Abstract
The viscosity and break temperature of La2O3–SiO2-FeO slag was investigated to develop low-Al2O3 or Al2O3-free slag for the effective recovery of rare-earth metals. When La2O3 content is fixed (45, 50 and 55 mass%), the viscosity and break temperature of La2O3–SiO2-FeO slag decrease with an increase in FeO content and a decrease in SiO2 content. A higher La2O3 content in the La2O3-SiO2-FeO ternary slag yields a lower slag viscosity but a higher break temperature. Individual minor components of Al2O3, MnO and B2O3 does not affect, or decreases slightly the viscosity of La2O3–SiO2-FeO slag, whereas the slag break temperature is reduced so that the reduction ability order is ranked as B2O3 > Al2O3 > MnO. A small amount of two components Al2O3 + MnO and Al2O3 + B2O3 has little effect on the viscosity of the slag but it has an additive effect on the slag break-temperature reduction.
1 Introduction
Rare-earth hydrogen-storage alloys and NdFeB magnets are used in various applications. Large amounts of waste from these material production processes and end-of-life rare-earth materials become significant secondary sources of rare-earth and other valuable metal elements. The development of a cost-effective recycling method is required urgently. Hydrometallurgical methods, pyrometallurgical methods and a combination of the two have been developed to recycle rare-earths. In general, industrial hydrometallurgical extraction proceeds by a hydrochloricacid selective-dissolution method, such as is used in NdFeB waste treatment. This method dissolves rare-earth oxides by hydrochloric acid preferentially from the oxidizing roasting NdFeB waste, and then separates different rare earths using solvent extraction [1, 2, 3]. Despite its high rare-earth recovery and the high-purity rare-earth products that form, hydrometallurgy is unable to collect other valuable elements very well and results in excessive acid consumption. Single pyrometallurgical method can recover rare-earth metals together with valuable metal elements and is more environmentally friendly [4, 5, 6]. The combination of pyrometallurgical and hydrometallurgical advantages could be used to recycle rare-earth and valuable elements simultaneously and reduce environmental pollution. A selected suitable slag system for the former pyrometallurgical step is critical to concentrate rare-earth oxides, for the smooth separation of slag-metal and for the efficiency of subsequent hydrometallurgical process.
Many types of flux have been used to recycle rare-earth oxides, such as CaO-CaF2 [7], CaO-SiO2-CaF2 [8], CaO-SiO2 [9], Al2O3-CaO-MgO-SiO2-(P2O5) [10] and SiO2-Al2O3 [11]. However, melting-separation characteristics for slags that are formed by most of the fluxes examined were not good. The purity and added value of the alloy have also been poorly considered. The authors [11] used gas-selective reduction-oxidation to produce high-added-value alloy from the Ni-metal-hydride battery electrode waste and subsequently accomplished excellent slag-metal separation by SiO2-Al2O3 flux addition. This method yielded a ~50 mass% RexOy-containing slag, which contributed to a saving in hydrochloric-acid consumption in the acid-leaching process.
In addition to the high rare-earth-oxides content and appropriate slag-metal separation characteristics for the formed slag, the selection of flux should be considered to ensure that other slag components do not enter the leaching solution or are separated easily and purified even if they enter the leaching solution. Besides, the latter extraction separation of different rare-earth elements cannot be affected by additive slag components. Some studies [12, 13] have indicated that the aluminum ion is an important factor that leads to the emulsification of extractant in the extraction process of different types of rare-earth elements. Our previous SiO2-Al2O3 flux is effective for single rare-earth elements. So, there is no emulsification problem of the leaching agent results. However, different types of rare-earth elements usually coexist in rare-earth waste materials, and the application of SiO2-Al2O3 flux gets some limitations. It is urgent to develop aluminum-free or low aluminum flux to reduce or prevent emulsification in the latter hydrometallurgical step in addition to separate the slag-metal effectively and recycle other valuable elements in the former pyrometallurgical step.
In this paper, La2O3 was selected as a representative of the rare earth oxides. Acidic SiO2 does not consume hydrochloric acid in the acid-leaching process. Although FeO can dissolve in the leaching solution, the iron ion is removed easily as a Fe(OH)3 precipitate by adjusting the pH of the leaching solution to ~3–4. SiO2 and FeO were considered as the main components of slag. The viscosity and break temperature of the La2O3-SiO2-FeO slag system, which are key parameters for effective separation of rare earth oxide-rich slag and valuable metal, were evaluated.
2 Experimental
2.1 Experimental Slag Component Preparation
The based slag system consisted of La2O3-SiO2-FeO ternary slag, which contains a small amount of one or two minor components of Al2O3, MnO and B2O3. All samples were prepared using analytical reagent grade La2O3, SiO2, Al2O3, MnO2 powder. La2O3 (99.95 mass%) was provided by Baogang Rare Earth Science and Technology Co. (Baotou, China). Other analytical reagents were provided by Tianjin Chemical Reagent Co. (Tianjin, China). La2O3, SiO2 and Al2O3 reagents were heated in a muffle furnace at 1123 K for 2 h to eliminate adherent moisture and B2O3 (99.9 mass%) was dehydrated in vacuum at 393 K for 4 h. MnO was produced by MnO2 reduction at 1123 K for 2 h in high-purity hydrogen in a tube atmosphere furnace. FeO was prepared by thermal decomposition of FeC 2O4·2H2O in argon (with a purity of above 99.995 mass%) at 1123 K for 2 h. The final product was confirmed to be a single FeO phase by X-ray diffraction as shown in Figure 1.

XRD pattern of thermal decomposition product for FeC2O4·2H2O in argon at 850∘C.
2.2 Viscosity and Break-Temperature Test
The slag viscosity was measured by the internal rotating-cylinder method. The viscosity test principle and instrument were the same as was used in our previous work [14].
According to the designed slag composition, ~160 g of prepared reagents were mixed, briquetted and placed into a molybdenum crucible with a 40-mm diameter and a 75-mm height, and the molybdenum crucible was placed inside the constant-temperature zone of the high-temperature resistance furnace. The temperature of the heating furnace was controlled by a Pt-PtRd thermocouple and the temperature-control accuracy was approximately ± 1 K. The sample was heated to 1823 K and held for 1 h in argon at 0.2 NL/min to obtain a homogeneous melt. The molybdenum spindle was immersed into a liquid slag bath and rotated. The viscosity at a constant temperature of 1823 K was obtained. The viscosity was measured continuously while the slag temperature reduction was controlled at 3 K/ min.When the viscosity increased to 6.0 Pa·s, the molybdenum spindle stopped rotating and the furnace was heated again to 1823 K so that the molybdenum spindle was removed from the melted slag. The slag break temperature was identified as the temperature at which there is a significant change in viscosity during the cooling cycle.
3 Results and Disscussion
3.1 Viscosity and Break Temperature of La2O3-SiO2-FeO Ternary Slag
The composition, constant-temperature viscosity at 1823 K and break temperature of the La2O3-SiO2-FeO ternary basic slag are given in Table 1. The viscosity curves are shown in Figure 2. For a fixed La2O3 mass content, the viscosity at 1823 K and the break temperature is lowered with a decrease in SiO2 content from 40 to 30 mass% and an increase in FeO content from 15 to 25 mass%. When the La2O3 content is 50 mass%, the viscosity-temperature curve of the 10 mass% FeO is supplied to observe better the effect of FeO content change on the break temperature of the slag. For a certain FeO content, the viscosity at 1823 K decreases, but the break temperature of the La2O3-SiO2-FeO ternary slag increases as the La2O3 content increases from 45, to 50 to 55 mass% as shown in Table 1.

Viscosity curves of La2O3-SiO2-FeO ternary basic slags. (a) 45 mass% La2O3-SiO2-FeO slags, (b) 50 mass% La2O3-SiO2-FeO slags, (c) 55 mass% La2O3-SiO2-FeO slags.
Constant-Temperature Viscosity and Break Temperature of La2O3-SiO2-FeO Ternary Basic Slag.
| Sample No. | Slag Composition (mass%) | Constant-Temperature | Break Temperature | ||
|---|---|---|---|---|---|
| La2O3 | SiO2 | FeO | Viscosity (η) (Pa·s) | [K] | |
| 1 | 45 | 40 | 15 | 0.4598 | 1727 |
| 2 | 45 | 35 | 20 | 0.2806 | 1692 |
| 3 | 45 | 30 | 25 | 0.1443 | 1674 |
| 4 | 50 | 40 | 10 | 1.0447 | 1797 |
| 5 | 50 | 35 | 15 | 0.3009 | 1758 |
| 6 | 50 | 30 | 20 | 0.1082 | 1761 |
| 7 | 50 | 25 | 25 | 0.1376 | 1715 |
| 8 | 55 | 30 | 15 | 0.1961 | 1810 |
| 9 | 55 | 25 | 20 | 0.0541 | 1784 |
| 10 | 55 | 20 | 25 | 0.0811 | 1780 |
The decrease in slag viscosity is related closely to a depolymerization of the silicate structure. The free oxygen ions could break the bridged oxygen in the Si-O network structure and simplify the complex network silicate structure to decrease the slag viscosity. The availability of free oxygen ions possibly increases with increasing La2O3 content and thus the viscosity of the La2O3-SiO2-FeO slag decreases. Toop and Samis have reported that FeO supplies free oxygen ions in CaO-SiO2-FeO melts [15]. Moreover, FeO contributes to the formation of a low-melting-point substance, such as fayalite (Fe2SiO4). As a result, increasing FeO content decrease the slag viscosity and melting temperature. The lower viscosity and melting temperature of slag as an FeO additive has been already practiced in steelmaking process. The variation behavior of the viscosity and break temperature for the La2O3-SiO2-FeO slag and the CaO-SiO2-FeO slag is similar with an increase in FeO content. It may be that the basic properties of La2O3 and CaO are some similar. However, the reported proof for the similar basic character of La2O3 and CaO is scarce at present.
3.2 Effect of Single Minor Component on the Based Slag System Viscosity and Break Temperature
In a LaNi5-type hydrogen-storage alloy, a small amount of Al and Mn are doped to replace Ni to improve the electrochemical properties [16, 17]. In a NdFeB permanent magnet, Co and Al are added to replace Fe to optimize the magnetic performance and corrosion behavior [18, 19]. After gas-selective reduction-oxidation, the active elements Mn, Al, B in the hydrogen-storage alloy and in the NdFeB waste are easily oxidized to MnO, Al2O3 and B2O3 [11]. They can remain in the rare-earth-oxide slag and may affect the viscosity and break temperature of the La2O3-SiO2-FeO based slag significantly.
The influence of single minor components Al2O3,MnO and B2O3 on the viscosity and break temperature of the La2O3-SiO2-FeO slag was studied to establish which component has a greater impact on the above values. So that proper viscosities and break temperatures can be obtained by adjusting the slag composition. The high content of rare-earth oxide in the La2O3-SiO2-FeO slag would reduce the acid consumption for the same amount of leaching rare-earth oxides although a high rare-earth-oxide content results in a high break temperature of the slag (shown in Table 1), which is undesirable for slag-metal separation. Therefore, the mass fraction ratio of the main components in the La2O3-SiO2-FeO-Al2O3 (MnO, B2O3) slag is fixed at 55:30:15.
3.2.1 Effect of Al2O3 on La2O3-SiO2-FeO slag viscosity and break temperature
The composition, constant-temperature viscosity at 1823 K and break temperature of the La2O3-SiO2-FeO-Al2O3 slag are listed in Table 2. The viscosity curves are shown in Figure 3. The viscosity change at 1823 K is insignificant but the break temperature is lowered substantially. The break temperature of the La2O3-SiO2-FeO slag that is doped with 0, 2, 4 and 6 mass% Al2O3 is reduced from 1810 to 1800, 1748 and 1719 K, respectively. This result implies that the dissolution of a small amount of Al2O3 into the slag, even from low-cost corundum-crucible corrosion, does not cause melting problems in the slag–metal separation process. The break temperature decrease with the an increase in Al2O3 content from 0 to 14.35 mass% in CaO-7 mass% MgO-SiO2-20 mass% FetO-Al2O3 slags has been reported [20], in which the slag-composition range and ternary basicity ((WCaO + WMgO)/WSiO2 = 1.5) was approximately same as those in this study (WLa2O3 /WSiO2 = 1.83).
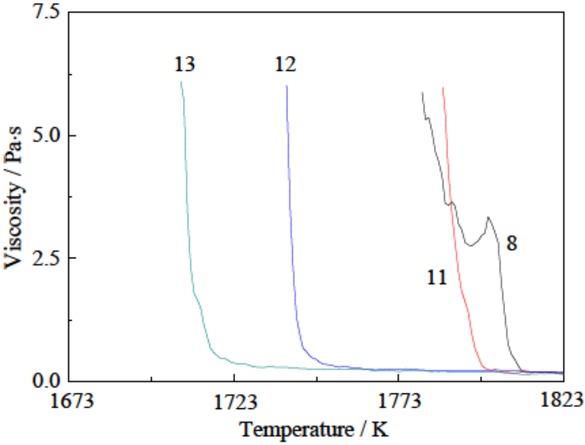
Viscosity curves of La2O3-SiO2-FeO-Al2O3 slags.
Constant-Temperature Viscosity and Break Temperature of La2O3-SiO2-FeO-Al2O3 Slag.
| Sample No. | Slag Composition (mass%) | Constant-Temperature | Break Temperature | |||
|---|---|---|---|---|---|---|
| La2O3 | SiO2 | FeO | Al2o3 | Viscosity (η) (Pa·s) | [K] | |
| 8 | 55 | 30 | 15 | 0 | 0.1961 | 1810 |
| 11 | 53.9 | 29.4 | 14.7 | 2 | 0.1812 | 1800 |
| 12 | 52.8 | 28.8 | 14.4 | 4 | 0.1913 | 1748 |
| 13 | 51.7 | 28.2 | 14.1 | 6 | 0.1476 | 1719 |
3.2.2 Effect of MnO on La2O3-SiO2-FeO slag viscosity and break temperature
Table 3 presents the composition, constant-temperature viscosity at 1823 K and break temperature of La2O3-SiO2-FeO-MnO slag. Figure 4 shows the curves of viscosity versus temperature for different MnO contents. An increase in MnO addition results in a small decrease in slag viscosity at 1823 K and the break temperature decreases. When the MnO content increases from 0 to 6 and 8 mass%, the slag break temperature is reduced from 1810 to 1772 and 1754 K, respectively. It has been reported that a 0–3 mass% MnO addition lowers the initial and complete melting temperature of the CaO-Al2O3 system mold flux because MnO formed low-melting compounds CaMnSiO6 and Mn2SiO4 with CaO and SiO2 [21].

Viscosity curves of La2O3-SiO2-FeO-MnO slags.
Constant-Temperature Viscosity and Break Temperature of La2O3-SiO2-FeO-MnO Slag.
| Sample No. | Slag Composition (mass%) | Constant-Temperature | Break Temperature | |||
|---|---|---|---|---|---|---|
| La2O3 | SiO2 | FeO | Mno | Viscosity (η) (Pa·s) | [K] | |
| 8 | 55 | 30 | 15 | 0 | 0.1961 | 1810 |
| 14 | 52.8 | 28.8 | 14.4 | 4 | 0.1242 | 1804 |
| 15 | 51.7 | 28.2 | 14.1 | 6 | 0.1208 | 1772 |
| 16 | 50.6 | 27.6 | 13.8 | 8 | 0.1007 | 1754 |
3.2.3 Effect of B2O3 on La2O3-SiO2-FeO slag viscosity and break temperature
The composition, constant-temperature viscosity at 1823 K and break temperature of La2O3-SiO2-FeO-B2O3 slag and the viscosity curves are given in Table 4 and Figure 5. The slag viscosity at 1823 K does not change visibly, but the break temperature of the slag is reduced significantly with an increase in B2O3 content, which is similar to the viscosity variation with the addition of Al2O3 in the slag as shown in Table 2. When B2O3 content changes from 0 to 2, 3 and 4 mass%, the break temperature of the slag decreases from 1810 to 1731, 1727 and 1697 K, respectively. This finding agrees with many reports [22, 23] although the slag compositions differ.

Viscosity curves of La2O3-SiO2-FeO-B2O3 slags.
Constant-Temperature Viscosity and Break Temperature of La2O3-SiO2-FeO–B2O3 Slag.
| Sample No. | Slag Composition (mass%) | Constant-Temperature | Break Temperature | |||
|---|---|---|---|---|---|---|
| La2O3 | SiO2 | FeO | B2O3 | Viscosity (η) (Pa·s) | [K] | |
| 8 | 55 | 30 | 15 | 0 | 0.1961 | 1810 |
| 17 | 53.9 | 29.4 | 14.7 | 2 | 0.1476 | 1731 |
| 18 | 53.35 | 29.1 | 14.55 | 3 | 0.1141 | 1727 |
| 19 | 52.8 | 28.8 | 14.4 | 4 | 0.1443 | 1697 |
B2O3 is a typical acidic oxide, which behaves as a network former in the current La2O3-SiO2-FeO alkaline slag. Amphoteric oxide Al2O3 also tends to behave as a network former in the alkaline slag. Consequently, the same viscosity-change result in the La2O3-SiO2-FeO slag with B2O3 and Al2O3 addition appears as expected. B2O3 is used often in metallurgy as a fluxing agent. The reasons for reducing the break temperature of the La2O3-SiO2-FeO slag by B2O3 additive could be as follows: (1) The melting point of B2O3 is inherently low (723 K). (2) The slag crystallization maybe suppressed by B2O3 addition [24]. (3) B2O3 reacts with some oxides to form a eutectic of low melting temperature, such as LaB3O6 (melting point 1414 K) [25] or FeB2O4 (melting point 1223 K) [26], which decreases the break temperature.
The decrease in constant-temperature viscosity at 1823 K by single minor component Al2O3, MnO and B2O3 is not obvious, and varies from 0.1961 to 0.1007 Pa·s. However, all three components enlarge the temperature range of the low viscosity and obviously decrease the break temperature of the La2O3-SiO2-FeO based slag. The break-temperature reduction ability of the single components is ranked as B2O3>Al2O3>MnO. For example, when the content of any of the three components in the slag is 4 mass%, B2O3, Al2O3 and MnO lower the break temperature of the La2O3-SiO2-FeO based slag by 113, 62 and 6 K, respectively. It has been reported that using some B2O3 to substitute for Al2O3 as a fluxing agent decreased the melting temperature of the CaO-based refining flux [27].
3.3 Effect of Complex Components Al2O3+MnO and Al2O3+B2O3 on La2O3-SiO2-FeO slag viscosity and break temperature
After AB5-type hydrogen-storage alloy and NdFeB waste were selectively oxidized and reduced, Al2O3+MnO and Al2O3+B2O3 could coexist in the rare-earth oxide slag as minor components, respectively. The effect of two components Al2O3+MnO and Al2O3+B2O3, which is close to the real rare-earth waste situation, on the viscosity and break temperature of the La2O3-SiO2-FeO slag was investigated. Here, the Al2O3, MnO and B2O3 compositions were selected to be 6, 6 and 3 mass%, respectively.
As shown in Table 5 and Figure 6, the coexistence of 6 mass% Al2O3 + 6 mass% MnO or 6 mass% Al2O3 + 3 mass% B2O3 has no effect on the slag viscosity, whereas they continue to reduce the break temperature to 1662 or 1585 K. For the La2O3-SiO2-FeO-6 mass% Al2O3-6 mass% MnO slag, the reduction in break temperature is 57 and 110 K compared with La2O3-SiO2-FeO-6 mass% Al2O3 and La2O3-SiO2-FeO-6 mass% MnO slags. For La2O3-SiO2-FeO-6 mass% Al2O3-3 mass% B2O3 slag, the reduction level is 134 and 142 K compared with La2O3-SiO2-FeO-6 mass% Al2O3 and La2O3-SiO2-FeO-3 mass% B2O3 slags, respectively. It can be concluded that the melting speed of the slag is increased by the coexistence of Al2O3+MnO or Al2O3+B2O3 because of the low break temperature, and the improved stability of the slag viscosity varies with temperature, which is favorable for slag-metal separation. The inflection point of the viscosity curves becomes unapparent.

Viscosity curves of La2O3-SiO2-FeO-Al2O3-MnO and La2O3-SiO2-FeO-Al2O3-B2O3 slags.
Constant-Temperature Viscosity and Break Temperature of La2O3-SiO2-FeO-Al2O3-MnO and La2O3-SiO2-FeO-Al2O3-B2O3 Slags.
| Sample No. | Slag Composition (mass%) | Constant-Temperature | Break Temperature | |||||
|---|---|---|---|---|---|---|---|---|
| La2O3 | SiO2 | FeO | Al2O3 | MnO | B2O3 | Viscosity (η) (Pa·s) | [K] | |
| 13 | 51.7 | 28.2 | 14.1 | 6 | 0 | 0 | 0.1476 | 1719 |
| 15 | 51.7 | 28.2 | 14.1 | 0 | 6 | 0 | 0.1208 | 1772 |
| 18 | 53.35 | 29.1 | 14.55 | 0 | 0 | 3 | 0.1141 | 1727 |
| 20 | 48.4 | 26.4 | 13.2 | 6 | 6 | 0 | 0.1476 | 1662 |
| 21 | 50.05 | 27.3 | 13.65 | 6 | 0 | 3 | 0.1544 | 1585 |
For slag-metal separation, the critical point was the selection of a suitable slag system that fulfils demands such as a low viscosity, and a moderate or low break temperature. In general, the break temperature of the slag is lower by 150 K or more than the melting temperature of the metal. The melting temperature of the recycled metal is expected to reach up to 1773 K, so the break temperature of the slag is best below 1623 K. In this experiment, the low viscosity meets the slag-metal separation requirement for all La2O3-SiO2-FeO slag systems. However, the break temperatures of the La2O3-SiO2-FeO based slag and the slag doped with single Al2O3, MnO and B2O3 are high, although these oxides could reduce the break temperature of the La2O3-SiO2-FeO based slag to different extents. Two residual components 6 mass% Al2O3+6 mass% MnO decrease the break temperature of the slag to close to 1623 K, and the break temperature decreases to below 1623 K when the Al2O3 and B2O3 contents in the slag are 6 and 3 mass% as shown in Table 5. Rare-earth waste often yields two or more minor components in the slag. As a result, the efficient separation of valuable metals and rich rare-earth oxide-containing slag must be achieved using the developed La2O3-SiO2-FeO slag system and simultaneously the slag composition does not affect or affects only slightly the subsequent extraction of rare-earth elements.
4 Conclusions
In this study, the viscosity and break temperature of La2O3-SiO2-FeO ternary slag and the variation as affected by one or two minor components Al2O3, MnO and B2O3 were investigated. The following conclusions were made:
For 45, 50 or 55 mass% La2O3, the viscosity at 1823 K and the break temperature of La2O3-SiO2-FeO ternary slag decreased with an increase in FeO content from 15 to 25 mass% and a decrease in SiO 2 content from 40 to 30 mass%. When the FeO content was fixed at 15, 20 or 25 mass%, the slag viscosity decreased, but the break temperature of the La2O3-SiO2-FeO ternary slag increased with an increase in La2O3 content from 45 to 55 mass%.
With an increase in individual minor components Al2O3 (0–6 mass%), MnO (0–8 mass%) and B2O3 (0–4 mass%), the constant-temperature viscosity at 1823 K of the La2O3-SiO2-FeO based slag decreased slightly or remained unchanged, but the break temperature of the slag was reduced significantly. B2O3 was most influential, followed by Al2O3 and MnO.
The coexistence of Al2O3+MnO or Al2O3+B2O3 reduces the break temperature of the La2O3-SiO2-FeO slag significantly compared with single Al2O3, MnO and B2O3 and the slag still maintains a low-viscosity feature, which benefits the slag-metal separation.
Acknowledgement
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 51364029) and the scientific innovation fund of Inner Mongolia University of Science and Technology (No. 2016 QDL-B17 and 2016 QDL-B16).
References
[1] L. Pietrelli, B. Bellomo, D. Fontana, and M. R. Montereali, Hydrometallurgy, 66 (2002) 135-139.10.1016/S0304-386X(02)00107-XSearch in Google Scholar
[2] Y. J. Wang, Y. H. Liu, G. Q. Weng, S. Y. Li, R. L. Liu and S. L. Wang, Rare Met. Cem. Carbides,35 (2007) 25-27.10.1016/S1002-0721(07)60072-6Search in Google Scholar
[3] Y. J. Chen, China Resources Comprehensive Utilization, (2004) 10-12.Search in Google Scholar
[4] T. Saito, H. Sato, and T. Motegi, J. Mater. Res., 18 (2003) 28142819.10.1557/JMR.2003.0392Search in Google Scholar
[5] T. Saito, H. Sato, S. Ozawa, J. Yu, and T. Motegi, J. Alloy Compd., 353 (2003) 189-193.10.1016/S0925-8388(02)01202-1Search in Google Scholar
[6] Y. Y. Bian, S. Q. Guo, L. Jiang, K. Tang, and W. Z. Ding, J. Sustain. Metall., 1(2) (2015) 151-160.10.1007/s40831-015-0009-5Search in Google Scholar
[7] H. Hagen, X. Guo, and L. Xiao, Metal. Ore Dress Abroad, 6 (2006) 34-38.Search in Google Scholar
[8] T. Muller and B. Friedrich. J. Power Sources, 158 (2006) 1498-1509.10.1016/j.jpowsour.2005.10.046Search in Google Scholar
[9] K. Tanga, A. Ciftjaa, C. van der Eijka, S. Wilsona, and G. Tranellb, J. Min. Metall. Sect. B-Metall., 49 (2013) 233-236.10.2298/JMMB120808004TSearch in Google Scholar
[10] T. Elwert, D. Goldmann, T. Schirmer, and K. Strauß, Chem. Ing. Tech., 86 (2014) 840-847.10.1002/cite.201300168Search in Google Scholar
[11] Y. J. Jiang, Y. C. Deng, and W. G Bu, Metall. Mater. Trans. B, 46 (2015) 2153-2157.10.1007/s11663-015-0362-6Search in Google Scholar
[12] M. L. Qian, Master Dissertation, Northeastern University, Shenyang, (2010).Search in Google Scholar
[13] J. T. Jia, Y. W. Zhang, S. Wu, et al. Rare Earths, 22 (2001) 10-13.Search in Google Scholar
[14] Y. C. Deng, S. L. Wu, Y. J. Jiang, S. Q. Jia, Metall. Mater. Trans. B, 47(4) (2016) 2433-2439.10.1007/s11663-016-0711-0Search in Google Scholar
[15] G. W. Toop and C. S. Samis, Trans. TMS-AIME, 224 (1962) 878-887.Search in Google Scholar
[16] L. Kong, B. Chen, K. Young, J. Koch, A. Chan, and W. Li, J. Power Sources, 213 (2012) 128-139.10.1016/j.jpowsour.2012.03.099Search in Google Scholar
[17] Q. R. Yao, H. Y. Zhou, Z. M. Wang, S. K. Pan, and G. H. Ra J. Alloy Compd., 606 (2014) 81-85.10.1016/j.jallcom.2014.04.026Search in Google Scholar
[18] J. Jakubowicza and M. Giersigb, J. Alloy Compd., 349 (2003) 311-315.10.1016/S0925-8388(02)00907-6Search in Google Scholar
[19] F. E. Camp and A. S. Kim, J. Appl. Phys., 70 (1991) 6348-6350.10.1063/1.349938Search in Google Scholar
[20] Z. J. Wang, Y. Q. Sun, S. Sridhar et al. Metall. Mater. Trans. B, 46 (2015) 537-541.10.1007/s11663-015-0303-4Search in Google Scholar
[21] H. Zhao, W. L. Wang, L. J. Zhou, B. Lu, and Y. B. Kang, Metall. Mater. Trans. B, 45(4) (2014) 1510-1519.10.1007/s11663-014-0043-xSearch in Google Scholar
[22] L. Wang, Y. R. Cui, J. Yang, C. Zhang, D. X. Cai, J. Q. Zhang, et al., Steel Res. Int., 86(6) (2015) 670-677.10.1002/srin.201400353Search in Google Scholar
[23] L. Zhang, W. L. Wang, S. L. Xie, K. X. Zhang, and I. Sohn, J. Non-cryst. Solids, 460 (2017)113-118.10.1016/j.jnoncrysol.2017.01.031Search in Google Scholar
[24] L. J. Zhou, W. L. Wang, B. X. Lu, G. H. Wen, and J. Yang, Met. Mater. Int., 21(1) (2015)126-133.10.1007/s12540-015-1015-7Search in Google Scholar
[25] D.S. Pytalev, D. Caurant , O. Majérus, H. Trégouët, T. Charpentier, and B. N. Mavrin, J. Alloy Compd., 641 (2015) 43-55.10.1016/j.jallcom.2015.03.244Search in Google Scholar
[26] L. K. Jakobsson, G, Tranell, and I. Jung. Metall. Mater. Trans. B, 48 (2017) 60-72.10.1007/s11663-016-0748-0Search in Google Scholar
[27] H. M. Wang, T. W. Zhang, H. Zhu, G. Li, Y. Yan, and J. Wang, ISIJ Int., 51 (2011) 702-706.10.2355/isijinternational.51.702Search in Google Scholar
© 2019 W. Xin et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

