Abstract
A novel homogenous braid-reinforced (HBR) poly (p-phenylene terephthamide) (PPTA) hollow fiber membrane was prepared in this study. The effects of PPTA concentration on the morphologies and properties of the membranes were further investigated. The results showed that when the PPTA concentration was 2.0 wt%, the cross-sectional morphology of satisfactory interfacial bonding was achieved and the permeation was still maintained at about 200 (L·m−2·h−1) after ultrasonic vibration. In addition, the tensile force exceeded 600 N, which indicated that the homogeneous effect effectively improved the poor mechanical properties and interfacial bonding. In addition, the HBR PPTA hollow fiber membranes were applied to simulate the membrane bioreactor system to explore the control factors of membrane fouling. The results demonstrated that the average removal of total phosphorus and NH4 +–N was above 49% and 96%, respectively; meanwhile, the operating time was up to 120 days. Furthermore, it was easier to nearly remove the pollutants by chemical cleaning.
Nomenclature
- BSA
-
bovine serum albumin
- CHCl3
-
trichloromethane
- HBR
-
homogenous braid-reinforced
- MBR
-
membrane bioreactor
- NF
-
nanofiltration
- NH4 +–N
-
ammonia nitrogen
- NMP
-
N-methyl-2-pyrrolidone
- PEG
-
polyethylene glycol
- PFAs
-
pore-forming agents
- PPTA
-
poly (p-phenylene terephthamide)
- PVP
-
polyvinylpyrrolidone
- THF
-
tetrahydrofuran
- TMP
-
transmembrane pressure difference
- TP
-
total phosphorus
- UF
-
ultrafiltration
1 Introduction
The membrane bioreactor (MBR) system is a new wastewater treatment technology combining traditional biotechnology and membrane separation technology, with a high solid–liquid separation rate, stable effluent quality, and high treatment efficiency, which has become the widely used technology for wastewater resource utilization and water environment treatment (1,2). However, MBR technology requires high performance of hollow fiber membranes, which not only requires membrane materials with excellent separation performance but also be able to withstand the effects of high-intensity water impact and aeration disturbance during MBR operation, which was difficult to be applied for traditional homogenous hollow fiber membranes (3,4).
The preparation methods of reinforced hollow fiber membranes are divided into two types: polymeric-reinforced membrane and fibrous-reinforced membrane (5,6,7,8). In the former, the hollow fiber membrane prepared by the thermally induced phase separation method or melt spinning-cold stretching method was used as the base membrane, and the separation layer was combined with its surface to prepare a polymeric-reinforced hollow fiber membrane. In the latter, the fiber or the fiber bundle was used as reinforcement, and the separation layer was combined with its surface to prepare a fibrous-reinforced hollow fiber membrane. In the process of fibrous-reinforced hollow fiber membrane preparation, it is important to solve the problem of interfacial bonding between the reinforcement and the surface separation layer for the preparation of high-performance-reinforced hollow fiber membranes (9,10).
According to the relationship of interfacial bonding between the reinforcement and the surface separation layer, the reinforced hollow fiber membrane could be divided into homogeneous BR (HBR) hollow fiber membrane and heterogeneous BR (HTR) hollow fiber membrane. Homogeneous reinforcement indicated that the fibrous reinforcement and the surface separation layer were of the same material and they were no thermodynamic compatibility differences so that an ideal interface structure could be easily formed between the surface separation layer and the reinforcement. Thus, there was an increasing demand for fabricated HBR hollow fiber membranes that combined high separation accuracy (smaller average pore size and narrower distribution) with high strength (fiber reinforcement) and high throughput (high porosity) for widely harsh environment applications (11,12,13).
Currently, the tubular braids-reinforced (BR) hollow fiber ultrafiltration (UF) membrane has been the most widely used in the MBR system instead of traditional homogeneous hollow fiber membrane, and its preparation process is similar to the concentric circle composite method (14); the inner layer is tubular braids and the outer layer is the polymer separation layer and the interfacial layer between them. For this reason, many researchers at home and abroad have developed and applied HTR hollow fiber membranes. Especially, the HTR PVDF hollow fiber membranes are made of PET or PP tubular braids as reinforcement and have become the most used UF membrane for the MBR system because of their high permeance, easy scale production, and engineering applications (15,16,17).
However, most polymeric materials lacked stability, including the PVDF membrane under extreme conditions, such as high temperatures, processes coupled to acid and alkali solutions, or in the presence of organic solvents. Meanwhile, the inorganic membranes were very expensive and hard to manufacture though they were suitable to be used under such requirements (18). Therefore, there was an urgent demand to develop high-performance membrane materials.
Poly (p-phenylene terephthamide) (PPTA) is a kind of rigid linear chain aromatic polyamide, which has superior mechanical strength, is acid and alkali-resistant, and is thermal and solvent resistant (19,20,21). Nevertheless, there were few relevant researchers in this field of PPTA membranes. Zschocke and Strathman (22) have reported a solvent-resistant membrane from PPTA. Wang et al. (23,24,25) have published a series of high-performance PPTA UF/nanofiltration membranes. Therefore, based on previous research studies (26,27,28,29,30), in this study, the HBR PPTA hollow fiber membranes consisting of PPTA hollow tubular braids and a PPTA separation layer were fabricated by the concentric circle extrusion spinning method. The influence of the PPTA concentration on the structure and performance of HBR PPTA hollow fiber membranes was investigated in terms of membrane morphologies, mechanical properties, and permeation/rejection performance. Then, it was applied in the MBR system, which was a simulation of urban domestic sewage to observe and further investigate the results on membrane performances such as transmembrane pressure difference (TMP), total phosphorus (TP) concentration, and ammonia nitrogen (NH4 +–N), aimed to verify the anti-fouling properties of HBR PPTA hollow fiber membranes.
2 Materials and methods
2.1 Materials
PPTA resin (PPTA, fiber grade, η inh = 5.5−6.5 dL·g−1) and filament yarn (PPTA, single fiber denier = 244 dtex, modulus = 590.48 CN‧dtex−1) were purchased from Zhonghua High-performance Fiber Material Co., Ltd. Concentrated sulfuric acid (H2SO4, 98%, AR) was purchased from Shanghai Titan Chemical Reagent Technologies Co. Ltd. Silica (SiO2) particles with an average size of 30 nm were purchased from Beijing Boyu Gaoke Advanced Materials Technical Co. Ltd. Bovine serum albumin (BSA, M w = 68,000) was purchased from Beijing Solarbio Science and Technology Co. Ltd. Polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP) were purchased from Tianjin Kemel Reagent Co. Ltd. N,N-Dimethylacetamide (AR), N,N-dimethylformamide (AR), trichloromethane (CHCl3, AR), tetrahydrofuran (THF, AR), and N-methyl-2-pyrrolidone (AR) were purchased from Tianjin Guangfu Fine Chemical Institute.
2.2 Membrane preparation
First, the PPTA tubular braids were prepared by the two-dimensional braided method. Then, they were coated with the PPTA casting solutions and guided through a coagulation bath, where the HBR PPTA hollow fiber membranes were formed. The casting solution was prepared with the mixtures of different compositions consisting of PPTA resin, pore-forming agents (PFAs, composed of PEG/PVP), SiO2, and H2SO4, and they were blended in a special weight homogeneously under high-speed agitation for 2–3 h at 80–90℃. After the formation of HBR PPTA hollow fiber membranes, they were stored in clean water to remove the residual solvents and PFAs.
The samples of HBR PPTA hollow fiber membranes with different PPTA resin concentrations are shown in Table 1 and the spinning parameters are shown in Table 2.
Samples of HBR PPTA hollow fiber membranes
| Sample | PPTAp (%) | H2SO4 (%) | PFAs (%) | SiO2 (%) |
|---|---|---|---|---|
| PPTA-1# | 1.5 | 88 | 10 | 0.5 |
| PPTA-2# | 1.75 | 87.75 | 10 | 0.5 |
| PPTA-3# | 2.0 | 87.5 | 10 | 0.5 |
| PPTA-4# | 2.5 | 87 | 10 | 0.5 |
Spinning parameters of HBR PPTA hollow fiber membranes
| Spinning parameter | |
|---|---|
| Spinning temperature (℃) | 80.0 ± 5.0 |
| Coagulation bath | Water |
| Coagulation temperature (℃) | 30.0 ± 5.0 |
| Air gap (cm) | 5.0 ± 0.5 |
| Take-up speed (m·h−1) | 10.0 ± 1.0 |
2.3 Characterization
2.3.1 Scanning electron microscopy (SEM)
The surface and cross-section morphology of membranes were observed by field emission SEM (Nano-230, FEI, Netherlands). The membrane was freeze-dried before cryogenically fractured in liquid N2, and they were sputtered with gold.
2.3.2 Mechanical performances
Mechanical properties were measured using an electronic stretching machine (AG-250kNE, Japan) at room temperature. The tensile rate was 50 mm·min−1 and the gripping length was 50 mm. The OD/ID values were about 1.8 mm/1.6 mm. The average value of five tests was chosen.
2.3.3 Permeation and interfacial bonding
Membrane permeation was characterized by pure water flux and organic solution flux. The pressure difference across the membrane was 0.1 MPa. The permeance was determined by the following equation (1):
where J w1 is the permeation (L·m−2·h−1), V is the total quantity of the filtrate (L), A is the tested membrane area (m2), and t is the testing time (h).
2.3.4 BSA protein separation
All experiments were carried out at room temperature. Before the test, each membrane was initially compacted for 10 min at 0.15 MPa and then the feed solution and filtrate were recorded at 0.1 MPa every 10 min until 60 min. The original BSA protein solution (1 g·L−1) was prepared in 0.03 wt% NaOH solution (pH = 11). The rejection rate was calculated by the following equation (2):
where C p and C f are the filtrate and the feed solution density, respectively. The absorbances of feed solution and filtrate were determined by UV spectroscopy at 280 nm, using a Beijing Purkinje UV-1901 spectrophotometer.
2.3.5 Simulation of the MBR system
The HBR PPTA-3# hollow fiber membrane was tested in the MBR system, which was a simulation of urban domestic sewage. The volume of the biochemical reactor was 50 L, and the flux was controlled at a constant rate of 40 mL·min−1. The level of the reactor was controlled by a liquid level controller. The raw water was controlled by the inlet pump and the outlet water was controlled by the suction pump. The intermittent pumping mode of 9 min pumping and 1 min stop was used. The aeration rate was 3.6 L·min−1 with air compressor, the air/water ratio was about 50:1, and the effective area of the membrane module was 0.1 m2. The experimental period was 120 days. The sludge concentration was about 8,500 mg·L−1, dissolved oxygen was about 6.5–8.5 mg·L−1, the pH was about 6.5–7.5, and the water temperature was about 18–20℃.
The membrane surface was observed by SEM before and after pollution, the TMP before and after pollution was observed by a pressure flow meter, and changes in parameters including TP and NH4 +–N were tested by standard methods (33,34,35).
3 Results and discussion
3.1 Morphology of the membrane
The outer surface and cross-sectional morphologies of the HBR PPTA-3# hollow fiber membranes are shown in Figure 1a–d. As shown in Figure 1a, a dense and smooth coating layer was formed at the outer surface of the tubular braids, which resulted typically from the solution phase separation via the solvent and non-solvent mutual diffusion (36). This was beneficial to obtain good rejection properties. As shown in Figure 1b–d, compared with the HTR PPTA-3# hollow fiber membrane (PPTA-3# was the outer separation layer and PET tubular braids were fibrous reinforced), the cross-section of the HBR PPTA-3# hollow fiber membrane separation layer had a finger-like pore structure, and as both were homogeneous materials, the concentrated sulfuric acid would etch the surface of the PPTA braided tube reinforcement to enable the surface separation layer casting solution to penetrate the fiber spacing, which improved the bonding strength between the hollow braided tube and the surface separation layer (shown in Figures A1 and A2 in Appendix). Moreover, the forming process adopted the concentric circle extrusion spinning method, which could effectively regulate the uniformity of the surface separation layer and reduced the eccentricity of the surface separation layer.

Outer surface and cross-sectional morphologies of the braids-reinforced PPTA hollow fiber membranes. (a) The outer surface of HBR PPTA-3#, (b) the cross-section of HBR PPTA-3#, (c) HBR PPTA-3# membrane, and (d) HTR PPTA-3# membrane (PET braids/PPTA).
3.2 Interfacial bonding
Interfacial bonding is important for BR hollow fiber membranes because it would restrict the operation life of the membranes. Ultrasonic vibration analysis is a typical method to evaluate the interfacial bonding state. Thus, the effect of ultrasonic treatment on pure water flux of HBR PPTA hollow fiber membranes is shown in Figure 2. The results showed that HBR PPTA-3# and 4#, with nearly no change in their pure water fluxes, possessed a stable interfacial bonding state. There was relatively strong interfacial bonding between the separation layer and the tubular braids because of the infiltration of the casting solution. Besides, the part of fiber bundles that were in contact with the separation layer was swollen to a certain extent and adhered to each other (shown in Figure A2). It was obvious that the pure water flux of PPTA-1# and 2# had a relatively higher vibration due to the lower PPTA concentration, which indicated that the interfacial bonding was weaker so there was a significant breakage phenomenon between the two interfaces.
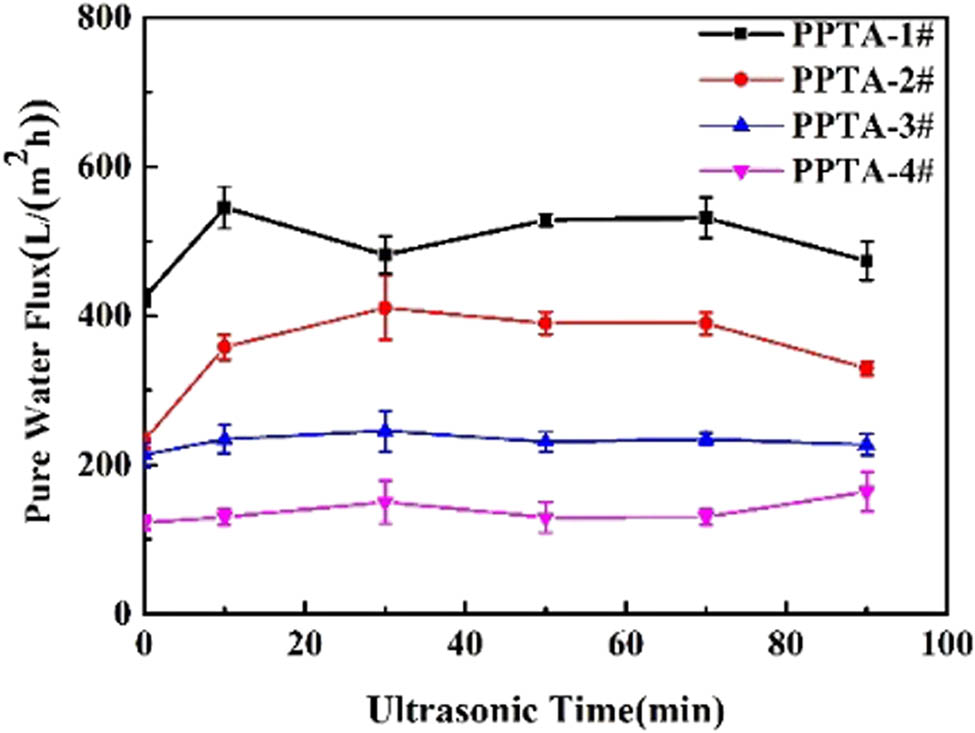
The effect of ultrasonic treatment on the interfacial bonding of HBR PPTA hollow fiber membranes.
3.3 Permeation properties
Figure 3 shows the pure water flux of the HBR PPTA hollow fiber membranes and the solvent flux of the HBR-3# PPTA hollow fiber membranes for five kinds of common organic solvents. As shown in Figure 3a, it is obvious that the pure water flux of the HBR PPTA hollow fiber membrane exhibits four very linear relationship because PPTA is a rigidly linear chain macromolecule, which could prevent the pore structure from deformation, and then the flux is stable under constant pressure conditions. This indicated that the swelling and adhering of the tubular braids increase the intrinsic resistance of the membranes. Therefore, there was a balanced state between the separation layer porosity and bonding strength, which only resulted in a little change in permeation. Figure 3b shows that the shrinking effect due to solvent interaction was considered to be insignificant. The HBR-3# PPTA hollow fiber membranes were proved to be stable in these organic solvents.

The pure water flux of HBR PPTA hollow fiber membranes (a) and the solvent flux of the HBR PPTA-3# hollow fiber membranes (b).
3.4 BSA rejection
Figure 4 shows the BSA rejection of the prepared HBR PPTA hollow fiber membranes. According to Figure 4, with the increase of the PPTA concentration in casting solutions, it was obvious that all the rejection ratios could reach above 80% depending on the test time; especially, with the increase of PPTA concentration, the higher rejection was more quickly achieved (such as HBR PPTA-4#), which indicated the denser separation layer (that was higher transmembrane pressure) and stable membrane pore structure. Besides, the results also showed that the rejection rate was stable. On the one hand, the membrane surface was of dense layer and narrow pore distribution, which could effectively intercept the protein spherical molecules. On the other hand, the strong polar amide groups (–NH–CO–) in the PPTA macromolecular chain caused strong electronegativity in the membranes, which was due to protein absorption and concentration polarization on the membrane surfaces (37,38).
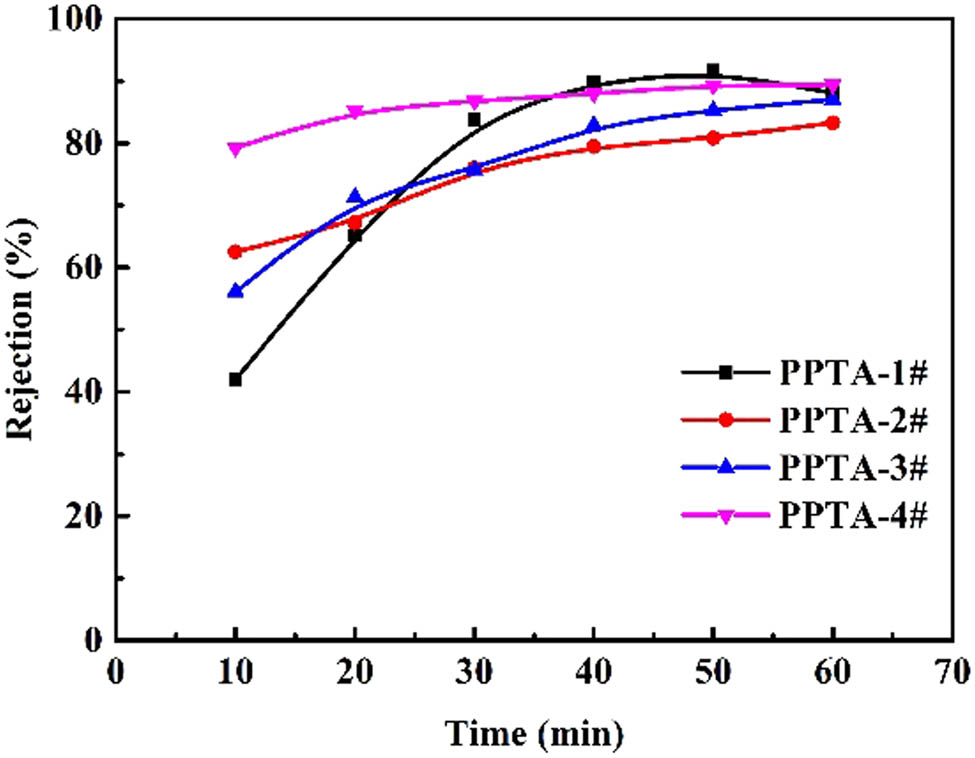
The BSA rejection of HBR PPTA hollow fiber membranes.
3.5 Mechanical properties
Figure 5 shows the tensile stress–strain behavior of the HBR PPTA-3# hollow fiber membrane. Different from the homogeneous PPTA hollow fiber membrane, the tensile force of HBR PPTA hollow fiber membranes mainly depended on the tubular braids while it hardly depended on the PPTA concentration. As shown in Figure 5, the curve exhibited a kind of stepwise fracture, and the maximum number of tensile stress was 0.6 kN while the elongation at break was about 20%. The curves exhibited a very linear relationship; the tensile strength quickly dropped to zero when it reached the breaking point and the yield area did not appear. The slope of the linear curve gave Young’s modulus, and the failure point was the ultimate strength of the membrane along their axial direction. It was because the PPTA had a high crystalline macromolecule structure. When elongation at break occurred, the areas of lamellar crystal bedded slip (39).

The mechanical properties of HBR PPTA-3# hollow fiber membranes.
3.6 Simulate MBR separation
3.6.1 Surface elements
Figure 6 shows the surface morphology of the homogeneous HBR PPTA-3# hollow fiber membrane in the MBR system after activation of sludge pollution and citric acid backwashing. Table 3 shows the surface elemental analysis. As shown in Table 3, after a period of MBR operation, different contents of N, P, Ca, Mg, Al, K, and Na, and other elements appeared on the membrane surface. This was because the feed solution contains NaCl, MgSO4, MnSO4, (NH4)2SO4, KH2PO4, etc., Mg, Al, Ca, and other elements in the form of cations, which could easily form precipitates with OH−, CO3 2−, PO4 3−, and other anions in the water. When operating for some time, a biofilm layer was formed on the membrane surface and these cations were present in the biopolymer, which could easily combine with metal ions to form a dense filter cake layer on the membrane surface. The elemental content of the membrane surface was significantly reduced after hydrodynamic backwashing, and the inorganic elements were removed after a shorter period of citric acid washing with a better cleaning effect, which was consistent with the results of the morphology diagram in Figure 6b, indicating that the resulting HBR PPTA hollow fiber membranes were easy to clean and had better anti-fouling performance.

The outer surface morphologies of HBR PPTA-3# hollow fiber membranes (a) after sludge pollution; (b) citric acid backwashing.
Surface element content on the HBR PPTA-3# hollow fiber membrane surface
| Element | Original (%) | Fouled 1 (%) | Water (%) | Fouled 2 (%) | Chemical (%) |
|---|---|---|---|---|---|
| C | 77.36 | 64.74 | 77.91 | 65.16 | 77.78 |
| O | 20.59 | 24.08 | 16.57 | 22.47 | 10.62 |
| N | 0 | 1.00 | 1.13 | 2.21 | 0.02 |
| P | 0 | 0.43 | 0.21 | 0.59 | 0.02 |
| Ca | 0.35 | 3.92 | 2.44 | 3.21 | 0.45 |
| Mg | 0 | 0.09 | 0.02 | 0.09 | 0 |
| Al | 0 | 0.12 | 0.23 | 0.33 | 0 |
| Cl | 0.90 | 4.17 | 1.08 | 4.59 | 0 |
| K | 0 | 0.45 | 0.01 | 0.68 | 0 |
| Na | 0 | 1.00 | 0.40 | 0.67 | 0 |
3.6.2 TMP
In this study, the HBR PPTA-3# hollow fiber membrane module was operated at a constant flux of 40 mL·min−1 for 120 days while monitoring the change of TMP. Figure 7 shows the TMP variation with the operation time of the HBR PPTA-3# hollow fiber membrane module. As shown in Figure 7, due to the strong hydrophilic separation layer of the HBR PPTA-3# hollow fiber membrane, the membrane was running for a longer period without cleaning. During the initial 56-day operation, the TMP showed a stable growing trend and the TMP difference gradually increased from 0.04 to 0.08 MPa with time. Meanwhile, the membrane surface pollution worsened. Subsequently, it was hydrodynamically backwashed, the TMP basically recovered to 0.04 MPa, and the membrane performance recovered well. This was because the woven pitch gap of tubular braids was larger and the internal pressure of backwash water through the pitch gap was lower, which could directly affect the surface separation layer and thus the cleaning effect was significant.

Variation in the TMP with time.
After 30 days of stable operation, the TMP increased to 0.08 MPa. Then, the TMP only recovered to 0.07 MPa when the membrane was backwashed again and the TMP quickly reached 0.08 MPa after a week of operation, which greatly shortened the operation period. This was because most of the organic pollutants deposited on the membrane surface had been removed, while metal inorganic pollutants were still attached to the membrane surface and were difficult to be removed by hydraulic cleaning. Through citric acid cleaning, TMP could be restored to 0.046 MPa. After1 month, the TMP again increased to 0.08 MPa. The membrane module was cleaned by citric acid cleaning again and the TMP decreased to 0.07 MPa.
In 10 consecutive days, TMP was more stable. It could be inferred that the HBR PPTA-3# hollow fiber membranes exhibited the surface compression resistance and structural stability of membrane pores because of the excellent mechanical properties of PPTA molecules. The membrane surface is not easily compacted, and organic contaminants such as proteins only caused membrane pore blockage resistance, while inorganic contaminants caused filter cake layer resistance forming deposition contamination (24,40,41). Overall, the HBR PPTA hollow fiber membranes had longer operating times and longer cleaning cycles, which exhibited excellent anti-pollution performance.
3.6.3 TP
Figure 8 shows the removal rate of phosphorus concentration (TP) from the supernatant and the filtrate of the HBR PPTA-3# hollow fiber membrane module. As shown in Figure 8, during the initial operation period, the number of phosphorus-polymerizing bacteria was low and the TP removal rate was only about 10%. With the increase of the operation time, the TP removal rate gradually increased and the TP removal rate reached a maximum value at 40 days of operation. After active sludge removal and washing, the TP removal rate started to increase again and when the system was running stable, the TP removal rate fluctuated in a wide range. The TP removal rate of the supernatant ranged from 12% to 83%; meanwhile, the TP removal rate of the HBR PPTA-3# hollow fiber membrane module ranged from 13% to 85%. After the active sludge removal at 96 days, the TP removal rate gradually increased again. This was mainly due to the long sludge retention time, which led to an increase in the sludge concentration, a decrease in sludge activity, and a sludge accumulation zone at the bottom of the reactor, where the dissolved oxygen was insufficient and turned anaerobic. In the anaerobic zone, polyphosphate bacteria fully released polyphosphates in their bodies, leading to an increase in the TP content in the system. After increasing the aeration to eliminate the anaerobic zone, the TP removal rate increased. After hydrodynamic backwashing and chemical cleaning, the TP removal rate of the membrane module was slightly higher than that of the supernatant, and the cleaning effect of the HBR PPTA-3# hollow fiber membrane module could be obtained.

The TP removal rate in the supernatant and HBR PPTA-3# hollow fiber membranes.
3.6.4 NH4 +–N
Figure 9 shows the removal ratio of ammonia nitrogen (NH4 +–N) in the supernatant and filtrate of the HBR PPTA-3# hollow fiber membrane module. As shown in Figure 9, they were more stable and the removal effect was better. When the simulated MBR system was running stable, the average NH4 +–N removal rate of the supernatant was about 94%, and the average NH4 +–N removal rate of the HBR PPTA-3# hollow fiber membrane module was about 96%. When the system was running until 96 days, the NH4 +–N removal rate dropped to the minimum. Afterwards, the NH4 +–N removal rates increased rapidly after the active sludge elimination. This was attributed mainly to the decrease in bacterial activity under long operation times.
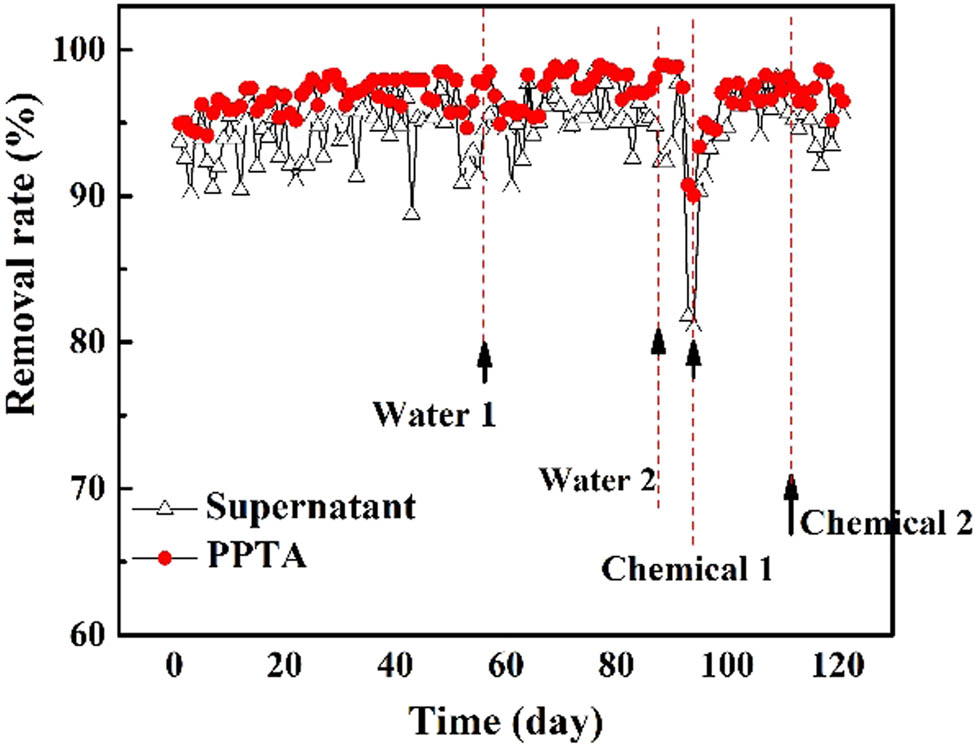
The NH4 +–N removal rate in the supernatant and HBR PPTA-3# hollow fiber membranes.
Compared with the NH4 +–N removal rate after two hydraulic backwashes, the results showed that there was no significant decrease in the removal rate after the second cleaning. After chemical cleaning, the NH4 +–N removal rate decreased, which indicated that a better cleaning effect for the HBR PPTA-3# hollow fiber membrane was achieved at this time.
4 Conclusion
The HBR PPTA hollow fiber membranes were fabricated by the concentric circle extrusion spinning method, which consisted of a PPTA separation layer and PPTA hollow tubular braids. With the increase of the PPTA concentration in the casting solution, the separation layer of the HBR PPTA hollow fiber membranes became dense and the pure water flux decreased from about 300 to 100 L·m−2·h−1. Then, the tensile force of HBR PPTA hollow fiber membranes reached above 600 N, which mainly depended on the tubular braids. Moreover, there was an effective interfacial bonding state between the separation layer and the tubular braids when the PPTA concentration was higher than 2 wt%. The HBR PPTA-3# hollow fiber membrane had a high BSA rejection rate, which could be achieved above 90%. Meanwhile, in different organic solvents, the solvents’ permeation of HBR PPTA-3# hollow fiber membranes was about 150–200 L·m−2·h−1. Especially, the THF permeation was above 270 L·m−2·h−1, which was nearly similar to the PWF of HBR PPTA-1# hollow fiber membranes. Moreover, the HBR PPTA-3# hollow fiber membranes applied in the MBR system showed stable removal rates of NH4 +–N and TP; the average removal rates were 96.71% and 49.81%, respectively, and the filtrate quality was stable. Based on the changes in the elemental mass fraction on the membrane surface after hydraulic backwashing and chemical cleaning, the inorganic elements were removed after a short period of citric acid cleaning, and the HBR PPTA-3# hollow fiber membrane was more resistant to pollution.
-
Funding information: This research was financially supported by the National Natural Science Foundation of China (Grant No. 21808165, 52173038) and Jiangsu Provincial Science and Technology R&D Projects of China – Key Core Technology Tackling (BE2022140).
-
Author contributions: Chun Wang: conceptualization, methodology, writing – original draft; Jingjing Yan: methodology, supervision, project administration, writing – review and editing; Dinghe Yan: investigation, visualization; Haolong Xue: investigation; and Shubin Song, Changfa Xiao: supervision, funding acquisition.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Data will be made available and shared on request.
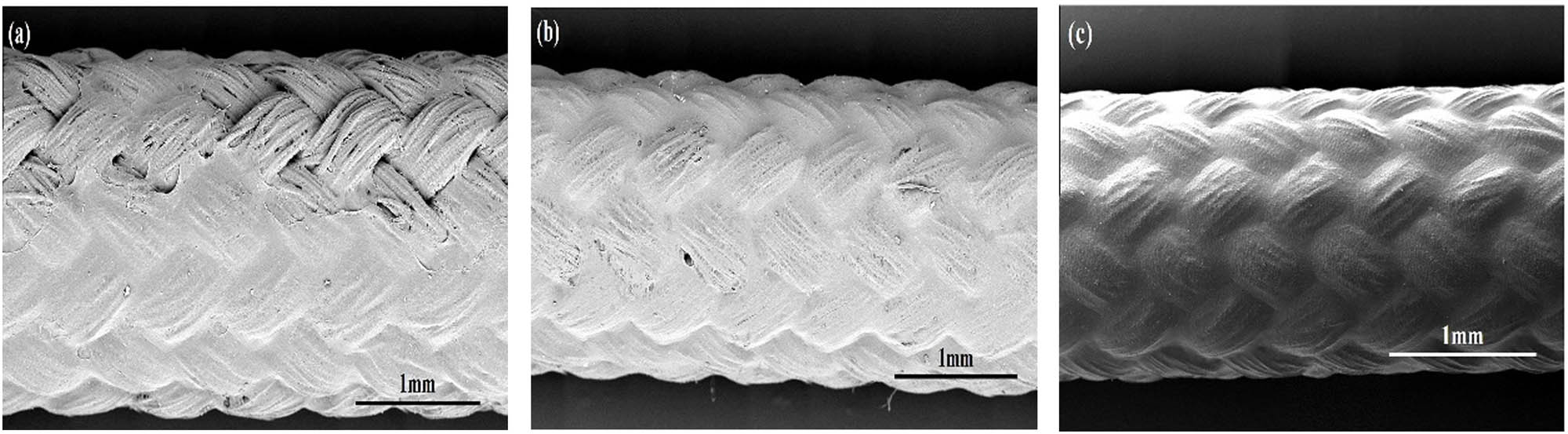
The outer surface morphologies of HBR PPTA hollow fiber membranes after ultrasonic treatment: (a) HBR PPTA-1#, (b) HBR PPTA-2#, and (c) HBR PPTA-3#.

The cross-sectional morphologies of HBR PPTA hollow fiber membranes: (a) partial cross-section of HBR PPTA-1#, (b) partial cross-section of HBR PPTA-2#, (c) partial cross-section of HBR PPTA-3#, and (d) partial cross-section of HBR PPTA-4#.
References
(1) Hoinkis J, Deowan SA, Panten V, Figoli A, Huang RR, Drioli E. Membrane bioreactor (MBR) technology – a promising approach for industrial water reuse. Procedia Eng. 2012;33:234–41. 10.1016/j.proeng.2012.01.1199.Search in Google Scholar
(2) Tang K, Xie J, Pan Y, Zou X, Sun F, Yu Y, et al. The optimization and regulation of energy consumption for MBR process: A critical review. J Environ Chem Eng. 2022;10(5):108406. 10.1016/j.jece.2022.108406.Search in Google Scholar
(3) Liu H, Xiao C, Huang Q, Hu X. Structure design and performance study on homogeneous-reinforced polyvinyl chloride hollow fiber membranes. Desalination. 2013;331:35–45. 10.1016/j.desal.2013.10.015.Search in Google Scholar
(4) Liu J, Li P, Li Y, Xie L, Wang S, Wang Z. Preparation of PET threads reinforced PVDF hollow fiber membrane. Desalination. 2009;249(2):453–7. 10.1016/j.desal.2008.11.010.Search in Google Scholar
(5) Zhang X, Xiao C, Hu X, Bai Q. Preparation and properties of homogeneous-reinforced polyvinylidene fluoride hollow fiber membrane. Appl Surf Sci. 2013;264:801–8. 10. 10.1016/j.apsusc.2012.10.135.Search in Google Scholar
(6) Quan Q, Xiao C, Liu H, Huang Q, Zhao W, Hu X, et al. Preparation and characterization of braided tube reinforced polyacrylonitrile hollow fiber membranes. J Appl Polym Sci. 2015;132(14):4175. 10.1002/app.41795.Search in Google Scholar
(7) Fan Z, Xiao C, Liu H, Huang Q, Zhao J. Structure design and performance study on braid-reinforced cellulose acetate hollow fiber membranes. J Membr Sci. 2015;486:248–56. 10.1016/j.memsci.2015.03.066.Search in Google Scholar
(8) Chen M, Xiao C, Wang C, Liu H. Study on the structural design and performance of novel braid-reinforced and thermostable poly(m-phenylene isophthalamide) hollow fiber membranes. RSC Adv. 2017;7(33):20327–35. 10.1039/C7RA01171G.Search in Google Scholar
(9) Hao J, Xiao C, Zhang T, Zhao J, Fan Z, Chen L. Preparation and performance of PET-braid-reinforced poly(vinylidene fluoride)/graphene hollow-fiber membranes. Ind Eng Chem Res. 2016;55(7):2174–82. 10.1021/acs.iecr.5b04428.Search in Google Scholar
(10) Jeon S, Rajabzadeh S, Okamura R, Ishigami T, Hasegawa S, Kato N, et al. The effect of membrane material and surface pore size on the fouling properties of submerged membranes. Water. 2016;8(12):602. 10.3390/w8120602.Search in Google Scholar
(11) Pendergast MM, Hoek EMV. A review of water treatment membrane nanotechnologies. Energy Environ Sci. 2011;4(6):1946–71. 10.1039/C0EE00541J.Search in Google Scholar
(12) Aslan T, Arslan S, Eyvaz M, Güçlü S, Yüksel E, Koyuncu İ. A novel nanofiber microfiltration membrane: Fabrication and characterization of tubular electrospun nanofiber (TuEN) membrane. J Membr Sci. 2016;520:616–29. 10.1016/j.memsci.2016.08.014.Search in Google Scholar
(13) Yan J, Xiao C, Wang C. Robust preparation of braid-reinforced hollow fiber membrane covered by PVDF nanofibers and PVDF/SiO2 micro/nanospheres for highly efficient emulsion separation. Sep Purif Technol. 2022;298:121593. 10.1016/j.seppur.2022.121593.Search in Google Scholar
(14) Fan Z, Xiao C, Liu H, Huang Q. Preparation and performance of homogeneous braid reinforced cellulose acetate hollow fiber membranes. Cellulose. 2015;22(1):695–707. 10.1007/s10570-014-0466-1.Search in Google Scholar
(15) Moattari RM, Mohammadi T, Rajabzadeh S, Dabiryan H, Matsuyama H. Reinforced hollow fiber membranes: A comprehensive review. J Taiwan Inst Chem Eng. 2021;122:284–3. 10. 10.1016/j.jtice.2021.04.052.Search in Google Scholar
(16) Turken T, Sengur-Tasdemir R, Ates-Genceli E, Tarabara VV, Koyuncu I. Progress on reinforced braided hollow fiber membranes in separation technologies: A review. J Water Process Eng. 2019;32:100938. 10.1016/j.jwpe.2019.100938.Search in Google Scholar
(17) Quan Q, Xiao CF, Liu HL, Zhao W, Hu XY, Huan GL. Preparation and properties of two-dimensional braid heterogeneous-reinforced polyvinylidene fluoride hollow fiber membrane. Adv Mater Res. 2014;936:218–25. 10.4028/www.scientific.net/AMR.936.218.Search in Google Scholar
(18) Rajabzadeh S, Maruyama T, Sotani T, Matsuyama H. Preparation of PVDF hollow fiber membrane from a ternary polymer/solvent/nonsolvent system via thermally induced phase separation (TIPS) method. Sep Purif Technol. 2008;63(2):415–23. 10.1016/j.seppur.2008.05.027.Search in Google Scholar
(19) Rao Y, Waddon AJ, Farris RJ. Structure–property relation in poly(p-phenylene terephthalamide) (PPTA) fibers. Polymer. 2001;42(13):5937–46. 10.1016/S0032-3861(00)00905-8.Search in Google Scholar
(20) Singh TJ, Samanta S. Characterization of Kevlar fiber and its composites: a review. Mater Today: Proc. 2015;2(4):1381–7. 10.1016/j.matpr.2015.07.057.Search in Google Scholar
(21) Yang B, Lu Z, Zhang M, Liu Y, Liu G. A ductile and highly fibrillating PPTA-pulp and its reinforcement and filling effects of PPTA-pulp on properties of paper-based materials. J Appl Polym Sci. 2016;133(13):43209. 10.1002/app.43209.Search in Google Scholar
(22) Zschocke P, Strathmann H. Solvent resistant membranes from poly-(p-phenylene-terephthalamide. Desalination. 1980;34(1):69–75. 10.1016/S0011-9164(00)88581-1.Search in Google Scholar
(23) Fu M, Wang C, Sun G, Xiao C, Ding Y. Controllable preparation of acid and alkali resistant 3D flower-like UiO-66-NH2/ZiF-8 imbedding PPTA composite nanofiltration membrane for dye wastewater separation. J Water Process Eng. 2022;50:103320. 10.1016/j.jwpe.2022.103320.Search in Google Scholar
(24) Wang C, Xiao C, Chen M, Huang Q, Liu H, Li N. Unique performance of poly(p-phenylene terephthamide) hollow fiber membranes. J Mater Sci. 2016;51(3):1522–31. 10.1007/s10853-015-9473-3.Search in Google Scholar
(25) Wang C, Lai X, Fu M, Wang L, Xiao C. Controllable preparation of novel “ridge-valley shaped” poly(p-phenylene terephthamide) (PPTA) hollow fiber nanofiltration membrane for thermal dye/salt wastewater separation. J Water Process Eng. 2022;50:103251. 10.1016/j.jwpe.2022.103251.Search in Google Scholar
(26) Huang Y, Xiao C, Huang Q, Liu H, Zhao J. Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development. Chem Eng J. 2021;403:126295. 10.1016/j.cej.2020.126295.Search in Google Scholar
(27) Koyuncu I, Eryildiz B, Kaya R, Karakus Y, Zakeri F, Khataee A, et al. Modification of reinforced hollow fiber membranes with WO3 nanosheets for treatment of textile wastewater by membrane bioreactor. J Environ Manag. 2023;326:116758. 10.1016/j.jenvman.2022.116758.Search in Google Scholar PubMed
(28) Zhao B, Shi GM, Lai J-Y, Chung T-S. Braid-reinforced polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration (OSN). Sep Purif Technol. 2022;290:120811. 10.1016/j.seppur.2022.120811.Search in Google Scholar
(29) Lu X, Chen Q, Lu J, Xu H, Ji J. Investigation of reinforced braided hollow fiber membrane containing silver-based butanediol for methyl linolenate separation: Better penetration rate, higher stability. J Membr Sci. 2022;642:119954. 10.1016/j.memsci.2021.119954.Search in Google Scholar
(30) Zhou Z, Rajabzadeh S, Fang L, Miyoshi T, Kakihana Y, Matsuyama H. Preparation of robust braid-reinforced poly (vinyl chloride) ultrafiltration hollow fiber membrane with antifouling surface and application to filtration of activated sludge solution. Mater Sci Eng C. 2017;77:662–71. 10.1016/j.msec.2017.03.277.Search in Google Scholar PubMed
(31) Xu H, Liu H, Huang Y, Xiao C. Three-dimensional structure design of tubular polyvinyl chloride hybrid nanofiber membranes for water-in-oil emulsion separation. J Membr Sci. 2021;620:118905. 10.1016/j.memsci.2020.118905.Search in Google Scholar
(32) Yang S, Wu C, Ji D, Xi Z, Chen K, Zhang X, et al. Preparation and characterization of fiber braided tube reinforced polyethylene hollow fiber membranes via thermally induced phase separation. J Environ Chem Eng. 2023;11(2):109375. 10.1016/j.jece.2023.109375.Search in Google Scholar
(33) Sengar A, Vijayanandan A. Effects of pharmaceuticals on membrane bioreactor: Review on membrane fouling mechanisms and fouling control strategies. Sci Total Environ. 2022;808:152132. 10.1016/j.scitotenv.2021.152132.Search in Google Scholar PubMed
(34) Chen K, Zhao W, Xiao C, Zhu H, Wang Q. Membrane fouling mechanism of HTR-PVDF and HMR-PVDF hollow fiber membranes in MBR system. Water. 2022;14(16):2576. 10.3390/w14162576.Search in Google Scholar
(35) Ahsani M, Hazrati H, Javadi M, Ulbricht M, Yegani R. Preparation of antibiofouling nanocomposite PVDF/Ag-SiO2 membrane and long-term performance evaluation in the MBR system fed by real pharmaceutical wastewater. Sep Purif Technol. 2020;249:116938. 10.1016/j.seppur.2020.116938.Search in Google Scholar
(36) Lai X, Wang C, Wang L, Xiao C. A novel PPTA/PPy composite organic solvent nanofiltration (OSN) membrane prepared by chemical vapor deposition for organic dye wastewater treatment. J Water Process Eng. 2022;45:102533. 10.1016/j.jwpe.2021.102533.Search in Google Scholar
(37) Wang C, Xiao C, Huang Q, Pan J. A study on structure and properties of poly(p-phenylene terephthamide) hybrid porous membranes. J Membr Sci. 2015;474:132–9. 10.1016/j.memsci.2014.09.055.Search in Google Scholar
(38) Wright TM, Carr CM, Grant CA, Lilladhar V, Russell SJ. Strength of hydroentangled fabrics manufactured from photo-irradiated poly para-phenylene terephthalamide (PPTA) fibres. Polym Degrad Stab. 2015;121:193–9. 10.1016/j.polymdegradstab.2015.08.01.Search in Google Scholar
(39) Yang B, Wang L, Zhang M, Luo J, Lu Z, Ding X. Fabrication, applications, and prospects of aramid nanofiber. Adv Funct Mater. 2020;30(22):2000186. 10.1002/adfm.202000186.Search in Google Scholar
(40) Bao X, Wu Q, Tian J, Shi W, Wang W, Zhang Z, et al. Fouling mechanism of forward osmosis membrane in domestic wastewater concentration: Role of substrate structures. Chem Eng J. 2019;370:262–73. 10.1016/j.cej.2019.03.174.Search in Google Scholar
(41) Lu X, Wang J, Han Y, Zhou Y, Song Y, Dong K, et al. Unrevealing the role of in-situ Fe(II)/S2O82− oxidation in sludge solid–liquid separation and membrane fouling behaviors of membrane bioreactor (MBR). Chem Eng J. 2022;434:134666. 10.1016/j.cej.2022.134666.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites

