Abstract
The recent study reports the fabrication and corrosion behavior of two Ti alloys, 88% Ti–12% Zr and 84% Ti–12% Zr–4% Ta, in 3.5% NaCl electrolyte. These alloys were manufactured using powder metallurgy, where the powders were mixed, ball milled, and sintered. The corrosion behavior of these alloys was examined using various electrochemical and spectroscopic tests. Cyclic polarization experiments indicated that adding 4% Ta reduces corrosion of the TiZr alloy by suppressing anodic dissolution, resulting in a lower corrosion rate. The Nyquist and Bode impedance spectra for the tested alloys revealed that the presence of 4% Ta within TiZr alloy highly decreases the corrosion by increasing the impedance of the interface, the maximum degree of phase angle, and polarization resistance. The chronoamperometric current measured at −0.10 V (Ag/AgCl) proved that the presence of 4% Ta powerfully alleviates both uniform and pitting corrosion for TiZr alloy by lowering the obtained absolute currents. The surface investigation using scanning electron microscopy confirmed the homogeneity of the surfaces. The elemental analysis performed on the surface using energy dispersive spectroscopy revealed that the surface of TiZr alloy forms a top film including different oxides such as TiO2 and ZrO2, and for TiZrTa alloy, the surface has TiO2 + ZrO2 plus TaO2. Experiments demonstrated that Ta has the ability to increase the corrosion passivation of TiZr alloy.
1 Introduction
Ti-base alloys have unique characteristics that make them suitable for a wide range of applications [1,2,3], including in the biomedical field, automotive main and spare parts, aerospace industries, etc. [4,5,6,7]. Ti-base alloys are distinguished by their superior biocompatibility and biodegradability, great corrosion resistance, and exceptional mechanical and tribological properties. Several scientists [7,8,9] have reported the use of titanium aluminum vanadium (Ti6Al4V) alloy as a biomedical material in the replacement of fractured bones, tissue repair, and transplantation procedures in the human bodies. Conversely, some side effects were found while using Ti6Al4V alloy in the human body [8,9,10,11,12]. It was reported that the alloy contains Al and V, which react with the medium in which it is implanted, releasing toxic radicals of Al3+ and V5+. When these active species are released into the patients' bloodstreams, they trigger hazardous reactions and may increase the risk of Alzheimer’s disease [13,14,15].
Regardless of research and development efforts in the field of artificial tissues for human bodies, there is a need for developing new alloys and composites to overcome the shortcomings of the currently used materials. Several trials have been conducted to avoid the presence of active radicals such as the toxic Al3+ and V5+ cations [16,17]. Wu et al. developed Ti–12Nb–12Zr–12Sn alloy using the machine learning method, namely, an artificial neural network [18]. The addition of Zr to form various Zr percentages of Ti–xZr alloys has been reported by numerous researchers [17,18,19,20,21]. The binary has the same α structure as commercially pure Ti (cp Ti) and is highly compatible with biological fluids [19]. This is because Ti–Zr binary is considered an ideal implant material due to its high resistance to corrosion resistance, outstanding mechanical properties, exceptional biocompatibility, strength, and osseointegration characteristics [21].
It was found that adding 15% Zr to pure Ti enhances corrosion resistance when exposed to simulated body fluid (SBF), and that this effect significantly magnifies the mechanical characteristics of Ti [1]. The effect of adding Zr and Ta to pure Ti, 15% Zr + 2% Ta, has also been reported [2], and it was found to significantly improve the corrosion resistance as compared to Ti and the Ti15% Zr alloy. The addition of Zr and Ta to Ti not only enhances corrosion resistance in SBF solution but also strongly increases the hardness of the alloy [22]. The high corrosion resistance of many Ti-base alloys is due to the development of an oxide (TiO2) film [23,24,25]. For example, TiZr and TiZrTa, TiAlV alloys, it forms not only TiO2 but also other oxides such as ZrO2, TaO2, and VO2 that highly increase their passivation [1,2,26].
The implantation of Zr, Ta, and other alloying elements with Ti, such as Hf, Nb, Sn, and so on, has been investigated to produce different series of alloys from TiZrTaHf and TiTaNbZr [20,26,27,28,29,30,31,32]. The current research aims at the fabrication of two alloys with the following compositions: 88% Ti–12% Zr alloy and 84% Ti–12% Zr–4% Ta alloy. The corrosion of these alloys in solution (3.5% NaCl) was tested after two exposure periods of 30 min and 3 days at room temperature. Various methods were used such as cyclic polarization, impedance spectroscopy, and chronoamperometric currents vs. time. Surface investigations were carried out using scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) analyses.
2 Materials and methods
Pure sodium chloride (NaCl, 99.9 purity) was supplied by Merck. To make a liter of 3.5% NaCl solution, weigh 35 g of the NaCl salt and dissolve it in distilled water. A 99.9% pure Ti powder, 99% pure Zr powder, and 99.9% pure Ta were used as received. The particle size of the powders is 5–7, 10, and 10 µm for Ti, Zr, and Ta, respectively. All these powders were obtained from Nanochemazone, Leduc, Alberta, Canada. Different powders were weighed to the desired weight and mixed in a ball mill (high energy). The weighed powders and balls (ball-to-powder ratio was 90:10) were mixed at 150 rpm for 30 min. The mixtures of powders were placed in graphite dies and sintered in a high-frequency heat induction furnace following the procedures described in the earlier studies [1,2].
For corrosion testing, the TiZr alloy and TiZrTa alloy electrodes were welded onto one face using copper wire. The electrodes were placed in an electrically inert epoxy resin. The other face of the mounted electrode was ground with emery sheets of various grits up to 1,000. The corrosion tests were conducted using a three-electrode electrochemical cell with a solution capacity of 60 mL. The three electrodes were the fabricated alloys, Ag/AgCl, and a platinum sheet, which served as the working, reference, and counter, respectively. The Metrohm Autolab model PGSTAT302N was used.
The cyclic polarization plots were obtained from −970 mV to +170 mV, starting from the forward potential, then in the backward directions, and back again at a scan rate of 0.1667 mV/s. The spectra of electrochemical impedance spectroscopy (EIS) were recorded at the steady potential values ranging from 100 kHz to 100 mHz as the scanning frequency. The EIS points were collected using Powersine software, with a point rate of 10 for every decade variation in frequency. ZSimpWin v.3.1 software was used to fit the EIS data. Chronoamperometric curves were collected at fixed voltage of −100 mV for 30 min. The morphology of the surface was investigated using a JEOL model JSM-7400F SEM (Tokyo, Japan). An attached unit of EDS was used to report the compositions of the surface produced film(s).
3 Results and discussion
3.1 Cyclic potentiodynamic polarization (CPP)
Figure 1 shows the CPP curves for (1) TiZr alloy and (2) TiZrTa alloy after immersion in 3.5% NaCl solution for 30 min. The CPP method has been widely recognized as the most successful technique for reporting corrosion parameters; besides, it can provide information on the occurrence and intensity of pitting corrosion [1,2,8,33,34,35]. To report the effect of longer immersion times, measurements were repeated for the same alloys after 3 day, as shown in Figure 2. The polarization curves were used to obtain the corrosion parameters listed in Table 1. The extrapolated tangents to the linearized cathodic and anodic current regions were used to calculate the values of the cathodic (β c) and anodic (β a) Tafel slopes, respectively. Other values for E Corr, corrosion potential, and j Corr, corrosion current, were reported in previous studies [33,34,35]. The values of corrosion rate (R Corr) and polarization resistance (R P) were obtained as follows [33,34,35]:
where k is a constant that defines the unit of corrosion rate, k = 3,272 mm (amp/cm/year), E W is the equivalent weight, d is the density, and A is the area. It is worth mentioning that the density of both 88Ti–12Zr alloy and 88Ti–12Zr–4Ta alloy was calculated to be 4.672 and 4.818 g/cm3, respectively. The relative densities for both 88Ti–12Zr alloy and 88Ti–12Zr–4Ta alloy were 99.42 and 97.86%, respectively.

CPP curves for (1) TiZr alloy and (2) TiZrTa alloy after 30 min exposure to the NaCl solutions.

CPP curves for (1) TiZr alloy and (2) TiZrTa alloy in NaCl solutions for 3 days.
Values obtained from CPP measurements
| Alloy/time | β c (mV/dec) | E Corr (mV) | β a (mV/dec) | j Corr (μA/cm2) | R P (kΩ/cm2) | R Corr (mm/y) |
|---|---|---|---|---|---|---|
| TiZr alloy/30 min | 165 | −735 | 188 | 6.5 | 5.88 | 0.0643 |
| TiZrTa alloy/30 min | 170 | −647 | 190 | 3.2 | 12.2 | 0.0328 |
| TiZr alloy/3 days | 190 | −632 | 180 | 5.5 | 7.31 | 0.0544 |
| TiZrTa alloy/3 days | 192 | −745 | 184 | 2.7 | 15.1 | 0.0277 |
The cathodic branch for all alloys starts with a decrease in current as a result of oxygen reduction. This reaction can be expressed as follows [32,33]:
The current continues to decrease till it reaches the j Corr value. Then, the anodic reaction starts, which is known to be a dissolution reaction that might lead to the increase of current. Figure 1 shows that both cathodic and anodic currents for TiZr alloy (curve 1) have higher currents; these currents are highly reduced for TiZrTa alloy (curve 2). The increase of currents for both TiZr and TiZrTa alloys is expected due to the increased application of potential. The higher recorded currents for TiZr alloy as compared to the ones obtained for TiZrTa alloy is due to the presence of Ta, which allows the formation of TaO2 in addition to the formation of TiO2 and ZrO2 that could also present on the surface of TiZr alloy. Table 1 also confirmed that where the value of j Corr (and in accordance R Corr) was higher, as well as the R P value was lower for TiZr alloy. Moreover, the value of E Corr was always less negative for TiZrTa alloy than for TiZr alloy, indicating that the presence of Ta passivates the anodic reactions more than cathodic ones.
Figure 2 shows the polarization behavior of the tested alloys after extending the immersion time in NaCl solution to 3 days, which is nearly identical. The currents (cathodic and anodic) recorded lower values for TiZrTa alloy than the values for TiZr alloy. This was also reflected in j Corr, R P, and R Corr, with TiZrTa alloy having lower j Corr and R Corr values but a larger R P value. The presence of Ta increases the corrosion passivation of TiZr alloy. The effect of increasing immersion duration from 30 min to 3 day can be elucidated by comparing Figures 1 and 2, as well as the values listed in Table 1. The tested alloys showed decreased anodic and cathodic currents versus potential after 3 days of immersion in NaCl solution, compared to their values after 30 min. The j Corr and R Corr for TiZr alloy measured lower values after 3 days of immersion, whereas TiZrTa alloy recorded even lower values after 3 days of immersion. The values of RP in turn scored higher values for both alloys when the time of exposure was extended to 3 days. Prolonging the immersion time has a positive effect on the passivation of TiZr alloy and TiZrTa alloy by the formation and thickening of a mixture of oxide film(s) of TiO2, ZrO2, and TaO2.
3.2 Chronoamperometry
The chronoamperometric current versus time at an anodic potential technique is effective for analyzing corrosion, particularly pitting, in most active metallic materials in aggressive media [36,37,38,39,40]. Figure 3 shows the change in current for 30 min at −0.1 V for (1) TiZr and (2) TiZrTa alloys in NaCl solutions. Figure 3 displays that the current begins with low values and rapidly increases in the first few seconds. This results from the dissolution of a formed surface oxide film during the immersion of the alloys, which is caused by the aggressive action of the chloride solution along with the applied active voltage. It is seen from Figure 3 (curve 1) that the obtained current for TiZr alloy is the highest, where it is abruptly shifted to the higher values and then slightly increased with time. The current fluctuates up and down with an average value of 750 µA/cm2. The increase and then the decrease in the currents result from the dissolution and subsequent formation of oxide(s), and it may also indicate pitting corrosion. TiZrTa alloy performs similarly, where the current started with an abrupt increase followed by a gradual decrease. The overall absolute currents obtained for TiZrTa alloy were far lower than those collected for TiZr alloy. This difference in the absolute currents between the two alloys results from the presence of 4% Ta, which forms an additional TaO2 film. The formation of this oxide, along with the formation of TiO2 and ZrO2, passivates the surface further, which is more than the case in TiZr alloy [1,2,40].

Current recorded at −0.1 V for (1) TiZr alloy and (2) TiZrTa alloy exposed to NaCl solutions for 30 min.
Figure 4 shows the trends for (1) TiZr alloy and (2) TiZrTa alloy after their exposure to 3.5% NaCl solution for 3 days. The currents increased significantly in the first 30 s due to surface integration caused by the immersion of the alloys for 3 days. Here, the recorded currents for TiZr alloy show an increase followed by a decrease, with an increase in overall currents until the end of the run. The measured current for TiZrTa alloy decreased significantly with a gradual increase over time. This confirms the efficiency of adding Ta to TiZr alloy in reducing absolute current values, indicating a large passivation of the alloy by Ta. After 3 days of immersion in the solution (Figure 3), the current values decreased from ∼750 to ∼300 µA/cm2 in the case of TiZr alloy and from ∼450 to ∼150 µA/cm2 in the case of TiZrTa alloy. This is because of the formation of a thick layer of TiO2 and ZrO2 in the case of TiZr alloy, and TiO2, ZrO2, and TaO2 for TiZrTa alloy due to the long period of exposure before applying the active potential, −0.1 V [2,40].

Current recorded at −0.1 V for (1) TiZr alloy and (2) TiZrTa exposed to NaCl solutions for 3 days.
3.3 Surface investigation
To support corrosion experiments, the surfaces of TiZr and TiZrTa alloys were investigated using SEM after being exposed to 3.5% NaCl solutions for 3 days before stepping a potential of −0.1 V for 30 min. EDS was used to analyze the compositions of the layer(s) formed on those surfaces under the same conditions. Figure 5 presents the SEM image and EDS profile for the TiZr alloy surface following the experiments shown in Figure 4, curve 1. It clearly visualized that the surface is homogeneous and does not have shallow or deep pits. The EDS profile analysis shows that the surface contains 68.03 wt% Ti, 21.78 wt% O, and 10.19 wt% Zr. The presence of a lower percentage of Ti than its original percent, 88%, indicates that the alloy surface is covered with a film that reduces the percentage of Ti. Also, the presence of a high percentage of O, 21.78, and 10.19% Zr gives an indication that the surface of the alloy is covered with TiO2 and ZrO2 layer(s). The presence of such kind of oxides increases its corrosion resistance in the chloride solution.

The SEM image and EDS analysis for the TiZr alloy surface after performing the experiments are shown in Figure 4, curve 1.
Figure 6 shows the SEM image and EDS analysis of the TiZrTa alloy surface following the experiments shown in Figure 4, curve 2. The SEM image of Figure 6 shows homogeneous surfaces with no pits. This means that a top layer formed on the surface while the alloy was immersed in the test solution. Figure 6 shows the EDS profile of the same surface, which has 64.45 wt% Ti, 24.35 wt% O, 8.86 wt% Zr, and 2.34% Ta. Again, the presence of a lower percentage of Ti, even less than the detected percentage for TiZr alloy than its original percent (88% as a starting wt%) and a high percentage of O within the surface of the alloy indicates that some oxide layer(s) cover the alloy surface. The detected weight percentages of Ti, O, Zr, and Ta indicate that the possible existing oxides on the TiZrTa alloy surface are TiO2, ZrO2, and TaO2. The presence of such oxides on the surface of the alloy reveals higher corrosion passivation of the TiZrTa alloy as compared to TiZr alloy and confirms that the presence of Ta increases the corrosion passivation.

The SEM image and EDS analysis for the TiZrTa alloy surface after performing the experiments are shown in Figure 4, curve 2.
3.4 EIS
EIS has been reported to provide an important explanation for investigating the relationship between the material’s interface and the test electrolyte [36,37,38,39,40,41,42]. The Nyquist plots for (1) TiZr alloy and (2) TiZrTa alloy after their exposure to the NaCl solutions for 30 min are shown in Figure 7. Also, the Nyquist plots were obtained for the same two alloys after being exposed to the solution for 3 days, as depicted in Figure 8. The obtained impedance results were matched to the best circuit , as shown in Figure 9. This circuit, which has varied impedance parameters, has been successfully used in numerous studies [2,40,43,44,45,46,47]. The elements of this model are defined as R S is the solution resistance, Q 1 and Q 2 are constant phase elements, and R P1 and R P2 are polarization resistances. All these impedance variables with their values are listed in Table 2.
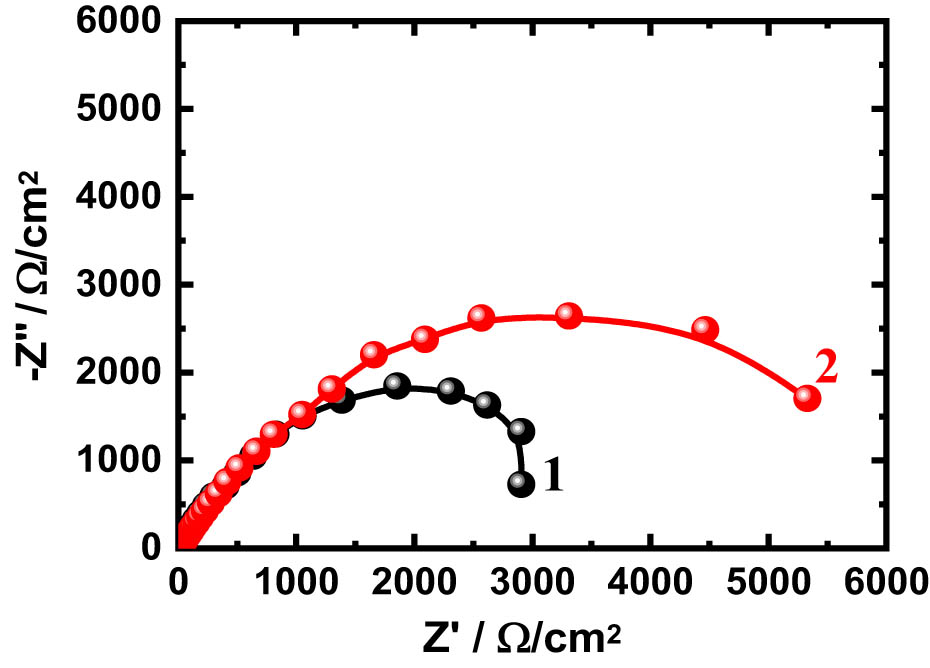
Nyquist plots for the (1) TiZr alloy and (2) TiZrTa alloy after their exposure to the NaCl solutions for 30 min.

Nyquist plots for the (1) TiZr alloy and (2) TiZrTa alloy after their exposure to the NaCl solutions for 3 days.

Circuits used to fit EIS measurements.
EIS parameters obtained from the fitted EIS data
| Alloy/time | R S (Ω/cm2) | Q 1 | R P1 (Ω/cm2) | Q 2 | R P2 (Ω/cm2) | ||
|---|---|---|---|---|---|---|---|
| Y Q1 (F/cm2) | n 1 | Y Q2 (F/cm2) | n 2 | ||||
| TiZr alloy/30 min | 42.4 | 0.00295 | 0.92 | 791 | 0.002864 | 0.89 | 1,089 |
| TiZrTa alloy/30 min | 48.9 | 0.00054 | 1.0 | 1,407 | 0.00216 | 0.77 | 3,702 |
| TiZr alloy/3 days | 49.7 | 0.00199 | 1.0 | 1,487 | 0.003765 | 0.77 | 1,547 |
| TiZrTa alloy/3 days | 52.1 | 0.00035 | 1.0 | 1,910 | 0.000988 | 0.77 | 4,037 |
The Nyquist plots of Figure 7 exhibited one semicircle, whose diameter increased in the presence of Ta. However, the diameter obtained for TiZr is small and increases with Ta addition. This indicates that the TiZrTa alloy has improved corrosion passivation, which was confirmed by the high values of R S, R P1, and R P2 when compared to TiZr alloy. Q 1, Y Q1, and its “n” component represent a double-layer capacitor because the value of “n” is nearly “1.” The lower value of Y Q1 for TiZrTa alloy as compared to TiZr alloy confirms that 4% Ta significantly increases the surface corrosion passivation. This was further evidenced by the presence of Q 2, which acts as a second double-layer capacitor with small pores.
Prolonging the exposure time to 3 days as shown in Figure 8 increased the size of the diameter of the semicircles obtained for TiZr alloy and TiZrTa alloy. This indicates that the corrosion passivation for both alloys improved over time, owing to the possibility for the alloys to form a protective layer, mostly oxide, which thickened with increasing immersion time. The coverage of the surfaces with this oxide(s) minimizes the ability of the corrosive chloride ions to attack the surface and thus reduces the corrosion of the alloys. Prolonging the immersion time from 30 min to 3 days highly increases the values of all resistances for both TiZr alloy and TiZrTa alloy and confirm that TiZrTa alloy has higher resistances than those obtained for TiZr alloy. As aforementioned, the presence and decrease of the values of Q 1 and Q 2, which act as double-layer capacitors, enhance the principle that extending immersion time enhances corrosion prevention. The presence of Ta in the alloy that was immersed for 3 days in NaCl solution further increases the passivation of the alloy, as confirmed by the wider semicircle (Figure 8), higher values of resistances, and lower values of the CPEs.
To support the obtained results by Nyquist plots, the Bode impedance, |Z|, and Bode phase angle degree, Φ, were plotted versus the frequency. Figure 10 shows the Bode plots for the change of |Z| with frequency, which was measured for (1) TiZr alloy and (2) TiZrTa alloy in NaCl solutions after (a) 30 min and (b) 3 days, respectively. It is known that the values of |Z| give important information on the corrosion of the alloys under investigation. The plots of Figure 10 show that the higher values of |Z| are recorded for TiZrTa alloy, whether the immersion time is 30 min or 3 days. This indicates that alloying TiZr alloy with 4% Ta passivates the corrosion of the alloy even after 3 days of its exposure to NaCl solution before measurement.

Bode impedance |Z| for (1) TiZr and (2) TiZrTa alloys after exposure to NaCl solutions for (a) 30 min and (b) 3 days.
The Bode Φ plots for the (1) TiZr alloy and (2) TiZrTa one immersed in NaCl solutions for (a) 30 min and (b) 3 days immersion are shown in Figure 11. The maximum value of Φ is always recorded for TiZrTa alloy, which confirms that the presence of Ta magnifies the resistance of TiZr alloy to corrosion both after 30-min and 3-day exposure to NaCl solution. The impedance results of both Nyquist and Bode plots thus reveal that Ta has the ability to suppress the corrosion of TiZr alloy and that the prolonging of the time of the exposure period has an additional impact on decreasing the corrosion and increasing the passivation of the test Ti-base alloy.

Bode F plots for (1) TiZr and (2) TiZrTa alloys after exposure to NaCl solutions for (a) 30 min and (b) 3 days.
4 Conclusions
Two Ti alloys, Ti–12% Zr and Ti–12% Zr–4% Ta, were produced using the powder metallurgy technique. The corrosion and passivation behavior of these alloys were reported after 30 min and 3 days of exposure to 3.5% NaCl solution. Various techniques such as CPP, chronoamperometric current, and EIS were employed as electrochemical methods. After 3 days of exposure to NaCl solution, the surfaces were evaluated using SEM and EDS. The polarization, CPP, readings revealed that the addition of Ta to TiZr alloy suppresses the corrosion reactions via reducing the corrosion current and increases the corrosion resistance, regardless of immersion time (30 min or 3 days). The alloys were immersed for 30 min with an active anodic potential of −0.1 V. The current was measured after 3 days. Ta lowers the overall currents by increasing the corrosion resistance. SEM images and EDS profiles of the tested surfaces revealed homogenous surfaces without indication of the presence of pits. Nyquist and Bode plots confirmed CPP and chronoamperometric current experiments, which showed that the Ti alloy has increased resistances, solution, and polarization in the presence of Ta. All results also indicated that the corrosion passivation increases when prolonging the time of exposure to 3 days by thickening the formed oxides of TiO2, ZrO2, and TiO2 on the alloy’s top surface.
-
Funding information: This work was financially supported by the Researchers Supporting Project Number (RSP2024R33), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: The author confirms the sole responsibility for the conception of the study, presented results, and manuscript preparation.
-
Conflict of interest: The author states no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available within the manuscript.
References
[1] Alharbi HF, Bahri YA, Sherif ESM. Influence of zirconium on the corrosion passivation of titanium in simulated body fluid. Crystals. 2021;11:1391. 10.3390/cryst11111391.Suche in Google Scholar
[2] Sherif ESM, Bahri YA, Alharbi HF, Ijaz MF, Alnaser IA. Influence of tantalum addition on the corrosion passivation of titanium-zirconium alloy in simulated body fluid. Materials. 2022;15:8812. 10.3390/ma15248812.Suche in Google Scholar PubMed PubMed Central
[3] Aghdam MM, Morsali SM. Effects of manufacturing environments on the residual stresses in a SiC/Ti metal-matrix composite. J Sci Eng Compos Mater. 2017;24(6):817–24. 10.1515/secm-2015-0089.Suche in Google Scholar
[4] Sherif ESM. A comparative study on the corrosion of pure titanium and titanium-12% zirconium alloy after different exposure periods of time in sodium chloride solution. AIP Advances. 2024;14(3):035314. 10.1063/5.0192701.Suche in Google Scholar
[5] Abdel-Hady M, Fuwa H, Hinoshita K, Kimura H, Shinzato Y, Morinaga M. Phase stability change with Zr content in β-type Ti–Nb alloys. Scr Mater. 2007;57(11):1000–3.10.1016/j.scriptamat.2007.08.003Suche in Google Scholar
[6] Sass JO, Sellin ML, Kauertz E, Johannsen J, Weinmann M, Stenzel M, et al. Advanced Ti–Nb–Ta alloys for bone implants with improved functionality. J Funct Biomater. 2024;15(2):46. 10.3390/jfb15020046.Suche in Google Scholar PubMed PubMed Central
[7] Li Y, Yang C, Zhao H, Qu Y, Li X, Li Y. New developments of Ti-based alloys for biomedical applications. Materials. 2014;7:1709–800.10.3390/ma7031709Suche in Google Scholar PubMed PubMed Central
[8] Sherif ESM, Ragab SA, Abdo HS. Role of vanadium additions on the corrosion mitigation of Ti-6Al-xV alloy in simulated body fluid. Metals. 2020;10:903. 10.3390/met10070903.Suche in Google Scholar
[9] Dai N, Zhang LZ, Zhang J, Chen Q, Wu M. Corrosion behavior of selective laser melted Ti-6Al-4 V alloy in NaCl solution. Corros Sci. 2016;102:484–9.10.1016/j.corsci.2015.10.041Suche in Google Scholar
[10] Chong Y, Bhattacharjee T, Tsuji N. Bi-lamellar microstructure in Ti–6Al–4V: Microstructure evolution and mechanical properties. Mater Sci Eng A. 2019;762:138077.10.1016/j.msea.2019.138077Suche in Google Scholar
[11] Dai N, Zhang LZ, Zhang J, Chen Q, Wu M, Yang C. Distinction in corrosion resistance of selective laser melted Ti-6Al-4Valloy on different planes. Corros Sci. 2016;111:703–10.10.1016/j.corsci.2016.06.009Suche in Google Scholar
[12] Abdo HS, Sherif ESM, El-Serehy HA. Manufacturing of Ti-6%Al and Ti-6%Al-4%V alloys and their corrosion in sodium chloride solutions. Crystals. 2020;10:181. 10.3390/cryst10030181.Suche in Google Scholar
[13] Krawiec H, Vignal V, Loch J, Erazmus-Vignal P. Influence of plastic deformation on the microstructure and corrosion behaviour of Ti–10Mo–4Zr and Ti–6Al–4V alloys in the Ringer’s solution at 37°C. Corros Sci. 2015;96:160–70.10.1016/j.corsci.2015.04.006Suche in Google Scholar
[14] Kawahara M, Kato-Negishi M. Link between aluminum and the pathogenesis of alzheimer’s disease: the integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimer’s Dis. 2011;2011:276393.10.4061/2011/276393Suche in Google Scholar PubMed PubMed Central
[15] Kandimalla R, Vallamkondu J, Corigat EB, Gill KD. Understanding aspects of aluminum exposure in alzheimer’s disease development. Brain Pathol. 2016;26:139–54.10.1111/bpa.12333Suche in Google Scholar PubMed PubMed Central
[16] Correa DRN, Kuroda PAB, Lourenço ML, Buzalaf MAR, Grandini CR. Adjustment of the microstructure and selected mechanical properties of biomedical Ti-15Zr-Mo alloys through oxygen doping. J Alloys Compd. 2019;775:158–67.10.1016/j.jallcom.2018.10.105Suche in Google Scholar
[17] da Silva MR, Gargarella P, Plaine AH, Contieri RJ, Pauly S, Kühn U, et al. Influence of the deformation rate on phase stability and mechanical properties of a Ti–29Nb–13Ta–4.6 Zr–xO alloy analyzed by in situ high-energy X-ray diffraction during compression tests. J Mater Res. 2020;35(14):1777–89.10.1557/jmr.2020.150Suche in Google Scholar
[18] Wu CT, Chang HT, Wu CY, Chen SW, Huang SY, Huang M, et al. Machine learning recommends affordable new Ti alloy with bone-like modulus. Materialstoday. 2020;34:41–50.10.1016/j.mattod.2019.08.008Suche in Google Scholar
[19] Bernhard N, Berner S, De Wild M, Wieland M. The binary TiZr alloy – A newly developed Ti alloy for use in dental implants. Forum Implantol. 2009;5:30–9.Suche in Google Scholar
[20] Grandin HM, Berner S, Dard M. A review of titanium zirconium (TiZr) alloys for use in endosseous dental implants. Materials. 2012;5:1348–60.10.3390/ma5081348Suche in Google Scholar
[21] Lin J, Ozan S, Li Y, Ping D, Tong X, Li G, Wen C. Novel Ti-Ta-Hf-Zr alloys with promising mechanical properties for prospective stent applications. Sci Rep. 2016;6:1–11.10.1038/srep37901Suche in Google Scholar PubMed PubMed Central
[22] Ijaz MF, Alharbi HF, Bahri YA, Sherif ESM. Alloy design and fabrication of duplex titanium-based alloys by spark plasma sintering for biomedical implant applications. Mater. 2022;15:8562.10.3390/ma15238562Suche in Google Scholar PubMed PubMed Central
[23] Samuel SG. Titanium – The material of choice? Periodontol 2000. Jun 1998;17:7–21. 10.1111/j.1600-0757.1998.tb00119.x.Suche in Google Scholar PubMed
[24] Vannoort R. Titanium – The implant material of today. J Mater Sci. 1987;22:3801–11.10.1007/BF01133326Suche in Google Scholar
[25] Williams DF. Titanium for medical applications. In: Brunette D, Tengvall P, Textor M, Thomson P, editors. Titanium in Medicine. Berlin, Germany: Springer; 2001. p. 14–24.Suche in Google Scholar
[26] Hu QM, Hao SJ, Yang YL, Johansson R, Vitos B, L. Phase stability and elastic modulus of Ti alloys containing Nb, Zr, and/or Sn from first-principles calculations. Appl Phys Lett. 2008;93:121902.10.1063/1.2988270Suche in Google Scholar
[27] Kim J, Park HW. Influence of a large pulsed electron beam (LPEB) on the corrosion resistance of Ti6Al7Nb alloys. Corros Sci. 2015;96:153–60.10.1016/j.corsci.2014.10.008Suche in Google Scholar
[28] Tahara M, Kim HY, Hosoda H, Miyazaki S. Cyclic deformation behavior of a Ti-26 at.% Nb alloy. Acta Mater. 2009;57:2461–9.10.1016/j.actamat.2009.01.037Suche in Google Scholar
[29] Stenlund P, Omar O, Brohede U, Norgren S, Norlindh B, Johansson A, et al. Bone response to a novel Ti-Ta-Nb-Zr alloy. Acta Biomater. 2015;20:165–75.10.1016/j.actbio.2015.03.038Suche in Google Scholar PubMed
[30] Kurtz MA, Wessinger AC, Mace A, Moreno-Reyes A, Gilbert JL. Additively manufactured Ti-29Nb-21Zr shows improved oxide polarization resistance versus Ti-6Al-4V in inflammatory simulating solution. J Biomed Mater Res. 2023;111:1538–53.10.1002/jbm.a.37552Suche in Google Scholar PubMed
[31] Mohan L, Anandan C, Rajendran N. Electrochemical behavior and effect of heat treatment on morphology, crystalline structure of self-organized TiO2 nanotube arrays on Ti–6Al–7Nb for biomedical applications. Mater Sci Eng C. 2015;50:394–401.10.1016/j.msec.2015.02.013Suche in Google Scholar PubMed
[32] Fojt J, Joska L, Malek J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros Sci. 2013;71:78–83.10.1016/j.corsci.2013.03.007Suche in Google Scholar
[33] Ammar HR, Sherif ESM, Sivasankaran S, Almufadi FA, Mekky AbH. Developing improved corrosion-resistant AA5083—BN/WC composites for tribological applications. Mater. 2023;16:1663. 10.3390/ma16041663.Suche in Google Scholar PubMed PubMed Central
[34] Alshammri GA, Fathy N, Al-Shomar SM, Alshammari AH, Sherif ESM, Ramadan M. Effect of Al2O3 and NiO Nanoparticle additions on the structure and corrosion behavior of Sn – 4% Zn alloy coating carbon steel. Sustainability. 2023;15:2511. 10.3390/su15032511.Suche in Google Scholar
[35] Sivasankaran S, Sherif ESM, Ammar HR, Alaboodi AS, Mekky AbH. Influence of oxide dispersions (Al2O3, TiO2, and Y2O3) in CrFeCuMnNi high-entropy alloy on microstructural changes and corrosion resistance. Crystals. 2023;13:605. 10.3390/cryst13040605.Suche in Google Scholar
[36] Alharthi N, Sherif ESM, Abdo HS, Alharbi HF, Misiolek WZ. Effect of extrusion welding locations on the corrosion of AM30 alloy extrudate. J Mater Res Technol. 2019;8:2280–9. 10.1016/j.jmrt.2019.03.008.Suche in Google Scholar
[37] Sherif ESM, Park SM. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim Acta. 2006;51:1313–21. 10.1016/j.electacta.2005.06.018.Suche in Google Scholar
[38] Sherif ESM, Potgieter JH, Comins JD, Cornish L, Olubambi PA, Machio CN. The beneficial effect of ruthenium additions on the passivation of duplex stainless-steel corrosion in sodium chloride solutions. Corros Sci. 2009;51:1364–71.10.1016/j.corsci.2009.03.022Suche in Google Scholar
[39] Latief FH, Sherif ESM, Almajid AA, Junaedi H. Fabrication of exfoliated graphite nanoplatelets-reinforced aluminum composites and evaluating their mechanical properties and corrosion behavior. J Anal Appl Pyrol. 2011;92:485–92.10.1016/j.jaap.2011.09.003Suche in Google Scholar
[40] Sherif ESM, Bahri YA, Alharbi HF, Ijaz MF. Corrosion passivation in simulated body fluid of Ti-Zr-Ta-xSn alloys as biomedical materials. Materials. 2023;16:4603. 10.3390/ma16134603.Suche in Google Scholar PubMed PubMed Central
[41] Afzali P, Ghomashchi R, Oskouei RH. On the corrosion Behaviour of low modulus titanium alloys for medical implant applications: A review. Metals. 2019;9:878.10.3390/met9080878Suche in Google Scholar
[42] Sherif ESM, Abdo HS, Latief FH, Alharthi NH, Zein El Abedin S. Fabrication of Ti–Al–Cu new alloys by inductive sintering, characterization, and corrosion evaluation. J Mater Res Technol. 2019;8:4302–11. 10.1016/j.jmrt.2019.07.040.Suche in Google Scholar
[43] Alam MA, Samad UA, Seikh A, Mohammed JA, Al-Zahrani SM, Sherif ESM. Development and characterization of PA 450 and PA 3282 epoxy coatings as anti-corrosion materials for offshore applications. Mater. 2022;15:2562. 10.3390/ma15072562.Suche in Google Scholar PubMed PubMed Central
[44] Alam MA, Samad UA, Anis A, Sherif ESM, Abdo HS, Al-Zahrani SM. The effect of zirconia nanoparticles on thermal, mechanical, and corrosion behavior of nanocomposite epoxy coatings on steel substrates. Mater. 2023;16:4813. 10.3390/ma16134813.Suche in Google Scholar PubMed PubMed Central
[45] Samad UA, Alam MA, Seikh AH, Mohammed JA, Al-Zahrani SM, Sherif ESM. Corrosion resistance performance of epoxy coatings incorporated with unmilled micro aluminium pigments. Crystals. 2023;13:558. 10.3390/cryst13040558.Suche in Google Scholar
[46] Alam MA, Samad UA, Sherif ESM, Seikh AH, Al-Zahrani SM, Alharthi NH, et al. Synergistic effect of Ag and ZnO nanoparticles on polyaniline incorporated epoxy/2pack coatings for splash zone applications. J Coat Technol Res. 2019;16:835–45.10.1007/s11998-018-00156-4Suche in Google Scholar
[47] Alam MA, Sherif ESM, Al-Zahrani SM. Mechanical properties and corrosion behavior of different coatings fabricated by diglycidyl ether of bisphenol-a epoxy resin and Aradur®-3282 curing agent. Int J Electrochem Sci. 2013;8:8388–400.10.1016/S1452-3981(23)12896-3Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Research on damage evolution mechanisms under compressive and tensile tests of plain weave SiCf/SiC composites using in situ X-ray CT
- Structural optimization of trays in bolt support systems
- Continuum percolation of the realistic nonuniform ITZs in 3D polyphase concrete systems involving the aggregate shape and size differentiation
- Multiscale water diffusivity prediction of plain woven composites considering void defects
- The application of epoxy resin polymers by laser induction technologies
- Analysis of water absorption on the efficiency of bonded composite repair of aluminum alloy panels
- Experimental research on bonding mechanical performance of the interface between cementitious layers
- A study on the effect of microspheres on the freeze–thaw resistance of EPS concrete
- Influence of Ti2SnC content on arc erosion resistance in Ag–Ti2SnC composites
- Cement-based composites with ZIF-8@TiO2-coated activated carbon fiber for efficient removal of formaldehyde
- Microstructure and chloride transport of aeolian sand concrete under long-term natural immersion
- Simulation study on basic road performance and modification mechanism of red mud modified asphalt mixture
- Extraction and characterization of nano-silica particles to enhance mechanical properties of general-purpose unsaturated polyester resin
- Roles of corn starch and gellan gum in changing of unconfined compressive strength of Shanghai alluvial clay
- A review on innovative approaches to expansive soil stabilization: Focussing on EPS beads, sand, and jute
- Experimental investigation of the performances of thick CFRP, GFRP, and KFRP composite plates under ballistic impact
- Preparation and characterization of titanium gypsum artificial aggregate
- Characteristics of bulletproof plate made from silkworm cocoon waste: Hybrid silkworm cocoon waste-reinforced epoxy/UHMWPE composite
- Experimental research on influence of curing environment on mechanical properties of coal gangue cementation
- Multi-objective optimization of machining variables for wire-EDM of LM6/fly ash composite materials using grey relational analysis
- Synthesis and characterization of Ag@Ni co-axial nanocables and their fluorescent and catalytic properties
- Beneficial effect of 4% Ta addition on the corrosion mitigation of Ti–12% Zr alloy after different immersion times in 3.5% NaCl solutions
- Study on electrical conductive mechanism of mayenite derivative C12A7:C
- Fast prediction of concrete equivalent modulus based on the random aggregate model and image quadtree SBFEM
- Research on uniaxial compression performance and constitutive relationship of RBP-UHPC after high temperature
- Experimental analysis of frost resistance and failure models in engineered cementitious composites with the integration of Yellow River sand
- Influence of tin additions on the corrosion passivation of TiZrTa alloy in sodium chloride solutions
- Microstructure and finite element analysis of Mo2C-diamond/Cu composites by spark plasma sintering
- Low-velocity impact response optimization of the foam-cored sandwich panels with CFRP skins for electric aircraft fuselage skin application
- Research on the carbonation resistance and improvement technology of fully recycled aggregate concrete
- Study on the basic properties of iron tailings powder-desulfurization ash mine filling cementitious material
- Preparation and mechanical properties of the 2.5D carbon glass hybrid woven composite materials
- Improvement on interfacial properties of CuW and CuCr bimetallic materials with high-entropy alloy interlayers via infiltration method
- Investigation properties of ultra-high performance concrete incorporating pond ash
- Effects of binder paste-to-aggregate ratio and polypropylene fiber content on the performance of high-flowability steel fiber-reinforced concrete for slab/deck overlays
- Interfacial bonding characteristics of multi-walled carbon nanotube/ultralight foamed concrete
- Classification of damping properties of fabric-reinforced flat beam-like specimens by a degree of ondulation implying a mesomechanic kinematic
- Influence of mica paper surface modification on the water resistance of mica paper/organic silicone resin composites
- Impact of cooling methods on the corrosion behavior of AA6063 aluminum alloy in a chloride solution
- Wear mechanism analysis of internal chip removal drill for CFRP drilling
- Investigation on acoustic properties of metal hollow sphere A356 aluminum matrix composites
- Uniaxial compression stress–strain relationship of fully aeolian sand concrete at low temperatures
- Experimental study on the influence of aggregate morphology on concrete interfacial properties
- Intelligent sportswear design: Innovative applications based on conjugated nanomaterials
- Research on the equivalent stretching mechanical properties of Nomex honeycomb core considering the effect of resin coating
- Numerical analysis and experimental research on the vibration performance of concrete vibration table in PC components
- Assessment of mechanical and biological properties of Ti–31Nb–7.7Zr alloy for spinal surgery implant
- Theoretical research on load distribution of composite pre-tightened teeth connections embedded with soft layers
- Coupling design features of material surface treatment for ceramic products based on ResNet
- Optimizing superelastic shape-memory alloy fibers for enhancing the pullout performance in engineered cementitious composites
- Multi-scale finite element simulation of needle-punched quartz fiber reinforced composites
- Thermo-mechanical coupling behavior of needle-punched carbon/carbon composites
- Influence of composite material laying parameters on the load-carrying capacity of type IV hydrogen storage vessel
- Review Articles
- Effect of carbon nanotubes on mechanical properties of aluminum matrix composites: A review
- On in-house developed feedstock filament of polymer and polymeric composites and their recycling process – A comprehensive review
- Research progress on freeze–thaw constitutive model of concrete based on damage mechanics
- A bibliometric and content analysis of research trends in paver blocks: Mapping the scientific landscape
- Bibliometric analysis of stone column research trends: A Web of Science perspective
Artikel in diesem Heft
- Regular Articles
- Research on damage evolution mechanisms under compressive and tensile tests of plain weave SiCf/SiC composites using in situ X-ray CT
- Structural optimization of trays in bolt support systems
- Continuum percolation of the realistic nonuniform ITZs in 3D polyphase concrete systems involving the aggregate shape and size differentiation
- Multiscale water diffusivity prediction of plain woven composites considering void defects
- The application of epoxy resin polymers by laser induction technologies
- Analysis of water absorption on the efficiency of bonded composite repair of aluminum alloy panels
- Experimental research on bonding mechanical performance of the interface between cementitious layers
- A study on the effect of microspheres on the freeze–thaw resistance of EPS concrete
- Influence of Ti2SnC content on arc erosion resistance in Ag–Ti2SnC composites
- Cement-based composites with ZIF-8@TiO2-coated activated carbon fiber for efficient removal of formaldehyde
- Microstructure and chloride transport of aeolian sand concrete under long-term natural immersion
- Simulation study on basic road performance and modification mechanism of red mud modified asphalt mixture
- Extraction and characterization of nano-silica particles to enhance mechanical properties of general-purpose unsaturated polyester resin
- Roles of corn starch and gellan gum in changing of unconfined compressive strength of Shanghai alluvial clay
- A review on innovative approaches to expansive soil stabilization: Focussing on EPS beads, sand, and jute
- Experimental investigation of the performances of thick CFRP, GFRP, and KFRP composite plates under ballistic impact
- Preparation and characterization of titanium gypsum artificial aggregate
- Characteristics of bulletproof plate made from silkworm cocoon waste: Hybrid silkworm cocoon waste-reinforced epoxy/UHMWPE composite
- Experimental research on influence of curing environment on mechanical properties of coal gangue cementation
- Multi-objective optimization of machining variables for wire-EDM of LM6/fly ash composite materials using grey relational analysis
- Synthesis and characterization of Ag@Ni co-axial nanocables and their fluorescent and catalytic properties
- Beneficial effect of 4% Ta addition on the corrosion mitigation of Ti–12% Zr alloy after different immersion times in 3.5% NaCl solutions
- Study on electrical conductive mechanism of mayenite derivative C12A7:C
- Fast prediction of concrete equivalent modulus based on the random aggregate model and image quadtree SBFEM
- Research on uniaxial compression performance and constitutive relationship of RBP-UHPC after high temperature
- Experimental analysis of frost resistance and failure models in engineered cementitious composites with the integration of Yellow River sand
- Influence of tin additions on the corrosion passivation of TiZrTa alloy in sodium chloride solutions
- Microstructure and finite element analysis of Mo2C-diamond/Cu composites by spark plasma sintering
- Low-velocity impact response optimization of the foam-cored sandwich panels with CFRP skins for electric aircraft fuselage skin application
- Research on the carbonation resistance and improvement technology of fully recycled aggregate concrete
- Study on the basic properties of iron tailings powder-desulfurization ash mine filling cementitious material
- Preparation and mechanical properties of the 2.5D carbon glass hybrid woven composite materials
- Improvement on interfacial properties of CuW and CuCr bimetallic materials with high-entropy alloy interlayers via infiltration method
- Investigation properties of ultra-high performance concrete incorporating pond ash
- Effects of binder paste-to-aggregate ratio and polypropylene fiber content on the performance of high-flowability steel fiber-reinforced concrete for slab/deck overlays
- Interfacial bonding characteristics of multi-walled carbon nanotube/ultralight foamed concrete
- Classification of damping properties of fabric-reinforced flat beam-like specimens by a degree of ondulation implying a mesomechanic kinematic
- Influence of mica paper surface modification on the water resistance of mica paper/organic silicone resin composites
- Impact of cooling methods on the corrosion behavior of AA6063 aluminum alloy in a chloride solution
- Wear mechanism analysis of internal chip removal drill for CFRP drilling
- Investigation on acoustic properties of metal hollow sphere A356 aluminum matrix composites
- Uniaxial compression stress–strain relationship of fully aeolian sand concrete at low temperatures
- Experimental study on the influence of aggregate morphology on concrete interfacial properties
- Intelligent sportswear design: Innovative applications based on conjugated nanomaterials
- Research on the equivalent stretching mechanical properties of Nomex honeycomb core considering the effect of resin coating
- Numerical analysis and experimental research on the vibration performance of concrete vibration table in PC components
- Assessment of mechanical and biological properties of Ti–31Nb–7.7Zr alloy for spinal surgery implant
- Theoretical research on load distribution of composite pre-tightened teeth connections embedded with soft layers
- Coupling design features of material surface treatment for ceramic products based on ResNet
- Optimizing superelastic shape-memory alloy fibers for enhancing the pullout performance in engineered cementitious composites
- Multi-scale finite element simulation of needle-punched quartz fiber reinforced composites
- Thermo-mechanical coupling behavior of needle-punched carbon/carbon composites
- Influence of composite material laying parameters on the load-carrying capacity of type IV hydrogen storage vessel
- Review Articles
- Effect of carbon nanotubes on mechanical properties of aluminum matrix composites: A review
- On in-house developed feedstock filament of polymer and polymeric composites and their recycling process – A comprehensive review
- Research progress on freeze–thaw constitutive model of concrete based on damage mechanics
- A bibliometric and content analysis of research trends in paver blocks: Mapping the scientific landscape
- Bibliometric analysis of stone column research trends: A Web of Science perspective

