Abstract
Heat stabilizers are crucial additives for enhancing the thermal stability of polyvinyl chloride (PVC) during processing. Among the various heat stabilizers available, organic tin compounds have shown remarkable effectiveness. In this study, we investigated the use of dimethyltin dineodecanoate (DMTDN), dibutyltin dineodecanoate (DBTDN), and dioctyltin dineodecanoate (DOTDN) as heat stabilizers for PVC. These compounds were combined with calcium stearate (CaSt2) and zinc stearate (ZnSt2) to improve the thermal stability of PVC materials. The results demonstrated that the thermal stabilization effects of the three tin neodecanoates, when used as standalone heat stabilizers, followed the order: DOTDN > DBTDN > DMTDN. Notably, the thermal stability and lubricity of the three-component heat stabilizer (MTN5-C4Z1, BTN5-C4Z1, and OTN5-C4Z1, respectively), which consisted of the three types of tin neodecanoate, CaSt2, and ZnSt2 in a ratio of 5:4:1, outperformed the use of tin neodecanoate alone. This study offered potential formulations to reduce the application cost of tin neodecanoate as a PVC heat stabilizer.
1 Introduction
Polyvinyl chloride (PVC) is a popular polymer due to its excellent characteristics such as corrosion resistance, abrasion resistance, incombustibility, self-extinguishing properties, and electrical insulation (1,2,3). However, PVC needs to be modified by adding various additives, such as heat stabilizers and plasticizers, to enhance its usage characteristics (4,5,6). One of the main issues with PVC is its low decomposition temperature, which causes thermal decomposition during processing, leading to the release of hydrogen chloride and a degradation of its physical and chemical properties (7,8,9). To improve the thermal stability of PVC, two general ideas are usually implemented. The first is to replace the chlorine atoms on the PVC molecular chain with certain compounds (10,11), while the second is to directly add heat stabilizers to improve PVC thermal stability. The latter method is simple and less costly and involves the addition of appropriate heat stabilizers to inhibit PVC thermal decomposition (12).
There are various types of PVC heat stabilizers, which include lead salts (13), organic tin (14,15,16), metal soaps (17), antimony-based (18), organic auxiliaries (19), rare earth (20), etc. Organic tin heat stabilizers are one of the most widely used and effective heat stabilizers for PVC, although they are gradually being replaced by more eco-friendly and non-toxic alternatives such as calcium–zinc heat stabilizers (21,22). However, calcium–zinc heat stabilizers need to be compounded with other heat stabilizers such as organic tin and auxiliary heat stabilizers to form a multi-component heat stabilizer (23,24). Therefore, organic tin still has a significant market share due to its excellent thermal stability, and the development of composite multi-functional organic tin heat stabilizer products for the PVC market remains a significant development trend (25,26).
Recently, some metal compounds of neodecanoic acid for PVC heat stabilizers are also reported (27). This study aims to investigate the thermal stability of PVC using different types of tin neodecanoate as heat stabilizers. Tin neodecanoate is a promising alternative to traditional organic tin heat stabilizers due to its low odor and cost. Neodecanoic acid, the precursor of tin neodecanoate, has a highly branched structure that gives the derivative good thermal stability. The study also examines the effects of compounding tin neodecanoate with calcium stearate and zinc stearate in different proportions to reduce the cost of the formulation. Two-component and three-component heat stabilizers are formulated and compared to determine the experimental formulation with better thermal stability. The results of this study will fill the research gap on the thermal stability of tin neodecanoate as a PVC heat stabilizer. It will provide a fundamental basis for developing high-efficiency, high-end, and cost-effective PVC heat stabilizer formulations.
2 Experimental
2.1 Materials
The PVC used in this study was PVC (SG-5) supplied by Tianye (Group) Co., Ltd., Xinjiang, China. Dioctyl terephthalate (DOTP), dioctyl phthalate (DOP), dioctyl adipate (DOA), and epoxidized soybean oil (ESO) (Industrial Grade, from Blue Sail Chemical Co., Ltd., Shandong, China) was used as a plasticizer. Dimethyltin dineodecanoate (DMTDN), dibutyltin dineodecanoate (DBTDN), and dioctyltin dineodecanoate (DOTDN) (Industrial Grade, tin content was 24.02%, 20.76%, and 17.41%, respectively, from Yunxi Tinchem [Nanjing] Technology Co., Ltd., Nanjing, China) were used as heat stabilizers. CaSt2 (AR, from Aladdin Biochemical Technology Co., Ltd., Shanghai, China) and ZnSt2 (AR, from Aladdin Biochemical Technology Co., Ltd., Shanghai, China) were also used as heat stabilizers. Heavy calcium carbonate (CaCO3) (Industrial Grade, from Yousuo Chemical Technology Co., Ltd., Shandong, China) was used as a filler. Fischer–Tropsch (FT) wax (Industrial Grade, from Tianyu Chemical Co., Ltd., Hebei, China) was used as a lubricant.
2.2 Preparation of PVC samples for thermal stability tests
Table 1 illustrates the PVC formulation used for the Congo red test and thermogravimetric (TG) analysis. The components were accurately weighed according to the formula in Table 1 and stirred for 3 min. Subsequently, the mixture was preplasticized in a hot oven at 120°C for 20 min and cooled to obtain a dry mix for the Congo red test and TG analysis. Table 2 presents the PVC formulations utilized for the thermal aging test and double-roller mill plasticizing test. After precisely weighing the components according to the formula in Table 2, each component was mixed at high speed for 3 min. The thermal aging test and double-roller mill plasticizing test were then conducted.
PVC formulations for Congo red test and TG
| Sample | PVC (phr) | DOTP (phr) | DMTDN (phr) | DBTDN (phr) | DOTDN (phr) | CaSt2 (phr) | ZnSt2 (phr) |
|---|---|---|---|---|---|---|---|
| S0 | 100 | 10 | |||||
| CZ | 100 | 10 | 1.6 | 0.4 | |||
| M1 | 100 | 10 | 2 | ||||
| M2 | 100 | 10 | 1 | 1 | |||
| M3 | 100 | 10 | 1.8 | 0.2 | |||
| M4 | 100 | 10 | 1 | 0.8 | 0.2 | ||
| B1 | 100 | 10 | 2 | ||||
| B2 | 100 | 10 | 1 | 1 | |||
| B3 | 100 | 10 | 1.8 | 0.2 | |||
| B4 | 100 | 10 | 1 | 0.8 | 0.2 | ||
| O1 | 100 | 10 | 2 | ||||
| O2 | 100 | 10 | 1 | 1 | |||
| O3 | 100 | 10 | 1.8 | 0.2 | |||
| O4 | 100 | 10 | 1 | 0.8 | 0.2 |
PVC formulations for thermal aging test and double-roller mill plasticizing test
| Sample | PVC (phr) | DOTP (phr) | DMTDN (phr) | DBTDN (phr) | DOTDN (phr) | CaSt2/ZnSt2 (phr) | CaCO3 (phr) | FT (phr) |
|---|---|---|---|---|---|---|---|---|
| S0 | 100 | 10 | 35 | 1.5 | ||||
| CZ | 100 | 10 | 1.6/0.4 | 35 | 1.5 | |||
| M1 | 100 | 10 | 2 | 35 | 1.5 | |||
| M4 | 100 | 10 | 1 | 0.8/0.2 | 35 | 1.5 | ||
| B1 | 100 | 10 | 2 | 35 | 1.5 | |||
| B4 | 100 | 10 | 1 | 0.8/0.2 | 35 | 1.5 | ||
| O1 | 100 | 10 | 2 | 35 | 1.5 | |||
| O4 | 100 | 10 | 1 | 0.8/0.2 | 35 | 1.5 |
2.3 Congo red test
The static thermal stability of PVC samples was tested according to the Congo red method outlined in the GB/T 2917.1-2002 standard. A heat stability tester (SHK-H112, Suzhou Jianzhuo Instrument Technology Co., Ltd., Suzhou) was used for the test at a temperature of 180°C. First, the PVC mixture was accurately weighed and added 3.6 g to a test tube. The lower end of the Congo red test paper was placed 2.5 cm from the PVC powder. The test tube was then immersed in 5 cm of glycerol. Each sample was tested twice, and the average of the two results was used as the final result.
2.4 Thermal aging test
The PVC mixture was processed on a double roller for 4 min, ensuring that the thickness of the resulting sample was 1 mm. The PVC sheet was then cut into a square shape with a side length of 15 mm. These PVC squares were then placed in an oven set to a temperature of 190°C. The samples were removed from the oven every 10 min, and any changes in the color of the PVC sheets were recorded.
2.5 TG
The PVC samples were analyzed under a nitrogen atmosphere using a TG analyzer (TA Instruments, SDT2960) with the following conditions: a nitrogen flow rate of 100 mL‧min−1, a sampling mass ranging from 1 to 10 mg, a temperature range of 25–600°C, and a temperature rise rate of 10°C‧min−1.
2.6 Double-roller mill plasticizing test
The dynamic thermal stability of the PVC samples was measured using a double-roller mill (JG-3010, Yangzhou Jinggang Machinery Factory, Yangzhou) at a front roll temperature of 190°C and a back roll temperature of 180°C. The change in color of the PVC samples was also measured using an X-rite colorimeter (SP62-161, X-Rite Color Management Co., Ltd., Shanghai) to compare their dynamic thermal stability.
2.7 Viscosity test and dispersion analysis
The effect of different tin neodecanoate viscosities on dispersibility and thermal stability was studied using a digital viscometer (NDJ-99, Shanghai Yixing Scientific Instrument Co., Ltd., Shanghai, China). The viscosity of each sample was tested at 25°C using rotor No. 2, with the speed adjusted according to the sample’s viscosity.
2.8 Torque rheometer test
The effect of tin neodecanoate and calcium–zinc heat stabilizers on the dynamic rheological properties of PVC was evaluated using a torque rheometer (RM-200A, Harbin Harp Electric Technology Co., Ltd., Harbin, China) according to the ASTM D 2538-02 standard. The test formulation primarily consisted of PVC (100 phr), DOTP (10 phr), and heat stabilizers (2 phr). The test temperature was set at 185°C, and the rotor speed was maintained at 35 rpm. The PVC sample mass used in the test was 70 g.
3 Results and discussion
3.1 Congo red test
3.1.1 Effect of the composition of heat stabilizers on the thermal stability of PVC
Table 3 shows the results of the Congo red test for each PVC sample. The results of the Congo red test showed that the static thermal stability of PVC samples was improved by using tin neodecanoate as a heat stabilizer. The improvement in thermal stability was observed in all three groups (M, B, and O), and the effect was highest for DOTDN, followed by DBTDN and DMTDN. However, when tin neodecanoate was combined with CaSt2 in a 1:1 ratio to form a two-component heat stabilizer (MTN1-C1, BTN1-C1, and OTN1-C1), the static thermal stabilization time of some samples improved, while others decreased slightly, compared to using tin neodecanoate alone. This indicated that one part of CaSt2 could replace one part of tin neodecanoate, but the overall improvement in thermal stability was minor.
Congo red test results
| Sample | Composition of heat stabilizers | Feed ratio (phr) | T i (min) | T s (min) | T c (min) |
|---|---|---|---|---|---|
| S0 | Black | 0 | 6 | 7 | 9 |
| CZ | C4Z1 | 1.6/0.4 | 40 | 44 | 48 |
| M1 | DMTDN | 2 | 22 | 30 | 71 |
| M2 | MTN1-C1 | 1/1 | 21 | 31 | 68 |
| M3 | MTN9-Z1 | 1.8/0.2 | 26 | 26.5 | 27 |
| M4 | MTN5-C4Z1 | 1/0.8/0.2 | 41 | 47 | 73 |
| B1 | DBTDN | 2 | 37 | 49 | 75 |
| B2 | BTN1-C1 | 1/1 | 37 | 47 | 72 |
| B3 | BTN9-Z1 | 1.8/0.2 | 43 | 43.5 | 44 |
| B4 | BTN5-C4Z1 | 1/0.8/0.2 | 54 | 60 | 76 |
| O1 | DOTDN | 2 | 50 | 56 | 75 |
| O2 | OTN1-C1 | 1/1 | 52 | 57 | 68 |
| O3 | OTN9-Z1 | 1.8/0.2 | 45 | 45.5 | 46 |
| O4 | OTN5-C4Z1 | 1/0.8/0.2 | 55 | 63 | 78 |
Note: T i – the initial bluing time of Congo red test paper (initial thermal stabilization time), T s – the obvious bluing time of Congo red test paper (static thermal stability time), T c – the complete bluing time of Congo red test paper (long term thermal stability time).
When tin neodecanoate was compounded with ZnSt2 separately to form a two-component heat stabilizer (MTN9-Z1, BTN9-Z1, and OTN9-Z1), the static thermal stabilization time of some samples decreased compared to using tin neodecanoate alone. This was likely due to the phenomenon of “zinc burning,” which cannot be inhibited by this combination of stabilizers.
However, when tin neodecanoate, CaSt2, and ZnSt2 were combined to form a three-component heat stabilizer, the static thermal stability of PVC samples was greatly improved. This was evident in the enhanced Ti, Ts, and Tc values of the samples, indicating improved stability in both the early and late stages of thermal degradation. Overall, the results suggest that a three-component heat stabilizer system may be more effective in stabilizing PVC than a two-component and single-component system.
3.1.2 Effect of different plasticizers on the thermal stability of PVC
Previous studies have shown that plasticizers, lubricants, and fillers, in addition to heat stabilizers, can all impact the properties of polymers (28,29,30,31). In a study by Fu et al. (32), the effect of bio-based plasticizer EACO on the mechanical properties, plasticizing effect, and thermal stability of PVC was investigated. The thermal stability times of PVC-DOP-DMTDN, PVC-DOA-DMTDN, and PVC-ESO-DMTDN ternary composites were compared to those of PVC-DOTP-DMTDN ternary composites using the Congo red test to evaluate the impact of different plasticizers on the thermal stability of the tin neodecanoate system. Table 4 displays the static thermal stabilization times of PVC ternary composites plasticized with three different plasticizers.
Effect of different plasticizers on the thermal stability of tin neodecanoate system
| Sample | Amount of component addition (phr) | T i | T s | T c |
|---|---|---|---|---|
| PVC–DOP–DMTDN | 100–10–2 | 20 | 28 | 67 |
| PVC–DOA–DMTDN | 100–10–2 | 22 | 30 | 70 |
| PVC–ESO–DMTDN | 100–10–2 | 18 | 35 | 86 |
From Table 4, it can be seen that when DOP and DOA were used as plasticizers for the PVC-DMTDN system, the difference in static heat stabilization time did not exceed 4 min. However, the T i, T s, and T c of the PVC-ESO-DMTDN ternary composites were 18, 35, and 86 min, respectively, and the static long-term thermal stability time was significantly improved compared to that of the PVC-DOTP-DMTDN ternary composites. This suggests that ESO as a plasticizer enhances the thermal stability of the tin neodecanoate system, as the epoxy group on ESO can capture the HCl produced by the degradation of PVC. In addition, ESO and DOTP, DOP, and DOA are different types of plasticizers with distinct molecular structures. DOTP, DOP, and DOA have smaller molecular weights and less interaction force with the PVC molecular chain, which results in less thermal stability when subjected to heat. It is important to note that ESO products are more expensive than DOTP, DOP, and DOA. As a result, ESO should be used carefully in production, taking into account cost considerations.
3.2 Thermal aging test
The results of the Congo red test study indicate that the three-component heat stabilizer composed of tin neodecanoate, CaSt2, and ZnSt2 in the ratio of 5:4:1 is superior to the tin neodecanoate and calcium–zinc heat stabilizer in terms of thermal stability. Ali et al. (33) investigated the properties of PVC-WF-CaCO3 ternary stable composites. In this study, CaCO3 was used as the filler for PVC, and the three-component heat stabilizer was compared with the single-component heat stabilizer using an oven thermal aging test. The color changes in the PVC sheets with different stabilizers were recorded at various time intervals, and the results are presented in Table 5. The PVC sheets with the three-component heat stabilizer showed a significantly lighter color than those with only the single-component and C4Z1 heat stabilizer at the same test time. Furthermore, the three-component heat stabilizer maintained a better initial color than the single-component heat stabilizer at 10 min, with B4 and O4 appearing the clearest. In contrast, the PVC sheets with the single-component and C4Z1 heat stabilizer showed significant color deepening from 40 min onwards. The degree of color deepening was M1 > B1 > O1. However, the PVC sheets with three-component heat stabilizers did not show this phenomenon at 40 min. The study also suggests that the functional group in tin neodecanoate, although absorbing HCl, could not effectively replace the Cl atom on PVC, which leads to the initial degradation and coloring of PVC. However, when CaSt2 and ZnSt2 were added to the compound with tin neodecanoate, the synergistic effect improved the initial and long-term thermal stability of PVC. ZnSt2 played its initial heat stabilization function, and “zinc burning” was also inhibited by CaSt2.
Color changes of different PVC sheets in the thermal aging test
| Sample | Composition of heat stabilizers | Thermal aging time (min) | |||||
|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | ||
| CZ | C4Z1 |

|

|

|

|

|

|
| M1 | DMTDN |

|

|

|

|

|

|
| M4 | MTN5-C4Z1 |

|

|

|

|

|

|
| B1 | DBTDN |

|

|

|

|

|

|
| B4 | BTN5-C4Z1 |

|

|

|

|

|

|
| O1 | DOTDN |

|

|

|

|

|

|
| O4 | OTN5-C4Z1 |

|

|

|

|

|

|
3.3 TG
The thermal stability properties of PVC were investigated through TG analysis of three different groups of formulations, namely Group M, Group B, and Group O. The study also examined the effects of tin neodecanoate, CaSt2, and ZnSt2 on PVC’s thermal stability. The TG and DTG curves of various PVC samples in Group M were analyzed and presented in Figure 1. Figure 1a shows the TG curves of different PVC formulations in Group M from 100°C to 600°C. The PVC samples exhibited two stages of weight loss, with residue rates of M1, M2, M3, and M4 being 6.8, 7.1, 10.2, and 10.6, respectively, when the temperature reached 600°C. Figure 1b shows the curve of mass loss versus temperature for different PVC formulations in Group M from 240°C to 270°C. It was observed that when the weight of the PVC sample was less than 97.6%, M4 had higher heat resistance than M1, indicating that M4 had better thermal stability at the start of PVC decomposition. This result explained the longer initial bluing time of the Congo red test paper for M4 than for M1 during the Congo red test. In addition, the heat resistance of M4 was higher than that of CZ, indicating that the three heat stabilizers had a favorable synergistic effect when compounded. Figure 1c shows the curve of mass loss versus temperature for different PVC formulations in Group M from 340°C to 400°C. It was found that when the temperature was higher than 360.1°C, the weight of the PVC sample was CZ > M4 > M1, indicating that calcium–zinc heat stabilizer improved the thermal stability of PVC in the second phase of thermal degradation. Figure 1d shows the DTG curves of different PVC formulations for Group M. The maximum decomposition rates of each sample were observed to be M1 > M2 > M3 > M4 > CZ. It was further observed that the maximum decomposition rate of M4 was significantly lower than that of M1, indicating that MTN5-C4Z1 could effectively inhibit the release of HCl. This result was attributed to the greater effect of CaSt2 and ZnSt2 on the maximum decomposition rate of PVC degradation than that of DMTDN.

TG and DTG curves of different PVC samples in Group M: (a) TG, (b) TG (240–270℃), (c) TG (340–400℃) and (d) DTG.
Figure 2 displays the TG and DTG curves of various PVC samples in Group B. Figure 2a shows the TG curves of different PVC formulations of Group B between 100°C and 600°C, revealing that the PVC samples experienced two stages of weight loss. At 600°C, the residue rates for B1, B2, B3, and B4 were 6.4%, 7.2%, 7.7%, and 9.4%, respectively. Figure 2b presents the curves of mass loss versus temperature for the five different PVC formulations of Group B from 245°C to 270°C. When the weight of the PVC sample exceeded 98.2%, the heat resistance followed the order B4 > B1, indicating that the thermal stability of B4 was superior to that of B1 at the initial stage of PVC decomposition. This result also explained the longer initial bluing time of the Congo red test paper for B4 compared to B1 during the Congo red test. In addition, the heat resistance of B4 was higher than that of CZ, demonstrating favorable synergistic effects among the three heat stabilizers when compounded. Figure 2c presents the curves of mass loss versus temperature for the five different PVC formulations of Group B between 340°C and 400°C. When the temperature exceeded 360.1°C, the weight of the PVC sample followed the order CZ > B4 > B1, indicating that the calcium–zinc heat stabilizer improved the second stage of thermal stability of PVC decomposition more than DBTDN. Figure 2d presents the DTG curves for the different PVC formulations of Group B, showing that the maximum decomposition rates of each sample followed the order B2 > B1 > B3 > B4 > CZ. Notably, the maximum decomposition rate of B4 was significantly lower than that of B1, indicating that BTN5-C4Z1 effectively inhibited the release of HCl. This result was attributed to the greater effect of the calcium–zinc heat stabilizer on the maximum decomposition rate of PVC degradation than that of DBTDN.

TG and DTG curves of different PVC samples in Group B: (a) TG, (b) TG (250–275℃), (c) TG (340–400℃) and (d) DTG.
Figure 3 displays the TG and DTG curves of various PVC samples from Group O. The graph in Figure 3a presents the TG curves of different PVC formulations from 100°C to 600°C for Group O. The PVC samples exhibited two stages of weight loss, and the residue rates of O1, O2, O3, and O4 were 7.2, 6.4, 9.4, and 9.7, respectively, when the temperature reached 600°C. Figure 3b shows the curves of mass loss versus temperature for the five different PVC formulations in Group O from 245°C to 270°C. It is evident from the figure that when the weight of the PVC sample was above 97.3%, the heat resistance was highest for O4 followed by O1 and CZ, which indicated that the thermal stability of O4 was superior to that of O1 and CZ at the beginning of PVC decomposition. This was the primary reason for the longer initial bluing time of the Congo red test paper for O4 compared to O1 and CZ. Figure 3c demonstrates the curves of mass loss versus temperature for the five different PVC formulations from 340 to 400°C for Group O. The weight of the PVC sample was CZ > O4 > O1 when the temperature was above 360.1°C, indicating that the calcium–zinc heat stabilizer improved the second stage of thermal stability of PVC decomposition more than DOTDN. Figure 3d illustrates the derivative TG curves for the different PVC formulations in Group O. The maximum decomposition rates of each sample were O2 > O4 > O3 > O1 > CZ, with the maximum decomposition rate of O4 slightly larger than that of O1, but they were significantly lower than that of O2. It demonstrated that OTN5-C4Z1 could effectively inhibit the release of HCl, and the inhibiting effect was better than OTN1-C1. The addition of zinc stearate to the formulation was the primary factor in reducing the maximum decomposition rate of PVC.
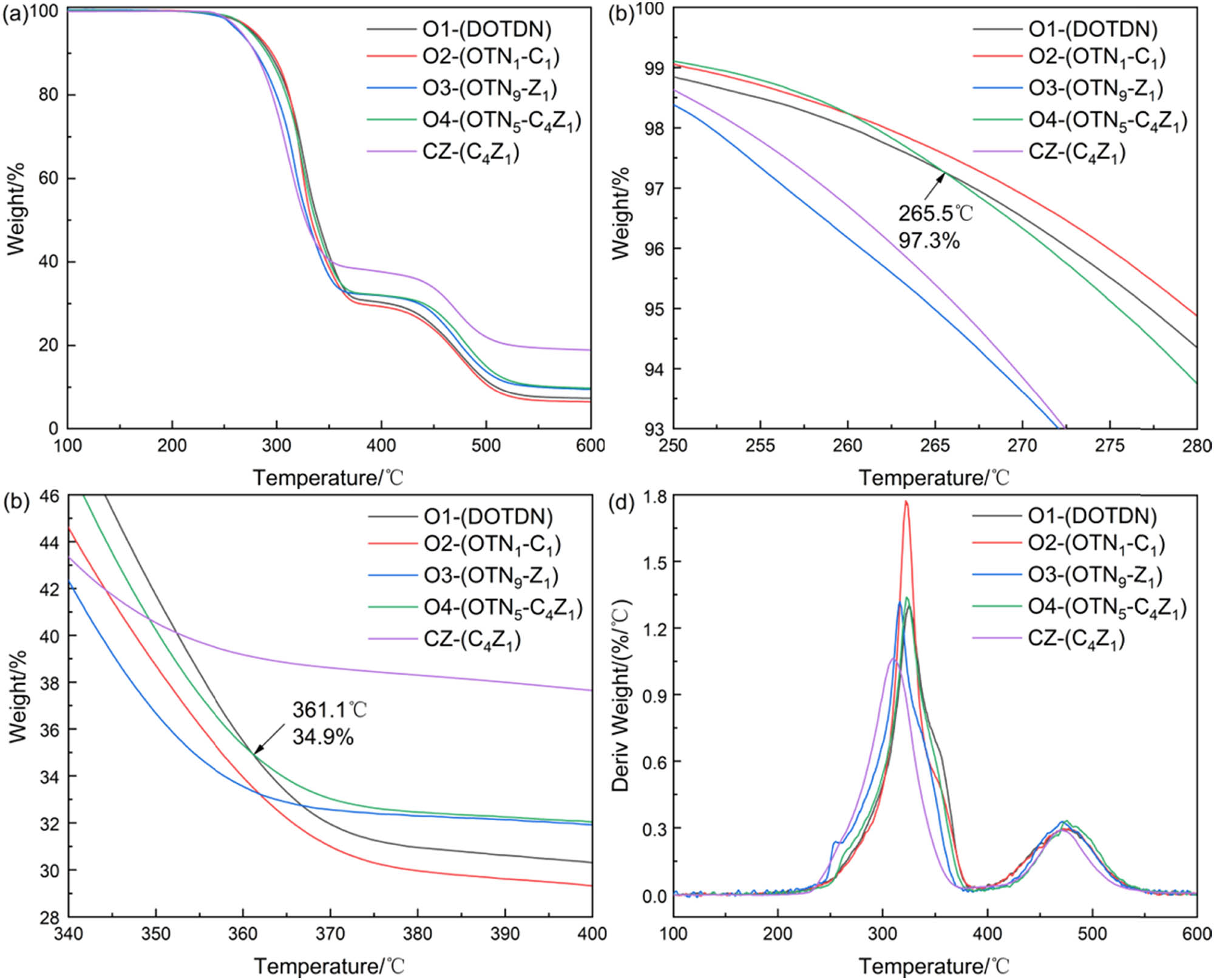
TG and DTG curves of different PVC samples in Group O: (a) TG, (b) TG (250–280℃), (c) TG (340–400℃) and (d) DTG.
Table 6 provides information on the thermal stability of different PVC samples from Groups M, B, and O at different levels of weight loss. It included the thermal loss temperature, peak temperature, maximum decomposition rate of the first stage, and final residue rate. It can be seen from the table that for T 1% and T 5%, O1 > B1 > M1, which indicated that the thermal stability effect of tin neodecanoate as a single component heat stabilizer was as follows: DOTDN > DBTDN > DMTDN, which was consistent with the experimental results of Congo red test. Based on the temperatures at one and five percent weight loss (T1% and T5%), the corresponding temperatures for samples in groups M, B, and O were M2 > M1, B1 > B2, and O2 > O1. Although B1 corresponds to a weight loss temperature greater than B2, the difference between them was only 0.4°C, which revealed that MTN1-C1, BTN1-C1, and OTN1-C1 could replace DMTDN, DBTDN, and DOTDN, respectively. According to T 1% and T 5%, we could also get the corresponding temperatures of each sample at this time M1 > M3, B1 > B3, and O1 > O3, which indicated that MTN9-Z1, BTN9-Z1, and OTN9-Z1 harmed thermal stability. Also for T1%, the samples correspond to temperatures with M4 > M1, B4 > B1, and O4 > O1, while for T5%, the samples had M1 > M4, B1 > B4, and O1 > O4. It showed that MTN5-C4Z1, BTN5-C4Z1, and OTN5-C4Z1 had better initial thermal stability compared with the single-component heat stabilizer. Moreover, for both T 1% and T 5%, the weight loss temperatures of M4, B4, and O4 were greater than those of CZ, which indicated that the combination of tin neodecanoate and calcium-zinc heat stabilizer in a proportion could improve thermal stability with a synergistic effect. The final residual rates were 18.9 for CZ, 6.8 for M1, 10.6 for M4, 6.4 for B1, 9.4 for B4, 7.2 for O1 and 9.7 for O4. It showed that CZ > M4 > M1, CZ > B4 > B1, and CZ > O4 > O1, which demonstrated that calcium-zinc heat stabilizer could improve the residual rate.
TG-DTG analysis data of different PVC samples
| Sample | Composition of heat stabilizers | T 1% (°C) | T 5% (°C) | T P1 (°C) | M 1 (%·℃-1) | R (%) |
|---|---|---|---|---|---|---|
| CZ | C4Z1 | 245.1 | 266.3 | 309.8 | 1.06 | 18.9 |
| M1 | DMTDN | 241.0 | 275.3 | 324.6 | 1.46 | 6.8 |
| M2 | MTN1-C1 | 244.6 | 276.2 | 324.2 | 1.38 | 7.1 |
| M3 | MTN9-Z1 | 240.8 | 264.9 | 318.8 | 1.16 | 10.2 |
| M4 | MTN5-C4Z1 | 245.8 | 269.5 | 321.2 | 1.11 | 10.6 |
| B1 | DBTDN | 246.2 | 276.1 | 321.4 | 1.43 | 6.4 |
| B2 | BTN1-C1 | 245.8 | 275.7 | 319.4 | 1.70 | 7.2 |
| B3 | BTN9-Z1 | 237.0 | 258.9 | 316.2 | 1.35 | 7.7 |
| B4 | BTN5-C4Z1 | 246.9 | 273.7 | 324.6 | 1.16 | 9.4 |
| O1 | DOTDN | 247.4 | 277.1 | 324.8 | 1.30 | 7.2 |
| O2 | OTN1-C1 | 250.7 | 279.4 | 322.0 | 1.77 | 6.4 |
| O3 | OTN9-Z1 | 242.3 | 264.7 | 316.2 | 1.32 | 9.4 |
| O4 | OTN5-C4Z1 | 251.8 | 275.3 | 322.6 | 1.34 | 9.7 |
3.4 Double-roller mill plasticizing test
Based on the results of the dynamic thermal stability test, it was found that the addition of the three-component heat stabilizer could significantly improve the dynamic thermal stability of PVC compared to single-component and C4Z1 heat stabilizers. Table 7 shows that when DMTDN, DBTDN, and DOTDN are used as single-component heat stabilizers, the PVC sheets had distinct differences in dynamic thermal stability, with M1 showing significant coloration at 4 min and B1 and O1 at 6 min. In terms of overall PVC coloring, the M1 group PVC sheet had the darkest color, the B1 group PVC sheet had the second darkest color, and the O1 group PVC sheet had the shallowest color, indicating that the dynamic thermal stability was DOTDN > DBTDN > DMTDN. In contrast, the overall color of the PVC sheet with the three-component heat stabilizer added in the ratio of 5:4:1 was significantly lighter than that of the PVC sheet with only tin neodecanoate and C4Z1 heat stabilizer. This suggested that the dynamic thermal stability performance of the three-component heat stabilizer was better than that of the single-component and C4Z1 heat stabilizers.
Color change in dynamic thermal stability test for different PVC sheets
| Sample | Composition of heat stabilizers | Dynamic thermal stabilization time (min) | |||||
|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 15 | 20 | ||
| CZ | C4Z1 |

|

|

|

|

|

|
| M1 | DMTDN |

|

|

|

|

|

|
| M4 | MTN5-C4Z1 |

|

|

|

|

|

|
| B1 | DBTDN |

|

|

|

|

|

|
| B4 | BTN5-C4Z1 |

|

|

|

|

|

|
| O1 | DOTDN |

|

|

|

|

|

|
| O4 | OTN5-C4Z1 |

|

|

|

|

|

|
To better compare the dynamic thermal stability of different heat stabilizers, the researchers used an X-rite colorimeter to measure the color change of each PVC sheet. The results showed that the redness value of the PVC sheets in each group gradually increased with the extension of the test time, as did the yellowness value. Meanwhile, the lightness value of the PVC sheets in each group gradually decreased with the extension of the test time (as shown in Figure 4).

Variation of chromatic aberration value for different PVC sheetssheets: (a) redness value, (b) yellowness value, and (c) lightness value.
Table 8 presents the redness, yellowness, and lightness values of each group of PVC sheets at 4 and 20 min. The three tin neodecanoate heat stabilizers showed distinct differences in thermal stability: DOTDN > DBTDN > DMTDN. The redness value was highest in M1, followed by B1 and O1, and the redness value of the three-component heat stabilizer decreased significantly compared to the single-component heat stabilizers. The yellowness value was also highest in M1, followed by B1 and O1, and the yellowness value of the three-component heat stabilizers decreased significantly compared to the single-component heat stabilizers. The lightness value was lowest in M1, followed by B1 and O1, and the lightness value of the three-component heat stabilizers increased significantly compared to the single-component heat stabilizers. Overall, the dynamic thermal stability of the three-component heat stabilizers was better than that of the single-component heat stabilizers.
Chromatic aberration value of PVC sheets at 4 and 20 min
| Sample | Composition of heat stabilizers | Redness value | Yellowness value | Lightness value | |||
|---|---|---|---|---|---|---|---|
| 4 min | 20 min | 4 min | 20 min | 4 min | 20 min | ||
| CZ | C4Z1 | 1.92 | 23.45 | 21.37 | 45.87 | 82.21 | 59.12 |
| M1 | DMTDN | 2.96 | 23.32 | 24.66 | 45.15 | 80.62 | 59.43 |
| M4 | MTN5-C4Z1 | −1.12 | 15.79 | 20.26 | 42.27 | 83.73 | 70.91 |
| B1 | DBTDN | 2.01 | 18.40 | 22.87 | 41.41 | 82.57 | 67.70 |
| B4 | BTN5-C4Z1 | −0.13 | 9.98 | 16.77 | 37.71 | 83.87 | 72.70 |
| O1 | DOTDN | 1.55 | 12.50 | 19.57 | 39.27 | 82.97 | 70.55 |
| O4 | OTN5-C4Z1 | −1.92 | 9.55 | 16.64 | 36.62 | 85.25 | 73.74 |
Furthermore, the redness values at 4 and 20 min were found to be O4 < B4 < M4 < CZ, the yellowness values were O4 < B4 < M4 < CZ, and the lightness values were O4 > B4 > M4 > CZ. It indicated that the dynamic thermal stability of MTN5-C4Z1, BTN5-C4Z1, and OTN5-C4Z1 was better than that of C4Z1, which showed that the tin neodecanoate, CaSt2, and ZnSt2 had a beneficial synergistic effect when compounded together.
3.5 Viscosity test and dispersion analysis
In the above study, the thermal stability effects of the three tin neodecanoates were found to be as follows: DOTDN > DBTDN > DMTDN. To further explored the reasons for this, the following two possibilities were proposed: on the one hand, the molecular chain length was octyl > butyl > methyl, which made DOTDN the most compatible with the PVC molecular chain, so the thermal stability performance of DOTDN-stabilized PVC blends would be better; on the other hand, the viscosity size of the tin neodecanoate products would also have an impact on the thermal stability of the PVC blends, as the difference in viscosity would make a difference in the dispersion effect of the heat stabilizer in the PVC blends, thus affecting the thermal stability of the PVC blends to a certain extent. Table 9 shows the viscosities of the different tin neodecanoate. The viscosities of the three tin neodecanoates were 2,244.1, 272.9, and 217.6 mPa·s, respectively, which resulted in the dispersion of tin neodecanoate in PVC as DMTDN < DBTDN < DOTDN.
Viscosity size of tin neodecanoate
| Heat stabilizer | Viscosity (mPa·s) |
|---|---|
| DMTDN | 2,244.1 |
| DBTDN | 272.9 |
| DOTDN | 217.6 |
To investigate the impact of heat stabilizer product viscosity on the thermal stability of PVC blends, the yellowness values of DMTDN, DBTDN, and DOTDN stabilized PVC sheets were measured at three different locations. As shown in Figure 5, the yellowness values of DMTDN stabilized PVC sheets exhibited more obvious variations at different positions, while the yellowness values of DBTDN stabilized PVC sheets showed fewer differences, and the yellowness values of DOTDN stabilized PVC sheets showed almost no variation. This phenomenon indicated that the viscosity of tin neodecanoate had an effect on its dispersion in PVC, which in turn influenced the thermal stability of the PVC blends to some extent.

Yellowness value of different PVC sheets at 4 min.
3.6 Torque rheology test
It is widely known that when the ester groups are the same in fatty acid tin compounds, the processing lubricity follows the order of octyl tin > butyl tin > methyl tin. To examine the effect of the calcium–zinc heat stabilizer compounded with tin neodecanoate on the processability of PVC, we conducted a torque rheology test to compare the rheology diagrams of PVC stabilized by DBTDN, C4Z1, and BTN5-C4Z1. As shown in Figure 6, both PVC samples exhibited the feed peak and the plasticized peak. The plasticization peaks of DBTDN-, BTN5-C4Z1-, and C4Z1-stabilized PVC samples occurred at 65, 89, and 292 s, respectively. The plasticization efficiency of the PVC sample stabilized solely by the C4Z1 system was slow. However, the DBTDN-stabilized PVC sample was effectively plasticized, and the addition of BTN5-C4Z1 accelerated the plasticization time by 203 s, resulting in a significant improvement in the plasticization efficiency compared to the use of C4Z1 alone. The torque magnitudes of the different PVC samples followed the order of CZ < B4 < B1. This indicated that the combination of tin neodecanoate and calcium–zinc heat stabilizers improved the lubricity compared to tin neodecanoate alone. This improvement was mainly due to the better lubricity of the calcium–zinc heat stabilizer compared to that of tin neodecanoate.

The results of DBTDN, BTN5-C4Z1, and C4Z1 torque rheology tests.
3.7 Analysis of thermal stability mechanism
The thermal degradation of PVC occurs when the allyl chloride and tert-butyl chloride structures on PVC remove the chlorine atom and combine with a neighboring hydrogen atom, resulting in the formation of hydrogen chloride and the creation of the –C═C– structure. Further thermal degradation leads to the production of double bonds with conjugate structures, which can cause the color of PVC to change to yellow, brown, or even black. To prevent this, Frye et al. (34,35) proposed that heat stabilizers could inhibit the growth of conjugated polyene sequences by replacing the unstable groups such as allyl chloride or tert-butyl chloride on the PVC molecular chain with stabilizers. However, the dynamic thermal stability test results showed that the PVC sheets with only DMTDN, DBTDN, and DOTDN as single-component heat stabilizers had deep coloration in the first several minutes of the test, indicating that these three tin neodecanoates were unable to replace the unstable groups on the PVC molecular chain and effectively inhibit PVC coloration. Instead, based on their initial coloration and structure (36), it can be inferred that they improved the thermal stability of PVC mainly by absorbing the HCl released from the PVC molecular chain. Therefore, as shown in Figure 7, these three tin neodecanoates mainly inhibit the degradation of PVC by absorbing HCl generated from the degradation of the PVC molecular chain.

Mechanism of HCl absorption by tin neodecanoate.
Figure 8 illustrates that the presence of calcium soap leads to the regeneration of harmful zinc chloride (ZnCl2) into zinc soap. This process activates the zinc soap and significantly reduces its catalytic capacity, while simultaneously converting itself into harmless calcium chloride (CaCl2), thus inhibiting the destructive effect of ZnCl2. Therefore, when CaSt2, ZnSt2, and tin neodecanoate were used in combination in the experiment, ZnSt2 replaced the unstable chlorine atoms on PVC, inhibited the initial coloration of PVC sheets, and improved the initial thermal stability of PVC sheets. CaSt2 absorbed and reconverted the harmful ZnCl2 into ZnSt2, suppressing the zinc burning phenomenon and realizing the regeneration of ZnSt2. Furthermore, adding tin neodecanoate improved the poor long-term thermal stability of calcium–zinc heat stabilizers and enhanced the long-term thermal stability of PVC sheets. The combined effect of these heat stabilizers resulted in an overall improvement in the thermal stability of PVC materials.
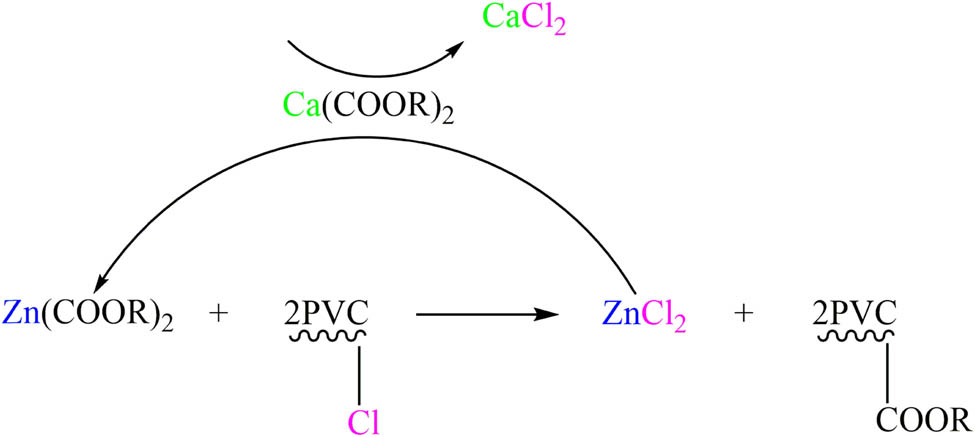
Calcium and zinc soap synergistic mechanism of action.
4 Conclusions
In this study, we synthesized a range of modified PVC materials with enhanced thermal stability by blending tin neodecanoate and calcium–zinc heat stabilizers with PVC at varying ratios. We conducted a comprehensive investigation of the static and dynamic thermal stability of these PVC materials. Our findings demonstrated that tin neodecanoate, as a standalone PVC heat stabilizer, significantly improved the thermal stability of PVC. Among the four plasticizers tested (DOTP, DOP, DOA, and ESO), the combination of ESO and tin neodecanoate exhibited a synergistic effect on PVC’s thermal stability. We also observed that different types of tin neodecanoate had varying impacts on PVC’s thermal stability, with DOTDN being the most effective, followed by DBTDN and DMTDN. This discrepancy could be attributed to the compatibility of the heat stabilizer with the PVC molecular chain and its dispersion within the PVC matrix. In addition, our investigation using Congo red and heat aging tests indicated that MTN5-C4Z1, BTN5-C4Z1, and OTN5-C4Z1 extended the static heat stabilization time and maintained better color stability. TG analysis revealed that MTN5-C4Z1, BTN5-C4Z1, and OTN5-C4Z1 improved the heat resistance of PVC materials. In the double-roller mill plasticizing test, we found that MTN5-C4Z1, BTN5-C4Z1, and OTN5-C4Z1 reduced the redness and yellowness values of PVC sheets while increasing their lightness values compared to DMTDN, DBTDN, DOTDN, and C4Z1. Finally, the torque rheology test demonstrated that the superior lubricity of CaSt2 and ZnSt2 rendered the three-component heat stabilizer superior to the single-component tin neodecanoate heat stabilizer. In conclusion, the three-component heat stabilizer comprising tin neodecanoate and calcium–zinc heat stabilizer exhibited enhanced thermal stability and lubricity compared to the single-component tin neodecanoate heat stabilizer.
Acknowledgements
The authors would like to express thanks to all the staff at the College of Materials Science and Engineering, Nanjing Tech University, and the Yunxi Tinchem (Nanjing) Technology Co., Ltd.
-
Funding information: The authors state that no funding is involved.
-
Author contributions: Xiang Wang: writing – original draft, methodology; Tingwei Wang: writing – review and editing, formal analysis; Chao Di: formal analysis, project administration.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Available upon request.
References
(1) Endo K. Synthesis and structure of poly(vinyl chloride). Prog Polym Sci. 2002;27(10):2021–54. 10.1016/s0079-6700(02)00066-7.Search in Google Scholar
(2) Li Y, Li D, Han W, Zhang M, Ai B, Zhang L, et al. Facile synthesis of di-mannitol adipate ester-based zinc metal alkoxide as a bi-functional additive for poly(vinyl chloride). Polymers (Basel). 2019;11(5):813. 10.3390/polym11050813.Search in Google Scholar PubMed PubMed Central
(3) Song LF, Huo HW, Zhang WS, Xia HY, Niu YH. The facile strategy of improving the long-term stability of highly transparent polyvinyl chloride by introducing unsaturated Zn oleate and uracil derivatives. Materials. 2022;15(7):2672. 10.3390/ma15072672.Search in Google Scholar PubMed PubMed Central
(4) Safronov A, Somova T, Suvorova A, Fisch MH, Stewen U, Bacaloglu R, et al. Mechanism of organotin stabilization of poly(vinyl Chloride). 6. Compatibility of organotin stabilizers with PVC. J Vinyl Addit Technol. 2003;9(3):127–37. 10.1002/vnl.10074.Search in Google Scholar
(5) Borukaev TA, Kitieva LI, Sultigova ZK, Martazova RM. Complex stabilizer based on calcium and zinc stearates for PVC compounds. Key Eng Mater. 2020;869:218–23. 10.4028/www.scientific.net/KEM.869.218.Search in Google Scholar
(6) Yu J, Sun LS, Ma C, Qiao Y, Yao H. Thermal degradation of PVC: A review. Waste Manage. 2016;48:300–14. 10.1016/j.wasman.2015.11.041.Search in Google Scholar PubMed
(7) Folarin OM, Sadiku ER. Thermal stabilizers for poly(vinyl chloride): A review. Int J Phys. 2011;6(18):4323–30. 10.5897/IJPS11.654.Search in Google Scholar
(8) Jubsilp C, Asawakosinchai A, Mora P, Saramas D, Rimdusit S. Effects of organic based heat stabilizer on properties of polyvinyl chloride for pipe applications: A comparative study with Pb and CaZn systems. Polymers (Basel). 2021;14(1):133. 10.3390/polym14010133.Search in Google Scholar PubMed PubMed Central
(9) Liu YB, Liu WQ, Hou MH. Metal dicarboxylates as thermal stabilizers for PVC. Polym Degrad Stabil. 2007;92(8):1565–71. 10.1016/j.polymdegradstab.2007.05.003.Search in Google Scholar
(10) Wang M, Fan XZ, Bu Q, Jia PY, Yuan SQ. Synergistic modification of PVC with nitrogen-containing heterocycle and tung-oil based derivative for enhanced heat stabilization and plasticization behavior. J Renew Mater. 2023;11(4):2015–31. 10.32604/jrm.2023.026063.Search in Google Scholar
(11) Wang M, Fan XZ, Song XH, Bu Q. The promoting effect of multifunctional groups on the thermal and mechanical properties of PVC materials. J Renew Mater. 2023;11(2):867–80. 10.32604/jrm.2022.022996.Search in Google Scholar
(12) Mohamed NA, Al-Magribi WM. N-(Substituted phenyl)itaconimides as organic stabilizers for plasticized poly(vinyl chloride) against thermal degradation. Polym Degrad Stabil. 2003;80(2):275–91. 10.1016/s0141-3910(02)00408-1.Search in Google Scholar
(13) Kalouskova R, Novotna M, Vymazal Z. Investigation of thermal stabilization of poly(vinyl chloride) by lead stearate and its combination with synthetic hydrotalcite. Polym Degrad Stabil. 2004;85(2):903–9. 10.1016/j.polymdegradstab.2004.04.008.Search in Google Scholar
(14) Han WY, Zhang MQ, Kong YF, Li DG, Liu LH, Tang S, et al. Pentaerythritol stearate ester-based tin (ii) metal alkoxides: A tri-functional organotin as poly(vinyl chloride) thermal stabilizers. Polym Degrad Stabil. 2020;175:109129. 10.1016/j.polymdegradstab.2020.109129.Search in Google Scholar
(15) Wang M, Xu JY, Wu H, Guo SY. Effect of pentaerythritol and organic tin with calcium/zinc stearates on the stabilization of poly(vinyl chloride). Polym Degrad Stabil. 2006;91(9):2101–9. 10.1016/j.polymdegradstab.2006.01.011.Search in Google Scholar
(16) Arkış E, Balköse D. Thermal stabilisation of poly(vinyl chloride) by organotin compounds. Polym Degrad Stabil. 2005;88(1):46–51. 10.1016/j.polymdegradstab.2004.02.021.Search in Google Scholar
(17) Wang GB, Yang SS, Xia JJ. Thermal degradation kinetics of calcium stearate/PVC composite. Results Phys. 2020;8:100123. 10.1016/j.rinma.2020.100123.Search in Google Scholar
(18) Liu YN, Li HB, Shu WG, Chen QY. Synthesis and application of antimony pent (isooctyl thioglycollate). J Cent South Univ Technol. 2005;12(1):64–7. 10.1007/s11771-005-0205-8.Search in Google Scholar
(19) Gao JG, Liu XQ, Yang JB, Zhu FL. Influence of polyols as a co-stabilizer on stabilization efficiency of calcium/zinc stabilizers to PVC. Adv Mat Res. 2012;549:251–4. 10.4028/www.scientific.net/AMR.549.251.Search in Google Scholar
(20) Liu Z, Fan J, Feng J, Li M, Hu Y, Hao W, et al. Study on the use of rare earth stabilizer as poly(vinyl chloride) stabilizer. J Vinyl Addit Technol. 2020;26(4):536–47. 10.1002/vnl.21768.Search in Google Scholar
(21) Lu YH, Wang B, Xue MY, Lu YW. Kinetics of thermal oxidative degradation of poly(vinyl chloride) containing Ca and Sn at low temperature. Waste Manage. 2021;121:52–8. 10.1016/j.wasman.2020.11.019.Search in Google Scholar PubMed
(22) Wang M, Jiang JC, Xia JL, Li SH, Li M. Phosphate ester groups-containing ricinoleic acid-based Ca/Zn: Preparation and application as novel thermal stabilizer for PVC. J Appl Polym Sci. 2018;135(10):45940. 10.1002/app.45940.Search in Google Scholar
(23) Jiang YN, Yang ZH, Su QS, Chen LL, Wu J, Meng JL. Preparation of magnesium-aluminum hydrotalcite by mechanochemical method and its application as heat stabilizer in poly(vinyl chloride). Materials. 2020;13(22):5223. 10.3390/ma13225223.Search in Google Scholar PubMed PubMed Central
(24) Ye F, Guo XJ, Zhan HH, Lin JX, Lou WC, Ma XT, et al. The synergistic effect of zinc urate with calcium stearate and commercial assistant stabilizers for stabilizing poly(vinyl chloride). Polym Degrad Stabil. 2018;156:193–201. 10.1016/j.polymdegradstab.2018.08.012.Search in Google Scholar
(25) Wang B, Lu YH, Lu YW. Organic tin, calcium–zinc and titanium composites as reinforcing agents and its effects on the thermal stability of polyvinyl chloride. J Therm Anal Calorim. 2020;142(2):671–83. 10.1007/s10973-020-09767-9.Search in Google Scholar
(26) Xue MY, Lu YH, Li K, Wang B, Lu YW. Thermal characterization and kinetic analysis of polyvinyl chloride containing Sn and Zn. J Therm Anal Calorim. 2019;139(2):1479–92. 10.1007/s10973-019-08505-0.Search in Google Scholar
(27) Liu HL, Jiang PP, Zhang K, Dong YM. Synthesis of Bismuth(iii) Neodecanoate and its application to poly(vinyl chloride) as a thermal stabilizer. Polym-Plast Technol. 2017;57(16):1657–64. 10.1080/03602559.2017.1410848.Search in Google Scholar
(28) Hayajneh M, Al-Shrida M, AL-Oqla F. Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review. e-Polymers. 2022;22(1):641–63. 10.1515/epoly-2022-0062.Search in Google Scholar
(29) Zhang Z, Jiang P, Liu D, Feng S, Zhang P, Wang Y, et al. Research progress of novel bio-based plasticizers and their applications in poly(vinyl chloride). J Mater Sci. 2022;56(17):10155–82. 10.1007/s10853-021-05934-x.Search in Google Scholar
(30) Pereira VA, Fonseca AC, Costa CSMF, Ramalho A, Coelho JFJ, Serra AC. End-capped biobased saturated polyesters as effective plasticizers for PVC. Polym Test. 2020;85:106406. 10.1016/j.polymertesting.2020.106406.Search in Google Scholar
(31) Wang Y, Jiang F, Zhang L. Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene. e-Polymers. 2023;23(1):20230008. 10.1515/epoly-2023-0008.Search in Google Scholar
(32) Fu Q, Long Y, Gao Y, Ling Y, Qian H, Wang F, et al. Synthesis and properties of castor oil based plasticizers. RSC Adv. 2019;9(18):10049–57. 10.1039/c8ra10288k.Search in Google Scholar PubMed PubMed Central
(33) Ali SFA, El Batouti M, Abdelhamed M, El-Rafey E. Formulation and characterization of new ternary stable composites: Polyvinyl chloride-wood flour-calcium carbonate of promising physicochemical properties. J Mater Res Technol. 2020;9(6):12840–54. 10.1016/j.jmrt.2020.08.113.Search in Google Scholar
(34) Frye AH, Horst RW, Paliobagis MA. The chemistry of poly(vinyl chloride) Stabilization. V. Organotin stabilizers having radioactively tagged Y groups. J Polym Sci. 1964;2(4):1801–14. 10.1002/pol.1964.100020421.Search in Google Scholar
(35) Frye AH, Horst RW. The mechanism of poly(vinyl chloride) stabilization by barium, cadmium, and zinc carboxylates. I. Infrared studies. J Polym Sci. 1959;40(137):419–31. 10.1002/pol.1959.1204013712.Search in Google Scholar
(36) da Silva JPV, Brito YC, Fragoso DMdA, Mendes PR, Barbosa ASL, Bortoluzzi JH, et al. Influence of different alkyl and carboxylate substituents on Sn(iv) organometallic catalysts during fatty acid methyl ester production. Catal Commun. 2015;58:204–8. 10.1016/j.catcom.2014.09.010.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites


