Abstract
Aluminium alloys are a material that is increasingly used in industry. This is due to very good strength properties with low specific weight and low production costs. The disadvantage of kinematic system aluminium elements is their surface’s susceptibility to adhesive wear. One method of eliminating the adverse impact of adhesive tacks on the surfaces of cooperating aluminium components of machinery is the application of the method based on the anodic oxidation of alloys surface. The layers obtained by this method are widely used in sliding connections of kinematic machine parts. The modification of anodic oxide layers with admixtures has been an uninterrupted area of interest since the 1990s. This article is a review of selected methods of modifying the structure and properties of aluminium oxide layers on aluminium alloys.
1 Introduction
Anodic aluminium oxide (AAO) has played an important role in surface engineering for many decades. Initially, it was mainly used as a protective layer against corrosion or a decorative layer [1−3]. Over the years, AAO has become increasingly important in the use of technical components, often in extreme operating conditions requiring coatings with high resistance to corrosion and wear [4−7], and recently also in nanotechnology [8−17].
Thanks to its ability to form a natural aluminium oxide layer, called the passive layer (5−100 nm), aluminium is a corrosion-resistant metal. Aluminium alloys, enriched with elements of copper, silicon, zinc, magnesium, manganese, nickel, chromium, or titanium, significantly expand aluminium’s areas of application, primarily increasing its mechanical properties compared to pure Al. However, aluminium alloys with Si, Cu, Mn, and Mg have lower corrosion resistance than pure Al. Aluminium alloys used in technology are divided into casting and forming alloys [18−25]. A modern method of modifying the surface layers of aluminium alloys is surface heat treatment using concentrated heat sources, such as a laser beam or a plasma stream [26−31]. To protect the surface of aluminium alloys from adverse environmental influences and to improve the properties of the surface layer, aluminium and its alloys are subjected to technological treatments such as plating or anodizing. The technological thin oxide layers or oxide coatings formed in this way take on a protective, decorative, or functional character [32−34].
This review article looks at the latest advances in the technology of obtaining and applying anodic oxide layers on aluminium alloys; in particular, attention is paid to the modification of the structure and properties of oxide layers and their possible applications in tribology. This overview is divided into three sections. Section 1 explains the reasons for undertaking this study and carries out a review of the literature on the main techniques of obtaining aluminium oxide along with the areas of its application. Section 2, which is the most important part of the review, contains a discussion and commentary on modifications of oxide layers on aluminium alloys as well as selected properties of layers for tribological applications. The final section is devoted to potential future research directions in the presented topic, and is followed by a summary.
1.1 Reasons for undertaking this study
According to Holmberg and Erdemir [35], a total of ∼23% (119 EJ) of the world energy consumption comes from tribological contacts. Of this, 20% (103 EJ) is used to overcome friction and 3% (16 EJ) is used to regenerate worn parts and spare equipment due to wear and wear-related failures. By using new surfaces, materials, and lubrication technologies to reduce friction and protect against wear in vehicles, machinery, and other equipment around the world, we can potentially decrease energy losses due to friction and wear by 40% in the long term (15 years) and by 18% in the short term (8 years). On a global scale, these savings would amount to 1.4% of GDP per year and 8.7% of total energy consumption in the long term. Requirements for the development of friction and wear control technology are an important part of the 4D industry issues. Kato [36], keeping in mind the importance of the idea of sustainability for the industrial energy revolution, reported the need to develop friction and wear control technologies. Renewable lubricants, water, nitrogen or hydrogen lubrication, on-demand lubrication, anti-wear coatings, surface texturing, surface modification, non-metallic bulk materials, and life cycle tribology have since been identified as major future research goals in tribology [36−38]. The use of lightweight aluminium and its alloys, among other materials, is one of the major requirements of today’s industry. Currently, the European Union is well advanced in achieving the goal of reducing national greenhouse gas emissions by at least 40% by 2030 compared to 1990. The European Aluminium Industry Association in Brussels has contributed to the debate, assuming, among other things, that increasing the production of recycled aluminium, rather than importing more primary aluminium from third countries, would reduce CO2 emissions by 880 to 1,500 million tons of CO2 between 2020 and 2050 [39]. All industries that use the high strength-to-weight ratio of aluminium and its alloys, i.e., aerospace, defence, architecture, automotive, electronics, plumbing, medical, and semiconductor equipment, consider the anodizing process as a very important finishing process for various components, allowing for a solid (sometimes decorative) anodic oxide to provide protection against corrosion and wear. The structure and properties of oxide layers on aluminium can be modified in various ways, such as changing the pore size, inter-pore spacing, and thickness of the porous oxide layer. This type of modification involves controlling the operating parameters of the technological process (i.e., voltage, temperature, and time) and carefully selecting the electrolyte for anodizing (i.e., its type and concentration). It also involves adding modifiers that affect the structure and properties of the oxides. In recent years, many solutions have been proposed to enrich the surface of aluminium and its alloys, many of them having an important impact on the development of technology for controlling friction and wear of machine and device components. For all these reasons, the modification of the surface of the oxide layer on aluminium alloys finds a special place in the work of many research groups in the world. This has also been my motivation in undertaking the research presented in this article.
2 Technological surface layers on aluminium alloys
Figure 1 shows a simplified diagram of the structure of the surface layer and anodic oxide coating. Under natural conditions, aluminium or its alloys are covered during passivation by a thin surface layer of aluminium oxide. After the anodizing process, an anodic oxide layer is formed, which starts below the physical limit of the starting aluminium alloy and increases above this limit [39]. In the available literature, the resulting layer is interchangeably referred to as aluminium oxide layer/film, anodic oxide coating on aluminium, or anodic surface layer on the aluminium.

Simplified diagram of the form of the surface layer and oxide coating on an aluminium alloy substrate.
2.1 Methods of obtaining and using AAO
A natural, passive oxide layer on aluminium and its alloys provides limited protection in aggressive environments. The protective properties of the oxide can be enhanced by further surface oxidation, e.g., thermally, chemically, or electrochemically [40]. The surface treatment of aluminium and its alloys is used to increase corrosion or wear resistance or to create special electrical, optical, hydrophilic, or adhesive properties [41]. The basic types of anode oxides (type I, II, and III), as defined in the U.S. Military Specification MIL-A-8625, have become the basis of many of the general international specifications [42]. Due to their morphology, anodic oxide layers are also divided into barrier-type non-porous oxide layers and porous-type oxide layers (Figure 2).
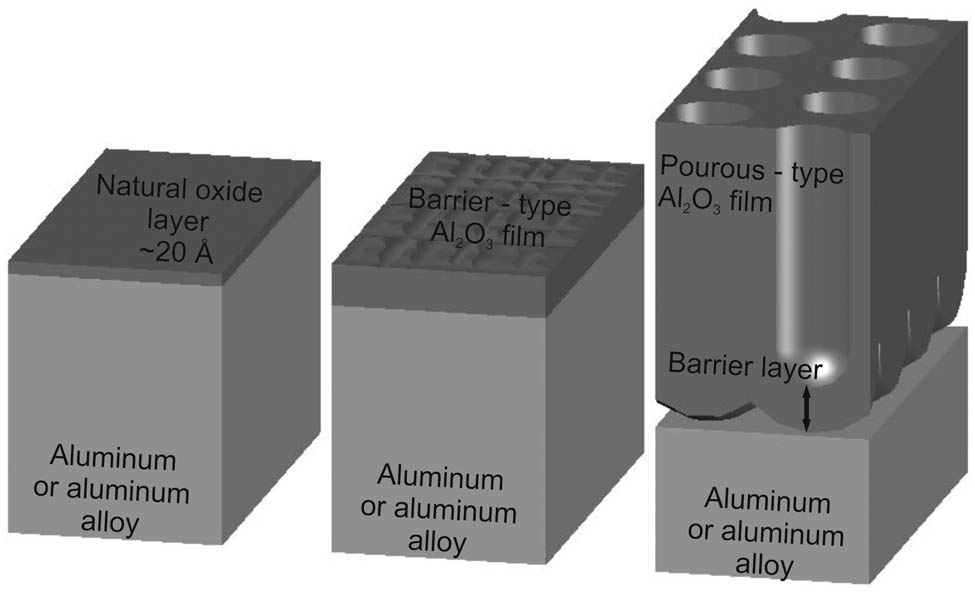
Types of the alumina oxide layer.
Compact, non-porous barrier-type AAO layers can be formed in neutral electrolytes (pH 5−7) of a solution of borate, oxalate, citrate, phosphate, adipate, tungstate, etc., in which the anode oxide is practically insoluble. Porous-type AAO is formed in acid electrolytes of selenic, sulphuric, oxalic, phosphoric, chromic, malonic, tartaric, citric, and malic acid, in which the anode oxide is poorly soluble [43−45].
2.1.1 Type I anodizing
Anodizing processes using chromic acid as the electrolyte were patented in 1923 by Bangough and Stuart [46] and are classified as type I anodizing. This process has been used to protect components made of aluminium and its alloys, especially duralumin, used in the aerospace and marine industry against corrosion [47] and as a base for paint. The advantage of this process, i.e., the fact that it does not cause corrosion even when the electrolyte remains in the cavities of the workpiece, has been used in components such as riveted or welded assemblies, where it is difficult or impossible to remove all the anodizing solutions. The initial parameters of the anodizing process were as follows: 2.5−3.5% CrO3 electrolyte at a temperature of 40 ± 2°C and voltage of 0−50 V. The process has been modified over the years [48−51]. The thickness of the oxide produced in chromic acid is about 3−5 µm. The irregular, oblique, and compact structure of type I oxides, with no pronounced unidirectional pores but with fine inter-lamellar vesicles above the continuous oxide layer at the interface with the substrate, which is usually identified as a “barrier layer”, makes it difficult for particles of the external environment to penetrate. The use of such a thin oxide in the finished element as a primer for varnish or gaskets further increases its corrosion resistance [52,53]. Environmental regulations in many areas encourage the use of chromium-free processes to avoid chromate ion toxicity. These processes used electrolytes made of organic acids such as tartaric acid, maleic acid, boric acid [54], and/or mixed electrolytes such as boric-sulfuric acid [55] or combinations of an organic acid with sulfuric acid [56−63].
2.1.2 Technical anodizing
Most of the technical anodizing processes are carried out in sulfuric acid electrolytes [64−72]. The variables to be controlled are as follows: acid concentration (usually 5–25 wt% of sulfuric acid), impurity in the anodizing bath, electrolyte temperature, anodizing current voltage and density, electrolyte mixing, and the composition and condition of the anodized alloy [16,40]. Type II anodizing is referred to as technical anodizing, “clearcoat”, decorative anodizing, and/or mild anodizing. Type II anodizing produces a fine, highly ordered, unidirectional, nanoporous oxide column microstructure. A columnar cell with a rounded bottom has a central pore that points parallel to the direction of the growth of the oxide. About 60% of the oxide layer grows into the anodized element, and 40% exceeds the physical surface before anodizing [54]. At low current density at ambient temperature, most anode layers are highly porous [73−75]. Porous oxide layers easily absorb and retain dyes, creating deeply coloured surfaces [76]; therefore, they are an effective base layer for ink, paint, varnish, or glue [53]. Porous oxide layers formed on metals provide a good adhesive base for electroplating, painting, and semipermanent decorative colouring to obtain a specific gloss, reflection, and clarity of the image. Many sulfuric acid anodized components can be found in electronic gadgets, electrolytic capacitors, cookware, outdoor products, plasma equipment, vehicles, architectural materials, machine parts, etc. [43,77]. The high adhesion of the oxide layer to the substrate means that the oxide does not flake and has a high level of durability. A technique called “flash anodizing” (exposure time less than a minute), based on the Type II anodizing process, produces AAO thin finishes that are only a few nanometres thick [78]. High gloss thin oxides are also used in headlamps in automotive, aerospace, and aviation applications [53,79].
2.1.3 Hard anodizing
Hard anodizing, also known as type III anodizing, is carried out, among others, in sulfuric acid electrolytes with a higher concentration and lower temperature than in type II anodizing. Type III hard anode oxides which are used in general engineering for components require a very wear-resistant surface, such as pistons, cylinders, and hydraulic gears [40,52,80,81,82]. The anodizing process is usually performed in sulfuric acid electrolytes, but other electrolytes can be used, including phosphoric acid, oxalic acid, and acid mixtures [51,52,61,65,83,84,85,86,87,88,89,90,91,92,93,94,95,96]. Hard anodic oxide coatings are usually thick according to normal anodizing standards (>25 µm), with higher hardness typically (>600 HV), and are produced under special anodizing conditions (very low temperature, high current density, special electrolytes). The 5xxx and 6xxx series alloys respond well to hard anodizing, while the 2xxx, 7xxx, and other alloys, including high copper and silicon casting alloys, do not. In the case of alloys with a higher content of silicon and copper, the anodized layer tends to have high porosity and low hardness [40]. Industrially, electrolyte concentrations of up to 350 g·l−1 of sulfuric acid are used. To obtain a thick oxide down to 125 μm, high current densities of about 2.2−3.5 A·dm−2 should be used depending on the method of producing the component and the complexity of the alloy. Processes with higher current density generate more resistive heat, resulting in a higher process temperature. As the temperature increases, the solubility of the growing oxide increases, which may affect the technical parameters of the oxide. Therefore, to obtain a thicker oxide during hard anodizing, the temperature of the anodizing bath should be lowered, usually to the range from about 0 to 10°C, and the bath should be mixed during anodizing [53,97,98].
2.1.4 Anodic oxide layers produced in other acids
Anodizing in organic acids began in the 1930s in Europe and Japan [52,54,99]. Anodizing in oxalic acid electrolytes was developed in Japan in 1923 [100], along with the subsequent sealing process [2], and it was widely used for kitchen utensils and electrical insulation [52]. Due to the problems with the stability of the oxalic acid electrolyte in Europe and the United States, they are used industrially only to a limited extent [53]. Obtaining of an anodic oxide coatings in various acids has been described, among others: by [75,101−109] and in formic acid [110], and mixed water solutions of sulfuric, oxalic, and phthalic acids [111], sulfuric and oxalic acid [16,112], in ionic liquids based on choline dihydrogen citrate eutectic mixtures both with oxalic acid and isopropyl alcohol and ethylene glycol [113]. Phosphoric acid anodizing is used as a pre-treatment for structural bonding in high-humidity environments in the aerospace industry. Aluminium alloys with a high level of metallurgical purity are hard anodized in phosphoric acid electrolytes to obtain a relatively thick anodic oxide structure with regular spacing and pore size for forming templates for nanoscale wire fabrication or for other applications that require a regular, highly ordered nanostructure [16,107,114−121]. In recent decades, anodic alumina has assumed the role of a self-organizing template for nanowires, nanotubes, and nano-indenters in medicine, the computer, and aerospace industries. AAO membranes are useful components of future and advanced sensing, separation, filtration, or controlled-release devices [122−126] or optical biosensors, light filters, vertical cavity surface emitting laser, and electro-absorptive reflection modulators [127−131]. AAO has become a platform for hydrogen and temperature sensors [53]. Due to their high dielectric strength, anodic oxide layers are also used in the production of metal-insulator-metal nanocapacitor arrays. Such solutions offer the possibility of creating, among other systems, cost-effective energy storage systems that provide both high energy density and high power density [132−136]. Hard anodizing process carried out in mixed electrolytes containing organic acids with sulfuric acid for the applications of oxide layers requiring increased smoothness and hardness, such as automotive and machine parts has also gained great popularity [26,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158].
2.1.5 Conversion coatings
The basis of the process of creating the conversion coating on aluminium and its alloy substrates is the formation of a compact and impermeable passive layer through an artificially induced and directed corrosion process. Chemical conversion of the outer oxide structure takes place by adsorbing an additional anion, giving different or additional properties to the passive layer on the aluminium surface. This process takes place in chemical equilibrium and without an external driving force such as electric power [159]. Preparations of conversion coatings, including chromates [160,161], phosphates [162,163], and silicates [161], are used to increase corrosion and wear resistance, and also serve as a primer for paints, adhesives, or polymers [53,164,165]. Since Cr(vi) has been identified as a carcinogen, it has become necessary to investigate alternative and more environmentally friendly surface treatments. As substitutes, apart from phosphates and silicates, there are also cerium [162], zirconium, titanium, vanadium [166,167,168], and trivalent chromium [167].
2.1.6 Anodic barrier layers
For the formation of the anode oxides of the barrier layer, weakly acidic or weakly basic electrolytes having 5 < pH < 7 are selected, which do not corrode the developing oxide, i.e. the oxide is not dissolved in the electrolyte. The resulting barrier-type anodic oxide layer has a compact, non-porous, continuous morphology, adheres strongly to the substrate, and is non-conductive. Consequently, it is not chemically influenced by a mild electrolyte, and the oxide remains practically insoluble in the anodizing process. These foils are extremely thin and dielectrically compact [53,169]. There has been a recognition that features such as vacancies, dislocations, and atomic packing densities might have a significant effect on anodic coatings [170]. Barrier layer oxides are typically formed in aqueous solutions of boric acid, ammonium borate, ammonium tartrate, and aqueous phosphate solutions, tetraborate in ethylene glycol, perchloric acid with ethanol, some organic electrolytes such as citric acid, malic acid, succinic acid, and glycolic acid [171−175], and ionic liquids [176]. Barrier-type oxides are used in dielectric capacitors. Scientific studies have shown that there is no clear difference in the choice of electrolyte used to form a barrier or porous layers, and the key factor responsible for the growth of the porous oxide structure on the previously formed barrier layer is time [169,177,178,179,180]
2.1.7 Plasma electrolytic oxidation (PEO)
PEO, also known as micro oxidation (MAO), spark anodic deposition, or plasma chemical oxidation, is the production of oxide-ceramic coatings on metals such as aluminium, titanium, magnesium, zircon, and their alloys. PEO is a process of electrochemical oxidation, in an electrolyte from dilute pH-neutral silicate or phosphate silicate solutions, using a high-voltage arc to form a coherent insulating oxide deposit. The produced oxide of the barrier layer type shows high hardness, excellent adhesion to the substrate, and resistance to wear and corrosion. It is real ceramics, mainly consisting of γ-Al2O3 [53,181−186]. Anodic alumina produced by PEO can be machined and polished to a high-quality surface finish, allowing a variety of applications that take advantage of its ability to withstand high levels of cyclic fatigue, abrasion, and impact. The process is more environmentally friendly because a non-aggressive electrolyte is used [115]. PEO coatings so far have only niche applications in industry, mainly due to the high energy consumption associated with the process. However, ongoing research may reduce energy consumption and optimize PEO coatings for aviation applications [158,187,188]. PEO/MAO coatings have been proposed as corrosion and abrasion protection [189–194], cavitation erosion protection [195], decorative coating [196,197], and thermal insulation improvement [198].
2.2 Modifiers in anodic coatings on aluminium alloys
In recent decades, much attention has been paid to various modifications aimed at improving the properties of anodic tribological coatings on aluminium alloys. Surface oxide layers formed in the process of hard anodizing belong to materials with a highly developed surface, and their properties can be very diverse and depend mainly on the conditions under which they are produced [199]. It was found that the porosity of anodic oxide layers can be used as a reservoir of lubricants to create self-lubricating structures, improving friction and wear properties [200,201]. Gyu-Sun Lee et al. [202] have produced porous anodic alumina templates with three types of nanometre pores to study the effect of pores on an anodized aluminium surface as a lubricant reservoir. The pores have been found to act as a reservoir of lubricant since the water stored in the pores is released to the surface because of elastoplastic deformation.
Takaya et al. [200] have prepared impregnated oxide layer in 0.5 mass% aqueous solutions of PVP–iodine (I) by re-anodizing. They also plated a 3 μm thickness Sn layer on anodic oxide coating of A1050, A6061-T4, and ADC12 boards and impregnated with polytetrafluoroethylene (PTFE) fluoro resin, namely, TUFRAM, on the anodic oxide on the A1050 board. The I compound was found in micro-pores as I of 0.1 mass% and formed an iodophor of an amorphous structure. They also concluded that the size of solid lubricant powder particles is larger than the pore size of the coatings. Although it is impossible to impregnate the lubricant powder directly inside the pores, it is possible to solidify a liquid lubricant electro-chemically inside the pores. Maejima et al. [201] have prepared impregnated oxide layer in molybdenum sulphide by re-anodizing it in 0.3 wt% aqueous solution of (NH4)2MoS4. Molybdenum sulphide and compounds filled the 20 nm diameter pores of the film. In previous works [200,201], Takaya et al. and Maejima et al. concluded that the size of solid lubricant powder particles is larger than the pore size of the coatings, making it impossible to impregnate the lubricant powder directly inside the pores. However, as the reports explain, it is possible to solidify a liquid lubricant electro-chemically inside the pores.
As Xu Tao et al. [203] have found, since the pores of the oxide coating are too small to be filled with grease, it is necessary to enlarge the pores prior to self-lubricating treatment. However, the process of expanding the pores and simultaneously reducing the thickness of the aluminium oxide cell walls is the key factor affecting the hardness and wear resistance of the anodic oxide layer. The minimum cell wall thickness necessary to maintain a relatively high hardness and good abrasion resistance of the coating is found to be 25 nm.
The work of Skeldon et al. [204,205] uses the duplex anodizing process to create self-lubricating MoS2 precursor layers on aluminium. The initial formation of the porous layer of alumina on aluminium was accomplished by anodizing in sulfuric acid; then, the development of MoS2 precursors in the pores was performed by subsequent anodic treatment in the ammonium tetra thiomolybdate (ATT) electrolyte (Figure 3a and b). In-depth Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analyses combined with energy dispersive X-ray (EDX) and X-ray photoelectron spectroscopy (XPS) analyses (Figure 3c and d) show that re-anodizing treatment results in a thickening of the barrier layer at the metal/film interface and precipitation of molybdenum sulphides, mainly amorphous MoS or a mixture of MoS and S, within pores and at the film surface.
![Figure 3
TEM micrograph (a) for an ultra-micro porous film (b) porous film containing incorporated lubricant; EDX spectra for modified oxide of the pore region (c) and pore wall region (d), reprinted from [204] with permission from Elsevier.](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_003.jpg)
TEM micrograph (a) for an ultra-micro porous film (b) porous film containing incorporated lubricant; EDX spectra for modified oxide of the pore region (c) and pore wall region (d), reprinted from [204] with permission from Elsevier.
The research of Posmyk et al. [155,206,207] gives examples of anodic oxide pore filling with glassy carbon to obtain a composite material (AHC+GC). According to these authors, the preparation of the samples was performed in compliance with research [208], and a multiwalled carbon nanotube (CNT) synthesis was carried out by Poly(furfuryl alcohol) carbonization in the channels of a porous oxide matrix with a thickness of 50 µm. As a result of the synthesis, a composite coating consisting of a very hard, wear-resistant oxide coating was obtained, containing nanotubes with a length of about 40–50 µm. Layers with larger pore diameters allow for the introduction of a greater amount of glassy carbon, which reduces frictional resistance. However, too large pore diameters reduce the wall thickness and the hardness of the alumina oxide. The amount of the introduced precursor depends on the time of the carbonization process. Only carbon particles that are smaller than the size of the nanopore can be introduced into nanopores. As the authors emphasize, the selection of substances is not simple and was not the subject of their work. However, it is difficult to identify carbon in the photos of the AHG+GC layer as shown in Figure 4.
![Figure 4
AHC+GC-composite layer: (a) fresh structure by SEM, (b) picture of nanopores filled with glassy carbon as a solid lubricant by HR-TEM, (c) surface nanopores view by SEM, and (d) fresh structure by SEM [207] (courtesy of Journals PAS).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_004.jpg)
AHC+GC-composite layer: (a) fresh structure by SEM, (b) picture of nanopores filled with glassy carbon as a solid lubricant by HR-TEM, (c) surface nanopores view by SEM, and (d) fresh structure by SEM [207] (courtesy of Journals PAS).
Bara et al. [199,209–211] have studied the preparation of aluminium oxide-graphite composite surface layers. Skoneczny and Bara [209,210] present a method of obtaining composite layers of alumina formed by hard anodizing in an electrolyte composed of an aqueous solution of acids and graphite. The addition of phthalic acid to the electrolyte ensured obtaining layers with the largest possible pores and conducting the process at room temperature. Examination of the chemical composition confirmed the possibility of incorporating graphite into the entire cross-section of the composite layers, which predisposes the alumina/graphite layers to tribological interaction. Previous studies [199,211,212] show how the alumina coating, produced by hard anodizing in a ternary electrolyte, was used as the base of the composite material (Figure 5). The use of carboxylic acids in the composition of the electrolyte enables anodizing at room temperature and causes adsorption of carbon from the electrolyte onto the oxide layer, which is used as a diffusion activator in the subsequent thermo-chemical treatment. The depth of the carbonized layer depends primarily on the time and temperature of carbonization.
![Figure 5
(a) Microstructure and (b) XPS spectra of a composite layer carbonized for 36 h [212] (courtesy of Journals PAS).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_005.jpg)
(a) Microstructure and (b) XPS spectra of a composite layer carbonized for 36 h [212] (courtesy of Journals PAS).
Hu et al. [213] have described the possibility of deposition of C60 particles using ultrasonic impregnation technology to obtain a self-lubricating surface. Their article presents an analysis of the results of research on the effect of pore-enlarging treatment on the porous structure and tribological properties of aluminium foil. Spherical C60 nanoparticles can be embedded in the nanopores of the anodic oxide layer. They have found that nanopores on the anodic surface of the oxide film can serve as a reservoir of C60 nanoparticles, due to which the porous anodic oxide layer can maintain a low coefficient of friction for a much longer time compared to the non-porous layer.
Escobar et al. [214] have prepared an anode film to incorporate PTFE nanoparticles into the porous structure of the film. Using an improved sedimentation technique, nanoparticles have been successfully incorporated into and onto the porous structure (Figure 6). Nanoparticles are able to enter the pores reaching right down to the bottom of the structure. The pores are filled with PTFE particles and a deposit is obtained on the surface with an average thickness of 2 μ. In the work of Tu et al. [216], a coating of amorphous carbon nanowires has been obtained on a porous aluminium oxide membrane by chemical vapour deposition. SEM/energy dispersive X-ray spectroscopy (EDS) results indicated the presence of Co within the pores on the membrane. The SEM image of carbon nanorod arrays embedded in the porous AAO membrane is shown in Figure 8a, and the thickness of the aligned film was about 3 μm. The Raman spectrum (Figure 8b) indicated that the carbon nanorods were mainly present as an amorphous structure of 2 μm. Wie and Deng [215] have obtained a self-lubricating PTFE composite anodic coating on aluminium alloy 6061 by anodizing in sulphuric acid. After anodization, the film pores were appropriately enlarged. PTFE particles were introduced into the pores by an electrophoretic process, in the water-soluble electrophoretic PTFE emulsion (Figure 7). SEM and EDX analyses revealed that the PTFE was present within the pores of the anodic film and extend from the base to the surface of the film.
![Figure 6
Field emission gun SEM pictures of an anodized film after drying sedimentation with 90 nm-sized PTFE particles. (Reprinted from [214] with permission from Elsevier).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_006.jpg)
Field emission gun SEM pictures of an anodized film after drying sedimentation with 90 nm-sized PTFE particles. (Reprinted from [214] with permission from Elsevier).
![Figure 7
SEM image of PTFE self-lubricating anodic film: (a) surface and (b) cross section [215] (copyright © Institute of Materials, Minerals and Mining., reprinted by permission of Taylor & Francis Ltd).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_007.jpg)
SEM image of PTFE self-lubricating anodic film: (a) surface and (b) cross section [215] (copyright © Institute of Materials, Minerals and Mining., reprinted by permission of Taylor & Francis Ltd).
![Figure 8
SEM image of carbon nanorod arrays embedded in the porous AAO membrane (a) and Raman spectrum of amorphous carbon (b) (Reprinted from [216], with permission from Elsevier).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_008.jpg)
SEM image of carbon nanorod arrays embedded in the porous AAO membrane (a) and Raman spectrum of amorphous carbon (b) (Reprinted from [216], with permission from Elsevier).
Zubillaga et al. [217] have obtained anodic oxide layers rich in polyaniline and TiO2 or ZrO2 nanoparticles. XPS analysis showed the presence of an impurity in the thickness ranging from 150 to 250 nm. The anode layer containing TiO2 nanoparticles showed a slightly lower surface roughness and a greater thickness of the layer rich in nanoparticles. In their research, Wang et al. [218] have produced hydrophobic AAO/Ni coatings and superhydrophobic AAO/Ni/Ni coatings based on aluminium alloys. The needle-like surface structure and organic modification gave rise to super hydrophobicity.
Shirmohammadi Yazdi et al. [219] have used the anodic oxide layer as a substrate for the Ni-P galvanic coating. Nanoporous anodic alumina enabled Ni−P particles to penetrate and fill the surface. In this way, a layer with good adhesion to the substrate was obtained. Regarding surface modification with the use of Ni−P particles, Kocabaş et al. [220] have proposed to deposit a nickel–phosphorus coating on the anodic oxide layer using nickel fluoride tetrahydrate as a surface activator (Figure 9). Activation was carried out by immersion in a nickel fluoride solution, followed by coating with a layer of Ni−P. The use of the activator has been shown to result in more efficient Ni−P deposition.
![Figure 9
Surface morphology of the Ni−P coating (a) and (b) (Reprinted from [220] with permission from Elsevier).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_009.jpg)
Surface morphology of the Ni−P coating (a) and (b) (Reprinted from [220] with permission from Elsevier).
Kamali et al. [221] have prepared silver-doped anodic oxide layers, confirming the embedding of Ag nanoparticles in the matrix of the oxide layer (Figure 10). It was found that the amount of porosity was increased by co-deposition of silver nanoparticles in the composite coating. A higher concentration of silver in the anodizing solution has led to an increase in its amount in the microstructure of the layer. The microhardness of the silver-modified layer decreases with the silver content in the layer. Silver co-deposition improves the optical properties (absorption and emission).
![Figure 10
(a) SEM image and (b) EDS spectra of composite anodic film + Ag [221] (copyright © Institute of Materials, Minerals and Mining, reprinted by permission of Taylor & Francis Ltd).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_010.jpg)
(a) SEM image and (b) EDS spectra of composite anodic film + Ag [221] (copyright © Institute of Materials, Minerals and Mining, reprinted by permission of Taylor & Francis Ltd).
Gordovskaya et al. [222] have modified the anodic oxide layer on an aluminium alloy with cerium. It has been shown that it is possible to obtain a cerium-containing layer with a typical thickness of 50−100 nm. The morphology of the layer depends to a large extent on the composition of the electrolyte used for anodizing. Anodizing in an electrolyte containing tartaric acid leads to a significant increase in the thickness of the cerium-containing layer, as well as an improvement in its uniformity on the surface of the anode layer. In the layer prepared in this way, cerium oxide particles were noticed in the pores of the anodic coating (Figure 11). EDX and XPS studies confirm the quantitative participation of cerium in the anodic oxide layer. The authors emphasize the need for further research to explain the effect of the addition of tartaric acid to the electrolyte on the quality of the modified oxide layer.
![Figure 11
(a) TEM and (b) HAADF images of the Ce-rich layer formed on the aluminium oxide [222] (CC BY 4.0).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_011.jpg)
(a) TEM and (b) HAADF images of the Ce-rich layer formed on the aluminium oxide [222] (CC BY 4.0).
The possibility of filling the nanopores of the Al2O3 oxide layer with IF-WS2 solid lubricant has been shown in the work of Korzekwa et al. (Figures 12 and 13) [223,224]. The first stage consists in obtaining a gradient Al2O3 oxide layer. The work shows that the pore diameter decreases with the increasing depth from the surface, causing a gradient of pore size distribution. In the second step, IF-WS2 nanoparticles are introduced into the pores by immersion, which facilitates ultrasonic excitation. The NPs form a concentration gradient along the depth of the porous alumina layer. The Al2O3/WS2 composite layer prepared in this way can improve the anti-wear and anti-friction properties during tribological cooperation (Figure 12).
![Figure 12
SEM/YAG-BSE image of a fresh fracture cross-section (a), quantitative results (b), and microhardness HV (a) for Al2O3/IFWS2 [223] (courtesy of John Wiley & Sons, Inc.).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_012.jpg)
SEM/YAG-BSE image of a fresh fracture cross-section (a), quantitative results (b), and microhardness HV (a) for Al2O3/IFWS2 [223] (courtesy of John Wiley & Sons, Inc.).
![Figure 14
(a) Surface morphologies and (b) cross-section of composite aluminium oxide coating with fly ash [225] (CC BY).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_014.jpg)
(a) Surface morphologies and (b) cross-section of composite aluminium oxide coating with fly ash [225] (CC BY).
Mat Tahir et al. [225] have prepared anodic oxide layers with the addition of fly ash (Figure 14). On the basis of the presented tests, they have found that the oxide surface was completely covered with dense composite films, which proves that the fly ash could penetrate into the pores and cause greater surface roughness. The addition of fly ash increases the thickness and hardness of the oxide coating.
Table 1 summarizes selected modifications of the oxide layers on aluminium/aluminium alloy.
Summary of available experimental data on modification of the oxide layer on aluminium/aluminium alloy
| Base | Electrolyte | Typical condition: temperature, current density (A·dm−2)/voltage (V) | Film properties: thickness (mm) and pore size (nm) | Modification |
|---|---|---|---|---|
| Al 1050, Al 6061-T4 and ADCl2 [200] | 5 wt% sulfuric acid | 7°C, constant current density 3 A∙dm−2, 30 min | 30 μm | Re-anodized in 0.5 wt% aqueous solutions of PVP–I, 5 min at 20°C, constant voltage 150 V, plated a 3 μm thickness Sn layer on anodic oxide coating of Al 1050, Al 6061-T4 and ADC12 and impregnated with PTFE fluoro resin, namely, TUFRAM, on the anodic oxide on Al 1050 |
| Al 1050 [201] | 22 wt% sulfuric acid | 10°C, constant current density A∙dm−2, 30 min | 30 μm | Impregnation of molybdenum sulphide by re-anodized in 0.3 wt% aqueous solution of (NH4)2MoS4, 6 min at 20°C, the constant current density of 50 m A∙dm−2 |
| 99.99% Al [203] | 0,78 mol·l−1 Oxalic acid | 20°C, constant current density 2.5 A·dm−2, 15 min | 20 μm, 25−100 nm | Pore-enlargement treatment − immersed in 0.78 mol·l−1 in oxalic acid solution at ∼40°C for 1, 2, 3 and 4 h modificatory – immersed in 1 mol·l−1 (NH4)2MoS4 water solution for 4 h |
| 99.97% Al [204,205] | 1.2 M sulfuric acid | 25°C, constant current density 0.5−2 A·dm−2 for different times | 10 nm for 0.5 A∙dm−2 or 20 nm for 0.5 A∙dm−2 | Re-anodized at the same current density and temperature to incorporate molybdenum sulphide into the as-formed porous films, in 10−2 M ATT, (NH4)2MoS4, electrolyte |
| EN-AW6061 [206,207] | n/a | n/a | 50 μm, 25−50 nm | As the authors wrote, the samples were prepared according to research [208]. Synthesis was carried out CNTs by PFA carbonization |
| PA2 (AlMg2) EN-AW-5251 [209,210] | Sulfuric, oxalic, and phthalic acids | 20 and 30°C, constant current density 2 and 3 A·dm−2, 60 min | 29−41, 47 μm | Water solutions of the SFS acids with the addition of loose graphite to produce composite layers |
| EN-AW-5251 EN-AW-6063 alloy [199,212] | Sulfuric, oxalic, and phthalic acids | 30°C, constant current density 3 A∙dm−2, 60 min | 44−47 μm | Carbonization of the oxide layers was conducted in ceramic boxes with graphite dust. Boxes were closed tightly and soaked in an electric furnace at 70−130°C for 24–48 h |
| 99.99% Al [213] | 0.3 mol·l−1 oxalate acid | 40 V stable electric source | n/a | Immersing in 5% (mass fraction) phosphoric acid solution at 30°C for 20, 30, and 40 min, respectively, for pore-enlargement treatment. Immersing in C60-toluol solution at room temperature in the environment of ultrasonic for 10 min |
| 1050 Al alloy [214] | 0.4 M phosphoric acid | 15−35°C, 1.2−1.5 A∙dm−2, 30−45 min, 1.4 A∙dm−2, 34 min, 20°C | 8−18 μm, 120−160 nm, 10 μm, 200 nm | Soaking in a cell containing a phosphoric acid solution (5 wt%) from 0 to 30 min thermally regulated at 30°C to increase the anodic pore diameter. Immersion of anodic oxide in a beaker containing dispersed PTFE (10–30 mL) |
| 6061 Al alloy [215] | 22 wt% sulfuric acid | −3°C, constant pulse current density 2, 3, 4, 5, 6 A∙dm−2, 60 min | 35−89, 0.8−1.5 μm (pores) | Immersing in phosphoric acid (5 wt%) for 5–7 min. for pore-enlargement treatment. Anodic films with PTFE was obtained by electrophoretic process in PTFE emulsion with particles <100 nm |
| 99.99% Al [216] | 0.3 M oxalic acid | 40 V, 15°C, 40 min (first), 2 h (second) | 2 μm, 90 nm | Immersing in phosphoric acid (5 wt%) at 50°C for 20 min for pore-enlargement treatment. First transition metal Co was electrodeposited and then the film of amorphous carbon nanorods was synthesized by catalytic chemical vapour deposition at 650°C |
| AA 20224T3 [217] | 0.5 M oxalic acid | Constant voltage 8 V | 2∙5 μm | Single-step anodizing process carried out in oxalic acid electrolyte containing aniline and nanoparticles of TiO2 or ZrO2 |
| AA6061 [218] | 300 g·l−1 phosphoric acid solution | 30°C, 60 V, 15 min | n/a | Immersing in phosphoric acid for 20 min for pore-enlargement treatment. Ni was electrodeposit in electrolyte (NiSO4∙6H2O, NiCl∙6H2O, NH2SO4, Na3C6H5O7∙2H2O, C6H11NaO7) at 60°C in first layer of Ni −20 min at 1.5 A·dm−2; second layer of Ni −5 min at 3 A·dm−2. Immersing into stearic acids (0.01 mol·l−1) with ethanol at room temperature for 60 min |
| 3004 Al alloy [219] | 165 g·l−1 sulfuric acid, 2 g·l−1 dissolved aluminium | 40 min., 17°C, DC voltage of 16 V | n/a | Ni was electrodeposit in electrolyte (NiSo4∙6H2O, MgSO4∙7H2O, H3BO3, Na4SO4) in 25°C at 0.4 A∙dm−2. Immersing in Ni−P solution for 60 min at 90°C |
| 1050 Al alloy [220] | 2 M sulfuric acid | Constant voltage 15 V at 20°C for 300 s | Immersing in 5 g·l−1 nickel fluoride tetrahydrate (NiF2∙4H2O) solution at 30°C and pH 6 for 60, 120, and 240 s. Immersing in Ni−P solution ((NiSO4∙6H2O, Na2H2PO2∙2H2O, C3H6O3, C3H6O2, CH4N2S), for 1 h at 85°C | |
| 1100 Al [221] | 20 wt% sulphuric acid | 25 V, 45 min | 37 μm | Single-step anodizing process carried out in electrolyte mixed with colloidal solution consisted of Ag nanoparticles (15 nm) |
| 99.99 wt% Al AA7075T6 [222] | 0.46 M sulfuric acid and 0.46 M sulfuric acid +0.53 M tartaric acid | Constant voltage of 14 V at room temperature | 94 and 97 μm | Immersion treatment in a solution of 0.015 M hydrated Ce(NO3)3 and 0.029 M H2O2 and deionized water, 30 min, 37°C |
| EN AW 5251 [223,224] | Sulfuric, oxalic, and phthalic acids | 30°C, constant current density 3 A∙dm−2, 60 min | 47−48 μm, 90 nm | Soaking in 35 vol% C2H6O/water or 35/IF-WS2 vol% C2H6O2/water/IF-WS2 mixture at room temperature in ultrasonic bath |
| AA2017-T4 [225] | 20 wt% sulfuric, acids | 24°C, constant current density 15 A∙dm−2 | 29−35 μm | Single-step anodizing process carried out in electrolyte mixed with fly-ash content (50 g·l−1) |
Note: n/a stands for not available.
3 Selected properties of anodic oxide layers for corrosion and tribological associations
Important tribological phenomena related to relative motion between two solid surfaces that lead to material degradation and energy dissipation are friction and wear [226]. As it has been shown [227], the increase in contact pressure and temperature causes changes in the wear mechanism. The authors, observing the mechanical properties, related them to the morphology and chemical composition of the layer and found that the increase in load and sliding velocity increases the friction coefficient in their oxide layers. Excellent wear and corrosion resistance are ensured by, for example, anodic oxide layers. The mechanical and tribological properties of oxide layers depend on the entire process of their production, including the selection of the electrolyte, its temperature, and current-time conditions [90,135,228–231] and also etching of aluminium alloy before anodizing [111,232]. The conditions of obtaining the oxide layers influence the physical condition of the created layer and its structure. This, in turn, determines the properties of the oxide layer on a macroscopic scale, e.g. microhardness, abrasion resistance, the nature of tribological cooperation with the counter-sample, and the value of the friction coefficient between them. The available research literature includes, inter alia, information on the influence of technological condition, porosity, substrate, or thickness of Al2O3 layers on their mechanical and tribological properties [213,233–238]. Generally, an increase in electrolyte temperature and electrolysis time causes an increase in the porosity of the oxide layer, which in turn results in a decrease in microhardness towards the anode layer surface. The change in wear resistance with increasing anodizing temperature indicates that the degradation of the wear resistance depends not only on the porosity of the oxide but also on its other properties. The tribological properties of the friction junction depend not only on the properties of the oxide layer and the conditions of the tribological process but also on the microstructural properties of the tribopartner. The laboratory tests showing how the morphology of anodic oxide layers can be used in tribological applications to create self-lubricating structures with different tribopartner can be found in previous studies [6,199,227,239–243]. A series of tests of abrasion resistance of porous anodic oxide layers filled with solid or liquid lubricants were also carried out in previous studies [194,211,220,244] . The lubrication mechanism has been shown to reduce friction and wear on solid surfaces. The abrasion properties of anodic oxide layers in dry conditions have been shown, among others, in previous studies [235,238,246–250]. However, ensuring better protection against corrosion by doping the oxide layer on aluminium alloys with nanoparticles, e.g., TiO2, ZrO2, Ni, or Ce, has been shown in previous works [217,218,222].
Lee et al. [202] have shown how the pores of the oxide layer act as a reservoir for water, which is stored in the pores and is released to the surface as a result of elastic–plastic deformation. In the case of dry tests, after immersing the layer in water, a hydroxide layer containing a large amount of hydrogen forms on the surface. They have observed that, in particular, for an aluminium oxide matrix with 260 nm pores, previously immersed in water and then tested in dry lubricant conditions, more hydroxide layers were formed, which resulted in a decrease in friction. Takaya et al. [200] have found that an attempt was made to improve the lubricity of the coating by attempting to physically impregnate the coating pores with PVP–I or PTFE fluororesin. They have shown that the coefficient of friction of the coating impregnated with PVP–I is lower than that of the non-impregnated coating and that modified anodic oxide coating was durable for a long period of time in comparison with anodic oxide coating non-impregnated and impregnated with PTFE fluororesin. Wang et al. [204,205] have found that the obtained hard aluminium foil in combination with the incorporated solid lubricant can impart much better abrasive properties to the aluminium. A sulphide-containing anodic aluminium coating has exhibited superior tribological properties compared with a normal porous alumina film. Their tests showed that the wear ratio of aluminium in tribological contact with the steel mandrel was lowered by the introduced solid lubricant.
Xu Tao et al. [203] have noted that the MoS2-modified oxide layer has a limited lubricating effect. The limitation is the lack of a transfer film between the rubbing surfaces. They have found that the mechanism of the friction process is related to the possibility of the release of MoS2 particles stored in the pores of the oxide coating. MoS2 particles transferred during friction reduce the coefficient of friction until they are completely exhausted. Not all MoS2 molecules in the pores can be easily transferred to the friction surface. At this point, the friction between the layer and the steel ball increases. However, the amount of MoS2 that is supplied to the friction surface is not sufficient to form a sliding film between the surfaces. They have also found that the MoS2 present in the pores of the oxide layer reduces the wear of the steel ball. Based on the tribological test conducted by Bara et al. [245], it can be concluded that the modification of aluminium oxide coatings with carbon by vacuum sputtering leads to a decrease in the coefficient of friction and wear intensity of the PEEK/BG and T5W polymers.
Tribological tests performed by Tu et al. [216] have demonstrated that oxide layers with the addition of amorphous nanorods show a lower steady-state friction coefficient in humid air and oxygen-rich environments compared to tests carried out in vacuum. However, the layer tested in vacuum has better wear resistance due to the lack of the effect of tribochemical reaction accelerating layer mass loss. Unfortunately, there is no comparison with the unmodified layer.
Korzekwa et al. [251] have proposed aluminium oxide coatings modified with IF-WS2 as a material with good properties for dry sliding contact. Non-parametric statistical analysis of variance has shown that Al2O3/IF-WS2 layers obtained in glycol ethylene had a higher dispersion of the results of both the friction coefficient and the wear intensity of TG15 material while they were in contact. A lower value of wear intensity and a smaller spread of data predispose the Al2O3/IF-WS2 layer produced in ethanol move to a sliding contact compared to the layer obtained in glycol ethylene. Regardless of the method, the modified layer had a lower coefficient of friction and wear intensity of TG15 material compared to the unmodified layer.
Hu et al. [213] in their research have demonstrated that the nanopores on the anodic oxide coating surface can serve as the reservoirs for the C60 modifiers; thus the porous anodic oxide film can hold the low frictional coefficient for a much longer period compared with the non-porous.
Escobar et al. [214] have conducted an experiment that showed how PTFE particles can be incorporated into the nanopores of the oxide layer, and then, during tribological tests, they showed that the PTFE-doped layer obtained in this way delays the wear of the composite coating, allowing for a twofold reduction in total wear and 75 − multiplying the lifetime of the coating.
Mat Tahir et al. [225] have found that with the highest amount of fly ash admixture (50 g·l−1) in the oxide layer, the coefficient of friction of the doped layer is lower (μ = 0.5) compared to the undoped oxide layer (μ = 0.6). Doping the oxide layer with fly ash significantly reduces the rate of wear of these layers compared to the unmodified layer. It has been suggested that fly ash particles have a high content of SiO2 and Al2O3 and can be used as inexpensive reinforcing particles that can increase the wear resistance and microhardness of the composite material.
Self-lubricating structures can arise when one or both relative surfaces wear during sliding in a mechanical process. Friction without a lubricant between the friction surfaces occurs in brakes, friction gears, and machine nodes of the textile, food, and chemical industries. The lubricant is also not used in the friction nodes of machines operating in high-temperature conditions, where the lubricant is unsuitable [252]. Table 2 summarizes selected experimental data concerning tribological properties of anodic oxide coating and modified anodic oxide coatings.
Summary of available, selected experimental data concerning tribological properties of anodic oxide coating and modified anodic oxide coatings
| Coating/method | Measurement of friction/wear | Comments |
|---|---|---|
| PEO on Al 99.5, AlCu4Mg1, AlMgSi1, AlZn5.5MgCu [189] | Rubber-wheel test according to ASTM G65, force – 130 N | All the coatings except of Al 99.5 show an excellent wear behaviour after the initial abrasion of the technological layer |
| MAO on 7075 Al alloy [194] | MDW-02 high-frequency reciprocating fatigue friction and wear testing machine with a reciprocating length of 6 mm, force – 5 N | With an increase in voltage, the slope of the friction coefficient first decreased and then increased. With the increase in MAO time, the friction coefficient decreased first and then increased μ (0.35−0.65). The long reaction time and the suitable reaction voltage resulted in a large thickness of the MAO film, a high binding strength, large roughness, a small friction coefficient, and a small wear volume. The higher the binding strength, the smaller the friction coefficient and the wear volume |
| MAO on 5065 and 7075 Al alloy [195] | Micro-scratch testing; a conical diamond tip with 5 μm radius; increasing load 0−1,500 mN; scan velocity 3 μm∙s−1 and loading rate: 9 mN∙s−1 | Changes in the friction coefficient as a function of increasing the normal force applied to the diamond cone are in the range of 0.047−0.32 for coating on 5056 Al alloy and 0.057−0.24 for coating on 7075 Al alloy |
| Anodic oxide coating on A1050, A6061-T4, and ADCl2 impregnated with I compound [200] | Ball-on-disk type rotational friction and wear tester; SUJ2 ball bearing steel of 5 mm; rotational friction –30 m with a load of 0.49, 0.98, and 1.4 N | The coefficient of friction of the coating impregnated with PVP–I is lower μ∼ (0.42−0.52) than that of the non-impregnated coating μ ∼ (0.75−0.82). Obtained anodic oxide coating was durable for a long period in comparison with anodic oxide coating non-impregnated |
| Re-anodized 99.97% al at the electrolyte of ATT and (NH4)2MoS4 to incorporate molybdenum sulphide [204,205] | Dry, reciprocating pin-on-plate tests on a Cameron- Plant Tribometer (En24 steel pin (∼550 HV) of 8 mm diameter, wear track of 50 mm length, a load of 5−20 N, and frequency of 1 Hz) | A sulphide-containing anodic aluminium coating exhibited superior tribological properties compared with a normal porous alumina film. Wear tests revealed a significant reduction in the coefficient of friction, by about a factor of 5−10, for the self-lubricating films |
| Anodic oxide coating on 99.99 % Al [202] | Ball-on-disk type sliding wear tests with commercial AISI52100; bearing steel with a diameter of 6 mm as a ball specimen; frequency of 5 Hz, sliding speed −120 rpm; distilled water was used as a lubricant | The nanopores of the oxide layer become reservoirs for water, which during friction is released to the surface as a result of elastic–plastic deformation forming like a lubricating film which minimized direct contact between the surfaces and results in low friction |
| Anodic oxide coating on 99.99 % Al immersed in 1 mol·l−1 (NH4)2MoS4 water solution for 4 h [203] | Ball-on-disk, oscillating sliding, and dry friction conditions; the ball – GCr15 bearing steel with a diameter of 10 mm; frequency of 15 Hz, sliding speed of 0.06 m·s−1; load 5 N | Self-lubrication treatment causes the friction coefficient to decrease from 0.87 to 0.65, and the diameter of the wear track on the steel ball reduced from 0.83 to 0.62 mm, which indicates that the wear rate of the steel ball was reduced |
| Sputtering carbon onto the oxide coating on EN AW5251 by Jeol IEE-4B vacuum sputtering machine [245] | Tester T-17 pin-plate type, in reciprocating motion; the pressure of 1 MPa; sliding speed 0.2 m·s−1; tribological partner − a mandrel with a diameter of 9 × 10−3 m, made of PEEK/BG and T5W | Modification of aluminium oxide coatings with carbon by vacuum sputtering leads to a decrease in the coefficient of friction and wear intensity of the PEEK/BG and T5W polymer. Oxide coatings have a favourable surface topography, which predisposes them to slide contact with polymeric materials in conditions of technically dry friction |
| Transition metal Co was electrodeposited and then the film of amorphous carbon nanorods was synthesized by CVD on anodic oxide coating on 99.99% Al [216] | WM-2002 ball-on-disk tribometer; ball GCr15 steel, 3 mm in diameter, load of 980 mN and a sliding velocity of 0.2 m·s−1 | The mean friction coefficients under the steady state in humid air, oxygen-rich and vacuum environment were 0.23, 0.24, and 0.25 in turn. The wear resistance in a vacuum enhanced for three to six times as compared to that in humid air and oxygen-rich environments |
| Soaking the anodic oxide coating on EN AW5251 in 35 vol% C2H6O/water/IF-WS2 or 35 vol% C2H6O2/water/IF-WS2 mixture at room temperature in ultrasonic bath [251] | Tester T-17 pin-plate type, in reciprocating motion; the pressure of 0.5 MPa; sliding speed −0.2 m∙s−1; tribological partner − a mandrel with a diameter of 9×10−3 m, made of TG15 | The modified Al2O3/IF-WS2 layers have a lower friction coefficient and rather lower value of TG15 wear intensity compared to the unmodified layer |
| Anodic oxide coating on 99.99% Al immersed in C60-toluol solution [213] | UMT-2 type CETR tribological tester with a self-made pin-on-disk; steel pin − GCr15 bearing steel, the diameter of 4 mm, load −5 N | The C60 nanoparticles embedded into the nanoholes of the anodic oxide film can decrease the friction between the AAO template and the steel pin and reducing the counterpart wear. Its frictional coefficient is decreased to 0.18, which is lower than that of the AAO template |
| Immersion of anodic oxide on 1050Al alloy in a beaker containing PTFE aqueous dispersions | CSM pin-on-disc tribometer; rotational mode, with a spherical alumina counterface of 6 mm in diameter; load of 1 N | The incorporated PTFE particles, by delaying the wear of the composite film, enabled a decrease of the total wear by a factor of two and a significant increase of the coating lifetime 75-fold |
| The oxide coating on AA2017/anodizing process carried out in electrolyte mixed with fly-ash content [225] | Ball-on-disk sliding tester by referring to ASTM G99; load of 1 N; speed of 30 rpm under dry-sliding conditions; silicon nitride (Si3N4) ball with a diameter of 8 mm | The addition of fly ash reduces the coefficient of friction at an early stage (below 150 revolutions) to less than μ = 0.06. The wear rate at 50 g·l−1 was the lowest of all surfaces and 79.3% better than the unanodized aluminium alloy and 28.8% better than the unmodified oxide layer |
4 Areas for further research
The general structure and properties of oxide layers on aluminium alloys have been known for many decades, as has the process of creating these layers in various electrolytes. The production of hard oxide layers on pure aluminium and its alloys allows us to improve both the hardness and strength of the layer as well as the anti-corrosion properties. In recent years, it has been found that the hydrophobic properties of the oxide layers can be modified. Both the physical phenomena and the basic principles of the formation of the oxide layer on aluminium and its alloys have been investigated and explained, including the influence of the main process parameters such as temperature, time, and current–voltage conditions. This knowledge, however, shows us how many parameters ultimately affect the properties of the oxide layer, while giving a wide field for future research. Due to the motivation of this work, related to the use of the new surface, material, and lubrication technologies to reduce energy losses due to friction and wear in vehicles, machines and other devices containing kinematic friction nodes, some open issues related to oxide layers on aluminium and its alloys are presented below as worth addressing in the coming years.
Due to the criteria of a high ratio of strength to material weight, corrosion resistance, and price, an increasing range of aluminium alloys is considered in the construction of machinery and equipment in the automotive, aviation, food, and pharmaceutical industries. Along with new modifications of the substrate material, i.e., aluminium alloys, new challenges arise in the selection and optimization of the parameters of the process of obtaining oxide layers on these alloys. When creating future articles, special attention should be paid to researching and discussing the micro-porosity and nano-porosity of the oxide layer, as both are present in the layer and can be used as reservoirs for modifiers, e.g., the need to obtain the desired tribological properties between the two materials. In particular, researchers should investigate which porosity is more important for the tribological properties of the oxide.
Another challenge is the development of computer, quantitative image analysis, which will consider, apart from nano and micro-porosity, also the shape and size of visible craters and lamellae, which look like lines of protruding hills on the surface of the oxide layer.
Another issue in future work is to check whether the test results on one alloy can be transferred and repeated for a larger number of aluminium alloys. And should that turn out to be impossible, we will need to look for the factors responsible.
It is necessary to search for the possibility to fragment the modifiers, which involves the breaking of agglomerates. It is also necessary to find carriers that will enable the formation of a homogeneous suspension enabling the introduction of the modifiers into a nanoporous oxide layer. This, in current research, still causes many difficulties. One solution here may be to combine the layer formation process in the electrolyte with ultrasounds and vibrations; another may be to use ultrasounds and vibrations in the second stage of layer formation with a modifier. It is also possible to develop technologies for the synthesis of nanopowders in the previously created oxide layer, as a way to produce nanocomposites.
An interesting direction for future research may also be the combination of tribological properties of layers filled with nanopowders with the possibility of heat/energy dissipation through nanoparticles outside the kinematic system exposed to higher temperatures from the friction process. This energy may be captured and reused. The combination of tribological, hydrophobic, and hydrophilic properties also seems to be an interesting issue.
Related to the surface engineering of oxide layers on aluminium alloys and alongside the process of their production and modification, there is a wide research area related to the study of the phenomena accompanying the rubbing pair and obtaining the desired operational effects of the tribological pair. Lying behind this are the processes of selecting and tribological testing of the cooperating pair. These processes involve the selection of a tribopartner for the modified oxide layer, the possibility of creating a sliding film, and testing its physicochemical and mechanical properties. Obviously, the goal is to reduce energy losses caused by friction and wear. It is recommended here to devote sufficient attention to understanding and trying to combine test results from different tribology testers operating under different test conditions.
Research should raise the issue of the repeatability of the process or research results, and this issue must be supported by an appropriate number of experiments performed and by the computer-assisted design of experimental research and its analysis. Research may also turn to potential difficulties related to the transfer of laboratory results to target operating conditions in the industry, and to attempts to solve them.
A great challenge in obtaining the surface of oxide layers with specific modifiers can also be the difficulty of computer numerical modelling of the formation of such oxide layers, as well as computer modelling predicting the tribological properties of the selected combination. How much simpler would surface engineering be if, after entering the selected aluminium alloy, the technological process conditions, and the modifications applied to the oxide layers into the computer, we could receive on the screen a simulation of the results of the mechanical properties of a yet unknown layer? This seems to be a daunting task due to the great number of process parameters, but often we find publications that present results of computer models that correlate well with reality. However, these models are always based on basic research data, so it seems impossible to simulate future reality accurately without performing laboratory tests.
5 Summary
This review article shows the options for modifying the oxide layers produced on aluminium alloys. Descriptions of examples of modifying the oxide layer with modifiers were preceded by the motivation to write this article and a review of the existing literature on the main techniques of obtaining aluminium oxide along with the areas of its application. The article discusses surface properties of modified oxide layers, including various surface morphologies, selected results of X-ray diffraction, SEM, and XPS phase analysis, as well as tribological properties of selected combinations. This article emphasizes the detailed contribution of scientists to the study of the possibility of filling the nanopores of the oxide layer with various modifiers, including nano-lubricants. It also familiarizes the reader with the impact of such modification on the tribological properties of the presented friction pair. Readers will also be interested in advice on the directions of future research, which, according to the author, may fill the current research gap and may enrich future research on newly created oxide layers on aluminium alloys. The versatility of the use of porous oxide layers, including its capacity of protecting against corrosion and wear, and of reducing the coefficient of friction in specific tribological associations, shows that the oxide layers in question can have a positive impact on the development of friction and wear control technologies, which must consider sustainable development for the energy revolution in the industry. The author of this article fully agrees with the words written in 1932 by Setoh and Miyata [3] that anodic alumina has been extensively studied, and yet the mechanism of anodic oxidation of aluminium is still not fully understood, as quoted by Runge [53]. In this dimension, the modification of anodic oxide layers with admixtures has not lost its validity, and, in combination with new methods of applying and introducing modifiers into the oxide structure, offers new perspectives on the application of oxide layers.
Acknowledgments
The author would like to express her gratitude to the Institute of Materials Engineering, University of Silesia in Katowice, for supporting the research in the field of materials engineering.
-
Funding information: The support from the Institute of Materials Engineering, University of Silesia in Katowice.
-
Author contributions: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The author states no conflict of interest.
References
[1] Wernick, S., R. Pinner, and P. G. Sheasby. The Surface Treatment and Finishing of Aluminium and its Alloys. Finishing Publications Ltd. and ASM International, United States. 5th ed., 1987.Search in Google Scholar
[2] Setoh, S. and A. Miyata. Electrolytic oxydation of aluminium and its industrial applications. Proceedings of World Engineering Congress Tokyo, Vol. 22, 1929, pp. 73–100.Search in Google Scholar
[3] Setoh, S. and A. Miyata. Researches on the anodic film of aluminium, II. Anodic behaviours of aluminium in aqueous solutions of oxalic acid. Scientific Papers of the Institute of Physical and Chemical Research Tokyo, Vol. 19, 1932, pp. 237–291.Search in Google Scholar
[4] Lampke, T., D. Meyer, G. Alisch, B. Wielage, H. Pokhmurska, M. Klapkiv, et al. Corrosion and wear behavior of alumina coatings obtained by various methods. Materials Science, Vol. 46, 2011, pp. 591–598.10.1007/s11003-011-9328-2Search in Google Scholar
[5] Remešová, M., S. Tkachenko, D. Kvarda, I. Ročňáková, B. Gollas, M. Menelaou, et al. Effects of anodizing conditions and the addition of Al2O3/PTFE particles on the microstructure and the mechanical properties of porous anodic coatings on the AA1050 aluminium alloy. Applied Surface Science, Vol. 513, 2020, id. 145780.10.1016/j.apsusc.2020.145780Search in Google Scholar
[6] Guezmil, M., W. Bensalah, A. Khalladi, K. Elleuch, M. Depetris-Wery, and H. F. Ayedi. Friction coefficient and microhardness of anodized aluminum alloys under different elaboration conditions. Transactions of Nonferrous Metals Society of China, Vol. 25, 2015, pp. 1950–1960.10.1016/S1003-6326(15)63803-1Search in Google Scholar
[7] Mezlini, S., K. Elleuch, and P. Kapsa. The effect of sulphuric anodisation of aluminium alloys on contact problems. Surface and Coatings Technology, Vol. 200, 2006, pp. 2852–2856.10.1016/j.surfcoat.2005.01.105Search in Google Scholar
[8] Stępniowski, W. J. and Z. Bojar. Nanoporous anodic aluminum oxide: Fabrication, characterization, and applications. In: Handbook of Nanoelectrochemistry, M. Aliofkhazraei, (Ed.), Springer International Publishing, Cham, pp. 593–645.10.1007/978-3-319-15266-0_19Search in Google Scholar
[9] Wang, X. W., Z. H. Yuan, and J. S. Li. (110) Orientation growth of magnetic metal nanowires with face-centered cubic structure using template synthesis technique. Materials Characterization, Vol. 62, 2011, pp. 642–646.10.1016/j.matchar.2011.04.005Search in Google Scholar
[10] Zaraska, L., G. D. Sulka, J. Szeremeta, and M. Jaskuła. Porous anodic alumina formed by anodization of aluminum alloy (AA1050) and high purity aluminum. Electrochimica Acta, Vol. 55, 2010, pp. 4377–4386.10.1016/j.electacta.2009.12.054Search in Google Scholar
[11] Sulka, G. D., L. Zaraska, and W. J. Stępniowski. Anodic porous alumina as a template for nanofabrication. Encyclopedia of Nanoscience and Nanotechnology, Vol. 11, 2011, pp. 261–349.Search in Google Scholar
[12] Sulka, G. D., A. Brzózka, and L. Liu. Fabrication of diameter-modulated and ultrathin porous nanowires in anodic aluminum oxide templates. Electrochimica Acta, Vol. 56, 2011, pp. 4972–4979.10.1016/j.electacta.2011.03.126Search in Google Scholar
[13] Ruiz-Clavijo, A., O. Caballero-Calero, and M. Martín-González. Revisiting anodic alumina templates: From fabrication to applications. Nanoscale, Vol. 13, 2021, pp. 2227–2265.10.1039/D0NR07582ESearch in Google Scholar PubMed
[14] Santos, A., T. Kumeria, and D. Losic. Nanoporous anodic alumina: A versatile platform for optical biosensors. Materials (Basel), Vol. 7, 2014, pp. 4297–4320.10.3390/ma7064297Search in Google Scholar PubMed PubMed Central
[15] Stepniowski, W. J., M. Moneta, K. Karczewski, M. Michalska-Domanska, T. Czujko, J. M. C. Mol, et al. Fabrication of copper nanowires via electrodeposition in anodic aluminum oxide templates formed by combined hard anodizing and electrochemical barrier layer thinning. Journal of Electroanalytical Chemistry, Vol. 809, 2018, pp. 59–66.10.1016/j.jelechem.2017.12.052Search in Google Scholar
[16] Michalska-DomańskaMarta Norek, M. Stępniowski, J. Wojciech, and B. Budner. Fabrication of high quality anodic aluminum oxide (AAO) on low purity aluminium – A comparative study with the AAO produced on high purity aluminum. Electrochimica Acta, Vol. 105, 2013, pp. 424–432.10.1016/j.electacta.2013.04.160Search in Google Scholar
[17] Ali, H. O. Review of porous anodic aluminium oxide (AAO) applications for sensors, MEMS and biomedical devices. Transactions of the Institute of Metal Finishing, Vol. 95, 2017, pp. 290–296.10.1080/00202967.2017.1358514Search in Google Scholar
[18] Huang, S., B. Zhu, Y. Zhang, H. Liu, S. Wu, and H. Xie. Microstructure comparison for AlSn20Cu antifriction alloys prepared by semi-continuous casting, semi-solid die casting, and spray forming. Metals (Basel). Epub ahead of print. Vol. 12, 2022, id. 1552.10.3390/met12101552Search in Google Scholar
[19] Fan, X., X. Chen, and S. Yuan. Novel forming process for aluminum alloy thin shells at ultra-low temperature gradient. International Journal of Machine Tools Manufacture, Vol. 185, 2023, id. 103992.10.1016/j.ijmachtools.2022.103992Search in Google Scholar
[20] Shen, P., B. Zhang, Z. Li, X. Pang, and W. Deng. Forming mechanism, mechanical properties, and corrosion properties of aluminum alloy sheet with gradient structure processed by plastic flow machining. Journal of Alloys and Compounds, Vol. 933, 2023, id. 167800.10.1016/j.jallcom.2022.167800Search in Google Scholar
[21] Ciemiorek, M., A. Ambroziak, K. Majchrowicz, M. Lewandowska, and J. Goliński. Ductility and formability of ultrafine-grained 5754 aluminium alloy under various strain rates and temperatures. Materials Science & Engineering, A: Structural Materials: Properties, Microstructure and Processing, Vol. 848, 2022, id. 7.10.1016/j.msea.2022.143375Search in Google Scholar
[22] Kaczyński, P., M. Skwarski, and K. Jaśkiewicz. Development of the technology for press-forming of energy-absorbing elements made of 7075 aluminum alloy. Journal of Manufacturing Processes, Vol. 50, 2020, pp. 676–683.10.1016/j.jmapro.2020.01.023Search in Google Scholar
[23] Venugopal, L. and K. Satyanarayana. Effect of heat treatment process on end forming behavior of 2014 aluminium alloy tubes. Advances Materials and Processing Technologies, Vol. 8, 2021, pp. 2141–2151.10.1080/2374068X.2021.1888498Search in Google Scholar
[24] Satish, D. R., F. Feyissa, and D. R. Kumar. Cryorolling and warm forming of AA6061 aluminum alloy sheets. Materials Manufacturing Processing, Vol. 32, 2017, pp. 1345–1352.10.1080/10426914.2017.1317352Search in Google Scholar
[25] Gopalan, R. and N. K. Prabhu. Oxide bifilms in aluminium alloy castings - A review. Materials Science and Technology, Vol. 27, 2011, pp. 1757–1769.10.1179/1743284711Y.0000000033Search in Google Scholar
[26] Posmyk, A. Influence of aluminium oxidation on insulation properties of oxide coating. Surface Engineering, Vol. 35, 2019, pp. 573–577.10.1080/02670844.2018.1542054Search in Google Scholar
[27] Iwaszko, J. and K. Kudła. Surface remelting treatment of 7075 aluminum alloy - Microstructural and technological aspects. Materials Research Express, Vol. 7, id. 016523.10.1088/2053-1591/ab625dSearch in Google Scholar
[28] Chan, C. P., T. M. Yue, and H. C. Man. The effect of excimer laser surface treatment on the pitting corrosion fatigue behaviour of aluminium alloy 7075. Journal of Materials Science, Vol. 38, 2003, pp. 2689–2702.Search in Google Scholar
[29] Benedetti, A., M. Cabeza, G. Castro, I. Feijoo, R. Mosquera, and P. Merino. Surface modification of 7075-T6 aluminium alloy by laser melting. Surface and Interface Analysis, Vol. 44, 2012, pp. 977–981.10.1002/sia.4840Search in Google Scholar
[30] Liu, Z., P. H. Chong, A. N. Butt, P. Skeldon, and G. E. Thompson. Corrosion mechanism of laser-melted AA 2014 and AA 2024 alloys. Applied Surface Science, Vol. 247, 2005, pp. 294–299.10.1016/j.apsusc.2005.01.067Search in Google Scholar
[31] Li, Y., J. Liu, W. Huang, and S. Zhang. Microstructure related analysis of tensile and fatigue properties for sand casting aluminum alloy cylinder head. Engineering Failure Analysis, Vol. 136, 2022, id. 106210.10.1016/j.engfailanal.2022.106210Search in Google Scholar
[32] Dobrzański, L. Podstawy nauki o materiałach i metaloznawstwo. Wydawnictwa Naukowo-Techniczne, 2002.Search in Google Scholar
[33] Surowska, B. Wybrane zagadnienia z korozji i ochrony przed korozją. Wydaw Politech Lub, 2002, pp. 6–144.Search in Google Scholar
[34] Henley, V. F. Anodic Oxidation of Aluminium and its Alloys, Epub ahead of print 1982, Pergamon, 1982.Search in Google Scholar
[35] Holmberg, K. and A. Erdemir. Influence of tribology on global energy consumption, costs and emissions. Friction, Vol. 5, 2017, pp. 263–284.10.1007/s40544-017-0183-5Search in Google Scholar
[36] Kato, K. Industrial tribology in the past and future. Tribol Online, Vol. 6, 2011, pp. 1–9.10.2474/trol.6.1Search in Google Scholar
[37] Ciulli, E. Tribology and industry: From the origins to 4.0. Frontieres Mechanical Engineering, Vol. 5, 2019, pp. 1–12.10.3389/fmech.2019.00055Search in Google Scholar
[38] Luo, J. and X. Zhou. Superlubricitive engineering – Future industry nearly getting rid of wear and frictional energy consumption. Friction, Vol. 8, 2020, pp. 643–665.10.1007/s40544-020-0393-0Search in Google Scholar
[39] European Aluminium. VISION 2050 | A Vision for Strategic, Low Carbon and Competitive Aluminium, 2022.Search in Google Scholar
[40] Yerokhin, A. and R. H. U. Khan. Anodising of light alloys. In: Woodhead Publishing Series in Metals and Surface Engineering, Surface Engineering of Light Alloys, H. Dong, (Ed.), Woodhead Publishing, 2010, pp. 83–109.10.1533/9781845699451.2.83Search in Google Scholar
[41] Terryn, H. and J. Vereecken. Surface engineering of aluminium and its alloys. EMC ’91 Non-Ferrous Metall Futur, 1991, pp. 473–480.10.1007/978-94-011-3684-6_51Search in Google Scholar
[42] Military Specification (MIL) – A – 8625F: Anodic Coatings for Aluminum and aluminum Alloys, http://everyspec.com/MIL-SPECS/MIL-SPECS-MIL-A/MIL-A-8625F_2377/ (1993).Search in Google Scholar
[43] Lee, W. and S. J. Park. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chemical Reviews, Vol. 114, 2014, pp. 7487–7556.10.1021/cr500002zSearch in Google Scholar PubMed
[44] Stępniowski, W. J., G. Cieślak, M. Norek, K. Karczewski, M. Michalska-Domańska, D. Zasada, et al. Nanoporous alumina formed by self-organized two-step anodization of Ni 3 Al intermetallic alloy in citric acid. Applied Surface Science, Vol. 264, 2013, pp. 605–610.10.1016/j.apsusc.2012.10.074Search in Google Scholar
[45] Wu, Y., W. Zhao, W. Wang, L. Wang, and Q. Xue. Novel anodic oxide film with self-sealing layer showing excellent corrosion resistance. Scientific Reports, Vol. 7, 2017, pp. 1–9.10.1038/s41598-017-01549-ySearch in Google Scholar PubMed PubMed Central
[46] Bengough, G. and J. M. Stuart. Improved process of protecting surfaces of aluminium of aluminium alloys. Brit Pat, Vol. GB223994A, 1923.Search in Google Scholar
[47] Bengough, G. D. and H. Sutton. The protection of aluminium and its alloys against corrosion by anodic oxidation. Engineering, Vol. 122, 1926, id. 274.Search in Google Scholar
[48] Modic, F. Neuere erfahrungen auf dem gebiet der anodischen oxydation von aluminium in chromsaure. Aluminium (Budapest), Vol. 39, 1963, id. 169.Search in Google Scholar
[49] Brace, A. W. and R. Peek. Production and properties of opaque coating by anodic oxidation in chromic acid. Transactions of the Institute of Metal Finishing, Vol. 34, No. 1, 1956, pp. 232–252.10.1080/00202967.1956.11869727Search in Google Scholar
[50] Lewsey, B. C. Anodic oxidation and colouring of aluminium. Electroplat Met Spray, Vol. 8.Search in Google Scholar
[51] Mahmoud, E. R. I., A. Algahtani, and V. Tirth. Study on microstructure characterisation of three different surface coating techniques on 6082 ‑ T6 aluminum alloy. Metals And Materials International, Vol. 27, 2021, pp. 4002–4013.10.1007/s12540-020-00825-3Search in Google Scholar
[52] Tajima, S. Anodic oxidation of aluminum. In: Advances in Corrosion Science and Technology, M. G. Fontana and Staehle, R. W., (Eds.), Plenum Press, New York, 1970, pp. 229–362.10.1007/978-1-4615-8252-6_4Search in Google Scholar
[53] Runge, J. M. The metallurgy of anodizing aluminum: connecting science to practice, Springer, 2018.10.1007/978-3-319-72177-4Search in Google Scholar
[54] Runge, J. M. Continued Development in Chrome-Free Anodic Oxide Finishes for Aluminum: Evaluation of Selected Mechanical Properties. Argonne, IL, http://compcote.com/_papers/download/Aerospace 2002.pdf (2002).Search in Google Scholar
[55] Wong, C. and Y. Moji. Method for anodizing aluminum. 4894127, Seattle, Waszyngton, www.freepatentsonline.com/4894127.html. 1990.Search in Google Scholar
[56] Fang, Z., J. Cao, and Y. Guan. Corrosion prevention and control during manufacturing. Springer Nature Singapore Pvt Ltd., Singapore, 2020.10.1007/978-981-15-1932-1_9Search in Google Scholar
[57] Sieber, M., R. Morgenstern, I. Scharf, and T. Lampke. Effect of nitric and oxalic acid addition on hard anodizing of AlCu4Mg1 in sulphuric acid. Metals (Basel), Vol. 8, 2018, pp. 1–15.10.3390/met8020139Search in Google Scholar
[58] Curioni, M., P. Skeldon, E. Koroleva, G. E. Thompson, and J. Ferguson. Role of tartaric acid on the anodizing and corrosion behavior of AA 2024 T3 aluminum alloy. Journal of Electrochemical Society. The Electrochemical Society, Inc., Vol. 156, 2009, id. C147.10.1149/1.3077602Search in Google Scholar
[59] Di Franco, F., V. Fiore, R. Miranda, D. Badagliacco, M. Santamaria, and A. Valenza. Influence of anodizing surface treatment on the aging behavior in salt-fog environment of aluminum alloy 5083 to fiber reinforced composites adhesive joints. Journal of Adhesion, Vol. 99, 2021, pp. 277–296.10.1080/00218464.2021.2011240Search in Google Scholar
[60] Marzocchi, V., L. Iglesias-Rubianes, G. E. Thompson, and F. Bellucci,. The influence of tartaric acid additions on the anodizing behaviour of AA2024-T3 alloy in sulphuric acid. Corrosion Reviews, Vol. 25, 2007, pp. 461–474.10.1515/CORRREV.2007.25.3-4.461Search in Google Scholar
[61] Ma, Y., X. Zhou, Y. Liao, X. Chen, C. Zhang, H. Wu, et al. Effect of anodizing parameters on film morphology and corrosion resistance of AA2099 aluminum-lithium alloy. Journal of the Electrochemical Society, Vol. 163, 2016, pp. C369–C376.10.1149/2.1081607jesSearch in Google Scholar
[62] Curioni, M., P. Skeldon, J. Ferguson, and G. E. Thompson. Reducing the energy cost of protective anodizing. Journal of Applied Electrochemistry, Vol. 41, 2011, pp. 773–785.10.1007/s10800-011-0295-ySearch in Google Scholar
[63] Carangelo, A., M. Curioni, A. Acquesta, T. Monetta, and F. Bellucci. Cerium-based sealing of anodic films on AA2024T3: effect of pore morphology on anticorrosion performance. Journal of the Electrochemical Society, Vol. 163, 2016, pp. C907–C916.10.1149/2.1001614jesSearch in Google Scholar
[64] Mason, R. B. and C. J. Slender. Anodic reactions of aluminum and its alloys in sulfuric and oxalic acid electrolytes. Industrial and Engineering Chemistry, Vol. 39, 1947, pp. 1602–1607.10.1021/ie50456a020Search in Google Scholar
[65] Arurault, L. Pilling-bedworth ratio of thick anodic aluminium porous films prepared at high voltages in h2so4 based electrolyte. Transactions of the Institute of Metal Finishing, Vol. 86, 2008, pp. 51–54.10.1179/174591908X264365Search in Google Scholar
[66] Maksymuk, M., K. Zazakowny, A. Lis, A. Kosonowski, T. Parashchuk, and K. T. Wojciechowski. Development of the anodized aluminum substrates for thermoelectric energy converters. Ceramics International, Vol. 49, 2022, pp. 4816–4825.10.1016/j.ceramint.2022.09.371Search in Google Scholar
[67] Hariprasad, C. P. S. and A. S. E. Lokeshkumar. Assessment of Corrosion and Scratch Resistance of Plasma Electrolytic Oxidation and Hard Anodized Coatings Fabricated on AA7075-T6. Transactions of the Indian Institute of Metals, Vol. 74, 2021, pp. 1991–2002.10.1007/s12666-021-02289-4Search in Google Scholar
[68] Walmsley, J. C., C. J. Simensen, A. Bjørgum, F. Lapique, and K. Redford. The structure and impurities of hard DC anodic layers on AA6060 aluminium alloy. Journal of Adhesion, Vol. 84, 2008, pp. 543–561.10.1080/00218460802161590Search in Google Scholar
[69] Buczko, Z., K. Olkowicz, J. Krasucki, K. Grabowiecki, E. Osuchowska, and P. Tomassi. Superhydrophobic properties of aluminium produced by surface abrasive blasting, anodic oxidation and fatty acid impregnation. Transactions of the Institute of Metal Finishing, Vol. 99, 2021, pp. 73–79.10.1080/00202967.2021.1877473Search in Google Scholar
[70] Mahović Poljaček, S., T. Tomašegović, S. Theohari, D. Kapović, and S. Orfanoudakis. Adhesion and colorimetric analysis of acrylate resin-based screen-printed inks on anodized aluminium substrate. Journal of Adhesion Science and Technology, Vol. 0, 2022, pp. 1–22.10.1080/01694243.2022.2117586Search in Google Scholar
[71] Chilimoniuk, P., M. Michalska-Domańska, and T. Czujko. Formation of nanoporous mixed aluminum-iron oxides by self-organized anodizing of FeAl3 intermetallic alloy. Materials (Basel), Vol. 12, 2019, pp. 6–13.10.3390/ma12142299Search in Google Scholar PubMed PubMed Central
[72] Gombár, M., A. Vagaská, M. Harničárová, J. Valíček, M. Kušnerová, A. Czán, and J. Kmec. Experimental analysis of the influence of factors acting on the layer thickness formed by anodic oxidation of aluminium. Coatings, Vol. 9, 2019, pp. 1–20.10.3390/coatings9010057Search in Google Scholar
[73] Masuda, H., F. Hasegwa, and S. Ono. Self‐Ordering of Cell Arrangement of Anodic Porous Alumina Formed in Sulfuric Acid Solution. Journal of the Electrochemical Society, Vol. 144, 1997, pp. L127–L130.10.1149/1.1837634Search in Google Scholar
[74] Li, A. P., F. Müller, A. Birner, K. Nielsch, and U. Gösele. Hexagonal pore arrays with a 50-420 nm interpore distance formed by self-organization in anodic alumina. Journal of Applied Physics, Vol. 84, 1998, pp. 6023–6026.10.1063/1.368911Search in Google Scholar
[75] Masuda, H. and K. Fukuda. Ordered metal nanohole arrays made. Science, Vol. 80–268, 1995, pp. 1466–1468.10.1126/science.268.5216.1466Search in Google Scholar PubMed
[76] Harakas, G. N. Production of colorful aluminum keepsakes and gas sensing smart materials: Anodizing, dyeing, and etching small aluminum parts on a budget. Journal of Chemical Education, Vol. 95, 2018, pp. 1187–1191.10.1021/acs.jchemed.7b00817Search in Google Scholar
[77] Lee, W. Structural engineering of porous anodic aluminum oxide. In: Nanoporous Alumina, Springer Series in Materials Science, D. Losic and A. Santos (Eds.), Springer International Publishing Switzerland, vol. 219, 2015.10.1007/978-3-319-20334-8_4Search in Google Scholar
[78] Levendusky, T. L., A. L. Askin, J. D. Guthrie, L. F. Vega, K. M. Robare, and C. Zediak. Corrosion resistant aluminum alloy substrates and methods of producing the same. 8309237. 2012.Search in Google Scholar
[79] Schenk, M. Werkstoff Aluminium und seine Anodische Oxydation : Ein Handbuch und Ratgeber fur den Praktiker, Bern: A. Francke Verlag, 1948.Search in Google Scholar
[80] Biestek, T. and J. Weber. Powłoki konwersyjne, Wydawnictwa Naukowo-Techniczne, Warszawa, 1968.Search in Google Scholar
[81] Gabe, D. R. Hard anodizing - What do we mean by hard? Metal Finishing, Vol. 100, 2002, pp. 52–58.10.1016/S0026-0576(02)80936-9Search in Google Scholar
[82] Posmyk, A., M. Cholewa, J. Wieczorek, and D. Scelina. Influence of electrolytical oxidising of silumine surfaces on the quality of bonding with epoxy resin. Archives of Metallurgy and Materials, Vol. 61, 2016, pp. 1255–1260.10.1515/amm-2016-0261Search in Google Scholar
[83] Tomashov, N. D. Anodizing and its uses in engine construction. Light Metals, Vol. 9, 1946, pp. 429–438.Search in Google Scholar
[84] Rummel, T. Uber Wachstum und Aufbau elektrolytisch erzeugter Aluminiumoxydschichten. Zeitschrift fuer Physik, Vol. 99, 1936, pp. 518–551.10.1007/BF01338807Search in Google Scholar
[85] Setoh, S. and A. Miyata. Researches on anodic film of aluminum II, anodic behaviors of aluminum in aq. solutions of oxalic acid. Scientific Papers of the Institute of Physical and Chemical Research, Vol. 19, 1932, id. 237.Search in Google Scholar
[86] Zhao, X. X., G. Y. Wei, X. F. Meng, and A. Zhang. High performance alumina films prepared by direct current plus pulse anodisation. Surface Engineering, Vol. 30, 2014, pp. 455–459.10.1179/1743294414Y.0000000258Search in Google Scholar
[87] Skoneczny, W. Model of structure of Al2O3 layer obtained via hard anodising method. Surface Engineering, Vol. 17, 2001, pp. 389–392.10.1179/026708401101518060Search in Google Scholar
[88] Rajendra, A., B. J. Parmar, A. K. Sharma, H. Bhojraj, M. M. Nayak, and K. Rajanna. Hard anodisation of aluminium and its application to sensorics. Surface Engineering, Vol. 21, 2005, pp. 193–197.10.1179/174329405X50000Search in Google Scholar
[89] Michal, P., A. Vagaská, M. Gombár, J. Kmec, E. Spišák, and M. Badida. Prediction of the effect of chemical composition of electrolyte on the thickness of anodic aluminium oxide layer. International Journal of Mathematical Models and Methods in Applied Sciences, Vol. 8, 2014, pp. 152–155.Search in Google Scholar
[90] Kwolek, P., K. Krupa, A. Obłój, P. Kocurek, M. Wierzbińska, and J. Sieniawski. Tribological properties of the oxide coatings produced onto 6061-T6 aluminum alloy in the hard anodizing process. Journal of materials Engineering and Performance, Vol. 27, 2018, pp. 3268–3275.10.1007/s11665-018-3421-8Search in Google Scholar
[91] Bara, M., S. Kulig, and J. Korzekwa. The influence of distance between electrodes used in anodizing process on the properties of aluminum oxide coatings. Met. 2019 - 28th Int Conf Metall Mater Conf Proc, 2019, pp. 991–996.Search in Google Scholar
[92] Sheasby, P. G. and B. A. Scott. Coatings produced by anodic oxidation. Corros Third Ed, Vol. 3–15, No. 21, 2013.10.1016/B978-0-08-052351-4.50118-7Search in Google Scholar
[93] Palibroda, E. and E. Indrea. On the aluminium hard anodization and the critical value V* of the barrier layer voltage. Thin Solid Films, Vol. 240, 1994, pp. 88–91.10.1016/0040-6090(94)90700-5Search in Google Scholar
[94] Csokán, P. Some Observations on the Growth Mechanism of Hard Anodic Oxide Coatings on. Aluminium Trans IMF, Vol. 41, 1964, pp. 51–56.10.1080/00202967.1964.11869884Search in Google Scholar
[95] Elssner, G. Schichtwachstum und Massenveranderung bei der elektrolytische oxydation des aluminiums. Aluminium, Vol. 25, 1943, id. 310.Search in Google Scholar
[96] Csokán, P. Beitrag zur Frage des Bildungsmechanisms von anodische erzeugten Hartoxydschichten. Werk u Korr, Vol. 12, 1961, pp. 288–295.10.1002/maco.19610120505Search in Google Scholar
[97] Gastón-García, B., E. García-Lecina, M. Díaz-Fuentes, J. A. Díez, and C. Müller. Sulphuric acid anodising of EN AC-46500 cast aluminium alloy. Transactions of the Institute of Metal Finishing, Vol. 89, 2011, pp. 312–319.10.1179/174591911X13167804921037Search in Google Scholar
[98] Sadeler, R. Effect of a commercial hard anodizing on the fatigue property of a 2014-T6 aluminium alloy. J Mater Sci, Vol. 41, 2006, pp. 5803–5809.10.1007/s10853-006-0725-0Search in Google Scholar
[99] Brace, A. W. The Technology of Anodizing Aluminium. (3rd ed.). Interall Srl, Modena, Italy, 2000.Search in Google Scholar
[100] Kujirai, T. and S. Ueki. Jap. P. 61,920 (Aug. 15, 1924, app! Dec. 28, 1923). Jap P, Vol. 61, 1924, id. 920.Search in Google Scholar
[101] Leontiev, A. P., I. V. Roslyakov, and K. S. Napolskii. Complex influence of temperature on oxalic acid anodizing of aluminium. Electrochimica Acta, Vol. 319, 2019, pp. 88–94.10.1016/j.electacta.2019.06.111Search in Google Scholar
[102] Sulka, G. D. and W. J. Stepniowski. Structural features of self-organized nanopore arrays formed by anodization of aluminum in oxalic acid at relatively high temperatures. Electrochimica Acta, Vol. 54, 2009, pp. 3683–3691.10.1016/j.electacta.2009.01.046Search in Google Scholar
[103] Sieber, M., R. Morgenstern, and T. Lampke. Anodic oxidation of the AlCu4Mg1 aluminium alloy with dynamic current control. Surface and Coatings Technology, Vol. 302, 2016, pp. 515–522.10.1016/j.surfcoat.2016.06.043Search in Google Scholar
[104] Schwirn, K., W. Lee, R. Hillebrand, M. Steinhart, K. Nielsch, and U. Gösele. Self-ordered anodic aluminum oxide formed by H2SO4 hard anodization. ACS Nano, Vol. 2, 2008, pp. 302–310.10.1021/nn7001322Search in Google Scholar PubMed
[105] Chi, C. S., J. H. Lee, I. Kim, and H. J. Oh. Effects of microstructure of aluminum substrate on ordered nanopore arrays in anodic alumina. Journal of Materials Science & Technology, Vol. 31, 2015, pp. 751–758.10.1016/j.jmst.2014.09.019Search in Google Scholar
[106] Qin, X., J. Zhang, X. Meng, C. Deng, L. Zhang, G. Ding, et al. Preparation and analysis of anodic aluminum oxide films with continuously tunable interpore distances. Applied Surface Science, Vol. 328, 2015, pp. 459–465.10.1016/j.apsusc.2014.12.048Search in Google Scholar
[107] Michalska-Domańska, M., W. J. Stępniowski, and M. Salerno. Effect of inter-electrode separation in the fabrication of nanoporous alumina by anodization. Journal of Electroanalytical Chemistry, Vol. 823, 2018, pp. 47–53.10.1016/j.jelechem.2018.05.038Search in Google Scholar
[108] Donahue, C. J. and J. A. Exline. Anodizing and coloring aluminum alloys. Journal of Chemical Education, Vol. 91, 2014, pp. 711–715.10.1021/ed3005598Search in Google Scholar
[109] Skominas, V., A. Timinskas, A. Jagminas, A. Češūnienė, M. Kurtinaitienė, and E. Matulionis. The use of fluoborate immersion solution in preparation of anodised aluminium for metal deposition in the oxide pores. Transactions of the Institute of Metal Finishing, Vol. 80, 2002, pp. 150–153.10.1080/00202967.2002.11871456Search in Google Scholar
[110] Klimas, V., A. Naujokaitis, S. Jankauskas, and A. Jagminas. Anodising of aluminium in formate solutions with formation of porous alumina arrays. Transactions of the Institute of Metal Finishing, Vol. 100, 2022, pp. 333–341.10.1080/00202967.2022.2110305Search in Google Scholar
[111] Bara, M. and M. Kubica. Influence of substrate preparation on the shaping of the topography of the surface of nanoceramic oxide layers. Applied Surface Science, Vol. 293, 2014, pp. 306–311.10.1016/j.apsusc.2013.12.158Search in Google Scholar
[112] Michal, P., A. Vagaská, M. Gombár, J. Kmec, E. Spišák, and D. Kučerka. Usage of neural network to predict aluminium oxide layer thickness. Scientific World Journal, Vol. 2015, 2015, pp. 1–10. Epub ahead of print 2015.10.1155/2015/253568Search in Google Scholar PubMed PubMed Central
[113] Fernandes, P. M. V., O. Brincoveanu, A. Pantazi, et al. Nanoporous anodic alumina layers obtained from novel deep eutectic solvent formulations. Transactions of the Institute of Metal Finishing, 2022, pp. 1–10.10.1080/00202967.2022.2105571Search in Google Scholar
[114] Bruera, F., G. Kramer, M. Vera, and A. Ares. Synthesis and morphological characterization of nanoporous aluminum oxide films by using a single anodization step. Coatings, Vol. 9, No. 2, 2019, pp. 1–12.10.3390/coatings9020115Search in Google Scholar
[115] Kwolek, P. Hard anodic coatings on aluminum alloys. Advances Manufacturing Science and Technology, Vol. 41, 2017, pp. 35–46.Search in Google Scholar
[116] Roy, P., S. Berger, and P. Schmuki. TiO2 nanotubes: Synthesis and applications. Angew Chemie - Int Ed, Vol. 50, 2011, pp. 2904–2939.10.1002/anie.201001374Search in Google Scholar PubMed
[117] Brzózka, A., A. Brudzisz, K. Hnida, and G. D. Sulka. Chemical and Structural Modifications of Nanoporous Alumina and its Optical Properties. In: Electrochemically Engineered Nanoporous Materials, Springer Series in Materials Science, D. Losic and Santos, A, (Eds.), Springer, Cham, Vol. 220, 2015.10.1007/978-3-319-20346-1_8Search in Google Scholar
[118] Zaraska, L., G. D. Sulka, and M. Jaskuła. Anodic alumina membranes with defined pore diameters and thicknesses obtained by adjusting the anodizing duration and pore opening/widening time. Journal of Solid State Electrochemistry, Vol. 15, 2011, pp. 2427–2436.10.1007/s10008-011-1471-zSearch in Google Scholar
[119] Furneaux, R. C., W. R. Rigby, and A. P. Davidson. The formation of controlled-porosity membranes from anodically oxidized aluminium. Nature, Vol. 337, 1989, pp. 147–149.10.1038/337147a0Search in Google Scholar
[120] Akinci, Z. B. and M. Ürgen. A simple method for the production of AAO templates for DC electrodeposition of nanostructures. ECS Electrochemistry Letters, Vol. 3, 2014, pp. 1–5.10.1149/2.0011411eelSearch in Google Scholar
[121] Tomassi, P. and Z. Buczko. Aluminum anodic oxide AAO as a template for formation of metal nanostructures. In: Electroplating of Nanostructures, M. Aliofkhazraei, (Ed.), IntechOpen, Rijeka, 2015.10.5772/61263Search in Google Scholar
[122] Jani, A. M. M., I. M. Kempson, D. Losic, and N. H. Voelcker. Dressing in layers: Layering surface functionalities in nanoporous aluminum oxide membranes. Angew Chemie - Int Ed, Vol. 49, 2010, pp. 7933–7937.10.1002/anie.201002504Search in Google Scholar PubMed
[123] Santos, A., J. Ferré-Borrull, J. Pallarès, and L. F. Marsal. Hierarchical nanoporous anodic alumina templates by asymmetric two-step anodization. Phys Status Solidi Applied Materials Sciences, Vol. 208, 2011, pp. 668–674.10.1002/pssa.201026435Search in Google Scholar
[124] Li, D., L. Zhao, C. Jiang, and J. G. Lu. Formation of anodic aluminum oxide with serrated nanochannels. Nano Letters, Vol. 10, 2010, pp. 2766–2771.10.1021/nl1004493Search in Google Scholar PubMed
[125] Losic, D. Preparation of porous anodic alumina with periodically perforated pores. Langmuir, Vol. 25, 2009, pp. 5426–5431.10.1021/la804281vSearch in Google Scholar PubMed
[126] Kim, Y. D., S. Choi, A. Kim, and W. Lee. Ionic current rectification of porous Anodic Aluminum Oxide (AAO) with a barrier oxide layer. ACS Nano, Vol. 14, 2020, pp. 13727–13738.10.1021/acsnano.0c05954Search in Google Scholar PubMed
[127] Kumeria, T., M. M. Rahman, A. Santos, J. Ferré-Borrull, L. F. Marsal, and D. Losic. Structural and optical nanoengineering of nanoporous anodic alumina rugate filters for real-time and label-free biosensing applications. Analytical Chemistry, Vol. 86, 2014, pp. 1837–1844.10.1021/ac500069fSearch in Google Scholar PubMed
[128] Sulka, G. D. and K. Hnida. Distributed Bragg reflector based on porous anodic alumina fabricated by pulse anodization. Nanotechnology, Vol. 23, Epub ahead of print 2012, id. 075303.10.1088/0957-4484/23/7/075303Search in Google Scholar PubMed
[129] Zheng, W. J., G. T. Fei, B. Wang, and L. De Zhang. Modulation of transmission spectra of anodized alumina membrane distributed bragg reflector by controlling anodization temperature. Nanoscale Research Letters, Vol. 4, 2009, pp. 665–667.10.1007/s11671-009-9289-7Search in Google Scholar PubMed PubMed Central
[130] Rahman, M. M., L. F. Marsal, J. Pallarès, and J. Ferré-Borrull. Tuning the photonic stop bands of nanoporous anodic alumina-based distributed bragg reflectors by pore widening. ACS Applied Materials & Interfaces, Vol. 5, 2013, pp. 13375–13381.10.1021/am4043118Search in Google Scholar PubMed
[131] Yanagishita, T., T. Kondo, K. Nishio, and H. Masuda. Optimization of antireflection structures of polymer based on nanoimprinting using anodic porous alumina. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena, Vol. 26, 2008, pp. 1856–1859.10.1116/1.2998731Search in Google Scholar
[132] Das, B. and C. Garman. Capacitance-voltage characterization of thin film nanoporous alumina templates. Microelectronics Journals, Vol. 37, 2006, pp. 695–699.10.1016/j.mejo.2005.12.004Search in Google Scholar
[133] Behzadi, F., M. Moradi, H. R. Karimi-Alavijeh, and A. Gharavi. The effect of anodization voltage and surface morphology on the capacitance properties of Al-Al2O3-Al nanocapacitors. Vacuum, Vol. 99, 2014, pp. 204–210.10.1016/j.vacuum.2013.05.025Search in Google Scholar
[134] Banerjee, P., I. Perez, L. Henn-Lecordier, S. B. Lee, and G. W. Rubloff. Nanotubular metal–insulator–metal capacitor arrays for energy storage. National Nanotechnology, Vol. 4, 2009, pp. 292–296.10.1038/nnano.2009.37Search in Google Scholar PubMed
[135] Md Jani, A. M., D. Losic, and N. H. Voelcker. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Progress in Materials Science, Vol. 58, 2013, pp. 636–704.10.1016/j.pmatsci.2013.01.002Search in Google Scholar
[136] Despic, A. and V. Parkhutik. Electrochemistry of aluminum in aqueous solutions and physics of its anodic oxide. In: Modern Aspects of Electrochemistry, J. O. Bockris, Conway, B. E. and White, R. E., (Eds.), Springer Science+Business Media, LLC, New York, 1989, pp. 487–492.Search in Google Scholar
[137] Niedźwiedź, M., W. Skoneczny, and M. Bara. The Influence of anodic alumina coating nanostructure produced on EN AW-5251 alloy on type of tribological wear process. Coatings, Vol. 10, No. 2, 2020, id. 105.10.3390/coatings10020105Search in Google Scholar
[138] Bara, M. and R. Dwornicka. Tribological properties of oxide coatings produced on EN AW-5251 alloy using different distances between electrodes. Qual Prod Improv - QPI, Vol. 1, 2019, pp. 400–405.10.2478/cqpi-2019-0054Search in Google Scholar
[139] Kessentini, I., S. Zouari, A. Bakir, and M. Bargui. Comparative study of mechanical and tribological properties of alumina coatings formed on 5754 aluminium alloy under various conditions. Surface Engineering Appl Electrochem, Vol. 54, 2018, pp. 524–533.10.3103/S106837551805006XSearch in Google Scholar
[140] Niedźwiedź, M., M. Bara, W. Skoneczny, S. Kaptacz, and G. Dercz. Influence of anodizing parameters on tribological properties and wettability of Al2O3 layers produced on the EN AW-5251 aluminum alloy. Materials (Basel), Vol. 15, 2022, id. 7732.10.3390/ma15217732Search in Google Scholar PubMed PubMed Central
[141] Niedźwiedź, M., W. Skoneczny, M. Bara, and V. Bulej. Tribological properties of Al2O3 layers after thermo-chemical treatment. Tribol - Finnish Journal Tribology, Vol. 39, 2022, pp. 4–16.10.30678/fjt.109312Search in Google Scholar
[142] Bara, M., M. Niedźwiedź, W. Skoneczny, and A. Barylski. Nanostructure and morphology of the surface as well as micromechanical and sclerometric properties of Al2O3 layers subjected to thermo-chemical treatment. Materials (Basel), Vol. 15, 2022, pp. 1–15.10.3390/ma15031051Search in Google Scholar PubMed PubMed Central
[143] Niedźwiedź, M., W. Skoneczny, M. Bara, and G. Dercz. Thin Al2O3 coatings produced by electrochemical method, subjected to thermo-chemical treatment. Coatings, Vol. 11, 2021, pp. 1–14.10.3390/coatings11111294Search in Google Scholar
[144] Niedźwiedź, M. Type of wear of aluminium oxide layers depending on manufacturing parameters. Tribologia, Vol. 294, 2020, pp. 39–44.10.5604/01.3001.0014.8334Search in Google Scholar
[145] Niedźwiedź, M., W. Skoneczny, and M. Bara. Influence of conditions for production and thermo-chemical treatment of Al2O3 coatings on wettability and energy state of their surface. Coatings, Vol. 10, 2020, id. 16.10.3390/coatings10070681Search in Google Scholar
[146] Bara, M., M. Niedźwiedź, and W. Skoneczny. Influence of anodizing parameters on surface morphology and surface-free energy of Al2O3 layers produced on EN AW-5251 alloy. Materials, Vol. 12, No. 5, 2019, id. 695.10.3390/ma12050695Search in Google Scholar PubMed PubMed Central
[147] Niedźwiedź, M., M. Bara, and W. Skoneczny. The influence of surface free energy on tribological properties of oxide layer formed on aluminium alloy EN AW-5251. Tribologia, Vol. 280, 2018, pp. 71–79.10.5604/01.3001.0012.7536Search in Google Scholar
[148] Skoneczny, W. Kształtowanie właściwości warstw wierzchnich aluminium i jego stopów metodą anodowania twardego. Bielsko-Biała: Wydawnicwto Politechniki Łódzkiej filii w Bielsku-Białej, 2001.Search in Google Scholar
[149] Skoneczny, W., M. Niedźwiedź, and M. Bara. The effect of production parameters of oxide layers on their nanostructure, nanomorphology, and surface free energy. Applied Sciences, Vol. 8, No. 11, 2018, id. 2251.10.3390/app8112251Search in Google Scholar
[150] Niedźwiedź, M., M. Bara and W. Skoneczny. Comparative study on the energy condition of the Al2O3 oxide layer on the aluminum alloy. In: Proceedings 28th International Conference on Metallurgy and Materials, 2019, pp. 1115–1120.10.37904/metal.2019.883Search in Google Scholar
[151] Niedźwiedź, M., M. Bara, and A. Barylski. Dependence of the surface morphology and micromechanical and sclerometric properties of Al2O3 layers on the parameters of anodizing aluminum alloy. Materials (Basel), Vol. 15, 2022, pp. 1–17.10.3390/ma15238482Search in Google Scholar PubMed PubMed Central
[152] Skoneczny, W. Effect of the base material condition on the structure and properties of Al2O3 oxide layers. Indian Journal of Engineering & Materials Sciences, Vol. 27, 2020, pp. 128–132.10.56042/ijems.v27i1.45882Search in Google Scholar
[153] Skoneczny, W., S. Kaptacz, A. Barylski, and T. KMITA. Analysis of tribological properties of selected PTFE-based polymer composites in a sliding interaction with aluminium oxide (Al2O3). Tribologia, Vol. 280, 2018, pp. 107–112.10.5604/01.3001.0012.7550Search in Google Scholar
[154] Kmita, T. and W. Skoneczny. Increase of operational durability of a plastic material-oxide coating couple as a result of the application of a pulsed anodizing process. Eksploat i Niezawodn, Vol. 45, 2010, pp. 77–82.Search in Google Scholar
[155] Posmyk, A. Kształtowanie Właściwości Tribologicznych Warstw Wierzchnich na Bazie Aluminium, Wydawnictwo Politechniki Śląskiej, Gliwice, 2002.Search in Google Scholar
[156] Tomassi, P., Z. Buczko, and K. Olkowicz. The influence of anodic oxidation parameters on the growth rate of oxide coatings on aluminium. Inżynieria Powierzchni, Vol. 23, No. 1, 2018, pp. 43–49.10.5604/01.3001.0011.8030Search in Google Scholar
[157] Bara, M., S. Kulig and J. Korzekwa. The influence of distance between electrodes used in anodizing process on the properties of aluminum oxide coatings. In: METAL 2019 - 28th International Conference on Metallurgy and Materials, Conference Proceedings, 2019.10.37904/metal.2019.904Search in Google Scholar
[158] Paz martínez-Viademonte, M., S. T. Abrahami, T. Hack, M. Burchardt, and H. Terryn. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings, Vol. 10, 2020, pp. 1–30.10.3390/coatings10111106Search in Google Scholar
[159] Runge, J. M. Enhancing Anodic Aluminum Oxide for Bonding Applications San Diego , California. Journal of the Electrochemical Society, Vol. 3, 2015, pp. 233–240.Search in Google Scholar
[160] Kendig, M., S. Jeanjaquet, R. Addison, and J. Waldrop. Role of hexavalent chromium in the inhibition of corrosion of aluminum alloys. Surface and Coatings Technology, Vol. 140, 2001, pp. 58–66.10.1016/S0257-8972(01)01099-4Search in Google Scholar
[161] Hamdy, A. S. Corrosion protection of aluminum composites by silicate/cerate conversion coating. Surface and Coatings Technology, Vol. 200, 2006, pp. 3786–3792.10.1016/j.surfcoat.2005.03.012Search in Google Scholar
[162] Kolics, A. Enhancement and inhibition of phosphate deposition on aluminum. Electrochemical and Solid State Letters, Vol. 3, 2000, pp. 369–372.10.1149/1.1391151Search in Google Scholar
[163] Arango, D. and J. Liu. Investigation of the initiation and propagation of filiform corrosion on aluminum alloys by electrochemical techniques. Canadian Metallurgical Quarterly, 2022, pp. 1–13.10.1080/00084433.2022.2160898Search in Google Scholar
[164] Kwiatkowski, L. and P. Tomassi. Powłoki konwersyjne na blachach aluminiowych - technologie wytwarzania i metody badań. Inżynieria Powierzchni, Vol. 1, 2009, pp. 36–45.Search in Google Scholar
[165] Harvey, T. G. Cerium-based conversion coatings on aluminium alloys: A process review. Corrosion Engineering Science and Technology, Vol. 48, 2013, pp. 248–269.10.1179/1743278213Y.0000000089Search in Google Scholar
[166] Yi, A., W. Li, J. Du, and S. Mu. Preparation and properties of chrome-free colored Ti/Zr based conversion coating on aluminum alloy. Applied Surface Science, Vol. 258, 2012, pp. 5960–5964.10.1016/j.apsusc.2011.12.045Search in Google Scholar
[167] Šekularac, G., J. Kovač, and I. Milošev. Prolonged protection, by zirconium conversion coatings, of AlSi7Mg0.3 aluminium alloy in chloride solution. Corrosion Science, Vol. 169, 2020, id. 108615.10.1016/j.corsci.2020.108615Search in Google Scholar
[168] Zhong, X., X. Wu, Y. Jia, and Y. Liu. Self-repairing vanadium-zirconium composite conversion coating for aluminum alloys. Applied Surface Science, Vol. 280, 2013, pp. 489–493.10.1016/j.apsusc.2013.05.015Search in Google Scholar
[169] Sulka, G. D. Highly Ordered Anodic Porous Alumina Formation by Self-Organized Anodizing. In: Nanostructured Materials in Electrochemistry, A. Eftekhari, (Ed.), Wiley-VCH Verlag GmbH & Co. KGaA, 2008.10.1002/9783527621507.ch1Search in Google Scholar
[170] Brace, A. W. Lattice defects model of anodic coating formation on aluminium. Transactions of the Institute of Metal Finishing, Vol. 89, 2011, pp. 155–161.10.1179/174591911X13013062414016Search in Google Scholar
[171] Thompson, G. E., H. Habazaki, K. Shimizu, M. Sakairi, P. Skeldon, X. Zhou, et al. Anodizing of aluminium alloys. Aircraft Engineering and Aerospace Technology, Vol. 71, 1999, pp. 228–238.10.1108/00022669910270709Search in Google Scholar
[172] Despic, A. and V. P. Parkhutik. Electrochemistry of aluminum in aqueous solutions and physics of its anodic oxide. In: Modern Aspects of Electrochemistry, J. O. Bockris, White, R. E. and Conway, B. E., (Eds.), Springer, Boston, MA, Vol. 20, 1989.Search in Google Scholar
[173] Diggle, J. W., T. C. Downie, and C. W. Goulding. Anodic oxide films on aluminum I. Introduction Journal of Electrochemicalv Society, Vol. 41, 1968, pp. 1–41.Search in Google Scholar
[174] Habazaki, H., R. Nishimura, K. Okitsu, H. Inoue, I. Kiriyama, F. Kataoka, et al. The effects of film thickness and incorporated anions on pitting corrosion of aluminum with barrier-type oxide films formed in neutral borate and phosphate electrolytes. Journal of Solid State Electrochemistry, Vol. 18, 2014, pp. 369–376.10.1007/s10008-013-2192-2Search in Google Scholar
[175] Schneider, M., O. Yezerska, and M. M. Lohrengel. Anodic oxide formation on AA2024: Electrochemical and microstructure investigation. Corrosion Engineering, Science and Technology, Vol. 43, 2008, pp. 304–312.10.1179/174327808X286211Search in Google Scholar
[176] Tateishi, K., A. Waki, H. Ogino, T. Ohishi, and M. Murakami. Formation of Al2O3 film and AIF3 containing Al2O3 film by an anodic polarization of aluminum in ionic liquids. Electrochemistry, Vol. 80, 2012, pp. 556–560.10.5796/electrochemistry.80.556Search in Google Scholar
[177] Despić, A. and V. P. Parkhutik. Electrochemistry of aluminum in aqueous solutions and physics of its anodic oxide. Modern Aspects of Electrochemistry, Vol. 20, 1989, pp. 401–503.10.1007/978-1-4684-8762-6_6Search in Google Scholar
[178] Shingubara, S., K. Morimoto, H. Sakaue, and T. Takahagi. Self-organization of a porous alumina nanohole array using a sulfuric/oxalic acid mixture as electrolyte. Electrochem Electrochem Solid-State Lett, Vol. 7, 2004, pp. E15–E17.10.1149/1.1644353Search in Google Scholar
[179] Ono, S. Nanostructure analysis of anodic films formed on aluminum-focusing on the effects of electric field strength and electrolyte anions. Molecules, Vol. 26, No. 23, 2021, id. 7270. 10.3390/molecules26237270Search in Google Scholar PubMed PubMed Central
[180] Han, H., S. J. Park, J. S. Jang, H. Ryu, K. J. Kim, S. Baik, et al. In situ determination of the pore opening point during wet-chemical etching of the barrier layer of porous anodic aluminum oxide: Nonuniform impurity distribution in anodic oxide. ACS Applied Materials & Interfaces, Vol. 5, 2013, pp. 3441–3448.10.1021/am400520dSearch in Google Scholar PubMed
[181] Simchen, F., M. Sieber, A. Kopp, and T. Lampke. Introduction to plasma electrolytic oxidation-an overview of the process and applications. Coatings, Vol. 10, No. 7, 2020, id. 628.10.3390/coatings10070628Search in Google Scholar
[182] Zhuang, J. J., Y. Q. Guo, N. Xiang, X. Y. Lu, Q. Hu, and R. G. Song. Sliding wear behaviour and microstructure of PEO coatings formed on aluminium alloy. Mater Sci Technol (United Kingdom), Vol. 32, 2016, pp. 1559–1566.10.1080/02670836.2015.1132031Search in Google Scholar
[183] Wang, P., J. P. Li, Y. C. Guo, Z. Yang, and J. L. Wang. Ceramic coating formation on high Si containing Al alloy by PEO process. Surfaces Engineering, Vol. 32, 2016, pp. 428–434.10.1179/1743294415Y.0000000003Search in Google Scholar
[184] Gȩbarowski, W. and S. Pietrzyk. Growth characteristics of the oxide layer on aluminium in the process of plasma electrolytic oxidation. Archives of Metallurgy and Materials, Vol. 59, 2014, pp. 407–411.10.2478/amm-2014-0070Search in Google Scholar
[185] Walsh, F. C., C. T. Low, R. J. Wood, K. T. Stevens, J. Archer, A. R. Poeton, et al. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, mg, Ti) alloys. Transactions of the IMF, Vol. 87, 2009, pp. 122–135.10.1179/174591908X372482Search in Google Scholar
[186] Lu, C., P. Shi, J. Yang, J. Jia, E. Xie, and Y. Sun. Effects of surface texturing on the tribological behaviors of PEO/PTFE coating on aluminum alloy for heavy-load and long-performance applications. Journal of Materials Research and Technology, Vol. 9, 2020, pp. 12149–12156.10.1016/j.jmrt.2020.09.008Search in Google Scholar
[187] Matykina, E., R. Arrabal, M. Mohedano, B. Mingo, J. Gonzalez, A. Pardo, et al. Recent advances in energy efficient PEO processing of aluminium alloys. Trans Nonferrous Met Soc China (English Ed), Vol. 27, 2017, pp. 1439–1454.10.1016/S1003-6326(17)60166-3Search in Google Scholar
[188] del Olmo, R., M. Mohedano, P. Visser, E. Matykina, and R. Arrabal. Flash-PEO coatings loaded with corrosion inhibitors on AA2024. Surface and Coatings Technology, Vol. 402, 2020, id. 126317.10.1016/j.surfcoat.2020.126317Search in Google Scholar
[189] Sieber, M., F. Simchen, R. Morgenstern, I. Scharf, and T. Lampke. Plasma electrolytic oxidation of high-strength aluminium alloys – substrate effect on wear and corrosion performance. Metals (Basel), Vol. 8, 2018, pp. 1–17.10.3390/met8050356Search in Google Scholar
[190] Jędrusik, M., A. Dębowska, A. Kopia, P. Petrzak, D. Koclęga, and I. Kalemba-Rec. Characterization of oxide layers made on aluminum alloy 7075 by different methods. Metallurgy and Foundry Engineering, Vol. 42, 2016, id. 187.10.7494/mafe.2016.42.3.187Search in Google Scholar
[191] Sowa, M., A. Olesiński, B. Szumski, A. Maciej, M. Bik, P. Jeleń, et al. Electrochemical characterization of anti-corrosion coatings formed on 6061 aluminum alloy by plasma electrolytic oxidation in the corrosion inhibitor-enriched aqueous solutions. Electrochimica Acta, Vol. 424, 2022, id. 140652.10.1016/j.electacta.2022.140652Search in Google Scholar
[192] Xiang, N., R. G. Song, C. Wang, Q. Z. Mao, Y. J. Ge, and J. H. Ding. Formation of corrosion resistant plasma electrolytic oxidation coatings on aluminium alloy with addition of sodium tungstate species. Corros Engineering Science and Technology, Vol. 51, 2016, pp. 146–154.10.1179/1743278215Y.0000000040Search in Google Scholar
[193] Duan, Y. F., J. W. Liu, and P. Wang. Effect of In2(SO4)3 on characteristics of Al–Li alloy MAO coating. Surfaces Engineering, Vol. 38, 2022, pp. 440–447.10.1080/02670844.2022.2087955Search in Google Scholar
[194] Li, W., S. Lei, Y. Xia, A. Amirfazli, and Y. Lu. Effects of technological parameters on the morphological, microstructural and mechanical behavior of micro-arc oxidation coatings on al substrates. Materiali in Tehnologije, Vol. 54, 2020, pp. 283–292.10.17222/mit.2019.024Search in Google Scholar
[195] Szkodo, M., Stanisławska A., Komarov A., and Bolewski Ł. Effect of MAO coatings on cavitation erosion and tribological properties of 5056 and 7075 aluminum alloys. Wear, Vol. 474–475, 2021, id. 203709.10.1016/j.wear.2021.203709Search in Google Scholar
[196] Kurze, P., W. Krysmann, J. Schreckenbach, T. Schwarz, and K. Rabending. Coloured ANOF Layers on Aluminium. Crystal Research and Technology, Vol. 22, 1987, pp. 53–58.10.1002/crat.2170220115Search in Google Scholar
[197] Wang, J.-M., D.-S. Tsai, J. T. J. Tsai, and C. C. Chou. Coloring the aluminum alloy surface in plasma electrolytic oxidation with the green pigment colloid. Surface and Coatings Technology, Vol. 321, 2017, pp. 164–170.10.1016/j.surfcoat.2017.04.038Search in Google Scholar
[198] Curran, J. A., H. Kalkancı, Y. Magurova, and T. W. Clyne. Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications. Surface and Coatings Technology, Vol. 201, 2007, pp. 8683–8687.10.1016/j.surfcoat.2006.06.050Search in Google Scholar
[199] Kmita, T. and M. Bara. Surface oxide layers with an increased carbon content for applications in oil-Less tribological systems. Chem Process Eng - Inz Chem i Proces, Vol. 33, 2012, pp. 479–486.10.2478/v10176-012-0040-zSearch in Google Scholar
[200] Takaya, M., K. Hashimoto, Y. Toda, and M. Maejima. Novel tribological properties of anodic oxide coating of aluminum impregnated with iodine compound. Surface and Coatings Technology, Vol. 169170, 2003, pp. 160–162.10.1016/S0257-8972(03)00218-4Search in Google Scholar
[201] Maejima, M., K. Saruwatari, and M. Takaya. Friction behaviour of anodic oxide film on aluminum impregnated with molybdenum sulfide compounds. Surface and Coatings Technology, Vol. 132, 2000, pp. 105–110.10.1016/S0257-8972(00)00849-5Search in Google Scholar
[202] Lee, G. S., J. Choi, Y. C. Choi, S. D. Bu, and Y. Z. Lee. Tribological effects of pores on an anodized Al alloy surface as lubricant reservoir. Current Applied Physics, Vol. 11, 2011, pp. S182–S186.10.1016/j.cap.2011.03.057Search in Google Scholar
[203] Tao, X., C. Jianmin, Z. Jiazheng, D. Hongxin. The pore-enlargement and self-lubrication treatment of anodic oxide film of aluminum. Wear, Vol. 196, 1996, pp. 214–218.10.1016/0043-1648(95)06904-6Search in Google Scholar
[204] Skeldon, P., H. W. Wang, and G. E. Thompson. Formation and characterization of self-lubricating MoS2 precursor films on anodized aluminium. Wear, Vol. 206, 1997, pp. 187–196.10.1016/S0043-1648(96)07350-4Search in Google Scholar
[205] Wang, H. W., P. Skeldon, and G. E. Thompson. Development and tribological assessment of self-lubricating anodic films on aluminium. Surface and Coatings Technology, Vol. 88, 1997, pp. 269–273.10.1016/S0257-8972(96)02935-0Search in Google Scholar
[206] Posmyk, A. and H. Wistuba. Ceramic composite layers with glassy nanocarbon. Composites, Vol. R8, 2008, pp. 31–35.Search in Google Scholar
[207] Posmyk, A. and H. Wistuba. Composite layers with ceramic matrix modified with glassy carbon destined for oil-less sliding pairings. Archives of Metallurgy and Materials, Vol. 56, 2011, pp. 909–917.10.2478/v10172-011-0100-2Search in Google Scholar
[208] Lee, J. S., G. H. Gu, H. Kim, K. S. Jeong, J. Bae, and J. S. Suh. Growth of carbon nanotubes on anodic aluminum oxide templates: Fabrication of a tube-in-tube and linearly joined tube. Chemistry of Materials, Vol. 13, 2001, pp. 2387–2391.10.1021/cm0014076Search in Google Scholar
[209] Skoneczny, W. and M. Bara. Aluminium oxide composite layers obtained by the electrochemical method in the presence of graphite. Materials Science-Poland, Vol. 25, 2007, pp. 1053–1062.Search in Google Scholar
[210] Bara, M. and W. Skoneczny. Properties of composite alluminium oxide-graphite layers obtained by an electrolytic method. Advance Manufacturing Science and Technology, Vol. 32, 2008, pp. 61–68.Search in Google Scholar
[211] Bara, M. Struktura i właściwości ceramiczno-grafitowych warstw powierzniowych otrzymywanych metodą duplex. Praca doktorska. Katowice, 2009, https://www.sbc.org.pl/dlibra/publication/23746.Search in Google Scholar
[212] Bara, M., W. Skoneczny, and M. Hajduga Ceramic-graphite surface layers obtained by the duplex method on an aluminium alloy substrate. Chem Process Eng - Inz Chem i Proces, Vol. 30, No. 3, 2009, pp. 431–442.Search in Google Scholar
[213] Hu, N., S. Ge, and L. Fang. Tribological properties of nano-porous anodic aluminum oxide template. Journal of Central South University of Technology, Vol. 18, 2011, pp. 1004–1008.10.1007/s11771-011-0794-3Search in Google Scholar
[214] Escobar, J., L. Arurault, and V. Turq. Improvement of the tribological behavior of PTFE-anodic film composites prepared on 1050 aluminum substrate. Applied Surface Science, Vol. 258, 2012, pp. 8199–8208.10.1016/j.apsusc.2012.05.022Search in Google Scholar
[215] Wei, X. W. and L. H. Deng. Preparation of PTFE composite anodic film on aluminium alloy 6061 using electrophoretic process. Tribol - Mater Surfaces Interfaces, Vol. 4, 2010, pp. 74–76.10.1179/175158310X12678019274327Search in Google Scholar
[216] Tu, J. P., C. X. Jiang, S. Y. Guo, X. B. Zhao, and M. F. Fu. Tribological properties of aligned film of amorphous carbon nanorods on AAO membrane in different environments. Wear, Vol. 259, 2005, pp. 759–764.10.1016/j.wear.2005.02.015Search in Google Scholar
[217] Zubillaga, O., F. J. Cano, I. Azkarate, G. Imbuluzqueta, and M. Insausti. Polyaniline and nanoparticle containing anodic films for corrosion protection of 2024T3 aluminium alloy. Transactions of the Institute of Metal Finishing, Vol. 87, 2009, pp. 315–319.10.1179/174591909X12550026459529Search in Google Scholar
[218] Wang, Y., L. Guan, Z. He, J. Tan, H. Singh, M. D. Hayat, et al. Preparation and characterisation of AAO/Ni/Ni superhydrophobic coatings on aluminium alloys. Surfaces Engineering, Vol. 37, 2021, pp. 1246–1254.10.1080/02670844.2021.1902110Search in Google Scholar
[219] Shirmohammadi Yazdi, S., F. Ashrafizadeh, and A. Hakimizad. Improving the grain structure and adhesion of Ni-P coating to 3004 aluminum substrate by nanostructured anodic film interlayer. Surface and Coatings Technology, Vol. 232, 2013, pp. 561–566.10.1016/j.surfcoat.2013.06.028Search in Google Scholar
[220] Kocabaş, M., C. Örnek, M. Curioni, and N. Cansever. Nickel fluoride as a surface activation agent for electroless nickel coating of anodized AA1050 aluminum alloy. Surface and Coatings Technology, Vol. 364, 2019, pp. 231–238.10.1016/j.surfcoat.2019.03.003Search in Google Scholar
[221] Kamali, S., F. Dehghan, H. S. Mardanpour, and S. Alirezaei. The microstructure and optical properties of novel Al2O3–Ag composite coating. Surface Engineering, Vol. 37, 2021, pp. 953–962.10.1080/02670844.2020.1840826Search in Google Scholar
[222] Gordovskaya, I. V., T. Hashimoto, J. Walton, M. Curioni, G. E. Thompson, and P. Skeldon. Development of cerium-rich layers on anodic films formed on pure aluminium and AA7075 T6 alloy. Journal of the Electrochemical Society, Vol. 161, 2014, pp. C601–C606.10.1149/2.0091501jesSearch in Google Scholar
[223] Korzekwa, J., R. Tenne, W. Skoneczny, and G. Dercz. Two-step method for preparation of Al2O3/IF-WS2 nanoparticles composite coating. Phys Status Solidi Applied Material Sciences, Vol. 210, 2013, pp. 2292–2297.10.1002/pssa.201329320Search in Google Scholar
[224] Korzekwa, J., A. Gądek-Moszczak, and M. Zubko. Influence of the size of nanoparticles on the microstructure of oxide coatings. Materials Science, Vol. 53, 2018, pp. 709–716.10.1007/s11003-018-0127-xSearch in Google Scholar
[225] Mat Tahir, N. A., S. Liza, K. Fukuda, S. Mohamad, M. Z. Hashimi, M. S. Yunus, et al. Surface and tribological properties of oxide films on aluminium alloy through fly-ash reinforcement. Coatings, Vol. 12, 2022, pp. 1–19.10.3390/coatings12020256Search in Google Scholar
[226] Eyre, T. S. Wear characteristics of metals. Tribology International, Vol. 9, 1976, pp. 203–212.10.1016/0301-679X(76)90077-3Search in Google Scholar
[227] Guezmil, M., W. Bensalah, A. Khalladi, K. Elleuch, M. De-Petris Wery, and H. F. Ayedi. Effect of test parameters on the friction behaviour of anodized aluminium alloy. International Scholarly Research Notices, Vol. 2014, 2014, pp. 1–9.10.1155/2014/795745Search in Google Scholar PubMed PubMed Central
[228] Fratila-Apachitei, L. E., J. Duszczyk, and L. Katgerman. AlSi(Cu) anodic oxide layers formed in H2SO4 at low temperature using different current waveforms. Surface and Coatings Technology, Vol. 165, 2003, pp. 232–240.10.1016/S0257-8972(02)00733-8Search in Google Scholar
[229] Fratila-Apachitei, L. E., J. Duszczyk, and L. Katgerman. Vickers microhardness of AlSi(Cu) anodic oxide layers formed in H2SO4 at low temperature. Surface and Coatings Technology, Vol. 165, 2003, pp. 309–315.10.1016/S0257-8972(02)00750-8Search in Google Scholar
[230] Kozhukhova, A. E., S. P. du Preez, and D. G. Bessarabov. Preparation of anodized aluminium oxide at high temperatures using low purity aluminium (Al6082). Surface and Coatings Technology, Vol. 378, 2019, id. 124970.10.1016/j.surfcoat.2019.124970Search in Google Scholar
[231] Jia, Y., H. Zhou, P. Luo, S. Luo, J. Chen, and Y. Kuang. Preparation and characteristics of well-aligned macroporous films on aluminum by high voltage anodization in mixed acid. Surface and Coatings Technology, Vol. 201, 2006, pp. 513–518.10.1016/j.surfcoat.2005.11.144Search in Google Scholar
[232] Bara, M. Ocena wpływu przygotowania podłoża na właściwości tribologiczne nanoceramicznych warstw tlenkowych. Tribologia, Vol. 4, 2014, pp. 9–20.Search in Google Scholar
[233] Aerts, T., T. Dimogerontakis, I. De Graeve, J. Fransaer, and H. Terryn. Influence of the anodizing temperature on the porosity and the mechanical properties of the porous anodic oxide film. Surface and Coatings Technology, Vol. 201, 2007, pp. 7310–7317.10.1016/j.surfcoat.2007.01.044Search in Google Scholar
[234] Roshani, M., A. Sabour Rouhaghdam, M. Aliofkhazraei, and A. Heydari Astaraee. Optimization of mechanical properties for pulsed anodizing of aluminum. Surface and Coatings Technology, Vol. 310, 2017, pp. 17–24.10.1016/j.surfcoat.2016.12.046Search in Google Scholar
[235] Kim, H., D. Kim, W. Lee, S. J. Cho, J. H. Hahn, and H. S. Ahn. Tribological properties of nanoporous anodic aluminum oxide film. Surface and Coatings Technology, Vol. 205, 2010, pp. 1431–1437.10.1016/j.surfcoat.2010.07.056Search in Google Scholar
[236] Korzekwa, J., W. Skoneczny, and L. Wojnar. Wpływ parametrów elektroosadzania na zmiany nanostruktury warstw Al2O3/WS2 o przeznaczeniu tribologicznym. Czas Tech, Vol. 15, 2011, pp. 11–19.Search in Google Scholar
[237] Bensalah, W., K. Elleuch, M. Feki, M. DePetris-Wery, and H. F. Ayedi. Comparative study of mechanical and tribological properties of alumina coatings formed on aluminium in various conditions. Materials & Design, Vol. 30, 2009, pp. 3731–3737.10.1016/j.matdes.2009.02.005Search in Google Scholar
[238] Santecchia, E., M. Cabibbo, A. M. S. Hamouda, F. Musharavati, A. Popelka, and S. Spigarelli. Dry sliding tribological properties of a hard anodized AA6082 aluminum alloy. Metals, Vol. 10, 2020, id. 207.10.3390/met10020207Search in Google Scholar
[239] Duda, P., M. Bara, and S. Kaptacz. The efect of electron beam irradiation on tribological characteristics of PTFE, POM and PA in combination with an anodic-oxide coatings. Tribologia, Vol. 3, 2012, pp. 53–60.Search in Google Scholar
[240] Bara, M., P. Duda, and S. Kaptacz. Wear resistance of constructional materials in cooperation with the oxide coating. Tribologia, Vol. 4, 2011, pp. 21–32.Search in Google Scholar
[241] Kmita, T. Wpływ parametrów procesu anodowania impulsowego na właściwości tribologiczne nanoceramicznych warstw tlenkowych Al2O3. Tribologia, Vol. 4, 2014, pp. 53–61.Search in Google Scholar
[242] Bara, M., T. Kmita, and J. Korzekwa. Microstructure and properties of composite coatings obtained on aluminium alloys. Archives of Metallurgy and Materials, Vol. 61, 2016, pp. 1453–1458.10.1515/amm-2016-0238Search in Google Scholar
[243] Korzekwa, J., W. Skoneczny, G. Dercz, and M. Bara. Wear mechanism of Al2O3/WS2 with PEEK/BG plastic. Journal of Tribology, Vol. 136, No. 1, 2014, id. 011601.10.1115/1.4024938Search in Google Scholar
[244] Liew, K. W., S. Y. Chia, C. K. Kok, and K. O. Low. Evaluation on tribological design coatings of Al2O3, Ni-P-PTFE and MoS2 on aluminium alloy 7075 under oil lubrication. Materials & Design, Vol. 48, 2013, pp. 77–84.10.1016/j.matdes.2012.08.010Search in Google Scholar
[245] Bara, M., G. Służałek, and H. Wistuba. Tribological properties of aluminium oxide layer modified by carbon. Tribologia, Vol. 3, 2010, pp. 11–20.Search in Google Scholar
[246] Picas, J. A., A. Forn, E. Rupérez, M. T. Baile, and E. Martín. Hard anodizing of aluminium matrix composite A6061/ (Al2O3)p for wear and corrosion resistance improvement. Plasma Processes and Polymers (Print), Vol. 4, 2007, pp. 579–583.10.1002/ppap.200731409Search in Google Scholar
[247] Sieber, M., T. Mehner, D. Dietrich, G. Alisch, D. Nickel, D. Meyer, et al. Wear-resistant coatings on aluminium produced by plasma anodising-A correlation of wear properties, microstructure, phase composition and distribution. Surface Coatings Technology, Vol. 240, 2014, pp. 96–102.10.1016/j.surfcoat.2013.12.021Search in Google Scholar
[248] Malayoglu, U., K. C. Tekin, U. Malayoglu, and S. Shrestha. An investigation into the mechanical and tribological properties of plasma electrolytic oxidation and hard-anodized coatings on 6082 aluminum alloy. Materials Science & Engineering, A: Structural Materials: Properties, Microstructure and Processing, Vol. 528, 2011, pp. 7451–7460.10.1016/j.msea.2011.06.032Search in Google Scholar
[249] Tsyntsaru, N., B. Kavas, J. Sort, M. Urgen, and J. P. Celis. Mechanical and frictional behaviour of nano-porous anodized aluminium. Materials Chemistry and Physics, Vol. 148, 2014, pp. 887–895.10.1016/j.matchemphys.2014.08.066Search in Google Scholar
[250] Benea, L. and V. Dumitrascu. Enhancement in sustained friction and wear resistance of nanoporous aluminum oxide films obtained by controlled electrochemical oxidation process. RSC Advances, Vol. 9, 2019, pp. 25056–25063.10.1039/C9RA05702ASearch in Google Scholar
[251] Korzekwa, J., M. Bara, J. Pietraszek, and P. Pawlus. Tribological behaviour of Al2O3/ inorganic fullerene-like WS2 composite layer sliding against plastic. International Journal of Surface Science and Engineering, Vol. 10, 2016, pp. 570–584.10.1504/IJSURFSE.2016.081035Search in Google Scholar
[252] Paszeczko M., M. Kindrachuk, V. Lubunets, K. Dziedzic, O. Radko, Y. Korbut. Tribologia. Politechnika Lubelska, Lublin, 2017.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Review Articles
- Progress in preparation and ablation resistance of ultra-high-temperature ceramics modified C/C composites for extreme environment
- Solar lighting systems applied in photocatalysis to treat pollutants – A review
- Technological advances in three-dimensional skin tissue engineering
- Hybrid magnesium matrix composites: A review of reinforcement philosophies, mechanical and tribological characteristics
- Application prospect of calcium peroxide nanoparticles in biomedical field
- Research progress on basalt fiber-based functionalized composites
- Evaluation of the properties and applications of FRP bars and anchors: A review
- A critical review on mechanical, durability, and microstructural properties of industrial by-product-based geopolymer composites
- Multifunctional engineered cementitious composites modified with nanomaterials and their applications: An overview
- Role of bioglass derivatives in tissue regeneration and repair: A review
- Research progress on properties of cement-based composites incorporating graphene oxide
- Properties of ultra-high performance concrete and conventional concrete with coal bottom ash as aggregate replacement and nanoadditives: A review
- A scientometric review of the literature on the incorporation of steel fibers in ultra-high-performance concrete with research mapping knowledge
- Weldability of high nitrogen steels: A review
- Application of waste recycle tire steel fibers as a construction material in concrete
- Wear properties of graphene-reinforced aluminium metal matrix composite: A review
- Experimental investigations of electrodeposited Zn–Ni, Zn–Co, and Ni–Cr–Co–based novel coatings on AA7075 substrate to ameliorate the mechanical, abrasion, morphological, and corrosion properties for automotive applications
- Research evolution on self-healing asphalt: A scientometric review for knowledge mapping
- Recent developments in the mechanical properties of hybrid fiber metal laminates in the automotive industry: A review
- A review of microscopic characterization and related properties of fiber-incorporated cement-based materials
- Comparison and review of classical and machine learning-based constitutive models for polymers used in aeronautical thermoplastic composites
- Gold nanoparticle-based strategies against SARS-CoV-2: A review
- Poly-ferric sulphate as superior coagulant: A review on preparation methods and properties
- A review on ceramic waste-based concrete: A step toward sustainable concrete
- Modification of the structure and properties of oxide layers on aluminium alloys: A review
- A review of magnetically driven swimming microrobots: Material selection, structure design, control method, and applications
- Polyimide–nickel nanocomposites fabrication, properties, and applications: A review
- Design and analysis of timber-concrete-based civil structures and its applications: A brief review
- Effect of fiber treatment on physical and mechanical properties of natural fiber-reinforced composites: A review
- Blending and functionalisation modification of 3D printed polylactic acid for fused deposition modeling
- A critical review on functionally graded ceramic materials for cutting tools: Current trends and future prospects
- Heme iron as potential iron fortifier for food application – characterization by material techniques
- An overview of the research trends on fiber-reinforced shotcrete for construction applications
- High-entropy alloys: A review of their performance as promising materials for hydrogen and molten salt storage
- Effect of the axial compression ratio on the seismic behavior of resilient concrete walls with concealed column stirrups
- Research Articles
- Effect of fiber orientation and elevated temperature on the mechanical properties of unidirectional continuous kenaf reinforced PLA composites
- Optimizing the ECAP processing parameters of pure Cu through experimental, finite element, and response surface approaches
- Study on the solidification property and mechanism of soft soil based on the industrial waste residue
- Preparation and photocatalytic degradation of Sulfamethoxazole by g-C3N4 nano composite samples
- Impact of thermal modification on color and chemical changes of African padauk, merbau, mahogany, and iroko wood species
- The evaluation of the mechanical properties of glass, kenaf, and honeycomb fiber-reinforced composite
- Evaluation of a novel steel box-soft body combination for bridge protection against ship collision
- Study on the uniaxial compression constitutive relationship of modified yellow mud from minority dwelling in western Sichuan, China
- Ultrasonic longitudinal torsion-assisted biotic bone drilling: An experimental study
- Green synthesis, characterizations, and antibacterial activity of silver nanoparticles from Themeda quadrivalvis, in conjugation with macrolide antibiotics against respiratory pathogens
- Performance analysis of WEDM during the machining of Inconel 690 miniature gear using RSM and ANN modeling approaches
- Biosynthesis of Ag/bentonite, ZnO/bentonite, and Ag/ZnO/bentonite nanocomposites by aqueous leaf extract of Hagenia abyssinica for antibacterial activities
- Eco-friendly MoS2/waste coconut oil nanofluid for machining of magnesium implants
- Silica and kaolin reinforced aluminum matrix composite for heat storage
- Optimal design of glazed hollow bead thermal insulation mortar containing fly ash and slag based on response surface methodology
- Hemp seed oil nanoemulsion with Sapindus saponins as a potential carrier for iron supplement and vitamin D
- A numerical study on thin film flow and heat transfer enhancement for copper nanoparticles dispersed in ethylene glycol
- Research on complex multimodal vibration characteristics of offshore platform
- Applicability of fractal models for characterising pore structure of hybrid basalt–polypropylene fibre-reinforced concrete
- Influence of sodium silicate to precursor ratio on mechanical properties and durability of the metakaolin/fly ash alkali-activated sustainable mortar using manufactured sand
- An experimental study of bending resistance of multi-size PFRC beams
- Characterization, biocompatibility, and optimization of electrospun SF/PCL composite nanofiber films
- Morphological classification method and data-driven estimation of the joint roughness coefficient by consideration of two-order asperity
- Prediction and simulation of mechanical properties of borophene-reinforced epoxy nanocomposites using molecular dynamics and FEA
- Nanoemulsions of essential oils stabilized with saponins exhibiting antibacterial and antioxidative properties
- Fabrication and performance analysis of sustainable municipal solid waste incineration fly ash alkali-activated acoustic barriers
- Electrostatic-spinning construction of HCNTs@Ti3C2T x MXenes hybrid aerogel microspheres for tunable microwave absorption
- Investigation of the mechanical properties, surface quality, and energy efficiency of a fused filament fabrication for PA6
- Experimental study on mechanical properties of coal gangue base geopolymer recycled aggregate concrete reinforced by steel fiber and nano-Al2O3
- Hybrid bio-fiber/bio-ceramic composite materials: Mechanical performance, thermal stability, and morphological analysis
- Experimental study on recycled steel fiber-reinforced concrete under repeated impact
- Effect of rare earth Nd on the microstructural transformation and mechanical properties of 7xxx series aluminum alloys
- Color match evaluation using instrumental method for three single-shade resin composites before and after in-office bleaching
- Exploring temperature-resilient recycled aggregate concrete with waste rubber: An experimental and multi-objective optimization analysis
- Study on aging mechanism of SBS/SBR compound-modified asphalt based on molecular dynamics
- Evolution of the pore structure of pumice aggregate concrete and the effect on compressive strength
- Effect of alkaline treatment time of fibers and microcrystalline cellulose addition on mechanical properties of unsaturated polyester composites reinforced by cantala fibers
- Optimization of eggshell particles to produce eco-friendly green fillers with bamboo reinforcement in organic friction materials
- An effective approach to improve microstructure and tribological properties of cold sprayed Al alloys
- Luminescence and temperature-sensing properties of Li+, Na+, or K+, Tm3+, and Yb3+ co-doped Bi2WO6 phosphors
- Effect of molybdenum tailings aggregate on mechanical properties of engineered cementitious composites and stirrup-confined ECC stub columns
- Experimental study on the seismic performance of short shear walls comprising cold-formed steel and high-strength reinforced concrete with concealed bracing
- Failure criteria and microstructure evolution mechanism of the alkali–silica reaction of concrete
- Mechanical, fracture-deformation, and tribology behavior of fillers-reinforced sisal fiber composites for lightweight automotive applications
- UV aging behavior evolution characterization of HALS-modified asphalt based on micro-morphological features
- Preparation of VO2/graphene/SiC film by water vapor oxidation
- A semi-empirical model for predicting carbonation depth of RAC under two-dimensional conditions
- Comparison of the physical properties of different polyimide nanocomposite films containing organoclays varying in alkyl chain lengths
- Effects of freeze–thaw cycles on micro and meso-structural characteristics and mechanical properties of porous asphalt mixtures
- Flexural performance of a new type of slightly curved arc HRB400 steel bars reinforced one-way concrete slabs
- Alkali-activated binder based on red mud with class F fly ash and ground granulated blast-furnace slag under ambient temperature
- Facile synthesis of g-C3N4 nanosheets for effective degradation of organic pollutants via ball milling
- DEM study on the loading rate effect of marble under different confining pressures
- Conductive and self-cleaning composite membranes from corn husk nanofiber embedded with inorganic fillers (TiO2, CaO, and eggshell) by sol–gel and casting processes for smart membrane applications
- Laser re-melting of modified multimodal Cr3C2–NiCr coatings by HVOF: Effect on the microstructure and anticorrosion properties
- Damage constitutive model of jointed rock mass considering structural features and load effect
- Thermosetting polymer composites: Manufacturing and properties study
- CSG compressive strength prediction based on LSTM and interpretable machine learning
- Axial compression behavior and stress–strain relationship of slurry-wrapping treatment recycled aggregate concrete-filled steel tube short columns
- Space-time evolution characteristics of loaded gas-bearing coal fractures based on industrial μCT
- Dual-biprism-based single-camera high-speed 3D-digital image correlation for deformation measurement on sandwich structures under low velocity impact
- Effects of cold deformation modes on microstructure uniformity and mechanical properties of large 2219 Al–Cu alloy rings
- Basalt fiber as natural reinforcement to improve the performance of ecological grouting slurry for the conservation of earthen sites
- Interaction of micro-fluid structure in a pressure-driven duct flow with a nearby placed current-carrying wire: A numerical investigation
- A simulation modeling methodology considering random multiple shots for shot peening process
- Optimization and characterization of composite modified asphalt with pyrolytic carbon black and chicken feather fiber
- Synthesis, characterization, and application of the novel nanomagnet adsorbent for the removal of Cr(vi) ions
- Multi-perspective structural integrity-based computational investigations on airframe of Gyrodyne-configured multi-rotor UAV through coupled CFD and FEA approaches for various lightweight sandwich composites and alloys
- Influence of PVA fibers on the durability of cementitious composites under the wet–heat–salt coupling environment
- Compressive behavior of BFRP-confined ceramsite concrete: An experimental study and stress–strain model
- Interval models for uncertainty analysis and degradation prediction of the mechanical properties of rubber
- Preparation of PVDF-HFP/CB/Ni nanocomposite films for piezoelectric energy harvesting
- Frost resistance and life prediction of recycled brick aggregate concrete with waste polypropylene fiber
- Synthetic leathers as a possible source of chemicals and odorous substances in indoor environment
- Mechanical properties of seawater volcanic scoria aggregate concrete-filled circular GFRP and stainless steel tubes under axial compression
- Effect of curved anchor impellers on power consumption and hydrodynamic parameters of yield stress fluids (Bingham–Papanastasiou model) in stirred tanks
- All-dielectric tunable zero-refractive index metamaterials based on phase change materials
- Influence of ultrasonication time on the various properties of alkaline-treated mango seed waste filler reinforced PVA biocomposite
- Research on key casting process of high-grade CNC machine tool bed nodular cast iron
- Latest research progress of SiCp/Al composite for electronic packaging
- Special Issue on 3D and 4D Printing of Advanced Functional Materials - Part I
- Molecular dynamics simulation on electrohydrodynamic atomization: Stable dripping mode by pre-load voltage
- Research progress of metal-based additive manufacturing in medical implants
Articles in the same Issue
- Review Articles
- Progress in preparation and ablation resistance of ultra-high-temperature ceramics modified C/C composites for extreme environment
- Solar lighting systems applied in photocatalysis to treat pollutants – A review
- Technological advances in three-dimensional skin tissue engineering
- Hybrid magnesium matrix composites: A review of reinforcement philosophies, mechanical and tribological characteristics
- Application prospect of calcium peroxide nanoparticles in biomedical field
- Research progress on basalt fiber-based functionalized composites
- Evaluation of the properties and applications of FRP bars and anchors: A review
- A critical review on mechanical, durability, and microstructural properties of industrial by-product-based geopolymer composites
- Multifunctional engineered cementitious composites modified with nanomaterials and their applications: An overview
- Role of bioglass derivatives in tissue regeneration and repair: A review
- Research progress on properties of cement-based composites incorporating graphene oxide
- Properties of ultra-high performance concrete and conventional concrete with coal bottom ash as aggregate replacement and nanoadditives: A review
- A scientometric review of the literature on the incorporation of steel fibers in ultra-high-performance concrete with research mapping knowledge
- Weldability of high nitrogen steels: A review
- Application of waste recycle tire steel fibers as a construction material in concrete
- Wear properties of graphene-reinforced aluminium metal matrix composite: A review
- Experimental investigations of electrodeposited Zn–Ni, Zn–Co, and Ni–Cr–Co–based novel coatings on AA7075 substrate to ameliorate the mechanical, abrasion, morphological, and corrosion properties for automotive applications
- Research evolution on self-healing asphalt: A scientometric review for knowledge mapping
- Recent developments in the mechanical properties of hybrid fiber metal laminates in the automotive industry: A review
- A review of microscopic characterization and related properties of fiber-incorporated cement-based materials
- Comparison and review of classical and machine learning-based constitutive models for polymers used in aeronautical thermoplastic composites
- Gold nanoparticle-based strategies against SARS-CoV-2: A review
- Poly-ferric sulphate as superior coagulant: A review on preparation methods and properties
- A review on ceramic waste-based concrete: A step toward sustainable concrete
- Modification of the structure and properties of oxide layers on aluminium alloys: A review
- A review of magnetically driven swimming microrobots: Material selection, structure design, control method, and applications
- Polyimide–nickel nanocomposites fabrication, properties, and applications: A review
- Design and analysis of timber-concrete-based civil structures and its applications: A brief review
- Effect of fiber treatment on physical and mechanical properties of natural fiber-reinforced composites: A review
- Blending and functionalisation modification of 3D printed polylactic acid for fused deposition modeling
- A critical review on functionally graded ceramic materials for cutting tools: Current trends and future prospects
- Heme iron as potential iron fortifier for food application – characterization by material techniques
- An overview of the research trends on fiber-reinforced shotcrete for construction applications
- High-entropy alloys: A review of their performance as promising materials for hydrogen and molten salt storage
- Effect of the axial compression ratio on the seismic behavior of resilient concrete walls with concealed column stirrups
- Research Articles
- Effect of fiber orientation and elevated temperature on the mechanical properties of unidirectional continuous kenaf reinforced PLA composites
- Optimizing the ECAP processing parameters of pure Cu through experimental, finite element, and response surface approaches
- Study on the solidification property and mechanism of soft soil based on the industrial waste residue
- Preparation and photocatalytic degradation of Sulfamethoxazole by g-C3N4 nano composite samples
- Impact of thermal modification on color and chemical changes of African padauk, merbau, mahogany, and iroko wood species
- The evaluation of the mechanical properties of glass, kenaf, and honeycomb fiber-reinforced composite
- Evaluation of a novel steel box-soft body combination for bridge protection against ship collision
- Study on the uniaxial compression constitutive relationship of modified yellow mud from minority dwelling in western Sichuan, China
- Ultrasonic longitudinal torsion-assisted biotic bone drilling: An experimental study
- Green synthesis, characterizations, and antibacterial activity of silver nanoparticles from Themeda quadrivalvis, in conjugation with macrolide antibiotics against respiratory pathogens
- Performance analysis of WEDM during the machining of Inconel 690 miniature gear using RSM and ANN modeling approaches
- Biosynthesis of Ag/bentonite, ZnO/bentonite, and Ag/ZnO/bentonite nanocomposites by aqueous leaf extract of Hagenia abyssinica for antibacterial activities
- Eco-friendly MoS2/waste coconut oil nanofluid for machining of magnesium implants
- Silica and kaolin reinforced aluminum matrix composite for heat storage
- Optimal design of glazed hollow bead thermal insulation mortar containing fly ash and slag based on response surface methodology
- Hemp seed oil nanoemulsion with Sapindus saponins as a potential carrier for iron supplement and vitamin D
- A numerical study on thin film flow and heat transfer enhancement for copper nanoparticles dispersed in ethylene glycol
- Research on complex multimodal vibration characteristics of offshore platform
- Applicability of fractal models for characterising pore structure of hybrid basalt–polypropylene fibre-reinforced concrete
- Influence of sodium silicate to precursor ratio on mechanical properties and durability of the metakaolin/fly ash alkali-activated sustainable mortar using manufactured sand
- An experimental study of bending resistance of multi-size PFRC beams
- Characterization, biocompatibility, and optimization of electrospun SF/PCL composite nanofiber films
- Morphological classification method and data-driven estimation of the joint roughness coefficient by consideration of two-order asperity
- Prediction and simulation of mechanical properties of borophene-reinforced epoxy nanocomposites using molecular dynamics and FEA
- Nanoemulsions of essential oils stabilized with saponins exhibiting antibacterial and antioxidative properties
- Fabrication and performance analysis of sustainable municipal solid waste incineration fly ash alkali-activated acoustic barriers
- Electrostatic-spinning construction of HCNTs@Ti3C2T x MXenes hybrid aerogel microspheres for tunable microwave absorption
- Investigation of the mechanical properties, surface quality, and energy efficiency of a fused filament fabrication for PA6
- Experimental study on mechanical properties of coal gangue base geopolymer recycled aggregate concrete reinforced by steel fiber and nano-Al2O3
- Hybrid bio-fiber/bio-ceramic composite materials: Mechanical performance, thermal stability, and morphological analysis
- Experimental study on recycled steel fiber-reinforced concrete under repeated impact
- Effect of rare earth Nd on the microstructural transformation and mechanical properties of 7xxx series aluminum alloys
- Color match evaluation using instrumental method for three single-shade resin composites before and after in-office bleaching
- Exploring temperature-resilient recycled aggregate concrete with waste rubber: An experimental and multi-objective optimization analysis
- Study on aging mechanism of SBS/SBR compound-modified asphalt based on molecular dynamics
- Evolution of the pore structure of pumice aggregate concrete and the effect on compressive strength
- Effect of alkaline treatment time of fibers and microcrystalline cellulose addition on mechanical properties of unsaturated polyester composites reinforced by cantala fibers
- Optimization of eggshell particles to produce eco-friendly green fillers with bamboo reinforcement in organic friction materials
- An effective approach to improve microstructure and tribological properties of cold sprayed Al alloys
- Luminescence and temperature-sensing properties of Li+, Na+, or K+, Tm3+, and Yb3+ co-doped Bi2WO6 phosphors
- Effect of molybdenum tailings aggregate on mechanical properties of engineered cementitious composites and stirrup-confined ECC stub columns
- Experimental study on the seismic performance of short shear walls comprising cold-formed steel and high-strength reinforced concrete with concealed bracing
- Failure criteria and microstructure evolution mechanism of the alkali–silica reaction of concrete
- Mechanical, fracture-deformation, and tribology behavior of fillers-reinforced sisal fiber composites for lightweight automotive applications
- UV aging behavior evolution characterization of HALS-modified asphalt based on micro-morphological features
- Preparation of VO2/graphene/SiC film by water vapor oxidation
- A semi-empirical model for predicting carbonation depth of RAC under two-dimensional conditions
- Comparison of the physical properties of different polyimide nanocomposite films containing organoclays varying in alkyl chain lengths
- Effects of freeze–thaw cycles on micro and meso-structural characteristics and mechanical properties of porous asphalt mixtures
- Flexural performance of a new type of slightly curved arc HRB400 steel bars reinforced one-way concrete slabs
- Alkali-activated binder based on red mud with class F fly ash and ground granulated blast-furnace slag under ambient temperature
- Facile synthesis of g-C3N4 nanosheets for effective degradation of organic pollutants via ball milling
- DEM study on the loading rate effect of marble under different confining pressures
- Conductive and self-cleaning composite membranes from corn husk nanofiber embedded with inorganic fillers (TiO2, CaO, and eggshell) by sol–gel and casting processes for smart membrane applications
- Laser re-melting of modified multimodal Cr3C2–NiCr coatings by HVOF: Effect on the microstructure and anticorrosion properties
- Damage constitutive model of jointed rock mass considering structural features and load effect
- Thermosetting polymer composites: Manufacturing and properties study
- CSG compressive strength prediction based on LSTM and interpretable machine learning
- Axial compression behavior and stress–strain relationship of slurry-wrapping treatment recycled aggregate concrete-filled steel tube short columns
- Space-time evolution characteristics of loaded gas-bearing coal fractures based on industrial μCT
- Dual-biprism-based single-camera high-speed 3D-digital image correlation for deformation measurement on sandwich structures under low velocity impact
- Effects of cold deformation modes on microstructure uniformity and mechanical properties of large 2219 Al–Cu alloy rings
- Basalt fiber as natural reinforcement to improve the performance of ecological grouting slurry for the conservation of earthen sites
- Interaction of micro-fluid structure in a pressure-driven duct flow with a nearby placed current-carrying wire: A numerical investigation
- A simulation modeling methodology considering random multiple shots for shot peening process
- Optimization and characterization of composite modified asphalt with pyrolytic carbon black and chicken feather fiber
- Synthesis, characterization, and application of the novel nanomagnet adsorbent for the removal of Cr(vi) ions
- Multi-perspective structural integrity-based computational investigations on airframe of Gyrodyne-configured multi-rotor UAV through coupled CFD and FEA approaches for various lightweight sandwich composites and alloys
- Influence of PVA fibers on the durability of cementitious composites under the wet–heat–salt coupling environment
- Compressive behavior of BFRP-confined ceramsite concrete: An experimental study and stress–strain model
- Interval models for uncertainty analysis and degradation prediction of the mechanical properties of rubber
- Preparation of PVDF-HFP/CB/Ni nanocomposite films for piezoelectric energy harvesting
- Frost resistance and life prediction of recycled brick aggregate concrete with waste polypropylene fiber
- Synthetic leathers as a possible source of chemicals and odorous substances in indoor environment
- Mechanical properties of seawater volcanic scoria aggregate concrete-filled circular GFRP and stainless steel tubes under axial compression
- Effect of curved anchor impellers on power consumption and hydrodynamic parameters of yield stress fluids (Bingham–Papanastasiou model) in stirred tanks
- All-dielectric tunable zero-refractive index metamaterials based on phase change materials
- Influence of ultrasonication time on the various properties of alkaline-treated mango seed waste filler reinforced PVA biocomposite
- Research on key casting process of high-grade CNC machine tool bed nodular cast iron
- Latest research progress of SiCp/Al composite for electronic packaging
- Special Issue on 3D and 4D Printing of Advanced Functional Materials - Part I
- Molecular dynamics simulation on electrohydrodynamic atomization: Stable dripping mode by pre-load voltage
- Research progress of metal-based additive manufacturing in medical implants

![Figure 13
The possibility of filling the nanopores of the Al2O3 oxide layer with IF-WS2 solid lubricant 3 (a) [223] (courtasy of John Wiley & Sons, Inc.) and (b) [224] (courtesy of Springer Nature).](/document/doi/10.1515/rams-2023-0108/asset/graphic/j_rams-2023-0108_fig_013.jpg)
