Ammonia gas-sensing behavior of uniform nanostructured PPy film prepared by simple-straightforward in situ chemical vapor oxidation
-
Khong Van Nguyen
Abstract
A highly uniform nanostructured polypyrrole (PPy) film prepared by a simple, straightforward in situ route of chemical vapor oxidation has been demonstrated as a sensitive substrate for NH3 gas sensing. The structure of PPy film was investigated by scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), and X-ray diffraction (XRD). The binding characteristics of the functional groups of the PPy film were examined by Fourier transform infrared and Raman spectroscopy. NH3 sensing properties of the PPy film were evaluated by its resistive response to gas concentrations from 45 to 350 ppm at different temperatures ranging from 25 to 100°C. The sensing response maximum value was 142.6% when exposed to 350 ppm of NH3 gas at room temperature (25°C). The sensing response of PPy film shows an excellent linear relationship and high selectivity toward NH3. The NH3 sensing mechanism is due to the physisorption and chemisorption interactions of NH3 molecules and the adsorptive sites of PPy (polaron and bipolaron charging carriers).
1 Introduction
Ammonia gas is well known as one of the most widely produced compounds and has been utilized in numerous fields of the industry as an indispensable raw material [1,2,3,4]. Recently, an estimated 20% of the NH3 produced has been used for medicines, explosives, cleaning products, and refrigeration; meanwhile, the remaining 80% is used for nitrogen-based fertilizer [5]. However, the risk of NH3 gas leakage from such industrial activities into the atmosphere is a significant issue. The NH3 gas causes air pollution, which is hazardous to human health and even carries the possibility of an NH3 gas explosion. The permissible exposure limit for NH3 gas is only 25 ppm averaged over 8 h, as established by the U.S. Occupational Safety and Health Administration (OSHA) [6,7]. Moreover, NH3-related aerosols such as ammonium nitrate and ammonium sulfate can negatively impact the greenhouse balance worldwide [6,7]. Especially, the formation of synergistic particles of HNO3–H2SO4–NH3 in the upper free troposphere was found. It has been demonstrated that nitric acid, sulfuric acid, and ammonia form particles synergistically faster and in a greater order of magnitude than any two of the three mentioned components [8]. Recently, a daily breath analyzer that monitors NH3 concentration from exhaled human breath to identify lung or renal disorders in patients with non-invasiveness has been an attractive potential field for health diagnosis [9,10,11]. Therefore, the accurate measurement of NH3 gas has attracted significant attention and demand.
There are various nanomaterials and technologies used for the fabrication of ammonia gas sensors and shown in many reports [12,13,14,15]. For example, ammonia sensors have been developed using nanostructured metal oxides such as SnO2, NiO, ZnO, W18O9, and MoO3 [16]. The high surface to volume of the metal oxides nanostructured exhibits good sensitivities; nevertheless, a significant disadvantage of metal oxide NH3 sensors is the high working temperature. Therefore, to extend the practical application of NH3 sensors, conductive polymers [PANI and polypyrrole (PPy)] are considered potential candidates due to their low operating temperature. In this regard, PPy is a regularly utilized material for NH3-sensitive layers [17,18,19,20]. The charge carriers are known as soliton (having a spin of ½ and can either exist in neutral or charged states), polaron (possessing spin of ½ and charge of +1), and bipolaron (possessing spin of zero and charge of +2). They can move through several consecutive steps of odd electron extraction per step arising from the intercalation/expulsion of ions and solvent molecules or the insertion of some dopants driven by oxidation/reduction [21]. It can be considered that the states of these charge carriers are two key factors that strongly affect the NH3 gas-sensing performance. For example, Zhang et al. [22] revealed that a high-density and small-diameter (about 50 nm) of nanowire arrays of PPy had contributed to a significant increase in their surface-to-volume ratio, leading to high NH3 gas responses changing from 10 to 26% for the gas concentration increased from 1.5 to 77 ppm, respectively. However, this PPy response dependence on the NH3 gas concentration only exhibited a small linear range from 1.5 to 12 ppm, corresponding to a change from 10 to 22%. In other works, a porous network PPy thin film was synthesized by microwave-assisted bath deposition [23]. When tested toward 200 ppm, the NH3-sensing response of this PPy film was about 85%, and the response/recovery times were 70 and 110 s, respectively. Zhang et al. [24] showed two different sensors based on PPy/NS@silk-fiber and PPyNS@sponge fabricated by using a simple in situ chemical oxidation polymerization. The NH3-sensing response of the PPy@sponges (14.51%) was far lower than that of the PPy/NS@silkfibers (73.25 %) when recorded at 100 ppm under room temperature and relative humidity (RH) of 68%. The PPy/NS@silk fiber disclosed long-term stability and short response/recovery times of 24 and 69 s, respectively. These results were explained by attributing the high surface area of the flexible PPy/NS@silk fiber due to the relatively rough hill-like shapes. However, the sensing mechanisms in previous studies have already been examined and mentioned as the complex redox reactions of gas-adsorbed active sites that decreased the conductivity of these materials. Nonetheless, there has not been a fulfilled and clear explanation for the gas-sensing performance of the conducting polymers, particularly under different nanostructure morphologies.
In this study, the PPy nanoparticle film with a highly uniform morphology was investigated. The PPy film significantly improves the NH3-sensing performance operating at room temperature. Influences of RH and operating temperature (25–100°C) on the sensing performance of the films were evaluated in detail. Furthermore, the mechanisms of polaron and bipolaron states’ formation and adsorptive interactions between NH3 molecule and adsorptive sites were also proposed.
2 Experimental
Reagents pyrrole monomer, ferric chloride (FeCl3·6H2O), and ethanol purchased from Sigma-Aldrich were used without further treatment. The Al2O3 substrates (6.3 mm × 6.3 mm × 0.25 mm) with two Pt electrodes were used for fabricating the nanostructured PPy films as shown in Figure 1. A Pt micro-heat stove integrated on the bottom of the Al2O3 substrate [25] was used to modulate the operating temperature.

Schematic representation of Pt/Al2O3 substrate (a) and SEM image of Al2O3 surface (b).
The PPy nanostructured film was synthesized by a chemical vapor-phase polymerization using FeCl3 as an oxidant; the fabrication procedure was described in a previous publication [25]. In detail, ferric chloride salt FeCl3·6H2O was dissolved into 50% ethanol and 50% distilled water to obtain a 0.02 M FeCl3 solution. The Pt/Al2O3 substrates were covered with FeCl3 oxidant by a spray-coating technique (electrospinning machine NTEC, Nantong) with a spraying flow of 0.2 ml h−1, a distance from the injector to the surface of the Al2O3 substrate of 15 cm, and a spray duration within 30 min at 45°C. The FeCl3-coated Al2O3 substrate was then transferred to a polymerization chamber (1 l) containing a pyrrole monomer source. The vapor-phase polymerization process took place for 60 min at atmospheric pressure. The sample was washed with ethanol and distilled water several times to remove contaminants and then dried in an oven at 50°C for 1 h to receive the nanostructured PPy film.
Morphologies and analyzing elements components of the synthesized PPy film were investigated using a scanning electron microscope with energy-dispersive X-ray spectroscopy (SEM-4800 Hitachi). Crystal structural and bonding characteristics of the film were analyzed via X-ray diffraction (SIEMENS-D5005), spectra of Fourier transform infrared (FTIR) (Shimazu Fourier transform infrared spectrometer, IRAffinity-1S), and Raman scattering spectra (Horiba Raman spectrometer, Labram HR 800).
Gas-sensing performances of the synthesized PPy film were investigated with NH3 concentrations from 45 to 350 ppm in dry air (80% N2 + 20% O2) by flow-through mixing method [26] and at operating temperatures of 25, 45, 60, 80, and 100°C. To examine the influences of humidity on the sensing characteristics of the synthesized PPy film, a humidifier with a working principle based on the saturated water vapor pressure was used. The RH in the measuring chamber was selected with 11, 16, 33, 75, 84, and 94 %RH by using saturated salt solutions including LiCl, KOH, MgCl2, NaCl, KCl, and Sr(NO3)2, respectively. The sensing response (S) was calculated by the following formula:
3 Results and discussion
3.1 Material characteristics
The surface morphologies of the FeCl3-coated layer and the synthesized PPy film on the Al2O3 substrate are shown in Figure 2a and b, respectively. It could be seen that the surface morphology of the synthesized PPy film is a highly ordered and uniform structure consisting of nanoparticles with a diameter of about 30–50 nm. The result exhibited a cross-linked 3D network morphology, different from the conventional PPy structures like nanoparticles-cluster, nanofibers-matrix, or nano-slabs [27].

SEM images of FeCl3 particles (a) and PPy nanoparticles (b) on Al2O3 substrate and EDS spectrum (c) and XRD pattern (d) of the synthesized PPy film.
The EDS spectrum of the synthesized PPy film (Figure 2c) showed peaks assigned for component elements of C, N, Cl, Fe, Al, and O at appropriate energy levels. Al and O elements belonged to the Al2O3 substrate, and C, N, Fe, and Cl were the components of the PPy film. An excess oxygen atom ratio (∼2.55%) compared with the stoichiometric composition ratio of 2/3 was ascribed to the absorption of water molecules into the PPy film. The EDS and XRD (showed Figure 2c and d, respectively) results of the PPy film also revealed that Fe and Cl elements could contribute as doping reagents (Fe2+ and Cl−) in PPy structure, as previously reported [18,28]. A typical nearly amorphous structure of the PPy film could be seen as a broad characteristic peak centered at approximately 2θ = 23.5°, which should be assigned the inter-chain spacing order of PPy [29]. The peaks at 2θ = 32.2, 33.4, 62.8, and 40.3° in the XRD pattern (Figure 2d) indexed (104), (110), (214), and (113) could be a reflection of the FeCl2 structure (JCPDF 77-0044) and implied that existence of Fe2+ doping in the synthesized PPy film. The behavior is caused by iron ions attached to the Brönsted pyrrole monomers during the polymerization process [30].
The FTIR spectra in the range from 500 to 4,000 cm−1 of the PPy films under conditions of as synthesized, NH3 adsorbed, and NH3 desorbed are presented in Figure 3a. The characteristic vibrations of specific functional groups of PPy structure were disclosed. The peaks at 1,543 and 1,482 cm−1 attributed to C═C and C–C stretching bonds of main chains PPy [31,32]. Furthermore, the relative intensity of two peaks of 1,543/1,482 cm−1 increases from 0.95 to 1.14 for the as-synthesized PPy and the NH3-adsorbed PPy, respectively, indicating that a preferential form of the aromatic increased while the quinoid form decreased when conducted the NH3 adsorption. Consequently, doping levels of the NH3-adsorbed PPy could be reduced, and electron movement in the PPy network was hindered due to an electron trapping effect that induced NH3 adsorption.

FTIR spectra of as-synthesized PPy, NH3-adsorbed PPy, and NH3 desorbed-PPy.
The small peaks at 3,240 cm−1 and the shoulder at 1,612 cm−1 were assigned to the presence of the N–H deformation vibrations in secondary amine in pyrrole rings [33,34] and stretching and bending modes of vibrations of –OH groups, respectively. O–H vibrations mainly originated from absorbed water molecules on the PPy film. The characteristic peak at 3,125 cm−1 corresponded to the stretching vibrations of hydrogen-bonded surface water molecules [35,36]. Notably, the peak around 1,093 cm−1 was ascribed to in-plane bending vibration of
For further insight into the relevance of the PPy film, Raman spectroscopic technique was employed in a wave number range of 600–2,000 cm−1. As shown in Figure 4a, the relative intensities in regions of 924–931, 1,047–1,050, and 1,608–1,614 cm−1 could be assigned to the oxidized states, and they decreased under the NH3 adsorption (Figure 4b). Besides a sharp peak at 1,612 cm−1 belonged the characteristic of C═C in-ring vibrations of the quinoid form, there is a shoulder at 1,556 cm−1, which presents inter-ring C–C vibrations of the short conjugation length [46]. The relative intensity

Raman spectra of as-synthesized PPy and NH3-adsorbed PPy (a), and the decrease of Raman intensities under NH3 adsorption (b).
From the FTIR and Raman results, the mechanisms for the formation of the polaron and bipolaron structures; and

Illustration of polaron and bipolaron structures and
3.2 Gas-sensing performance
To examine the gas-sensing behaviors of the PPy film, the resistance response at two temperatures of 25 and 100°C when exposed to four cycles of 350 ppm NH3 gas in dry air was measured, as shown in Figure 6. It was observed that the resistance increased when responded to NH3 gas and decreased to its initial value when recovered to dry air. The results showed that the PPy film had good reversibility and stability; thus, this material could be suitable for utilizing the NH3 gas sensor.
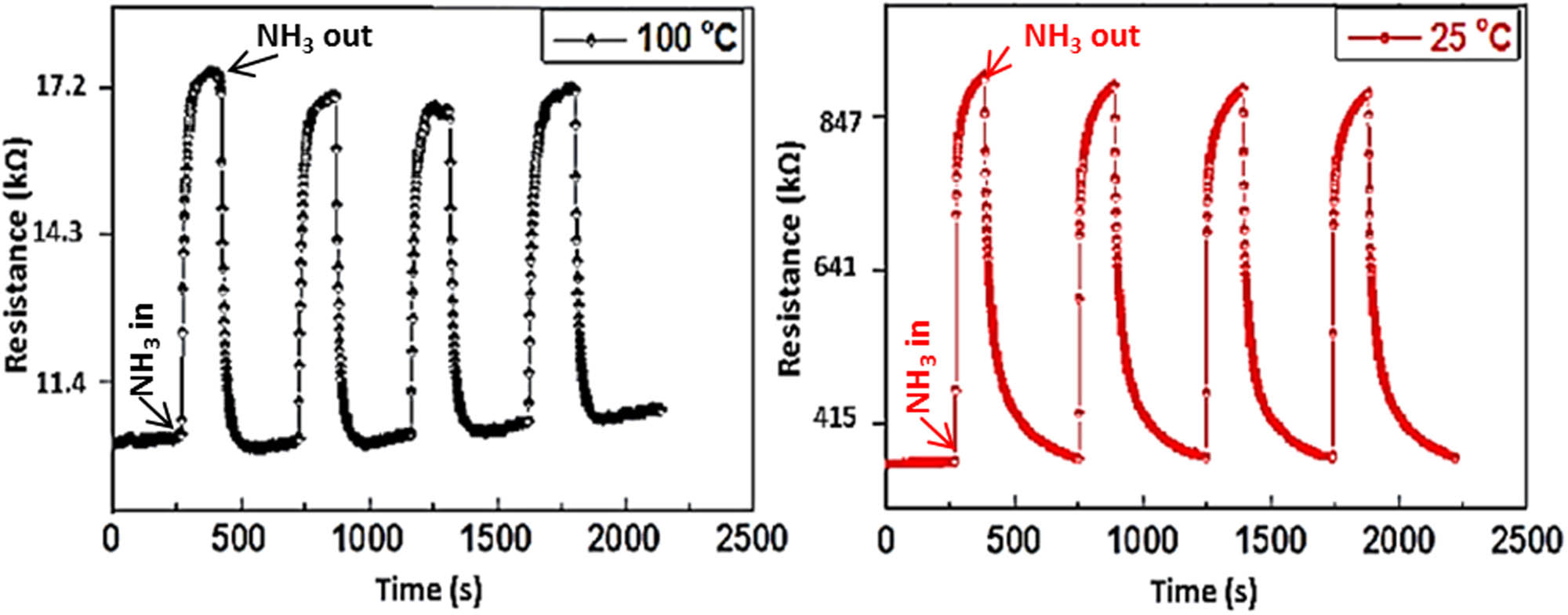
Resistance of the PPy film responding to four cycles of 350 ppm NH3/dry air.
NH3-sensing responses of the PPy film were investigated for different concentrations from 45 to 350 ppm at temperatures of 25, 45, 60, 80, and 100°C, as presented in Figure 7a. From the results, it was obvious that the resistance increased upon increasing the NH3 gas concentration. As shown in Figure 7a and b, the PPy film revealed a similar gas-sensing characteristic at different operating temperatures. It also demonstrated that the PPy film exhibited well temperature-driven reversibility to all the tested NH3 gas concentrations. The sensing responses at 25, 45, 60, 80, and 100°C (Figure 7b) showed that the dependence between the response and NH3 concentration is almost linear, with a standard deviation under 5%. With each NH3 gas concentration, the sensing response decreased with increasing the operating temperature and reached saturate values at the high operating temperature. For example, at 350 ppm NH3 (Figure 7c), the sensing response decreased significantly by about 80% when increased the operating temperature from 25 to 100°C.

Resistance (a) of the PPy film responding to cycles of NH3/dry air and its sensing response dependences on NH3 concentration (b) and operating temperature (c).
Figure 8 shows the response/recovery times of the PPy film. The response times exhibited a similar tendency with high in the low temperatures and low in the high temperatures for all the tested NH3 gas concentrations (Figure 8a). Nevertheless, the response times decreased according to increasing the NH3 concentration (Figure 8b). These behaviors could be considered to reveal the competition of the temperature-driven convective diffusion and the adsorption/desorption processes involving physical adsorption and chemisorption at the active sites. At 25 and 100°C, with the low NH3 concentration, the collisions of NH3 molecules per unit surface area per unit time were slow. Then, the number of the NH3 molecules with their kinetic energy having a larger adsorption enthalpy and their striking onto active sites are still low. Thus, a long time was needed to achieve a full surface coverage of the PPy film. Meanwhile, at higher NH3 concentration, NH3 molecules moved and overcame a certain thickness of the diffusion-convection layer to reach the effective thickness, where collisions between NH3 molecules the kinetic energy of NH3 molecules were kept a most constant and still larger adsorption enthalpy to make more sticking collisions, leading the decreases in response time. For each NH3 concentration (Figure 8a), when the temperature was raised, the kinetic energies of NH3 molecules and active sites were increased so that there were more collisions between them. This concluded that the mean lifetime of collisions was decreased, and the initial sticking probability of collisions was due to a decrease in the physical adsorption. With sticking collisions having a larger lifetime, they could be converted into physical adsorption states. As a result, the response time increased and reached a maximum value of 45°C. If the temperature continued to increase, the speed and kinetic energies of NH3 molecules increased intensively. Under the condition, the speed movement of the NH3 molecules increased, resulting in more frequent and more forceful collisions with the adsorptive sites. The increased sticking probability of collisions led to decreased response time for complete physical adsorption. In the high temperatures from 60 to 100°C, the initial stage of the physical adsorption occurred at the surface coverage reached a maximum, and the active interaction sites for chemisorption were activated with a high activation energy. At this stage, a number of the NH3 molecules with kinetic energies larger enthalpy of chemisorption were small. For a near-saturation coverage of the active interaction sites of chemisorption, the response time could increase and reach a maximum value at the high temperature, as observed at 80°C in this work. After that, the higher temperature, the greater fraction of sticking collisions of NH3 molecules with energies larger enthalpy; thus, the response time decreased. In contrast to a complicated response time evolution, the recovery time’s dependence on the temperature and the NH3 concentration was simple. As shown in Figure 8c, an exception from the lowest NH3 concentration (45 ppm), the recovery times at the higher concentrations decreased with increasing the operating temperature.

Response/recovery times of the PPy film depending on operating temperature (a and c) and NH3 concentration (b and d).
With the lowest NH3 gas concentration of 45 ppm, the thickness of the diffusion layer could be the greatest when compared to the other gas concentrations. Thus, from 25 to 45°C, NH3 molecules adsorbed on the active sites were released from the PPy surface and took a long time to exist in the diffusion layer. As a result, a gradient of the NH3 gas concentration gradually decreased, and then, the recovery time increased with increasing the operating temperature and reached a maximum. As the high operating temperature, the average speed and kinetic energy of NH3 molecules could increase intensively; thus, the NH3 molecules rapidly moved away from the diffusion layer to promote the desorption, as observed in Figure 8d. On the dependence of the recovery time on the NH3 concentration, there were two tendencies. In the low range of 25–45°C, the recovery time increased with increasing the NH3 gas concentration and reached a maximum of 90 ppm. In the high range of 60–100°C, the fashion of the recovery time and the NH3 concentration changed from convex (60–80°C) to concave (100°C). This change showed that when the NH3 gas concentration increased in the concave, the recovery time also increased and reached a constant value. In contrast, in the convex, the recovery time decreased and reached a minimum value. The NH3 molecules that were physically adsorbed on the top of the film surface were more quickly released into the diffusion layer when exposed to dry air. With the NH3 concentration increase, the number of NH3 molecules that were physically and chemically adsorbed could occur on the film surface; therefore, a layer of molecules could be physically adsorbed on top of the underlying chemisorbed layer, resulting in the recovery time increased again. However, in the higher operating temperature (100°C), the NH3 molecules’ speed that was diffused into/out of the film surface was high enough to form a higher number of chemisorbed NH3 molecules. Consequently, the recovery time increased with increasing the NH3 concentration.
The selectivity tests were carried out by evaluating the influence of some interfering gases (Figure 9a). The interfering gases included NO2, H2S, CO, and H2 with concentrations 100, 100, 1,000, and 1,000 ppm, respectively. The concentrations were many folds compared to the NH3 concentration of 45 ppm. However, the NH3 sensing response value of the PPy film was substantially greater for the other interfering gases. The results indicated that the film had an excellent specific identification capacity for NH3 gas. It was also obvious that the RH was as high as 94%, which could cause a capillary condensation on the PPy surface; the NH3-sensing response was much higher for responding to the high RH concentration. An effect of RH fluctuations on the NH3-sensing performance was conducted at various humidity concentrations of 11, 16, 33, 75, 84, and 94 %RH under the sensing response to 45 ppm NH3 (Figure 9b and c). The results showed that the sensing responses increased from 3.34 to 8.95% with the RH increase.

Selectivity of the PPy film at 25 and 100°C (a) and its influence of humidity on NH3 gas sensing (b and c).
3.3 NH3-sensing mechanism of PPy
In general, the interaction mechanism of PPy with proton-adsorbed
In more detail, Figure 10 represents the adsorption and desorption of NH3 molecules on adsorptive sites within the PPy backbone. Herein, the adsorptive interactions were depicted by dashed lines and arrows that presented the movement of electrons. The physical adsorption of NH3 molecules by the electrostatic interaction of hydrogen atoms of NH3 molecule and π electrons of the conjugated system decrease π electrons’ density at the adsorbed sites, so the double bonds were represented by one slight line and one bold line (before the adsorption represented by two bold lines). The NH3 chemisorption was conducted at the positive holes in polaron and bipolaron structures of the PPy chains.

Illustration of mechanism of adsorption/desorption of NH3 molecules at adsorptive sites of PPy with formation of
4 Conclusion
The PPy film with highly ordered and uniform nanoparticles with the diameter of about 30–50 nm was successfully prepared by a chemical vapor-phase polymerization using FeCl3 oxidant. The sensing responses of the PPy film to different concentrations of NH3 gas were tested at the operating temperatures from 25 to 100°C. The PPy film displayed the highest sensing response of 143% for 350 ppm NH3 at 25°C, and the lowest sensing response of 37% for 45 ppm NH3 at 100°C. The results demonstrated a good linear relationship between the sensing response and the NH3 concentration. For the result of other interfering gases, the sensing response of the PPy film was significantly greater and exhibited an outstanding specific identification capacity for detecting NH3 gas.
The interaction of PPy with NH3 molecules was revealed when examining electron transfer between the adsorptive sites of PPy chains and NH3 molecules, caused increasing the film’s resistance. The interaction mechanism between NH3 molecules and the adsorptive sites of the PPy chains was considered to be the formation of polaron and bipolaron structures, as well as the mechanism of adsorption/desorption of NH3 molecules.
Acknowledgments
The authors are thankful for financial support by the project (code UTEHY.L.2020.09) from Hung Yen University of Technology and Education Technology.
-
Funding information: This work was financially supported by the project (code UTEHY.L.2020.09) from Hung Yen University of Technology and Education Technology.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Ghavam S, Vahdati M, Wilson IAG, Styring P. Sustainable ammonia production processes. Front Energy Res. 2021;9(March):1–19.10.3389/fenrg.2021.580808Search in Google Scholar
[2] Shohaimi NAM, Bakar WAWA, Jaafar J. Catalytic neutralization of acidic crude oil utilizing ammonia in ethylene glycol basic solution. J Ind Eng Chem. 2014;20(4):2086–94.10.1016/j.jiec.2013.09.037Search in Google Scholar
[3] Wu G, Geng J, Xu K, Ren H. Removal of pharmaceuticals by ammonia oxidizers during nitrification. Appl Microbiol Biotechnol. 2021;105(3):909–21.10.1007/s00253-020-11032-wSearch in Google Scholar PubMed
[4] Comotti M, Frigo S. Hydrogen generation system for ammonia-hydrogen fuelled internal combustion engines. Int J Hydrogen Energy. 2015;40(33):10673–86.10.1016/j.ijhydene.2015.06.080Search in Google Scholar
[5] Giddey S, Badwal SPS, Kulkarni A. Review of electrochemical ammonia production technologies and materials. Int J Hydrogen Energy. 2013;38(34):14576–94.10.1016/j.ijhydene.2013.09.054Search in Google Scholar
[6] Sutton MA, Erisman JW, Dentener F, Möller D. Ammonia in the environment: From ancient times to the present. Env Pollut. 2008;156(3):583–604.10.1016/j.envpol.2008.03.013Search in Google Scholar PubMed
[7] Skjøth CA, Geels C. The effect of climate and climate change on ammonia emissions in Europe. Atmos Chem Phys. 2013;13(1):117–28.10.5194/acp-13-117-2013Search in Google Scholar
[8] Wang M, Xiao M, Bertozzi B, Marie G, Rörup B, Schulze B, et al. Synergistic HNO3–H2SO4–NH3 upper tropospheric particle formation. Nature. 2022;605(7910):483–9.10.1038/s41586-022-04605-4Search in Google Scholar PubMed PubMed Central
[9] Gouma P, Kalyanasundaram K, Yun X, Stanaćević M, Wang L. Nanosensor and breath analyzer for ammonia detection in exhaled human breath. IEEE Sens J. 2010;10(1):49–53.10.1109/JSEN.2009.2036050Search in Google Scholar
[10] Krishnan ST, Devadhasan JP, Kim S. Recent analytical approaches to detect exhaled breath ammonia with special reference to renal patients. Anal Bioanal Chem. 2017;409(1):21–31.10.1007/s00216-016-9903-3Search in Google Scholar PubMed
[11] Broza YY, Mochalski P, Ruzsanyi V, Amann A, Haick H. Hybrid Volatolomics and Disease Detection. Angew Chem - Int Ed. 2015;54(38):11036–48.10.1002/anie.201500153Search in Google Scholar PubMed
[12] Wang D, Zhang D, Guo J, Hu Y, Yang Y, Sun T, et al. Multifunctional poly(vinyl alcohol)/Ag nanofibers-based triboelectric nanogenerator for self-powered MXene/tungsten oxide nanohybrid NO2 gas sensor. Nano Energy. 2021;89:106410. https://www.sciencedirect.com/science/article/pii/S2211285521006650.10.1016/j.nanoen.2021.106410Search in Google Scholar
[13] Wang D, Zhang D, Yang Y, Mi Q, Zhang J, Yu L. Multifunctional Latex/Polytetrafluoroethylene-based triboelectric nanogenerator for self-powered organ-like MXene/Metal–organic framework-derived CuO nanohybrid ammonia sensor. ACS Nano. 2021 Feb 23;15(2):2911–9. 10.1021/acsnano.0c09015 Search in Google Scholar PubMed
[14] Wang X, Zhang D, Zhang H, Gong L, Yang Y, Zhao W, et al. In situ polymerized polyaniline/MXene (V2C) as building blocks of supercapacitor and ammonia sensor self-powered by electromagnetic-triboelectric hybrid generator. Nano Energy. 2021;88:106242. https://www.sciencedirect.com/science/article/pii/S2211285521004973.10.1016/j.nanoen.2021.106242Search in Google Scholar
[15] Zhang D, Yang Y, Xu Z, Wang D, Du C. An eco-friendly gelatin based triboelectric nanogenerator for a self-powered PANI nanorod/NiCo2O4 nanosphere ammonia gas sensor. J Mater Chem A. 2022;10(20):10935–49. 10.1039/D2TA01788A Search in Google Scholar
[16] Zappa D, Galstyan V, Kaur N, Munasinghe Arachchige HMM, Sisman O, Comini E. “Metal oxide -based heterostructures for gas sensors”- A review. Anal Chim Acta. 2018;1039:1–23. https://www.sciencedirect.com/science/article/pii/S0003267018310894.10.1016/j.aca.2018.09.020Search in Google Scholar PubMed
[17] Šetka M, Bahos FA, Matatagui D, Potoček M, Kral Z, Drbohlavová J, et al. Love wave sensors based on gold nanoparticle-modified polypyrrole and their properties to ammonia and ethylene. Sens Actuators B Chem. 2020;304Ref. 17 and 20: Please provide the page number.10.1016/j.snb.2019.127337Search in Google Scholar
[18] Hien HT, Van Tuan C, Anh Thu DT, Ngan PQ, Thai GH, Doanh SC, et al. Influence of surface morphology and doping of PPy film simultaneously polymerized by vapour phase oxidation on gas sensing. Synth Met. 2019;250(December 2018):35–41.10.1016/j.synthmet.2019.02.013Search in Google Scholar
[19] Zhang T, Li W, Shi Y, Li C. Polyaniline-based room temperature ammonia gas sensor employing hybrid organic-inorganic substrate. Mater Chem Phys. 2022;288(March):126404.10.1016/j.matchemphys.2022.126404Search in Google Scholar
[20] Hao L, Dong C, Zhang L, Zhu K, Yu D. Polypyrrole nanomaterials: Structure, preparation and application. Polymers. 2022;14.10.3390/polym14235139Search in Google Scholar PubMed PubMed Central
[21] Harima Y, Jiang X, Patil R, Komaguchi K, Mizota H. Influence of film structure on mobilities of charge carriers in conducting polymers. Electrochim Acta. 2007;52(28):8088–95.10.1016/j.electacta.2007.07.007Search in Google Scholar
[22] Zhang L, Meng F, Chen Y, Liu J, Sun Y, Luo T, et al. A novel ammonia sensor based on high density, small diameter polypyrrole nanowire arrays. Sens Actuators B Chem. 2009;142(1):204–9.10.1016/j.snb.2009.07.042Search in Google Scholar
[23] Yadav AA, Kulkarni SB, Lokhande CD. Synthesis and characterization of polypyrrole thin film by MW-CBD method for NH3 gas sensor. Polym Bull. 2018;75(10):4547–53.10.1007/s00289-018-2282-5Search in Google Scholar
[24] She C, Li G, Zhang W, Xie G, Zhang Y, Li L, et al. A flexible polypyrrole/silk-fiber ammonia sensor assisted by silica nanosphere template. Sens Actuators A Phys. 2021;317:112436.10.1016/j.sna.2020.112436Search in Google Scholar
[25] Thi Hien H, Thi Anh Thu D, Quang Ngan P, Hong Thai G, Thanh Trung D, Trung T, et al. High NH3 sensing performance of NiO/PPy hybrid nanostructures. Sens Actuators B Chem. 2021;340(April):129986.10.1016/j.snb.2021.129986Search in Google Scholar
[26] Endres HE, Jander HD, Göttler W. A test system for gas sensors. Sens Actuators B Chem. 1995;23(2–3):163–72.10.1016/0925-4005(94)01272-JSearch in Google Scholar
[27] Cho S, Lee JS. Recent development of morphology controlled conducting polymer nanomaterial-based biosensor. Appl Sci. 2020;10(17):5889.10.3390/app10175889Search in Google Scholar
[28] Tu J, Li N, Yuan Q, Wang R, Geng W, Li Y, et al. Humidity-sensitive property of Fe2 + doped polypyrrole. Synth Met. 2009;159(23–24):2469–73.10.1016/j.synthmet.2009.08.014Search in Google Scholar
[29] Nagare AB, Harale NS, Dhas SD, Shembade UV, Ghatage SR, Patil PS, et al. Facile synthesis of nanogranular PPy thin films for sensitive and selective detection of toxic NO2 gas. Inorg Chem Commun. 2022;146(October):110067.10.1016/j.inoche.2022.110067Search in Google Scholar
[30] Battiston AA, Bitter JH, De Groot FMF, Overweg AR, Stephan O, Van Bokhoven JA, et al. Evolution of Fe species during the synthesis of over-exchanged Fe/ZSM5 obtained by chemical vapor deposition of FeCl3. J Catal. 2003;213(2):251–71.10.1016/S0021-9517(02)00051-9Search in Google Scholar
[31] Wang JG, Yang Y, Huang ZH, Kang F. MnO2/polypyrrole nanotubular composites: Reactive template synthesis, characterization and application as superior electrode materials for high-performance supercapacitors. Electrochim Acta. 2014;130:642–9.10.1016/j.electacta.2014.03.082Search in Google Scholar
[32] Šetka M, Drbohlavová J, Hubálek J. Nanostructured polypyrrole-based ammonia and volatile organic compound sensors. Sens (Switz). 2017;17(3):562.10.3390/s17030562Search in Google Scholar PubMed PubMed Central
[33] Yalçinkaya S, Demetgül C, Timur M, Çolak N. Electrochemical synthesis and characterization of polypyrrole/chitosan composite on platinum electrode: Its electrochemical and thermal behaviors. Carbohydr Polym. 2010;79(4):908–13.10.1016/j.carbpol.2009.10.022Search in Google Scholar
[34] Tian B, Zerbi G. Lattice dynamics and vibrational spectra of polypyrrole. J Chem Phys. 1990;92(6):3886–91.10.1063/1.457794Search in Google Scholar
[35] Liu L, Zhao C, Zhao Y, Jia N, Zhou Q, Yan M, et al. Characteristics of polypyrrole (PPy) nano-tubules made by templated ac electropolymerization. Eur Polym J. 2005;41(9):2117–21.10.1016/j.eurpolymj.2005.03.025Search in Google Scholar
[36] Ramesan MT. Synthesis, characterization, and conductivity studies of polypyrrole/copper sulfide nanocomposites. J Appl Polym Sci. 2013;128(3):1540–6.10.1002/app.38304Search in Google Scholar
[37] Ramesan MT, Santhi V. In situ synthesis, characterization, conductivity studies of polypyrrole/silver doped zinc oxide nanocomposites and their application for ammonia gas sensing. J Mater Sci Mater Electron. 2017;28(24):18804–14.10.1007/s10854-017-7830-5Search in Google Scholar
[38] Zhang W, Wen X, Yang S. Synthesis and characterization of uniform arrays of copper sulfide nanorods coated with nanolayers of polypyrrole. Langmuir. 2003;19(10):4420–6.10.1021/la020894wSearch in Google Scholar
[39] Jang J, Bae J. Carbon nanofiber/polypyrrole nanocable as toxic gas sensor. Sens Actuators B Chem. 2007;122(1):7–13.10.1016/j.snb.2006.05.002Search in Google Scholar
[40] Basavaraja C, Kim WJ, Kim DG, Huh DS. Synthesis and characterization of soluble polypyrrole-poly(ε- caprolactone) polymer blends with improved electrical conductivities. Mater Chem Phys. 2011;129(3):787–93.10.1016/j.matchemphys.2011.05.057Search in Google Scholar
[41] Kang HC, Geckeler KE. Enhanced electrical conductivity of polypyrrole prepared by chemical oxidative polymerization: Effect of the preparation technique and polymer additive. Polym (Guildf). 2000;41(18):6931–4.10.1016/S0032-3861(00)00116-6Search in Google Scholar
[42] Gemeiner P, Kuliček J, Mikula M, Hatala M, Švorc Ľ, Hlavatá L, et al. Polypyrrole-coated multi-walled carbon nanotubes for the simple preparation of counter electrodes in dye-sensitized solar cells. Synth Met. 2015;210:323–31.10.1016/j.synthmet.2015.10.020Search in Google Scholar
[43] Samadi A, Ahmadi R, Hosseini SM. Influence of TiO2-Fe3O4-MWCNT hybrid nanotubes on piezoelectric and electromagnetic wave absorption properties of electrospun PVDF nanocomposites. Org Electron. 2019;75(April):105405.10.1016/j.orgel.2019.105405Search in Google Scholar
[44] Lei C, Zhou Z, Chen W, Xie J, Huang B. Polypyrrole supported Pd/Fe bimetallic nanoparticles with enhanced catalytic activity for simultaneous removal of 4-chlorophenol and Cr(VI). Sci Total Env. 2022;831:154754.10.1016/j.scitotenv.2022.154754Search in Google Scholar PubMed
[45] Wang L, Jiang R. Investigation on the ammonia sensitivity mechanism of conducting polymer polypyrroles using In-Situ FT-IR. Mater Sci Appl. 2019;10(7):497–508.10.4236/msa.2019.107036Search in Google Scholar
[46] Furukawa Y, Tazawa S, Fujii Y, Harada I. Raman spectra of polypyrrole and its 2,5-13C-substituted and C-deuterated analogues in doped and undoped states. Synth Met. 1988;24(4):329–41.10.1016/0379-6779(88)90309-8Search in Google Scholar
[47] Šetka M, Calavia R, Vojkůvka L, Llobet E, Drbohlavová J, Vallejos S. Raman and XPS studies of ammonia sensitive polypyrrole nanorods and nanoparticles. Sci Rep. 2019;9(1):1–10.10.1038/s41598-019-44900-1Search in Google Scholar PubMed PubMed Central
[48] Duchet J, Legras R, Demoustier-Champagne S. Chemical synthesis of polypyrrole: Structure-properties relationship. Synth Met. 1998;98(2):113–22.10.1016/S0379-6779(98)00180-5Search in Google Scholar
[49] Liu X, Zhang D, Wang D, Li T, Song X, Kang Z. A humidity sensing and respiratory monitoring system constructed from quartz crystal microbalance sensors based on a chitosan/polypyrrole composite film. J Mater Chem A. 2021;9(25):14524–33. 10.1039/D1TA02828F.Search in Google Scholar
[50] Zhang D, Wu Z, Zong X, Zhang Y. Fabrication of polypyrrole/Zn2SnO4 nanofilm for ultra-highly sensitive ammonia sensing application. Sens Actuators B Chem. 2018;274:575–86. https://www.sciencedirect.com/science/article/pii/S0925400518314278.10.1016/j.snb.2018.08.001Search in Google Scholar
[51] Joulazadeh M, Navarchian AH. Ammonia detection of one-dimensional nano-structured polypyrrole/metal oxide nanocomposites sensors. Synth Met. 2015;210:404–11. https://www.sciencedirect.com/science/article/pii/S0379677915301302.10.1016/j.synthmet.2015.10.026Search in Google Scholar
[52] Pirsa S. Chemiresistive gas sensors based on conducting polymers. In Mater Sci Eng;2017. p. 543–74. IGI Global. https://www.igi-global.com/chapter/chemiresistive-gas-sensors-based-on-conducting-polymers/166410 10.4018/978-1-5225-1798-6.ch022.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Dynamic properties of the attachment oscillator arising in the nanophysics
- Parametric simulation of stagnation point flow of motile microorganism hybrid nanofluid across a circular cylinder with sinusoidal radius
- Fractal-fractional advection–diffusion–reaction equations by Ritz approximation approach
- Behaviour and onset of low-dimensional chaos with a periodically varying loss in single-mode homogeneously broadened laser
- Ammonia gas-sensing behavior of uniform nanostructured PPy film prepared by simple-straightforward in situ chemical vapor oxidation
- Analysis of the working mechanism and detection sensitivity of a flash detector
- Flat and bent branes with inner structure in two-field mimetic gravity
- Heat transfer analysis of the MHD stagnation-point flow of third-grade fluid over a porous sheet with thermal radiation effect: An algorithmic approach
- Weighted survival functional entropy and its properties
- Bioconvection effect in the Carreau nanofluid with Cattaneo–Christov heat flux using stagnation point flow in the entropy generation: Micromachines level study
- Study on the impulse mechanism of optical films formed by laser plasma shock waves
- Analysis of sweeping jet and film composite cooling using the decoupled model
- Research on the influence of trapezoidal magnetization of bonded magnetic ring on cogging torque
- Tripartite entanglement and entanglement transfer in a hybrid cavity magnomechanical system
- Compounded Bell-G class of statistical models with applications to COVID-19 and actuarial data
- Degradation of Vibrio cholerae from drinking water by the underwater capillary discharge
- Multiple Lie symmetry solutions for effects of viscous on magnetohydrodynamic flow and heat transfer in non-Newtonian thin film
- Thermal characterization of heat source (sink) on hybridized (Cu–Ag/EG) nanofluid flow via solid stretchable sheet
- Optimizing condition monitoring of ball bearings: An integrated approach using decision tree and extreme learning machine for effective decision-making
- Study on the inter-porosity transfer rate and producing degree of matrix in fractured-porous gas reservoirs
- Interstellar radiation as a Maxwell field: Improved numerical scheme and application to the spectral energy density
- Numerical study of hybridized Williamson nanofluid flow with TC4 and Nichrome over an extending surface
- Controlling the physical field using the shape function technique
- Significance of heat and mass transport in peristaltic flow of Jeffrey material subject to chemical reaction and radiation phenomenon through a tapered channel
- Complex dynamics of a sub-quadratic Lorenz-like system
- Stability control in a helicoidal spin–orbit-coupled open Bose–Bose mixture
- Research on WPD and DBSCAN-L-ISOMAP for circuit fault feature extraction
- Simulation for formation process of atomic orbitals by the finite difference time domain method based on the eight-element Dirac equation
- A modified power-law model: Properties, estimation, and applications
- Bayesian and non-Bayesian estimation of dynamic cumulative residual Tsallis entropy for moment exponential distribution under progressive censored type II
- Computational analysis and biomechanical study of Oldroyd-B fluid with homogeneous and heterogeneous reactions through a vertical non-uniform channel
- Predictability of machine learning framework in cross-section data
- Chaotic characteristics and mixing performance of pseudoplastic fluids in a stirred tank
- Isomorphic shut form valuation for quantum field theory and biological population models
- Vibration sensitivity minimization of an ultra-stable optical reference cavity based on orthogonal experimental design
- Effect of dysprosium on the radiation-shielding features of SiO2–PbO–B2O3 glasses
- Asymptotic formulations of anti-plane problems in pre-stressed compressible elastic laminates
- A study on soliton, lump solutions to a generalized (3+1)-dimensional Hirota--Satsuma--Ito equation
- Tangential electrostatic field at metal surfaces
- Bioconvective gyrotactic microorganisms in third-grade nanofluid flow over a Riga surface with stratification: An approach to entropy minimization
- Infrared spectroscopy for ageing assessment of insulating oils via dielectric loss factor and interfacial tension
- Influence of cationic surfactants on the growth of gypsum crystals
- Study on instability mechanism of KCl/PHPA drilling waste fluid
- Analytical solutions of the extended Kadomtsev–Petviashvili equation in nonlinear media
- A novel compact highly sensitive non-invasive microwave antenna sensor for blood glucose monitoring
- Inspection of Couette and pressure-driven Poiseuille entropy-optimized dissipated flow in a suction/injection horizontal channel: Analytical solutions
- Conserved vectors and solutions of the two-dimensional potential KP equation
- The reciprocal linear effect, a new optical effect of the Sagnac type
- Optimal interatomic potentials using modified method of least squares: Optimal form of interatomic potentials
- The soliton solutions for stochastic Calogero–Bogoyavlenskii Schiff equation in plasma physics/fluid mechanics
- Research on absolute ranging technology of resampling phase comparison method based on FMCW
- Analysis of Cu and Zn contents in aluminum alloys by femtosecond laser-ablation spark-induced breakdown spectroscopy
- Nonsequential double ionization channels control of CO2 molecules with counter-rotating two-color circularly polarized laser field by laser wavelength
- Fractional-order modeling: Analysis of foam drainage and Fisher's equations
- Thermo-solutal Marangoni convective Darcy-Forchheimer bio-hybrid nanofluid flow over a permeable disk with activation energy: Analysis of interfacial nanolayer thickness
- Investigation on topology-optimized compressor piston by metal additive manufacturing technique: Analytical and numeric computational modeling using finite element analysis in ANSYS
- Breast cancer segmentation using a hybrid AttendSeg architecture combined with a gravitational clustering optimization algorithm using mathematical modelling
- On the localized and periodic solutions to the time-fractional Klein-Gordan equations: Optimal additive function method and new iterative method
- 3D thin-film nanofluid flow with heat transfer on an inclined disc by using HWCM
- Numerical study of static pressure on the sonochemistry characteristics of the gas bubble under acoustic excitation
- Optimal auxiliary function method for analyzing nonlinear system of coupled Schrödinger–KdV equation with Caputo operator
- Analysis of magnetized micropolar fluid subjected to generalized heat-mass transfer theories
- Does the Mott problem extend to Geiger counters?
- Stability analysis, phase plane analysis, and isolated soliton solution to the LGH equation in mathematical physics
- Effects of Joule heating and reaction mechanisms on couple stress fluid flow with peristalsis in the presence of a porous material through an inclined channel
- Bayesian and E-Bayesian estimation based on constant-stress partially accelerated life testing for inverted Topp–Leone distribution
- Dynamical and physical characteristics of soliton solutions to the (2+1)-dimensional Konopelchenko–Dubrovsky system
- Study of fractional variable order COVID-19 environmental transformation model
- Sisko nanofluid flow through exponential stretching sheet with swimming of motile gyrotactic microorganisms: An application to nanoengineering
- Influence of the regularization scheme in the QCD phase diagram in the PNJL model
- Fixed-point theory and numerical analysis of an epidemic model with fractional calculus: Exploring dynamical behavior
- Computational analysis of reconstructing current and sag of three-phase overhead line based on the TMR sensor array
- Investigation of tripled sine-Gordon equation: Localized modes in multi-stacked long Josephson junctions
- High-sensitivity on-chip temperature sensor based on cascaded microring resonators
- Pathological study on uncertain numbers and proposed solutions for discrete fuzzy fractional order calculus
- Bifurcation, chaotic behavior, and traveling wave solution of stochastic coupled Konno–Oono equation with multiplicative noise in the Stratonovich sense
- Thermal radiation and heat generation on three-dimensional Casson fluid motion via porous stretching surface with variable thermal conductivity
- Numerical simulation and analysis of Airy's-type equation
- A homotopy perturbation method with Elzaki transformation for solving the fractional Biswas–Milovic model
- Heat transfer performance of magnetohydrodynamic multiphase nanofluid flow of Cu–Al2O3/H2O over a stretching cylinder
- ΛCDM and the principle of equivalence
- Axisymmetric stagnation-point flow of non-Newtonian nanomaterial and heat transport over a lubricated surface: Hybrid homotopy analysis method simulations
- HAM simulation for bioconvective magnetohydrodynamic flow of Walters-B fluid containing nanoparticles and microorganisms past a stretching sheet with velocity slip and convective conditions
- Coupled heat and mass transfer mathematical study for lubricated non-Newtonian nanomaterial conveying oblique stagnation point flow: A comparison of viscous and viscoelastic nanofluid model
- Power Topp–Leone exponential negative family of distributions with numerical illustrations to engineering and biological data
- Extracting solitary solutions of the nonlinear Kaup–Kupershmidt (KK) equation by analytical method
- A case study on the environmental and economic impact of photovoltaic systems in wastewater treatment plants
- Application of IoT network for marine wildlife surveillance
- Non-similar modeling and numerical simulations of microploar hybrid nanofluid adjacent to isothermal sphere
- Joint optimization of two-dimensional warranty period and maintenance strategy considering availability and cost constraints
- Numerical investigation of the flow characteristics involving dissipation and slip effects in a convectively nanofluid within a porous medium
- Spectral uncertainty analysis of grassland and its camouflage materials based on land-based hyperspectral images
- Application of low-altitude wind shear recognition algorithm and laser wind radar in aviation meteorological services
- Investigation of different structures of screw extruders on the flow in direct ink writing SiC slurry based on LBM
- Harmonic current suppression method of virtual DC motor based on fuzzy sliding mode
- Micropolar flow and heat transfer within a permeable channel using the successive linearization method
- Different lump k-soliton solutions to (2+1)-dimensional KdV system using Hirota binary Bell polynomials
- Investigation of nanomaterials in flow of non-Newtonian liquid toward a stretchable surface
- Weak beat frequency extraction method for photon Doppler signal with low signal-to-noise ratio
- Electrokinetic energy conversion of nanofluids in porous microtubes with Green’s function
- Examining the role of activation energy and convective boundary conditions in nanofluid behavior of Couette-Poiseuille flow
- Review Article
- Effects of stretching on phase transformation of PVDF and its copolymers: A review
- Special Issue on Transport phenomena and thermal analysis in micro/nano-scale structure surfaces - Part IV
- Prediction and monitoring model for farmland environmental system using soil sensor and neural network algorithm
- Special Issue on Advanced Topics on the Modelling and Assessment of Complicated Physical Phenomena - Part III
- Some standard and nonstandard finite difference schemes for a reaction–diffusion–chemotaxis model
- Special Issue on Advanced Energy Materials - Part II
- Rapid productivity prediction method for frac hits affected wells based on gas reservoir numerical simulation and probability method
- Special Issue on Novel Numerical and Analytical Techniques for Fractional Nonlinear Schrodinger Type - Part III
- Adomian decomposition method for solution of fourteenth order boundary value problems
- New soliton solutions of modified (3+1)-D Wazwaz–Benjamin–Bona–Mahony and (2+1)-D cubic Klein–Gordon equations using first integral method
- On traveling wave solutions to Manakov model with variable coefficients
- Rational approximation for solving Fredholm integro-differential equations by new algorithm
- Special Issue on Predicting pattern alterations in nature - Part I
- Modeling the monkeypox infection using the Mittag–Leffler kernel
- Spectral analysis of variable-order multi-terms fractional differential equations
- Special Issue on Nanomaterial utilization and structural optimization - Part I
- Heat treatment and tensile test of 3D-printed parts manufactured at different build orientations
Articles in the same Issue
- Regular Articles
- Dynamic properties of the attachment oscillator arising in the nanophysics
- Parametric simulation of stagnation point flow of motile microorganism hybrid nanofluid across a circular cylinder with sinusoidal radius
- Fractal-fractional advection–diffusion–reaction equations by Ritz approximation approach
- Behaviour and onset of low-dimensional chaos with a periodically varying loss in single-mode homogeneously broadened laser
- Ammonia gas-sensing behavior of uniform nanostructured PPy film prepared by simple-straightforward in situ chemical vapor oxidation
- Analysis of the working mechanism and detection sensitivity of a flash detector
- Flat and bent branes with inner structure in two-field mimetic gravity
- Heat transfer analysis of the MHD stagnation-point flow of third-grade fluid over a porous sheet with thermal radiation effect: An algorithmic approach
- Weighted survival functional entropy and its properties
- Bioconvection effect in the Carreau nanofluid with Cattaneo–Christov heat flux using stagnation point flow in the entropy generation: Micromachines level study
- Study on the impulse mechanism of optical films formed by laser plasma shock waves
- Analysis of sweeping jet and film composite cooling using the decoupled model
- Research on the influence of trapezoidal magnetization of bonded magnetic ring on cogging torque
- Tripartite entanglement and entanglement transfer in a hybrid cavity magnomechanical system
- Compounded Bell-G class of statistical models with applications to COVID-19 and actuarial data
- Degradation of Vibrio cholerae from drinking water by the underwater capillary discharge
- Multiple Lie symmetry solutions for effects of viscous on magnetohydrodynamic flow and heat transfer in non-Newtonian thin film
- Thermal characterization of heat source (sink) on hybridized (Cu–Ag/EG) nanofluid flow via solid stretchable sheet
- Optimizing condition monitoring of ball bearings: An integrated approach using decision tree and extreme learning machine for effective decision-making
- Study on the inter-porosity transfer rate and producing degree of matrix in fractured-porous gas reservoirs
- Interstellar radiation as a Maxwell field: Improved numerical scheme and application to the spectral energy density
- Numerical study of hybridized Williamson nanofluid flow with TC4 and Nichrome over an extending surface
- Controlling the physical field using the shape function technique
- Significance of heat and mass transport in peristaltic flow of Jeffrey material subject to chemical reaction and radiation phenomenon through a tapered channel
- Complex dynamics of a sub-quadratic Lorenz-like system
- Stability control in a helicoidal spin–orbit-coupled open Bose–Bose mixture
- Research on WPD and DBSCAN-L-ISOMAP for circuit fault feature extraction
- Simulation for formation process of atomic orbitals by the finite difference time domain method based on the eight-element Dirac equation
- A modified power-law model: Properties, estimation, and applications
- Bayesian and non-Bayesian estimation of dynamic cumulative residual Tsallis entropy for moment exponential distribution under progressive censored type II
- Computational analysis and biomechanical study of Oldroyd-B fluid with homogeneous and heterogeneous reactions through a vertical non-uniform channel
- Predictability of machine learning framework in cross-section data
- Chaotic characteristics and mixing performance of pseudoplastic fluids in a stirred tank
- Isomorphic shut form valuation for quantum field theory and biological population models
- Vibration sensitivity minimization of an ultra-stable optical reference cavity based on orthogonal experimental design
- Effect of dysprosium on the radiation-shielding features of SiO2–PbO–B2O3 glasses
- Asymptotic formulations of anti-plane problems in pre-stressed compressible elastic laminates
- A study on soliton, lump solutions to a generalized (3+1)-dimensional Hirota--Satsuma--Ito equation
- Tangential electrostatic field at metal surfaces
- Bioconvective gyrotactic microorganisms in third-grade nanofluid flow over a Riga surface with stratification: An approach to entropy minimization
- Infrared spectroscopy for ageing assessment of insulating oils via dielectric loss factor and interfacial tension
- Influence of cationic surfactants on the growth of gypsum crystals
- Study on instability mechanism of KCl/PHPA drilling waste fluid
- Analytical solutions of the extended Kadomtsev–Petviashvili equation in nonlinear media
- A novel compact highly sensitive non-invasive microwave antenna sensor for blood glucose monitoring
- Inspection of Couette and pressure-driven Poiseuille entropy-optimized dissipated flow in a suction/injection horizontal channel: Analytical solutions
- Conserved vectors and solutions of the two-dimensional potential KP equation
- The reciprocal linear effect, a new optical effect of the Sagnac type
- Optimal interatomic potentials using modified method of least squares: Optimal form of interatomic potentials
- The soliton solutions for stochastic Calogero–Bogoyavlenskii Schiff equation in plasma physics/fluid mechanics
- Research on absolute ranging technology of resampling phase comparison method based on FMCW
- Analysis of Cu and Zn contents in aluminum alloys by femtosecond laser-ablation spark-induced breakdown spectroscopy
- Nonsequential double ionization channels control of CO2 molecules with counter-rotating two-color circularly polarized laser field by laser wavelength
- Fractional-order modeling: Analysis of foam drainage and Fisher's equations
- Thermo-solutal Marangoni convective Darcy-Forchheimer bio-hybrid nanofluid flow over a permeable disk with activation energy: Analysis of interfacial nanolayer thickness
- Investigation on topology-optimized compressor piston by metal additive manufacturing technique: Analytical and numeric computational modeling using finite element analysis in ANSYS
- Breast cancer segmentation using a hybrid AttendSeg architecture combined with a gravitational clustering optimization algorithm using mathematical modelling
- On the localized and periodic solutions to the time-fractional Klein-Gordan equations: Optimal additive function method and new iterative method
- 3D thin-film nanofluid flow with heat transfer on an inclined disc by using HWCM
- Numerical study of static pressure on the sonochemistry characteristics of the gas bubble under acoustic excitation
- Optimal auxiliary function method for analyzing nonlinear system of coupled Schrödinger–KdV equation with Caputo operator
- Analysis of magnetized micropolar fluid subjected to generalized heat-mass transfer theories
- Does the Mott problem extend to Geiger counters?
- Stability analysis, phase plane analysis, and isolated soliton solution to the LGH equation in mathematical physics
- Effects of Joule heating and reaction mechanisms on couple stress fluid flow with peristalsis in the presence of a porous material through an inclined channel
- Bayesian and E-Bayesian estimation based on constant-stress partially accelerated life testing for inverted Topp–Leone distribution
- Dynamical and physical characteristics of soliton solutions to the (2+1)-dimensional Konopelchenko–Dubrovsky system
- Study of fractional variable order COVID-19 environmental transformation model
- Sisko nanofluid flow through exponential stretching sheet with swimming of motile gyrotactic microorganisms: An application to nanoengineering
- Influence of the regularization scheme in the QCD phase diagram in the PNJL model
- Fixed-point theory and numerical analysis of an epidemic model with fractional calculus: Exploring dynamical behavior
- Computational analysis of reconstructing current and sag of three-phase overhead line based on the TMR sensor array
- Investigation of tripled sine-Gordon equation: Localized modes in multi-stacked long Josephson junctions
- High-sensitivity on-chip temperature sensor based on cascaded microring resonators
- Pathological study on uncertain numbers and proposed solutions for discrete fuzzy fractional order calculus
- Bifurcation, chaotic behavior, and traveling wave solution of stochastic coupled Konno–Oono equation with multiplicative noise in the Stratonovich sense
- Thermal radiation and heat generation on three-dimensional Casson fluid motion via porous stretching surface with variable thermal conductivity
- Numerical simulation and analysis of Airy's-type equation
- A homotopy perturbation method with Elzaki transformation for solving the fractional Biswas–Milovic model
- Heat transfer performance of magnetohydrodynamic multiphase nanofluid flow of Cu–Al2O3/H2O over a stretching cylinder
- ΛCDM and the principle of equivalence
- Axisymmetric stagnation-point flow of non-Newtonian nanomaterial and heat transport over a lubricated surface: Hybrid homotopy analysis method simulations
- HAM simulation for bioconvective magnetohydrodynamic flow of Walters-B fluid containing nanoparticles and microorganisms past a stretching sheet with velocity slip and convective conditions
- Coupled heat and mass transfer mathematical study for lubricated non-Newtonian nanomaterial conveying oblique stagnation point flow: A comparison of viscous and viscoelastic nanofluid model
- Power Topp–Leone exponential negative family of distributions with numerical illustrations to engineering and biological data
- Extracting solitary solutions of the nonlinear Kaup–Kupershmidt (KK) equation by analytical method
- A case study on the environmental and economic impact of photovoltaic systems in wastewater treatment plants
- Application of IoT network for marine wildlife surveillance
- Non-similar modeling and numerical simulations of microploar hybrid nanofluid adjacent to isothermal sphere
- Joint optimization of two-dimensional warranty period and maintenance strategy considering availability and cost constraints
- Numerical investigation of the flow characteristics involving dissipation and slip effects in a convectively nanofluid within a porous medium
- Spectral uncertainty analysis of grassland and its camouflage materials based on land-based hyperspectral images
- Application of low-altitude wind shear recognition algorithm and laser wind radar in aviation meteorological services
- Investigation of different structures of screw extruders on the flow in direct ink writing SiC slurry based on LBM
- Harmonic current suppression method of virtual DC motor based on fuzzy sliding mode
- Micropolar flow and heat transfer within a permeable channel using the successive linearization method
- Different lump k-soliton solutions to (2+1)-dimensional KdV system using Hirota binary Bell polynomials
- Investigation of nanomaterials in flow of non-Newtonian liquid toward a stretchable surface
- Weak beat frequency extraction method for photon Doppler signal with low signal-to-noise ratio
- Electrokinetic energy conversion of nanofluids in porous microtubes with Green’s function
- Examining the role of activation energy and convective boundary conditions in nanofluid behavior of Couette-Poiseuille flow
- Review Article
- Effects of stretching on phase transformation of PVDF and its copolymers: A review
- Special Issue on Transport phenomena and thermal analysis in micro/nano-scale structure surfaces - Part IV
- Prediction and monitoring model for farmland environmental system using soil sensor and neural network algorithm
- Special Issue on Advanced Topics on the Modelling and Assessment of Complicated Physical Phenomena - Part III
- Some standard and nonstandard finite difference schemes for a reaction–diffusion–chemotaxis model
- Special Issue on Advanced Energy Materials - Part II
- Rapid productivity prediction method for frac hits affected wells based on gas reservoir numerical simulation and probability method
- Special Issue on Novel Numerical and Analytical Techniques for Fractional Nonlinear Schrodinger Type - Part III
- Adomian decomposition method for solution of fourteenth order boundary value problems
- New soliton solutions of modified (3+1)-D Wazwaz–Benjamin–Bona–Mahony and (2+1)-D cubic Klein–Gordon equations using first integral method
- On traveling wave solutions to Manakov model with variable coefficients
- Rational approximation for solving Fredholm integro-differential equations by new algorithm
- Special Issue on Predicting pattern alterations in nature - Part I
- Modeling the monkeypox infection using the Mittag–Leffler kernel
- Spectral analysis of variable-order multi-terms fractional differential equations
- Special Issue on Nanomaterial utilization and structural optimization - Part I
- Heat treatment and tensile test of 3D-printed parts manufactured at different build orientations

