Abstract
MgO–C refractory was polarized across the slag-refractory interface at a voltage of 8 V in a synthetic CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K. A deposition layer mainly composed of dicalciumsilicate (2CaO.SiO2) or spinel (MgO.Al2O3) with several hundred microns thick was achieved by cathodic polarization. However, the anodic decay was accelerated in comparison to the cathodic refractories and nonpolarized reference MgO–C refractories. The electrodeposition layer was mainly caused by the reduction of silicate anions, in which a shift of slag composition to the dicalciumsilicate (C2S) or spinel (MA) primary phase field was induced. Furthermore, the rapid migration of mobile Ca2+ and Mg2+ ions can also lead to the enrichment of CaO and MgO at the slag/refractory interface. The present voltage level (8 V) is acceptable for the economic considerations and the slag component also need a careful section for the corrosion protection of MgO–C refractories.
Introduction
MgO–C refractories are widely used as furnace and ladle lining due to their high melting point, corrosion resistance and shock resistance [1, 12, 3]. However, a decrease of their service life is often caused by the damage because of chemical corrosion, thermal shock, and mechanical erosion in steelmaking operations. Accelerated corrosion can decrease not only the steel quality through an increase in the number of nonmetallic inclusions but also the service life of refractories. In order to improve the service life of lining materials and steel quality, some measures must be carried out to reduce the dissolution of MgO–C refractories. In general, the damage of refractory is evaluated based on the penetration depth (defined here as “physical penetration” through the open pore without chemical reaction), and attack of slag (defined here as “chemical penetration” involving interface reactions between the refractories and slag) [4]. Recent studies have shown that the penetration rate of melt into the refractories can be hampered either by using extremely low porosity of refractories, such as density MgO-based ceramic, or by applying a voltage across the slag-refractory interface [5, 6, 7]. This finding can be explained using the integrated form of Poiseulle’s law [8], shown in eq. (1)
where r is the open pore radius of refractory, γ is the interfacial tension, θ is the contact angle between the melt and refractory, η is the slag viscosity, t is corrosion time and l is the penetration depth. The extremely low porosities of refractories can reduce the penetration depth by reducing the parameter r for a given time. An application of an electrode potential across the slag-refractory interface can also reduce the penetration depth by changing γ and θ for a given time. Moreover, the directional shift of cation to the cathode can also lead to an increase of slag viscosity (η) surrounding the cathode. All operations can reduce the penetration depth (l) of slag into the refractories as explained in formula (1). Recent studies of MgO refractories immersed in slag have found that a spinel (MgO.Al2O3) layer is formed on the MgO surface, significantly slowing the further dissolution of MgO [6, 9, 10]. As is known, liquid oxides are ionic in nature at high temperature and the formation of spinel involves the counter-diffuse of ions, which can be controlled by using applied voltage. Unfortunately, no further studies involving the slag component and the amplitude of applied voltage are investigated aiming at the formation process of deposition layer.
In the present work, a deposition layer formed on the MgO–C refractory surface in contact with a CaO–Al2O3–SiO2 – (MgO) slag under an applied voltage has been studied and reported. Dicalciumsilicate (C2S) was prone to be deposited on the cathode surface in the CaO–Al2O3–SiO2 slag and spinel (MA) was achieved easily in the CaO–Al2O3–SiO2-10% MgO slag
Experimental
A piece of MgO–C refractory was drilled a φ 3×5 mm2 blind-hole in one of the electrode faces. The electrode was fastened to a φ 3×1000 mm2 molybdenum rod by applying a drop of high temperature cement into the blind-hole and hardening at 100 ℃ for 24 h. Similarly to standard practice, MgO–C refractories were used as the cathode and anode in the experiment, respectively. The electrodes were all dipped into the slag for 60 min at 1773 K, and the distance between anode and cathode as well as the immersion depth was 10 mm. A RLD-3020 potentiostat was used as a power source. After removal and air-cooling, all samples were prepared for SEM/EDS analysis by sectioning, grinding and polishing using standard procedures. All the experiments were carried out under Ar atmosphere at 1773 K in a self-constructed test furnace (Figure 1).

The phase first depositing from a slag is termed primary phase and the composition range in which the primary phase remains stable is known as the primary phase field. In this work, a synthetic slag with the composition (Table 1) close to the primary phase field of dicalciumsilicate (2CaO.SiO2) or spinel (MgO.Al2O3) was chosen. Slag preparation commenced with a homogenization step by mixing the appropriate amounts of high-purity CaO, SiO2, Al2O3 and MgO in a ball mill for 8 h, followed by melting the mixture in a clay-bound graphite crucible at 1773 K in a muffle furnace. The slag was then ground and remelted several times to produce a homogeneous slag. After that, the homogenized slag was placed in a graphite crucible with φ 80×70 mm2 for the following experiments.
Composition of synthetic slags.
| Samples | Mass fraction % | R=(CaO + MgO)/SiO2 | |||
|---|---|---|---|---|---|
| No. | CaO | SiO2 | Al2O3 | MgO | |
| 1 | 40 | 48.5 | 11.5 | – | 0.8 |
| 2 | 35 | 43.5 | 11.5 | 10 | 1.0 |
The phase composition of cross-section for polarized MgO–C refractories was analyzed by means of X-ray diffractometer (XRD, PANalytical X’Pert PRO, Dutch PANalytical Corporation). The microstructure was determined by scanning electron microscope (SEM, SU-5000, Hitachi High-Technologies Corporation) equipped with Energy Dispersive Analysis System of X-ray (EDS, Pheoenix, EDAX Limited Corporation).
Results and discussion
Reduction potential of silicate and physical properties of synthetic slag
As mentioned above, the reduction of silicate anions can be the drive force for the formation of desired primary phase, explained as the known back rule in the ternary diagram. In order to determine the reduction potential of silicate anions in the present synthetic slag, a linear sweep voltammetric curve (LSV) was performed in a double-electrode electrochemical cell using a Princeton Versa STAT 4 workstation (Princeton Instruments, American). A molybdenum rod and the prepared MgO–C electrode were used as the counter electrode and working electrode, respectively. The LSV curve of No.1 slag is shown in Figure 2. As displayed in Figure 2, a pronounced current increase was observed around the voltage of –6.1 V, corresponding to the reduction reaction of silicate anions. The finding was in agreement with the result of Allanore’s report [11]. No other recognizable current increase was observed, suggesting that the reduction of Ca2+or Al3+ was very difficult in the present canning range. Notably, the reduction potential of silicate anions measured here is more negative than that of silicon dioxide calculated from the thermodynamic data and Nernst formula (−1.2 V) due to the electrode over-potential, IR drop and the conductor drop and etc. Comparing to those of Ca2+ and Al3+, the reduction potential of Mg2+ in No. 2 slag is more negative, and thus the No. 2 slag presumably shows the similar electrochemical phenomenon displayed in Figure 2 between 0 and –8 V. Therefore, all the experiments were conducted at voltages of 8 V across the anode and cathode interface, which could ensure the reduction of silicate anions.
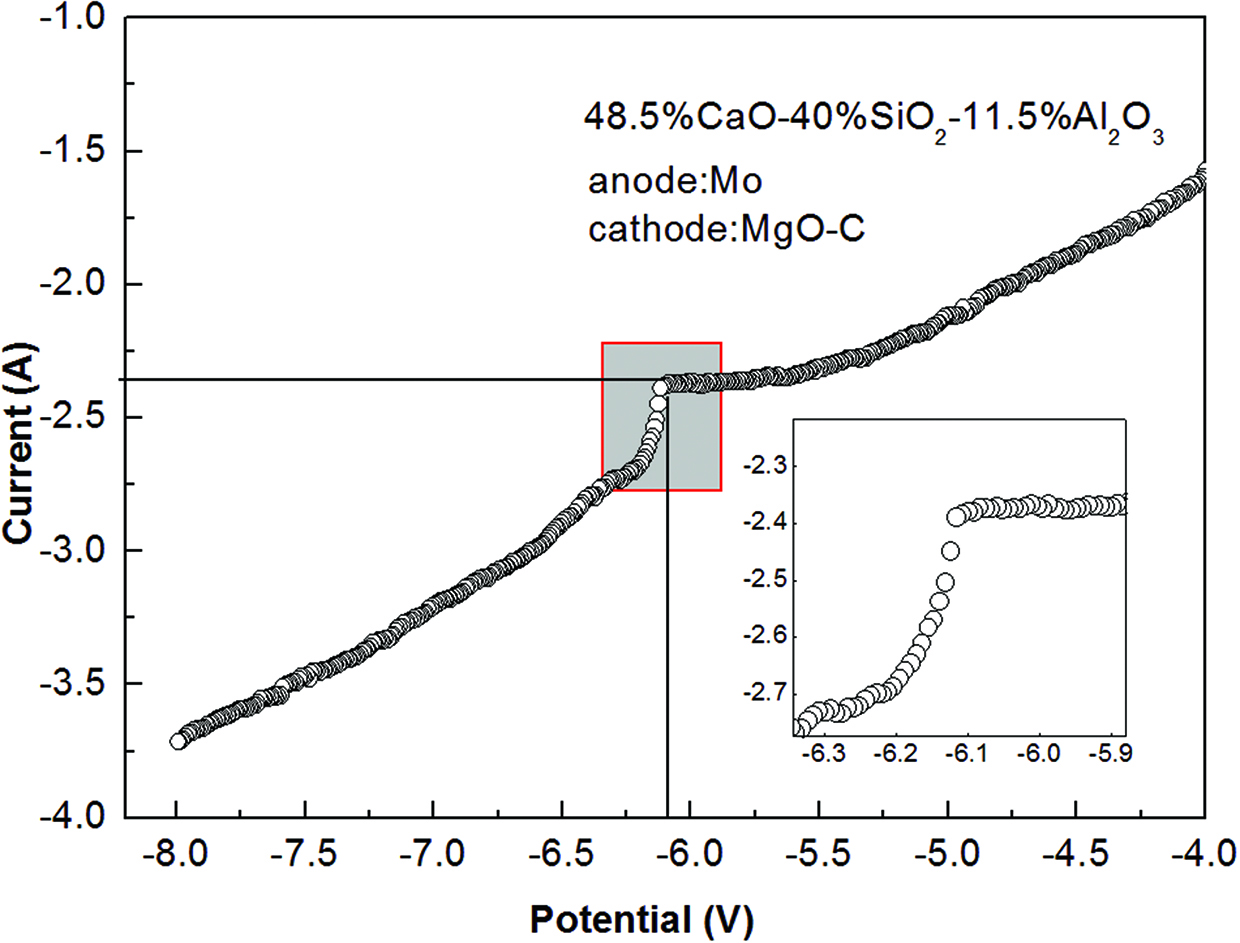
As known, the viscosity of synthetic slag is also an important index for the penetration depth. The viscosity curves of synthetic slag are shown in Figure 3. The viscosities of synthetic slags decreased with an increasing temperature, and the values of No. 1 and No. 2 slags were about 0.64 and 0.21 Pa.s at 1773 K, respectively. The results showed that the No. 2 slag involving 10% MgO could be more aggressive than that of No. 1 slag into the refractories due to its low viscosity. According to the reference [12], the conductivity of synthetic slag is around 0.12 Ω−1 cm−1 at 1773 K, and thus the IR drop between the electrodes was negligible.

Appearance, micrograph and phase component of polarized MgO–C refractory
Figure 4 exhibits the appearances of polarized electrodes and reference electrodes. As shown in Figure 4(a0), the reference sample covered with a viscous slag exhibited a decreasing cross-section, indicating the slag penetration into the MgO–C refractory was inevitable, and then a portion of refractory were peeled off from the body surface due to the temperature-dependent thermal expansion coefficient between the slag and refractory. However, a remarkable difference was detected for the polarized electrodes. The anode polarized at 8 V was attacked significantly after 60 min and exhibited a recognizable and discrete appearance (Figure 4(a1)), deriving from a complete slag penetration, interfacial chemical and electrochemical reactions. On the other hand, the cathode polarized at −8 V showed a negligible damage due to the formation of solid deposition layer (Figure 4(a2)). The deposition layer was attached tightly on the surface of cathode, leading to a smooth and regular surface. Also, the reference sample corroded in CaO–SiO2–Al2O3–10% MgO slag displayed no obvious appearance damage but an exfoliated edge marked in red circle at the sample bottom (Figure 4(b0)). Although, the viscosity of slag containing MgO was low, the dissolution of MgO–C refractory was hampered due to the relatively low concentration gradient of MgO between the slag and refractory. Comparing to that corroded in CaO–SiO2–Al2O3 slag, the anode corroded in the slag involving 10% MgO also displayed a less corrosion, indicating that the addition of MgO into the slag was favorite for the corrosion protection of MgO–C refractory, even at an extreme condition (Figure 4(b1)). Furthermore, the cathode corroded in the slag containing 10% MgO exhibited a complete and regular appearance, suggesting no corrosion happened at the surface (Figure 4(b2)). Notably, both the reference sample and polarized samples exhibited a good corrosion resistance in the slag involving 10% MgO, indicating that the addition of MgO in molten slag was very beneficial to the corrosion protection of basic refractory materials, such as MgO-base refractories.

SEM-micrographs of the slag/MgO–C interfacial regions in Figure 5 confirmed this impression shown in Figure 4 for the reference and polarized samples. In order to obtain the penetration depth of slag into the refractory, a small amount of ZrO2 was used as a tracer. An obvious penetration layer approximately 150 to 200 μm in depth was detected close to the boundary according to the trace of tracer element Zr, indicating that the reference sample was corroded through diffusion dissolution among the boundary layer (Figure 5(a0)). A recognizable and clean boundary was observed near the sample-slag interface due to the active dissolution of MgO–C refractory in a CaO–SiO2–Al2O3 ternary system (Figure 5(a1)). The anode dissolution is accelerated presumably by the change of interfacial tension at the slag-refractory boundary under applied voltage [13]. However, no visible penetration was related to the reference sample immersed in CaO–SiO2–Al2O3–MgO quarterly system (Figure 5(b0)), in agreement with the relative complete appearance observed in Figure 4(b0). According to the trace of Zr, the penetration depth of reference sample was about 60 to 100 μm, which is less than that immersed in CaO–SiO2–Al2O3 system. The appearance of anode immersed in slag containing 10% MgO also exhibited the same phenomenon as that immersed in CaO–SiO2–Al2O3 system (Figure 5(b1)). A several hundred micron thick deposition layer was observed on the surface of MgO–C cathodes obtained both in CaO–SiO2–Al2O3 ternary system and CaO–SiO2–Al2O3–MgO quaternary system (Figures 5(a2) and 5(b2)). Further investigation was performed to identify the solid layer deposited on the MgO–C cathode surface according to the mapping scanning and EDS phase analysis. EDS analysis of the light yellow area shown in Figure 6(a1) confirmed that the deposition layer consisted of dicalciumsilicate (2CaO.SiO2, C2S) (Table 2). The observed layer disintegration during the cooling process is a well-known behavior of dicalciumsilicate (C2S) caused by temperature-dependent thermal expansion coefficient changed with an associated volume expansion of 12% (Figure 5(a2)). Additionally, small amounts of silicon metals were also visually identified in the C2S. A spinel-matrix involving a rather low CaO and SiO2 content was observed adherent to the cathode surface in CaO–SiO2–Al2O3–MgO quarterly system (Figure 6(b2)). The spinel has a nominal composition of MgO.Al2O3 (Table 2). Still farther out obviously higher amounts of silicon metals were visually determined close to the sample-slag interface (Figure 6(b2)). Table 2 exemplarily showed that the gross slag composition in the interfacial region was a bit different from that of the bulk composition, further confirming that the accumulation of Ca2+ and Al3+ was produced under applied voltage. Because of the uncertainties of EDS-measurements, more evidences should be pursued to confirm the results.

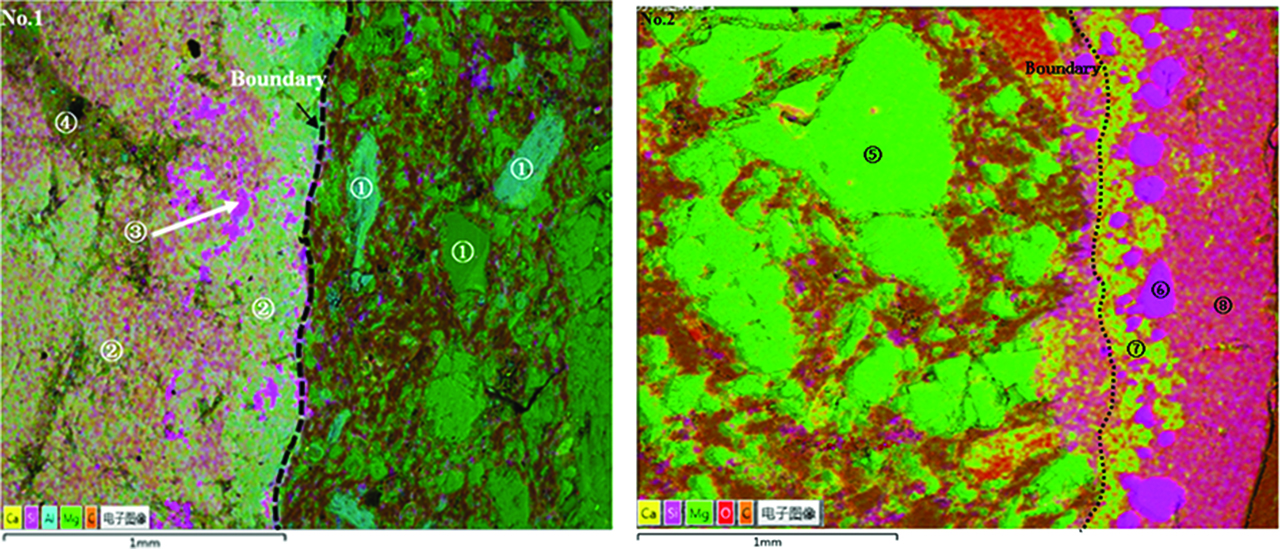
Cathodic sediment phase composition of different slag.
| Sample No. | Phase | Mass fraction % | Main component | |||||
|---|---|---|---|---|---|---|---|---|
| C | Mg | O | Ca | Si | Al | |||
| 1 | ① | 0.8 | 67.3 | 37.1 | 0.5 | 0.3 | – | MgO |
| ② | – | 7.6 | 25.8 | 47.3 | 14.0 | 5.3 | 2CaO.SiO2 | |
| ③ | 0.5 | 0.6 | 12.2 | – | 84.5 | 2.1 | Si | |
| ④ | 1.2 | 12 | 31.2 | 27.8 | 14.6 | 13.2 | Slag | |
| 2 | ⑤ | 1.8 | 67.4 | 30.1 | – | – | – | MgO |
| ⑥ | 2.7 | 0.1 | 4.1 | 0.3 | 91.7 | 0.6 | Si | |
| ⑦ | 5.3 | 17.0 | 37.5 | 1.2 | 2.3 | 36.7 | MgO.Al2O3 | |
| ⑧ | 1.2 | 6.5 | 38.0 | 11.2 | 22.9 | 20.2 | Slag | |
XRD analysis of the reference sample, anode and cathode is displayed in Figure 7. As shown in Figure 7(a), the reference sample was mainly composed of uncorroded MgO, C crystals and some negligible spinel grain, indicating the active dissolution of MgO–C refractory and the inevitable interface reaction between the molten slag and MgO–C refractory. However, a different phenomenon was related to the reference sample corroded in the slag containing 10% MgO. In addition to uncorroded MgO-grains, more diffraction peaks of spinel are observed (Figure 7(b)), suggesting that the addition of MgO into slag facilitated the formation of spinel crystals. Compared to the spinel formed under applied voltage (Figure 6(b2)), the spinel crystals formed here were discrete and were peeled off easily from the surface. Similar phase components were obtained on the anode surface both in CaO–SiO2–Al2O3 slag and CaO–SiO2–Al2O3–MgO slag. Complete C2S diffraction peaks were detected on the cathode surface immersed in CaO–SiO2–Al2O3 slag, in agreement with the results observed in Figure 5(a2). Spinel was the main phase close to the cathode interface, along with some Si metals displayed in the brighter part in Figure 5(b2). These findings further confirmed the positive effect of applied voltage on the cathode by forming a deposition layer, which contributed to the corrosion resistance of MgO–C refractory.

Electrochemical deposition mechanism
As mentioned above, Si metal existed around the cathode range, which was confirmed by the SEM/EDS analysis. The shift of silicate complex-anions under applied voltage was hampered because of their big ions radius, and then they were reduced into Si metal near the cathodes. This could be depicted as eq. (2)
Oxygen ions were also obtained due to the ionic nature at high temperature, and were promoted to shift in the anode direction. Therefore, the supposed anodic reaction was the oxidation of oxygen ions to oxygen and then was consumed by carbon oxidation involved in the MgO-C refractory, which accelerated the damage of anode. The supposed anodic reactions could be expressed as following:
During the reduction of silicate anions to silicon metals, the slag near the cathode was partly deprived of the oxide composition, as displayed in Figure 8. Electrochemical reduction of silicate anions led to a shift of local slag composition towards the dicalciumsilicate (C2S) or spinel (MA) primary phase field, marked from the dot “a” to dot “b” or from dot “c” to dot “d” through a known back rule in the CaO–SiO2–Al2O3 ternary diagram or CaO–SiO2–Al2O3–10% MgO quaternary diagram conducted using Factsage software at the present experiment temperature (Figures 9(a) and 9(b)). The increasing thickness of deposition layer was related to the on-going reduction of silicate anions. The induced deposition layer on the cathode surface effectively cut off the connection between the slag and MgO–C refractory. It should be noted that the deposition layer attached tightly on the surface of MgO–C refractory and had a higher melting-point than that of slag, which contributed to the corrosion protection of MgO–C refractory. However, the continuous oxidization of C involved in MgO–C refractory could result in a porous nature in the anode, which led to an active dissolution in the anode, in agreement with the discrete appearance exhibited in Figure 4(a1). On the other hand, because of the electrostatic alteration of the slag/refractory interface, the rapid migration of mobile Ca2+ ions and their accumulation at the cathode range could result in a locally increased CaO-content. This action was also beneficial to the formation of dicalciumsilicate (C2S). Additional, the hampered diffusion of Mg2+ ions produced through electrode dissolution into the slag was also to be expected under the applied voltage. Given the natural convection of ions, a voltage of 8 V was required for the immobilization of Mg2+ ions at the cathode’s edges, quickly resulting in a local MgO-saturation. As the thermodynamic calculations can accurately mirror the experimental findings in Figure 6, Factsage calculations were also carried out in the MgO-saturated slag at 1773 K. First deposition was predicted for a CaO concentration of app. 51 %, gradually increasing the amount of C2S, along with the reduction of silicate anions and accumulation of Ca2+ ions around the cathode. Therefore, the phase composition of deposition layer was mainly dependent on the slag composition. If the slag composition deviated the C2S primary phase field, even the CaO content exceeding up 51%, no expecting deposition layer (C2S) was observed on the cathode surface. However, a different fact was detected in the slag consisting of 10% MgO, and the deposition layer was composed of spinel even at a low CaO content. The electrochemical process was unclear and further investigation must be proceeded.


Conclusions
In the present work, the induced electro-deposition layer (C2S or MA) was achieved by cathodic polarization at an applied voltage of 8 V in different slags. The corrosion resistance of cathode was improved because of the deposition layer, whereas the anode degradation was substantially accelerated because of the damaged electrochemical reaction. The applied voltage exerted on not only the reduction of silicate anions, but also the accumulation of Ca2+ and Mg2+ ions. The reduction of silicate anions could result in a shift of slag composition to the dicalciumsilicate (C2S) or spinel (MA) primary phase field. Basic cations, such as Ca2+ and Mg2+, were most susceptible to the applied voltage, and shifted to the cathode, which led to enrichment of CaO or MgO around the cathode range. The saturated MgO and CaO content around the cathode could protect the cathode involved in basic oxides, such as MgO-matrix refractories. Conversely, the anode decay was accelerated due to the oxidation of carbon involved in refractory and the decreased interfacial tension. Therefore, the cathodic polarization was beneficial to the basic oxide refractory. It also should be noted that the present voltage level (8 V) is acceptable for the economic considerations and the slag composition also needs a careful section for the corrosion protection of MgO–C refractories.
Acknowledgments
This work was supported for the National Natural Science Foundation of China (Grant Numbers: 51604179, 51674172, 51774208 & 51704200) and Natural Science Foundation of Jiangsu Province (Grant Numbers: BK20150336 and BK20150334). Their supports enable us to complete this work.
References
[1] S.A. Nightingale, G.A. Brooks and B.J. Monaghan, Metall. Mater. Trans. B., 36 (2005) 453–461.10.1007/s11663-005-0036-xSearch in Google Scholar
[2] P. Zhang and S. Seetharaman, J. Am. Ceram. Soc., 77 (1994) 970–976.10.1111/j.1151-2916.1994.tb07254.xSearch in Google Scholar
[3] W.E. Lee and S. Zhang, Int. Mater. Rev., 40 (1999) 77–104.10.1179/095066099101528234Search in Google Scholar
[4] S.W. Li, B. Gao, S.H. Yin, G.F. Tu, et al, Appl. Surf. Sci., 357 (2015) 2004–2012.10.1016/j.apsusc.2015.09.172Search in Google Scholar
[5] X.C. Li, B.Q. Zhu and T.X. Wang, Ceram. Int., 38 (2012) 2105–2109.10.1016/j.ceramint.2011.10.049Search in Google Scholar
[6] R.K. Sauerbrey, G. Mori, C. Majcenovic and H. Harmuth, Corros. Sci., 51 (2009) 1–5.10.1016/j.corsci.2008.11.009Search in Google Scholar
[7] B.J. Monaghan, S.A. Nightingale, Q. Dong and M. Funcik, Eng., 2 (2010) 496–501.10.4236/eng.2010.27065Search in Google Scholar
[8] A.A. Kazakov, Russ. Metall., 6 (1997) 25–29.10.1007/BF02463357Search in Google Scholar
[9] D.Y. Wang, X.B. Li, H.H. Wang, Y. Min, et al, J. Non-Cryst. Solids, 358 (2012) 1196–1201.10.1016/j.jnoncrysol.2012.02.014Search in Google Scholar
[10] H. Wang, B. Glaser and S. Du, Metall. Mater Trans. B., 46 (2015) 749–757.10.1007/s11663-014-0277-7Search in Google Scholar
[11] A.A. Allanore, Nature, 5 (2013) 353–355.10.1038/nature12134Search in Google Scholar PubMed
[12] Schlackenatlas, M.B.H.Verlag, Stahleisen, Dusseldorf (1981) pp. 267.Search in Google Scholar
[13] A.A. Kazakov, Y.V. Matveev, L.A. Arykova and V.V. Ryabov, Russ. Metall., 4 (1993) 94–96.Search in Google Scholar
© 2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

