Abstract
The rhodium 0,5 μm thick layer was deposited on pure nickel and CMSX 4 Ni-based superalloy using the electroplating method. The rhodium coated substrates were aluminized by the CVD method. Oxidation resistance of nonmodified and rhodium modified coatings deposited both on nickel and CMSX 4 superalloy was compared. The triple-layer structure of rhodium modified coatings deposited on pure nickel was found. The β-(Ni,Rh)Al, rhodium doped γ'-Ni3Al and rhodium doped γ-Ni(Al) phases were the main components of the coatings on pure nickel. Two layers – additive and interdiffusion ones were identified in coatings deposited on CMSX 4 superalloy. TEM, SEM and XRD analysis revealed that β-(Ni,Rh)Al phase was the main component of the additive layer. Moreover Topologically Closed-Pack σ phases containing refractory elements in the β-(Ni,Rh)Al matrix of the interdiffusion layer were found. The rhodium modified aluminide coatings have better oxidation resistance than the nonmodified ones both on the pure nickel and CMSX 4 superalloy.
1 Introduction
Diffusion aluminide coatings have been widely applied on turbine engine hot section blades and vane segments as protection from the aggressive conditions of industrial and aero-gas turbines [1]. This protection derives from the promotion of a slow growing Al2O3 oxide scale that acts as a barrier against oxidation and hot corrosion [2].
It is well known that platinum addition significantly improves resistance of aluminide coatings on high temperature oxidation and hot corrosion. It is reported that platinum is effective in reducing oxide growth stresses, enhancing Al concentration in the coating and suppressing void formation in the oxide scale [3]. Platinum encourages formation of pure alumina oxide and reduces oxide growth rate, moreover it acts as NiAl phase stabilizer and prevents the β to γ' phase transformation [4]. Pint et al. [5] showed, that platinum improves the scale adhesion in cyclic oxidation test at 1150 and 1200◦C.Addition of platinum to aluminide coatings deposited by the CVD method reduced or eliminated interfacial void growth thereby improving contact between metal and scale [6]. According to Schneider et al. [7] superalloys coated by platinum modified aluminide coatings have poor high cyclic fatigue performance. Therefore, it is necessary to replace platinum in aluminide coatings by other metals. Literature data indicate that small amounts of reactive elements (zirconium, hafnium, yttrium or cerium) in the aluminide coatings improves their oxidation resistance [8]. Zirconium migrates via β-NiAl grain boundaries towards the surface during oxidation and distributes homogeneous in the whole oxide scale. Zirconium delays oxide spalling during oxidation of coatings, inhibits also the formation of cavities at the β-NiAl/oxide scale interface. Zirconium locates in the sub-lattice of NiAl, contributes to faster annihilation of aluminum vacancies and to their fast diffusion toward the metal/oxide interface and inhibits the cavities formation and growth. The absence of cavities improves thermally grown alumina scale adhesion on the β-NiAl phase coating. Hafnium addition to the aluminide coatings increases oxide scale adhesion to the coating and decreases scale growth rate during oxidation [9]. According to Li et al. [10] hafnium ions first segregate to the scale/coating interface during oxidation. Then diffuse to the oxide scale surface
along oxide grain boundaries. The slow outward diffusion of large hafnium ions stands against the outward diffusion of aluminum ions and inward diffusion of oxygen. Thus the alumina scale growth rate decreases. In spite of the beneficial effect of reactive elements on aluminide coatings’ oxidation resistance, there are some difficulties to incorporate small amount of reactive elements to aluminide coatings.
Improvement of the aluminide coated superalloys oxidation resistance might be achieved by modifying of coated superalloys with other elements. Oxidation resistance of the Ni-8Cr-6Al alloy with 5 wt.% of rhodium was investigated by Felten [11]. The addition of 5wt.%rhodium to the Ni–8Cr–6Al alloy is approximately equivalent to 10 wt.% of platinum. Such small amount of rhodium in the Ni–8Cr–6Al alloy increases oxide scale adherence to the alloy and improves its oxidation resistance. Rhodium addition to the aluminide coating also improves oxidation resistance of the coated superalloy [12].
In spite of good oxidation resistance of the rhodium modified aluminide coating, mechanism of its formation is unknown. Therefore in this paper, the influence of rhodium on the microstructure and phase composition of aluminide diffusion coatings deposited on the nickel substate and CMSX 4 Ni-based superalloy by the CVD metod is analysed. The explanation of mechanism of the rhodium modified aluminide coating formation on pure nickel and CMSX 4 Ni-based superalloy was attempted. Oxidation behaviour of rhodium modified aluminide coatings and nonmodified one on pure nickel and CMSX 4 Ni-based superalloy was compared.
2 Experimental procedure
The commercial nickel of 99.95 wt% purity and CMSX 4 superalloy (monocrystal) were cut and grounded up to SiC No 1000, degreased in ethanol, ultrasonically cleaned and finally coated by the rhodium layer (0.5 μm thick). The chemical composition of the CMSX 4 superalloy is presented in Table 1. The rhodium layer was deposited by the electroplating method. Rhodium electroplating process was conducted in the bath of rhodium sulphate Rh2(SO4)3 – 0.1 g/dm3, sulphuric acid H2SO4 – 0.15 g/dm3 and selenium acid H2SeO4 – 0.010 g/dm3 at 50◦C. The current density during the electroplating process was about 0.2 A/dm2. The samples were cleaned in water heated to 70◦C after electroplating process [13].
Chemical composition of the CMSX 4 superalloy, % wt.
| Ni | Cr | Mo | Nb | Ta | Al | Ti | Co | W | Hf | Re |
|---|---|---|---|---|---|---|---|---|---|---|
| 61.7 | 6.5 | 0.6 | - | 6.5 | 5.6 | 1 | 9 | 6 | 0.1 | 3 |
Afterwards, aluminide coatings were deposited in the low activity CVD process using the CVD BPXPR0325S equipment manufactured by the IonBond company [13, 14, 15].
The microstructure of the cross-sections of the coatings were investigated by the optical microscope Nikon Epiphot 300, the scanning electron microscope (SEM) Hitachi S-3400N and energy dispersive spectroscope (EDS). Phase composition of the nonmodified and rhodium modified aluminide coatings was investigated using ARL X’TRA X-ray diffractometer, equipped with a filtered copper lamp with a voltage of 45 kV. The rhodium modified as well as nonmodified aluminide coatings on the pure nickel and CMSX 4 Ni-based superalloy were oxidized in the air atmosphere at 1100◦C. Samples were placed in the furnace heated to 1100◦C, removed from the furnace after 20 h and cooled in the air atmosphere to the room temperature. Specimens were weighed after each cycle of oxidation.
3 Results
3.1 Rhodium modified aluminide coatings deposited on pure nickel
SEM investigation and EDS analysis of rhodium modified aluminide coatings on pure nickel revealed their triple-layer structure (Figure 1, Table 2). In the first layer, on the top of the coating, the proportion of Ni to Al corresponds to the β-NiAl phase (Table 2, point 1-2). Rhodium
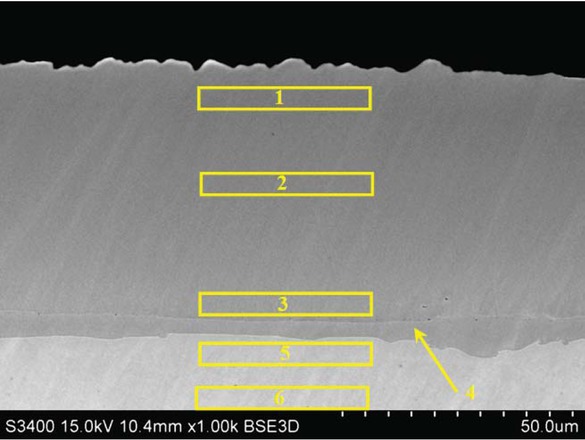
Microstructure on the cross-section of the rhodium modified aluminide coating deposited on pure nickel.
EDS results of the spots of Figure 1 in at.%.
| Spot | Al | Ni | Rh |
|---|---|---|---|
| 1 | 39.6 | 60.4 | <0.1 |
| 2 | 37.9 | 62.1 | <0.1 |
| 3 | 35.1 | 63.5 | 1.4 |
| 4 | 23.1 | 76.8 | 0.1 |
| 5 | 12.9 | 87.1 | 0.1 |
| 6 | - | 100 | - |
content is low (< 0.1 at.%). Therefore, it may be assumed that rhodium doped β-NiAl phase is being formed. The thickness of the top-layer is about 55 μm. The second layer, below is thinner (about 5-10 μm) and consists of the γ'-Ni3Al phase (Table 2, point 4). Rhodium content in the γ'-Ni3Al phase is < 0.1 at.%. It suggests that rhodium doped γ'-Ni3Al phase is being formed. The chemical composition of the third, inner layer, corresponds to the rhodium doped γ-Ni(Al) phase, i.e. the rhodium doped aluminum solid solution in nickel (Table 2, point 5). Chemical composition of the zone below rhodium doped γ-Ni(Al) phase is the same as the matrix composition, that is pure nickel (Table 2, point 6). Rhodium content between the first and second layer is about 1.4 at.%. (Table 2, point 3). Therefore the β-(Ni,Rh)Al phase is being formed between the first and second layers. The EDS analysis of the cross-section of the rhodium modified aluminide coating (Figure 2) suggests nickel outward diffusion from the substrate and aluminum inward diffusion from the surface. The decrease of aluminum content and increase of nickel content from the surface to the material substrate is typical for the low-activity aluminizing process [14]. EDS cross-section results confirmed that the largest rhodium content (1.4 at.%) is between the first and the second layer. The XRD surface analysis revealed that peaks of the β-NiAl phase in the rhodium modified coating are shifted to bigger diffraction angles in comparison to the β-NiAl phase peaks of the nonmodified coating (Figure 3). This is probably due to the incorporation of rhodium to the β-NiAl phase. Rhodium modified aluminide coating deposited on pure nickel has better oxidation resistance than nonmodified ones (Figure 4). The weight loss of the nonmodified coatings is about −18 mg/cm2 just after four cycles of oxidation, while for the
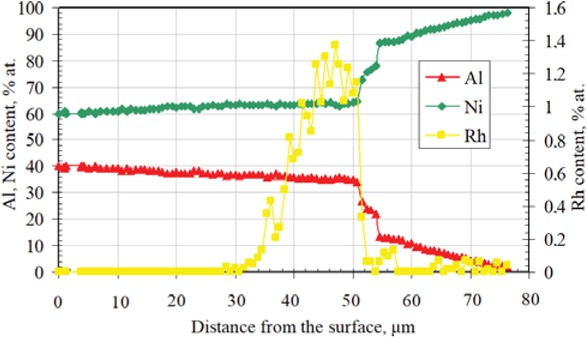
EDS analysis on the cross-section of the rhodium modified aluminide coating deposited on pure nickel.
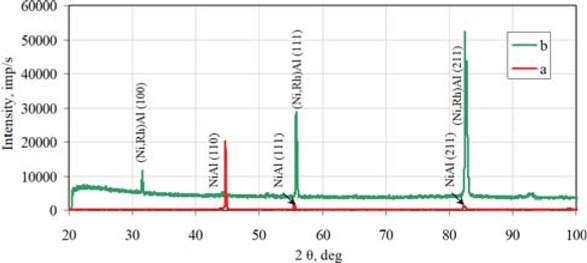
X-ray diffraction of the nonmodified (a) and rhodium modified (b) aluminide coating deposited on pure nickel.
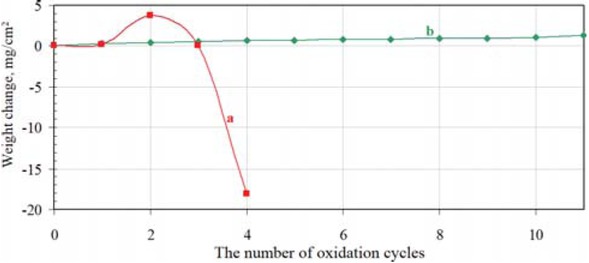
Oxidation test results of the nonmodified (a) and rhodium modified (b) aluminide coating deposited on pure nickel.
rhodium modified coatings the wieght chage is about +1 mg/cm2 after 11 cycles of oxidation (Figure 4).
3.2 Rhodium modified aluminide coatings deposited on CMSX 4 Ni-based superalloy
The SEM investigation and the EDS analysis of the rhodium modified aluminide coating on CMSX 4 superalloy revealed the double layer (additive and interdiffusion) structure (Figure 5). In the first additive layer, on the top of the coating, the proportion of Ni do Al corresponds to the β-
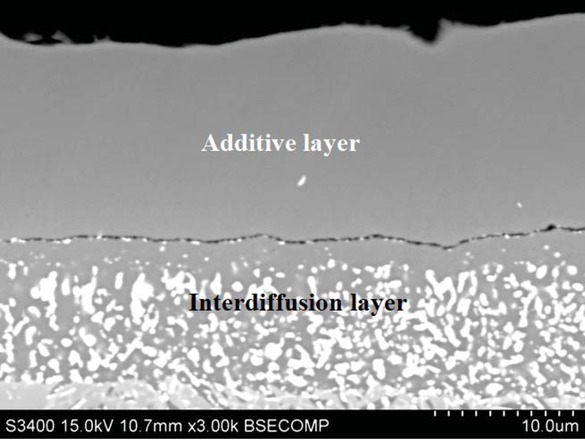
Microstructure on the cross-section of the rhodium modified aluminide coating deposited on CMSX 4 Ni-based superalloy.
NiAl phase (Table 3, point 1). Small rhodium (0.3 at.%) content was also identified. Such chemical composition indicates that the rhodium doped β-NiAl phase in the additive layer is being formed. The thickness of the additive layer is about 15 μm. The EDS analysis on the cross-section confirms the presence of the β-NiAl phase in the interdiffusion layer (Figure 6). The largest (3.2 at.%) rhodium content between the additive and interdiffusion layer was identified. No rhodium-rich particles were observed, so rhodium incorporates into the β-NiAl phase. Rhodium content in the interdiffusion layer is about 2.7 at.%. Therefore it may
![Figure 6 EDS analysis on the cross-section of the rhodium modified aluminide coating deposited on CMSX 4 Ni-based superalloy [13].](/document/doi/10.1515/htmp-2019-0008/asset/graphic/j_htmp-2019-0008_fig_006.jpg)
EDS analysis on the cross-section of the rhodium modified aluminide coating deposited on CMSX 4 Ni-based superalloy [13].
EDS results of the spots of Figure 5 in at.%.
| Spot | Al | Cr | Ni | Rh | Re |
|---|---|---|---|---|---|
| Additive layer | 43.7 | 2.6 | 53.1 | 0.3 | 0.3 |
| Additive/Interdiffusion | 41.4 | 3.0 | 52.0 | 3.2 | 0.4 |
| layer | |||||
| Interdiffusion layer | 36.2 | 7.3 | 51.7 | 2.7 | 2.0 |
be assumed that rhodium modified β-NiAl phase is being formed. The thickness of the interdiffusion layer is about 15 μm. The XRD surface analysis revealed that peaks of the β-NiAl phase of the rhodium modified coating are shifted to
bigger diffraction angles in comparison to the β-NiAl phase peaks of the nonmodified coating (Figure 7). This is probably due to incorporation of rhodium to the β-NiAl phase.
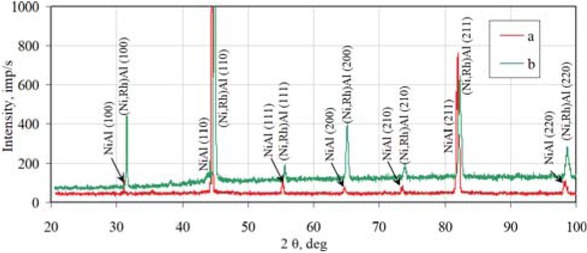
X-ray diffraction of the nonmodified (a) and rhodium modified (b) aluminide coating deposited on CMSX 4 Ni-based superalloy.
TEM investigations of the cross-section also showed a double layers structure of rhodium modified aluminide coatings. β-(Ni,Rh)Al phase was identified in the additive layer (Figure 8a). Moreover α-Al2O3 oxide was formed at the interface of additive/interdiffusion layer (Figure 8b). The grains of β-(Ni,Rh)Al phase were revealed in the interdiffusion layer (Figure 8b). Numerous inclusions of σ-phase containing tungsten, chromium and cobalt in the interdiffusion layer were found (Figure 8c-9). The maps of chemical composition revealed uniform distribution of nickel and aluminum both in the additive and interdiffusion layers. Such distribution confirms the same phase composition of both layers (Figure 9). Rhodium was found only in the β-NiAl phase. This indicates, that it dissolves in the coating (Figure 9).
![Figure 8 TEM image and SAED patterns of the cross-section of the rhodium modified aluminide coating on CMSX 4 superalloy: additive layer (a), additive/interdiffusion layer (b) and interdiffusion layer (c-d) [15].](/document/doi/10.1515/htmp-2019-0008/asset/graphic/j_htmp-2019-0008_fig_008.jpg)
TEM image and SAED patterns of the cross-section of the rhodium modified aluminide coating on CMSX 4 superalloy: additive layer (a), additive/interdiffusion layer (b) and interdiffusion layer (c-d) [15].
![Figure 9 STEM HAADF image of the cross-section of the rhodium modified aluminide coating on CMSX 4 superalloy with corresponding maps of Al, Co, Cr, Ni, W and Rh [15].](/document/doi/10.1515/htmp-2019-0008/asset/graphic/j_htmp-2019-0008_fig_009.jpg)
STEM HAADF image of the cross-section of the rhodium modified aluminide coating on CMSX 4 superalloy with corresponding maps of Al, Co, Cr, Ni, W and Rh [15].
Weight loss of the nonmodified coatings is about −1.1 mg/cm2 just after eight cycles of oxidation, while wieght loss to about −0.8 mg/cm2 in the rhodium modified coatings falls down after 21 cycles of oxidation (Figure 10).
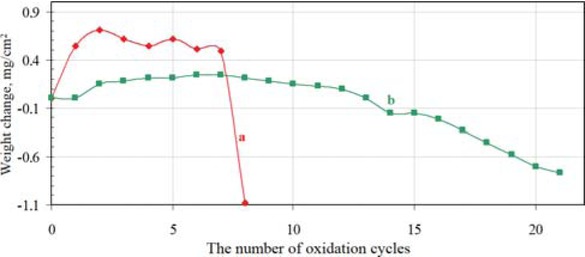
Oxidation test results of the nonmodified (a) and rhodium modified (b) aluminide coating deposited on CMSX 4 Ni-based superalloy.
4 Discussion
The EDS analysis on the crosss-section of the rhodium modified aluminide coating on pure nickel showed outward nickel diffusion from the substrate and the inward aluminum diffusion from the surface to the nickel substrate (Figure 2). XRD surface analysis of the rhodium modified aluminide coating on pure nickel confirmed that rhodium was successively introduced to the coating (Figure 3). No rhodium-rich parties were observed, so rhodium is dissolved in the coating. Rhodium modification of aluminide coating improves oxidation resistance of coated nickel (Figure 4).
Mechanism of nonmodified aluminide coating formation on pure nickel was proposed by Gowad and Boone [16]. It was found, that nickel prefentially diffuses outward and combines with aluminum to form β-NiAl phase during low activity aluminizing of pure nickel.
During the aluminizing process of rhodium coated nickel substrate, rhodium probably dissolves in the nickel substrate, whereas nickel preferentially diffuses out from the substrate and combines with aluminum to form rhodium doped β-NiAl, γ'-Ni3Al and γ-Ni(Al) phases (Figure 11). According to Wanget al. [17] enthalpy of the β-NiAl phase formation at 1000◦C is about −67 kJ/mol, while enthalpy of the γ'-Ni3Al phases formation is −42 kJ/mol. So it may be assumed that rhodium doped β-NiAl phase forms earlier than rhodium doped γ'-Ni3Al phase. The coating grows outward. The largest rhodium content (about 1.4 at.%) between the first and second zones in the β-NiAl phase provides β-(Ni,Rh)Al phase formation.
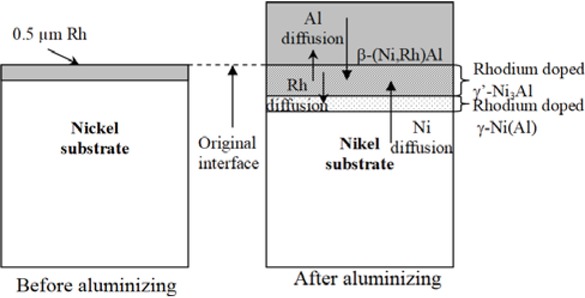
Rhodium modified aluminide coating formation on pure nickel.
The XRD surface analysis of the rhodium modified aluminide coating on CMSX 4 superalloy confirmed that rhodium was successively introduced to the coating (Figure 7). No rhodium-rich parties were observed. The rhodium content between the additive and interdiffusion layers is 3-4 at.%. According to the Al–Ni–Rh phase diagram, this is too low to form rhodium-rich precipitations [18]. So rhodium is dissolved in the coating.
Formation of the rhodium-modified aluminide coating on CMSX 4 superalloy takes place in several steps: firstly rhodium probably dissolves in γ+γ' phases near the surface of CMSX 4 superalloy; secondly nickel, chromium, rhenium and other superalloys’ elements diffuses from the substrate to the surface, leading to formation of the interdiffusion layer; thirdly aluminum—during aluminizing in the CVD process arrives at the surface. Since aluminum has more affinity to nickel than rhodium, it forms the (Ni,Rh)Al phase layer, similarly to the case of the platinum-modified aluminide coating [19] (Figure 12).
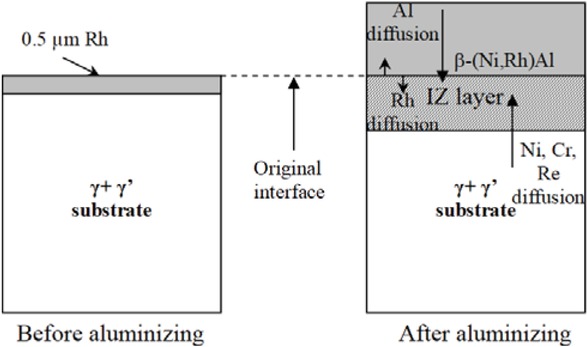
Rhodium modified aluminide coating formation on CMSX 4 Ni-based superalloy.
A small rhodium content in aluminide coatings (3 – 4 at.%) improves oxidation resistance of CMSX 4 superalloy. Moreover rhodium modified aluminide coating deposited on CMSX 4 Ni-based superalloy has better oxidation resistance than the nonmodified one.
5 Conclusions
Rhodium modified aluminide coatings were successfully deposited on pure nickel and CMSX-4 nickel superalloy. Coatings deposited on pure nickel have triple-layer structure. The top layer of coatings consists of the β-(Ni,Rh)Al phase, whereas the second layer consists of the rhodium doped γ'-Ni3Al phase. The rhodium doped γ-Ni(Al) phase of the third layer of the coating was identified. While coatings deposited on CMSX 4 Ni-base superalloy consist of two layers – additive and interdiffusion ones. TEM, SEM and XRD analysis proved that rhodium incorporates into the β-NiAl phase and the β-(Ni,Rh)Al phase is being formed in the additive layer. Topologically Closed-Pack σ phases containing refractory elements distribute in the β-(Ni,Rh)Al matrix of the interdiffusion layer. Small rhodium addition to the aluminide coating improves oxidation resistance of both pure nickel and CMSX 4 Ni-based superalloy.
Acknowledgement
This research was supported by the National Science Centre, Poland (NCN), project number 2016/21/D/ST8/01684.
References
[1] Y. Tamarin, Protective Coatings for Turbine Blades, AMS International, Materials Park, (2002).Search in Google Scholar
[2] G.W. Goward, Materials Science and Technology, 2 (1986) 194-200.10.1179/mst.1986.2.3.194Search in Google Scholar
[3] Y. Zhang, J. A. Haynes,W. Lee, I.G.Wright, B. A. Pint, K.M. Cooley, and P. K. Liaw, Metall. Mater. Trans. A, 32 (2001) 1727-1741.10.1007/s11661-001-0150-6Search in Google Scholar
[4] M. Zagula-Yavorska and J. Sieniawski. Journal of Materials Engineering and Performance, 23 (2014) 918-926.10.1007/s11665-013-0841-3Search in Google Scholar
[5] B.A. Pint, I.G. Wright, W.Y. Lee, Y. Zhang, K. Prüßner, and K.B. Alexander, Materials Science and Engineering A, 245 (1998) 201-211.10.1016/S0921-5093(97)00851-4Search in Google Scholar
[6] B.A. Pint and I.G. Wright, Electrochem Soc. Proc., 98-99 (1998) 263-274.Search in Google Scholar
[7] K. Schneider, H. Arnim, and H. Grünling, Thin Solid Films, 84 (1981) 29-36.10.1016/0040-6090(81)90006-7Search in Google Scholar
[8] B. Pint, T. Haynes, and T. Besmann, Surf. Coat. Technol., 205 (2010) 3287-3293.10.1016/j.surfcoat.2010.03.040Search in Google Scholar
[9] J. Haynes, B. Pint, K. More, and Y. Zhang, Oxidation of metals, 58 (2002) 513-544.10.1023/A:1020525123056Search in Google Scholar
[10] D. Li, H. Guo, D. Wang, T. Zhang, S. Gong, and X. Hubin, Corros. Sci., 66 (2013) 125-135.10.1016/j.corsci.2012.09.010Search in Google Scholar
[11] E. Felten, Oxid. Met., 10 (1976) 23-28.10.1007/BF00611696Search in Google Scholar
[12] M. Zagula-Yavorska, M. Wierzbińska, K. Gancarczyk, and J. Sieniawski, Journal of Microscopy, 23 (2016) 118-123.10.1111/jmi.12401Search in Google Scholar PubMed
[13] M. Zagula-Yavorska, M. Wierzbińska, and J. Sieniawski, Metals, 7 (2017) 548.10.3390/met7120548Search in Google Scholar
[14] J. Romanowska, M. Zagula-Yavorska, and J. Sieniawski, Bull. Mater. Sci., 36(6) (2013) 1043-1048.10.1007/s12034-013-0579-4Search in Google Scholar
[15] M. Zagula-Yavorska, J. Morgiel, J. Romanowska, and J. Sieniawski, Journal of Microscopy, 261(3) (2016) 320-345.10.1111/jmi.12344Search in Google Scholar
[16] G. Goward and D. Bone, Oxidation of Metals, 3 (1971) 475-495.10.1007/BF00604047Search in Google Scholar
[17] Y. Wang, Z. Liu, and L. Chen, Acta Materialia, 52 (2004) 2665-2671.10.1016/j.actamat.2004.02.014Search in Google Scholar
[18] B. Przepiórzyński, S. Mi, B. Grushko, and M. Surowiec, Intermetallics, 15 (2007) 918-928.10.1016/j.intermet.2006.10.051Search in Google Scholar
[19] J. Benoist, K. Badawi, A. Malie, C. Ramade, Surf. Coat. Technol., 182 (2004) 14.10.1016/S0257-8972(03)00871-5Search in Google Scholar
© 2019 M. Zagula-Yavorska, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites
Articles in the same Issue
- Frontmatter
- Review Article
- Research on the Influence of Furnace Structure on Copper Cooling Stave Life
- Influence of High Temperature Oxidation on Hydrogen Absorption and Degradation of Zircaloy-2 and Zr 700 Alloys
- Correlation between Travel Speed, Microstructure, Mechanical Properties and Wear Characteristics of Ni-Based Hardfaced Deposits over 316LN Austenitic Stainless Steel
- Factors Influencing Gas Generation Behaviours of Lump Coal Used in COREX Gasifier
- Experiment Research on Pulverized Coal Combustion in the Tuyere of Oxygen Blast Furnace
- Phosphate Capacities of CaO–FeO–SiO2–Al2O3/Na2O/TiO2 Slags
- Microstructure and Interface Bonding Strength of WC-10Ni/NiCrBSi Composite Coating by Vacuum Brazing
- Refill Friction Stir Spot Welding of Dissimilar 6061/7075 Aluminum Alloy
- Solvothermal Synthesis and Magnetic Properties of Monodisperse Ni0.5Zn0.5Fe2O4 Hollow Nanospheres
- On the Capability of Logarithmic-Power Model for Prediction of Hot Deformation Behavior of Alloy 800H at High Strain Rates
- 3D Heat Conductivity Model of Mold Based on Node Temperature Inheritance
- 3D Microstructure and Micromechanical Properties of Minerals in Vanadium-Titanium Sinter
- Effect of Martensite Structure and Carbide Precipitates on Mechanical Properties of Cr-Mo Alloy Steel with Different Cooling Rate
- The Interaction between Erosion Particle and Gas Stream in High Temperature Gas Burner Rig for Thermal Barrier Coatings
- Permittivity Study of a CuCl Residue at 13–450 °C and Elucidation of the Microwave Intensification Mechanism for Its Dechlorination
- Study on Carbothermal Reduction of Titania in Molten Iron
- The Sequence of the Phase Growth during Diffusion in Ti-Based Systems
- Growth Kinetics of CoB–Co2B Layers Using the Powder-Pack Boriding Process Assisted by a Direct Current Field
- High-Temperature Flow Behaviour and Constitutive Equations for a TC17 Titanium Alloy
- Research on Three-Roll Screw Rolling Process for Ti6Al4V Titanium Alloy Bar
- Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships
- Formation Mechanism and Influence Factors of the Sticker between Solidified Shell and Mold in Continuous Casting of Steel
- Casting Defects in Transition Layer of Cu/Al Composite Castings Prepared Using Pouring Aluminum Method and Their Formation Mechanism
- Effect of Current on Segregation and Inclusions Characteristics of Dual Alloy Ingot Processed by Electroslag Remelting
- Investigation of Growth Kinetics of Fe2B Layers on AISI 1518 Steel by the Integral Method
- Microstructural Evolution and Phase Transformation on the X-Y Surface of Inconel 718 Ni-Based Alloys Fabricated by Selective Laser Melting under Different Heat Treatment
- Characterization of Mn-Doped Co3O4 Thin Films Prepared by Sol Gel-Based Dip-Coating Process
- Deposition Characteristics of Multitrack Overlayby Plasma Transferred Arc Welding on SS316Lwith Co-Cr Based Alloy – Influence ofProcess Parameters
- Elastic Moduli and Elastic Constants of Alloy AuCuSi With FCC Structure Under Pressure
- Effect of Cl on Softening and Melting Behaviors of BF Burden
- Effect of MgO Injection on Smelting in a Blast Furnace
- Structural Characteristics and Hydration Kinetics of Oxidized Steel Slag in a CaO-FeO-SiO2-MgO System
- Optimization of Microwave-Assisted Oxidation Roasting of Oxide–Sulphide Zinc Ore with Addition of Manganese Dioxide Using Response Surface Methodology
- Hydraulic Study of Bubble Migration in Liquid Titanium Alloy Melt during Vertical Centrifugal Casting Process
- Investigation on Double Wire Metal Inert Gas Welding of A7N01-T4 Aluminum Alloy in High-Speed Welding
- Oxidation Behaviour of Welded ASTM-SA210 GrA1 Boiler Tube Steels under Cyclic Conditions at 900°C in Air
- Study on the Evolution of Damage Degradation at Different Temperatures and Strain Rates for Ti-6Al-4V Alloy
- Pack-Boriding of Pure Iron with Powder Mixtures Containing ZrB2
- Evolution of Interfacial Features of MnO-SiO2 Type Inclusions/Steel Matrix during Isothermal Heating at Low Temperatures
- Effect of MgO/Al2O3 Ratio on Viscosity of Blast Furnace Primary Slag
- The Microstructure and Property of the Heat Affected zone in C-Mn Steel Treated by Rare Earth
- Microwave-Assisted Molten-Salt Facile Synthesis of Chromium Carbide (Cr3C2) Coatings on the Diamond Particles
- Effects of B on the Hot Ductility of Fe-36Ni Invar Alloy
- Impurity Distribution after Solidification of Hypereutectic Al-Si Melts and Eutectic Al-Si Melt
- Induced Electro-Deposition of High Melting-Point Phases on MgO–C Refractory in CaO–Al2O3–SiO2 – (MgO) Slag at 1773 K
- Microstructure and Mechanical Properties of 14Cr-ODS Steels with Zr Addition
- A Review of Boron-Rich Silicon Borides Basedon Thermodynamic Stability and Transport Properties of High-Temperature Thermoelectric Materials
- Siliceous Manganese Ore from Eastern India:A Potential Resource for Ferrosilicon-Manganese Production
- A Strain-Compensated Constitutive Model for Describing the Hot Compressive Deformation Behaviors of an Aged Inconel 718 Superalloy
- Surface Alloys of 0.45 C Carbon Steel Produced by High Current Pulsed Electron Beam
- Deformation Behavior and Processing Map during Isothermal Hot Compression of 49MnVS3 Non-Quenched and Tempered Steel
- A Constitutive Equation for Predicting Elevated Temperature Flow Behavior of BFe10-1-2 Cupronickel Alloy through Double Multiple Nonlinear Regression
- Oxidation Behavior of Ferritic Steel T22 Exposed to Supercritical Water
- A Multi Scale Strategy for Simulation of Microstructural Evolutions in Friction Stir Welding of Duplex Titanium Alloy
- Partition Behavior of Alloying Elements in Nickel-Based Alloys and Their Activity Interaction Parameters and Infinite Dilution Activity Coefficients
- Influence of Heating on Tensile Physical-Mechanical Properties of Granite
- Comparison of Al-Zn-Mg Alloy P-MIG Welded Joints Filled with Different Wires
- Microstructure and Mechanical Properties of Thick Plate Friction Stir Welds for 6082-T6 Aluminum Alloy
- Research Article
- Kinetics of oxide scale growth on a (Ti, Mo)5Si3 based oxidation resistant Mo-Ti-Si alloy at 900-1300∘C
- Calorimetric study on Bi-Cu-Sn alloys
- Mineralogical Phase of Slag and Its Effect on Dephosphorization during Converter Steelmaking Using Slag-Remaining Technology
- Controllability of joint integrity and mechanical properties of friction stir welded 6061-T6 aluminum and AZ31B magnesium alloys based on stationary shoulder
- Cellular Automaton Modeling of Phase Transformation of U-Nb Alloys during Solidification and Consequent Cooling Process
- The effect of MgTiO3Adding on Inclusion Characteristics
- Cutting performance of a functionally graded cemented carbide tool prepared by microwave heating and nitriding sintering
- Creep behaviour and life assessment of a cast nickel – base superalloy MAR – M247
- Failure mechanism and acoustic emission signal characteristics of coatings under the condition of impact indentation
- Reducing Surface Cracks and Improving Cleanliness of H-Beam Blanks in Continuous Casting — Improving continuous casting of H-beam blanks
- Rhodium influence on the microstructure and oxidation behaviour of aluminide coatings deposited on pure nickel and nickel based superalloy
- The effect of Nb content on precipitates, microstructure and texture of grain oriented silicon steel
- Effect of Arc Power on the Wear and High-temperature Oxidation Resistances of Plasma-Sprayed Fe-based Amorphous Coatings
- Short Communication
- Novel Combined Feeding Approach to Produce Quality Al6061 Composites for Heat Sinks
- Research Article
- Micromorphology change and microstructure of Cu-P based amorphous filler during heating process
- Controlling residual stress and distortion of friction stir welding joint by external stationary shoulder
- Research on the ingot shrinkage in the electroslag remelting withdrawal process for 9Cr3Mo roller
- Production of Mo2NiB2 Based Hard Alloys by Self-Propagating High-Temperature Synthesis
- The Morphology Analysis of Plasma-Sprayed Cast Iron Splats at Different Substrate Temperatures via Fractal Dimension and Circularity Methods
- A Comparative Study on Johnson–Cook, Modified Johnson–Cook, Modified Zerilli–Armstrong and Arrhenius-Type Constitutive Models to Predict Hot Deformation Behavior of TA2
- Dynamic absorption efficiency of paracetamol powder in microwave drying
- Preparation and Properties of Blast Furnace Slag Glass Ceramics Containing Cr2O3
- Influence of unburned pulverized coal on gasification reaction of coke in blast furnace
- Effect of PWHT Conditions on Toughness and Creep Rupture Strength in Modified 9Cr-1Mo Steel Welds
- Role of B2O3 on structure and shear-thinning property in CaO–SiO2–Na2O-based mold fluxes
- Effect of Acid Slag Treatment on the Inclusions in GCr15 Bearing Steel
- Recovery of Iron and Zinc from Blast Furnace Dust Using Iron-Bath Reduction
- Phase Analysis and Microstructural Investigations of Ce2Zr2O7 for High-Temperature Coatings on Ni-Base Superalloy Substrates
- Combustion Characteristics and Kinetics Study of Pulverized Coal and Semi-Coke
- Mechanical and Electrochemical Characterization of Supersolidus Sintered Austenitic Stainless Steel (316 L)
- Synthesis and characterization of Cu doped chromium oxide (Cr2O3) thin films
- Ladle Nozzle Clogging during casting of Silicon-Steel
- Thermodynamics and Industrial Trial on Increasing the Carbon Content at the BOF Endpoint to Produce Ultra-Low Carbon IF Steel by BOF-RH-CSP Process
- Research Article
- Effect of Boundary Conditions on Residual Stresses and Distortion in 316 Stainless Steel Butt Welded Plate
- Numerical Analysis on Effect of Additional Gas Injection on Characteristics around Raceway in Melter Gasifier
- Variation on thermal damage rate of granite specimen with thermal cycle treatment
- Effects of Fluoride and Sulphate Mineralizers on the Properties of Reconstructed Steel Slag
- Effect of Basicity on Precipitation of Spinel Crystals in a CaO-SiO2-MgO-Cr2O3-FeO System
- Review Article
- Exploitation of Mold Flux for the Ti-bearing Welding Wire Steel ER80-G
- Research Article
- Furnace heat prediction and control model and its application to large blast furnace
- Effects of Different Solid Solution Temperatures on Microstructure and Mechanical Properties of the AA7075 Alloy After T6 Heat Treatment
- Study of the Viscosity of a La2O3-SiO2-FeO Slag System
- Tensile Deformation and Work Hardening Behaviour of AISI 431 Martensitic Stainless Steel at Elevated Temperatures
- The Effectiveness of Reinforcement and Processing on Mechanical Properties, Wear Behavior and Damping Response of Aluminum Matrix Composites

