Abstract
The present study focuses on the evaluation of a novel prolonged action fertilizer that has been encapsulated by a combination of carboxymethyl cellulose (CMC) and humic acid (HA). The aim of this research was to investigate the release of the essential plant nutrients: phosphorus, nitrogen, and potassium. This study investigated the material composition and nutrient release properties of a novel prolonged action fertilizer encapsulated by CMC and HA. The release of these nutrients was determined by quantifying their concentrations over time using various analytical techniques, such as photometric, titrimetric, and flame photometric methods. The results revealed that the CMC/HA matrix effectively controlled the nutrient release, with extended-release durations observed under acidic (pH 5.0) and neutral (pH 7.0) conditions compared to alkaline environments (pH 8.5). This indicates that the encapsulated fertilizer is well suited for a variety of soil pH conditions, particularly in acidic and neutral soils. This information could have important implications for the development of more sustainable and environmentally friendly fertilizer technologies.
1 Introduction
The encapsulation process is used to improve the functionality, presentation, and quality of fertilizer products. It consists of a shell and a core, which isolate and protect the encapsulated substance (1). Depending on the substance, the core’s aggregate position may differ. Encapsulation offers the possibility to modify the color, strength, granulating composition, density, and shelf life of the substance. It can also reduce reactivity. Mineral fertilizer encapsulation aims to slow down the release of nutrients to prevent soil erosion and control the release of target components (2). This is especially important due to the potential accumulation of nitrites and nitrates in plants, which can result from excessive nutrient application. There are currently different types of encapsulation and production methods available, allowing for the regulation of mineral fertilizer granule dissolution rates. The spherical shape of capsule particles has proven to be successful due to the absence of a flat surface and reduced channels. The initial granule of mineral fertilizer undergoes additional processing to obtain a high-quality capsule that can be used under various environmental conditions. To prevent significant increases in particle size during the encapsulation process, a protective film is applied either before or after the granulation stage. This process results in increased dissolution time and the strength of self-granulation (3). Granulation is a physical and physico-chemical process that shapes and structures substances. The relevance of studying this area is increasing as the number and forms of dispersed products required for encapsulation or granulation continue to grow. Encapsulation is known to reduce the release of active substances and promote their real-time release, prolong the shelf life of unstable and perishable substances, reduce the toxicity of products, prevent the interaction of immiscible and mutually influencing substances, and improve physical and physico-chemical properties. Environmental factors may not affect the encapsulation of various materials (4). Currently, encapsulation is a widely used process for a variety of materials, including chemicals, oil, raw materials, pesticides, fertilizers, and medicines (5). This process involves coating the surface of particles with a strong and dense film that alters the properties of the material, a phenomenon referred to as encapsulation. In the case of fertilizers, the encapsulation process involves covering the particles with a shell that makes it difficult for aqueous solutions to pass through, resulting in the production of mineral fertilizers with delayed action. To create this shell, a range of substances such as paraffin, polyethylene suspensions, sulfur compounds, acrylic resin, and polyacrylic acids are utilized (6,7). The encapsulation process improves the physico-chemical properties of fertilizers by coating them with a film. This results in increased hygroscopicity, mechanical strength, and extended shelf life. The frequency of nutrient supply varies depending on the composition and thickness of the coating layers. The biological conditions of the coating materials and the continuous nutrient supply are essential in determining the effectiveness of fertilizers used in agriculture. Some fertilizers are developed with two-layer granules where the core is easily soluble and coated with a shell that quickly affects fertilizers. The outer protective layer may have varying thicknesses (8).
Encapsulation is a process that can be achieved through various methods including polycondensation and polymerization, melt extrusion in liquid media, spray drying, physical adsorption, thermal encapsulation, spraying, pressing, extrusion, and more. These methods can be grouped into three main categories: physico-chemical, chemical, and physical methods, based on the nature of the encapsulation process (9). In practice, a combination of different methods is often used to produce the desired encapsulated material. In addition to these methods, aerosol encapsulation methods also exist, which involve the transformation of chemical and physical particles and belong to the first and second groups. The choice of encapsulation method depends on various factors such as the quality of the final product, economic efficiency, and other influencing factors (10,11). Despite the various encapsulation methods available, the technical base of this area of chemical technology has not yet been fully developed and is currently being actively researched.
In recent years, the demand for sustainable agricultural practices has grown exponentially, driven by the need to increase crop productivity and ensure global food security while minimizing environmental impacts (12,13). One of the major challenges faced by modern agriculture is the efficient management of fertilizers, which play a crucial role in supplying essential nutrients to crops (14,15). Conventional fertilizers, however, often suffer from limitations such as rapid nutrient leaching, environmental pollution, and reduced nutrient use efficiency. To address these issues, the development of innovative fertilization strategies, such as controlled-release fertilizers, has become imperative.
Controlled-release fertilizers offer numerous advantages over their traditional counterparts, including improved nutrient use efficiency, decreased application frequency, and reduced environmental impact. By encapsulating nutrients within protective coatings, these fertilizers enable a gradual and controlled release of nutrients, ensuring their availability to plants over an extended period. Consequently, this approach has the potential to enhance crop productivity while mitigating the adverse effects associated with conventional fertilizers.
In this context, the present study aims to develop a novel prolonged action fertilizer encapsulated by carboxymethyl cellulose (CMC) and humic acid (HA). Both CMC and HA are biodegradable, eco-friendly, and cost-effective materials that have garnered increasing attention for their potential in encapsulation applications. CMC, a cellulose derivative, is known for its excellent film-forming and water retention properties, while HA, a naturally occurring organic compound, has been shown to facilitate nutrient uptake and improve soil fertility. By combining these two materials, we hypothesize that the resulting encapsulation system will protect nutrients from premature degradation and leaching while ensuring a controlled and sustained release of nutrients to plants.
The decision to combine HA with CMC in our encapsulation system was driven by the complementary properties and benefits that each material offers, which we believed would result in a more effective and environmentally friendly encapsulation system for prolonged action fertilizers.
Synergistic effects: The combination of CMC and HA capitalizes on the strengths of each material to create a robust and effective encapsulation system. CMC, a cellulose derivative, possesses excellent film-forming properties, which contribute to the structural integrity of the encapsulation layer (16). It also exhibits strong water retention capabilities, which help regulate the release of nutrients by controlling the rate of water penetration. On the other hand, HA, a naturally occurring organic compound, enhances nutrient availability and uptake by plants (17), owing to its ability to form stable complexes with various nutrients, such as nitrogen, phosphorus, and potassium.
Biodegradability and eco-friendliness: Both CMC and HA are biodegradable and environmentally benign materials (18). CMC, being a cellulose derivative, is biocompatible and non-toxic, while HA is a natural component of soil organic matter. By using these materials in our encapsulation system, we aim to minimize the environmental impact associated with conventional fertilizers, such as pollution from nutrient leaching and degradation.
In summary, the decision to use HA in conjunction with CMC was based on their complementary properties, which, when combined, offer a promising encapsulation system that can effectively address the challenges associated with conventional fertilizers while promoting sustainable agricultural practices.
2 Materials and methods
2.1 Preparation of the fertilizer sample
The encapsulated fertilizer was obtained by acid decomposition. As a raw material, solid waste from the phosphorus industry was used – phosphorus sludge and cottrell dust (CD). A mixture of phosphoric sludge (PS) and CD in a ratio of 1:2 was decomposed using HA. Optimal decomposition conditions: time – 70 min, temperature – 80°C, and the ratio of the mixture PS-CD:HA – 1:2. The resulting pulp in the process of HA decomposition of phosphorus sludge and CD was filtered into liquid and solid phases under vacuum 0.06 MPa. The resulting liquid phase-filtrate is phosphoric acid (PA) of the composition presented in Table 1.
Average chemical composition of PA (n = 8)
| P2O5 (%) | K2O (%) | CaO (%) | MgO (%) | Al2O3 (%) | Fe2O3 (%) |
|---|---|---|---|---|---|
| 29.19 | 18.14 | 10.07 | 0.19 | 0.07 | 0.06 |
The resulting PA was then used for the subsequent decomposition of a mixture of PS and CD taken in a ratio of 1:2. At the same time, the optimal decomposition conditions were as follows: time – 60 min, temperature – 70°C, the ratio of the mixture PS-CD:PA – 1:1. The resulting pulp in the process of PA decomposition of PS and CD was cooled to room temperature. The resulting pulp had a total P2O5 – 46.28% and assimilable P2O5 – 32.17%.
2.2 Preparation of HA
HA was obtained from brown coal waste from the Lenger deposit (South Kazakhstan) by alkaline extraction of humates at 80°C for 100 min, where potassium hydroxide with a concentration of 1% is used as an extraction reagent, followed by acid deposition at room temperature using hydrochloric acid with a concentration of 4% for 30 min. The acidic characteristics of HA are given in Table 2.
Acid characteristics and yield of HAs during their hydrochloric acid deposition (average values, n = 8)
| pH | Number of COOH groups (%) | Acid value (mg·g−1) | HA yield, α (%) |
|---|---|---|---|
| 1.92 | 1.72 | 21.49 | 75.15 |
2.3 Preparation of the encapsulated solution
The encapsulated solution was prepared by dissolving carboxymethylcellulose (CMC) in deionized water and further heating it at 70°C. Then, the pulp obtained in the 2.1 stage is mixed with the encapsulated solution in the ratio pulp:encapsulated solution – 2:1. Finally, HA is added drop by drop to complete the crosslinking reaction. In the future, the fertilizer coated with CMC is granulated and dried to a constant weight at 110°C.
2.4 Assessing the release performance of the CMC/HA coated fertilizer
To evaluate the ability of the CMC/HA matrix to control the release of nutrients, the amounts of nitrogen, phosphorus, and potassium were measured over a 30-day period. Three buffer solutions with pH levels of 5.0, 7.0, and 8.5 were prepared and 5 mL samples were taken every 5 days. The NPK concentrations were determined using standard techniques, and the percent release of each nutrient was plotted over time under different pH conditions.
2.5 Chemical and instrumental methods of analysis
2.5.1 Chemical analysis of nutrients
The concentration of phosphorus was measured using a photometric method with Trilon B as the reagent. This method is based on the reaction between Trilon B and phosphorus to form a complex with a characteristic color. The intensity of this color is proportional to the concentration of phosphorus, and the absorption can be measured using a spectrophotometer at a specific wavelength (λ = 440 nm). The photometric method provides a sensitive and reliable means of determining the concentration of phosphorus in a sample, making it well suited for use in this study.
The mass fraction of nitrogen in fertilizers containing nitrogen in nitrate form was determined using a titrimetric method based on the reduction of nitrate nitrogen with a solution of iron sulfate(ii) in an acidic medium in the presence of ammonium molybdenum as a catalyst, followed by titration of the excess iron sulfate(ii) with a solution of potassium permanganate. The reaction between nitrates and iron sulfate(ii) in the presence of an acidic medium and ammonium molybdenum results in the reduction of nitrates to nitric oxide, which can then be titrated with potassium permanganate. The volume of potassium permanganate required to neutralize the iron sulfate(ii) is proportional to the amount of nitrogen in the fertilizer sample. The results of the titration are then used to calculate the mass fraction of nitrogen in the sample, providing an accurate measure of the nitrogen content.
The mass fraction of potassium in a sample was determined using a flame photometric method. The method is based on a comparison of the radiation intensity of resonant potassium lines formed in a flame when the sample and a comparison solution are introduced into it. The most intense resonant potassium lines at 766.5 and 769.9 nm were used in photometry. A flame photometer of the FPA brand was used to perform the analysis. The sample and the comparison solution were introduced into the flame, and the radiation intensity of the resonant potassium lines was measured. The results of the measurement were used to calculate the mass fraction of potassium in the sample, providing an accurate and precise measure of the potassium content.
2.5.2 Scanning electron microscope (SEM)
The JSM6490 LV SEM was used to obtain high-resolution images of the sample surface. The SEM operates at a low voltage, allowing for imaging of delicate or soft materials without causing significant damage. The instrument was equipped with a backscattered electron detector, an energy-dispersive X-ray spectroscopy (EDS) system, and a cathodoluminescence detector for imaging and chemical analysis.
Images were acquired using the backscattered electron detector, which provides information on the surface and sub-surface structure of the sample. Chemical analysis was performed using the EDS system, which detects X-rays emitted from the sample when it is bombarded by the electron beam. The cathodoluminescence detector was used to visualize light emission from the sample in response to electron bombardment. The results of the imaging and analysis provide insights into the surface and sub-surface structure, as well as the chemical composition, of the sample.
2.5.3 X-ray diffraction (XRD) analysis
XRD analysis was performed on the D8 Advance equipment from Bruker. XRD is a powerful tool for characterizing the crystalline structure of materials and provides information on the crystal structure, crystallinity, and preferred orientation of the sample.
The resulting diffractograms were processed using the EVA software. The software was used to identify the crystalline phases present in the sample and to determine the lattice parameters, such as the unit cell dimensions and crystal symmetry. The software also provided information on the preferred orientation of the crystals in the sample and the degree of crystallinity.
2.6 Statistical analysis
In this study, statistical analysis was performed to evaluate the significance of the results. The statistical analysis was performed using Microsoft Excel. The data collected from nutrient release measurements were subjected to normal distribution tests to ensure that the data met the assumptions of parametric statistical analysis. A p-value of less than 0.05 was considered statistically significant, indicating that there was a 95% confidence level that the results were not due to chance. The results of the statistical analysis were used to support the conclusions of the study.
3 Results and discussion
3.1 Description of prolonged action fertilizer encapsulated in a CMC/HA matrix
Prolonged action fertilizer encapsulated in a CMC/HA matrix is a type of fertilizer that is coated with a combination of CMC and HA. This combination forms a matrix around the fertilizer particles that controls the release of nutrients over an extended period of time. The CMC component of the matrix serves as a slow-release barrier, allowing water to penetrate and dissolve the fertilizer particles gradually over time. The HA component enhances the overall efficacy of the fertilizer by improving soil structure, water-holding capacity, and nutrient uptake by plants (19). The use of this type of fertilizer has several benefits. First, it reduces the frequency of fertilizer application, saving time and effort for farmers and gardeners. Second, it minimizes the risk of nutrient loss due to leaching, ensuring that the nutrients are available to plants over an extended period of time. Finally, it helps to improve soil quality and plant health, resulting in higher yields and better crop quality.
Overall, the use of a CMC/HA matrix in fertilizer production can provide a more efficient and sustainable approach to fertilization, leading to improved crop productivity and reduced environmental impact.
Table 3 displays the typical chemical composition of the fertilizer that is enclosed in a matrix.
The average chemical composition of the encapsulated fertilizer (n = 8)
| P2O5 (%) | K2O (%) | CaO (%) | MgO (%) | NO3 (%) |
|---|---|---|---|---|
| 46.28 ± 1.19 | 14.81 ± 0.86 | 8.49 ± 0.77 | 0.27 ± 0.12 | 8.73 ± 0.48 |
The results of the chemical analysis of the encapsulated fertilizer show that it contains a significant amount of P2O5, with an average concentration of 46.28 ± 1.19%. This value is higher than the levels reported in several other studies (20) and highlights the potential efficacy of the encapsulated fertilizer as a source of phosphorous. It is important to take into account that the phosphorus base at the same time consists of phosphorus-containing solid waste – phosphorus sludge and CD. The presence of K2O at 14.81 ± 0.86% further supports this conclusion and suggests that the fertilizer may provide both primary macronutrients required for plant growth. The amount of CaO in the fertilizer, at 8.49 ± 0.77%, is also noteworthy. This value is in line with other research (21), which highlights the importance of calcium in plant development. MgO, although present in trace amounts (0.27 ± 0.12%), has been shown to play a critical role in plant metabolism, and its presence in the encapsulated fertilizer should not be overlooked.
Finally, the concentration of NO3 in the fertilizer, at 8.73 ± 0.48%, is comparable to other studies and suggests (22) that the fertilizer may provide an additional source of nitrogen for plant growth.
These values indicate the average amounts of each component present in the encapsulated fertilizer, with the ± values indicating a margin of error.
The results of the element-weight analysis, as well as the micrographs of the surface (40×) of the encapsulated fertilizer, are displayed in Table 4 and Figure 1.
Element-weight composition of encapsulated fertilizer
| Element | C | O | Na | Mg | P | S | Cl | K | Ca | Mn |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight composition (%) | 10.24 | 47.73 | 1.75 | 1.03 | 20.46 | 0.35 | 0.52 | 7.79 | 9.82 | 0.30 |
| In terms of oxides (%) | — | — | 2.35 | 1.70 | 46.87 | — | — | 9.38 | 13.73 | 0.38 |
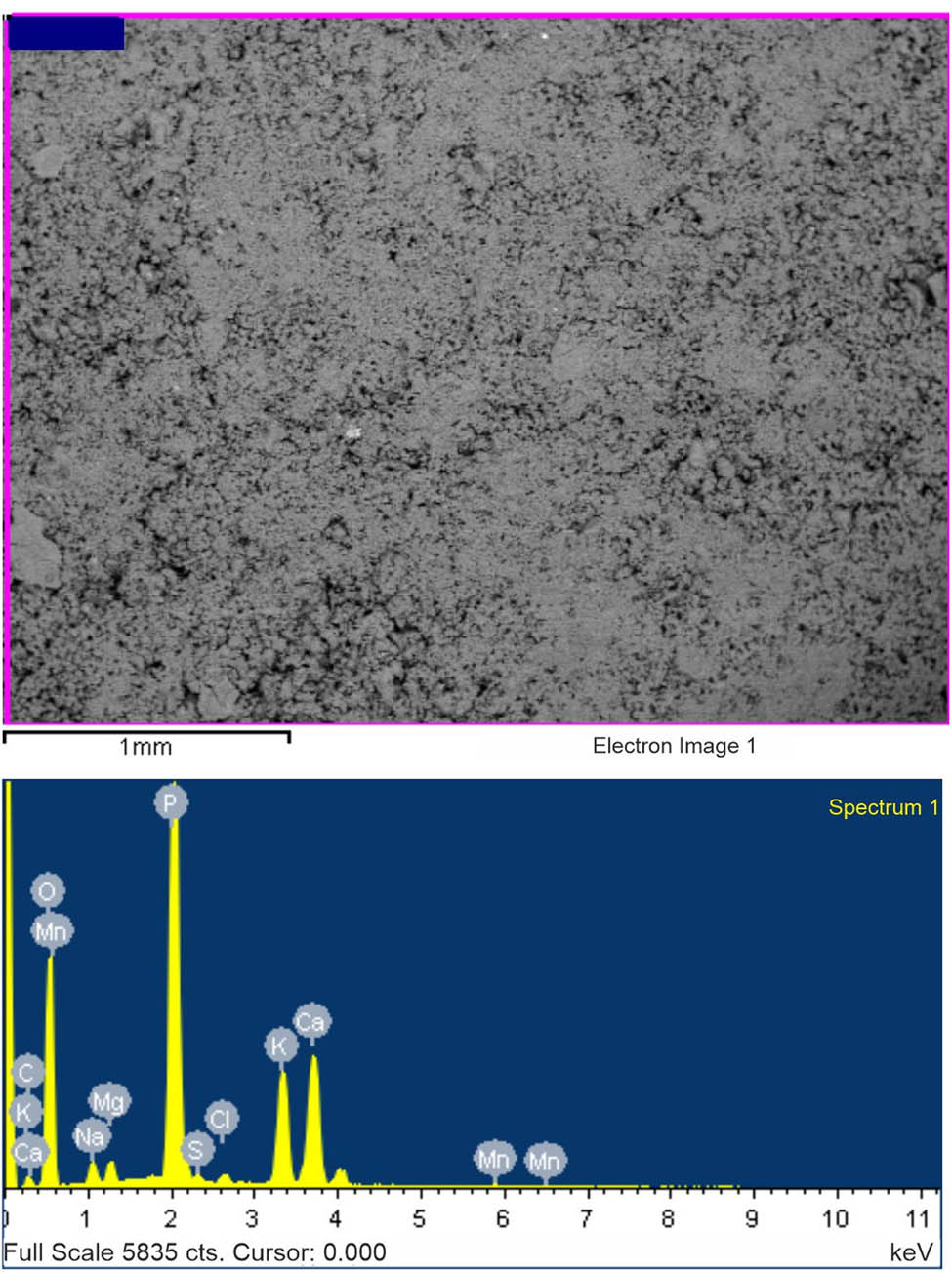
SEM image (top) and EDX analysis (bottom) of encapsulated fertilizer.
The element-weight composition analysis of the encapsulated fertilizer revealed the presence of several important elements, including Na2O at 2.35%, MgO at 1.70%, and P2O5 at 46.87%. These results demonstrate that the fertilizer contains important micronutrients, as well as a significant source of phosphorous. The presence of sodium is associated with the use of the sodium salt of CMC. In addition, the analysis showed the presence of S at 0.35% and Cl at 0.52%. The presence of sulfur in the fertilizer can be attributed to the complex chemical composition of the feedstock used in the production of fertilizer, specifically PS and CD. Meanwhile, the presence of chlorine is linked to the utilization of hydrochloric acid in the process of precipitating HA. The concentration of K2O in the fertilizer at 9.38% also highlights its potential as a source of this essential macronutrient.
The SEM image of the encapsulated fertilizer displays a highly porous, spherical structure with a rough surface. The individual particles are well defined and appear to be uniform in size and shape, indicating a consistent production process. The porous nature of the particles suggests that they have the potential to provide a large surface area for the release of nutrients over time. The surface of the particles appears to be free of cracks or defects, which could potentially compromise the stability of the fertilizer.
The XRD analysis results, displayed in Figure 2, offer additional information regarding the compounds present in the composition of the encapsulated fertilizer. It appears to be a mixture of various minerals, including decacalcium tetrakis(ortho-phosphate) bis(carbonate) tetrahydroxide (Ca10(PO4)4(CO3)2(OH)4), which makes up 28.7% of the mixture. This compound is a complex calcium phosphate-carbonate material and is very similar to apatite hydroxycarbonate. This, in turn, is explained by the fact that the PA obtained at stage 1 did not fully decompose the PS-CD mixture. Potassium dihydrogen triphosphate hydrate (K3H2P3O10H2O), which makes up 22.4% of the mixture. It is an inorganic compound consisting of potassium cations and a pyrophosphate anion. It serves as a buffer and chelating agent. In addition, it has the ability to retain moisture. This compound is a source of both potassium and phosphorous, which are essential plant nutrients. Quartz (SiO2), which makes up 19.4% of the mixture, its presence may be related to the chemical composition of the initial raw materials. 9.7% sodium-calcium phosphate (Na2CaP2O7) proves the reaction between the CMC and the phosphate part of the encapsulated fertilizer.
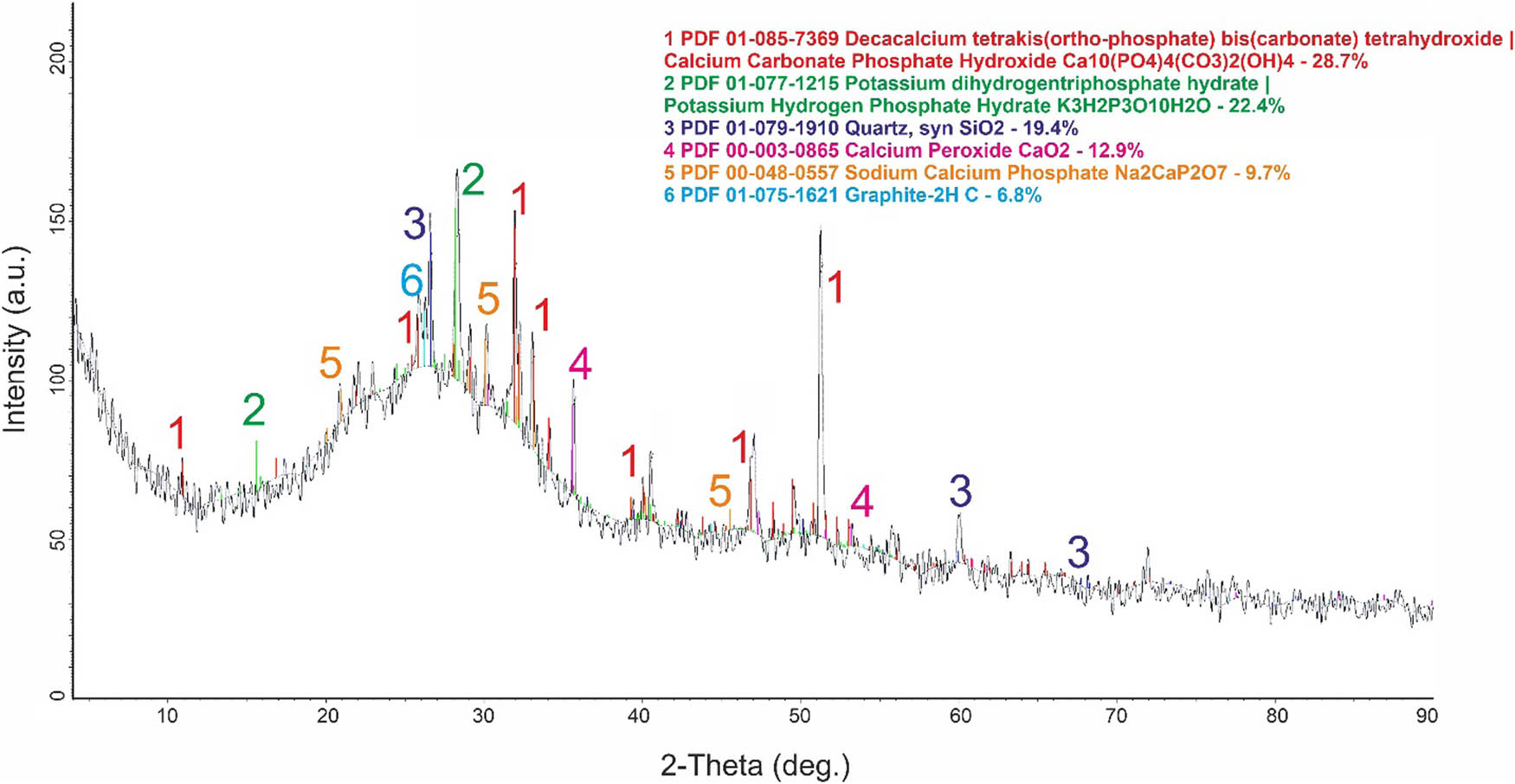
XRD pattern of encapsulated fertilizer.
12.9% of the mixture is calcium peroxide (CaO2), which can release oxygen. However, the isolation of such a compound raises some doubts. Although having such a complex chemical composition, identification of substances can be difficult. It is widely recognized that XRD analysis is not a suitable method for identifying organic compounds. Instead, infrared (IR) spectroscopy is used to determine the composition and structure of organic compounds, and this is demonstrated in Figure 3 and Table 5.
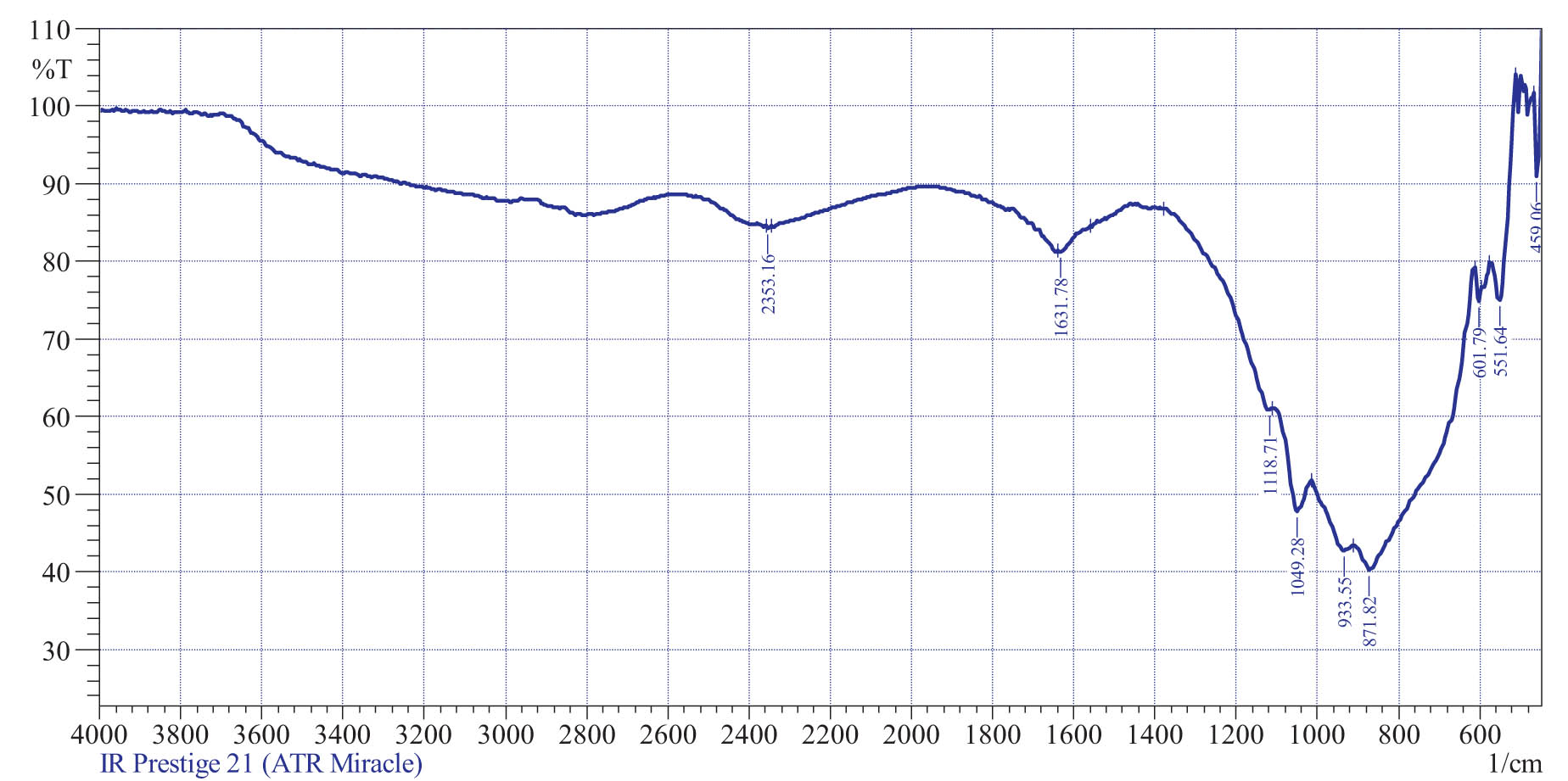
IR spectrogram of encapsulated fertilizer.
Decoding of the IR spectrogram
| No. | Peak | Intensity | Corr. intensity | Base (H) | Base (L) | Area | Corr. area |
|---|---|---|---|---|---|---|---|
| 1 | 459.06 | 91.043 | 14.228 | 466.77 | 447.49 | 0.203 | 0.696 |
| 2 | 551.64 | 74.977 | 13.956 | 574.79 | 513.07 | 5.015 | 2.534 |
| 3 | 601.79 | 74.811 | 2.949 | 613.36 | 594.08 | 2.264 | 0.183 |
| 4 | 871.82 | 40.295 | 7.806 | 910.40 | 613.36 | 86.654 | 17.894 |
| 5 | 933.55 | 42.759 | 2.561 | 1,014.56 | 910.40 | 35.131 | 1.428 |
| 6 | 1,049.28 | 47.837 | 7.360 | 1,111.00 | 1,014.56 | 26.247 | 2.188 |
| 7 | 1,118.71 | 60.890 | 0.980 | 1,377.17 | 1,111.00 | 31.969 | −4.683 |
| 8 | 1,631.78 | 81.263 | 0.383 | 1,639.49 | 1,558.48 | 6.571 | −0.015 |
| 9 | 2,353.16 | 84.297 | 0.261 | 2,357.01 | 2,345.44 | 0.852 | 0.010 |
The IR spectrogram peaks for encapsulated fertilizer that you have provided are characteristic of certain chemical functional groups. Here is a tentative assignment of the peaks based on their wavenumbers (23,24): the peak at 459.06 cm−1 may be due to the stretching of nitrogen–hydrogen bonds found in nitrogen-based fertilizers. The peak at 551.64 cm−1 may be due to the bending of carbon–hydrogen bonds found in many organic compounds. Wavenumbers at 601.79 cm−1 may be related to the stretching of carbon–oxygen bonds in compounds containing carbonyl groups, such as carboxylic acids and esters. Stretching vibrations at 871.82 cm−1 may be due to the bending of nitrogen–oxygen bonds found in nitrogen-containing compounds, such as amides or nitrates. If the peak corresponding to 933.55 cm−1 can be due to the stretching of carbon–nitrogen bonds found in nitrogen-containing compounds, then wave numbers of 1,049.28 cm−1 characterize a hydrogen sulfide bond. Stretching vibrations 1,118.71 and 1,631.78 cm−1 indicate the presence of C–C and C═O bonds, respectively (25,26). The presence of a peak at 2,353.16 cm−1 indicates carbon–hydrogen bonds in aromatic rings found in compounds with aromatic functional groups.
3.2 Investigation of NPK nutrients release
Studies on the release of nutrients were conducted following the methodology outlined in Section 2.4, which involved sampling placed in buffer solutions every 5 days. The results are shown in Figure 4.
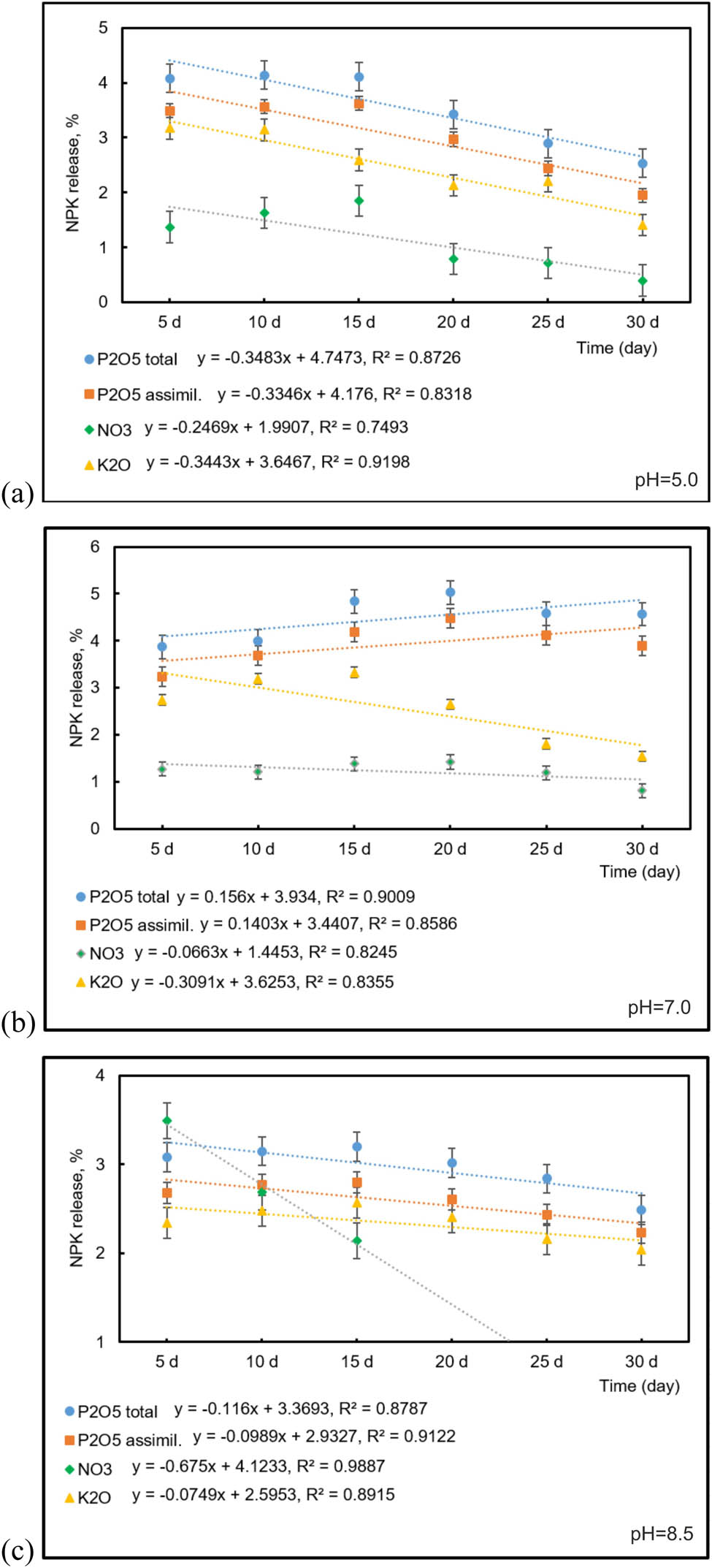
Plots of NPK release vs. time (day) at various pH value: (a) pH = 5.0, (b) pH = 7.0, and (c) pH = 8.5.
The study found that the release of nitrogen at pH 5.0 followed a specific pattern. It gradually increased over time, reaching a peak at day 15, and then slowly decreased. In contrast, the amounts of total and assimilable phosphorus tended to decrease, as did potassium levels.
Regarding nutrient release, the study found that significant amounts of nitrogen, phosphorus, and potassium were released within a specified time period. The release of nitrogen from the coated fertilizer was significant, with 77% of the initial composition being released in the first 30 days. However, the nitrogen content may have been lower due to dilution and volatilization during the coating process. This suggests that the use of CMC as a coating material could affect the nutrient analysis of the coated fertilizer. This finding is consistent with a previous study (27) that also used CMC as a coating material, along with starch. That study reported similar observations regarding the impact of coating on nutrient content.
For phosphorus, two types were released at notable levels: 46% and 70%, respectively. The highest value was recorded for potassium, with 98% of its content being released in the same timeframe. The XRD analysis confirmed the presence of potassium dihydrogen triphosphate in the coated fertilizer composition. This compound is highly soluble in acidic environments, which leads to the release of significant amounts of both potassium and phosphorus. As a result, these two nutrients have higher mobility than other nutrients present in the fertilizer.
Studying the release of nitrogen, phosphorus, and potassium in a neutral medium, it was found that the behavior of these nutrients followed similar patterns. Nitrogen release increased gradually over 20 days before decreasing, with 80% being exhausted during the study period. The release pattern of phosphorus, similar to nitrogen, is characterized by a gradual increase followed by a decrease. During the study period, 52% and 90% of the total and assimilable forms of phosphorus were released, respectively. Although the trend indicates a further growth in the release of these compounds, only half of the phosphorus was distributed. It can be argued that the remaining proportion of phosphorus will be released further.
Research by Broschat and Moore (28) suggests that phosphorus in controlled-release fertilizers is released more slowly than nitrogen. Moreover, studies conducted by Mengel and Kirkby (29) have found that the mobility of phosphates is generally low. Understanding the release pattern and mobility of phosphorus is essential for managing soil fertility and optimizing crop production.
Potassium release, on the other hand, followed a gradual decrease over 10–15 days before being completely consumed in 30 days. In comparison to an acidic medium, the release of nutrients in a neutral medium occurs at a faster rate. The intense release of potassium and nitrogen can be attributed to the use of CMC material for coating, which facilitates a faster release of these elements (30,31).
These findings indicate that the behavior of nitrogen, phosphorus, and potassium in a neutral medium is characterized by gradual increases followed by decreases in release. While the trend for phosphorus suggests that more of it will be released in the future, potassium is rapidly consumed in a neutral medium.
Research conducted in an alkaline environment with pH 8.5 indicates increased nitrogen activity. However, as shown in the figure, nitrogen is fully consumed by the 15th day. This behavior can be explained by the accelerated loss of nitrogen that occurs in alkaline environments. Phosphorus shows a similar pattern of behavior to that observed at pH 7.0, but in this case, the release of total and assimilable forms of phosphorus is slower than in acidic and neutral media. In alkaline environments, the solubility of phosphorus is primarily dependent on the amount of calcium present, which is the main element in alkaline media. Orthophosphates react with calcium to form calcium phosphates, which have low solubility in alkaline environments (32). Despite the decreased solubility of phosphorus in alkaline media, it remains available to crops. The release of the total and assimilable forms of phosphorus was 36% and 57%, respectively, which is much lower than in acidic and neutral environments. Researchers suggest that HAs may slow down or smooth out nutrient release (33). Potassium also exhibits a similar trend of gradual decrease, with a release of 94%, which can be attributed to its presence in the form of potassium-phosphorus salt and its connection with phosphorus.
Therefore, the study suggests that the nature of nutrient release can differ depending on the specific conditions, such as pH level.
4 Conclusion
In conclusion, the CMC/HA encapsulation system for prolonged action fertilizer offers a promising approach to sustainable nutrient management in agriculture. The encapsulation technology not only extends the availability of nutrients to crops by minimizing nutrient losses through leaching and volatilization but also modulates the release dynamics, potentially leading to enhanced nutrient uptake and crop productivity. The adoption of such encapsulated fertilizers could contribute to increased crop yields, reduced environmental pollution from fertilizer runoff, and decreased fertilization costs.
As the effectiveness of the encapsulation may vary depending on soil conditions and crop-specific nutrient requirements, it is essential to consider these factors when implementing this technology. By tailoring the encapsulation system to the specific needs of different crops and soil environments, the potential benefits of CMC/HA encapsulated fertilizers can be maximized. Future research should continue to explore and optimize this encapsulation system, building upon the insights gained from the current study, to further advance sustainable agricultural practices and contribute to global food security.
-
Funding information: This research was funded by Ministry of Higher Education and Science of the Republic of Kazakhstan, grant number AP09057884.
-
Author contributions: Ulzhalgas Nazarbek: writing – original draft, methodology, project administration; Saule Nazarbekova: data curation, visualization; Yerkebulan Raiymbekov: writing – original draft, methodology, data curation, investigation; Maksat Kambatyrov: software, validation, formal analysis; Perizat Abdurazova: writing – review and editing, investigation.
-
Conflict of interest: The authors state no conflict of interest.
References
(1) Sonawane SH, Bhanvase BA, Sivakumar M, Potdar SB. 1 - Current overview of encapsulation. In: Sonawane SH, Bhanvase BA, Sivakumar M, editors. Encapsulation of active molecules and their delivery system. Netherlands: Elsevier; 2020. p. 1–8. 10.1016/B978-0-12-819363-1.00001-6.Search in Google Scholar
(2) Yanovska A, Artyukhov A, Vakal S, Vakal V, Shkola V. Encapsulated organic–mineral fertilizers with nanoporous structure. Appl Nanosci. 2020;12:1275–83. 10.1007/s13204-021-01893-6.Search in Google Scholar
(3) Ostroha R, Yukhymenko M, Lytvynenko O, Lytvynenko A. Production of encapsulated organo-mineral fertilizers in a fluidized bed granulator. Acta Mechanica Slov. 2020;24(2):50–5. 10.21496/ams.2020.031.Search in Google Scholar
(4) Nagurskyy O, Krylova H, Vasiichuk V, Kachan S, Dziurakh Y, Nahursky A, et al. Safety usage of encapsulated mineral fertilizers based on polymeric waste. Ecol Eng Environ Technol. 2022;23(1):156–61. 10.12912/27197050/143139.Search in Google Scholar
(5) Huertas HJ. The particle encapsulation process. In: Huertas HJ, editor. Montecarlo simulation of two component aerosol processes. London: IntechOpen; 2016. p. 150. 10.5772/62019.Search in Google Scholar
(6) Timilsena Y, Haque M, Adhikari B. Encapsulation in the food industry: A brief historical overview to recent developments. Food Nutr Sci. 2020;11:481–508. 10.4236/fns.2020.116035.Search in Google Scholar
(7) Aboudzadeh MA, Hamzehlou S. Special issue on function of polymers in encapsulation process. Polymers. 2022;14(6):1178. 10.3390/polym14061178.Search in Google Scholar PubMed PubMed Central
(8) Al-Rawajfeh AE, Alrbaihat MR, AlShamaileh EM. Chapter 4 - Characteristics and types of slow- and controlled-release fertilizers. In: Lewu FB, Volova T, Rakhimol KR, editors. Controlled Release Fertilizers for Sustainable Agriculture. Netherlands: Elsevier; 2020. p. 57–78. 10.1016/B978-0-12-819555-0.00004-2.Search in Google Scholar
(9) Choudhury N, Meghwal M, Das K. Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Front. 2021;2(4):426–42. 10.1002/fft2.94.Search in Google Scholar
(10) Yuvaraj M, Subramanian KS. Novel slow release nanocomposite fertilizers. In: Sen M, editor. Nanotechnology and the environment. London: IntechOpen; 2020. p. 170. 10.5772/intechopen.93267.Search in Google Scholar
(11) Anderson K. Economic impacts of policies affecting crop biotechnology and trade. N Biotechnol. 2010;27(5):558–64. 10.1016/j.nbt.2010.05.012.Search in Google Scholar PubMed
(12) Moradi-Pour M, Saberi-Riseh R, Esmaeilzadeh-Salestani K, Mohammadinejad R, Loit E. Evaluation of Bacillus velezensis for biological control of rhizoctonia solani in bean by alginate/gelatin encapsulation supplemented with nanoparticles. J Microbiol Biotechnol. 2021;31(10):1373–82. 10.4014/jmb.2105.05001.Search in Google Scholar PubMed PubMed Central
(13) Moradi-Pour M, Saberi-Riseh R, Mohammadinejad R, Hosseini A. Investigating the formulation of alginate- gelatin encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 strains) for controlling Fusarium solani on potato. Int J Biol Macromol. 2019;133:603–13. 10.1016/j.ijbiomac.2019.04.071.Search in Google Scholar PubMed
(14) Abd El-Aziz M, Salama D, Morsi S, Youssef A, El-Sakhawy M. Development of polymer composites and encapsulation technology for slow-release fertilizers. Rev Chem Eng. 2022;38(5):603–16. 10.1515/revce-2020-0044.Search in Google Scholar
(15) Salama DM, Abd El-Aziz ME, El-Naggar ME, Shaaban EA, Abd El-Wahed MS. Synthesis of an eco-friendly nanocomposite fertilizer for common bean based on carbon nanoparticles from agricultural waste biochar. Pedosphere. 2021;31(6):923–33. 10.1016/S1002-0160(21)60024-3.Search in Google Scholar
(16) Yildirim-Yalcin M, Tornuk F, Toker OS. Recent advances in the improvement of carboxymethyl cellulose-based edible films. Trends Food Sci Technol. 2022;129:179–83. 10.1016/j.tifs.2022.09.022.Search in Google Scholar
(17) Ampong K, Thilakaranthna MS, Gorim LY. Understanding the role of humic acids on crop performance and soil health. Front Agron. 2022;4:848621. 10.3389/fagro.2022.848621.Search in Google Scholar
(18) Miroshnichenko D, Lebedeva K, Cherkashina A, Lebedev V, Tsereniuk O, Krygina N. Study of hybrid modification with humic acids of environmentally safe biodegradable hydrogel films based on hydroxypropyl methylcellulose. C. 2022;8(4):71. 10.3390/c8040071.Search in Google Scholar
(19) Tavares OCH, Santos LA, de Araujo OJL, Bucher CPC, Garcia AC, Arruda LN, et al. Humic acid as a biotechnological alternative to increase N-NO3− or N-NH4 + uptake in rice plants. Biocatal Agric Biotechnol. 2019;20:101226. 10.1016/j.bcab.2019.101226.Search in Google Scholar
(20) Qian T, Ong WS, Lu D, Zhou Y. A potential phosphorus fertilizer to alleviate the coming “phosphorus crisis”-biochar derived from enhanced biological phosphorus removal sludge. Sci Total Environ. 2022;838(4):156559. 10.1016/j.scitotenv.2022.156559.Search in Google Scholar PubMed
(21) Lambers H, Barrow NJ. The pervasive use of P2O5, K2O, CaO, MgO and other molecules that do not exist in soil or fertiliser bags. N Phytologist. 2021;232(5):1901–3. 10.1111/nph.17715.Search in Google Scholar PubMed
(22) Finch HJS, Samuel AM, Lane GPF. 4 - Fertilisers and manures. In: Finch HJS, Samuel AM, Lane GPF, editors. Lockhart & wiseman’s crop husbandry including grassland. Netherlands: Elsevier; 2014. p. 63–91. 10.1533/9781782423928.1.63.Search in Google Scholar
(23) Fischer P, McDowell C. The infrared absorption spectra of urea-hydrocarbon adducts. Can J Chem. 1960;38(2):187–93.10.1139/v60-025Search in Google Scholar
(24) Larkin P. Infrared and Raman spectroscopy; principles and spectral interpretation. Netherlands: Elsevier; 2011.10.1016/B978-0-12-386984-5.10002-3Search in Google Scholar
(25) Griffiths P, de Haseth J. Fourier transform infrared spectrometry. 2nd edn. New York, New York: Wiley; 2007.10.1002/047010631XSearch in Google Scholar
(26) Smith BC. Fundamentals of fourier transform infrared spectroscopy. 2nd edn. Boca Raton, Florida: CRC Press; 2011.10.1201/b10777Search in Google Scholar
(27) Hashim N, Misuan NS, Isa IM, Abu Bakar S, Mustafar S, Mamat M, et al. Carboxymethylcellulose-coated magnesium-layered hydroxide nanocomposite for controlled release of 3-(4-methoxyphenyl)propionic acid. Arab J Chem. 2020;13(2):3974–87. 10.1016/j.arabjc.2019.04.004.Search in Google Scholar
(28) Broschat TK, Moore KK. Release rates of ammonium‐nitrogen, nitrate‐nitrogen, phosphorus, potassium, magnesium, iron, and manganese from seven controlled‐release fertilizers. Commun Soil Sci Plant Anal. 2007;38(7–8):843–50. 10.1080/00103620701260946.Search in Google Scholar
(29) Mengel K, Kirkby EA, Kosegarten H, Appel T. Principles of plant nutrition. Dordrecht: Springer; 2012.Search in Google Scholar
(30) Mizuta K, Taguchi S, Sato S. Soil aggregate formation and stability induced by starch and cellulose. Soil Biol Biochem. 2015;87:90–6. 10.1016/j.soilbio.2015.04.011.Search in Google Scholar
(31) Jarosiewicz A, Tomaszewska M. Controlled-release NPK fertilizer encapsulated by polymeric membranes. J Agric Food Chem. 2003;51(2):413–7. 10.1021/jf020800o.Search in Google Scholar PubMed
(32) Dorozhkin SV. Calcium orthophosphates. Biomatter. 2011;1(2):121–64. 10.4161/biom.18790.Search in Google Scholar PubMed PubMed Central
(33) Magrini-Bair KA, Czernik S, Pilath HM, Evans RJ, Maness PC, Leventhal J. Biomass derived, carbon sequestering, designed fertilizers. Ann Environ Sci. 2010;3:2017–225.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites
Articles in the same Issue
- Research Articles
- Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles
- Effect of Zr-doped CaCu3Ti3.95Zr0.05O12 ceramic on the microstructure, dielectric properties, and electric field distribution of the LDPE composites
- Effects of dry heating, acetylation, and acid pre-treatments on modification of potato starch with octenyl succinic anhydride (OSA)
- Loading conditions impact on the compression fatigue behavior of filled styrene butadiene rubber
- Characterization and compatibility of bio-based PA56/PET
- Study on the aging of three typical rubber materials under high- and low-temperature cyclic environment
- Numerical simulation and experimental research of electrospun polyacrylonitrile Taylor cone based on multiphysics coupling
- Experimental investigation of properties and aging behavior of pineapple and sisal leaf hybrid fiber-reinforced polymer composites
- Influence of temperature distribution on the foaming quality of foamed polypropylene composites
- Enzyme-catalyzed synthesis of 4-methylcatechol oligomer and preliminary evaluations as stabilizing agent in polypropylene
- Molecular dynamics simulation of the effect of the thermal and mechanical properties of addition liquid silicone rubber modified by carbon nanotubes with different radii
- Incorporation of poly(3-acrylamidopropyl trimethylammonium chloride-co-acrylic acid) branches for good sizing properties and easy desizing from sized cotton warps
- Effect of matrix composition on properties of polyamide 66/polyamide 6I-6T composites with high content of continuous glass fiber for optimizing surface performance
- Preparation and properties of epoxy-modified thermosetting phenolic fiber
- Thermal decomposition reaction kinetics and storage life prediction of polyacrylate pressure-sensitive adhesive
- Effect of different proportions of CNTs/Fe3O4 hybrid filler on the morphological, electrical and electromagnetic interference shielding properties of poly(lactic acid) nanocomposites
- Doping silver nanoparticles into reverse osmosis membranes for antibacterial properties
- Melt-blended PLA/curcumin-cross-linked polyurethane film for enhanced UV-shielding ability
- The affinity of bentonite and WO3 nanoparticles toward epoxy resin polymer for radiation shielding
- Prolonged action fertilizer encapsulated by CMC/humic acid
- Preparation and experimental estimation of radiation shielding properties of novel epoxy reinforced with Sb2O3 and PbO
- Fabrication of polylactic acid nanofibrous yarns for piezoelectric fabrics
- Copper phenyl phosphonate for epoxy resin and cyanate ester copolymer with improved flame retardancy and thermal properties
- Synergistic effect of thermal oxygen and UV aging on natural rubber
- Effect of zinc oxide suspension on the overall filler content of the PLA/ZnO composites and cPLA/ZnO composites
- The role of natural hybrid nanobentonite/nanocellulose in enhancing the water resistance properties of the biodegradable thermoplastic starch
- Performance optimization of geopolymer mortar blending in nano-SiO2 and PVA fiber based on set pair analysis
- Preparation of (La + Nb)-co-doped TiO2 and its polyvinylidene difluoride composites with high dielectric constants
- Effect of matrix composition on the performance of calcium carbonate filled poly(lactic acid)/poly(butylene adipate-co-terephthalate) composites
- Low-temperature self-healing polyurethane adhesives via dual synergetic crosslinking strategy
- Leucaena leucocephala oil-based poly malate-amide nanocomposite coating material for anticorrosive applications
- Preparation and properties of modified ammonium polyphosphate synergistic with tris(2-hydroxyethyl) isocynurate for flame-retardant LDPE
- Thermal response of double network hydrogels with varied composition
- The effect of coated calcium carbonate using stearic acid on the recovered carbon black masterbatch in low-density polyethylene composites
- Investigation of MXene-modified agar/polyurethane hydrogel elastomeric repair materials with tunable water absorption
- Damping performance analysis of carbon black/lead magnesium niobite/epoxy resin composites
- Molecular dynamics simulations of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) and TKX-50-based PBXs with four energetic binders
- Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications
- Study on by-products synthesis of powder coating polyester resin catalyzed by organotin
- Ab initio molecular dynamics of insulating paper: Mechanism of insulating paper cellobiose cracking at transient high temperature
- Effect of different tin neodecanoate and calcium–zinc heat stabilizers on the thermal stability of PVC
- High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing
- Impacts of micro-size PbO on the gamma-ray shielding performance of polyepoxide resin
- Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites
- Fiber-reinforced polyvinyl alcohol hydrogel via in situ fiber formation
- Preparation and performance of homogenous braids-reinforced poly (p-phenylene terephthamide) hollow fiber membranes
- Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance
- Impact of WO3 and BaO nanoparticles on the radiation shielding characteristics of polydimethylsiloxane composites
- Comprehensive study of the radiation shielding feature of polyester polymers impregnated with iron filings
- Preparation and characterization of polymeric cross-linked hydrogel patch for topical delivery of gentamicin
- Mechanical properties of rCB-pigment masterbatch in rLDPE: The effect of processing aids and water absorption test
- Pineapple fruit residue-based nanofibre composites: Preparation and characterizations
- Effect of natural Indocalamus leaf addition on the mechanical properties of epoxy and epoxy-carbon fiber composites
- Utilization of biosilica for energy-saving tire compounds: Enhancing performance and efficiency
- Effect of capillary arrays on the profile of multi-layer micro-capillary films
- A numerical study on thermal bonding with preheating technique for polypropylene microfluidic device
- Development of modified h-BN/UPE resin for insulation varnish applications
- High strength, anti-static, thermal conductive glass fiber/epoxy composites for medical devices: A strategy of modifying fibers with functionalized carbon nanotubes
- Effects of mechanical recycling on the properties of glass fiber–reinforced polyamide 66 composites in automotive components
- Bentonite/hydroxyethylcellulose as eco-dielectrics with potential utilization in energy storage
- Study on wall-slipping mechanism of nano-injection polymer under the constant temperature fields
- Synthesis of low-VOC unsaturated polyester coatings for electrical insulation
- Enhanced apoptotic activity of Pluronic F127 polymer-encapsulated chlorogenic acid nanoparticles through the PI3K/Akt/mTOR signaling pathway in liver cancer cells and in vivo toxicity studies in zebrafish
- Preparation and performance of silicone-modified 3D printing photosensitive materials
- A novel fabrication method of slippery lubricant-infused porous surface by thiol-ene click chemistry reaction for anti-fouling and anti-corrosion applications
- Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation
- Tribological characterization of sponge gourd outer skin fiber-reinforced epoxy composite with Tamarindus indica seed filler addition using the Box–Behnken method
- Stereocomplex PLLA–PBAT copolymer and its composites with multi-walled carbon nanotubes for electrostatic dissipative application
- Enhancing the therapeutic efficacy of Krestin–chitosan nanocomplex for cancer medication via activation of the mitochondrial intrinsic pathway
- Variation in tungsten(vi) oxide particle size for enhancing the radiation shielding ability of silicone rubber composites
- Damage accumulation and failure mechanism of glass/epoxy composite laminates subjected to repeated low velocity impacts
- Gamma-ray shielding analysis using the experimental measurements for copper(ii) sulfate-doped polyepoxide resins
- Numerical simulation into influence of airflow channel quantities on melt-blowing airflow field in processing of polymer fiber
- Cellulose acetate oleate-reinforced poly(butylene adipate-co-terephthalate) composite materials
- Radiation shielding capability and exposure buildup factor of cerium(iv) oxide-reinforced polyester resins
- Recyclable polytriazole resins with high performance based on Diels-Alder dynamic covalent crosslinking
- Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane
- Comprehensive performance evaluation based on electromagnetic shielding properties of the weft-knitted fabrics made by stainless steel/cotton blended yarn
- Review Articles
- Preparation and application of natural protein polymer-based Pickering emulsions
- Wood-derived high-performance cellulose structural materials
- Flammability properties of polymers and polymer composites combined with ionic liquids
- Polymer-based nanocarriers for biomedical and environmental applications
- A review on semi-crystalline polymer bead foams from stirring autoclave: Processing and properties
- Rapid Communication
- Preparation and characterization of magnetic microgels with linear thermosensitivity over a wide temperature range
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Synthesis and characterization of proton-conducting membranes based on bacterial cellulose and human nail keratin
- Fatigue behaviour of Kevlar/carbon/basalt fibre-reinforced SiC nanofiller particulate hybrid epoxy composite
- Effect of citric acid on thermal, phase morphological, and mechanical properties of poly(l-lactide)-b-poly(ethylene glycol)-b-poly(l-lactide)/thermoplastic starch blends
- Dose-dependent cytotoxicity against lung cancer cells via green synthesized ZnFe2O4/cellulose nanocomposites

