Abstract
Combustion process of bituminous coal, steam coal, anthracite (AC) and semi-coke were investigated through thermogravimetric analysis method and influence of metamorphic grade as well as heating rate on combustion characteristics were analyzed. Results show that combustion performance could not be represented by single combustion characteristic parameter. Through analysis of comprehensive combustion characteristic indexes, with increase of metamorphic grade combustion performance of coal is lowered, and combustion performance of semi-coke and AC are close to each other. With increase of heating rate, combustion curves move into high temperature region and comprehensive combustion characteristic indexes are increased, which show that the combustion performance is improved. Random pore model (RPM), unreacted shrinking core model (URCM) and volume model were used to calculate kinetic parameters of combustion process. Results show that RPM has the best performance to represent combustion process of the four samples and through calculation by RPM kinetic energy of combustion process for all samples are between 43.08 and 99.43 kJ/mol, and there is compensation effect during combustion process.
Introduction
In traditional iron and steel making process energy consumed in iron making accounts for 70% of total energy consumption and the ratio is the same for pollutant emission, so iron making process takes priority in energy conservation and emission reduction [1]. Coke in blast
furnace works as heat resource, reductant and carburizer, and at the same time in lower region of blast furnace coke is the only solid material serving as skeleton to guarantee gas permeability [2]. However, pollutant emission in the coking process is the most serious and the coking process also has disadvantages of high energy consumption and high capital expenditure, so to lower coke consumption is a continuous main goal for iron making process [3]. Pulverized coal injection is one of effective ways to lower energy consumption and pollutant emission in blast furnace iron making. For quite a long time, anthracite (AC) is the most common coal injected into blast furnace in China, but after decades of development the mixture of bituminous coal (BC) and AC is injected now to lower cost, and further for some enterprises and research units the injection of steam coal (SC) and semi coke is also proposed [4, 5, 6, 7].
Thermogravimetric analysis method has been widely used in study of pulverized coal injection in blast furnace [8, 9, 10, 11]. For thermogravimetric analysis the isothermal method focuses on reactivity for the whole combustion process and the non-isothermal method focuses on change of reactivity during combustion process. Compared with isothermal method, the non-isothermal method is program controlled heating and has advantages of less experiment work and more information [12]. He et al. [13] studied combustion characteristics and reactivity of blast furnace pulverized coal through thermogravimetric analysis method and came up with optimal proposal for Pansteel Company. Wang et al. [14] studied the combustion characteristics of brown coal, AC and their blends. It could be seen that adding brown coal could improve the combustion reactivity of coal blends, and meanwhile a double parallel reactions random pore model (RPM) provided a good prediction of the blends co-combustion process. Co-combustion of flash ash from coke dry quenching and pulverized coal were studied by Liu et al. [15] through thermogravimetric method and results showed that with increase of flash ash content in blends the ignition temperature and burnout temperature both lowered and combustion properties were improved. It could be found from above research that the thermogravimetric analysis method is an effective way to study combustion process of pulverized coal.
Four samples of normally used BC, SC, AC and semi-coke were selected from practical iron making plant, and through thermogravimetric analysis method the combustion of these samples and kinetics were investigated under different heating rate conditions. RPM [16, 17], unreacted shrinking core model (UCRM) [18], volume model (VM) [19, 20] were selected to calculate reaction kinetic parameters by linear fitting method, hoping to offer reference for blast furnace to inject more kinds of fuel.
Experimental
Raw materials
Four samples of BC, SC, AC and semi-coke (LC) were collected from work site of steel company. Before experiment samples were grinded and screened to make sure particle size smaller than 74 μm. Then samples were dried under 105°C for 4 h in drying oven to eliminate moisture. Contents of carbon, hydrogen and nitrogen were ascertained through LECO CHN600 elemental analyzer. Sulfur content was ascertained by sulfur determination device and content of oxygen was determined by subtraction method. Under standard heating rate and temperature condition contents of volatile, solid carbon and ash in samples were ascertained through LECO MAC500 coal analyzer. Proximate and ultimate analysis of different samples is shown in Table 1.
Proximate and ultimate analysis of different samples.
| Sample | Proximate analysis, wt% | Ultimate analysis, wt% | ||||||
|---|---|---|---|---|---|---|---|---|
| Vd | FCd | Ad | Cd | Hd | Nd | Od | Sd | |
| BC | 31.17 | 61.25 | 7.58 | 79.32 | 4.72 | 0.8 | 5.76 | 0.16 |
| SC | 23.35 | 66.54 | 10.11 | 70.56 | 3.9 | 0.78 | 9.34 | 0.24 |
| AC | 7.91 | 81.15 | 10.94 | 83.37 | 1.69 | 1.55 | 2.02 | 0.43 |
| LC | 5.42 | 87.29 | 7.29 | 88.98 | 0.98 | 1.08 | 2.47 | 0.41 |
Notes: FCd, Vd, Ad are mass fractions of fixed carbon, volatile content and ash, respectively; Cd, Hd, Od, Nd, Sd are mass fractions of carbon, hydrogen, oxygen, nitrogen and sulfur of different samples, respectively.
Combustion experiment
Combustion of samples was carried out through STA4009C thermogravimetric analyzer. For each experiment 5mg sample was put into Φ3mm× 1.5mm alumina crucible and rested on balance pan, at the same time keeping minimum distance between sample and thermal couple to make temperature tested with low deviation. During experiment pressed gas with flow of 100ml/min was introduced into thermal balance. Compared with so small amount of sample the gas flow is excessive, and the aim of it is to reduce the influence of external diffusion into a very low level. Non-isothermal method was chosen to investigate combustion characteristics of samples and heating rates were set as 2.5°C/min, 5°C/min, 10°C/min and 20°C/min, respectively. Samples were heated from room temperature to 900°C then keeping constant for 10 min to make sure total combustion. To ensure accuracy of experiment data each test was repeated at least for three times until differences among three tests were less than 3°C.
During experiment weight loss curve was obtained automatically by computer with frequency 1 s for one record and combustion rate curve was obtained through differentiation of weight loss data. In order to compare combustion characteristics and for kinetics analysis under different conditions the weight loss data was pretreated to get conversion ratio curve. Conversion ratio α is calculated by formula (1):
In the formula: m0 is initial sample weight, mg; m∞ is sample weight after total combustion, mg; mt is sample weight at time t, mg.
Characteristic parameters of combustion
To quantitatively analyze combustion process of different samples, characteristic parameters of Ti, Tf, Tm, Tg and Rmax were defined. The initial combustion temperature Ti, burn out temperature Tf, peak combustion rate Rmax and peak combustion rate temperature (Tm) could be obtained from TG-DTG curves as shown in Figure 1. Tg is combustion time and it is defined as Tg = Tf − Ti. The comprehensive combustion characteristic index S is determined by the equation as follow [21]:
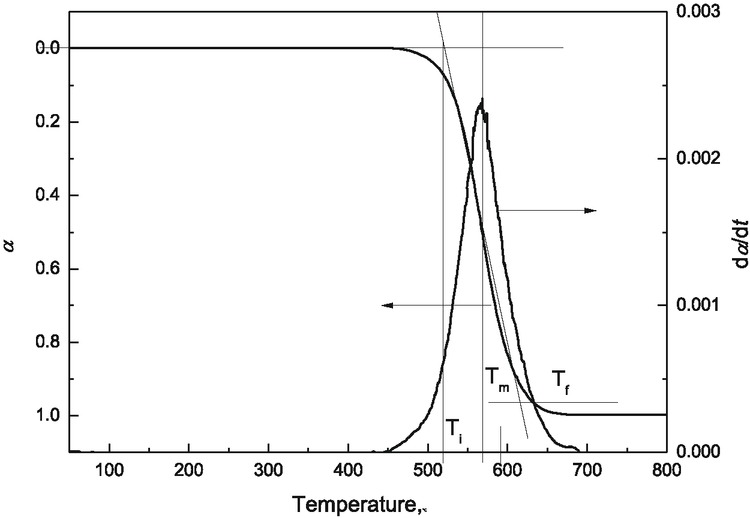
Determine characterization parameter of combustion.
where da/dtmax is the maximum combustion rate; da/dtmean represents mean value for combustion rate.
Results and discussion
Analysis of combustion curves
It could be concluded from Figure 2 that for different samples the conversion curves and conversion rate curves have similar trend, from which the total combustion process could be divided into three stages of preheating, quick combustion and burn out. In the first stage sample was heated and with temperature rising the weight of sample kept nearly constant. In the second stage, the sample weight lost quickly and the combustion of volatile and combustion of solid carbon overlapped with each other, so in conversion rate curve only one peak was presented though volatile content in different samples differed. In the third stage samples burned out. In thermogravimetric analysis device, compared with the sample amount the gas flow was surplus so it could be thought that all combustible matter like volatile and solid carbon was exhausted only with residue ash left. Through comparison of initial weight loss temperature range for different samples it could be concluded that for BC and SC the range was 300–400°C but for AC and semi-coke it was 400–500°C, and with temperature further rising quick weight loss happened. From this aspect obvious difference was existed for different samples. In order to quantitatively analyze combustion characteristics for all samples the TG-DTGmethod was used to ascertain combustion characteristic parameters including ignition temperature (Ti), burn out temperature (Tf), peak combustion rate (Rmax), peak combustion rate temperature (Tm), mean combustion rate (Rmean), comprehensive combustion index (S), combustion time (tg), and through comparison of these parameters the difference of combustion property for all samples could be obtained. Combustion characteristic parameters are shown in Table 2. From comparison of Ti for BC, SC and AC the Ti increases with increase of metamorphic grade. Ti is an important index to represent combustion property of coal and normally the lower is Ti the better is combustion property. In experiment two conditions are needed for ignition of coal, one is certain concentration of combustible matter at surface of coal particle, and the other is certain degree of superheat temperature. BC and SC has low metamorphic grade with fairly high content of volatile so in preheating process at relatively low temperature with volatilization of volatile content the concentration of combustible matter around particle surface could reach to high level and get ignition. For Tf of different samples, SC is the lowest, BC follows and AC is the highest, for which the order is different from that of Ti, and at the same time Rmax and Rmean also have the same trend. Semi-coke is produced through middle temperature pyrolysis with
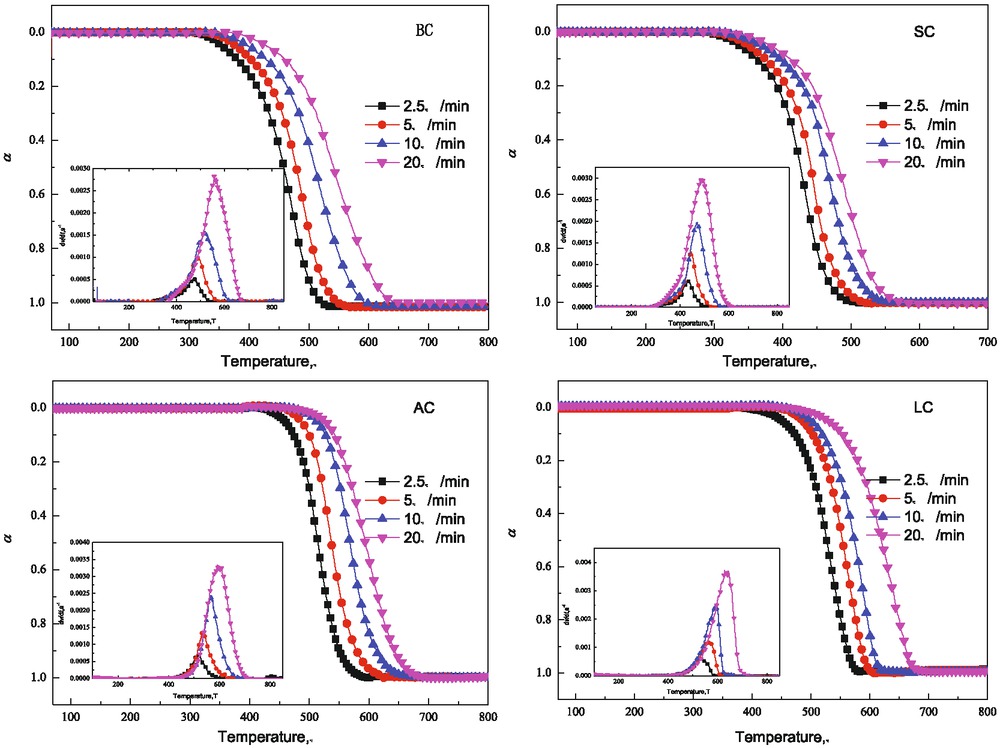
Conversion curves and conversion rate curves for different samples under different heating rates.
Combustion characteristic parameters of different samples under different heating rates.
| Samples | β, °C/min | Ti, °C | Tm, °C | Tf, °C | Rmax, s−1 | Rmean, s−1 | S × 1014 | tg, min |
|---|---|---|---|---|---|---|---|---|
| 2.5 | 319.80 | 467.02 | 519.67 | 0.53E−03 | 0.232E−03 | 0.04 | 62.35 | |
| BC | 5 | 326.55 | 488.85 | 534.02 | 0.84E−03 | 0.323E−03 | 0.36 | 31.49 |
| 10 | 349.62 | 519.67 | 590.32 | 1.58E−03 | 0.990E−03 | 0.41 | 16.87 | |
| 20 | 363.88 | 548.63 | 628.06 | 2.77E−03 | 1.030E−03 | 0.77 | 5.61 | |
| 2.5 | 337.32 | 426.00 | 475.66 | 0.60E−03 | 0.220E−03 | 0.24 | 55.34 | |
| SC | 5 | 348.10 | 441.40 | 493.39 | 1.28E−03 | 0.430E−03 | 0.92 | 29.06 |
| 10 | 368.16 | 463.96 | 520.64 | 1.98E−03 | 0.740E−03 | 2.07 | 15.25 | |
| 20 | 382.37 | 482.85 | 545.38 | 2.98E−03 | 1.180E−03 | 4.41 | 8.15 | |
| 2.5 | 722.20 | 796.44 | 839.67 | 6.60E−04 | 3.26E−04 | 0.05 | 46.99 | |
| AC | 5 | 755.76 | 822.88 | 866.10 | 1.32E−03 | 5.48E−04 | 0.15 | 22.07 |
| 10 | 772.54 | 842.19 | 882.88 | 2.36E−03 | 1.00E−03 | 0.45 | 11.03 | |
| 20 | 806.10 | 890.24 | 940.59 | 3.30E−03 | 1.41E−03 | 0.76 | 6.72 | |
| 2.5 | 724.12 | 796.44 | 839.82 | 5.70E−04 | 2.08E−04 | 0.03 | 46.28 | |
| 5 | 758.31 | 822.88 | 864.35 | 1.19E−03 | 5.08E−04 | 0.12 | 21.21 | |
| LC | 10 | 772.23 | 842.20 | 881.99 | 2.49E−03 | 9.84E−04 | 0.47 | 10.98 |
| 20 | 805.34 | 887.71 | 940.19 | 3.71E−03 | 1.57E−03 | 0.95 | 6.74 |
most of volatile content removed, so the combustion process of it is close to that of AC, which could also be concluded from combustion characteristic parameters. Combustion property of different samples could not be ascertained only through parameters of Tf, Ti, Rmax and Rmean. According to literature review the comprehensive combustion index S calculated based on above four parameters is used to represent combustion property of different samples. For S of different samples, BC has the highest value, SC follows and for AC and semi-coke the S of them are close to each other with the lowest value. This illustrates that combustion property of BC is the highest, SC follows and the combustion property of AC and semi-coke is the lowest.
For different samples through comparison of weight loss curves under different heating rates, with increase of heating rate the conversion curves and conversion rate curves all moved into high temperature range. The reason could be attributed to two aspects. One is that with increase of heating rate the combustion time in certain temperature range is shortened with less combustion weight loss and more sample remained for combustion in high temperature range. The other reason is that with increase of heating rate the temperature difference between the inside and surface of sample also increases. At the same time it could be found that with increase of heating rate the peak weight loss rate Rmax and combustion comprehensive index S both increases, and combustion time is shortened. Take BC for example when heating rate is increased from 2.5°C/min to 20°C/min, Ti is increased from 319.80°C to 363.88°C, Rmax is increased from 0.53 × 10−3 s−1 to 2.77 × 10−3 s−1, combustion time tg is shortened from 62.35 min to 5.61 min and comprehensive combustion index S is increased from 0.04 × 10−14 to 0.77 × 10−14. So it could be ascertained that with increase of heating rate the combustion rate is increased and the combustion time is shortened, which showed that the combustion property of samples were improved.
Kinetic analysis
Kinetic study of combustion process has great meaning in selection of injection coal and design of combustion device. In order to illustrate kinetic combustion behaviors of coal under different heating rates different kinetic models were chosen to analyze combustion process through nonlinear fitting method. Pyrolysis, combustion and gasification of coal are typical gas-solid multi-phase reaction. Though combustion of coal is a complicated process including evaporation of water, volatilization of volatile and combustion of residue carbon, however through analysis of weight loss curve of different samples in this paper only one peak weight loss rate is existed, so the main reaction process could also be represented by typical gas-solid reaction formula:
In the formula, k is apparent combustion rate constant, which is related with temperature and pressure; f(α) is kinetic mechanism function for combustion of coal.
During experiment the outlet of gas flow is open to atmosphere so gas pressure is constant during combustion process, so the apparent reaction rate constant k is determined by temperature, which could be performed according to Arrhenius formula:
In the formula, k0 and E are pre-exponential factor and reaction activation energy, respectively.
For combustion of coal many kinetic models have been founded by former researchers and in this paper three models are chosen to analyze combustion process, and they are RPM, URCM and VM. In different models kinetic mechanism function f(α) is different. RPM is proposed by Bhatia and Perlmutter [16, 17] and has been widely applied in study of gas-solid reaction for porous carbonaceous materials. When reaction is controlled by interface reaction the model could be expressed as:
In the formula: kRPM is reaction rate constant; Ψ is parameter for particle size and shape, with expression Ψ=4πL0(1 − ε0)/S02, S0 representing specific reaction surface area, L0 representing initial pore length in per unit volume, ε0 representing initial porosity of particle.
UCRM is proposed by Szekely and Evans [18], and during reaction process reaction starts from particle surface and gradually moves into inner particle. An unreacted core is existed and reaction could only happen at reaction interface. When particle is sphere and reaction is controlled by chemical reaction, the model could be expressed as:
In the formula: kURCM is reaction rate constant.
In VM, the reaction process is independent of particle structure and reaction happens simultaneous across the particle, and during reaction process the reaction interface decreased with reaction progressing [19, 20]. The formula could be expressed as:
In the formula, kVM is reaction rate constant.
Under program controlled heating process combustion temperature T, heating rate β and reaction time t has the following relation:
In the formula, T0 is room temperature, 25°C.
Put eq. (5) into eq. (8), and after integration the relation between conversion rate and reaction temperature could be obtained.
where C is concentration of reaction gas, and n is reaction order.
In the formula:
The same could be obtained from eqs. (6) and (7):
Eqs. (10), (11) and (12) are integration form of RPM, URCM and VM, respectively. According to study of Miura and Silveston [22], when using program controlled heating method to study gasification kinetics arbitrary result could be obtained only through single heating rate, and at least three groups of experiment data under different heating rates could guarantee accurate and unique result. So in this study four different heating rates were chosen to calculate combustion kinetic parameters. Using the relation of α and T from eqs. (10), (11) and (12), the kinetic parameters k0 and E can be calculated by nonlinear fitting method.
Different models are established based on different assumptions, and when different models are used to represent combustion process of different samples deviation between calculated value and experiment data are inevitable. In order to accurately determine the description of the coal char and biomass char combustion kinetics with different kinetic models eq. (13) can be used to calculate the deviation of the results for different models.
where DEV(α)(%) is relative error, αexp , i, αcalc, i is the experimental data and calculated value by model. max (α)exp is maximum conversion rate of experiment, N is the number of experimental points.
Combustion kinetics of samples were investigated through RPM, URCM and VM models, and according to eqs. (10), (11) and (12) kinetic parameters E, k0 and ψ were calculated and shown in Table 3. From Table 3 it could be found that the fitting performance of these models are all in good level with R2 over 0.9600, and among them RPM has the best performance with R2 all over 0.9900 which is higher than that of URCM and UM. When using RPM to simulate reaction process the coefficient ψ has significant influence. When sample porosity is high gas diffusion would become easy in combustion process, and if reaction happens simultaneously across the particle to certain level the reaction process would become close to VM. With increase of ψ, particle porosity becomes low and resistance for gas to get into inner particle becomes strong, and at this situation combustion reaction happens in surface of particle and the process would become close to URCM. Volatile content in BC and SC is high and in combustion process large amount of volatilization happens both inside and outside leading to production of high porosity in particle, and it could be found in Table 3 that ψ of them is small. When ψ is 0 RPM and VM are the same and this is the reason for activation energy calculated from them is close to each other. For different models the changing rule of activation energy for different samples is the same, and from high to low the order is BC, semi-coke, AC and SC, and activation energy calculated by RMP are 99.43, 57.13, 54.06 and 43.08 kJ/mol, respectively. Normally for reaction with high activation energy high temperature is needed to activate molecules into active state, so at the same temperature sample with high activation energy should have low reactivity.
Kinetic parameters under different heating rates for RPM, URCM and VM.
| Sample | RPM | URCM | VM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E, kJ/mol | k0, min−1 | ψ | R2 | E, kJ/mol | k0, min−1 | R2 | E, kJ/mol | k0, min−1 | R2 | |
| BC | 99.4 | 832.05 | 3.05 | 0.9978 | 75.8 | 95.79 | 0.9922 | 105.9 | 2732.23 | 0.9977 |
| SC | 43.1 | 19.77 | 0.01 | 0.9985 | 40.1 | 17.98 | 0.9893 | 43.1 | 47.70 | 0.9985 |
| AC | 54.1 | 23.43 | 26.72 | 0.9937 | 54.5 | 39.36 | 0.9851 | 64.7 | 453.49 | 0.9861 |
| LC | 57.1 | 40.06 | 14.69 | 0.9925 | 57.6 | 56.36 | 0.9622 | 66.3 | 460.85 | 0.9853 |
However through comparison of relation between reaction rate and activation energy for different samples it could be found in this study the rule is abnormal because BC has the highest reactivity but its activation energy is also the highest. So there must be compensation effect during combustion process.
For reaction with compensation effect, the activation energy E is contained in pre-exponential factor A0 and exponential term of Arrhenius formula. Compensation effect is often observed in gasification and combustion process of carbonaceous materials. Some researchers thought the compensation effect was purely attributed to mathematical reasons, while others gave several theoretical interpretations for it. Xie et al. [23] thought compensation effect could be attributed to similar mechanism with gasification reaction, especially for the oxygen-containing surface complexes C(O). For reactions with lower E values, it is easier for the free active sites to bond with CO2 to produce C(O). If the C(O) bond is strong, the movement of C(O) would be limited and the char structure would be stable, leading to low value of A0. Activation energy is the minimum energy needed for molecule to get active in reaction and pre-exponential factor is the number of molecules with effective collision. Increase of activation energy make reaction become hard to happen but increase of pre-exponential factor would quicken reaction rate, so there is compensation effect between pre-exponential factor and activation energy. In recent years many theoretical explanations have been done to compensation effect. Through study of Sosnovski [24], in reaction process compensation effect is caused by existing of two kinds of matter with different activation energy. Through work of Galway et al. [25] compensation effect is due to non-uniformity of surface. Through work of Ding et al. [26, 27], unstable transition phase is formed during gasification and combustion of coal. In initial stage of reaction carbon oxide is first formed, because in surface of coal active site is easy to react with gasification agent so activation energy to form carbon oxide is small. At the same time connection between gasification agent and coal is strong and the formed carbon oxide is hard to move presenting low activation entropy so pre-exponential factor is lowered with decrease of activation energy. Through different models, relationship between activation energy and pre-exponential factor for different samples is shown in Figure 3 and obvious compensation effect could be observed.
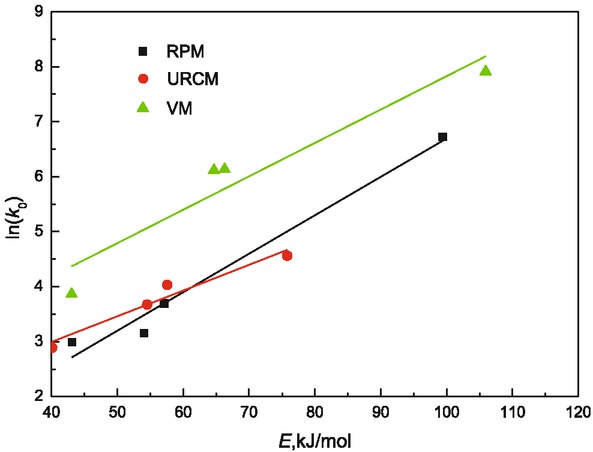
Relation between ln(k0) and E.
Figure 4 shows the relationship between the calculated values and experimental data of different samples at the different heating rates. In order to quantify the errors produced by the kinetic models in predicting the values of conversion, the experimental and calculated α values are compared by calculating the deviation (DEV) between the experimental data and calculated curves using eq. (13). The results obtained from the best fitting models for all samples are summarized in Table 4. The lowest deviation from the calculated values of the conversion rates are obtained by using the RPM model for all samples and VM model for SC sample can obtain the same value as RPM model. Meanwhile, what needs to be pointed out is that experiments in this study are carried out under laboratory low heating rate condition which is significantly different from working state in blast furnace raceway, but theoretical reference could also be offered for optimization of coal selection and simulation study.
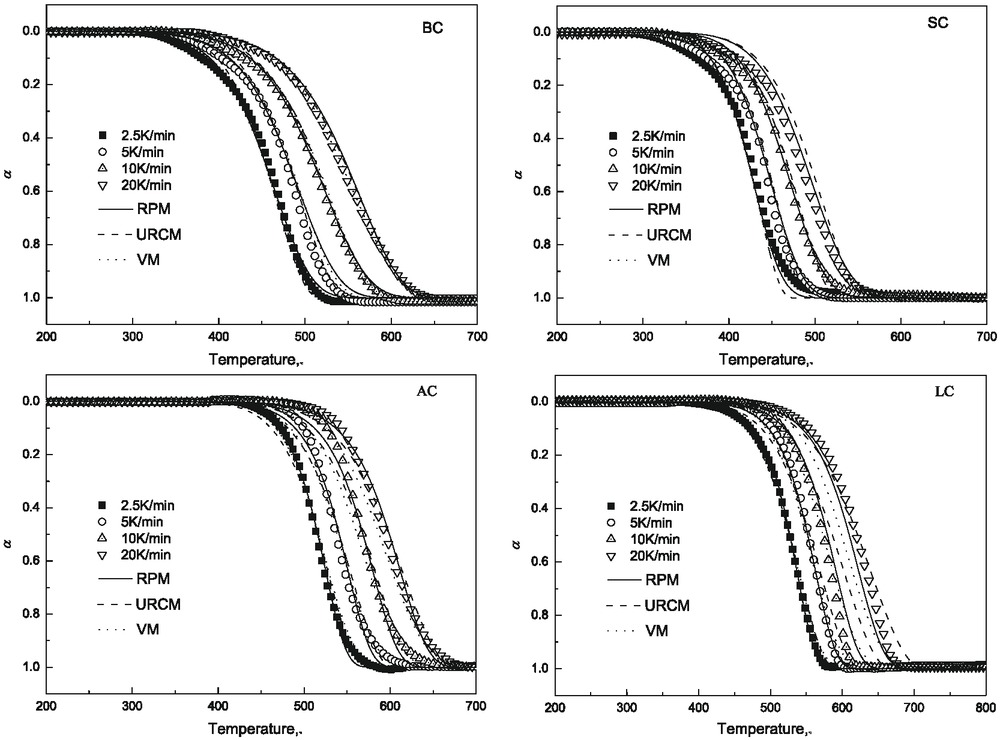
Relationship between calculated value and experimental data under different heating rates.
Deviation between the experimental and calculated conversion.
| Char | DEV (α)% | ||
|---|---|---|---|
| RPM | URCM | VM | |
| BC | 1.32 | 1.87 | 1.36 |
| SC | 1.18 | 2.15 | 1.18 |
| AC | 1.65 | 2.43 | 2.27 |
| LC | 1.97 | 3.67 | 2.49 |
Conclusions
Non isothermal thermogravimetric analysis method was used to investigate combustion behaviors and kinetics of pulverized coal with different metamorphic grades and following conclusions are obtained:
Combustion property of coal is lowered with increase of metamorphic grade and combustion characteristics of semi-coke and BC are close to each other. Heating rate has obvious influence on combustion process and with increase of heating rate combustion process moves into high temperature region with higher combustion rate and shorter combustion time.
Combustion process of different samples could all be presented by RPM, URCM and VM with good accuracy. For BC, AC and semi-coke model calculation and experiment data has the best fitting performance by RPM. For SC the fitting performance of RPM and VM are close to each other.
Through RPM the calculated activation energy for BC, SC, AC and semi-coke are 99.43, 43.08, 54.06 and 57.13 kJ/mol, respectively, and there is no relevance between activation energy and combustion property which could be explained by existing of kinetics compensation effect between activation energy and pre-exponential factor.
Acknowledgements
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20125314110011), Grant of National Natural Science Foundation (Grant No. 51004056) and Foundation Research Project of Yunnan Province (2014FA024).
References
[1] R.S. Xu, J.L. Zhang, G.W. Wang, T.F. Song and H.Y. Wang, J. Chongqing Univ., 38 (2015) 1–9.Search in Google Scholar
[2] G.W. Wang, J.L. Zhang, G.H. Zhang, H.Y. Wang and D. Zhao, ISIJ Int., 57 (2017) 1373–1382.10.2355/isijinternational.ISIJINT-2016-630Search in Google Scholar
[3] G.W. Wang, J.L. Zhang, J.G. Shao, Z.J. Liu, G.H. Zhang, T. Xu, J. Guo, H.Y. Wang, R.S. Xu and H. Lin, Energy Convers. Manage., 124 (2016) 414–426.10.1016/j.enconman.2016.07.045Search in Google Scholar
[4] Y.Z. Wang, C.M. Lu and H.T. Liu, J. Shandong Univ., 36 (2006) 26–31.Search in Google Scholar
[5] G.W. Wang, J.L. Zhang, J.G. Shao, Z.J. Liu, H.Y. Wang, X.Y. Li, P.C. Zhang, W.W. Geng and G.H. Zhang, Energy, 114 (2014) 143–154.10.1016/j.energy.2016.08.002Search in Google Scholar
[6] P. Wang, G.W. Wang, J.L. Zhang, J.Y. Lee, Y.J. Li and C. Wang, Appl. Therm. Eng., 137 (2018) 678–688.10.1016/j.applthermaleng.2018.04.026Search in Google Scholar
[7] G.W. Wang, J.L. Zhang, H.B. Zuo and Q.H. Pang, Energ Metall. Ind., 33 (2014) 49–53.Search in Google Scholar
[8] G.W. Wang, J.L. Zhang, J.G. Shao and S. Ren, Thermochim. Acta., 591 (2014) 68–74.10.1016/j.tca.2014.07.019Search in Google Scholar
[9] G.W. Wang, J.L. Zhang, J.G. Shao, H. Sun and H.B. Zuo, J. Iron Steel Res. Int., 21 (2014) 897–904.10.1016/S1006-706X(14)60159-XSearch in Google Scholar
[10] G.W. Wang, J.L. Zhang, X.M. Hou, J.G. Shao and W.W. Geng, Bioresour. Technol., 177 (2014) 66–73.10.1016/j.biortech.2014.11.063Search in Google Scholar PubMed
[11] S.P. Zou, Y.L. Wu, M.D. Yang, C. Li and J.M. Tong, Bioresour. Technol., 101 (2010) 359–365.10.1016/j.biortech.2009.08.020Search in Google Scholar PubMed
[12] Z.J. Zhou, X.L. Fan, W. Zhang, F.C. Wang and Z.H. Yu, J. China Coal Soc., 31 (2006) 219–220.Search in Google Scholar
[13] X.J. He, J.L. Zhang, C.L. Qi, Y.C. Wen and W.G. Fu, Chin. J. Process Eng., 11 (2011) 951–957.Search in Google Scholar
[14] H.Y. Wang, J.L. Zhang, G.W. Wang, R.S. Xu, P.C. Zhang, S.Y. Liu and T.F. Song, J. Therm. Anal Calorim., 126 (2016) 447–454.10.1007/s10973-016-5557-9Search in Google Scholar
[15] G.Q. Liu, Q.C. Liu and L. Yao, Chin. J. Process Eng., 15 (2015) 272–276.10.1016/j.powtec.2014.12.015Search in Google Scholar
[16] S.K. Bhatia and D.D. Perlmutter, AIChE J., 26 (1980) 379–385.10.1002/aic.690260308Search in Google Scholar
[17] G.W. Wang, J.L. Zhang, G.H. Zhang, X.J. Ning, X.Y. Li, Z.J. Liu and J. Guo, Energy, 131 (2017) 27–40.10.1016/j.energy.2017.05.023Search in Google Scholar
[18] J. Ochoa, M.C. Cassanello, P.R. Bonelli and A.L. Cukierman, Fuel Process Technol., 74 (2001) 161–176.10.1016/S0378-3820(01)00235-1Search in Google Scholar
[19] S. Kasaoka, Y. Sakata and S. Kayano, Int. Chem. Eng., 25 (1984) 160–175.Search in Google Scholar
[20] G.W. Wang, J.L. Zhang, W.W. Chang, R.P. Li, Y.J. Li and C. Wang, Energy, 147 (2018) 25–35.10.1016/j.energy.2018.01.025Search in Google Scholar
[21] Y.G. Xu, C. Zhang, Y.H. Duan, J.J. Yin and G. Chen, Asia-Pacific J. Chem. Eng., 9 (2010) 435–440.Search in Google Scholar
[22] K. Miura and P.L. Silveston, Energy Fuels, 6 (1989) 243–249.10.1021/ef00014a020Search in Google Scholar
[23] K.C. Xie, Coal Structure and Its Reactivity, Science Publishing House, Beijing (2002).Search in Google Scholar
[24] H.H. Sosnovski, J. Phys. Chem. Solids., 10 (1959) 304–310.10.1016/0022-3697(59)90005-8Search in Google Scholar
[25] A.K. Galway and M.E. Brown, J. Catal., 60 (1979) 335–338.10.1016/0021-9517(79)90154-4Search in Google Scholar
[26] H. Ding, Y. Jiang, Y.F. Chen and W.H. Li, J. China Coal Soc., 35 (2010) 3–4.Search in Google Scholar
[27] R.G. Yu, Y. Chen, C. Lin and J.Y. Zhang, J. Combust. Sci. Technol., 18 (2012) 85–89.Search in Google Scholar
© 2019 Yang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

