Abstract
The chromium was deposited on the surface of 0.45 C medium carbon steel by high current pulsed electron beam (HCPEB) alloying treatment to obtain a high quality alloying layer. The microstructure of the alloying layer was studied by X-ray diffraction, optical microscopy, scanning electron microscopy (SEM), and transmission electron microscopy. The hardness of the surface was measured by Vickers durometer. The corrosion resistance of samples before and after HCPEB irradiation was also measured by an electrochemical workstation. The results showed that the alloying layer with a dept of about 4–9 μm on the surface was formed after HCPEB alloying treatment. TEM results revealed that the Cr element is dissolved on the surface and alloyed with C element in the substrate to form Cr23C6 enhanced particles. The microhardness and corrosion resistance of the medium carbon steel subjected to a HCPEB alloying processing were remarkably improved compared with the original one.
Introduction
The 0.45 C medium carbon steel is one of the most used structural materials, such as shafts and gears in the manufacture, some of the main force components and steel structures [1, 2, 3]. However, its hardness and corrosion resistance are relatively poor. In many cases, it is often difficult to meet the requirements of practical use, in particular the abrasion and corrosion resistance, which limits its applications [4, 5]. Surface alloying technology used on the steel surface to obtain a desired alloying layer is an effective method to improve the surface properties [6, 7, 8]. Basically, this technology involves interactions between a solvent (substrate) and a solute (alloying elements) when the alloying elements are uniformly distributed in the alloying layer. Therefore, significant improvements in the surface hardness, strength, and corrosion resistance can be achieved [9, 10]. Furthermore, the resistance of thermal fatigue can be increased, and the service life of the workpiece can be improved [11], as well. Nowadays, there are several kinds of surface alloying technologies that are widely used, such as double glow plasma surface alloying technology, evaporative pattern casting technology, laser beam surface alloying, and ion beam implantation [12, 13, 14, 15, 16]. However, these methods have some disadvantages, such as high cost, complicated operation, long cycle, and low efficiency. For instance, the laser beam results in the coarse microstructure with relative long duration and the irradiated surface is easy to oxidize in a non-vacuum environment [17, 18].
High current pulsed electron beam (HCPEB) is a new type of surface modification technology [19, 20, 21]. During the instantaneous process of HCPEB bombardment, the higher energy (107–109 W/cm2) acts on the surface of the material in a very short time (several microseconds), resulting in extremely rapid heating, cooling, melting, and even evaporation, followed by a rapid solidification. As a result, the formation of a dense remelted layer can be obtained. Many researchers focus on the change of mechanical properties of the materials after HCPEB treatment and the thermo-mechanical coupling mechanism involved in the materials surface modification by HCPEB irradiation [22, 23, 24]. However, the surface alloying on the medium carbon steel by HCPEB treatment is still occasionally applied. The HCPEB technology has a huge potential in surface alloying and unique advantages due to the local high energy density, instantaneous heating-cooling effect, and specific modified characteristics [25]. The substrate and coating can be simultaneously melted resulting in their combination at the interface, which can significantly improve the performance of materials.
In this paper, 0.45 C medium carbon steel was used as an experimental substrate in the annealed state. Chromium (Cr) powder was precoated on the surface. The surface alloying of medium carbon steel was carried out by HCPEB irradiation. The microstructural characterization and performance test were investigated, and the alloying mechanism after HCPEB treatment was studied in detail.
Materials and characterization techniques
The chemical composition of 0.45 C medium carbon steel used in the current investigation is 0.42–0.50 C wt %, 0.17–0.37 Si wt. %, 0.5–0.80 Mn wt. %, ≤ 0.035 P wt. %, ≤ 0.035 S wt. %, ≤ 0.25 Ni wt. %, ≤ 0.25 Cr wt. %, ≤ 0.25 Cu wt. %, and the balance Fe. The samples were cut into 10 × 10×10 mm3 by wire electrical discharge machining. Before testing, the annealing process was carried out in a high temperature furnace, as follows. The sample was heated at 500 °C, then hold for 4 h, and finally cooled at 25 °C. The sample surface was grounded by using metallographic sandpapers and polished by diamond paste, and then washed with anhydrous ethanol. Cr powder (99.9 %, 30–40 μm particle size) was selected as the alloying material. A slurry was obtained by mixing 10 g Cr powder with an organic binder (100 mL, nitrocellulose lacquer : solvent=1 : 2) and deposited on the polished surface with a thickness of about 0.05–0.1 mm by using a spraying gun. After drying, the polished surfaces of samples were irradiated using HOPE-I type source at room temperature. The irradiation was performed under vacuum of 10–5 torr, and using an electron energy of 27 keV, an energy density of 4 J/cm2, a target distance of 150 mm, a current pulse duration of 1.5 μs, and an irradiated pulse number of 10, 20, and 30.
X-ray diffraction (XRD) with CuKα radiation was used for phase identification on a RigakuD/max-2500/pc X-ray diffractometer. The microstructural evolution was carefully analyzed by using LEICA DM-2500M optical microscope, JEOL JSM-7100F scanning electron microscope (SEM), and JEOL-2100 transmission electron microscope (TEM).
Microhardness was measured by a HVS-1000 device. To ensure the reliability of the measurements, five test points were installed in each sample. The electrochemical corrosion was measured using a Bio-Logic VMP2 electrochemical workstation with saturated calomel electrode as a reference electrode and platinum sheet as a counter electrode. The electrolyte was a 3.5 wt. % NaCl water solution. The cyclic polarization was done with a sweep rate of 0.333 mV/s. The tested specimen was exposed to the solution with an area of 1 cm2, and the rest was sealed by vulcanized silicone rubber.
Results and discussion
XRD analysis
Figure 1 shows the XRD patterns of the samples before and after HCPEB alloying treatment. It can be seen that the original sample with no Cr powder was composed mainly of ferrite (α-Fe) phase. After HCPEB treatment, the peaks of martensite and austenite were detected, which indicated that the phase transformation occurred due to the thermostress coupling effect induced by HCPEB irradiation. Besides, compared to the initial one, the diffraction peak of austenite (γ-Fe) appeared on the irradiated surface, and the corresponding diffraction intensity was gradually increased with the increasing of the HCPEB pulses. This result indicates that the content of γ-Fe phase was increased, and Cr element has been dissolved into the substrate to form a solid solution. The width of diffraction peaks shows a remarkable broadening in comparison with the untreated one in Figure 1(b), which could result from the refined grain size caused by HCPEB irradiation. Previous reports have shown that HCPEB irradiation can induce a rapid remelting of the metal surface, as well as a the formation of a high number of nucleation sites, which have no enough time to grow during the next rapid cooling process [26]. Therefore, ultrafine crystals are developed on the metal surface, which are reflected by very broad and less intense diffraction peaks.
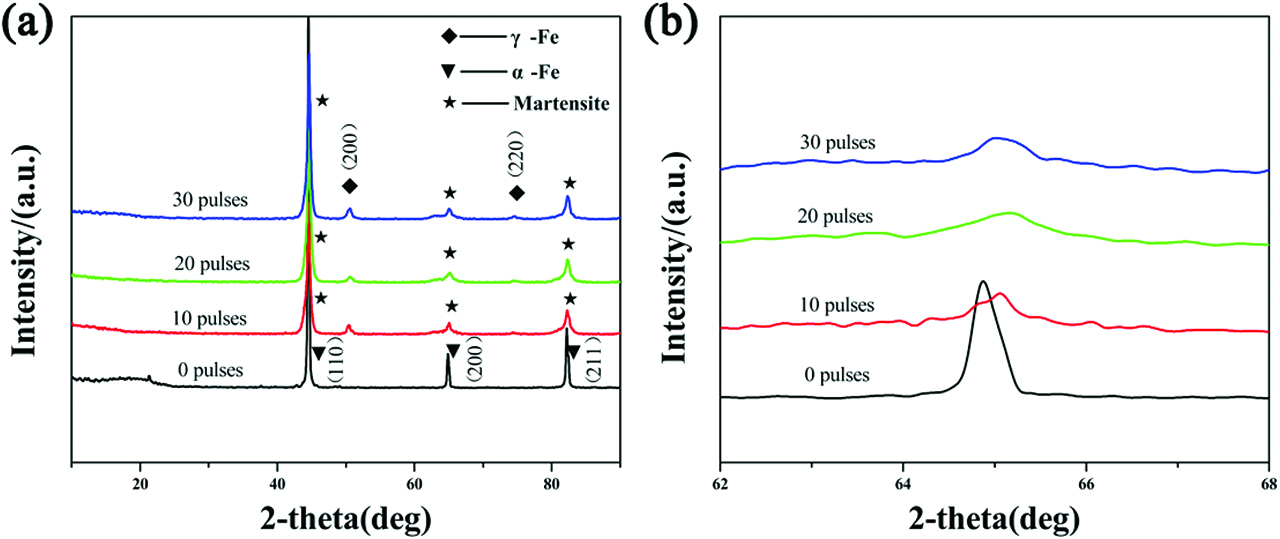
XRD analysis of medium carbon steel before and after HCPEB alloying. (a) XRD patterns, (b) enlarged view of the (200) diffraction peak.
Morphology of the surface and cross section
Figure 2(a) shows the metallographic structure of the initial sample. It can be seen that, before HCPEB irradiation, the sample is composed of the lamellar pearlite and coarse ferrite. Figure 2(b)–(d) presents the surface morphologies of HCPEB-alloyed samples prepared with 10, 20, and 30 pulses, respectively. It can be seen that volcano-like craters were formed on the irradiated surface while the precoated Cr film disappeared. This result suggests that the surface was remelted after HCPEB alloying treatment, which resulted in the redistribution of elements that were uniformly dispersed in the material [27]. As the number of pulses increased, the crater density was gradually decreased. The volcano-like craters were formed due to the non-homogeneous local melting, and subsequent eruptions occurred near the subsurface when the matrix reached the melting point. Many previous studies have shown that the inclusions and second phases on the surface were more likely acted as nucleation sites for crater eruption [28, 29, 30]. The crater density was decreased with the number of pulses due to the gradual eruption of the inclusions, which could bring about a significant effect, the so-called “selective purification” effect [28, 31]. This effect is crucial for improving the corrosion resistance of the materials.
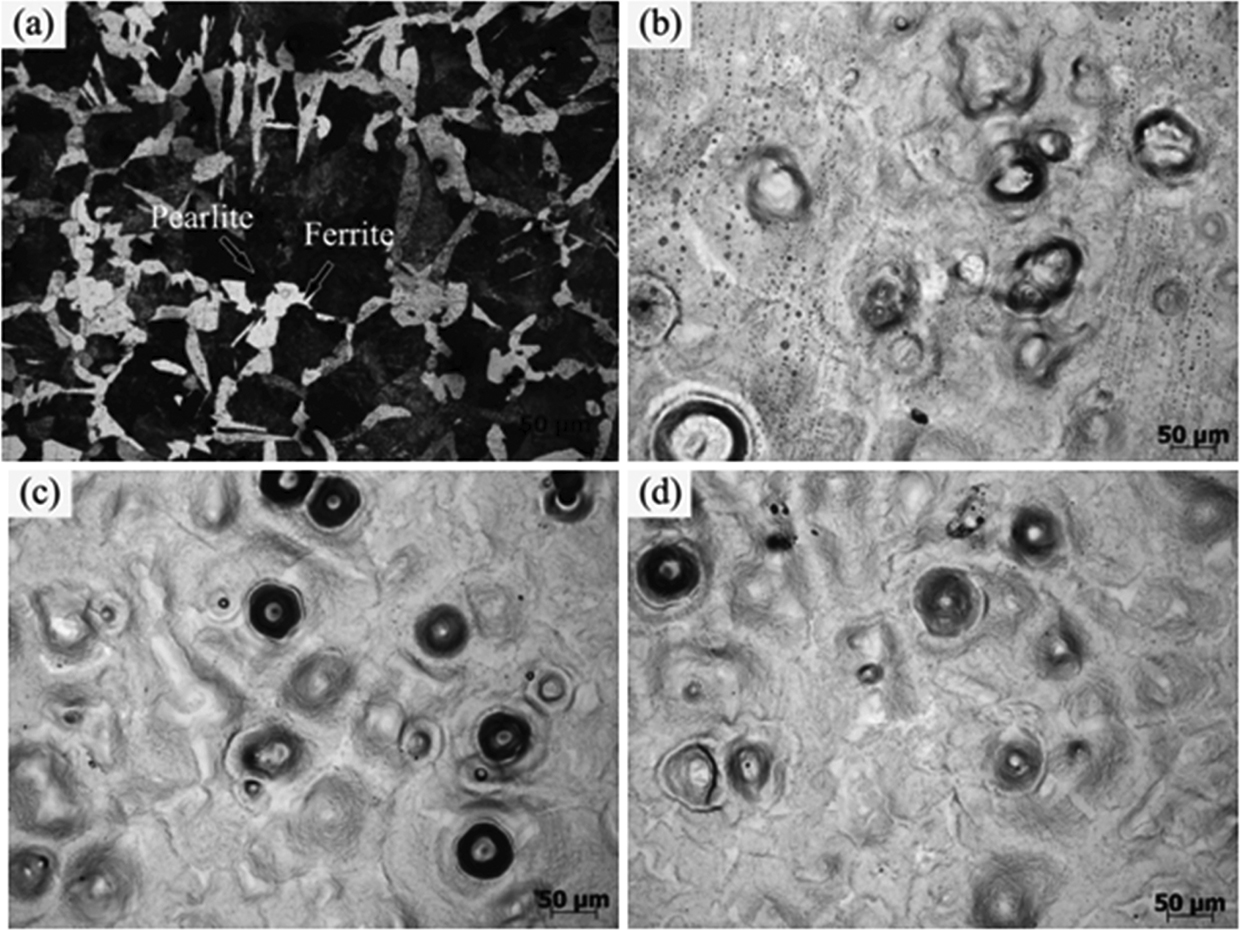
Metallographic morphologies of medium carbon steel before and after HCPEB alloying treatment. (a) initial, (b) 10, (c) 20, and (d) 30 pulses.
Figure 3(a) and (b) shows the representative SEM images of the surface of HCPEB samples alloys with 20 and 30 pulses, respectively. Obviously, the surface was remelted, and the precoated Cr powders completely disappeared on the irradiated surface of the samples alloys. As seen in Figure 3(a), the crater had a deep center-dimpling shape with a small bottom hole (marked with a red arrow), which confirmed the melting and eruption of local subsurface layer. After 30-pulsed HCPEB irradiation (Figure 3(b)), the number of craters was decreased visibly, whereas the depth was shallow. In other words, the surface became smoother, which is consistent with the results obtained by light microscopy. Besides, the EDS analysis (inset in Figure 3(b)) indicates that the surface is rich in Cr after HCPEB alloying. The Cr-rich solid solution in the steel will have a crucial role in improving the surface strength and corrosion resistance of the material [32, 33]. High resolution SEM images of samples alloys are given in Figure 3(c) and (d). One can see that, in addition to the typical morphology, two other visible features can be also obtained on the alloying surface. The first one is associated with the formation of martensitic structures in some areas of 20-pulsed sample alloy (Figure 3(c)), which was caused by the rapid heating and cooling processes. Another one was related to the nano-structures i. e. refined austenite grains, which are generated on the alloying surface after 30-pulsed HCPEB irradiation due to the “self-cooling hardening” effect. Extremely fast solidification and cooling processes favor the formation of large numbers of nucleus, which do not have enough time to grow. Consequently, the nanoscale grains were formed on the irradiated surface. It is evident that the phase transformation occurred after HCPEB alloying. The formation of these nano grains is defining for the global properties of the material surface, which will be improved [34].
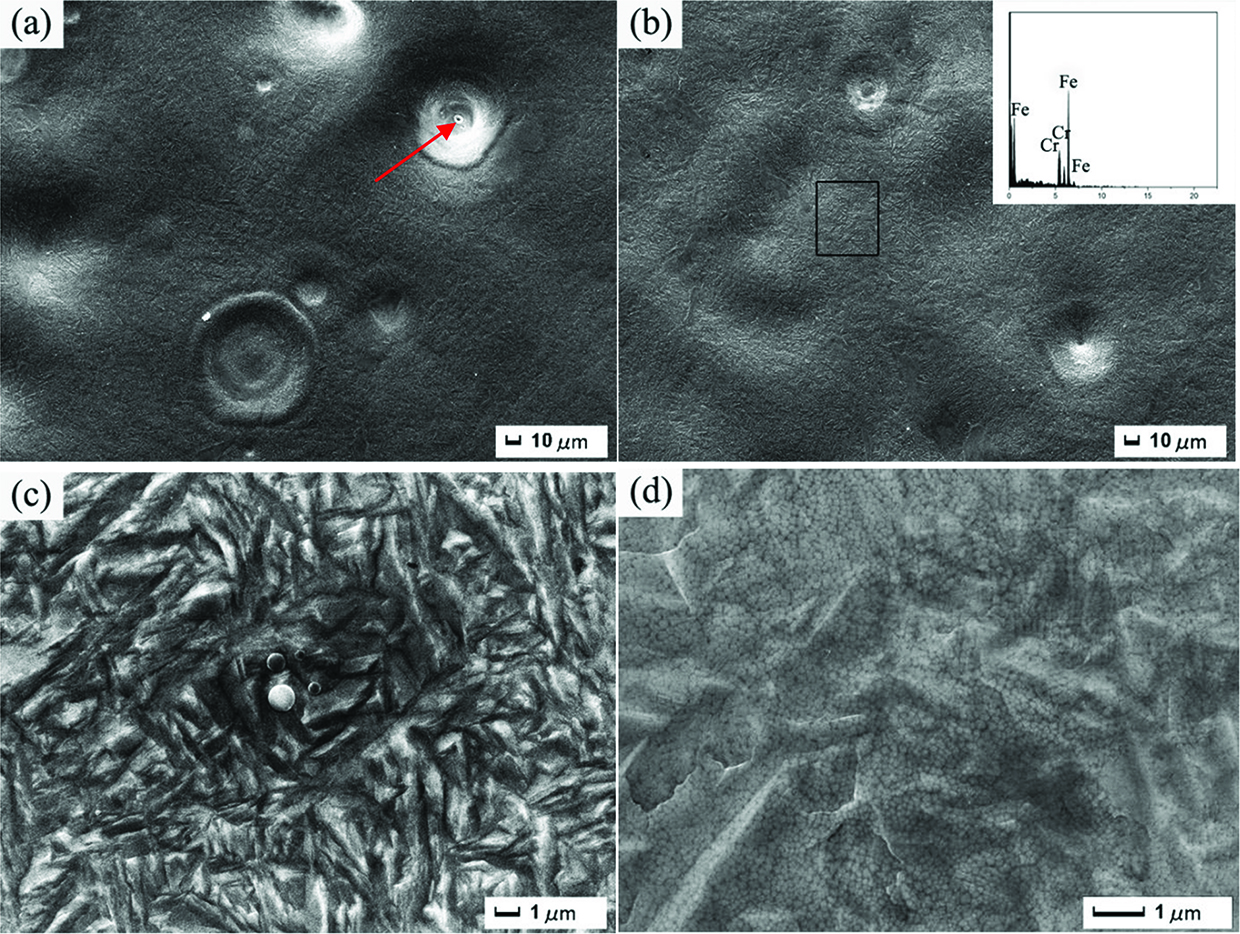
Surface SEM images of the alloyed samples. (a) and (c) 20 pulses, (b) and (d) 30 pulses.
In Figure 4, the cross-sectional SEM images of the samples after HCPEB alloying treatment with different pulses are displayed. As noticed, an alloyed layer was formed on the top surface, which was obviously different from the substrate. The modified surface exhibits a stratified structure composed of a remelted layer, a heat affected zone, and a substrate matrix. The corresponding thickness of this mixed alloy layer increased with the increasing of the number of HCPEB pulses, and it was about 4–9 μm (Figure 4(a)–(c)). Punctual EDS analysis on the image in Figure 4(c) shows that the content of Cr element in the remelted layer was much higher than that of the substrate, which was about 8 μm length (Figure 4(d)). This result sustains the formation of the Cr-rich alloyed layer after HCPEB alloying.
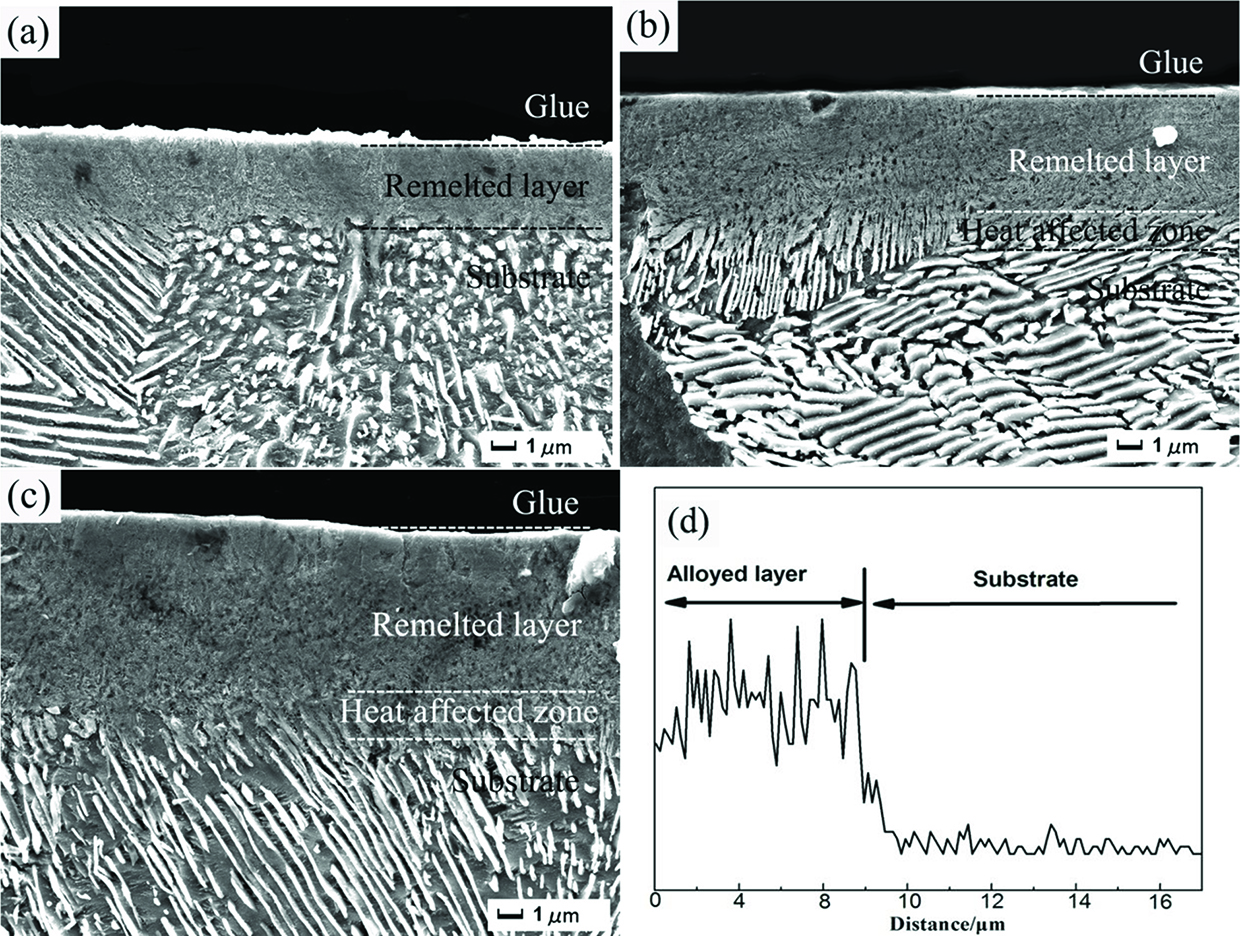
Cross-sectional SEM images of the alloying samples. (a) 10, (b) 20, (c) 30 pulses, and (d) the EDS line scanning of the alloying sample after 30 pulses.
Figure 5(a)-(c) shows the TEM bright field images of the irradiated samples with 20 pulses. Figure 5(a) reveals that the lath-like martensitic structures were formed in certain regions of the irradiated layer while Figure 5(b) shows the dislocation cells in ferrite, which was a typical structure of the strongly textured metal material formed by the entanglement of the high density of dislocations. The dislocation needed a rearrangement to reduce energy while the entanglement of dislocation prevented the further dislocation slip. The dislocation subgrains were formed because of its lower energy [35]. Besides, there were many particles with an average size of about 0.24 μm found in the alloying layer (Figure 5(c)), which can be identified as Cr23C6 based on the corresponding selected area electron diffraction (SAED). Figure 5(d)–(f) shows the TEM images of the 30-pulsed alloyed sample. The grains with average sizes of about 186 nm were clearly observed (Figure 5(d)), which can be considered as austenite grains according to the result provided by SAED. This result is in good agreement with the SEM result illustrated in Figure 3. The micro-twinning was formed on the alloying surface as shown in Figure 5(c), revealing that the twinning deformation occurred after HCPEB irradiation. The micro-twinning consisted of a rapid and strongly deformation, which indicates that the HCPEB irradiation caused a severe plastic deformation [36], followed by the appearance of deformed structures, such as twins and complicated dislocation configurations. The diffusion of Cr element can be promoted by these structures, whereas the grain boundaries of nano-crystalline structures are generated by a rapid solidification. Finally, a thick and compact alloying layer was formed. It was believed that the ultrafine grain size and dense solution of alloying elements in the austenite phase would inhibit the natural transformation of martensite. Thus, the degree of carbon alloying is a key factor for the stabilization of the austenite crystalline phase [37]. In Figure 5(f), Cr-rich carbides with smaller average size of about 10 nm were also formed in the alloying layer. These Cr-rich carbides can effectively prevent the movement of dislocation and subgrain boundaries improving the material strength by the effect of dispersion strengthening. In addition, the formation of the refined carbides is crucial for the austenite stabilization. Moreover, these carbides can improve the thermal fatigue properties of the material, as well [38, 39].

TEM images of medium carbon steel after HCPEB alloying with 20 pulses (a), (b), and (c) as well as 30 pulses (d), (e), and (f).
Microhardness testing
Figure 6 illustrates the surface microhardness of the samples before and after HCPEB surface alloying after 10, 20, and 30 pulses. Compared with the original one (0 pulses), the surface microhardness of the irradiated samples was significantly increased as the number of pulses increased. The value of surface microhardness of 30-pulsed sample was the highest, which reached to 726 HV (about four times of the original one). It can be noticed that the sample prepared with 20 pulses displays a surface microhardness very close to that of the sample irradiated with 30 pulses. On this finding, it could be affirmed that an irradiation with 20 pulses could be as optimum to obtain a material with enhanced microhardenss. However, the results of the morphology and corrosion resistance clearly indicated that the 30-pulse sample shows improved properties reinforcing the idea that a number of 30 pulses is needed to provide a high performance alloyed material. After HCPEB irradiation, the modified layer surface of the medium carbon steel was transformed in a complex microstructure composed of martensite with very refined austenite phase. Besides, as the HCPEB pulses increases, the alloying element is better incorporated into the carbon lattice, which promotes the formation of Cr-rich carbides. These strengthening mechanisms, including martensitic hardening, fine grain strengthening, and solid solution strengthening are very important to improve the hardness of surface alloying layer by HCPEB treatment.

The surface microhardness of medium carbon steel before and after HCPEB alloying.
Corrosion resistance testing
The polarization curves of the medium carbon steel in 3.5 wt. % NaCl solution before and after HCPEB alloying are displayed in Figure 7. The corrosion potential (Ecorr) and corrosion current (Icorr) density measured by Tafel extrapolation are listed in Table 1. The Ecorr of alloyed samples was higher than that of the initial one, and the maximum value of –0.854 V was found after 30-pulsed irradiation. Besides, the Icorr density was decreased gradually after HCPEB surface alloying. The corrosion potential reflects the trend and thermodynamics of the reactions occurring on the alloyed surface while the corrosion current reflects the dynamics of corrosion, which is proportional to the rate of corrosion. The results showed that the corrosion resistance of the samples is improved significantly after HCPEB alloying treatment. The enhanced corrosion resistance was attributed to several factors, as follows. First, the presence of second phases or inclusions was prone to act as sites of corrosion pitting. The undesirable inclusions were erupted or dissolved due to the formation of craters induced by HCPEB irradiation, which is known as selective purification effect. Therefore, the reduction of corrosion pitting density can stimulate the formation of a smooth and denser protective oxide film. Secondly, the content of Cr in the alloying layer obtained by irradiation was much higher than that of the initial one. The Cr was highly reactive, which allowed the formation of the passive films on the surface. Therefore, the increased Cr content was essential to improve the corrosion resistance. Thirdly, the defect structures, such as grain boundaries, subgrain boundaries, and dislocations were formed, which can provide numerous channels by which Cr can diffuse and form the protective film. Hence, a thick and dense chromium oxidation film (passive film) on the surface can be easily formed and it plays a noteworthy role in surface passivation. However, there are some differences regarding the corrosion resistance from a sample to another depending on the number of pulses. These differences are related to the formation of craters. As shown in Figure 2, for 10- and 20-pulsed samples, the numerous craters can hinder the formation of a continuous and dense protective passive film, which is detrimental for the corrosion resistance. The crater density of 30-pulsed sample was rather lower compared to that of other samples, which was reflected by the formation of a thicker, denser, and more stable passive layer. Under these abovementioned factors, the corrosion resistance of the 30-pulsed sample was greatly improved.
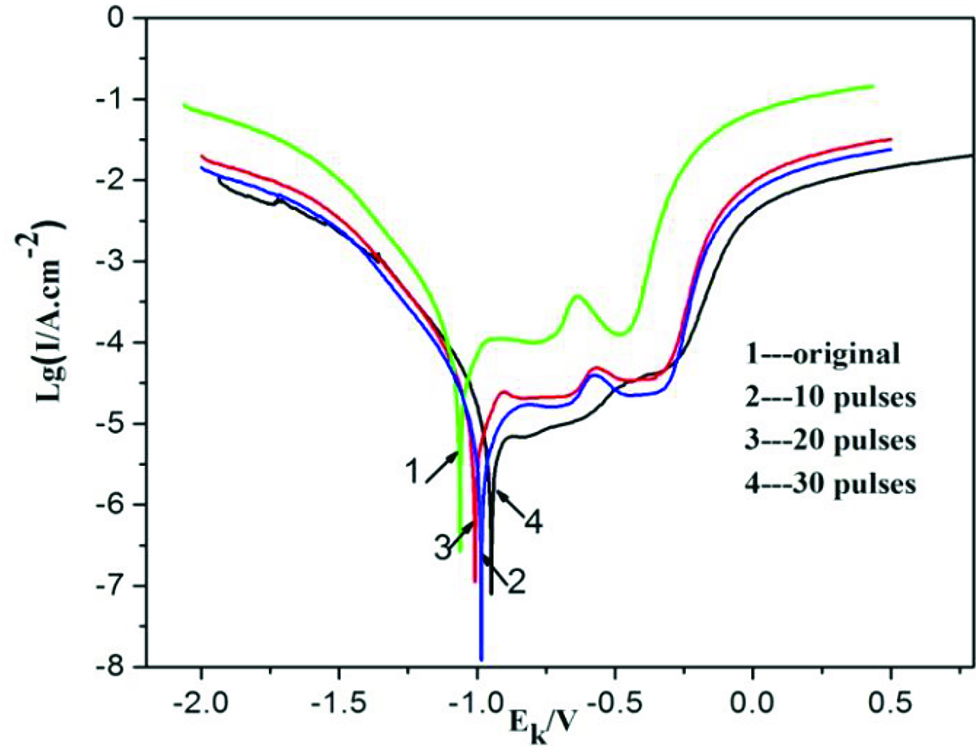
Polarization curve of medium carbon steel before and after HCPEB alloying.
Electrochemical corrosion performance measurement.
| Samples | Icorr/μA·cm-2 | Ecorr/V |
|---|---|---|
| Initial | 88.03 | –1.252 |
| 10 pulses | 37.64 | –0.999 |
| 20 pulses | 42.27 | –1.008 |
| 30 pulses | 25.93 | –0.854 |
Conclusion
In conclusion, a systematic study on the surface chromium alloying processing of 0.45 C carbon steel by HCPEB experiments was performed. The main findings are summarized, as follows.
After HCPEB alloying treatment, the thickness of the remelted layer, composed of lath martensite and ferrite, was about 4–9 μm. The Cr element diffused on the surface, and particles of tiny Cr23C6 were formed in the substrate.
The surface microhardness of the material increased after HCPEB irradiation, and a dependence of the microhardness on the number of the irradiated pulses was noticed.
The HCPEB alloying treatment significantly improved the corrosion resistance of the medium carbon steel. The solid solution, surface cleaning effect, and formation of nano-crystals in the alloying layer greatly improved the corrosion resistance of the materials.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (Nos. 51601071, 51601072), Jiangsu Province Natural Science Foundation for Youths (No. BK20160530) and Youth Talent Development Program of Jiangsu University.
References
[1] M. Szkodo, Surf. Coat. Tech., 296 (2016) 117–123.10.1016/j.surfcoat.2016.04.032Suche in Google Scholar
[2] P. Xue, W.D. Li and D. Wang, Mater. Sci. Eng. A, 670 (2016) 153–158.10.1016/j.msea.2016.06.014Suche in Google Scholar
[3] S. Kumar, A. Bhattacharyya and D.K. Mondal, Wear, 270 (5–6) (2011) 413–421.10.1016/j.wear.2010.12.007Suche in Google Scholar
[4] N. Liu, Sci. Technol. Association Forum, 8 (2009) 69–70.10.1016/S0739-5930(09)79419-XSuche in Google Scholar
[5] S.X. Liu, Y. Chen, G.Q. Liu, X.F. Liu, Y.G. Zhang and J.K. Huang, Mater. Sci. Eng. A, 499 (1-2) (2009) 83–87.10.1016/j.msea.2007.11.146Suche in Google Scholar
[6] J.C. Oh, D.K. Choo and S. Lee, Surf. Coat. Tech., 127 (1) (2000) 76–85.10.1016/S0257-8972(99)00664-7Suche in Google Scholar
[7] K.V. Acker, D. Vanhoyweghen, R. Persoons and J. Vangrunderbeek, Wear, 258 (1–4) (2005) 194–202.10.1016/j.wear.2004.09.041Suche in Google Scholar
[8] X.W. Qiu, J. Netshape Forming Eng., 7 (3) (2015) 58–61.Suche in Google Scholar
[9] Y.H. Zhang and H. Zhang, Hot Working Tech., 4 (2000) 6–8.Suche in Google Scholar
[10] R.G. Davies, Metall. Trans. A, 9 (5) (1978) 671–679.10.1007/BF02659924Suche in Google Scholar
[11] M. Moonesan, A.H. Raouf and F. Madah, J. Alloy Compd., 520 (4) (2012) 226–231.10.1016/j.jallcom.2012.01.027Suche in Google Scholar
[12] L. Qin and C. Liu, Mater. Lett., 82 (2012) 127–129.10.1016/j.matlet.2012.05.069Suche in Google Scholar
[13] P.Z. Zhang, Z. Xu and G.H. Zhang, Trans. Nonferrous Met. Soc. China, 16 (s3) (2006) 2100–2103.10.1016/S1003-6326(06)60225-2Suche in Google Scholar
[14] D. Chen, X. Dong and Z. Fan, China Foundry, 7 (1) (2010) 13–18.Suche in Google Scholar
[15] J. Zhu, M. Xu and W. Yang, Coatings, 7 (11) (2017) 191.10.3390/coatings7110191Suche in Google Scholar
[16] Y. Chi, G. Gu and H. Yu, Opt. Laser Eng., 100 (2018) 23–37.10.1016/j.optlaseng.2017.07.006Suche in Google Scholar
[17] M. Ansari, M.H. Sohi and R. Soltani, Int. J. Adv. Manuf. Tech., 83 (1-4) (2016) 285–291.10.1007/s00170-015-7516-1Suche in Google Scholar
[18] A. Almeida and R. Vilar, Scr. Mater., 63 (8) (2010) 811–814.10.1016/j.scriptamat.2010.06.022Suche in Google Scholar
[19] J. Cai, P. Lv, Q.F. Guan and A.C.S. Appl, Mater. Interfaces, 8 (2016) 32541–32556.10.1021/acsami.6b11129Suche in Google Scholar
[20] C. Dong, A. Wu and S. Hao, Surf. Coat. Tech., 163-164 (2) (2003) 620–624.10.1016/S0257-8972(02)00657-6Suche in Google Scholar
[21] Q.F. Guan, H. Zou and G.T. Zou, Surf. Coat. Tech., 196 (1–3) (2005) 145–149.10.1016/j.surfcoat.2004.08.104Suche in Google Scholar
[22] Y. Qin, A.M. Wu and J.X. Zou, High Power Laser Part. Beams, 15 (7) (2003) 701–704.Suche in Google Scholar
[23] Y. Qin, X.G. Wang, C. Dong and A. Phys, Sin., 52 (12) (2003) 3043–3048.10.7498/aps.52.3043Suche in Google Scholar
[24] J. Zou, Y. Qin and A. Wu, Nucl. Tech., 27 (7) (2004) 519–524.10.1016/j.jmpt.2004.08.006Suche in Google Scholar PubMed
[25] S.Z. Hao, D.Y. He and M.C. Li, Mater. Sci. Forum. Trans. Tech. Publ., 675 (2011) 1205–1208.10.4028/www.scientific.net/MSF.675-677.1205Suche in Google Scholar
[26] C.S. Liu, S.Y. Chen and L.J. Shang, Chinese Journal of Lasers, 29 (3) (2002) 277–280.Suche in Google Scholar
[27] Q.F. Guan, G.T. Zhou and J. Lin, Jilin University.Suche in Google Scholar
[28] J. Zou, K. Zhang and C. Dong, Appl. Phys. Lett., 89 (4) (2006) 041913.10.1063/1.2234306Suche in Google Scholar
[29] C. Zhang, J. Cai, P. Lv, Y. Zhang, H. Xia and Q. Guan, J. Alloy Compd., 697 (2016) 69–103.10.1016/j.jallcom.2016.07.160Suche in Google Scholar
[30] C. Zhang, P. Lv, J. Cai, C.T. Peng, Y. Jin and Q. Guan, Appl. Surf. Sci., 422 (2017) 582–590.10.1016/j.apsusc.2017.06.049Suche in Google Scholar
[31] K.M. Zhang, J.X. Zou, T. Grosdidier, C. Dong and D. Yang, Surf. Coat. Tech., 201 (3–4) (2006) 1393–1400.10.1016/j.surfcoat.2006.02.008Suche in Google Scholar
[32] L. Xu, B. Wang and J. Zhu, Appl. Sur. Sci., 379 (2016) 39–46.10.1016/j.apsusc.2016.04.049Suche in Google Scholar
[33] S. Jiang, F. Chai and H. Su, Corros. Sci., 123 (2017) 217–227.10.1016/j.corsci.2017.04.024Suche in Google Scholar
[34] J. Zou, T. Grosdidier and K. Zhang, Acta Mater., 54 (20) (2006) 5409–5419.10.1016/j.actamat.2006.05.053Suche in Google Scholar
[35] Q.F. Guan, D.Q. Cheng and D.H. Qiu, Acta Phys. Sin., 58 (7) (2009) 4846–4852.10.7498/aps.58.4846Suche in Google Scholar
[36] Q.F. Guan, Q. Zhang and C. Dong, Isij Int., 48 (2) (2008) 235–239.10.2355/isijinternational.48.235Suche in Google Scholar
[37] S. Hao, H. Wang and L. Zhao, Nucl. Instrum. Meth. Phys. Res. B, 368 (2016) 81–85.10.1016/j.nimb.2015.11.039Suche in Google Scholar
[38] H. Wang, W. Yan and S.V. Zwaag, Acta Mater., 134 (2017) 143–154.10.1016/j.actamat.2017.05.069Suche in Google Scholar
[39] K. Fukaura, Y. Yokoyama and D. Yokoi, Metall. Mater. Trans. A, 35 (4) (2004) 1289–1300.10.1007/s11661-004-0303-5Suche in Google Scholar
© 2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Artikel in diesem Heft
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

