Polymer-based nanocarriers for biomedical and environmental applications
-
Dahir Sagir Idris
, Soumya Pandit
Abstract
Polymer-based nanocarriers are created from natural or synthetic polymers that are modified to form submicroscopic particles. The polymer matrix can be customized to provide specific properties, such as surface chemistry and flexibility. This allows the particles to be designed and used in different fields. They are promising nanomaterials that are used as therapeutic and diagnostic agents, and they have potential biomedical and environmental applications. These nanocarriers are polymers that can be engineered with other types of nanomaterials with different sizes, shapes, and compositions. They can deliver drugs or their cargo to a specific site with precisely controlled release. They have many advantages compared to traditional drug delivery carriers, including improved biocompatibility, reduced toxicity, and increased efficacy. In biomedical applications, polymer nanocarriers have been used as drug delivery carriers, cancer therapy, and gene therapy. In environmental applications, polymer nanocarriers are able to remove heavy metals and other contaminants from air and water bodies. In this review, a summary of recent fabrication, design, synthesis, characterisation, and various applications in the biomedical and environmental fields has been provided. The review also highlights the current challenges and prospects of evolving polymer nanocarriers.
1 Introduction
In recent years, the use of nanotechnology aimed at developing cancer drugs and nanocarriers has increased rapidly. Nanotechnology has a very wide range of applications and also has the potential to make a significant impact in biotechnology, healthcare, drug delivery, electronics, storage systems, and industries (1). However, the history of nanotechnology dates back as far back as 1959 when the physicist Richard P. Feynman once said “There is plenty of room at the bottom” in a lecture, forecasting that materials can be reduced to the nanoscale level. He suggested that shrinking materials to the nanoscale level and building them back up is the key to future technological advancement (2). Nanomaterials are considered to be in the range of 1–100 nm which allow these particles to communicate or interact with the surface of a cell in such a way that the physiological and biochemical properties of the cell can be decoded. Nanoparticles can be incorporated into a form of composites with improved properties, strength, toughness, or thermal conductivity. Of course, sometimes we cannot deny the fact that the newly formed composite possesses an unknown property that is not known to the parent constituent material (3). For example, in nanomedicine, there is an increasing research interest in the development of various systems, such as nanocarriers for drug delivery to targeted cells such as cancer cells or infections/neurodegenerative disorders (4,5,6). Among the various types of nanomaterials, polymer-based nanocarriers and colloids have attracted more attention because they have interesting properties such as flexibility, diversity, and unique composition, and also easy to functionalise with specific ligands that are intended to be recognised by a particular cell receptor, thus paving the way for active targeting (6). Subject to the type, nature, ligand involved, and coupling reaction of the selected polymer, nanocarriers can be designed to possess the ability to load drugs in them and deliver them to cells of specific site in a biological system (7). Subsequently, polymeric nanomaterials used for the delivery of agrochemicals such as pesticides, antibiotics, growth promoters, and other essential nutrients, considering the impact of biodegradability and biocompatibility of polymeric nanomaterials, have gained great interest in research from many researchers (8). They contribute to less environmental impact and higher efficiency for their use (8). Some of these polymeric nanocarriers are designed to act as smart systems in the sense that they respond or react to certain stimuli such as light, sound, temperature, pH, or even potential differences in triggers and release the drug or cargo content at the preferred location or destination (9). This review will examine the latest trend in polymer-based nanocarriers, their types, synthesis approach, characterisation, and biomedical and environmental applications among others.
2 Types of nanomaterials
Nanomaterials are of different types depending on their size, shape, and functional group, or physical, chemical, biological, and optical properties. These types include carbon-based, metal-based, polymer-based, ceramic-based, semiconductor-based, dendrimers, and nanocomposites (Figure 1).

Types of nanomaterials.
2.1 Carbon-based nanoparticles
Carbon is one of the most abundant elements in the universe that is allotropic in nature. It can assume different forms based on how the atoms are arranged. Diamonds, fullerenes, graphene, and graphite are considered to be four allotropes of pure carbon. In nanotechnology, carbon nanostructures are making a significant contribution in different research fields (10). They have been used as catalysts or support for catalysts, additives, sensors or substrates for sensors, adsorbents, filtering media, active materials in energy systems (i.e. supercapacitors, batteries, and energy), additives for metals and their alloys, glass, ceramics, polymer textiles, and composite nanomaterials (11). Nanocomposites of carbon derivatives may exhibit superior performance, like electrical and mechanical properties, which are useful for various applications. There are other forms of carbon nanomaterials as well.
2.2 Metal-based nanomaterials
Metal-based nanomaterials are submicrometre nanomaterials that are synthesised from pure metal salts or precursor of pure metals. Their synthesis approach is usually classified under the bottom-up technique of synthesising nanoparticles. Almost all of the metal-based nanoparticles available can be synthesised from their respective metal salts/precursors. Gold, silver, platinum, palladium, zinc, iron, nickel, copper, cobalt, aluminium, lead, cadmium, and manganese salts/precursors have all been used in fabricating various metallic nanoparticles. These nanoparticles are known to possess unique properties such as biocompatibility, crystallinity, pore size, electrical conductivity, optoelectrical characteristics, high surface area, and sensitivity (12,13,14,15). To precisely tailor the unique size, shape, and other properties of nanoparticles, some synthesis constraints need to be adjusted accordingly. These constraints include the pH of the reaction chamber, concentration, and temperature. Furthermore, metal oxide has also been exploited and found its application in many fields, including sensors, super-strong materials, antimicrobial activity, catalysts, tissue engineering, and electrochemical detection of biomolecules (16,17,18,19).
2.3 Ceramic nanoparticles
Ceramic nanoparticles are made of tiny particles that are primarily of ceramic materials such as oxides, nitrides, carbides, and carbonates of metals and metalloids such as silicon, titanium, and calcium. Ceramic nanomaterials possess some characteristics and properties such as increased surface area, increased reactivity, high heat resistance, chemical inertness, and improved mechanical strength (20). They have been used for different applications in many fields including biomedicine, electronics, catalysis, energy storage, and environmental remediation (21,22,23). For example, ceramic nanoparticles can be used in electronics as conductive fillers in polymer composites to improve their electrical conductivity (24). Another example is their use in the fabrication of electrodes for lithium ion batteries to improve their energy storage capacity and density (22). In the case of catalysis, they are used to increase the efficacy of chemical reactions (25,26). Ceramic nanocarriers also found their use in the field of biomedicine as nanocarriers to enhance targeted cargo release at the required site (20). However, the synthesis and processing can be challenging due to their very small size and their tendency to aggregate or even form unwanted structures (27). Therefore, a thorough understanding of the synthesis methods and behaviour of ceramic nanoparticles is crucial for their successful application in various fields.
2.4 Semiconductor nanoparticles
Semiconductor nanoparticles are fluorescent materials that are generally metal compounds with non-metallic properties (28). These nanomaterials are made from different compounds, such as II–VI semiconductor nanocrystals. III–V and IV–VI semiconductor nanocrystals have been used (29). Although these nanomaterials are still in the early stage of research, they have promising application in many fields, including light-emitting diodes, laser technology, solar cells, waveguides, nanoscale electronic devices, chemicals, and biosensors (30). With the advancement of nanotechnology, the semiconductor industry may witness a significant breakthrough in the near future.
2.5 Nanocomposites
Nanocomposites, as the name implies, are heterogeneous/hybrid complexes that are made up of two or more different materials combined to blend their properties, in which at least one of these materials is in the dimension of 10−9 m. Nanocomposites usually consist of one or more discontinuous phases (reinforcements; e.g., metals, clay) distributed along a continuous phase (matrix; e.g., polymer). They can be polymer base (biocomposites base [e.g., elastin-collagen], polymer–polymer base [e.g., poly(p-phenylene oxide)], polymer-layered silica base [e.g., nylon-6], polymer–ceramics base [e.g., barium titanate], inorganic–organic polymer base [e.g., nanofibers], inorganic–organic hybrid base [e.g., nanocrystals]), or non-polymer base (metal–metal nanocomposites; either in the form of alloy or shell [e.g., Pt-Ru], metal–ceramics nanocomposites; either in the form of nanotubes or complicated nanostructures [e.g., polysiloxane/polysiloxane], ceramic–ceramic nanocomposite; alloy or ceramics [e.g., zirconia]) (31,32,33,34,35).
2.6 Polymer-based nanoparticles
Polymer-based nanoparticles are an exciting field of study in nanotechnology. The development of these particles has the potential to revolutionise many industries, from medical applications to energy production/storage to the automobile industry. Polymer-based nanoparticles are created from natural or synthetic polymers that are modified to form submicroscopic particles. The polymer matrix can be customized to provide specific properties, such as surface chemistry and flexibility. This allows the particles to be designed and used in different fields. For example, polymeric nanoparticles have been used to deliver drugs or genes to a specific site and are also used in tissue engineering (36). Biodegradable and low-toxic poly(lactic acid) (PLA), chitosan, and poly(ethylene glycol) (PEG) are the common polymers used in the synthesis process of polymeric nanocarriers. PEG-based nanoparticles are especially advantageous because they can be easily modified and are hydrophilic in nature, thereby, making them ideal for drug delivery (37,38). PLA-based nanoparticles are preferred for nanofabrication due to their excellent optical properties and their ability to form stable complexes with other materials (39). The main advantages of using polymer-based nanoparticles are their versatility and biocompatibility, which means that they can be safely used in medical applications. However, polymer-based nanoparticles in terms of cost and complexity. The synthesis of these particles requires sophisticated equipment, and the cost can be prohibitive for some applications (40). Furthermore, the particles need to be carefully characterised to ensure that their properties match the desired specifications.
3 Types of polymeric nanomaterials
Biocompatible and biodegradable polymer-based nanomaterials are becoming increasingly popular because of their remarkable properties, such as their small size and their ease of incorporation into various materials. They are designed to be used for different applications in fields such as biomedical imaging, drug delivery, catalytic activities, and sensing. They are usually designed to have a very large surface area and a high degree of accessibility, and can also be tailored to exhibit various biological, mechanical, and optical properties (41,42). There are several types of polymeric nanomaterials (Figure 2).
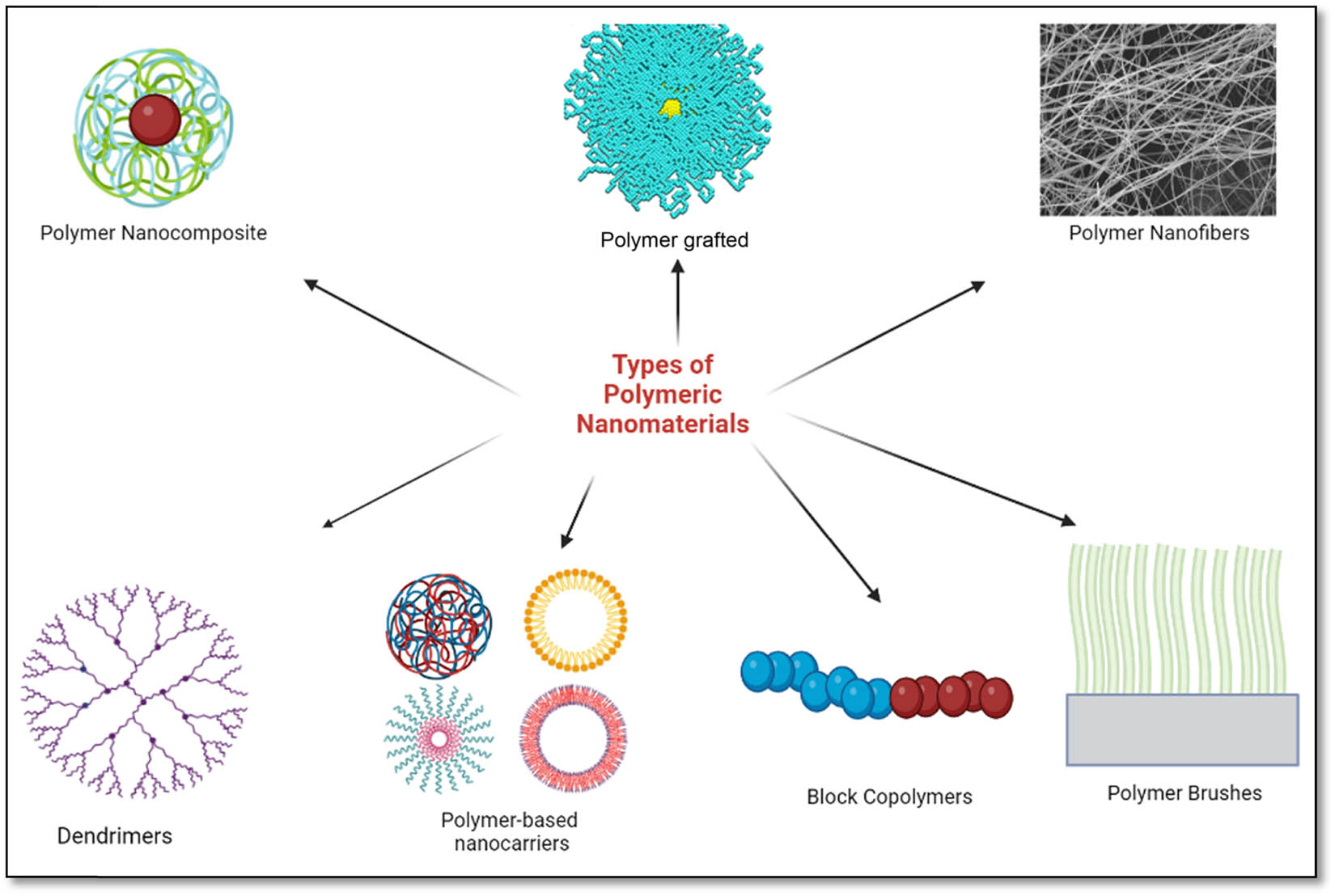
Types of polymeric nanomaterials.
3.1 Polymer nanocomposites
Polymer nanocomposites are materials that consist of two distinct components: a polymer matrix, which is a continuous phase combined with nanofillers, which is a discontinuous phase or reinforcement (in the form of nanoparticles, such as ceramics, metals/metal oxide, or carbon-based nanoparticles). The addition of these nanofillers creates unique structures with advanced characteristics and properties such as mechanical, electrical, thermal, and chemical properties of the nanocomposite (43). For example, polymers that are reinforced with carbon nanotubes have been shown to have increased tensile strength and stiffness compared to traditional polymeric material (44). Polymer nanocomposite also shows excellent barrier properties that make them suitable for application in food packaging, coatings, and medical devices (45,46). Among the promising applications of polymer nanocomposites, polymer nanocarriers that are used to encapsulate and deliver drugs have gained more attention due to the increased efficacy of drug loading/encapsulation (47). They have also been used for biomedical imaging applications (48).
3.2 Polymer nanofibers
These are a class of nanomaterials composed of nanofibrils that have properties and characteristics that make them ideal for applications in many fields. They are made from a combination of polymers and nanotechnology and possess some remarkable properties that are not found in conventional materials. These nanofibers with a diameter in the nanoscale range (1–100 nm) can be synthesised using different synthesis methods to create fibres of various shapes, sizes, and compositions. They can be made by spinning polymer solutions or melting through a nozzle or spinneret (49). The unique properties of the polymer nanofiber, such as its flexibility, lightweight, high strength, and corrosion resistance, make them suitable for application in many industries ranging from biomedical to automotive. For example, polymer nanofibers have been used as scaffolds (50), wound dressings (51,52), and delivering of drugs (53) in the biomedical field. They are also used in lithium-ion recovery (54) which is very useful for battery-related industries, filtration systems such as water and air purification systems (55,56), and also used in the production of fuel cells (57). They can be used to create conductive materials, as well as to store and transport liquids, gases, and biological molecules (54). Furthermore, they can be used to create flexible and transparent displays, as well as to create nanosensors for use in different industries (58,59). The automotive industry has also taken advantage of the unique characteristics of polymer nanofibers, using them to create lightweight, fuel-efficient vehicle components (60). With further research, the potential for polymer nanofibers is sure to expand and provide new and exciting opportunities for researchers and engineers.
3.3 Polymer-grafted nanoparticles
These are nanoparticles that are a relatively recent addition to the field of materials science, and their potential for technological development is immense. Polymer-grafted nanoparticles are nanomaterials that have been coated/attached with a thin layer of polymer on their surface which can improve their stability and compatibility with other materials (61). This concept of polymers attached to nanoparticles has been studied extensively and has resulted in many potential applications. The most impressive applications of polymer-grafted nanoparticles are in drug delivery systems. By having polymers and nanoparticles attached, the loading capacity of the drug can be increased by up to five times, allowing for more drugs to be administered effectively and efficiently (62,63). This impressive and amazing phenomenon has the potential to revolutionise the way drugs are administered and distributed, as many drugs can now be efficiently tested and administered without having to worry about being wasted or lost. Polymer-grafted nanoparticles have also been used in other fields, such as tissue engineering and sensors (64,65). The hybrid combination of nanoparticles attached to the surface of polymers can make them more resistant to mechanical and chemical damage, and they can also interact more effectively and efficiently with biological cells and other molecules. This can be used to create better tissue scaffolds or better biosensors, leading to better and more reliable medical treatments. Moreover, the application of polymer-grafted nanoparticles in dielectric capacitors has also been exploited (66). With more research, polymer-grafted nanoparticles could be used in a variety of ways and revolutionise the field of materials science.
3.4 Dendrimers
These are a type of specialised nanomaterial with a highly branched 3-dimensional structure, and a well-defined molecular architecture that are usually used as nanocarriers (67), imaging agents (69), sensors (70), for water purification (68), and to deliver drugs (71) in a biological system because of their unique size, shape, functional groups, and chemical functionalities. They consist of a central core that is characterised by a highly branched tree-like structure with many functional groups. This unique structure gives dendrimers a special opportunity to engage in a multivalent reaction. Dendrimers are made of polymers with a repeating structure and they can be several nanometres in size. They are generally synthesised through a step-by-step iterative approach known as the divergent or convergent approach. In the divergent approach, dendrimer formation grows outwards from the multifunctional core molecule, which serves as the central scaffold for dendrimer growth. The core molecule is functionalised with a branching unit containing one reactive site and two dormant groups. The core molecule reacts with these branching units to form the first-generation dendrimers. The new periphery of the molecules is then reactivated for a new reaction with monomers, thereby forming the second-generation dendrimers, and the iteration keeps repeating itself, forming multiple generations. As for the convergence approach, the synthesis/formation starts from the exterior, starting with a single unit that eventually becomes the outermost structure of the final dendrimer. Once the desired generation is achieved, dendrimer growth is terminated by attaching specific functional groups which are very useful for different applications such as biomedical imaging, drug encapsulation, or targeting ligands (72). Each dendrimer is made up of many branches of polymer, forming a spherical structure with several functional groups spread along the surface. These functional groups can be designed to interact with different molecules, and can also contain drug molecules or other materials (73). Dendrimers have a high degree of solubility due to their highly branched structure and surface area, which enables them to be applied in a field of study. For example, the dendrimer surface can be decorated with functional groups to target specific cells or organs in the body (74). They can also be used to transport other materials, such as drugs, genes, or proteins (75).
3.5 Polymer brushes
When thin layers of polymer are grafted onto the surface of another core layer, they form a structure known as a polymer brush. They are usually composed of a core layer of polymers, which are densely packed together, and surrounded by an outer layer of polymers, which are loosely packed together. This combination creates a brush like structure that is flexible, strong, and biocompatible (76). Polymer brushes have been used in different fields, from materials science to medical implants. In materials science, for example, they can be used to increase surface tension or modify surface energy. These modifications allow for improved adhesion of coatings and improved wear resistance (77). In medical applications, polymer brushes can be used as an antibacterial coating, hip and knee joint replacement coating, or implant coatings such as orthopaedic implants and dental implants. The brush like structure increases the surface area of the implant, thereby allowing for a more secure attachment to the bone or other tissues (78,79,80). In addition, its unique properties can provide a barrier to bacterial adhesion, thus reducing the risk of infection. Polymer brushes are also used in the pharmaceutical industry as nanocarriers that can be tailored to specific needs. Brushes can be designed to release the drug at a programmed rate or to shield and protect the drug from degradation or premature release as a result of external forces such as light or heat, or enzymes (81). As such, the properties of polymer brushes are attractive for many different applications.
3.6 Block copolymers
These are polymers that are made up of two or more polymeric chains that are chemically distinct but covalently bonded together. They can self-assemble into nanoscale structures. Block copolymers, in general, comprise two distinct types of blocks. The first type is the “hard” block, which is made of molecules that are not as flexible or as easily deformable as the “soft” block molecules. The second type is the “soft” block, which is made of molecules that are more flexible and deformable. The combination of the two types of blocks allows the formation of a variety of structures such as cylindrical or spherical depending on the ratios and proportions of the two blocks (82). Block copolymers have had multiple applications in medicine and in the automotive industry. In medical applications, block copolymers are often used to create medical microspheres, which are small vehicles that are used for drug delivery purposes (83). They have also been used for nanolithography (84). In industrial applications, they are used to create some kind of hybrid materials that have unique properties, such as the ability to resist heat or chemical degradation (85). In addition, block copolymers can also be used to create biodegradable materials, such as plastics and composites (86). The biodegradable materials created by block copolymers can be used in the food and construction industries. Block copolymers are also used in the production of additives (87), biosensors (88,89), pharmaceuticals, and cosmetics (90).
3.7 Polymer-based nanocarriers
Polymeric nanocarriers are a unique, novel, and fascinating type of nanocarriers that possess the potential to transform medicine. These nanocarriers are extremely small vehicles that can transport drugs, vaccines, and other molecules to a specific area or cell in the body. Depending on their application, these nanocarriers consist of different types of materials, including lipids, proteins, and polymers. Among these, polymer-based nanocarriers are considered a better option due to their flexibility, biocompatibility, and ease of synthesis (91). Polymeric nanocarriers are made up of biocompatible polymers, which are materials that do not cause harm to living tissues. They have many advantages over traditional drug delivery systems. First, they can deliver drugs to a specific target, bypassing the need to administer high doses of a drug to the entire body. This can result in fewer side effects and better efficacy. For instance, a study designed a nanocarrier using luteinizing hormone-releasing hormone peptide with specific targeting ligands for cancer cells to minimise the effect of chemotherapy drugs on healthy cells while simultaneously enhancing drug inflow and retention of the drug in cancer cells (92). Second, the nanocarriers can protect drugs/cargo from the body’s natural metabolic processes, thus, increasing the drug’s shelf life and decreasing the need for continuous administration of the drug to the entire body. Afrouz et al. (93) designed a fluorescence correlation spectroscopy (FCS) method to track and monitor the circulation time, stability, and premature release of drugs from the nanocarrier in a highly dense and complex environment in the body. Third, the design and targeting of specific types of cells, such as cancer cells, can be achieved through modification of the surface of the nanocarrier surface with the help of ligands, which can be effective in delivering drugs directly to the diseased cells (92). This implies that the drugs can be programmed to be released over a certain period, thus allowing a lower overall dose of the drug to be administered to the whole body. This has the potential and possibility of decreasing the cost of treatment and also improving patient compliance. In addition, polymer-based nanocarriers are also being explored for other applications in the field of tissue engineering and gene therapy. For example, they could be used to deliver genes to a specific tissue, allowing the expression of desired proteins in the target tissue (94). Polymer-based nanocarriers are a promising technology that could revolutionise the field of medicine. They offer a more targeted and cost-effective form of drug delivery, with the potential to improve patient outcomes. They could also be used in a variety of other applications, such as energy conversions, making them an exciting technology for the future.
4 Design, fabrication, and characterisation of polymer-based nanocarriers
4.1 Design of polymer-based nanocarriers
Polymer-based nanocarriers are noble, specialised, and incredibly small vehicles that are engineered to transport and deliver materials or cargo such as drugs, genes, vaccines, and other therapeutic molecules in the body. They are designed to be biocompatible and biodegradable, which means they can be used safely in vivo, and can also target specific areas of the body without causing harm to healthy tissues or cells. The design of polymer-based nanocarriers is a critical step in developing effective nanocarriers. This section will detail the most important aspect of designing polymer-based nanocarriers, including the selection of suitable polymers, the preparation of nanocarriers, surface modification, loading of cargo (drug or genes), and cargo release kinetics.
4.1.1 Selection of the appropriate material
The first and most important step in the fabrication and design of polymer-based nanocarriers is selecting the best material. Selecting the polymer is based on the intended properties that the nanocarrier should possess. These properties can be biocompatibility, biodegradability, stability, drug loading capacity, nanocarrier release kinetics, or ability/properties that enable them to interact with and deliver their cargo to targeted sites (91). They also provide the nanocarrier with its structural and functional properties. Natural polymers are known to be biocompatible, less toxic, and biodegradable. Chitosan, alginate, and hyaluronic acid have been used in the fabrication of polymer nanocarriers for various types of drugs and biomolecules, including proteins, peptides, and genes. For example, chitosan, which is derived from chitin, is a biopolymer that is known to possess good mucoadhesive properties (95). Mucoadhesion is the ability of a substance to attach to mucosal surfaces, such as those found in the gastrointestinal tract, respiratory tract, and urinary tract. So, fabricating a chitosan-based nanocarrier to target respiratory or gastrointestinal tract infection will prove to be more effective than a traditional nanocarrier. Lee and Mooney (96) fabricated a chitosan nanocarrier that is functionalised with graphene oxide (GO) and used for the delivery of genes and water-soluble anticancer drugs. The nanocarrier shows a higher loading capacity and remarkably high cytotoxicity in Hela cells and HepG2 cells. The same functionalised chitosan graphene nanocarrier was able to contract plasmid DNA into a stable nanosized complex. Alginate is a biocompatible, inexpensive, and low-toxic type of biopolymer derived from brown seaweed that is known for its excellent gel-forming properties. They are used as drug carriers and have been applied in tissue engineering to support the growth of cells (97,98). One of the advantages of natural polymers is their unique ability to interact with biological systems, but the physical and chemical characteristics of the polymer might be challenging to control, which might limit their applications in certain fields.
Poly(lactic-co-glycolic acid) (PLGA), polyethyleneimine (PEI), and PEG are considered as synthetic polymers that are biocompatible, biodegradable, and have great tunability and control over their physicochemical properties. PLGA is a copolymer of glycolic and lactic acid that is used extensively in drug delivery applications (4). It can also be produced with varying molecular weights and monomer ratios, which can affect the efficiency of loading drugs, as well as the release kinetics of the nanocarrier. Similarly, PEI is a type of synthetic polymer that has found its use as a gene delivery vehicle due to its unique ability to encapsulate and protect the content of its cargo like DNA (94). PEG is also another example of a synthetic polymer used as a nanocarrier. It helps to increase the circulation time of nanocarriers by reducing their recognition and uptake by the body’s immune system (99). However, synthetic polymers can have high toxicity and immunogenicity, which can limit their use in certain applications (100).
A hybrid polymer from natural and synthetic polymers can be formed that has the properties and advantages of both natural and synthetic polymers. For example, the combination of chitosan and PLGA can enhance the biocompatibility and drug release kinetics of the nanocarrier (101). Hybrid polymers fabricated with varying ratios of natural and synthetic polymers can have an impact on the physicochemical properties and drug release kinetics of the nanocarrier to a great length. Once the polymer has been selected, the next step is to modify the polymer so that it has the desired properties. For example, Lee et al. (102) fabricated a chitosan gold nanocarrier functionalised with PLGA to improve oral insulin uptake. The nanocarrier was found to be a highly effective vehicle for the loading and release of insulin at various pH levels.
4.1.2 Preparation of the nanocarriers
The preparation of polymer-based nanocarriers involves several steps, including polymer synthesis, nanoparticle formation, and drug/cargo loading. There are various techniques available for the preparation of polymer-based nanocarriers with specific physicochemical properties. Most of them are explained in Section 4.2.
4.1.3 Nanocarrier surface modification for active targeting
Surface modification is the process of changing the surface properties and characteristics of a material to improve its performance or functionality. In terms of polymer-based nanocarriers, modifying their surface can improve their stability, biocompatibility, and targeting capabilities. Modification can be done by attaching ligands or other types of macromolecules and functional groups to the polymer surface or coating the surface with a layer of another material thereby changing the surface properties (103). An example of this is a lipid-based nanocarrier that is coated with a thin layer of PEG to enhance the circulation time of the nanocarrier in the bloodstream while reducing the possibility of its clearance by the immune system (104). A common method of surface modification techniques used in polymer-based nanocarriers is the attachment of ligands that can interact with specific receptors that are found on the surface of cells for active targeting. Active targeting is achieved through the process of molecular recognition of the target tissue or cell via different molecular signatures that are overexpressed at the affected site either through antigen–antibody interaction, targeting with the help of aptamers, or through the ligand–receptor interaction. Ligands employed for active targeting include carbohydrates (such as galactose, mannose, hyaluronan, glycyrrhizin, and lactose), small molecules (such as vitamin B7 and B9, biotin, selegiline, alendronate, selectin, and curcumin), peptides and proteins (such as octapeptide octreotide, lectin, glutathione, cysteine and glycine, tripeptide Arg-Gly-Asp (RGD) with their derivatives, cell-penetrating peptides, epidermal growth factor receptor, A665, A666, Tet1, hNgf EE, and transferrin), mini aptamers, and antibodies (91). These ligands have been used to target brain, breast, lung, and ovarian cancer cells. The modification mechanism includes physical adsorption, covalent binding, and encapsulation. Physical adsorption involves the noncovalent surface binding of molecules to the nanocarrier through electrostatic, hydrophobic, or van der Waals force of attraction. Covalent binding involves a chemical bond in between the surface of the polymeric nanocarrier and the modified functional group or ligand. Encapsulation involves the entrapment of the functional group or ligand within the nanocarrier matrix. Ligands can be decorated on the surface of the nanocarrier through physical adsorption or covalent binding (105,106,107). Ligands can be used to improve the targeting capabilities of the nanocarrier, enabling it to precisely deliver drugs to specific cells. Furthermore, the nanocarriers should possess specific physicochemical properties that enable them to evade immune surveillance and prolong their circulation in the body.
4.1.4 Loading of cargo/drug
After nanoparticle formation, the drug is loaded into the nanocarrier and must be chosen on its solubility and stability in the chosen polymer matrix. There are various mechanisms for loading drugs into the nanocarrier, including physical entrapment, chemical conjugation, and electrostatic interactions.
4.1.4.1 Physical entrapment/encapsulation
Physical entrapment is a relatively simple, versatile, and reproducible method used to load drugs into polymer-based nanocarriers that are employed for active targeted drug delivery applications. The physical entrapment/encapsulation method involves physically trapping the drug molecules or cargo within the polymer matrix of the nanocarriers during the synthesis process. This is usually done through a non-covalent interaction and can be used for both hydrophilic and hydrophobic loading of drugs (108). The physical entrapment method is known for its simplicity. The process of loading drugs into nanocarriers through physical entrapment is relatively straightforward and does not require complex chemical reactions or expensive equipment. This makes it a cost-effective method for drug loading. For instance, Trofymchuk et al. (109) incorporated an anticancer drug (adriamycin – ADR) into polymeric micelles, thus forming a block copolymer nanocarrier. The structural stability of the block copolymer was found to depend on the physical entrapment of the anticancer drug and the anti-tumour activity was also found to be high against the C 26 tumour. Similarly, ADR entrapment in the AB block copolymer was also investigated by Park and Na (110). Fluorescence spectroscopy and gel permeation chromatography were used to determine the successful entrapment of the ADR. However, there are also some limitations associated with the physical entrapment. One of the main limitations is the potential for drug leakage from the nanocarrier, especially during storage or transportation (111). This can be addressed by modifying the polymer matrix to improve its stability and prevent drug leakage. Another limitation is the potential for premature drug release from the nanocarrier, which can lead to suboptimal drug discharge at the target site. However, this problem can be addressed by controlling the release kinetics of the nanocarrier using methods like modifying the polymer matrix or incorporating stimuli-responsive components (112,113).
4.1.4.2 Chemical conjugation
In this method, drug molecules or the cargo of the nanocarrier are covalently attached to the polymer matrix of the nanocarrier through chemical reactions (109). Chemical conjugation offers several advantages over other drug loading, including high drug loading efficacy, specificity, controlled drug release kinetics, and improved stability of the drug (114). One of the main benefits of chemical conjugation is its high drug loading efficiency. Since the cargo is covalently attached to the polymer matrix, a high degree of drug loading can be achieved, which can result in a more efficient drug delivery system (114). Furthermore, the controlled chemical conjugation of drugs to the polymer matrix allows for precise control over the number of drug molecules attached to each nanocarrier, resulting in a more uniform drug distribution. For instance, Sharp and Honig (115) synthesised a polyN-(2-hydroxypropyl)methacrylamide (HPMA) nanocarrier functionalised with polyclonal and monoclonal antibodies to deliver anticancer drug doxorubicin. Another advantage of chemical conjugation is the ability to control the release kinetics. This is achieved through modification of the chemical structure of the drug molecule and/or polymer matrix, which can affect the strength and stability of the covalent bonds. When the strength and stability of these bonds are controlled, the release of the drug from the nanocarrier can be tailored to specific conditions, such as pH or temperature. A study tested the hypothesis of the pharmacokinetics of poly(2-oxazoline) drug conjugate nanocarriers with drugs like tetrahydrocannabinol, cannabigerol, buprenorphine, cannabidiol, dronabinol, and rotigotine attached to the nanocarrier through releasable linkages (116). Evidence supports the hypothesis and demonstrated how it can be used to tune drug release kinetics. Chemical conjugation also offers improved stability of the drug, as the covalent bonds between the drug and the polymer matrix prevent the drug from being released prematurely. This can be particularly important for drugs that are unstable or rapidly degraded in the body. However, there are some limitations associated with chemical conjugation. One of the main limitations is the potential toxicity and reduced bioactivity of the drug after conjugation. Chemical conjugation can alter the chemical structure of the drug, which can affect its bioactivity and reduce its efficacy. This can be addressed by optimising chemical conjugation conditions to minimise the impact on the bioactivity of the drug (116).
4.1.4.3 Electrostatic interactions
Electrostatic interactions are a type of noncovalent interaction that can be used to load drugs into polymeric nanocarriers. In this method, drug molecules are attracted to the surface of the nanocarrier through electrostatic forces between the charged groups on the drug and the polymer matrix of the nanocarrier (117). Electrostatic interactions can be particularly useful for loading charged drugs, such as nucleic acids, proteins, and peptides, into polymer-based nanocarriers. A study proposed a perspective that uses electrostatic interaction to enhance drug penetration into cartilaginous tissues due to the inefficiency of current traditional methods, such as intra-articular drug delivery methods, to deliver adequate drugs into cartilage tissue at the desired concentration to trigger a biological response (118). An advantage of electrostatic interactions for drug loading is their simplicity and ease of use. Electrostatic interactions have been employed for loading different types of drugs into various types of nanocarriers without the need for complex chemical reactions or modifications, and they also possess the ability of controlled release kinetics. This can be achieved by modifying the charge density or pH of the surrounding environment, which can affect the strength of the electrostatic interaction between the drug and the nanocarrier. For instance, a study examines the release behaviour of the lovastatin drug from polycaprolactone (PCL) coated β-tricalcium phosphate (119). The lovastatin drug was incorporated into a water-soluble and biodegradable PCL coating where a controlled release was observed when there is a variation in the pH medium. The hydrophilic–hydrophobic interaction between the PLC and the drug was the main factor controlling the release kinetics. So, by controlling the strength of these interactions, the release of the drug from the nanocarrier can be tailored to specific conditions, such as drug release at the targeted site. However, there are also some limitations associated with electrostatic interactions. One of the main limitations is their sensitivity. Some changes in the surrounding environment such as pH or ionic strength can weaken electrostatic interactions thereby, resulting in premature release of the drug, as in the case of lovastatin drug loaded in PCL (119). Another limitation is the potential for reduced drug loading efficiency. Charge density and surface area can influence the electrostatic interactions, which ultimately limit the amount of drug that can be loaded onto the nanocarrier. This can be addressed by optimising the charge density and ionic strength, as well as the pH of the surrounding environment (120).
4.1.5 Cargo release kinetics
Drug loading and release studies are used to determine the amount of drug to be loaded and the release kinetics. The amount of drug that is to be loaded is a measure of the amount of drug that can be incorporated into the nanocarrier. The kinetics is a measure of the rate and extent to which the drug is released from the nanocarrier (121). These parameters can be affected by several characteristics, including polymer properties, nanoparticle size, and pH (122). How the nanocarrier is designed can influence the release kinetics of the nanocarrier. For example, hydrophobic groups at the surface of a nanocarrier can delay the release of hydrophilic drugs by slowing the penetration of water into the nanocarrier (123). Furthermore, stimuli-responsive polymers such as pH- or temperature-sensitive polymers can allow controlled release of cargo in response to changes in environmental conditions at the target site (119). The type of cargo that is loaded into the nanocarrier can also influence the cargo release. For example, the charge of the cargo and size can affect the rate of release from the nanocarrier. Small, highly charged molecules may be released more quickly than larger, less charged molecules (124). The environmental conditions at the target site can also influence the kinetics of cargo release kinetics. The condition of the surrounding environment, such as changes in pH or ionic strength, can affect the electrostatic interactions between the cargo and the nanocarrier, which can influence the release kinetics. For example, a decrease in pH can weaken the electrostatic interactions, leading to a faster release of cargo from the nanocarrier (122). The overall cargo release kinetics from polymer-based nanocarriers is a complex interplay between the properties of the nanocarrier, the cargo, and the target site environment. The complex design and optimisation of nanocarriers to be used as drug delivery vehicles require a thorough understanding of these factors to achieve optimal drug release kinetics and also improve the efficacy of drug delivery. After the fabrication process and drug loading are complete, the nanocarriers are tested to ensure that they possess the desired properties.
4.1.6 Biocompatibility essay
Biocompatibility, as the name implies, is the ability of a material to interact with biological systems without causing adverse effects. For polymer-based nanocarriers, biocompatibility is assessed in terms of their potential toxicity, immunogenicity, and interactions with biological barriers, such as cell membranes and the immune system, which can be done via any of the in vivo or in vitro studies. These studies are specially designed to ensure the safety and effectiveness of the nanocarrier (7). Toxicity is a major concern when designing polymer-based nanocarriers, which can result from many factors such as the size, composition, or surface modification of the nanocarriers. Toxicity is known to cause adverse side effects such as inflammation, cell death, and organ damage. To minimise toxicity, biodegradable and biocompatible polymers PLGA or PEG are commonly employed in fabricating nanocarriers (125). These polymers degrade into nontoxic by-products in the body, minimising the risk of toxicity. Additionally, purification techniques and characterisation of the nanocarriers can be used to remove impurities and ensure the biocompatibility of the nanocarrier. Immunogenicity is another factor that can affect the biocompatibility of a polymer-based nanocarrier. The presence of foreign materials in the body can trigger an immune response, leading to the clearance of the nanocarrier and to decreased efficacy of drug delivery. To minimise immunogenicity, biocompatible polymers that are less likely to elicit an immune response, such as PEG, are commonly used to coat the nanocarrier surface of the nanocarrier (126). Similarly, stimuli-responsive polymers, such as pH-sensitive or temperature-sensitive polymers, can minimise the exposure of the nanocarrier to the immune system and increase circulation time in the body (126). Interactions with biological barriers, such as cell membranes and the immune system, are also critical considerations for the biocompatibility of polymer-based nanocarriers. The surface characteristics of the nanocarrier, such as the surface charge and hydrophobicity, can affect the interaction between the nanocarrier and biological barriers. For example, hydrophobic moieties on the nanocarrier surface can facilitate interactions with cell membranes, promoting cellular uptake of the nanocarrier (127). Similarly, the surface charge of the nanocarrier can influence interactions with the immune system, with neutral or negatively charged nanocarriers less likely to elicit an immune response (128). Overall, the biocompatibility of polymer-based nanocarriers is a critical factor that must be carefully considered during their design and fabrication. Biodegradable and biocompatible polymers, purification and characterisation techniques, and surface modifications can be used to minimise the toxicity, immunogenicity, and interactions with biological barriers of the nanocarrier. When the biocompatibility of polymer-based nanocarriers is ensured, the safety and efficacy of targeted drug delivery can be improved, which will subsequently lead to better therapeutic outcomes for patients.
4.2 Fabrication of polymer-based nanocarrier
Fabrication of nanoparticles can be achieved by either breaking down of bulk material into nanoparticles or building the nanoparticles from their respective atoms. With regard to nanocarriers, the top-down approach usually employs a preprepared polymer material to synthesise the NPs. Such a technique includes emulsion diffusion/evaporation, electrospinning, salting out, and the nanoprecipitation method. Bottom-up methods, on the other hand, normally start from a monomer and build up to form a polymer. Precipitation polymerisation, chemical vapour deposition (CVD), interfacial polymerisation, thermal, and emulsion polymerisation, and solution-based techniques are the specific forms of synthesis.
4.2.1 Emulsion evaporation method
Emulsion evaporation is a process used to produce polymer nanocarriers using the emulsion polymerisation technique. This method involves the preparation of an emulsion of monomers, stabilisers, and water, followed by the initiation of the polymerisation with the help of a catalyst or initiator. The polymerisation reaction takes place within the emulsion droplets, which results in forming polymer nanoparticles. The polymer nanoparticles are obtained by evaporating the water from the emulsion during emulsion evaporation, thus leaving behind the solid nanoparticles. The nanoparticle properties such as size and shape can be affected by simply adjusting the synthesis parameters such as the concentration of the polymer, the type of organic solvent, the stirring rate, and the temperature of the evaporation process. For example, when the concentration of the polymer is increased, it can result in larger nanoparticles, while the use of a different organic solvent can affect the surface chemistry. As such, optimisation of the synthesis parameters is very necessary to obtain nanoparticles with the desired properties (129,130,131).
4.2.2 Emulsion diffusion method
Polymeric nanoparticles can be synthesised by emulsion of polymer solution in a nonsolvent, followed by the diffusion of the nonsolvent into the polymer solution, thereby inducing precipitation and formation of the polymer nanomaterial. In simpler terms, a mixture of polymer and water-immiscible organic solvent is added to an aqueous solution in a dropwise manner. The aqueous solution containing the stabiliser or surfactant is then sonicated or stirred to form an emulsion containing the dispersed polymer droplets. Then, the nonsolvent is added dropwise to the emulsion. As the nonsolvent diffuses with the polymer solution, it induces precipitation that forms the polymer nanoparticles. This method can be viewed as a variation in the solvent evaporation technique used in the solution-based synthesis of polymeric nanoparticles. Like emulsion evaporation, polymeric nanoparticle properties such as size, morphology, and surface chemistry can be regulated by adjusting the synthesis condition, such as polymer concentration, organic solvent, the type and concentration of the non-solvent, and the stirring rate (126).
4.2.3 Electrospinning method
Electrospinning is a process of fabricating nanofibers. It involves using an electric field with a melt or polymer solution. The process begins with applying a high voltage through the polymer solution or melt, which subsequently creates an electrically charged jet of polymer that is then drawn out into a fine fibre by an opposing electric field or collector plate, or rotating drum, thus forming a nonwoven mat or a fabric-like structure. The result is a continuous nanoscale fibre with a high surface area-to-volume ratio (132). The electrospinning process can be used to fabricate nanofibers using different types of synthetic and natural polymers, including polyethylene, polypropylene, nylon, polystyrene (PS), and chitosan (133,134). The properties of nanofibers, such as diameter, morphology, and surface chemistry, can be regulated by adjusting various synthesis conditions such as polymer concentration, the electric field strength, the space between the needle and the collector, and the temperature and humidity of the surrounding environment (135). This method has some advantages. First, it is a relatively simple and inexpensive process that only requires a voltage source, a syringe, and a collector plate. Second, it can create nanofibers with different diameters, from nm to mm. Third, the surface area of the electrospun nanofibers can be useful for several applications ranging from biomedical applications to engineering applications. Electrospinning has many potential applications. For example, electrospun nanofibers have been used to create scaffolds, drug delivery systems, and high-performance battery electrodes (136,137). However, there are still some challenges that need to be addressed to make electrospinning a more practical and scalable process for industrial applications, including improving the consistency and reproducibility of nanofibers and increasing the production rate (138).
4.2.4 Salting out
Fabricating a polymer nanocarrier via the salting-out method involves adding salt to a polymer solution, thereby causing it to precipitate polymer nanoparticles. The salt causes the polymer to become less soluble and to form aggregates, which then form nanoparticles. The salting process for polymeric nanomaterial synthesis typically involves the following steps: first, the dissolution of a well-suitable polymer solvent to form a clear solution. The amount of dissolved polymer can affect the size and morphology. Second, a high concentration of salt is added to the polymer solution. The salt concentration and type of salt used will depend on the specific polymer being used and the desired nanoparticle properties. Third, the polymer solution is stirred and incubated for some time, allowing the salt to interact with the polymer and induce nanoparticle formation. Then finally, the resulting nanoparticles were separated from the solution by centrifugation and washed to remove any residual salt or impurities (139,140). The salting-out method for polymeric nanoparticle synthesis has several advantages. It is quite simple and inexpensive and has the potential to produce polymer nanomaterial in large quantities (141). The salting method is suitable for synthesising different types of polymeric nanoparticles, including those made from PS, poly(methyl methacrylate), and PEG, which has potential for application in the biomedical field (142).
4.2.5 Nanoprecipitation
This method involves the rapid mixing of two immiscible liquids, typically a polymer solution and an antisolvent. The mixture of the solution results in precipitation of the polymer. It is a simple method for fabricating polymeric nanoparticles with desirable size and surface properties. The basic principle of nanoprecipitation is to use a polymer that is soluble in a solvent but not in a non-solvent, also called an antisolvent. When the antisolvent is added to the polymer solution, it becomes supersaturated, leading to the formation of nanoparticles. The nanoprecipitation process generally involves the preparation of a clear solution mixture of polymer and a suitable organic solvent. The polymer concentration can vary depending on the desired size and morphology of the nanoparticles. Then, an antisolvent is added rapidly to the polymer solution while the mixture continues to be stirred vigorously. The antisolvent causes the polymer to precipitate as a result of the decreasing solubility of the polymer. Finally, the resulting nanoparticle suspension was then collected by centrifugation or filtration and washed to remove any residual solvent or impurities. This method can be used to synthesise a variety of polymeric nanoparticles, including those made of PS, poly(methyl methacrylate), and PEG (143,144). Nanoprecipitation has potential applications in many biomedical fields, such as imaging and sensing, among others (145). This method is easy and scalable, and control of size and surface modification is achievable by adjusting the ratio of polymer to antisolvent, the concentration of the polymer, and the stirring speed (141).
4.2.5.1 CVD
CVD is a technique for depositing thin films of materials on the surface of a substrate. This process involves the reactions of highly reactive gas molecules with the surface of the substrate to form a thin film. The CVD process begins when the reactant gases enter the deposition chamber and are brought to a high temperature. At this temperature, the reactants break down into their component atoms which then react with the substrate surface. Depending on the reactants used, this reaction can result in the formation of either a single- or multiple-layer film. On the basis of the properties of the substrate, the film may be deposited as a solid, an oxide, or a combination of both. Once the layer has been deposited, the film is then subjected to post-deposition processing, such as oxidation, etching, and baking, to refine its properties (146,147). This method has been used to synthesise many polymeric nanomaterials including polymer films (148). For example, the growth of carbon nanotubes involves the catalytic decomposition of a hydrocarbon gas, such as methane or ethylene, on a metal substrate, typically iron, nickel, or cobalt. CNTs can be grown in a vertical or horizontal orientation which depends on the substrate type and growth conditions (149). Another instance is the deposition of a thin polymer film on a substrate. This process involves the use of a polymer precursor, such as a monomer or polymerizable gas, which is introduced into the reaction chamber along with a reactive gas. The reactive gas can be used to initiate the polymerisation reaction which results in the formation of a thin polymer film on the substrate (148). The properties of the resulting film can be customized by varying reaction conditions like temperature, pressure, and gas flow rate. CVD synthesis of polymeric nanomaterials has a wide range of applications, including the production of electronic devices, sensors, and biomedical materials. For example, CNTs have been used as conductive additives in composites and as electrodes in batteries and supercapacitors (150). Polymer thin films have been used as coatings to improve the biocompatibility of medical devices, antireflux, and protective coatings for electronic devices (151,152,153). CVD is often used to produce high-quality components, due to its ability to produce uniform and homogeneous films and is also used instead of wet chemical processes, as it can produce films with greater control of their physical and chemical properties (154). Furthermore, CVD is often used to produce large-scale components, such as integrated circuits and flat panel displays, due to its ability to create large-area films quickly (155). Types of CVD include thermal CVD, plasma-enhanced CVD, low-pressure CVD, and atomic layer deposition (156).
4.2.5.2 Interfacial polymerisation
Interfacial polymerisation is used to synthesise polymeric nanoparticles at an interface between two immiscible liquids. Monomer polymerisation occurs in between the two phases (usually an aqueous phase and an organic phase), thereby resulting in nanoparticle formation. The polymerisation process typically involves a monomer and initiator dissolved in one of the immiscible liquids, typically the organic phase. The choice of monomer and initiator depends on the intended characteristics of the resulting nanoparticles. Then, the immiscible liquids are brought into contact, forming an interface between them. The interface can be stabilised using a surfactant or emulsifying agent. The monomers at the interface are polymerised by adding a catalyst or by a chemical reaction triggered by heat or light. The polymerisation reaction occurs at the interface and the growing polymer chains form nanoparticles. Finally, the nanoparticles are collected by separating them from the bulk solution using centrifugation, filtration, or other methods. They are then purified to remove any unreacted monomers, surfactants, or other impurities (157,158). Interfacial polymerisation can be used to synthesise a wide range of polymeric materials such as core, nanocapsules, and nanospheres (159,160). These nanomaterials have found applications in drug delivery, catalysis, and imaging (160). They have several advantages, such as producing well-defined narrow-sized particles and control over the composition and morphology (158). However, it also has some limitations, such as the need for careful control of reaction conditions and the difficulty of scaling up the process (161).
4.2.5.3 Solution-based techniques
Solution-based synthesis of polymeric nanomaterials is a commonly used technique for producing nanoparticles with controlled size, morphology, and properties. This method involves dissolving monomers or prepolymers in a solvent, followed by the addition of a crosslinker and initiator to initiate the polymerisation reaction. The reaction proceeds in the solution, resulting in the formation of polymeric nanoparticles (162). One common approach to solution-based synthesis is known as the “solvent evaporation” method. In this method, a monomer or a prepolymer is dissolved in a solvent, and a crosslinker and initiator are added. The mixture is then stirred or sonicated to ensure homogeneity. The solution is then added dropwise to a nonsolvent or anti-solvent, which causes the polymer to precipitate out of the solution and form nanoparticles. The resulting nanoparticles are collected by filtration or centrifugation and can be further characterised and processed (163). Another solution-based synthesis method is known as “microemulsion polymerisation.” This method involves the formation of a microemulsion, which is a thermodynamically stable system consisting of droplets of a dispersed phase (i.e., monomer) surrounded by a continuous surfactant-stabilised phase (i.e., water). A cross-linker and initiator are added to the microemulsion, and the polymerisation reaction occurs within the droplets. The resulting polymeric nanoparticles are then recovered by various methods, such as filtration or centrifugation (164). Solution-based synthesis allows for the production of nanoparticles with a high degree of control over size, morphology, and composition. This technique can be used to produce a wide range of polymeric nanoparticles, including core-shell, dendrimer, and block-copolymer nanoparticles. Additionally, solution-based synthesis can be scaled up, making it suitable for large-scale production (165). However, this method typically requires the use of organic solvents, which can be hazardous to handle and dispose of. Therefore, the development of green synthesis approaches for polymeric nanoparticles is an area of active research.
4.2.5.4 Self-assembly-based approach
Self-assembly is a technique that involves the continuous formation of molecules into a specific structure without the need for external forces. This can be achieved through hydrogen bonding mechanisms and electrostatic and hydrophobic interactions. One method of a self-assembly-based approach for fabricating polymer-based nanocarriers is the use of block copolymers. Block copolymers are made up of two or more chemically distinct polymer blocks that are covalently linked together. As a result of the incompatibility between the different polymer blocks, they tend to spontaneously organise into well-defined nanostructures, such as micelles, vesicles, and nanoparticles. Self-assembly can be initiated by stimuli such as pH, temperature, and ion concentration and does not require the use of organic solvents or surfactants, making it a simple and environmentally friendly method for nanocarrier preparation (166). For example, self-assembled nanocarriers can be prepared by mixing cationic and anionic polymers in a solution, which results in the formation of nanoparticles through electrostatic interactions (167). Self-assembly can also be used to prepare nanocarriers with specific targeting properties, such as incorporating ligands that can interact with specific receptors on the surface of cells (168).
4.2.5.5 Layer-by-layer assembly (LbL)
LbL is a sequential deposition of alternating layers of oppositely charged polymers onto a substrate, creating a multilayer film or coating. The resulting nanocarrier can be customized for a variety of applications, including drug delivery, sensing, and tissue engineering (169). The LbL assembly allows for precise control over the width and composition of the multilayered film, which can be customized to optimise the properties of the nanocarrier. The process is versatile and can be used for several substrates and templates, including nanoparticles, fibres, and surfaces. Finally, LbL assembly can be performed using water-based solutions, which is advantageous for biological applications as it minimises the use of organic solvents. The process of assembly of LbL starts with the depositing of a charged polymer layer onto the substrate or template. This can be accomplished by immersing the substrate in a solution of the charged polymer or by using a spray-coating or spin-coating technique. Once the first layer has been deposited, the substrate is rinsed with a buffer solution to remove any unbound polymer. The process is repeated with a solution of the opposite charge, creating a multilayered film. The choice of polymer is critical in LbL assembly because it affects the properties of the produced nanocarrier (169). Common polymers used in LbL assembly include polyelectrolytes PEI and poly(sodium 4-styrene sulfonate), and biopolymers, such as chitosan and alginate. The charge of the polymer can be adjusted by modifying the pH or ionic strength of the solution, allowing for precise control over the deposition process (170). The properties of the resulting nanocarrier can be further tailored by incorporating functional groups or biomolecules into the multilayered film. For example, peptides, proteins, and nucleic acids can be incorporated into the nanocarrier to facilitate targeted drug delivery or gene therapy. Additionally, polymers that respond to stimuli, such as temperature-sensitive or pH-sensitive polymers, can be incorporated into the nanocarrier to facilitate triggered drug release. The assembly of LbL has been applied in biomedical applications and tissue engineering scaffolds (171,172).
4.2.5.6 Thermal and emulsion polymerisation
Emulsion polymerisation and thermal polymerisation are two common methods for synthesising polymeric nanomaterials. Emulsion polymerisation involves dispersing the monomer in an aqueous solution containing a surfactant, resulting in the formation of nanometre-sized droplets. Polymerisation is triggered within the droplets by the addition of an initiator, leading to the formation of polymeric nanoparticles. Surfactant plays an important role in stabilising the droplets and controlling the size and morphology of the resulting polymeric nanomaterials. The formation of these polymers can be easily controlled by the synthesis condition (173). The first step in emulsion polymerisation is the formation of an emulsion. The monomer is dispersed in an aqueous medium containing a surfactant, which stabilises the droplets and prevents them from coalescing. Surfactant also serves as a template for polymer particles, influencing their size, shape, and surface properties. The choice of surfactant can, therefore, have a significant impact on the properties of the resulting nanoparticles. Next a polymerisation initiator is added to the emulsion. The initiator can be water-soluble or oil-soluble and can be added in a single shot or gradually over time. The initiator generates free radicals, which initiate the polymerisation reaction and promote the growth of the polymer particles. The polymerisation reaction proceeds as the monomer droplets grow and coalesce to form larger polymer particles. The surfactant stabilises the growing particles, preventing them from sticking together, and promoting their growth in size. The reaction can be stopped at a desired point by quenching the reaction with a quenching agent. The size and morphology of nanoparticles can be characterised using techniques such as dynamic light scattering, electron microscopy, and X-ray diffraction (XRD) (174). Emulsion polymerisation is a versatile and widely used technique for synthesising polymeric nanomaterials, with applications in fields such as drug delivery, coatings, and electronics (175). The technique allows precise control over the size and morphology of the resulting nanoparticles, and the use of surfactants and other additives can further tailor the properties of the particles. It is a relatively inexpensive process.
Thermal polymerisation, on the other hand, involves the initiation of polymerisation by heating the monomer under pressure or a mixture of monomers above their melting point or glass transition temperature. The reaction is typically initiated using a thermal initiator, which generates free radicals or other reactive species that trigger the polymerisation reaction. During the process, cross-linking occurs between monomer units to create a polymer chain (176). Homopolymers, copolymers, block copolymers, and cross-linked polymers can be produced using this method. The resulting polymer properties can be controlled by varying the polymerisation conditions, like temperature, duration of the reaction, and type and concentration of initiator used to trigger the polymerisation reaction. An example of thermal polymerisation synthesis of polymeric nanomaterials is the production of PS nanoparticles. PS is a widely used polymer in many applications, including insulation, electronics, and the production of packaging materials. The thermal polymerisation process of styrene monomers using a radical initiator, such as benzoyl peroxide, produces PS nanoparticles with a narrow size distribution and a controlled morphology (177). Cross-linked polymeric nanoparticles are another example of thermal polymerisation. The process involves the polymerisation of a cross-linking monomer, such as divinylbenzene, along with a primary monomer. The resulting cross-linked polymer nanoparticles have improved mechanical strength and thermal stability compared to their linear counterparts (178). Thermal polymerisation synthesis of polymeric nanomaterials has a wide range of applications, including the production of coatings, adhesives, and composites.
One key difference between the two techniques is the reaction environment. Emulsion polymerisation is typically performed in an aqueous solution which allows for more precise control over particle size and morphology and uses surfactants and other additives to control particle properties, while thermal polymerisation is carried out in a nonaqueous environment and may result in a broader size distribution, and also relies on the choice of thermal initiator to control the properties of the resulting nanoparticles. This can have a significant impact on the properties of the resulting nanoparticles, as the presence of water can affect the stability of the polymer particles and their physical properties. Furthermore, the choice of monomers and initiators can differ between the two techniques. In emulsion polymerisation, the choice of surfactant and monomer significantly influences the properties of the resulting nanoparticles. In thermal polymerisation, the type and concentration of the thermal initiator can impact the properties of the nanoparticles.
4.2.6 Green synthesis approaches to polymeric nanomaterial synthesis
Polymeric nanomaterial synthesis via the green approach/method explores the production of nanoparticles without the use of hazardous chemicals and solvents. The increasing concern over the environmental impact of chemical synthesis methods led to the development of more sustainable approaches for the synthesis of polymeric nanoparticles. Green synthesis approaches for polymeric nanoparticle synthesis use eco-friendly, nontoxic, and renewable materials and solvents such as plant extracts. These phytochemicals are used to replace toxic chemicals and their by-products. Here are some examples of green synthesis approaches for polymeric nanoparticles.
4.2.6.1 Biopolymer-based synthesis
Biopolymer-based synthesis refers to the use of naturally occurring polymers, such as proteins, polysaccharides, and nucleic acids, as templates for the synthesis of polymeric nanomaterials. Biopolymers are eco-friendly and attractive materials for nanoparticle synthesis. There are several approaches to the biopolymer-based synthesis of polymeric nanoparticles. One method involves the use of protein-based templates for the synthesis of inorganic nanoparticles. For example, bovine serum albumin has been used to synthesise gold nanoparticles, where the protein reduced and stabilised the nanoparticles (179). Polysaccharides, such as hyaluronic acid, chitosan, and starch have also been employed as starting materials for synthesising polymer nanomaterials. Chitosan is a natural polymer derived from chitin; a biopolymer found in crustacean shells. It has been used to prepare nanoparticles through a variety of techniques, including emulsion crosslinking, ionic gelation, and polyelectrolyte complexation. Chitosan can also be used to reduce silver nitrate to produce chitosan-silver nanoparticles on linen fabric for antibacterial activity (180). Starch can be used to reduce gold ions to produce starch-gold nanoparticles (181). Hyaluronic acid is a glycosaminoglycan that is widely distributed in the human body and has been used to prepare nanoparticles for drug delivery applications (182). Another approach to biopolymer-based synthesis involves the use of nucleic acids, such as DNA and RNA, as templates for the synthesis of nanoparticles. For example, DNA has been used as a template for the synthesis of gold and silver nanoparticles, where nucleotide bases act as reducing agents, and the DNA strands provide stability to the nanoparticles (183,184). The biopolymer-based synthesis of polymeric nanoparticles offers several advantages, including the use of renewable and biodegradable materials, the potential for targeted drug delivery, and reduced toxicity compared to synthetic polymers (185). However, it also presents some challenges, such as the need for careful control of the synthesis conditions to ensure reproducibility and the potential for low yields or low drug loading capacity (186). Despite these challenges, biopolymer-based synthesis of polymeric nanoparticles is an active area of research and holds promise for a wide range of biomedical and environmental applications.
4.2.6.2 Microbial synthesis
The microorganisms are used to produce nanoparticles that are made up of biopolymers or biocompatible polymers. This method has the potential for green and sustainable synthesis to produce nanomaterials. One approach to the microbial synthesis of polymeric nanomaterials is through the use of biopolymer-producing microorganisms. For example, bacterial strains such as Acinetobacter (187), Pseudomonas (188), and Azotobacter (189) have been used for the synthesis of biopolymer nanoparticles such as polysaccharides. By the use of specific growth conditions and modifying the nutrient media, the production of biopolymers can be optimised for nanoparticle synthesis. Another approach is through the use of genetically modified microorganisms. By engineering the microorganisms to express specific enzymes or proteins, polymeric nanoparticle synthesis can be controlled and targeted toward specific applications. For example, fungus (190), Escherichia coli (191), and Bacillus subtilis (192) have been genetically modified to produce nanoparticles, such as gold and silver, with controlled size and shape. Another bacterium can be used to synthesise biodegradable polyhydroxyalkanoates, which can serve as a precursor for the synthesis of polymeric nanoparticles (193). Fungi and yeast have also been explored for the microbial synthesis of polymeric nanoparticles. For example, the Fusarium oxysporum has been used for the synthesis of chitosan nanoparticles (194), while the yeast Saccharomyces cerevisiae has been used for the production of polystyrene nanoparticles, respectively (195). Algae have also been used for the microbial synthesis of polymeric nanoparticles. For example, Chlamydomonas reinhardtii microalgae have been used for the synthesis of biocompatible polymeric nanoparticles, such as chitosan and alginate (196). One of the advantages of microbial synthesis of polymeric nanoparticles is the potential for sustainable and eco-friendly synthesis. For example, the use of biopolymer-producing microorganisms can utilise waste materials as nutrient sources, reducing the environmental impact of nanoparticle synthesis. Additionally, microbial synthesis can be easily scaled up for large-scale nanoparticle production and has great potential for sustainable and controlled synthesis of nanoparticles with unique properties (197,198). Although this method is still in its early stages, further research into the use of different microorganisms and their genetic modification can unlock the full potential of microbial synthesis for polymeric nanoparticle synthesis.
4.2.6.3 Plant extract-based synthesis
Plant extract-based polymeric nanomaterial synthesis is an emerging approach for green and sustainable nanoparticle synthesis. This method involves the use of plant extracts, which contain various phytochemicals, such as flavonoids, terpenoids, and phenolic acids, as reducing agents and stabilisers for nanoparticle synthesis (199). This approach has many advantages, including its simplicity, low cost, and absence of toxic chemicals, making it environmentally friendly and sustainable for nanoparticle synthesis. The synthesis approach using plant extracts is typically similar to that used for the synthesis of metal nanoparticles where the reduction of metal ions leads to the formation of metal nanoparticles. The plant extracts can act as reducing agents by donating electrons to the metal ions, leading to their reduction and formation of nanoparticles, and also act as stabilisers by providing a kind of protective layer on the nanoparticle surface to prevent aggregation and improve stability. Similarly, the synthesis of polymeric nanoparticles involves adding plant extract to a solution containing the monomer and initiator, and the process is allowed to proceed. For example, chitosan has been synthesised using different types of extracts from garlic, ginger, and basil (200,201). Chitosan is commonly used for biomedical applications because of its biodegradability and biocompatibility. The resulting polymeric nanoparticles can have enhanced bioactivity due to the presence of phytochemicals.
The main advantage of plant extract-based synthesis is its low-cost and scalable production. Plant extracts used in the synthesis are often readily available and can be easily extracted using simple methods, making this approach a cost-effective alternative to traditional chemical methods (202). Plant extract-based synthesis also offers the potential for numerous applications due to the variable biomolecule compositions in the plant extract. When the plant extract composition and extraction method are modified, the properties of the synthesised nanoparticles can be tuned to specific applications. Polymeric nanomaterials synthesis based on plant extracts is a promising approach for green and sustainable nanoparticle synthesis (203).
4.2.6.4 Supercritical fluid-based synthesis
Supercritical fluid-based synthesis is a relatively new approach to the synthesis of polymeric nanomaterials. It involves the use of supercritical fluids, which are substances that are heated and pressurised to a state where they exhibit unique properties, such as low viscosity, high solvent power, and high diffusivity. Supercritical carbon dioxide (scCO2) is one of the most widely used supercritical fluids in this approach due to its low toxicity, low cost, and wide availability. In supercritical fluid-based synthesis, the polymeric precursor and other chemicals are dissolved in scCO2 in the presence of high temperature and pressure. The dissolved precursor and chemicals are then allowed to react and form nanoparticles. The properties of the nanoparticles can be controlled by varying the reaction conditions such as pressure, temperature, and time, as well as the precursor and chemical composition. The resulting nanoparticles are then recovered by depressurising the system, allowing the supercritical fluid to evaporate, leaving behind the nanoparticles (204). The advantages of supercritical fluid-based synthesis for polymeric nanoparticles include the absence of toxic solvents, a high degree of control over the nanoparticle properties, and the potential for facile scale-up. Furthermore, scCO2 is a “green” solvent that is easily removed from the reaction mixture by depressurisation, leaving behind a clean product. One of the main challenges of supercritical fluid-based synthesis is the requirement for high-pressure equipment, which can be expensive and difficult to operate. However, advances in technology have led to the development of more affordable and user-friendly supercritical fluid systems, making this approach more accessible to researchers and industries (205). Supercritical fluid-based synthesis has been used to synthesise a variety of polymeric nanoparticles, including drug delivery systems, biodegradable nanoparticles, and nanocomposites. For example, scCO2 has been used to prepare PLA used in drug delivery. The resulting nanoparticles exhibited excellent drug loading capacity and controlled release profiles (206). This method offers many advantages over traditional solvent-based methods, including the absence of toxic solvents, a high degree of control over nanoparticle properties, and the potential for facile scale-up. While this approach is still in its early stages, further research and development is expected to develop and increase the scope and applications of supercritical fluid-based synthesis in the field of nanomaterials.
4.2.7 Role of reducing and capping agents in polymeric nanoparticle synthesis
The role of reducing and capping agents in the synthesis of polymer nanomaterials is similar to that of their role in the synthesis of metallic and metal oxide nanoparticles but with some differences in the specific mechanisms involved. In the case of metallic nanoparticle synthesis, a reducing agent is a substance that donates electrons to the metal ions, reducing them to their zero-valence state, thereby facilitating the formation of nanoparticles. In many cases, the reduction of metal ions to form nanoparticles requires a strong reducing agent, such as sodium borohydride, hydrazine, or ascorbic acid. Similarly, in the synthesis of polymer nanomaterials, a reducing agent is often used to initiate the polymerisation reaction, which involves the reduction of monomers or pre-polymers to their active radical state (207,208). The reducing agent can also be a strong reducing agent, such as sodium borohydride, or a weak reducing agent, such as ascorbic acid or hydrogen peroxide. The reducing agent can also be a photoreducing agent, which is activated by light to produce free radicals that initiate polymerisation (209). The choice of reducing agent depends on the specific polymerisation mechanism and the nature of the monomers or prepolymers used, as it can affect the size, shape, and composition of the nanoparticles.
Capping agents in the synthesis of polymer nanomaterials serve a similar function as that in the synthesis of metallic and metal oxide nanoparticles. A capping agent, also known as a stabilising agent or surfactant, is a substance that forms a coating around the nanoparticles to prevent them from aggregating or coalescing and stabilises them in solution (210,211). It can be organic, inorganic, or a combination of both, depending on the nature of the nanomaterial being synthesised. For example, organic and inorganic capping agents such as cetyltrimethylammonium bromide and oleic acid or trioctylphosphine oxide are commonly used in the synthesis of gold and semiconductor nanoparticles (212,213). They can influence the size and shape of nanoparticles by controlling the growth rate of crystal planes. The capping agent can also be a surfactant, an emulsifier that coats the nanomaterials, or a ligand that coordinates with the polymer chains to form a stable complex. The selection of a capping agent depends on the specific polymerisation mechanism and the nature of the nanomaterials being synthesised (214). In some cases, reducing and capping agents can be combined into a single molecule, known as a dual functional agent, which can perform both functions simultaneously. For example, a polymeric reducing agent can be used to initiate polymerisation and also serve as a stabilising agent for the resulting nanomaterials (215).
In summary, the role of reducing and capping agents for the synthesis of polymer nanomaterials is similar to that of the synthesis of metallic and metal oxide nanoparticles, but with some differences in the specific mechanisms involved. The reducing agent initiates the polymerisation reaction while controlling the growth and stabilisation of the nanomaterial is done by the stabiliser. However, the choice of reducing and capping agents depends on the specific polymerisation mechanism and the nature of the nanomaterials that are being synthesised.
4.2.8 Factors affecting the fabrication of polymeric nanomaterials
Polymeric nanomaterials are increasingly used in a variety of applications due to their unique properties. However, the fabrication of polymeric nanomaterials is a complex process involving several factors that must be taken into account. Understanding these factors is crucial to obtain materials with the desired properties for specific applications (216).
The first factor is the type of polymeric material used. Different polymers have different properties, such as the molecular weight, particle size, chemical composition, the melting point, surface tension, and the solubility in various solvents, which can affect the fabrication process. Therefore, the selection of polymeric material is essential for successful fabrication (217). The choice of polymer affects the stability and functionality of the resulting nanomaterials, as different polymers have varying degrees of solubility and chemical reactivity. Therefore, choosing the right polymer for the desired application is of paramount importance. The second factor to consider is the solvent properties. The choice of solvent used to dissolve the polymer and the solvent removal can significantly impact the size, morphology, and stability of the resulting nanomaterials. The boiling point, surface tension, and solubility parameter of the solvent all play a role in the fabrication process, as they can affect the rate of evaporation of the solvent and the extent of polymer aggregation (218). Additionally, the choice of solvent can also impact the environmental sustainability of the fabrication process, as some solvents are not environmentally friendly and can be harmful to human health or the environment. The third factor is the fabrication technique and processing conditions being used. Different fabrication techniques have different advantages and disadvantages, and the choice of technique will depend on the desired properties of the polymeric nanomaterial (219). For example, electrospinning may be more suitable for the fabrication of polymers with high surface area, while higher temperatures may be used for the fabrication of polymers with higher mechanical strength. Higher temperatures and pressures can also lead to materials with smaller particle sizes, while slower mixing rates can result in materials with larger particle sizes. Therefore, selecting the processing conditions and the fabrication technique is critical to achieving the desired properties of the resulting nanomaterials (218). The fourth factor is the addition of additives. Certain additives can be used to modify the properties of the polymer, such as increasing its mechanical strength or reducing its surface tension. Surfactants and stabilisers are typically used to control the size, stability, and morphology of the resulting nanomaterials. These compounds can affect the rate of solvent evaporation and can also influence the surface energy and surface charge of the resulting nanomaterials. Therefore, the selection of surfactants and stabilisers can therefore significantly impact the properties of the resulting nanomaterials. The choice of additive will depend on the desired properties of the polymeric nanomaterial (220). The fifth factor is post-fabrication processing. This is a critical step in the fabrication process, which can affect the physical and chemical properties of the polymeric nanomaterial (221). For example, heat treatment or chemical treatment can be used to modify the surface structures of the polymer, while sintering can be used to increase mechanical strength (222,223). Thus, the fabrication of polymeric nanomaterials is a complex process that involves several factors including selecting the correct polymeric material, the fabrication technique, additives, and post-fabrication processing, which are all essential for successful fabrication. When these all factors are present, it is possible to fabricate polymeric nanomaterials with the desired properties for a variety of applications.
4.3 Characterisation
Polymeric nanomaterials, which are composed of polymers on a nanometre scale, are an important class of materials with a wide range of applications. Characterisation of polymeric nanomaterials is critical for understanding their properties and behaviour so that they can be used effectively in various applications. To understand the behaviour of polymeric nanomaterials, it is advised to characterise them on a molecular level. This involves studying the structure, composition, and physical and chemical properties of the polymers (224).
4.3.1 Structural analysis
The structural characterisation of polymeric nanomaterials can be done using techniques such as XRD, transmission electron microscopy (TEM), scanning electron microscopy (SEM), atomic force microscopy (AFM), and nuclear magnetic resonance (NMR). These techniques can provide information about the size, shape, and morphology of polymers, their surface topography, and the orientation of the polymeric chains (225,226,227).
4.3.2 Chemical composition analysis
Chemical characterisation involves studying the chemical composition and functional groups present in polymeric nanomaterials, which can be performed using techniques such as spectroscopy (fourier-transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy, and Raman spectroscopy) and chromatographic techniques (228).
4.3.3 Surface charge analysis
It is very important to be able to understand the interactions that exist between polymeric nanomaterials and other materials, such as proteins, cells, and other polymeric materials. This can be done by using characterisation techniques like surface plasmon resonance (SPR), zeta potential, FCS, and AFM. These techniques can provide information about the binding affinities, surface energies, and surface topographies of polymeric nanomaterials (229,230).
4.3.4 Mechanical, electrical, and thermal properties
In addition to studying the structure and composition of polymeric nanomaterials, it is also important to characterise their physical properties, such as their thermal, electrical, and optical properties. This can be done using techniques such as differential scanning calorimetry (DSC), thermogravimetric analysis, dynamic mechanical analysis, and electrical impedance spectroscopy. These techniques can provide information on the thermal stability, electrical conductivity, and optical properties of polymeric nanomaterials (231,232).
4.3.5 Biological properties
Polymeric nanomaterials can be evaluated for their interactions with biological systems, including cytotoxicity, cell uptake, and bio-distribution, using techniques such as in vitro and in vivo studies (233,234). In summary, the characterisation of polymeric nanomaterials is a critical step in understanding their behaviour and properties so that they can be used effectively in various applications. Structural, chemical, physical, and interaction studies of polymeric nanomaterials can be done using techniques such as TEM, SEM, AFM, NMR, spectroscopy, chromatography, and DSC.
5 Applications of polymer-based nanocarriers
5.1 Biomedical applications
Polymer-based nanocarriers have revolutionised the field of biomedical research by providing a platform for targeted and efficient drug delivery. These nanocarriers are composed of polymers with unique physicochemical properties that enable them to encapsulate and transport drugs to specific sites in the body. This section will discuss the biomedical applications of polymer-based nanocarriers as drug delivery vehicles, gene therapy, and imaging, including their advantages, challenges, and prospects (Figure 3).

Applications of polymeric nanomaterials.
5.1.1 Drug delivery
Efficient drug delivery in the biomedical field is among the most important aspects that has been studied in-depth. Nanocarriers can efficiently encapsulate drugs/cargo and be able to successfully transport them to specific parts of the body to deliver their cargo load to the desired tissues or cells (235). An advantage of polymer-based nanocarriers is their ability to target specific cells and protect drugs from being degraded or destroyed by the body’s defence mechanism or immune response. This is possible by modification of the surface of the nanocarrier with ligands that can precisely and selectively bind to the targeted cell receptors (236). They can also be engineered to have specific properties that allow targeted delivery, such as response to stimuli such as changes in temperature, pH, or concentration in the microenvironment (237). For example, a nanocarrier designed for cancer therapy could be engineered to respond to the acidic tumour microenvironment by selectively releasing the drug at the tumour site (4). Polymer-based nanocarriers can also improve the pharmacokinetic and pharmacodynamic properties of drugs, reducing the frequency and dose of administration and enhancing therapeutic efficacy. For example, polymeric micelles can enhance the circulation and solubility time of hydrophobic in nature, while the polymer matrix can allow for sustained and prolonged release of the drugs (238).
5.1.2 Application in biomedical imaging
Nanocarriers possess the ability to have unique imaging properties, such as X-ray contrast, magnetic resonance, or fluorescence, which makes them highly attractive for in vivo imaging and the diagnosis of diseases. Nanocarriers can be loaded with a range of imaging agents, such as dyes, metals, or radioisotopes, that enable sensitive and specific detection of diseases in tissues or cells (239). Liposomes, for example, are spherical vesicles that are made up of phospholipid bilayers that can encapsulate magnetic resonance contrast agents. They can be tailored for optimal imaging performance and enhance their circulation time in the body to target specific tissues by modifying their surface properties (240). Likewise, dendrimers can be used to encapsulate radionuclides or fluorescent dyes for positron emission tomography and optical imaging (241). PLGA nanoparticles have been investigated to encapsulate near-infrared dyes for fluorescence imaging (242). Theranostic applications of polymer-based nanocarriers have also been investigated. Iron oxide, for example, has been coated with a biocompatible polymer and tested for drug delivery and MRI imaging (243). The nanocarriers can also be designed to enable multimodal imaging, combining different imaging modalities for improved accuracy and sensitivity.
5.1.3 Application in gene therapy
Nanocarriers have found their application in gene therapy, which can enhance the efficient delivery of genetic materials, such as DNA or RNA, to specific cells or tissues for the expression of a certain protein. Nanocarriers can protect genetic materials from being degraded or destroyed by the body’s defence mechanism, thus, facilitating their uptake and expression by the target cells (244). Also, nanocarriers can be engineered to have specific properties such as temperature or pH sensitivity that enable targeted delivery and controlled release of genetic materials, thereby, improving the safety and efficacy of gene therapy. For example, lipid-based nanocarriers possess the ability to encapsulate and protect nucleic acids. Their surface can be engineered and functionalised with stimuli-responsive materials such as pH-sensitive polymers that can trigger the release of nucleic acids in an acidic environment (245). Additionally, cationic polymers such as PEI and polyamidoamine (PAMAM) dendrimers are used in gene therapy. Their surface can be modified to enhance their cellular uptake by specific cells (246) and also possess the ability to bind to negatively charged nucleic acids and form a stable complex to protect their cargo. In addition, polymer-based nanocarriers have also been investigated for the delivery of gene editing tools, such as CRISPR-Cas9. These tools can be delivered to target cells using nanocarriers to facilitate gene editing and correct genetic mutations (247). However, despite the fascinating and significant potential of polymer-based nanocarriers, several challenges are hindering their usability, which still need to be addressed. One of the main challenges is the biocompatibility of the nanocarriers. The potential toxicity of nanocarriers can arise from their size, surface chemistry, and biodegradability. This can lead to adverse biological effects, such as inflammation. Thus, the safety and toxicity of nanocarriers need to be carefully evaluated before clinical translation (238).
5.2 Environmental application of polymer-based nanomaterial
Polymer-based nanomaterials have emerged as a promising platform for environmental applications, including environmental remediation, water treatment, and energy conversion. These nanomaterials possess unique physicochemical properties that enable them to interact with pollutants and contaminants in the environment and facilitate their removal, providing a highly efficient and cost-effective solution for environmental challenges. The environmental applications of polymer-based nanomaterials are discussed below.
5.2.1 Water purification/treatment
Contamination of the ecosystem due to the continuous discharge of industrial waste has posed a significant threat to public health. Many industrial wastes have been linked to contamination of groundwater sources with toxic waste or heavy metals. However, nanomaterials such as polymer-based nanocarriers have had a significant impact on water purification applications. These nanocarriers can be engineered to selectively bind to specific contaminants/pollutants, such as heavy metals, organic compounds, or microplastics, and facilitate their removal from water (248). Nanomaterials can be designed to have a high surface area, porosity, and reactivity, enhancing their pollutant adsorption and catalytic activity. For example, polymer-coated magnetic nanoparticles can be functionalised with specific ligands that selectively bind to pollutants in water. Once the nanoparticles have bound to the pollutants, they can be magnetically separated from the water, facilitating their removal (249,250). Another example is polymer-based adsorbents. These adsorbents are composed of porous polymer particles that can selectively adsorb pollutants from water. The efficiency of adsorption depends on the size and surface chemistry of the particles which can be optimised for maximum adsorption capacity and selectivity (251). Polymeric membranes have also been investigated for water purification applications. These membranes can be designed to selectively allow certain molecules to pass through while blocking others. For example, polymeric membranes can be used to remove salt from seawater, making it suitable for drinking and agricultural use (252). Polymer-based nanocarriers can also be used for water disinfection. For example, nanoparticles composed of antimicrobial polymers, such as polyhexamethylene biguanide, can be used to kill bacteria and viruses in water (253,254). Moreover, the nanomaterials can be easily recovered and reused, making them a highly sustainable and efficient solution for water pollution remediation.
5.2.2 Air purification
Another major application of polymer-based carriers explored is air purification. They can selectively bind and remove pollutants from air, making them an attractive option for air purification. These nanocarriers can be designed to selectively remove specific pollutants, such as volatile organic compounds, nitrogen oxides (NOx), and particulate matter (255,256,257,258). These adsorbents can be designed to selectively adsorb specific pollutants from the air and can also be incorporated into air filters, which can then be used in air purification systems to remove pollutants from the air. Another application of polymer-based nanocarriers for air purification is the use of photocatalytic materials. Photocatalytic nanocarriers, such as titanium dioxide nanoparticles, can be incorporated into air filters or coatings. When exposed to light, these photocatalytic nanocarriers can generate reactive oxygen species, which can then react with pollutants in the air and degrade them (259). Polymeric membranes have also been explored for air purification applications. Membranes can be designed to selectively allow certain molecules, such as pollutants, to pass through while blocking others (260,261). Polymer-based nanocarriers can also be used for air disinfection (262). Antimicrobial polymers can be incorporated into air filters or coatings, which can then be used to kill bacteria and viruses in the air (263,264).
5.2.3 Energy conversion
Another application of polymer-based nanocarriers is in energy conversions. Nanomaterials can be designed to have specific properties that enable them to absorb sunlight and convert it into electrical or chemical energy (solar and/or fuel cells), providing a highly sustainable and clean solution for energy generation (265). As for solar cells, polymer-based nanocarriers are used as electrode material in dye-sensitised solar cells (DSSCs). These solar cells use a photoactive dye to absorb light and generate electrons, which are then transported through a series of materials to generate an electrical current. Polymer-based nanocarriers can be incorporated into this type of DSSC to enhance their performance. For example, polymer-based nanocarriers can be used to improve electron transport between the dye and electrode or to increase the surface area of the electrode to enhance the absorption of light (266). Another example is the case of organic photovoltaic (OPV) devices. OPVs use organic materials to generate electrical current from sunlight. Polymer-based nanocarriers can be incorporated into these OPVs to enhance the performance and device stability while simultaneously stabilising the system. For example, polymer-based nanocarriers can be used to improve the morphology of organic materials, which can improve the absorption of light and improve the transport of electrons and holes (267). Fuel cells are another energy conversion device that can benefit from the use of polymer-based nanocarriers. Fuel cells generate electricity by oxidising a fuel, such as hydrogen or methanol. Polymer-based nanocarriers can be used to improve the performance and durability of fuel cell components, such as electrodes and electrolyte membranes. For example, polymer-based nanocarriers can be used to improve fuel and oxygen through the electrodes or to enhance the stability of the electrolyte membrane (268).
Despite the significant potential of polymer-based nanomaterials in environmental applications, several challenges still need to be addressed. One of the main challenges is potential toxicity. These nanomaterials can be discharged into the environment and pose a potential threat to health. The potential toxicity and environmental impact of nanomaterials can arise from their size, surface chemistry, and biodegradability, and can also lead to adverse environmental effects. The safety and environmental impact of nanomaterials needs to be carefully evaluated before widespread use. Agricultural applications of polymer nanocarriers have also been reported (269).
5.3 Other applications
5.3.1 3D printing
Polymer-based nanomaterials have also found application in 3D printing for biomedical applications. For example, Kim et al. (270) printed a 3D bionic ear using an extrusion-based Fab@Home 3D printer and a chondrocyte-seeded alginate hydrogel matrix. With the aid of a computerised model (CAD) drawing and an intertwined electrically conducting polymer that is infused with silver nanoparticles, the anatomical geometry of the bionic ear was printed. The ear was subsequently cultured in vitro to allow the continued growth of cartilaginous tissue to form a cyborg ear. The cyborg ear was then tested and was found to have improved sensing capability of electromagnetic signals in the radio frequency range.
Similarly, Wang et al. (271) fabricated a hybrid 3D printed metal-polymer scaffold that is capable of delivering dual antibiotics to treat periprosthetic joint infection that is caused by antibiotic-resistant bacteria. The scaffold design was in two parts: cylindrical scaffold and circular stainless steel. The cylindrical scaffold was fabricated by homogeneously mixing tobramycin, vancomycin, and melted PLC. The mixture was printed in a cylindrical shape using a 3DX printer and the product served as the dual antibiotic while the circular stainless steel was printed by using a metal 3D printer. The fabricated parts were then assembled to create the hybrid scaffold. To investigate the susceptibility and release profile of the antibiotics, the hybrid scaffold was immersed in normal saline and sampled for 28 days using high performance liquid chromatography and disk diffusion method. The results showed that the hybrid scaffold has antimicrobial effects against Gram-negative and Gram-positive bacteria. Consequently, there was no cytotoxicity in the fabricated scaffold.
Moreover, Banik et al. (272) also carried out an investigation on the anisotropic properties of 3D printed biocompatible thermoplastic polyurethane (TPU)/PLA/GO metal–polymer nanocomposites and its potential application as a biocompatible material in tissue engineering. This nanocomposite was prepared by the solvent-based mixing method and then extruded into the FDM printing filament. The FTIR and SEM characterisation images show relatively good dispersion of GO in the polymer matrix. The addition of GO significantly enhanced the mechanical property and thermal stability. The result shows improved mechanical properties of the polymer matrix with a significant increase of 75.5% in the tensile modulus and 167% in the compression modulus. The printing orientation during 3D printing leads to weak adhesion strength between layers, and interestingly, it is found that the mechanical responses are highly dependent on the 3D printing orientation. The printed TPU/PLA/GO nanocomposite is biocompatible with NIH3T3 cells, indicating a promising scaffold biomaterial for tissue engineering applications.
6 Conclusion
Polymer nanocarriers have shown great potential for a wide range of biomedical and environmental applications. These nanocarriers offer numerous advantages over traditional drug delivery systems available, including improved bioavailability, controlled release, and improved therapeutic efficacy. Functionalisation of the nanocarriers can be achieved leading to targeted drug delivery to specific tissues or cells, reducing potential side effects. Polymer nanocarriers have also shown promising environmental applications in water treatment and environmental remediation. They possess the ability to efficiently adsorb pollutants and contaminants from water and soil, making them a valuable tool in addressing environmental challenges. However, despite their promising and unique properties, there are still challenges to be addressed in the development of polymer nanocarriers, such as improving their stability and biocompatibility. Further research and development are needed to optimise these systems for clinical and environmental applications. In general, polymer nanocarriers represent a promising and rapidly advancing field with the potential to revolutionise drug delivery and environmental remediation. As our understanding of these systems continues to grow, we can expect to see even more innovative applications of polymer nanocarriers in the future.
7 Future perspective
The future of polymer nanocarriers for biomedical and environmental applications is promising, with ongoing research focused on improving their functionality and effectiveness. In the biomedical field, researchers are exploring ways to enhance the targeting capabilities of polymer nanocarriers by incorporating molecular targeting agents such as antibodies or ligands. This could lead to more precise and effective drug delivery to specific cells or tissues, resulting in better treatment outcomes and fewer side effects. In addition, the development of biodegradable and biocompatible polymers is a promising area of research, as these materials could eliminate the need for the removal of the nanocarrier after drug delivery. This could reduce the risk of toxicity and improve patient comfort and compliance. In the environmental field, the potential applications of polymer nanocarriers are also expanding. Researchers are investigating the use of nanocarriers to remove heavy metals, pesticides, and other pollutants from water and soil. This could lead to more efficient and cost-effective remediation of contaminated environments, reducing the impact of these pollutants on human health and the environment. In general, the future of polymer nanocarriers is bright, with continued advances in materials science, drug development, and environmental remediation. As these technologies become more refined and widely adopted, we can expect to see significant improvements in human health and environmental sustainability.
-
Funding information: This work was supported by the Research University Grant, Universiti Kebangsaan Malaysia, Geran Translasi, UKM-TR2022-05.
-
Author contributions: Arpita Roy: conceptualisation and manuscript design; Dahir Sagir Idris, Arpita Roy, and Soumya Pandit: writing – original draft; Saad Alghamdi, Mazen Almehmadi, Ahad Amer Alsaiari, Osama Abdulaziz, Abdulaziz Alsharif, Mayeen Uddin Khandake, and Mohammad Rashed Iqbal Faruque: writing – review and editing, formal analysis, and resource; Mayeen Uddin Khandaker and Mohammad Rashed Iqbal Faruque: fund acquisition. The final manuscript has been approved by all the authors.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this article.
References
(1) Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J Drug Deliv Sci Technol. 2019;53:101174. 10.1016/j.jddst.2019.101174.Suche in Google Scholar
(2) Leon L, Chung EJ, Rinaldi C. Chapter 1 - A brief history of nanotechnology and introduction to nanoparticles for biomedical applications. In: Chung EJ, Leon L, Rinaldi C, editors. Nanoparticles for Biomedical Applications. Netherlands: Elsevier; 2020. p. 1–4. 10.1016/B978-0-12-816662-8.00001-1.Suche in Google Scholar
(3) Sen M. Nanocomposite materials. London, England: IntechOpen. 2020. 10.5772/intechopen.93047.Suche in Google Scholar
(4) Avramović N, Mandić B, Savić-Radojević A, Simić T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics. 2020;12:298. 10.3390/pharmaceutics12040298.Suche in Google Scholar PubMed PubMed Central
(5) Din FUd, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, et al. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed. 2017;12:7291–309. 10.2147/IJN.S146315.Suche in Google Scholar PubMed PubMed Central
(6) Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B, Emiliani C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J Funct Biomater. 2019;10:4. 10.3390/jfb10010004.Suche in Google Scholar PubMed PubMed Central
(7) Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41:2545–61. 10.1039/C2CS15327K.Suche in Google Scholar PubMed PubMed Central
(8) Shakiba S, Astete CE, Paudel S, Sabliov CM, Rodrigues DF, Louie SM. Emerging investigator series: polymeric nanocarriers for agricultural applications: synthesis, characterization, and environmental and biological interactions. Environ Sci: Nano. 2020;7:37–67. 10.1039/C9EN01127G.Suche in Google Scholar
(9) Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv Drug Deliv Rev. 2012;64:866–84. 10.1016/j.addr.2012.01.020.Suche in Google Scholar PubMed
(10) Spirescu VA, Chircov C, Grumezescu AM, Vasile BȘ, Andronescu E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int J Mol Sci. 2021;22(9):4595. 10.3390/ijms22094595.Suche in Google Scholar PubMed PubMed Central
(11) Misra M, Vivekanandhan S, Mohanty AK, Denault J. 4.09 - Nanotechnologies for Agricultural Bioproducts. In: Moo-Young M, editor. Comprehensive biotechnology. 2nd edn. Burlington: Academic Press; 2011. p. 111–9. 10.1016/B978-0-08-088504-9.00260-9.Suche in Google Scholar
(12) Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J Phys Chem B. 2003;107:668–77. 10.1021/jp026731y.Suche in Google Scholar
(13) Miller MM, Lazarides AA. Sensitivity of metal nanoparticle surface plasmon resonance to the dielectric environment. J Phys Chem B. 2005;109:21556–65. 10.1021/jp054227y.Suche in Google Scholar PubMed
(14) Augustine R, Hasan A. Emerging applications of biocompatible phytosynthesized metal/metal oxide nanoparticles in healthcare. J Drug Deliv Sci Technol. 2020;56:101516. 10.1016/j.jddst.2020.101516.Suche in Google Scholar
(15) Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R, et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials. 2020;10:292. 10.3390/nano10020292.Suche in Google Scholar PubMed PubMed Central
(16) Eivazzadeh-Keihan R, Bahojb Noruzi E, Khanmohammadi Chenab K, Jafari A, Radinekiyan F, Hashemi SM, et al. Metal-based nanoparticles for bone tissue engineering. J Tissue Eng Regener Med. 2020;14:1687–714. 10.1002/term.3131.Suche in Google Scholar PubMed
(17) Aderibigbe BA. Metal-based nanoparticles for the treatment of infectious diseases. Molecules. 2017;22:1370. 10.3390/molecules22081370.Suche in Google Scholar PubMed PubMed Central
(18) Kumar A, Choudhary A, Kaur H, Mehta S, Husen A. Metal-based nanoparticles, sensors, and their multifaceted application in food packaging. J Nanobiotechnol. 2021;19:256. 10.1186/s12951-021-00996-0.Suche in Google Scholar PubMed PubMed Central
(19) Thomas SC, Harshita, Kumar Mishra P, Talegaonkar S. Ceramic nanoparticles: Fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21:6165–88.10.2174/1381612821666151027153246Suche in Google Scholar PubMed
(20) Moreno-Vega A-I, Gómez-Quintero T, Nuñez-Anita R-E, Acosta-Torres L-S, Castaño V. Polymeric and ceramic nanoparticles in biomedical applications. J Nanotechnol. 2012;2012:e936041. 10.1155/2012/936041.Suche in Google Scholar
(21) Chang Y, Sun X, Ma M, Mu C, Li P, Li L, et al. Application of hard ceramic materials B4C in energy storage: Design B4C@C core-shell nanoparticles as electrodes for flexible all-solid-state micro-supercapacitors with ultrahigh cyclability. Nano Energy. 2020;75:104947. 10.1016/j.nanoen.2020.104947.Suche in Google Scholar
(22) Liu K, Marwat MA, Ma W, Wei T, Li M, Fan P, et al. Enhanced energy storage performance of nanocomposites filled with paraelectric ceramic nanoparticles by weakening the electric field distortion. Ceram Int. 2020;46:21149–55. 10.1016/j.ceramint.2020.05.192.Suche in Google Scholar
(23) Messler RW. 9 - Ceramic and glass attachment schemes and attachments. In: Messler RW, editor. Integral Mechanical Attachment. Burlington: Butterworth-Heinemann; 2006. p. 239–77. 10.1016/B978-075067965-7/50027-4.Suche in Google Scholar
(24) Vernoux P, Lizarraga L, Tsampas MN, Sapountzi FM, De Lucas-Consuegra A, Valverde J-L, et al. Ionically conducting ceramics as active catalyst supports. Chem Rev. 2013;113:8192–260. 10.1021/cr4000336.Suche in Google Scholar PubMed
(25) Wang J, Gili A, Grünbacher M, Praetz S, Dirk Epping J, Görke O, et al. Silicon oxycarbonitride ceramic containing nickel nanoparticles: from design to catalytic application. Mater Adv. 2021;2:1715–30. 10.1039/D0MA00917B.Suche in Google Scholar
(26) Rohrer GS, Affatigato M, Backhaus M, Bordia RK, Chan HM, Curtarolo S, et al. Challenges in Ceramic Science: A Report from the Workshop on Emerging Research Areas in Ceramic Science. J Am Ceram Soc. 2012;95:3699–3712. 10.1111/jace.12033.Suche in Google Scholar
(27) Sharma R, Raghavarao KSMS. Chapter 6 - Nanoparticle-based aptasensors for food contaminant detection. In: López Rubio A, Fabra Rovira MJ, Martínez Sanz M, Gómez-Mascaraque LG, editors. Nanomaterials for food applications. Netherlands: Elsevier; 2019. p. 123–45. 10.1016/B978-0-12-814130-4.00006-3.Suche in Google Scholar
(28) Suresh S. Semiconductor nanomaterials, methods and applications: A review. Nanosci Nanotechnol. 2013;3:62–74, http://article.sapub.org/ When the size of semiconductor materials is reduced to nanoscale, their physical and chemical properties change drastically, resulting in unique properties due to their large surface area or quantum size effect. Currently, semiconductor nanomaterials and devices are still in the research stage, but they are promising for applications in many fields, such as solar cells, nanoscale electronic devices, light-emitting nano devices, laser technology, waveguide, chemicals and biosensors. Further development of nanotechnology will certainly lead to significant breakthroughs in the semiconductor industry. This paper deals with the some of the current initiatives and critical issues in the improvement of semiconductors based on nanostructures and nanodevices. (accessed February 12, 2023).Suche in Google Scholar
(29) Sahu MK. Semiconductor nanoparticles theory and applications. Int J Appl Eng Res. 2019;14:491–4.Suche in Google Scholar
(30) Ezzat HA, Hegazy MA, Nada NA, Osman O, Ibrahim MA. Development of natural polymer/metal oxide nanocomposite reinforced with graphene oxide for optoelectronic applications. NRIAG J Astron Geophysics. 2021;10:10–22. 10.1080/20909977.2020.1846246.Suche in Google Scholar
(31) Hashim A, Hadi Q. Synthesis of novel (polymer blend-ceramics) nanocomposites: Structural, optical and electrical properties for humidity sensors. J Inorg Organomet Polym. 2018;28:1394–401. 10.1007/s10904-018-0837-4.Suche in Google Scholar
(32) George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int J Pharmaceutics. 2019;561:244–64. 10.1016/j.ijpharm.2019.03.011.Suche in Google Scholar PubMed
(33) Giliopoulos D, Zamboulis A, Giannakoudakis D, Bikiaris D, Triantafyllidis K. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules. 2020;25:185. 10.3390/molecules25010185.Suche in Google Scholar PubMed PubMed Central
(34) Anancharoenwong E, Chueangchayaphan W, Rakkapao N, Marthosa S, Chaisrikhwun B. Thermo-mechanical and antimicrobial properties of natural rubber-based polyurethane nanocomposites for biomedical applications. Polym Bull. 2021;78:833–48. 10.1007/s00289-020-03137-z.Suche in Google Scholar
(35) Nitta SK, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. Int J Mol Sci. 2013;14:1629–54. 10.3390/ijms14011629.Suche in Google Scholar PubMed PubMed Central
(36) Wang Y, Li P, Truong-Dinh Tran T, Zhang J, Kong L. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials. 2016;6:26. 10.3390/nano6020026.Suche in Google Scholar PubMed PubMed Central
(37) Sharma M. Chapter 18 - Transdermal and intravenous nano drug delivery systems: Present and future. In: Mohapatra SS, Ranjan S, Dasgupta N, Mishra RK, Thomas S, editors. Applications of targeted nano drugs and delivery systems. Netherlands: Elsevier; 2019. p. 499–550. 10.1016/B978-0-12-814029-1.00018-1.Suche in Google Scholar
(38) Fukushima K, Kimura Y. Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application. Polym Int. 2006;55:626–42. 10.1002/pi.2010.Suche in Google Scholar
(39) Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M. Scale-up polymeric-based nanoparticles drug delivery systems: Development and challenges. OpenNano. 2022;7:100048. 10.1016/j.onano.2022.100048.Suche in Google Scholar
(40) Hasan AMA, Abdel-Raouf ME-S. Cellulose-based superabsorbent hydrogels. In: Mondal MdIH, editor. Cellulose-based superabsorbent hydrogels. Cham: Springer International Publishing; 2019. p. 245–67. 10.1007/978-3-319-77830-3_11.Suche in Google Scholar
(41) Zoppe JO, Ataman NC, Mocny P, Wang J, Moraes J, Klok H-A. Surface-initiated controlled radical polymerization: State-of-the-art, opportunities, and challenges in surface and interface engineering with polymer brushes. Chem Rev. 2017;117:1105–1318. 10.1021/acs.chemrev.6b00314.Suche in Google Scholar PubMed
(42) Winey KI, Vaia RA. Polymer nanocomposites. MRS Bull. 2007;32:314–22. 10.1557/mrs2007.229.Suche in Google Scholar
(43) Kumar A, Sharma K, Dixit AR. A review on the mechanical properties of polymer composites reinforced by carbon nanotubes and graphene. Carbon Lett. 2021;31:149–65. 10.1007/s42823-020-00161-x.Suche in Google Scholar
(44) Luna J, Vílchez A. Chapter seven - Polymer nanocomposites for food packaging. In: Busquets R, editor. Emerging nanotechnologies in food science. Boston: Elsevier; 2017. p. 119–47. 10.1016/B978-0-323-42980-1.00007-8.Suche in Google Scholar
(45) Feldman D. Polymer nanocomposites in medicine. J Macromol Sci, Part A. 2016;53:55–62. 10.1080/10601325.2016.1110459.Suche in Google Scholar
(46) DeLeon VH, Nguyen TD, Nar M, D’Souza NA, Golden TD. Polymer nanocomposites for improved drug delivery efficiency. Mater Chem Phys. 2012;132:409–15. 10.1016/j.matchemphys.2011.11.046.Suche in Google Scholar
(47) Jia L, Zhou T, Xu J, Xu Z, Zhang M, Wang Y, et al. Visible light-induced lanthanide polymer nanocomposites based on clays for bioimaging applications. J Mater Sci. 2016;51:1324–32. 10.1007/s10853-015-9450-x.Suche in Google Scholar
(48) Li C, Mu J, Song Y, Chen S, Xu F. Highly aligned cellulose/polypyrrole composite nanofibers via electrospinning and in situ polymerization for anisotropic flexible strain sensor. ACS Appl Mater Interfaces. 2023;15:9820–9. 10.1021/acsami.2c20464.Suche in Google Scholar PubMed
(49) Wang Q, Ma J, Chen S, Wu S. Designing an innovative electrospinning strategy to generate PHBV nanofiber scaffolds with a radially oriented fibrous pattern. Nanomaterials. 2023;13:1150. 10.3390/nano13071150.Suche in Google Scholar PubMed PubMed Central
(50) Alyamani AA, Al-Musawi MH, Albukhaty S, Sulaiman GM, Ibrahim KM, Ahmed EM, et al. Electrospun polycaprolactone/chitosan nanofibers containing cordia myxa fruit extract as potential biocompatible antibacterial wound dressings. Molecules. 2023;28:2501. 10.3390/molecules28062501.Suche in Google Scholar PubMed PubMed Central
(51) Rajati H, Alvandi H, Rahmatabadi SS, Hosseinzadeh L, Arkan E. A nanofiber-hydrogel composite from green synthesized AgNPs embedded to PEBAX/PVA hydrogel and PA/Pistacia atlantica gum nanofiber for wound dressing. Int J Biol Macromol. 2023;226:1426–43. 10.1016/j.ijbiomac.2022.11.255.Suche in Google Scholar PubMed
(52) Liu Y, Wang L, Huang Y, Hou C, Xin B, Li T, et al. Electrospun poly(l-lactide-co-ε-caprolactone)/gelatin core–shell nanofibers encapsulated with doxorubicin hydrochloride as a drug delivery system. Polym Int. 2023;72:166–75. 10.1002/pi.6458.Suche in Google Scholar
(53) Jo H, Le T-H, Lee H, Lee J, Kim M, Lee S, et al. Macrocyclic ligand-embedded graphene-in-polymer nanofiber membranes for lithium ion recovery. Chem Eng J. 2023;452:139274. 10.1016/j.cej.2022.139274.Suche in Google Scholar
(54) Ma W, Lu T, Cao W, Xiong R, Huang C. Bioinspired nanofibrous aerogel with vertically aligned channels for efficient water purification and salt-rejecting solar desalination. Adv Funct Mater. 2023;33:2214157. 10.1002/adfm.202214157.Suche in Google Scholar
(55) Sohrabi M, Abbasi M, Sadighzadeh A. Fabrication and evaluation of electrospun polyacrylonitrile/silver nanofiber membranes for air filtration and antibacterial activity. Polym Bull. 2023;80:5481–99. 10.1007/s00289-022-04311-1.Suche in Google Scholar PubMed PubMed Central
(56) Wei P, Huang D, Luo C, Sui Y, Li X, Liu Q, et al. High-performance sandwich-structure PI/SPEEK + HPW nanofiber composite membrane with balanced proton conductivity and stability. Polymer. 2023;271:125800. 10.1016/j.polymer.2023.125800.Suche in Google Scholar
(57) Im H-G, An BW, Jin J, Jang J, Park Y-G, Park J-U, et al. A high-performance, flexible and robust metal nanotrough-embedded transparent conducting film for wearable touch screen panels. Nanoscale. 2016;8:3916–22. 10.1039/C5NR07657A.Suche in Google Scholar
(58) Asadnia M, Kottapalli AGP, Karavitaki KD, Warkiani ME, Miao J, Corey DP, et al. From biological cilia to artificial flow sensors: Biomimetic soft polymer nanosensors with high sensing performance. Sci Rep. 2016;6:32955. 10.1038/srep32955.Suche in Google Scholar PubMed PubMed Central
(59) Ikromov N, Alijonov A, Soliyev B, Mamajonov Y, Mahammadjonov N, Meliqoziyev A. Analysis of mechanical properties of polymer bushing used in automobile industry. Asian J Multidimens Res. 2021;10:560–3. 10.5958/2278-4853.2021.00170.1.Suche in Google Scholar
(60) Chancellor AJ, Seymour BT, Zhao B. Characterizing polymer-grafted nanoparticles: From basic defining parameters to behavior in solvents and self-assembled structures. Anal Chem. 2019;91:6391–402. 10.1021/acs.analchem.9b00707.Suche in Google Scholar PubMed
(61) Peralta ME, Jadhav SA, Magnacca G, Scalarone D, Mártire DO, Parolo ME, et al. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J Colloid Interface Sci. 2019;544:198–205. 10.1016/j.jcis.2019.02.086.Suche in Google Scholar PubMed
(62) Dutta S, Parida S, Maiti C, Banerjee R, Mandal M, Dhara D. Polymer grafted magnetic nanoparticles for delivery of anticancer drug at lower pH and elevated temperature. J Colloid Interface Sci. 2016;467:70–80. 10.1016/j.jcis.2016.01.008.Suche in Google Scholar PubMed
(63) Zhang Y, Zhu T, Xu Y, Yang Y, Sheng D, Ma Q. Quaternized salicylaldehyde Schiff base side-chain polymer-grafted magnetic Fe3O4 nanoparticles for the removal and detection of Cu2+ ions in water. Appl Surf Sci. 2023;611:155632. 10.1016/j.apsusc.2022.155632.Suche in Google Scholar
(64) Pal A, Das Karmakar P, Vel R, Bodhak S. Synthesis and characterizations of bioactive glass nanoparticle-incorporated triblock copolymeric injectable hydrogel for bone tissue engineering. ACS Appl Bio Mater. 2023;6:445–57. 10.1021/acsabm.2c00718.Suche in Google Scholar PubMed
(65) Tawade BV, Singh M, Apata IE, Veerasamy J, Pradhan N, Karim A, et al. Polymer-grafted nanoparticles with variable grafting densities for high energy density polymeric nanocomposite dielectric capacitors. JACS Au. 2023;3:1365–75. 10.1021/jacsau.3c00022.Suche in Google Scholar PubMed PubMed Central
(66) Mekuria SL, Li J, Song C, Gao Y, Ouyang Z, Shen M, et al. Facile formation of PAMAM dendrimer nanoclusters for enhanced gene delivery and cancer gene therapy. ACS Appl Bio Mater. 2021;4:7168–75. 10.1021/acsabm.1c00743.Suche in Google Scholar PubMed
(67) Yuan B, Zhao S, Hu P, Cui J, Niu QJ. Asymmetric polyamide nanofilms with highly ordered nanovoids for water purification. Nat Commun. 2020;11:6102. 10.1038/s41467-020-19809-3.Suche in Google Scholar PubMed PubMed Central
(68) Mohammadzadeh P, Shafiee Ardestani M, Mortazavi-Derazkola S, Bitarafan-Rajabi A, Ghoreishi SM. PEG-citrate dendrimer second generation: Is this a good carrier for imaging agents in vitro and in vivo? IET Nanobiotechnol. 2019;13:560–4. 10.1049/iet-nbt.2018.5360.Suche in Google Scholar PubMed PubMed Central
(69) Li P, Zhang M, Sun X, Guan S, Zhang G, Baumgarten M, et al. A dendrimer-based highly sensitive and selective fluorescence-quenching sensor for Fe3+ both in solution and as film. Biosens Bioelectron. 2016;85:785–91. 10.1016/j.bios.2016.05.046.Suche in Google Scholar PubMed
(70) Yuan H, Luo K, Lai Y, Pu Y, He B, Wang G, et al. A novel poly(l-glutamic acid) dendrimer based drug delivery system with both pH-sensitive and targeting functions. Mol Pharm. 2010;7:953–62. 10.1021/mp1000923.Suche in Google Scholar PubMed
(71) Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, et al. Dendrimers: Synthesis, applications, and properties. Nanoscale Res Lett. 2014;9:247. 10.1186/1556-276X-9-247.Suche in Google Scholar PubMed PubMed Central
(72) Vögtle F, Gestermann S, Hesse R, Schwierz H, Windisch B. Functional dendrimers. Prog Polym Sci. 2000;25:987–1041. 10.1016/S0079-6700(00)00017-4.Suche in Google Scholar
(73) Wang SH, Shi X, Van Antwerp M, Cao Z, Swanson SD, Bi X, et al. Dendrimer-functionalized iron oxide nanoparticles for specific targeting and imaging of cancer cells. Adv Funct Mater. 2007;17:3043–50. 10.1002/adfm.200601139.Suche in Google Scholar
(74) Jevprasesphant R, Penny J, Attwood D, McKeown NB, D’Emanuele A. Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm Res. 2003;20:1543–50. 10.1023/A:1026166729873.Suche in Google Scholar PubMed
(75) Hadjesfandiari N, Bajgai MP, Yu K, Brooks DE, Kizhakkedathu JN. Polymer brushes. In Encyclopedia of Polymer Science and Technology. United States: John Wiley & Sons, Ltd.; 2013. 10.1002/0471440264.pst529.pub2.Suche in Google Scholar
(76) Ayres N. Polymer brushes: Applications in biomaterials and nanotechnology. Polym Chem. 2010;1:769–77. 10.1039/B9PY00246D.Suche in Google Scholar
(77) Krishnamoorthy M, Hakobyan S, Ramstedt M, Gautrot JE. Surface-initiated polymer brushes in the biomedical field: Applications in membrane science, biosensing, cell culture, regenerative medicine and antibacterial coatings. Chem Rev. 2014;114:10976–1026. 10.1021/cr500252u.Suche in Google Scholar PubMed
(78) Liu T, Yan S, Zhou R, Zhang X, Yang H, Yan Q, et al. Self-adaptive antibacterial coating for universal polymeric substrates based on a micrometer-scale hierarchical polymer brush system. ACS Appl Mater Interfaces. 2020;12:42576–85. 10.1021/acsami.0c13413.Suche in Google Scholar PubMed
(79) Wei Q, Liu H, Zhao X, Zhao W, Xu R, Ma S, et al. Bio-inspired hydrogel-polymer brush bi-layered coating dramatically boosting the lubrication and wear-resistance. Tribol Int. 2023;177:108000. 10.1016/j.triboint.2022.108000.Suche in Google Scholar
(80) Bai S, Jia D, Ma X, Liang M, Xue P, Kang Y, et al. Cylindrical polymer brushes-anisotropic unimolecular micelle drug delivery system for enhancing the effectiveness of chemotherapy. Bioact Mater. 2021;6:2894–904. 10.1016/j.bioactmat.2021.02.011.Suche in Google Scholar PubMed PubMed Central
(81) Tyrrell ZL, Shen Y, Radosz M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog Polym Sci. 2010;35:1128–43. 10.1016/j.progpolymsci.2010.06.003.Suche in Google Scholar
(82) Kim MP, Kang DJ, Jung D-W, Kannan AG, Kim K-H, Ku KH, et al. Gold-decorated block copolymer microspheres with controlled surface nanostructures. ACS Nano. 2012;6:2750–7. 10.1021/nn300194z.Suche in Google Scholar PubMed
(83) Ding S-P, Zhang Z-K, Ye Z, Xia D-L, Xu J-T. Electrostatic crosslinking-enabled highly asymmetric lamellar nanostructures of polyzwitterionic block copolymers for lithography. Nanoscale. 2023;15:4553–60. 10.1039/D3NR00073G.Suche in Google Scholar
(84) Jiang H, Liu Y, Muhammad Y, Pei R, Guo R, Li J. Preparation and evaluation of performance and mechanism of gallic acid–rubber powder–microalgae bio-oil/styrene block copolymers composite modified asphalt. Arab J Sci Eng. 2023;48:5229–42. 10.1007/s13369-022-07366-4.Suche in Google Scholar
(85) Oliveira ASR, Pereira P, Mendonça PV, Fonseca AC, Simões S, Serra AC, et al. Novel degradable amphiphilic 4-arm star PLA-b-POEOA and PLGA-b-POEOA block copolymers: synthesis, characterization and self-assembly. Polym Chem. 2023;14:161–71. 10.1039/D2PY01216B.Suche in Google Scholar
(86) Leitner L-C, Schneider R, Steiner T, Stenzel MH, Freitag R, Greiner A. Efficient synthesis and wetting characteristics of amphiphilic galactose–PLA block copolymers: A potential additive for the accelerated biodegradation of micro- and nanoplastics. Macromol Chem Phys. 2023;224:2100431. 10.1002/macp.202100431.Suche in Google Scholar
(87) Suthar J, Alvarez-Fernandez A, Osarfo-Mensah E, Angioletti-Uberti S, Williams GR, Guldin S. Amplified EQCM-D detection of extracellular vesicles using 2D gold nanostructured arrays fabricated by block copolymer self-assembly. Nanoscale Horiz. 2023;8:460–72. 10.1039/D2NH00424K.Suche in Google Scholar PubMed PubMed Central
(88) Chang Y-X, Wang C-F, Chang C-J, Lu C-H, Chen J-K. Fabrication of scalable poly(N-isopropylacrylamide)/gold nanoparticles composite ring array as LSPR sensor for label-free biosensor application. Sens Actuators B: Chem. 2023;375:132875. 10.1016/j.snb.2022.132875.Suche in Google Scholar
(89) Viola M, Migliorini C, Matricardi PC, Di Meo. Synthesis and characterization of a novel amphiphilic polyacrylate-cholesterol derivative as promising material for pharmaceutical and cosmetic applications. Eur Polym J. 2023;184:111774. 10.1016/j.eurpolymj.2022.111774.Suche in Google Scholar
(90) Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–235. 10.1039/C2CS35265F.Suche in Google Scholar PubMed
(91) Dharap SS, Wang Y, Chandna P, Khandare JJ, Qiu B, Gunaseelan S, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc Natl Acad Sci. 2005;102:12962–7. 10.1073/pnas.0504274102.Suche in Google Scholar PubMed PubMed Central
(92) Schmitt S, Huppertsberg A, Klefenz A, Kaps L, Mailänder V, Schuppan D, et al. Fluorescence correlation spectroscopy monitors the fate of degradable nanocarriers in the blood stream. Biomacromolecules. 2022;23:1065–74. 10.1021/acs.biomac.1c01407.Suche in Google Scholar PubMed PubMed Central
(93) Afrouz M, Ahmadi-Nouraldinvand F, Amani A, Zahedian H, Elias SG, Arabnejad F, et al. Preparation and characterization of magnetic PEG-PEI-PLA-PEI-PEG/Fe3O4-PCL/DNA micelles for gene delivery into MCF-7 cells. J Drug Deliv Sci Technol. 2023;79:104016. 10.1016/j.jddst.2022.104016.Suche in Google Scholar
(94) Wang T, Fleming E, Luo Y. An overview of the biochemistry, synthesis, modification, and evaluation of mucoadhesive polymeric nanoparticles for oral delivery of bioactive compounds. Adv Compos Hybrid Mater. 2022;6:6. 10.1007/s42114-022-00586-0.Suche in Google Scholar
(95) Bao H, Pan Y, Ping Y, Sahoo NG, Wu T, Li L, et al. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small. 2011;7:1569–78. 10.1002/smll.201100191.Suche in Google Scholar PubMed
(96) Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37:106–26. 10.1016/j.progpolymsci.2011.06.003.Suche in Google Scholar PubMed PubMed Central
(97) Karim A, Rehman A, Feng J, Noreen A, Assadpour E, Kharazmi MS, et al. Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds. Adv Colloid Interface Sci. 2022;307:102744. 10.1016/j.cis.2022.102744.Suche in Google Scholar PubMed
(98) van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res. 2007;24:1405–14. 10.1007/s11095-007-9284-6.Suche in Google Scholar PubMed
(99) Marisca OT, Kantner K, Pfeiffer C, Zhang Q, Pelaz B, Leopold N, et al. Comparison of the in vitro uptake and toxicity of collagen- and synthetic polymer-coated gold nanoparticles. Nanomaterials. 2015;5:1418–30. 10.3390/nano5031418.Suche in Google Scholar PubMed PubMed Central
(100) Dash R, Yadav M, Biswal J, Chandra A, Goel VK, Sharma T, et al. Modeling of chitosan modified PLGA atorvastatin-curcumin conjugate (AT-CU) nanoparticles, overcoming the barriers associated with PLGA: An approach for better management of atherosclerosis. Int J Pharmaceutics. 2023;640:123009. 10.1016/j.ijpharm.2023.123009.Suche in Google Scholar PubMed
(101) Asal HA, Shoueir KR, El-Hagrasy MA, Toson EA. Controlled synthesis of in-situ gold nanoparticles onto chitosan functionalized PLGA nanoparticles for oral insulin delivery. Int J Biol Macromol. 2022;209:2188–96. 10.1016/j.ijbiomac.2022.04.200.Suche in Google Scholar PubMed
(102) Kim CH, Lee SG, Kang MJ, Lee S, Choi YW. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J Pharm Investigation. 2017;47:203–27. 10.1007/s40005-017-0329-5.Suche in Google Scholar
(103) Amgoth C, Phan C, Banavoth M, Rompivalasa S, Tang G, Amgoth C, et al. Polymer properties: Functionalization and surface modified nanoparticles. IntechOpen. 2019. 10.5772/intechopen.84424.Suche in Google Scholar
(104) Petrovic B, Gorbounov M, Masoudi Soltani S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021;312:110751. 10.1016/j.micromeso.2020.110751.Suche in Google Scholar
(105) Pujari SP, Scheres L, Marcelis ATM, Zuilhof H. Covalent Surface Modification of Oxide Surfaces. Angew Chem Int Ed. 2014;53:6322–56. 10.1002/anie.201306709.Suche in Google Scholar PubMed
(106) Machado MGC, Pound-Lana G, de Oliveira MA, Lanna EG, Fialho MCP, de Brito ACF, et al. Labeling PLA-PEG nanocarriers with IR780: Physical entrapment versus covalent attachment to polylactide. Drug Deliv Transl Res. 2020;10:1626–43. 10.1007/s13346-020-00812-6.Suche in Google Scholar PubMed
(107) Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, et al. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J Control Rel. 1998;50:79–92. 10.1016/S0168-3659(97)00115-6.Suche in Google Scholar
(108) Kwon GS, Naito M, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Physical entrapment of adriamycin in AB block copolymer micelles. Pharm Res. 1995;12:192–5. 10.1023/A:1016266523505.Suche in Google Scholar
(109) Trofymchuk K, Valanciunaite J, Andreiuk B, Reisch A, Collot M, Klymchenko AS. BODIPY-loaded polymer nanoparticles: chemical structure of cargo defines leakage from nanocarrier in living cells. J Mater Chem B. 2019;7:5199–5210. 10.1039/C8TB02781A.Suche in Google Scholar PubMed
(110) Park W, Na K. Advances in the synthesis and application of nanoparticles for drug delivery. WIREs Nanomed Nanobiotechnol. 2015;7:494–508. 10.1002/wnan.1325.Suche in Google Scholar PubMed
(111) Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:219–33. 10.1002/wnan.1157.Suche in Google Scholar PubMed PubMed Central
(112) Hu B, Wang Y, Xie M, Hu G, Ma F, Zeng X. Polymer nanoparticles composed with gallic acid grafted chitosan and bioactive peptides combined antioxidant, anticancer activities and improved delivery property for labile polyphenols. J Funct Foods. 2015;15:593–603. 10.1016/j.jff.2015.04.009.Suche in Google Scholar
(113) Ulbrich K, Šubr V, Strohalm J, Plocová D, Jelı́nková M, Řı́hová B. Polymeric drugs based on conjugates of synthetic and natural macromolecules: I. Synthesis and physico-chemical characterisation. J Control Rel. 2000;64:63–79. 10.1016/S0168-3659(99)00141-8.Suche in Google Scholar PubMed
(114) Harris JM, Bentley MD, Moreadith RW, Viegas TX, Fang Z, Yoon K, et al. Tuning drug release from polyoxazoline-drug conjugates. Eur Polym J. 2019;120:109241. 10.1016/j.eurpolymj.2019.109241.Suche in Google Scholar
(115) Sharp KA, Honig B. Electrostatic interactions in macromolecules: Theory and applications. Annu Rev Biophys Biophys Chem. 1990;19:301–32. 10.1146/annurev.bb.19.060190.001505.Suche in Google Scholar PubMed
(116) Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: can electrostatic interactions help? Nat Rev Rheumatol. 2017;13:183–93. 10.1038/nrrheum.2016.210.Suche in Google Scholar PubMed
(117) Tarafder S, Nansen K, Bose S. Lovastatin release from polycaprolactone coated β-tricalcium phosphate: Effects of pH, concentration and drug–polymer interactions. Mater Sci Eng: C. 2013;33:3121–8. 10.1016/j.msec.2013.02.049.Suche in Google Scholar PubMed PubMed Central
(118) Zhang L, Zhang P, Zhao Q, Zhang Y, Cao L, Luan Y. Doxorubicin-loaded polypeptide nanorods based on electrostatic interactions for cancer therapy. J Colloid Interface Sci. 2016;464:126–36. 10.1016/j.jcis.2015.11.008.Suche in Google Scholar PubMed
(119) Yu M, Yuan W, Li D, Schwendeman A, Schwendeman SP. Predicting drug release kinetics from nanocarriers inside dialysis bags. J Control Rel. 2019;315:23–30. 10.1016/j.jconrel.2019.09.016.Suche in Google Scholar PubMed
(120) Aydin RST, Pulat M. 5-fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: evaluation of controlled release kinetics. J Nanomaterials. 2012;2012:42. 10.1155/2012/313961.Suche in Google Scholar
(121) Wang X, Liu K, Fu S, Wu X, Xiao L, Yang Y, et al. Silk nanocarrier with tunable size to improve transdermal capacity for hydrophilic and hydrophobic drugs. ACS Appl Bio Mater. 2023;6:74–82. 10.1021/acsabm.2c00666.Suche in Google Scholar PubMed
(122) Son MJ, Kim T, Lee S-W. Facile synthesis of fluorescent mesoporous nanocarriers with pH-sensitive controlled release of naturally derived dieckol. Colloids Surf A: Physicochem Eng Asp. 2023;657:130535. 10.1016/j.colsurfa.2022.130535.Suche in Google Scholar
(123) Sheykhisarem R, Dehghani H. In vitro biocompatibility evaluations of pH-sensitive Bi2MoO6/NH2-GO conjugated polyethylene glycol for release of daunorubicin in cancer therapy. Colloids Surf B: Biointerfaces. 2023;221:113006. 10.1016/j.colsurfb.2022.113006.Suche in Google Scholar PubMed
(124) Jeshvaghani PA, Pourmadadi M, Yazdian F, Rashedi H, Khoshmaram K, Nigjeh MN. Synthesis and characterization of a novel, pH-responsive sustained release nanocarrier using polyethylene glycol, graphene oxide, and natural silk fibroin protein by a green nano emulsification method to enhance cancer treatment. Int J Biol Macromol. 2023;226:1100–15. 10.1016/j.ijbiomac.2022.11.226.Suche in Google Scholar PubMed
(125) Guzman-Villanueva D, Mendiola MR, Nguyen HX, Yambao F, Yu N, Weissig V. Conjugation of triphenylphosphonium cation to hydrophobic moieties to prepare mitochondria-targeting nanocarriers. In: Weissig V, Elbayoumi T, editors. Pharmaceutical nanotechnology: Basic protocols. New York, NY: Springer; 2019. p. 183–9. 10.1007/978-1-4939-9516-5_12.Suche in Google Scholar PubMed
(126) Bonaccorso A, Musumeci T, Serapide MF, Pellitteri R, Uchegbu IF, Puglisi G. Nose to brain delivery in rats: Effect of surface charge of rhodamine B labeled nanocarriers on brain subregion localization. Colloids Surf B: Biointerfaces. 2017;154:297–306. 10.1016/j.colsurfb.2017.03.035.Suche in Google Scholar PubMed
(127) Hoa LTM, Chi NT, Triet NM, Nhan LNT, Chien DM. Preparation of drug nanoparticles by emulsion evaporation method. J Phys: Conf Ser. 2009;187:012047. 10.1088/1742-6596/187/1/012047.Suche in Google Scholar
(128) Nava-Arzaluz MG, Pinon-Segundo E, Ganem-Rondero A, Lechuga-Ballesteros D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat Drug Deliv Formul. 2012;6:209–23. 10.2174/187221112802652633.Suche in Google Scholar PubMed
(129) Liu R, Ma G-H, Wan Y-H, Su Z-G. Influence of process parameters on the size distribution of PLA microcapsules prepared by combining membrane emulsification technique and double emulsion-solvent evaporation method. Colloids Surf B: Biointerfaces. 2005;45:144–53. 10.1016/j.colsurfb.2005.08.004.Suche in Google Scholar PubMed
(130) Zhou H, Modi S, Biswas P. Controlled synthesis of charged lignin nanocarriers by electrospray. Colloids Surf A: Physicochem Eng Asp. 2022;648:129314. 10.1016/j.colsurfa.2022.129314.Suche in Google Scholar
(131) Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17:R89. 10.1088/0957-4484/17/14/R01.Suche in Google Scholar PubMed
(132) Agarwal S, Greiner A, Wendorff JH. Functional materials by electrospinning of polymers. Prog Polym Sci. 2013;38:963–91. 10.1016/j.progpolymsci.2013.02.001.Suche in Google Scholar
(133) Theron SA, Zussman E, Yarin AL. Experimental investigation of the governing parameters in the electrospinning of polymer solutions. Polymer. 2004;45:2017–30. 10.1016/j.polymer.2004.01.024.Suche in Google Scholar
(134) Fang J, Niu H, Lin T, Wang X. Applications of electrospun nanofibers. Chin Sci Bull. 2008;53:2265–86. 10.1007/s11434-008-0319-0.Suche in Google Scholar
(135) Xue J, Xie J, Liu W, Xia Y. Electrospun nanofibers: New concepts, materials, and applications. Acc Chem Res. 2017;50:1976–87. 10.1021/acs.accounts.7b00218.Suche in Google Scholar PubMed PubMed Central
(136) Taemeh MA, Shiravandi A, Korayem MA, Daemi H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr Polym. 2020;228:115419. 10.1016/j.carbpol.2019.115419.Suche in Google Scholar PubMed
(137) Balakrishanan MH, Rajan M. Size-controlled synthesis of biodegradable nanocarriers for targeted and controlled cancer drug delivery using salting out cation. Bull Mater Sci. 2016;39:69–77. 10.1007/s12034-015-0946-4.Suche in Google Scholar
(138) Tok KC, Gumustas M, Sengel-Turk CT, Amasya G, Bayram B, Arioglu-Inan E. Development of salting-out extraction methodology for the determination of piroxicam from polymeric based nanocarriers and biological samples. J Pharm Biomed Anal. 2022;219:114966. 10.1016/j.jpba.2022.114966.Suche in Google Scholar PubMed
(139) Galindo-Rodriguez S, Allémann E, Fessi H, Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21:1428–39. 10.1023/B:PHAM.0000036917.75634.be.Suche in Google Scholar
(140) Mendoza-Munoz N, Quintanar-Guerrero D, Allemann E. The impact of the salting-out technique on the preparation of colloidal particulate systems for pharmaceutical applications. Recent Pat Drug Deliv Formul. 2012;6:236–49. 10.2174/187221112802652688.Suche in Google Scholar PubMed
(141) Bilati U, Allémann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24:67–75. 10.1016/j.ejps.2004.09.011.Suche in Google Scholar PubMed
(142) Almoustafa HA, Alshawsh MA, Chik Z. Technical aspects of preparing PEG-PLGA nanoparticles as carrier for chemotherapeutic agents by nanoprecipitation method. Int J Pharm. 2017;533:275–84. 10.1016/j.ijpharm.2017.09.054.Suche in Google Scholar PubMed
(143) Abu-Thabit N. Nano- and microencapsulation: Techniques and applications. London, England: BoD – Books on Demand; 2021.10.5772/intechopen.88590Suche in Google Scholar
(144) Park J-H, Sudarshan TS. Chemical vapor deposition. United States: ASM International; 2001.Suche in Google Scholar
(145) Odian G. Principles of polymerization. United States: John Wiley & Sons; 2004.10.1002/047147875XSuche in Google Scholar
(146) Alf ME, Asatekin A, Barr MC, Baxamusa SH, Chelawat H, Ozaydin-Ince G, et al. Chemical vapor deposition of conformal, functional, and responsive polymer films. Adv Mater. 2010;22:1993–2027. 10.1002/adma.200902765.Suche in Google Scholar PubMed
(147) Kumar M, Ando Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J Nanosci Nanotechnol. 2010;10:3739–58. 10.1166/jnn.2010.2939.Suche in Google Scholar PubMed
(148) Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes–the route toward applications. Science. 2002;297:787–92. 10.1126/science.1060928.Suche in Google Scholar PubMed
(149) Li X, Yu X, Han Y. Polymer thin films for antireflection coatings. J Mater Chem C. 2013;1:2266–85. 10.1039/C2TC00529H.Suche in Google Scholar
(150) Ikawa M, Yamada T, Matsui H, Minemawari H, Tsutsumi J, Horii Y, et al. Simple push coating of polymer thin-film transistors. Nat Commun. 2012;3:1176. 10.1038/ncomms2190.Suche in Google Scholar PubMed PubMed Central
(151) LaPorte RJ. Hydrophilic polymer coatings for medical devices. New York: Routledge; 2017. 10.1201/9780203751381.Suche in Google Scholar
(152) Colomer J-F, Stephan C, Lefrant S, Van Tendeloo G, Willems I, Kónya Z, et al. Large-scale synthesis of single-wall carbon nanotubes by catalytic chemical vapor deposition (CCVD) method. Chem Phys Lett. 2000;317:83–9. 10.1016/S0009-2614(99)01338-X.Suche in Google Scholar
(153) Nguyen SV. High-density plasma chemical vapor deposition of silicon-based dielectric films for integrated circuits. IBM J Res Dev. 1999;43:109–26. 10.1147/rd.431.0109.Suche in Google Scholar
(154) Pierson HO. Handbook of chemical vapor deposition: Principles, technology and applications. Netherlands: William Andrew; 1999.10.1016/B978-081551432-9.50005-XSuche in Google Scholar
(155) Raaijmakers MJT, Benes NE. Current trends in interfacial polymerization chemistry. Prog Polym Sci. 2016;63:86–142. 10.1016/j.progpolymsci.2016.06.004.Suche in Google Scholar
(156) Zhang F, Fan J, Wang S. Interfacial polymerization: From chemistry to functional materials. Angew Chem Int Ed. 2020;59:21840–56. 10.1002/anie.201916473.Suche in Google Scholar
(157) Kuypers S, Kumar Pramanik S, D’Olieslaeger L, Reekmans G, Peters M, D’Haen J, et al. Interfacial thiol–isocyanate reactions for functional nanocarriers: A facile route towards tunable morphologies and hydrophilic payload encapsulation. Chem Commun. 2015;51:15858–61. 10.1039/C5CC05258K.Suche in Google Scholar
(158) Aboubakar M, Puisieux F, Couvreur P, Deyme M, Vauthier C. Study of the mechanism of insulin encapsulation in poly(isobutylcyanoacrylate) nanocapsules obtained by interfacial polymerization. J Biomed Mater Res. 1999;47:568–76. 10.1002/(SICI)1097-4636(19991215)47:4<568::AID-JBM14>3.0.CO;2-X.Suche in Google Scholar
(159) Lu X, Elimelech M. Fabrication of desalination membranes by interfacial polymerization: history, current efforts, and future directions. Chem Soc Rev. 2021;50:6290–307. 10.1039/D0CS00502A.Suche in Google Scholar
(160) Zhu H, Liang C, Yan W, Overbury SH, Dai S. Preparation of highly active silica-supported Au catalysts for CO oxidation by a solution-based technique. J Phys Chem B. 2006;110:10842–48. 10.1021/jp060637q.Suche in Google Scholar
(161) O’Donnell PB, McGinity JW. Preparation of microspheres by the solvent evaporation technique. Adv Drug Deliv Rev. 1997;28:25–42. 10.1016/S0169-409X(97)00049-5.Suche in Google Scholar
(162) Pavel FM. Microemulsion polymerization. J Dispers Sci Technol. 2004;25:1–16. 10.1081/DIS-120027662.Suche in Google Scholar
(163) Chavan RB, Lodagekar A, Yadav B, Shastri NR. Amorphous solid dispersion of nisoldipine by solvent evaporation technique: Preparation, characterization, in vitro, in vivo evaluation, and scale up feasibility study. Drug Deliv Transl Res. 2020;10:903–18. 10.1007/s13346-020-00775-8.Suche in Google Scholar
(164) Li Z, Fan Q, Yin Y. Colloidal self-assembly approaches to smart nanostructured materials. Chem Rev. 2022;122:4976–5067. 10.1021/acs.chemrev.1c00482.Suche in Google Scholar PubMed
(165) Mueller E, Himbert S, Simpson MJ, Bleuel M, Rheinstadter MC, Hoare T. Cationic, anionic, and amphoteric dual pH/temperature-responsive degradable microgels via self-assembly of functionalized oligomeric precursor polymers. Macromolecules. 2021;54:351–63. 10.1021/acs.macromol.0c02304.Suche in Google Scholar
(166) Zhang W-J, Hong C-Y, Pan C-Y. Polymerization-induced self-assembly of functionalized block copolymer nanoparticles and their application in drug delivery. Macromol Rapid Commun. 2019;40:1800279. 10.1002/marc.201800279.Suche in Google Scholar PubMed
(167) Qi A, Chan P, Ho J, Rajapaksa A, Friend J, Yeo L. Template-free synthesis and encapsulation technique for layer-by-layer polymer nanocarrier fabrication. ACS Nano. 2011;5:9583–91. 10.1021/nn202833n.Suche in Google Scholar PubMed
(168) Sellergren B, Rückert B, Hall AJ. Layer-by-layer grafting of molecularly imprinted polymers via iniferter modified supports. Adv Mater. 2002;14:1204–8. 10.1002/1521-4095(20020903)14:17<1204::AID-ADMA1204>3.0.CO;2-O.Suche in Google Scholar
(169) Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP. Layer-by-layer self-assembled shells for drug delivery. Adv Drug Deliv Rev. 2011;63:762–71. 10.1016/j.addr.2011.03.016.Suche in Google Scholar
(170) Ai H, Jones SA, Lvov YM. Biomedical applications of electrostatic layer-by-layer nano-assembly of polymers, enzymes, and nanoparticles. Cell Biochem Biophys. 2003;39:23–43. 10.1385/CBB:39:1:23.Suche in Google Scholar
(171) Chern CS. Emulsion polymerization mechanisms and kinetics. Prog Polym Sci. 2006;31:443–86. 10.1016/j.progpolymsci.2006.02.001.Suche in Google Scholar
(172) Noh MW, Lee DC. Synthesis and characterization of PS-clay nanocomposite by emulsion polymerization. Polym Bull. 1999;42:619–26. 10.1007/s002890050510.Suche in Google Scholar
(173) Chern C-S. Principles and applications of emulsion polymerization. United States: John Wiley & Sons; 2008.10.1002/9780470377949Suche in Google Scholar
(174) Chrissafis K, Bikiaris D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim Acta. 2011;523:1–24. 10.1016/j.tca.2011.06.010.Suche in Google Scholar
(175) Wu X, Qiu J, Liu P, Sakai E, Lei L. Polystyrene grafted carbon black synthesis via in situ solution radical polymerization in ionic liquid. J Polym Res. 2013;20:167. 10.1007/s10965-013-0167-8.Suche in Google Scholar
(176) Li A, Luehmann HP, Sun G, Samarajeewa S, Zou J, Zhang S, et al. Synthesis and in vivo pharmacokinetic evaluation of degradable shell cross-linked polymer nanoparticles with poly(carboxybetaine) versus poly(ethylene glycol) surface-grafted coatings. ACS Nano. 2012;6:8970–82. 10.1021/nn303030t.Suche in Google Scholar PubMed PubMed Central
(177) AL-Jawad SMH, Taha AA, Al-Halbosiy MMF, AL-Barram LFA. Synthesis and characterization of small-sized gold nanoparticles coated by bovine serum albumin (BSA) for cancer photothermal therapy. Photodiagn Photodyn Ther. 2018;21:201–10. 10.1016/j.pdpdt.2017.12.004.Suche in Google Scholar PubMed
(178) Shahid-ul-Islam, Butola BS, Verma D. Facile synthesis of chitosan-silver nanoparticles onto linen for antibacterial activity and free-radical scavenging textiles. Int J Biol Macromolecules. 2019;133:1134–41. 10.1016/j.ijbiomac.2019.04.186.Suche in Google Scholar PubMed
(179) Tajammul Hussain S, Iqbal M, Mazhar M. Size control synthesis of starch capped-gold nanoparticles. J Nanopart Res. 2009;11:1383–91. 10.1007/s11051-008-9525-6.Suche in Google Scholar
(180) Choi KY, Min KH, Na JH, Choi K, Kim K, Park JH, et al. Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: synthesis, characterization, and in vivo biodistribution. J Mater Chem. 2009;19:4102–7. 10.1039/B900456D.Suche in Google Scholar
(181) Zinchenko A, Miwa Y, Lopatina LI, Sergeyev VG, Murata S. DNA hydrogel as a template for synthesis of ultrasmall gold nanoparticles for catalytic applications. ACS Appl Mater Interfaces. 2014;6:3226–32. 10.1021/am5008886.Suche in Google Scholar PubMed
(182) Li J, Zhu Z, Liu F, Zhu B, Ma Y, Yan J, et al. DNA-mediated morphological control of silver nanoparticles. Small. 2016;12:5449–87. 10.1002/smll.201601338.Suche in Google Scholar PubMed
(183) Taherimehr M, YousefniaPasha H, Tabatabaeekoloor R, Pesaranhajiabbas E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr Rev Food Sci Food Saf. 2021;20:5321–44. 10.1111/1541-4337.12832.Suche in Google Scholar PubMed
(184) Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules. 2011;12:1387–408. 10.1021/bm200083n.Suche in Google Scholar PubMed
(185) Shaker MA, Shaaban MI. Synthesis of silver nanoparticles with antimicrobial and anti-adherence activities against multidrug-resistant isolates from Acinetobacter baumannii. J Taibah Univ Med Sci. 2017;12:291–7. 10.1016/j.jtumed.2017.02.008.Suche in Google Scholar PubMed PubMed Central
(186) Oliva-Arancibia B, Órdenes-Aenishanslins N, Bruna N, Ibarra PS, Zacconi FC, Pérez-Donoso JM, et al. Co-synthesis of medium-chain-length polyhydroxyalkanoates and CdS quantum dots nanoparticles in Pseudomonas putida KT2440. J Biotechnol. 2017;264:29–37. 10.1016/j.jbiotec.2017.10.013.Suche in Google Scholar PubMed
(187) Jose AA, Hazeena SH, Lakshmi NM, Arun KB, Madhavan A, Sirohi R, et al. Bacterial biopolymers: From production to applications in biomedicine. Sustain Chem Pharm. 2022;25:100582. 10.1016/j.scp.2021.100582.Suche in Google Scholar
(188) Mishra A, Tripathy SK, Yun S-I. Fungus mediated synthesis of gold nanoparticles and their conjugation with genomic DNA isolated from Escherichia coli and Staphylococcus aureus. Process Biochem. 2012;47:701–11. 10.1016/j.procbio.2012.01.017.Suche in Google Scholar
(189) Du L, Jiang H, Liu X, Wang E. Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem Commun. 2007;9:1165–70. 10.1016/j.elecom.2007.01.007.Suche in Google Scholar
(190) Alsamhary KI. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J Biol Sci. 2020;27:2185–91. 10.1016/j.sjbs.2020.04.026.Suche in Google Scholar PubMed PubMed Central
(191) Doi Y. Microbial synthesis, physical properties, and biodegradability of polyhydroxyalkanoates. Macromol Symposia. 1995;98:585–99. 10.1002/masy.19950980150.Suche in Google Scholar
(192) Boruah S, Dutta P. Fungus mediated biogenic synthesis and characterization of chitosan nanoparticles and its combine effect with Trichoderma asperellum against Fusarium oxysporum, Sclerotium rolfsii and Rhizoctonia solani. Indian Phytopathology. 2021;74:81–93. 10.1007/s42360-020-00289-w.Suche in Google Scholar
(193) Miyazaki J, Kuriyama Y, Miyamoto A, Tokumoto H, Konishi Y, Nomura T. Adhesion and internalization of functionalized polystyrene latex nanoparticles toward the yeast Saccharomyces cerevisiae. Adv Powder Technol. 2014;25:1394–7. 10.1016/j.apt.2014.06.014.Suche in Google Scholar
(194) Rahman A, Kumar S, Bafana A, Lin J, Dahoumane SA, Jeffryes C. A mechanistic view of the light-induced synthesis of silver nanoparticles using extracellular polymeric substances of Chlamydomonas reinhardtii. Molecules. 2019;24:3506. 10.3390/molecules24193506.Suche in Google Scholar PubMed PubMed Central
(195) Kitching M, Ramani M, Marsili E. Fungal biosynthesis of gold nanoparticles: Mechanism and scale up. Microb Biotechnol. 2015;8:904–17. 10.1111/1751-7915.12151.Suche in Google Scholar PubMed PubMed Central
(196) Natarajan K, Selvaraj S, Murty VR. Microbial production of silver nanoparticles. Dig J Nanomater Biostructures. 2010;5:135–40.Suche in Google Scholar
(197) Dauthal P, Mukhopadhyay M. Noble metal nanoparticles: Plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind Eng Chem Res. 2016;55:9557–77. 10.1021/acs.iecr.6b00861.Suche in Google Scholar
(198) Al-Ali RM, Al-Hilifi SA, Rashed MMA. Fabrication, characterization, and anti‐free radical performance of edible packaging‐chitosan film synthesized from shrimp shell incorporated with ginger essential oil. Food Measure. 2021;15:2951–62. 10.1007/s11694-021-00875-0.Suche in Google Scholar
(199) Masood F, Makhdoom MA, Channa IA, Gilani SJ, Khan A, Hussain R, et al. Development and characterization of chitosan and chondroitin sulfate based hydrogels enriched with garlic extract for potential wound healing/skin regeneration applications. Gels. 2022;8:676. 10.3390/gels8100676.Suche in Google Scholar PubMed PubMed Central
(200) Parveen K, Banse V, Ledwani L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf Proc. 2016;1724:020048. 10.1063/1.4945168.Suche in Google Scholar
(201) Leitner W. Supercritical carbon dioxide as a green reaction medium for catalysis. Acc Chem Res. 2002;35:746–56. 10.1021/ar010070q.Suche in Google Scholar PubMed
(202) Han X, Poliakoff M. Continuous reactions in supercritical carbon dioxide: problems, solutions and possible ways forward. Chem Soc Rev. 2012;41:1428–36. 10.1039/C2CS15314A.Suche in Google Scholar
(203) Urbanczyk L, Ngoundjo F, Alexandre M, Jérôme C, Detrembleur C, Calberg C. Synthesis of polylactide/clay nanocomposites by in situ intercalative polymerization in supercritical carbon dioxide. Eur Polym J. 2009;45:643–8. 10.1016/j.eurpolymj.2008.11.033.Suche in Google Scholar
(204) Hoppe CE, Lazzari M, Pardiñas-Blanco I, López-Quintela MA. One-step synthesis of gold and silver hydrosols using poly(N-vinyl-2-pyrrolidone) as a reducing agent. Langmuir. 2006;22:7027–34. 10.1021/la060885d.Suche in Google Scholar PubMed
(205) Maximiano P, Mendes JP, Mendonça PV, Abreu CMR, Guliashvili T, Serra AC, et al. Cyclopentyl methyl ether: A new green co-solvent for supplemental activator and reducing agent atom transfer radical polymerization. J Polym Sci Part A: Polym Chem. 2015;53:2722–9. 10.1002/pola.27736.Suche in Google Scholar
(206) Zhang Y, Chen D, Guo Z, Wei Z, Zhang X, Xing H. Visible-light-induced controlled radical polymerization of methacrylates mediated by zirconium-porphryinic metal–organic frameworks. N J Chem. 2020;44:5235–42. 10.1039/D0NJ00476F.Suche in Google Scholar
(207) Campisi S, Schiavoni M, Chan-Thaw CE, Villa A. Untangling the role of the capping agent in nanocatalysis: Recent advances and perspectives. Catalysts. 2016;6:185. 10.3390/catal6120185.Suche in Google Scholar
(208) Corbierre MK, Cameron NS, Sutton M, Laaziri K, Lennox RB. Gold nanoparticle/polymer nanocomposites: Dispersion of nanoparticles as a function of capping agent molecular weight and grafting density. Langmuir. 2005;21:6063–72. 10.1021/la047193e.Suche in Google Scholar PubMed
(209) Smith DK, Korgel BA. The importance of the CTAB surfactant on the colloidal seed-mediated synthesis of gold nanorods. Langmuir. 2008;24:644–9. 10.1021/la703625a.Suche in Google Scholar PubMed
(210) Liu H, Owen JS, Alivisatos AP. Mechanistic study of precursor evolution in colloidal group II−VI semiconductor nanocrystal synthesis. J Am Chem Soc. 2007;129:305–12. 10.1021/ja0656696.Suche in Google Scholar PubMed
(211) Javed R, Zia M, Naz S, Aisida SO, Ain NU, Ao Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J Nanobiotechnol. 2020;18:172. 10.1186/s12951-020-00704-4.Suche in Google Scholar PubMed PubMed Central
(212) Liu S, Tian J, Wang L, Li H, Zhang Y, Sun X. Stable aqueous dispersion of graphene nanosheets: Noncovalent functionalization by a polymeric reducing agent and their subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. Macromolecules. 2010;43:10078–83. 10.1021/ma102230m.Suche in Google Scholar
(213) Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26:1025–58. 10.1007/s11095-008-9800-3.Suche in Google Scholar PubMed
(214) Kirillova A, Yeazel TR, Asheghali D, Petersen SR, Dort S, Gall K, et al. Fabrication of biomedical scaffolds using biodegradable polymers. Chem Rev. 2021;121:11238–304. 10.1021/acs.chemrev.0c01200.Suche in Google Scholar PubMed
(215) Rao JP, Geckeler KE. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog Polym Sci. 2011;36:887–913. 10.1016/j.progpolymsci.2011.01.001.Suche in Google Scholar
(216) Krishnamoorthy K, Mahalingam M. Selection of a suitable method for the preparation of polymeric nanoparticles: multi-criteria decision making approach. Adv Pharm Bull. 2015;5:57–67.10.4103/2231-4040.137410Suche in Google Scholar PubMed PubMed Central
(217) Baghbanzadeh M, Rana D, Lan CQ, Matsuura T. Effects of inorganic nano-additives on properties and performance of polymeric membranes in water treatment. Sep Purif Rev. 2016;45:141–67. 10.1080/15422119.2015.1068806.Suche in Google Scholar
(218) Lee Y, Zhou H, Lee T-W. One-dimensional conjugated polymer nanomaterials for flexible and stretchable electronics. J Mater Chem C. 2018;6:3538–50. 10.1039/C7TC05927B.Suche in Google Scholar
(219) Rahaman MSA, Ismail AF, Mustafa A. A review of heat treatment on polyacrylonitrile fiber. Polym Degrad Stab. 2007;92:1421–32. 10.1016/j.polymdegradstab.2007.03.023.Suche in Google Scholar
(220) Wey M-Y, Tseng H-H, Chiang C. Improving the mechanical strength and gas separation performance of CMS membranes by simply sintering treatment of α-Al2O3 support. J Membr Sci. 2014;453:603–13. 10.1016/j.memsci.2013.11.039.Suche in Google Scholar
(221) Campbell D, Pethrick RA, White JR. Polymer characterization: Physical techniques. 2nd edn. London: CRC Press; 2017. 10.1201/9781315274706.Suche in Google Scholar
(222) Murthy NS. Recent developments in polymer characterization using x-ray diffraction. Rigaku J. 2004;21:15–24.Suche in Google Scholar
(223) Jagtap RN, Ambre AH. Overview literature on atomic force microscopy (AFM): Basics and its important applications for polymer characterization. IJEMS. August 2006;13(4):363–84. http://nopr.niscpr.res.in/handle/123456789/7570 (accessed February 27, 2023).Suche in Google Scholar
(224) Alqaheem Y, Alomair AA. Microscopy and spectroscopy techniques for characterization of polymeric membranes. Membranes. 2020;10:33. 10.3390/membranes10020033.Suche in Google Scholar PubMed PubMed Central
(225) Philipsen HJA. Determination of chemical composition distributions in synthetic polymers. J Chromatogr A. 2004;1037:329–50. 10.1016/j.chroma.2003.12.047.Suche in Google Scholar PubMed
(226) Fornaguera C, Solans C. Analytical methods to characterize and purify polymeric nanoparticles. Int J Polym Sci. 2018;2018:e6387826. 10.1155/2018/6387826.Suche in Google Scholar
(227) Fornaguera C, Solans C. Characterization of polymeric nanoparticle dispersions for biomedical applications: Size, surface charge and stability. Pharm Nanotechnol. 2018;6:147–64. 10.2174/2211738506666180706121515.Suche in Google Scholar PubMed
(228) Jayanarayanan K, Rasana N, Mishra RK. Chapter 6 - Dynamic mechanical thermal analysis of polymer nanocomposites. In: Thomas S, Thomas R, Zachariah AK, Mishra RK, editors. Thermal and rheological measurement techniques for nanomaterials characterization. Netherlands: Elsevier; 2017. p. 123–57. 10.1016/B978-0-323-46139-9.00006-2.Suche in Google Scholar
(229) Abdullah OGh, Hanna RR, Salman YAK. Structural, optical, and electrical characterization of chitosan: methylcellulose polymer blends based film. J Mater Sci: Mater Electron. 2017;28:10283–94. 10.1007/s10854-017-6796-7.Suche in Google Scholar
(230) Dankers PYW, van Leeuwen ENM, van Gemert GML, Spiering AJH, Harmsen MC, Brouwer LA, et al. Chemical and biological properties of supramolecular polymer systems based on oligocaprolactones. Biomaterials. 2006;27:5490–501. 10.1016/j.biomaterials.2006.07.011.Suche in Google Scholar PubMed
(231) Fornaguera C, Solans C. Methods for the in vitro characterization of nanomedicines—Biological component interaction. J Pers Med. 2017;7:2. 10.3390/jpm7010002.Suche in Google Scholar PubMed PubMed Central
(232) Jana S, Kumar Sen K, Gandhi A. Alginate based nanocarriers for drug delivery applications. Curr Pharm Des. 2016;22:3399–410.10.2174/1381612822666160510125718Suche in Google Scholar PubMed
(233) He Z, Bao K, Zhang J, Ju D, Luo M, Liu L, et al. Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment. e-Polymers. 2022;22(1):342–56.10.1515/epoly-2022-0020Suche in Google Scholar
(234) Wang X-Q, Zhang Q. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur J Pharm Biopharm. 2012;82:219–29. 10.1016/j.ejpb.2012.07.014.Suche in Google Scholar PubMed
(235) Chen W, Zhou S, Ge L, Wu W, Jiang X. Translatable high drug loading drug delivery systems based on biocompatible polymer nanocarriers. Biomacromolecules. 2018;19:1732–45. 10.1021/acs.biomac.8b00218.Suche in Google Scholar PubMed
(236) Parekh G, Shi Y, Zheng J, Zhang X, Leporatti S. Nano-carriers for targeted delivery and biomedical imaging enhancement. Ther Deliv. 2018;9:451–68. 10.4155/tde-2018-0013.Suche in Google Scholar PubMed
(237) Zheng J, Liu J, Dunne M, Jaffray DA, Allen C. In vivo performance of a liposomal vascular contrast agent for CT and MR-based image guidance applications. Pharm Res. 2007;24:1193–201. 10.1007/s11095-006-9220-1.Suche in Google Scholar PubMed
(238) Lesniak WG, Chu C, Jablonska A, Behnam Azad B, Zwaenepoel O, Zawadzki M, et al. PET imaging of distinct brain uptake of a nanobody and similarly-sized PAMAM dendrimers after intra-arterial administration. Eur J Nucl Med Mol Imaging. 2019;46:1940–51. 10.1007/s00259-019-04347-y.Suche in Google Scholar PubMed PubMed Central
(239) Wang L, Qian Y. Modification of a SOCT-ISC type triphenylamine-BODIPY photosensitizer by a multipolar dendrimer design for photodynamic therapy and two-photon fluorescence imaging. Biomater Sci. 2023;11:1459–69. 10.1039/D2BM01838A.Suche in Google Scholar PubMed
(240) Kemal E, Fedatto Abelha T, Urbano L, Peters R, Owen DM, Howes P, et al. Bright, near infrared emitting PLGA–PEG dye-doped CN-PPV nanoparticles for imaging applications. RSC Adv. 2017;7:15255–64. 10.1039/C6RA25004A.Suche in Google Scholar
(241) Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S. Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. J Am Chem Soc. 2006;128:7383–9. 10.1021/ja061529k.Suche in Google Scholar PubMed
(242) Arya G, Mankamna Kumari R, Sharma N, Gupta N, Chandra R, Nimesh S. Chapter 18 - Polymeric nanocarriers for site-specific gene therapy. In: Grumezescu AM, editor. Drug targeting and stimuli sensitive drug delivery systems. Elsevier, Netherlands: William Andrew Publishing; 2018. p. 689–714. 10.1016/B978-0-12-813689-8.00018-5.Suche in Google Scholar
(243) Liang J, Guo S, Bai M, Huang M, Qu Y, Zhao Y, et al. Stimulus-responsive hybrid nanoparticles based on multiple lipids for the co-delivery of doxorubicin and Sphk2-siRNA and breast cancer therapy. Food Chem Toxicol. 2023;171:113532. 10.1016/j.fct.2022.113532.Suche in Google Scholar PubMed
(244) Li J, Liang H, Liu J, Wang Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 2018;546:215–25. 10.1016/j.ijpharm.2018.05.045.Suche in Google Scholar PubMed
(245) Xiu K, Saunders L, Wen L, Ruan J, Dong R, Song J, et al. Delivery of CRISPR/Cas9 plasmid DNA by hyperbranched polymeric nanoparticles enables efficient gene editing. Cells. 2023;12:156. 10.3390/cells12010156.Suche in Google Scholar PubMed PubMed Central
(246) Pan B, Pan B, Zhang W, Lv L, Zhang Q, Zheng S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J. 2009;151:19–29. 10.1016/j.cej.2009.02.036.Suche in Google Scholar
(247) Zhang S, Zhang Y, Bi G, Liu J, Wang Z, Xu Q, et al. Mussel-inspired polydopamine biopolymer decorated with magnetic nanoparticles for multiple pollutants removal. J Hazard Mater. 2014;270:27–34. 10.1016/j.jhazmat.2014.01.039.Suche in Google Scholar PubMed
(248) Ge F, Ye H, Li M-M, Zhao B-X. Efficient removal of cationic dyes from aqueous solution by polymer-modified magnetic nanoparticles. Chem Eng J 198–. 2012;199:11–7. 10.1016/j.cej.2012.05.074.Suche in Google Scholar
(249) Alzahrani FM, Alsaiari NS, Katubi KM, Amari A, Ben Rebah F, Tahoon MA. Synthesis of polymer-based magnetic nanocomposite for multi-pollutants removal from water. Polymers. 2021;13:1742. 10.3390/polym13111742.Suche in Google Scholar PubMed PubMed Central
(250) Bhran A, Shoaib A, Elsadeq D, El-gendi A, Abdallah H. Preparation of PVC/PVP composite polymer membranes via phase inversion process for water treatment purposes. Chin J Chem Eng. 2018;26:715–22. 10.1016/j.cjche.2017.09.003.Suche in Google Scholar
(251) Ng I-S, Ooi CW, Liu B-L, Peng C-T, Chiu C-Y, Chang Y-K. Antibacterial efficacy of chitosan-and poly (hexamethylene biguanide)-immobilized nanofiber membrane. Int J Biol Macromol. 2020;154:844–54.10.1016/j.ijbiomac.2020.03.127Suche in Google Scholar PubMed
(252) Rembe J-D, Fromm-Dornieden C, Schäfer N, Böhm JK, Stuermer EK. Comparing two polymeric biguanides: Chemical distinction, antiseptic efficacy and cytotoxicity of polyaminopropyl biguanide and polyhexamethylene biguanide. J Med Microbiology. 2016;65:867–76.10.1099/jmm.0.000294Suche in Google Scholar PubMed
(253) Liu Z, Qin L, Liu S, Zhang J, Wu J, Liang X. Superhydrophobic and highly moisture-resistant PVA@EC composite membrane for air purification. RSC Adv. 2022;12:34921–30. 10.1039/D2RA05798K.Suche in Google Scholar PubMed PubMed Central
(254) Cui J, Lu T, Li F, Wang Y, Lei J, Ma W, et al. Flexible and transparent composite nanofibre membrane that was fabricated via a “green” electrospinning method for efficient particulate matter 2.5 capture. J Colloid Interface Sci. 2021;582:506–14. 10.1016/j.jcis.2020.08.075.Suche in Google Scholar PubMed
(255) Thi HPN, Thi KTP, Tran NT, Mai TH, Naqvi SR, Chung WJ, et al. Graphene-integrated nonwoven polypropylene fabric for simultaneous filtering of particulate matter and volatile organic compounds. Waste Biomass Valor. 2023;14:479–86. 10.1007/s12649-022-01841-7.Suche in Google Scholar
(256) Majidi R, Parhizkar J, Karamian E. Photocatalytic removal of NOx gas from air by TiO2/polymer composite nanofibers. Nanochem Res. 2018;3:212–8. 10.22036/ncr.2018.02.011.Suche in Google Scholar
(257) Rao H, Zhang Z, Tian Y. Preparation and high oxygen-enriching properties of cross-linking polydimethylsiloxane/SiO2 nanocomposite membranes for air purification. AIChE J. 2013;59:650–5. 10.1002/aic.13860.Suche in Google Scholar
(258) Gao X, Li Z-K, Xue J, Qian Y, Zhang L-Z, Caro J, et al. Titanium carbide Ti3C2Tx (MXene) enhanced PAN nanofiber membrane for air purification. J Membr Sci. 2019;586:162–9. 10.1016/j.memsci.2019.05.058.Suche in Google Scholar
(259) Fu Y, Jiang J, Zhang Q, Zhan X, Chen F. Robust liquid-repellent coatings based on polymer nanoparticles with excellent self-cleaning and antibacterial performances. J Mater Chem A. 2017;5:275–84. 10.1039/C6TA06481G.Suche in Google Scholar
(260) Jung JH, Hwang GB, Lee JE, Bae GN. Preparation of airborne Ag/CNT hybrid nanoparticles using an aerosol process and their application to antimicrobial air filtration. Langmuir. 2011;27:10256–64. 10.1021/la201851r.Suche in Google Scholar PubMed
(261) Lv D, Wang R, Tang G, Mou Z, Lei J, Han J, et al. Ecofriendly electrospun membranes loaded with visible-light-responding nanoparticles for multifunctional usages: Highly efficient air filtration, dye scavenging, and bactericidal activity. ACS Appl Mater Interfaces. 2019;11:12880–89. 10.1021/acsami.9b01508.Suche in Google Scholar PubMed
(262) Grodniski DC, Benatto L, Gonçalves JP, de Oliveira CC, Pacheco KRM, Adad LB, et al. High photothermal conversion efficiency for semiconducting polymer/fullerene nanoparticles and its correlation with photoluminescence quenching. Mater Adv. 2023;4:486–503. 10.1039/D2MA00912A.Suche in Google Scholar
(263) Gallah H, Mighri F, Ajji A, Bandyopadhyay J. Flexible PET/(PET-TiO2) core/shell nanofibrous mats as potential photoanode layer for dye-sensitized solar cells, DSSCs. Mater Chem Phys. 2023;305:127911. 10.1016/j.matchemphys.2023.127911.Suche in Google Scholar
(264) Mohsenzadegan N, Nouri E, Mohammadi MR. Efficient quasi-solid-state dye-sensitized solar cells aided by mesoporous TiO2 beads and a non-volatile gel polymer electrolyte. CrystEngComm. 2023;25:3210–21. 10.1039/D3CE00126A.Suche in Google Scholar
(265) Bessette A, Hanan GS. Design, synthesis and photophysical studies of dipyrromethene-based materials: insights into their applications in organic photovoltaic devices. Chem Soc Rev. 2014;43:3342–3405. 10.1039/C3CS60411J.Suche in Google Scholar
(266) Hirano T, Tsuboi T, Cao KLA, Tanabe E, Ogi T. High specific surface area niobium-doped tin oxide nanoparticles produced in spray flames as catalyst supports in polymer electrolyte fuel cells. J Nanopart Res. 2022;25:1. 10.1007/s11051-022-05649-3.Suche in Google Scholar
(267) Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21:1166–70. 10.1038/nbt875.Suche in Google Scholar PubMed
(268) Zhang Y, Martinez MR, Sun H, Sun M, Yin R, Yan J, et al. Charge, aspect ratio, and plant species affect uptake efficiency and translocation of polymeric agrochemical nanocarriers. Env Sci Technol. 2023;57:8269–79. 10.1021/acs.est.3c01154.Suche in Google Scholar PubMed PubMed Central
(269) Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, et al. 3D printed bionic ears. Nano Lett. 2013;13:2634–9. 10.1021/nl4007744.Suche in Google Scholar PubMed PubMed Central
(270) Kim J-Y, Kim J-H, Ahn G, An S-H, Ryu R-H, Kim J-S, et al. In vitro study of three-dimensional printed metal-polymer hybrid scaffold incorporated dual antibiotics for treatment of periprosthetic joint infection. Mater Lett. 2018;212:263–6. 10.1016/j.matlet.2017.10.039.Suche in Google Scholar
(271) Wang Y, Lei M, Wei Q, Wang Y, Zhang J, Guo Y, et al. 3D printing biocompatible l-Arg/GNPs/PLA nanocomposites with enhanced mechanical property and thermal stability. J Mater Sci. 2020;55:5064–78. 10.1007/s10853-020-04353-8.Suche in Google Scholar
(272) Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: The future of nanomedicine. WIREs Nanomed Nanobiotechnol. 2016;8:271–99. 10.1002/wnan.1364.Suche in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Artikel in diesem Heft
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites

