linezolid-resistant S. aureus suggested that new antibiotics are in demand in the treatment of nosocomial infections.
Two limitations were included in this present study. First, the sample size for these comparisons was small (n = 638) and studies with larger cohorts should be performed. Second, only five studies were included, and four [1–4] were published by the same research team. Accordingly, this study might reflect the situation of a local hospital. Third, our results showed that there was no difference in the efficacy between linezolid and vancomycin in treating Gram-positive bacterial infections in children under 12 years. However, our present analysis showed that linezolid had a superior safety to vancomycin for resistant Gram-positive bacterial infections. Patients who received linezolid had lower incidence rates of rash, red man syndrome, and alanine aminotransferase increase than vancomycin.
5 Conclusion
This systematic review suggested the efficacy and safety of linezolid in the treatment of resistant Gram-positive bacterial infections in children <12 years. Linezolid might be prescribed safely by neonatologists and pediatricians in the treatment of Gram-positive bacterial pathogens. Further studies providing evidence with a larger size of patients should be performed to validate the efficacy of linezolid.
Abbreviations
- CI
-
confidence interval
- CoNS
-
coagulase-negative Staphylococci
- FDA
-
Food and Drug Administration
- MRSA
-
methicillin-resistant S. aureus
- OR
-
odds ratio
- RCT
-
randomized controlled trial
- VRE
-
vancomycin-resistant enterococci
Acknowledgments
None.
-
Funding information: None.
-
Author contributions: Study conception and design: Jianyang Jiang, Qian Wu, Mingqing Tian, and Xiaohua Xu. Data collection and analysis: Jianyang Jiang and Qian Wu. Manuscript drafting: Qian Wu. Previous and final revisions of the manuscript: Xiaohua Xu, Mingqing Tian, and Jianyang Jiang. All authors read and approved the final manuscript.
-
Conflict of interest: The authors declare that there is no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Jantausch B, Deville J, Adler S, Morfin M, Lopez P, Edge-Padbury B, et al. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant gram-positive bacterial pathogens. Pediatr Infect Dis J. 2003;22(9 Suppl):164–71. 10.1097/01.inf.0000086956.45566.55.Search in Google Scholar PubMed
[2] Kaplan S, Deville J, Yogev R, Morfin M, Wu E, Alder S, et al. Linezolid versus vancomycin for treatment of resistant Gram-positive infections in children. Pediatr Infect Dis J. 2003;22(8):677–86. 10.1097/01.inf.0000078160.29072.42.Search in Google Scholar PubMed
[3] Deville J, Adler S, Azimi P, Jantausch B, Morfin M, Beltran S, et al. Linezolid versus vancomycin in the treatment of known or suspected resistant gram-positive infections in neonates. Pediatr Infect Dis J. 2003;22(9 Suppl):S158–S63. 10.1097/01.inf.0000086955.93702.c7.Search in Google Scholar PubMed
[4] Kaplan S, Afghani B, Lopez P, Wu E, Fleishaker D, Edge-Padbury B, et al. Linezolid for the treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2003;22(9 Suppl):S178–S85. 10.1097/01.inf.0000087020.75886.93.Search in Google Scholar PubMed
[5] Jacqz-Aigrain E, Zhao W, Sharland M, van den Anker JN. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med. 2013;18(1):28–34.10.1016/j.siny.2012.10.003Search in Google Scholar PubMed
[6] Linder N, Lubin D, Hernandez A, Amit L, Ashkenazi S. Duration of vancomycin treatment for coagulase-negative Staphylococcus sepsis in very low birth weight infants. Br J Clin Pharmacol. 2013;76(1):58–64.10.1111/bcp.12053Search in Google Scholar PubMed PubMed Central
[7] Dawoud TH, Khan N, Afzal U, Varghese N, Rahmani A, Abu S, et al. Assessment of initial vancomycin trough levels and risk factors of vancomycin-induced nephrotoxicity in neonates. Eur J Hosp Pharm. 2022;29:44–9. 10.1136/ejhpharm-2019-002181.Search in Google Scholar PubMed PubMed Central
[8] Martini S, Alessandroni R, Arcuri S, Faldella G. Vancomycin-induced red man syndrome presentation in a preterm infant. Pediatr Dermatol. 2018;35(6):e408–9.10.1111/pde.13654Search in Google Scholar PubMed
[9] Hashemian S, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;12:1759–67. 10.147/DDDT.S164515.Search in Google Scholar
[10] Zahedi-Bialvaei A, Rahbar M, Yousefi M, Asgharzadeh M, Samadi-Kafil H. Linezolid: a promising option in the treatment of Gram-positives. J Antimicrob Chemother. 2017;72(2):354–64. 10.1093/jac/dkw450.Search in Google Scholar PubMed
[11] Meissner H, Townsend T, Wenman W, Kaplan S, Morfin M, Edge-Padbury B, et al. Hematologic effects of linezolid in young children. Pediatr Infect Dis J. 2003;22(9 Suppl):S186–92. 10.1097/01.inf.0000087021.20838.d9.Search in Google Scholar
[12] Shibata Y, Yamagishi Y, Mikamo H, Kato H, Nishiyama N, Asai N, et al. Comparative study on safety of linezolid and vancomycin in the treatment of infants and neonates for Gram-positive bacterial infections. J Infect Chemother. 2018;27(9):695–701. 10.1016/j.jiac.2018.04.006.Search in Google Scholar
[13] Bayram N, Düzgöl M, Kara A, Özdemir F, Devrim İ. Linezolid-related adverse effects in clinical practice in children. Arch Argent Pediatr. 2017;115(5):470–5.10.5546/aap.2017.eng.470Search in Google Scholar
[14] Ioannidou M, Apostolidou-Kiouti F, Haidich A, Niopas I, Roilides E. Efficacy and safety of linezolid for the treatment of infections in children: a meta-analysis. Eur J Pediatr. 2014;173(9):1179–86. 10.007/s00431-014-2307-5.Search in Google Scholar
[15] Itani KMF, Dryden MS, Bhattacharyya H, Kunkel MJ, Baruch AM, Weigelt JA. Efficacy and safety of linezolid versus vancomycin for the treatment of complicated skin and soft-tissue infections proven to be caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2010;199(6):810–6. 10.1016/j.amjsurg.2009.08.045.Search in Google Scholar
[16] Liang B, Cai Y, Chen M-L, Bai N, Yu X-H, Wang R. Linezolid versus vancomycin for the treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2010;35(1):3–12. 10.1016/j.ijantimicag.2009.09.013.Search in Google Scholar
[17] Zhang W, Xu N, Bai T, Huang Z-H, Lv J, Wu W-J, et al. Efficacy and safety of linezolid versus vancomycin for methicillin-resistant Staphylococcus aureus (MRSA)-related pneumonia: updated systematic review and meta-analysis. Int J Clin Exp Med. 2019;12(4):3185–200.Search in Google Scholar
[18] Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Rev Esp Nutr Huma Diet. 2009;18(3):e123.10.1371/journal.pmed.1000097Search in Google Scholar
[19] Dersimonian R, Nan L. Meta-analysis in clinical trials. Controll Clin Trials. 1986;7(3):177–88.10.1016/0197-2456(86)90046-2Search in Google Scholar
[20] Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controll Clin Trials. 1996;17(1):1–12.10.1016/0197-2456(95)00134-4Search in Google Scholar
[21] Jacqueline C, Broquet A, Roquilly A, Davieau M, Caillon J, Altare F, et al. Linezolid dampens neutrophil-mediated inflammation in methicillin-resistant Staphylococcus aureus-induced pneumonia and protects the lung of associated damages. J Infect Dis. 2014;210(5):814–23. 10.1093/infdis/jiu145.Search in Google Scholar PubMed
[22] van Rensburg L, van Zyl J, Smith J. Deposition and transport of linezolid mediated by a synthetic surfactant Synsurf® within a pressurized metered dose inhaler: a Calu-3 model. Drug Des Devel Ther. 2018;12:1107–18. 10.2147/DDDT.S147035.Search in Google Scholar PubMed PubMed Central
[23] Li J, Zhao Q-H, Huang K-C, Zhang L-Y, Yin D-Y, Pan F, et al. Linezolid vs vancomycin in treatment of methicillin-resistant staphylococcus aureus infections: a meta-analysis. Eur Rev Med Pharmacol Sci. 2017;21(17):3974–9.Search in Google Scholar
[24] Garazzino S, Tovo P. Clinical experience with linezolid in infants and children. J Antimicrob Chemother. 2011;66(4s):iv23–41. 10.1093/jac/dkr074.Search in Google Scholar
[25] An D, Mi YL, Jeong TD, Sung H, Kim MN, Hong SB. Co-emergence of linezolid-resistant Staphylococcus aureus and Enterococcus faecium in a patient with methicillin-resistant S. aureus pneumonic sepsis. Diagn Microbiol Infect Dis. 2011;69(2):232–3. 10.1016/j.diagmicrobio.2010.09.002.Search in Google Scholar
[26] Besier S, Ludwig A, Zander J, Brade V, Wichelhaus TA. Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob Agents Chemother. 2008;52(4):1570–2.10.1128/AAC.01098-07Search in Google Scholar
[27] Toh S, Xiong L, Arias C, Villegas M, Lolans K, Quinn J, et al. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol Microbiol. 2007;64(6):1506–14. 10.111/j.365-2958.007.05744.x.Search in Google Scholar
[28] Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet. 2001;358(9277):207–8.10.1016/S0140-6736(01)05410-1Search in Google Scholar
[29] Sánchez-García M, De la Torre M, Morales G, Peláez B, Tolón M, Domingo S, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303(22):2260–4. 10.1001/jama.2010.757.Search in Google Scholar PubMed
© 2022 Qian Wu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- AMBRA1 attenuates the proliferation of uveal melanoma cells
- A ceRNA network mediated by LINC00475 in papillary thyroid carcinoma
- Differences in complications between hepatitis B-related cirrhosis and alcohol-related cirrhosis
- Effect of gestational diabetes mellitus on lipid profile: A systematic review and meta-analysis
- Long noncoding RNA NR2F1-AS1 stimulates the tumorigenic behavior of non-small cell lung cancer cells by sponging miR-363-3p to increase SOX4
- Promising novel biomarkers and candidate small-molecule drugs for lung adenocarcinoma: Evidence from bioinformatics analysis of high-throughput data
- Plasmapheresis: Is it a potential alternative treatment for chronic urticaria?
- The biomarkers of key miRNAs and gene targets associated with extranodal NK/T-cell lymphoma
- Gene signature to predict prognostic survival of hepatocellular carcinoma
- Effects of miRNA-199a-5p on cell proliferation and apoptosis of uterine leiomyoma by targeting MED12
- Does diabetes affect paraneoplastic thrombocytosis in colorectal cancer?
- Is there any effect on imprinted genes H19, PEG3, and SNRPN during AOA?
- Leptin and PCSK9 concentrations are associated with vascular endothelial cytokines in patients with stable coronary heart disease
- Pericentric inversion of chromosome 6 and male fertility problems
- Staple line reinforcement with nebulized cyanoacrylate glue in laparoscopic sleeve gastrectomy: A propensity score-matched study
- Retrospective analysis of crescent score in clinical prognosis of IgA nephropathy
- Expression of DNM3 is associated with good outcome in colorectal cancer
- Activation of SphK2 contributes to adipocyte-induced EOC cell proliferation
- CRRT influences PICCO measurements in febrile critically ill patients
- SLCO4A1-AS1 mediates pancreatic cancer development via miR-4673/KIF21B axis
- lncRNA ACTA2-AS1 inhibits malignant phenotypes of gastric cancer cells
- circ_AKT3 knockdown suppresses cisplatin resistance in gastric cancer
- Prognostic value of nicotinamide N-methyltransferase in human cancers: Evidence from a meta-analysis and database validation
- GPC2 deficiency inhibits cell growth and metastasis in colon adenocarcinoma
- A pan-cancer analysis of the oncogenic role of Holliday junction recognition protein in human tumors
- Radiation increases COL1A1, COL3A1, and COL1A2 expression in breast cancer
- Association between preventable risk factors and metabolic syndrome
- miR-29c-5p knockdown reduces inflammation and blood–brain barrier disruption by upregulating LRP6
- Cardiac contractility modulation ameliorates myocardial metabolic remodeling in a rabbit model of chronic heart failure through activation of AMPK and PPAR-α pathway
- Quercitrin protects human bronchial epithelial cells from oxidative damage
- Smurf2 suppresses the metastasis of hepatocellular carcinoma via ubiquitin degradation of Smad2
- circRNA_0001679/miR-338-3p/DUSP16 axis aggravates acute lung injury
- Sonoclot’s usefulness in prediction of cardiopulmonary arrest prognosis: A proof of concept study
- Four drug metabolism-related subgroups of pancreatic adenocarcinoma in prognosis, immune infiltration, and gene mutation
- Decreased expression of miR-195 mediated by hypermethylation promotes osteosarcoma
- LMO3 promotes proliferation and metastasis of papillary thyroid carcinoma cells by regulating LIMK1-mediated cofilin and the β-catenin pathway
- Cx43 upregulation in HUVECs under stretch via TGF-β1 and cytoskeletal network
- Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey
- Histopathologic findings on removed stomach after sleeve gastrectomy. Do they influence the outcome?
- Analysis of the expression and prognostic value of MT1-MMP, β1-integrin and YAP1 in glioma
- Optimal diagnosis of the skin cancer using a hybrid deep neural network and grasshopper optimization algorithm
- miR-223-3p alleviates TGF-β-induced epithelial-mesenchymal transition and extracellular matrix deposition by targeting SP3 in endometrial epithelial cells
- Clinical value of SIRT1 as a prognostic biomarker in esophageal squamous cell carcinoma, a systematic meta-analysis
- circ_0020123 promotes cell proliferation and migration in lung adenocarcinoma via PDZD8
- miR-22-5p regulates the self-renewal of spermatogonial stem cells by targeting EZH2
- hsa-miR-340-5p inhibits epithelial–mesenchymal transition in endometriosis by targeting MAP3K2 and inactivating MAPK/ERK signaling
- circ_0085296 inhibits the biological functions of trophoblast cells to promote the progression of preeclampsia via the miR-942-5p/THBS2 network
- TCD hemodynamics findings in the subacute phase of anterior circulation stroke patients treated with mechanical thrombectomy
- Development of a risk-stratification scoring system for predicting risk of breast cancer based on non-alcoholic fatty liver disease, non-alcoholic fatty pancreas disease, and uric acid
- Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway
- circ_0062491 alleviates periodontitis via the miR-142-5p/IGF1 axis
- Human amniotic fluid as a source of stem cells
- lncRNA NONRATT013819.2 promotes transforming growth factor-β1-induced myofibroblastic transition of hepatic stellate cells by miR24-3p/lox
- NORAD modulates miR-30c-5p-LDHA to protect lung endothelial cells damage
- Idiopathic pulmonary fibrosis telemedicine management during COVID-19 outbreak
- Risk factors for adverse drug reactions associated with clopidogrel therapy
- Serum zinc associated with immunity and inflammatory markers in Covid-19
- The relationship between night shift work and breast cancer incidence: A systematic review and meta-analysis of observational studies
- LncRNA expression in idiopathic achalasia: New insight and preliminary exploration into pathogenesis
- Notoginsenoside R1 alleviates spinal cord injury through the miR-301a/KLF7 axis to activate Wnt/β-catenin pathway
- Moscatilin suppresses the inflammation from macrophages and T cells
- Zoledronate promotes ECM degradation and apoptosis via Wnt/β-catenin
- Epithelial-mesenchymal transition-related genes in coronary artery disease
- The effect evaluation of traditional vaginal surgery and transvaginal mesh surgery for severe pelvic organ prolapse: 5 years follow-up
- Repeated partial splenic artery embolization for hypersplenism improves platelet count
- Low expression of miR-27b in serum exosomes of non-small cell lung cancer facilitates its progression by affecting EGFR
- Exosomal hsa_circ_0000519 modulates the NSCLC cell growth and metastasis via miR-1258/RHOV axis
- miR-455-5p enhances 5-fluorouracil sensitivity in colorectal cancer cells by targeting PIK3R1 and DEPDC1
- The effect of tranexamic acid on the reduction of intraoperative and postoperative blood loss and thromboembolic risk in patients with hip fracture
- Isocitrate dehydrogenase 1 mutation in cholangiocarcinoma impairs tumor progression by sensitizing cells to ferroptosis
- Artemisinin protects against cerebral ischemia and reperfusion injury via inhibiting the NF-κB pathway
- A 16-gene signature associated with homologous recombination deficiency for prognosis prediction in patients with triple-negative breast cancer
- Lidocaine ameliorates chronic constriction injury-induced neuropathic pain through regulating M1/M2 microglia polarization
- MicroRNA 322-5p reduced neuronal inflammation via the TLR4/TRAF6/NF-κB axis in a rat epilepsy model
- miR-1273h-5p suppresses CXCL12 expression and inhibits gastric cancer cell invasion and metastasis
- Clinical characteristics of pneumonia patients of long course of illness infected with SARS-CoV-2
- circRNF20 aggravates the malignancy of retinoblastoma depending on the regulation of miR-132-3p/PAX6 axis
- Linezolid for resistant Gram-positive bacterial infections in children under 12 years: A meta-analysis
- Rack1 regulates pro-inflammatory cytokines by NF-κB in diabetic nephropathy
- Comprehensive analysis of molecular mechanism and a novel prognostic signature based on small nuclear RNA biomarkers in gastric cancer patients
- Smog and risk of maternal and fetal birth outcomes: A retrospective study in Baoding, China
- Let-7i-3p inhibits the cell cycle, proliferation, invasion, and migration of colorectal cancer cells via downregulating CCND1
- β2-Adrenergic receptor expression in subchondral bone of patients with varus knee osteoarthritis
- Possible impact of COVID-19 pandemic and lockdown on suicide behavior among patients in Southeast Serbia
- In vitro antimicrobial activity of ozonated oil in liposome eyedrop against multidrug-resistant bacteria
- Potential biomarkers for inflammatory response in acute lung injury
- A low serum uric acid concentration predicts a poor prognosis in adult patients with candidemia
- Antitumor activity of recombinant oncolytic vaccinia virus with human IL2
- ALKBH5 inhibits TNF-α-induced apoptosis of HUVECs through Bcl-2 pathway
- Risk prediction of cardiovascular disease using machine learning classifiers
- Value of ultrasonography parameters in diagnosing polycystic ovary syndrome
- Bioinformatics analysis reveals three key genes and four survival genes associated with youth-onset NSCLC
- Identification of autophagy-related biomarkers in patients with pulmonary arterial hypertension based on bioinformatics analysis
- Protective effects of glaucocalyxin A on the airway of asthmatic mice
- Overexpression of miR-100-5p inhibits papillary thyroid cancer progression via targeting FZD8
- Bioinformatics-based analysis of SUMOylation-related genes in hepatocellular carcinoma reveals a role of upregulated SAE1 in promoting cell proliferation
- Effectiveness and clinical benefits of new anti-diabetic drugs: A real life experience
- Identification of osteoporosis based on gene biomarkers using support vector machine
- Tanshinone IIA reverses oxaliplatin resistance in colorectal cancer through microRNA-30b-5p/AVEN axis
- miR-212-5p inhibits nasopharyngeal carcinoma progression by targeting METTL3
- Association of ST-T changes with all-cause mortality among patients with peripheral T-cell lymphomas
- LINC00665/miRNAs axis-mediated collagen type XI alpha 1 correlates with immune infiltration and malignant phenotypes in lung adenocarcinoma
- The perinatal factors that influence the excretion of fecal calprotectin in premature-born children
- Effect of femoral head necrosis cystic area on femoral head collapse and stress distribution in femoral head: A clinical and finite element study
- Does the use of 3D-printed cones give a chance to postpone the use of megaprostheses in patients with large bone defects in the knee joint?
- lncRNA HAGLR modulates myocardial ischemia–reperfusion injury in mice through regulating miR-133a-3p/MAPK1 axis
- Protective effect of ghrelin on intestinal I/R injury in rats
- In vivo knee kinematics of an innovative prosthesis design
- Relationship between the height of fibular head and the incidence and severity of knee osteoarthritis
- lncRNA WT1-AS attenuates hypoxia/ischemia-induced neuronal injury during cerebral ischemic stroke via miR-186-5p/XIAP axis
- Correlation of cardiac troponin T and APACHE III score with all-cause in-hospital mortality in critically ill patients with acute pulmonary embolism
- LncRNA LINC01857 reduces metastasis and angiogenesis in breast cancer cells via regulating miR-2052/CENPQ axis
- Endothelial cell-specific molecule 1 (ESM1) promoted by transcription factor SPI1 acts as an oncogene to modulate the malignant phenotype of endometrial cancer
- SELENBP1 inhibits progression of colorectal cancer by suppressing epithelial–mesenchymal transition
- Visfatin is negatively associated with coronary artery lesions in subjects with impaired fasting glucose
- Treatment and outcomes of mechanical complications of acute myocardial infarction during the Covid-19 era: A comparison with the pre-Covid-19 period. A systematic review and meta-analysis
- Neonatal stroke surveillance study protocol in the United Kingdom and Republic of Ireland
- Oncogenic role of TWF2 in human tumors: A pan-cancer analysis
- Mean corpuscular hemoglobin predicts the length of hospital stay independent of severity classification in patients with acute pancreatitis
- Association of gallstone and polymorphisms of UGT1A1*27 and UGT1A1*28 in patients with hepatitis B virus-related liver failure
- TGF-β1 upregulates Sar1a expression and induces procollagen-I secretion in hypertrophic scarring fibroblasts
- Antisense lncRNA PCNA-AS1 promotes esophageal squamous cell carcinoma progression through the miR-2467-3p/PCNA axis
- NK-cell dysfunction of acute myeloid leukemia in relation to the renin–angiotensin system and neurotransmitter genes
- The effect of dilution with glucose and prolonged injection time on dexamethasone-induced perineal irritation – A randomized controlled trial
- miR-146-5p restrains calcification of vascular smooth muscle cells by suppressing TRAF6
- Role of lncRNA MIAT/miR-361-3p/CCAR2 in prostate cancer cells
- lncRNA NORAD promotes lung cancer progression by competitively binding to miR-28-3p with E2F2
- Noninvasive diagnosis of AIH/PBC overlap syndrome based on prediction models
- lncRNA FAM230B is highly expressed in colorectal cancer and suppresses the maturation of miR-1182 to increase cell proliferation
- circ-LIMK1 regulates cisplatin resistance in lung adenocarcinoma by targeting miR-512-5p/HMGA1 axis
- LncRNA SNHG3 promoted cell proliferation, migration, and metastasis of esophageal squamous cell carcinoma via regulating miR-151a-3p/PFN2 axis
- Risk perception and affective state on work exhaustion in obstetrics during the COVID-19 pandemic
- lncRNA-AC130710/miR-129-5p/mGluR1 axis promote migration and invasion by activating PKCα-MAPK signal pathway in melanoma
- SNRPB promotes cell cycle progression in thyroid carcinoma via inhibiting p53
- Xylooligosaccharides and aerobic training regulate metabolism and behavior in rats with streptozotocin-induced type 1 diabetes
- Serpin family A member 1 is an oncogene in glioma and its translation is enhanced by NAD(P)H quinone dehydrogenase 1 through RNA-binding activity
- Silencing of CPSF7 inhibits the proliferation, migration, and invasion of lung adenocarcinoma cells by blocking the AKT/mTOR signaling pathway
- Ultrasound-guided lumbar plexus block versus transversus abdominis plane block for analgesia in children with hip dislocation: A double-blind, randomized trial
- Relationship of plasma MBP and 8-oxo-dG with brain damage in preterm
- Identification of a novel necroptosis-associated miRNA signature for predicting the prognosis in head and neck squamous cell carcinoma
- Delayed femoral vein ligation reduces operative time and blood loss during hip disarticulation in patients with extremity tumors
- The expression of ASAP3 and NOTCH3 and the clinicopathological characteristics of adult glioma patients
- Longitudinal analysis of factors related to Helicobacter pylori infection in Chinese adults
- HOXA10 enhances cell proliferation and suppresses apoptosis in esophageal cancer via activating p38/ERK signaling pathway
- Meta-analysis of early-life antibiotic use and allergic rhinitis
- Marital status and its correlation with age, race, and gender in prognosis of tonsil squamous cell carcinomas
- HPV16 E6E7 up-regulates KIF2A expression by activating JNK/c-Jun signal, is beneficial to migration and invasion of cervical cancer cells
- Amino acid profiles in the tissue and serum of patients with liver cancer
- Pain in critically ill COVID-19 patients: An Italian retrospective study
- Immunohistochemical distribution of Bcl-2 and p53 apoptotic markers in acetamiprid-induced nephrotoxicity
- Estradiol pretreatment in GnRH antagonist protocol for IVF/ICSI treatment
- Long non-coding RNAs LINC00689 inhibits the apoptosis of human nucleus pulposus cells via miR-3127-5p/ATG7 axis-mediated autophagy
- The relationship between oxygen therapy, drug therapy, and COVID-19 mortality
- Monitoring hypertensive disorders in pregnancy to prevent preeclampsia in pregnant women of advanced maternal age: Trial mimicking with retrospective data
- SETD1A promotes the proliferation and glycolysis of nasopharyngeal carcinoma cells by activating the PI3K/Akt pathway
- The role of Shunaoxin pills in the treatment of chronic cerebral hypoperfusion and its main pharmacodynamic components
- TET3 governs malignant behaviors and unfavorable prognosis of esophageal squamous cell carcinoma by activating the PI3K/AKT/GSK3β/β-catenin pathway
- Associations between morphokinetic parameters of temporary-arrest embryos and the clinical prognosis in FET cycles
- Long noncoding RNA WT1-AS regulates trophoblast proliferation, migration, and invasion via the microRNA-186-5p/CADM2 axis
- The incidence of bronchiectasis in chronic obstructive pulmonary disease
- Integrated bioinformatics analysis shows integrin alpha 3 is a prognostic biomarker for pancreatic cancer
- Inhibition of miR-21 improves pulmonary vascular responses in bronchopulmonary dysplasia by targeting the DDAH1/ADMA/NO pathway
- Comparison of hospitalized patients with severe pneumonia caused by COVID-19 and influenza A (H7N9 and H1N1): A retrospective study from a designated hospital
- lncRNA ZFAS1 promotes intervertebral disc degeneration by upregulating AAK1
- Pathological characteristics of liver injury induced by N,N-dimethylformamide: From humans to animal models
- lncRNA ELFN1-AS1 enhances the progression of colon cancer by targeting miR-4270 to upregulate AURKB
- DARS-AS1 modulates cell proliferation and migration of gastric cancer cells by regulating miR-330-3p/NAT10 axis
- Dezocine inhibits cell proliferation, migration, and invasion by targeting CRABP2 in ovarian cancer
- MGST1 alleviates the oxidative stress of trophoblast cells induced by hypoxia/reoxygenation and promotes cell proliferation, migration, and invasion by activating the PI3K/AKT/mTOR pathway
- Bifidobacterium lactis Probio-M8 ameliorated the symptoms of type 2 diabetes mellitus mice by changing ileum FXR-CYP7A1
- circRNA DENND1B inhibits tumorigenicity of clear cell renal cell carcinoma via miR-122-5p/TIMP2 axis
- EphA3 targeted by miR-3666 contributes to melanoma malignancy via activating ERK1/2 and p38 MAPK pathways
- Pacemakers and methylprednisolone pulse therapy in immune-related myocarditis concomitant with complete heart block
- miRNA-130a-3p targets sphingosine-1-phosphate receptor 1 to activate the microglial and astrocytes and to promote neural injury under the high glucose condition
- Review Articles
- Current management of cancer pain in Italy: Expert opinion paper
- Hearing loss and brain disorders: A review of multiple pathologies
- The rationale for using low-molecular weight heparin in the therapy of symptomatic COVID-19 patients
- Amyotrophic lateral sclerosis and delayed onset muscle soreness in light of the impaired blink and stretch reflexes – watch out for Piezo2
- Interleukin-35 in autoimmune dermatoses: Current concepts
- Recent discoveries in microbiota dysbiosis, cholangiocytic factors, and models for studying the pathogenesis of primary sclerosing cholangitis
- Advantages of ketamine in pediatric anesthesia
- Congenital adrenal hyperplasia. Role of dentist in early diagnosis
- Migraine management: Non-pharmacological points for patients and health care professionals
- Atherogenic index of plasma and coronary artery disease: A systematic review
- Physiological and modulatory role of thioredoxins in the cellular function
- Case Reports
- Intrauterine Bakri balloon tamponade plus cervical cerclage for the prevention and treatment of postpartum haemorrhage in late pregnancy complicated with acute aortic dissection: Case series
- A case of successful pembrolizumab monotherapy in a patient with advanced lung adenocarcinoma: Use of multiple biomarkers in combination for clinical practice
- Unusual neurological manifestations of bilateral medial medullary infarction: A case report
- Atypical symptoms of malignant hyperthermia: A rare causative mutation in the RYR1 gene
- A case report of dermatomyositis with the missed diagnosis of non-small cell lung cancer and concurrence of pulmonary tuberculosis
- A rare case of endometrial polyp complicated with uterine inversion: A case report and clinical management
- Spontaneous rupturing of splenic artery aneurysm: Another reason for fatal syncope and shock (Case report and literature review)
- Fungal infection mimicking COVID-19 infection – A case report
- Concurrent aspergillosis and cystic pulmonary metastases in a patient with tongue squamous cell carcinoma
- Paraganglioma-induced inverted takotsubo-like cardiomyopathy leading to cardiogenic shock successfully treated with extracorporeal membrane oxygenation
- Lineage switch from lymphoma to myeloid neoplasms: First case series from a single institution
- Trismus during tracheal extubation as a complication of general anaesthesia – A case report
- Simultaneous treatment of a pubovesical fistula and lymph node metastasis secondary to multimodal treatment for prostate cancer: Case report and review of the literature
- Two case reports of skin vasculitis following the COVID-19 immunization
- Ureteroiliac fistula after oncological surgery: Case report and review of the literature
- Synchronous triple primary malignant tumours in the bladder, prostate, and lung harbouring TP53 and MEK1 mutations accompanied with severe cardiovascular diseases: A case report
- Huge mucinous cystic neoplasms with adhesion to the left colon: A case report and literature review
- Commentary
- Commentary on “Clinicopathological features of programmed cell death-ligand 1 expression in patients with oral squamous cell carcinoma”
- Rapid Communication
- COVID-19 fear, post-traumatic stress, growth, and the role of resilience
- Erratum
- Erratum to “Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway”
- Erratum to “Effect of femoral head necrosis cystic area on femoral head collapse and stress distribution in femoral head: A clinical and finite element study”
- Erratum to “lncRNA NORAD promotes lung cancer progression by competitively binding to miR-28-3p with E2F2”
- Retraction
- Expression and role of ABIN1 in sepsis: In vitro and in vivo studies
- Retraction to “miR-519d downregulates LEP expression to inhibit preeclampsia development”
- Special Issue Computational Intelligence Methodologies Meets Recurrent Cancers - Part II
- Usefulness of close surveillance for rectal cancer patients after neoadjuvant chemoradiotherapy
Articles in the same Issue
- Research Articles
- AMBRA1 attenuates the proliferation of uveal melanoma cells
- A ceRNA network mediated by LINC00475 in papillary thyroid carcinoma
- Differences in complications between hepatitis B-related cirrhosis and alcohol-related cirrhosis
- Effect of gestational diabetes mellitus on lipid profile: A systematic review and meta-analysis
- Long noncoding RNA NR2F1-AS1 stimulates the tumorigenic behavior of non-small cell lung cancer cells by sponging miR-363-3p to increase SOX4
- Promising novel biomarkers and candidate small-molecule drugs for lung adenocarcinoma: Evidence from bioinformatics analysis of high-throughput data
- Plasmapheresis: Is it a potential alternative treatment for chronic urticaria?
- The biomarkers of key miRNAs and gene targets associated with extranodal NK/T-cell lymphoma
- Gene signature to predict prognostic survival of hepatocellular carcinoma
- Effects of miRNA-199a-5p on cell proliferation and apoptosis of uterine leiomyoma by targeting MED12
- Does diabetes affect paraneoplastic thrombocytosis in colorectal cancer?
- Is there any effect on imprinted genes H19, PEG3, and SNRPN during AOA?
- Leptin and PCSK9 concentrations are associated with vascular endothelial cytokines in patients with stable coronary heart disease
- Pericentric inversion of chromosome 6 and male fertility problems
- Staple line reinforcement with nebulized cyanoacrylate glue in laparoscopic sleeve gastrectomy: A propensity score-matched study
- Retrospective analysis of crescent score in clinical prognosis of IgA nephropathy
- Expression of DNM3 is associated with good outcome in colorectal cancer
- Activation of SphK2 contributes to adipocyte-induced EOC cell proliferation
- CRRT influences PICCO measurements in febrile critically ill patients
- SLCO4A1-AS1 mediates pancreatic cancer development via miR-4673/KIF21B axis
- lncRNA ACTA2-AS1 inhibits malignant phenotypes of gastric cancer cells
- circ_AKT3 knockdown suppresses cisplatin resistance in gastric cancer
- Prognostic value of nicotinamide N-methyltransferase in human cancers: Evidence from a meta-analysis and database validation
- GPC2 deficiency inhibits cell growth and metastasis in colon adenocarcinoma
- A pan-cancer analysis of the oncogenic role of Holliday junction recognition protein in human tumors
- Radiation increases COL1A1, COL3A1, and COL1A2 expression in breast cancer
- Association between preventable risk factors and metabolic syndrome
- miR-29c-5p knockdown reduces inflammation and blood–brain barrier disruption by upregulating LRP6
- Cardiac contractility modulation ameliorates myocardial metabolic remodeling in a rabbit model of chronic heart failure through activation of AMPK and PPAR-α pathway
- Quercitrin protects human bronchial epithelial cells from oxidative damage
- Smurf2 suppresses the metastasis of hepatocellular carcinoma via ubiquitin degradation of Smad2
- circRNA_0001679/miR-338-3p/DUSP16 axis aggravates acute lung injury
- Sonoclot’s usefulness in prediction of cardiopulmonary arrest prognosis: A proof of concept study
- Four drug metabolism-related subgroups of pancreatic adenocarcinoma in prognosis, immune infiltration, and gene mutation
- Decreased expression of miR-195 mediated by hypermethylation promotes osteosarcoma
- LMO3 promotes proliferation and metastasis of papillary thyroid carcinoma cells by regulating LIMK1-mediated cofilin and the β-catenin pathway
- Cx43 upregulation in HUVECs under stretch via TGF-β1 and cytoskeletal network
- Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey
- Histopathologic findings on removed stomach after sleeve gastrectomy. Do they influence the outcome?
- Analysis of the expression and prognostic value of MT1-MMP, β1-integrin and YAP1 in glioma
- Optimal diagnosis of the skin cancer using a hybrid deep neural network and grasshopper optimization algorithm
- miR-223-3p alleviates TGF-β-induced epithelial-mesenchymal transition and extracellular matrix deposition by targeting SP3 in endometrial epithelial cells
- Clinical value of SIRT1 as a prognostic biomarker in esophageal squamous cell carcinoma, a systematic meta-analysis
- circ_0020123 promotes cell proliferation and migration in lung adenocarcinoma via PDZD8
- miR-22-5p regulates the self-renewal of spermatogonial stem cells by targeting EZH2
- hsa-miR-340-5p inhibits epithelial–mesenchymal transition in endometriosis by targeting MAP3K2 and inactivating MAPK/ERK signaling
- circ_0085296 inhibits the biological functions of trophoblast cells to promote the progression of preeclampsia via the miR-942-5p/THBS2 network
- TCD hemodynamics findings in the subacute phase of anterior circulation stroke patients treated with mechanical thrombectomy
- Development of a risk-stratification scoring system for predicting risk of breast cancer based on non-alcoholic fatty liver disease, non-alcoholic fatty pancreas disease, and uric acid
- Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway
- circ_0062491 alleviates periodontitis via the miR-142-5p/IGF1 axis
- Human amniotic fluid as a source of stem cells
- lncRNA NONRATT013819.2 promotes transforming growth factor-β1-induced myofibroblastic transition of hepatic stellate cells by miR24-3p/lox
- NORAD modulates miR-30c-5p-LDHA to protect lung endothelial cells damage
- Idiopathic pulmonary fibrosis telemedicine management during COVID-19 outbreak
- Risk factors for adverse drug reactions associated with clopidogrel therapy
- Serum zinc associated with immunity and inflammatory markers in Covid-19
- The relationship between night shift work and breast cancer incidence: A systematic review and meta-analysis of observational studies
- LncRNA expression in idiopathic achalasia: New insight and preliminary exploration into pathogenesis
- Notoginsenoside R1 alleviates spinal cord injury through the miR-301a/KLF7 axis to activate Wnt/β-catenin pathway
- Moscatilin suppresses the inflammation from macrophages and T cells
- Zoledronate promotes ECM degradation and apoptosis via Wnt/β-catenin
- Epithelial-mesenchymal transition-related genes in coronary artery disease
- The effect evaluation of traditional vaginal surgery and transvaginal mesh surgery for severe pelvic organ prolapse: 5 years follow-up
- Repeated partial splenic artery embolization for hypersplenism improves platelet count
- Low expression of miR-27b in serum exosomes of non-small cell lung cancer facilitates its progression by affecting EGFR
- Exosomal hsa_circ_0000519 modulates the NSCLC cell growth and metastasis via miR-1258/RHOV axis
- miR-455-5p enhances 5-fluorouracil sensitivity in colorectal cancer cells by targeting PIK3R1 and DEPDC1
- The effect of tranexamic acid on the reduction of intraoperative and postoperative blood loss and thromboembolic risk in patients with hip fracture
- Isocitrate dehydrogenase 1 mutation in cholangiocarcinoma impairs tumor progression by sensitizing cells to ferroptosis
- Artemisinin protects against cerebral ischemia and reperfusion injury via inhibiting the NF-κB pathway
- A 16-gene signature associated with homologous recombination deficiency for prognosis prediction in patients with triple-negative breast cancer
- Lidocaine ameliorates chronic constriction injury-induced neuropathic pain through regulating M1/M2 microglia polarization
- MicroRNA 322-5p reduced neuronal inflammation via the TLR4/TRAF6/NF-κB axis in a rat epilepsy model
- miR-1273h-5p suppresses CXCL12 expression and inhibits gastric cancer cell invasion and metastasis
- Clinical characteristics of pneumonia patients of long course of illness infected with SARS-CoV-2
- circRNF20 aggravates the malignancy of retinoblastoma depending on the regulation of miR-132-3p/PAX6 axis
- Linezolid for resistant Gram-positive bacterial infections in children under 12 years: A meta-analysis
- Rack1 regulates pro-inflammatory cytokines by NF-κB in diabetic nephropathy
- Comprehensive analysis of molecular mechanism and a novel prognostic signature based on small nuclear RNA biomarkers in gastric cancer patients
- Smog and risk of maternal and fetal birth outcomes: A retrospective study in Baoding, China
- Let-7i-3p inhibits the cell cycle, proliferation, invasion, and migration of colorectal cancer cells via downregulating CCND1
- β2-Adrenergic receptor expression in subchondral bone of patients with varus knee osteoarthritis
- Possible impact of COVID-19 pandemic and lockdown on suicide behavior among patients in Southeast Serbia
- In vitro antimicrobial activity of ozonated oil in liposome eyedrop against multidrug-resistant bacteria
- Potential biomarkers for inflammatory response in acute lung injury
- A low serum uric acid concentration predicts a poor prognosis in adult patients with candidemia
- Antitumor activity of recombinant oncolytic vaccinia virus with human IL2
- ALKBH5 inhibits TNF-α-induced apoptosis of HUVECs through Bcl-2 pathway
- Risk prediction of cardiovascular disease using machine learning classifiers
- Value of ultrasonography parameters in diagnosing polycystic ovary syndrome
- Bioinformatics analysis reveals three key genes and four survival genes associated with youth-onset NSCLC
- Identification of autophagy-related biomarkers in patients with pulmonary arterial hypertension based on bioinformatics analysis
- Protective effects of glaucocalyxin A on the airway of asthmatic mice
- Overexpression of miR-100-5p inhibits papillary thyroid cancer progression via targeting FZD8
- Bioinformatics-based analysis of SUMOylation-related genes in hepatocellular carcinoma reveals a role of upregulated SAE1 in promoting cell proliferation
- Effectiveness and clinical benefits of new anti-diabetic drugs: A real life experience
- Identification of osteoporosis based on gene biomarkers using support vector machine
- Tanshinone IIA reverses oxaliplatin resistance in colorectal cancer through microRNA-30b-5p/AVEN axis
- miR-212-5p inhibits nasopharyngeal carcinoma progression by targeting METTL3
- Association of ST-T changes with all-cause mortality among patients with peripheral T-cell lymphomas
- LINC00665/miRNAs axis-mediated collagen type XI alpha 1 correlates with immune infiltration and malignant phenotypes in lung adenocarcinoma
- The perinatal factors that influence the excretion of fecal calprotectin in premature-born children
- Effect of femoral head necrosis cystic area on femoral head collapse and stress distribution in femoral head: A clinical and finite element study
- Does the use of 3D-printed cones give a chance to postpone the use of megaprostheses in patients with large bone defects in the knee joint?
- lncRNA HAGLR modulates myocardial ischemia–reperfusion injury in mice through regulating miR-133a-3p/MAPK1 axis
- Protective effect of ghrelin on intestinal I/R injury in rats
- In vivo knee kinematics of an innovative prosthesis design
- Relationship between the height of fibular head and the incidence and severity of knee osteoarthritis
- lncRNA WT1-AS attenuates hypoxia/ischemia-induced neuronal injury during cerebral ischemic stroke via miR-186-5p/XIAP axis
- Correlation of cardiac troponin T and APACHE III score with all-cause in-hospital mortality in critically ill patients with acute pulmonary embolism
- LncRNA LINC01857 reduces metastasis and angiogenesis in breast cancer cells via regulating miR-2052/CENPQ axis
- Endothelial cell-specific molecule 1 (ESM1) promoted by transcription factor SPI1 acts as an oncogene to modulate the malignant phenotype of endometrial cancer
- SELENBP1 inhibits progression of colorectal cancer by suppressing epithelial–mesenchymal transition
- Visfatin is negatively associated with coronary artery lesions in subjects with impaired fasting glucose
- Treatment and outcomes of mechanical complications of acute myocardial infarction during the Covid-19 era: A comparison with the pre-Covid-19 period. A systematic review and meta-analysis
- Neonatal stroke surveillance study protocol in the United Kingdom and Republic of Ireland
- Oncogenic role of TWF2 in human tumors: A pan-cancer analysis
- Mean corpuscular hemoglobin predicts the length of hospital stay independent of severity classification in patients with acute pancreatitis
- Association of gallstone and polymorphisms of UGT1A1*27 and UGT1A1*28 in patients with hepatitis B virus-related liver failure
- TGF-β1 upregulates Sar1a expression and induces procollagen-I secretion in hypertrophic scarring fibroblasts
- Antisense lncRNA PCNA-AS1 promotes esophageal squamous cell carcinoma progression through the miR-2467-3p/PCNA axis
- NK-cell dysfunction of acute myeloid leukemia in relation to the renin–angiotensin system and neurotransmitter genes
- The effect of dilution with glucose and prolonged injection time on dexamethasone-induced perineal irritation – A randomized controlled trial
- miR-146-5p restrains calcification of vascular smooth muscle cells by suppressing TRAF6
- Role of lncRNA MIAT/miR-361-3p/CCAR2 in prostate cancer cells
- lncRNA NORAD promotes lung cancer progression by competitively binding to miR-28-3p with E2F2
- Noninvasive diagnosis of AIH/PBC overlap syndrome based on prediction models
- lncRNA FAM230B is highly expressed in colorectal cancer and suppresses the maturation of miR-1182 to increase cell proliferation
- circ-LIMK1 regulates cisplatin resistance in lung adenocarcinoma by targeting miR-512-5p/HMGA1 axis
- LncRNA SNHG3 promoted cell proliferation, migration, and metastasis of esophageal squamous cell carcinoma via regulating miR-151a-3p/PFN2 axis
- Risk perception and affective state on work exhaustion in obstetrics during the COVID-19 pandemic
- lncRNA-AC130710/miR-129-5p/mGluR1 axis promote migration and invasion by activating PKCα-MAPK signal pathway in melanoma
- SNRPB promotes cell cycle progression in thyroid carcinoma via inhibiting p53
- Xylooligosaccharides and aerobic training regulate metabolism and behavior in rats with streptozotocin-induced type 1 diabetes
- Serpin family A member 1 is an oncogene in glioma and its translation is enhanced by NAD(P)H quinone dehydrogenase 1 through RNA-binding activity
- Silencing of CPSF7 inhibits the proliferation, migration, and invasion of lung adenocarcinoma cells by blocking the AKT/mTOR signaling pathway
- Ultrasound-guided lumbar plexus block versus transversus abdominis plane block for analgesia in children with hip dislocation: A double-blind, randomized trial
- Relationship of plasma MBP and 8-oxo-dG with brain damage in preterm
- Identification of a novel necroptosis-associated miRNA signature for predicting the prognosis in head and neck squamous cell carcinoma
- Delayed femoral vein ligation reduces operative time and blood loss during hip disarticulation in patients with extremity tumors
- The expression of ASAP3 and NOTCH3 and the clinicopathological characteristics of adult glioma patients
- Longitudinal analysis of factors related to Helicobacter pylori infection in Chinese adults
- HOXA10 enhances cell proliferation and suppresses apoptosis in esophageal cancer via activating p38/ERK signaling pathway
- Meta-analysis of early-life antibiotic use and allergic rhinitis
- Marital status and its correlation with age, race, and gender in prognosis of tonsil squamous cell carcinomas
- HPV16 E6E7 up-regulates KIF2A expression by activating JNK/c-Jun signal, is beneficial to migration and invasion of cervical cancer cells
- Amino acid profiles in the tissue and serum of patients with liver cancer
- Pain in critically ill COVID-19 patients: An Italian retrospective study
- Immunohistochemical distribution of Bcl-2 and p53 apoptotic markers in acetamiprid-induced nephrotoxicity
- Estradiol pretreatment in GnRH antagonist protocol for IVF/ICSI treatment
- Long non-coding RNAs LINC00689 inhibits the apoptosis of human nucleus pulposus cells via miR-3127-5p/ATG7 axis-mediated autophagy
- The relationship between oxygen therapy, drug therapy, and COVID-19 mortality
- Monitoring hypertensive disorders in pregnancy to prevent preeclampsia in pregnant women of advanced maternal age: Trial mimicking with retrospective data
- SETD1A promotes the proliferation and glycolysis of nasopharyngeal carcinoma cells by activating the PI3K/Akt pathway
- The role of Shunaoxin pills in the treatment of chronic cerebral hypoperfusion and its main pharmacodynamic components
- TET3 governs malignant behaviors and unfavorable prognosis of esophageal squamous cell carcinoma by activating the PI3K/AKT/GSK3β/β-catenin pathway
- Associations between morphokinetic parameters of temporary-arrest embryos and the clinical prognosis in FET cycles
- Long noncoding RNA WT1-AS regulates trophoblast proliferation, migration, and invasion via the microRNA-186-5p/CADM2 axis
- The incidence of bronchiectasis in chronic obstructive pulmonary disease
- Integrated bioinformatics analysis shows integrin alpha 3 is a prognostic biomarker for pancreatic cancer
- Inhibition of miR-21 improves pulmonary vascular responses in bronchopulmonary dysplasia by targeting the DDAH1/ADMA/NO pathway
- Comparison of hospitalized patients with severe pneumonia caused by COVID-19 and influenza A (H7N9 and H1N1): A retrospective study from a designated hospital
- lncRNA ZFAS1 promotes intervertebral disc degeneration by upregulating AAK1
- Pathological characteristics of liver injury induced by N,N-dimethylformamide: From humans to animal models
- lncRNA ELFN1-AS1 enhances the progression of colon cancer by targeting miR-4270 to upregulate AURKB
- DARS-AS1 modulates cell proliferation and migration of gastric cancer cells by regulating miR-330-3p/NAT10 axis
- Dezocine inhibits cell proliferation, migration, and invasion by targeting CRABP2 in ovarian cancer
- MGST1 alleviates the oxidative stress of trophoblast cells induced by hypoxia/reoxygenation and promotes cell proliferation, migration, and invasion by activating the PI3K/AKT/mTOR pathway
- Bifidobacterium lactis Probio-M8 ameliorated the symptoms of type 2 diabetes mellitus mice by changing ileum FXR-CYP7A1
- circRNA DENND1B inhibits tumorigenicity of clear cell renal cell carcinoma via miR-122-5p/TIMP2 axis
- EphA3 targeted by miR-3666 contributes to melanoma malignancy via activating ERK1/2 and p38 MAPK pathways
- Pacemakers and methylprednisolone pulse therapy in immune-related myocarditis concomitant with complete heart block
- miRNA-130a-3p targets sphingosine-1-phosphate receptor 1 to activate the microglial and astrocytes and to promote neural injury under the high glucose condition
- Review Articles
- Current management of cancer pain in Italy: Expert opinion paper
- Hearing loss and brain disorders: A review of multiple pathologies
- The rationale for using low-molecular weight heparin in the therapy of symptomatic COVID-19 patients
- Amyotrophic lateral sclerosis and delayed onset muscle soreness in light of the impaired blink and stretch reflexes – watch out for Piezo2
- Interleukin-35 in autoimmune dermatoses: Current concepts
- Recent discoveries in microbiota dysbiosis, cholangiocytic factors, and models for studying the pathogenesis of primary sclerosing cholangitis
- Advantages of ketamine in pediatric anesthesia
- Congenital adrenal hyperplasia. Role of dentist in early diagnosis
- Migraine management: Non-pharmacological points for patients and health care professionals
- Atherogenic index of plasma and coronary artery disease: A systematic review
- Physiological and modulatory role of thioredoxins in the cellular function
- Case Reports
- Intrauterine Bakri balloon tamponade plus cervical cerclage for the prevention and treatment of postpartum haemorrhage in late pregnancy complicated with acute aortic dissection: Case series
- A case of successful pembrolizumab monotherapy in a patient with advanced lung adenocarcinoma: Use of multiple biomarkers in combination for clinical practice
- Unusual neurological manifestations of bilateral medial medullary infarction: A case report
- Atypical symptoms of malignant hyperthermia: A rare causative mutation in the RYR1 gene
- A case report of dermatomyositis with the missed diagnosis of non-small cell lung cancer and concurrence of pulmonary tuberculosis
- A rare case of endometrial polyp complicated with uterine inversion: A case report and clinical management
- Spontaneous rupturing of splenic artery aneurysm: Another reason for fatal syncope and shock (Case report and literature review)
- Fungal infection mimicking COVID-19 infection – A case report
- Concurrent aspergillosis and cystic pulmonary metastases in a patient with tongue squamous cell carcinoma
- Paraganglioma-induced inverted takotsubo-like cardiomyopathy leading to cardiogenic shock successfully treated with extracorporeal membrane oxygenation
- Lineage switch from lymphoma to myeloid neoplasms: First case series from a single institution
- Trismus during tracheal extubation as a complication of general anaesthesia – A case report
- Simultaneous treatment of a pubovesical fistula and lymph node metastasis secondary to multimodal treatment for prostate cancer: Case report and review of the literature
- Two case reports of skin vasculitis following the COVID-19 immunization
- Ureteroiliac fistula after oncological surgery: Case report and review of the literature
- Synchronous triple primary malignant tumours in the bladder, prostate, and lung harbouring TP53 and MEK1 mutations accompanied with severe cardiovascular diseases: A case report
- Huge mucinous cystic neoplasms with adhesion to the left colon: A case report and literature review
- Commentary
- Commentary on “Clinicopathological features of programmed cell death-ligand 1 expression in patients with oral squamous cell carcinoma”
- Rapid Communication
- COVID-19 fear, post-traumatic stress, growth, and the role of resilience
- Erratum
- Erratum to “Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway”
- Erratum to “Effect of femoral head necrosis cystic area on femoral head collapse and stress distribution in femoral head: A clinical and finite element study”
- Erratum to “lncRNA NORAD promotes lung cancer progression by competitively binding to miR-28-3p with E2F2”
- Retraction
- Expression and role of ABIN1 in sepsis: In vitro and in vivo studies
- Retraction to “miR-519d downregulates LEP expression to inhibit preeclampsia development”
- Special Issue Computational Intelligence Methodologies Meets Recurrent Cancers - Part II
- Usefulness of close surveillance for rectal cancer patients after neoadjuvant chemoradiotherapy


