Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
Abstract
This work investigated the thermal, morphological, and tensile properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide) (PLLA-PEG-PLLA)/thermoplastic starch (TPS) blends with 3 wt% citric acid (CA) treatment of TPS. The blends with PLLA-PEG-PLLA/CA-TPS ratios of 100/0, 90/10, 80/20, and 60/40 (w/w) were investigated and compared with PLLA-PEG-PLLA/CA-free TPS blends. Crystallizability of the blends decreased and thermal stability increased as the TPS content increased. The thermal stability of the blends was found to improve after CA treatment of TPS. The PLLA-PEG-PLLA/CA-TPS blends showed better phase compatibility than those of the PLLA-PEG-PLLA/CA-free TPS blends. The tensile properties of the blends were improved by CA treatment of TPS. In conclusion, improvement in thermal stability, phase compatibility, and tensile properties of the PLLA-PEG-PLLA/TPS blends was obtained by CA treatment of TPS. The resulting PLLA-PEG-PLLA/CA-TPS blends could potentially be used to prepare biodegradable and flexible bioplastics.
1 Introduction
An important synthetic bioplastic is poly(l-lactic acid) or poly(l-lactide) (PLLA) because of its bio-renewability, biocompatibility, and biodegradability (1,2,3,4,5,6). The utilization of PLLA in plastic applications is considered to solve the problem of non-biodegradable petroleum-based plastic waste (7). However, low flexibility and high production cost of PLLA are the main drawbacks compared with traditional petroleum-based plastics which restrict its range of applications (8,9,10,11,12).
Poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide) (PLLA-PEG-PLLA) block copolymers are more flexible and more hydrophilic than PLLA due to the characteristics of PEG blocks (13,14,15). PLLA-PEG-PLLA reacts with a chain extender by post melt blending (14,15) and by in situ ring-opening polymerization (16) to control its melt flow ability for conventional melt processing. PLLA-PEG-PLLA is blended with low-cost thermoplastic starch (TPS) to reduce its production cost and to increase its biodegradation rate (17,18). The results showed that the PLLA-PEG-PLLA/TPS blends exhibited good phase compatibility compared with the PLLA/TPS blends. However, blending of TPS decreased the flexibility of PLLA-PEG-PLLA due to poor mechanical properties of TPS (12,19,20). Thus, the improvement in flexibility of PLLA-PEG-PLLA/TPS blends emerged as a challenge in this work.
Wang et al. (21,22) prepared PLLA/cornstarch blends in the presence of glycerol and citric acid (CA) by one-step extrusion processing. They found that the starch was degraded by CA treatment to improve the dispersion and compatibility in the blends. Kahar et al. (23) systematically investigated the effect of CA on the blend morphology and tensile properties of polyethylene/natural rubber/TPS blends. They found that the phase compatibility improved and the tensile properties increased after CA modification of TPS. Ibrahim et al. (24) treated TPS with CA to improve compatibility in PLLA/TPS blends. The results demonstrated that tensile stress, strain at break, and Young’s modulus of the PLLA/TPS blends increased when the TPS was treated with CA. The tensile stress and Young’s modulus of the blends increased with the CA content until CA content was 3 wt%. However, PLLA-PEG-PLLA/TPS treated with CA blends have not been reported so far. Thus, we hypothesized that CA treatment of TPS could improve phase compatibility and tensile properties of the PLLA-PEG-PLLA/TPS blends.
The objective of this work was to improve phase compatibility and tensile properties of the PLLA-PEG-PLLA/TPS blends by CA treatment. For this purpose, TPS was treated with CA before melt blending with PLLA-PEG-PLLA. The effects of CA treatment and blend ratio on the thermal, morphological, and tensile properties of the PLLA-PEG-PLLA/TPS blends were determined and discussed. The PLLA-PEG-PLLA/CA-free TPS blends were also prepared for comparison.
2 Materials and methods
2.1 Materials
A chain-extended PLLA-PEG-PLLA with melt flow index of 26 g·10 min−1 (determined at 190°C under 2.16 kg load force) was synthesized in our research unit, as described in our previous work (16). TPS, (TapioplastTM) prepared from tapioca starch (79% amylopectin) was supplied by SMS Corporation Co., Ltd. (Pathum Thani, Thailand). CA was obtained from Merck.
2.2 CA treatment of TPS
TPS and CA were dried at 50°C under vacuum overnight before melt blending using a HAAKE PolyLab OS Rheomix batch-mixer (Thermo Scientific, USA) at 150°C for 10 min with a rotor speed of 100 rpm. TPS with and without 3 wt% CA treatment was prepared and designed as 3% CA-TPS and CA-free TPS, respectively.
2.3 Preparation of PLLA-PEG-PLLA/TPS blends
PLLA-PEG-PLLA and 3% CA-TPS were dried at 50°C under vacuum overnight before melt blending in a HAAKE PolyLab OS Rheomix batch-mixer (Thermo Scientific, USA) at 180°C for 5 min with a rotor speed of 100 rpm. PLLA-PEG-PLLA/3% CA-TPS blends with blend ratios of 100/0, 90/10, 80/20, and 60/40 (w/w) were investigated. PLLA-PEG-PLLA/CA-free TPS blends were also prepared by the same method for comparison.
2.4 Characterization of treated TPS and PLLA-PEG-PLLA/TPS blends
The reaction between TPS and CA was characterized with a Fourier-transform infrared (FTIR) spectrometer (Invenio-S, Bruker) equipped with an attenuated total reflectance accessory in the range of 4,000−500 cm−1 with a 4 cm−1 resolution and 32 accumulated scans.
The thermal transition properties of the blends were determined with a Pyris Diamond differential scanning calorimeter (DSC, Perkin Elmer) over the temperature range 0–200°C with a heating rate of 10°C·min−1 under a nitrogen gas flow. Thermal history of the blends was erased by melting at 200°C for 3 min followed with fast quenching to 0°C with cooling rate of 100°C·min−1 before the DSC heating scan. The degree of crystallinity (X c) of the blend was calculated using Eq. 1 (9). Each DSC result was the average from three measurements.
where ∆H m and ∆H cc are enthalpies of melting and cold crystallization, respectively. The ΔH m of 100% X c PLLA is 93.6 J·g−1 (9,25). W PLLA is the PLLA weight fraction.
The thermal decomposition properties of the blends were determined with a SDT Q600 thermogravimetric analyzer (TGA, TA-Instrument) over the temperature range of 50–1,000°C at a heating rate of 20°C·min−1 under a nitrogen gas flow.
The phase morphology of cryofractured surfaces for the blend films were examined with a JSM-6460LV scanning electron microscope (SEM, JEOL) at an acceleration potential of 15 kV. Cryofracture surfaces were prepared by fracturing under liquid nitrogen. The samples were sputter-coated with gold prior to scan.
The tensile properties of the blend films (100 mm × 10 mm) were measured with a LY-1066B universal mechanical tester (Dongguan Liyi Environmental Technology Co., Ltd) at 25°C with a 100 kg load cell, a 50 mm·min−1 crosshead speed, and a 50 mm gauge length. Each tensile property was averaged from at least five samples.
3 Results and discussion
3.1 FTIR analysis
The FTIR spectra of the CA-free TPS and 3% CA-TPS are shown in Figure 1. They showed broad peaks in the range of 3,700−3,000 cm−1 referred to the stretching hydroxyl groups, the peaks at 2,925 cm−1 attributed to the C–H stretching groups, and the peaks at 1,640 cm−1 related to the bending hydroxyl groups of starch (17,18,26). For 3% CA-TPS in Figure 1(b), an additional peak at 1,742 cm−1 assigned to the ester carbonyl groups of the TPS treated with CA was detected (23,27). This confirmed that an acetylation reaction between the hydroxyl groups of starch and carboxylic acid groups of CA had occurred (23,24).

FTIR spectra of (a) CA-free TPS and (b) 3% CA-TPS.
Figure 2 shows expanded FTIR spectra of TPS in the region of C–O stretching vibration. The peaks at 1,150 cm−1 referred to C–O stretching vibration of C–O–H groups of starch (23). The height of this peak for 3% CA-TPS in Figure 2 (red line) was higher than that of the CA-free TPS in Figure 2 (black line) using the peak at 758 cm−1 (C–O–C ring vibration in starch) as the reference peak. This indicates the content of C–O–H groups of 3% CA-TPS was more than the CA-free TPS. The results implied that glycosidic bonds of starch were hydrolyzed with CA to form new hydroxyl groups (23). The molar mass of starch then decreased by acid-catalyzed hydrolysis with CA (28).

Expanded FTIR spectra in C–O stretching region (700−1,200 cm−1) of CA-free TPS and 3% CA-TPS.
3.2 Thermal transition properties
The thermal transition properties of the blends with and without 3% CA treatment including glass transition temperature (T g), cold crystallization temperature (T cc), melting temperature (T m), and degree of crystallinity (X c) were investigated from DSC heating curves as shown in Figure 3. The DSC results are summarized in Table 1. All the blends had similar T g values in the range of 30−33°C. The T cc and T m values of pure PLLA-PEG-PLLA were 71.3°C and 159.6°C, respectively. The T cc peaks shifted to higher temperatures (73.8−78.0°C) and the T m peaks shifted to lower temperatures (156.4−157.8°C) when the TPS was incorporated. This shifting of T cc and T m peaks suggested that the cold crystallization of PLLA end-blocks was suppressed by the addition of TPS and induced formation of imperfect PLLA crystallites (17). The T cc peaks slightly shifted to higher temperature as the TPS content increased. However, the TPS content did not significantly affect shifting of the T m peaks.

DSC heating curves of (top) PLLA-PEG-PLLA/CA-free TPS and (bottom) PLLA-PEG-PLLA/3% CA-TPS blends for blend ratios of (a) 100/0, (b) 90/10, (c) 80/20, and (d) 60/40 (w/w).
Thermal transition properties of PLLA-PEG-PLLA/TPS blends with and without CA treatment
| PLLA-PEG-PLLA/TPS (w/w) | T g (°C) | T cc (°C) | T m (°C) | X c (%) |
|---|---|---|---|---|
| Pure PLLA-PEG-PLLA | 30.0 ± 0.6 | 71.3 ± 0.3 | 159.6 ± 0.2 | 25.2 ± 0.4 |
| PLLA-PEG-PLLA/CA-free TPS | ||||
| 90/10 | 31.4 ± 0.2 | 73.8 ± 0.2 | 157.3 ± 0.1 | 22.4 ± 0.5 |
| 80/20 | 32.2 ± 0.2 | 74.1 ± 0.3 | 156.6 ± 0.2 | 19.8 ± 0.4 |
| 60/40 | 33.0 ± 0.3 | 76.4 ± 0.1 | 156.4 ± 0.3 | 17.6 ± 0.7 |
| PLLA-PEG-PLLA/3% CA-TPS | ||||
| 90/10 | 32.5 ± 0.4 | 76.2 ± 0.2 | 157.8 ± 0.4 | 19.4 ± 0.6 |
| 80/20 | 32.3 ± 0.2 | 77.3 ± 0.3 | 157.3 ± 0.2 | 18.2 ± 0.5 |
| 60/40 | 30.4 ± 0.7 | 78.0 ± 0.1 | 157.4 ± 0.1 | 13.9 ± 0.4 |
All the blends had X c values (13.9−22.4%) lower than the pure PLLA-PEG-PLLA (25.2%). The X c values steadily decreased as the TPS content increased for both the blend series. It should be noted that the X c values of the PLLA-PEG-PLLA/3% CA-TPS blends were lower than the PLLA-PEG-PLLA/CA-free TPS blends for the same TPS content as shown in Figure 4. This indicates that the shorter CA-treated TPS chains showed more suppression of the crystallization of PLLA end-blocks in the blends than the longer CA-free TPS chains.

Effect of CA treatment on X c of PLLA-PEG-PLLA/TPS blends.
3.3 Thermal decomposition properties
The thermal decomposition properties of the blends were studied from thermogravimetric (TG) and derivative TG (DTG) curves as shown in Figures 5 and 6, respectively. The TG and DTG results including residue weight at 1,000°C, temperature at maximum decomposition rate of PLLA blocks (PLLA-T d,max), and temperature at maximum decomposition rate of PEG blocks (PEG-T d,max), are summarized in Table 2. The TG curve of pure PLLA-PEG-PLLA exhibited two weight loss steps in the ranges of 250−350°C and 350−450°C due to thermal decompositions of PLLA end-blocks and PEG middle-blocks, respectively (14,15,16,17). The pure PLLA-PEG-PLLA showed complete decomposition at a temperature around 450°C. From Table 2, the PLLA-T d,max and PEG-T d,max peaks of the pure PLLA-PEG-PLLA are seen to have been 294°C and 416°C, respectively.

TG curves of (top) PLLA-PEG-PLLA/CA-free TPS and (bottom) PLLA-PEG-PLLA/3% CA-TPS blends for various blend ratios.

DTG curves of (top) PLLA-PEG-PLLA/CA-free TPS and (bottom) PLLA-PEG-PLLA/3% CA-TPS blends for various blend ratios (T d,max peaks as shown).
Thermal decomposition properties of PLLA-PEG-PLLA/TPS blends with and without CA treatment
| PLLA-PEG-PLLA/TPS (w/w) | Residue weight at 1,000°C (%)a | PLLA-Td,max (°C)b | PEG-Td,max (°C)b |
|---|---|---|---|
| Pure PLLA-PEG-PLLA | 0.3 | 294 | 416 |
| PLLA-PEG-PLLA/CA-free TPS | |||
| 90/10 | 1.6 | 342 | 414 |
| 80/20 | 3.4 | 355 | 415 |
| 60/40 | 7.0 | 359 | 415 |
| PLLA-PEG-PLLA/3% CA-TPS | |||
| 90/10 | 1.9 | 346 | 417 |
| 80/20 | 3.1 | 361 | 416 |
| 60/40 | 6.6 | 367 | 418 |
aObtained from TG curves.
bObtained from DTG curves.
From TG curves, it was found that all the blends also exhibited two weight loss steps in the ranges 100−380°C and 380−450°C. The temperature ranges for main thermal decompositions of PLLA end-blocks in the blends significantly shifted to higher temperature for both the blend series. From DTG curves, the PLLA-T d,max peaks of both the blend series dramatically shifted to higher temperature as the TPS content increased, indicating that the thermal stability of PLLA end-blocks in the blends increased with the increase in the TPS content. Hydrogen bonding could occur between the oxygen atoms of PEG middle-blocks and hydroxyl groups of starch to improve thermal stability of the PLLA end-blocks in PLLA-PEG-PLLA/starch blends (17). In addition, products from pyrolysis of starch induced a shielding effect that enhanced thermal stability of the compatible polymer/starch blends (17,19,30). However, the PEG-T d,max did not change significantly when the TPS was blended.
This indicates that the addition of TPS with and without CA treatment improved thermal stability of the PLLA end-blocks. However, the weight losses in the temperature range 100−300°C of the blends increased with the TPS content. This was due to lower thermal stability of the TPS than PLLA-PEG-PLLA (17,29). As would be expected, residue weight at 1,000°C of the blends increased as the TPS content increased because of increase in the TPS ashes.
From Table 2, it can be seen that the PLLA-T d,max peaks of PLLA-PEG-PLLA/3% CA-TPS blends were at higher temperature than the PLLA-PEG-PLLA/CA-free TPS blends for the same TPS content. This may be explained by the content of hydroxyl groups in 3% CA-TPS being more than the CA-free TPS as described in FTIR analysis reported above. Therefore, higher content of hydrogen bonds in the PLLA-PEG-PLLA/3% CA-TPS blends enhanced the higher thermal stability of the blends.
3.4 Phase morphology
The phase morphology of the blends was investigated from SEM images of the film cryo-fractured surfaces as shown in Figure 7. The cryo-fractured surface of pure PLLA-PEG-PLLA film in Figure 7(a) was homogeneous with rough surfaces suggesting it had a ductile character (13,14). All the blend films in Figure 7(b)−(g) showed that many voids which occurred from TPS phases fell out during film fractionation indicating phase separation between PLLA-PEG-PLLA matrices and dispersed TPS. The number and size of these voids increased as the TPS content increased.
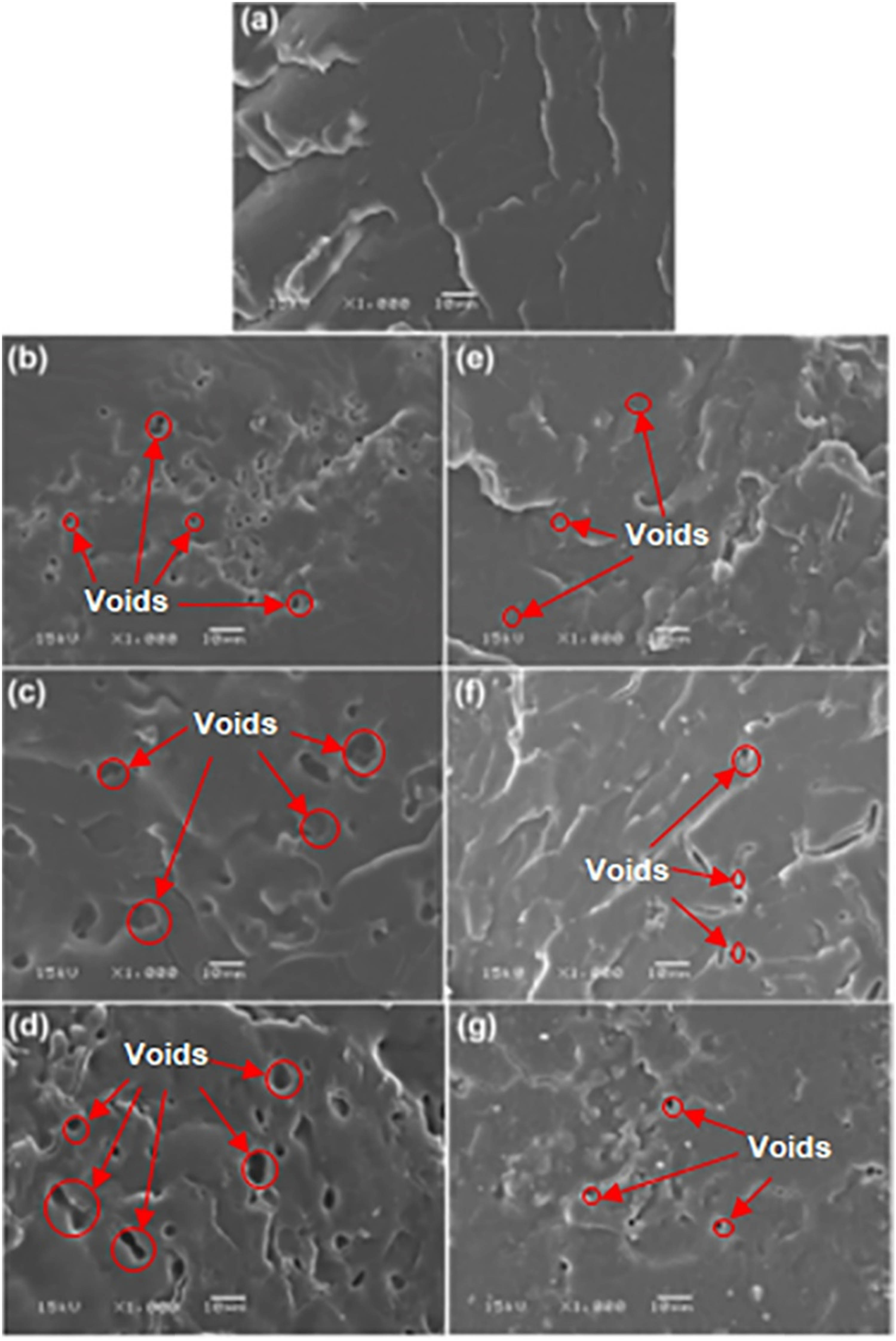
Cryo-fractured surfaces of (a) pure PLLA-PEG-PLLA and PLLA-PEG-PLLA/CA-free TPS blends for blend ratios of (b) 90/10, (c) 80/20, and (d) 60/40 (w/w) as well as PLLA-PEG-PLLA/3% CA-TPS blends for blend ratios of (e) 90/10, (f) 80/20, and (g) 60/40 (w/w) (all bar scales = 10 µm).
It is clearly seen that the void sizes in the blends that contained 3% CA-TPS (Figure 7(e)−(g)) were smaller than in the blends containing CA-free TPS (Figure 7(b)−(d)) which suggested that the phase compatibility in the blends containing 3% CA-TPS was better than that in CA-free TPS (17,31,32). It has been reported that modification of TPS with CA improved the dispersion and phase compatibility of TPS in the polyethylene (33) and PLLA (24). This may be explained by acidic hydrolysis of starch with CA through melt processing leading to decreased molar mass of starch (23,28,34) and development of hydroxyl groups (23) to improve the phase compatibility between PLLA-PEG-PLLA and TPS. In addition, the unreacted CA could develop an in situ compatibilization effect by reacting with both the PLLA end-chains and TPS during melt blending (35) and could create hydrogen bonding (36) to improve the interfacial interaction between PLLA and TPS.
3.5 Tensile properties
The tensile properties of the blend films were determined from stress–strain curves as shown in Figure 8 and the averaged tensile properties are summarized in Table 3. The ultimate tensile strength, elongation at break, and Young’s modulus of the pure PLLA-PEG-PLLA films were 17.4 MPa, 258%, and 243 MPa, respectively. These tensile properties decreased when both the CA-free TPS and 3% CA-TPS were blended and the TPS content was increased. This is due to the poor mechanical properties of the TPS (12,19,20). However, the tensile properties of the blend films containing 3% CA-TPS were higher than the blend films that contained CA-free TPS for the same TPS content as shown in Figure 9. This may be due to the good phase compatibility in the blend films that contained 3% CA-TPS as described above in the SEM analysis. The CA treatment can increase the tensile properties of the PLLA/TPS blends by improving its phase compatibility (24). The tensile results support a conclusion that the tensile properties of the PLLA-PEG-PLLA/TPS blends were improved by CA treatment of TPS.

Tensile curves of (top) PLLA-PEG-PLLA/CA-free TPS and (bottom) PLLA-PEG-PLLA/3% CA-TPS blends for various blend ratios.
Tensile properties of PLLA-PEG-PLLA/TPS blends with and without CA treatment
| Sample | Ultimate tensile strength (MPa) | Elongation at break (%) | Young’s modulus (MPa) |
|---|---|---|---|
| Pure PLLA-PEG-PLLA | 17.4 ± 1.1 | 258 ± 24 | 243 ± 31 |
| PLLA-PEG-PLLA/CA-free TPS | |||
| 90/10 | 12.9 ± 2.3 | 156 ± 21 | 232 ± 28 |
| 80/20 | 11.5 ± 1.4 | 86 ± 14 | 192 ± 24 |
| 60/40 | 6.1 ± 0.7 | 24 ± 6 | 84 ± 15 |
| PLLA-PEG-PLLA/3% CA-TPS | |||
| 90/10 | 13.1 ± 2.4 | 184 ± 27 | 234 ± 26 |
| 80/20 | 12.4 ± 1.5 | 142 ± 18 | 205 ± 27 |
| 60/40 | 7.5 ± 1.2 | 73 ± 7 | 103 ± 14 |

Effect of CA treatment on tensile properties of PLLA-PEG-PLLA/TPS blends.
4 Conclusion
In this study, TPS was treated with 3 wt% CA (3% CA) before melt blending with the PLLA-PEG-PLLA for PLLA-PEG-PLLA/TPS blend ratios of 90/10, 80/20, and 60/40 (w/w). The FTIR analysis indicated that both the acetylation and acidic hydrolysis reactions of TPS with CA had occurred. For PLLA-PEG-PLLA/TPS blends, the crystallization ability of PLLA-PEG-PLLA matrices decreased and the thermal stability increased as the TPS content increased, as determined from DSC and TGA analyses, respectively. The addition of 3% CA-TPS showed more suppression in crystallization of the PLLA end-blocks and more improvement in thermal stability of the PLLA end-blocks for the PLLA-PEG-PLLA/TPS blends than with the addition of CA-free TPS. The blends containing 3% CA-TPS showed better phase compatibility and higher tensile properties than those of the blends containing CA-free TPS for same blend ratio as investigated from SEM analysis and tensile test, respectively. PLLA-PEG-PLLA/TPS treated with CA blends can be used as fully biodegradable and flexible bioplastics.
Acknowledgements
The authors like to thank the Centre of Excellence for Innovation in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education, Thailand for providing the tensile test.
-
Funding information: This research project is financially supported by Thailand Science Research and Innovation (TSRI).
-
Author contributions: Yaowalak Srisuwan: conceptualization, methodology, investigation, writing – original draft, and funding acquisition; Prasong Srihanam: validation, investigation, writing – review and editing; Theeraphol Phromsopha: validation, investigation, and writing – review and editing; Yodthong Baimark: conceptualization, methodology, investigation, writing – original draft, writing – review and editing, supervision, project administration, and funding acquisition.
-
Conflict of interest: The authors have no conflict of interest.
References
(1) Silva D, Kaduri M, Poley M, Adir O, Krinsky N, Shainsky-Rotiman J, et al. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem Eng J. 2018;340:9–14.10.1016/j.cej.2018.01.010Search in Google Scholar PubMed PubMed Central
(2) Mishra RK, Ha SK, Verma K, Tiwari SK. Recent progress in selected bio-nanomaterials and their engineering applications: An overview. J Sci: Adv Mater Dev. 2018;3:263–88.10.1016/j.jsamd.2018.05.003Search in Google Scholar
(3) Standau T, Castellón SM, Delavoie A, Christian Bonten C, Altstädt V. Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion. e-Polymers. 2019;19:297–304.10.1515/epoly-2019-0030Search in Google Scholar
(4) Donate R, Monzón M, Alemán-Domínguez ME. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers. 2020;20:571–99.10.1515/epoly-2020-0046Search in Google Scholar
(5) Huang D, Hu Z-D, Liu T-Y, Lu B, Zhen Z-C, Wang G-X, et al. Seawater degradation of PLA accelerated by water-soluble PVA. e-Polymers. 2020;20:759–72.10.1515/epoly-2020-0071Search in Google Scholar
(6) Albuquerque RQ, Brütting C, Tobias Standau T, Ruckdäschel H. A machine learning investigation of low-density polylactide batch foams. e-Polymers. 2022;22:318–31.10.1515/epoly-2022-0031Search in Google Scholar
(7) Kumari SVG, Pakshirajan K, Pugazhenthi G. Recent advances and future prospects of cellulose, starch, chitosan, polylactic acid and polyhydroxyalkanoates for sustainable food packaging applications. Int J Biol Macromol. 2022;221:163–82.10.1016/j.ijbiomac.2022.08.203Search in Google Scholar PubMed
(8) Jin FL, Hu RR, Park SJ. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: a review. Compos B. 2019;164:287–96.10.1016/j.compositesb.2018.10.078Search in Google Scholar
(9) Song Q. Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology. e-Polymers. 2022;22:1007–20.10.1515/epoly-2022-8097Search in Google Scholar
(10) Fu X, Zhang T, Zhang W, Zhong Y, Fang S, Wang G, et al. Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability. e-Polymers. 2023;23:20230009.10.1515/epoly-2023-0009Search in Google Scholar
(11) Akrami M, Ghasemi I, Azizi H, Karrabi M. A new approach in compatibilization of the poly(lactic acid)/thermoplastic starch (PLA/TPS) blends. Carbohyd Polym. 2016;144:254–62.10.1016/j.carbpol.2016.02.035Search in Google Scholar PubMed
(12) Chotiprayon P, Chaisawad B, Yoksan R. Thermoplastic cassava starch/poly(lactic acid) blend reinforced with coir fibres. Int J Biol Macromol. 2020;156:960–8.10.1016/j.ijbiomac.2020.04.121Search in Google Scholar PubMed
(13) Yun X, Li X, Jin Y, Sun W, Dong T. Fast crystallization and toughening of poly(L-lactic acid) by incorporating with poly(ethylene glycol) as a middle block chain. Polym Sci Ser A. 2018;60:141–55.10.1134/S0965545X18020141Search in Google Scholar
(14) Baimark Y, Rungseesantivanon W, Prakymoramas N. Improvement in melt flow property and flexibility of poly(L-Lactide)-b-poly(ethylene glycol)-b-poly(L-Lactide) by chain extension reaction for potential use as flexible bioplastics. Mater Des. 2018;154:73–80.10.1016/j.matdes.2018.05.028Search in Google Scholar
(15) Baimark Y, Srisuwan Y. Thermal and mechanical properties of highly flexible poly(L-Lactide)-b-poly(ethylene glycol)-b-poly(L-Lactide) bioplastics: effects of poly(ethylene glycol) block length and chain extender. J Elastom Plast. 2020;52(2):142–58.10.1177/0095244319827993Search in Google Scholar
(16) Baimark Y, Rungseesantivanon W, Prakymoramas N. Synthesis of flexible poly(L-lactide)-b-polyethylene glycol-b-poly(L-lactide) bioplastics by ring-opening polymerization in the presence of chain extender. e-Polymers. 2020;20(1):423–29.10.1515/epoly-2020-0047Search in Google Scholar
(17) Srisuwan Y, Baimark Y. Thermal, morphological and mechanical properties of flexible poly(L-lactide)-b-polyethylene glycol-b-poly(L-lactide)/thermoplastic starch blends. Carbohyd Polym. 2022;283:119155.10.1016/j.carbpol.2022.119155Search in Google Scholar PubMed
(18) Thongsomboon W, Srihanam P, Baimark Y. Preparation of flexible poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/talcum/thermoplastic starch ternary composites for use as heat-resistant and single-use bioplastics. Int J Biol Macromol. 2023;230:123172.10.1016/j.ijbiomac.2023.123172Search in Google Scholar PubMed
(19) Sessini V, Arrieta MP, Raquez J-M, Dubois P, Kenny JM, Peponi L. Thermal and composting degradation of EVA/Thermoplastic starch blends and their nanocomposites. Polym Degrad Stab. 2019;159:184–98.10.1016/j.polymdegradstab.2018.11.025Search in Google Scholar
(20) Zaaba NF, Ismail H. A review on tensile and morphological properties of poly(lactic acid) (PLA)/thermoplastic starch (TPS) blends. Polym-Plast Tech Mat. 2019;58:1945–64.10.1080/25740881.2019.1599941Search in Google Scholar
(21) Wang N, Yu J, Chang PR, Ma X. Influence of citric acid on the properties of glycerol-plasticized dry starch (DTPS) and DTPS/poly(lactic acid) blends. Polym Comps. 2007;59(9):409–17.10.1002/star.200700617Search in Google Scholar
(22) Wang N, Yu J, Ma X. Preparation and characterization of compatible thermoplastic dry starch/poly(lactic acid). Starke. 2008;29(5):551–9.10.1002/pc.20399Search in Google Scholar
(23) Kahar AWM, Ismail H, Othman N. Morphology and tensile properties of high-density polyethylene/natural rubber/thermoplastic tapioca starch blends: The effect of citric acid-modified tapioca starch. J Appl Polym Sci. 2012;125:768–75.10.1002/app.35057Search in Google Scholar
(24) Ibrahim N, Wahab MKA, Uylan DN, Ismail H. Physical and degradation properties of polylactic acid and thermoplastic starch blends – effect of citric acid treatment on starch structures. Bioresour. 2017;12(2):3076–87.10.15376/biores.12.2.3076-3087Search in Google Scholar
(25) Liu W, Wu X, Ou Y, Liu H, Zhang C. Electrically conductive and light-weight branched polylactic acid-based carbon nanotube foams. e-Polymers. 2021;21:96–107.10.1515/epoly-2021-0013Search in Google Scholar
(26) Cuevas-Carballo ZB, Duarte-Aranda S, Canch´ e-Escamilla G. Properties and biodegradation of thermoplastic starch obtained from grafted starches with poly(lactic acid). J Polym Environ. 2019;27:2607–17.10.1007/s10924-019-01540-wSearch in Google Scholar
(27) Reddy N, Yang Y. Citric acid cross-linking of starch films. Food Chem. 2010;118(3):702–11.10.1016/j.foodchem.2009.05.050Search in Google Scholar
(28) Carvalho AJF, Zambon MD, Curvelo AA, Gandini A. Thermoplastic starch modification during melt processing: Hydrolysis catalyzed by carboxylic acids. Carbohyd Polym. 2005;62(4):387–90.10.1016/j.carbpol.2005.08.025Search in Google Scholar
(29) Ferri JM, Garcia-Garcia D, Carbonell-Verdu A, Fenollar O, Balart R. Poly(lactic acid) formulations with improved toughness by physical blending with thermoplastic starch. J Appl Polym Sci. 2017;134:45751.10.1002/app.45751Search in Google Scholar
(30) Nguyen DM, Vu TT, Grillet A-C, Ha Thuc H, Ha, Thuc CN. Effect of organoclay on morphology and properties of linear low density polyethylene and Vietnamese cassava starch biobased blend. Carbohyd Polym. 2016;2016(136):163–70.10.1016/j.carbpol.2015.09.020Search in Google Scholar PubMed
(31) Wootthikanokkhan J, Kasemwananimit P, Sombatsompop N, Kositchaiyong A, Isarankura, na Ayutthaya S, Kaabbuathong N. Preparation of modified starch-grafted poly(lactic acid) and a study on compatibilizing efficacy of the copolymers in poly(lactic acid)/thermoplastic starch blends. J Appl Polym Sci. 2012;126:388–95.10.1002/app.36896Search in Google Scholar
(32) Noivoil N, Yoksan R. Oligo(lactic acid)-grafted starch: A compatibilizer for poly(lactic acid)/thermoplastic starch blend. Int J Biol Macromol. 2020;160:506–17.10.1016/j.ijbiomac.2020.05.178Search in Google Scholar PubMed
(33) Wang N, Yu JG, Ma XF, Han CM. High performance modified thermoplastic starch/linear low-density polyethylene blends in one-step extrusion. Polym Compos. 2007;28(1):89–97.10.1002/pc.20266Search in Google Scholar
(34) Da Róz AL, Zambon MD, Curvelo AAS, Carvalho AJF. Thermoplastic starch modified during melt processing with organic acids: The effect of molar mass on thermal and mechanical properties. Ind Crops Prod. 2011;33(1):152–7.10.1016/j.indcrop.2010.09.015Search in Google Scholar
(35) Murillo EA. In situ compatibilization of thermoplastic starch/polylactic acid blends using citric acid. Macromol Res. 2023;31(2):157–69.10.1007/s13233-023-00127-8Search in Google Scholar
(36) Chabrat E, Abdillahi H, Rouilly A, Rigal L. Influence of citric acid and water on thermoplastic wheat flour/poly(lactic acid) blends. I: Thermal, mechanical and morphological properties. Ind Crops Prod. 2012;37(1):238–46.10.1016/j.indcrop.2011.11.034Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites

