Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
-
Karma Albalawi
, Mamdoh S. Moawadh
Abstract
Surgery, chemotherapy, and radiation therapy are all forms of cancer treatment, as well as more recent methods including interventional radiology and immunotherapy. In this study, we synthesize a novel chitosan (CH) nanocomplex (NC)-based polysaccharide Krestin (PSK) for drug delivery. This technique was used to develop PSK@CH@NC. According to the study, PSK@CH@NC had a particle size of around 500 nm, slight polydispersity as observed under a scanning electron microscope, and a strong positive surface charge of 18 mV. Investigation into the in vitro growth inhibition of the MCF-7 cell line after treatment with CH, PSK, and PSK@CH@NC was followed by morphological changes. Compared to other treatment groups, PSK@CH@NC therapy dramatically reduced the fraction of apoptotic cells, cancer cell survival, and proliferation. Fluorescence analysis was used to examine how PSK@CH@NC affected the distribution of cell cycle phases. This study also shows that a promising foundation for creating cancer nanomedicine can be established by employing new polysaccharides.
1 Introduction
Breast cancer growth affected 2.3 million individuals globally in 2020, with a mortality rate of 6,85,000 individuals (1). By the end of 2023, 8.3 million more women will have breast cancer than in the previous 5 years, making it the most prevalent cancer worldwide (2). The gender of the woman is the strongest risk factor for breast cancer. Men make up about 0.5–1% of the population, and they have an average rate of breast cancer. The same standards apply to men’s breast cancer treatment as to women’s (3). Certain genetic high penetrance quality transformations, the most well-known of which are modifications in the BRCA1, BRCA2, and PALB-2, will significantly enhance the risk of breast cancer (4). Breast cancer therapy can be quite effective, especially if the disease is detected early. Breast cancer is generally treated with a combination of various treatments (5), including radiation treatment (6), drug therapy involving hormonal treatment, chemotherapy (7), as well as with designed chemo complexes (8). Such a treatment can stop cancer from spreading and growing but has negative effects on the system over time.
A protein-bound polysaccharide or “proteoglycan 3,” called SK, also known as polysaccharide K or Krestin, has been used in Japan for more than 30 years as an adjuvant immunotherapy for many cancer types, including lung cancer. The effects of polysaccharide Krestin (PSK) when combined with conventional surgery, chemotherapy, and radiation therapy have been researched in Japan since it was first isolated from the Coriolus versicolor (also known as Trametes versicolor or Yun Zhi) mushroom in 1971. Extensive survival durations have been linked to the use of PSK in controlled human studies for several cancer types, including breast, colorectal, and other gastrointestinal cancers; however, the impact of PSK, particularly on breast cancer, has not been well examined (9).
As a result, it is critical to ensure early cancer detection and treatment in order to prevent disease spread and mortality. Nanotechnology is one of the most extensively employed strategies in cancer research today (10). A few fascinating applications of nanotechnology involve disease diagnosis and treatment including medication administration, gene therapy, identification and diagnostics, drug transportation, biomarker planning, therapeutic targets, and sub-atomic imaging (11–13). Nanotechnology-based molecular diagnostics, such as the discovery of biomarkers, can reliably and swiftly diagnose malignancies (14). Treatments based on nanotechnology, such as the creation of nanoscale medication delivery, can enable accurate malignant tissue targeting while minimizing adverse effects (15). Following that, other studies have looked at various types of nanomaterials, such as lipid nanoparticles, polymeric, microspheres, and antibodies, with the hope of achieving a balance between improving efficacies and lowering toxicity by combining these nanomaterials in disease treatment configurations (16). One of the most promising methods for providing target specificity for intracellular imaging, diagnosis, and therapy is the coupling of nanocarriers with aptamers (17).
One of the most often used mushroom extracts is PSK (18,19). In 1977, it was recognized in Japan as a preparation medicine for the handling of cancer. By 1987, PSK had accounted for more than a quarter of Japan’s entire anticancer drug expenditure (20). It is a highly pure CM-101Trametes (Coriolus) versicolor extract that is commercially accessible. Beta-glucan molecules, which range in size from 94 to 100 kDa, are the most important component (21). PSK has been shown in clinical trials to increase the chances of surviving malignancies of colorectal, lung, and stomach cancer (22). According to several studies, PSK appears to be an immunological modulator, enhancing IL-8 gene expression in mononuclear cells in the peripheral blood after oral treatment, stimulating T-cell proliferation, and increasing CD4+ T cell activation in lymphoid tissue related to the gut. Polymer nanoparticles have the ability to pack large amounts of anticancer medicines or other compounds and can cause selective toxicity. Nanogels or hydrogels, poly(-caprolactone), poly(lactic-co-glycolic acid), chitosan (CH) dendrimers, etc., are some examples of biodegradable polymeric drug delivery methods (23,24). Because of its significant mucoadhesion qualities, superior biocompatibility, biodegradability, and potential to create membranes, chitosan, a linear polysaccharide, was chosen as a natural polymer for the present study as a carrier (25,26).
As a result of these characteristics, the focus of the present study is mostly on the possible cytotoxic and antiproliferative effect of the Krestin conjugate chitosan nanocomplex (NC) PSK@CH@NCMCF-7 human breast cancer cells in vitro. Furthermore, our findings suggest that the Kerstin conjugate chitosan NC could be a source of natural anticancer chemicals in the fight against human cancer.
2 Materials and methods
2.1 Krestin polysaccharide
The test compound Krestin polysaccharide was purchased from Sigma (Merck CAS Number 122965-43-9) and stored in a cool and dry place.
2.2 Preparation of PSK@CH@NC
The PSK@CH@NC was prepared following the protocol given in the study of Zhang et al. (27). The Krestin polysaccharide was first dissolved in a freshly made 0.1% w/v chitosan solution in 1% acetic acid before being suspended in 15 mL of double distilled water. Until a reaction suspension formed, the mixture was repeatedly stirred. Aqueous sodium TPP solution was used to create the nanoparticles after the reaction solution was ultrasonically processed to diminish the nanoparticles. Next, the chitosan and Krestin were added to the polymer solution. The coating procedure was completed using the microvolume flow titration technique. After the resulting product was dehydrated using the spray pyrolysis process, the NC powder formed was collected. With the developed PSK@CH@NC, various characterization techniques were applied.
2.3 Characterization of PSK@CH@NC
The analysis was done using dynamic light scattering (DLS) distribution, and the average size of the formed PSK@CH@NC was determined. The Zeta potential was used to test the PSK@CH@NC that had been developed (Malvern Zeta Analyser). Fourier transform-infrared (FT-IR) analysis was used to analyse the formed PSK@CH@NC and its functional groups that were surface-bound. Using an FT-IR machine, the prepared PSK@CH@NC sample was evaluated (IR Prestige-21; Shimadzu, Kyoto, Japan). After that, the polymer NC was blended with KBr to form pellets under a vacuum pressure of 60 kN for 15 min before being evaluated at 4,000–500 cm–1. A scanning electron microscope (SEM; Hitachi S-4800) was used to examine the size, shape, and content of the manufactured PSK@CH@NC.
2.4 Animal cell culture
The human breast cancer MCF-7 cells were procured from NCCS and cultured on Dulbecco’s modified Eagle’s medium enriched with 10% foetal bovine serum and penicillin/streptomycin concentrations (100 IU per 100 g) adjusted to 1 mL·L−1 and kept at 37°C in a 5% CO2 incubator. The cells were trypsinized after they had reached 80% confluency (28).
2.5 Evaluation of cytotoxicity
To determine the inhibitory concentration (IC50), a cell cytotoxicity assay (MTT, Hi-Media) was used. MCF-7 cells were allowed to grow for 48 h in a 96-well plate (1 × 104 cells per well) to achieve 80% cell confluency. The culture medium was changed with chitosan, PSK, and PSK@CH@NC containing fresh medium at varied concentrations, and the cells were incubated for 24 h. Then, the culture medium and the supernatant were removed, followed by the addition of 100 µL of the MTT solution mixed with the wells and incubated at 37°C for 4 h. After that, 100 µL of DMSO was added to each well, and they were incubated for 15 min to disperse the formazan crystals. A Biotek multimode plate reader (USA) was used to calculate the optical density (OD) of MCF-7 cells at 570 nm:
2.6 Morphological study
The MCF-7 breast cancer cells (1 × 105) were treated with various dosages of the desired IC50 concentrations of the compounds PSK and PSK@CH@NC with chitosan at 100 µg·mL−1 for 24 h before being fixed in acetic acid/ethanol (1:3; v/v) solution (29). For the morphologic examination, the coverslips were carefully placed on glass slides. Three monolayers were micrographic in each experimental group. A bright field inverted light microscope (Nikon, Japan) at 40× magnifications was used to investigate the morphological alterations of the cells.
2.7 Apoptotic cell death analysis
As a working solution, 1 mL of the dye mixture from the 100× stock solution −15 mg of acridine orange (AO) and 50 mg of ethidium bromide (EtBr) in 1 mL of 95% ethanol and 49 mL of distilled water was diluted in PBS for about 10 times. The MCF-7 cells were treated with the desired IC50 concentration of c PSK and PSK@CH@NC along with chitosan at 100 µg·mL−1 and were collected and rinsed in PBS. After that, 10 µL of AO/EtBr were stained. The cells were rinsed twice with PBS after 2 min. A fluorescence microscope uses light to produce images at a magnification of 20×. The cells were seeded on glass microscope slides in a 24-well plate and cultured for 24 h. After permeabilizing the frozen cells at room temperature for 10 min with 0.2% Triton X-100 (50 µL), the cells were treated for 3 min with 10 µL of DAPI. Glass coverslips were used to ensure that the stain spread evenly. A fluorescence microscope was used to examine the cells.
2.8 Measurement of mitochondrial transmembrane potential
Rhodamine 123 was used to respond to variations in the membrane potentials of mitochondria. The MCF-7 breast cancer cells were then treated with the desired IC50 concentration of PSK and PSK@CH@NC, respectively, with chitosan at 100 µg·mL−1 before being stained with 5 µg·mL−1 Rhodamine 123 staining solutions and analysed for 15 min at 37°C under a fluorescence microscope over fluorescent levels. The intensity of fluorescence of Rhodamine 123 in cells was measured using fluorescence microscopy.
2.9 Cell cycle analysis
Regarding the cell cycle investigation, MCF-7 (1 × 105) breast cancer cells were kept in a six-well plate for 48 h with the desired IC50 concentration of PSK, and PSK@CH@NC, respectively, and chitosan at 100 µg·mL−1. The cells were then extracted and treated at room temperature for 30 min in 500 mL of PBS solution containing 25 µg of propidium iodide and 50 µg of RNase. Flow cytometry was used to analyse the samples by BD FACS Verse USA.
2.10 Detection of apoptosis by Annexin-V/FITC-Flow cytometer
In a six-well culture plate, MCF-7 cells (1 × 105) were cultured. PSK and PSK@CH@NC were included in the medium at their IC50 concentrations and chitosan at 100 µg·mL−1. After about 24 h of culture, the cells were taken out using trypsin, washed with PBS, 70% ethanol fixation, and 1 h storage at −20°C. The nuclear DNA of the cells was stained with Annexin V-FITC before being incubated for 30 min at 37°C. A BD FACS Verse flow cytometer was used in duplicate. The fluorescence intensity was determined and assessed using Cell Quest and Modifit.
2.11 Data analysis
All the experiments were done in triplicate. The SPSS statistical program version 16.0 edition was used to conduct all analyses. The IC50 for cell apoptosis and maximal inhibition results were calculated using probability analysis.
3 Results and discussion
3.1 Characterization of formulated PSK@CH@NC
PSK@CH@NC production revealed the patterns of distribution and size limitation of synthesized PSK@CH@NC, which were investigated using DLS analysis. The results of the DLS investigation revealed unambiguous peaks, confirming the size and distribution of CH@NC ranging from 200 nm (Figure 1a) to 500 nm (Figure 1b) after PSK conjugation. Furthermore, the zeta potentials of CH@NC are + 31 mV (Figure 2a), which is conjugated with PSK, whose zeta potential is less positive +18 mV (Figure 2b), indicating that PSK was successfully linked to the formation of NC. The FT-IR was used to assess the presence of functional groups in the formulated PSK@CH@NC, and the results are shown in Figure 3. The FT-IR spectral analysis of the formed PSK@CH@NC revealed several different peaks at distinct frequencies. The spectra of chitosan display the typical chitosan absorptions at 952 cm−1 (N–H stretch) and 1,625 cm−1 (C═O stretch). The stretching vibrations of N–H and O–H are attributed to the peaks at 3,376 cm−1, whereas hydroxyl groups are attributed to the peaks at 1,396 cm−1. Figure 3b displays the FTIR spectra of Krestin with the peaks at 904 cm−1 (the glycosidic group’s C–O–C symmetrical stretching band), and 1,512 (asymmetric stretching of S═O) and 904 cm−1 (sulphate ester), respectively. However, Figure 3c indicates that there were peaks for Krestin and chitosan, demonstrating their interaction and binding, respectively. Our investigation’s results are in line with those of another study (30,31), which discovered that biological elements influence the chitosan conjugation process through their functional groups. In order to identify the PSK@CH@NC NC, the size and appearance of the synthesized PSK@CH@NC were examined using SEM analysis. Figure 4a and b shows SEM microphotographs of the constructed PSK@CH@NC, which showed tetragonal and agglomerated morphological appearances. The latter may be attributable to the SEM analysis sample preparation methodology’s control of physical PSK@CH@NC dryness (32).

DLS analysis of the particle size distribution of synthesized NC: (a) chitosan alone and (b) Krestin –chitosan nanoparticles.
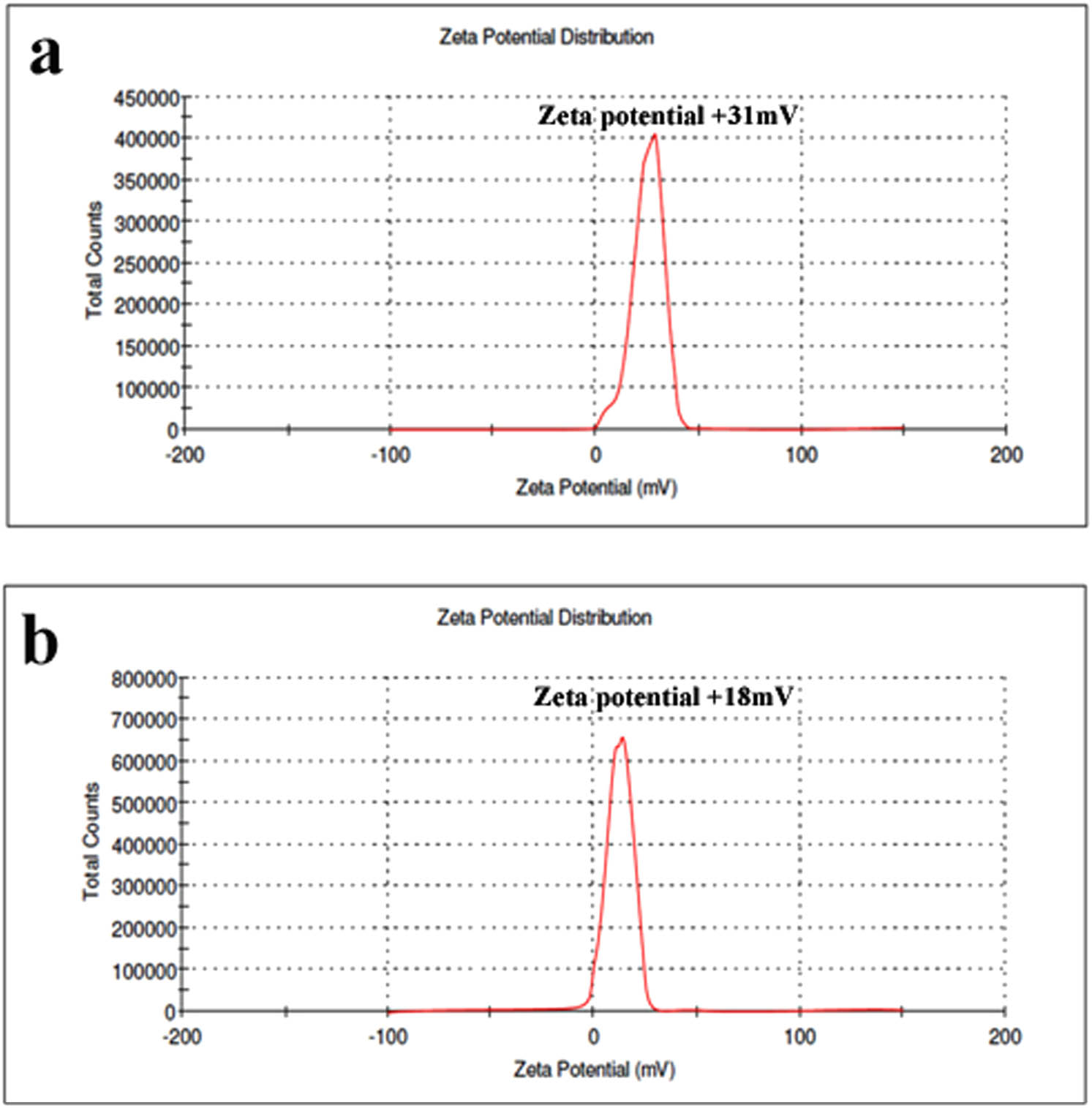
Zeta potential analysis of the synthesized NC: (a) chitosan alone and (b) Krestin–chitosan nanoparticles.

FTIR spectra of (a) Krestin–chitosan nanoparticles, (b) Krestin, and (c) chitosan alone.

SEM micrograph of the synthesised NC: (a) Chitosan NPs and (b) PSK@CH@NC.
3.2 Cytotoxic assay
Chitosan, PSK, and PSK@CH@NC were tested for cytotoxicity on MCF-7 human breast cancer cells using the MTT bioassay for 24 h, and the IC50 values are tabulated in Table 1. As shown in Figure 5, PSK@CH@NC prevents the growth of MCF-7 human breast cancer cells at an IC50 concentration of 19.5 µg·mL−1 significantly when compared to PSK and chitosan, demonstrating the efficacy of PSK@CH@NC towards the treatment, as evidenced by the low pH response level in normal cells (33). From the cytotoxic study, we assumed that the PSK@CH@NC molecules demonstrated considerable inhibitory efficacy against breast cell proliferation (34).
Cytotoxic activity of compounds (µg·mL−1)
| Compound name | IC50 |
|---|---|
| PSK@CH@NC | 19.5 ± 0.6 |
| PSK | 23.8 ± 0.2 |
| Chitosan | Insignificant toxicity up to 200 µg·mL−1 |

MTT cytotoxicity analysis: in vitro cell viability of breast cancer cells after treatment with Krestin–Chitosan nanoparticles.
3.3 Morphological analysis
The morphological alterations in MCF-7 cells after 24 h of treatment with an IC50 concentration of PSK@CH@NC are shown in Figure 6. The MCF-7 cell morphology is affected by the PSK and PSK@CH@NC treatment. The cytotoxicity of PSK@CH@NC was enhanced significantly when compared to other treatment groups. This NC caused cell shrinkage, cell adjustment, and a reduction in the number of viable cells. On the other hand, the untreated control cells did not demonstrate any effect. The chitosan did not exhibit significant change throughout the treatment.

Morphometric analysis of breast cancer cells: (a) control, (b) chitosan 100 µg·mL−1, (c) IC50 of PSK, and (d) IC50 of PSK@CH@NC.
3.4 AO/EtBr staining investigation
Fluorescence microscopy was used to investigate the effect of PSK@CH@NC apoptogenic activity on cancer cells. Because most of the PSK@CH@NC was diffused at all these low concentrations, no aggregation of PSK@CH@NC was seen during the experiment. Fluorescence microscopy stained with AO/EtBr was used to analyse the induction of apoptosis in MCF-7 cells after treatment with the IC50 concentration of PSK@CH@NC. Live cells fluoresced green, while dead cells fluoresced acridine orange, according to these findings (35). Figure 7 shows that untreated control cells had a large number of viable cells. Figure 7b–d shows orangish bodies with nuclear shrinkage, membrane blebbing, and nucleus disintegration in MCF-7 cells treated with PSK and PSK@CH@NC. The change is significant in PSK@CH@NC when compared to other groups.

AO/EtBr staining of the Krestin–chitosan NC in breast cancer cells: (a) control, (b) chitosan 100 µg·mL−1, (c) IC50 of PSK, and (d) IC50 of PSK@CH@NC.
3.5 Fluorescence microscopic analysis of DAPI staining
Further, we also used the DAPI staining approach to analyse PSK@CH@NC. Figure 8 shows a fluorescence microscopic image of DAPI-stained cells after 24 h in the proximity and absence of PSK@CH@NC. Figure 8a shows no significant changes in the cells, whereas Figure 8b–d exhibits bright signals because of the treatment with PSK and PSK@CH@NC treatment in MCF-7 cells, showing the structure of condensed chromatins and nuclear fragmentations (36). A brighter blue stain is produced as DAPI enters the cell and damages the apoptotic cell membrane. The visual identification of apoptotic cells labelled with DAPI is also aided by the different nuclear morphology of apoptotic cells, such as chromosome condensation and disintegration. DNA damage is the hallmark of the apoptotic process. As shown in Figure 8, it is assumed that the PSK@CH@NC could induce apoptosis. Thus, PSK@CH@NC could be used as an effective cancer therapy agent, according to the results of the fluorescence microscopic study.

Krestin–chitosan NC staining with DAPI in breast cancer cells: (a) control, (b) chitosan 100 µg·mL−1, (c) IC50 of PSK, and (d) IC50 of PSK@CH@NC.
3.6 Mitochondrial membrane potential analysis
The depolarization of the mitochondrial membrane during cell death is substantial and distinct since it leads to increased external membrane permeability and pore formation (37). PSK@CH@NC was used to treat breast cancer cells at its IC50 concentration. After 24 h of treatment, the PSK@CH@NC reduces cell count in cells with complete mitochondrial membrane orientation while increasing cell count in cells with inadequate mitochondrial membrane polarity, and vice versa. Fluorescence intensity was exposed to calculate the decrease in mitochondrial integrity. When compared to the untreated control cells, the fixed IC50 of PSK@CH@NC significantly increases mitochondrial disintegration (Figure 9).

Effect of Krestin–chitosan NC on the mitochondrial membrane potential in breast cancer cells. Cells treated with chitosan, PSK, and PSK@CH@NC for 24 h and were incubated with Rhodamine 123 and measured using a fluorescence microscope: (a) control, (b) chitosan 100 µg·mL−1, (c) IC50 of PSK, and (d) IC50 of PSK@CH@NC.
3.7 Cell cycle analysis
Flow cytometry was used to assess the stage of a cell’s life cycle at which the cells were arrested (38). In the G0–G1, S, and G2M phases, the cells in the control group (untreated group) were evenly distributed. PSK and PSK@CH@NC were administered to breast cancer cells at their IC50 doses. Thus, on treatment, the cells were arrested in the G2M phase on PSK treatment but for PSK@CH@NC the cells were arrested in the S phase. As demonstrated in Figure 10, PSK@CH@NC had stopped breast cancer cells from proliferation upon treatment.

Cell cycle analysis of breast cancer cells after treatment with (a) control, (b) chitosan 100 µg·mL−1, (c) IC50 of PSK, and (d) IC50 of PSK@CH@NC.
3.8 Apoptosis via flow cytometry
Flow cytometry and Annexin V/FITC were used to determine the fraction of cells that are dead (39). Throughout the late apoptotic stage, the percentage of surviving cells in the control group remained constant; on the other hand, the fraction of dead cells increased dramatically in the PSK- and PSK@CH@NC-treated cells. Figure 11 demonstrates the effect of PSK@CH@NC with the proportion of viable cells reduced significantly when compared to other groups.

Analysis of breast cancer cells using dual parameter flow cytometry. Necrotic cells are represented by Quadrant 4, late apoptotic cells are represented by Quadrant 3, pro-apoptotic cells are represented by Quadrant 2, and viable cells are represented by Quadrant 1. (a) Control, (b) chitosan 100 µg·mL−1, (c) IC50 of PSK, and (d) IC50 of PSK@CH@NC.
4 Conclusions
In combination, our findings demonstrated that the proposed PSK@CH@NC therapy had effective antitumor activity towards in vitro human breast cancer MCF-7 cells. In MCF-7 cells, the PSK@CH@NC treatment promoted cell shrinkage, altered the cells, decreased the number of live cells, and induced apoptosis. PSK@CH@NC also revealed that cells were inhibited during the S phase. As a result, it was obvious that PSK@CH@NC was effective against breast cancer and that it might be used as an anticancer NC to treat human breast cancer in the future.
-
Funding information: No funding was involved.
-
Author contributions: CP, HAA, SKM, KA, and MSM designed the research plan, and drafted, revised, and formatted the manuscript. CP, ZMM, and AIA performed the experimental work and data compilation. MAA, AAK, MMA, AHA, MA, and SK coordinated the work and discussed the results. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: All the authors declare no conflict of interest.
-
Data availability statement: Not applicable.
References
(1) Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2020;71:209–49. 10.3322/caac.21660.Search in Google Scholar PubMed
(2) Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43–6. 10.7314/apjcp.2016.17.s3.43.Search in Google Scholar PubMed
(3) Yalaza M, İnan A, Bozer M. Male breast cancer. J Breast Health. 2016;12:1–8. 10.5152/tjbh.2015.2711.Search in Google Scholar PubMed PubMed Central
(4) Tsaousis GN, Papadopoulou E, Apessos A, Agiannitopoulos K, Pepe G, Kampouri S, et al. Analysis of hereditary cancer syndromes by using a panel of genes: novel and multiple pathogenic mutations. BMC cancer. 2019;19(1):1–9. 10.21873/cgp.20304.Search in Google Scholar PubMed PubMed Central
(5) Riis M. Modern surgical treatment of breast cancer. Ann Med Surg (Lond). 2020;56:95–107. 10.1016/j.amsu.2020.06.016.Search in Google Scholar PubMed PubMed Central
(6) Haussmann J, Corradini S, Nestle-Kraemling C, Bölke E, Njanang FJ, Tamaskovics B, et al. Recent advances in radiotherapy of breast cancer. Rad Oncol. 2020;15:1–0. 10.1186/s13014-020-01501-x.Search in Google Scholar PubMed PubMed Central
(7) Schiavon G, Tonini G. Hormone-biological therapy in breast cancer: preclinical evidence, clinical studies, and future directions. Curr Cancer Drug Targets. 2010;10:3–18. 10.2174/156800910790980278.Search in Google Scholar PubMed
(8) Riggio AI, Varley KE, Welm AL. The lingering mysteries of metastatic recurrence in breast cancer. British J Cancer. 2021;124(1):13–26. 10.1038/s41416-020-01161-4.Search in Google Scholar PubMed PubMed Central
(9) Fritz H, Kennedy DA, Ishii M, Fergusson D, Fernandes R, Cooley K, et al. Polysaccharide K and Coriolus versicolor extracts for lung cancer: a systematic review. Integr Cancer Ther. 2015;14(3):201–11.10.1177/1534735415572883Search in Google Scholar PubMed
(10) Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964–73. 10.7150/ijms.49801.Search in Google Scholar PubMed PubMed Central
(11) Parvanian S, Mostafavi SM, Aghashiri M. Multifunctional nanoparticle developments in cancer diagnosis and treatment. Sens Bio-Sens Res. 2017;13:81–7. 10.1016/j.sbsr.2016.08.002.Search in Google Scholar
(12) Zhang Y, Li M, Gao X, Chen Y, Liu T. Nanotechnology in cancer diagnosis: progress, challenges and opportunities. J Hematol Oncol. 2019;12:137. 10.1186/s13045-019-0833-3.Search in Google Scholar PubMed PubMed Central
(13) Kemp JA, Kwon YJ. Cancer nanotechnology: current status and perspectives. Nano Convergence. 2021;8:34. 10.1186/s40580-021-00282-7.Search in Google Scholar PubMed PubMed Central
(14) Combes GF, Vučković AM, Perić Bakulić M, Antoine R, Bonačić-Koutecky V, Trajković K. Nanotechnology in tumor biomarker detection: the potential of iganded nanoclusters as nonlinear optical contrast agents for molecular diagnostics of cancer. Cancers (Basel). 2021;13:4206. 10.3390/cancers13164206.Search in Google Scholar PubMed PubMed Central
(15) Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y, et al. Nanoparticle-Based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci. 2020;7:193. 10.3389/fmolb.2020.00193.Search in Google Scholar PubMed PubMed Central
(16) Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12:908–31.10.1016/j.arabjc.2017.05.011Search in Google Scholar
(17) Rata DM, Cadinoiu AN, Atanase LI, Popa M, Mihai CT, Solcan C, et al. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil – An innovative concept for the skin cancer therapy. Mater Sci Eng C Mat Biolog Appl. 2021;119:111591.10.1016/j.msec.2020.111591Search in Google Scholar PubMed
(18) Lu H, Yang Y, Gad E, Wenner CA, Chang A, Larson ER, et al. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin Cancer Res. 2011;17:67–76. 10.1158/1078-0432.CCR-10-1763.Search in Google Scholar PubMed PubMed Central
(19) Sun C, Rosendahl AH, Wang XD, Wu DQ, Andersson R. Polysaccharide-K (PSK) in cancer – old story, new possibilities. Curr Med Chem. 2012;19:757–62. 10.2174/092986712798992020.Search in Google Scholar PubMed
(20) Ito A, Munakata K, Imazu Y, Watanabe K. First nationwide attitude survey of Japanese physicians on the use of traditional Japanese medicine (Kampo) in cancer treatment. Evid based Complement Altern Med. 2012;2012:957082.10.1155/2012/957082Search in Google Scholar PubMed PubMed Central
(21) Habtemariam S. Trametes versicolor (Synn) polysaccharides in cancer therapy: Targets and efficacy. Biomedicines. 2020;8:135.10.3390/biomedicines8050135Search in Google Scholar PubMed PubMed Central
(22) Maehara Y, Tsujitani S, Saeki H, Oki E, Yoshinaga K, Emi Y, et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN®): review of development and future perspectives. SurgToday. 2012;42:8–28.10.1007/s00595-011-0075-7Search in Google Scholar PubMed PubMed Central
(23) Rabha B, Bharadwaj KK, Pati S, Choudhury BK, Sarkar T, Kari ZA, et al. Development of polymer-based nanoformulations for glioblastoma brain cancer therapy and diagnosis: an update. Polymers. 2021;13:4114.10.3390/polym13234114Search in Google Scholar PubMed PubMed Central
(24) Puluhulawa LE, Joni IM, Elamin KM, Mohammed AF, Muchtaridi M, Wathoni N. Chitosan-hyaluronic acid nanoparticles for active targeting in cancer therapy. Polymers. 2022;14:3410.10.3390/polym14163410Search in Google Scholar PubMed PubMed Central
(25) Rață DM, Cadinoiu AN, Atanase LI, Bacaita SE, Mihalache C, Daraba OM, et al. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater Sci Eng C Mat Biolog Appl. 2019;103:109828.10.1016/j.msec.2019.109828Search in Google Scholar PubMed
(26) Dubey SK, Bhatt T, Agrawal M, Saha RN, Saraf S, Saraf S, et al. Application of chitosan modified nanocarriers in breast cancer. Inter J Biol Macromol. 2022;194:521–38. 10.1016/j.ijbiomac.2021.11.095.Search in Google Scholar PubMed
(27) Zhang J, Wang Y, Li J, Zhao W, Yang Z, Feng Y. α-Santalol functionalized chitosan nanoparticles as efficient inhibitors of polo-like kinase in triple negative breast cancer. RSC Adv. 2020;10:5487–5501. 10.1039/c9ra09084c.Search in Google Scholar PubMed PubMed Central
(28) Rejeeth C, Vivek R, NipunBabu V, Sharma A, Ding X, Qian K. Cancer nanomedicine: from PDGF targeted drug delivery. Med Chem Comm. 2017;8:2055–9. 10.1016/j.diamond.2018.06.024.Search in Google Scholar
(29) Rejeeth C, Vivek R, Kannan S. A novel magnetic drug delivery nanocomplex with a cisplatin-conjugated Fe3O4 core and a PEG-functionalized mesoporous silica shell for enhancing cancer drug delivery efficiency. RSC Adv. 2015;5:94534–8. 10.1039/C7NJ02860A.Search in Google Scholar
(30) Malik NS, Ahmad M, Minhas MU, Tulain R, Barkat K, Khalid I, et al. Chitosan/Xanthan gum based hydrogels as potential carrier for an antiviral drug: fabrication, characterization, and safety evaluation. Front Chem. 2020;8:50. 10.3389/fchem.2020.00050.Search in Google Scholar PubMed PubMed Central
(31) Mathew SA, Praveena P, Dhanavel S, Manikandan R, Senthilkumar S, Stephen A. Luminescent chitosan/carbon dots as an effective nano-drug carrier for neurodegenerative diseases. RSC Adv. 2020;10:24386–96. 10.1039/D1NA00722J.Search in Google Scholar
(32) Zhou H, Li Q, Wei L, Huang S, Zhao S. A comparative scanning electron microscopy evaluation of smear layer removal with chitosan and MTAD. NigerJ Clin Pract. 2018;21:76–80. 10.4103/1119-3077.224798.Search in Google Scholar PubMed
(33) Xu Y, Zhao Z, Tong W, Ding Y, Liu B, Shi Y, et al. An acid-tolerance response system protecting exponentially growing Escherichia coli. Nat Commun. 2020;11:1496. 10.1038/s41467-020-15350-5.Search in Google Scholar PubMed PubMed Central
(34) Jiménez-Medina E, Berruguilla E, Romero I, Algarra I, Collado A, Garrido F, et al. The immunomodulator PSK induces in vitro cytotoxic activity in tumour cell lines via arrest of cell cycle and induction of apoptosis. BMC Cancer. 2008;8:78–8. 10.1186/1471-2407-8-78.Search in Google Scholar PubMed PubMed Central
(35) Liu K, Liu PC, Liu R, Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res. 2015;21:15–20. 10.12659/MSMBR.893327.Search in Google Scholar PubMed PubMed Central
(36) Monier B, Suzanne M. Orchestration of force generation and nuclear collapse in apoptotic cells. Int J Mol Sci. 2021;22:10257. 10.3390/ijms221910257.Search in Google Scholar PubMed PubMed Central
(37) Vyssokikh MY, Holtze S, Averina OA, Lyamzaev KG, Panteleeva AA, Marey MV, et al. Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program. Proc Natl Acad Sci. 2020;117:6491–501. 10.1073/pnas.1916414117.Search in Google Scholar PubMed PubMed Central
(38) Vanzyl EJ, Rick KR, Blackmore AB, MacFarlane EM, McKay BC. Flow cytometric analysis identifies changes in S and M phases as novel cell cycle alterations induced by the splicing inhibitor isoginkgetin. Plos One. 2018;13:e0191178. 10.1371/journal.pone.0191178.10.1371/journal.pone.0191178.Search in Google Scholar
(39) Demchenko AP. Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology. 2013;65:157–72. 10.1007/s10616-012-9481-y.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites

