Abstract
The structure of antioxidants significantly affects the anti-migration and thermal-oxidative aging properties of nitrile butadiene rubber (NBR) composites, which are crucial for applications in aerospace, biomedical, and fuel cell stack sealing industries. The findings reveal that the migration rate of N-(4-anilino phenyl) maleic imide (MC), with a reactive double bond, is only 15.6%, exhibiting the best extraction resistance. 2,4,6-Tris-(N-1,4-dimethylpentyl-p-phenylenediamino)-1,3,5-triazine (TMPPD), with a greater weight and dendritic structure, follows at 47.7%, while N-isopropyl-N′-phenyl-p-phenylenediamine (4010NA) performs the worst, with 77.8%. Furthermore, after thermal-oxidative exposure, the cross-link density of NBR composites increases, generating oxygenated substances, such as ethers, aldehydes, and acids. The addition of antioxidants in the composites improves the thermal-oxidative aging properties compared to those without any antioxidants. Moreover, antioxidants 4010NA and TMPPD display superior resistance to thermal-oxidative aging properties compared to antioxidant MC.
1 Introduction
Nitrile butadiene rubber (NBR) is widely used in aerospace, automotive, biomedical, and other industries (1,2,3,4) owing to its excellent physical and mechanical properties (5), wear resistance (6), and solvent resistance (7). However, the carbon–carbon double bonds in rubber’s molecular chains are susceptible to deterioration when exposed to oxygen and heat (8,9,10). Antioxidants are commonly incorporated into the rubber formulation to solve this problem and extend its lifespan (11,12,13).
Numerous researchers have studied the thermal oxygen aging properties of N-isopropyl-N′-phenyl-p-phenylenediamine (4010NA) (14), N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (4020) (15), and N-(1,4-dimethylamyl)-N′-phenyl-p-phenylenediamine (7PPD) (16), and other commercial diphenylamine antioxidants. The results show that antioxidant structure will significantly affect antioxidants’ anti-aging properties. However, few studies have investigated the relationship between the structure of diphenylamine antioxidants and their thermo-oxidative aging properties. Besides, the small molecular-weight commercial diphenylamine antioxidants, such as 4010NA (IPPD) and 4020 (6PPD), are vulnerable to volatilization, which decreases the efficiency of antioxidants and raises environmental concerns (17). This issue significantly limits the application of rubber products in many fields, such as biomedical, food packaging, and fuel cell stack sealing (18,19,20). Many researchers have studied different structure antioxidants’ anti-migration properties (21,22,23). Song et al. (24) qualitatively investigated the anti-migration properties of 4010NA and graphene oxide grafted 4010NA (GO-g-4010NA) by molecular simulation (MD). The results showed that 4010NA grafted on graphite oxide could improve the anti-migration of 4010NA. Sulekha and Joseph (25) investigated the effect of chlorinated paraffin-bound paraphenylenediamine on the anti-migration properties of NBR. According to the results, the polymer-grafted antioxidants still maintained excellent anti-aging properties following acetone extraction compared to 4020 antioxidants. However, the relationship between antioxidant structure and anti-migration is rarely studied quantitatively by instrumentation.
In this study, three antioxidants with different structures were selected, namely 4010NA, MC, and TMPPD. Among them, 4010NA is the most affordable, MC falls within the moderate price range, while TMPPD is the most expensive option. The relationship between the antioxidant structure and the migration rate was obtained, quantitatively, by UV–visible spectra (UV–vis) and high-performance liquid chromatography (HPLC). Following the aging process, the changes in mechanical properties, thermal-oxidative stability, internal groups, and surface characterizations were investigated by mechanical testing, thermogravimetric analysis (TGA), attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy, and X-ray photoelectron spectroscopy (XPS), respectively, aiming to investigate the effect of antioxidant structure on thermal-oxidative aging performance of NBR composites.
2 Experimental
2.1 Materials
The NBR230S nitrile rubber with an acrylonitrile mass fraction of 35% was supplied by JSR Corporation of Japan. N-(4-Anilino phenyl) maleic imide (MC, C16H12N2O2, ≥99.7% pure, 1.35 g·cm−3), N-isopropyl-N′-phenyl-p-phenylenediamine (4010NA, C15H18N2, ≥99.7% pure, 1.17 g·cm−3), 2,4,6-tris-(N-1,4-dimethylpentyl-p-phenylenediamino)-1,3,5-triazine (TMPPD, C42H63N9, ≥99.7% pure, 1.05 g·cm−3), ethanol (C2H6O, 46.1 g·mol−1, ≥99.7% pure, 0.789 g·cm−3), acetonitrile (C2H3N, 41.1 g·mol−1, ≥99.9% pure, 0.786 g·cm−3), and acetone (C3H6O, 58.1 g·mol−1, ≥99.7% pure, 0.789 g·cm−3) were obtained from China National Pharmaceutical Group Chemical Reagent Co., Ltd. The other materials are commercially available products.
2.2 Preparation of NBR composites
The 100phr NBR230S material was masticated through a two-roll open mill for 2 min. Next, 30 phr of carbon black (N550), 5 phr of ZnO, 1 phr of stearic acid, 2 phr of antioxidant, and 2.5 phr of dicumyl peroxide were added into the NBR. This process produced four samples: NBR without antioxidant, NBR/4010NA, NBR/MC, and NBR/TMPPD, with 2 phr of 4010NA, MC, and TMPPD added into NBR composites. After the compounds were mixed thoroughly, they were cured at 170°C for T 90 + 2 min to produce NBR composites.
2.3 Accelerated aging
NBR composites were placed in an accelerated aging chamber at 100°C for 24, 48, 72, 96, and 168 h, respectively, to obtain the thermal-oxidative sample.
2.4 Characterizations
2.4.1 FTIR spectra
ATR-FTIR and FTIR spectra were obtained using a VERTEX 70 FTIR spectrometer (Germany), with wavenumbers scanned from 400 to 4,000 cm−1.
2.4.2 Liquid chromatograph mass spectrometer (LC-MS)
LC-MS (Xevo G2 Qtof, Waters) was used to determine the mass-to-charge ratio m/z of the antioxidant. The anode ionization mode was used to ionize the sample.
2.4.3 UV–vis
The absorption strength of the migration solution at 200–400 nm was determined by an Agilent-8453 UV–vis spectrophotometer. To obtain the migration solution, 0.5 g of NBR composites were soaked in 30 mL of ethanol at 60℃ for 24 h. The intensity of the absorption peak was related to the number of antioxidants that had migrated into the migration solution.
2.4.4 HPLC
The migration rate of the antioxidant in an ethanol solution was quantified using HPLC (LC-20AD, Shimadzu, Japan) (26). A C18-5 column was chosen for the analysis. The mobile phase was water (A) and acetonitrile (B). Binary linear gradients were applied: starting with 80% A for 6 min, followed by a transition from 20% to 100% B over 20 min, and finally maintained at 100% B for 20 min, totaling 40 min. The wavelength of UV detection is 289 nm, and the temperature of the column is 40°C.
The concentration of antioxidants in the migration solutions was determined by inferring the analytical curve after determining the peak area of the migration solutions. Finally, the migration rate was calculated using the following equation:
where
2.4.5 Characterization of curing parameters
The curing characteristics of NBR compounds were analyzed using an M-3000A rotorless rheometer (High-Tech Testing Instruments Co., Ltd., Dongguan, China) at 170℃.
According to the curing curve, M H, M L, T 90, and T 10 parameters are obtained, representing the maximum and minimum torque, the optimal vulcanization time, and scorch time, respectively. T 90 and T 10 correspond to the points time when the torque value reaches M L + (M H – M L) × 90% and M L + (M H – M L) × 10% on the curing curve, respectively (27). The curing rate index (CRI), representing the vulcanization speed of NBR compounds, was calculated as follows (28):
2.4.6 Mechanical properties
The mechanical properties of NBR composites were measured by the AI-7000S1 electronic tensile testing machine (High-Tech Testing Instruments Co., Ltd., Dongguan, China) at a speed of 500 mm·min−1 following ASTM D412.
2.4.7 Cross-link density measurement
To determine the cross-link density (
where Vr and Vs refer to the volume fraction of the sample that reaches swelling equilibrium and the molar volume (acetone: 73.7 cm3·mol−1).
where
2.4.8 TGA
TGA of NBR composites was conducted by a TGA-60A instrument (Shimadzu, Japan). Each sample, weighing approximately 10 mg, was placed in the sample chamber, heating from 40°C to 800°C under an air atmosphere. The heating rate is 10°C·min−1.
2.4.9 Differential scanning calorimeter (DSC)
NBR composites of the temperature at oxidation exothermic were determined by a NETZSCH instrument DSC (Germany) from 40°C to 500°C at a scanning rate of 10°C·min−1 under an air atmosphere.
2.4.10 XPS
XPS (Thermo Scientific K-Alpha, America) was used to analyze the effect of thermal-oxidative aging on the surface characterization of NBR composites.
3 Results and discussion
3.1 Effect of antioxidant structure on migrant resistance
The structure of antioxidants significantly impacts the solvent extraction resistance and thermal-oxidative aging performance of NBR composites (30). Figure 1a shows that the absorption peaks at 1,570 and 1,760 cm−1 for antioxidant MC correspond to the C═C and C═O stretching vibrations, respectively (23). Antioxidant TMPPD, on the contrary, has a characteristic absorption peak at 1,560 and 1,410 cm−1, corresponding to the triazine ring of stretching vibrations (31,32). Additionally, the three antioxidants exhibit a stretching vibration absorption peak of N–H and Ar–H at 3,370 and 1,600, 1,500 cm−1, respectively. The LC-MS results show that the molecular weights of 4010NA, MC, and TMPPD correspond to 227, 265, and 694 g·mol−1, respectively. In summary, MC contains reactive double bonds, TMPPD has a high molecular weight, and 4010NA has a low molecular weight and does not contain reactive functional groups.

(a) FTIR spectra of three antioxidants and (b) LC-MS result of three antioxidants.
UV spectroscopy and HPLC were employed to investigate the impact of the antioxidant structure on solvent extraction resistance (18). As shown in Figure 2d, all antioxidant migration solutions exhibit strong UV absorption peaks at 289 nm. A higher UV absorption intensity at 289 nm of the migration solution indicates a higher concentration of antioxidants in the solution.

HPLC analytical curves: (a) 4010NA, (b) MC, (c) TMPPD, (d) UV–vis spectra of four migration solutions, (e) HPLC of four migration solutions, and (f) the migration rate of antioxidants.
To plot the analytical curves, a series of standard acetonitrile solutions containing different nominal values of antioxidants (0.1 mg·10 mL−1, 0.2 mg·10 mL−1, 0.3 mg·10 mL−1, and 0.4 mg·10 mL−1) were analyzed using HPLC, generating a graph of antioxidant concentration versus peak area, as shown in Figure 2 (33). As the coefficient of determination R 2 > 0.99, the equation of the analytical curve was used to quantify the antioxidant concentrations in the migration solution. Finally, the migration rate of the three antioxidants was quantitatively calculated using Eq. 1. The obtained data, presented in Table 1, revealed the migration rate of 4010NA, MC, and TMPPD as 77.8%, 15.6%, and 47.7%, respectively.
Retention time and migration rate of antioxidant
| NBR/4010NA | NBR/MC | NBR/TMPPD | |
|---|---|---|---|
| Retention time (min) | 9.9 | 16.3 | 12.9 |
| Migration rate (%) | 77.8 | 15.6 | 47.7 |
A comprehensive analysis revealed that antioxidant MC, containing a reactive double bond capable of being grafted onto the molecular chain during the vulcanization process of NBR, exhibits the best extraction resistance. Antioxidant TMPPD has a higher molecular weight and a dendritic structure, which enables it to intertwine within the cross-link network of rubber, making it difficult to extract. Consequently, TMPPD exhibits a moderate level of extraction resistance. In contrast, antioxidant 4010NA has a low molecular weight and a simple structure, causing it to be easily extracted by solvents.
3.2 Effect of antioxidant on the curing characteristics of NBR composites
The curing curves and parameters of NBR compounds at 170°C are shown in Figure 3 and Table 2. The M H – M L values of the four samples follow the relationship: NBR > NBR/MC > NBR/TMPPD > NBR/4010NA. As is illustrated in Figure 3b, the amine group (R-NH) in diphenylamine acts as a free radical acceptor and deactivates radical (RO·) released by peroxide during the vulcanization process. Higher amine group activity leads to a more significant effect on the vulcanization reaction (34,35). Both steric hindrance and electronic effects influence the activity of the amine functional groups, with electronic effects being more impactful (36). TMPPD features a dendritic structure with long carbon chains, which generates steric hindrance, reducing the amine group’s activity. As a result, TMPPD exerts a lower influence on the vulcanization of NBR composites compared to 4010NA. The MC molecular structure contains a strong electron-absorbing group, C═O, which exhibits a huge electronic effect and significantly reduces the amine group’s activity, leading to the lowest influence on the vulcanization reaction.

(a) The curing curves of NBR composites at 170°C and (b) mechanism of diphenylamine antioxidant inhibiting curing reaction.
The curing parameters of NBR composites at 170°C
| M H (dNm) | M L (dNm) | M H − M L (dNm) | T 10 (m:s) | T 90 (m:s) | CRI (s−1) | |
|---|---|---|---|---|---|---|
| NBR | 12.8 | 1.0 | 11.8 | 0:43 | 5:37 | 0.34 |
| NBR/4010NA | 7.5 | 0.8 | 6.7 | 0:52 | 6:00 | 0.32 |
| NBR/MC | 12.3 | 0.8 | 11.5 | 0:48 | 6:46 | 0.28 |
| NBR/TMPPD | 11.4 | 0.9 | 10.5 | 0:47 | 5:31 | 0.35 |
3.3 Effect of antioxidant on the thermal-oxidative stability of NBR composites
The thermogravimetry (TG) and differential thermogravimetry (DTG) of four NBR composites recorded by TGA are shown in Figure 4. The TG curve reveals that the NBR composites’ degradation in the air is separated into two stages. The first stage (390–530℃) corresponds to the oxidation chain-breaking of NBR composites, while the second stage (550–680℃) is related to the oxidation reaction of oxidation products and carbon black (37). Meanwhile, compared to NBR composites without antioxidants, the addition of 4010NA, MC, and TMPPD increases T 5 of NBR composites by 18.1℃, 5.8℃, and 12.1℃, respectively, suggesting an improvement in NBR’s initial thermal-oxygen stability, as shown in Table 3. It is possible that the aniline radicals can terminate the free oxygen radicals generated in the system initially (38). Figure 4b shows that the peak area of the DTG curve of NBR composites incorporating TMPPD and MC is larger than that of the control sample, whereas the area of 4010NA is smaller. This result indicates that antioxidants TMPPD and MC accelerate the chain-breaking reaction of NBR after the beginning of the decomposition reaction, while antioxidant 4010NA does the opposite. Besides, the char residue for 4010NA is significantly higher than others, being 16.9%. In short, the TGA in air demonstrates that MC and TMPPD can improve initial thermal stability without preventing the chain-breaking reaction after degradation begins. 4010NA promotes the carbon formation reaction of NBR, enhancing thermal oxygen stability.

(a) TG curve of NBR composites in air and (b) DTG curve of NBR composites in air.
Effect of antioxidants on the thermal stability of NBR composites in air
| Sample | Temperature at 5% weight loss T 5 (°C) | Temperature at 50% weight loss T 50 (°C) | Maximum degradation temperature T max (°C) | Char residue weight (%) |
|---|---|---|---|---|
| NBR | 390.9 | 480.6 | 475.0 | 6.6 |
| NBR/4010NA | 409.6 | 493.9 | 475.0 | 16.9 |
| NBR/MC | 396.7 | 482.0 | 475.0 | 6.6 |
| NBR/TMPPD | 403.0 | 475.9 | 466.4 | 6.6 |
3.4 Effect of antioxidant on mechanical properties of NBR composites after thermal-oxidative aging
Figure 5 illustrates the variations in mechanical properties of NBR composites after aging at 100°C for 168 h. All three antioxidants improve the thermal-oxidative aging performance of NBR composites compared to the control sample. In particular, TMPPD shows the most substantial improvement, exhibiting the highest retention rate for tensile strength and elongation at break after aging, followed by 4010NA. Furthermore, the increase in hardness and M100 after aging at 100°C for 168 h suggests that the cross-linking reaction rate surpasses the chain-breaking reaction. Moreover, Figure 5e displays the effects of thermal-oxidative aging time on cross-linking densities of NBR composites. Interestingly, MC and TMPPD increased the rubber’s cross-linking density before aging, while 4010NA decreased it. Furthermore, the cross-linking density of NBR composites exhibits a rising trend with aging time, but the increase is slower with added antioxidants 4010NA and TMPPD. In conclusion, TMPPD and 4010NA demonstrate more excellent thermal-oxidative aging properties, compared with MC. This can be attributed to the fact that MC is grafted onto the molecular chain; it cannot move freely to remove the radicals generated by oxidation reaction on the rubber surface.

Influences of aging time on (a) tensile strength retention, (b) elongation at break retention, (c) modulus at 100% elongation, (d) cross-linking density of NBR composites, and (e) hardness.
3.5 Structural changes of NBR composites after aging
Figure 6a presents the ATR-FTIR spectra of the NBR composites before and after thermal-oxidative aging. The intensities of absorption for the stretching of C═O,
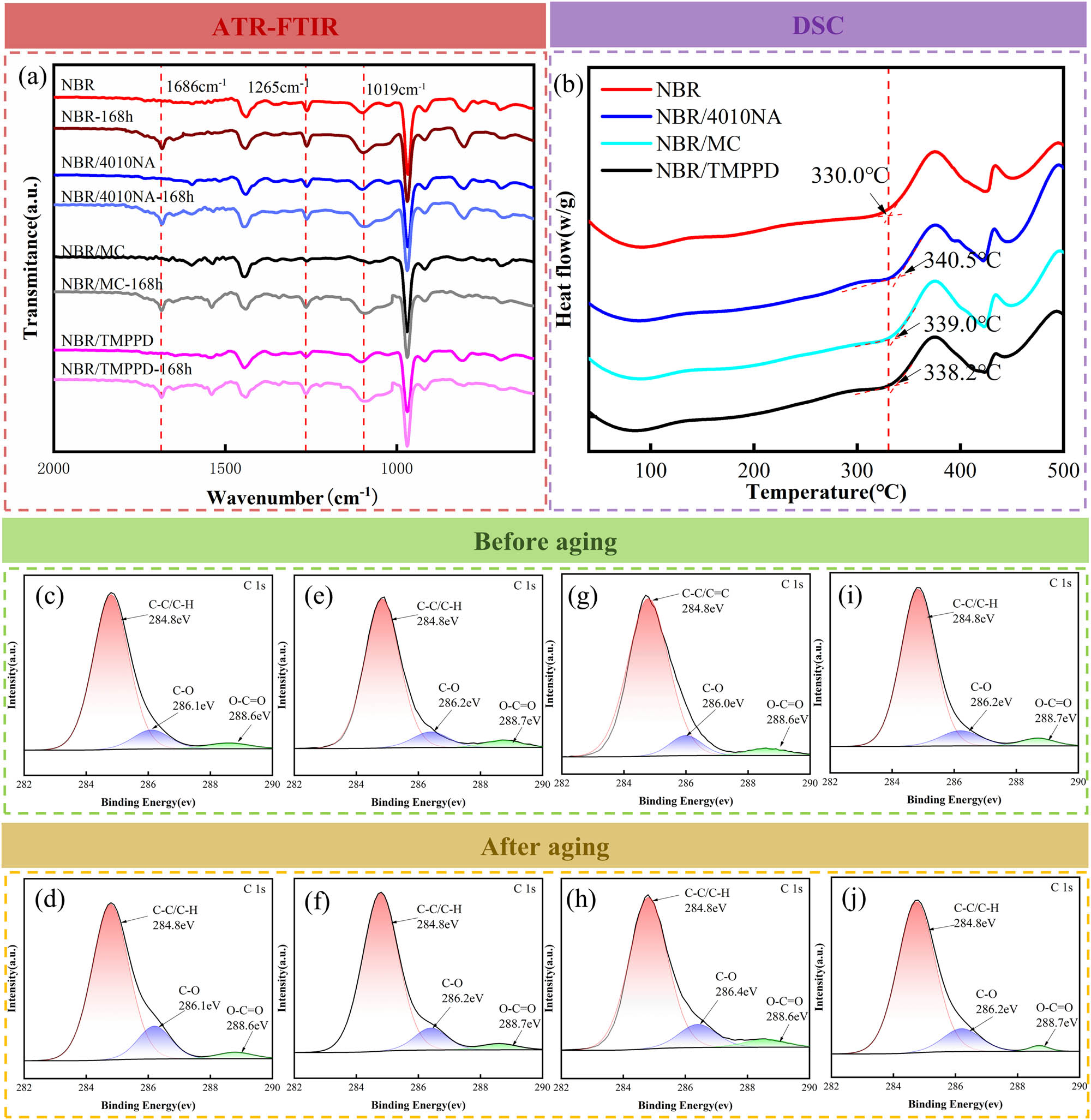
(a) ATR-FTIR spectra of the NBR composites before and after thermal oxygen aging at 100°C for 168 h. (b) The oxidation induction temperatures of NBR composites. High-resolution C1s XPS spectra of NBR composites after aging at 100°C for 168 h: (c) NBR, (d) NBR aging 168 h, (e) NBR/4010NA, (f) NBR/4010NA aging 168 h, (g) NBR/MC, (h) NBR/MC aging 168 h, (i) NBR/TMPPD, and (j) NBR/TMPPD aging 168 h.
As illustrated in Figure 6b, the oxidation induction temperatures of the control sample and NBR composites with added antioxidants 4010NA, MC, and TMPPD were 330°C, 340.5°C, 339.0°C, and 338.2°C, respectively. This analysis indicates that incorporation of three antioxidants enhances the oxidation resistance of the NBR composites, which is also confirmed by TGA in the air (23).
To further analyze the surface chemistry changes and aging degree of NBR composites, high-resolution C1s XPS spectra were obtained by the XPS measurement, as shown in Figure 6. The result reveals that three types of carbon atoms, C–C and C–H (284.8 eV), C–O (286.2 eV), and O–C═O (288.7 eV), were generated in NBR composites (40). Table 4 reveals that the peak area ratio of oxygenic carbon atoms (C–O and O–C═O) increased for all NBR composites after thermal-oxidative aging, signifying the generation of oxygenated substances, such as ethers, aldehydes, and acids. Notably, the control sample without any antioxidant exhibits the highest increase of 39.3%, followed by NBR/MC with a 36.5% increase, NBR/TMPPD with a 12.4% increase, and NBR/4010NA composites with a mere 3.65% increase. This result is related to the activity ability of antioxidants in rubber cross-linking networks (41). Antioxidant 4010NA, a small molecular-weight antioxidant, cannot graft onto the rubber molecular chain. This structure allows it to easily migrate to the rubber surface, where it scavenges free radicals produced by oxidation, leading to the lowest oxygen-containing groups on the surface. Antioxidant TMPPD, which features a larger molecular mass and dendritic molecular structure, exhibits moderate molecular mobility within the rubber cross-linking network, resulting in a moderate oxygen-containing group on the surface. Antioxidant MC is grafted onto the rubber macromolecular chains and cannot freely move to the surface of NBR composite materials to remove the radicals generated by an oxidation reaction, resulting in the highest oxygen-containing groups on the surface.
XPS analyses for the surface characterizations of NBR composites before and after aging at 100°C for 168 h
| Sample | NBR | NBR/4010NA | NBR/MC | NBR/TMPPD | ||||
|---|---|---|---|---|---|---|---|---|
| Before aging | After aging | Before aging | After aging | Before aging | After aging | Before aging | After aging | |
| C–C, C–H | 0.837 | 0.773 | 0.863 | 0.857 | 0.874 | 0.828 | 0.863 | 0.846 |
| C–O | 0.143 | 0.190 | 0.094 | 0.108 | 0.088 | 0.124 | 0.099 | 0.139 |
| O–C═O | 0.020 | 0.037 | 0.043 | 0.035 | 0.038 | 0.048 | 0.038 | 0.015 |
| Increase ratea (%) | 39.3 | 3.65 | 36.5 | 12.4 | ||||
a Increase rate (%): increased peak area (C–O and O–C═O)/initial peak area (C–O and O–C═O).
3.6 Possible antioxidative mechanisms
This study demonstrates that diphenylamine antioxidants 4010NA, MC, and TMPPD effectively improve the thermal-oxidative aging property, as evidenced by the TGA, FTIR, DSC, and XPS test results. The possible mechanism for the anti-thermal-oxidative aging of diphenylamine antioxidants in NBR composites is presented in Figure 7a. Cycle II in Figure 7a illustrates the decomposition of the rubber molecular chain (RH) under heat and force, producing macromolecular radicals (R·) that combine with oxygen to generate peroxyl radicals (ROO·). These peroxyl radicals then further attack RH to form new R· and hydrogen peroxide compounds (ROOH), which continue to attack new RH. Simultaneously, the rapid decomposition of ROOH further accelerates the aging process. The experimental results of ATR-FTIR spectroscopy and XPS confirm that NBR composites produce significant amounts of oxygenated material during thermal-oxidative aging. However, when diphenylamine antioxidants are added, the two molecules RO·, generated by ROOH decomposition, are used up to form two molecules of stable ROH molecules, effectively disrupting Cycle II. Furthermore, the aniline antioxidant undergoes circular transformation, as shown in Cycle I, allowing it to continuously scavenge free radicals in the system and improve the aging resistance of NBR composites.

(a) Thermal-oxidative aging mechanism of three antioxidants and (b) mechanism of antioxidant MC graft reaction.
Figure 7b illustrates the mechanism for the antioxidant MC grafts onto the NBR molecular chain during the vulcanization reaction. This explains why MC exhibits superior solvent extraction resistance, prolongs the NBR CRI, and improves the cross-linking density of NBR composites. However, since antioxidant MC is grafted onto the molecular chain, it cannot move freely to remove the radicals generated by oxidation on the rubber surface. Consequently, the anti-thermal-oxidative efficiency of MC is lower than that of 4010NA and TMPPD.
4 Conclusion
In this study, we investigated the effect of phenylamine antioxidant chemical structures on solvent extraction resistance and thermal-oxidative aging performance of NBR composites. The results revealed that the small molecular-weight antioxidant, 4010NA, exhibited weak anti-migration properties but displayed strong thermal oxygen aging characteristics because its higher mobility within the rubber helps it remove radicals generated during thermal oxygen aging. TMPPD, with its larger molecular mass and dendritic structure, demonstrated moderate anti-migration and anti-aging properties owing to its moderate molecular mobility within the rubber cross-linking network. Antioxidant MC, containing reactive double bonds, can be grafted to the rubber molecular chain during the rubber curing process, causing the lowest mobility among the rubber cross-linking network. As a result, it exhibits excellent extraction resistance but the poorest thermal oxygen aging properties. Furthermore, all four NBR composites exhibited increased cross-linking densities and generated oxygenated substances such as ethers, aldehydes, and acids following thermal-oxidative exposure. These changes were observed through variations in mechanical properties, ATR-FTIR, and XPS analyses. Overall, this study highlights the significant influence of phenylamine antioxidant structures on the anti-migration and anti-aging performance of NBR composites, providing valuable insights for the optimization and application of antioxidants in organic media resistance rubber materials.
Acknowledgements
The authors acknowledge funding supports provided by the National Natural Science Foundation of China, which has significantly contributed to the success of this research project.
-
Funding information: This research was supported by the National Natural Science Foundation of China (No. 52127804).
-
Author contributions: Wei Liu: writing – original draft, investigation, experiment, data analysis, and plotting; Hua Zou: data analysis, methodology, project administration, and resource. Baotong Xing: experiment, writing – review, and editing; Shuqi Wang: methodology and data analysis; Hongda Mao: data analysis and methodology; Jiyang Zhang: methodology and resource.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The experimental data and analysis results exhibited in this study are original and have been contributed by all authors. The corresponding author can make this information available to interested parties upon providing a reasonable justification.
References
(1) Akinlabi AK, Okieimen FE, Aigbodion AI. Thermal aging properties and chemical resistance of blends of natural rubber and epoxidized low molecular weight natural rubber. J Appl Polym Sci. 2005;98(4):1733–9.10.1002/app.22314Search in Google Scholar
(2) Chou HW, Huang JS, Lin ST. Effects of thermal aging on fatigue of carbon black-reinforced EPDM rubber. J Appl Polym Sci. 2007;103(2):1244–51.10.1002/app.24967Search in Google Scholar
(3) Sulekha PB, Joseph R, Manjooran KB. New oligomer-bound antioxidants in natural rubber/polybutadiene rubber and natural rubber/styrene-butadiene rubber blends. J Appl Polym Sci. 2004;93(1):437–43.10.1002/app.20427Search in Google Scholar
(4) Li S, Ke YC, Xie LY, Zhao ZZ, Huang XY, Wang YC, et al. Study on the aging of three typical rubber materials under high and low-temperature cyclic environment. E-Polymers. 2023;23(1):80–9.10.1515/epoly-2022-8089Search in Google Scholar
(5) Gao Q, Hou D, Ji Z, Yu X. Study on the property of HNBR/NBR blends. Chem Ind. 2013;42(10):1872–4.Search in Google Scholar
(6) Liu X, Zhou X, Kuang F, Zuo H, Huang J. Mechanical and tribological properties of nitrile rubber reinforced by nano-SiO2: Molecular dynamics simulation. Tribol Lett. 2021;691–11.10.21203/rs.3.rs-194024/v1Search in Google Scholar
(7) Ge B, Yin Y, Wang Y, Zhang H, Yuan P. Study of solvent effect on the dissolution, size, structure and catalytic hydrogenation of nitrile butadiene rubber. Ciesc J. 2021;72(1):543–54.Search in Google Scholar
(8) Han R, Wu Y, Quan X, Niu K. Effects of cross-linking densities on mechanical properties of nitrile rubber composites in thermal oxidative aging environment. J Appl Polym Sci. 2020;137(36):49076.10.1002/app.49076Search in Google Scholar
(9) Wu WJ, Li HQ, Yang SY, Lai XJ, Fan HB, Zeng XR. Thermo-oxidative aging resistance and mechanism of a macromolecular hindered phenol antioxidant for natural rubber. J Elastomers Plast. 2017;50(4):372–87.10.1177/0095244317729556Search in Google Scholar
(10) Hu D, Luo Y, Chen Y, Lin J, Jia D. Mesoporous silica as nanocarrier of antioxidant for highly anti-aging elastomer composites. Polym Degrad Stab. 2019;169:108987.10.1016/j.polymdegradstab.2019.108987Search in Google Scholar
(11) Kandil H, El Nashar DE, Ward AA, Khalaf AI. Jojoba seed powder as eco-friendly antioxidant for rubber products. J Appl Polym Sci. 2022;139(29):562542.10.1002/app.52642Search in Google Scholar
(12) Oncel S, Kurtoglu B, Karaagac B. An alternative antioxidant for sulfur-vulcanized natural rubber: henna. J Elastomers Plast. 2019;51(5):440–56.10.1177/0095244318796594Search in Google Scholar
(13) Zhao W, He J, Yu P, Jiang X, Zhang L. Recent progress in the rubber antioxidants: a review. Polym Degrad Stab. 2023;207:110223.10.1016/j.polymdegradstab.2022.110223Search in Google Scholar
(14) Colom X, Andreu-Mateu F, Cañavate FJ, Mujal R, Carrillo F. Study of the influence of IPPD on thermo‐oxidation process of elastomeric hose. J Appl Polym Sci. 2009;114(5):2011–8.10.1002/app.30746Search in Google Scholar
(15) Osswald K, Reincke K, Schossig M, Sökmen S, Langer B. Influence of different types of antioxidants on the aging behavior of carbon-black filled NR and SBR vulcanizates. Polym Test. 2019;79(8):106053.10.1016/j.polymertesting.2019.106053Search in Google Scholar
(16) Huntink NM, Datta RN, Noordermeer JWM. Addressing durability of rubber compounds. Rubber Chem Technol. 2004;77(3):476–511.10.5254/1.3547833Search in Google Scholar
(17) Ignatz-Hoover F, To BH, Datta RN, De Hoog AJ, Huntink NM, Talma AG. Chemical additives migration in rubber. Rubber Chem Technol. 2003;76(3):747–68.10.5254/1.3547765Search in Google Scholar
(18) Fu Y, Zhao D, Yao P, Wang W, Zhang L, Lvov Y. Highly aging-resistant elastomers doped with antioxidant-loaded clay nanotubes. ACS Appl Mater Interfaces. 2015;7(15):8156–65.10.1021/acsami.5b00993Search in Google Scholar PubMed
(19) Lu L, Cheng C, Xu L, Pan L, Xia HF, Lu L. Migration of antioxidants from food-contact rubber materials to food simulants. J Food Eng. 2022;318(2):110904.10.1016/j.jfoodeng.2021.110904Search in Google Scholar
(20) Kwon T, Lim Y, Cho J, Lawler R, Min BJ, Goddard WA, et al. Antioxidant technology for durability enhancement in polymer electrolyte membranes for fuel cell applications. Mater Today. 2022;58:135–63.10.1016/j.mattod.2022.06.021Search in Google Scholar
(21) Zhang L, Liu J, Fan X, Cai Z, Zhu M. Thermal antioxidation behavior of a new hindered phenol with branched structure for nitrile rubber. Int J Mod Phys B. 2019;33:1940058.10.1142/S0217979219400587Search in Google Scholar
(22) Giurginca M, Ivan G, Herdan J. Grafting antioxidants VII. Grafting of antioxidants containing aminic and thiol groups to epoxidized natural rubber. Polym Degrad Stab. 1994;44(1):79–83.10.1016/0141-3910(94)90036-1Search in Google Scholar
(23) Luo K, Ye X, Zhang H, Liu J, Luo Y, Zhu J, et al. Vulcanization and antioxidation effects of accelerator modified antioxidant in styrene-butadiene rubber: experimental and computational studies. Polym Degrad Stab. 2020;177:109181.10.1016/j.polymdegradstab.2020.109181Search in Google Scholar
(24) Song M, Yue X, Chang C, Cao F, Yu G, Wang X. Investigation of the compatibility and damping performance of graphene oxide grafted antioxidant/nitrile-butadiene rubber composite: insights from experiment and molecular simulation. Polymers. 2022;14(4):736.10.3390/polym14040736Search in Google Scholar PubMed PubMed Central
(25) Sulekha PB, Joseph R. Studies on polymer bound antioxidants in NBR vulcanizates. Int J Polym Mater Polym Biomater. 2005;54(5):333–45.10.1080/00914030390251788Search in Google Scholar
(26) Dopico MS, López VJM, Bouza R, Abad MJ, González E, González MV. Extraction and quantification of antioxidants from low-density polyethylene by microwave energy and liquid chromatography. Anal Chim Acta. 2004;521(2):179–88.10.1016/j.aca.2004.05.087Search in Google Scholar
(27) Huangfu S, Jin G, Sun Q, Li L, Yu P, Wang R, et al. The use of crude carbon dots as novel antioxidants for natural rubber. Polym Degrad Stab. 2021;186:109506.10.1016/j.polymdegradstab.2021.109506Search in Google Scholar
(28) Jiang Y, Zhang Y. Improving thermal oxidative aging resistance and anti‐reversion property of natural rubber by adding a cross-linking agent. J Appl Polym Sci. 2022;139(14):51882.10.1002/app.51882Search in Google Scholar
(29) Campos GN, Ribeiro Coimbra AC, Da Silva AA, Dutra Da Rocha EB, Linhares FN, Guimaraes Furtado CR, et al. Cross-link density measurement of nitrile rubber vulcanizates using dynamic shear test (a). Polimeros. 2022;32(1):e2022011.10.1590/0104-1428.20220031Search in Google Scholar
(30) Wakil AE, Mogy S, Halim SF, Hakim A. Enhancement of aging resistance of EPDM rubber by natural rubber‐g‐N (4‐phenylenediamine) maleimide as a grafted antioxidant. J Vinyl Addit Techn. 2022;28(2):367–78.10.1002/vnl.21875Search in Google Scholar
(31) Qi M-H, Gao M-L, Liu L, Han Z-B. Robust bifunctional core-shell catalyst for one-pot tandem reaction. Inorg Chem. 2018;57(23):14467.10.1021/acs.inorgchem.8b02303Search in Google Scholar PubMed
(32) Ke C-H, Li J, Fang K-Y, Zhu Q-L, Zhu J, Yan Q, et al. Synergistic effect between a novel hyperbranched charring agent and ammonium polyphosphate on the flame retardant and anti-dripping properties of polylactide. Polym Degrad Stab. 2010;95(5):763–70.10.1016/j.polymdegradstab.2010.02.011Search in Google Scholar
(33) Avila LM, Dos Santos APF, De Mattos DIM, De Souza CG, De Andrade DF, D’avila LA. Determination of ethanol in gasoline by high-performance liquid chromatography. Fuel. 2018;212:236–9.10.1016/j.fuel.2017.10.039Search in Google Scholar
(34) Li ZH, Chen SJ, Zhang J, Shi DQ. Influence of different antioxidants on cure kinetics and aging behaviours of ethylene propylene diene rubber/low density polyethylene blends. Plast Rubber Compos. 2009;38(5):187–94.10.1179/174328909X435311Search in Google Scholar
(35) Sirisinha C, Phoowakeereewiwat S, Saeoui P. Cure and dynamic mechanical properties in peroxide-cured isoprene rubber: effects of stearic acid and amine-based antioxidant. Eur Polym J. 2004;40(8):1779–85.10.1016/j.eurpolymj.2004.03.002Search in Google Scholar
(36) Ferradino AG. Antioxidant selection for peroxide cure elastomer applications. Rubber Chem Technol. 2003;76(3):694–718.10.5254/1.3547763Search in Google Scholar
(37) Ding P, Zou Y, He J, Sun Y, Liu F. Effects of a novel chitosan based macromolecule antioxidant cos-GMMP on the thermo-oxidative aging of styrene-butadiene rubber/silica composites. Polym Degrad Stab. 2022;195:109813.10.1016/j.polymdegradstab.2021.109813Search in Google Scholar
(38) Ning N, Ma Q, Zhang Y, Zhang L, Wu H, Tian M. Enhanced thermo-oxidative aging resistance of EPDM at high temperature by using synergistic antioxidants. Polym Degrad Stab. 2014;102:1–8.10.1016/j.polymdegradstab.2014.01.037Search in Google Scholar
(39) Zhou MZ, Wang HR, Guo X, Wei YC, Liao SQ. Synergistic effect of thermal oxygen and UV aging on natural rubber. E-Polymers. 2023;23(1):20230016.10.1515/epoly-2023-0016Search in Google Scholar
(40) Zheng W, Jia Z, Zhang Z, Yang W, Zhang L, Wu S. Improvements of lanthanum complex on the thermal-oxidative stability of natural rubber. J Mater Sci. 2016;51(1):9043–56.10.1007/s10853-016-0157-4Search in Google Scholar
(41) Wang X, Yang K, Zhang P. The influence of amine antioxidant D37 on the ozone aging process of SIBR. Polymer. 2022;238(3):124425.10.1016/j.polymer.2021.124425Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites

