Abstract
The amount of dust produced from the blast furnace is very large, and this paper proposed a new method for comprehensive utilization of blast furnace dust. Firstly, cold bonded agglomerates directly put into the iron groove were made by blast furnace dust. The cold bonded agglomerates were reduced and melted by the energy of molten iron, and the valuable elements such as Fe reduced into molten iron and zinc existed in rich-Zn dust in the cold bonded agglomerates could be recovered. In order to simulate this process, the reduction behavior of cold bonded agglomerate in the iron bath was studied, and the reduction mechanism were analyzed by Factsage calculation and SEM-EDS. The results showed that: in the slag phase, there were small metallic iron particles dispersed and hard to gather. The main reason for this phenomenon is due to the hindrance role of the carbon residue in the agglomerates, and the problem could be solved through adding magnetite concentrate in cold bonded agglomerates.
1 Introduction
With the development of the metallurgical industry and improvement of environmental protection awareness, a great deal of attention has been paid to the recovery and treatment of metallurgical dusts. Most of the domestic iron and steel enterprises put the dusts directly back to the iron and steel plant recycling in China. However, due to circulation and enrichment of zinc in the blast furnace, it would shorten the life of lining in a blast furnace and affect the normal operation of the blast furnace [1, 2, 3, 4]. At present, there were a number of methods for treating the blast furnace dusts put forward at home and abroad. B. Asadi Zeydabadi leached selectively the valuable elements of the dust by sulfuric acid at low acid concentration and room temperature, and achieved high recovery of zinc. It also could separate zinc and alkali metals from blast furnace flue dust, which zinc as a valuable metal is produced. Secondly, scaffolds and the other similar irregularities in the blast furnaces were controlled [5]. Junwei Han proposed a novel method to recover zinc and iron by reduction roasting-low acid leaching-magnetic separation [6]. Das. B analyzed the characterization, beneficiation and utilization aspects of blast furnace flue dust, blast furnace sludge, LD sludge and LD slag generated at modern steel plants, and the cell or column flotation techniques to recover the carbon values from blast furnace flue dust were used [7]. A. Lopez-Delgado evaluated the use of blast furnace sludge as an adsorbent for the elimination of heavy metal ions from aqueous effluents [8]. Manish Kumar Sinha dealt with the recovery of the zinc and iron from the waste chloride solution of steel industry using solvent extraction and precipitation-stripping process for the synthesis of high purity value added products [9]. However, all of the above methods have not been widely applied in China. So it was a great significance to develop green environmental protection and efficient recycling technology for blast furnace dust.
In this paper, the blast furnace dust was pressed into cold bonded agglomerates using horizontal twinroler machine and then directly put into the simulation device of iron bath, and the metallic oxides would be reduced by carbon in the blast furnace dust using the energy of molten iron. In this way, iron oxides in blast furnace were reduced to metallic iron into molten iron, and zinc oxide was reduced to metallic zinc which would volatilized and collected by the dust remover. Other components entered into slag. It can achieve the purpose of recycling the iron and
zinc in blast furnace dust. This paper also analyzed the reason that the iron and slag did not gather after the high temperature reaction of the cold bonded agglomerates in order to finally realize the effective separation between the iron and slag.
2 Experiment
2.1 Materials
The blast furnace dust and pig iron used in the experiments were provided by a steel company in China. The chemical compositions of them are listed in Table 1 and Table 2, respectively.
Chemical composition of blast furnace dust, %
| TFe | ZnO | C | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | Pb | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| 33.94 | 3.57 | 16.30 | 5.54 | 1.90 | 6.52 | 2.82 | 1.64 | 0.92 | 0.29 | 13.04 |
Chemical composition of pig iron, %
| Fe | Si | Mn | P | S | Ti | V |
|---|---|---|---|---|---|---|
| 94.78 | 0.34 | 0.21 | 0.080 | 0.049 | 0.124 | 0.061 |
It could be found that the content of carbon in the blast furnace dust is high, and it can be the main reducing agent during the reduction of iron and zinc oxide, which means the external carbon is not needed during the reduction process. The iron oxide will be reduced into metallic iron and other compositions will form the slag phase, and the Zn is transformed into steam and volatile to be removed.
2.2 Experimental equipment
The simulation device of iron bath was used as shown in Figure 1. In the experiment, pig iron was put into the crucible. When the furnace was heated to the test temperature, the pig iron melted, which could simulate the situation of the iron groove in front of the blast furnace. The main structures of the device were the heating furnace and temperature controller. The hearth of the furnace was corundum tube, and U-type silicon molybdenum rod distributing around the hearth was as a heating element. The furnace temperature was measured by a double platinum and rhodium thermocouple, and the program is controlled by PID. The maximum temperature of the device was 1873 K and power rating was 8000W.
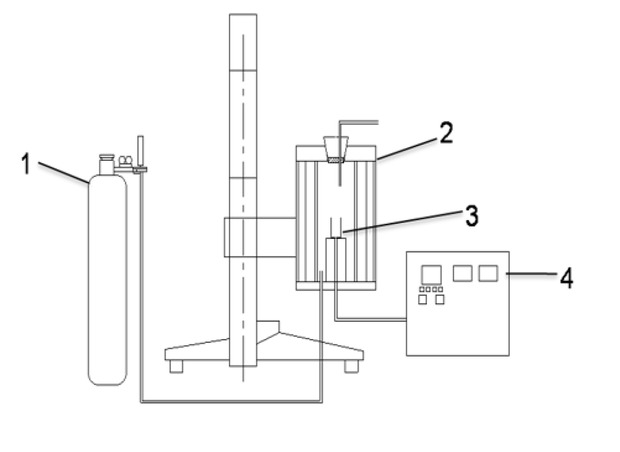
Simulation device of iron bath (1-Ar gas cylinder; 2-heating furnace; 3-crucible; 4-temperature controller)
2.3 Experimental methods
The blast furnace dust and binder were mixed intensively in a mortar, then pressed into ϕ20mm×30mm cylindrical cold bonded agglomerates with water under the pressure of 20 MPa. The addition of binder was 2%. The agglomerates were then dried in a thermostatic oven for 8 hours at 378 K. The crucible with the iron block was placed in the simulation device of the iron bath, then raised the device’s temperature to 1773 K and kept for 30min in order to ensure the iron block melt completely. After that, the cold bonded agglomerates were added into the iron bath crucible, and the reaction product was taken out after 1 hour. The proportion of the cold bonded agglomerates was 15% of molten iron. The reaction products were investigated by means of chemical analysis and SEM-EDS analysis. In order to clarify the effect of molten iron composition on the melting effect of the cold bonded agglomerates, the cold bonded agglomerates were added into the crucible directly and reacted at 1773 K to compare the reaction products under the two conditions.
3 Results and Discussions
3.1 Reduction process of cold bonded agglomerates
Figure 2 showed the metallization rate and the zinc oxide content in the agglomerates. It could be seen from Figure 2 that the metallization rate of the cold bonded agglomerates increased gradually with the reaction time prolonged. After the reaction time exceeding to 15 min, the metallization rate decreased and tended to a balance. The content of ZnO in the cold bonded agglomerates was very low, which basically maintained at around 0.001%. In the reduction process, the iron oxide in the cold bonded agglomerates could be reduced to metal iron, and the metallization rate could reach more than 83%, while ZnO could be reduced to metal Zn, which could ensure that the iron recovery rate and removal of zinc [10, 11, 12, 13, 14].
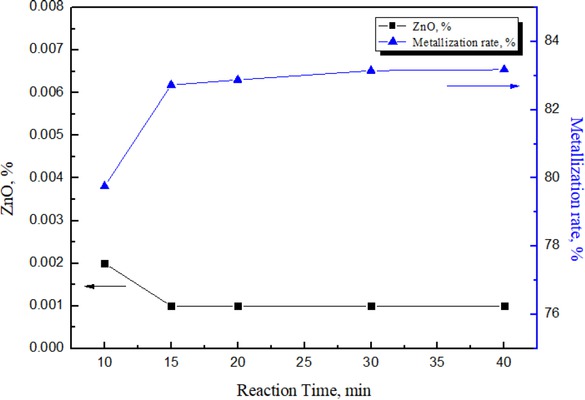
Effect of reaction time on the metallization rate and ZnO content of cold bonded agglomerates
Figure 3 demonstrates the morphology of the cold bonded agglomerates reduced in the crucible (a) and in the iron bath(b). The reaction temperature was 1773 K and the reaction time was 30 min.
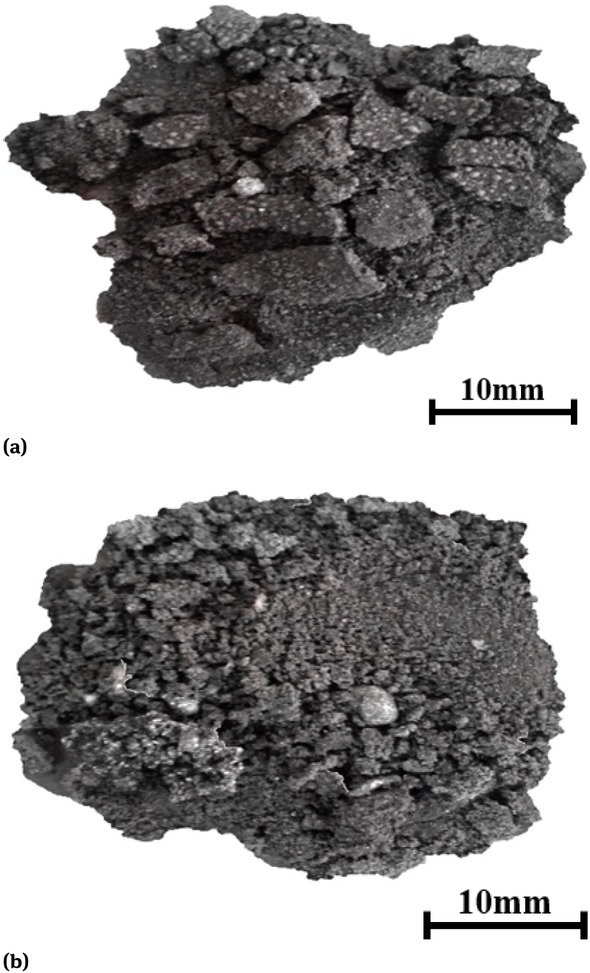
Morphology of briquetting after reaction
As shown in Figure 3, it could be seen that the cold bonded agglomerate put directly into the crucible or into the iron bath, the iron oxide in the cold bonded agglomerate could be reduced, but it was still in a dispersed state after reacting for 30 min in the 1773 K condition. There were iron particles attached or mixed to the surface of the cold bonded agglomerate. The iron phase and slag phase were not gathered separately, which was not conducive to the recovery of iron and the formation of slag. Therefore, it is important to analyze the reason of this phenomenon for the efficient handling of blast furnace dust.
3.2 Thermodynamic analysis of molten slag
According to the chemical composition of the blast furnace dust in Table 1, it was assumed that only CaO, MgO, SiO2 and Al2O3 were in the slag phase when the reaction finished. The composition containing the four components was normalized to determine the percentage of each component, and the CaO-MgO-SiO2-Al2O3 quaternary phase diagram was plotted with FactSage [15, 16]. The phase diagram of which was showed in Figure 4.
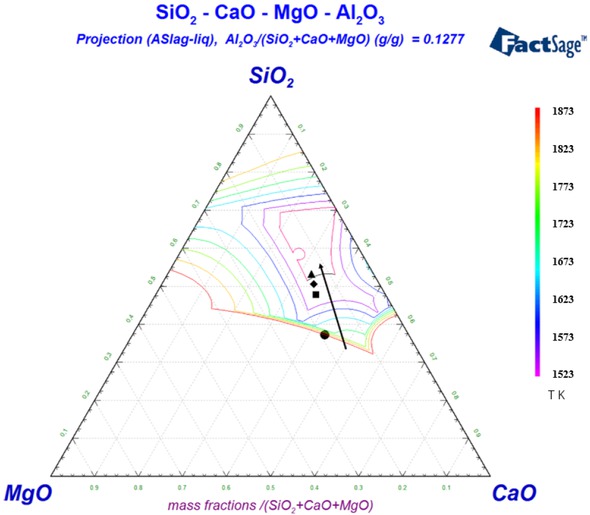
Quaternary phase diagram of CaO-MgO-SiO2-Al2O3
Figure 4 indicates the melting temperature of the slag phase formed after the reaction of the cold bonded agglomerate exceeds 1873 K. The melting point of the slag was higher, and the temperature of the reduction process cannot
reach its melting point, which causes that the cold bonded agglomerate could not melt and still in dispersed state, hindering the aggregation between the iron particles. As a result, the iron phase and slag phase cannot be separated. From Figure 4 it could be found that if the content of SiO2 in the slag phase was increased by 4%, the melting point of the slag formed after the briquetting reaction could be reduced to about 1523 K. Therefore, in order to reduce the melting point of the slag phase, 4% of SiO2 reagent was added. The blast furnace dust and 4% of SiO2 reagent were mixed intensively in a mortar, then pressed into cold bonded agglomerates. The dried cold bonded agglomerate was placed in an iron bath crucible and reacted at 1500∘C for 30 min. Figure 5 shows the morphology of the reaction product.

Morphology of reaction product containing SiO2
There was no obvious difference of cold bonded agglomerate that contains SiO2 or not as shown in Figure 5. The reaction product in the slag phase was dispersed, and it did not gather together. The iron particles were reduced in the middle of the slag phase, which could not achieve the separation of slag iron, recover the valuable element iron in the blast furnace dust. It was not consistent with the results of the four-phase phase diagram analysis of CaO-MgO-SiO2-Al2O3 in Figure 4. So the proportion of the slag-forming phase components in the cold bonded agglomerate was not the main factor affecting the melting and accumulation of the slag in the reaction product.
3.3 Analysis of SEM-EDS
In order to determine the presence state of metallic iron and slag in the reaction product after the high temperature reduction of the cold bonded agglomerate, the reaction product was subjected to SEM-EDS analysis. Figure 6 indicates the morphology and energy spectrum analysis of the Fe phase (a) and slag phase (b) in the reaction product.
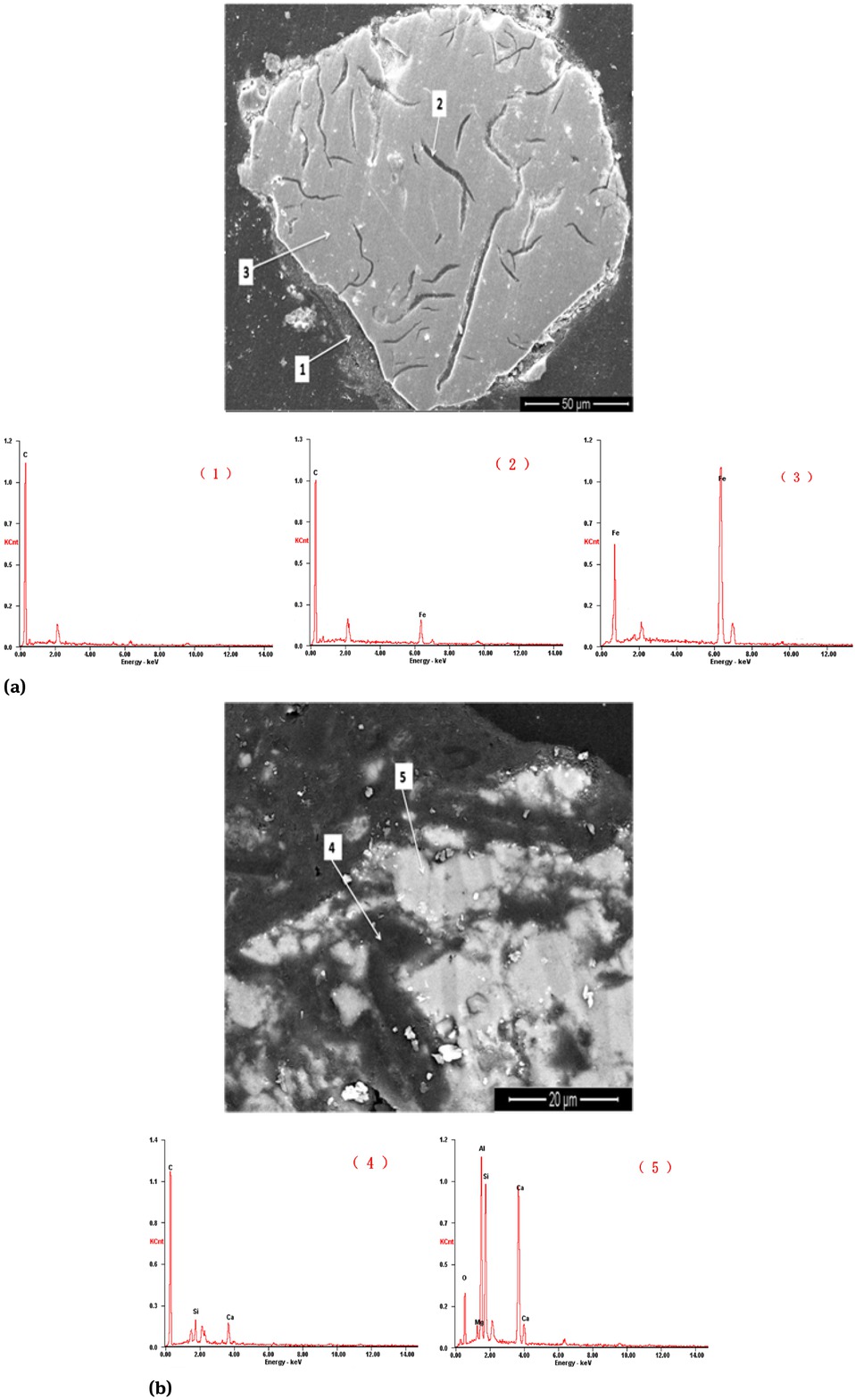
The morphology and energy spectrum analysis of Fe phase (a) and slag phase (b) of the reaction product
Table 1 showed that the content of TFe of the cold bonded agglomerate is 33.94% and C is 16.30%. According to the reduction of C and iron oxides, the content of C is superfluous to the reduction of iron and zinc oxide. There is still no depletion of residual carbon in the reaction product, as shown in Figure 6.
From Figure6(a) it could be seen that the iron oxide in the reaction product was reduced at the high temperature, but the metal iron particles were surrounded by the residual carbon in the blast furnace dust. Iron particles were also mixed with unreacted carbon inside resulting in the iron difficult to gather. Because iron particles were lighter, they would only be mixed in the slag phase, floating in the upper part of the molten iron, which affect the recovery of valuable elements in the cold bonded agglomerate. It cannot achieve the purpose of comprehensive utilization of Blast furnace dust.
The energy spectrum analysis results of the mark in Figure 6(b) show that the slag phase which is also wrapped or isolated from the residual carbon in the cold bonded agglomerate is formed by other components, and it cannot be gathered together The slag phase in the reaction product is macroscopically dispersed and mixed with many of the reduced iron particles.
3.4 Validation of results
In order to verify the above points of view, in the course of the experiment, the cold bonded agglomerates were pressed using blast furnace dust and a certain amount of magnetite added to ensure that the carbon could be expended completely. The cold bonded agglomerates contained magnetite concentrate were placed in an iron bath for 30min at 1773 K. Figure 7 shows the morphology of the reaction product.

Morphology of reaction product containing magnetite concentrate
From Figure 7, it can be seen that the reaction product of metallic iron and slag phase were gathered together when the cold bonded agglomerate contained magnetite concentrate, and the slag phase formed a solid phase, which was no longer dispersed. It was conducive to the realization of the separation of iron and slag, and iron could be restored in molten iron easy. Then the goal of recovering the valuable elements in the blast furnace dust would be achieved.
4 Conclusions
Putting the cold bonded agglomerate into the molten iron, and relying on the heat provided by molten iron, the iron oxide in the cold bonded agglomerate can be reduced into a metal iron in the case of its own carbon as a reducing agent, without an additional reducing agent.
When the cold bonded agglomerate is directly placed into the molten iron, the reduction of iron oxide cannot completely consume its own carbon, and the reaction product also retains part of the carbon. The reduced iron is still mixed with the slag phase, affecting the recovery of iron.
Adding a certain amount of high oxidizing materials during the briquetting process of blast furnace dust, the carbon in the blast furnace dust can be completely consumed without any residue after the high temperature reaction. The iron oxide is reduced to metallic iron into the hot metal, and the other components form slag phase which floats on the molten iron, which achieve the separation of iron and slag.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (51874025) and National Key R&D Program of China (2017YFB0304300 & 2017YFB0304302)
References
[1] X.F. She, Q.G. Xue, J.J. Dong, J.S. Wang and H. Zeng, Chin. J. Process Eng., 9 (2009) 7-10.Search in Google Scholar
[2] S.M.Hay and W.J.Rankin, Miner. Eng., 18 (1994) 987-1001.Search in Google Scholar
[3] V Glady and VS Filippov, Steel in Translation, 31 (2001) 1-4.Search in Google Scholar
[4] F. Kukurugya, T. Vindt and T. Havlik, Hydrometallurgy, 154 (2015) 20-32.10.1016/j.hydromet.2015.03.008Search in Google Scholar
[5] B. Asadi Zeydabadi, D. Mowla and M.H.Shriat, Hydrometallurgy, 47 (1997) 113-125.10.1016/S0304-386X(97)00039-XSearch in Google Scholar
[6] J.W. Han, W. Liu, W.Q. Qin, B. Peng, K. Yang and Y.X. Zheng, J. Ind. Eng. Chem., 22 (2015) 272-279.10.1016/j.jiec.2014.07.020Search in Google Scholar
[7] B Das., S Prakash., P. S R. Reddy and V.N. Misra, Resources Conservation and Recycling, 50 (2007) 40-57.10.1016/j.resconrec.2006.05.008Search in Google Scholar
[8] A. Lopez-Delgado, C. Perez and F. A. Lopez, Water Res., 32 (1998) 989-996.10.1016/S0043-1354(97)00304-7Search in Google Scholar
[9] MK Sinha, S Pramanik, SK Sahu, LB Prasad, MK Jha and B D Pandey, Sep. Purif. Technol., 167 (2016) 37-44.10.1016/j.seppur.2016.04.049Search in Google Scholar
[10] J.L. Zhang, Y.F. Yan, M. Xu, X.H. Zhao and X.D. Zhang, Iron and Steel, 41 (2006) 78-81.Search in Google Scholar
[11] X.D. Xing, J.L. Zhang, M.M. Cao, K.X. Jiao and S. Ren, Mining and Metallurgical Engineering, 32 (2012) 86-91.Search in Google Scholar
[12] M Sethurajan, D Huguenot, R Jain, P N.L. Lens, H A. Horn L H.A. Figueiredo and E D. Hullebusch, J. Hazard. Mater., 324 Part A (2017) 71-82.10.1016/j.jhazmat.2016.01.028Search in Google Scholar
[13] Ryan Robinson, Thermochim. Acta, 432 (2005) 112-123.10.1016/j.tca.2005.04.015Search in Google Scholar
[14] WP Jin, C A Joong and S Hocheol, Resources Conservation and Recycling, 34 (2002) 129-140.10.1016/S0921-3449(01)00098-2Search in Google Scholar
[15] JR Kim, Y S Lee and DJ Min, ISIJ Int., 44 (2004) 1291-1297.10.2355/isijinternational.44.1291Search in Google Scholar
[16] B J Monaghan, L Chen and J Sorbe, Ironmaking Steelmaking, 32 (2005) 258-264.10.1179/174328105X28793Search in Google Scholar
© 2019 X.-L. Liu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

