Abstract
Chromium carbide (Cr3C2) coatings on the diamond were fabricated using the mixtures of molten salt of NaCl and BaCl2, powders of metallic chromium and diamond as raw materials in the temperature range of 750–900 °C by the microwave-assisted molten-salt synthesis. The morphology, microstructure and phase composition of the surface layer were characterized by Scanning Electron Microscopy, Energy-dispersive Spectrometry and X-ray diffraction. The results show that the surface of the diamond particles could be coated with Cr, forming a uniform and continuous Cr3C2-coated layer. The coatings thickness on the diamond can be controlled by adjusting the heating temperature and time. The coating thickness expanded from 0.73 to 2.30 µm when the temperature was increased from 750 °C to 900 °C, and increasing rapidly during the temperature range of 800 °C–850 °C, the coating thickness expanded from 1.30 to 2.80 µm when the holding time was increased from 0.5 h to 4 h. The results illustrate that the microwave-assisted molten salt synthesis plays a positive role in chromium coatings on the diamond, offering a potent method for the surface metallization of diamond.
Introduction
For the diamond compacts, diamond particles are usually bonded with substrates using a binder. In order to increase the bonding strength of the diamond particles, it is useful to coat them with a material having high affinity to both of the diamond and the binder [1, 2]. The transition metal carbides have properties of very high melting points (~1727–3727 °C), high hardness (1200–3000 kg/mm2), high elastic modulus (300–700 GPa), good heat and electrical conductivity, and anti-erosion and corrosion, making them widely being used in a variety of industrial applications. Metal carbides coatings can extend the lives of the materials by allowing mechanical properties of the substrate materials to be maintained while protecting them against wear or corrosion. The coating can protect the diamond particles from graphitization at high temperature and promote the interfacial bonding without deteriorating the thermal conductivity of the metal matrix. Metal carbides can be prepared by using chemical vapor deposition, physical vapor deposition, precipitation of salts containing metal, carbon and oxygen followed by reduction and annealing, mechanical alloying and high energy milling from mixed powders of metal and carbon, etc [3, 4].
Thermal treatments have been proposed to coat the diamond particles with carbides. In the treatments, the diamond particles react with elemental substances or oxides at a higher temperature, resulting in the surface of the diamond being coated with the carbides generated from the reaction [2, 5, 6]. However, diamond and metals are chemically incompatible, it is very difficult to make the diamond to be soaked by metal substrate, resulting in a lower adhesive strength between the diamond particles and the metal support, and a loss of diamond particles from the metal support during the grinding and cutting [7, 8]. It is well established that the diamond surface metallization is an effective method to improve the adhesive properties between diamond and surface carbide layer [9].
In order to improve the adhesive force between the metal layer and the diamond, several diamond surface metallization approaches, such as dc magnetron sputtering technique, electroless plating, electroplating, vacuum micro-deposition technology, vacuum plating, and salt bath plating, have been reported in recent years [5, 9, 10, 11, 12]. Active elements, such as Cr, B, Ti, Mo, and W, are coated on the surface of diamond particles before sintering and infiltration to improve the interface bonding property diamond/metal composites [2, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. These metal elements can act as middle layers that strengthen the interface and protect the diamond powder from the atmosphere and reduce the degree of graphitization at high temperatures. However, the conventional powder metallurgy methods still have drawbacks of longer sintering time, higher temperature and higher energy consumption, which will produce defects in the diamond structure or form excessive interface products of a chemical reaction between the coating elements and diamond or matrix, causing a significant decrease of thermal conductivity of diamond or matrix, or even resulting in the reduced thermal conductivity of the composite.
Molten salt synthesis is now a well-established low-temperature technique, attracting increasing interest in recent years, in which the molten salts (e. g. alkali chlorides and sulphates) are utilized as a solvent or reacting species, or sometimes both, has been demonstrated to be one of the simplest methods to prepare pure and stoichiometric powders of multicomponent oxides. Since the diffusion rate of the components in molten salts are much higher than those in the solid state reaction, various oxide powders and the transition metal carbides can be prepared at relatively lower temperatures (about 950 °C compared to the more typical synthesis temperature of 1400–1700 °C for carbothermic reduction) and shorter reaction times ( about 1 h compared to the more typical synthesis time of over 4 h for carbothermic reduction) by the molten salt synthesis method. The salt bath plating by the molten salt method not only can form a good combined layer, but also can control the thickness of coating by adjusting the plating temperature and holding time, having advantages of lower energy consumption, and lower cost [2, 22, 24, 25, 26, 27, 28].
Microwave heating has gained growing attention during the past decade, a number of potential applications of microwave heating in different fields have been thoroughly reviewed [29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]. It is well-established that intrinsic advantages in utilizing microwave technologies for processing materials compared with conventional heating include penetrating radiation, controlled electric field distribution, and selective and volumetric heating due to the difference of heating mechanism between them. For example, microwave sintering technology has unique attributes of higher heating rate, shorter processing time, it is possible to reduce internal stresses, which contribute to cracking of parts during sintering, and to create a more uniform microstructure, resulting in the improved mechanical properties and reliability by eliminating temperature gradients, and has been developed as a new technique for controlling microstructure to improve the properties of sintered materials, which has been widely used in many fields [33, 35, 38, 39, 40].
Crystalline Cr3C2 has wide applications in coatings and structural reinforcement, attracting wide research due to its excellent properties of high melting point, hardness, strength, toughness, chemical stability, and corrosion resistance [41]. The chromium carbide was usually synthesized by using the carbon thermal reduction method using chromium oxide and carbon powders as raw materials. For chromium carbide produced in the molten salt, Dai et al. [42] investigated the production of nano-sized chromium carbide powders from Cr2O3/C precursors by direct electrochemical reduction in molten calcium chloride. The results showed that the single phase Cr7C3 powders with a mean diameter of<200nm were prepared after electrolysis at 2.8 V for 5 h in 850 °C CaCl2 melt. For chromium carbide fabricated by microwave processing, Zhao et al. [43] and Zhao and Hu [44] reported the synthesis of chromium carbide nanopowders from nanometer Cr2O3 and nanometer carbon black by microwave heating technique, and using micron-sized chromic oxide (Cr2O3) and nano-sized carbon black as raw materials via mechanical alloying assisted microwave heating route, concluding that the synthesis temperature and time required by the microwave heating method is at least 400 °C lower and 3 h shorter than those required by the conventional approaches for preparing chromium carbide, making the distribution of particle size more uniform, Gunnewiek and Kiminami [45] reported the fast synthesis of porous chromium carbide by microwave-assisted carbothermal reduction from Cr2O3 and carbon black in only 20 min of reaction time, further proved the advantages of microwave heating for the preparation of chromium carbide coatings.
Although the copper-diamond composites with chromium carbide coatings on diamond particles by vacuum pressure infiltration, spark plasma sintering technique, or conventional sintering method [12, 17, 46, 47], silicon carbide coating on the diamond particles [48], TiC coating on the diamond particles [20] by microwave heating have been reported; and silicon carbide (SiC)-coated graphite composite powders was synthesized using graphite flakes and silicon powders as raw materials by the microwave-assisted molten salt synthesis [49], there has been little discussion about the chromium carbide coatings on the diamond particles by microwave sintering process in a molten salt mixture.
Herein, on the premise of making full use of the advantages of microwave sintering and molten salt synthesis mentioned above, a facile microwave-assisted molten salts synthesis was developed for the low-temperature preparation of chromium carbide coatings on the diamond particles. In order to evaluate the effects of plating temperature and time on the coating thickness, the Cr/diamond samples were heated at temperatures of 750, 800, 850, and 900 °C for 1 h, respectively, and also heated at 850 °C for 0.5 h, 2 h, and 4 h, respectively. Scanning Electron Microscopy (SEM), Energy-dispersive Spectrometry (EDS), X-ray diffraction (XRD) was adopted to analyze the morphology, microstructure and phase composition of the surface layer.
Experimental
Materials and apparatus
Diamonds with average diameters of 125 µm (Henan Huanghe Whirlwind CO., LTD.), Chromium powder (reagent grade, 99 % pure, Reagent Chemicals Co., Ltd Tianjin.) NaCl (reagent grade, Reagent Chemicals Co., Ltd Tianjin.) and BaCl2 (reagent grade, Reagent Chemicals Co., Ltd Tianjin.) were used for the salt molten coating process by microwave and conventional heating. Figure 1 shows an SEM image of the uncoated diamond.

SEM micrographs of uncoated diamond.
High temperature microwave furnace (HAMiLab-V3000, SYNOTHERM Co., Ltd Changsha, China) was used for heating the material to the salt-melt temperature, and the Multimode cavity of high temperature microwave furnace with 2.45 GHz of maximum 3 kW output power was illustrated in Figure 2, which was cooled via water circulating in the double wall. SiC slice was used to increase the overall heating rate in the microwave cavity. Dilute hydrochloric acid (reagent grade, Reagent Chemicals Co., Ltd Tianjin.) and Acetone (reagent grade, Xin Guan Chemical Co., Ltd Wei Fang) were used for washing the diamond. Thermostatic water bath (HH-1, Hangzhou David Scientific Instrument Co., Ltd.) and Ultrasonic cleaning (PS-1001T, Hefei climbed Ultrasonic Technology Co., Ltd ) were used for removing excess powder and dissolving the molten plating

The cavity of high temperature microwave furnace (1-Rotating stage, 2-Loading ring, 3-Alumina insulating sheath, 4-SiC slice, 5-Corundum crucible, 6-Quartz glass, 7-microwave generator, 8-Infrared pyrometer, 9-Samples).
Pretreatment of diamond
Before the diamond deposition, the pretreatment of the diamond was carried out to increase the surface activity The diamonds were immersed in dilute hydrochloric acid for half an hour, and then cleaned with acetone to remove the remaining wax, catalysts and other impurities, and then washed with distilled water two times and dried in digital blast oven for obtaining the suitable experimental samples
Preparation of chromium carbide coating on diamond powders
The diamond coating was prepared by heating the well-blended mixtures of 20 g molten salt of NaCl and BaCl2 (1:1), 10 g metallic chromium and 10 g diamond powder by a microwave furnace at the heating rate about 30℃/min The chromium was embedded in the salt mixture, because this procedure was assumed to be protective against a reaction of chromium with the oxygen in the atmosphere. The gaseous precursor chosen for diamond coating was a gas mixture of Ar, H2 (volume ratio: Ar 85%, H215%). The flow rates of Ar and H2 were 1700 sccm (standard cm3 min−1) and 300 sccm, respectively. The coating pressure was maintained at the standard atmospheric pressure. The coating temperature was determined by an infrared pyrometer (Optris IRtec 10:1 M, Germany). In order to study the effects of plating temperature and time on the coating thickness, the Cr/diamond samples were heated at temperatures of 750, 800, 850, and 900 °C for 1 h, respectively, and also heated at a temperature of 850 °C for 0.5 h, 2 h, 4 h, respectively.
Characterization of diamond coatings
Scanning electron microscope(XL30 ESEM-TMP, Dutch Philips CO., LTD.) was used to characterize the surface and cross-sectional morphologies of the diamond coatings. An energy dispersive X-ray spectrometer (EDS) (PHOENIX, American EDAX CO., LTD.) was utilized to perform Microanalysis of the selected areas of the samples.
The diamond coatings were analyzed with an X-ray diffraction (XRD, Germany BRUKER CO., LTD) apparatus at a grazing angle of 2°(scan step size: 0.02°). The cross-sectional material component analysis was determined by EDS Liner Scanning (PHOENIX, American EDAX CO., LTD.).
Results and discussions
Heating chromium powder by microwaves
The microwave coupling capability of materials depends on their dielectric properties: coupling increases when the dielectric loss is more significant [36]. Theoretically, bulk metals with higher conductivity will reflect microwaves or microwaves cause sparking of metallic materials at low temperature, which cannot be effectively heated by microwaves, however, it is well established that metal powders can be readily heated by microwaves [40]. Therefore, the microwave absorption ability of the chromium powders and the temperature rising curve of chromium powders were investigated. Figure 3 shows the temperature rising curve of chromium powders heated by microwaves, illustrating that the chromium powders can be heated up rapidly. The temperature of the chromium powders increases slowly from 0 °C to 600 °C in about 300 s, while increasing rapidly from 600 °C to 1000 °C in just 100s, suggesting that 600 °C is the critical point for chromium powders absorbing the microwave energy. The results demonstrate that it is feasible to coat diamond with chromium in a molten salt mixture heated by microwaves.

The temperature rising curve of chromium powder heating in the 2.45 GHz microwave furnace.
Coating diamond with chromium in a molten salt mixture by microwave heating
The Cr-coated diamond was prepared by using the well-blended mixtures of 20 g molten salt of NaCl and BaCl2 (1:1), 10 g metallic chromium and 10 g diamond powder as raw materials heated by microwaves at the heating rate about 30 °C /min. Figure 4 exhibits the SEM micrographs with and without Cr coating on diamond surfaces. It can be seen in Figure 4(a) that the surface of the uncoated diamond was clean and smooth. In contrast, it was observed in Figure 4(b) that the surface of the coated diamond was rough and a large amount of residues was retained, which confirms that chemical reactions occurred at the diamond interface. Compared with the transparent yellow color of the uncoated synthetic diamond, the color of coated diamond is opaque dark gray after the formation of Cr layer. The Cr coating is uniform and macroscopically homogeneous on the surface of diamond via the molten salt technique by microwave heating. It can be observed that the diamond particles still keep their initial shapes (regular polyhedrons) after coating deposition and is not damaged by high temperature burning. The surface defects of uncoated diamond such as stairs, pits, cracks are repaired during the salt-melting deposition under microwave heating.

SEM micrographs of diamonds: (a) without coating and (b) with Cr coating.
Figure 5 shows the EDS of Cr-coated diamond by microwave heating at the temperature of 850 °C for 1 h, indicating that the surface coating mainly contains chromium and carbon and the ratio of the two atoms is close to 3:2, and suggesting that the elemental Cr in the surface layer has been reacted to form the Cr3C2 phases. It is well documented that chromium carbides show complex structures, presenting three stable phases: cubic Cr23C6, two orthorhombic Cr7C3 and Cr3C2 [3, 50]. Cr3C2 is the most stable transition metal carbide exhibiting high hardness, good strength, high Young’s modulus, high corrosion and erosion properties, good chemical stability and high oxidation resistance [41].

The EDS of coated diamond by microwave heating at the temperature of 850 °C for 1 h.
Figure 6 shows the XRD pattern of the Cr-coated diamond under microwave heating at a temperature of 850 °C for 1 h. The patterns contain peaks corresponding to diamond and chromium carbide, indicating that the coating on the diamond particles exhibits high purity by microwave heating at the temperature of 850 °C under the argon-hydrogen atmosphere. Meanwhile, Cr3C2 are formed on the surface of diamond particles, revealing that interfacial chemical reaction between Cr and C elements has occurred during the microwave salt-bath process.

X-ray diffraction pattern of coated diamond by microwave heating at the temperature of 850 °C for 1 h.
Based on the above mentioned XRD, EDS, and SEM analyses, the result indicates that the surface of diamond particles could be successfully coated with Cr using microwave-assisted molten-salt synthesis method, and Cr2C3 was generated during coating simultaneously. The present result is almost similar to the result of the copper-diamond composites with chromium carbide coatings on the diamond particles by vacuum pressure infiltration technique, the coatings on the diamond particles were Cr7C3, which were formed with a reaction medium of chromium in mixed molten salt of NaCl and KCl (1:1) [6], and were in good agreement with the result of Cr2C3 prepared from nanometer Cr2O3 and nanometer carbon black by microwave heating technique, in which chromium carbide nanopowders were prepared by a microwave heating method at 1000 °C for 1 h [43, 44].
Molten salt investigation revealed that transport reactions occur because metals dissociated to mobile cations and delocalized electrons, a state which is considered to be intermediate between ionic and metallic, facilitating the dissolution and transport of the chromium, and hence the formation of the chromium carbide coatings, through the diffusion of chromium cations from the molten salt to the surface of the diamond particles with subsequent reaction, demonstrating that the chemical reaction in molten salt system was easier and more uniform [25, 42]. Prior research showed that the advantages of salt bath plating are simple to operate, easy to control the coating thickness, and carbide layer can be directly formed on the diamond surface uniform and compact.
Meanwhile, the non-thermal effects of microwave show that the electromagnetic fields can reduce sintering activation energy of materials, improve diffusion of grain boundary and bring an extra driving force for mass transport, which result in low sintering temperature and short sintering time required for full densification of materials. Therefore, the microwave sintering is an energy and time saving sintering technology, and can be used to fabricate the materials with fine grains and homogeneous microstructure [33, 38, 39]. For the present study discussed above, Cr powders can absorb the microwaves, accelerating the motion and distribution of Cr metal and resulting in the coatings more uniform and compact [40]. Furthermore, it has been demonstrated that Cr3C2 can also couple effectively with microwaves [43, 44], which results in a more uniform temperature gradient within the billet, making the carbide layer formed on the diamond surface more uniform and compact.
Figure 7 illustrates the SEM images of Cr-coated diamond by (a) microwave heating and (b) conventional heating at the temperature of 850 °C for 1 h. It can be found in Figure 7(a) that the diamond plating by microwave heating is more uniform and homogeneous than that of conventional heating (Figure 7(b)), illustrating that the chromium carbide coating can be synthesized at 850 °C for 1 h by the microwave-assisted molten-salt synthesis. The reaction temperature of the present study was about 150 °C lower than the work reported by Zhao et al [43]., in which chromium carbide nanopowders were prepared by a microwave heating method at a temperature of 1000 °C for 1 h, highlighting the advantages of microwave-assisted molten-salt synthesis method developed in the present study.

The SEM images of coated diamond by (a) microwave heating and (b) conventional heating at temperature of 850 °C for 1 h.
Coating thickness of diamond by microwave heating
In order to evaluate the effects of heating temperature and time on the coating thickness, the Cr/diamond samples were heated at temperatures of 750, 800, 850, and 900 °C for 1 h, respectively, and also heated at a temperature of 850 °C for 0.5 h, 2 h, 4 h, respectively. Figure 8. shows the coating thickness of SEM images by microwave heating at different temperatures of 750, 800, 850, and 900 °C for 1 h, and Figure 9 shows the coating thickness of SEM images by microwave heating at the temperature of 850 °C for different time (a) 0.5 h, (b) 2 h, and (c) 4 h. It can be seen in Figure 9(a) that the coating adheres to the surface of the diamond which confirms a strong interfacial bonding between diamond, while it appeared obviously cracking phenomenon in Figure 9(c), because too thick coating has large thermal stress during the cooling process.

The coating thickness of SEM images by microwave heating at different temperatures for 1 h.
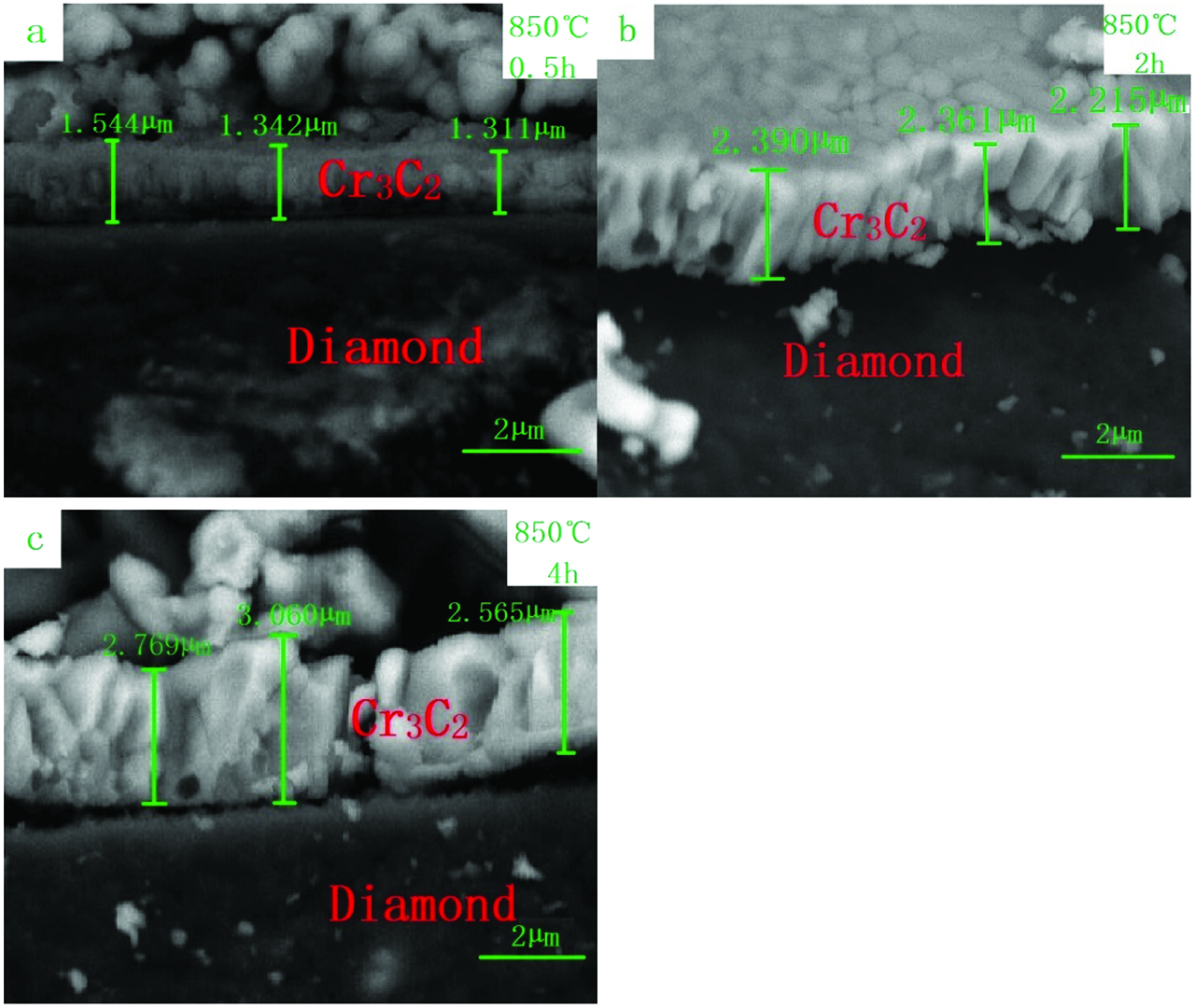
The coating thickness of SEM images by microwave heating at temperature of 850 °C for different time (a) 0.5 h, (b) 2 h and (c) 4 h.
Figure 10(a) exhibits the relationship between coating thickness and plating temperature, which indicated the interlayer expanded from 0.73 to 2.30 µm, when the temperature was increased from 750 °C to 900 °C, suggesting that the formation of Cr3C2 interface species could be promoted by the heating time. Moreover, the coating thickness increased rapidly in the temperature range of 800 ~850 °C, because the interface chemical reaction and the formation of Cr3C2 species were greatly affected by temperature in this range.

The relationship between coating thickness and (a) plating temperature and (b) holding time.
In order to reveal the effect of heating time on the coating thickness, the samples were heated by microwaves at a temperature of 850 °C for 0.5 h, 1 h, 2 h and 4 h (Figure 10(b)). Figure 10(b) shows the relationship between coating thickness and holding time, which exhibits that the coating thickness expanded from 1.30 to 2.80 µm when the holding time was increased from 0.5 to 4 h. In addition, when thick carbide film formed on diamonds, the diffusion of Cr atoms goes through the carbide film became difficult, resulting in the coating thickness increased more and more slowly with the increasing of holding time. Chromium coating on diamond belongs to solid - solid phase reaction and the reaction rates are affected by the diffusion rate of chromium atoms going through a phase [6, 46].
The results demonstrated that the plating temperature and time had substantial impact on the thickness of Cr coating, suggesting the thickness of coating on the diamond by microwave heating can be controlled by adjusting the heating temperature and holding time.
Compared with the methods of carbothermic reduction or via molten-salt synthesis by conventional heating, the microwave-assisted molten-salt synthesis has the advantages of lower reaction temperature and shorter reaction time. In a word, the chromium carbide (Cr3C2) coatings on the diamond particles can be prepared by making full use of the advantages of microwave heating and molten salt synthesis.
Conclusions
A continuous chromium coating on the diamond was achieved using a molten salt technique by microwave heating in the temperature range of 750–900 °C. The thickness of coating on the diamond by microwave heating can be controlled by adjusting the heating temperature and holding time. The interlayer expands from 0.73 to 2.30 µm, when the annealing temperature increases from 750 °C to 900 °C, the coating thickness increases rapidly during the temperature range of 800~850 °C, because the interface chemical reaction and the formation of Cr3C2 species are greatly affected during this temperature range, while, the coating thickness expands from 1.30 to 2.80 µm when the holding time increases from 0.5 to 4 h. The results suggest that the molten salt synthesis by microwave heating maybe a technically viable option for the diamond surface metallization.
Acknowledgements
The authors would like to express their gratitude for the financial supports of the National Natural Science Foundation of China (Grant No. 51204081); the Joint Fund of National Natural Science Foundation of China and Yunnan (Grant No. U1502273); Yunnan Provincial Science and Technology Innovation Talents scheme - Technological Leading Talent of China (NO. 2013HA002); Applied Basic Research Project of Yunnan Province of China (Grant No. 2013FZ008), Yunnan Provincial Department of Education Research Fund of China (Grant No. 2013Z118) and the State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization,Kunming 650093 (Grant No. CNMRCUKF1606).
References
[1] S.P. Lawrence and R.K. Don, Diamond: Electronic Properties and Applications, Springer Science & Business Media, New York (1995).Search in Google Scholar
[2] T. Okada, K. Fukuoka, Y. Arata, S. Yonezawa, H. Kiyokawa and M. Takashima, Diamond Relat. Mater., 52 (2015) 11–17.10.1016/j.diamond.2014.11.008Search in Google Scholar
[3] L.E. Toth, Transition Metal Carbides and Nitrides, Academic Press, New York (1971).Search in Google Scholar
[4] H.O. Pierson, Handbook of Refractory Carbide and Nitrides – Properties, Characteristics, Processing and Applications First ed., Noyes, New Jersey (1996).Search in Google Scholar
[5] E. Breval, J.P. Cheng and D.K. Agrawal, J. Am. Ceram. Soc., 83 (2000) 2106–2108.10.1111/j.1151-2916.2000.tb01524.xSearch in Google Scholar
[6] Q.P. Kang, X.B. He, S.B. Ren, L. Zhang, M. Wu, C.Y. Guo, W. Cui and X.H. Qu, Appl. Therm. Eng., 60 (2013) 423–429.10.1016/j.applthermaleng.2013.05.038Search in Google Scholar
[7] T. Schubert, L. Ciupinski, W. Zielinski, A. Michalski, T. Weißgarber and B. Kieback, Scripta. Mater., 58 (2008) 263–266.10.1016/j.scriptamat.2007.10.011Search in Google Scholar
[8] T. Schubert, B. Trindade, T. Weisgarber and B. Kieback, Mater. Sci. Eng. A., 475 (2008) 39–44.10.1016/j.msea.2006.12.146Search in Google Scholar
[9] Y.F. Zhu, L. Wang, W.Q. Yao and L.L. Cao, Appl. Surf. Sci., 171 (2001) 143–150.10.1016/S0169-4332(00)00555-9Search in Google Scholar
[10] G.A. Shafeev, S.M. Pimenov and E.N. Loubnin, Appl. Surf. Sci., 86 (1995) 392–397.10.1016/0169-4332(94)00423-4Search in Google Scholar
[11] S.M. Pimenov, G.A. Shafeev, V.I. Konov and E.N. Loubnin, Diamond Relat. Mater., 5 (1996) 1042–1047.10.1016/0925-9635(95)00494-7Search in Google Scholar
[12] S.B. Ren, X.Y. Shen, C.Y. Guo, N. Liu, J.B. Zhang and X.B. He, Compos. Sci. Technol., 71 (2011) 1550–1555.10.1016/j.compscitech.2011.06.012Search in Google Scholar
[13] A.M. Abyzov, M.J. Kruszewski, L. Ciupinski, M. Mazurkiewicz, A. Michalski and K.J. Kurzydłowski, Mater. Des., 76 (2015) 97–109.10.1016/j.matdes.2015.03.056Search in Google Scholar
[14] A.H. Ras, F.D. Auret and J.M. Nel, Diamond Relat. Mater., 19 (2010) 1411–1414.10.1016/j.diamond.2010.08.013Search in Google Scholar
[15] J. Hell, C. Chirtoc, C. Eisenmenger-Sittner, H. Hutter, N. Kornfeind, P. Kijamnajsuk, M. Kitzmantel, K. Neubauer and K. Zellhofer, Surf. Coat. Technol., 208 (2012) 24–31.10.1016/j.surfcoat.2012.07.068Search in Google Scholar PubMed PubMed Central
[16] X.Y. Shen, X.B. He, S.B. Ren, H.M. Zhang and X.H. Qu, J. Alloys Compd., 529 (2012) 134–139.10.1016/j.jallcom.2012.03.045Search in Google Scholar
[17] C. Zhao and J. Wang, Mater. Sci. Eng A., 588 (2013) 221–227.10.1016/j.msea.2013.09.034Search in Google Scholar
[18] Q.L. Che, J.J. Zhang, X.K. Chen, Y.Q. Ji, Y.W. Li, L.X. Wang, S.Z. Cao, L. Guo, S.W. Wang, Z.K. Zhang and Y.G. Jiang, Mater. Sci. Semiconductor Process., 3 (2015) 67–75.10.1016/j.mssp.2015.01.041Search in Google Scholar
[19] H. Feng, J.K. Yu and W. Tan, Mater. Chem. Phys., 124 (2010) 851–855.10.1016/j.matchemphys.2010.08.003Search in Google Scholar
[20] Q.C. Gu, J.H. Peng, L. Xu, C. Srinivasakannan, L.B. Zhang, Y. Xia, Q.T. Wu and H.Y. Xia, Appl. Surf. Sci., 390 (2016) 909–916.10.1016/j.apsusc.2016.08.168Search in Google Scholar
[21] X.Y. Liu, W.G. Wang, D. Wang, D.R. Ni, L.Q. Chen and Z.Y. Ma, Mater. Chem. Phys., 182 (2016) 256–262.10.1016/j.matchemphys.2016.07.030Search in Google Scholar
[22] S.D. Ma, N.Q. Zhao, C.S. Shi, E.Z. Liu, C.N. He, F. He and L.Y. Ma, Appl. Surf. Sci., 402 (2017) 372–383.10.1016/j.apsusc.2017.01.078Search in Google Scholar
[23] Z.F. Che, J.W. Li, Q.X. Wang, L.H. Wang, H.L. Zhang, Y. Zhang, X.T. Wang, J.G. Wang and M.J. Kim, Composites A., 107 (2018) 164–170.10.1016/j.compositesa.2018.01.002Search in Google Scholar
[24] G.Z. Chen, D.J. Fray and T.W. Farthing, Nature, 407 (2000) 361–364.10.1038/35030069Search in Google Scholar PubMed
[25] X.K. Li, Z.J. Dong, A. Westwood, A. Brown, S.W. Zhang, R. Brydson, N. Li and B. Rand, Carbon, 46 (2008) 305–309.10.1016/j.carbon.2007.11.020Search in Google Scholar
[26] J. Ding, D. Guo, C.J. Deng, H.X. Zhu and C. Yu, Appl. Surf. Sci., 407 (2017) 315–321.10.1016/j.apsusc.2017.02.196Search in Google Scholar
[27] X.Q. Kan, J. Ding, C. Yu, H.X. Zhu, C.J. Deng and G.Y. Li, Ceramics Int., 43 (2017) 6377–6384.10.1016/j.ceramint.2017.02.048Search in Google Scholar
[28] K. Zhang, Z.Q. Shi, X.Y. Zhang, Z.J. Zhang, B.Z. Ge, H.Y. Xia, Y.J. Guo and G.J. Qiao, Ceramics Int., 43 (2017) 8089–8097.10.1016/j.ceramint.2017.03.131Search in Google Scholar
[29] S.D. Luo and M. Qian, Mater. Manuf. Processes., 33 (2018) 35–49.10.1080/10426914.2016.1257800Search in Google Scholar
[30] A.J. Buttress, J. Katrib, D.A. Jones, A.R. Batchelor, D.A. Craig, T.A. Royal, C. Dodds and S.W. Kingman, Miner. Eng., 109 (2017) 169–183.10.1016/j.mineng.2017.03.006Search in Google Scholar
[31] A.R. Batchelor, A.J. Buttress, D.A. Jones, J. Katrib, D. Way, T. Chenje, D. Stoll, C. Dodds and S.W. Kingman, Miner. Eng., 111 (2017) 5–24.10.1016/j.mineng.2017.05.003Search in Google Scholar
[32] M. Bhattacharya and T. Basak, Energy, 97 (2016) 306–338.10.1016/j.energy.2015.11.034Search in Google Scholar
[33] P.R. Matli, R.A. Shakoor, A.M.A. Mohamed and M. Gupta, Metals, 6 (2016) 143–162.10.3390/met6070143Search in Google Scholar
[34] R.R. Mishra and A.K. Sharma, Composites A., 81 (2016) 78–97.10.1016/j.compositesa.2015.10.035Search in Google Scholar
[35] R.R. Mishra and A.K. Sharma, Crit. Rev. Solid State Mate. Sci., 41 (2016) 217–255.10.1080/10408436.2016.1142421Search in Google Scholar
[36] Z.W. Peng and J.Y. Hwang, Int. Mater. Rev., 60 (2015) 30–63.10.1179/1743280414Y.0000000042Search in Google Scholar
[37] S. Singh, D. Gupta, V. Jain and A.K. Sharma, Mater. Manuf. Process., 30 (2015) 1–29.10.1080/10426914.2014.952028Search in Google Scholar
[38] K.I. Rybakov, E.A. Olevsky and E.V. Krikun, J. Am. Ceram. Soc., 96 (2013) 1003–1020.10.1111/jace.12278Search in Google Scholar
[39] M. Oghbaei and O. Mirzaee, J. Alloys Compd., 494 (2010) 175–189.10.1016/j.jallcom.2010.01.068Search in Google Scholar
[40] R. Roy, D. Agrawal, J.P. Cheng and S. Gedevanishvili, Nature, 399 (1999) 668–670.10.1038/21390Search in Google Scholar
[41] K. Hirota, K. Mitani, M. Yoshinaka and O. Yamaguchi, Mater. Sci. Eng. A., 399 (2005) 154–160.10.1016/j.msea.2005.02.062Search in Google Scholar
[42] L. Dai, Y. Lu, X.Y. Wang, J. Zhu, Y.H. Li and L. Wang, Int. J. Refract. Met. Hard Mater., 51 (2015) 153–159.10.1016/j.ijrmhm.2015.03.012Search in Google Scholar
[43] Z.W. Zhao, F.X. Chen, M.C. Wang and H.J. Zheng, Int. J. Refract. Met. Hard Mater., 51 (2015) 212–215.10.1016/j.ijrmhm.2015.04.010Search in Google Scholar
[44] Z.W. Zhao and W.M. Hu, Int. J. Refract. Met. Hard Mater., 58 (2016) 206–210.10.1016/j.ijrmhm.2016.05.003Search in Google Scholar
[45] R.F.K. Gunnewiek and R.H.G.A. Kiminami, Ceramics Int., 43 (2017) 10614–10618.10.1016/j.ceramint.2017.05.062Search in Google Scholar
[46] K. Chu, Z.F. Liu, C.C. Jia, H. Chen, X.B. Liang, W.J. Gao, W.H. Tian and H. Guo, J. Alloys Comp., 490 (2010) 453–458.10.1016/j.jallcom.2009.10.040Search in Google Scholar
[47] Ł. Ciupiński, M.J. Kruszewski, J. Grzonka, M. Chmielewski, R. Zielińs, D. Moszczyńska and A. Michalski, Mater. Des., 120 (2017) 170–185.10.1016/j.matdes.2017.02.005Search in Google Scholar
[48] S. Leparoux, C. Diot, A. Dubach and S. Vaucher, Scripta. Mater., 57 (2007) 595–597.10.1016/j.scriptamat.2007.06.016Search in Google Scholar
[49] Y.B. Bi, H.F. Wang, J.H. Liu, M. Wang, S.T. Ge, H.J. Zhang and S.W. Zhang, Surf. Coat. Technol., 337 (2018) 217–222.10.1016/j.surfcoat.2018.01.017Search in Google Scholar
[50] A.H. Cottrell, Chemical Bonding in Transition Metal Carbides 1–97, The Institute of Materials, London (1995).Search in Google Scholar
© 2019 Walter de Gruyter GmbH, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

