Abstract
Permittivity is a vitally important parameter for describing the absorbing and heating characteristics of a material under microwave irradiation, and it is also strongly dependent on temperature. However, the literature contains little information on this topic and even less particular permittivity data at elevated temperatures. In this paper, the permittivity of a CuCl residue at temperatures from 13 to 450 °C at 2.45 GHz was measured using the cavity perturbation method. The relationship of its real part (ε′) and imaginary part (ε″) with temperature (T) was deduced. In addition, the temperature-dependent tangent (tan δ) and the penetration depth (d) of microwaves into the material were calculated. The results of the permittivity study show that the dielectric constant (ε′) of the CuCl residue increased linearly with increasing temperature. In contrast, the dielectric loss factor (ε″) and loss tangent first maintained on a steady value between 13 and 300 °C and then substantially increased from 300 to 450 °C. The positive interaction of the dielectric property and temperature showed the reasonableness of our earlier metallurgy process, where the CuCl residue for dechlorination was roasted at 350–450 °C under microwave irradiation.
Introduction
In recent years, because of its special advantages, which include selective heating, internal heating and fast heating with concentrated energy, microwave energy has found applications in an increasing number of processes and areas (e.g. the food, medical and petrochemical industries), where it has led to gigantic profits [1, 2, 3, 4]. In addition, in the mineral processing and metallurgy fields, additional research on mineral grinding, sintering, melting, thermal reduction and oxidation has recently been carried out, which has led to less energy consumption and higher efficiency compared with conventional technology [5, 6, 7]. Thus, the advantages of microwave energy may efficiently solve the problems facing traditional factories. However, the lack of the fundamental physical data, especially permittivity data, and the uncertainty of the microwave enhancement mechanism are two substantial obstacles that continue to impede final application this technology [8]. Permittivity is critically important as a parameter for characterizing the absorption and heating properties of a material under microwave irradiation. It is also strongly dependent on temperature [9, 10]. The literature contains few reports on this topic, and specific data on high-temperature permittivity are even less common.
In our previous work, we proposed a new process for dechlorinating a hydrometallurgical residue (i.e. CuCl residue) using microwave energy. Initially, we measured the dielectric properties of the CuCl residue as a function of its moisture content and density at room temperature [11]. The sticky and wet CuCl residue containing 33 % water was then dried efficiently under microwave irradiation and was ground into a powder with a moisture of approximately 5 % [12]. The dried CuCl residue powder was subsequently roasted under an atmosphere of steam and oxygen for dechlorination in a tubular microwave furnace at 350–450 °C, the efficiency of the dechlorination process was greater than 95 % [13]. We also found that, compared with the dechlorination efficiency in a conventional electric tubular furnace, the speed and efficiency under microwave irradiation conditions were both greatly enhanced [14, 15].
In the present work, we first measured the dielectric constant (ε′) and the dielectric loss factor (ε″) of the CuCl residue under different temperatures (from 13 to 450 °C) at 2450 MHz. We then calculated the loss tangent (tanδ) and penetration depth of the CuCl residue at high temperatures. The heating curves of the CuCl residue in microwave field generated at different microwave power levels (1200–2400 W) were also studied. Finally, on the basis of our comparison of the dechlorination results with those obtained using a conventional electric furnace, SEM analysis results for the roasting product, and the roasting product’s permittivity data, we deduced an intensification mechanism of microwave roasting.
Experimental
Materials
The raw material, CuCl residue, was obtained from a zinc hydrometallurgical plant in Yunnan Province, China. The main chemical composition and phase of the CuCl residue after complete drying is shown in Table 1 and Figure 1.

XRD pattern of the raw material.
Chemical composition of the CuCl residue from a zinc hydrometallurgy process.
| Composition | Cu | Cl | O | S | Zn |
|---|---|---|---|---|---|
| Content (%) | 54.68 | 17.27 | 3.34 | 4.74 | 6.55 |
Figure 1 shows that copper(I) chloride (CuCl) and copper(II) oxide (Cu2O) were the major components of the CuCl residue and that a minor amount of ZnS was also present.
Experimental devices
High-temperature permittivity measurement system
A microwave cavity perturbation method was used to measure the dielectric parameters of the CuCl residue at high temperature. The testing equipment was developed by the Institute of Applied Electromagnetics at Sichuan University, China. A schematic of this system is shown in Figure 2.

Schematic of the high-temperature permittivity measurement system.
The device consists of a vector network analyser, a waveguide–coax transition, a directional coupler, an electromagnetic induction heater, a water recirculator, a gas lift and a cavity resonator. Microwave is launched from one port of the vector network analyser, enter the analysing cavity through the waveguide–coax transition and directional coupler. The resonator is used to hold in the analysing cavity. The microwave signal returns to the signal receiver of the vector network analyser through the waveguide–coax transition and directional coupler after interacting with the material in the cavity. The test control unit is connected to a computer via a USB data cable; a software system is responsible for calculating the dielectric parameters (Figure 2). The system error of the high-temperature permittivity measurement system is estimated to be only 3–5 %.
Microwave oven used in heating-curve experiments
A microwave reactor with a power of 3 kW and a frequency of 2450 MHz was used during heating-curve experiments. Its schematic is presented in Figure 3. The microwave system consists of two magnetrons, a multi-mode cavity and a quartz glass tube. It is equipped with a water-cooled condenser and a temperature controller for adjusting the microwave input power level for a preset temperature. A thermocouple pyrometer was inserted into the centre of the sample for measuring the heating temperature.

Schematic of the microwave oven used in this work.
Dechlorination devices
To illustrate the mechanism by which microwaves intensify dechlorination, a series of experiments were carried out by conventional and microwave heating. A tubular resistance furnace and a tubular microwave oven were used in the conventional and the microwave experiments, respectively. The microwave heating schematic is illustrated in Figure 4.

Schematic of the microwave oven used in this work.
Experimental procedures
A four-step process was used to measure the dielectric properties: (1) The test sample was placed in a small quartz tube, which was subsequently placed into the heater of the high-temperature permittivity measurement system. (2) When the temperature of the sample reached a preset temperature, it was lifted into the cavity resonator quickly through an opening via a gas lift; the sample’s influence on the microwave distribution was detected by the sensors. (3) The software rapidly calculated the dielectric parameters on the basis of test-cavity perturbation theory. (4) The sample then was lowered to the heater and heated to a higher preset temperature for measurements of its permittivity at a different temperature.
During the microwave heating-curve experiments, a certain amount (100 g) of dried and ground sample loaded into a mullite crucible was placed inside the microwave oven and heated to 500–600 °C at a certain microwave power (1200 , 1800 or 2400 W).
For roasting experiments, a thin layer of 20 g of dried and ground sample was placed in a mullite boat, which was, in turn, inserted into a furnace and heated to 300–500 °C under oxygen flowing at 150 mL/min. The sample was maintained at the desired temperature for 120 min. Absorbing bottles were used to treat the off-gas.
Results and discussion
Dielectric constant
The variation of the real permittivity of the CuCl residue with increasing temperature and at 2.45 GHz is shown in Figure 5.
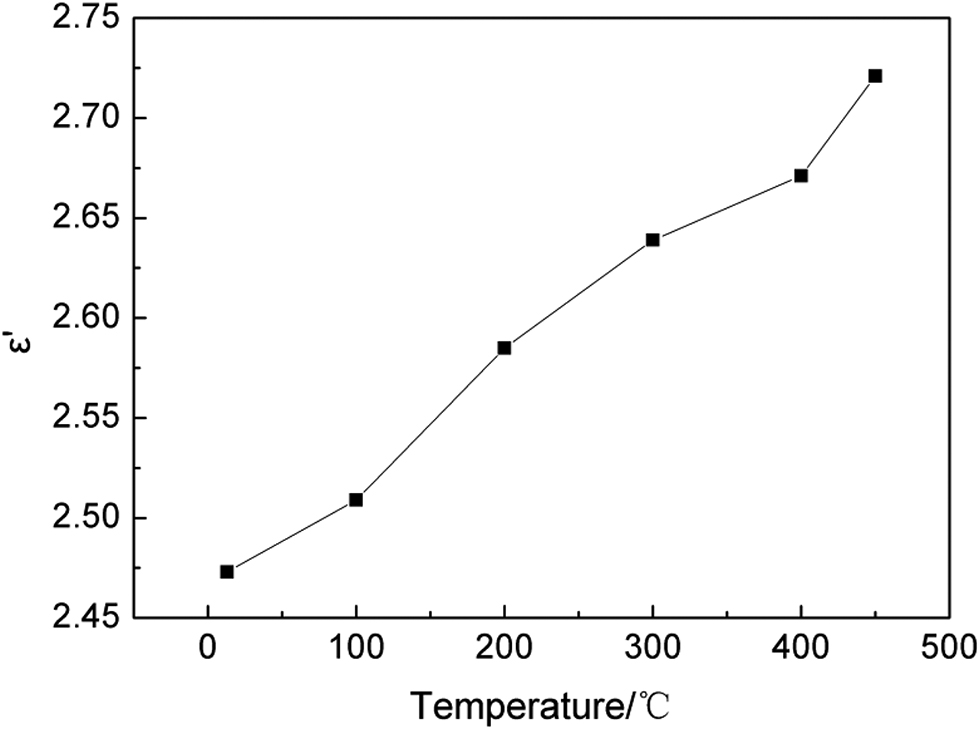
Variation of dielectric constant of the CuCl residue as a function of temperature.
The dielectric constant of the CuCl residue was 2.473 at room temperature (13 °C) and increased with increasing temperature. The real permittivity slightly increased to 2.721 at 450 °C, and the change in the dielectric constant was approximately linear in this temperature range. The curve fitting results in Table 2 indicate the changes in the relationship between temperature and the dielectric constant of the CuCl residue. Although the temperature range of the experiments was limited to 13–450 °C, the tendency of the dielectric constant of the CuCl residue to increase with increasing temperature is the same tendency exhibited by the dielectric constants of other ores and minerals [16]. Compared with many other minerals such as kaolin ores [16] and natural ilmenite [17], the CuCl residue exhibits a lower dielectric constant.
The variation of dielectric properties of the CuCl residue with temperature.
| Result | R2 |
|---|---|
| ε′=2.4639 + 5.5698×10−4T | 0.9847 |
Loss factor
Figure 6 shows the variation of the loss factor of the CuCl residue as a function of temperature at 2.45 GHz. The loss factor was lower than the dielectric constant in the range of tested temperatures, whereas the trend of increasing temperature differed from that of the dielectric constant. The loss factor reached a stable level of 0.0182−0.0258 at temperatures below 300 °C. However, when the temperature was greater than 300 °C, it rapidly increased with increasing sample temperature; the value of the loss factor almost tripled relative to the average value at temperatures between 13 and 400 °C, reaching a maximum value of 0.0621.

The variation of loss factor of CuCl residue with temperature.
The residue in our work is composed of CuCl, Cu2O and a minor amount of ZnS. The crystal structure of the CuCl residue is ionic crystals. On the interfaces between various compounds a large number of ions and electrons exist, most of which will induce a relaxation phenomenon under a microwave field [18]. Because the melting point of CuCl is approximately 430 °C, when the temperature was above 400 °C, the number of active ions and electrons of CuCl residue will sharply increase. The loss factor of a material is directly proportional to its conductivity; thus, the loss factor increases with increasing temperature [19].
Loss tangent
The values of loss tangent at the frequency of 2.45 GHz were calculated using the measured data (ε′ and ε″); the results are shown in Figure 7.

Variation of the loss tangent of the CuCl residue with temperature.
The values of loss tangent of the CuCl residue at different temperatures exhibit the same tendency as the loss factor. From the formula tan δ=ε″/ε′, an increase in tanδ is a result of a more rapid increase in ε″ than in ε′. At temperatures between 13 and 300 °C, the loss tangent reached a stable level in the range 0.0069–0.0104 and then rapidly increased when the temperature was greater than 300 °C; the value of the loss tangent almost doubled compared with its average value in the range 13–400 °C, reaching a maximum value of 0.023.
Penetration depth
The power penetration depth (Dp) is defined as the depth where the strength of an applied microwave field is reduced to 1/e of its surface value and is expressed with the equation
where λ0 is the wavelength (λ0=12.24 cm at 2.45 GHz) and π is a constant. The penetration depth of the CuCl residue can be calculated by eq. (1) on the basis of the measured dates.
Figure 8 shows the variation of the penetration depth of the CuCl residue with temperature at 2.45 GHz.

The variation of penetration depth of the CuCl residue with temperature.
As shown in Figure 8, the optimum thickness for uniform heat varied from 8 to 6 cm at room temperature to 450 °C. The penetration depth stabilized between 7.8 and 8.4 cm in the temperature range 13–300 °C and then decreased to 6.1 cm at 450 °C. When the penetration depth is less than the size of the heated sample, obvious temperature gradients will be observed. In the test of microwave roasting of CuCl residue, the dechlorination process of the sample was diminished; thus, the material thickness had no effect on the uniformity of microwave heating. However, in expanding experiments and industrialization trial production processes, extra attention should be paid to the material mixing to ensure high efficiency of microwave heating.
Microwave heating curves
To reveal the real heating behaviour of the residue in various microwave fields, we recorded a series of heating-rate curves for the CuCl residue at different microwave power levels; the results are shown in Figure 8.
A nonlinear relationship exists between the temperature of the sample and the microwave heating time. Figure 9 shows that the sample temperature increased from room temperature to approximately 600 °C within 15 min, which indicates that the CuCl residue exhibits a strong ability to absorb microwave energy and convert it into heat.

Heating-rate curves of the CuCl residue at different microwave power levels.
Figure 9 shows that the increasing tendency can be divided into two parts. The sample temperature slowly increased in the temperature range 25–300 °C and rapidly increased in the range 300–500 °C. This result is in good agreement with the dielectric properties of the CuCl residue. At 25–300 °C, with lower ε′, ε″ and tanδ values, the CuCl residue can absorb only a small portion of the microwave energy. Therefore, only a small fraction of the radiation energy was converted into thermal energy and the material temperature increased slowly. When the temperature was greater than 300 °C, the values of ε′, ε″ and tanδ increased swiftly and the material temperature increased rapidly within a relatively short time. The interplay of the dielectric characteristics and temperature explains the mechanism whereby the CuCl residue can be heated to a high temperature in a short time in microwave field because the sample can absorb a much larger fraction of microwave energy at higher temperatures.
Intensification mechanism of its dechlorination process by microwave energy
Comparison of dechlorination effect with elect microwave
Many scholars have studied the reaction process of copper chloride at different temperatures. A new phase of Cu2OCl2 was found during the oxidation of CuCl, and the sequence of chemical reactions at various temperatures is shown in Table 3.
| Temperature (°C) | Chemical reaction |
|---|---|
| <300 | The volatilization of free water and crystal water |
| Cu2O(s) + O2(g) ⇌ CuO(s) | |
| About 375 | 4CuCl(s) + O2(g) ⇌ 2Cu2OCl2(s) |
| 400–470 | 3Cu2OCl2(s) ⇌ CuCl2(s) + 2CuCl(s or l) + 3CuO(s) + Cl2(g) |
| >464 | 2Cu2OCl2(s) + O2 ⇌ 4CuO(s) + 2Cl2(g) |
| >550 | CuCl(l) ⇌ CuCl(g) |
| CuCl(l) + O2(g) ⇌ CuO(s) + Cl2(g) |
The changes of the product phase in the experiment were analysed by XRD. Figure 10(b) and (c) shows the major phases of samples treated by conventional and microwave roasting, respectively. After conventional roasting, phases containing chloride (CuCl and Cu2OCl2) were still present. The XRD analysis results demonstrate that the dechlorination rate during microwave roasting was higher than that during conventional roasting.

XRD patterns of products roasted by conventional and microwave roasting: a: raw material; b: conventional heating; c: microwave roasting.
Microwave intensification mechanism
The power absorbed by the material is directly proportional to the loss factor, the frequency and the intensity of the electric field, as shown in eq. (1), which is derived from eq. (2):
where ε0 is the permittivity of free space (8.854 pF m−1), ε″ is the imaginary part of the complex permittivity, also known as the loss factor (F m−1), Ei is the internal electric field (V· m−1), f denotes the frequency (Hz) and Pd is the power density dissipated in the heated material (W m−3).
From eqs (1) and (2), we can find that microwave heating depends on two main parameters: E and ε″. Therefore, the microwave roasting mechanism mainly comprises the following steps:
(1) At low temperatures, the dielectric loss of a mineral is relatively low and the penetration depth is high. Thus, the energy of microwaves can be concentrated into an electric peak. As a consequence, a state of flash heating and reaction occur inside the material because of the highly localized electric field, as shown in Figure 11(a).
(2) As the temperature of the hot core area reaches the reaction stage (approximately 450 °C), CuCl residue particles in the core quickly react with the surrounding air. Actually, the CuCl contained in the residue becomes a floatable liquid, as its melting point is approximately 427 °C. The microwaves can reverse CuCl molecules, which exhibit strong polar characteristics when melted, like water molecules. This reversing effect will intensively enhance and accelerate the oxidation of CuCl into CuO. Correspondingly, this mechanism coincides with the observed trend of a dramatic increase of dielectric loss from 350 to 450 °C, as shown in Figure 6.
Figure 12 shows that the microwave roasting products have a very porous structure; thus, the gas–solid reaction can easily occur.

The deduced microwave heating mechanics: (a) hot core induced by microwaves; (b) as heat conducts outside, outward microwave heating is enhanced; (c) a state of homogeneous heating is reached.

SEM images of the products after removal of chlorine; red circles show the reaction channels.
(3) Some shrinking surfaces or reaction layers inside the particles appear because of the difference in physical properties such as hardness and density between the CuCl and Cu2O in the residue and in the product. The gaps between these shrinking surfaces or reaction layers function as channels for air transmission, thereby facilitating the reaction of CuCl molecules inside the particles with oxygen.
(4) The heat in the hot core region conducts to the nearby area because of the good thermal conductivity of molten CuCl and CuO produced during the CuCl and Cu2O oxidation, as shown in Figure 11(b), which can dramatically raise the dielectric loss of the material in the heated region, as shown in Figure 6. Thus, this nearby region will absorb more microwave energy and undergo rapid heating, as indicated by eq. (1). In the meanwhile, the increase in dielectric loss will decrease the penetration depth of the microwaves, as eq. (2) shows. The energy absorbed by the hot core region decreases, so its rate of temperature increase decreases and its heat is continuously passed outwards for conduction. The layer-by-layer reaction process continues until a uniform temperature and reaction state are reached, as shown in Figure 11(c).
Conclusion
The dielectric constant of the CuCl residue increased with increasing temperature, and the change tended to exhibit a linear relationship. The dielectric loss factor and loss tangent maintained steady values at temperatures between 13 and 300 °C and then significantly increased from 300 to 450 °C. The penetration depth stabilized between 7.8 and 8.4 cm in the range 13–300 °C and then decreased to 6.1 cm from 300 to 450 °C.
In 15 min, the material temperature could reach more than 500 °C under a microwave field. The increasing temperature trend was divided into two parts: the region where the sample temperature slowly increased from 25 to 300 °C, and the region where the temperature rapidly increased to 300–500 °C.
The microwave and conventional roasting of CuCl residue were compared. The results demonstrated that the dechlorination rate during microwave roasting was higher than that during conventional roasting.
The microwave roasting mechanism was evaluated through modern analysis methods such as chemical analysis, XRD and SEM.
Acknowledgements
The authors gratefully acknowledge the foundation of Henan educational committee (No. 18A530002) and key scientific and technological project of Henan province (No. 182102310896) for funding this work.
References
[1] C. Saucier, M.A. Adebayo, E.C. Lima, R. Cataluña, P.S. Thue, L.D.T. Prola, M.J. Puchana-Rosero, F.M. Machado, F.A. Pavan and G. Dotto, J. Hazard. Mater., 289 (2015) 18–27.10.1016/j.jhazmat.2015.02.026Search in Google Scholar PubMed
[2] N. Wang and P. Wang, Chem. Eng. J., 283 (2016) 193–214.10.1016/j.cej.2015.07.046Search in Google Scholar
[3] F. Zhu, L. Li and J. Xing, J. Hazard. Mater., 321 (2017) 103–110.10.1016/j.jhazmat.2016.09.012Search in Google Scholar PubMed
[4] Y.C. Chou, S.L. Lo, J. Kuo and C.J. Yeh, J. Hazard. Mater., 284 (2015) 83–91.10.1016/j.jhazmat.2014.10.043Search in Google Scholar PubMed
[5] S. Singh and D. Gupta, Mater. Manuf. Process., 30 (2015) 1–29.10.1080/10426914.2014.952028Search in Google Scholar
[6] K. Yang, S. Li, L. Zhang, J. Peng, W. Chen, F. Xie and A. Ma, Hydrometallurgy, 166 (2016) 243–251.10.1016/j.hydromet.2016.07.012Search in Google Scholar
[7] J. Chang, L.B. Zhang, C.J. Yang, Q. Ye, J. Chen, J.H. Peng, C. Srinivasakannan and W. Li, Chem. Eng. Process., 97 (2015) 75–83.10.1016/j.cep.2015.09.006Search in Google Scholar
[8] C. Liu, L. Zhang, J. Peng, C. Srinivasakannan, B. Liu, H. Xia and L. Xu, J. Microwave Power E.E., 47 (2013) 199–209.10.1080/08327823.2013.11689858Search in Google Scholar
[9] D. Ding, W. Zhou, B. Zhang, F. Luo and D. Zhu, J. Mater. Sci., 46 (2011) 2709–2714.10.1007/s10853-010-5140-xSearch in Google Scholar
[10] Y. Wang, F. Luo, L. Zhang, D. Zhu and W. Zhou, Ceram. Int., 39 (2013) 8723–8727.10.1016/j.ceramint.2013.04.056Search in Google Scholar
[11] Z.Y. Guo, S.H. Ju, J.H. Peng, W.W. Qu, L.B. Zhang and C.H. Liu, Mater. Rev. (China), 27 (2013) 141–144.Search in Google Scholar
[12] S. Lu, S. Ju, J. Peng, X. Zhu, C. Srinivasakannan, L. Zhang and G. Tu, High Temp. Mat. Pr-Is., 34 (2015) 147–154.Search in Google Scholar
[13] Z.Y. Guo, T. Lei, W. Li, H.L. Luo, S.H. Ju, J.H. Peng and L.B. Zhang, Chem. Eng. Process., 92 (2015) 67–73.10.1016/j.cep.2015.03.024Search in Google Scholar
[14] Z. Guo, S. Ju, J. Peng, L. Zhang and T. Lei, Optimization of microwave roasting for dechlorination of CuCl residue under oxygen enriched condition, High Temp. Mat. Pr-Is., 35 (2016) 135–143.10.1515/htmp-2014-0177Search in Google Scholar
[15] Z. Guo, W. Li, S. Ju, J. Peng and T. Lei, High Temp. Mat. Pr-Is., 35 (2016) 479–485.10.1515/htmp-2014-0206Search in Google Scholar
[16] C.A. Pickles, Miner. Eng., 17 (2004) 775–784.10.1016/j.mineng.2004.01.007Search in Google Scholar
[17] C. Chiteme and A.F. Mulaba-Bafubiandi, J. Mater. Sci., 41 (2006) 2365–2372.10.1007/s10853-006-1819-4Search in Google Scholar
[18] C.H. Liu, L.B. Zhang, J.H. Peng, B.G. Liu, H.Y. Xia, G.U. Xiao-Chun, X.C. Gu and Y.F. Shi, T. Nonferr. Metal. Soc., 23 (2013) 3462–3469.10.1016/S1003-6326(13)62889-7Search in Google Scholar
[19] C. Liu, L. Zhang, J. Peng, W. Qu, B. Liu, H. Xia and J. Zhou, High Temp. Mat. Pr-Is., 32 (2013) 587–596.10.1515/htmp-2013-0008Search in Google Scholar
[20] J.B. Sharkey and S.Z. Lewin, Thermochim. Acta., 3 (1972) 189–201.10.1016/0040-6031(72)85029-9Search in Google Scholar
[21] A. Nixon, M. Ferrandon, M.H. Kaye and L. Trevani, J. Therm. Anal. Calori., 110 (2012) 1095–1105.10.1007/s10973-011-1998-3Search in Google Scholar
[22] R.C. Wang and H.Y. Lin, Mater. Chem. Phys., 136 (2012) 661–665.10.1016/j.matchemphys.2012.07.039Search in Google Scholar
© 2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

